SUMMARY
Cercarial dermatitis (swimmer's itch) is a condition caused by infective larvae (cercariae) of a species-rich group of mammalian and avian schistosomes. Over the last decade, it has been reported in areas that previously had few or no cases of dermatitis and is thus considered an emerging disease. It is obvious that avian schistosomes are responsible for the majority of reported dermatitis outbreaks around the world, and thus they are the primary focus of this review. Although they infect humans, they do not mature and usually die in the skin. Experimental infections of avian schistosomes in mice show that in previously exposed hosts, there is a strong skin immune reaction that kills the schistosome. However, penetration of larvae into naive mice can result in temporary migration from the skin. This is of particular interest because the worms are able to migrate to different organs, for example, the lungs in the case of visceral schistosomes and the central nervous system in the case of nasal schistosomes. The risk of such migration and accompanying disorders needs to be clarified for humans and animals of interest (e.g., dogs). Herein we compiled the most comprehensive review of the diversity, immunology, and epidemiology of avian schistosomes causing cercarial dermatitis.
INTRODUCTION
Cercarial dermatitis is a condition caused by both mammalian and avian schistosomes (Trematoda: Schistosomatidae). Which of those species is more prevalent in a dermatitis outbreak depends on where you are in the world and how humans and birds/mammals (and, by association, snails) come into contact with a particular type of aquatic environment. The name “cercarial dermatitis” is derived from the term “cercaria,” the last larval stage developing in an aquatic snail. Cercaria is the infective stage that, after leaving the snail, searches for and invades a warm-blooded vertebrate host via skin penetration. Besides the official name, “cercarial dermatitis,” many local terms are used (“sawah itch,” “koganbyo,” etc.), with the most widely used name being “swimmer's itch.”
Schistosome cercariae were disclosed as the causative agent of cercarial dermatitis in the United States in 1928 (1). Since that time, numerous reports of cercarial dermatitis have been documented from different parts of the world. Global economic losses due to outbreaks of cercarial dermatitis are not known, as there is no systematic method of reporting either the number of cases or incurred economic losses in terms of recreation or person work hours. Furthermore, what data do exist that estimate local costs are usually not available to the public domain, but it is accepted that outbreaks can have considerable impacts on local, tourism-based economies in the areas of recreational lakes (2). For example, in the recreational area of Naroch Lake (Belarus), 4,737 cases of cercarial dermatitis were recorded between 1995 and 2006 (3). In addition, cercarial dermatitis may represent a debilitating occupational disease among rice farmers (4) and may incur costs in terms of lost person work hours. Older reports refer to 75% or more of the population experiencing the characteristic symptoms of “koganbyo” in the areas of Japan where the disease is most highly endemic (5). Recent reviews (6–9) agree that in some regions cercarial dermatitis has appeared as a new problem, either because the dermatitis was previously unknown (e.g., the U.S. Southwest and Chile) or because the number of reports of outbreaks increased (8, 10, 11). Consequently, cercarial dermatitis is now regarded as an emerging disease. Besides human schistosomes (Schistosoma spp.), no animal (e.g., avian) schistosomes have any other presently known pathogenic effects on humans. Thus, the use of animal models to study the potential risk of animal (avian) schistosomes to human health is invaluable.
The last decade has revealed diverse avian schistosome species and biology, as well as the snails that host them. These discoveries have outpaced the equally essential host-parasite biological, immunological, pathological, and epidemiological studies of species diversity in terms of incorporating the results of such studies into the current known diversity of schistosomes. Such studies are difficult and time-consuming, and consequently, only a few species have been adapted to experimental conditions. Nevertheless, such studies are crucial to understanding the current and future roles that these species might play in the frequency and distribution of cercarial dermatitis, as well as understanding how to break the life cycle to prevent outbreaks. What has been documented, and is detailed in the following sections, points to an understanding of the avian schistosome-host relationships and thus offers the foundation on which future studies will be modeled.
DIVERSITY OF SCHISTOSOMES CAUSING DERMATITIS
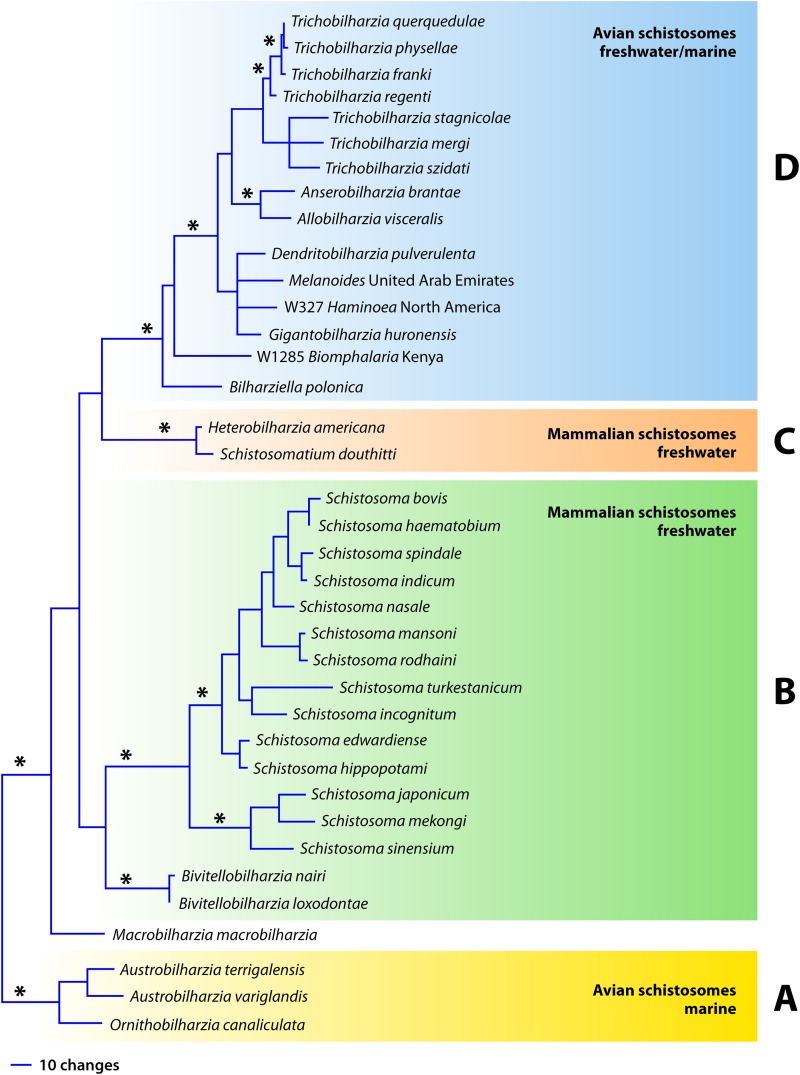
Considering only the named species in the literature, there are 4 schistosome genera from mammals and 10 from birds, with about 30 described species from mammals and about 67 from birds (12). The total is close to 100 species, with ∼70% of them being avian schistosomes distributed around the world that may initiate cercarial dermatitis. The role of some of the species of avian schistosomes as dermatitis agents has not been studied sufficiently, as they are not often found in areas where people most commonly are in contact with water and snails. To discuss the distribution and diversity of schistosomes causing dermatitis within the phylogenetic framework of the family Schistosomatidae, we refer to Fig. 1 (12–14).
FIG 1.
Phylogenetic tree showing generic and species positions based on Bayesian analysis of the nuclear ribosomal DNA 28S region (1,200 bp) of Schistosomatidae. Panels A to D refer to the clades discussed in the text. This tree is based on genetic data, not morphological data, and as such, there are more species that have been described morphologically than genetically. Asterisks denote significant posterior probabilities (>0.95).
The basal clade of the family tree (Fig. 1, clade A) comprises the exclusively marine avian schistosomes Austrobilharzia (4 species) and Ornithobilharzia (2 species); the species shown in the tree are those for which there are genetic data. Species of these two genera are associated with outbreaks of dermatitis in shallow marine environments (15, 16). Infection often occurs in people who are swimming, playing in tidal pools, or working, for example, collecting tidal invertebrates in the sand (15, 17–27). Both of these genera have robust, large worms as adults and are common schistosomes of marine birds, particularly gulls. Species of Austrobilharzia are more often implicated as a cause of dermatitis outbreaks (25).
The next main clade includes the remaining schistosomes (Fig. 1, clades B, C, and D). Clades B and C are exclusively freshwater mammalian schistosomes. The largest clade of mammalian schistosomes includes the genus Schistosoma, with ∼25 species (clade B). In particular, three of these species (Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum) cause one of the most devastating helminth diseases in humans, schistosomiasis, affecting about 220 million people, mainly in the tropical and subtropical latitudes around the world (WHO). All but one species (S. mansoni) occur exclusively in the Eastern Hemisphere. These species are not typically implicated in dermatitis outbreaks, yet there is a mild eruption of dermatitis following penetration by all schistosomes (28). Most reported cases of dermatitis caused by the genus Schistosoma are from parasites that infect domesticated work animals, such as cattle and buffalo, mainly in Asia. For example, in countries such as India and Nepal, the species Schistosoma turkestanicum, Schistosoma nasale, Schistosoma indicum, and Schistosoma spindale are often implicated in outbreaks of dermatitis (29–38). This relationship may not be a surprise, as bovids are the definitive host, and the people in these areas depend upon these animals for their livelihood in farming. Additionally, the snail host for the major species causing dermatitis (S. nasale, S. indicum, and S. spindale) is Indoplanorbis exustus, a widespread and abundant snail that is found mainly in Nepal and India, to the exclusion of Biomphalaria and Bulinus, snail hosts for a majority of the African transmitted species of Schistosoma.
The genus Bivitellobilharzia is considered a schistosome of elephants, but it has also been reported from wild rhinoceroses in Nepal (38–41). There are no known reports of cercarial dermatitis in humans from areas inhabited by African elephants (with the Bivitellobilharzia loxodontae schistosome), but in areas where domesticated Asian elephants are used, there have been cases of dermatitis in the mahouts, or elephant handlers, when the elephants are taken for bathing (e.g., in Sri Lanka [40]). In Nepal, Bivitellobilharzia nairi has thus far been found in wild, not domesticated, elephants (38). The snail host remains unknown but is likely a pulmonate snail (42). At least two species of Schistosoma from Biomphalaria snails infect the African hippopotamus, but these species have not been implicated directly in dermatitis outbreaks, despite the presence of humans working on lakeshores where there are hippopotamuses (43–45). Given the prevalence of human schistosomiasis in these areas, however, dermatitis caused by hippopotamus schistosomes may easily go undetected.
The small clade C (Fig. 1) has two species of mammalian schistosomes that, as far as we know, are found only in North America and are not frequently associated with dermatitis outbreaks, though they both produce a skin reaction (46–50). These two species are parasites of lymnaeid snails (often Stagnicola elodes), usually with raccoons and muskrats as mammalian hosts. Schistosomatium douthitti adults inhabit aquatic and semiaquatic rodents in more northern latitudes or at high elevations (48, 51, 52). Heterobilharzia americana has been reported from a wide range of mammalian hosts (rivaling Schistosoma japonicum), including horses, in the southern regions of North America (49, 53, 54).
Perhaps the most remarkable clade of schistosomes responsible for dermatitis is clade D, a large clade of avian schistosomes whose adults are long and threadlike (except Dendritobilharzia and Bilharziella) and that includes both freshwater and marine species. In particular, the genus Trichobilharzia has achieved notoriety as the primary etiological agent for dermatitis outbreaks around the world. The diversity of aquatic environments, host use, morphology, definitive host habitat, and cercarial behavior is unparalleled in any other group of schistosomes, and probably most other groups of trematodes (12, 14). Figure 1 includes a molecular phylogeny of all the known genera of schistosomes except one. Jilinobilharzia has not been reported since the original paper reporting it from the duck Anas crecca in northeastern China; its snail host remains unknown (55). Morphological characteristics and host use suggest that Jilinobilharzia belongs in the large clade of avian schistosomes (Fig. 1, clade D), perhaps even to Trichobilharzia.
At the base of clade D is an unresolved group of avian schistosomes, most of which have been implicated in dermatitis outbreaks and comprise the most diverse range of both bird and snail (9 families) host use (13, 40, 56–66). Current results based on all of the available sequence data in GenBank for the internal transcribed spacer (ITS) region indicate that there are about nine distinct lineages, only two of which are described: Gigantobilharzia and Dendritobilharzia (40, 64–69). Most of the lineages in this part of clade D have one to a few species and have been seen in only a few cases, many related to dermatitis outbreaks (12) (Table 1). Thus far, the literature suggests that species in this clade cause dermatitis in more local areas, whereas Trichobilharzia causes cercarial dermatitis globally. For example, in the San Francisco Bay area (California), one beach in particular has annual cases of dermatitis (64). The prevalence of dermatitis caused by schistosomes from Valvata or Melanoides snails (63, 65, 66) depends on how often people use areas where these snails release cercariae.
TABLE 1.
Summary of general host use of known genera of schistosomes, reflecting current knowledge, habitat in the definitive host, and broad geographic locality
| Genus | Snail host | Mammalian/avian host | Definitive host habitat | Locality | Aquatic habitat | Major areas for outbreaks |
|---|---|---|---|---|---|---|
| Austrobilharzia | Nassariidae, Batillaridae, Littoriniidae, Potamididae | Charadriiformes | Visceral | Global | Marine | Shallow marine areas, tidal pools |
| Ornithobilharzia | Batillaridae | Charadriiformes | Visceral | Global | Marine | Shallow marine areas, tidal pools |
| Macrobilharzia | Unknown | Suliformes (Anhinga) | Visceral | North America, Africa | Unknown | Unknown if causes dermatitis |
| Bivitellobilharzia | Unknown | Elephantidae, Rhinocerotidae | Visceral | Africa, Asia | Freshwater | Probably freshwater rivers |
| Schistosoma | Planorbidae, Lymnaeidae, Pomatiopsidae | Mammalia | Visceral, Nasal | Eurasia, Africa, South America | Freshwater | Mostly eutrophic ponds |
| Heterobilharzia | Lymnaeidae | Mammalia | Visceral | North America | Freshwater | Marshy areas |
| Schistosomatium | Lymnaeidae | Rodentia | Visceral | North America | Freshwater | Marshy areas |
| Bilharziella | Planorbidae | Anseriformes, Gruiformes, Ciconiformes, Podicipediformes | Visceral | Europe | Freshwater | Eutrophic ponds |
| Species isolated from Haminoea | Haminoeidae | Charadriiformes, Pelicaniformes | Visceral | North America | Marine | Shallow marine areas, tidal pools |
| Gigantobilharziaa | Physidae | Passeriformes | Visceral | North America | Freshwater | Marshy areas, usually with cattails |
| Dendritobilharzia | Planorbidae | Anseriformes, Gruiformes, Pelicaniformes, Gaviiformes | Visceral | Global | Freshwater | Unknown if reports of dermatitis |
| Jilinobilharzia | Unknown | Anseriformes (Anatidae) | Visceral | China | Unknown | Unknown if reports of dermatitis |
| Allobilharzia | Unknown | Anseriformes (swans) | Visceral | Northern Hemisphere | Unknown | Unknown if causes dermatitis |
| Anserobilharzia | Planorbidae | Anseriformes (geese) | Visceral | Northern Hemisphere | Freshwater | Eutrophic ponds, reservoirs |
| Trichobilharzia | Lymnaeidae, Physidae | Anseriformes (Anatidae) | Visceral, nasal | Global | Freshwater | Eutrophic ponds, glacial lakes, reservoirs |
Since Gigantobilharzia is not a monophyletic genus, the information listed here is for G. huronensis only.
Species of Trichobilharzia (Fig. 1, clade D) are globally distributed and cause the majority of recreational and occupational reports of dermatitis found in the literature, especially in the temperate latitudes. In North America and Europe, where most of the research has been focused, outbreaks occur in recreational ponds and reservoirs. These outbreaks have been reviewed extensively (7, 8, 70, 71). Species of Trichobilharzia have been reported to cause dermatitis from other areas as well, such as Rwanda-Burundi (72), South Africa (73, 74), New Zealand and Australia (75–79), Malaysia/Indonesia (80–82), Iran (65, 83–86), United Arab Emirates (66), Thailand (87), and China (88, 89) in the Eastern Hemisphere and Argentina (57, 90, 91), Chile (11), and El Salvador (92) in the Western Hemisphere. We are just beginning to better understand the significant disease components for dermatitis as a global problem.
Cercarial dermatitis is also recognized as an occupational hazard in many areas of the world, especially in areas where rice is grown (82, 93). Rice fields are areas where snails, domestic and wild ducks, cattle, and humans seasonally use the water, so the life cycle is maintained consistently (5, 84, 94–104). Species of Trichobilharzia are identified most often, though not exclusively (5). Rice fields are plowed by water buffalo and cattle in areas where Indoplanorbis exustus occurs, and hence, dermatitis may be caused by one of the species of Schistosoma, as noted above. Nonetheless, Trichobilharzia is still by far the most common etiological agent.
MOLLUSCAN AND AVIAN HOST SPECIFICITY
Schistosomes have colonized many families of snails as first intermediate hosts (12, 62). Mammalian schistosomes use 3 families of snails, compared to 15 families used by avian schistosomes, as summarized in Table 1. The majority of schistosome species are transmitted by the pulmonate snail families Physidae, Lymnaeidae, and Planorbidae (10, 70, 105). Interestingly, two snail families contain species (e.g., Biomphalaria and Indoplanorbis in the Planorbidae family and Stagnicola in the Lymnaeidae family) that can host both avian and mammalian schistosomes that cause dermatitis (40, 51, 70, 104, 106).
Previous papers have reviewed the details of host specificity in mammalian schistosomes (Fig. 1, clades B and C) (107, 108). Because avian schistosomes are the major group of schistosomes causing dermatitis, our own discussion focuses on their avian and snail hosts. For two reasons, these avian schistosome species comprise most of the dermatitis reports: first, many avian hosts seasonally migrate, consequently disseminating avian schistosomes as they fly (domestic ducks can serve as definitive hosts particularly for Trichobilharzia); and second, some snail hosts are habitat generalists (e.g., Physa [syn. Physella] acuta and Lymnaea stagnalis) that are now globally distributed. As a result, the opportunities for birds and snails to come into contact across time and space are vast. For example, P. acuta (host to Gigantobilharzia huronensis, Trichobilharzia physellae, and Trichobilharzia querquedulae) thrives in both natural and altered environments, with a wide tolerance for water temperature and chemistry, including the conditions found in ponds, drainage ditches, rivers, marshes, and ephemeral water (109–111). In Europe, the lymnaeid snail L. stagnalis (host of Trichobilharzia szidati) has also been linked to cases of dermatitis in people who acquired it while working with aquaria (112, 113), providing evidence of the snail's ability to persist, in addition to its local global presence.
From an evolutionary perspective, the vagility and habitat specificity of most bird hosts, in concert with the availability of snails in aquatic habitats, are likely mechanisms for widespread host switching in snails, and thus for diversification of avian schistosomes (12, 114). Our knowledge of the current schistosome-snail associations indicates that once a schistosome is hosted by a particular species of snail, it possesses little ability to utilize more than a few species within that genus (e.g., Radix, Stagnicola, and Physa) (115). Two exceptions are Dendritobilharzia pulverulenta, which uses Anisus vortex in Europe (116) and Gyraulus parvus in North America (117), both of which are small, related planorbid snails, and Trichobilharzia regenti, which employs lymnaeid snails of the genus Radix in Europe and Austropeplea tomentosa in New Zealand (118). There is little evidence of schistosome species crossing snail families, naturally or experimentally (for an exception, see references 119 and 120). Trichobilharzia franki was reported to be widespread across Europe, but detailed molecular studies are showing that it may represent several species related to snail host use (70, 121, 122).
Cercarial dermatitis is caused not only by species of schistosomes from indigenous snails but also by those from invasive or introduced snails. For example, Haminoea japonica (originally from Japan but now off the California coast) and Ilyanassa obsoleta (originally from the east coast but now on the west coast of North America) are responsible for annual dermatitis outbreaks at marine swimming beaches (25, 64). In freshwater, L. stagnalis is found commonly in the northern Eastern hemisphere, yet in the northern Western hemisphere it is only locally common. When L. stagnalis is found to be infected, the infecting species is related to a common European species, Trichobilharzia szidati (70, 123). Interestingly, P. acuta is one of the most invasive pulmonate snails and can host at least four species of avian schistosomes in North America (10, 70), yet there are no reports of this snail hosting schistosomes in their invasive range (outside North America). It is also noteworthy that most of the schistosome species transmitted by physid snails have thus far been found only in North America (at least based on genetic comparisons) (e.g., G. huronensis, T. physellae, and one undescribed lineage of schistosome [10]). The lymnaeid snail genera Stagnicola (found in North America) and Radix (found in the Eastern Hemisphere) are the main snail hosts for most species of Trichobilharzia; in fact, thus far, Radix maintains most of the reported species diversity of Trichobilharzia (122). Trichobilharzia regenti has been recognized to cause dermatitis in Lake Wanaka in New Zealand, and it may have been introduced from Europe in wild duck breeds (Anas platyrhynchos) used for hunting. It is now found commonly in the endemic nonmigratory scaup Aythya novaeseelandiae and the snail Austropeplea tomentosa (118). Schistosomes seem to be specific to particular snail hosts at the species or genus level, but not as much to their avian or mammalian hosts, though loose specificity of definitive host use exists at higher taxonomic levels (Table 1) (e.g., Schistosoma haematobium in humans, Bivitellobilharzia in elephants, and Allobilharzia in swans).
The diversity of avian schistosomes found around the world is in no small part due to the ability of thousands of migratory birds to carry their parasites across several latitudes and longitudes, exposing commonly encountered snails. This propensity to migrate large distances distinguishes avian schistosomes from mammalian schistosomes in terms of distribution, diversification, and host use, perhaps with the exception of Schistosoma mansoni (in terms of long-distance migration only) (124). Yet the schistosome species found in a wide range of avian host orders (e.g., Bilharziella and Dendritobilharzia) (Table 1) are not the ones recurrently responsible for outbreaks and are also species that are not genetically diverse compared to other species (69, 125, 126). Currently, D. pulverulenta might be the most widespread single species of avian schistosome, crossing both the Northern and Southern Hemispheres (117, 125, 127, 128).
