SUMMARY
Legionnaires' disease (LD) is an often severe and potentially fatal form of bacterial pneumonia caused by an extensive list of Legionella species. These ubiquitous freshwater and soil inhabitants cause human respiratory disease when amplified in man-made water or cooling systems and their aerosols expose a susceptible population. Treatment of sporadic cases and rapid control of LD outbreaks benefit from swift diagnosis in concert with discriminatory bacterial typing for immediate epidemiological responses. Traditional culture and serology were instrumental in describing disease incidence early in its history; currently, diagnosis of LD relies almost solely on the urinary antigen test, which captures only the dominant species and serogroup, Legionella pneumophila serogroup 1 (Lp1). This has created a diagnostic “blind spot” for LD caused by non-Lp1 strains. This review focuses on historic, current, and emerging technologies that hold promise for increasing LD diagnostic efficiency and detection rates as part of a coherent testing regimen. The importance of cooperation between epidemiologists and laboratorians for a rapid outbreak response is also illustrated in field investigations conducted by the CDC with state and local authorities. Finally, challenges facing health care professionals, building managers, and the public health community in combating LD are highlighted, and potential solutions are discussed.
INTRODUCTION
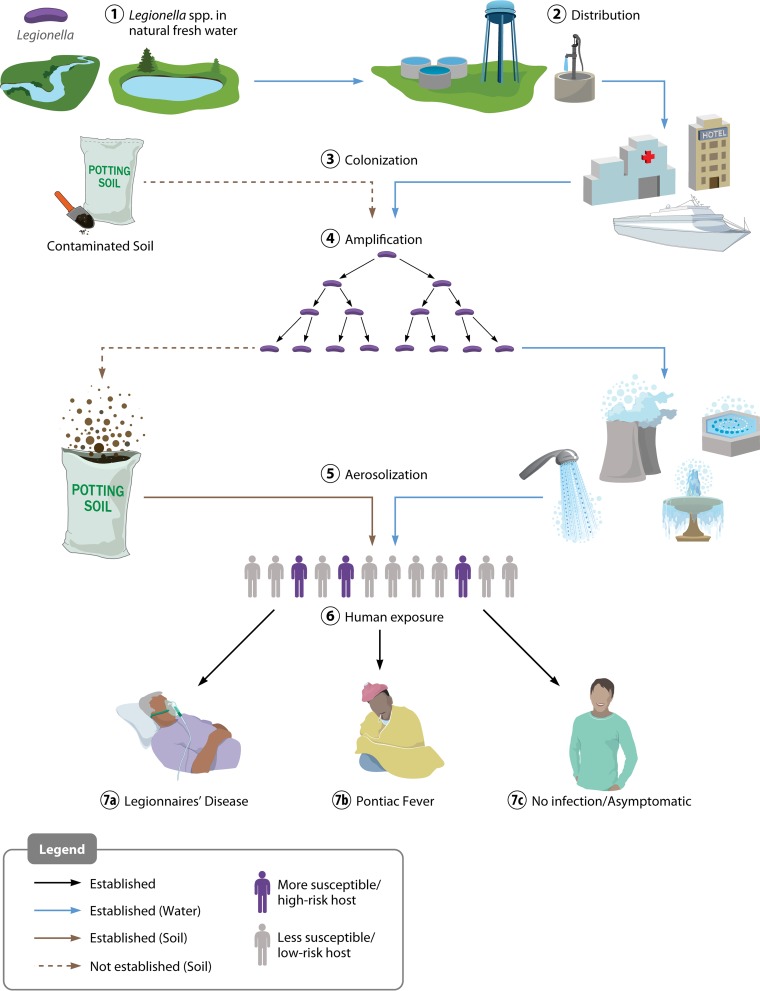
In the summer of 1976, the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, responded to a sudden, explosive epidemic of febrile illness with pneumonia among attendees of the American Legion conference in Philadelphia, PA (1). With heightened public awareness due to “swine flu” earlier that year and mass vaccinations potentially on the way (2), front-page headlines dubbed this new threat “Legionnaires' disease” (LD) (3). A total of 32 people, with at least 20 epidemiologists, led by David Fraser, were mobilized from the CDC, the largest team sent to the field for any outbreak in the center's history to that date, to work with local and state agencies (4). The investigation uncovered 221 suspected cases of this unusual respiratory disease from conference attendees and bystanders in and around the convention hotel (including cases originally labeled “Broad Street pneumonia”); ultimately, 34 individuals died (5, 6). Amid widespread speculation on the nature of this idiopathic disease, scientists ruled out toxicity from >30 heavy metals and infection by 77 known pathogens; however, attempts at growing the culprit organism on 14 different media and in 13 virologic hosts were initially unsuccessful (7). By December of that year, Joseph McDade and coworkers isolated what proved to be a new genus of bacteria from guinea pigs exposed to patient lung tissue, subsequently naming it Legionella for the American veterans' association (i.e., the American Legion) (7–9). Culturing and detection of Legionella were originally hampered by fastidious growth requirements and variable bacterial staining in infected tissues (2, 6, 10), but once the organism was isolated, scientists at the CDC developed tools and methods to reexamine historical collections and past outbreaks with similar presentations. Those scientists found clinically associated Legionella isolates from as far back as 1947 (11, 12) as well as patient seroconversion in two previously unsolved disease clusters: the first was in Washington, DC, in 1965, where 14 of 81 infected individuals died (7, 13), and the second was a nonpneumonic outbreak that occurred in Pontiac, MI, in 1968, where no deaths were reported among 144 cases (7, 14). The latter condition became the clinically and epidemiologically distinct “Pontiac fever,” an acute, shorter-duration, self-limiting, flu-like illness with a high attack rate, which accounts for <1% of Legionella infections reported in the United States (6, 10, 15). The term “legionellosis” is commonly used to describe both the pneumonic and nonpneumonic forms of this disease. As we now know, these two syndromes may coexist within an exposed population (Fig. 1) (16–18), but it is unclear whether Pontiac fever is one potential outcome in the spectrum of disease severity or whether it is due to the presence of nonviable legionellae, amoebal pathogens, and/or high levels of bacterial endotoxin (19–23).
FIG 1.
Route of Legionella dissemination from natural waters to development of Legionnaires' disease and/or Pontiac fever. Legionella from freshwater sources (1) is distributed at low concentrations from points of water purification (2) to colonize downstream local plumbing networks and cooling systems (among other sites) (3) and amplifies under permissive environmental conditions (4). Subsequent aerosolization (5) exposes a human population, which may include individuals with increased susceptibility (6), leading to a potential disease spectrum. More susceptible individuals (due to age or underlying medical conditions) are at a higher risk of LD than those less susceptible, and both groups are at risk for Pontiac fever. The route of LD caused by contaminated soil is less well understood but also appears to involve aerosol exposure.
The 1976 Philadelphia outbreak spurred the swift development of serological methods for LD diagnosis and laboratory techniques for cultivating and isolating the bacterium. Today, many of these original diagnostic tests are still commonly used in laboratories; however, current and emerging proteomics- and nucleic acid-based methods afford significant improvements and expanded capabilities in this area. The goals of this review are to (i) briefly provide background for the physiology and ecology of legionellae, (ii) examine the historical and current state of Legionella detection and diagnosis in clinical and nonclinical laboratory settings and identify gaps and areas in need of improvement or modernization, (iii) highlight advances in molecular-based detection methodologies developed in the last decade that are being applied and implemented in clinical and research settings, (iv) describe recent and past Legionella outbreaks to capture their complexities and diversity while emphasizing the importance of cooperation between epidemiologists and laboratorians during these intensive investigations, and (v) discuss current challenges facing health care professionals and administrators, facility managers, public health officials, and laboratorians in addressing rising LD rates in the coming decades.
PHYSIOLOGY AND ECOLOGY
Legionellae are aerobic, Gram-negative, non-spore-forming gammaproteobacteria. Legionella pneumophila, the most widely studied species, undergoes a phenotypically distinct biphasic life cycle that alternates between a nonmotile, replicative phase and a virulent, flagellated, transmissive phase (23–25). The bacterium displays dramatic pleomorphism, demonstrating coccoid, bacillary (∼0.3- to 0.6-μm by ∼3-μm), and/or long filamentous (∼8- to 50-μm) forms that are influenced by temperature, available nutrients or metabolites, growth environment (e.g., inside amoebae), and medium type (7, 23, 26–29). Legionella spp. are ubiquitous in freshwater habitats, including rivers, lakes, streams, ponds, hot springs, and subsurface waters, and are naturally part of microbial ecosystems (Fig. 1) (30–33). Several species have also been recovered from composts and potting mixes in the United States, Australia, and the United Kingdom and in the soil of Thai farmland (34–41). At present, there are ∼56 distinct Legionella species (and many unnamed species) encompassing at least 70 serogroups, approximately half of which have been isolated from, or detected in, clinical specimens, but all species are regarded as potential human pathogens (42, 43).
In the environment, legionellae can be associated with complex biofilm communities, where the bacterium likely transitions to a motile, planktonic stage; all legionellae studied have the ability to infect and replicate inside freshwater amoebae, which commonly consume biofilms (44–49). The fastidious in vitro nutritional requirements of L. pneumophila (27, 50) originally contradicted findings of its recovery from low-nutrient, highly competitive, polymicrobial environments (23, 31, 51, 52). However, it soon became clear that the unique physiology of legionellae was primarily adapted for survival and replication within numerous protozoan genera, including Acanthamoeba, Naegleria, Hartmannella, and Tetrahymena (24, 53, 54), and secondarily as a free-living or biofilm-associated aquatic bacterium. Their association with amoebae, in the vegetative or cyst form, may induce virulent bacterial phenotypes, assist in distribution, and provide protection from harsh or bactericidal environmental conditions, such as excessive heat and chlorine (55–65).
Legionellae thrive in tepid water (25°C to 37°C) but may propagate at temperatures above and below this range and may even survive at growth-restrictive temperatures of <20°C and >55°C (66, 67). A recent controlled, pilot-scale hot water distribution study confirms what many hospital, hotel, and cruise ship operators have reported: legionellae may persist and quickly recolonize potable water networks even after multiple rounds of heat shock (70°C for 30 min) and biocide treatment (68). An extensive collection of case studies and research articles dating back >30 years demonstrates the adaptability and potential of legionellae to colonize man-made aquatic environments (Fig. 1), from the initial point of water treatment (69–73) to private homes (74–78), hospitals (79–83), restaurants (84, 85), bath houses (86–89), hotels (90–92), and, eventually, wastewater facilities (93, 94). These studies underscore the resiliency and persistence of legionellae.
EPIDEMIOLOGY AND DISEASE
Legionnaires' disease is a respiratory illness caused by inhalation of Legionella-containing aerosols generated by showers, faucets, air-conditioning cooling towers, whirlpool spas, and fountains, among others (Fig. 1). Legionellae are frequently isolated from natural waters, but these sources are typically not implicated in direct transmission, with one possible exception being natural hot springs adapted for human bathing, such as public baths, which are popular in Japan and Taiwan, among other locations (86, 95–101). Aspiration of water containing Legionella has also been suggested to be a common transmission route (102), although the frequency with which this occurs is unclear. Reports have suggested that immunocompromised patients in health care settings may be at risk from contaminated respiratory equipment (103–108); in these specific instances, the use of sterile potable water may be advised (109–111).
Humans are considered incidental (and dead-end) hosts, whereby legionellae infect and replicate within alveolar macrophages. The resulting illness may manifest as a febrile disease characterized by pneumonia and possible bacteremia (112–115). Together, Legionella spp., Mycoplasma pneumoniae, and Chlamydophila pneumoniae represent the “atypical” branch (i.e., not Streptococcus) of nonzoonotic bacterial respiratory pathogens, responsible for ∼22% of cases of community-acquired pneumonias (CAP) in the United States and Canada and up to 28% of cases worldwide (116). Legionellae alone are responsible for at least 8,000 to 18,000 hospitalizations every year in the United States, accounting for 2 to 9% of all pneumonias, a statistic also reflected in international studies (e.g., CAPNETZ), and yet, it may be an underestimation (116–119). The majority of LD cases occur in the summer and fall and more commonly affect males >50 years of age who have lung disease or immunosuppression (Fig. 1) (15, 82, 120, 121). Additional risk factors for legionellosis include smoking; recent travel; and underlying medical conditions such as diabetes, cancer, AIDS, end-stage renal disease (121, 122), and, potentially, human cellular Toll-like receptor 6 (TLR6) mutations (123). Notably, the reported LD incidence has increased substantially since 2000 for all age groups and U.S. geographic regions, with the median age for disease trending younger between 1990 and 2005 (15, 124–126). While this increase may in part reflect a true rise in the number of LD cases, a combination of factors may be contributing, including increased diagnostic testing, changes in case reporting methods, and expansion of the vulnerable elderly population. The influence of changes to national water quality standards or adjustments in medical insurance reimbursement patterns is likely minimal (15, 124). Interestingly, sporadic LD has been linked to higher-than-average atmospheric temperatures and increased rainfall (or humidity) in several studies, and while a mechanism is not clear, standing road water may play some part in infection (127–134). A similar increase in the overall European LD incidence has also been documented over the past 20 years but with a possible plateau being reached in the late 2000s (120, 135).
Clinical and radiographic presentations of LD are virtually indistinguishable from those of other, more common forms of pneumonia (136). Rapid, laboratory-based or point-of-care (POC) testing is crucial for accurate diagnosis and improved detection of LD outbreaks, and it allows for confirmation of LD-inclusive empirical treatment, changes in drug dosage or duration, or targeting of alternative antibiotics active against Legionella spp. (10, 137–139). In addition, despite the sensitivity of L. pneumophila to commonly available antibiotics, LD is associated with greater CAP severity and a higher case-fatality rate (up to ∼30%) than CAP from other atypical pathogens (121, 140–145). The U.S. LD case-fatality rate has decreased steadily since the mid-1980s (125), from a high of ∼34% to 8% on average in the 2000s (15). Although European LD case-fatality data were not compiled before the mid-1990s, current estimates place these rates on par with those for the United States during the previous decade (135, 146–149). L. pneumophila serogroup 1 (sg1 or Lp1) is responsible for 70 to 92% of laboratory-detected legionellosis cases in the United States and Europe and ∼50% of the cases in Australia (120, 125, 150). The remaining species and L. pneumophila serogroups, such as L. pneumophila sg6, L. longbeachae, L. micdadei, and L. bozemanii [sic], account for most of the remaining disease, with the exception that L. longbeachae is the source of ∼30 to 55% of LD cases in Australia and New Zealand; however, proportions vary greatly depending on the state or territory (119, 125, 150–152).
Generally, the high percentage of Lp1 clinical cases is not reflected in the local environmental distribution of Legionella. Indeed, seroprevalence studies in many countries have demonstrated potential ongoing exposure to diverse Legionella serogroups and species, which typically contrasts with the greater clinical prevalence of Lp1 (153–160). As such, substantial effort has been devoted to characterizing Lp1 virulence determinants, such as secreted effectors or surface factors. The Lp1 lipopolysaccharide (LPS) serves as the basis for traditional serogrouping, and it contains a virulence-associated epitope (recognized by monoclonal antibody 2 [MAb2] or MAb3/1 of the internationally used monoclonal panels) which is dependent on a functional lag-1 gene for synthesis (161, 162). This LPS modification is strongly associated with Lp1 clinical disease and predominates in outbreak strains but is less frequently found in environmental Lp1 isolates (163–166; CDC, unpublished data). At present, it is not completely understood why this single serogroup and the lag-1 genotype are responsible for the majority of clinical cases; potentially, some strains of Legionella may be especially pathogenic to humans, easily aerosolized, or more suited to colonization of anthropogenic water distribution systems (33, 163–170). One notable exception to the dominance of lag-1-expressing strains in LD cases is the Lp1 OLDA/Oxford (lag-1-negative) subgroup, which currently makes up 43% of all clinical isolates in Israel (171).
Unlike Mycoplasma and Chlamydophila, person-to-person Legionella transmission has never been reported, and community-acquired legionellosis is typically associated with man-made structures that generate water aerosols (Fig. 1), such as fountains (84, 85, 172–176), building water systems (75, 79, 177–184), cooling towers (185–193), and whirlpool spas (194–198), among others. U.S. surveillance data from 2001 to 2010 have consistently included Legionella as one of the leading causes of drinking water-associated outbreaks, accounting for 58% of cases in 2010 (199). While large-scale outbreaks, such as the 1976 Philadelphia epidemic or the recent and likely recurring Pittsburgh Veterans Health Administration Hospital incident (200, 201), attract national attention, the majority of LD cases are isolated and sporadic; from 2000 to 2009, outbreak-associated LD accounted for only 4% and 9.3% of all legionellosis cases reported in the United States and Europe, respectively (15, 114, 135, 146–149). Domestic and international travel-related disease is an increasingly recognized and significant category of infection; travel-associated legionellosis represented 19 to 24% of LD cases reported in the United States and Europe during the previous decade (15, 135, 146–149, 202, 203), with Lp1 predominating in both regions (204–213).
DETECTION AND DIAGNOSIS
Procedures for the diagnosis and management of LD require important methodological distinctions from the identification of Legionella spp., serogroups, and sequence types (STs) for epidemiological investigations. While complementary, these related activities serve different objectives. Properly informed LD treatment does not necessitate discrimination beyond the genus level because all Legionella species tested are sensitive to commonly prescribed macrolides and fluoroquinolones (e.g., azithromycin and levofloxacin), which are active against and recommended for community- and hospital-acquired infections (214–220). Unlike CAP caused by M. pneumoniae or Streptococcus pneumoniae, acquired antibiotic resistance has never been reported for any Legionella strain, although a recent study reported a single clinical isolate displaying azithromycin and ciprofloxacin resistance outside the wild-type range (221–223). A follow-up study detailing the molecular basis for this resistance was not able to determine if mutations (in the gyrA gene) arose before or after the antibiotic was administered (224). Of particular interest, a next-generation macrolide (the first fluoroketolide), solithromycin, is currently in phase III clinical trials and, at least in vitro, appears to be highly active against Lp1 (225). In contrast, while timely processing is still important, epidemiological investigations that link one or more disease cases to common environmental exposures must employ more thorough approaches for identifying shared phylogenies between clinical and environmental strains. These techniques may include traditional antibody-based assays or more recently developed nucleic acid amplification tests (NAATs), such as PCR.
Early in the field's history, a limited set of culture- and antibody-based methods was used for both clinical and epidemiological investigations (e.g., direct fluorescent-antibody [DFA] assay); the later commercial development and widespread adoption of a Legionella urine antigen test (UAT) largely eliminated many of these assays from the clinical repertoire. Fortunately, nucleic acid molecular technologies introduced in the late 1980s and early 1990s, such as PCR and DNA sequencing, proved valuable for advancing both LD diagnostic and epidemiological capabilities (21, 226–229). Current LD case classification is based upon a combination of factors, including displaying clinically compatible symptoms (e.g., fever, myalgia, cough, and pneumonia), supporting epidemiological information, and positive laboratory findings (Fig. 2). In the United States and Europe, a positive laboratory result from a UAT, bacterial culture, and/or paired serology (i.e., indirect fluorescent-antibody [IFA] assay or enzyme-linked immunosorbent assay [ELISA]) for Lp1 defines a clinical case. Furthermore, detection of Legionella antigen or whole bacteria in respiratory secretions, tissues, or fluids by DFA, detection of seroconversion (4-fold or higher increase in titer) to non-sg1 serogroups, non-pneumophila Legionella species, or multiple species using pooled antigens, and/or detection of Legionella nucleic acid supports a suspected or probable case in the United States (230–233). Definitions for a probable case assignment in the European Union differ slightly from those in the United States, because European standards do not specify the minimum titer increase for seroconversion and they allow a single high antibody titer for assessing disease status.
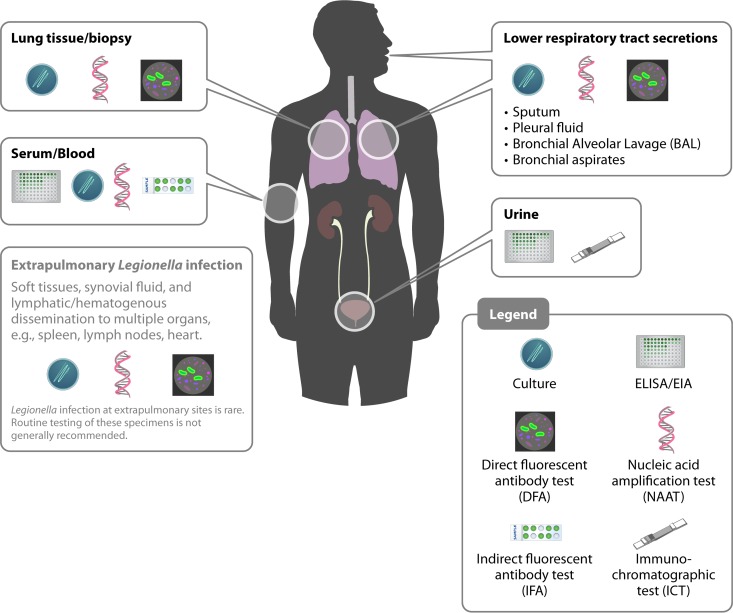
FIG 2.
Specimen types, diagnostic tests, and anatomical locations for determining a potential current or recent Legionella infection. Some assays are applicable to multiple specimen types, such as culture and nucleic acid amplification. In general, the success of detecting Legionella is dependent on the severity of disease, specimen integrity, technical proficiency of the laboratory, and particular test characteristics, as listed in Table 1. Additional recent emerging methods and technologies may also be used, such as mass spectrometry, but they may not be widely available or accessible. Note that Legionella infection at extrapulmonary sites, such as soft tissues or organs (e.g., spleen and heart), is rare.
Prospective and retrospective epidemiological studies may also use these case-defining laboratory techniques in addition to slide/serum/latex agglutination methods, monoclonal antibody (MAb) typing, and nucleic acid molecular methods such as mip gene sequencing, sequence-based typing (SBT) (234, 235), and/or PCR, both conventional and real time. Evidenced by its popularity in the peer-reviewed literature, Legionella nucleic acid detection is being increasingly recognized, standardized (236), and implemented in the laboratory for rapid LD diagnostics and detection. The following sections detail the major categories of Legionella tests (Fig. 2) offered in clinical settings and at reference laboratories such as the CDC, emphasizing their purpose, benefits, and drawbacks as well as highlighting emerging technologies and procedures (Table 1). Information for clinicians and health departments, preferred diagnostic assays and collection procedures, and detailed protocols for most environmental techniques described here can be found on the CDC Legionella website, as listed in Table 3.
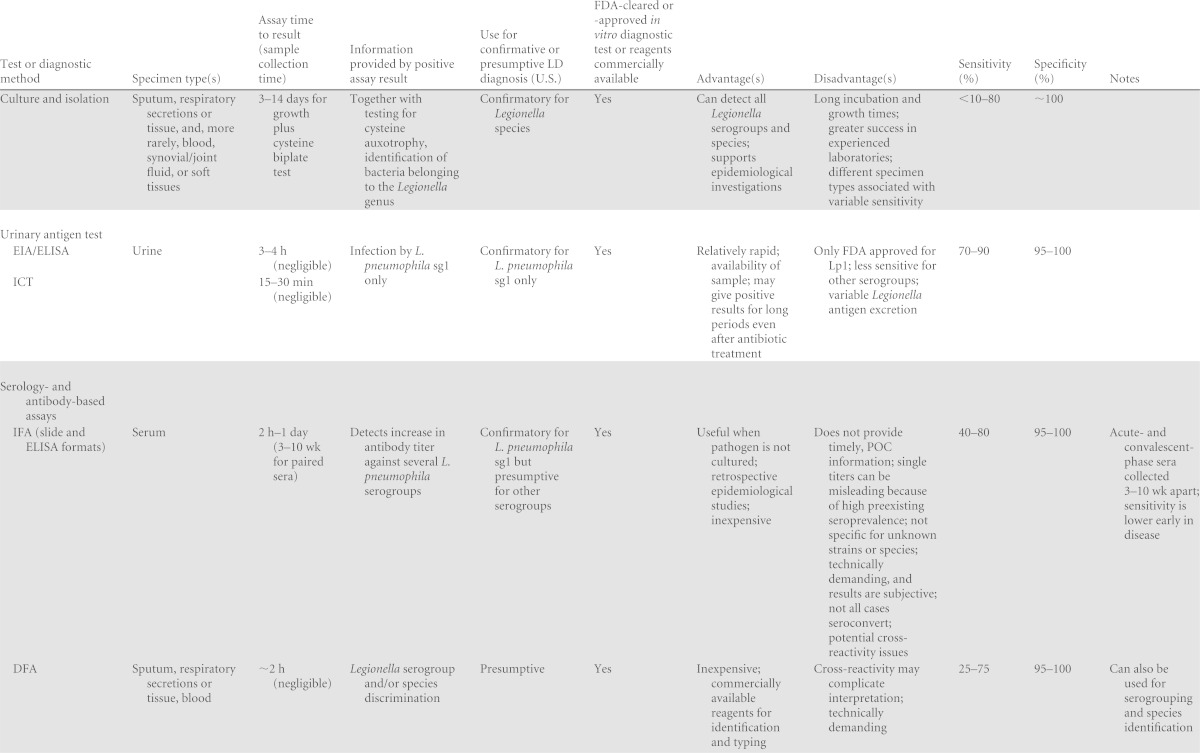
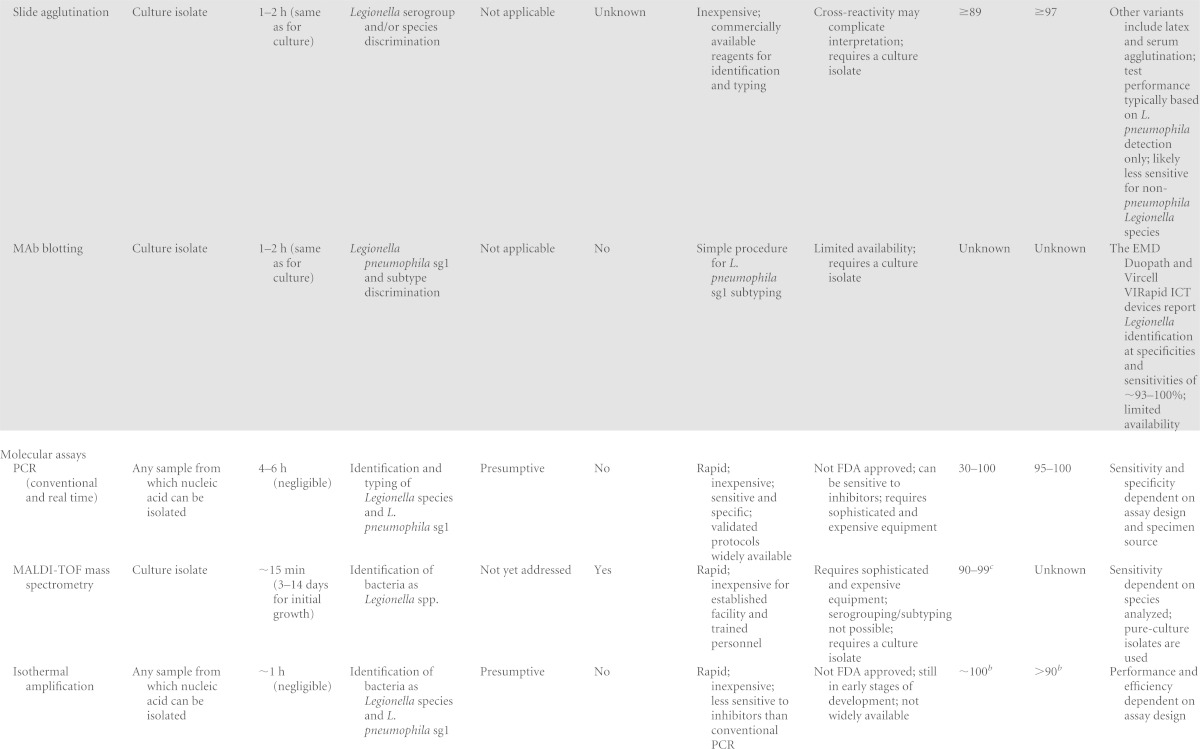
TABLE 1.
Past, current, and emerging diagnostic tests for Legionella and LDa
ICT, immunochromatographic test; IFA, indirect fluorescent antibody; DFA, direct fluorescent antibody; EIA, enzyme immunoassay; MAb, monoclonal antibody, MALDI-TOF, matrix-assisted laser desorption ionization–time of flight; sg, serogroup.
Very few studies for comparison.
Compared to mip sequencing.
TABLE 3.
Selected online Legionella resourcesa
| Organization(s) and/or institution(s) | Resource or reference | Topic(s) covered | Website URL |
|---|---|---|---|
| CDC Legionella Web pages | Main page has information for the public, clinicians, the media, health departments, and building and environmental professionals | Multiple topics, including disease facts, clinical indications, diagnostic tests, investigation tools, sampling protocols, position statements, and the ELITE program | http://www.cdc.gov/legionella/index.html |
| ELITE program Web page | Details of the ELITE certification process for laboratories isolating Legionella from environmental water | http://www.cdc.gov/legionella/elite.html | |
| Epidemiological investigation tools | Diagnostic tests, case verification, and patient interviews; environmental assessment and sampling; decontamination (including hot tubs and cruise ships); and CDC assistance | http://www.cdc.gov/legionella/health-depts/inv-tools.html | |
| Environmental specimen collection and management | Procedures and protocols for environmental sampling and processing | http://www.cdc.gov/legionella/specimen-collect-mgmt/index.html | |
| CDC and HICPAC | Guidelines for preventing health-care-associated pneumonia, 2003 (111) | Prevention of health care-associated legionellosis and other pneumonias | http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5303a1.htm |
| Guidelines for environmental infection control in health-care facilities (560) | Infection control in health care facilities, with some focus on Legionella | http://www.cdc.gov/mmwr/Preview/mmwrhtml/rr5210a1.htm | |
| Guideline for disinfection and sterilization in health care facilities, 2008 (587) | General health care disinfection and sterilization procedures, with notes on Legionella and amoebae | http://www.cdc.gov/hicpac/Disinfection_Sterilization/acknowledg.html | |
| EWGLI/ESGLI Sequence Based Typing Database for Legionella pneumophila | Main page has links to multiple SBT resources | Multiple topics, including methods and protocols, SBT locus data, strain submission, sequence quality tools, and various query functions | http://www.hpa-bioinformatics.org.uk/legionella/legionella_sbt/php/sbt_homepage.php |
| EPA | Legionella: human health criteria document (588) | Multiple topics, including general information on Legionella, occurrence, human health effects, risk assessment, and environmental analysis | http://water.epa.gov/action/advisories/drinking/upload/2009_02_03_criteria_humanhealth_microbial_legionella.pdf |
| WHO | Legionella and prevention of legionellosis (589) | Multiple topics, including water safety plans, in-building distribution, and exterior cooling system assessment | http://www.who.int/water_sanitation_health/emerging/legionella.pdf |
| Guidelines for drinking water quality, 4th ed. (590) | Drinking water risk assessment, with some attention toward Legionella | http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf | |
| Water safety in buildings (591) | Water safety plans, risk management, and supporting information | http://whqlibdoc.who.int/publications/2011/9789241548106_eng.pdf?ua=1 | |
| Guidelines for safe recreational water environments, vol 2 (592) | Risk assessment and health hazards associated with water environments, including legionellosis | http://www.who.int/water_sanitation_health/bathing/bathing2/en/index.html | |
| ASHRAE | Guideline 12-2000 (595)b | Guidelines for minimizing Legionella in building water systems | |
| Proposed new standard 188 (593)c | Standard practices and operating procedures for building operators to prevent legionellosis associated with building water systems | https://osr.ashrae.org/Public%20Review%20Draft%20Standards%20Lib/Std-188P-PPR2%20Final%206%2010%202011.pdf | |
| ECDC | ECDC legionellosis health topic website | Disease facts, news, epidemiological data, surveillance reports, recent publications, external resources, and Legionella-related events | http://ecdc.europa.eu/en/healthtopics/legionnaires_disease/Pages/index.aspx |
| ELDSNet | ELDSNet information, case definitions, surveillance activities, operating procedures, forms, investigation guidelines, and an outbreak toolbox | http://ecdc.europa.eu/en/activities/surveillance/ELDSNet/Pages/index.aspx | |
| Health and Safety Executive, United Kingdom | Legionella and Legionnaires' disease Web page | Multiple topics, including disease and treatment information, workplace risk exposure, employer responsibilities, recent news, and further Legionella-related resources | http://www.hse.gov.uk/legionnaires/index.htm |
| Legionnaires’ disease: the control of Legionella bacteria in water systems, L8, 4th ed. (594) | Approved code of practice and guidance for Legionella risk assessment, prevention, management, control, and monitoring processes | http://www.hse.gov.uk/pubns/priced/l8.pdf | |
| Public Health England (currently merging with the Health Protection Agency) | Legionnaires' disease Web page | Multiple topics, including general disease and organism information, sampling and investigation guidelines, surveillance and epidemiological data, external proficiency testing scheme for Legionella (from a linked site), case definitions, and recent publications and reports | http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/LegionnairesDisease/ |
SBT, sequence-based typing; ELITE, Environmental Legionella Isolation Techniques Evaluation; EWGLI, European Working Group on Legionella Infections; ESGLI, European Society for Clinical Microbiology Study Group.
Currently undergoing an update.
Proposed standard not yet finalized.
