SUMMARY
Kingella kingae is a common etiology of pediatric bacteremia and the leading agent of osteomyelitis and septic arthritis in children aged 6 to 36 months. This Gram-negative bacterium is carried asymptomatically in the oropharynx and disseminates by close interpersonal contact. The colonized epithelium is the source of bloodstream invasion and dissemination to distant sites, and certain clones show significant association with bacteremia, osteoarthritis, or endocarditis. Kingella kingae produces an RTX (repeat-in-toxin) toxin with broad-spectrum cytotoxicity that probably facilitates mucosal colonization and persistence of the organism in the bloodstream and deep body tissues. With the exception of patients with endocardial involvement, children with K. kingae diseases often show only mild symptoms and signs, necessitating clinical acumen. The isolation of K. kingae on routine solid media is suboptimal, and detection of the bacterium is significantly improved by inoculating exudates into blood culture bottles and the use of PCR-based assays. The organism is generally susceptible to antibiotics that are administered to young patients with joint and bone infections. β-Lactamase production is clonal, and the local prevalence of β-lactamase-producing strains is variable. If adequately and promptly treated, invasive K. kingae infections with no endocardial involvement usually run a benign clinical course.
INTRODUCTION
In the 1960s Elizabeth O. King, working at the U.S. Centers for Disease Control (CDC) in Atlanta, GA, described a novel bacterial species isolated from human respiratory secretions, blood, and bone and joint exudates (1). The organism, initially assigned to the genus Moraxella and designated Moraxella kingii in honor of King's seminal research, was later placed in a separate genus and renamed Kingella kingae (2).
The interest in K. kingae was initially limited (3–10), and only 55 reports on the organism were published in the medical literature until 1990. The number, however, has sharply increased in recent years, jumping to 105 in the following decade and 83 in the short period from January 2010 through August 2014 (determined by a PubMed search with “Kingella kingae” and “Moraxella kingii”), and has firmly established the status of K. kingae as a common agent of bacteremia with no focus (also called occult bacteremia) (11, 12) and the predominant etiology of joint and bone infections in 6- to 36-month-old children (13–16). This increasing detection of K. kingae does not indicate that the bacterium is really a novel human pathogen. It appears, rather, than this rapidly enlarging body of information is the result of improvement in detection methods, particularly the observation that inoculation of skeletal system exudates into blood culture vials (BCVs) enhances the isolation of the organism (17), and the development and increasing usage of sensitive nucleic acid amplification (NAA) assays (16).
The vast majority of publications on K. kingae infections have originated in countries in the developed world, particularly the United States, France, Switzerland, and Israel (18), whereas reports from the developing world are still scarce (19–25), probably reflecting the unavailability of expensive automated blood culture systems and NAA technology needed for detecting the bacterium in resource-poor countries.
Coinciding with the recognition of K. kingae as a major cause of pediatric disease, the growing attention of the scientific community to this pathogen has resulted in the rapid accumulation of knowledge in the fields of the epidemiology, pathogenesis, diagnosis, and treatment of invasive K. kingae infections (Fig. 1), which are the subject of this review.
FIG 1.
Timeline of milestone research studies on K. kingae.
BACTERIOLOGY
Taxonomy
The genus Kingella belongs to the Neisseriaceae family in the beta subclass of the Proteobacteria and comprises four recognized species: K. denitrificans, which has been implicated in cases of bacteremia, endocarditis, pleural empyema, pediatric vaginitis, chorioamnionitis, and granulomatous disease in AIDS patients (26–29); K. oralis, which is a commensal dweller of the human buccal cavity and is associated with dental plaque and periodontitis (30, 31); K. potus, a zoonotic organism recovered from an infected bite (32); and K. kingae. Although K. kingae's taxonomic place remained uncertain for many years (1, 2), subsequent analysis of its biochemical profile and fatty acid composition (33–39) and genotypic studies (33, 40–44) have led to the conclusion that K. kingae constitutes a separate species, only distantly linked to other Neisseriaceae (44).