The most derived clade, or most recently evolved clade, in clade D has three genera: Allobilharzia, Anserobilharzia, and Trichobilharzia (Fig. 1). Allobilharzia, from swans, and Anserobilharzia, from geese, both have a circumpolar distribution (69, 70, 105, 129, 130). Anserobilharzia brantae, which is common in North America (in the Canada goose [Branta canadensis] and the snow goose [Chen caerulescens]) but also found in Europe (in the greylag goose [Anser anser]), has been identified in at least one outbreak of dermatitis in the United States (10). The area was a eutrophic municipal lake/pond with a dense population of Gyraulus parvus snails and Canada geese. The third genus, Trichobilharzia, the most species-rich genus in the family, is found almost exclusively in ducks. It should be noted that there are several species of Trichobilharzia reported from other avian families (Trichobilharzia corvi from passeriforms, along with other species [131, 132]), but based on the morphology of adults and eggs and snail host use (when known), these probably represent new genera, or these avian hosts are not the primary (competent) hosts or might be aberrant cases (70, 78). Within the genus Trichobilharzia, several clades are specific to certain groups of ducks: for example, Trichobilharzia stagnicolae and Trichobilharzia mergi are found in mergansers (Merginae) (70, 133), T. querquedulae in the “blue-winged duck” clade (Anas clypeata, Anas discors, and Anas cyanoptera) (70, 134), T. physellae in an ecological group of diving ducks that includes ducks of the Aythinae and Merginae, and a common, undescribed species of Trichobilharzia, species A, in Anas americana (70). It is not yet clear which duck groups (phylogenetic or ecological) are more specific for T. franki or T. szidati. Interestingly, T. regenti has probably been reported from the most diverse duck species (135) and does not appear to have a preferred host within the Anatidae. In North America, T. stagnicolae and T. physellae are most often identified in dermatitis outbreaks (70).
INTRAMOLLUSCAN DEVELOPMENT OF AVIAN SCHISTOSOMES
As noted above, Trichobilharzia is the most diverse schistosome genus and has most often been implicated in outbreaks of cercarial dermatitis. As a result, studies on the avian schistosome-snail intermediate host relationship have focused primarily on species of the genus Trichobilharzia. Additionally, long-term laboratory maintenance of T. szidati and L. stagnalis enabled the experiments that uncovered the intimate molecular interactions of avian schistosomes and their snail hosts. (The Trichobilharzia ocellata organism used as an experimental model in European laboratories for the last few decades is identical to T. szidati, and the latter name is preferred and is used here [123]. If T. ocellata is used in the text body, then it refers to the non-European isolates of the parasite.)
After hatching from eggs in an aquatic environment, schistosome miracidia search for and invade an appropriate snail host species. This behavior must be accomplished quickly, as miracidia have a temperature-dependent limited life span of around 20 h, as reported for, e.g., T. stagnicolae (136). Studies on the miracidial behavior of T. szidati have shown a progression of steps from host finding to penetration and migration. Miracidia respond to environmental stimuli, such as light or gravity, that direct them to the microhabitat occupied by the host snails (137). Snails release various chemical compounds that form an “active space” around them and serve as chemoattractants for the miracidia. Miracidia recognize macromolecular glycoconjugates, termed miracidium-attracting glycoproteins (MAGs) or miraxones, that consist of a protein core and carbohydrate chains linked O-glycosidically via N-acetyl-d-galactosamine and serine/threonine (138, 139). The attractant for miracidia is encoded in these carbohydrate moieties. Upon entering the “active space” of the snail, miracidia modify their movement by increased random turns within the increasing attractant gradient and by a turn-back form of swimming within the decreasing attractant gradient (140). This mode of orientation (chemokinesis), observed, e.g., in T. szidati or T. franki (115, 138), results in the first contact of the miracidia with the snail, which is then followed by repeated investigation/probing of the snail surface and, finally, miracidial attachment (140). Penetration of the snail surface follows, although the factors contributing to this process, such as the components of miracidial penetration glands, remain largely unknown.
After penetrating the surface epithelium of the snail, miracidia of avian schistosomes transform into mother sporocysts that give rise to daughter sporocysts, which migrate to the snail hepatopancreas, where production of the final larval stage, the cercariae, takes place (13). The prepatent period lasts about 3 to 10 weeks, depending on several factors, such as the miracidial dose or temperature (13, 141). Infection by avian schistosomes may lead to alterations of the snail internal defense system (IDS), metabolism, and endocrine functions. Such alterations have been studied widely in L. stagnalis, the intermediate host of T. szidati (142–145).
The snail IDS is based solely on innate immune mechanisms composed of humoral and cellular limbs. Lectins are essential humoral components, whereas hemocytes represent the main effector cells (146, 147). Both limbs of the L. stagnalis IDS appear to be activated and then suppressed during early and late stages, respectively, of a T. szidati infection (143). In vitro, hemocytes failed to encapsulate and destroy T. szidati sporocysts (148). It has been suggested that parasite excretory-secretory products participate in the modulation of hemocyte activities (149). Disruption of hemocyte signaling pathways, such as protein kinase C (PKC) and extracellular signal-regulated kinase (ERK) pathways, may also influence hemocyte activities (150–152). For example, carbohydrates known to be present on larval surfaces of T. szidati and T. regenti, such as d-galactose and l-fucose (153–156), affected hemocyte PKC and ERK signaling in L. stagnalis, which suggests an immunosuppressive role (157, 158). However, experiments investigating the direct effect of Trichobilharzia larvae on hemocyte signaling have not been performed. Alterations of humoral defense components also occur during infections by avian schistosomes, and at least two molecules, molluscan defense molecule (MDM) and granularin, have been investigated in this respect (159, 160). Both molecules, produced in L. stagnalis by granular cells of connective tissue, are related to phagocytic activity of hemocytes. MDM enhances phagocytosis of hemocytes, and the expression of a corresponding gene for MDM is downregulated in L. stagnalis infected with T. szidati (159, 161). In contrast, treatment of hemocytes with granularin decreases phagocytic activity, and the encoding gene is upregulated in parasitized snails (160, 161).
In the snail host, avian schistosomes also interfere with metabolism, such as causing abnormal body growth, and endocrine functions, such as causing a reduction of egg laying (161). In L. stagnalis, these processes are regulated by neuroendocrine cells in the central nervous system (CNS) (162, 163). Trichobilharzia szidati releases an undescribed substance that induces the snail host to produce schistosomin (a peptide of 8.7 kDa consisting of 79 amino acids) from its connective tissue and hemocytes (145). Schistosomin acts as a neuropeptide that interferes with some hormones, such as calfluxin, a neuropeptide that stimulates the influx of Ca2+ into the mitochondria of albumen gland cells (164, 165). As a consequence, ovulation and egg laying are inhibited in the snail. Excitability of neuroendocrine cells (light green cells [LGCs]) responsible for growth (162) increases in response to schistosomin (166). As a result, the body size of infected snails may become considerably larger than that of uninfected snails (142, 161). Other neuropeptides (FMRFamide-related peptides) are also upregulated during infection of L. stagnalis by T. szidati, and these peptides, via inhibition of neuroendocrine cells, may be responsible for the suppression of snail metabolism and reproduction (167). All these changes in infected snails may provide energy resources and space that can be exploited by the schistosomes for development (145, 161).
The survival rate of infected snails releasing schistosome cercariae as the agent of cercarial dermatitis varies among species. A limited number of studies have focused on survival rates of avian schistosome-infected versus uninfected snails. As an example, 90% of L. stagnalis snails infected experimentally with a single miracidium of T. ocellata (North American isolate) were alive at 28 weeks of age (three infected snails were alive for 19 months), whereas all uninfected snails were dead (168). In contrast, L. stagnalis or Planorbarius corneus snails naturally infected with T. szidati or Bilharziella polonica, respectively, lived a shorter time, on average, than the corresponding uninfected individuals (169, 170).
VERTEBRATE HOST FINDING AND PENETRATION
Cercariae emerging from the snail intermediate host are the infective stage to the definitive host and are also the stage responsible for causing cercarial dermatitis. A cercaria is a multicellular larva comprised of an oblong body and a slender tail that is bifurcated (furcocercous) at the posterior end (Fig. 2). Cercariae of schistosomes leave their snail hosts actively. For this purpose, they employ a pair of specialized unicellular escape glands that are obvious in mature cercariae within sporocysts. Once the cercariae have emerged, only their ducts lined by microtubules are visible, suggesting a release of granular gland content likely containing histolytic enzymes during their migration through the snail tissue (171).
FIG 2.
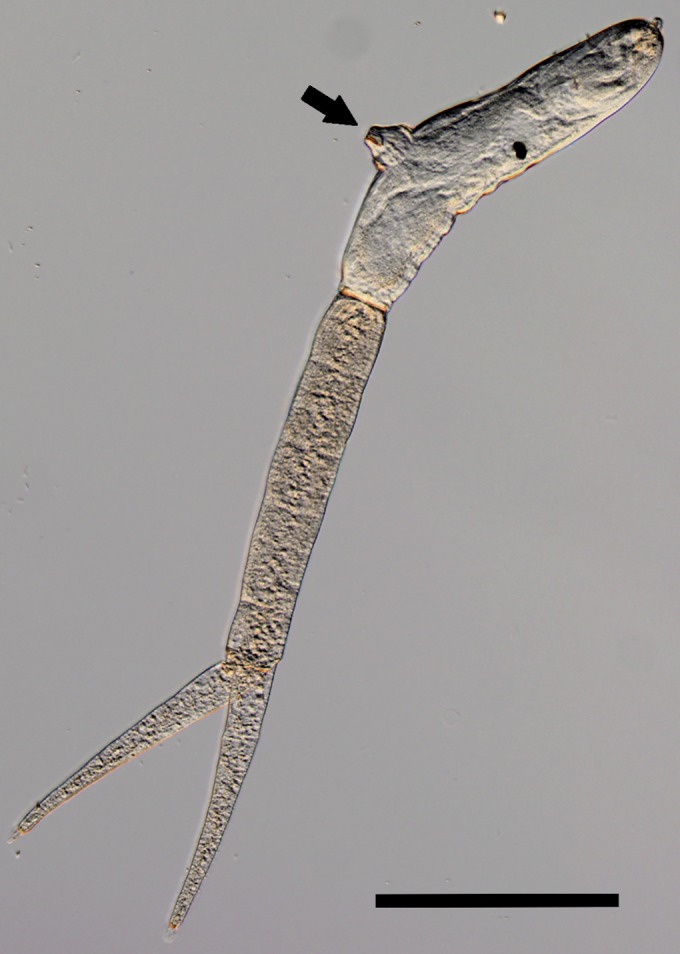
Furcocercaria of Trichobilharzia regenti with protruded acetabulum (arrow), lateral view. Bar, 200 μm. (Courtesy of J. Bulantová, reproduced with permission.)
Once in the water, schistosome cercariae express a complex pattern of behaviors that is composed of movement cycles that are repeated in defined frequencies (172). In general, they express negative geotaxy and positive phototaxy, which result in the concentration of cercariae just beneath the water surface, where appropriate definitive hosts may occur (173). Surface tension of the water enables clinging of cercariae via their ventral sucker (acetabulum) (173). This resting phase is interrupted by phases of active swimming (173). The effect of light on the behavior of swimming cercariae was studied in detail for Trichobilharzia szidati. Cercariae are able to react to a moving shadow stimulus (produced by a potential host) by a burst of forward swimming (body first) away from the source of light, to deeper levels, where they can encounter the feet of duck definitive hosts. A shadow stimulus applied in the active phase inhibits swimming and prolongs the following passive phase (173). Two types of photoreceptors, located near the dorsal surface of the body, were described for this species: a pair of lens-covered pigment cup ocelli and a special type of unpigmented, rhabdomeric photoreceptors, composed of three cells arranged in a three-dimensional (3-D) configuration. The lens-covered pigment cup ocelli probably serve to detect the direction of incoming light and to control the direction of swimming in relation to a light source, while the unpigmented photoreceptors serve as monitors for light intensity (174). The pigmented ocelli are also present in other genera of avian (Bilharziella, Dendritobilharzia, Gigantobilharzia, and Austrobilharzia) and mammalian (Schistosomatium and Heterobilharzia) schistosomes but are absent in Schistosoma.
Moving shadows also trigger a readiness for cercarial attachment to substrates, further stimulated by thermal and chemical host cues (172). As for the latter signals, compounds in the host skin (ceramides and cholesterol) stimulate enduring contact of cercariae of T. szidati with the host skin (173, 175). There is variability among schistosome species in responses to light, shadow, physical, and chemical cues, such that for different species, some of the signals may not work or may include some additional ones, such as touch, water turbulence, and/or additional chemical compounds (172, 176). The variation among the different avian schistosome species reflects the diversity in biology and ecology of schistosomes and their adaptations to the spectra of avian hosts (172, 176).
Invasion of the bird or mammal skin is initiated by the cercariae receiving the proper signal. Surprisingly, there were few differences between the avian T. szidati and human S. mansoni organisms in their pattern of invasive behavior toward living human skin; most cercariae did not penetrate the skin immediately after attachment but performed a leech-like creeping which lasted 0 to 80 s for T. szidati and 15 s to 5.58 min for S. mansoni (177). Such behavior guided the cercariae to skin wrinkles or hair follicles, where most penetration sites were located (178) (Fig. 3). Penetration behavior and production of secretions stimulate neighboring cercariae to use the same entry site on the skin (177, 178). Invasion of the skin is facilitated by secretions of cercarial penetration glands released from openings at the apex of the muscular head organ by spasmodic contractions of cercarial body musculature (179). The head organ performs concurrent thrust movements against the skin surface while the cercaria is firmly attached by the ventral sucker (176, 178, 180). Signals for skin invasion seem to be universal for schistosomes—fatty acids, especially polyunsaturated fatty acids containing 18 carbons and two or three cis double bonds (linoleic and linolenic acids), which are bound in cell membranes and occur as free molecules on the surface of human and bird skin (176).
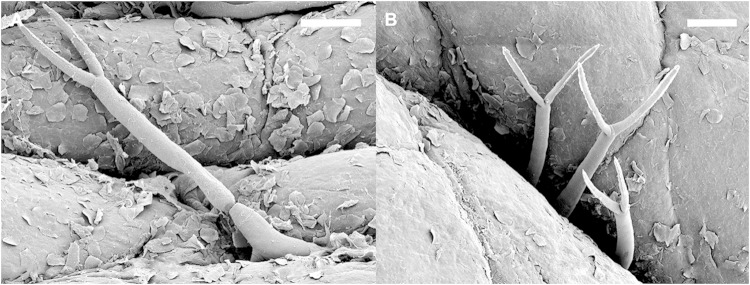
FIG 3.
Scanning electron micrographs of cercariae of Trichobilharzia regenti penetrating the skin of a duck leg. (A) An individual larva entering the skin; the tail is still preserved. (B) Tails of three cercariae penetrating the skin in a group. Bars, 100 μm. (Courtesy of J. Bulantová, reproduced with permission.)
For T. szidati, the tail is shed within 0 to 105 s after the onset of penetration, sometimes during creeping. This shedding seems to be generated at least partially by contractions of a muscular collar at the body-tail junction, which plays a role in the closure of the cercarial hind body after tail shedding. Cercarial penetration occurs in a nearly surface-parallel direction, while the spined ventral sucker supports squeezing of the cercarial body into the opening in the skin caused by histolytic gland secretions. Full penetration of living human skin was achieved within a mean of 4 min (83 s to 13.3 min), which was significantly faster than the case for the human parasite S. mansoni (6.58 min, on average) (177). Faster penetration of avian schistosome cercariae might be a consequence of these parasites' adaptation to lower concentrations of fatty acids in duck skin; therefore, reaction to higher concentrations in human skin may induce faster invasion (177, 178). Another explanation may be that different histolytic enzymes are used for penetration (see below). Skin penetration success rates may vary greatly. In experiments with T. szidati and human volunteers, the highest penetration success rate was 49% underneath the forearm (181).
The ultrastructure and chemical composition of avian schistosome cercarial penetration glands and their secretions have been poorly studied relative to the case for human schistosomes (Schistosoma). In fact, most of our knowledge is based on only two species of Trichobilharzia. There are five pairs of unicellular penetration glands: three pairs are located behind the ventral sucker (postacetabular glands), and two pairs are located around the ventral sucker (circumacetabular or preacetabular glands). These glands are filled with secretory vesicles that are released through the gland processes at the surface of the head organ. A 3-D model of T. regenti acetabular glands shows that they occupy more than one-fourth of the cercarial body volume (postacetabular glands, ca. 15%; and circumacetabular glands, ca. 12%). Differences were observed in the appearance of granular material/secretory vesicles contained in the glands, pH value, and the ability to bind various dyes and fluorescent markers (180, 182).
In T. szidati, proteolytic activity was detected in cercarial gland secretions induced by linoleic acid. This activity was linked with an orthologue of a chymotrypsin-like serine peptidase, named cercarial elastase and characterized from S. mansoni and S. haematobium cercariae. A protein on blots of T. szidati cercarial secretions as well as in histological sections of penetration glands immunologically cross-reacted with antibodies against elastases of S. mansoni and S. haematobium (183, 184). However, in another study, the reaction of antibodies raised against elastase of S. mansoni was observed neither with the penetration glands of T. szidati nor with the cercarial secretions on Western blots (180). In contrast, high activities of cysteine peptidases were noticed in induced cercarial secretions and homogenates for T. szidati and T. regenti (180, 185). In addition, the presence of cercarial elastase in the latter species was not confirmed by screening of a cDNA library (186). On the other hand, a papain-like cysteine peptidase, termed cathepsin B2, was found in the postacetabular penetration glands of T. regenti. This enzyme was shown to cleave proteins of the host skin, similar to the case with S. japonicum (187, 188). Its expression was even higher in intravertebrate stages (schistosomula and adults), suggesting that there are multiple roles of this enzyme during the life cycle (189). It seems that the use of particular peptidase families for skin penetration and tissue invasion is diverse among schistosomes (190) and may confer host specificity.
In the postacetabular penetration glands of T. szidati and T. regenti cercariae, a lectin(s) specific for β-1,3- and β-1,4-linked saccharide chains and their sulfated derivatives is present, though its biological function is still unknown (180, 191). It is interesting that there are high concentrations of calcium in the circumacetabular glands of both species (180). Several hypotheses suggest that the role(s) of calcium in the glands (including those of S. mansoni) may be to regulate gland peptidase activity, stimulate glycocalyx removal, interact with connective tissue proteoglycans, regulate host blood coagulation, or polymerize the adhesive substance from postacetabular glands. However, none of these hypotheses (except for a regulation of peptidase activity) has been confirmed adequately (180, 192, 193). Following contact with the host skin or a linoleic acid-coated surface (L. Mikeš, unpublished data), cercariae start to expel small amounts of gland content during the creeping movement, which is “printed” as the cercariae touch the surface—these “kissing marks” are made of a sticky substance. In S. mansoni, this substance is a product of the postacetabular glands and is composed of neutral and acidic mucosubstances (194). This product might serve adhesive or enzyme-directive functions. Similar material is produced by cercariae of Trichobilharzia spp. yet differs in chemical composition (180). Finally, the production of three types of eicosanoids by T. szidati cercariae is stimulated by linoleate (195). Eicosanoids may have a role in host invasion (vasodilatation), and their involvement in immune evasion was proven by the inhibition of superoxide production by human neutrophils (195).
During cercarial penetration, dramatic changes of surface structures and metabolism lead to the transformation of the cercaria to a schistosomulum. The thick glycocalyx that served as a protective layer for the free-living cercaria is shed, as its carbohydrate-rich composition is a target of the host complement cascade. In the schistosome species studied so far, the glycocalyx is markedly rich in fucose residues (154, 196–198). There is an obvious loss of saccharide moieties at the surfaces of transformed schistosomula of T. szidati and T. regenti, leading to reduced immunoreactivity and attractiveness for fucose-specific lectins (154, 155, 199, 200). Also, similar to the case for human schistosomes and members of other families of blood flukes, the trilaminar surface membrane of the outer cercarial tegument gradually changes to the doubled heptalaminar membrane of the schistosomulum, which has a protective function against the host immune system (199). In T. regenti stimulated by linoleate in vitro, shedding of the glycocalyx starts at the anterior of the cercaria, surrounding the openings of penetration glands (J. Chaloupecká and L. Mikeš, unpublished data). Sticky products of the glands adhere to the surface of the body, and as the cercaria crawls forward, the glycocalyx, with the gland products, is shed in a sleeve-like manner, until it is detached at the end of the hind body (Fig. 4). Transformed cercariae/schistosomula then lose their osmotic resistance toward water and become dependent on isosmotic conditions of the host (Chaloupecká and Mikeš, unpublished data). Whether any compounds of gland secretions take part directly (e.g., enzymatically) in the process of glycocalyx shedding is still unclear.
FIG 4.
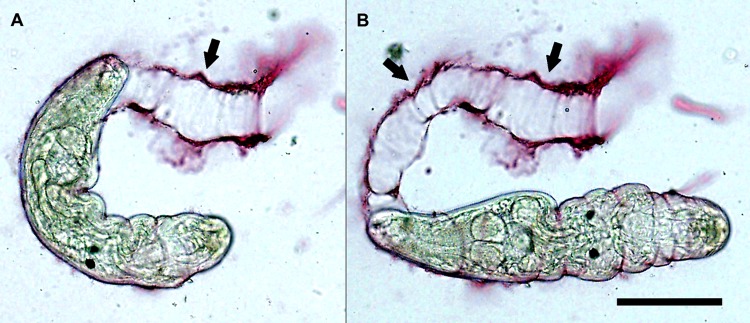
Living Trichobilharzia regenti cercaria in vitro, shedding its glycocalyx upon stimulation by linoleate. The sticky products of the penetration glands, stained with lithium carmine, adhere to the surface of the body, and as the cercaria crawls forward by periodical constrictions (A) and extensions (B), the glycocalyx and bound secretions are removed from the surface in a sleeve-like manner. Arrows indicate detached sleeve-like remnants of glycocalyx and gland products. Bar, 100 μm. (Courtesy of J. Chaloupecká, reproduced with permission.)
It should be mentioned that cercariae of avian schistosomes readily penetrate other (soft) tissues, and peroral infections of birds (definitive hosts) and mice (accidental hosts) with cercariae of T. regenti and T. szidati have been confirmed (L. Kolářová, K. Blažová, V. Pech, and P. Horák, unpublished data). This phenomenon is also known for mammalian schistosomes of the genus Schistosoma (201–203). It is not clear, however, how much these peroral infections contribute to the transmission of schistosomes under natural conditions. Due to the features of the esophageal mucosa, the penetration of Trichobilharzia cercariae does not require the penetration glands to be emptied completely, and their tails may be preserved for some time. The stimuli triggering this penetration behavior remain unknown.
PATHOGENICITY OF AND IMMUNE REACTIONS AGAINST AVIAN SCHISTOSOMES
Survival and Migration in Avian Hosts
Once the cercariae have transformed into schistosomula and reached their final location, it is not clear how long avian schistosomes live in their definitive hosts. For example, in experimental infections of ducks, adults of Trichobilharzia parocellata were found at 86 days postinfection (p.i.) (204). Whereas experimental infections with T. szidati and T. regenti last for about 3 to 5 weeks (13, 205), the adult worms of a Canadian isolate of T. ocellata were found in the liver at 370 days p.i. (206). Data describing the schistosome life span and length of time for egg release in a bird host will be necessary in considering the epidemiology of dermatitis. Nonetheless, avian schistosomes have a preference for two major habitats within the avian host: the visceral venous system (mesenteric, renal, cloacal, and portal vessels) and the nasal passages (except for Dendritobilharzia, which is found in the arterial system) (13).