Microbiological Culture
Culture and isolation remain the “gold standard” for Legionella detection and LD diagnosis (51, 114). Acceptable culture specimens include those from the lower respiratory tract, such as sputum, pleural fluid, bronchial aspirates, and bronchial alveolar lavage (BAL) fluid (Fig. 2) (237). Lung tissue and biopsy specimens are also appropriate for attempting culture. Less conventional specimens include those from extrapulmonary sites, such as soft tissues, joint fluids, and blood (51, 113, 238). Reports of Legionella infection at these sites are rare, as is recovery of an isolate. In these instances, culture should be attempted only when other etiologies have been ruled out. Among all potential specimens for culture, sputum is generally most commonly sought, although a significant proportion of LD patients produce little or no sputum for culture analysis (51, 239). The sensitivity of detection of Legionella by culturing of clinical specimens is highly variable, ranging from <10% to 80%, and recovery is dependent on the sample type as well as the experience and technical proficiency of laboratory personnel (239). Legionellae grow on several types of complex artificial media; however, the most successful medium and procedure include buffered charcoal yeast extract (BCYE) agar containing 0.1% α-ketoglutarate with l-cysteine incubated at 35°C in a humidified, 2.5% CO2 atmosphere (50, 240, 241). Most isolates demonstrate growth in 3 to 5 days, but non-pneumophila Legionella species and occasionally primary-specimen isolates may require considerably longer incubation times, sometimes up to 2 weeks (238, 242, 243). Despite extended growth periods, obtaining an isolate provides numerous advantages in allowing greater characterization and further epidemiological studies.
Not surprisingly, it is generally easier to isolate bacteria from patients with severe LD (due to increased bacterial burden) (119), and several methods can be employed to isolate Legionella from nonsterile specimens, such as sputum, or from heavily contaminated environmental sources, such as air-conditioning cooling towers (239, 244). Semiselective procedures enhance Legionella recovery in the presence of competing flora (from both clinical and environmental samples), including brief acid and heat exposure and/or the addition of glycine, polymyxin B, cycloheximide, and vancomycin to the growth media, to which legionellae are naturally resistant. Most legionellae are cysteine auxotrophs (the exceptions being L. oakridgensis, L. jordanis, and L. nagasakiensis, all of which may adapt to cysteine-deficient media after serial passage [245, 246]); thus, cysteine biplates can be used to quickly screen potential Legionella-like colonies (50). To date, at least 16 named and 6 undesignated species exhibit yellow-green, blue-white, or red-pink autofluorescence under long-wave (365-nm) UV light (238, 247–251; CDC, unpublished) when cultivated on BCYE or non-charcoal-containing medium. Most reported cases of LD are associated with L. pneumophila; however, currently, 8 of the 12 named species exhibiting blue-white and red fluorescence are also linked to human disease (81, 249, 252–256). Therefore, even uncommon environmental species should be considered potential human pathogens, especially for at-risk populations.
Clinical and hospital-based laboratories are a critical link in the chain of Legionella detection, diagnosis, and possible remediation. Ideally, attempting Legionella culture in all suspected cases for confirmation and further analyses should be the desired goal (214). Additionally, the concomitant development of alternative, culture-independent diagnostics should continue. Given its central role in LD investigations, the ongoing exchange of training and knowledge is encouraged, to sustain Legionella culture proficiency in the laboratory, especially since a sharp decline in the frequency of culture diagnosis has been reported in the United States, from >60% in the early 1990s to 5% on average from 2005 to 2009 (15, 125). The remarkable lack of laboratory expertise for Legionella isolation was confirmed by a College of American Pathologists survey, in which one-third of clinical laboratories were unable to grow a pure Legionella culture (138). The European Union has also experienced a large drop in overall culture-based detection, from 18% in 1996 to 12% in 2011 (120, 257). These statistical averages can be misleading since culture recovery can vary significantly among European Union member states. For instance, from 2007 to 2008, LD was diagnosed by culture in Spain and Italy in 0.45% and 1.7% of cases, respectively, while a higher culture confirmation rate of 15 to 40% was reported in Austria, France, Denmark, and the Netherlands (135, 203). A concerted effort by laboratorians and administrators is clearly needed to improve culture-based confirmation practices, preferably in conjunction with other tests based on nucleic acid amplification or UATs.
Until recently, there was no formal external laboratory accreditation program in the United States for environmental Legionella detection, making it difficult to assess the competency and proficiency of testing laboratories. To fill this gap, the CDC established the Environmental Legionella Isolation Techniques Evaluation (ELITE) program (see Table 3) (258). This free program, which began in late 2008, enables commercial, governmental, and hospital-based laboratories in the United States and abroad to evaluate their Legionella isolation techniques by using standardized, blind samples. Certification as an ELITE member requires biannual proficiency testing, whereby laboratories must successfully isolate and identify Legionella from heterogeneous aqueous mixtures. There are also similar programs in Europe for assessment of competency in the detection of Legionella in water samples (such as the “Legionella Scheme” administered by the Health Protection Agency of the United Kingdom). Given the complexity of patient specimens and existing regulatory oversight, there is no equivalent U.S. proficiency program specifically for clinical laboratory Legionella culture; however, third-party proficiency testing and certification for clinical bacteriology laboratories, required by the Clinical Laboratory Improvement Act (CLIA), may include Legionella as an unknown for culture and identification.
Urinary Antigen Test
The UAT has dramatically outpaced other laboratory methods for diagnosis, representing 82% and 97% of the diagnostic tools used for LD confirmation in Europe and the United States, respectively (15, 125, 144, 146, 257). The popularity and ubiquity of the UAT are attributed to its speed, relatively low cost, uncomplicated procedure, ease of sample collection, commercial availability, and FDA clearance (CE marking in the European Union). Legionella-specific urinary antigens can be detected in the majority of L. pneumophila infections shortly after clinical symptoms appear (2 to 3 days) and may be excreted for several days to >10 months, even during antibiotic treatment and after disease resolution (244, 259, 260). For most cases, however, Legionella antigen is no longer detected in urine 1 to 2 months after therapy. The UAT was initially an in-house method for Legionella antigen detection (261–263) and was commercially developed as a radioimmunoassay in the late 1980s and early 1990s (264, 265).
Currently, the Legionella UAT is available from several vendors in two main formats: a 96-well plate-based enzyme immunoassay (EIA), or an ELISA, and a rapid, immunochromatographic test (ICT), in a card- or strip-based format, similar to a home pregnancy test (also known as a lateral-flow test). The most commonly used rapid ICT and EIA formats are highly specific for L. pneumophila (between 95 and 100%), with sensitivities from 70 to 90%, depending on whether urine is artificially concentrated; however, only five tests are FDA cleared for sale in the United States (Alere BinaxNOW Legionella urinary antigen card, Binax Legionella urinary antigen EIA, SAS Legionella test, Bartels Legionella urinary antigen ELISA, and Meridian Tru Legionella) and only for the detection of Lp1 (http://www.fda.gov/) (266–269). It is widely recognized that EIAs from some manufacturers exhibit cross-reactivity for various non-sg1 L. pneumophila serogroups and thus may detect a wider variety of pathogens. However, sensitivities among commercially available tests for non-Lp1 LD are highly variable and generally much lower than those for Lp1-associated disease when assayed with urine from patients with confirmed LD (267, 270–275). Importantly, the sensitivities of most similar-format UATs are generally equivalent, regardless of the manufacturer (267–269, 276–279), and they all allow for rapid assessment and patient treatment (280–282), unlike culture or serology, which may take days to weeks (139). Overall, the card-based ICT is a rapid, simple, qualitative assay for basic laboratory or POC use, while the EIA format is quantitative, may offer comparatively higher sensitivity and specificity, and is more suited for larger clinical, reference, or research laboratories (283).
Over the past 30 years, the development and implementation of UATs have greatly benefited LD patients by significantly improving diagnosis rates and thus allowing timely treatment (146, 257, 280). The LD-associated mortality rate decreased dramatically (∼77%) in the United States from 1985 to 2009, along with a rapid increase in DFA and culture-based detection in the early 1980s, the mainstream introduction of the Legionella UAT (in the 1980s and 1990s), and updated guidelines (in the 1990s) by the American Thoracic Society and the Infectious Diseases Society of America for coverage of Legionella in empirical antibiotic therapy for CAP (15, 125, 284, 285). Several developments may have contributed to this changing diagnostic and treatment landscape, including standardized Legionella growth media, more readily available and sensitive DFA reagents, novel and rapid urine-based assays, and greater LD awareness by health care practitioners; additional data suggest that the superior sensitivity of the UAT over that of culture may have also allowed the detection of cases with milder disease and an inherently higher survival rate (125). In parallel with declining mortality, disease attributed to non-sg1 and non-pneumophila Legionella species decreased by 79% (125), suggesting that Lp1 is overrepresented in current estimates of LD. While the Legionella UAT is a valuable tool, sole reliance on this one diagnostic test may result in significant numbers of undetected LD cases (51, 125, 271). It is unclear whether this dramatic decrease in non-sg1-associated infections is in any way attributable to fewer actual cases. Surveillance conducted in the United States before and during the increase in UAT popularity suggests that the proportion of LD associated with Lp1 is variable year to year and ranges from 50 to 91% when only culture-confirmed cases are included (121, 125). More recent international surveillance and laboratory data suggest that a simple decrease in the non-Lp1 burden is not to blame. Denmark employs a more comprehensive diagnostic and testing regimen that relies on culture isolation and NAATs at levels well above the rest of the European Union and U.S. averages (120, 135). In the period from 1996 to 2006, approximately one-third of LD cases were culture confirmed in Denmark, which revealed Lp1 in only 60% of cases on average, similar to U.S. rates before the decline of culture techniques (271, 286). At present, it is uncertain if Denmark is burdened with higher-than-average enviromental levels of non-Lp1 (170); seroprevalence studies are suggestive of wide-ranging and diverse Legionella exposure in this country but fail to clarify questions surrounding environmental distribution (160, 287, 288). Regardless, enhanced surveillance and identification in Denmark likely detect a broader spectrum of LD caused by less common clinical serogroups and species, for which the current Lp1-specific UATs are not sensitive.
A closer analysis of Danish research also reveals an alarming trend: mortality rates for all non-sg1 LD patient groups were higher than those for any Lp1-infected population (i.e., MAb2 positive or negative) (271). Similarly high levels of mortality were observed in the United States between 1980 and 1989 for patients infected with L. pneumophila sg6 (121). Moreover, the reported survival rate is low (73%) for cases who are culture positive but UAT negative (125), a pattern which is more likely for non-Lp1 infections. Three plausible explanations for the apparently higher mortality rate in these subgroups include (i) preexisting patient immunosuppression leading to increased susceptibility to all legionellae and potentially higher inherent mortality, (ii) misdiagnosis and treatment delay due to the previously discussed UAT serogroup limitations (286), and/or (iii) increased non-sg1 Legionella pathogenicity. Given this historical overview of shifting diagnostic trends in the United States and recent international data suggesting a higher non-Lp1 clinical prevalence, it is reasonable to assume that significant underdiagnosis of non-Lp1 LD has occurred due to an overreliance on current-generation UATs.
Healthy populations across geographically diverse parts of the United States, Europe, the Middle East, Australia, Japan, South Korea, China, and former Soviet states display a large variation in seroprevalence for all legionellae, which additionally supports a reexamination of local Legionella exposures and the value of Lp1-specific UATs for diagnosing most LD cases (153–159, 289–303). This underscores a pressing need for the development of more inclusive Legionella rapid diagnostics; to be useful in a basic laboratory or POC setting, a pan-Legionella UAT would ostensibly be as simple to perform as current tests while detecting additional species and serogroups with similar efficiencies. Such a test would be invaluable, especially in regions with potentially higher environmental levels of non-sg1 L. pneumophila (e.g., the southwestern United States) and in countries where non-pneumophila Legionella species are documented in a significant proportion of LD cases (e.g., Thailand, Australia, and New Zealand) (271).
Regardless of the species or serogroup, clinicians should be particularly aware of in vitro diagnostic limitations when ruling out LD. A negative UAT does not necessarily exclude LD from consideration (304), because severe disease is more likely to yield a positive test (as opposed to mild LD), and results can vary greatly with time since exposure (141, 259, 305–307). These complications were confirmed in several research studies, where presumably low antigen excretion presented “delayed positive” ICT results (using the BinaxNOW Legionella ICT) observed at later time points (e.g., after 1 to 4 h of incubation) for samples initially giving borderline EIA absorbance measurements (267, 268, 276, 308). However, with some commercial products, this procedure may occasionally yield false-positive results, and manufacturers typically do not endorse this method of use (with some exceptions, e.g., Oxoid Xpect); in any case, results should be interpreted with caution if this method is performed (278, 309). In general, if initial UAT results are negative but the index of suspicion for LD remains high, clinicians are encouraged to perform testing multiple times over a longer period and/or to employ alternative testing modalities, such as PCR (283, 305), and additionally, concentrating urine can increase sensitivity without decreasing specificity (266, 279, 283, 306); however, boiling to reduce nonspecific interactions may be advised. These additional steps would likely abrogate the time advantages of the rapid ICT. Also, while results from a UAT may be sufficient for initial LD treatment, a culture from a patient specimen is still invaluable for epidemiological studies to mitigate further exposure from an environmental point source (214). From a public health standpoint, this cannot be overemphasized. Many outbreaks unfortunately result in a greater number of casualties due to the lack of an isolate from the initial patient(s), thus hindering an effective and timely public health response.
Serological and Antibody-Based Assays
Serological testing for IgG and IgM antibodies against Legionella is a diagnostic tool that was critical in the original Philadelphia outbreak investigation (7, 9, 51) and one of the principal methods used for LD diagnosis in the early 1980s (125). While once popular, the number and scope of serological tests performed in the modern clinical laboratory have dropped significantly with the rise of standardized culture media and techniques and faster, more definitive analyses such as the rapid UAT and molecular methods (125). According to the European Centre for Disease Prevention and Control (ECDC) and the World Health Organization (WHO), the use of serology for LD confirmation in Europe declined from 61% to 6% on average in the period from 1995 to 2010, displaced by the faster, less technically demanding UAT (146, 257). Data from the U.S. passive surveillance system since 1980 and the Supplemental Legionnaires' Disease Surveillance System (SLDSS) between 2005 and 2009 highlight an equally dramatic 60% decrease in the use of serology, with <1% of case diagnoses currently relying on serology or DFA assays (15, 125). A similar trend was found in parts of Canada, where the probability of detection by serology or DFA has fallen precipitously with increased UAT usage (310). There are several obvious reasons for this change; even with the commercial availability of IFA assays and ELISAs for detecting patient seroconversion, serology is not a timely indicator of disease. Reliance on a 4-fold increase in antibody titer (to 1:128) between acute- and convalescent-phase serum samples taken 4 to 8 weeks apart means that the window for treatment has long passed (113). Furthermore, underlying medical conditions or immunosuppression may occasionally delay or prevent a 4-fold increase in titer from actually occurring, despite the existence of a bona fide infection (311–313). The majority (5.5% out of a total of 7%) of serology testing in the European Union is performed with a single, high convalescent-phase titer (120), which can be problematic, since prior exposure cannot be ruled out, even at titers of >1:256 (238, 314). A growing list of studies suggests that elevated Ig titers for Legionella can be detected in <1% to almost 30% of healthy individuals, depending on age, location, work environment, and, occasionally, gender (154, 155, 158, 288, 293, 297, 315). Of potential importance, the use of different in-house-developed and commercially developed IFA and EIA antigen preparations may complicate the interpretation of antibody titers for Legionella, especially across time and from different studies.
There are further challenges for serological assays: cross-reactivity may complicate the interpretation of results for non-pneumophila and non-sg1 Legionella infections (316–320), 20 to 30% of individuals with culture-confirmed LD never seroconvert based on the 4-fold rule (113), and proper interpretation of serological tests, such as IFA assays, requires extensive training and experience because results are often subjective and semiquantitative. As such, commercially available ELISA kits for detection of Legionella seroconversion may be increasing in popularity because they abrogate interpretational ambiguity through automation; however, the diagnostic accuracy of these tests is not yet established or agreed upon, and any trend toward their adoption has not been fully evaluated (216). Acknowledging these limitations, serology is still relevant for LD confirmation when the infectious agent cannot be isolated, and serology provides supportive data when corroborated by additional tests such as DFA or other immunohistochemical assays. Serology can also be valuable for retrospective epidemiological investigations, to identify patterns of disease, potential ongoing outbreaks, and general seroprevalence.
DFA assays, slide agglutination tests (SATs), and MAb screens are antibody based but not generally considered “serology” in the traditional sense because patient serum is not directly tested. SATs and MAb screens require a pure-culture isolate, while DFA assays can be performed on cultures, patient tissues, or secretions. Overall, their use in the clinical laboratory for Legionella respiratory antigen detection appears to be minimal, decreasing from a rate of 1% in 1996 to <1/10 of 1% in 2010 (146, 257). Interestingly, among ELITE member laboratories (n = 141), approximately half of clinical (43%) and commercial (54%) laboratories and most public health laboratories (65%) rely on MAbs or SATs for isolate or specimen confirmation, and a subset of laboratories use DFA assays for subtyping purposes (C. Lucas, personal communication). These numbers are not surprising given that ELITE-certified laboratories have a specific interest in Legionella environmental detection and typing.
DFA assays, SATs, and MAb blotting are useful for qualitative Legionella identification and typing at the species and serogroup levels. The tests benefit from being relatively rapid, inexpensive, and reliable, allowing strain comparisons across time with commercially available reagents (113), but similar to the IFA assay, they require a moderate-to-high level of laboratory expertise. Of particular note, MAb panels, such as the one developed by Joly et al., that allow Lp1 subtype discrimination can be useful in research or clinical laboratories for epidemiological investigations (321–323). While this panel of MAbs is not widely available or sold commercially in the United States, a comparable collection, known as the Dresden panel, is distributed by a single research laboratory in Dresden, Germany (317). The CDC routinely employs all three methods alongside more recently developed nucleic acid-based amplification techniques for initial isolate screening and molecular typing for epidemiological studies. For instance, our laboratory now uses assays that rely on high-resolution melt (HRM) technology, along with alternative chemistries, to effectively identify clinically relevant Legionella species (CDC, unpublished data). We believe that these newer approaches support a more focused identification scheme than solely targeting conserved regions, such as 16S. One notable limitation to this, and all NAATs, is the inability to detect unknown or novel strains that may be present, and like the UAT, a negative DFA or IFA result does not necessarily exclude LD from the diagnosis and should not preclude attempted isolation by culture (113).
Nucleic Acid-Based Molecular Diagnostics
Nucleic acid-based research for Legionella detection, diagnostics, and typing began in the mid-1980s (324, 325). Prior to the widespread adoption of PCR, scientists experimented with Legionella-specific DNA probes and commercial 125I-labeled Legionella DNA-RNA precipitations (Gen-Probe kit) (326, 327). The first report of PCR as a tool for Legionella detection came in 1989, when researchers from Stanford University combined PCR with Southern blot analysis to detect Legionella DNA spiked in water (227). Progress with PCR-based strategies continued into the 1990s for epidemiological studies with environmental samples. Both retrospective and prospective clinical diagnostic and epidemiological research validated this powerful new method in a variety of matrices, including water from cooling towers, rivers, and hot tubs as well as sputum, BAL fluid, serum, and urine (21, 229, 328–334). Real-time PCR gained popularity in the early 2000s, and although it requires technical expertise and complex, expensive thermal cyclers and software, many commercially marketed rapid environmental Legionella detection assays now employ this technology (Table 2).
TABLE 2.
Commercial nucleic acid amplification tests for Legionella DNA
| Company | Product name or description | Information provided by assay | Technologya | Sample source | Assay run time | Assay target(s) | Limit of detection listed | Availability |
|---|---|---|---|---|---|---|---|---|
| Qiagen | Mericon Quant Legionella species kit | Presence and quantification of Legionella species DNA | Real-time qPCR; probe based | Concentrated water | ∼2 h | Not specified | 10 genomic equivalents/well | U.S. |
| Minerva Biolabs | Aquascreen Legionella pneumophila and Legionella species for real-time PCR | Presence and quantification of Legionella species and L. pneumophila DNA | Real-time qPCR; probe based | Concentrated water | ∼2 h | 16S rRNA genes for Legionella species; mip for L. pneumophila | 10 genomic equivalents/well | International |
| ielab/Life Technologies | Kit for detection and quantification of Legionella | Presence and quantification of Legionella species and L. pneumophila DNA | Real-time qPCR; detection method unknown | Water | Not specified | Unknown | Not specified | Unknown |
| TIB MolBiol/Roche | LightMix kit for Legionella pneumophila and Legionella species | Presence of Legionella species and L. pneumophila DNA | Real-time PCR; Legionella detection probe based, species detection method unknown | Not specified | 1–2 h | 16S rRNA genes for Legionella species; mip for L. pneumophila | 10 genomic equivalents/well | International |
| Diagenode Diagnostics | Legionella species and Legionella pneumophila real-time PCR | Presence of Legionella species or L. pneumophila DNA | Real-time PCR; probe based | Extracted human respiratory specimen | ∼2 h | 16S rRNA genes for Legionella species; mip for L. pneumophila | 180 CFU/ml | International |
| Diateva | Legionella species quantitative kit | Presence and quantification of Legionella species DNA | Real-time qPCR; intercalating dye based | Not specified | ∼2 h | Not specified | 5 cells/well | International |
| bioMérieux/Argene | Chlamylege kit | Presence of C. pneumoniae, M. pneumoniae, and Legionella species DNA | Conventional PCR | Not specified | ∼1 day | 5S-23S intergenic rRNA gene spacer | Not specified | Unknown |
| AES Chemunex/bioMérieux | Adiacontrol Legionella | Unknown | Real-time qPCR; probe based | Water | <5 h | Not specified | 5 genomic equivalents/reaction | Unknown |
| Bio-Rad | iQ-Check Quanti Legionella species and L. pneumophila | Presence and quantification of L. pneumophila and Legionella species DNA | Real-time qPCR; probe based | Concentrated water | ∼4 h | Not specified | Not specified | U.S. |
| Eiken Chemical Co., Ltd. | Loopamp Legionella detection kit E | Presence of Legionella species DNA | Loop-mediated isothermal amplification | Concentrated environmental samples | ∼1.5 h | 16S rRNA genes | 60 CFU/test | U.S. and international |
| Genekam Biotechnology AG | Legionella PCR kits | Presence of Legionella species and L. pneumophila DNA | Conventional and real-time PCR; detection method unknown | Various | Not specified | rRNA genes | 2 CFU/reaction | U.S. and international |
| Pall Corporation | GeneDisk Rapid Microbiology system | Presence and quantification of L. pneumophila and Legionella species DNA | Real-time qPCR GeneDisk system | Water | ∼3 h | Not specified | 5 genomic equivalents/well | U.S. and international |
| Vircell Microbiologists | Speed-oligo Legionella pneumophila | Presence of L. pneumophila DNA | Conventional PCR combined with oligochromatographic test | Clinical samples | ∼2 h | mip | Not specified | Unknown |
| Genycell | Duplicα real-time Legionella pneumophila and Legionella species detection kit | Presence of Legionella species or L. pneumophila DNA | Real-time qPCR; probe based | Not specified | Not specified | 16S rRNA genes for Legionella species; 23S rRNA genes for L. pneumophila | Not specified | International |
| Life Technologies | TrueScience RespiFinder pathogen and viral identification panels | Presence of up to 19 different respiratory pathogens, including L. pneumophila | Multiplex ligation-dependent probe amplification and capillary electrophoresis | Multiple clinical sample types | Not specified | mip | Not specified | International |
| Genesig | PrimerDesign Genesig kit for Legionella pneumophila | Presence and quantification of L. pneumophila DNA | Real-time qPCR; probe based | Not specified | ∼2–3 h | 16S rRNA genes | <100 target copies/reaction | U.S. and international |
qPCR, quantitative PCR.
The benefits of NAATs, including high sensitivity and specificity, rapid turnaround time, and widespread use, have validated this technology as a probable indication for clinical LD diagnosis (230–232). Isothermal amplification, conventional PCR, and real-time PCR (single and multiplex) protocols have been developed for Legionella detection and characterization, the latter enabling target quantification and bacterial enumeration. Since the mainstream introduction of real-time PCR, numerous groups have evaluated the efficacy of nucleic acid detection alongside culture and other established methods. Assuming proper bioinformatics and primer/probe design and stringency, most NAAT-based assays are highly specific (close to 100%), and the growing consensus is that the sensitivity of PCR (both conventional and real time) is equal to or greater than that of culture-based detection using specimens from the lower respiratory tract or environmental water samples (286, 335–344). Notably, the success of both PCR and culture for LD diagnosis is positively correlated with disease severity. However, culture demonstrates a greater decrease in sensitivity over the course of infection (due to antibiotic treatment and disease resolution) than PCR-based methods; thus, nucleic acid detection may be superior for diagnosing milder LD cases or detecting prior exposures (119, 336, 343). Additionally, PCR does not exhibit the apparent culture medium bias where BCYE or its selective variants favor the growth of particular L. pneumophila serogroups or Legionella species (345–348), and thus, NAATs may be more sensitive for the detection of all legionellae (339).
There is a growing list of commercially developed assays for Legionella nucleic acid detection (Table 2); however, only one has FDA clearance for clinical LD detection in the United States. A single test from Becton, Dickinson (BD Probetec ET Legionella pneumophila) that uses strand displacement amplification received FDA clearance in 2004 but is not currently available for sale in the United States (http://www.fda.gov). Commercial and in-house-developed NAATs are used in both clinical and environmental laboratories; however, the list of Legionella genes amplified is limited. The most common targets include a conserved segment of the rRNA genes for the 5S and 16S subunits, the 16S-23S spacer, and/or the macrophage inhibitor protein mip, found primarily in the genus Legionella and highly conserved in all L. pneumophila isolates (226, 332–334, 349–358). Several studies have also examined alternative chromosomal targets, such as dotA, gyrB, dnaJ, wzm, and wzt (340).
There is currently no consensus on the value of one gene or marker over another for Legionella NAAT development, with the exception that mip is typically used for L. pneumophila detection or general species identification. Ultimately, the selection of gene targets is influenced by the specific objectives of the testing laboratory, and thus, NAAT standardization may not be necessary or possible. One example in this respect is the CDC Legionella multiplex real-time PCR assay that was developed in response to the need for a prevalidated Legionella NAAT at U.S. state public health departments and partner laboratories in Thailand, Egypt, Kenya, and South Africa as part of the Global Disease Detection Program. The test was designed to simplify laboratory workflow for the simultaneous detection and typing of culture isolates, specimens, and contaminated environmental samples, with an internal control target (359, 360). This single-tube assay targets the ssrA (for all Legionella species), mip (for L. pneumophila), and wzm (for Lp1) genes.
In addition to detection and diagnosis, NAATs are commonly used for Legionella typing, mainly in conjunction with traditional MAb use or serology. Former and current nucleic acid typing methods include plasmid profiling, restriction fragment length polymorphism (RFLP) detection, pulsed-field gel electrophoresis (PFGE), ribotyping, arbitrarily primed PCR (AP-PCR) (or random amplified polymorphism DNA [RAPD] analysis), repetitive element PCR (rep-PCR), RFLP plus PCR (infrequent restriction site PCR [IRS-PCR]/amplified fragment length polymorphism [AFLP] analysis), and phylogenetic comparison of various Legionella species- and strain-specific genes, including ftsZ and sidA, among others (192, 228, 325, 361–370). Legionella species identification has relied largely on 16S rRNA gene or mip sequencing, while the common L. pneumophila sg1 Paris subtype can be further characterized by strain-specific, short, regularly spaced, palindromic sequences (spoligotyping) (333, 371, 372).
The current gold-standard L. pneumophila genotyping assay for epidemiological investigations is sequence-based typing (SBT), developed as a variant of multilocus sequence typing (234, 235, 373). SBT-based strain discrimination relies on the sequences of an ordered seven-gene collection (flaA, pilE, asd, mip, mompS, proA, and neuA), with the option of including a neuA homologue (neuAh) when the standard SBT primers fail to amplify the target in non-sg1 strains. SBT has the advantage of direct sequence comparison, which eliminates the interpretational subjectivity of non-sequence-based methods such as PFGE, which are prone to banding ambiguities over time or between laboratories (374). In support of SBT, the European Society for Clinical Microbiology Study Group on Legionella Infections (ESGLI) (formerly the European Working Group on Legionella Infections [EWGLI]) maintains an allele database (currently version 3.0) that allows querying of large sets of raw sequence data, delivering both an allelic profile and a final combined sequence type (ST) for each isolate (Table 3). The database is dynamic and continually updated with the addition of new allele sequences and STs.
SBT is traditionally performed on DNA extracted from culture isolates; however, several studies have demonstrated some success when standard or nested SBT was performed directly on nucleic acids extracted from patient tissues or fluids (375–378). This adds a much-needed tool to the Legionella typing repertoire. However, caution is stressed because the efficiency of SBT on culture-independent preparations varies widely with sample origin (e.g., sputum versus BAL fluid) and quality and also is typically much lower than that of pure isolate extractions. Laboratory expertise and the use of high-quality media can maximize bacterial growth from otherwise low-quality samples, yet if an isolate is not obtained because of prior antibiotic therapy or suboptimal shipping and storage, culture-independent SBT offers a potentially viable alternative.
Legionella nucleic acid-based detection offers significant advantages over serology and culture in terms of sensitivity and speed. However, there are several notable disadvantages and limitations. PCR may not be ideal for testing non-lower respiratory tract samples (e.g., urine and serum); at best, PCR sensitivity in these specimens only approaches that of the L. pneumophila UAT (286, 336, 343, 379–383). One inherent complication with all nucleic acid amplification methods is the difficulty in assessing bacterial viability. These methods do not discriminate between free nucleic acids, either in solution or amoeba associated; nucleic acids from dead or dying bacteria; and/or viable but nonculturable (VBNC) legionellae. This is evidenced in environmental studies that detected a higher level of bacterial DNA than was corroborated by culture and where the persistence of Legionella DNA after multiple rounds of remediation resulted in no detectable culture growth (183, 338, 341, 342, 384). As a consequence, PCR has a low positive predictive value (PPV) for legionellae compared to that of culture methods in environmental settings; conversely, PCR has a high negative predictive value for viable Legionella in the same samples (≥97%) (341, 343). Interestingly, PCR in clinical applications may have a higher PPV (than for environmental samples) despite the lower sensitivity with nonrespiratory specimens (379). Two potential remedies for the low PPV with samples from environmental sources include reverse transcription-PCR, amplifying labile RNA targets present in metabolically active bacteria, and the use of a cell-impermeant chemical, such as ethidium monoazide (EMA) or propidium monoazide (PMA), to inhibit PCR amplification from nonviable cells or extracellular nucleic acids (385–390). Notably, neither alternative protocol alone will discriminate VBNC legionellae; however, this cell population still poses a potential human health risk (391–394).
The expansion and development of nucleic acid amplification methods offer important real and potential benefits to the field of Legionella detection and diagnosis. In comparison, serological or antibody-dependent assays create cross-reactivity and stability issues and require expensive investments in manpower, animal care, and time. Nucleic acid amplification technologies still necessitate specially trained personnel and sophisticated machines but are increasingly accessible to a wider array of laboratories on a moderate budget. The commercial availability of environmental Legionella nucleic acid detection kits (Table 2) and the abundance of research and methodology (244) mean that laboratories need not design, optimize, and implement a complex, “home-grown” strategy for testing. Additionally, there are some problems that nucleic acid molecular methods are more apt to solve; PCR is the only approach currently suitable for diagnosis of LD due to non-sg1 and non-pneumophila Legionella species in a time frame that could positively influence patient management (51).
Collectively, nucleic acid-based methods are valuable additions to LD diagnostic and detection schemes; however, the limitations inherent to NAATs (395), as discussed above, support the concurrent use of multiple testing modalities to increase the probability of successful detection. Combined with traditional confirmatory techniques, NAATs can augment diagnostic sensitivity for LD in clinical and epidemiological settings (119, 344), especially for less severe disease with lower bacterial loads, and can help define the full extent of disease burden (119).
Emerging Methods and Technologies
Advancements in Legionella in vitro diagnostics are often derived from the application of novel approaches to existing assays or through de novo development of innovative technologies. Representing both methodologies are several established (mass spectrometry and real-time PCR-based TaqMan array cards) and emerging (immunomagnetic separation [IMS], isothermal nucleic acid amplification, high-resolution melt analysis, and whole-genome sequencing [WGS]) techniques that may enhance Legionella detection and characterization in various clinical settings. This will ostensibly improve outcomes during outbreak responses and epidemiological investigations.
Isothermal nucleic acid amplification is a general classification for DNA or RNA amplification at a constant temperature with minimal or no cycling, as is required for PCR (for reviews, see references 396 and 397). The major advantage of most isothermal techniques is rapid target detection (within 15 to 60 min) without the need for expensive, complex, and energy-demanding thermal cyclers. Among the various methodologies, nucleic acid sequence-based amplification (NASBA) and loop-mediated isothermal amplification (LAMP) have been used to detect Legionella DNA in clinical and environmental samples (130, 398–402). Numerous studies have employed isothermal amplification for detection and identification of viral (403–405), bacterial (406–408), and parasitic (409–411) pathogens, and various commercial kits and components are available. In particular, one company (Eiken Chemical Co., Japan) offers a Legionella-specific LAMP kit that is gaining popularity in environmental research and monitoring (130, 401, 402).
Microfluidic TaqMan low-density microarray cards (TAC), developed in the mid-2000s by Life Technologies, can quickly interrogate gene expression in various disease states (412–416). Researchers at the CDC adapted TACs for the simultaneous detection of >20 respiratory pathogens, including Legionella (417, 418); this custom array card is now the principal tool for identifying unknown respiratory disease outbreaks and is being piloted for population-based surveillance programs at several U.S. and international sites to define respiratory disease etiology and burden (419). TACs offer increased real-time PCR throughput (384 individual reactions) in a rapid, reproducible, and simple setup containing prespotted primer-and-probe combinations. This format has since been customized for larger field evaluations and for the detection of nonrespiratory syndromes (420–424).