Identification
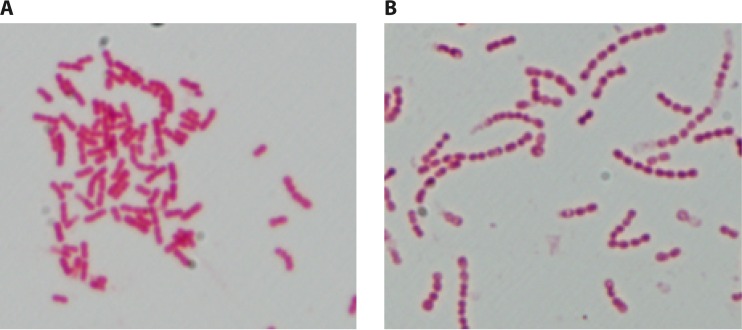
Kingella kingae appears as pairs or chains of 4 to 8 plump (0.6 to 1 μm by 1 to 3 μm) coccobacilli (Fig. 2A). Kingella kingae cells tend to resist decolorization, and thus the organism may be erroneously identified as Gram positive (45), but electron microscopic examination discloses a characteristic Gram-negative cell wall structure. The bacterium is beta-hemolytic, nonmotile, and non-spore forming, exhibits negative catalase, urease, and indol tests, and, with rare exceptions, has oxidase activity. Kingella kingae produces acid from glucose and usually from maltose (46, 47), hydrolyzes indoxyl phosphate and l-prolyl-β-naphthylamide, and exhibits positive alkaline and acid phosphatase reactions. The fatty acid content of the organism resembles that of K. denitrificans and comprises a high percentage of myristic acid and lesser concentrations of palmitic, lauric, palmitoleic, linoleic, oleic, 3-hydroxilauric, 3-hydroximyristic, and cis-vaccenic acids (35, 36).
FIG 2.
(A) Typical Gram stain of K. kingae organisms, depicting short Gram-negative coccobacilli with tapered ends arranged in pairs or short chains. (B) Gram stain of K. kingae small-colony variant, showing atypical long chains.
The identification of K. kingae in the clinical microbiology laboratory can be accomplished with a wide array of commercial systems, such as the quadFERM+ kit (48), API NH card, Vitek 2 instrument (49, 50), matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) (50, 51), and 16S rRNA gene sequencing (52), but the organism is completely misidentified by the Remel RapID NH kit (50).
Culture
Kingella kingae is a facultative anaerobic bacterium that grows on conventional Trypticase-soy agar supplemented with 5% hemoglobin (blood agar medium), chocolate agar, Columbia-based blood agar (53), and GC-based media. Similar to the case for other Neisseriaceae, most K. kingae strains can be recovered on Thayer-Martin medium but do not develop on MacConkey or Krigler agar. Many isolates show poor growth on the cation-supplemented Mueller-Hinton medium used to determine the antibiotic susceptibility of the species. Similar to the case for other bacteria that inhabit the respiratory tract, growth is improved in a 5% CO2 atmosphere, but only a minute proportion of isolates are strictly capnophilic (18).
Growth on solid media is characterized by marked pitting of the agar surface, which is best seen after removal of the colony (34). Kingella kingae strains produce three different colony types that are associated with the degree of pilus expression: a spreading-corroding morphology distinguished by a small central colony encircled by a wide fringe, a nonspreading/noncorroding type consisting of a flat colony surrounded by a narrow fringe, and a dome-shaped colony with no noticeable fringe. The first two morphologies are associated with the presence of long fimbriae, whereas strains growing as domed colonies are nonpiliated (54–57). The ability to produce the spreading-corroding type of morphology can be irreversibly lost after repeated subculture (47).
Small-Colony Variants
In recent years, small-colony variants (SCVs) of K. kingae, characterized by growth as pinpoint colonies on blood agar plates and no growth on Thayer-Martin medium, have been isolated in approximately 15% of healthy pharyngeal carriers in southern Israel (58). These strains frequently fail to produce acid from maltose and tend to form atypical long chains of coccobacilli, indicating impaired cell separation (Fig. 2B). Although SCVs detected in other bacterial pathogens such as Staphylococcus aureus or Pseudomonas aeruginosa are commonly associated with chronic or difficult-to-eradicate infections, K. kingae SCVs have been only exceptionally isolated from patients with invasive disease, suggesting decreased virulence. Reversion to a rapidly growing phenotype, but without modification of the long-chain configuration, is obtained by seeding K. kingae SCVs on GC-based medium, but not on chocolate agar, implying a defect in electron transport, menadione metabolism, or thymidine uptake (59). Kingella kingae cultures in general, and SCVs in particular, should be subcultured frequently (every 2 to 3 days) to keep the organism viable.