Migration and localization have been characterized for a few visceral schistosomes and only one (T. regenti) of the eight nasal schistosomes (205, 207). Visceral schistosomes in birds have a migration pattern similar to those of Schistosoma spp. in mammals. After skin penetration, schistosomula of T. szidati navigate toward deeper skin layers by following dark and higher concentrations of d-glucose and l-arginine (208, 209). Once a blood capillary is found, the worms penetrate it and migrate to the heart and lungs. In the lungs, the worms enter free air space and then reenter the blood system (206, 210). Finally, visceral blood vessels (usually portal and mesenteric veins) are the preferred habitat (13). However, there are at least two exceptions, as follows: (i) the adults of T. szidati/T. ocellata leave the blood system and enter the layers of the host intestinal wall and mucosa (206, 211) and (ii) Dendritobilharzia pulverulenta prefers the arterial system of its hosts, where it is found in the lower dorsal aorta and the femoral arteries (212). For the nasal schistosome T. regenti, migration is dramatically different. Schistosomula leave the skin and then seek and penetrate peripheral nerves (Fig. 5) to migrate to the spinal cord and brain of their host. From there, the adult worms appear intra- and extravascularly in the nasal mucosa (207).
FIG 5.
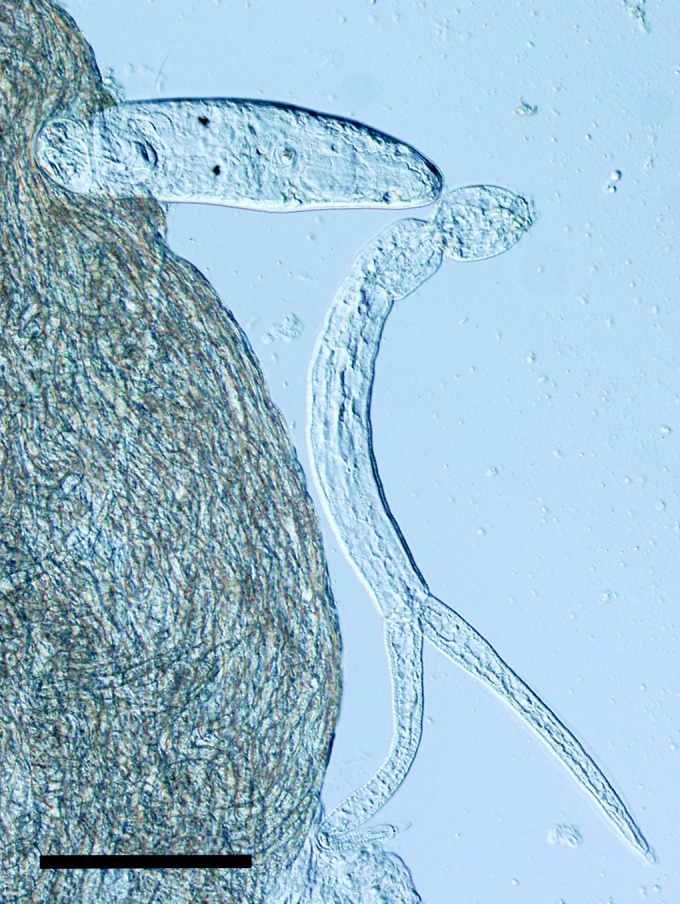
Cercaria of Trichobilharzia regenti in vitro, penetrating a peripheral nerve isolated from a duck. The tail is already detached, and the head organ burrows into the nerve. Bar, 200 μm. (Courtesy of J. Bulantová, reproduced with permission.)
Pathology Caused by Visceral Species in Birds and Mammals
Most pathological studies of avian schistosomes in the avian host have been detailed for experimental birds, though there are a few reports from wild birds. As for accidental mammalian hosts, only experimental infections have been evaluated. The schistosomula migrate through the heart and lungs of birds and mammals, and only in the avian host do they reach their final destination. Migration through host lungs has been shown to cause damage (16, 210, 213). Infections of duck and mouse lungs by T. szidati are accompanied by hemorrhages in the periphery of the lungs (210). Migration of schistosomula in the lungs of ducks leads to formation of lymphocytic lesions and an influx of macrophages, heterophils, and eosinophils into the afflicted tissue (210). However, in mouse lungs, schistosomula do not evoke a specific inflammatory reaction; only alveolar congestion and edema are observed (210). Damage to the lung tissue in general, and formation of alveolar congestion in particular, might be caused by schistosomula that leave the blood system and localize extravascularly in the alveolar walls. Their inability to reenter the blood system might be linked to their relative size and loss of orientation in a noncompatible mouse host (210). Similarly, migrations to mammalian lungs and accompanied hemorrhages have been observed in hamsters, guinea pigs, rabbits, and rhesus monkeys experimentally exposed to three species of Trichobilharzia (213). Pulmonary infections of chickens and pigeons with the marine schistosome Ornithobilharzia canaliculata led to the development of lesions in the arterial and venous vascular systems (lymphocytic endarteritis, periarteritis, and segmental proliferation of the vascular endothelium), hyperplasia of smooth muscles in tertiary bronchi, and thickening of alveolar septa. Cellular infiltrates consisted mainly of histiocytes, heterophils, and lymphocytes (16). In contrast to the case with visceral schistosomes, infections of duck and mouse lungs with the nasal species T. regenti probably represent an ectopic localization of schistosomula (207, 214, 215).
As for patent infections (exclusively in birds), major pathology is caused by granulomas around eggs and only partly by adult worms. Obliterative endophlebitis caused by adult schistosomes (probably Trichobilharzia filiformis) in the intestinal veins of mute swans (Cygnus olor) has been recorded (216). The intestinal surface showed various stages of villous atrophy with intestinal mucosal lesions, associated with infiltration of the lamina propria of the jejunum and ileum by lymphocytes and plasma cells and by a smaller number of heterophils and eosinophils. Eggs were observed multifocally in the lamina propria of the small and large intestines, and their presence triggered mild to severe granulomatous reactions (216). Eggs laid by the adult worms of Austrobilharzia variglandis in experimentally infected chickens caused edema, cellular infiltration, and hyperplasia of smooth muscle of the muscularis externa of the intestine. Around the eggs, mononuclear cells formed early granulomas with a few eosinophils and heterophils, followed by accumulation of giant and epithelioid cells (217).
Similarly, examination of Atlantic brant geese (Branta bernicla hrota) infected with Trichobilharzia sp. (probably Anserobilharzia brantae, based on current taxonomy) revealed the development of granulomas around eggs located in the colon (218). Granulomas were also observed in the duodenum and small intestine of pigeons infected with Ornithobilharzia canaliculata (16). Three species of ducks infected with T. physellae showed no associated tissue reaction in the vicinity of adults located in mesenteric veins, but a granulomatous reaction around the eggs was detected occasionally in the mucosa and submucosa of the intestine (219). On the other hand, the most serious lesions and fibroplasia of the portal triads and adjacent parenchyma were observed in the livers of those ducks and were attributed to mature T. physellae (219). In the case of pigeons infected with O. canaliculata, granulomatous lesions surrounding collapsed eggs were observed in the liver parenchyma (16). Intestinal pathology may be accompanied by poor nutritional conditions, as noticed in pigeons infected with O. canaliculata (16). Exceptionally, a more serious manifestation of the infection in naturally infected wild ducks was reported where the adults and eggs of T. physellae caused partial to complete paralysis of the cervical, wing, and leg muscles, foul-smelling diarrhea, and half-closed pasted eyelids (219).
Pathology Caused by Nasal Species in Birds and Mice
Histological examination of the nasal tissue of birds showed the extra- and intravascular locations of adult worms of T. regenti (220), with their first appearance on day 13 p.i. (205). Immature eggs appeared from day 15 p.i., and at day 19 p.i., eggs were fully developed and observed extravascularly in the nasal mucosa, with the maximum number of eggs seen on day 22 p.i. (221). The area surrounding the eggs was infiltrated by numerous eosinophils, heterophils, histiocytes, and multinucleated giant cells and a few plasma cells and mononucleated cells (220). The formation of granulomas around the eggs was noted from day 22 p.i. (221). Miracidia that hatched directly in the host tissue were surrounded by lymphocytes, eosinophils, and heterophils, without granuloma formation (221). While an infected bird is drinking/feeding, only the miracidia leave the tissue to enter the water, which represents an exceptional mode of transmission among schistosomes (205). The presence of adults only did not initiate an influx of immune cells to their vicinity, but the presence of large worms and eggs caused the development of focal hemorrhages throughout the nasal mucosa (207, 221). Probably the more devastating aspect of the pathology of this species is the effect on the CNS of hosts (birds and experimental mammals). As stated above, cercariae penetrate the skin and migrate to the nasal passages via the CNS rather than the circulatory system.
CNS infections of ducks and mice by T. regenti may lead to the development of various transient or permanent neuromotor symptoms, such as weak to severe leg paralysis and balance/orientation disorders (207, 214, 215). In avian hosts, schistosomula in the CNS initiated an accumulation of inflammatory cells, such as eosinophils and heterophils, that represented the most abundant cell infiltrates, yet minimal damage to nervous system cells was detected (220). In a few cases, however, damage to the CNS was observed in birds with visceral schistosomes. For example, granulomatous encephalitis in mute swans, caused by Dendritobilharzia sp., has been described (222, 223). Schistosome eggs found in the cerebrums and cerebellums of naturally infected swans were surrounded by giant cells, macrophages, lymphocytes, and, to a lesser extent, heterophils and fibroblasts (223). The presence of Dendritobilharzia eggs in the CNS represents an ectopic localization.
Because of the pathology caused by T. regenti, and thus the implications for human health, most experimental work has been done in mice rather than birds. For up to 3 days p.i., migration of schistosomula through the murine nervous tissue did not evoke inflammation or tissue damage, and all detected parasites were intact (215, 224). A host reaction to the infection was visible on days 6 and 7 p.i. The presence of parasites led to the accumulation of immune cells, predominantly microglia, macrophages, and neutrophils and, to a lesser extent, CD3+ lymphocytes (215, 224). Proliferating astrocytes formed “glial scars” at the sites of previously migrating schistosomula (215). Ongoing infection was associated with a more intense inflammatory reaction in white and gray matter of the spinal cord. Microglia, macrophages, neutrophils, eosinophils, and CD3+ lymphocytes participated in the formation of inflammatory lesions surrounding the disintegrating schistosomula, and damage to the axons was detected (215) (Fig. 6). The localization of schistosomula outside the solid tissue, in the subarachnoidal space of the spinal cord and the brain and in the cavity of the 4th ventricle of the brain, led neither to damage nor to inflammation of the adjacent nervous tissue (215). It seems that schistosomula located in the cavities of CNS were able to delay destruction by the immune cells. Nevertheless, most of the worms were eliminated by 21 days p.i. (215). Challenge infections triggered a strong immune response, which efficiently and rapidly eliminated the schistosomes (215, 224).
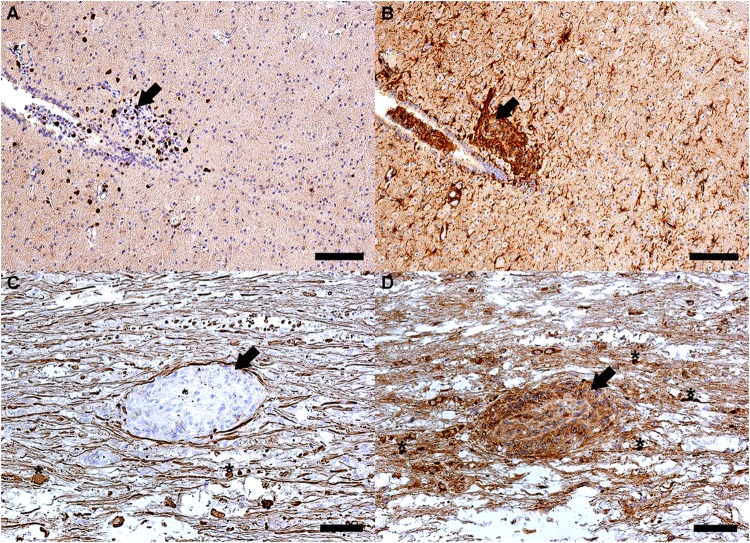
FIG 6.
Destruction of a schistosomulum (arrows) in the thoracic part of the spinal cord of a BALB/c mouse at 21 days p.i. (longitudinal sections). (A and B) Inflammatory lesion consisting of CD3+ lymphocytes (dark spots) (A) and microglia cells (brown-stained ramified cells) (B) that were detected by use of anti-mouse CD3+ and anti-mouse Iba-1 antibodies, respectively. Nuclei of other cells were stained blue by hematoxylin. (C and D) Tissue around the schistosomulum contains damaged axons. Axonal damage was accompanied by formation of spheroids (asterisks) in the site of axonal disruption and was visualized immunohistochemically by use of anti-mouse nonphosphorylated and phosphorylated neurofilament antibodies (SMI-311 and SMI-312, respectively) (C) and anti-mouse β-amyloid precursor protein antibodies (D). Bars, 100 μm (A and B) and 50 μm (C and D). (The figure was created by L. Lichtenbergová.)
In immunodeficient SCID mice, primary infections as well as reinfections did not evoke a significant skin reaction, and the schistosomula often escaped from the skin to the CNS (224), where migrating schistosomula caused axonal damage and an influx of immune cells (215). In comparison to the case with immunocompetent mice, the schistosomula survived longer in the CNS, probably due to the absence of T and B lymphocytes (215, 224), cells that may represent important effectors in destruction of schistosomula. Larger numbers of schistosomula in the CNS and their extended time of migration via nervous tissue resulted in a higher rate of occurrence of paralysis of immunodeficient SCID mice (215).
The above-mentioned damage to the nervous tissues of birds and mice demonstrates that not only the eggs but also the other stages of schistosomes are responsible for major pathology. In this particular case, just the schistosomula (migrating juveniles) of T. regenti can be regarded as the most pathogenic stage of the parasite.
Skin Immune Response and Cercarial Dermatitis
Skin immune reactions of birds to the penetration of avian schistosome cercariae are insufficiently described. In any case, birds do respond to penetrating cercariae, as shown in a histological observation of chicken skin infected by Ornithobilharzia canaliculata. Severe infiltrations of the dermis by histiocytes and heterophils and aggregation of lymphocytes around dilated capillaries in the dermis were recorded (16). Dead and destructed schistosomula surrounded by heterophils and histiocytes were found in the epidermises of chickens at 12 h p.i. At 24 h p.i., lymphocytes, histiocytes, and heterophils still persisted in the dermis, but the number of immune cells decreased (16).
Immunohistopathology of cercarial dermatitis in humans recognized three phases of cellular responses (leucocytic, lymphocytic, and histiocytic) against Trichobilharzia larvae (225). However, because studies on humans are rarely performed (112, 225, 226), a mouse model was established to provide more-detailed studies on immunohistopathology. Primary infection of mice with T. regenti causes an acute inflammatory reaction with edema and vasodilatation (227). Parasites located in the dermis are surrounded by large inflammatory cellular foci that are formed by neutrophils, macrophages, mast cells, major histocompatibility complex class II (MHC II) antigen-presenting cells, and a small number of CD4+ lymphocytes (227). Repeated infections cause perivasculitis, folliculitis, and substantially more influx of the same cell types that are noted after primary infection (227). Extensive skin inflammation leads to the formation of large abscesses and subsequently to dermal and epidermal necrosis. Sites of previous cercarial penetration are characterized by intraepidermal pustulae and parakeratosis (227).
In vitro culture of skin biopsy specimens from primary mouse infections by T. regenti revealed a release of the acute-phase cytokines interleukin-1β (IL-1β) and IL-6 and an increased production of IL-12 (227). Larger amounts of IL-12 correlated with elevated production of gamma interferon (IFN-γ) in the cell culture supernatant (antigen-stimulated lymphocytes) from the skin draining lymph nodes (227). IFN-γ and IL-12 are associated with Th1 cell differentiation, and IL-1β and IL-6 are important in Th17 polarization (228). Although the role of Th17 in host tissue immunopathology has been described for some infections by helminths (e.g., human schistosomes) (228), participation of Th17 cells in the processes associated with skin penetration by avian schistosomes needs to be clarified.
Reinfections of mice with T. regenti were accompanied by edema that developed as a consequence of local vascular permeability caused by histamine released from activated mast cells (227). Histamine has a regulatory function in the Th1 and Th2 polarization of the immune response (229). Mast cells also rapidly released a large amount of IL-4, which has been detected in the supernatants of skin biopsy specimens from T. regenti-reinfected mice (227). Like mast cells, basophils degranulate and release IL-4 as a response to the presence of T. regenti antigens (230). Elevation of total serum IgE levels implies that histamine and IL-4 production by mast cells and basophils occurs in an IgE-dependent manner (227, 230). Dominance of the Th2 response was also supported by an elevation of antigen-specific IgG1 antibodies and a decrease of IgG2b antibodies (Th1 associated) in the sera of mice reinfected with T. regenti (230). Cercarial dermatitis in reinfected mice is therefore Th2 polarized, with a response comprised of an early type I hypersensitivity reaction and late-phase skin inflammation (227).
It has been shown in several cases that mammals (including humans) are unsuitable hosts for avian schistosomes, such that the worms cannot mature and reproduce (except for Austrobilharzia variglandis, which is able to reach sexual maturity in the lungs of Meriones unguiculatus gerbils [231]). Nevertheless, cercarial dermatitis is probably not the only interaction of avian schistosomes and mammals. Dermatitis develops as an immune (allergic) reaction of the already sensitized person; it represents a powerful protection of the body against worms in the skin. However, in a naive (nonsensitized) or immunodeficient experimental host, at least some worms survive, leave the skin, and migrate throughout the body (213, 232). Mild to severe consequences of such migration may appear (see above); most importantly, T. regenti is neurotropic and can cause damage to the central nervous system (155, 207, 214, 215, 220, 224). To date, no information about migration of avian schistosomes in human bodies is available. It therefore seems that laboratory animals (mice, rats, etc.) are indispensable for assessing all risks associated with infections of mammals (humans) by avian schistosomes.
DETECTION AND IDENTIFICATION OF AVIAN SCHISTOSOMES
Prior to or during the dermatitis season, especially following a dermatitis outbreak, a standard protocol for the detection of schistosome cercariae involves the collection of and screening for cercarial emergence in snails. Usually, in a laboratory, individual snails are placed in a beaker/small wells with clean water and exposed to a lamp to stimulate shedding of cercariae. Subsequently, cercariae are collected and identified under a light microscope. If no cercariae emerge from the snails, there are three options: the snails can be dissected to find the schistosomes, the snail tissue can be pooled and molecular techniques applied to detect a schistosome infection, and/or additional snails can be collected from affected and surrounding areas (233, 234). Detection of microorganisms directly from water samples is developing rapidly, but among schistosomes, these techniques have been optimized for human schistosomes (235, 236). Methods thus far to detect avian schistosomes, Trichobilharzia in particular (237, 238), involve concentrating the cercariae from a water sample and using a PCR assay to detect a single cercaria in plankton (0.5 g) and snail tissues (0.25 g) (239). Using definitive host-seeking behavior, cercariae can be lured to a trap that contains linoleic acid, a known stimulus for cercarial penetration (240). Irrespective of a dermatitis outbreak, identifying which adult worms can be found in the habitat can be completed by examination of feces for eggs by using sedimentation/flotation (241) or Kato-Katz fecal smear methods (242) and/or postmortem examination of the arterial/venous system, nasals, liver, or kidney of the host. For nasal schistosome species, lavage of the nasal cavity of birds represents a method of choice. After rinsing out the nasal cavity with saline or water, freely moving miracidia (and partly the liberated eggs) can be detected in the wash fluid (13). The above-described examination of snails and birds for avian schistosomes is thoroughly summarized in reference 243.
Examination of water or the snail or bird host is important for species delineation. Not only is there a highly diverse population of schistosomes, especially avian schistosomes, that are responsible for dermatitis, but these species differ markedly in their morphology and pathogenicity in birds and experimental animals (12, 13). Identification of schistosomes, particularly avian schistosomes, is challenging. Traditional methods using morphology (adults mostly, but also eggs and cercariae) are usually not sufficient to separate species, particularly within a genus, as these characteristics can be missing or minute, and their recognition often depends on the experience of the observer. However, some characteristics have been found to be informative for rough species identification, such as the position of internal organs and tegument spination of adults (13), morphology of eggs (244), or distribution of sensory papillae on cercariae (68, 198).
In addition to the morphological features, there are some nonmorphological criteria that can be used to differentiate species, for example, the behavior of cercariae (245), compatibility with the snail hosts, and organ/tissue affinity of adults in birds (135). If eggs and adults from a host are examined, it is not always clear which adults/eggs are conspecific or which match cercariae from snails unless experimental trials are conducted. Experimental infections with cercariae from snails to obtain adult worms for morphological assessment are ideal, but these are time-consuming and often yield low prevalences of infection. Because of the reported species, morphological, and pathogenic diversity of avian schistosomes (12, 13), from an epidemiological perspective on dermatitis, there is a need for species delineation and host use, and molecular techniques have proven a necessary and excellent tool for more rapid identifications (63, 70, 135). Such techniques have allowed us to genetically connect larval stages from snails to adult stages from birds, greatly advancing the epizootology and epidemiology of avian schistosomes in particular (10, 40, 123).
Molecular identification of an avian schistosome provides clues to the host source and biology and possible targets for control. There have been steady efforts and testing of markers that have revealed some consistency and validity for species identification of schistosomes (123, 246, 247). Molecular phylogenetics has had a major impact on the taxonomy and discovery of new lineages of avian schistosomes, particularly the major etiological agents of cercarial dermatitis, i.e., species of Trichobilharzia (10, 40, 63, 67, 69, 70, 121–123, 125, 129, 135, 248–251). Most of the effort has been conducted using three gene regions. To date, the nuclear ribosomal DNA D1-D2 regions of 28S, the internal transcribed spacer (ITS) region, and the mitochondrial cox1 gene have been tested as molecular markers for systematics (40, 63, 67, 69, 70, 105, 123, 248, 249, 251), epidemiology (252), and diagnostics (121, 253). Sequencing of gene regions such as the nuclear ITS region and the mitochondrial cox1 gene not only has linked life cycle stages but also has suggested that our recognition of the diversity of avian schistosomes continues to grow (10, 12, 40, 65–67, 69, 70, 105, 129, 133, 135).
While the molecular identification of avian schistosomes is still in the early stages, these markers have thus far proved successful in attributing most samples to a known species. At the level of (infra)populations, genetic diversity of T. szidati cercariae from 7 snails collected at 3 localities in Russia was recently shown by use of randomly amplified polymorphic DNA (RAPD) (254). At this time, neither microsatellite markers nor next-generation sequencing protocols have been developed that would allow detection of specific populations or host strains. The development of genome-wide sequencing protocols for population genetic analyses would greatly aid in identifying the epidemiological determinants of cercarial dermatitis outbreaks. Although genetic identifications have provided a framework for circumscribing species and for rapid detection, caution must be exercised, since genetic identification alone is not sufficient in the absence of data on disease dynamics and morphology (255, 256). A species designation should ideally reflect all data available, i.e., host species, location, and morphological characteristics of adult worms and eggs. In addition, comparative molecular analyses must be performed to obtain reliable and convincing results of species identification.