Mass spectrometry (MS) is a mature yet still evolving technology adapted to the rapid identification and classification of clinically relevant pathogens (for a review, see reference 425). MS for Legionella identification was first performed in the late 1970s in combination with gas chromatography (426). The development of matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) MS has proven reliable for Legionella species identification and typing, although intraspecies serogroup discrimination is not yet possible (425, 427–430). Given a core facility with established expertise and equipment, MALDI-TOF MS is a fast (∼10-min), inexpensive method for isolate identification that has recently received FDA clearance. Reference spectral databases are critical for MS chromatogram comparisons, and several have been created or improved to aid in Legionella typing (e.g., modified Bruker and Biotyper databases) (427, 429, 431).
IMS combines specific, whole-cell antibody recognition with magnetic bead-based purification for bacterial concentration. Published IMS research demonstrates sensitivity for legionellae in environmental and clinical samples, with or without filter concentration or DFA, at or above standard culture or fluorescence detection levels (351, 432–438). Further IMS development may be useful for rapid field, laboratory, or POC detection, although the specificity limitations of antibody-based isolation must be addressed (see “Serological and Antibody-Based Assays,” above, for a discussion of limitations).
HRM curve analysis was originally proposed (439) and then developed to detect single-nucleotide polymorphisms (SNPs) within PCR amplicons (440–443). This technique may employ double-stranded intercalating dyes (e.g., SYBR-GreenER, EvaGreen, or SYTO9) or fluorescently labeled primers (Lux chemistry) in both initial real-time PCR and subsequent HRM analyses; alternatively, fluorescence resonance energy transfer (FRET)-based probes can be added after PCR for the secondary melt analysis. One variant of this technique employs a solid-state surface plasmon resonance sensor combined with gold-labeled probes (444). HRM analysis has been used in numerous studies since the mid-2000s on a wide range of human pathogens, including those within the genera Campylobacter, Brucella, Leishmania, Bordetella, Clostridium, Mycobacterium, Mycoplasma, Chlamydophila, Cryptosporidium, and Staphylococcus, among others (445–452). Recent studies have shown HRM analysis to be a powerful technique for characterizing antibiotic resistance and typing respiratory disease agents, including M. pneumoniae, C. pneumoniae, and Chlamydophila psittaci (453–459). To our knowledge, HRM analysis for intraspecies or interspecies Legionella discrimination has been included in only a few studies (460–463); however, this approach seems promising for future Legionella diagnostics or typing.
WGS is an increasingly popular and accessible technique with broad diagnostic potential for public health laboratories. The development of “next-generation” DNA sequencing platforms and reagents from a variety of manufacturers has dramatically decreased the time and cost of WGS over the past 10 years. A recent and growing pool of research demonstrates that these technologies are well suited for sequencing applications toward microbial identification and typing (464–470); examination of phylogenic relationships among pathogens for population-based, longitudinal, or retrospective epidemiological studies (471–488); identification of molecular bases for antibiotic resistance or virulence (489–501); discovery of DNA targets for diagnostic development (502, 503); and, less often, prospective surveillance or outbreak investigations (504–509). One of the largest hurdles to the adoption of WGS for a rapid response during public health emergencies is the development of an efficient bioinformatics pipeline for data analysis and interpretation, including comprehensive microbial reference libraries. Recently, WGS was employed in a retrospective United Kingdom pilot study and in real time during a Legionella outbreak investigation at an Australian hospital (510, 511). These initial studies confirm that WGS can provide high-quality typing and epidemiological data, although continued improvement in data analysis will undoubtedly be necessary to realize the maximum benefit of this approach. As part of the recently launched CDC Advanced Molecular Detection initiative (http://www.cdc.gov/amd), the CDC Legionella Laboratory Team is currently integrating next-generation sequencing technologies with enhanced bioinformatics capabilities for legionellosis detection and outbreak responses.
CDC OUTBREAK INVESTIGATIONS: THE SYNERGY OF EPIDEMIOLOGY AND LABORATORY SCIENCE
General Field and Laboratory Procedures for Outbreak Investigations
Between 1976 and 2013, CDC laboratories have assisted with the isolation, identification, and/or typing of legionellae in ∼170 domestic and international outbreaks and thousands of sporadic cases (CDC, unpublished). In that same time frame, CDC personnel have participated in at least 98 Legionella-focused coordinated epidemic assistance investigations (Epi-Aids) (512, 513; CDC, unpublished). Our understanding of Legionella ecology, epidemiology, and disease is informed, in large part, by these investigations. Since 1976, Epi-Aid field work and subsequent research studies have been instrumental in defining the clinical description of LD and risk factors for contracting the disease as well as detailing the environmental growth, persistence, and epidemiological transmission of legionellae to susceptible populations (512). Concurrently, the CDC Legionella laboratory team and others have helped characterize bacterial physiology while developing diagnostics and procedures for disease and environmental detection. In order to detail and share how laboratory diagnostic and field detection methods are integrated with epidemiological investigations during high-profile Legionella outbreaks, the following paragraphs outline the general field and laboratory workflow. This includes sample collection and analysis as well as culturing and typing of legionellae to identify culprit strains and sources of environmental contamination. Procedures and protocols for assessment, collection, and testing can be found in Table 3, on the CDC Legionella website, as well as in a recent publication (514) and accompanying reports in the same volume.
The basic activities common to all outbreak-associated epidemiological field investigations assisted by the CDC Legionella laboratory team include (i) environmental assessment and sample collection, (ii) culturing and testing of both clinical specimens and environmental samples, and (iii) phylogenetic and/or strain characterization of clinical and environmental Legionella isolates. During outbreak investigations, shared geography among disease cases typically defines the study area, while field personnel, led by epidemiologists and often including laboratorians, complete an extensive “on-the-ground” environmental assessment to identify potential sampling sites. The environmental assessment provides relevant information, such as the presence of water and air-handling systems that are capable of aerosol generation, but the specific number and type of samples collected are dependent on the size and complexity of the facility as well as the locations of reported LD cases. Investigations of localized LD clusters where patients share recent common exposures may require only 40 to 50 environmental samples from 30 to 40 sites, while outbreaks over larger, less defined areas in which potential exposure sources may not be obvious can necessitate >300 samples from 100 or more locations. As a standard practice, when possible, the CDC collects both 1-liter bulk water and biofilm swabs in sterile plastic containers from interior locations distal to incoming water, such as shower heads, faucets, hot water heaters, misters, decorative fountains, and spas, and from more central or proximal sites, such as incoming municipal or well water mains and hot recirculation supply lines. Air-conditioning cooling towers, which are historically associated with Legionella contamination (81, 245, 515–518) and LD outbreaks in the United States (187, 519–521) and internationally (186, 190, 521, 522), can support heavy bacterial growth, and all locations within the study zone are routinely sampled. At the time of collection, water is assessed for temperature, pH, and residual disinfectant (e.g., chlorine or bromine) before chemical neutralization with sodium thiosulfate. Samples are immediately packed and shipped in insulated containers to ensure minimal temperature fluctuation en route to the CDC Legionella Laboratory in Atlanta or an approved local testing facility.
In the laboratory, potable water is concentrated with 0.2-μm-pore-size polycarbonate filters, and nonpotable samples (e.g., from cooling towers or fountains) are both acid treated and directly plated onto BCYE solid medium with selective agents (e.g., antibiotics and/or glycine) (243, 514). Plates are incubated for up to 14 days, and primary colony isolates are replated and confirmed on cysteine biplates. If necessary, serogrouping is performed by MAb dot blotting, slide agglutination, and DFA analysis in some instances (238). L. pneumophila sg1 subgroups (e.g., Philadelphia and Benidorm, etc.) are assigned based on a previously developed MAb panel (321, 322). A representative subset of L. pneumophila isolates are typed by using the standardized ESGLI allelic sequence profiling method (234, 235, 321, 322). Non-pneumophila Legionella isolates and those of ambiguous type are identified by an in-house-developed real-time PCR assay followed by mip gene sequencing, if required (359, 371). Clinical specimens are interrogated by the same identification and typing procedures as those described above; an epidemiological linkage is established when SBT and MAb2 profiles match between patient and environmental isolates.
High-Profile Legionnaires' Disease Outbreaks
U.S. federal, state, and local authorities as well as international organizations and foreign health ministries may formally request assistance from the CDC when LD is detected. A comprehensive Epi-Aid involves on-site support to health agencies in determining the scope of infection and the potential source(s) of disease transmission. These resource-intensive investigations are typically in response to an urgent public health threat and can garner local or national attention (e.g., a recent outbreak in Pittsburgh, PA, led to congressional hearings) (85, 92, 523). The CDC Legionella laboratory team is an essential part of the outbreak response and supports Epidemic Intelligence Service (EIS) officers and epidemiologists both in field investigations and with laboratory expertise. Several well-documented Epi-Aid investigations carried out by the CDC in the 1990s and 2000s are discussed below to illustrate the interdisciplinary nature of these events and to highlight the complexities and nuances in each type of outbreak investigation and, where applicable, emphasize important laboratory advances, such as new diagnostic or detection methods, that aided in the identification of a transmission source.
Health care-associated outbreak in a long-term-care facility.
Older adults and persons with underlying medical conditions have an increased risk of Legionella infection; thus, hospital and nursing home settings are often implicated in outbreaks. In September 2004, the CDC responded to an LD outbreak in a long-term-care facility (LTCF) in Cherokee County, NC (193). In total, four residents of the LTCF and three local community cases were confirmed to have LD by the Legionella UAT; three of the seven cases were fatal. A comprehensive field investigation was undertaken at the LTCF, which centered on potential transmission from potable water; however, Legionella was not found in any sample collected from the facility. Focusing outside the LTCF, investigators conducted >250 interviews in the surrounding community in search of unidentified LD cases; three additional “confirmed” and two “possible” cases were detected. With the size and scope of the sampling area expanded, a local industrial cooling tower 0.4 km away from the LTCF was found to harbor multiple Legionella species and Lp1 monoclonal subgroups. In addition, Legionella DNA was detected (by PCR for the mip gene) on special filters fitted to rooftop air-handling units of the LTCF and was subsequently matched to the DNA of a cooling tower isolate. The proximity of the cooling tower to both the LTCF and the additional community cases was sufficient for a presumptive remediation of the site; unfortunately, patient respiratory specimens did not yield bacterial growth, which hindered the positive identification of a disease source. Notably, what was initially suspected to be institutional transmission (because two patients never left the LTCF) proved instead to be community based, requiring multiple rounds of interviews, sampling, and testing. The use of PCR for pathogen detection and typing provided laboratory flexibility in defining the likely transmission source and conduit (air-conditioning fresh-air intake). As highlighted in the published case description, illnesses at the LTCF represented sentinel events in a wider community exposure (193) and additionally underscored the complexities associated with Legionella outbreak investigations.
Health care-associated outbreak in a hospital.
Immunocompromised individuals, especially transplant recipients on immunosuppressive regimens, are at an increased risk of developing LD due to Lp1 or other species and serogroups (524–528). In addition, case-fatality rates for LD are higher among older males and the immunosuppressed (121). In the first half of 1996, a cluster of health care-associated LD cases was identified at a Southwestern U.S. regional transplant center (529, 530). An initial in-house investigation and attempted remediation were unsuccessful in identifying and controlling the problem, and by summer, the CDC was requested to assist state and local officials. LD surveillance was intensified, and a retrospective study for previously unrecognized cases was initiated. A total of 25 culture-confirmed or potential health care-associated cases were found: 17 were identified with disease onset between 1987 and 1995, and 8 were discovered since then (from 1996 to the present). The calculated LD attack rate for all transplant patients at the hospital was 6%, and 12 patients ultimately died, for a case-fatality rate of 48%.
Urine samples were available for many patients; however, a commercially available UAT failed to detect Legionella urine antigens. Fortunately, a hospital policy promoting bacterial culture and typing combined with increased surveillance revealed infections by L. pneumophila serogroups 1, 4, 5, and 10 as well as by sg6, which represented more than half of the disease burden. An extensive field investigation of hospital potable water from case patient room showers and faucets as well as water softeners, hot water tanks, and private supply wells, among other locations, was conducted. Environmental samples processed at the CDC reflected the bacterial composition and diversity in prior clinical isolates; the CDC Legionella laboratory found the above-mentioned serogroups, with the addition of serogroup 11, L. anisa, and an undetermined species. L. pneumophila sg6 was detected in both the shower vapor of a case patient's room (by a specialized Andersen air-sampling device) and a carpet cleaner reservoir tank used in the bone marrow transplant unit. Hospital water supply systems can be extensive and complex, providing optimal conditions for Legionella growth and distribution. The difficulties and risks associated with water distribution for transplant medicine places added importance to disease monitoring and a rapid response in specialized hospitals or units. This particular outbreak highlights how field and laboratory techniques were critical in defining the sources of infection and linking them to clinical disease; a thorough epidemiological investigation helped identify common exposures, and by using PFGE, CDC laboratorians confirmed that the L. pneumophila sg6 strain found in the shower vapor and carpet cleaner was also responsible for most of the health care-associated LD cases.
Commercially linked outbreak from an unlikely source.
Both potable and nonpotable water can support the amplification and transmission of legionellae to susceptible populations. During outbreak events, investigators face the daunting task of locating all aerosol-producing devices when relatively few common exposures are identified and disease is distributed over a seemingly wide area. Decorative water features are a frequent addition to public and private spaces and may serve as potential disease reservoirs, but they can be overlooked in water safety plans due to their unassuming size or lack of visible aerosol generation. In the fall of 2004, clinicians at Rapid City Hospital in South Dakota instituted increased diagnostic testing for seasonal CAP, which included a Legionella UAT, in order to more efficiently target antibiotic therapy. Eighteen cases of legionellosis were recognized in the summer and fall of 2005 due to this improved surveillance. The UAT was the primary method of diagnosis, while bacterial cultures were grown from respiratory specimens of 4 patients, and a 4-fold increase in serum titers was documented for one individual. Fourteen individuals were hospitalized, and one patient ultimately died. Beginning in June of the same year, the CDC began an extensive Epi-Aid response to this cluster of LD cases in Rapid City (85). After interviewing case patients and controls, ∼300 targeted 1-liter bulk water samples and biofilm swabs were collected from 123 sites around the city and immediately shipped to the CDC in Atlanta and a commercial Legionella laboratory. The list of sites tested was extensive and citywide, including cooling towers, chillers, condensers, supermarket misters, showers, sinks, hot water tanks, municipal water systems and treatment plants, hot tubs, and multiple fountains. Legionella contamination was widespread, being identified in 35% of the locations tested.
Legionella pneumophila sg1 was the most common environmental isolate recovered from the study region (from 43 of 123 sources); however, a number of other serogroups and non-pneumophila Legionella species were also cultured, including L. pneumophila sg8, L. anisa, L. bozemanii [sic], L. feeleii, L. rubrilucens, and L. spiritensis (CDC, unpublished). It is not uncommon to find multiple potential transmission sources during outbreak investigations. Importantly, a promising epidemiological link among half of all case patients was recognized, and MAb subtyping and SBT analysis confirmed that the only environmental match to previous clinical isolates came from a small, unassuming, plastic decorative fountain in a local restaurant lobby; the outbreak promptly ended when the fountain was removed. Clusters of LD cases have been linked to decorative fountains both before and after this reported outbreak (84, 172–176); however, the current case was the first report of a small, low-aerosol-generating, decorative fountain as the source. Notably, the success of this investigation was dependent on epidemiological clues and clinical isolates available from four case patients, which enabled laboratory typing methods (MAb subtyping and SBT) to identify the exact transmission source among the large number of potential sites.
Cruise ship-associated outbreak.
From 2005 to 2009, cruise-related LD cases accounted for ∼5% of travel-associated legionelloses reported to the U.S. SLDSS (15) and 7.6% of cases on average in Europe during the same time frame (207–209, 211, 212). As previously reported (531), Legionella has become a problem for cruise ships in a similar fashion as for hotels; both must manage complex air-handling networks as well as potable and recreational water distribution systems with the potential for Legionella growth and transmission. Defective or improperly maintained onboard water systems may present an increased LD risk for the older average cruising demographic, which is between 55 and 61 years of age (532–534).
Identification of the largest cruise ship-associated LD outbreak to date (535, 536) began in mid-July 1994, when a New Jersey physician reported a cluster of legionellosis cases among three individuals returning from the same Caribbean cruise (537). Upon learning of three additional cases, CDC and New York State health officials distributed an epidemiological questionnaire to offloading passengers from the same vessel and provided a preliminary health warning to boarding travelers. After consulting with the ship's staff and cruise line representatives onboard, the CDC team began an environmental investigation by collecting 1-liter bulk water samples and biofilm swabs from sinks, showers, fountains, water heaters, storage tanks, and the whirlpool spa, among other sites. In addition, a number of tourist destinations were sampled at the ship's international port of call; all samples were shipped back to the CDC Legionella laboratory in Atlanta. A then-recently developed, commercially available Legionella PCR dot blot assay (EnviroAmp; Perkin-Elmer Cetus) detected Legionella species DNA at the majority of sites on the cruise ship (CDC, unpublished), with L. pneumophila DNA in approximately half of the samples.
Among all sampled sites, the whirlpool spa and filtration system gave the strongest DNA hybridization signals, and a case-control study demonstrated a strong epidemiological disease association with the shipboard spa. Physical and chemical examination of the spa revealed large amounts of organic material in the sand filtration unit and no detectable bromine, which was required to prevent microbial growth. Importantly, Legionella culture growth was recovered from only this single location. State and international epidemiologists were immediately notified about the outbreak, and questionnaires were mailed to ∼3,000 recent passengers in an effort to identify additional cases of disease. In total, 50 cases of LD, with a median age of 63 years, were identified from 9 separate cruises on the same ship during the spring and summer of 1994; one individual died. Sixteen cases were confirmed by UAT, serology, or both, and one L. pneumophila sg1 isolate was grown from patient sputum. The MAb subtype and AP-PCR patterns were an exact match between the single clinical isolate and the strain grown from the shipboard whirlpool spa.
This cruise-borne LD outbreak investigation is notable for the following two unique developments. (i) Only one location demonstrated bacterial growth despite positive Legionella DNA test results for the majority of environmental samples, including potable water. As detailed in “Nucleic Acid-Based Molecular Diagnostics,” above, PCR is a powerful technique, but in most applications, it is not designed to discriminate live versus dead or VBNC bacteria. The exclusive use of this PCR-based method would have provided the false impression that most shipboard water was currently colonized, when, in fact, the whirlpool spa was the only detectable, and most likely, source of transmission at the time of sample collection, as confirmed by culture. (ii) It is a challenge to recognize cruise-related outbreaks due to the potentially long LD incubation period and the dispersal of passengers after a ship docks (531, 535, 538). Fortunately, during this event, a concerned physician prepared a careful medical and travel history for the first three identified LD cases (537), which allowed the recognition of wider disease transmission. The rapid reporting and response from local to state and federal levels mobilized resources to define the LD distribution and prevent further cases.
Legionella contamination onboard passenger and cargo vessels may be an unappreciated hazard, as detailed here and as documented in sporadic cases and outbreaks on smaller scales (531, 539–547). A recent survey of Norwegian naval vessels suggests that Legionella contamination is not isolated to passenger ships: researchers found Legionella species DNA in the onboard potable water of approximately one-half the naval vessels sampled, and they were able to culture L. pneumophila from a smaller subset of these ships (548). As described above, recreational baths and whirlpool spas can provide optimal conditions for bacterial growth and transmission and are among the nonpotable water sources most frequently associated with ship- and land-based outbreaks (18, 194–198, 531, 541, 549–556). To mitigate this waterborne hazard, the CDC offers public guidance for the safe operation and disinfection of recreational hot tubs, and the WHO provides literature for Legionella risk assessment in recreational environments (Table 3).
Summary of Field and Laboratory Operations for Outbreak Investigations
Each LD outbreak presents a separate and distinct set of challenges for laboratory and field personnel. Some general parameters and guidelines are common to all outbreak responses (e.g., environmental assessment and sample collection), but no two situations require identical resources or activities. Even sampling in similar types of environments or scenarios requires an appreciation of individual water and air management systems, usage patterns, and, in some cases, weather and prevailing winds. The complex and sometimes unmapped water distribution systems found in both old- and new-building construction during a sustained outbreak at a regional transplant center necessitated sampling at hundreds of potential sites; unlike a typical transmission point source, the outbreak strain was systemic and was found in multiple areas. Investigations also quickly change with new information; the focus of sampling efforts can be influenced by an individual's recall during interviews, and outcome success is variable depending on the availability of clinical specimens. Several case patients in the South Dakota investigation remembered their potential restaurant exposure only after a reinterview or hearing about the outbreak in the media, and the implicated decorative fountain was not originally sampled because it was turned off during the environmental investigation and therefore was considered an unlikely source (85). The lack of a clinical isolate for epidemiological comparison in the North Carolina LTCF meant that transmission via the nearby cooling tower was presumptive as opposed to confirmed.
The LD outbreak examples mentioned above together illustrate several key points: (i) rapid, accurate transmission source identification during an outbreak response requires seamless cooperation between state, federal, and local health authorities and continuous communication among epidemiologists and laboratorians; (ii) molecular methods for Legionella detection and typing, such as PFGE, AP-PCR, real-time PCR, and SBT, increase diagnostic sensitivity and enhance strain discriminatory power over traditional techniques (e.g., SAT), but these results must be interpreted with care; (iii) a complete and thorough epidemiological investigation that identifies common points of exposure to LD cases is critical for narrowing down and defining potential transmission sources; and (iv) a patient culture isolate, or sufficient quantity of high-quality DNA from a specimen when no isolate is obtained, is necessary for linking clinical disease with an exposure source to enable focused remediation and to initiate public health efforts to prevent further infections.
DISCUSSION AND FUTURE OF LEGIONELLA DIAGNOSTICS AND DETECTION
Education, Awareness, and Reporting
From 2000 to 2011, the LD incidence rate more than tripled in the United States, increasing in every U.S. geographical region, sometimes dramatically (e.g., a >7-fold increase in the New England region) (15, 126, 557). A similar trend was documented in Europe, with a 158% increase over 9 years starting in 2004 (146, 148). This increase is likely attributable, in part, to enhanced detection (due to the popularity of the rapid UAT) and more complete surveillance and reporting. In the United States, the increasing median age, and concomitant decreasing health, of a growing population may also promote this trend (534, 558). However, the actual disease burden is likely underestimated, given that LD cases presenting as nondescript CAP (especially mild cases) may be treated empirically using broad-spectrum antibiotics that are active against Legionella, leaving the disease, and its transmission source(s), unrecognized and unreported. Consequently, LD-inclusive empirical treatment has contributed to the trend of declining mortality while masking both the true extent of disease and potential outbreak-related disease clusters (15, 535). When CAP is diagnosed and LD is suspected, laboratory confirmation is central to defining disease etiology, detecting unidentified LD outbreaks, and contributing to the larger goal of defining the scope of legionellosis in the United States. Education and awareness of LD by health care professionals, not as an exotic disease but as a potentially deadly contributor to community- and health care-associated pneumonia, are critical to decreasing mortality among at-risk populations. This is a key first step to understanding and addressing legionellosis in the coming decades.
An additional, unintended consequence of undetected outbreaks is the missed opportunity for further description of disease risk and clinical presentation. Most LD risk factors were originally compiled during outbreak investigations, and ongoing research is needed to fully document, refine, and continually update this description. For example, a wide spectrum of clinical features was recently reported for full-term infants exposed to Legionella-containing aerosols from a cold-mist humidifier (559). Surveillance has also demonstrated sustained, high levels of travel-associated LD cases, which creates challenges for health care systems in all countries and states, regardless of the regional LD prevalence (15, 202, 210). Federal and international surveillance networks, such as the National Notifiable Diseases Surveillance System (NNDSS) and the Waterborne Disease and Outbreak Surveillance System (WBDOSS) in the United States, are vital for recognizing these patterns and clusters of disease. However, monitoring networks are valuable only when health practitioners are familiar with the symptoms and risk factors for LD, leading to laboratory confirmation, rapid treatment, and timely reporting by state agencies.
Controlling Disease Transmission
Legionellae are ubiquitous in natural ecosystems (both aquatic and terrestrial), and advances in building technologies, particularly since the 1950s (e.g., air-conditioning cooling towers, recreational water features, and complex water distribution systems), have provided ideal conditions for bacterial growth and persistence (6). High rates of morbidity and mortality may result when large human populations are brought into close proximity to respiratory pathogens, especially at locations that concentrate individuals in at-risk groups (e.g., transplant hospitals). Therefore, a second key to addressing legionellosis in the coming decades is minimizing or eliminating disease transmission through risk assessment, regular maintenance of potable and nonpotable water systems, and water monitoring and treatment in facilities that care for susceptible populations. Several informational resources are available to building managers, industrial hygienists, and administrators (as well as to clinicians, laboratorians, and the general public) to inform and guide their decisions in the design of a comprehensive plan to inhibit Legionella colonization or when contemplating action after a positive test result or disease is discovered. Links to the following resources are provided in Table 3. The American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) is an international organization that provides standards, guidelines, and best practices to the building technology field. Numerous ASHRAE publications address the issues of legionellosis prevention and response in nonresidential locales, such as guideline 12-2000 and Standard 188P, which deal with minimizing and preventing LD associated with building water systems. The Environmental Protection Agency (EPA) published the Legionella: Human Health Criteria document, detailing bacterial ecology and distribution, with sections on risk assessment, analysis, and treatment. The WHO commits several chapters in its guide Legionella and the Prevention of Legionellosis to water safety plans (WSPs) as well as in-building distribution and exterior cooling system assessment; additional WHO documents discuss risk assessment for drinking water and water safety plans in buildings. The ECDC provides two Legionella-related Web resources, the Legionellosis Health Topic Web page and the European Legionnaires' Disease Surveillance Network (ELDSNet) website, which together include disease facts, recent surveillance reports and publications, Legionella-focused events, case definitions, operating procedures, and an outbreak investigation toolbox. Two United Kingdom agencies, the Health and Safety Executive and Public Health England (currently merging with the Health Protection Agency), also provide useful informational resources. The CDC Morbidity and Mortality Weekly Report (MMWR) publishes two related guidelines, the first document, Guidelines for Preventing Healthcare-Associated Pneumonia, addresses specific recommendations on the topic of Legionella, and the second document, coordinated by the CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC), is entitled Guidelines for Environmental Infection Control in Health-Care Facilities (560). In addition, the above-mentioned CDC Legionella Web page provides tools and protocols for environmental assessment, sample collection, and Legionella testing and a list of ELITE-certified commercial laboratories. Finally, there are many additional documents and resources from organizations and government agencies not included here that may serve as important guidance and instruction for interested parties. Table 3 is not meant to be an exhaustive list but rather representative of the types and scope of resources available.
The issue of ongoing microbial water monitoring is controversial and thus warrants special attention. The CDC is not currently positioned to unilaterally mandate regular assessment for Legionella in potable and nonpotable water systems. However, it is clear that institutions frequented by or housing susceptible individuals should be aware of and mitigate the LD risk for their occupants and visitors, as detailed in previous CDC guidelines (Table 3) (111, 560). Large numbers of individuals in these high-risk categories are concentrated in organ transplant units, intensive care units (ICUs), cancer centers, infant nurseries, and sites that commonly care for chronically ill or immunocompromised patients, among others. These facilities should be vigilant in clinical surveillance for Legionella and maintain a high index of suspicion for disease even when results of environmental testing are negative (561). Outside this institutional population, general hospitals and facilities for seniors and the elderly (e.g., LTCFs) should, at the least, undertake an infection control risk assessment and conduct ongoing LD surveillance, while cruise ship medical providers are recommended to employ rapid UATs in standard practice. These locales should also understand their water distribution systems and monitor temperature and levels of disinfectant (and/or water quality parameters) at distal point-of-use sites. The importance of actively monitoring LD risk in non-health-care settings is less clear; this is not to say that other establishments shoulder little risk or bear no responsibility for the health of their patrons. As demonstrated by the original 1976 American Legion epidemic at a conference center hotel and the hundreds of LD clusters identified since then, complex water and air-handling systems require proper maintenance and disinfection regardless of their location or the typical clientele. An additional, distinct category is reserved for institutions that have experienced one or more LD outbreaks, especially in the past 5 years. Data from recently conducted CDC Epi-Aids suggest that disease transmission from a localized, potable water point source is typically symptomatic of wider, systemic plumbing network colonization (CDC, unpublished). In these instances, regular, short-term Legionella testing is advised, to monitor the success of remediation; yearly, long-term testing may also be warranted, to ensure that colonization does not recur.
Legionella Persistence and Remediation
Of particular interest to facility managers in areas with a high environmental burden of Legionella, complex water distribution, or at-risk populations is the issue of Legionella persistence. The problem of long-term colonization in anthropogenic water systems has arisen many times in environmental assessments, retrospective studies (183, 562–565), and outbreak investigations (75, 92, 530, 566–571). As illustrated in one recent outbreak report (75), ∼35 cases of LD in condominium residents were discovered over 9 years due to the mistaken belief that low-level potable water contamination did not pose a significant disease threat; seemingly arbitrary “action levels” for remediation laid out by facility managers and defined by bacterial concentration thresholds resulted in recurrent and prolonged transmission and disease. As data from the CDC ELITE program suggest, quantifying risk through such a strategy is problematic, because while most participating laboratories accurately determined the presence of bacteria (93% of samples correctly characterized), the precision in Legionella quantitation was very low (interlaboratory bacterial counts differed by up to 3 logs), with average 1.25-log underestimates of viable numbers (258). The difficulty and extreme variability in Legionella enumeration between different laboratories, sampling strategies, and culture methods, and even from the same source on different days, are reflected in previous reports as well (345, 561, 572, 573). Additionally, the numerical relationship between the colonization level and disease is at best complex and at worst misleading; for example, a recent metastudy evaluated an often cited metric for assessing LD risk that is based on an increased prevalence (≥30%) of hospital sampling sites being positive for Legionella (574, 575). Researchers identified 31 peer-reviewed journal articles representing 119 hospitals where reports of LD were temporally associated with environmental testing. The results indicate that the ≥30% positivity cutoff is neither sensitive (59%) nor specific (74%) for use in LD risk management within health care settings. While continued research is needed to confirm previous findings, at least two important points are clear: (i) there is currently no known safe concentration of Legionella in man-made potable and nonpotable water networks, which is due in part to unreliable bacterial enumeration in complex samples, potential day-to-day variability for Legionella detection at any individual source (258), and disease dependence on multiple individual-, environment-, and bacterium-specific factors (75), and (ii) risk assessment and environmental management must be a multilevel approach, based on proven science and best practices, and should account for complexities in system architecture, potential routes of exposure, and the susceptibility of present populations, among others. Legionella can persist for long periods under permissive control and monitoring policies. Quantification of risk based on arbitrary levels of detectable colonization (75, 574, 576) is currently a misinformed and ambiguous calculus at best; hence, the recommendation of unproven or incomplete approaches in response to positive environmental samples (577) is imprudent and ill-advised. Given these concerns, complete eradication of Legionella from man-made water and air-handling systems should be the stated goal, especially when cases of LD have been documented previously.
Future Approaches for the Advancement of Legionella Diagnostics
Legionnaires' disease is an underappreciated, mostly sporadic illness. This poses a serious challenge and highlights three significant approaches that are key to addressing legionellosis in the coming decades; these approaches include (i) employing all available, reliable diagnostic tools (286); (ii) improving existing assays; and (iii) developing new technologies that offer increased sensitivity, specificity, availability, and/or efficiency. The diversity of specimen types and availability of laboratory test platforms enable (and warrant) multiple, simultaneous detection strategies. Toward this end, several groups have assessed the “added value” of a coordinated approach for the detection of Legionella. They found overall increases in diagnostic sensitivity and specificity by employing more than one complementary assay (e.g., a combination of PCR, culture, UAT, and/or IFA assay) (335, 344, 578).
Most current clinical diagnostics for LD were developed and commercialized in the 1980s and 1990s (http://www.fda.gov/). Among them, card- and ELISA-based UATs were widely adopted by clinical laboratories, leading to increased detection and reporting of LD but with a bias toward recognizing L. pneumophila sg1 infections. A dramatic decrease in the use of culture methods for Legionella identification was also observed in the same time period both in the United States and abroad. A combination of these trends has potentially led to the underrepresentation of LD caused by non-sg1 legionellae. As mentioned above, within the past 6 years, two additional FDA-cleared Legionella UATs were marketed in the United States, with sensitivities comparable to those of existing assays. Additionally, at least 5 apparently equivalent Legionella UATs were developed and sold abroad (Table 4). While different test formats (e.g., dipstick/card/lateral flow) may increase choice and help lower laboratory costs, no significant improvements in detection efficiency or test sensitivity have been realized for alternative Legionella serogroups or species. At least two in-house, validated, broad-spectrum Legionella EIAs have been described (579, 580), but the only promising development is the discovery of a genus-wide common immunodominant antigen in legionellae, peptidoglycan-associated lipoprotein (PAL) (581–584). To date, one company has applied research toward a potential pan-Legionella rapid ICT (SD Bioline Legionella) using this antigen. Despite an initial negative review, this ICT was compared favorably to the Alere BinaxNOW Legionella UAT for Lp1 diagnosis; however, further evaluation is needed to assess its potential for non-sg1 disease diagnosis (309, 585).
TABLE 4.
Commercially marketed Legionella urinary antigen tests
| Company | Product name | Test format | FDA cleared | Commercial availability | Note |
|---|---|---|---|---|---|
| Alere | BinaxNOW Legionella urinary antigen card | ICT | Yes | U.S. | Approved only for Lp1 |
| Alere | Binax Legionella urinary antigen EIA | EIA/ELISA | Yes | U.S. | Approved only for Lp1 |
| Meridian Bioscience, Inc. | Tru Legionella | ICT | Yes | U.S. | Approved only for Lp1 |
| SA Scientific | SAS Legionella test | ICT | Yes | U.S. | Approved only for Lp1; based on >1 polyclonal antibody |
| Trinity Biotech | Bartels Legionella UAT | EIA/ELISA | Yes | U.S. | Approved only for Lp1 |
| Bio-Rad Medical Diagnostics GmbHb | Legionella urine antigen EIA | EIA/ELISA | No | Unknown | Reported to detect all L. pneumophila serogroups and some other species; however, this is unverified |
| ThermoFisher Scientific/Oxoid | Xpect Legionella UAT | ICT | No | Unknown | Reported to detect L. pneumophila sg1 and sg6 |
| Coris Bio | Legionella V-Test | ICT | No | Unknown | Reported to detect L. pneumophila sg1 |
| Trinity Biotech | Uni-Gold Legionella Urinary Antigen Plus | ICT | No | Unknown | Reported to detect L. pneumophila sg1 |
| Diamondialb | Rapid U Legionella Plus | Unknown | No | Unknown | |
| Standard Diagnosticsb | SD Bioline Legionella | ICT | No | Unknown | Marketed for Lp1, based on pan-Legionella PAL antigen |
ICT, immunochromatographic test; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; UAT, urine antigen test; FDA, U.S. Food and Drug Administration.
Very little information available.
Neither FDA approval nor clearance is required for in vitro diagnostic tests developed and implemented within a clinical or reference laboratory (although other regulatory considerations apply, e.g., CLIA). However, not all laboratories have the time and personnel resources for in-house assay development. In the United States, commercially available in vitro diagnostic tests must have FDA clearance and therefore represent a prevalidated, “ready-to-use” system for the identification of disease-causing agents. Nucleic acid-based molecular diagnostics offer rapid, accurate results for CAP etiology and are available for many disease agents; unfortunately, only one Legionella species NAAT has been FDA cleared, but it is not commercially available. A simple, inexpensive, FDA-cleared NAAT for most disease-associated Legionella species, based on proven PCR or emerging technologies such as LAMP, would greatly empower health care providers and laboratorians who currently rely on tests of prohibitive length or cost or of limited specificity.
Since 2004, respiratory infections represented the largest human disease category worldwide and one of the leading causes of mortality (586). Legionella is among the top nonzoonotic atypical agents of severe respiratory illness, and successful disease resolution requires swift treatment together with rapid diagnosis for informed and accurate antibiotic management and epidemiological awareness. Traditional techniques, such as culture and serology, will continue to offer value for research, epidemiology, and typing purposes. Despite clear challenges, the future of Legionella and legionellosis detection in the 21st century is promising; novel molecular approaches increase sensitivity, ease of use, and efficiency, while existing assays are updated to recognize a wider spectrum of pathogens. Ultimately, all facets of Legionella research and education will lead to better surveillance, enable earlier disease diagnosis, and decrease the LD burden.
ACKNOWLEDGMENTS
We thank Lauri Hicks and Claressa Lucas for their valuable insight and critical review of the manuscript and Meredith Boyter in the CDC Graphics Services Branch for her artistic eye and skill.
There are no disclosures or conflicts of interest to declare.
Biographies

Jeffrey W. Mercante is a microbiologist in the Pneumonia Response and Surveillance Laboratory at the Centers for Disease Control and Prevention in Atlanta, GA, where he develops and evaluates diagnostic and detection methods for Legionella laboratory and outbreak investigations. He earned a master's degree from Louisiana State University, studying virulence and methods of gene replacement in Brucella spp., and a Ph.D. in Microbiology and Molecular Genetics from Emory University, investigating the structure and function of the CsrA regulatory protein. Following a postdoctoral teaching and research fellowship at Emory, he came to the CDC to follow up his doctoral gene regulatory interests in Legionella. He works with a collaborative network of microbiologists and epidemiologists to better define Legionella physiology, ecology, and distribution, enabling the design of more accurate, rapid assays for detecting Legionella environmental contamination and human disease.

Jonas M. Winchell received his Ph.D. in Molecular Cell Biology from the University of Connecticut. After completing a postdoctoral appointment at the Dana-Farber Cancer Institute/Harvard Medical School, he began his career in public health, first at the Massachusetts Department of Public Health and then at the Centers for Disease Control and Prevention, where he has been since 2001. Since 2006, he has been the Laboratory Chief of the Pneumonia Response and Surveillance Laboratory within the Respiratory Diseases Branch of the Division of Bacterial Diseases in the National Center for Immunization and Respiratory Diseases. His laboratory heavily focuses on the development and implementation of advanced molecular diagnostics for detecting respiratory pathogens. This laboratory also plays a primary role during respiratory outbreak responses. Dr. Winchell has also been an adjunct faculty member since 2003 in the School of Biology at the Georgia Institute of Technology, teaching courses in Medical Microbiology and Virology.
REFERENCES
- 1.Sharrar RG, Parkin WE. 1976. Respiratory infection—Pennsylvania. MMWR Morb Mortal Wkly Rep 25:244. [Google Scholar]
- 2.Dowdle WR. 1993. 1976: lessons learned, p 1–7. In Barbaree JM, Breiman RF, Dufour AP (ed), Legionella: current status and emerging perspectives. American Society for Microbiology, Washington, DC. [Google Scholar]
- 3.Altman LK. 1 August 2006. In Philadelphia 30 years ago, an eruption of illness and fear. The New York Times; http://www.nytimes.com/2006/08/01/health/01docs.html?pagewanted=all&_r=0. [Google Scholar]
- 4.Fraser DW. 2005. The challenges were legion. Lancet Infect Dis 5:237–241. doi: 10.1016/S1473-3099(05)70054-2. [DOI] [PubMed] [Google Scholar]
- 5.Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG, Harris J, Mallison GF, Martin SM, McDade JE, Shepard CC, Brachman PS. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med 297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 6.McDade JE. 2002. Legionnaires' disease 25 years later: lessons learned, p 1–12. In Marre R, Kwaik YA, Bartlett C, Cianciotto NP, Fields BS, Frosch M, Hacker J, Luck PC (ed), Legionella. ASM Press, Washington, DC. [Google Scholar]
- 7.McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med 297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ, Steigerwalt AG, McDade JE. 1979. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann Intern Med 90:656–658. doi: 10.7326/0003-4819-90-4-656. [DOI] [PubMed] [Google Scholar]
- 9.Sharrar RG, Streiff E, Parkin WE. 1977. Special issue: follow up on respiratory illness—Philadelphia. MMWR Morb Mortal Wkly Rep 26:9–11. [Google Scholar]
- 10.Edelstein PH. 2008. Legionnaires' disease: history and clinical findings, p 1–19. In Heuner K, Swanson M (ed), Legionella: molecular microbiology. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 11.Jackson EB, Crocker TT, Smadel JE. 1952. Studies on two rickettsia-like agents probably isolated from guinea pigs. Bacteriol Proc 52:119. [Google Scholar]
- 12.McDade JE, Brenner DJ, Bozeman FM. 1979. Legionnaires' disease bacterium isolated in 1947. Ann Intern Med 90:659–661. doi: 10.7326/0003-4819-90-4-659. [DOI] [PubMed] [Google Scholar]
- 13.Thacker SB, Bennett JV, Tsai TF, Fraser DW, McDade JE, Shepard CC, Williams KH Jr, Stuart WH, Dull HB, Eickhoff TC. 1978. An outbreak in 1965 of severe respiratory illness caused by the Legionnaires' disease bacterium. J Infect Dis 138:512–519. doi: 10.1093/infdis/138.4.512. [DOI] [PubMed] [Google Scholar]
- 14.Glick TH, Gregg MB, Berman B, Mallison G, Rhodes WW Jr, Kassanoff I. 1978. Pontiac fever. An epidemic of unknown etiology in a health department: I. Clinical and epidemiologic aspects. Am J Epidemiol 107:149–160. [DOI] [PubMed] [Google Scholar]
- 15.Hicks L, Garrison L, Nelson GE, Hampton LM. 2011. Legionellosis—United States, 2000-2009. MMWR Morb Mortal Wkly Rep 60:1083–1086. [PubMed] [Google Scholar]
- 16.Hunt DA, Cartwright KA, Smith MC, Middleton J, Bartlett CL, Lee JV, Dennis PJ, Harper D. 1991. An outbreak of Legionnaires' disease in Gloucester. Epidemiol Infect 107:133–141. doi: 10.1017/S0950268800048767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas DL, Mundy LM, Tucker PC. 1993. Hot tub legionellosis. Legionnaires' disease and Pontiac fever after a point-source exposure to Legionella pneumophila. Arch Intern Med 153:2597–2599. [DOI] [PubMed] [Google Scholar]
- 18.Euser S, Pelgrim M, den Boer JW. 2010. Legionnaires' disease and Pontiac fever after using a private outdoor whirlpool spa. Scand J Infect Dis 42:910–916. doi: 10.3109/00365548.2010.509331. [DOI] [PubMed] [Google Scholar]
- 19.Castor ML, Wagstrom EA, Danila RN, Smith KE, Naimi TS, Besser JM, Peacock KA, Juni BA, Hunt JM, Bartkus JM, Kirkhorn SR, Lynfield R. 2005. An outbreak of Pontiac fever with respiratory distress among workers performing high-pressure cleaning at a sugar-beet processing plant. J Infect Dis 191:1530–1537. doi: 10.1086/428776. [DOI] [PubMed] [Google Scholar]
- 20.Fields BS, Haupt T, Davis JP, Arduino MJ, Miller PH, Butler JC. 2001. Pontiac fever due to Legionella micdadei from a whirlpool spa: possible role of bacterial endotoxin. J Infect Dis 184:1289–1292. doi: 10.1086/324211. [DOI] [PubMed] [Google Scholar]
- 21.Miller LA, Beebe JL, Butler JC, Martin W, Benson R, Hoffman RE, Fields BS. 1993. Use of polymerase chain reaction in an epidemiologic investigation of Pontiac fever. J Infect Dis 168:769–772. doi: 10.1093/infdis/168.3.769. [DOI] [PubMed] [Google Scholar]
- 22.Rowbotham TJ. 1981. Pontiac fever, amoebae, and legionellae. Lancet i:40–41. [DOI] [PubMed] [Google Scholar]
- 23.Rowbotham TJ. 1986. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci 22:678–689. [PubMed] [Google Scholar]
- 24.Rowbotham TJ. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol 33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molofsky AB, Swanson MS. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol Microbiol 53:29–40. doi: 10.1111/j.1365-2958.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 26.Mauchline WS, Araujo R, Wait R, Dowsett AB, Dennis PJ, Keevil CW. 1992. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. J Gen Microbiol 138:2371–2380. doi: 10.1099/00221287-138-11-2371. [DOI] [PubMed] [Google Scholar]
- 27.Pine L, George JR, Reeves MW, Harrell WK. 1979. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol 9:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greub G, Raoult D. 2003. Morphology of Legionella pneumophila according to their location within Hartmanella vermiformis. Res Microbiol 154:619–621. doi: 10.1016/j.resmic.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Katz SM, Hashemi S, Brown KR, Habib WA, Hammel JM. 1984. Pleomorphism of Legionella pneumophila. Ultrastruct Pathol 6:117–129. doi: 10.3109/01913128409018566. [DOI] [PubMed] [Google Scholar]
- 30.Fliermans CB. 1996. Ecology of Legionella: from data to knowledge with a little wisdom. Microb Ecol 32:203–228. [DOI] [PubMed] [Google Scholar]
- 31.Fliermans CB, Cherry WB, Orrison LH, Smith SJ, Tison DL, Pope DH. 1981. Ecological distribution of Legionella pneumophila. Appl Environ Microbiol 41:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin T, Yan G, Ren H, Zhou H, Wang H, Xu Y, Zhao M, Guan H, Li M, Shao Z. 2013. High prevalence, genetic diversity and intracellular growth ability of Legionella in hot spring environments. PLoS One 8:e59018. doi: 10.1371/journal.pone.0059018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortiz-Roque CM, Hazen TC. 1987. Abundance and distribution of Legionellaceae in Puerto Rican waters. Appl Environ Microbiol 53:2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travis TC, Brown EW, Peruski LF, Siludjai D, Jorakate P, Salika P, Yang G, Kozak NA, Kodani M, Warner AK, Lucas CE, Thurman KA, Winchell JM, Thamthitiwat S, Fields BS. 2012. Survey of Legionella species found in thai soil. Int J Microbiol 2012:218791. doi: 10.1155/2012/218791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pravinkumar SJ, Edwards G, Lindsay D, Redmond S, Stirling J, House R, Kerr J, Anderson E, Breen D, Blatchford O, McDonald E, Brown A. 2010. A cluster of Legionnaires' disease caused by Legionella longbeachae linked to potting compost in Scotland, 2008-2009. Euro Surveill 15(8):pii=19496 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19496. [DOI] [PubMed] [Google Scholar]
- 36.Casati S, Gioria-Martinoni A, Gaia V. 2009. Commercial potting soils as an alternative infection source of Legionella pneumophila and other Legionella species in Switzerland. Clin Microbiol Infect 15:571–575. doi: 10.1111/j.1469-0691.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 37.Koide M, Saito A, Okazaki M, Umeda B, Benson RF. 1999. Isolation of Legionella longbeachae serogroup 1 from potting soils in Japan. Clin Infect Dis 29:943–944. doi: 10.1086/520470. [DOI] [PubMed] [Google Scholar]
- 38.Koide M, Arakaki N, Saito A. 2001. Distribution of Legionella longbeachae and other legionellae in Japanese potting soils. J Infect Chemother 7:224–227. doi: 10.1007/s101560170017. [DOI] [PubMed] [Google Scholar]
- 39.Duchin JS, Koehler J, Rakita RM, Olson K, Hampson NB, Gilbert DN, Jackson JM, Stefonek KR, Kohn MA, Rosenberg J, Vugia D, Marchione-Mastroianni M. 2000. Legionnaires' disease associated with potting soil—California, Oregon, and Washington, May-June 2000. MMWR Morb Mortal Wkly Rep 49:777–778. [PubMed] [Google Scholar]
- 40.Steele TW, Lanser J, Sangster N. 1990. Isolation of Legionella longbeachae serogroup 1 from potting mixes. Appl Environ Microbiol 56:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currie SL, Beattie TK, Knapp CW, Lindsay DS. 2014. Legionella spp. in UK composts—a potential public health issue? Clin Microbiol Infect 20:O224–O229. doi: 10.1111/1469-0691.12381. [DOI] [PubMed] [Google Scholar]
- 42.Pearce MM, Theodoropoulos N, Mandel MJ, Brown E, Reed KD, Cianciotto NP. 2012. Legionella cardiaca sp. nov., isolated from a case of native valve endocarditis in a human heart. Int J Syst Evol Microbiol 62:2946–2954. doi: 10.1099/ijs.0.039248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muder RR, Yu VL. 2002. Infection due to Legionella species other than L. pneumophila. Clin Infect Dis 35:990–998. doi: 10.1086/342884. [DOI] [PubMed] [Google Scholar]
- 44.Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW. 1994. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl Environ Microbiol 60:1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121–3126. [DOI] [PubMed] [Google Scholar]
- 46.Walker JT, Sonesson A, Keevil CW, White DC. 1993. Detection of Legionella pneumophila in biofilms containing a complex microbial consortium by gas chromatography-mass spectrometry analysis of genus-specific hydroxy fatty acids. FEMS Microbiol Lett 113:139–144. doi: 10.1111/j.1574-6968.1993.tb06504.x. [DOI] [PubMed] [Google Scholar]
- 47.Declerck P. 2010. Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol 12:557–566. doi: 10.1111/j.1462-2920.2009.02025.x. [DOI] [PubMed] [Google Scholar]
- 48.Declerck P, Behets J, van Hoef V, Ollevier F. 2007. Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res 41:3159–3167. doi: 10.1016/j.watres.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan KB, Henson JM, Ferris MJ. 2005. Legionella species diversity in an acidic biofilm community in Yellowstone National Park. Appl Environ Microbiol 71:507–511. doi: 10.1128/AEM.71.1.507-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feeley JC, Gibson RJ, Gorman GW, Langford NC, Rasheed JK, Mackel DC, Baine WB. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol 10:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fliermans CB, Cherry WB, Orrison LH, Thacker L. 1979. Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl Environ Microbiol 37:1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor M, Ross K, Bentham R. 2009. Legionella, protozoa, and biofilms: interactions within complex microbial systems. Microb Ecol 58:538–547. doi: 10.1007/s00248-009-9514-z. [DOI] [PubMed] [Google Scholar]
- 54.Fields BS. 1996. The molecular ecology of legionellae. Trends Microbiol 4:286–290. doi: 10.1016/0966-842X(96)10041-X. [DOI] [PubMed] [Google Scholar]
- 55.Berk SG, Faulkner G, Garduno E, Joy MC, Ortiz-Jimenez MA, Garduno RA. 2008. Packaging of live Legionella pneumophila into pellets expelled by Tetrahymena spp. does not require bacterial replication and depends on a Dot/Icm-mediated survival mechanism. Appl Environ Microbiol 74:2187–2199. doi: 10.1128/AEM.01214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berk SG, Ting RS, Turner GW, Ashburn RJ. 1998. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol 64:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hojo F, Sato D, Matsuo J, Miyake M, Nakamura S, Kunichika M, Hayashi Y, Yoshida M, Takahashi K, Takemura H, Kamiya S, Yamaguchi H. 2012. Ciliates expel environmental Legionella-laden pellets to stockpile food. Appl Environ Microbiol 78:5247–5257. doi: 10.1128/AEM.00421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuchta JM, Navratil JS, Shepherd ME, Wadowsky RM, Dowling JN, States SJ, Yee RB. 1993. Impact of chlorine and heat on the survival of Hartmannella vermiformis and subsequent growth of Legionella pneumophila. Appl Environ Microbiol 59:4096–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kilvington S, Price J. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol 68:519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 60.Barker J, Brown MR, Collier PJ, Farrell I, Gilbert P. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol 58:2420–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker J, Scaife H, Brown MR. 1995. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother 39:2684–2688. doi: 10.1128/AAC.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cirillo JD, Cirillo SL, Yan L, Bermudez LE, Falkow S, Tompkins LS. 1999. Intracellular growth in Acanthamoeba castellani affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect Immun 67:4427–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neumeister B, Reiff G, Faigle M, Dietz K, Northoff H, Lang F. 2000. Influence of Acanthamoeba castellanii on intracellular growth of different Legionella species in human monocytes. Appl Environ Microbiol 66:914–919. doi: 10.1128/AEM.66.3.914-919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas V, Bouchez T, Nicolas V, Robert S, Loret JF, Levi Y. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J Appl Microbiol 97:950–963. doi: 10.1111/j.1365-2672.2004.02391.x. [DOI] [PubMed] [Google Scholar]
- 65.Bigot R, Bertaux J, Frere J, Berjeaud JM. 2013. Intra-amoeba multiplication induces chemotaxis and biofilm colonization and formation for Legionella. PLoS One 8:e77875. doi: 10.1371/journal.pone.0077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wadowsky RM, Wolford R, McNamara AM, Yee RB. 1985. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl Environ Microbiol 49:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arvand M, Jungkind K, Hack A. 2011. Contamination of the cold water distribution system of health care facilities by Legionella pneumophila: do we know the true dimension? Euro Surveill 16(16):pii=19844 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19844. [PubMed] [Google Scholar]
- 68.Farhat M, Moletta-Denat M, Frere J, Onillon S, Trouilhe MC, Robine E. 2012. Effects of disinfection on Legionella spp., eukarya, and biofilms in a hot water system. Appl Environ Microbiol 78:6850–6858. doi: 10.1128/AEM.00831-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz T, Hoffmann S, Obst U. 2003. Formation of natural biofilms during chlorine dioxide and u.v. disinfection in a public drinking water distribution system. J Appl Microbiol 95:591–601. doi: 10.1046/j.1365-2672.2003.02019.x. [DOI] [PubMed] [Google Scholar]
- 70.Thomas V, Loret JF, Jousset M, Greub G. 2008. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ Microbiol 10:2728–2745. doi: 10.1111/j.1462-2920.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 71.Wullings BA, Bakker G, van der Kooij D. 2011. Concentration and diversity of uncultured Legionella spp. in two unchlorinated drinking water supplies with different concentrations of natural organic matter. Appl Environ Microbiol 77:634–641. doi: 10.1128/AEM.01215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwon S, Moon E, Kim TS, Hong S, Park HD. 2011. Pyrosequencing demonstrated complex microbial communities in a membrane filtration system for a drinking water treatment plant. Microbes Environ 26:149–155. doi: 10.1264/jsme2.ME10205. [DOI] [PubMed] [Google Scholar]
- 73.Garcia A, Goni P, Cieloszyk J, Fernandez MT, Calvo-Begueria L, Rubio E, Fillat MF, Peleato ML, Clavel A. 2013. Identification of free-living amoebae and amoeba-associated bacteria from reservoirs and water treatment plants by molecular techniques. Environ Sci Technol 47:3132–3140. doi: 10.1021/es400160k. [DOI] [PubMed] [Google Scholar]
- 74.Arnow PM, Weil D, Para MF. 1985. Prevalence and significance of Legionella pneumophila contamination of residential hot-tap water systems. J Infect Dis 152:145–151. doi: 10.1093/infdis/152.1.145. [DOI] [PubMed] [Google Scholar]
- 75.Silk BJ, Moore MR, Bergtholdt M, Gorwitz RJ, Kozak NA, Tha MM, Brown EW, Winchester JL, Labus BJ, Rowley P, Middaugh JP, Fields BS, Hicks LA. 2012. Eight years of Legionnaires' disease transmission in travellers to a condominium complex in Las Vegas, Nevada. Epidemiol Infect 140:1993–2002. doi: 10.1017/S0950268811002779. [DOI] [PubMed] [Google Scholar]
- 76.Al-Matawah QA, Al-Zenki SF, Qasem JA, Al-Waalan TE, Ben Heji AH. 2012. Detection and quantification of Legionella pneumophila from water systems in Kuwait residential facilities. J Pathog 2012:138389. doi: 10.1155/2012/138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathys W, Stanke J, Harmuth M, Junge-Mathys E. 2008. Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int J Hyg Environ Health 211:179–185. doi: 10.1016/j.ijheh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Stout JE, Yu VL, Yee YC, Vaccarello S, Diven W, Lee TC. 1992. Legionella pneumophila in residential water supplies: environmental surveillance with clinical assessment for Legionnaires' disease. Epidemiol Infect 109:49–57. [PMC free article] [PubMed] [Google Scholar]
- 79.Cordes LG, Wiesenthal AM, Gorman GW, Phair JP, Sommers HM, Brown A, Yu VL, Magnussen MH, Meyer RD, Wolf JS, Shands KN, Fraser DW. 1981. Isolation of Legionella pneumophila from hospital shower heads. Ann Intern Med 94:195–197. doi: 10.7326/0003-4819-94-2-195. [DOI] [PubMed] [Google Scholar]
- 80.Stout J, Yu VL, Vickers RM, Zuravleff J, Best M, Brown A, Yee RB, Wadowsky R. 1982. Ubiquitousness of Legionella pneumophila in the water supply of a hospital with endemic Legionnaires' disease. N Engl J Med 306:466–468. doi: 10.1056/NEJM198202253060807. [DOI] [PubMed] [Google Scholar]
- 81.Gorman GW, Feeley JC, Steigerwalt A, Edelstein PH, Moss CW, Brenner DJ. 1985. Legionella anisa: a new species of Legionella isolated from potable waters and a cooling tower. Appl Environ Microbiol 49:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arnow PM, Chou T, Weil D, Shapiro EN, Kretzschmar C. 1982. Nosocomial Legionnaires' disease caused by aerosolized tap water from respiratory devices. J Infect Dis 146:460–467. doi: 10.1093/infdis/146.4.460. [DOI] [PubMed] [Google Scholar]
- 83.Alary M, Joly JR. 1992. Factors contributing to the contamination of hospital water distribution systems by legionellae. J Infect Dis 165:565–569. doi: 10.1093/infdis/165.3.565. [DOI] [PubMed] [Google Scholar]
- 84.Jones TF, Benson RF, Brown EW, Rowland JR, Crosier SC, Schaffner W. 2003. Epidemiologic investigation of a restaurant-associated outbreak of Pontiac fever. Clin Infect Dis 37:1292–1297. doi: 10.1086/379017. [DOI] [PubMed] [Google Scholar]
- 85.O'Loughlin RE, Kightlinger L, Werpy MC, Brown E, Stevens V, Hepper C, Keane T, Benson RF, Fields BS, Moore MR. 2007. Restaurant outbreak of Legionnaires' disease associated with a decorative fountain: an environmental and case-control study. BMC Infect Dis 7:93. doi: 10.1186/1471-2334-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsu BM, Chen CH, Wan MT, Cheng HW. 2006. Legionella prevalence in hot spring recreation areas of Taiwan. Water Res 40:3267–3273. doi: 10.1016/j.watres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Huang SW, Hsu BM, Wu SF, Fan CW, Shih FC, Lin YC, Ji DD. 2010. Water quality parameters associated with prevalence of Legionella in hot spring facility water bodies. Water Res 44:4805–4811. doi: 10.1016/j.watres.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 88.Koide M. 2002. Hot spring bath as the reservoir of Legionella bacterium. Intern Med 41:759. doi: 10.2169/internalmedicine.41.759. [DOI] [PubMed] [Google Scholar]
- 89.Lee HK, Shim JI, Kim HE, Yu JY, Kang YH. 2010. Distribution of Legionella species from environmental water sources of public facilities and genetic diversity of L. pneumophila serogroup 1 in South Korea. Appl Environ Microbiol 76:6547–6554. doi: 10.1128/AEM.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alexiou SD, Antoniadis A, Papapaganagiotou J, Stefanou T. 1989. Isolation of Legionella pneumophila from hotels of Greece. Eur J Epidemiol 5:47–50. doi: 10.1007/BF00145044. [DOI] [PubMed] [Google Scholar]
- 91.Dennis PJ, Taylor JA, Fitzgeorge RB, Bartlett CL, Barrow GI. 1982. Legionella pneumophila in water plumbing systems. Lancet i:949–951. [DOI] [PubMed] [Google Scholar]
- 92.Cowgill KD, Lucas CE, Benson RF, Chamany S, Brown EW, Fields BS, Feikin DR. 2005. Recurrence of Legionnaires disease at a hotel in the United States Virgin Islands over a 20-year period. Clin Infect Dis 40:1205–1207. doi: 10.1086/428844. [DOI] [PubMed] [Google Scholar]
- 93.Kusnetsov J, Neuvonen LK, Korpio T, Uldum SA, Mentula S, Putus T, Tran Minh NN, Martimo KP. 2010. Two Legionnaires' disease cases associated with industrial waste water treatment plants: a case report. BMC Infect Dis 10:343. doi: 10.1186/1471-2334-10-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fykse EM, Aarskaug T, Thrane I, Blatny JM. 2013. Legionella and non-Legionella bacteria in a biological treatment plant. Can J Microbiol 59:102–109. doi: 10.1139/cjm-2012-0166. [DOI] [PubMed] [Google Scholar]
- 95.Miyamoto H, Jitsurong S, Shiota R, Maruta K, Yoshida S, Yabuuchi E. 1997. Molecular determination of infection source of a sporadic Legionella pneumonia case associated with a hot spring bath. Microbiol Immunol 41:197–202. doi: 10.1111/j.1348-0421.1997.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 96.Ito I, Naito J, Kadowaki S, Mishima M, Ishida T, Hongo T, Ma L, Ishii Y, Matsumoto T, Yamaguchi K. 2002. Hot spring bath and Legionella pneumonia: an association confirmed by genomic identification. Intern Med 41:859–863. doi: 10.2169/internalmedicine.41.859. [DOI] [PubMed] [Google Scholar]
- 97.Kurosawa H, Fujita M, Kobatake S, Kimura H, Ohshima M, Nagai A, Kaneko S, Iwasaki Y, Kozawa K. 2010. A case of Legionella pneumonia linked to a hot spring facility in Gunma Prefecture, Japan. Jpn J Infect Dis 63:78–79. [PubMed] [Google Scholar]
- 98.Matsui M, Fujii S-I, Shiroiwa R, Amemura-Maekawa J, Chang B, Kura F, Yamauchi K. 2010. Isolation of Legionella rubrilucens from a pneumonia patient co-infected with Legionella pneumophila. J Med Microbiol 59:1242–1246. doi: 10.1099/jmm.0.016089-0. [DOI] [PubMed] [Google Scholar]
- 99.Nozue T, Chikazawa H, Miyanishi S, Shimazaki T, Oka R, Shimazaki S, Miyamoto S. 2005. Legionella pneumonia associated with adult respiratory distress syndrome caused by Legionella pneumophila serogroup 3. Intern Med 44:73–78. doi: 10.2169/internalmedicine.44.73. [DOI] [PubMed] [Google Scholar]
- 100.Chiba Y, Okamoto H, Nagatomo A, Kunikane H, Watanabe K. 1998. Legionnaires' disease diagnosed by bronchoalveolar lavage. Intern Med 37:153–156. doi: 10.2169/internalmedicine.37.153. [DOI] [PubMed] [Google Scholar]
- 101.Furuhata K, Hara M, Yoshida S, Fukuyama M. 2004. Distribution of Legionella spp. in hot spring baths in Japan. Kansenshogaku Zasshi 78:710–716. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 102.Yu VL. 2001. Legionnaires' disease: seek and ye shall find. Cleve Clin J Med 68:318–322. doi: 10.3949/ccjm.68.4.318. [DOI] [PubMed] [Google Scholar]
- 103.Blatt SP, Parkinson MD, Pace E, Hoffman P, Dolan D, Lauderdale P, Zajac RA, Melcher GP. 1993. Nosocomial Legionnaires' disease: aspiration as a primary mode of disease acquisition. Am J Med 95:16–22. doi: 10.1016/0002-9343(93)90227-G. [DOI] [PubMed] [Google Scholar]
- 104.Loeb M, Simor AE, Mandell L, Krueger P, McArthur M, James M, Walter S, Richardson E, Lingley M, Stout J, Stronach D, McGeer A. 1999. Two nursing home outbreaks of respiratory infection with Legionella sainthelensis. J Am Geriatr Soc 47:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venezia RA, Agresta MD, Hanley EM, Urquhart K, Schoonmaker D. 1994. Nosocomial legionellosis associated with aspiration of nasogastric feedings diluted in tap water. Infect Control Hosp Epidemiol 15:529–533. doi: 10.2307/30148403. [DOI] [PubMed] [Google Scholar]
- 106.Hosein IK, Hill DW, Tan TY, Butchart EG, Wilson K, Finlay G, Burge S, Ribeiro CD. 2005. Point-of-care controls for nosocomial legionellosis combined with chlorine dioxide potable water decontamination: a two-year survey at a Welsh teaching hospital. J Hosp Infect 61:100–106. doi: 10.1016/j.jhin.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 107.Bencini MA, Yzerman EP, Koornstra RH, Nolte CC, den Boer JW, Bruin JP. 2005. A case of Legionnaires' disease caused by aspiration of ice water. Arch Environ Occup Health 60:302–306. doi: 10.3200/AEOH.60.6.302-306. [DOI] [PubMed] [Google Scholar]
- 108.Johnson JT, Yu VL, Wagner RL, Best MG. 1985. Nosocomial Legionella pneumonia in a population of head and neck cancer patients. Laryngoscope 95:1468–1471. [DOI] [PubMed] [Google Scholar]
- 109.Marrie TJ, Haldane D, MacDonald S, Clarke K, Fanning C, Le Fort-Jost S, Bezanson G, Joly J. 1991. Control of endemic nosocomial Legionnaires' disease by using sterile potable water for high risk patients. Epidemiol Infect 107:591–605. doi: 10.1017/S0950268800049293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mastro TD, Fields BS, Breiman RF, Campbell J, Plikaytis BD, Spika JS. 1991. Nosocomial Legionnaires' disease and use of medication nebulizers. J Infect Dis 163:667–671. doi: 10.1093/infdis/163.3.667. [DOI] [PubMed] [Google Scholar]
- 111.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R, CDC Healthcare Infection Control Practices Advisory Committee. 2004. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recommend Rep 53:1–36. [PubMed] [Google Scholar]
- 112.Horwitz MA, Silverstein SC. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest 66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edelstein PH. 1987. The laboratory diagnosis of Legionnaires' disease. Semin Respir Infect 2:235–241. [PubMed] [Google Scholar]