Most importantly, specimens and other data, e.g., genetic data, should be archived in a permanent museum collection or an archivable Web-based database for data resulting from a specimen, such as the sequence archive GenBank. Voucher samples of any life cycle stage of these schistosomes (or any parasite and host) should be preserved and deposited in a permanent museum collection (257–259). This is imperative for several reasons: the most important is for the question that has not yet been asked. In the event that the parasite sample does not match known species or has odd features, further work may be necessary. Access to images and measurements, plus an additional sample(s), will be used in further sequencing. Moreover, documentation of any species of schistosome coming through a clinic is an important record that can contribute to epidemiological studies, especially if it is associated with people affected by dermatitis.
CLINICAL FEATURES, DIAGNOSIS, TREATMENT, AND PROPHYLAXIS OF HUMAN INFECTIONS
As for the clinical symptoms and signs, penetration of avian schistosome cercariae into mammalian (human) skin may initiate an immediate prickling sensation that persists for approximately 1 h (4, 260). The development and intensity of the subsequent allergic reaction depend on the number and duration of previous cercarial contacts, as well as individual susceptibility (4, 7, 260). The primary contact with cercariae may lead to either an imperceptible (7, 261) or mild skin reaction, with the development of small and transient macules, maculopapules, or inconspicuous papules of about 1 to 2 mm after 0.5 to 2 days p.i. A delayed reaction in the form of small papules can be observed in some persons as late as 8 days p.i. (7, 238).
Repeated infections cause a more pronounced cutaneous reaction followed by diffuse edema and development of erythematous papules or papulovesicles (4, 261). More specifically, the first transitory macules (up to 10 mm) and primary itching can appear as soon as 4 to 20 min after exposure. Thereafter (1 to 15 h p.i.), macules are replaced by papules (about 3 to 8 mm), and an intense itching (secondary itching) is experienced. In addition, erythema and edema may occur in the afflicted area for a few days. Vesicles of about 1 to 8 mm may form on papules at 2 to 3 days p.i. and may rupture as a result of scratching. As a consequence, bacterial superinfection may result in formation of pustules. Papules usually regress and disappear at 4 to 10 days p.i., leaving a pigmented spot (about 1 to 4 mm) on the skin for weeks (7, 225, 238); however, in some cases, the symptoms may persist for about 20 days p.i. (7, 238, 262, 263). Every macula/papula is a reaction against the penetrating cercaria(e) and thus represents the part of the body in direct contact with cercariae (Fig. 7). An attack by many cercariae may be accompanied by generalized reactions, such as limb and lymph node swelling, nausea, diarrhea, and fever (7, 238).
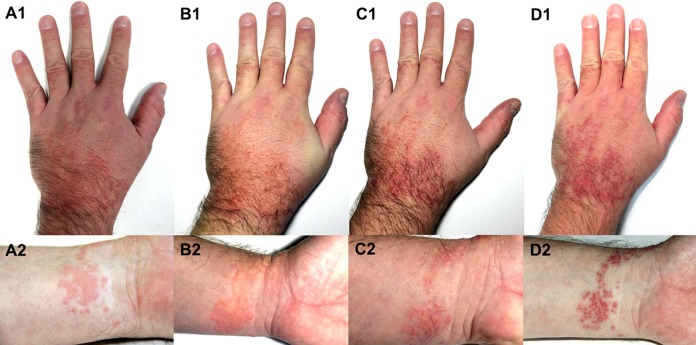
FIG 7.
Development of cercarial dermatitis on the dorsal (1) and ventral (2) parts of the left hand of a sensitized volunteer infected experimentally by Trichobilharzia szidati (the whole hand was immersed into a beaker containing water with cercariae). Images were taken at 1 (A), 2 (B), 3 (C), and 4 (D) days postexposure. Formation of macules (A) and papules with vesicles (B to D) can be seen. No penetration/reaction was recorded for the palm. Noticeable swelling of the hand is shown mainly in panel B1. (The figure was created by H. Kulíková and P. Horák.)
Diagnosis is rather problematic. Skin reactions to cercariae from freshwater or marine environments may resemble insect bites, bacterial dermatitis, contact dermatitis, or skin reactions against nematocysts of larval cnidarians (sea anemones, thimble jellyfish, etc.) (7). Anamnestic data (suggesting recent contact with water reservoirs) and maculopapular skin eruption on the body parts that were in contact with water are important indicators. Direct proof of schistosome infection may be shown by skin biopsy of the papulae no later than 48 h p.i. Individual papulae should be excised/shaved off under local anesthesia, put in Bouin's fixative, cut into 10-μm sections, and stained with hematoxylin-eosin (225, 226). As for basic laboratory tests, increased eosinophil counts and elevated levels of total IgE may indicate an attack by avian schistosomes (7, 230, 238). Specific immunological/serological assays (skin test, “Cercarienhüllenreaktion” [a precipitation reaction surrounding the cercarial body in the presence of a specific antibody], indirect fluorescent-antibody test [IFAT], enzyme-linked immunosorbent assay [ELISA], and complement fixation test) to detect penetration by avian schistosomes are not sufficiently specific or sensitive (7, 238). Exceptionally, some reaction has been obtained with sera of dermatitis patients and a heterologous antigen—Schistosoma mansoni cercariae—used in Cercarienhüllenreaktion and IFAT (263, 264). Selection of a reliable (recombinant) antigen or primer for serological or DNA-based tests, respectively, is in progress (our unpublished data).
Therapy for afflicted areas includes only symptomatic (not causal) treatment of the condition in the form of soothing agents. For example, water chestnut planters in India use mustard oil to relieve the itching and rash in mild cases of dermatitis (265). In serious cases, application of systemic antihistamines (tablets or gels, e.g., hydroxyzine) or mild corticosteroids (e.g., 0.1% triamcinolon cream or 1% hydrocortisone ointment) may be considered (7, 226, 262, 266).
There are several recommendations to protect individuals. Wearing rubber waders or gloves or neoprene diving suits is 100% reliable, although not always appropriate or realistic. Upon contact with cercariae in water, the number of penetrating larvae can be reduced if action is taken within seconds to a few minutes (see the part on vertebrate host penetration above). For example, thorough toweling and exposure of skin to the sun are recommended for bathers immediately after leaving water. In the case of an accidental exposure in the laboratory, skin can be washed with 70% ethanol or warm water (as much as one can tolerate) and soap (our personal experience). Several chemicals have been tested as barriers to cercarial penetration. For example, LipoDEET, a long-acting liposome formulation of DEET (N,N-diethyl-m-toluamide), a common, safe, and available insect repellent, has been used successfully to prevent penetration of S. mansoni (267). However, a cream formulation of DEET was poorly effective against T. szidati penetrating living human skin (181). Two other formulations were effective: (i) SafeSea lotion against jellyfish stings, where the effective compound may be H1-antihistamine diphenhydramine; and (ii) niclosamide in a dosage as low as 0.1% in water-resistant sunscreens. We hypothesize that sunscreens based on plant oils (nonmedicated) will trigger cercarial penetration due to a high content of unsaturated fatty acids. In this regard, a negative effect in terms of human protection was observed for dimethicones (polydimethylsiloxanes and silicone oils), which are common ingredients in many skin care products (181).
ECOLOGICAL FACTORS INFLUENCING THE OCCURRENCE OF AVIAN SCHISTOSOMES AND CERCARIAL DERMATITIS
Due to climate changes and land alterations, the seasonal window for parasite transmission may become longer, in addition to changes in the behavioral and physiological patterns of parasite hosts. For example, the accelerated growth of both snail and trematode larval populations (71) or changes in phenology of aquatic migratory birds to sedentary (268–270) can increase chances for outbreaks of cercarial dermatitis in both space and time.
Global Warming and Eutrophication
At least in temperate climates, schistosome cercariae occur seasonally once the ambient temperature is high enough for snail activity and thus cercarial emergence (271). Also, snails infected during late summer can survive through the winter season and serve as a source of cercariae in spring (13, 272, 273). Global warming is predicted to have a positive effect on trematode intramolluscan stages, because their development is strongly temperature dependent, leading to increased cercarial emission rates. For example, a shorter prepatent period in relation to higher temperatures was demonstrated in experimental infections with T. szidati (141, 274). A slight rise in temperature will increase developmental rates and transmission success (number of cercariae available to find the definitive host). For example, a 5-fold increase in cercarial emergence with a 10°C increase in temperature was recorded for Trichobilharzia sp. from Radix peregra (275). In addition, a link has been suggested between the rise of 0.8°C within the last century and the increased frequency of cercarial dermatitis and Trichobilharzia prevalence at higher latitudes in Europe (9, 276). What does not change is the life span of cercariae, which is limited to the restricted source of reserve glycogen (cercariae do not take up any food). In this regard, temperature strongly affects cercarial survival. For example, the half-life of T. szidati was 16 h at 16°C but only 7.8 h at 25°C (169), and the life span of Trichobilharzia arcuata was 72 h at 4°C but only 48 h at 26°C (277). Infected snails change their thermal microhabitat selection, e.g., Lymnaea stagnalis infected with T. szidati prefers a colder water temperature than that preferred by uninfected snails (19.97°C versus 25°C). This may be an adaptation for either the snail or the schistosome, because at these temperatures larval development is slower and the rate of cercarial emission is lower, leading to less tissue damage in the snail (169, 170).
Eutrophication promotes excessive plant growth and decay, causing alterations in the dynamics of freshwater communities and thus leading to an increased risk of acquiring cercarial dermatitis (11, 278–280). Greater biomass of primary producers is associated with faster development and growth of snails due to the increase in food availability (280–282). Dense snail populations may increase the probability that a miracidium will find a snail and lead to a higher prevalence of infection. In addition, aquatic birds are attracted to such nutrient-rich environments, also contributing to an increase in parasite transmission (283). Using data from a mark-recapture study of L. stagnalis in eutrophic fishponds, rapid trematode recruitment into the snail populations was demonstrated (284). Maximum annual rates of colonization/recruitment for T. szidati were shown to reach up to 300%, such that the odds of trematode establishment in an individual snail were 3 times per year versus once in 10 years in other, mainly marine trematode-snail systems (284, 285).
Large snail populations with a high prevalence of avian schistosomes have been reported for several localities in Europe (71, 286–288), North America (289, 290), and Australia (291). Usually, natural preserves support diverse and abundant populations of potential hosts of schistosomes, so these areas may serve as hot spots for outbreaks of cercarial dermatitis. Although eutrophication contributes to infection risk, cases of cercarial dermatitis or findings of avian schistosomes have also been reported from oligotrophic or mesotrophic systems (8). In addition, other abiotic and biotic factors and human-induced habitat alterations may influence the occurrence of schistosomes and cercarial dermatitis, such as altered hydrology conditions with water-level fluctuation, ice cover, acidification, or dam constructions (268, 292, 293), anthropogenic pollutants (268, 294–297), biodiversity change in terms of introducing nonindigenous species that may affect endemic parasites (298), host susceptibility or resistance (9), predation upon trematode free-swimming larval stages by fish and other aquatic animals (295, 299, 300), or interspecific competition of parasites within the same snail host (301–303).
Recreational Activities and Cercarial Dermatitis
The data in the literature suggest that lakes represent high-risk areas for cercarial dermatitis, as they are attractive for a large number of people, usually for recreational purposes (8). Cercarial dermatitis develops as a result of sensitization of the human immune system (232, 260), and repeated exposures to cercariae influence the occurrence and intensity of subsequent infections. For example, longer exposure in water increases cercarial penetration via frequent water visits and more time spent in shallow water (4, 304–306). While gender does not influence the risk of infection (4, 304, 305, 307), some studies found a higher risk among children of less than 15 years of age, since they tend to spend more time in shallow water (280, 308, 309). There are rare cases of nonsensitive individuals that may be due to host desensitization (310) or individual nonsusceptibility/nonattractivity to cercariae (4, 311).
Locally, the highest risk of infection usually occurs in shallow, warm, and vegetation-rich shore areas, where the snails accumulate and release cercariae (308, 309). Cercariae of avian schistosomes are concentrated just beneath the water surface (see the information on host finding), so swimming in deeper water may reduce the infection probability (280). However, snail preferences for habitat differ: Lymnaea stagnalis is found mostly in patches of aquatic vegetation, feeding on periphyton; Radix spp. accumulate in vegetation-free areas, on stones or muddy sediments; and physid snails prefer detritus as a source of food (312, 313). In addition, cercariae of avian schistosomes can be transported by wind and water currents for several kilometers (2, 304, 305). Therefore, taking the local situation into account is essential for risk assessment, because conditions unsuitable for one snail species may provide an ideal habitat for another.
Season and time of day were shown to have considerable effects. Most cases of cercarial dermatitis correlate with high air and water temperatures during the summer months, when schistosome development in snails is amplified and emission rates of cercariae are stimulated by sunlight (4, 11, 279, 280, 304, 305, 309, 314). Production can reach several thousands of cercariae/snail/day (169, 300, 315), and these emission rates may even counterbalance the loss of cercarial capability to survive and infect hosts at high temperatures (see above). Irrespective of the season, outbreaks of cercarial dermatitis have been reported in geothermally heated lakes and ponds in Iceland (71). Time of day proved to be another risk factor. Production of schistosome cercariae is usually discontinuous—particular species may differ in circadian rhythms of cercarial release. This is usually attributed to the peak activity of the preferred definitive hosts. Thus, Trichobilharzia and some other genera infecting aquatic birds leave the snail host during the light period of the day, mainly in the morning hours (2, 4, 280, 305). Increased illumination, temperature, and snail locomotory activity trigger cercarial emergence (169, 211, 316, 317). Patterns of cercarial emergence can be changed almost immediately by reversing the dark-light regimen, suggesting that light has a decisive influence on the direction of the process (317, 318). The number of cercariae released from snails may vary greatly depending on the parasite/host species and the phase (age) of infection; large species of snails represent a higher risk, as more cercariae are produced per snail and released into the environment (10, 275, 315). This may also explain why some species of avian schistosomes that use smaller species of snails are not often detected during an outbreak.
Control Measures Related to the Ecology of Avian Schistosomes
In order to reduce the risk of cercarial dermatitis, either water use during peak cercarial emergence should be avoided or the life cycle of avian schistosomes should be interrupted. Interruption of the schistosome life cycle has been tried on a number of occasions. One option is to reduce the prevalence of adult avian schistosomes in birds by eliminating ducks from high-risk areas. However, public acceptance of regular hunting or repelling of birds (ducks) at recreational lakes during summer months is uncertain. Another option is to treat birds with the antihelminthic drug praziquantel. Birds are captured in the field by use of modified dive traps and subsequently treated with praziquantel and then released. Recapture results showed a significant reduction of infections in mallards (319–321). Furthermore, in the subsequent year, there was a marked decline in prevalence of avian schistosomes in snails (320). However, treatment of the entire duck population is time-consuming and labor-intensive.
Reduction or elimination of snail populations is another strategy of cercarial dermatitis control by interrupting the life cycle. In the past, application of commercial molluscicides, such as niclosamide or copper sulfate, was common (319). However, repeated and unlimited treatments had a deleterious effect on animal communities. In addition, after repeated exposures, some snails may become resistant to copper sulfate or avoid the treatment by burrowing into the mud (322, 323). Manual collection of snails (309, 324) or destruction of their habitats by local removal of littoral vegetation (2) represents a less harmful approach to snail control. Large-scale destruction of snail populations by use of heavy machines along the shores of Annecy Lake (France) and Cultus Lake (Canada) led to substantial reductions of cercarial dermatitis (2). As for the biological control of snail populations, use of molluscivorous fish or prawns as natural snail predators is promising for long-term control (325–328), although it represents a risk for native flora and fauna if nonindigenous organisms are introduced.
Free-swimming miracidia and cercariae may represent a promising target for life cycle interruption. Snail finding and recognition by miracidia are based markedly on recognition of host molecules called miraxones (see the information on intramolluscan development), which led to a proposal to use miracidial traps containing miraxones (329). Unfortunately, due to the high level of diversity of avian schistosomes and presumed variability of snail recognition (139), this proposal proved unrealistic. Similarly, cercarial traps based on species-specific host-finding behavior (see the information on vertebrate host finding) might provide local protection (330). For example, fatty acid-stimulated transformation to the schistosomulum stage could be initiated in these traps, leading to the loss of resistance against a hypo-osmotic water environment (178, 331, 332). As far as trophic interactions of organisms are concerned, predation by small aquatic animals may represent a promising means of biological control. Schistosome miracidia and cercariae may serve as prey for larval aquatic insects, crustaceans, oligochaetes, shrimps, and fish (271, 333–338). In particular, the annelid Chaetogaster limnaei sensu lato, living commensally or parasitically on the shell surface or in the mantle and pulmonary cavities of freshwater snails, may act as an efficient predator and prevent penetration of miracidia (339, 340) or feed on cercariae as they are released from the snail (341, 342).
Finally, competitive interactions among trematode larval communities within an individual snail can substantially influence establishment and survival of schistosome intramolluscan stages. In terms of interspecific interactions, particular species of trematodes can be dominant, usually by producing competitively aggressive rediae, or subordinate because they have less aggressive sporocysts and no redial stage. Schistosomes belong to the latter group, and their sporocysts are eliminated via the predatory interactions of redia-producing trematodes, e.g., echinostomes (301, 343). Yet schistosomes may exert a dominant effect in some cases. The avian schistosome Trichobilharzia brevis persists in coinfections with two dominant echinostomes, Echinostoma audyi and Hypoderaeum dingeri (343), and can cause developmental suppression of the latter species. The nonpredatory exclusion of H. dingeri by T. brevis has also been shown experimentally (344). Intertrematode competition was recently identified as an important determinant in transmission of avian schistosomes. For example, competitive exclusion was estimated to result in an 18.0% reduction of Trichobilharzia szidati in Lymnaea stagnalis (345). Interestingly, T. szidati may represent a subordinate species, yet it frequently cooccurs in snails with other trematodes. Therefore, T. szidati may be either an obligate secondary invader of snails with a compromised immune system or a schistosome that can coexist in double infections, as was demonstrated for other species of Trichobilharzia and Austrobilharzia (302, 343, 346).
PERSPECTIVES
The more we know about avian schistosome diversity and distribution, the better we are positioned to understand their evolutionary history and potential for the future dynamics of dermatitis outbreaks. These days, we have practical means for their identification and can better gauge the likelihood that some of these species may emerge in new contexts to cause unexpected problems. An effort to describe the avian schistosome species diversity in their hosts in the circumpolar regions, where most migratory birds spend the summer (and young birds become infected), seems to be a critical component to understanding the epidemiology of cercarial dermatitis. In addition, the condition itself and the host-parasite interaction require further characterization at the molecular level. At least three examples of future applications can be mentioned. (i) A more detailed knowledge of cercarial penetration mechanisms may help in the prevention of dermatitis by introducing new formulations containing inhibitory molecules. (ii) Characterization of biologically active secretions produced by avian schistosomes may help us to understand the molecular basis of tissue pathology in avian and mammalian hosts. (iii) Newly designed primers or carefully selected antigens may be developed for reliable antibody- or DNA-based diagnostic tools. Such tools may be used to screen people or animals with health problems following recent water contact to assess the risk associated with exposure to cercariae of avian schistosomes.
ACKNOWLEDGMENTS
The University of New Mexico partially supported this study through a National Institutes of Health grant to Eric S. Loker (R01 AI101438) and a National Science Foundation grant to S.V.B. (DEB 1021427). M.S. is grateful for financial support from the Institute of Parasitology (RVO; grant 60077344), Biology Centre of the Academy of Sciences of the Czech Republic. The scientific activities of P.H., L.M., L.L., and V.S. were financially supported by Charles University in Prague (research program P41/PrFUK, UNCE grant 204017, GAUK grant 435911, and grant SVV 260074/2014) and the Czech Science Foundation (grant 13-29577S).
Biographies

Petr Horák, M.Sc., Ph.D., is a Professor of Parasitology and Head of the Department of Parasitology at the Faculty of Science, Charles University in Prague, Czech Republic. He obtained his parasitology/biology degree from the Charles University in Prague. The President of the Czech Republic appointed Petr Horák as a Professor of Parasitology in 2005. Professor Horák has presented and published on many topics, dealing with parasitic helminths in general and cercarial dermatitis/avian schistosomes in particular. His teaching activities lie in general parasitology and the biology of helminths. His research is focused on functional morphology of helminths, trematodes, and avian schistosomes (systematics, life cycles, and host-parasite interactions). Details of his personal data can be found at http://www.petrhorak.eu.

Libor Mikeš, M.Sc., Ph.D., is an Assistant Professor of Parasitology at the Department of Parasitology, Faculty of Science, Charles University in Prague, from which he received his master's and doctoral degrees. His international experience includes, e.g., stays at the Keele University, United Kingdom, and the Institute for the Biotechnology of Infectious Diseases, UTS, Sydney, Australia. Dr. Mikeš is currently the President of the Czech Society for Parasitology. His basic academic interests lie in the general biology of helminths (with a special focus on the trematode superfamilies Schistosomatoidea and Diplostomoidea). He is also involved in teaching molecular and cellular interactions between parasites and hosts and biochemical parasitology, and he leads several field courses in parasitology. His research and publication activities are focused mainly on the biochemistry of helminths (proteomics, peptidases of trematodes and monogeneans, and glycobiology of larval trematodes) and immune reactions of hosts against avian schistosomes.

Lucie Lichtenbergová, M.Sc., Ph.D., is a Postdoctoral Fellow at the Department of Parasitology, Faculty of Science, Charles University in Prague. She finished her master's study at the Masaryk University in Brno, Czech Republic, and obtained her Ph.D. from the Charles University in Prague, Czech Republic. Since her doctoral study, she has focused on the research of interactions of avian schistosomes and their natural avian and accidental mammalian hosts. Immunology and histopathology are her centers of interest.

Vladimír Skála, M.Sc., is a Ph.D. student at the Department of Parasitology, Faculty of Science, Charles University in Prague, Czech Republic. His master's degree was obtained at the same institution, and his master's thesis focused on the morphology and development of the monostome fluke Notocotylus attenuatus (Digenea: Notocotylidae). At present, he is investigating the defensive activity of hemocytes of two lymnaeid snails transmitting avian schistosomes and the immunomodulation caused by trematodes in their snail hosts. His research is carried out in collaboration with Anthony J. Walker from Kingston University, London, United Kingdom. Vladimír has presented the results of his research at several national and international scientific meetings.

Miroslava Soldánová, M.Sc., Ph.D., received a Ph.D. in parasitology at the Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic, in 2011. Simultaneously, she started as a Research Assistant at the Institute of Parasitology, Biology Centre, Academy of Sciences of the Czech Republic, where she currently continues as a Postdoctoral Fellow in the research team focused on trematode ecology and systematics. Her 1-year postdoctoral fellowship (2012–2013) at the University of Duisburg-Essen (Faculty of Biology, Aquatic Ecology, Germany) focused on the impact of eutrophication on the composition of trematode communities in large lake ecosystems in the Ruhr River, screening of freshwater snails in relation to trematode infection with avian schistosomes, and assessment of potential risks of cercarial dermatitis for humans. Her fields of interest comprise parasite ecology (trematode community structure and organization in freshwater molluscs), environmental parasitology (use of parasite communities in molluscs as bioindicators), and trematode taxonomy.