- 114.Heuner K, Swanson M. 2008. Legionella: molecular microbiology. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 115.Segal G, Shuman HA. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun 67:2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arnold FW, Summersgill JT, Lajoie AS, Peyrani P, Marrie TJ, Rossi P, Blasi F, Fernandez P, File TM Jr, Rello J, Menendez R, Marzoratti L, Luna CM, Ramirez JA, Community-Acquired Pneumonia Organization Investigators. 2007. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 175:1086–1093. doi: 10.1164/rccm.200603-350OC. [DOI] [PubMed] [Google Scholar]
- 117.Marston BJ, Plouffe JF, File TM Jr, Hackman BA, Salstrom SJ, Lipman HB, Kolczak MS, Breiman RF. 1997. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 157:1709–1718. [PubMed] [Google Scholar]
- 118.Von Baum H, Ewig S, Marre R, Suttorp N, Gonschior S, Welte T, Lück C, Competence Network for Community Acquired Pneumonia Study Group. 2008. Community-acquired Legionella pneumonia: new insights from the German Competence Network for Community Acquired Pneumonia. Clin Infect Dis 46:1356–1364. doi: 10.1086/586741. [DOI] [PubMed] [Google Scholar]
- 119.Murdoch DR, Podmore RG, Anderson TP, Barratt K, Maze MJ, French KE, Young SA, Chambers ST, Werno AM. 2013. Impact of routine systematic PCR testing on case finding for Legionnaires' disease: a pre-post comparison study. Clin Infect Dis 57:1275–1281. doi: 10.1093/cid/cit504. [DOI] [PubMed] [Google Scholar]
- 120.European Centre for Disease Prevention and Control. 2013. Legionnaires disease in Europe, 2011. ECDC, Stockholm, Sweden. doi: 10.2900/78974. [DOI] [Google Scholar]
- 121.Marston BJ, Lipman HB, Breiman RF. 1994. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch Intern Med 154:2417–2422. [PubMed] [Google Scholar]
- 122.Straus WL, Plouffe JF, File TM Jr, Lipman HB, Hackman BH, Salstrom SJ, Benson RF, Breiman RF. 1996. Risk factors for domestic acquisition of Legionnaires disease. Ohio Legionnaires Disease Group. Arch Intern Med 156:1685–1692. [PubMed] [Google Scholar]
- 123.Misch EA, Verbon A, Prins JM, Skerrett SJ, Hawn TR. 2013. A TLR6 polymorphism is associated with increased risk of Legionnaires' disease. Genes Immun 14:420–426. doi: 10.1038/gene.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neil K, Berkelman R. 2008. Increasing incidence of legionellosis in the United States, 1990-2005: changing epidemiologic trends. Clin Infect Dis 47:591–599. doi: 10.1086/590557. [DOI] [PubMed] [Google Scholar]
- 125.Benin AL, Benson RF, Besser RE. 2002. Trends in Legionnaires disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin Infect Dis 35:1039–1046. doi: 10.1086/342903. [DOI] [PubMed] [Google Scholar]
- 126.US Centers for Disease Control and Prevention. 2013. Summary of notifiable diseases—United States, 2011. MMWR Morb Mortal Wkly Rep 62:1–117. [PubMed] [Google Scholar]
- 127.Hicks LA, Rose CE Jr, Fields BS, Drees ML, Engel JP, Jenkins PR, Rouse BS, Blythe D, Khalifah AP, Feikin DR, Whitney CG. 2007. Increased rainfall is associated with increased risk for legionellosis. Epidemiol Infect 135:811–817. doi: 10.1017/S0950268806007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fisman DN, Lim S, Wellenius GA, Johnson C, Britz P, Gaskins M, Maher J, Mittleman MA, Spain CV, Haas CN, Newbern C. 2005. It's not the heat, it's the humidity: wet weather increases legionellosis risk in the greater Philadelphia metropolitan area. J Infect Dis 192:2066–2073. doi: 10.1086/498248. [DOI] [PubMed] [Google Scholar]
- 129.Garcia-Vidal C, Labori M, Viasus D, Simonetti A, Garcia-Somoza D, Dorca J, Gudiol F, Carratala J. 2013. Rainfall is a risk factor for sporadic cases of Legionella pneumophila pneumonia. PLoS One 8:e61036. doi: 10.1371/journal.pone.0061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sakamoto R, Ohno A, Nakahara T, Satomura K, Iwanaga S, Kouyama Y, Kura F, Kato N, Matsubayashi K, Okumiya K, Yamaguchi K. 2009. Legionella pneumophila in rainwater on roads. Emerg Infect Dis 15:1295–1297. doi: 10.3201/eid1508.090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schalk JA, Docters van Leeuwen AE, Lodder WJ, de Man H, Euser S, den Boer JW, de Roda Husman AM. 2012. Isolation of Legionella pneumophila from pluvial floods by amoebal coculture. Appl Environ Microbiol 78:4519–4521. doi: 10.1128/AEM.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ricketts KD, Charlett A, Gelb D, Lane C, Lee JV, Joseph CA. 2009. Weather patterns and Legionnaires' disease: a meteorological study. Epidemiol. Infect. 137:1003–1012. doi: 10.1017/S095026880800157X. [DOI] [PubMed] [Google Scholar]
- 133.Kanatani J-I, Isobe J, Kimata K, Shima T, Shimizu M, Kura F, Sata T, Watahiki M. 2013. Close genetic relationship between Legionella pneumophila serogroup 1 isolates from sputum specimens and puddles on roads, as determined by sequence-based typing. Appl Environ Microbiol 79:3959–3966. doi: 10.1128/AEM.00637-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conza L, Casati S, Limoni C, Gaia V. 2013. Meteorological factors and risk of community-acquired Legionnaires' disease in Switzerland: an epidemiological study. BMJ Open 3:e002428. doi: 10.1136/bmjopen-2012-002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joseph CA, Ricketts KD, European Working Group for Legionella Infections. 2010. Legionnaires disease in Europe 2007-2008. Euro Surveill 15(8):pii=19493 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19493. [PubMed] [Google Scholar]
- 136.Tan MJ, Tan JS, Hamor RH, File TM Jr, Breiman RF. 2000. The radiologic manifestations of Legionnaire's disease. The Ohio Community-Based Pneumonia Incidence Study Group. Chest 117:398–403. doi: 10.1378/chest.117.2.398. [DOI] [PubMed] [Google Scholar]
- 137.Granados A, Podzamczer D, Gudiol F, Manresa F. 1989. Pneumonia due to Legionella pneumophila and pneumococcal pneumonia: similarities and differences on presentation. Eur Respir J 2:130–134. [PubMed] [Google Scholar]
- 138.Edelstein PH. 1993. Legionnaires' disease. Clin Infect Dis 16:741–747. doi: 10.1093/clind/16.6.741. [DOI] [PubMed] [Google Scholar]
- 139.Dionne M, Hatchette T, Forward K. 2003. Clinical utility of a Legionella pneumophila urinary antigen test in a large university teaching hospital. Can J Infect Dis 14:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ishiguro T, Takayanagi N, Yamaguchi S, Yamakawa H, Nakamoto K, Takaku Y, Miyahara Y, Kagiyama N, Kurashima K, Yanagisawa T, Sugita Y. 2013. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med 52:317–324. doi: 10.2169/internalmedicine.52.8830. [DOI] [PubMed] [Google Scholar]
- 141.Torres A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Cobo E, Rodriguez-Roisin R. 1991. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 144:312–318. [DOI] [PubMed] [Google Scholar]
- 142.Falco V, Fernandez de Sevilla T, Alegre J, Ferrer A, Martinez Vazquez JM. 1991. Legionella pneumophila. A cause of severe community-acquired pneumonia. Chest 100:1007–1011. [DOI] [PubMed] [Google Scholar]
- 143.Heath CH, Grove DI, Looke DF. 1996. Delay in appropriate therapy of Legionella pneumonia associated with increased mortality. Eur J Clin Microbiol Infect Dis 15:286–290. doi: 10.1007/BF01695659. [DOI] [PubMed] [Google Scholar]
- 144.Viasus D, Di Yacovo S, Garcia-Vidal C, Verdaguer R, Manresa F, Dorca J, Gudiol F, Carratala J. 2013. Community-acquired Legionella pneumophila pneumonia: a single-center experience with 214 hospitalized sporadic cases over 15 years. Medicine (Baltimore, MD) 92:51–60. doi: 10.1097/MD.0b013e31827f6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gacouin A, Le Tulzo Y, Lavoue S, Camus C, Hoff J, Bassen R, Arvieux C, Heurtin C, Thomas R. 2002. Severe pneumonia due to Legionella pneumophila: prognostic factors, impact of delayed appropriate antimicrobial therapy. Intensive Care Med 28:686–691. doi: 10.1007/s00134-002-1304-8. [DOI] [PubMed] [Google Scholar]
- 146.Beaute J, Zucs P, de Jong B, European Legionnaires Disease Surveillance Network. 2013. Legionnaires disease in Europe, 2009-2010. Euro Surveill 18(10):pii=20417 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20417. [DOI] [PubMed] [Google Scholar]
- 147.Ricketts KD, Joseph CA, European Working Group for Legionella Infections. 2007. Legionnaires disease in Europe: 2005-2006. Euro Surveill 12(12):pii=753 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=753. [Google Scholar]
- 148.Joseph CA, European Working Group for Legionella Infections. 2004. Legionnaires' disease in Europe 2000-2002. Epidemiol Infect 132:417–424. doi: 10.1017/S0950268804002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ricketts KD, Joseph CA, European Working Group for Legionella Infections. 2005. Legionnaires' disease in Europe 2003-2004. Euro Surveill 10(12):256–259. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=588.16371692 [Google Scholar]
- 150.Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis 186:127–128. doi: 10.1086/341087. [DOI] [PubMed] [Google Scholar]
- 151.NNDSS Annual Report Writing Group, Corvisy R, Trungove M, Bright A, Fitzsimmons G, Knope K, Martin N, Gavin K, Bareja C, Sloan-Gardner T. 2013. Australia's notifiable disease status, 2011: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep 37:E313–E393. [DOI] [PubMed] [Google Scholar]
- 152.NNDSS Annual Report Writing Group, Milton A, Stirzaker S, Trungove M, Knuckey D, Martin N, Hastie C, Pennington K, Sloan-Gardner T, Fitzsimmons G, Knope K, Martinek S, Mills L, Barry C, Wright P, Power M. 2012. Australia's notifiable disease status, 2010: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep 36:1–69. [DOI] [PubMed] [Google Scholar]
- 153.Bornstein N, Janin N, Bourguignon G, Surgot M, Fleurette J. 1987. Prevalence of anti-Legionella antibodies in a healthy population and in patients with tuberculosis or pneumonia. Pathol Biol (Paris) 35:353–356. [PubMed] [Google Scholar]
- 154.Fotos PG, Westfall HN, Snyder IS, Miller RW, Mutchler BM. 1985. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J Dent Res 64:1382–1385. doi: 10.1177/00220345850640121101. [DOI] [PubMed] [Google Scholar]
- 155.Lee HK, Woo MK, Ju YI, Baek SJ, Song HJ, Choi JS, Kweon SS, Jeon DY, Kang YH. 2008. Prevalence of antibodies in response to Legionella species, analysis of a healthy population from Jeollanam-do Province, Korea. J Microbiol 46:160–164. doi: 10.1007/s12275-007-0181-9. [DOI] [PubMed] [Google Scholar]
- 156.Romano F, Ribera G, D'Errico MM, Grasso GM, Montanaro D. 1989. Levels of anti-Legionella antibodies in a healthy Neapolitan population. Ann Ig 1:629–636. (In Italian.) [PubMed] [Google Scholar]
- 157.Sampson IA. 1988. Prevalence of antibody to Legionella pneumophila in aborigines and non-aborigines in Western Australia. Med J Aust 148:16–19. [DOI] [PubMed] [Google Scholar]
- 158.Wang J, Brown-Schlumpf MS, Brown A, Xie XZ. 1988. Seroprevalence of Legionella in Shanxi Province, China. Infection 16:179–182. doi: 10.1007/BF01644097. [DOI] [PubMed] [Google Scholar]
- 159.Wewalka G. 1983. Sero-epidemiology of legionellosis in Austria. Zentralbl Bakteriol Mikrobiol Hyg A 255:15–19. [PubMed] [Google Scholar]
- 160.Rudbeck M, Molbak K, Uldum S. 2008. High prevalence of antibodies to Legionella spp. in Danish blood donors. A study in areas with high and average incidence of Legionnaires' disease. Epidemiol Infect 136:257–262. doi: 10.1017/S0950268807008606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Helbig JH, Luck PC, Knirel YA, Witzleb W, Zahringer U. 1995. Molecular characterization of a virulence-associated epitope on the lipopolysaccharide of Legionella pneumophila serogroup 1. Epidemiol Infect 115:71–78. doi: 10.1017/S0950268800058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zou CH, Knirel YA, Helbig JH, Zahringer U, Mintz CS. 1999. Molecular cloning and characterization of a locus responsible for O acetylation of the O polysaccharide of Legionella pneumophila serogroup 1 lipopolysaccharide. J Bacteriol 181:4137–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kozak NA, Benson RF, Brown E, Alexander NT, Taylor TH Jr, Shelton BG, Fields BS. 2009. Distribution of lag-1 alleles and sequence-based types among Legionella pneumophila serogroup 1 clinical and environmental isolates in the United States. J Clin Microbiol 47:2525–2535. doi: 10.1128/JCM.02410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dournon E, Bibb WF, Rajagopalan P, Desplaces N, McKinney RM. 1988. Monoclonal antibody reactivity as a virulence marker for Legionella pneumophila serogroup 1 strains. J Infect Dis 157:496–501. doi: 10.1093/infdis/157.3.496. [DOI] [PubMed] [Google Scholar]
- 165.Harrison TG, Afshar B, Doshi N, Fry NK, Lee JV. 2009. Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000-2008). Eur J Clin Microbiol Infect Dis 28:781–791. doi: 10.1007/s10096-009-0705-9. [DOI] [PubMed] [Google Scholar]
- 166.Harrison TG, Doshi N, Fry NK, Joseph CA. 2007. Comparison of clinical and environmental isolates of Legionella pneumophila obtained in the UK over 19 years. Clin Microbiol Infect 13:78–85. doi: 10.1111/j.1469-0691.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- 167.Doleans A, Aurell H, Reyrolle M, Lina G, Freney J, Vandenesch F, Etienne J, Jarraud S. 2004. Clinical and environmental distributions of Legionella strains in France are different. J Clin Microbiol 42:458–460. doi: 10.1128/JCM.42.1.458-460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Newton HJ, Ang DK, van Driel IR, Hartland EL. 2010. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 23:274–298. doi: 10.1128/CMR.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Montagna MT, Napoli C, Tato D, Spilotros G, Barbuti G, Barbuti S. 2006. Clinical-environmental surveillance of legionellosis: an experience in Southern Italy. Eur J Epidemiol 21:325–331. doi: 10.1007/s10654-006-0009-7. [DOI] [PubMed] [Google Scholar]
- 170.Helbig JH, Bernander S, Castellani Pastoris M, Etienne J, Gaia V, Lauwers S, Lindsay D, Luck PC, Marques T, Mentula S, Peeters MF, Pelaz C, Struelens M, Uldum SA, Wewalka G, Harrison TG. 2002. Pan-European study on culture-proven Legionnaires' disease: distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur J Clin Microbiol Infect Dis 21:710–716. doi: 10.1007/s10096-002-0820-3. [DOI] [PubMed] [Google Scholar]
- 171.Moran-Gilad J, Mentasti M, Lazarovitch T, Huberman Z, Stocki T, Sadik C, Shahar T, Anis E, Valinsky L, Harrison TG, Grotto I. 9 December 2013. Molecular epidemiology of Legionnaires' disease in Israel. Clin Microbiol Infect doi: 10.1111/1469-0691.12425. [DOI] [PubMed] [Google Scholar]
- 172.Correia AM, Goncalves G, Reis J, Cruz JM, Castro e Freitas JA. 2001. An outbreak of Legionnaires disease in a municipality in northern Portugal. Euro Surveill 6(7):121–124. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=228. [PubMed] [Google Scholar]
- 173.Fenstersheib MD, Miller M, Diggins C, Liska S, Detwiler L, Werner SB, Lindquist D, Thacker WL, Benson RF. 1990. Outbreak of Pontiac fever due to Legionella anisa. Lancet 336:35–37. doi: 10.1016/0140-6736(90)91532-F. [DOI] [PubMed] [Google Scholar]
- 174.Haupt TE, Heffernan RT, Kazmierczak JJ, Nehls-Lowe H, Rheineck B, Powell C, Leonhardt KK, Chitnis AS, Davis JP. 2012. An outbreak of Legionnaires disease associated with a decorative water wall fountain in a hospital. Infect Control Hosp Epidemiol 33:185–191. doi: 10.1086/663711. [DOI] [PubMed] [Google Scholar]
- 175.Hlady WG, Mullen RC, Mintz CS, Shelton BG, Hopkins RS, Daikos GL. 1993. Outbreak of Legionnaire's disease linked to a decorative fountain by molecular epidemiology. Am J Epidemiol 138:555–562. [DOI] [PubMed] [Google Scholar]
- 176.Palmore TN, Stock F, White M, Bordner M, Michelin A, Bennett JE, Murray PR, Henderson DK. 2009. A cluster of cases of nosocomial Legionnaires disease linked to a contaminated hospital decorative water fountain. Infect Control Hosp Epidemiol 30:764–768. doi: 10.1086/598855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schlech WF III, Gorman GW, Payne MC, Broome CV. 1985. Legionnaires' disease in the Caribbean. An outbreak associated with a resort hotel. Arch Intern Med 145:2076–2079. [PubMed] [Google Scholar]
- 178.Shands KN, Ho JL, Meyer RD, Gorman GW, Edelstein PH, Mallison GF, Finegold SM, Fraser DW. 1985. Potable water as a source of Legionnaires' disease. JAMA 253:1412–1416. doi: 10.1001/jama.1985.03350340064018. [DOI] [PubMed] [Google Scholar]
- 179.Hanrahan JP, Morse DL, Scharf VB, Debbie JG, Schmid GP, McKinney RM, Shayegani M. 1987. A community hospital outbreak of legionellosis. Transmission by potable hot water. Am J Epidemiol 125:639–649. [DOI] [PubMed] [Google Scholar]
- 180.Nechwatal R, Ehret W, Klatte OJ, Zeissler HJ, Prull A, Lutz H. 1993. Nosocomial outbreak of legionellosis in a rehabilitation center. Demonstration of potable water as a source. Infection 21:235–240. [DOI] [PubMed] [Google Scholar]
- 181.Graman PS, Quinlan GA, Rank JA. 1997. Nosocomial legionellosis traced to a contaminated ice machine. Infect Control Hosp Epidemiol 18:637–640. doi: 10.2307/30141491. [DOI] [PubMed] [Google Scholar]
- 182.Goeller D, Blyth D, Davenport M, Blackburn M, Flannery B, Lucas C, Fields B, Moore M, Castel AD, Hicks L. 2005. Legionnaires disease associated with potable water in a hotel—Ocean City, Maryland, October 2003-February 2004. MMWR Morb Mortal Wkly Rep 54:165–168. [PubMed] [Google Scholar]
- 183.Cooper IR, White J, Mahenthiralingam E, Hanlon GW. 2008. Long-term persistence of a single Legionella pneumophila strain possessing the mip gene in a municipal shower despite repeated cycles of chlorination. J Hosp Infect 70:154–159. doi: 10.1016/j.jhin.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 184.Cheng VC, Wong SS, Chen JH, Chan JF, To KK, Poon RW, Wong SC, Chan KH, Tai JW, Ho PL, Tsang TH, Yuen KY. 2012. An unprecedented outbreak investigation for nosocomial and community-acquired legionellosis in Hong Kong. Chin Med J (Engl) 125:4283–4290. [PubMed] [Google Scholar]
- 185.Band JD, LaVenture M, Davis JP, Mallison GF, Skaliy P, Hayes PS, Schell WL, Weiss H, Greenberg DJ, Fraser DW. 1981. Epidemic Legionnaires' disease. Airborne transmission down a chimney. JAMA 245:2404–2407. [PubMed] [Google Scholar]
- 186.Nordstrom K, Kallings I, Dahnsjo H, Clemens F. 1983. An outbreak of Legionnaires' disease in Sweden: report of sixty-eight cases. Scand J Infect Dis 15:43–55. [DOI] [PubMed] [Google Scholar]
- 187.Garbe PL, Davis BJ, Weisfeld JS, Markowitz L, Miner P, Garrity F, Barbaree JM, Reingold AL. 1985. Nosocomial Legionnaires' disease. Epidemiologic demonstration of cooling towers as a source. JAMA 254:521–524. [DOI] [PubMed] [Google Scholar]
- 188.Friedman S, Spitalny K, Barbaree J, Faur Y, McKinney R. 1987. Pontiac fever outbreak associated with a cooling tower. Am J Public Health 77:568–572. doi: 10.2105/AJPH.77.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mitchell E, O'Mahony M, Watson JM, Lynch D, Joseph C, Quigley C, Aston R, Constable GN, Farrand RJ, Maxwell S, Hutchinson DN, Craske J, Lee JV. 1990. Two outbreaks of Legionnaires' disease in Bolton Health District. Epidemiol Infect 104:159–170. doi: 10.1017/S095026880005932X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Castellani Pastoris M, Ciceroni L, Lo Monaco R, Goldoni P, Mentore B, Flego G, Cattani L, Ciarrocchi S, Pinto A, Visca P. 1997. Molecular epidemiology of an outbreak of Legionnaires' disease associated with a cooling tower in Genova-Sestri Ponente, Italy. Eur J Clin Microbiol Infect Dis 16:883–892. doi: 10.1007/BF01700554. [DOI] [PubMed] [Google Scholar]
- 191.Isozumi R, Ito Y, Ito I, Osawa M, Hirai T, Takakura S, Iinuma Y, Ichiyama S, Tateda K, Yamaguchi K, Mishima M. 2005. An outbreak of Legionella pneumonia originating from a cooling tower. Scand J Infect Dis 37:709–711. doi: 10.1080/00365540510012143. [DOI] [PubMed] [Google Scholar]
- 192.Gilmour MW, Bernard K, Tracz DM, Olson AB, Corbett CR, Burdz T, Ng B, Wiebe D, Broukhanski G, Boleszczuk P, Tang P, Jamieson F, Van Domselaar G, Plummer FA, Berry JD. 2007. Molecular typing of a Legionella pneumophila outbreak in Ontario, Canada. J Med Microbiol 56:336–341. doi: 10.1099/jmm.0.46738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Phares CR, Russell E, Thigpen MC, Service W, Crist MB, Salyers M, Engel J, Benson RF, Fields B, Moore MR. 2007. Legionnaires' disease among residents of a long-term care facility: the sentinel event in a community outbreak. Am J Infect Control 35:319–323. doi: 10.1016/j.ajic.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 194.Benkel DH, McClure EM, Woolard D, Rullan JV, Miller GB Jr, Jenkins SR, Hershey JH, Benson RF, Pruckler JM, Brown EW, Kolczak MS, Hackler RL, Rouse BS, Breiman RF. 2000. Outbreak of Legionnaires' disease associated with a display whirlpool spa. Int J Epidemiol 29:1092–1098. doi: 10.1093/ije/29.6.1092. [DOI] [PubMed] [Google Scholar]
- 195.Campese C, Roche D, Clement C, Fierobe F, Jarraud S, de Waelle P, Perrin H, Che D. 2010. Cluster of Legionnaires' disease associated with a public whirlpool spa, France, April-May 2010. Euro Surveill 15(26):pii=19602 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19602. [PubMed] [Google Scholar]
- 196.Coetzee N, Duggal H, Hawker J, Ibbotson S, Harrison TG, Phin N, Laza-Stanca V, Johnston R, Iqbal Z, Rehman Y, Knapper E, Robinson S, Aigbogun N. 2012. An outbreak of Legionnaires' disease associated with a display spa pool in retail premises, Stoke-on-Trent, United Kingdom, July 2012. Euro Surveill 17(37):pii=20271 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20271. [PubMed] [Google Scholar]
- 197.Den Boer JW, Yzerman EP, Schellekens J, Lettinga KD, Boshuizen HC, Van Steenbergen JE, Bosman A, Van den Hof S, Van Vliet HA, Peeters MF, Van Ketel RJ, Speelman P, Kool JL, Conyn-Van Spaendonck MA. 2002. A large outbreak of Legionnaires' disease at a flower show, the Netherlands, 1999. Emerg Infect Dis 8:37–43. doi: 10.3201/eid0801.010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ruscoe Q, Hill S, Blackmore T, McLean M. 2006. An outbreak of Legionella pneumophila suspected to be associated with spa pools on display at a retail store in New Zealand. N Z Med J 119:U2253. [PubMed] [Google Scholar]
- 199.Hilborn ED, Wade TJ, Hicks L, Garrison L, Carpenter J, Adam E, Mull B, Yoder J, Roberts V, Gargano JW. 2013. Surveillance for waterborne disease outbreaks associated with drinking water and other nonrecreational water—United States, 2009-2010. MMWR Morb Mortal Wkly Rep 62:714–720. [PMC free article] [PubMed] [Google Scholar]
- 200.Brown A, Yu VL, Elder EM, Magnussen MH, Kroboth F. 1980. Nosocomial outbreak of Legionnaire's disease at the Pittsburgh Veterans Administration Medical Center. Trans Assoc. Am Physicians 93:52–59. [PubMed] [Google Scholar]
- 201.Fábregas L, Wereschagin M, Smeltz A, Kilzer L. 23 February 2013, posting date Lapses at Pittsburgh VA stoked spread of Legionella bacteria. Trib Total Media. http://triblive.com/news/allegheny/3697655-74/cdc-legionella-system#axzz3I8ZY6EfM. [Google Scholar]
- 202.Smith P, Moore M, Alexander N, Hicks L, O'Loughlin RE. 2007. Surveillance for travel-associated Legionnaires disease—United States, 2005-2006. MMWR Morb Mortal Wkly Rep 56:1261–1263. [PubMed] [Google Scholar]
- 203.European Centre for Disease Prevention and Control. 2012. Legionnaires disease in Europe 2010. ECDC, Stockholm, Sweden. doi: 10.2900/62079. [DOI] [Google Scholar]
- 204.Lever F, Joseph CA, European Working Group for Legionella Infections. 2003. Travel associated Legionnaires' disease in Europe in 2000 and 2001. Euro Surveill 8(3):65–72. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=404.12766263 [Google Scholar]
- 205.Ricketts K, Joseph C, European Working Group for Legionella Infections. 2004. Travel associated Legionnaires' disease in Europe: 2002. Euro Surveill 9(2):6–9. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=444. [DOI] [PubMed] [Google Scholar]
- 206.Ricketts K, Joseph C, European Working Group for Legionella Infections. 2004. Travel associated Legionnaires' disease in Europe: 2003. Euro Surveill 9(10):40–43. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=480. [DOI] [PubMed] [Google Scholar]
- 207.Joseph CA, Yadav R, Ricketts KD, European Working Group for Legionella Infections. 2009. Travel-associated Legionnaires disease in Europe in 2007. Euro Surveill 14(18):pii=19196 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19196. [Google Scholar]
- 208.Ricketts K, Joseph CA, Yadav R, European Working Group for Legionella Infections. 2010. Travel-associated Legionnaires disease in Europe in 2008. Euro Surveill 15(21):pii=19578 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19578. [Google Scholar]
- 209.Joseph CA, Ricketts KD, Yadav R, Patel S, European Working Group for Legionella Infections. 2010. Travel-associated Legionnaires disease in Europe in 2009. Euro Surveill 15(41):pii=19683 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19683. [DOI] [PubMed] [Google Scholar]
- 210.de Jong B, Payne Hallstrom L, Robesyn E, Ursut D, Zucs P, ELDSNet. 2013. Travel-associated Legionnaires' disease in Europe, 2010. Euro Surveill 18(23):pii=20498 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20498. [DOI] [PubMed] [Google Scholar]
- 211.Ricketts K, McNaught B, Joseph C, European Working Group for Legionella Infections. 2007. Travel-associated Legionnaires disease in Europe: 2005. Euro Surveill 12(1):pii=680 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=680. [DOI] [PubMed] [Google Scholar]
- 212.Ricketts KD, Yadav R, Joseph CA, European Working Group for Legionella Infections. 2008. Travel-associated Legionnaires disease in Europe: 2006. Euro Surveill 13(29):pii=18930 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18930. [Google Scholar]
- 213.Ricketts KD, McNaught B, Joseph CA, European Working Group for Legionella Infections. 2006. Travel-associated Legionnaires' disease in Europe: 2004. Euro Surveill 11(4):107–110. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=617.16645242 [Google Scholar]
- 214.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lewis VJ, Thacker WL, Shepard CC, McDade JE. 1978. In vivo susceptibility of the Legionnaires disease bacterium to ten antimicrobial agents. Antimicrob Agents Chemother 13:419–422. doi: 10.1128/AAC.13.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Diederen BM. 2008. Legionella spp. and Legionnaires' disease. J Infect 56:1–12. doi: 10.1016/j.jinf.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 217.Garcia-Vidal C, Carratala J. 2006. Current clinical management of Legionnaires' disease. Expert Rev Anti Infect Ther 4:995–1004. doi: 10.1586/14787210.4.6.995. [DOI] [PubMed] [Google Scholar]
- 218.American Thoracic Society, Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 219.Edelstein PH. 1995. Antimicrobial chemotherapy for Legionnaires' disease: a review. Clin Infect Dis 21(Suppl 3):S265–S276. [DOI] [PubMed] [Google Scholar]
- 220.Lee TC, Vickers RM, Yu VL, Wagener MM. 1993. Growth of 28 Legionella species on selective culture media: a comparative study. J Clin Microbiol 31:2764–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bebear C, Pereyre S, Peuchant O. 2011. Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol 6:423–431. doi: 10.2217/fmb.11.18. [DOI] [PubMed] [Google Scholar]
- 222.Cornick JE, Bentley SD. 2012. Streptococcus pneumoniae: the evolution of antimicrobial resistance to beta-lactams, fluoroquinolones and macrolides. Microbes Infect 14:573–583. doi: 10.1016/j.micinf.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 223.Bruin JP, Ijzerman EP, den Boer JW, Mouton JW, Diederen BM. 2012. Wild-type MIC distribution and epidemiological cut-off values in clinical Legionella pneumophila serogroup 1 isolates. Diagn Microbiol Infect Dis 72:103–108. doi: 10.1016/j.diagmicrobio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 224.Bruin JP, Koshkolda T, IJzerman EPF, Lück C, Diederen BMW, Den Boer JW, Mouton JW. 4 June 2014. Isolation of ciprofloxacin-resistant Legionella pneumophila in a patient with severe pneumonia. J Antimicrob Chemother doi: 10.1093/jac/dku196. [DOI] [PubMed] [Google Scholar]
- 225.Mallegol J, Fernandes P, Melano RG, Guyard C. 2014. Antimicrobial activity of solithromycin against clinical isolates of Legionella pneumophila serogroup 1. Antimicrob Agents Chemother 58:909–915. doi: 10.1128/AAC.01639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Engleberg NC, Carter C, Weber DR, Cianciotto NP, Eisenstein BI. 1989. DNA sequence of mip, a Legionella pneumophila gene associated with macrophage infectivity. Infect Immun 57:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Starnbach MN, Falkow S, Tompkins LS. 1989. Species-specific detection of Legionella pneumophila in water by DNA amplification and hybridization. J Clin Microbiol 27:1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gomez-Lus P, Fields BS, Benson RF, Martin WT, O'Connor SP, Black CM. 1993. Comparison of arbitrarily primed polymerase chain reaction, ribotyping, and monoclonal antibody analysis for subtyping Legionella pneumophila serogroup 1. J Clin Microbiol 31:1940–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yamamoto H, Hashimoto Y, Ezaki T. 1993. Comparison of detection methods for Legionella species in environmental water by colony isolation, fluorescent antibody staining, and polymerase chain reaction. Microbiol Immunol 37:617–622. doi: 10.1111/j.1348-0421.1993.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 230.US Centers for Disease Control and Prevention. 2012. 2012 case definitions: nationally notifiable conditions infectious and non-infectious case. US Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 231.Council of State and Territorial Epidemiologists. 2009. Public health reporting and national notification for legionellosis. CSTE Position Statement 09-ID-45. Council of State and Territorial Epidemiologists, Atlanta, GA. [Google Scholar]
- 232.European Centre for Disease Control and Prevention. 2012. European Legionnaires' Disease Surveillance Network (ELDSNet): operating procedures. ECDC, Stockholm, Sweden. doi: 10.2900/19185. [DOI] [Google Scholar]
- 233.Cunha BA. 2010. Legionnaires' disease: clinical differentiation from typical and other atypical pneumonias. Infect Dis Clin North Am 24:73–105. doi: 10.1016/j.idc.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gaia V, Fry NK, Afshar B, Luck PC, Meugnier H, Etienne J, Peduzzi R, Harrison TG. 2005. Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J Clin Microbiol 43:2047–2052. doi: 10.1128/JCM.43.5.2047-2052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gaia V, Fry NK, Harrison TG, Peduzzi R. 2003. Sequence-based typing of Legionella pneumophila serogroup 1 offers the potential for true portability in legionellosis outbreak investigation. J Clin Microbiol 41:2932–2939. doi: 10.1128/JCM.41.7.2932-2939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, Bourbeau P, Carroll KC, Kehl SC, Dunne WM, Robinson-Dunn B, Schwartzman JD, Chapin KC, Snyder JW, Forbes BA, Patel R, Rosenblatt JE, Pritt BS. 2013. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis 57:e22–e121. doi: 10.1093/cid/cit278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Maiwald M, Helbig JH, Lück PC. 1998. Laboratory methods for the diagnosis of Legionella infections. J Microbiol Methods 33:59–79. doi: 10.1016/S0167-7012(98)00041-4. [DOI] [Google Scholar]
- 238.Wilkinson HW. 1988. Hospital-laboratory diagnosis of Legionella infection. Centers for Disease Control, Atlanta, GA. [Google Scholar]
- 239.Murdoch DR. 2003. Diagnosis of Legionella infection. Clin Infect Dis 36:64–69. doi: 10.1086/345529. [DOI] [PubMed] [Google Scholar]
- 240.Pasculle AW, Feeley JC, Gibson RJ, Cordes LG, Myerowitz RL, Patton CM, Gorman GW, Carmack CL, Ezzell JW, Dowling JN. 1980. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis 141:727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- 241.Edelstein PH. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol 14:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Blyth CC, Adams DN, Chen SC. 2009. Diagnostic and typing methods for investigating Legionella infection. N S W Public Health Bull 20:157–161. doi: 10.1071/NB08062. [DOI] [PubMed] [Google Scholar]
- 243.Fields BS. 2005. Procedures for the recovery of Legionella from the environment. Respiratory Disease Laboratory Section, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 244.Jarraud S, Descours G, Ginevra C, Lina G, Etienne J. 2013. Identification of Legionella in clinical samples. Methods Mol Biol 954:27–56. doi: 10.1007/978-1-62703-161-5_2. [DOI] [PubMed] [Google Scholar]
- 245.Orrison LH, Cherry WB, Tyndall RL, Fliermans CB, Gough SB, Lambert MA, McDougal LK, Bibb WF, Brenner DJ. 1983. Legionella oakridgensis: unusual new species isolated from cooling tower water. Appl Environ Microbiol 45:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yang G, Benson RF, Ratcliff RM, Brown EW, Steigerwalt AG, Thacker WL, Daneshvar MI, Morey RE, Saito A, Fields BS. 2012. Legionella nagasakiensis sp. nov., isolated from water samples and from a patient with pneumonia. Int J Syst Evol Microbiol 62:284–288. doi: 10.1099/ijs.0.027193-0. [DOI] [PubMed] [Google Scholar]
- 247.Edelstein PH, Brenner DJ, Moss CW, Steigerwalt AG, Francis EM, George WL. 1982. Legionella wadsworthii species nova: a cause of human pneumonia. Ann Intern Med 97:809–813. doi: 10.7326/0003-4819-97-6-809. [DOI] [PubMed] [Google Scholar]
- 248.Lo Presti F, Riffard S, Meugnier H, Reyrolle M, Lasne Y, Grimont PAD, Grimont F, Vandenesch F, Etienne J, Fleurette J, Freney J. 1999. Legionella taurinensis sp. nov., a new species antigenically similar to Legionella spiritensis. Int J Syst Bacteriol 49:397–403. doi: 10.1099/00207713-49-2-397. [DOI] [PubMed] [Google Scholar]
- 249.Thacker WL, Benson RF, Schifman RB, Pugh E, Steigerwalt AG, Mayberry WR, Brenner DJ, Wilkinson HW. 1989. Legionella tucsonensis sp. nov. isolated from a renal transplant recipient. J Clin Microbiol 27:1831–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wilkinson IJ, Sangster N, Ratcliff RM, Mugg PA, Davos DE, Lanser JA. 1990. Problems associated with identification of Legionella species from the environment and isolation of six possible new species. Appl Environ Microbiol 56:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Adeleke A, Pruckler J, Benson R, Rowbotham T, Halablab M, Fields B. 1996. Legionella-like amebal pathogens—phylogenetic status and possible role in respiratory disease. Emerg Infect Dis 2:225–230. doi: 10.3201/eid0203.960311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Griffith ME, Lindquist DS, Benson RF, Thacker WL, Brenner DJ, Wilkinson HW. 1988. First isolation of Legionella gormanii from human disease. J Clin Microbiol 26:380–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Harris PP, Aufdemorte T, Ewing EP Jr, Johnson JE, Tio FO. 1981. Fluorescent-antibody detection of Legionella dumoffii in a fatal case of pneumonia. J Clin Microbiol 13:778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lo Presti F, Riffard S, Vandenesch F, Reyrolle M, Ronco E, Ichai P, Etienne J. 1997. The first clinical isolate of Legionella parisiensis, from a liver transplant patient with pneumonia. J Clin Microbiol 35:1706–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Matsui M, Fujii S, Shiroiwa R, Amemura-Maekawa J, Chang B, Kura F, Yamauchi K. 2010. Isolation of Legionella rubrilucens from a pneumonia patient co-infected with Legionella pneumophila. J Med Microbiol 59:1242–1246. doi: 10.1099/jmm.0.016089-0. [DOI] [PubMed] [Google Scholar]
- 256.Parker MM, Macher AM, Shelhamer JH, Balow JE, Gill V, Parrillo JE. 1983. Unresponsiveness of Legionella bozemanii pneumonia to erythromycin administration despite in vitro sensitivity. Am Rev Respir Dis 128:955–956. [DOI] [PubMed] [Google Scholar]
- 257.World Health Organization. 1997. Legionnaires' disease in Europe, 1996. Introduction Wkly Epidemiol Rec 72:253–257. [PubMed] [Google Scholar]
- 258.Lucas CE, Taylor TH Jr, Fields BS. 2011. Accuracy and precision of Legionella isolation by US laboratories in the ELITE program pilot study. Water Res 45:4428–4436. doi: 10.1016/j.watres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 259.Kohler RB, Winn WC, Wheat LJ. 1984. Onset and duration of urinary antigen excretion in Legionnaires disease. J Clin Microbiol 20:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sopena N, Sabria M, Pedro-Botet ML, Reynaga E, Garcia-Nunez M, Dominguez J, Matas L. 2002. Factors related to persistence of Legionella urinary antigen excretion in patients with Legionnaires' disease. Eur J Clin Microbiol Infect Dis 21:845–848. [DOI] [PubMed] [Google Scholar]
- 261.Berdal BP, Farshy CE, Feeley JC. 1979. Detection of Legionella pneumonophila antigen in urine by enzyme-linked immunospecific assay. J Clin Microbiol 9:575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tilton RC. 1979. Legionnaires' disease antigen detected by enzyme-linked immunosorbent assay. Ann Intern Med 90:697–698. doi: 10.7326/0003-4819-90-4-697. [DOI] [PubMed] [Google Scholar]
- 263.Kohler RB, Zimmerman SE, Wilson E, Allen SD, Edelstein PH, Wheat LJ, White A. 1981. Rapid radioimmunoassay diagnosis of Legionnaires' disease: detection and partial characterization of urinary antigen. Ann Intern Med 94:601–605. doi: 10.7326/0003-4819-94-5-601. [DOI] [PubMed] [Google Scholar]
- 264.Aguero-Rosenfeld ME, Edelstein PH. 1988. Retrospective evaluation of the Du Pont radioimmunoassay kit for detection of Legionella pneumophila serogroup 1 antigenuria in humans. J Clin Microbiol 26:1775–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Oliverio MJ, Fisher MA, Vickers RM, Yu VL, Menon A. 1991. Diagnosis of Legionnaires' disease by radioimmunoassay of Legionella antigen in pleural fluid. J Clin Microbiol 29:2893–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dominguez J, Gali N, Matas L, Pedroso P, Hernandez A, Padilla E, Ausina V. 1999. Evaluation of a rapid immunochromatographic assay for the detection of Legionella antigen in urine samples. Eur J Clin Microbiol Infect Dis 18:896–898. doi: 10.1007/s100960050427. [DOI] [PubMed] [Google Scholar]
- 267.Helbig JH, Uldum SA, Luck PC, Harrison TG. 2001. Detection of Legionella pneumophila antigen in urine samples by the BinaxNOW immunochromatographic assay and comparison with both Binax Legionella Urinary Enzyme Immunoassay (EIA) and Biotest Legionella Urine Antigen EIA. J Med Microbiol 50:509–516. [DOI] [PubMed] [Google Scholar]
- 268.Diederen BM, Peeters MF. 2007. Evaluation of the SAS Legionella Test, a new immunochromatographic assay for the detection of Legionella pneumophila serogroup 1 antigen in urine. Clin Microbiol Infect 13:86–88. doi: 10.1111/j.1469-0691.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 269.Shimada T, Noguchi Y, Jackson JL, Miyashita J, Hayashino Y, Kamiya T, Yamazaki S, Matsumura T, Fukuhara S. 2009. Systematic review and metaanalysis: urinary antigen tests for legionellosis. Chest 136:1576–1585. doi: 10.1378/chest.08-2602. [DOI] [PubMed] [Google Scholar]
- 270.Harrison T, Uldum S, Alexiou-Daniel S, Bangsborg J, Bernander S, Draŝar V, Etienne J, Helbig J, Lindsay D, Lochman I, Marques T, de Ory F, Tartakovskii I, Wewalka G, Fehrenbach F. 1998. A multicenter evaluation of the Biotest Legionella urinary antigen EIA. Clin Microbiol Infect 4:359–365. doi: 10.1111/j.1469-0691.1998.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 271.St-Martin G, Uldum S, Molbak K. 2013. Incidence and prognostic factors for Legionnaires' disease in Denmark 1993-2006. ISRN Epidemiol 2013:8. doi: 10.5402/2013/847283. [DOI] [Google Scholar]
- 272.Olsen CW, Elverdal P, Jørgensen CS, Uldum SA. 2009. Comparison of the sensitivity of the Legionella urinary antigen EIA kits from Binax and Biotest with urine from patients with infections caused by less common serogroups and subgroups of Legionella. Eur J Clin Microbiol Infect Dis 28:817–820. doi: 10.1007/s10096-008-0697-x. [DOI] [PubMed] [Google Scholar]
- 273.Benson RF, Tang PW, Fields BS. 2000. Evaluation of the Binax and Biotest urinary antigen kits for detection of Legionnaires' disease due to multiple serogroups and species of Legionella. J Clin Microbiol 38:2763–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Svarrer CW, Luck C, Elverdal PL, Uldum SA. 2012. Immunochromatic kits Xpect Legionella and BinaxNOW Legionella for detection of Legionella pneumophila urinary antigen have low sensitivities for the diagnosis of Legionnaires' disease. J Med Microbiol 61:213–217. doi: 10.1099/jmm.0.035014-0. [DOI] [PubMed] [Google Scholar]
- 275.Chang FY, Jacobs SL, Colodny SM, Stout JE, Yu VL. 1996. Nosocomial Legionnaires' disease caused by Legionella pneumophila serogroup 5: laboratory and epidemiologic implications. J Infect Dis 174:1116–1119. doi: 10.1093/infdis/174.5.1116. [DOI] [PubMed] [Google Scholar]
- 276.Bruin JP, Diederen BMW. 2013. Evaluation of Meridian TRU Legionella, a new rapid test for detection of Legionella pneumophila serogroup 1 antigen in urine samples. Eur J Clin Microbiol Infect Dis 32:333–334. doi: 10.1007/s10096-012-1745-0. [DOI] [PubMed] [Google Scholar]
- 277.Harrison TG, Doshi N. 2001. Evaluation of the Bartels Legionella Urinary Antigen enzyme immunoassay. Eur J Clin Microbiol Infect Dis 20:738–740. doi: 10.1007/s100960100598. [DOI] [PubMed] [Google Scholar]
- 278.Diederen BM, Bruin JP, Scopes E, Peeters MF, IJzerman EP. 2009. Evaluation of the Oxoid Xpect Legionella test kit for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 47:2272–2274. doi: 10.1128/JCM.00397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Guerrero C, Toldos CM, Yague G, Ramirez C, Rodriguez T, Segovia M. 2004. Comparison of diagnostic sensitivities of three assays (Bartels enzyme immunoassay [EIA], Biotest EIA, and Binax NOW immunochromatographic test) for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 42:467–468. doi: 10.1128/JCM.42.1.467-468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Engel MF, van Manen L, Hoepelman AI, Thijsen S, Oosterheert JJ. 2013. Diagnostic, therapeutic and economic consequences of a positive urinary antigen test for Legionella spp. in patients admitted with community-acquired pneumonia: a 7-year retrospective evaluation. J Clin Pathol 66:797–802. doi: 10.1136/jclinpath-2012-201209. [DOI] [PubMed] [Google Scholar]
- 281.Garbino J, Bornand JE, Uckay I, Fonseca S, Sax H. 2004. Impact of positive Legionella urinary antigen test on patient management and improvement of antibiotic use. J Clin Pathol 57:1302–1305. doi: 10.1136/jcp.2004.018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lettinga KD, Verbon A, Weverling GJ, Schellekens JF, Den Boer JW, Yzerman EP, Prins J, Boersma WG, van Ketel RJ, Prins JM, Speelman P. 2002. Legionnaires' disease at a Dutch flower show: prognostic factors and impact of therapy. Emerg Infect Dis 8:1448–1454. doi: 10.3201/eid0812.020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Dirven K, Ieven M, Peeters MF, van der Zee A, De Schrijver K, Goossens H. 2005. Comparison of three Legionella urinary antigen assays during an outbreak of legionellosis in Belgium. J Med Microbiol 54:1213–1216. doi: 10.1099/jmm.0.45909-0. [DOI] [PubMed] [Google Scholar]
- 284.Niederman MS, Bass JB, Campbell GD, Fein AM, Grossman RF, Mandell LA, Marrie TJ, Sarosi GA, Torres A, Yu VL. 1993. Medical Section of the American Lung Association. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. Am Rev Respir Dis 148:1418–1426. doi: 10.1164/ajrccm/148.5.1418. [DOI] [PubMed] [Google Scholar]
- 285.Bartlett JG, Breiman RF, Mandell LA, File TM. 1998. Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis 26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 286.Jespersen S, Sogaard OS, Fine MJ, Ostergaard L. 2009. The relationship between diagnostic tests and case characteristics in Legionnaires' disease. Scand J Infect Dis 41:425–432. doi: 10.1080/00365540902946536. [DOI] [PubMed] [Google Scholar]
- 287.Rudbeck M, Molbak K, Uldum SA. 2009. Dynamics of Legionella antibody levels during 1 year in a healthy population. Epidemiol Infect 137:1013–1018. doi: 10.1017/S0950268808001684. [DOI] [PubMed] [Google Scholar]
- 288.Rudbeck M, Viskum S, Molbak K, Uldum SA. 2009. Legionella antibodies in a Danish hospital staff with known occupational exposure. J Environ Public Health 2009:812829. doi: 10.1155/2009/812829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Beer S, Boldur I, Kazak R, Avidan S, Kannai Y. 1985. Serum antibodies to Legionella agents in bronchial asthma. Arch Dis Child 60:225–230. doi: 10.1136/adc.60.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Boldur I, Ergaz M, Sompolinsky D. 1984. A prevalence study of antibodies to Legionella spp. in geriatric institutions. J Hyg (Lond) 92:37–43. doi: 10.1017/S0022172400064007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Borella P, Bargellini A, Marchesi I, Rovesti S, Stancanelli G, Scaltriti S, Moro M, Montagna MT, Tato D, Napoli C, Triassi M, Montegrosso S, Pennino F, Zotti CM, Ditommaso S, Giacomuzzi M. 2008. Prevalence of anti-Legionella antibodies among Italian hospital workers. J Hosp Infect 69:148–155. doi: 10.1016/j.jhin.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 292.Borobio V, Martinez C, Perea EJ. 1987. Prevalence of anti-Legionella pneumophila antibodies in various groups with different risk factors in Seville (Spain). Eur J Epidemiol 3:436–438. doi: 10.1007/BF00145658. [DOI] [PubMed] [Google Scholar]
- 293.Coniglio MA, Pignato S, Giammanco G. 2009. Prevalence of antibodies against Legionella spp. in HIV-infected subjects and blood donors. J Infect 59:423–425. doi: 10.1016/j.jinf.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 294.Franzin L, Scramuzza F. 1995. Prevalence of Legionella pneumophila serogroup 1 antibodies in blood donors. Eur J Epidemiol 11:475–478. doi: 10.1007/BF01721236. [DOI] [PubMed] [Google Scholar]
- 295.Haraldsson A, Rechnitzer C, Friis-Moller A, Briem H. 1990. Prevalence of IgM antibodies to nine Legionella species in Icelandic children. Scand J Infect Dis 22:445–449. doi: 10.3109/00365549009027076. [DOI] [PubMed] [Google Scholar]
- 296.Helms CM, Johnson W, Renner ED, Hierholzer WJ Jr, Wintermeyer LA, Viner JP. 1980. Background prevalence of microagglutination antibodies to Legionella pneumophila serogroups 1, 2, 3, and 4. Infect Immun 30:612–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Helms CM, Renner ED, Viner JP, Hierholzer WJ Jr, Wintermeyer LA, Johnson W. 1980. Indirect immunofluorescence antibodies to Legionella pneumophila: frequency in a rural community. J Clin Microbiol 12:326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Irie M, Miyamoto H, Ikeda M, Yoshida S. 2004. A 3-year follow-up study of anti-Legionella antibodies in users of Japanese 24-hour hot water baths. J Occup Health 46:68–77. doi: 10.1539/joh.46.68. [DOI] [PubMed] [Google Scholar]
- 299.Reinthaler FF, Mascher F, Stunzner D. 1988. Serological examinations for antibodies against Legionella species in dental personnel. J Dent Res 67:942–943. doi: 10.1177/00220345880670061001. [DOI] [PubMed] [Google Scholar]
- 300.Smalley DL, Ourth DD. 1980. Prevalence of antibodies to Legionella pneumophila in adults from Memphis. South Med J 73:1096. [DOI] [PubMed] [Google Scholar]
- 301.Snowman WJ, Holtzhauer FJ, Halpin TJ, Correa-Villasenor A. 1982. The role of indoor and outdoor occupations in the seroepidemiology of Legionella pneumophila. J Infect Dis 145:275. doi: 10.1093/infdis/145.2.275. [DOI] [PubMed] [Google Scholar]
- 302.Vasil'eva VI, Prozorovskii SV, Rusakova EV, Tartakovskii IS, Gotvianskaia TP. 1986. Immune structure of the population in some regions of the USSR with respect to Legionella pneumophila. Zh Mikrobiol Epidemiol Immunobiol 1986(7):75–79. (In Russian.) [PubMed] [Google Scholar]
- 303.Yonke CA, Stiefel HE, Wentworth BB, Wilson DL. 1982. Prevalence of antibody to serogroups 1-4 of Legionella pneumophila: a seroepidemiologic study using the indirect hemagglutination test. Am J Epidemiol 115:633–639. [DOI] [PubMed] [Google Scholar]
- 304.Waterer GW, Baselski VS, Wunderink RG. 2001. Legionella and community-acquired pneumonia: a review of current diagnostic tests from a clinician's viewpoint. Am J Med 110:41–48. doi: 10.1016/S0002-9343(00)00624-0. [DOI] [PubMed] [Google Scholar]
- 305.Bernander S, Gastrin B, Lofgren S, Olinder-Nielsen AM. 1994. Legionella urinary antigen in early disease. Scand J Infect Dis 26:777–778. doi: 10.3109/00365549409008653. [DOI] [PubMed] [Google Scholar]
- 306.Yzerman EP, den Boer JW, Lettinga KD, Schellekens J, Dankert J, Peeters M. 2002. Sensitivity of three urinary antigen tests associated with clinical severity in a large outbreak of Legionnaires' disease in The Netherlands. J Clin Microbiol 40:3232–3236. doi: 10.1128/JCM.40.9.3232-3236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Blazquez RM, Espinosa FJ, Martinez-Toldos CM, Alemany L, Garcia-Orenes MC, Segovia M. 2005. Sensitivity of urinary antigen test in relation to clinical severity in a large outbreak of Legionella pneumonia in Spain. Eur J Clin Microbiol Infect Dis 24:488–491. doi: 10.1007/s10096-005-1361-3. [DOI] [PubMed] [Google Scholar]
- 308.Held J. 2012. Increasing the sensitivity of the BinaxNOW Legionella urinary antigen immunochromatographic test by additional readings at later time points. J Med Microbiol 61:884–885. doi: 10.1099/jmm.0.041996-0. [DOI] [PubMed] [Google Scholar]
- 309.Diederen BM, Peeters MF. 2006. Evaluation of two new immunochromatographic assays (Rapid U Legionella antigen test and SD Bioline Legionella antigen test) for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 44:2991–2993. doi: 10.1128/JCM.00799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ng V, Tang P, Jamieson F, Guyard C, Low DE, Fisman DN. 2009. Laboratory-based evaluation of legionellosis epidemiology in Ontario, Canada, 1978 to 2006. BMC Infect Dis 9:68. doi: 10.1186/1471-2334-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Scerpella EG, Whimbey EE, Champlin RE, Bodey GP. 1994. Pericarditis associated with Legionnaires' disease in a bone marrow transplant recipient. Clin Infect Dis 19:1168–1170. doi: 10.1093/clinids/19.6.1168. [DOI] [PubMed] [Google Scholar]
- 312.Benz-Lemoine E, Delwail V, Castel O, Guilhot F, Robert R, Grollier G, Roblot-Casenave F, Giraud C, Tanzer J. 1991. Nosocomial Legionnaires' disease in a bone marrow transplant unit. Bone Marrow Transplant 7:61–63. [PubMed] [Google Scholar]
- 313.Bernat A, Garrigos E, Martin J, Moll R, Perez A. 1994. Legionnaires' disease outbreak in a haemodialysis unit. Nephrol Dial Transplant 9:217–218. [PubMed] [Google Scholar]
- 314.Plouffe JF, File TM Jr, Breiman RF, Hackman BA, Salstrom SJ, Marston BJ, Fields BS. 1995. Reevaluation of the definition of Legionnaires' disease: use of the urinary antigen assay. Community Based Pneumonia Incidence Study Group. Clin Infect Dis 20:1286–1291. [DOI] [PubMed] [Google Scholar]
- 315.Ngeow YF, Suwanjutha S, Chantarojanasriri T, Wang F, Saniel M, Alejandria M, Hsueh PR, Ping-Ing L, Park SC, Sohn JW, Aziah AM, Liu Y, Seto WH, Ngan CC, Hadiarto M, Hood A, Cheong YM. 2005. An Asian study on the prevalence of atypical respiratory pathogens in community-acquired pneumonia. Int J Infect Dis 9:144–153. doi: 10.1016/j.ijid.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 316.Ditommaso S, Giacomuzzi M, Gentile M, Zotti CM. 2008. Antibody detection and cross-reactivity among species and serogroups of Legionella by indirect immunofluorescence test. J Microbiol Methods 75:350–353. doi: 10.1016/j.mimet.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 317.Helbig JH, Kurtz JB, Pastoris MC, Pelaz C, Luck PC. 1997. Antigenic lipopolysaccharide components of Legionella pneumophila recognized by monoclonal antibodies: possibilities and limitations for division of the species into serogroups. J Clin Microbiol 35:2841–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Boswell TC, Marshall LE, Kudesia G. 1996. False-positive Legionella titres in routine clinical serology testing detected by absorption with campylobacter: implications for the serological diagnosis of Legionnaires' disease. J Infect 32:23–26. doi: 10.1016/S0163-4453(96)80005-3. [DOI] [PubMed] [Google Scholar]
- 319.Musso D, Raoult D. 1997. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol 4:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Raoult D, Dasch GA. 1995. Immunoblot cross-reactions among Rickettsia, Proteus spp and Legionella spp in patients with Mediterranean spotted fever. FEMS Immunol Med Microbiol 11:13–18. doi: 10.1111/j.1574-695X.1995.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 321.Joly JR, McKinney RM, Tobin JO, Bibb WF, Watkins ID, Ramsay D. 1986. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J Clin Microbiol 23:768–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Stout JE, Joly J, Para M, Plouffe J, Ciesielski C, Blaser MJ, Yu VL. 1988. Comparison of molecular methods for subtyping patients and epidemiologically linked environmental isolates of Legionella pneumophila. J Infect Dis 157:486–495. doi: 10.1093/infdis/157.3.486. [DOI] [PubMed] [Google Scholar]
- 323.Joly JR, Chen YY, Ramsay D. 1983. Serogrouping and subtyping of Legionella pneumophila with monoclonal antibodies. J Clin Microbiol 18:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nolte FS, Conlin CA, Roisin AJ, Redmond SR. 1984. Plasmids as epidemiological markers in nosocomial Legionnaires' disease. J Infect Dis 149:251–256. doi: 10.1093/infdis/149.2.251. [DOI] [PubMed] [Google Scholar]
- 325.van Ketel RJ, ter Schegget J, Zanen HC. 1984. Molecular epidemiology of Legionella pneumophila serogroup 1. J Clin Microbiol 20:362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Grimont PA, Grimont F, Desplaces N, Tchen P. 1985. DNA probe specific for Legionella pneumophila. J Clin Microbiol 21:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wilkinson HW, Sampson JS, Plikaytis BB. 1986. Evaluation of a commercial gene probe for identification of Legionella cultures. J Clin Microbiol 23:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Maiwald M, Schill M, Stockinger C, Helbig JH, Luck PC, Witzleb W, Sonntag HG. 1995. Detection of Legionella DNA in human and guinea pig urine samples by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis 14:25–33. doi: 10.1007/BF02112614. [DOI] [PubMed] [Google Scholar]
- 329.Lindsay DS, Abraham WH, Fallon RJ. 1994. Detection of mip gene by PCR for diagnosis of Legionnaires' disease. J Clin Microbiol 32:3068–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Matsiota-Bernard P, Pitsouni E, Legakis N, Nauciel C. 1994. Evaluation of commercial amplification kit for detection of Legionella pneumophila in clinical specimens. J Clin Microbiol 32:1503–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Koide M, Saito A, Kusano N, Higa F. 1993. Detection of Legionella spp. in cooling tower water by the polymerase chain reaction method. Appl Environ Microbiol 59:1943–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mahbubani MH, Bej AK, Miller R, Haff L, DiCesare J, Atlas RM. 1990. Detection of Legionella with polymerase chain reaction and gene probe methods. Mol Cell Probes 4:175–187. doi: 10.1016/0890-8508(90)90051-Z. [DOI] [PubMed] [Google Scholar]
- 333.Fry NK, Rowbotham TJ, Saunders NA, Embley TM. 1991. Direct amplification and sequencing of the 16S ribosomal DNA of an intracellular Legionella species recovered by amoebal enrichment from the sputum of a patient with pneumonia. FEMS Microbiol Lett 83:165–168. doi: 10.1111/j.1574-6968.1991.tb04434.x-i1. [DOI] [PubMed] [Google Scholar]
- 334.Jaulhac B, Nowicki M, Bornstein N, Meunier O, Prevost G, Piemont Y, Fleurette J, Monteil H. 1992. Detection of Legionella spp. in bronchoalveolar lavage fluids by DNA amplification. J Clin Microbiol 30:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Elverdal PL, Jorgensen CS, Krogfelt KA, Uldum SA. 2013. Two years' performance of an in-house ELISA for diagnosis of Legionnaires' disease: detection of specific IgM and IgG antibodies against Legionella pneumophila serogroup 1, 3 and 6 in human serum. J Microbiol Methods 94:94–97. doi: 10.1016/j.mimet.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 336.Mentasti M, Fry NK, Afshar B, Palepou-Foxley C, Naik FC, Harrison TG. 2012. Application of Legionella pneumophila-specific quantitative real-time PCR combined with direct amplification and sequence-based typing in the diagnosis and epidemiological investigation of Legionnaires' disease. Eur J Clin Microbiol Infect Dis 31:2017–2028. doi: 10.1007/s10096-011-1535-0. [DOI] [PubMed] [Google Scholar]
- 337.Stojek NM, Wojcik-Fatla A, Dutkiewicz J. 2012. Efficacy of the detection of Legionella in hot and cold water samples by culture and PCR. II. Examination of native samples from various sources. Ann Agric Environ Med 19:295–298. [PubMed] [Google Scholar]
- 338.Krojgaard LH, Krogfelt KA, Albrechtsen HJ, Uldum SA. 2011. Detection of Legionella by quantitative-polymerase chain reaction (qPCR) for monitoring and risk assessment. BMC Microbiol 11:254. doi: 10.1186/1471-2180-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lee JV, Lai S, Exner M, Lenz J, Gaia V, Casati S, Hartemann P, Luck C, Pangon B, Ricci ML, Scaturro M, Fontana S, Sabria M, Sanchez I, Assaf S, Surman-Lee S. 2011. An international trial of quantitative PCR for monitoring Legionella in artificial water systems. J Appl Microbiol 110:1032–1044. doi: 10.1111/j.1365-2672.2011.04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Merault N, Rusniok C, Jarraud S, Gomez-Valero L, Cazalet C, Marin M, Brachet E, Aegerter P, Gaillard JL, Etienne J, Herrmann JL, DELPH-I Study Group, Lawrence C, Buchrieser C. 2011. Specific real-time PCR for simultaneous detection and identification of Legionella pneumophila serogroup 1 in water and clinical samples. Appl Environ Microbiol 77:1708–1717. doi: 10.1128/AEM.02261-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Guillemet TA, Levesque B, Gauvin D, Brousseau N, Giroux JP, Cantin P. 2010. Assessment of real-time PCR for quantification of Legionella spp. in spa water. Lett Appl Microbiol 51:639–644. doi: 10.1111/j.1472-765X.2010.02947.x. [DOI] [PubMed] [Google Scholar]
- 342.Bonetta S, Bonetta S, Ferretti E, Balocco F, Carraro E. 2010. Evaluation of Legionella pneumophila contamination in Italian hotel water systems by quantitative real-time PCR and culture methods. J Appl Microbiol 108:1576–1583. doi: 10.1111/j.1365-2672.2009.04553.x. [DOI] [PubMed] [Google Scholar]
- 343.Maurin M, Hammer L, Gestin B, Timsit JF, Rogeaux O, Delavena F, Tous J, Epaulard O, Brion JP, Croize J. 2010. Quantitative real-time PCR tests for diagnostic and prognostic purposes in cases of legionellosis. Clin Microbiol Infect 16:379–384. doi: 10.1111/j.1469-0691.2009.02812.x. [DOI] [PubMed] [Google Scholar]
- 344.Nazarian EJ, Bopp DJ, Saylors A, Limberger RJ, Musser KA. 2008. Design and implementation of a protocol for the detection of Legionella in clinical and environmental samples. Diagn Microbiol Infect Dis 62:125–132. doi: 10.1016/j.diagmicrobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 345.Ta AC, Stout JE, Yu VL, Wagener MM. 1995. Comparison of culture methods for monitoring Legionella species in hospital potable water systems and recommendations for standardization of such methods. J Clin Microbiol 33:2118–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Roberts KP, August CM, Nelson JD Jr. 1987. Relative sensitivities of environmental legionellae to selective isolation procedures. Appl Environ Microbiol 53:2704–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wadowsky RM, Yee RB. 1981. Glycine-containing selective medium for isolation of Legionellaceae from environmental specimens. Appl Environ Microbiol 42:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ditommaso S, Gentile M, Giacomuzzi M, Zotti CM. 2011. Recovery of Legionella species from water samples using an internal method based on ISO 11731: suggestions for revision and implementation. Diagn Microbiol Infect Dis 70:200–206. doi: 10.1016/j.diagmicrobio.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 349.Palmer CJ, Tsai YL, Paszko-Kolva C, Mayer C, Sangermano LR. 1993. Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent-antibody, and plate culture methods. Appl Environ Microbiol 59:3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Bej AK, Mahbubani MH, Miller R, DiCesare JL, Haff L, Atlas RM. 1990. Multiplex PCR amplification and immobilized capture probes for detection of bacterial pathogens and indicators in water. Mol Cell Probes 4:353–365. doi: 10.1016/0890-8508(90)90026-V. [DOI] [PubMed] [Google Scholar]
- 351.Yanez MA, Carrasco-Serrano C, Barbera VM, Catalan V. 2005. Quantitative detection of Legionella pneumophila in water samples by immunomagnetic purification and real-time PCR amplification of the dotA gene. Appl Environ Microbiol 71:3433–3441. doi: 10.1128/AEM.71.7.3433-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ballard AL, Fry NK, Chan L, Surman SB, Lee JV, Harrison TG, Towner KJ. 2000. Detection of Legionella pneumophila using a real-time PCR hybridization assay. J Clin Microbiol 38:4215–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Hayden RT, Uhl JR, Qian X, Hopkins MK, Aubry MC, Limper AH, Lloyd RV, Cockerill FR. 2001. Direct detection of Legionella species from bronchoalveolar lavage and open lung biopsy specimens: comparison of LightCycler PCR, in situ hybridization, direct fluorescence antigen detection, and culture. J Clin Microbiol 39:2618–2626. doi: 10.1128/JCM.39.7.2618-2626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rantakokko-Jalava K, Jalava J. 2001. Development of conventional and real-time PCR assays for detection of Legionella DNA in respiratory specimens. J Clin Microbiol 39:2904–2910. doi: 10.1128/JCM.39.8.2904-2910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Herpers BL, de Jongh BM, van der Zwaluw K, van Hannen EJ. 2003. Real-time PCR assay targets the 23S-5S spacer for direct detection and differentiation of Legionella spp. and Legionella pneumophila. J Clin Microbiol 41:4815–4816. doi: 10.1128/JCM.41.10.4815-4816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhou G, Cao B, Dou Y, Liu Y, Feng L, Wang L. 2011. PCR methods for the rapid detection and identification of four pathogenic Legionella spp. and two Legionella pneumophila subspecies based on the gene amplification of gyrB. Appl Microbiol Biotechnol 91:777–787. doi: 10.1007/s00253-011-3283-6. [DOI] [PubMed] [Google Scholar]
- 357.Horng Y-T, Soo P-C, Shen B-J, Hung Y-L, Lo K-Y, Su H-P, Wei J-R, Hsieh S-C, Hsueh P-R, Lai H-C. 2006. Development of an improved PCR-ICT hybrid assay for direct detection of legionellae and Legionella pneumophila from cooling tower water specimens. Water Res 40:2221–2229. doi: 10.1016/j.watres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 358.Bencini MA, van den Brule AJ, Claas EC, Hermans MH, Melchers WJ, Noordhoek GT, Salimans MM, Schirm J, Vink C, van der Zee A, Jansen R. 2007. Multicenter comparison of molecular methods for detection of Legionella spp. in sputum samples. J Clin Microbiol 45:3390–3392. doi: 10.1128/JCM.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Benitez AJ, Winchell JM. 2013. Clinical application of a multiplex real-time PCR assay for simultaneous detection of Legionella species, Legionella pneumophila, and Legionella pneumophila serogroup 1. J Clin Microbiol 51:348–351. doi: 10.1128/JCM.02510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. 2011. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis 70:1–9. doi: 10.1016/j.diagmicrobio.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Ott M, Bender L, Marre R, Hacker J. 1991. Pulsed field electrophoresis of genomic restriction fragments for the detection of nosocomial Legionella pneumophila in hospital water supplies. J Clin Microbiol 29:813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Saunders NA, Harrison TG, Haththotuwa A, Taylor AG. 1991. A comparison of probes for restriction fragment length polymorphism (RFLP) typing of Legionella pneumophila serogroup 1 strains. J Med Microbiol 35:152–158. doi: 10.1099/00222615-35-3-152. [DOI] [PubMed] [Google Scholar]