Sara Vanessa Brant, Ph.D., is an Assistant Research Professor and Senior Collections Manager of the Museum of Southwestern Biology, Division of Parasites, at the University of New Mexico. She obtained her parasitology/biology M.S. and Ph.D. from the University of Nebraska-Lincoln. Her main research focus is the evolutionary and ecological diversity of trematodes, in particular those in the blood fluke families (Schistosomatidae, Spirorchiidae, Sanguinicolidae). Her recent efforts have been funded to address global avian schistosome diversity and their host interactions. She is also involved in efforts to motivate and promote the importance of placing both parasite and host vouchers in permanent museum collections for future studies.
REFERENCES
- 1.Cort WW. 1928. Schistosome dermatitis in the United States (Michigan). JAMA 90:1027–1029. doi: 10.1001/jama.1928.02690400023010. [DOI] [PubMed] [Google Scholar]
- 2.Leighton BJ, Nervos S, Webster JM. 2000. Ecological factors in schistosome transmission, and an environmentally benign method for controlling snails in a recreational lake with a record of schistosome dermatitis. Parasitol Int 49:9–17. doi: 10.1016/S1383-5769(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 3.Nesterenko SV, Malko NJ. 2007. Dynamics of human allergic dermatites caused by schistosomes in the Mjadel district (1995–2006), p 202–207. In Problems of cercarial dermatitis in the Naroch district. Scientific seminar. Medisont, Minsk, Belarus. [Google Scholar]
- 4.Chamot E, Toscani L, Rougemont A. 1998. Public health importance and risk factors for cercarial dermatitis associated with swimming in Lake Leman at Geneva, Switzerland. Epidemiol Infect 120:305–314. doi: 10.1017/S0950268898008826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter GW, Ritchie LS, Tanabe H. 1951. The epidemiology of schistosome dermatitis (‘koganbyo') in Japan. Trans R Soc Trop Med Hyg 45:103–112. doi: 10.1016/S0035-9203(51)90604-9. [DOI] [PubMed] [Google Scholar]
- 6.Beer SA, Voronin MV. 2007. Cercarioses in urbanized ecosystems. Nauka, Moscow, Russia. [Google Scholar]
- 7.Kolářová L, Horák P, Skírnisson K, Marečková H, Doenhoff M. 2013. Cercarial dermatitis, a neglected allergic disease. Clin Rev Allergy Immunol 45:63–74. doi: 10.1007/s12016-012-8334-y. [DOI] [PubMed] [Google Scholar]
- 8.Soldánová M, Selbach C, Kalbe M, Kostadinova A, Sures B. 2013. Swimmer's itch: etiology, impact, and risk factors in Europe. Trends Parasitol 29:65–74. doi: 10.1016/j.pt.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Horák P, Kolářová L. 2011. Snails, waterfowl and cercarial dermatitis. Freshwater Biol 56:779–790. doi: 10.1111/j.1365-2427.2010.02545.x. [DOI] [Google Scholar]
- 10.Brant SV, Loker ES. 2009. Schistosomes in the southwest United States and their potential for causing cercarial dermatitis or ‘swimmer's itch’. J Helminthol 83:191–198. doi: 10.1017/S0022149X09308020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdovinos C, Balboa C. 2008. Cercarial dermatitis and lake eutrophication in south-central Chile. Epidemiol Infect 136:391–394. doi: 10.1017/S0950268807008734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brant SV, Loker ES. 2013. Discovery-based studies of schistosome diversity stimulate new hypotheses about parasite biology. Trends Parasitol 29:449–459. doi: 10.1016/j.pt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horák P, Adema CM, Kolářová L. 2002. Biology of the schistosome genus Trichobilharzia. Adv Parasitol 52:155–233. doi: 10.1016/S0065-308X(02)52012-1. [DOI] [PubMed] [Google Scholar]
- 14.Loker ES, Brant SV. 2006. Diversification, dioecy and dimorphism in schistosomes. Trends Parasitol 22:521–528. doi: 10.1016/j.pt.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Grodhaus G, Keh B. 1958. The marine, dermatitis-producing cercariae of Austrobilharzia variglandis in California (Trematoda: Schistosomatidae). J Parasitol 44:633–638. doi: 10.2307/3274549. [DOI] [PubMed] [Google Scholar]
- 16.Morales GA, Helmboldt CF, Penner LR. 1971. Pathology of experimentally induced schistosome dermatitis in chickens: the role of Ornithobilharzia canaliculata (Rudolphi, 1819) Odhner 1912 (Trematoda: Schistosomatidae). Avian Dis 15:262–276. doi: 10.2307/1588696. [DOI] [PubMed] [Google Scholar]
- 17.Penner LR. 1950. Cercaria littorinalinae sp. nov., a dermatitis-producing schistosome larvae from the marine snail, Littorina planaxis Philippi. J Parasitol 36:466–472. doi: 10.2307/3273174. [DOI] [PubMed] [Google Scholar]
- 18.Orris L, Combes FC. 1952. Clam digger's dermatitis; schistosome dermatitis from sea water. Arch Derm Syphilol 66:367–370. doi: 10.1001/archderm.1952.01530280071011. [DOI] [PubMed] [Google Scholar]
- 19.Sindermann CJ, Gibbs RF. 1953. A dermatitis producing schistosome, which causes “clam diggers itch” along the central Maine coast. Maine Dept Sea Shore Fish Res Bull 12:143–162. [Google Scholar]
- 20.Bearup AJ. 1955. A schistosome larva from the marine snail Pyrazus australis as a cause of cercarial dermatitis in man. Med J Aust 1:955–960. [PubMed] [Google Scholar]
- 21.Sindermann CJ. 1960. Ecological studies of marine dermatitis-producing schistosome larvae in northern New England. Ecology 41:678–684. doi: 10.2307/1931801. [DOI] [Google Scholar]
- 22.Short RB, Holliman RB. 1961. Austrobilharzia penneri, a new schistosome from marine snails. J Parasitol 47:447–452. doi: 10.2307/3275378. [DOI] [Google Scholar]
- 23.Rohde K. 1977. The bird schistosome Austrobilharzia terrigalensis from the Great Barrier Reef, Australia. Z Parasitenkd 52:39–51. doi: 10.1007/BF00380557. [DOI] [PubMed] [Google Scholar]
- 24.Appleton CC. 1989. Translocation of an estuarine whelk and its trematodes parasites in Australia. Environ Conserv 16:172–182. doi: 10.1017/S0376892900008973. [DOI] [Google Scholar]
- 25.Leighton BJ, Ratzlaff D, McDougall D, Stewart G, Nadan A, Gustafson L. 2004. Schistosome dermatitis at Crescent Beach. Preliminary report. Environ Health Rev 48:5–13. [Google Scholar]
- 26.Walker JC. 2005. Medical importance: marine schistosome dermatitis, p 439–442. In Rhodes K. (ed), Marine parasitology. CSIRO Publishing, Collingwood, Victoria, Australia. [Google Scholar]
- 27.Al-Kandari WY, Al-Bustan SA, Isaac AM, George BA, Chandy BS. 2012. Molecular identification of Austrobilharzia species parasitizing Cerithidia cingulata (Gastropoda: Potamididae) from Kuwait Bay. J Helminthol 86:470–478. doi: 10.1017/S0022149X11000733. [DOI] [PubMed] [Google Scholar]
- 28.Boros DL. 1989. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev 2:250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley JJC. 1938. On a dermatitis in Malays caused by the cercariae of Schistosoma spindale Montgomery, 1906. J Helminthol 16:117–120. doi: 10.1017/S0022149X00018368. [DOI] [Google Scholar]
- 30.Anantaraman M. 1958. On schistosome dermatitis. I. Dermatitis in India caused by cercariae of Schistosoma spindale Montgomery, 1906. Indian J Helminthol 10:46–52. [Google Scholar]
- 31.Islam KS, Awal MA. 1991. Schistosome dermatitis in Bangladesh. Indian J Parasitol 15:165–169. [Google Scholar]
- 32.Narain K, Mahanta J, Dutta R, Dutta P. 1994. Paddy field dermatitis in Assam: a cercarial dermatitis. J Commun Dis 26:26–30. [PubMed] [Google Scholar]
- 33.Narain K, Rajguru SK, Mahanta J. 1998. Incrimination of Schistosoma spindale as a causative agent of farmer's dermatitis in Assam with a note on liver pathology in mice. J Commun Dis 30:1–6. [PubMed] [Google Scholar]
- 34.Agrawal MC, Rao VG, Vohra S, Bhondeley MK, Ukey MJ, Anvikar AR, Yadav R. 2007. Is active human schistosomiasis present in India? Curr Sci 92:889. [Google Scholar]
- 35.Rao VG, Dash AP, Agrawal MC, Yadav RS, Anvikar AR, Vohra S, Bhondeley MK, Ukey MJ, Das SK, Minocha RK, Tiwari BK. 2007. Cercarial dermatitis in central India: an emerging health problem among tribal communities. Ann Trop Med Parasitol 101:409–413. doi: 10.1179/136485907X176463. [DOI] [PubMed] [Google Scholar]
- 36.Wang CR, Chen J, Zhao JP, Chen AH, Zhai YQ, Li L, Zhu XQ. 2009. Orientobilharzia species: neglected parasitic zoonotic agents. Acta Trop 109:171–175. doi: 10.1016/j.actatropica.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal MC. 2012. Schistosomes and schistosomiasis in South Asia. Springer, New Delhi, India. [Google Scholar]
- 38.Devkota R, Brant SV, Thapa A, Loker ES. 2014. Sharing schistosomes: the elephant schistosome Bivitellobilharzia nairi also infects the greater one-horned rhinoceros (Rhinoceros unicornis) in Chitwan National Park, Nepal. J Helminthol 88:32–40. doi: 10.1017/S0022149X12000697. [DOI] [PubMed] [Google Scholar]
- 39.Mudaliar SV, Ramanujachary G. 1945. Schistosoma nairi n. sp. from an elephant. Indian Vet J 12:1–5. [Google Scholar]
- 40.Brant SV, Morgan JAT, Mkoji GM, Snyder SD, Rajapakse JRPV, Loker ES. 2006. An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including DNA sequence from single life cycle stages. J Parasitol 92:77–88. doi: 10.1645/GE-3515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brant SV, Pomajbíková K, Modrý D, Petrželková KJ, Loker ES. 2013. Molecular phylogenetics of the elephant schistosome, Bivitellobilharzia loxodontae (Trematoda: Schistosomatidae) from Central African Republic. J Helminthol 87:102–107. doi: 10.1017/S0022149X1200003X. [DOI] [PubMed] [Google Scholar]
- 42.Vogel H, Minning W. 1940. Bilharziose bei Elefanten. Arch Schiffs Trop Hyg 42:562–574. [Google Scholar]
- 43.Thurston JP. 1963. Schistosomes from Hippopotamus amphibious L. I. The morphology of Schistosoma hippopotami sp. nov. Parasitology 53:49–54. [DOI] [PubMed] [Google Scholar]
- 44.Thurston JP. 1964. Schistosomes from Hippopotamus amphibious L. II. The morphology of Schistosoma edwardiense sp. nov. Parasitology 54:67–72. [DOI] [PubMed] [Google Scholar]
- 45.Thurston JP. 1971. Further studies on Schistosoma hippopotami and Schistosoma edwardiense in Uganda. Rev Zool Bot Afr 84:145–152. [Google Scholar]
- 46.Cort WW. 1914. Larval trematodes from North American fresh-water snails. J Parasitol 1:65–84. doi: 10.2307/3271183. [DOI] [Google Scholar]
- 47.Cort WW. 1936. Studies on schistosome dermatitis. IV. Further information on distribution in Canada and the United States. Am J Hyg (Lond) 24:318–333. [Google Scholar]
- 48.Penner LR. 1942. Studies on dermatitis producing schistosomes in eastern Massachusetts, with emphasis on the status of Schistosomatium pathlocopticum Tanabe, 1923. J Parasitol 28:103–112. doi: 10.2307/3272720. [DOI] [Google Scholar]
- 49.Malek EA, Armstrong JC. 1967. Infection with Heterobilharzia americana in primates. Am J Trop Med Hyg 16:708–714. [PubMed] [Google Scholar]
- 50.Wall RC. 1968. “Swimmer's itch” in the Great Lakes region. Wards Bull 7:1–7. [Google Scholar]
- 51.Price HF. 1931. Life history of Schistosomatium douthitti (Cort). Am J Hyg (Lond) 13:685–727. [Google Scholar]
- 52.Malek EA. 1977. Geographical distribution, hosts, and biology of Schistosomatium douthitti (Cort, 1914) Price, 1931. Can J Zool 55:661–671. doi: 10.1139/z77-087. [DOI] [PubMed] [Google Scholar]
- 53.McKown RD, Veatch JK, Fox LB. 1991. New locality record for Heterobilharzia americana. J Wildl Dis 27:156–160. doi: 10.7589/0090-3558-27.1.156. [DOI] [PubMed] [Google Scholar]
- 54.Corapi WV, Snowden KF, Rodrigues A, Porter FB, Buote MA, Birch SM, Jackson ND, Eden KB, Whitley DB, Mansell J, Edwards JF, Hardy J, Chaffin MK. 2012. Natural Heterobilharzia americana infection in horses in Texas. Vet Pathol 49:552–556. doi: 10.1177/0300985811432346. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Bai G. 1976. On bird schistosomes from Jilin Province: Jilinobilharzia crecci gen. nov., sp. nov. (Schistosomatidae: Bilharziellinae) with a discussion on the taxonomy of the subfamily Bilharziellinae. Acta Zool Sin 22:385–392. [Google Scholar]
- 56.Leigh WH. 1955. The morphology of Gigantobilharzia huttoni (Leigh, 1953) an avian schistosome with marine dermatitis producing larvae. J Parasitol 41:262–269. doi: 10.2307/3274202. [DOI] [PubMed] [Google Scholar]
- 57.Szidat L. 1958. Investigaciones sobre Cercaria chascomusi n. sp. agente causal de una nueva enfermedad humana en la Argentina: ‘la dermatitis de los banistas de la laguna Chascomus.' Bol Museo Argent Cienc Nat 18:1–13. [Google Scholar]
- 58.Ito J. 1960. Studies on the morphology and life cycle of Pseudobilharziella corvi Yamaguti, 1941 (Trematoda: Schistosomatidae). Jpn J Med Sci Biol 13:53–58. doi: 10.7883/yoken1952.13.53. [DOI] [PubMed] [Google Scholar]
- 59.Ewers WH. 1961. A new intermediate host of schistosome trematodes from New South Wales. Nature 190:283–284. [DOI] [PubMed] [Google Scholar]
- 60.Leedom WS, Short RB. 1981. Cercaria pomaceae sp. n., a dermatitis producing schistosome cercaria from Pomacea paludosa, the Florida apple snail. J Parasitol 67:257–261. doi: 10.2307/3280646. [DOI] [Google Scholar]
- 61.Martorelli SR. 1984. Sobre una cercaria de la familia Schistosomatidae (Digenea) parasita de Chilina gibbosa Sowerby, 1841 en el Lago Pellegrini, Provincia de Rio Negro, Republica Argentina. Neotropica 30:97–106. [Google Scholar]
- 62.Blair D, Davis GM, Wu B. 2001. Evolutionary relationships between trematodes and snails emphasizing schistosomes and paragonimids. Parasitology 123:S229–S243. doi: 10.1017/S003118200100837X. [DOI] [PubMed] [Google Scholar]
- 63.Aldhoun JA, Faltýnková A, Karvonen A, Horák P. 2009. Schistosomes in the North: a unique finding from a prosobranch snail using molecular tools. Parasitol Int 58:314–317. doi: 10.1016/j.parint.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Brant SV, Cohen AN, James D, Hui L, Hom A, Loker ES. 2010. Cercarial dermatitis transmitted by an exotic marine snail. Emerg Infect Dis 16:1357–1365. doi: 10.3201/eid1609.091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karamian M, Aldhoun JA, Maraghi S, Hatam G, Farhangmehr B, Sadjjadi SM. 2011. Parasitological and molecular study of the furcocercariae from Melanoides tuberculata as a probable agent of cercarial dermatitis. Parasitol Res 108:955–962. doi: 10.1007/s00436-010-2138-x. [DOI] [PubMed] [Google Scholar]
- 66.Schuster RK, Aldhoun JA, O'Donovan D. 2014. Gigantobilharzia melanoidis n. sp. (Trematoda: Schistosomatidae) from Melanoides tuberculata (Gastropoda: Thiaridae) in the United Arab Emirates. Parasitol Res 113:959–972. doi: 10.1007/s00436-013-3728-1. [DOI] [PubMed] [Google Scholar]
- 67.Aldhoun JA, Kolářová L, Horák P, Skírnisson K. 2009. Bird schistosome diversity in Iceland: molecular evidence. J Helminthol 83:173–180. doi: 10.1017/S0022149X09289371. [DOI] [PubMed] [Google Scholar]
- 68.Aldhoun JA, Podhorský M, Holická M, Horák P. 2012. Bird schistosomes in planorbid snails in the Czech Republic. Parasitol Int 61:250–259. doi: 10.1016/j.parint.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Jouet D, Ferté H, Hologne C, Kaltenbach ML, Depaquit J. 2009. Avian schistosomes in French aquatic birds: a molecular approach. J Helminthol 83:181–189. doi: 10.1017/S0022149X09311712. [DOI] [PubMed] [Google Scholar]
- 70.Brant SV, Loker ES. 2009. Molecular systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. J Parasitol 95:941–963. doi: 10.1645/GE-1870.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skírnisson K, Aldhoun JA, Kolářová L. 2009. A review on swimmer's itch and the occurrence of bird schistosomes in Iceland. J Helminthol 83:165–171. doi: 10.1017/S0022149X09336408. [DOI] [PubMed] [Google Scholar]
- 72.Fain A. 1955. Sur une furcocercaire du groupe Ocellata produisant experimentalement la dermatitie des nageurs a Astrida (Ruanda-Urundi). Ann Soc Belg Med Trop 35:701–707. [PubMed] [Google Scholar]
- 73.De Meillon B, Stoffberg N. 1954. Swimmer's itch in South Africa. S Afr Med J 28:1062–1064. [PubMed] [Google Scholar]
- 74.Appleton CC, Brock K. 1986. The penetration of mammalian skin by cercariae of Trichobilharzia sp. (Trematoda: Schistosomatidae) from South Africa. Onderstepoort J Vet Res 53:209–211. [PubMed] [Google Scholar]
- 75.Johnston TH. 1941. Bather's itch (schistosome dermatitis) in the Murray swamps, South Australia. Trans R Soc S Aust 65:276–284. [Google Scholar]
- 76.Macfarlane WV. 1944. Schistosome dermatitis in the southern lakes: an investigation of swimmers' itch. N Z Med J 43:136–140. [Google Scholar]
- 77.Macfarlane WV. 1952. Schistosome dermatitis in Australia. Med J Aust 39:669–672. [DOI] [PubMed] [Google Scholar]
- 78.Blair D, Islam KS. 1983. The life cycle and morphology of Trichobilharzia australis n. sp. (Digenea: Schistosomatidae) from the nasal blood vessels of the black duck (Anas superciliosa) in Australia, with a review of the genus Trichobilharzia. Syst Parasitol 5:89–117. doi: 10.1007/BF00049237. [DOI] [Google Scholar]
- 79.Featherston DW, Weeks PJ, Featherston N. 1988. Schistosome dermatitis in Lake Wanaka: Cercaria longicauda prevalence in Lymnaea tomentosa, 1978–83. N Z J Zool 15:381–386. doi: 10.1080/03014223.1988.10422963. [DOI] [Google Scholar]
- 80.Sandosham AA. 1953. Malaysian parasites. XII. Cercarial dermatitis caused by a member of the “elvae” group. Malaya 26:195–198. [Google Scholar]
- 81.Margono SS. 1968. Cercaria of Trichobilharzia brevis Basch, 1966 in Djakarta as a possible cause of schistosome dermatitis. Med J Malaya 22:306–312. [PubMed] [Google Scholar]
- 82.Wooi CE, Hong SLL, Ambu S. 2007. Cercarial dermatitis in Kelantan, Malaysia an occupation related health problem. Int eJ Sci Med Educ 1:69–73. [Google Scholar]
- 83.Farahnak A, Essalat M. 2003. A study on cercarial dermatitis in Khuzestan province, southwestern Iran. BMC Public Health 3:35–38. doi: 10.1186/1471-2458-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Athari A, Gohar-Dehi S, Rostami-Jalilian M. 2006. Determination of definitive and intermediate hosts of cercarial dermatitis-producing agents in northern Iran. Arch Iran Med 9:11–15. [PubMed] [Google Scholar]
- 85.Maleki SH, Athari A, Haghighi A, Taghipour N, Gohardehi SH, Seyyed Tabaei S. 2012. Species identification of birds nasal Trichobilharzia in Sari, North of Iran. Iran J Parasitol 7:82–85. [PMC free article] [PubMed] [Google Scholar]
- 86.Gohardehi S, Fakhar M, Madjidaei M. 2013. Avian schistosomes and human cercarial dermatitis in a wildlife refuge in Mazandaran province, northern Iran. Zoonoses Public Health 60:442–447. doi: 10.1111/zph.12020. [DOI] [PubMed] [Google Scholar]
- 87.Kullavanijaya P, Wongwaisayawan H. 1993. Outbreak of cercarial dermatitis in Thailand. Int J Dermatol 32:113–115. doi: 10.1111/j.1365-4362.1993.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Minsheng C, Hui K, Lin L. 1980. A survey of the etiological agent of paddy field dermatitis with studies on the life cycle of Trichobilharzia jianensis Liu et al., 1977. Acta Zool Sin 26:153–159. [Google Scholar]
- 89.Li C, Ma C, Gu J, Lu W. 1998. An epidemiological survey of schistosome cercarial dermatitis among the residents living along the banks of the Huaihe River. Chin J Parasitol Parasit Dis 16:384–387. [PubMed] [Google Scholar]
- 90.Szidat L. 1951. Cercarias schistosomicas y dermatitis schistosomica humana en la Republica Argentina. Commun Inst Nac Invest Cienc Nat Museo Argent Cienc Nat Bernardino Rivadavia 2:129–150. [Google Scholar]
- 91.Bosq P, Szidat L, Soria MF. 1955. Dermatitis schistosomica por Cercaria chascomusi. Prensa Med Argent 42:3500–3504. [PubMed] [Google Scholar]
- 92.Reyes E. 1944. Dermatitis sistosomiasica en El Salvador. Arch Hosp Rosales 31:3–8. [Google Scholar]
- 93.Joshi SK, Phil M. 2002. Rice field work and the occupational hazards. Occup Med 4:111–114. [Google Scholar]
- 94.Tanaka M. 1960. Studies on Trichobilharzia physellae in Oki Islands. 2. Four kinds of schistosome cercariae parasitic in Lymnaea japonica in Oki Islands. Jpn J Parasitol 9:604–609. [Google Scholar]