- 363.Sandery M, Coble J, McKersie-Donnolley S. 1994. Random amplified polymorphic DNA (RAPD) profiling of Legionella pneumophila. Lett Appl Microbiol 19:184–187. doi: 10.1111/j.1472-765X.1994.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 364.Georghiou PR, Doggett AM, Kielhofner MA, Stout JE, Watson DA, Lupski JR, Hamill RJ. 1994. Molecular fingerprinting of Legionella species by repetitive element PCR. J Clin Microbiol 32:2989–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.van Belkum A, Struelens M, Quint W. 1993. Typing of Legionella pneumophila strains by polymerase chain reaction-mediated DNA fingerprinting. J Clin Microbiol 31:2198–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Riffard S, Lo Presti F, Vandenesch F, Forey F, Reyrolle M, Etienne J. 1998. Comparative analysis of infrequent-restriction-site PCR and pulsed-field gel electrophoresis for epidemiological typing of Legionella pneumophila serogroup 1 strains. J Clin Microbiol 36:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Valsangiacomo C, Baggi F, Gaia V, Balmelli T, Peduzzi R, Piffaretti JC. 1995. Use of amplified fragment length polymorphism in molecular typing of Legionella pneumophila and application to epidemiological studies. J Clin Microbiol 33:1716–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Aye T, Wachsmuth K, Feeley JC, Gibson RJ, Johnson SR. 1981. Plasmid profiles of Legionella species. Curr Microbiol 6:389–394. doi: 10.1007/BF01567017. [DOI] [Google Scholar]
- 369.Maher WE, Plouffe JF, Para MF. 1983. Plasmid profiles of clinical and environmental isolates of Legionella pneumophila serogroup 1. J Clin Microbiol 18:1422–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ratcliff RM. 2013. Sequence-based identification of Legionella. Methods Mol Biol 954:57–72. doi: 10.1007/978-1-62703-161-5_3. [DOI] [PubMed] [Google Scholar]
- 371.Ratcliff RM, Lanser JA, Manning PA, Heuzenroeder MW. 1998. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J Clin Microbiol 36:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Ginevra C, Jacotin N, Diancourt L, Guigon G, Arquilliere R, Meugnier H, Descours G, Vandenesch F, Etienne J, Lina G, Caro V, Jarraud S. 2012. Legionella pneumophila sequence type 1/Paris pulsotype subtyping by spoligotyping. J Clin Microbiol 50:696–701. doi: 10.1128/JCM.06180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ratzow S, Gaia V, Helbig JH, Fry NK, Luck PC. 2007. Addition of neuA, the gene encoding N-acylneuraminate cytidylyl transferase, increases the discriminatory ability of the consensus sequence-based scheme for typing Legionella pneumophila serogroup 1 strains. J Clin Microbiol 45:1965–1968. doi: 10.1128/JCM.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Scaturro M, Losardo M, De Ponte G, Ricci ML. 2005. Comparison of three molecular methods used for subtyping of Legionella pneumophila strains isolated during an epidemic of legionellosis in Rome. J Clin Microbiol 43:5348–5350. doi: 10.1128/JCM.43.10.5348-4350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Coscolla M, Gonzalez-Candelas F. 2009. Direct sequencing of Legionella pneumophila from respiratory samples for sequence-based typing analysis. J Clin Microbiol 47:2901–2905. doi: 10.1128/JCM.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Ginevra C, Lopez M, Forey F, Reyrolle M, Meugnier H, Vandenesch F, Etienne J, Jarraud S, Molmeret M. 2009. Evaluation of a nested-PCR-derived sequence-based typing method applied directly to respiratory samples from patients with Legionnaires' disease. J Clin Microbiol 47:981–987. doi: 10.1128/JCM.02071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Luck PC, Ecker C, Reischl U, Linde HJ, Stempka R. 2007. Culture-independent identification of the source of an infection by direct amplification and sequencing of Legionella pneumophila DNA from a clinical specimen. J Clin Microbiol 45:3143–3144. doi: 10.1128/JCM.00685-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Scaturro M, Fontana S, Ricci ML. 2011. Use of nested polymerase chain reaction based on sequence-based typing of clinical samples to determine the source of infection for hospital-acquired Legionnaires' disease. Infect Control Hosp Epidemiol 32:510–512. doi: 10.1086/659785. [DOI] [PubMed] [Google Scholar]
- 379.Diederen BM, de Jong CM, Marmouk F, Kluytmans JA, Peeters MF, Van der Zee A. 2007. Evaluation of real-time PCR for the early detection of Legionella pneumophila DNA in serum samples. J Med Microbiol 56:94–101. doi: 10.1099/jmm.0.46714-0. [DOI] [PubMed] [Google Scholar]
- 380.Murdoch DR, Walford EJ, Jennings LC, Light GJ, Schousboe MI, Chereshsky AY, Chambers ST, Town GI. 1996. Use of the polymerase chain reaction to detect Legionella DNA in urine and serum samples from patients with pneumonia. Clin Infect Dis 23:475–480. doi: 10.1093/clinids/23.3.475. [DOI] [PubMed] [Google Scholar]
- 381.Socan M, Kese D, Marinic-Fiser N. 2000. Polymerase chain reaction for detection of legionellae DNA in urine samples from patients with community-acquired pneumonia. Folia Microbiol (Praha) 45:469–472. doi: 10.1007/BF02817623. [DOI] [PubMed] [Google Scholar]
- 382.Alexiou-Daniel S, Stylianakis A, Papoutsi A, Zorbas I, Papa A, Lambropoulos AF, Antoniadis A. 1998. Application of polymerase chain reaction for detection of Legionella pneumophila in serum samples. Clin Microbiol Infect 4:144–148. doi: 10.1111/j.1469-0691.1998.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 383.Helbig JH, Engelstadter T, Maiwald M, Uldum SA, Witzleb W, Luck PC. 1999. Diagnostic relevance of the detection of Legionella DNA in urine samples by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis 18:716–722. doi: 10.1007/s100960050384. [DOI] [PubMed] [Google Scholar]
- 384.Touron-Bodilis A, Pougnard C, Frenkiel-Lebosse H, Hallier-Soulier S. 2011. Usefulness of real-time PCR as a complementary tool to the monitoring of Legionella spp. and Legionella pneumophila by culture in industrial cooling systems. J Appl Microbiol 111:499–510. doi: 10.1111/j.1365-2672.2011.05063.x. [DOI] [PubMed] [Google Scholar]
- 385.Chang B, Sugiyama K, Taguri T, Amemura-Maekawa J, Kura F, Watanabe H. 2009. Specific detection of viable Legionella cells by combined use of photoactivated ethidium monoazide and PCR/real-time PCR. Appl Environ Microbiol 75:147–153. doi: 10.1128/AEM.00604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Leskela T, Tilsala-Timisjarvi A, Kusnetsov J, Neubauer P, Breitenstein A. 2005. Sensitive genus-specific detection of Legionella by a 16S rRNA based sandwich hybridization assay. J Microbiol Methods 62:167–179. doi: 10.1016/j.mimet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 387.Bej AK, Mahbubani MH, Atlas RM. 1991. Detection of viable Legionella pneumophila in water by polymerase chain reaction and gene probe methods. Appl Environ Microbiol 57:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kumar S, Wang L, Fan J, Kraft A, Bose ME, Tiwari S, Van Dyke M, Haigis R, Luo T, Ghosh M, Tang H, Haghnia M, Mather EL, Weisburg WG, Henrickson KJ. 2008. Detection of 11 common viral and bacterial pathogens causing community-acquired pneumonia or sepsis in asymptomatic patients by using a multiplex reverse transcription-PCR assay with manual (enzyme hybridization) or automated (electronic microarray) detection. J Clin Microbiol 46:3063–3072. doi: 10.1128/JCM.00625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Yanez MA, Nocker A, Soria-Soria E, Murtula R, Martinez L, Catalan V. 2011. Quantification of viable Legionella pneumophila cells using propidium monoazide combined with quantitative PCR. J Microbiol Methods 85:124–130. doi: 10.1016/j.mimet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 390.Delgado-Viscogliosi P, Solignac L, Delattre JM. 2009. Viability PCR, a culture-independent method for rapid and selective quantification of viable Legionella pneumophila cells in environmental water samples. Appl Environ Microbiol 75:3502–3512. doi: 10.1128/AEM.02878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Al-Bana BH, Haddad MT, Garduno RA. 2014. Stationary phase and mature infectious forms of Legionella pneumophila produce distinct viable but non-culturable cells. Environ Microbiol 16:382–395. doi: 10.1111/1462-2920.12219. [DOI] [PubMed] [Google Scholar]
- 392.Allegra S, Berger F, Berthelot P, Grattard F, Pozzetto B, Riffard S. 2008. Use of flow cytometry to monitor Legionella viability. Appl Environ Microbiol 74:7813–7816. doi: 10.1128/AEM.01364-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Alleron L, Merlet N, Lacombe C, Frere J. 2008. Long-term survival of Legionella pneumophila in the viable but nonculturable state after monochloramine treatment. Curr Microbiol 57:497–502. doi: 10.1007/s00284-008-9275-9. [DOI] [PubMed] [Google Scholar]
- 394.Garcia MT, Jones S, Pelaz C, Millar RD, Abu Kwaik Y. 2007. Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ Microbiol 9:1267–1277. doi: 10.1111/j.1462-2920.2007.01245.x. [DOI] [PubMed] [Google Scholar]
- 395.Kern M, Bohm S, Deml L, Wolf H, Reischl U, Niller HH. 2009. Inhibition of Legionella pneumophila PCR in respiratory samples: a quantitative approach. J Microbiol Methods 79:189–193. doi: 10.1016/j.mimet.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 396.Chang CC, Chen CC, Wei SC, Lu HH, Liang YH, Lin CW. 2012. Diagnostic devices for isothermal nucleic acid amplification. Sensors (Basel) 12:8319–8337. doi: 10.3390/s120608319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Craw P, Balachandran W. 2012. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 12:2469–2486. doi: 10.1039/c2lc40100b. [DOI] [PubMed] [Google Scholar]
- 398.Loens K, Beck T, Goossens H, Ursi D, Overdijk M, Sillekens P, Ieven M. 2006. Development of conventional and real-time NASBA for the detection of Legionella species in respiratory specimens. J Microbiol Methods 67:408–415. doi: 10.1016/j.mimet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 399.Loens K, Beck T, Ursi D, Overdijk M, Sillekens P, Goossens H, Ieven M. 2008. Development of real-time multiplex nucleic acid sequence-based amplification for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella spp. in respiratory specimens. J Clin Microbiol 46:185–191. doi: 10.1128/JCM.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Chen Y, Tateda K, Fujita K, Ishii T, Ishii Y, Kimura S, Saga T, Annaka T, Yamada S, Zhao L, Li S, Azuma A, Gemma A, Kudoh S, Yamaguchi K. 2010. Sequential changes of Legionella antigens and bacterial load in the lungs and urines of a mouse model of pneumonia. Diagn Microbiol Infect Dis 66:253–260. doi: 10.1016/j.diagmicrobio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 401.Lu X, Mo Z-Y, Zhao H-B, Yan H, Shi L. 2011. LAMP-based method for a rapid identification of Legionella spp. and Legionella pneumophila. Appl Microbiol Biotechnol 92:179–187. doi: 10.1007/s00253-011-3496-8. [DOI] [PubMed] [Google Scholar]
- 402.Annaka T. 2003. Rapid and simple detection of Legionella species by LAMP, a new DNA amplification method. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 14:25–30. (In Japanese.) [PubMed] [Google Scholar]
- 403.Kievits T, van Gemen B, van Strijp D, Schukkink R, Dircks M, Adriaanse H, Malek L, Sooknanan R, Lens P. 1991. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods 35:273–286. doi: 10.1016/0166-0934(91)90069-C. [DOI] [PubMed] [Google Scholar]
- 404.Mainka C, Fuss B, Geiger H, Hofelmayr H, Wolff MH. 1998. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol 56:91–98. doi:. [DOI] [PubMed] [Google Scholar]
- 405.Wacharapluesadee S, Hemachudha T. 2001. Nucleic-acid sequence based amplification in the rapid diagnosis of rabies. Lancet 358:892–893. doi: 10.1016/S0140-6736(01)06041-X. [DOI] [PubMed] [Google Scholar]
- 406.van der Vliet GM, Schukkink RA, van Gemen B, Schepers P, Klatser PR. 1993. Nucleic acid sequence-based amplification (NASBA) for the identification of mycobacteria. J Gen Microbiol 139:2423–2429. doi: 10.1099/00221287-139-10-2423. [DOI] [PubMed] [Google Scholar]
- 407.Uyttendaele M, Schukkink R, van Gemen B, Debevere J. 1994. Identification of Campylobacter jejuni, Campylobacter coli and Campylobacter lari by the nucleic acid amplification system NASBAR. J Appl Bacteriol 77:694–701. doi: 10.1111/j.1365-2672.1994.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 408.Spears PA, Linn CP, Woodard DL, Walker GT. 1997. Simultaneous strand displacement amplification and fluorescence polarization detection of Chlamydia trachomatis DNA. Anal Biochem 247:130–137. doi: 10.1006/abio.1997.2043. [DOI] [PubMed] [Google Scholar]
- 409.Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. 2003. Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol 41:5517–5524. doi: 10.1128/JCM.41.12.5517-5524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ikadai H, Tanaka H, Shibahara N, Matsuu A, Uechi M, Itoh N, Oshiro S, Kudo N, Igarashi I, Oyamada T. 2004. Molecular evidence of infections with Babesia gibsoni parasites in Japan and evaluation of the diagnostic potential of a loop-mediated isothermal amplification method. J Clin Microbiol 42:2465–2469. doi: 10.1128/JCM.42.6.2465-2469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS. 2006. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem 52:303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]
- 412.Steg A, Wang W, Blanquicett C, Grunda JM, Eltoum IA, Wang K, Buchsbaum DJ, Vickers SM, Russo S, Diasio RB, Frost AR, LoBuglio AF, Grizzle WE, Johnson MR. 2006. Multiple gene expression analyses in paraffin-embedded tissues by TaqMan low-density array: application to hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J Mol Diagn 8:76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Krupinski J, Turu MM, Martinez-Gonzalez J, Carvajal A, Juan-Babot JO, Iborra E, Slevin M, Rubio F, Badimon L. 2006. Endogenous expression of C-reactive protein is increased in active (ulcerated noncomplicated) human carotid artery plaques. Stroke 37:1200–1204. doi: 10.1161/01.STR.0000217386.37107.be. [DOI] [PubMed] [Google Scholar]
- 414.Jiang Z, Hu J, Li X, Jiang Y, Zhou W, Lu D. 2006. Expression analyses of 27 DNA repair genes in astrocytoma by TaqMan low-density array. Neurosci Lett 409:112–117. doi: 10.1016/j.neulet.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 415.Steinbach D, Schramm A, Eggert A, Onda M, Dawczynski K, Rump A, Pastan I, Wittig S, Pfaffendorf N, Voigt A, Zintl F, Gruhn B. 2006. Identification of a set of seven genes for the monitoring of minimal residual disease in pediatric acute myeloid leukemia. Clin Cancer Res 12:2434–2441. doi: 10.1158/1078-0432.CCR-05-2552. [DOI] [PubMed] [Google Scholar]
- 416.Gallagher WM, Bergin OE, Rafferty M, Kelly ZD, Nolan IM, Fox EJ, Culhane AC, McArdle L, Fraga MF, Hughes L, Currid CA, O'Mahony F, Byrne A, Murphy AA, Moss C, McDonnell S, Stallings RL, Plumb JA, Esteller M, Brown R, Dervan PA, Easty DJ. 2005. Multiple markers for melanoma progression regulated by DNA methylation: insights from transcriptomic studies. Carcinogenesis 26:1856–1867. doi: 10.1093/carcin/bgi152. [DOI] [PubMed] [Google Scholar]
- 417.Diaz MH, Waller JL, Napoliello RA, Islam MS, Wolff BJ, Burken DJ, Holden RL, Srinivasan V, Arvay M, McGee L, Oberste MS, Whitney CG, Schrag SJ, Winchell JM, Saha SK. 2013. Optimization of multiple pathogen detection using the TaqMan Array Card: application for a population-based study of neonatal infection. PLoS One 8:e66183. doi: 10.1371/journal.pone.0066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Kodani M, Winchell JM. 2012. Engineered combined-positive-control template for real-time reverse transcription-PCR in multiple-pathogen-detection assays. J Clin Microbiol 50:1057–1060. doi: 10.1128/JCM.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Cieslak PR, Britt AS, Hicks L, Conklin L, Van Beneden C, Garrison L, Winchell JM, Schneider E, Erdman D, Fry AM, Jain S, Uyeki T, Finelli L, Lindstrom SL, Clark TA, Tondella ML, Shieh WJ, Zaki SR, Flemming-Dutra KE. 2012. Unexplained Respiratory Disease Outbreak Working Group activities—worldwide, March 2007-September 2011. MMWR Morb Mortal Wkly Rep 61:480–483. [PubMed] [Google Scholar]
- 420.Weinberg GA, Schnabel KC, Erdman DD, Prill MM, Iwane MK, Shelley LM, Whitaker BL, Szilagyi PG, Hall CB. 2013. Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol 57:254–260. doi: 10.1016/j.jcv.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Conklin L, Adjemian J, Loo J, Mandal S, Davis C, Parks S, Parsons T, McDonough B, Partida J, Thurman K, Diaz MH, Benitez A, Pondo T, Whitney CG, Winchell JM, Kendig N, Van Beneden C. 2013. Investigation of a Chlamydia pneumoniae outbreak in a federal correctional facility in Texas. Clin Infect Dis 57:639–647. doi: 10.1093/cid/cit357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Weller SA, Cox V, Essex-Lopresti A, Hartley MG, Parsons TM, Rachwal PA, Stapleton HL, Lukaszewski RA. 2012. Evaluation of two multiplex real-time PCR screening capabilities for the detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis in blood samples generated from murine infection models. J Med Microbiol 61:1546–1555. doi: 10.1099/jmm.0.049007-0. [DOI] [PubMed] [Google Scholar]
- 424.Rachwal PA, Rose HL, Cox V, Lukaszewski RA, Murch AL, Weller SA. 2012. The potential of TaqMan Array Cards for detection of multiple biological agents by real-time PCR. PLoS One 7:e35971. doi: 10.1371/journal.pone.0035971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Clark AE, Kaleta EJ, Arora A, Wolk DM. 2013. Matrix-assisted laser desorption ionization–time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev 26:547–603. doi: 10.1128/CMR.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Fisher-Hoch S, Hudson MJ, Thompson MH. 1979. Identification of a clinical isolate as Legionella pneumophila by gas chromatography and mass spectrometry of cellular fatty acids. Lancet ii:323–325. [DOI] [PubMed] [Google Scholar]
- 427.Moliner C, Ginevra C, Jarraud S, Flaudrops C, Bedotto M, Couderc C, Etienne J, Fournier PE. 2010. Rapid identification of Legionella species by mass spectrometry. J Med Microbiol 59:273–284. doi: 10.1099/jmm.0.014100-0. [DOI] [PubMed] [Google Scholar]
- 428.Fujinami Y, Kikkawa HS, Kurosaki Y, Sakurada K, Yoshino M, Yasuda J. 2011. Rapid discrimination of Legionella by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Microbiol Res 166:77–86. doi: 10.1016/j.micres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 429.Gaia V, Casati S, Tonolla M. 2011. Rapid identification of Legionella spp. by MALDI-TOF MS based protein mass fingerprinting. Syst Appl Microbiol 34:40–44. doi: 10.1016/j.syapm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 430.Pennanec X, Dufour A, Haras D, Rehel K. 2010. A quick and easy method to identify bacteria by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 24:384–392. doi: 10.1002/rcm.4404. [DOI] [PubMed] [Google Scholar]
- 431.He Y, Chang TC, Li H, Shi G, Tang YW. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry and database for identification of Legionella species. Can J Microbiol 57:533–538. doi: 10.1139/w11-039. [DOI] [PubMed] [Google Scholar]
- 432.Sethi S, Gore MT, Sethi KK. 2007. Increased sensitivity of a direct fluorescent antibody test for Legionella pneumophila in bronchoalveolar lavage samples by immunomagnetic separation based on BioMags. J Microbiol Methods 70:328–335. doi: 10.1016/j.mimet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 433.Fuchslin HP, Kotzsch S, Keserue HA, Egli T. 2010. Rapid and quantitative detection of Legionella pneumophila applying immunomagnetic separation and flow cytometry. Cytometry A 77:264–274. doi: 10.1002/cyto.a.20858. [DOI] [PubMed] [Google Scholar]
- 434.Allegra S, Girardot F, Grattard F, Berthelot P, Helbig JH, Pozzetto B, Riffard S. 2011. Evaluation of an immunomagnetic separation assay in combination with cultivation to improve Legionella pneumophila serogroup 1 recovery from environmental samples. J Appl Microbiol 110:952–961. doi: 10.1111/j.1365-2672.2011.04955.x. [DOI] [PubMed] [Google Scholar]
- 435.Reidt U, Geisberger B, Heller C, Friedberger A. 2011. Automated immunomagnetic processing and separation of Legionella pneumophila with manual detection by sandwich ELISA and PCR amplification of the ompS gene. J Lab Autom 16:157–164. doi: 10.1016/j.jala.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 436.Keserue HA, Baumgartner A, Felleisen R, Egli T. 2012. Rapid detection of total and viable Legionella pneumophila in tap water by immunomagnetic separation, double fluorescent staining and flow cytometry. Microb Biotechnol 5:753–763. doi: 10.1111/j.1751-7915.2012.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Rodriguez Albalat G, Bedrina Broch B, Jimenez Bono M. 2012. Validation of the Legipid Bioalarm Legionella assay. J AOAC Int 95:1440–1451. doi: 10.5740/jaoacint.12-146. [DOI] [PubMed] [Google Scholar]
- 438.Bedrina B, Macian S, Solis I, Fernandez-Lafuente R, Baldrich E, Rodriguez G. 2013. Fast immunosensing technique to detect Legionella pneumophila in different natural and anthropogenic environments: comparative and collaborative trials. BMC Microbiol 13:88. doi: 10.1186/1471-2180-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ririe KM, Rasmussen RP, Wittwer CT. 1997. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem 245:154–160. doi: 10.1006/abio.1996.9916. [DOI] [PubMed] [Google Scholar]
- 440.Lay MJ, Wittwer CT. 1997. Real-time fluorescence genotyping of factor V Leiden during rapid-cycle PCR. Clin Chem 43:2262–2267. [PubMed] [Google Scholar]
- 441.Bernard PS, Ajioka RS, Kushner JP, Wittwer CT. 1998. Homogeneous multiplex genotyping of hemochromatosis mutations with fluorescent hybridization probes. Am J Pathol 153:1055–1061. doi: 10.1016/S0002-9440(10)65650-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Germer S, Higuchi R. 1999. Single-tube genotyping without oligonucleotide probes. Genome Res 9:72–78. [PMC free article] [PubMed] [Google Scholar]
- 443.Gundry CN, Bernard PS, Herrmann MG, Reed GH, Wittwer CT. 1999. Rapid F508del and F508C assay using fluorescent hybridization probes. Genet Test 3:365–370. doi: 10.1089/gte.1999.3.365. [DOI] [PubMed] [Google Scholar]
- 444.Knez K, Janssen KP, Pollet J, Spasic D, Lammertyn J. 2012. Fiber-optic high-resolution genetic screening using gold-labeled gene probes. Small 8:868–872. doi: 10.1002/smll.201102209. [DOI] [PubMed] [Google Scholar]
- 445.van Blerk GN, Leibach L, Mabunda A, Chapman A, Louw D. 2011. Rapid and specific detection of Salmonella in water samples using real-time PCR and high resolution melt (HRM) curve analysis. Water Sci Technol 64(12):2453–2459. doi: 10.2166/wst.2011.838. [DOI] [PubMed] [Google Scholar]
- 446.Nasereddin A, Jaffe CL. 2010. Rapid diagnosis of Old World leishmaniasis by high-resolution melting analysis of the 7SL RNA gene. J Clin Microbiol 48:2240–2242. doi: 10.1128/JCM.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Chan WF, Maharjan RP, Reeves PR, Sintchenko V, Gilbert GL, Lan R. 2009. Rapid and accurate typing of Bordetella pertussis targeting genes encoding acellular vaccine antigens using real time PCR and high resolution melt analysis. J Microbiol Methods 77:326–329. doi: 10.1016/j.mimet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 448.Hadfield SJ, Chalmers RM. 2012. Detection and characterization of Cryptosporidium cuniculus by real-time PCR. Parasitol Res 111:1385–1390. doi: 10.1007/s00436-012-2874-1. [DOI] [PubMed] [Google Scholar]
- 449.Ajitkumar P, Barkema HW, Zadoks RN, Morck DW, van der Meer FJ, De Buck J. 2013. High-resolution melt analysis for species identification of coagulase-negative staphylococci derived from bovine milk. Diagn Microbiol Infect Dis 75:227–234. doi: 10.1016/j.diagmicrobio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 450.Grando D, Said MM, Mayall BC, Gurtler V. 2012. High resolution melt analysis to track infections due to ribotype 027 Clostridium difficile. J Microbiol Methods 89:87–94. doi: 10.1016/j.mimet.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 451.Winchell JM, Wolff BJ, Tiller R, Bowen MD, Hoffmaster AR. 2010. Rapid identification and discrimination of Brucella isolates by use of real-time PCR and high-resolution melt analysis. J Clin Microbiol 48:697–702. doi: 10.1128/JCM.02021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Ramirez MV, Cowart KC, Campbell PJ, Morlock GP, Sikes D, Winchell JM, Posey JE. 2010. Rapid detection of multidrug-resistant Mycobacterium tuberculosis by use of real-time PCR and high-resolution melt analysis. J Clin Microbiol 48:4003–4009. doi: 10.1128/JCM.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Robertson T, Bibby S, O'Rourke D, Belfiore T, Lambie H, Noormohammadi AH. 2009. Characterization of Chlamydiaceae species using PCR and high resolution melt curve analysis of the 16S rRNA gene. J Appl Microbiol 107:2017–2028. doi: 10.1111/j.1365-2672.2009.04388.x. [DOI] [PubMed] [Google Scholar]
- 454.Twin J, Stevens MP, Garland SM, Zaia AM, Tabrizi SN. 2012. Rapid determination of lymphogranuloma venereum serovars of Chlamydia trachomatis by quantitative high-resolution melt analysis (HRMA). J Clin Microbiol 50:3751–3753. doi: 10.1128/JCM.01670-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Li SL, Sun HM, Zhao HQ, Cao L, Yuan Y, Feng YL, Xue GH. 2012. A single tube modified allele-specific-PCR for rapid detection of erythromycin-resistant Mycoplasma pneumoniae in Beijing. Chin Med J (Engl) 125:2671–2676. [PubMed] [Google Scholar]
- 456.Mitchell SL, Wolff BJ, Thacker WL, Ciembor PG, Gregory CR, Everett KD, Ritchie BW, Winchell JM. 2009. Genotyping of Chlamydophila psittaci by real-time PCR and high-resolution melt analysis. J Clin Microbiol 47:175–181. doi: 10.1128/JCM.01851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wolff BJ, Thacker WL, Schwartz SB, Winchell JM. 2008. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high-resolution melt analysis. Antimicrob Agents Chemother 52:3542–3549. doi: 10.1128/AAC.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Schwartz SB, Mitchell SL, Thurman KA, Wolff BJ, Winchell JM. 2009. Identification of P1 variants of Mycoplasma pneumoniae by use of high-resolution melt analysis. J Clin Microbiol 47:4117–4120. doi: 10.1128/JCM.01696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Schwartz SB, Thurman KA, Mitchell SL, Wolff BJ, Winchell JM. 2009. Genotyping of Mycoplasma pneumoniae isolates using real-time PCR and high-resolution melt analysis. Clin Microbiol Infect 15:756–762. doi: 10.1111/j.1469-0691.2009.02814.x. [DOI] [PubMed] [Google Scholar]
- 460.Giglio S, Monis PT, Saint CP. 2005. Legionella confirmation using real-time PCR and SYTO9 is an alternative to current methodology. Appl Environ Microbiol 71:8944–8948. doi: 10.1128/AEM.71.12.8944-8948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Knez K, Janssen KPF, Spasic D, Declerck P, Vanysacker L, Denis C, Tran DT, Lammertyn J. 2013. Spherical nucleic acid enhanced FO-SPR DNA melting for detection of mutations in Legionella pneumophila. Anal Chem 85:1734–1742. doi: 10.1021/ac303008f. [DOI] [PubMed] [Google Scholar]
- 462.Reischl U, Linde HJ, Lehn N, Landt O, Barratt K, Wellinghausen N. 2002. Direct detection and differentiation of Legionella spp. and Legionella pneumophila in clinical specimens by dual-color real-time PCR and melting curve analysis. J Clin Microbiol 40:3814–3817. doi: 10.1128/JCM.40.10.3814-3817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Stolhaug A, Bergh K. 2006. Identification and differentiation of Legionella pneumophila and Legionella spp. with real-time PCR targeting the 16S rRNA gene and species identification by mip sequencing. Appl Environ Microbiol 72:6394–6398. doi: 10.1128/AEM.02839-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Gilbert GL, Sintchenko V. 2013. The use of mycobacterial interspersed repetitive unit typing and whole genome sequencing to inform tuberculosis prevention and control activities. N S W Public Health Bull 24:10–14. doi: 10.1071/NB12106. [DOI] [PubMed] [Google Scholar]
- 465.Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Moller N, Aarestrup FM. 2014. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol 52:139–146. doi: 10.1128/JCM.02452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Long SW, Williams D, Valson C, Cantu CC, Cernoch P, Musser JM, Olsen RJ. 2013. A genomic day in the life of a clinical microbiology laboratory. J Clin Microbiol 51:1272–1277. doi: 10.1128/JCM.03237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Cody AJ, McCarthy ND, Jansen van Rensburg M, Isinkaye T, Bentley SD, Parkhill J, Dingle KE, Bowler IC, Jolley KA, Maiden MC. 2013. Real-time genomic epidemiological evaluation of human Campylobacter isolates by use of whole-genome multilocus sequence typing. J Clin Microbiol 51:2526–2534. doi: 10.1128/JCM.00066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Major E, Rigo K, Hague T, Berces A, Juhos S. 2013. HLA typing from 1000 genomes whole genome and whole exome Illumina data. PLoS One 8:e78410. doi: 10.1371/journal.pone.0078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Jolley KA, Hill DM, Bratcher HB, Harrison OB, Feavers IM, Parkhill J, Maiden MC. 2012. Resolution of a meningococcal disease outbreak from whole-genome sequence data with rapid Web-based analysis methods. J Clin Microbiol 50:3046–3053. doi: 10.1128/JCM.01312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Barzon L, Pacenti M, Franchin E, Lavezzo E, Masi G, Squarzon L, Pagni S, Toppo S, Russo F, Cattai M, Cusinato R, Palu G. 2013. Whole genome sequencing and phylogenetic analysis of West Nile virus lineage 1 and lineage 2 from human cases of infection, Italy, August 2013. Euro Surveill 18(38):pii=20591 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20591. [DOI] [PubMed] [Google Scholar]