- 95.Yang K, Tchou T, T'ang CC, Ho T, Luo H. 1965. A study on dermatitis in rice farmers. Chin Med J 84:143–159. [PubMed] [Google Scholar]
- 96.Basch PF. 1966. The life cycle of Trichobilharzia brevis n. sp., an avian schistosome from Malaya. Z Parasitenkd 27:242–251. [DOI] [PubMed] [Google Scholar]
- 97.Bearup AJ, Langsford WA. 1966. Schistosome dermatitis in association with rice growing in the northern territory of Australia. Med J Aust 1:521–525. [DOI] [PubMed] [Google Scholar]
- 98.Kumada N, Ohya S, Makiya K. 1970. Paddy field dermatitis in Aichi Prefecture. II. Results of the survey of fresh-water snails and parasitic cercariae in the western region of the prefecture. Rep Aichi Inst Public Health 20:65–73. [Google Scholar]
- 99.Kumada N, Ohya S, Makiya K. 1971. Paddy field dermatitis in Aichi Prefecture. V. Eggs and adults of the genus Trichobilharzia recovered from resident waterfowls in the endemic area. Rep Aichi Inst Public Health 21:39–46. [Google Scholar]
- 100.Oda T. 1973. Schistosome dermatitis in Japan. Prog Med Parasitol Jpn 5:5–63. [Google Scholar]
- 101.Suzuki N, Kawanaka M. 1980. Trichobilharzia brevis Basch 1966, as a cause of an outbreak of cercarial dermatitis in Japan. Jpn J Parasitol 29:1–11. [Google Scholar]
- 102.Makiya K, Ishiguro T. 1982. Population studies on Austropeplea ollula (Gould), the snail intermediate host of dermatitis-producing avian schistosomes. Nagoya J Med Sci 44:47–55. [PubMed] [Google Scholar]
- 103.Hoque MA, Skerratt LF, Rahman MA, Alim MA, Grace D, Bummow B, Rabiul Alam Beg ABM, Debnath NC. 2011. Monitoring the health and production of household Jinding ducks on Hatia Island of Bangladesh. Trop Anim Health Prod 43:431–440. doi: 10.1007/s11250-010-9710-3. [DOI] [PubMed] [Google Scholar]
- 104.Fernandez MV, Hamann MI, Nafiez MO. 2013. Larval trematodes of Biomphalaria straminea (Mollusca: Planorbidae) in ricefield in Corrientes Province, Argentina. Rev Mex Biodivers 84:756–764. doi: 10.7550/rmb.33748. [DOI] [Google Scholar]
- 105.Brant SV, Jouet D, Ferté H, Loker ES. 2013. Anserobilharzia gen. n. (Digenea, Schistosomatidae) and redescription of A. brantae (Farr & Blankemeyer, 1956) comb. n. (syn. Trichobilharzia brantae), a parasite of geese (Anseriformes). Zootaxa 3670:193–206. doi: 10.11646/zootaxa.3670.2.5. [DOI] [PubMed] [Google Scholar]
- 106.Devkota R, Brant SV, Thapa A, Loker ES. 2014. Two avian schistosome cercariae from Nepal, including a Macrobilharzia-like species from Indoplanorbis exustus. Parasitol Int 63:374–380. doi: 10.1016/j.parint.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Loker ES. 1983. A comparative study of the life-histories of mammalian schistosomes. Parasitology 87:343–369. doi: 10.1017/S0031182000052689. [DOI] [PubMed] [Google Scholar]
- 108.Rollinson D, Southgate VR. 1987. The genus Schistosoma: a taxonomic appraisal, p 1–49. In Rollinson D, Simpson AJG (ed), The biology of schistosomes: from genes to latrines. Academic Press, United Kingdom. [Google Scholar]
- 109.Paraense WL, Pointier JP. 2003. Physa acuta Draparnaud, 1805 (Gastropoda: Physidae): a study of topotypic specimens. Mem Inst Oswaldo Cruz 98:513–517. doi: 10.1590/S0074-02762003000400016. [DOI] [PubMed] [Google Scholar]
- 110.Taylor DW. 2003. Introduction to Physidae (Gastropoda: Hygrophila); biogeography, classification, morphology. Rev Biol Trop 51(Suppl 1):1–263, 265–287. [PubMed] [Google Scholar]
- 111.Kock KN, Wolmarans CT. 2007. Distribution and habitats of the alien invader freshwater snail Physa acuta in South Africa. Water SA (Pretoria) 33:717–722. [Google Scholar]
- 112.Bastert J, Sing A, Wollenberg A, Korting HC. 1998. Aquarium dermatitis: cercarial dermatitis in an aquarist. Dermatology 197:84–86. doi: 10.1159/000017965. [DOI] [PubMed] [Google Scholar]
- 113.Folster-Holst R, Disko R, Rowert J, Bockeler W, Kreiselmaier I, Christophers E. 2001. Cercarial dermatitis contracted via contact with an aquarium: case report and review. Br J Dermatol 145:638–664. doi: 10.1046/j.1365-2133.2001.04414.x. [DOI] [PubMed] [Google Scholar]
- 114.Brant SV, Loker ES. 2005. Can specialized pathogens colonize distantly related hosts? Schistosome evolution as a case study. PLoS Pathog 1:e38. doi: 10.1371/journal.ppat.0010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kock S. 2001. Investigations of intermediate host specificity help to elucidate the taxonomic status of Trichobilharzia ocellata (Digenea: Schistosomatidae). Parasitology 123:67–70. doi: 10.1017/S0031182001008101. [DOI] [PubMed] [Google Scholar]
- 116.Khalifa R. 1976. Studies on Schistosomatidae Looss, 1899 (Trematoda) of aquatic birds of Poland. III. Notes on the morphology and life cycle of Dendritobilharzia pulverulenta (Braun, 1901). Acta Parasitol Pol 24:1–9. [Google Scholar]
- 117.Brant SV, Bochte CA, Loker ES. 2011. New intermediate host records for the avian schistosomes Dendritobilharzia pulverulenta, Gigantobilharzia huronensis, and Trichobilharzia querquedulae from North America. J Parasitol 97:946–949. doi: 10.1645/GE-2743.1. [DOI] [PubMed] [Google Scholar]
- 118.Davis NE. 2011. Avian schistosome research accomplished in New Zealand from 1991 through the present. Where to from here?, poster 179. 86th Meet Am Soc Parasitol, Anchorage, AK. [Google Scholar]
- 119.Leite ACR, Costa HMA, Costa JO. 1978. Trichobilharzia jequitibaensis sp. n. (Trematoda, Schistosomatidae) in Cairina moschata domestica (Anatidae). Rev Braz Biol 38:843–846. [Google Scholar]
- 120.Leite ACR, Costa HMA, Costa JO. 1979. Life cycle of Trichobilharzia jequitibaensis Leite, Costa and Costa, 1978 (Trematoda, Schistosomatidae). Rev Braz Biol 39:341–345. [Google Scholar]
- 121.Picard D, Jousson O. 2001. Genetic variability among cercariae of the Schistosomatidae (Trematoda: Digenea) causing swimmer's itch in Europe. Parasite 8:237–242. doi: 10.1051/parasite/2001083237. [DOI] [PubMed] [Google Scholar]
- 122.Jouet D, Skírnisson K, Kolářová L, Ferté H. 2010. Molecular diversity of Trichobilharzia franki in two intermediate hosts (Radix auricularia and Radix peregra): a complex of species. Infect Genet Evol 10:1218–1227. doi: 10.1016/j.meegid.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 123.Rudolfová J, Hampl V, Bayssade-Dufour C, Lockyer AE, Littlewood DTJ, Horák P. 2005. Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitol Res 95:79–89. doi: 10.1007/s00436-004-1262-x. [DOI] [PubMed] [Google Scholar]
- 124.Morgan JA, Dejong RJ, Adeoye GO, Ansa ED, Barbosa CS, Brémond P, Cesari IM, Charbonnel N, Corrêa LR, Coulibaly G, D'Andrea PS, De Souza CP, Doenhoff MJ, File S, Idris MA, Incani RN, Jarne P, Karanja DM, Kazibwe F, Kpikpi J, Lwambo NJ, Mabaye A, Magalhães LA, Makundi A, Moné H, Mouahid G, Muchemi GM, Mungai BN, Séne M, Southgate V, Tchuenté LA, Théron A, Yousif F, Zanotti-Magalhães EM, Mkoji GM, Loker ES. 2005. Origin and diversification of the human parasite Schistosoma mansoni. Mol Ecol 14:3889–3902. doi: 10.1111/j.1365-294X.2005.02709.x. [DOI] [PubMed] [Google Scholar]
- 125.Bayssade-Dufour C, Jouet D, Rudolfová J, Horák P, Ferté H. 2006. Seasonal morphological variations in bird schistosomes. Parasite 13:205–214. doi: 10.1051/parasite/2006133205. [DOI] [PubMed] [Google Scholar]
- 126.Chrisanfova GG, Lopatkin AA, Shestak AG, Mishchenkov VA, Zhukova TV, Akimova LN, Semyenova SK. 2011. Polymorphism of the cox1 mtDNA gene from cercarial isolates of the avian schistosome Bilharziella polonica (Trematoda: Schistosomatidae) from Belarussian lakes. Russ J Genet 47:603–609. doi: 10.1134/S1022795411050097. [DOI] [PubMed] [Google Scholar]
- 127.Rind S. 1989. Dendritobilharzia pulverulenta (Trematoda: Schistosomatidae) in New Zealand. N Z J Zool 16:215–220. doi: 10.1080/03014223.1989.10422571. [DOI] [Google Scholar]
- 128.Birmani NA, Dharejo AM, Khan MM, Shaikh AM. 2013. New record of Dendritobilharzia pulverulenta (Trematoda: Schistosomatidae) from Pakistan. J Anim Plant Sci 23:1215–1218. [Google Scholar]
- 129.Kolářová L, Rudolfová J, Hampl V, Skirnisson K. 2006. Allobilharzia visceralis gen. nov. sp. nov. (Schistosomatidae-Trematoda) from Cygnus cygnus (L.) (Anatidae). Parasitol Int 55:179–186. doi: 10.1016/j.parint.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 130.Brant SV. 2007. The occurrence of the avian schistosome Allobilharzia visceralis Kolářová, Rudolfová, Hampl et Skírnisson, 2006 (Schistosomatidae) in the tundra swan, Cygnus columbianus (Anatidae), from North America. Folia Parasitol 54:99–104. doi: 10.14411/fp.2007.013. [DOI] [PubMed] [Google Scholar]
- 131.Fain A. 1956. Les schistosomes d'oiseaux du genre Trichobilharzia Skrjabin et Zakharow, 1920 au Ruanda-Urundi. Rev Zool Bot Afr 54:147–178. [Google Scholar]
- 132.Rudolfová J, Sitko J, Horák P. 2006. Unusual finding of Trichobilharzia sp. in Motacilla alba in the Czech Republic. J Helminthol 80:83–85. doi: 10.1079/JOH2005321. [DOI] [PubMed] [Google Scholar]
- 133.Kolářová L, Skírnisson K, Ferté H, Jouet D. 2013. Trichobilharzia mergi sp. nov. (Trematoda: Digenea: Schistosomatidae), a visceral schistosome of Mergus serrator (L.) (Aves: Anatidae). Parasitol Int 62:300–308. doi: 10.1016/j.parint.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 134.Johnson KJ, Sorenson MD. 1999. Phylogeny and biogeography of the dabbling ducks (genus: Anas): a comparison of molecular and morphological evidence. Auk 116:792–805. doi: 10.2307/4089339. [DOI] [Google Scholar]
- 135.Jouet D, Skírnisson K, Kolářová L, Ferté H. 2010. Final hosts and variability of Trichobilharzia regenti under natural conditions. Parasitol Res 107:923–930. doi: 10.1007/s00436-010-1953-4. [DOI] [PubMed] [Google Scholar]
- 136.Murray JA. 2002. Effect of temperature on the longevity and infectivity of Trichobilharziastagnicolae miracidia in Stagnicola emarginata, p 13 7th Int Cong Med Appl Malacol, Los Banos, Laguna, Philippines. [Google Scholar]
- 137.Hertel J, Holweg A, Haberl B, Kalbe M, Haas W. 2006. Snail odour-clouds: spreading and contribution to the transmission success of Trichobilharzia ocellata (Trematoda: Digenea) miracidia. Oecologia 147:173–180. doi: 10.1007/s00442-005-0239-5. [DOI] [PubMed] [Google Scholar]
- 138.Kalbe M, Haberl B, Haas W. 1997. Miracidial host-finding in Fasciola hepatica and Trichobilharzia ocellata is stimulated by species-specific glycoconjugates released from the host snails. Parasitol Res 83:806–812. doi: 10.1007/s004360050344. [DOI] [PubMed] [Google Scholar]
- 139.Kalbe M, Haberl B, Haas W. 2000. Snail host finding by Fasciola hepatica and Trichobilharzia ocellata: compounds analysis of “miracidia-attracting glycoproteins.” Exp Parasitol 96:231–242. doi: 10.1006/expr.2000.4579. [DOI] [PubMed] [Google Scholar]
- 140.Haas W, Haberl B, Kalbe M, Körner M. 1995. Snail-host-finding by miracidia and cercariae: chemical host cues. Parasitology 11:468–472. [DOI] [PubMed] [Google Scholar]
- 141.Sluiters JE, Brussaard-Wust CM, Meuleman EA. 1980. The relationship between miracidial dose, production of cercariae, and reproductive activity of the host in the combination Trichobilharzia ocellata and Lymnaea stagnalis. Z Parasitenkd 63:13–26. doi: 10.1007/BF00927722. [DOI] [PubMed] [Google Scholar]
- 142.Joosse J, Van Elk R. 1986. Trichobilharzia ocellata: physiological characterization of giant growth, glycogen depletion, and absence of reproductive activity in the intermediate snail host, Lymnaea stagnalis. Exp Parasitol 62:1–13. doi: 10.1016/0014-4894(86)90002-0. [DOI] [PubMed] [Google Scholar]
- 143.Amen RI, Baggen JMC, Bezemer PD, de Jong-Brink M. 1992. Modulation of the activity of the internal defence system of the pond snail Lymnaea stagnalis by the avian schistosome Trichobilharzia ocellata. Parasitology 104:33–40. doi: 10.1017/S0031182000060777. [DOI] [PubMed] [Google Scholar]
- 144.Nuñez PE, Adema CM, de Jong-Brink M. 1994. Modulation of the bacterial clearance activity of haemocytes from the freshwater mollusc, Lymnaea stagnalis, by the avian schistosome, Trichobilharzia ocellata. Parasitology 109:299–310. doi: 10.1017/S0031182000078331. [DOI] [PubMed] [Google Scholar]
- 145.de Jong-Brink M. 1995. How schistosomes profit from the stress responses they elicit in their hosts. Adv Parasitol 35:177–256. doi: 10.1016/S0065-308X(08)60072-X. [DOI] [PubMed] [Google Scholar]
- 146.Horák P, van der Knaap WPW. 1997. Lectins in snail-trematode immune interactions: a review. Folia Parasitol 44:161–172. [Google Scholar]
- 147.van der Knaap WPW, Adema CM, Sminia T. 1993. Invertebrate blood cells: morphological and functional aspects of the haemocytes in the pond snail Lymnaea stagnalis. Comp Haematol Int 3:20–26. doi: 10.1007/BF00394923. [DOI] [Google Scholar]
- 148.Adema CM, van Deutekom-Mulder EC, van der Knaap WPW, Sminia T. 1994. Schistosomicidal activities of Lymnaea stagnalis haemocytes: the role of oxygen radicals. Parasitology 109:479–485. doi: 10.1017/S0031182000080732. [DOI] [PubMed] [Google Scholar]
- 149.Nuñez PE, de Jong-Brink M. 1997. The suppressive excretory-secretory product of Trichobilharzia ocellata: a possible factor for determining compatibility in parasite-host interactions. Parasitology 115:193–203. doi: 10.1017/S0031182097001169. [DOI] [PubMed] [Google Scholar]
- 150.Plows LD, Cook RT, Davies AJ, Walker AJ. 2004. Activation of extracellular-signal regulated kinase is required for phagocytosis by Lymnaea stagnalis haemocytes. Biochim Biophys Acta 1692:25–33. doi: 10.1016/S0167-4889(04)00042-4. [DOI] [PubMed] [Google Scholar]
- 151.Lacchini AH, Davies AJ, Mackintosh D, Walker AJ. 2006. Beta-1,3-glucan modulates PKC signalling in Lymnaea stagnalis defence cells: a role for PKC in H2O2 production and downstream ERK activation. J Exp Biol 209:4829–4840. doi: 10.1242/jeb.02561. [DOI] [PubMed] [Google Scholar]
- 152.Walker AJ, Lacchini AH, Sealey KL, Mackintosh D, Davies AJ. 2010. Spreading by snail (Lymnaea stagnalis) defence cells is regulated through integrated PKC, FAK and Src signaling. Cell Tissue Res 341:131–145. doi: 10.1007/s00441-010-0986-4. [DOI] [PubMed] [Google Scholar]
- 153.Gerhardus MJT, Baggen JMC, van der Knaap WPW, Sminia T. 1991. Analysis of surface carbohydrates of Trichobilharzia ocellata miracidia and sporocysts using lectin binding techniques. Parasitology 103:51–59. doi: 10.1017/S003118200005928X. [DOI] [PubMed] [Google Scholar]
- 154.Horák P. 1995. Developmentally regulated expression of surface carbohydrate residues on larval stages of the avian schistosome Trichobilharzia szidati. Folia Parasitol 42:255–265. [PubMed] [Google Scholar]
- 155.Blažová K, Horák P. 2005. Trichobilharzia regenti: the developmental differences in natural and abnormal hosts. Parasitol Int 54:167–172. doi: 10.1016/j.parint.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 156.Chanová M, Bulantová J, Máslo P, Horák P. 2009. In vitro cultivation of early schistosomula of nasal and visceral bird schistosomes (Trichobilharzia spp., Schistosomatidae). Parasitol Res 104:1445–1452. doi: 10.1007/s00436-009-1343-y. [DOI] [PubMed] [Google Scholar]
- 157.Plows LD, Cook RT, Davies AJ, Walker AJ. 2005. Carbohydrates that mimic schistosome surface coat components affect ERK and PKC signalling in Lymnaea stagnalis haemocytes. Int J Parasitol 35:293–302. doi: 10.1016/j.ijpara.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 158.Walker AJ. 2006. Do trematode parasites disrupt defence cell signalling in their snail host? Trends Parasitol 22:154–159. doi: 10.1016/j.pt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 159.Hoek RM, Smit AB, Frings H, Vink JM, de Jong-Brink M, Geraerts WPM. 1996. A new Ig-superfamily member, molluscan defence molecule (MDM) from Lymnaea stagnalis, is down-regulated during parasitosis. Eur J Immunol 26:939–944. doi: 10.1002/eji.1830260433. [DOI] [PubMed] [Google Scholar]
- 160.Smit AB, de Jong-Brink M, Li KW, Sassen MM, Spijker S, Van Elk R, Buijs S, Van Minnen J, Van Kesteren RE. 2004. Granularin, a novel molluscan opsonin comprising a single vWF type C domain is up-regulated during parasitation. FASEB J 18:845–847. doi: 10.1096/fj.03-0590fje. [DOI] [PubMed] [Google Scholar]
- 161.de Jong-Brink M, Bergamin-Sassen M, Solis Soto M. 2001. Multiple strategies of schistosomes to meet their requirements in the intermediate snail host. Parasitology 123:S129–S141. [DOI] [PubMed] [Google Scholar]
- 162.Geraerts WP. 1992. Neurohormonal control of growth and carbohydrate metabolism by the light green cells in Lymnaea stagnalis. Gen Comp Endocrinol 86:433–444. doi: 10.1016/0016-6480(92)90068-U. [DOI] [PubMed] [Google Scholar]
- 163.Hermann PM, de Lange RP, Pieneman AW, ter Maat A, Jansen RF. 1997. Role of neuropeptides encoded on CDCH-1 gene in the organization of egg-laying behavior in the pond snail, Lymnaea stagnalis. J Neurophysiol 78:2859–2869. [DOI] [PubMed] [Google Scholar]
- 164.de Jong-Brink M, Elsaadany M, Soto M. 1991. The occurrence of schistosomin, an antagonist of female gonadotropic hormones, is a general phenomenon in haemolymph of schistosome-infected freshwater snails. Parasitology 103:371–378. doi: 10.1017/S0031182000059886. [DOI] [PubMed] [Google Scholar]
- 165.Schallig HDFH, Sassen MJM, Hordijk PL, de Jong-Brink M. 1991. Trichobilharzia ocellata: influence of infection on the fecundity of its intermediate snail host Lymnaea stagnalis and cercarial induction of the release of schistosomin, a snail neuropeptide antagonizing female gonadotropic hormones. Parasitology 102:85–91. doi: 10.1017/S0031182000060376. [DOI] [PubMed] [Google Scholar]
- 166.Hordijk PL, de Jong-Brink M, ter Maat A, Pieneman AW, Lodder JC, Kits KS. 1992. The neuropeptide schistosomin and haemolymph from parasitized snails induce similar changes in excitability in neuroendocrine cells controlling reproduction and growth in a fresh-water snail. Neurosci Lett 136:193–197. doi: 10.1016/0304-3940(92)90047-B. [DOI] [PubMed] [Google Scholar]
- 167.Hoek RM, Li KW, van Minnen J, Lodder JC, de Jong-Brink M, Smit AB, van Kesteren RE. 2005. LFRFamides: a novel family of parasitation-induced -RFamide neuropeptides that inhibit the activity of neuroendocrine cells in Lymnaea stagnalis. J Neurochem 92:1073–1080. doi: 10.1111/j.1471-4159.2004.02927.x. [DOI] [PubMed] [Google Scholar]
- 168.McClelland G, Bourns TKR. 1969. Effects of Trichobilharzia ocellata on growth, reproduction, and survival of Lymnaea stagnalis. Exp Parasitol 24:137–146. doi: 10.1016/0014-4894(69)90150-7. [DOI] [PubMed] [Google Scholar]
- 169.Zbikowska E. 2005. Do larvae of Trichobilharzia szidati and Echinostoma revolutum generate behavioral fever in Lymnaea stagnalis individuals? Parasitol Res 97:68–72. doi: 10.1007/s00436-005-1394-7. [DOI] [PubMed] [Google Scholar]
- 170.Zbikowska E, Cichy A. 2012. Symptoms of behavioural anapyrexia—reverse fever as a defence response of snails to fluke invasion. J Invertebr Pathol 109:269–273. doi: 10.1016/j.jip.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 171.Stirewalt MA. 1974. Schistosoma mansoni: cercaria to schistosomule. Adv Parasitol 12:115–182. doi: 10.1016/S0065-308X(08)60388-7. [DOI] [PubMed] [Google Scholar]
- 172.Haas W. 1992. Physiological analysis of cercarial behavior. J Parasitol 78:243–255. doi: 10.2307/3283471. [DOI] [PubMed] [Google Scholar]
- 173.Feiler W, Haas W. 1988. Host-finding in Trichobilharzia ocellata cercariae—swimming and attachment to the host. Parasitology 96:493–505. doi: 10.1017/S0031182000080136. [DOI] [PubMed] [Google Scholar]
- 174.Sopott-Ehlers B, Haas W, Ehlers U. 2003. Ultrastructure of pigmented and unpigmented photoreceptors in cercariae of Trichobilharzia ocellata (Plathelminthes, Trematoda, Schistosomatidae): evidence for the evolution of parasitism in Neodermata. Parasitol Res 91:109–116. doi: 10.1007/s00436-003-0933-3. [DOI] [PubMed] [Google Scholar]
- 175.Feiler W, Haas W. 1988. Trichobilharzia ocellata—chemical stimuli of duck skin for cercarial attachment. Parasitology 96:507–517. doi: 10.1017/S0031182000080148. [DOI] [PubMed] [Google Scholar]
- 176.Haas W. 2003. Parasitic worms: strategies of host finding, recognition and invasion. Zoology 106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- 177.Haas W, Häberlein S. 2009. Penetration of cercariae into the living human skin: Schistosoma mansoni vs. Trichobilharzia szidati. Parasitol Res 105:1061–1066. doi: 10.1007/s00436-009-1516-8. [DOI] [PubMed] [Google Scholar]
- 178.Haas W, van de Roemer A. 1998. Invasion of the vertebrate skin by cercariae of Trichobilharzia ocellata: penetration processes and stimulating host signals. Parasitol Res 84:787–795. doi: 10.1007/s004360050489. [DOI] [PubMed] [Google Scholar]
- 179.Bulantová J, Chanová M, Houžvičková L, Horák P. 2011. Trichobilharzia regenti (Digenea: Schistosomatidae): changes of body wall musculature during the development from miracidium to adult worm. Micron 42:47–54. doi: 10.1016/j.micron.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 180.Mikeš L, Zídková L, Kašný M, Dvořák J, Horák P. 2005. In vitro stimulation of penetration gland emptying by Trichobilharzia szidati and T. regenti (Schistosomatidae) cercariae. Quantitative collection and partial characterization of the products. Parasitol Res 96:230–241. doi: 10.1007/s00436-005-1347-1. [DOI] [PubMed] [Google Scholar]
- 181.Wulff C, Häberlein S, Haas W. 2007. Cream formulations protecting against cercarial dermatitis by Trichobilharzia. Parasitol Res 101:91–97. doi: 10.1007/s00436-006-0431-5. [DOI] [PubMed] [Google Scholar]
- 182.Ligasová A, Bulantová J, Šebesta O, Kašný M, Koberna K, Mikeš L. 2011. Secretory glands in cercaria of the neuropathogenic schistosome Trichobilharzia regenti—ultrastructural characterization, 3-D modelling, volume and pH estimations. Parasit Vectors 4:162. doi: 10.1186/1756-3305-4-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bahgat M, Francklow K, Doenhoff MJ, Li YL, Ramzy RM, Kirsten C, Ruppel A. 2001. Infection induces antibodies against the cercarial secretions, but not against the cercarial elastases of Schistosoma mansoni, Schistosoma haematobium, Schistosoma japonicum and Trichobilharzia ocellata. Parasite Immunol 23:557–565. doi: 10.1046/j.1365-3024.2001.00417.x. [DOI] [PubMed] [Google Scholar]
- 184.Bahgat M, Ruppel A. 2002. Biochemical comparison of the serine protease (elastase) activities in cercarial secretions from Trichobilharzia ocellata and Schistosoma mansoni. Parasitol Res 88:495–500. doi: 10.1007/s00436-002-0597-4. [DOI] [PubMed] [Google Scholar]
- 185.Kašný M, Mikeš L, Dalton JP, Mountford AP, Horák P. 2007. Comparison of cysteine peptidase activities in Trichobilharzia regenti and Schistosoma mansoni cercariae. Parasitology 134:1599–1609. doi: 10.1017/S0031182007002910. [DOI] [PubMed] [Google Scholar]
- 186.Dolečková K, Kašný M, Mikeš L, Mutapi F, Stack C, Mountford AP, Horák P. 2007. Peptidases of Trichobilharzia regenti (Schistosomatidae) and its molluscan host Radix peregra s. lat. (Lymnaeidae): construction and screening of cDNA library from intramolluscan stages of the parasite. Folia Parasitol 54:94–98. doi: 10.14411/fp.2007.012. [DOI] [PubMed] [Google Scholar]
- 187.Dolečková K, Kašný M, Mikeš L, Cartwright J, Jedelský P, Schneider EL, Dvořák J, Mountford AP, Craik CS, Horák P. 2009. The functional expression and characterisation of a cysteine peptidase from the invasive stage of the neuropathogenic schistosome Trichobilharzia regenti. Int J Parasitol 39:201–211. doi: 10.1016/j.ijpara.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dvořák J, Mashiyama ST, Braschi S, Sajid M, Knudsen GM, Hansell E, Lim KC, Hsieh I, Bahgat M, Mackenzie B, Medzihradszky KF, Babbitt PC, Caffrey CR, McKerrow JH. 2008. Differential use of protease families for invasion by schistosome cercariae. Biochimie 90:345–358. doi: 10.1016/j.biochi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 189.Dolečková K, Albrecht T, Mikeš L, Horák P. 2010. Cathepsins B1 and B2 in the neuropathogenic schistosome Trichobilharzia regenti: distinct gene expression profiles and presumptive roles throughout the life cycle. Parasitol Res 107:751–755. doi: 10.1007/s00436-010-1943-6. [DOI] [PubMed] [Google Scholar]
- 190.Pleass RJ, Aboobaker AA, Schmitt AO. 2008. Piggybacking schistosome invasion: similarities are only skin deep. Trends Parasitol 24:153–156. doi: 10.1016/j.pt.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 191.Horák P, Grubhoffer L, Mikeš L, Tichá M. 1997. Lectins of Trichobilharzia szidati cercariae. Parasite 4:27–35. doi: 10.1051/parasite/1997041027. [DOI] [Google Scholar]
- 192.McKerrow JH, Pinoheiss S, Lindquist R, Werb Z. 1985. Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem 260:3703–3707. [PubMed] [Google Scholar]
- 193.Modha J, Redman CA, Thornhill JA, Kusel JR. 1998. Schistosomes: unanswered questions on the basic biology of the host-parasite relationship. Parasitol Today 14:396–401. doi: 10.1016/S0169-4758(98)01321-0. [DOI] [PubMed] [Google Scholar]
- 194.Linder E. 1986. Fluorochrome-labelled lectins reveal secreted glycoconjugates of schistosome larvae. Parasitol Today 2:219–221. doi: 10.1016/0169-4758(86)90085-2. [DOI] [PubMed] [Google Scholar]
- 195.Nevhutalu PA, Salafsky B, Haas W, Conway T. 1993. Schistosoma mansoni and Trichobilharzia ocellata—comparison of secreted cercarial eikosanoids. J Parasitol 79:130–133. [PubMed] [Google Scholar]
- 196.Horák P, Mikeš L. 1995. Cercarial surface saccharides of six trematode species from the pond snail, Lymnaea stagnalis. Parasite 2:419–421. [Google Scholar]
- 197.Hokke CH, Neeleman AP, Koeleman CAM, van den Eijnden DH. 1998. Identification of an alpha 3-fucosyltransferase and a novel alpha 2-fucosyltransferase activity in cercariae of the schistosome Trichobilharzia ocellata: biosynthesis of the Fuc alpha 1 → 2Fuc alpha 1 → 3[Gal(NAc)beta 1 → 4]GlcNAc sequence. Glycobiology 8:393–406. doi: 10.1093/glycob/8.4.393. [DOI] [PubMed] [Google Scholar]
- 198.Podhorský M, Hůzová Z, Mikeš L, Horák P. 2009. Cercarial dimensions and surface structures as a tool for species determination of Trichobilharzia spp. Acta Parasitol 54:28–36. doi: 10.2478/s11686-009-0011-9. [DOI] [Google Scholar]
- 199.Horák P, Kovář L, Kolářová L, Nebesářová J. 1998. Cercaria-schistosomulum surface transformation of Trichobilharzia szidati and its putative immunological impact. Parasitology 116:139–147. doi: 10.1017/S0031182097002059. [DOI] [PubMed] [Google Scholar]
- 200.Chanová M, Lichtenbergová L, Bulantová J, Mikeš L, Horák P. 2012. Trichobilharzia regenti: antigenic structures of intravertebrate stages. Cent Eur J Biol 7:83–90. doi: 10.2478/s11535-011-0074-0. [DOI] [Google Scholar]
- 201.Boulanger D, Schneider D, Sidikou F, Capron A, Chippaux J-P, Sellin B. 1999. The oral route as a potential way of transmission of Schistosoma bovis in goats. J Parasitol 85:464–467. doi: 10.2307/3285779. [DOI] [PubMed] [Google Scholar]
- 202.Oleaga A, Ramajo V. 2004. Efficiency of the oral, intramuscular and subcutaneous routes for the experimental infection of hamster and sheep with Schistosoma bovis. Vet Parasitol 124:43–53. doi: 10.1016/j.vetpar.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 203.Giver H, Johansen MV, Christensen NO, Bøgh H, Nansen P. 1999. Peroral infection of pigs with Schistosoma japonicum cercariae. Vet Parasitol 83:161–165. doi: 10.1016/S0304-4017(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 204.Islam KS, Copeman DB. 1986. The morphology and life cycle of Trichobilharzia parocellata (Johnston & Simpson, 1939) Islam & Copeman, 1980 from the visceral blood vessels of Australian anatids. Syst Parasitol 8:39–49. doi: 10.1007/BF00010308. [DOI] [Google Scholar]
- 205.Horák P, Kolářová L, Dvořák J. 1998. Trichobilharzia regenti n. sp. (Schistosomatidae, Bilharziellinae) a new nasal schistosome from Europe. Parasite 5:349–357. doi: 10.1051/parasite/1998054349. [DOI] [PubMed] [Google Scholar]
- 206.Bourns TKR, Ellis JC, Rau ME. 1973. Migration and development of Trichobilharzia ocellata (Trematoda: Schistosomatidae) in its duck hosts. Can J Zool 51:1021–1030. doi: 10.1139/z73-148. [DOI] [PubMed] [Google Scholar]
- 207.Horák P, Dvořák J, Kolářová L, Trefil L. 1999. Trichobilharzia regenti, a pathogen of the avian and mammalian central nervous system. Parasitology 119:577–581. doi: 10.1017/S0031182099005132. [DOI] [PubMed] [Google Scholar]
- 208.Grabe K, Haas W. 2004. Navigation within host tissues: Schistosoma mansoni and Trichobilharzia ocellata schistosomula respond to chemical gradients. Int J Parasitol 34:927–934. doi: 10.1016/j.ijpara.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 209.Grabe K, Haas W. 2004. Navigation within host tissues: cercariae orientate towards dark after penetration. Parasitol Res 93:111–113. doi: 10.1007/s00436-003-1045-9. [DOI] [PubMed] [Google Scholar]
- 210.Chanová M, Vuong S, Horák P. 2007. Trichobilharzia szidati: the lung phase of migration within avian and mammalian hosts. Parasitol Res 100:1243–1247. doi: 10.1007/s00436-006-0398-2. [DOI] [PubMed] [Google Scholar]
- 211.Neuhaus W. 1952. Biologie und Entwicklung von Trichobilharzia szidati n. sp. (Trematoda, Schistosomatidae), einem Erreger von Dermatitis bei Menschen. Z Parasitenkd 15:203–266. [DOI] [PubMed] [Google Scholar]
- 212.Vusse FJV. 1979. Host-parasite relations of Dendritobilharzia pulverulenta (Trematoda: Schistosomatidae) and anatids. J Parasitol 65:894–897. doi: 10.2307/3280244. [DOI] [Google Scholar]
- 213.Olivier L. 1953. Observations on the migrations of avian schistosomes in mammals previously unexposed to cercariae. J Parasitol 39:237–243. doi: 10.2307/3273943. [DOI] [PubMed] [Google Scholar]
- 214.Hrádková K, Horák P. 2002. Neurotropic behaviour of Trichobilharzia regenti in ducks and mice. J Helminthol 76:137–141. doi: 10.1079/JOH2002113. [DOI] [PubMed] [Google Scholar]
- 215.Lichtenbergová L, Lassmann H, Jones MK, Kolářová L, Horák P. 2011. Trichobilharzia regenti: host immune response in the pathogenesis of neuroinfection in mice. Exp Parasitol 128:328–335. doi: 10.1016/j.exppara.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 216.van Bolhuis GH, Rijks JM, Dorrestein GM, Rudolfová J, van Dijk M, Kuiken T. 2004. Obliterative endophlebitis in mute swans (Cygnus olor) caused by Trichobilharzia sp. (Digenea: Schistosomatidae) infection. Vet Pathol 41:658–665. doi: 10.1354/vp.41-6-658. [DOI] [PubMed] [Google Scholar]
- 217.Wood LM, Bacha WJ Jr. 1983. Distribution of eggs and the host response in chickens infected with Austrobilharzia variglandis (Trematoda). J Parasitol 69:682–688. doi: 10.2307/3281141. [DOI] [PubMed] [Google Scholar]
- 218.Wojcinski ZW, Barker IK, Hunter DB, Lumsden H. 1987. An outbreak of schistosomiasis in Atlantic brant geese, Branta bernicla hrota. J Wildl Dis 23:248–255. doi: 10.7589/0090-3558-23.2.248. [DOI] [PubMed] [Google Scholar]
- 219.Pence DB, Rhodes MJ. 1982. Trichobilharzia physellae (Digenea: Schistosomatidae) from endemic waterfowl on the high plains of Texas. J Wildl Dis 18:69–74. doi: 10.7589/0090-3558-18.1.69. [DOI] [PubMed] [Google Scholar]
- 220.Kolářová L, Horák P, Cada F. 2001. Histopathology of CNS and nasal infections caused by Trichobilharzia regenti in vertebrates. Parasitol Res 87:644–650. doi: 10.1007/s004360100431. [DOI] [PubMed] [Google Scholar]
- 221.Chanová M, Horák P. 2007. Terminal phase of bird schistosomiasis caused by Trichobilharzia regenti (Schistosomatidae) in ducks (Anas platyrhynchos f. domestica). Folia Parasitol 54:105–107. doi: 10.14411/fp.2007.014. [DOI] [PubMed] [Google Scholar]
- 222.Levine ND, Clark DT, Hanson LE. 1956. Encephalitis in a swan due to Dendritobilharzia sp. (Trematoda; Schistosomatidae). J Parasitol 42:496–500. doi: 10.2307/3274443. [DOI] [PubMed] [Google Scholar]
- 223.Wilson RB, New JC, Scholtens RG. 1982. Granulomatous encephalitis caused by schistosomiasis in swans. J Am Vet Med Assoc 181:1386–1387. [PubMed] [Google Scholar]
- 224.Kouřilová P, Syrůček M, Kolářová L. 2004. The severity of mouse pathologies caused by the bird schistosome Trichobilharzia regenti in relation to host immune status. Parasitol Res 93:8–16. doi: 10.1007/s00436-004-1079-7. [DOI] [PubMed] [Google Scholar]
- 225.Haemmerli U. 1953. Schistosomen-Dermatitis am Zürichsee. Dermatologica 107:301–341. [PubMed] [Google Scholar]
- 226.Tremaine AM, Whittemore DE, Gewirtzman AJ, Bartlett BL, Mendoza N, Rapini RP, Tyring SK. 2009. An unusual case of swimmer's itch. J Am Acad Dermatol 60:174–176. doi: 10.1016/j.jaad.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 227.Kouřilová P, Hogg KG, Kolářová L, Mountford AP. 2004. Cercarial dermatitis caused by bird schistosomes comprises both immediate and late phase cutaneous hypersensitivity reactions. J Immunol 172:3766–3774. doi: 10.4049/jimmunol.172.6.3766. [DOI] [PubMed] [Google Scholar]
- 228.Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, Stadecker MJ. 2012. Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol 34:873–888. doi: 10.1007/s00281-012-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. 2001. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 230.Lichtenbergová L, Kolbeková P, Kouřilová P, Kašný M, Mikeš L, Haas H, Schramm G, Horák P, Kolářová L, Mountford AP. 2008. Antibody responses induced by Trichobilharzia regenti antigens in murine and human hosts exhibiting cercarial dermatitis. Parasite Immunol 30:585–595. doi: 10.1111/j.1365-3024.2008.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bacha W Jr, Roush R Jr, Icardi S. 1982. Infection of the gerbil by the avian schistosome Austrobilharzia variglandis (Miller and Northup 1926; Pener 1953). J Parasitol 68:505–507. doi: 10.2307/3280970. [DOI] [PubMed] [Google Scholar]
- 232.Horák P, Kolářová L. 2001. Bird schistosomes—do they die in mammalian skin? Trends Parasitol 17:66–69. doi: 10.1016/S1471-4922(00)01770-0. [DOI] [PubMed] [Google Scholar]
- 233.Melo FL, Gomes ALD, Barbosa CS, Werkhauser RP, Abath FGC. 2006. Development of molecular approaches for the identification of transmission sites of schistosomiasis. Trans R Soc Trop Med Hyg 100:1049–1055. doi: 10.1016/j.trstmh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 234.Kane RA, Stothard JR, Rollinson D, Leclipteux T, Evraerts J, Standley CJ, Allan F, Betson M, Kaba R, Mertens P, Laurent T. 2013. Detection and quantification of schistosome DNA in freshwater snails using either fluorescent probes in real-time PCR or oligochromatographic dipstick assays targeting the ribosomal intergenic spacer. Acta Trop 128:241–249. doi: 10.1016/j.actatropica.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 235.Worrell C, Xiao N, Vidal JE, Chen L, Zhong B, Remais J. 2011. Field detection of Schistosoma japonicum cercariae in environmental water sample by quantitative PCR. Appl Environ Microbiol 77:2192–2195. doi: 10.1128/AEM.01561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Onichandran S, Kumar T, Lim YAL, Sawangjaroen N, Andiappan H, Salibay CC, Chye TT, Ithoi I, Dungca JZ, Sulaiman WYW, Ling LY, Nissapatorn V. 2013. Waterborne parasites and physico-chemical assessment of selected lakes in Malaysia. Parasitol Res 112:4185–4191. doi: 10.1007/s00436-013-3610-1. [DOI] [PubMed] [Google Scholar]
- 237.Schets FM, Lodder WJ, Husman AMD. 2010. Confirmation of the presence of Trichobilharzia by examination of water samples and snails following reports of cases of cercarial dermatitis. Parasitology 137:77–83. doi: 10.1017/S0031182009990849. [DOI] [PubMed] [Google Scholar]
- 238.Horák P, Schets FM, Kolářová L, Brant SV. 2013. Trichobilharzia, p 455–465. In Liu D. (ed), Molecular detection of human parasitic pathogens. Taylor and Francis, CRC Press, Boca Raton, FL. [Google Scholar]
- 239.Hertel J, Hamburger J, Haberl B, Haas W. 2002. Detection of bird schistosomes in lakes by PCR and filter-hybridization. Exp Parasitol 101:57–63. doi: 10.1016/S0014-4894(02)00036-X. [DOI] [PubMed] [Google Scholar]
- 240.Graczyk TK, Shiff CJ. 2000. Recovery of avian schistosome cercariae from water using penetration stimulant matrix with an unsaturated fatty acid. Am J Trop Med Hyg 63:174–177. [DOI] [PubMed] [Google Scholar]
- 241.Cringoli G, Rinaldi L, Utzinger J. 2010. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscope diagnosis of parasites in animals and humans. Nat Protoc 5:503–515. doi: 10.1038/nprot.2009.235. [DOI] [PubMed] [Google Scholar]
- 242.Katz N, Chavez A, Pellegrino J. 1972. A simple device for quantitative stool thicksmear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14:397–400. [PubMed] [Google Scholar]
- 243.Kolářová L, Horák P, Skírnisson K. 2010. Methodical approaches in the identification of areas with potential risk of infection by bird schistosomes causing cercarial dermatitis. J Helminthol 84:327–335. doi: 10.1017/S0022149X09990721. [DOI] [PubMed] [Google Scholar]
- 244.Skírnisson K, Kolářová L. 2008. Diversity of bird schistosomes in anseriform birds in Iceland based on egg measurements and egg morphology. Parasitol Res 103:43–50. doi: 10.1007/s00436-008-0925-4. [DOI] [PubMed] [Google Scholar]
- 245.Lu DB, Wang TP, Rudge JW, Donnelly CA, Fang GR, Webster JP. 2009. Evolution in a multi-host parasite: chronobiological circadian rhythm and population genetics of Schistosoma japonicum cercariae indicates contrasting definitive host reservoirs by habitat. Int J Parasitol 39:1581–1588. doi: 10.1016/j.ijpara.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 246.Nolan MJ, Cribb TJ. 2005. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv Parasitol 60:101–163. doi: 10.1016/S0065-308X(05)60002-4. [DOI] [PubMed] [Google Scholar]
- 247.Vilas R, Criscione C, Blouin M. 2005. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 131:839–846. doi: 10.1017/S0031182005008437. [DOI] [PubMed] [Google Scholar]
- 248.Dvořák J, Vaňáčová S, Hampl V, Flegr J, Horák P. 2002. Comparison of European Trichobilharzia species based on ITS1 and ITS2 sequences. Parasitology 124:307–313. doi: 10.1017/S0031182001001238. [DOI] [PubMed] [Google Scholar]
- 249.Chrisanfova GG, Lopatkin AA, Mishchenkov VA, Kheidorova EE, Dorozhenkova TE, Zhukova TV, Ryskov AP, Semyenova SK. 2009. Genetic variability of bird schistosomes (class Trematoda, family Schistosomatidae) of Naroch Lake: identification of a new species in the Trichobilharzia ocellata group. Dokl Biochem Biophys 428:268–272. doi: 10.1134/S1607672909050123. [DOI] [PubMed] [Google Scholar]
- 250.Jouet D, Ferté H, Depaquit J, Rudolfová J, Latour P, Zanella D, Kaltenbach ML, Léger N. 2008. Trichobilharzia spp. in natural conditions in Annecy Lake, France. Parasitol Res 103:51–58. doi: 10.1007/s00436-008-0926-3. [DOI] [PubMed] [Google Scholar]
- 251.Rizevsky SV, Cherviakovsky EM, Kurchenko VP. 2011. Molecular taxonomic identification of Schistosomatidae from Naroch Lake and Polonevichi Lake in Belarus. Biochem Syst Ecol 39:14–21. doi: 10.1016/j.bse.2010.12.014. [DOI] [Google Scholar]
- 252.Ferté H, Depaquit J, Carré S, Villena I, Léger N. 2005. Presence of Trichobilharzia szidati in Lymnaea stagnalis and T. franki in Radix auricularia in northeastern France: molecular evidence. Parasitol Res 95:150–154. doi: 10.1007/s00436-004-1273-7. [DOI] [PubMed] [Google Scholar]
- 253.Semyenova SK, Chrisanfova GG, Filippova EK, Beér SA, Voronin MV, Ryskov AP. 2005. Individual and population variation in cercariae of bird schistosomes of the Trichobilharzia ocellata species group as revealed with the polymerase chain reaction. Genetika 41:12–16. [PubMed] [Google Scholar]
- 254.Korsunenko A, Chrisanfova G, Lopatkin A, Beer SA, Voronin M, Ryskov AP, Semyenova SK. 2012. Genetic differentiation of cercariae infrapopulations of the avian schistosome Trichobilharzia szidati based on RAPD markers and mitochondrial cox1 gene. Parasitol Res 110:833–841. doi: 10.1007/s00436-011-2562-6. [DOI] [PubMed] [Google Scholar]