- 472.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Croucher NJ, Finkelstein JA, Pelton SI, Mitchell PK, Lee GM, Parkhill J, Bentley SD, Hanage WP, Lipsitch M. 2013. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet 45:656–663. doi: 10.1038/ng.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Didelot X, Eyre DW, Cule M, Ip CL, Ansari MA, Griffiths D, Vaughan A, O'Connor L, Golubchik T, Batty EM, Piazza P, Wilson DJ, Bowden R, Donnelly PJ, Dingle KE, Wilcox M, Walker AS, Crook DW, Peto TEA, Harding RM. 2012. Microevolutionary analysis of Clostridium difficile genomes to investigate transmission. Genome Biol 13:R118. doi: 10.1186/gb-2012-13-12-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Harrison EM, Paterson GK, Holden MT, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Bak Zeuthen A, Heltberg O, Harris SR, Zadoks RN, Parkhill J, Peacock SJ, Holmes MA. 2013. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med 5:509–515. doi: 10.1002/emmm.201202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Lavezzo E, Toppo S, Franchin E, Di Camillo B, Finotello F, Falda M, Manganelli R, Palu G, Barzon L. 2013. Genomic comparative analysis and gene function prediction in infectious diseases: application to the investigation of a meningitis outbreak. BMC Infect Dis 13:554. doi: 10.1186/1471-2334-13-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Price JR, Golubchik T, Cole K, Wilson DJ, Crook DW, Thwaites GE, Bowden R, Walker AS, Peto TE, Paul J, Llewelyn MJ. 2014. Whole-genome sequencing shows that patient-to-patient transmission rarely accounts for acquisition of Staphylococcus aureus on an intensive care unit. Clin Infect Dis 58:609–618. doi: 10.1093/cid/cit807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-beta-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 4(6):e00377–13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Underwood AP, Jones G, Mentasti M, Fry NK, Harrison TG. 2013. Comparison of the Legionella pneumophila population structure as determined by sequence-based typing and whole genome sequencing. BMC Microbiol 13:302. doi: 10.1186/1471-2180-13-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Van Immerseel F, Studholme DJ, Eeckhaut V, Heyndrickx M, Dewulf J, Dewaele I, Van Hoorebeke S, Haesebrouck F, Van Meirhaeghe H, Ducatelle R, Paszkiewicz K, Titball RW. 2013. Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine 31:4940–4945. doi: 10.1016/j.vaccine.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 481.Vaughan G, Xia G, Forbi JC, Purdy MA, Rossi LM, Spradling PR, Khudyakov YE. 2013. Genetic relatedness among hepatitis A virus strains associated with food-borne outbreaks. PLoS One 8:e74546. doi: 10.1371/journal.pone.0074546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Gardy JL, Johnston JC, Ho Sui SJ, Cook VJ, Shah L, Brodkin E, Rempel S, Moore R, Zhao Y, Holt R, Varhol R, Birol I, Lem M, Sharma MK, Elwood K, Jones SJ, Brinkman FS, Brunham RC, Tang P. 2011. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med 364:730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 483.Grad YH, Waldor MK. 2013. Deciphering the origins and tracking the evolution of cholera epidemics with whole-genome-based molecular epidemiology. mBio 4(5):e00670–13. doi: 10.1128/mBio.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Koser CU, Holden MT, Ellington MJ, Cartwright EJ, Brown NM, Ogilvy-Stuart AL, Hsu LY, Chewapreecha C, Croucher NJ, Harris SR, Sanders M, Enright MC, Dougan G, Bentley SD, Parkhill J, Fraser LJ, Betley JR, Schulz-Trieglaff OB, Smith GP, Peacock SJ. 2012. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med 366:2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Loman NJ, Constantinidou C, Christner M, Rohde H, Chan JZ, Quick J, Weir JC, Quince C, Smith GP, Betley JR, Aepfelbacher M, Pallen MJ. 2013. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli O104:H4. JAMA 309:1502–1510. doi: 10.1001/jama.2013.3231. [DOI] [PubMed] [Google Scholar]
- 486.Mather AE, Reid SW, Maskell DJ, Parkhill J, Fookes MC, Harris SR, Brown DJ, Coia JE, Mulvey MR, Gilmour MW, Petrovska L, de Pinna E, Kuroda M, Akiba M, Izumiya H, Connor TR, Suchard MA, Lemey P, Mellor DJ, Haydon DT, Thomson NR. 2013. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science 341:1514–1517. doi: 10.1126/science.1240578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.McDonnell J, Dallman T, Atkin S, Turbitt DA, Connor TR, Grant KA, Thomson NR, Jenkins C. 2013. Retrospective analysis of whole genome sequencing compared to prospective typing data in further informing the epidemiological investigation of an outbreak of Shigella sonnei in the UK. Epidemiol Infect 141:2568–2575. doi: 10.1017/S0950268813000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, NISC Comparative Sequencing Program Group, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Chen YT, Peng HL, Shia WC, Hsu FR, Ken CF, Tsao YM, Chen CH, Liu CE, Hsieh MF, Chen HC, Tang CY, Ku TH. 2012. Whole-genome sequencing and identification of Morganella morganii KT pathogenicity-related genes. BMC Genomics 13(Suppl 7):S4. doi: 10.1186/1471-2164-13-S7-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Clark TG, Mallard K, Coll F, Preston M, Assefa S, Harris D, Ogwang S, Mumbowa F, Kirenga B, O'Sullivan DM, Okwera A, Eisenach KD, Joloba M, Bentley SD, Ellner JJ, Parkhill J, Jones-Lopez EC, McNerney R. 2013. Elucidating emergence and transmission of multidrug-resistant tuberculosis in treatment experienced patients by whole genome sequencing. PLoS One 8:e83012. doi: 10.1371/journal.pone.0083012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Farhat MR, Shapiro BJ, Kieser KJ, Sultana R, Jacobson KR, Victor TC, Warren RM, Streicher EM, Calver A, Sloutsky A, Kaur D, Posey JE, Plikaytis B, Oggioni MR, Gardy JL, Johnston JC, Rodrigues M, Tang PK, Kato-Maeda M, Borowsky ML, Muddukrishna B, Kreiswirth BN, Kurepina N, Galagan J, Gagneux S, Birren B, Rubin EJ, Lander ES, Sabeti PC, Murray M. 2013. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nat Genet 45:1183–1189. doi: 10.1038/ng.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Flores AR, Sahasrabhojane P, Saldana M, Galloway-Pena J, Olsen RJ, Musser JM, Shelburne SA. 2014. Molecular characterization of an invasive phenotype of group A Streptococcus arising during human infection using whole genome sequencing of multiple isolates from the same patient. J Infect Dis 209:1520–1523. doi: 10.1093/infdis/jit674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Hill JA, Ammar R, Torti D, Nislow C, Cowen LE. 2013. Genetic and genomic architecture of the evolution of resistance to antifungal drug combinations. PLoS Genet 9:e1003390. doi: 10.1371/journal.pgen.1003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Khan MA, Knox N, Prashar A, Alexander D, Abdel-Nour M, Duncan C, Tang P, Amatullah H, Dos Santos CC, Tijet N, Low DE, Pourcel C, Van Domselaar G, Terebiznik M, Ensminger AW, Guyard C. 2013. Comparative genomics reveal that host-innate immune responses influence the clinical prevalence of serogroups. PLoS One 8:e67298. doi: 10.1371/journal.pone.0067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Linkevicius M, Sandegren L, Andersson DI. 2013. Mechanisms and fitness costs of tigecycline resistance in Escherichia coli. J Antimicrob Chemother 68:2809–2819. doi: 10.1093/jac/dkt263. [DOI] [PubMed] [Google Scholar]
- 496.Miller C, Kong J, Tran TT, Arias CA, Saxer G, Shamoo Y. 2013. Adaptation of Enterococcus faecalis to daptomycin reveals an ordered progression to resistance. Antimicrob Agents Chemother 57:5373–5383. doi: 10.1128/AAC.01473-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Ormerod KL, Morrow CA, Chow EW, Lee IR, Arras SD, Schirra HJ, Cox GM, Fries BC, Fraser JA. 11 March 2013. Comparative genomics of serial isolates of Cryptococcus neoformans reveals gene associated with carbon utilization and virulence. G3 (Bethesda) doi: 10.1534/g3.113.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Perichon B, Goussard S, Walewski V, Krizova L, Cerqueira G, Murphy C, Feldgarden M, Wortman J, Clermont D, Nemec A, Courvalin P. 2014. Identification of fifty class D beta-lactamases and of sixty-five Acinetobacter-derived cephalosporinases in Acinetobacter spp. Antimicrob Agents Chemother 58:936–949. doi: 10.1128/AAC.01261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Rolain JM, Diene SM, Kempf M, Gimenez G, Robert C, Raoult D. 2013. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob Agents Chemother 57:592–596. doi: 10.1128/AAC.01314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Kozak NA, Buss M, Lucas CE, Frace M, Govil D, Travis T, Olsen-Rasmussen M, Benson RF, Fields BS. 2010. Virulence factors encoded by Legionella longbeachae identified on the basis of the genome sequence analysis of clinical isolate D-4968. J Bacteriol 192:1030–1044. doi: 10.1128/JB.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Browall S, Norman M, Tangrot J, Galanis I, Sjostrom K, Dagerhamn J, Hellberg C, Pathak A, Spadafina T, Sandgren A, Battig P, Franzen O, Andersson B, Ortqvist A, Normark S, Henriques-Normark B. 2014. Intraclonal variations among Streptococcus pneumoniae isolates influence the likelihood of invasive disease in children. J Infect Dis 209:377–388. doi: 10.1093/infdis/jit481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Ioerger TR, O'Malley T, Liao R, Guinn KM, Hickey MJ, Mohaideen N, Murphy KC, Boshoff HI, Mizrahi V, Rubin EJ, Sassetti CM, Barry CE III, Sherman DR, Parish T, Sacchettini JC. 2013. Identification of new drug targets and resistance mechanisms in Mycobacterium tuberculosis. PLoS One 8:e75245. doi: 10.1371/journal.pone.0075245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Liu Z, Zheng H, Gottschalk M, Bai X, Lan R, Ji S, Liu H, Xu J. 2013. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS One 8:e72070. doi: 10.1371/journal.pone.0072070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Cheung MK, Kwan HS. 2012. Fighting outbreaks with bacterial genomics: case review and workflow proposal. Public Health Genomics 15:341–351. doi: 10.1159/000342770. [DOI] [PubMed] [Google Scholar]
- 505.Harris SR, Cartwright EJ, Torok ME, Holden MT, Brown NM, Ogilvy-Stuart AL, Ellington MJ, Quail MA, Bentley SD, Parkhill J, Peacock SJ. 2013. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis 13:130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Roetzer A, Diel R, Kohl TA, Ruckert C, Nubel U, Blom J, Wirth T, Jaenicke S, Schuback S, Rusch-Gerdes S, Supply P, Kalinowski J, Niemann S. 2013. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med 10:e1001387. doi: 10.1371/journal.pmed.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Rohde H, Qin J, Cui Y, Li D, Loman NJ, Hentschke M, Chen W, Pu F, Peng Y, Li J, Xi F, Li S, Li Y, Zhang Z, Yang X, Zhao M, Wang P, Guan Y, Cen Z, Zhao X, Christner M, Kobbe R, Loos S, Oh J, Yang L, Danchin A, Gao GF, Song Y, Li Y, Yang H, Wang J, Xu J, Pallen MJ, Wang J, Aepfelbacher M, Yang R, E. coli O104:H4 Genome Analysis Crowd-Sourcing Consortium. 2011. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med 365:718–724. doi: 10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- 508.Torok ME, Reuter S, Bryant J, Koser CU, Stinchcombe SV, Nazareth B, Ellington MJ, Bentley SD, Smith GP, Parkhill J, Peacock SJ. 2013. Rapid whole-genome sequencing for investigation of a suspected tuberculosis outbreak. J Clin Microbiol 51:611–614. doi: 10.1128/JCM.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Lee VJ, Yap J, Maurer-Stroh S, Lee RT, Eisenhaber F, Tay JK, Ting PJ, Loh JP, Wong CW, Tan BH, Koay ES, Kelly PM, Hibberd ML. 2011. Investigation of causes of oseltamivir chemoprophylaxis failures during influenza A (H1N1-2009) outbreaks. J Clin Virol 50:104–108. doi: 10.1016/j.jcv.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 510.Reuter S, Harrison TG, Koser CU, Ellington MJ, Smith GP, Parkhill J, Peacock SJ, Bentley SD, Torok ME. 2013. A pilot study of rapid whole-genome sequencing for the investigation of a Legionella outbreak. BMJ Open 3:e002175. doi: 10.1136/bmjopen-2012-002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Graham RM, Doyle CJ, Jennison AV. 27 February 2014. Real-time investigation of a Legionella pneumophila outbreak using whole genome sequencing. Epidemiol Infect doi: 10.1017/S0950268814000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Hadler SC, Castro KG, Dowdle W, Hicks L, Noble G, Ridzon R. 2011. Epidemic Intelligence Service investigations of respiratory illness, 1946-2005. Am J Epidemiol 174:S36–46. doi: 10.1093/aje/kwr309. [DOI] [PubMed] [Google Scholar]
- 513.Brunkard JM, Ailes E, Roberts VA, Hill V, Hilborn ED, Craun GF, Rajasingham A, Kahler A, Garrison L, Hicks L, Carpenter J, Wade TJ, Beach MJ, Yoder JS, CDC; 2011. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2007-2008. MMWR Surveill Summ 60:38–68. [PubMed] [Google Scholar]
- 514.Kozak NA, Lucas CE, Winchell JM. 2013. Identification of Legionella in the environment. Methods Mol Biol 954:3–25. doi: 10.1007/978-1-62703-161-5_1. [DOI] [PubMed] [Google Scholar]
- 515.Morris GK, Patton CM, Feeley JC, Johnson SE, Gorman G, Martin WT, Skaliy P, Mallison GF, Politi BD, Mackel DC. 1979. Isolation of the Legionnaires' disease bacterium from environmental samples. Ann Intern Med 90:664–666. doi: 10.7326/0003-4819-90-4-664. [DOI] [PubMed] [Google Scholar]
- 516.Orrison LH, Cherry WB, Milan D. 1981. Isolation of Legionella pneumophilia from cooling tower water by filtration. Appl Environ Microbiol 41:1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Buehler JW, Kuritsky JN, Gorman GW, Hightower AW, Broome CV, Sikes RK. 1985. Prevalence of antibodies to Legionella pneumophila among workers exposed to a contaminated cooling tower. Arch Environ Health 40:207–210. doi: 10.1080/00039896.1985.10545919. [DOI] [PubMed] [Google Scholar]
- 518.Ikedo M, Yabuuchi E. 1986. Ecological studies of Legionella species. I. Viable counts of Legionella pneumophila in cooling tower water. Microbiol Immunol 30:413–423. [DOI] [PubMed] [Google Scholar]
- 519.Dondero TJ Jr, Rendtorff RC, Mallison GF, Weeks RM, Levy JS, Wong EW, Schaffner W. 1980. An outbreak of Legionnaires' disease associated with a contaminated air-conditioning cooling tower. N Engl J Med 302:365–370. doi: 10.1056/NEJM198002143020703. [DOI] [PubMed] [Google Scholar]
- 520.Conwill DE, Werner SB, Dritz SK, Bissett M, Coffey E, Nygaard G, Bradford L, Morrison FR, Knight MW. 1982. Legionellosis—the 1980 San Francisco outbreak. Am Rev Respir Dis 126:666–669. [DOI] [PubMed] [Google Scholar]
- 521.Walser SM, Gerstner DG, Brenner B, Höller C, Liebl B, Herr CEW. 2014. Assessing the environmental health relevance of cooling towers—a systematic review of legionellosis outbreaks. Int J Hyg Environ Health 217:145–154. doi: 10.1016/j.ijheh.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 522.Timbury MC, Donaldson JR, McCartney AC, Fallon RJ, Sleigh JD, Lyon D, Orange GV, Baird DR, Winter J, Wilson TS. 1986. Outbreak of Legionnaires' disease in Glasgow Royal Infirmary: microbiological aspects. J Hyg (Lond) 97:393–403. doi: 10.1017/S0022172400063580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Black N, Griffin D. 15 January 2014, posting date VA under scrutiny after Legionnaires' cases in Pittsburgh. CNN, 14 December 2012. http://www.cnn.com/2012/12/13/health/legionnaires-hospital-water/.
- 524.Stallworth C, Steed L, Fisher MA, Nolte FS. 2012. Legionnaires' disease caused by Legionella londiniensis. J Clin Microbiol 50:4178–4179. doi: 10.1128/JCM.02182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wright AJ, Humar A, Gourishankar S, Bernard K, Kumar D. 2012. Severe Legionnaire's disease caused by Legionella longbeachae in a long-term renal transplant patient: the importance of safe living strategies after transplantation. Transpl Infect Dis 14:E30–E33. doi: 10.1111/j.1399-3062.2012.00755.x. [DOI] [PubMed] [Google Scholar]
- 526.Larru B, Gerber JS, Ota KV. 2012. Medical treatment failure and complete left pneumonectomy after Legionella pneumophila pneumonia in a bone marrow transplant recipient. Pediatr Infect Dis J 31:979–981. doi: 10.1097/INF.0b013e31825cb28f. [DOI] [PubMed] [Google Scholar]
- 527.Lee J, Caplivski D, Wu M, Huprikar S. 2009. Pneumonia due to Legionella feeleii: case report and review of the literature. Transpl Infect Dis 11:337–340. doi: 10.1111/j.1399-3062.2009.00390.x. [DOI] [PubMed] [Google Scholar]
- 528.Gudiol C, Garcia-Vidal C, Fernandez-Sabe N, Verdaguer R, Llado L, Roca J, Gil-Vernet S, Carratala J. 2009. Clinical features and outcomes of Legionnaires' disease in solid organ transplant recipients. Transpl Infect Dis 11:78–82. doi: 10.1111/j.1399-3062.2008.00337.x. [DOI] [PubMed] [Google Scholar]
- 529.Kioski C, Cage G, Johnson B, Rosales C, England B. 1997. Sustained transmission of nosocomial Legionnaires disease—Arizona and Ohio. MMWR Morb Mortal Wkly Rep 46:416–421. [PubMed] [Google Scholar]
- 530.Kool JL, Fiore AE, Kioski CM, Brown EW, Benson RF, Pruckler JM, Glasby C, Butler JC, Cage GD, Carpenter JC, Mandel RM, England B, Breiman RF. 1998. More than 10 years of unrecognized nosocomial transmission of Legionnaires' disease among transplant patients. Infect Control Hosp Epidemiol 19:898–904. doi: 10.2307/30142014. [DOI] [PubMed] [Google Scholar]
- 531.Rowbotham TJ. 1998. Legionellosis associated with ships: 1977 to 1997. Commun Dis Public Health 1:146–151. [PubMed] [Google Scholar]
- 532.Peake DE, Gray CL, Ludwig MR, Hill CD. 1999. Descriptive epidemiology of injury and illness among cruise ship passengers. Ann Emerg Med 33:67–72. doi: 10.1016/S0196-0644(99)70419-1. [DOI] [PubMed] [Google Scholar]
- 533.Dowling RK. 2006. Cruise ship tourism. CABI Publishing, Wallingford, United Kingdom. [Google Scholar]
- 534.Howden LM, Meyer JA. 2011. Age and sex composition: 2010. US Department of Commerce, Washington, DC. [Google Scholar]
- 535.Jernigan DB, Hofmann J, Cetron MS, Genese CA, Nuorti JP, Fields BS, Benson RF, Carter RJ, Edelstein PH, Guerrero IC, Paul SM, Lipman HB, Breiman R. 1996. Outbreak of Legionnaires' disease among cruise ship passengers exposed to a contaminated whirlpool spa. Lancet 347:494–499. doi: 10.1016/S0140-6736(96)91137-X. [DOI] [PubMed] [Google Scholar]
- 536.Genese C, Hung MJ, Paul S, Brook J, Finelli L, Spitalny KC. 1994. Outbreak of pneumonia associated with a cruise ship, 1994. MMWR Morb Mortal Wkly Rep 43:521. [PubMed] [Google Scholar]
- 537.Guerrero IC, Filippone C. 1996. A cluster of Legionnaires' disease in a community hospital: a clue to a larger epidemic. Infect Control Hosp Epidemiol 17:177–178. doi: 10.2307/30142380. [DOI] [PubMed] [Google Scholar]
- 538.Edelstein PH, Cetron MS. 1999. Editorial response: sea, wind, and pneumonia. Clin Infect Dis 28:39–41. doi: 10.1086/515084. [DOI] [PubMed] [Google Scholar]
- 539.Castellani Pastoris M, Lo Monaco R, Goldoni P, Mentore B, Balestra G, Ciceroni L, Visca P. 1999. Legionnaires' disease on a cruise ship linked to the water supply system: clinical and public health implications. Clin Infect Dis 28:33–38. doi: 10.1086/515083. [DOI] [PubMed] [Google Scholar]
- 540.Sedgwick J, Joseph C, Chandrakumar M, Harrison T, Lee J, de Jong B. 2007. Outbreak of respiratory infection on a cruise ship. Euro Surveill 12(8):pii=3246 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3246. [DOI] [PubMed] [Google Scholar]
- 541.Beyrer K, Lai S, Dreesman J, Lee JV, Joseph C, Harrison T, Surman-Lee S, Luck C, Brodhun B, Buchholz U, Windorfer A. 2007. Legionnaires' disease outbreak associated with a cruise liner, August 2003: epidemiological and microbiological findings. Epidemiol Infect 135:802–810. doi: 10.1017/S0950268806007473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Joseph C, van Vijngaarden J, Mshar P, Oravetz C, Fix AM, Genese C, Johnson GS, Kacica M, Weant B, Jenkins P, Baker N, Forney D, Ames J, Vaughan G, Schnoor J, Kim D, Guerra M, Fields B, Moore M, Newbern C, Thigpen M. 2005. Cruise-ship-associated Legionnaires disease, November 2003-May 2004. MMWR Morb Mortal Wkly Rep 54:1153–1155. [PubMed] [Google Scholar]
- 543.Kobayashi A, Yamamoto Y, Chou S, Hashimoto S. 2004. Severe Legionella pneumophila pneumonia associated with the public bath on a cruise ship in Japan. J Anesth 18:129–131. doi: 10.1007/s00540-003-0218-0. [DOI] [PubMed] [Google Scholar]
- 544.Cayla JA, Maldonado R, Gonzalez J, Pellicer T, Ferrer D, Pelaz C, Gracia J, Baladron B, Plasencia A. 2001. A small outbreak of Legionnaires' disease in a cargo ship under repair. Eur Respir J 17:1322–1327. doi: 10.1183/09031936.01.00046801. [DOI] [PubMed] [Google Scholar]
- 545.Christenson B, Lidin-Janson G, Kallings I. 1987. Outbreak of respiratory illness on board a ship cruising to ports in southern Europe and northern Africa. J Infect 14:247–254. doi: 10.1016/S0163-4453(87)93535-3. [DOI] [PubMed] [Google Scholar]
- 546.Hicken P, Johnson MA, Gourley T. 1979. Legionnaires' disease in Vancouver. J Can Assoc Radiol 30:179. [PubMed] [Google Scholar]
- 547.Christie P, Joseph C. 1998. Legionella on board a cruise ship. Euro Surveill 2(27):pii=1191. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=1191. [Google Scholar]
- 548.Ahlen C, Aas M, Nor A, Wetteland PI, Johansen H, Sorbo T, Sommerfelt-Pettersen JK, Iversen OJ. 2013. Legionella pneumophila in Norwegian naval vessels. Tidsskr Nor Laegeforen 133:1445–1448. doi: 10.4045/tidsskr.12.1459. [DOI] [PubMed] [Google Scholar]
- 549.Modi A, Gardner J, Lighton L, Coetzee N. 2008. Pontiac fever outbreak associated with a spa-pool, United Kingdom, April 2008. Euro Surveill 13(30):pii=18934 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18934. [PubMed] [Google Scholar]
- 550.Alsibai S, Bilo de Bernardi P, Janin C, Che D, Lee JV. 2006. Outbreak of legionellosis suspected to be related to a whirlpool spa display, September 2006, Lorquin, France. Euro Surveill 11(41):pii=3063. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3063. [DOI] [PubMed] [Google Scholar]
- 551.Foster K, Gorton R, Waller J. 2006. Outbreak of legionellosis associated with a spa pool, United Kingdom. Euro Surveill 11(38):pii=3053. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3053. [DOI] [PubMed] [Google Scholar]
- 552.Huhn GD, Adam B, Ruden R, Hilliard L, Kirkpatrick P, Todd J, Crafts W, Passaro D, Dworkin MS. 2005. Outbreak of travel-related Pontiac fever among hotel guests illustrating the need for better diagnostic tests. J Travel Med 12:173–179. doi: 10.2310/7060.2005.12401. [DOI] [PubMed] [Google Scholar]
- 553.Benin AL, Benson RF, Arnold KE, Fiore AE, Cook PG, Williams LK, Fields B, Besser RE. 2002. An outbreak of travel-associated Legionnaires disease and Pontiac fever: the need for enhanced surveillance of travel-associated legionellosis in the United States. J Infect Dis 185:237–243. doi: 10.1086/338060. [DOI] [PubMed] [Google Scholar]
- 554.McEvoy M, Batchelor N, Hamilton G, MacDonald A, Faiers M, Sills A, Lee J, Harrison T. 2000. A cluster of cases of Legionnaires' disease associated with exposure to a spa pool on display. Commun Dis Public Health 3:43–45. [PubMed] [Google Scholar]
- 555.Luttichau HR, Vinther C, Uldum SA, Moller J, Faber M, Jensen JS. 1998. An outbreak of Pontiac fever among children following use of a whirlpool. Clin Infect Dis 26:1374–1378. doi: 10.1086/516354. [DOI] [PubMed] [Google Scholar]
- 556.Fallon RJ, Rowbotham TJ. 1990. Microbiological investigations into an outbreak of Pontiac fever due to Legionella micdadei associated with use of a whirlpool. J Clin Pathol 43:479–483. doi: 10.1136/jcp.43.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Groseclose SL, Brathwaite WS, Hall PA, Knowles CM, Adams DA, Connor FJ, Hester M, Sharp P, Anderson WJ, Fagan RF, Aponte JJ, Jones GF, Nitschke DA, Vaughan J, Worsham CA, Chang M, Doyle T, Jajosky R. 2002. Summary of notifiable diseases—United States, 2000. MMWR Morb Mortal Wkly Rep 49:1–100. [PubMed] [Google Scholar]
- 558.National Center for Health Statistics. 2013. Health, United States, 2012: with special feature on emergency care. National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- 559.Yiallouros PK, Papadouri T, Karaoli C, Papamichael E, Zeniou M, Pieridou-Bagatzouni D, Papageorgiou GT, Pissarides N, Harrison TG, Hadjidemetriou A. 2013. First outbreak of nosocomial Legionella infection in term neonates caused by a cold mist ultrasonic humidifier. Clin Infect Dis 57:48–56. doi: 10.1093/cid/cit176. [DOI] [PubMed] [Google Scholar]
- 560.Sehulster L, Chinn RY. 2003. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 52:1–42. [PubMed] [Google Scholar]
- 561.Butler JC, Fields BS, Breiman RF. 1998. Issues in the control of nosocomial legionellosis. Infect Dis Clin Pract 7:117–118. doi: 10.1097/00019048-199802000-00012. [DOI] [Google Scholar]
- 562.Scaturro M, Dell'eva I, Helfer F, Ricci ML. 2007. Persistence of the same strain of Legionella pneumophila in the water system of an Italian hospital for 15 years. Infect Control Hosp Epidemiol 28:1089–1092. doi: 10.1086/519869. [DOI] [PubMed] [Google Scholar]
- 563.Garcia MT, Baladron B, Gil V, Tarancon ML, Vilasau A, Ibanez A, Elola C, Pelaz C. 2008. Persistence of chlorine-sensitive Legionella pneumophila in hyperchlorinated installations. J Appl Microbiol 105:837–847. doi: 10.1111/j.1365-2672.2008.03804.x. [DOI] [PubMed] [Google Scholar]
- 564.Triassi M, Di Popolo A, Ribera D'Alcala G, Albanese Z, Cuccurullo S, Montegrosso S, Crispino M, Borella P, Zarrilli R. 2006. Clinical and environmental distribution of Legionella pneumophila in a university hospital in Italy: efficacy of ultraviolet disinfection. J Hosp Infect 62:494–501. doi: 10.1016/j.jhin.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 565.Darelid J, Bernander S, Jacobson K, Lofgren S. 2004. The presence of a specific genotype of Legionella pneumophila serogroup 1 in a hospital and municipal water distribution system over a 12-year period. Scand J Infect Dis 36:417–423. doi: 10.1080/00365540410020749. [DOI] [PubMed] [Google Scholar]
- 566.Garcia-Nunez M, Sopena N, Ragull S, Pedro-Botet ML, Morera J, Sabria M. 2008. Persistence of Legionella in hospital water supplies and nosocomial Legionnaires' disease. FEMS Immunol Med Microbiol 52:202–206. doi: 10.1111/j.1574-695X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 567.Berthelot P, Grattard F, Ros A, Lucht F, Pozzetto B. 1998. Nosocomial legionellosis outbreak over a three-year period: investigation and control. Clin Microbiol Infect 4:385–391. doi: 10.1111/j.1469-0691.1998.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 568.Rangel-Frausto MS, Rhomberg P, Hollis RJ, Pfaller MA, Wenzel RP, Helms CM, Herwaldt LA. 1999. Persistence of Legionella pneumophila in a hospital's water system: a 13-year survey. Infect Control Hosp Epidemiol 20:793–797. doi: 10.1086/501586. [DOI] [PubMed] [Google Scholar]
- 569.Visca P, Goldoni P, Luck PC, Helbig JH, Cattani L, Giltri G, Bramati S, Castellani Pastoris M. 1999. Multiple types of Legionella pneumophila serogroup 6 in a hospital heated-water system associated with sporadic infections. J Clin Microbiol 37:2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Lepine LA, Jernigan DB, Butler JC, Pruckler JM, Benson RF, Kim G, Hadler JL, Cartter ML, Fields BS. 1998. A recurrent outbreak of nosocomial Legionnaires' disease detected by urinary antigen testing: evidence for long-term colonization of a hospital plumbing system. Infect Control Hosp Epidemiol 19:905–910. doi: 10.1086/647761. [DOI] [PubMed] [Google Scholar]
- 571.Prodinger WM, Bonatti H, Allerberger F, Wewalka G, Harrison TG, Aichberger C, Dierich MP, Margreiter R, Tiefenbrunner F. 1994. Legionella pneumonia in transplant recipients: a cluster of cases of eight years' duration. J Hosp Infect 26:191–202. doi: 10.1016/0195-6701(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 572.Boulanger CA, Edelstein PH. 1995. Precision and accuracy of recovery of Legionella pneumophila from seeded tap water by filtration and centrifugation. Appl Environ Microbiol 61:1805–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Napoli C, Iatta R, Fasano F, Marsico T, Montagna MT. 2009. Variable bacterial load of Legionella spp. in a hospital water system. Sci Total Environ 408:242–244. doi: 10.1016/j.scitotenv.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 574.Allen JG, Myatt TA, MacIntosh DL, Ludwig JF, Minegishi T, Stewart JH, Connors BF, Grant MP, McCarthy JF. 2012. Assessing risk of health care-acquired Legionnaires' disease from environmental sampling: the limits of using a strict percent positivity approach. Am J Infect Control 40:917–921. doi: 10.1016/j.ajic.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 575.Best M, Yu VL, Stout J, Goetz A, Muder RR, Taylor F. 1983. Legionellaceae in the hospital water-supply. Epidemiological link with disease and evaluation of a method for control of nosocomial Legionnaires' disease and Pittsburgh pneumonia. Lancet ii:307–310. [DOI] [PubMed] [Google Scholar]
- 576.Morris GK, Shelton BG. 1998. Legionella bacteria in environmental samples: hazard analysis and suggested remedial actions. PathCon Laboratories technical bulletin 1.5. Pathogen Control Associates, Inc, Norcross, GA. [Google Scholar]
- 577.Seenivasan MH, Yu VL, Muder RR. 2005. Legionnaires' disease in long-term care facilities: overview and proposed solutions. J Am Geriatr Soc 53:875–880. doi: 10.1111/j.1532-5415.2005.53270.x. [DOI] [PubMed] [Google Scholar]
- 578.Diederen BM, Kluytmans JA, Vandenbroucke-Grauls CM, Peeters MF. 2008. Utility of real-time PCR for diagnosis of Legionnaires' disease in routine clinical practice. J Clin Microbiol 46:671–677. doi: 10.1128/JCM.01196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Tang PW, Toma S. 1986. Broad-spectrum enzyme-linked immunosorbent assay for detection of Legionella soluble antigens. J Clin Microbiol 24:556–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Helbig JH, Luck PC, Witzleb W. 1989. Diagnosis of Legionella pneumonia by detection of antigenuria using an enzyme immunoassay with 6 antibody specificities. Z Gesamte Hyg 35:591–593. (In German.) [PubMed] [Google Scholar]
- 581.Kim MJ, Sohn JW, Park DW, Park SC, Chun BC. 2003. Characterization of a lipoprotein common to Legionella species as a urinary broad-spectrum antigen for diagnosis of Legionnaires' disease. J Clin Microbiol 41:2974–2979. doi: 10.1128/JCM.41.7.2974-2979.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Engleberg NC, Drutz DJ, Eisenstein BI. 1984. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun 44:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Engleberg NC, Pearlman E, Dixon D, Eisenstein BI. 1986. Antibodies isolated by using cloned surface antigens recognize antigenically related components of Legionella pneumophila and other Legionella species. J Immunol 136:1415–1417. [PubMed] [Google Scholar]
- 584.Engleberg NC, Pearlman E, Eisenstein BI. 1984. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol 160:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Bruin JP, Diederen BM. 2012. Evaluation of the SD Bioline test, a new assay for detecting Legionella pneumophila serogroup 1 antigen in urine. J Infect 64:113–114. doi: 10.1016/j.jinf.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 586.Mathers C, Fat DM, Boerma JT, World Health Organization. 2008. The global burden of disease: 2004 update. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 587.Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee. 2008. Guideline for disinfection and sterilization in health care facilities, 2008. CDC, Atlanta, GA: http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf. [Google Scholar]
- 588.EPA. 1999. Legionella: human health criteria document. EPA, Washington, DC: http://water.epa.gov/action/advisories/drinking/upload/2009_02_03_criteria_humanhealth_microbial_legionella.pdf. [Google Scholar]
- 589.World Health Organization. 2007. Legionella and prevention of legionellosis. World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/emerging/legionella.pdf. [Google Scholar]
- 590.World Health Organization. 2011. Guidelines for drinking water quality, 4th ed World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/. [Google Scholar]
- 591.Cunliffe D, Bartram J, Briand E, Chartier Y, Colbourne J, Drury D, Lee J, Schaefer B, Surman-Lee S (ed). 2011. Water safety in buildings. World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/publications/2011/9789241548106/en/. [Google Scholar]
- 592.World Health Organization. 2006. Guidelines for safe recreational water environments, vol 2 Swimming pools and similar environments. World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/bathing/bathing2/en/. [Google Scholar]
- 593.American Society of Heating, Refrigerating, and Air-Conditioning Engineers. 2011. Proposed new standard 188, prevention of legionellosis associated with building water systems. American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Atlanta, GA: https://osr.ashrae.org/Public%20Review%20Draft%20Standards%20Lib/Std-188P-PPR2%20Final%206%2010%202011.pdf. [Google Scholar]
- 594.Health and Safety Executive. 2013. Legionnaires' disease: the control of Legionella bacteria in water systems, L8, 4th ed Health and Safety Executive, London, United Kingdom: http://www.hse.gov.uk/pubns/books/l8.htm. [Google Scholar]
- 595.American Society of Heating, Refrigerating, and Air-Conditioning Engineers. 2000. Guideline 12-2000: minimizing the risk of legionellosis associated with building water systems. American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Atlanta, GA: http://www.techstreet.com/ashrae/products/232891. [Google Scholar]