- 255.Besansky NJ, Severson DW, Ferdig MT. 2003. DNA barcoding of parasites and invertebrate disease vectors: what you don't know can hurt you. Trends Parasitol 19:545–546. doi: 10.1016/j.pt.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 256.Hansen H, Bakke TA, Bachmann L. 2007. DNA taxonomy and barcoding of monogenean parasites: lessons from Gyrodactylus. Trends Parasitol 23:363–367. doi: 10.1016/j.pt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 257.Peterson AT, Moyle RG, Nyári AS, Robbins MB, Brumfield RT, Remsen JV Jr. 2007. The need for proper vouchering in phylogenetic studies of birds. Mol Phylogenet Evol 45:1042–4044. doi: 10.1016/j.ympev.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 258.Olsson U, Sundberg P, Alström P, Gelang M, Ericson PGP. 2008. What is the proper vouchering in phylogenetic studies of birds?—a reply to Peterson et al. (2007). Mol Phylogenet Evol 48:383–385. doi: 10.1016/j.ympev.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 259.Hoberg EP, Pilitt PA, Galbreath KE. 2009. Why museums matter: a tale of pinworms (Oxyuroidea: Heteroxynematidae) among pikas (Ochotona princeps and O. collaris) in the American West. J Parasitol 95:490–501. doi: 10.1645/GE-1823.1. [DOI] [PubMed] [Google Scholar]
- 260.Olivier L. 1949. Schistosome dermatitis, a sensitization phenomenon. Am J Hyg (Lond) 49:290–302. [DOI] [PubMed] [Google Scholar]
- 261.Augustine DL, Weller TH. 1949. Experimental studies on the specificity of skin tests for the diagnosis of schistosomiasis. J Parasitol 35:461–466. doi: 10.2307/3273648. [DOI] [PubMed] [Google Scholar]
- 262.Ulrich H, Landthaler M, Vogt T. 2008. Aquatic dermatoses. J Dtsch Dermatol Ges 6:133–146. doi: 10.1111/j.1610-0387.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 263.Kimmig P, Meier M. 1985. Parasitologische Untersuchungen, Diagnose und Klinik der Zerkariendermatitis-hygienische Bedeutung für Badegewässer gemässigter Zonen. Zentralbl Bakteriol Hyg I Abt Orig B 181:390–408. [PubMed] [Google Scholar]
- 264.Kouřilová P, Kolářová L. 2002. Variations in immunofluorescent antibody response against Trichobilharzia and Schistosoma antigens in compatible and incompatible hosts. Parasitol Res 88:513–521. doi: 10.1007/s00436-002-0607-6. [DOI] [PubMed] [Google Scholar]
- 265.Agrawal MC, Gupta S, George J. 2000. Cercarial dermatitis in India. Bull World Health Organ 78:278. [PMC free article] [PubMed] [Google Scholar]
- 266.Fraser SJ, Allan SJR, Roworth M, Smith HV, Holme SA. 2009. Cercarial dermatitis in the UK. Clin Exp Dermatol 34:344–346. doi: 10.1111/j.1365-2230.2008.02903.x. [DOI] [PubMed] [Google Scholar]
- 267.Salafsky B, Ramaswamy K, He YX, Li J, Shibuya T. 1999. Development and evaluation of LIPODEET, a new long-acting formulation of N,N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg 61:743–750. [DOI] [PubMed] [Google Scholar]
- 268.Marcogliese DJ. 2001. Implications of climate change for parasitism of animals in the aquatic environment. Can J Zool 79:1331–1352. doi: 10.1139/z01-067. [DOI] [Google Scholar]
- 269.Cotton PA. 2003. Avian migration phenology and global climate change. Proc Natl Acad Sci U S A 100:12219–12222. doi: 10.1073/pnas.1930548100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Suter W. 1994. Overwintering waterfowl on Swiss lakes: how are abundance and species richness influenced by trophic status and lake morphology? Hydrobiologia 279/280:1–14. [Google Scholar]
- 271.Morley NJ. 2012. Cercariae (Platyhelminthes: Trematoda) as neglected components of zooplankton communities in freshwater habitats. Hydrobiologia 1:7–19. doi: 10.1007/s10750-012-1029-9. [DOI] [Google Scholar]
- 272.Barber KE, Caira JN. 1995. Investigation of the life cycle and adult morphology of the avian blood fluke Austrobilharzia variglandis (Trematoda: Schistosomatidae) from Connecticut. J Parasitol 81:584–592. doi: 10.2307/3283857. [DOI] [PubMed] [Google Scholar]
- 273.Davis NE. 1998. Population dynamics of and larval trematode interactions with Lymnaea tomentosa and the potential for biological control of schistosome dermatitis in Bremner Bay, Lake Wanaka, New Zealand. J Helminthol 72:319–324. doi: 10.1017/S0022149X00016679. [DOI] [PubMed] [Google Scholar]
- 274.Sluiters JE. 1981. Development of Trichobilharzia ocellata in Lymnaea stagnalis and the effects of infection on the reproductive system of the host. Z Parasitenkd 64:303–319. [DOI] [PubMed] [Google Scholar]
- 275.Poulin R. 2006. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132:143–151. doi: 10.1017/S0031182005008693. [DOI] [PubMed] [Google Scholar]
- 276.Larsen AH, Bresciani J, Buchmann K. 2004. Increasing frequency of cercarial dermatitis at higher latitudes. Acta Parasitol 49:217–221. [Google Scholar]
- 277.Islam KS. 1986. The morphology and life-cycle of Trichobilharzia arcuata n. sp. (Schistosomatidae: Bilharziellinae) a nasal schistosome of water whistle ducks (Dendrocygna arcuata) in Australia. Syst Parasitol 8:117–128. doi: 10.1007/BF00009868. [DOI] [Google Scholar]
- 278.Allgöwer R. 1995. Zerkariendermatitis—ein Warnsignal für zunehmende Gewässereutrophierung? Limnol Aktuell 7:193–203. [Google Scholar]
- 279.de Gentile L, Picot H, Bourdeau P, Bardet R, Kerjan A, Piriou M, Le Guennic A, Bayssade-Dufour C, Chabasse D, Mott KE. 1996. Le dermatite cercarienne en Europe: un probleme de la santé publique nouveau? Bull World Health Organ 74:159–163. [PMC free article] [PubMed] [Google Scholar]
- 280.Lindblade KA. 1998. The epidemiology of cercarial dermatitis and its association with limnological characteristics of a Northern Michigan Lake. J Parasitol 84:19–23. doi: 10.2307/3284521. [DOI] [PubMed] [Google Scholar]
- 281.Chase JM. 2003. Strong and weak trophic cascades along a productivity gradient. Oikos 101:187–195. doi: 10.1034/j.1600-0706.2003.12062.x. [DOI] [Google Scholar]
- 282.McKenzie VJ, Townsend AR. 2007. Parasitic and infectious disease responses to changing global nutrient cycles. EcoHealth 4:384–396. doi: 10.1007/s10393-007-0131-3. [DOI] [Google Scholar]
- 283.Marcogliese DJ. 2008. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech 27:467–484. [PubMed] [Google Scholar]
- 284.Soldánová M, Kostadinova A. 2011. Rapid colonisation of Lymnaea stagnalis by larval trematodes in eutrophic ponds in Central Europe. Int J Parasitol 41:981–990. doi: 10.1016/j.ijpara.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 285.Curtis LA. 2002. Ecology of larval trematodes in three marine gastropods. Parasitology 124:S43–S56. doi: 10.1017/S0031182002001452. [DOI] [PubMed] [Google Scholar]
- 286.Müller V, Kimmig E. 1994. Trichobilharzia franki n. sp.—die Ursache für Badedermatitiden in südwestdeutschen Baggerseen. Appl Parasitol 35:12–31. [PubMed] [Google Scholar]
- 287.Väyrynen T, Siddall R, Valtonen ET, Taskinen J. 2000. Patterns of trematode parasitism in lymnaeid snails from northern and central Finland. Ann Zool Fenn 37:189–199. [Google Scholar]
- 288.Soldánová M, Faltýnková A, Scholz T, Kostadinova A. 2011. Parasites in a man-made landscape: contrasting patterns of trematode flow in a fishpond area in Central Europe. Parasitology 138:789–807. doi: 10.1017/S0031182011000291. [DOI] [PubMed] [Google Scholar]
- 289.Graham AL. 2003. Effects of snail size and age on the prevalence and intensity of avian schistosome infection: relating laboratory to field studies. J Parasitol 89:458–463. doi: 10.1645/0022-3395(2003)089[0458:EOSSAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 290.Coady NR, Muzzall PM, Burton TM, Snider RJ, Saxton J, Sergeant M, Sommers A. 2006. Ubiquitous variability in the prevalence of Trichobilharzia stagnicolae (Schistosomatidae) infecting Stagnicola emarginata in three northern Michigan lakes. J Parasitol 92:10–15. doi: 10.1645/GE-3336.1. [DOI] [PubMed] [Google Scholar]
- 291.Hurley M, Hearnden MN, Blair D, Kay BH. 1994. Larval trematodes in freshwater snails at the Ross River reservoir, northern Australia, with emphasis on Trichobilharzia sp(p)., causative agents of swimmer's itch. Aust J Mar Freshw Res 45:563–567. doi: 10.1071/MF9940563. [DOI] [Google Scholar]
- 292.Patz JA, Graczyk TK, Gelle N, Vittor AY. 2000. Effect of environmental change on emerging parasitic diseases. Int J Parasitol 30:1395–1405. doi: 10.1016/S0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 293.Mas-Coma S, Valero AV, Bargues MD. 2009. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet Parasitol 163:264–280. doi: 10.1016/j.vetpar.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 294.Lafferty KD. 1997. Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13:251–255. doi: 10.1016/S0169-4758(97)01072-7. [DOI] [PubMed] [Google Scholar]
- 295.Pietrock M, Marcogliese DJ. 2003. Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol 19:293–299. doi: 10.1016/S1471-4922(03)00117-X. [DOI] [PubMed] [Google Scholar]
- 296.Sures B. 2008. Environmental parasitology: interactions between parasites and pollutants in the aquatic environment. Parasite 15:434–438. doi: 10.1051/parasite/2008153434. [DOI] [PubMed] [Google Scholar]
- 297.Vidal-Martínez VM, Pech D, Sures B. 2010. Can parasites really reveal environmental impact? Trends Parasitol 26:44–51. doi: 10.1016/j.pt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 298.Poulin R, Paterson RA, Townsend C, Tompkins DM, Kelly DW. 2011. Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshw Biol 56:676–688. doi: 10.1111/j.1365-2427.2010.02425.x. [DOI] [Google Scholar]
- 299.Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, Poulin R, Thieltges DW. 2010. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol Evol 35:362–371. doi: 10.1016/j.tree.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 300.Thieltges DW, Jensen KT, Poulin R. 2008. The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135:407–426. doi: 10.1017/S0031182007000248. [DOI] [PubMed] [Google Scholar]
- 301.Lim HK, Heyneman D. 1972. Intramolluscan inter-trematode antagonism: a review of factors influencing the host-parasite system and its possible role in biological control. Adv Parasitol 10:191–268. doi: 10.1016/S0065-308X(08)60175-X. [DOI] [PubMed] [Google Scholar]
- 302.Kuris AM. 1990. Guild structure of larval trematodes in molluscan hosts: prevalence, dominance and significance of competition, p 69–100. In Esch GW, Bush AO, Aho JM (ed), Parasite communities: patterns and processes. Chapman & Hall, London, United Kingdom. [Google Scholar]
- 303.Sousa WP. 1992. Interspecific interactions among larval trematode parasites of freshwater and marine snails. Am Zool 32:583–592. [Google Scholar]
- 304.Verbrugge LM, Rainey JJ, Reimink RL, Blankespoor HD. 2004. Prospective study of swimmers itch incidence and severity. J Parasitol 90:697–704. doi: 10.1645/GE-237R. [DOI] [PubMed] [Google Scholar]
- 305.Verbrugge LM, Rainey JJ, Reimink RL, Blankespoor HD. 2004. Swimmer's itch: incidence and risk factors. Am J Public Health 94:738–741. doi: 10.2105/AJPH.94.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Schets FM, Lodder WJ, van Duynhoven YTHP, Husman AMD. 2008. Cercarial dermatitis in the Netherlands caused by Trichobilharzia spp. J Water Health 6:187–195. doi: 10.2166/wh.2008.028. [DOI] [PubMed] [Google Scholar]
- 307.Hörweg C, Sattman H, Auer H. 2006. Cercarial dermatitis in Austria: questionnaires as useful tools to estimate risk factors? Middle Eur J Med 118:77–80. doi: 10.1007/s00508-006-0674-2. [DOI] [PubMed] [Google Scholar]
- 308.Appleton CC, Lethbridge RC. 1979. Schistosome dermatitis in the swan estuary, western Australia. Med J Aust 131:141–144. [DOI] [PubMed] [Google Scholar]
- 309.Lévesque B, Giovenazzo P, Guerrier P, Laverdiére D, Prud'Homme H. 2002. Investigation of an outbreak of cercarial dermatitis. Epidemiol Infect 129:379–386. doi: 10.1017/S0950268802007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Berg K, Reiter HEH. 1960. Observations on schistosome dermatitis in Denmark. Acta Derm Venereol 40:369–380. [Google Scholar]
- 311.Kolářová L, Horák P, Sitko J. 1997. Cercarial dermatitis in focus: schistosomes in the Czech Republic. Helminthologia 34:127–139. [Google Scholar]
- 312.Thorp JH, Covich AP. 2001. Ecology and classification of North American freshwater invertebrates, 2nd ed, p 1056 Academic Press, New York, NY. [Google Scholar]
- 313.Dillon RT. 2004. The ecology of freshwater molluscs, p 509 Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 314.Allgöwer R, Effelsberg W. 1991. Swimmer's dermatitis in an excavation pool—an incentive for the status analysis of the water and for the preparation of an ecology-friendly utilization concept. Offentl Gesundheitswes 53:138–143. [PubMed] [Google Scholar]
- 315.Kagan IG, Short RB, Nez MM. 1954. Maintenance of Schistosomatium douthitti (Cort, 1914) in the laboratory (Trematoda: Schistosomatidae). J Parasitol 40:424–439. doi: 10.2307/3273890. [DOI] [PubMed] [Google Scholar]
- 316.Macy RW, Moore DJ, Price WS Jr. 1955. Studies on dermatitis-producing schistosomes in the Pacific Northwest, with special reference to Trichobilharzia oregonensis. Trans Am Microsc Soc 74:235–251. doi: 10.2307/3224097. [DOI] [Google Scholar]
- 317.Anderson PA, Nowosielsky LW, Croll NA. 1976. Emergence of cercariae of Trichobilharzia ocellata and its relationship to activity of its snail host Lymnaea stagnalis. Can J Zool 54:1481–1487. doi: 10.1139/z76-171. [DOI] [PubMed] [Google Scholar]
- 318.Olivier L. 1951. The influence of light on the emergence of Schistosomatium douthitti cercariae from their snail host. J Parasitol 37:201–204. doi: 10.2307/3273454. [DOI] [PubMed] [Google Scholar]
- 319.Blankespoor HD, Reimink RL. 1991. The control of swimmer's itch in Michigan: past, present and future. Mich Acad 24:7–23. [Google Scholar]
- 320.Reimink RL, DeGoede JA, Blankespoor HD. 1995. Efficacy of praziquantel in natural populations of mallards infected with avian schistosomes. J Parasitol 81:1027–1029. doi: 10.2307/3284067. [DOI] [PubMed] [Google Scholar]
- 321.Blankespoor CL, Reimink RL, Blankespoor HD. 2001. Efficacy of praziquantel in treating natural schistosome infections in common mergansers. J Parasitol 87:424–426. doi: 10.1645/0022-3395(2001)087[0424:EOPITN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 322.Blankespoor HD, Cameron SC, Cairns J Jr. 1985. Resistance of pulmonate snail populations to repeated treatments of copper sulfate. Environ Manage 9:455–458. doi: 10.1007/BF01866345. [DOI] [Google Scholar]
- 323.Mitchell AJ. 2002. A copper-sulfate-citric acid pond shoreline treatment to control the rams-horn snail Planorbella trivolvis. N Am J Aquacult 64:182–187. doi:. [DOI] [Google Scholar]
- 324.Graefe G, Aspöck H, Picher O. 1973. Auftreten von Bade-Dermatitis in Österreich und Möglichkeiten ihrer Bekämpfung. Zentralbl Bakteriol Hyg 225:398–405. [PubMed] [Google Scholar]
- 325.Ledford JJ, Kelly AM. 2006. A comparison of black carp Mylopharyngodon piceus, redear sunfish Lepomis microlophus, and blue catfish Ictalurus furcatus as biological controls of snail populations. N Am J Aquacult 68:339–347. doi: 10.1577/A05-062.1. [DOI] [Google Scholar]
- 326.Venable DL, Gaude AP, Klerks PL. 2000. Control of the trematode Bolbophorus confusus in channel catfish Ictalurus punctatus ponds using salinity manipulation and polyculture with black carp Mylopharyngodon piceus. J World Aquacult Soc 31:158–166. doi: 10.1111/j.1749-7345.2000.tb00349.x. [DOI] [Google Scholar]
- 327.Wui YS, Engle CR. 2007. The economic impact of restricting use of black carp for snail control on hybrid striped bass farms. N Am J Aquacult 69:127–138. doi: 10.1577/A05-088.1. [DOI] [Google Scholar]
- 328.Sokolow SH, Lafferty KD, Kuris AM. 2014. Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop 132:64–74. doi: 10.1016/j.actatropica.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Haas W, Haberl B, Kalbe M, Stoll K. 1998. Traps for schistosome miracidia/cercariae, p 359–363. In Tada I. (ed), IXth International Congress of Parasitology. Monduzzi Editore, Bologna, Italy. [Google Scholar]
- 330.Ahmed AA, Babiker A, Eltash LA, Shiff C. 2002. Development of a modified baited trap for detection of schistosome cercariae using natural oils rich in polyunsaturated fatty acids in Sudan. Acta Trop 82:363–368. doi: 10.1016/S0001-706X(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 331.Haas W. 1984. Schistosoma mansoni: cercaricidal effect of 2-tetradecenoic acid, a penetration stimulant. Exp Parasitol 58:215–222. doi: 10.1016/0014-4894(84)90037-7. [DOI] [PubMed] [Google Scholar]
- 332.Naples JM, Shiff C, Halden RU. 2005. Reduction of infectivity of schistosome cercariae by application of cercaricidal oil to water. Am J Trop Med Hyg 73:956–961. [PubMed] [Google Scholar]
- 333.Bunnag T, Freitas JR, De Scott HG. 1977. The predatory activity of Lebistes reticulatus (Peter, 1859) on Schistosoma mansoni miracidia in laboratory experiments. Trop Geogr Med 29:411–414. [PubMed] [Google Scholar]
- 334.Knight WB, Ritchie LS, Liard F, Chiriboga J. 1970. Cercariophagic activity of guppy fish (Lebistes reticulatus) detected by cercariae labelled with radioselenium (75Se). Am J Trop Med Hyg 19:620–625. [DOI] [PubMed] [Google Scholar]
- 335.Holliman RB, Mecham J. 1971. Macrostomum gigas (Turbellaria: Rhabdocoela), a predator of Schistosoma mansoni cercariae. J Parasitol 57:680–681. doi: 10.2307/3277941. [DOI] [Google Scholar]
- 336.Christensen NO. 1979. Schistosoma mansoni: interference with cercarial host-finding by various aquatic organisms. J Helminthol 53:7–14. doi: 10.1017/S0022149X00005678. [DOI] [PubMed] [Google Scholar]
- 337.Christensen NO, Frandsen F, Nansen P. 1980. The interaction of some environmental factors influencing Schistosoma mansoni cercarial host-finding. J Helminthol 54:203–205. doi: 10.1017/S0022149X0000660X. [DOI] [PubMed] [Google Scholar]
- 338.Thieltges DW, de Montaudouin X, Fredensborg B, Jensen KT, Koprivnikar J, Poulin R. 2008. Production of marine trematode cercariae: a potentially overlooked path of energy flow in benthic systems. Mar Ecol Prog Ser 372:147–155. doi: 10.3354/meps07703. [DOI] [Google Scholar]
- 339.Ibrahim MM. 2007. Population dynamics of Chaetogaster limnaei (Oligochaeta: Naididae) in the field population of freshwater snails and its implications as a potential regulator of trematode larvae community. Parasitol Res 101:25–33. doi: 10.1007/s00436-006-0436-0. [DOI] [PubMed] [Google Scholar]
- 340.Michelson EH. 1964. The protective action of Chaetogaster limnaei on snails exposed to Schistosoma mansoni. J Parasitol 50:441–444. doi: 10.2307/3275851. [DOI] [PubMed] [Google Scholar]
- 341.Wajdi N. 1964. The predation of Schistosoma mansoni by the oligochaete annelid Chaetogaster. J Helminthol 38:391–392. doi: 10.1017/S0022149X00033939. [DOI] [PubMed] [Google Scholar]
- 342.Hopkins SR, Wyderko JA, Sheehy RR, Belden LK, Wojdak JM. 2013. Parasite predators exhibit a rapid numerical response to increased parasite abundance and reduce transmission to hosts. Ecol Evol 3:4427–4438. doi: 10.1002/ece3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lie KJ. 1973. Larval trematode antagonism: principles and possible application as a control method. Exp Parasitol 33:343–349. doi: 10.1016/0014-4894(73)90038-6. [DOI] [PubMed] [Google Scholar]
- 344.Ow-Yang CK, Lie KJ. 1972. Interaction between Hypoderaeum dingeri and Trichobilharzia brevis in the Lymnaea intermediate host. Proc Twelfth Pac Sci Congr 1:203. [Google Scholar]
- 345.Soldánová M, Kuris AM, Scholz T, Lafferty KD. 2012. The role of spatial and temporal heterogeneity and competition in structuring trematode communities in the great pond snail, Lymnaea stagnalis (L). J Parasitol 98:460–471. doi: 10.1645/GE-2964.1. [DOI] [PubMed] [Google Scholar]
- 346.Walker JC. 1979. Austrobilharzia terrigalensis: a schistosome dominant in interspecific interactions with the molluscan host. Int J Parasitol 9:137–140. doi: 10.1016/0020-7519(79)90104-8. [DOI] [Google Scholar]