The SCVs of K. kingae belong to several pulsed-field gel electrophoresis (PFGE) clones, of which clone T is the most commonly carried by the healthy pediatric population in southern Israel and is associated with β-lactamase production (60). Although clone T isolates are readily identified as K. kingae by MALDI-TOF technology and share alleles of the rtxA and por genes (encoding the RTX toxin and the porin protein, respectively) found in some other K. kingae clones, attempts to type clone T strains by multilocus sequence typing (MLST) failed, because even when diverse couples of primers were employed, amplification succeeded for only three housekeeping genes (abcZ, adk, and cpn60) (61). In addition, examination of the 16S rRNA gene sequence indicates that clone T isolates share only 96.9% nucleotide identity with the ATCC 23330 type strain (61). Taken together, these genomic findings suggest that clone T is only remotely linked to other K. kingae clones and probably represents a separate clade or quasispecies (61).
GENOMICS
Comparison of the genomes of 22 K. kingae isolates from asymptomatic carriers and 28 derived from patients with different infections and diverse geographic origins revealed that the genome size of the species ranges between 1,990,794 bp and 2,096,758 bp, comprises 1,981 to 2,300 protein-encoding genes and between 43 and 52 RNA genes, and has a GC content between 46.8% and 46.9% (62, 63; L. Rouli, unpublished data).
Genomic studies in which K. kingae isolates from different geographic origins were studied by MLST of 6 housekeeping genes (abcZ, adk, aroE, cpn60, gdh, and recA) demonstrated that the species displays notable genomic variability, and to date, 64 MLST sequence types (STs) comprising strains sharing identical alleles combinations, 74 PFGE clones, and 18 rtxA and 11 different por alleles have been identified in the species (61, 64, 65). Three unrelated MLST sequence type complexes (STCs) comprising strains that differ at ≤1 locus from at least one other member of the group, namely, STC-6, STC-14, and STC-23, have been isolated from healthy individuals and patients with K. kingae disease in North America, Iceland, continental Europe, Australia, and Israel for over 2 decades, whereas other STCs show a more restricted temporal and spatial distribution or appear to be country specific and sporadic (61, 64, 65).
Similarly to other members of the Neisseriaceae family, K. kingae organisms are naturally transformable, and this phenomenon represents a potent driving force of genetic diversity. The uptake of exogenous DNA in Neisseriaceae is finely regulated by DNA uptake sequences (DUS), which facilitate transformation by homologous DNA and discriminate against horizontal transfer of heterologous and possibly harmful genomic sequences (66). DUS are short (12-nucleotide) DNA sequences which are present in the genome in multiple copies, consisting of a conserved 5′-CTG-3′ core flanked by variable sequences, resulting in DUS variants named “dialects” (66). Integrity of the core is strictly required for transformation, whereas the grade of genetic resemblance between the dialects of the donor and recipient organisms correlates with the efficiency of the transformation process (66). The DUS are, therefore, efficient obstacles to between-species recombination, contributing to the genetic stability and sexual isolation of the species.
Examination of K. kingae strain ATCC 23330 revealed that a DUS named king3DUS with the sequence 5′-AAGCAGAGCCTGCA-3′ is present in 2,787 copies in the genome (66). An identical sequence was identified in 3,603 copies in the taxonomically close K. denitrificans, which differs from the DUS detected in K. oralis and in other Neisseriaceae (66). Given that the genome size of K. kingae is approximately 2 Mb (62, 63), DUS should be randomly repeated, on average, every 500 to 1,000 bp. Because the number of DUS and their chromosomal location vary between strains, a novel PCR assay targeting this highly ubiquitous sequence was developed to investigate the genomic polymorphism of the species (65). The test amplifies sequences located between two consecutive DUS, resulting in multiple DNA bands that are unique and differ in length and thus can be separated in an electrophoretic gel. In an innovative study, DUS typing results showed excellent correlation with those obtained with the more costly and labor-intensive MLST method (65).
Despite the transformation competency of K. kingae, almost perfect correlation has been found between the different genotyping methods, disregarding the isolates' clinical, temporal, or geographic source, and strains are usually characterized by a unique combination of PFGE profiles and consistent allele content of housekeeping, rtxA, and por genes (61, 64, 65). For instance, all PFGE clone A isolates studied so far belong to the MLST STC-34 and share rtxA allele 3 or 13 and the por 4 allele, PFGE clone ψ organisms belong to MLST STC-29 and harbor the rtxA 4 and por 12 alleles, and PFGE clone K isolates belong to MLST STC-6 and possess the closely related rtxA 8 or 9 allele and the por 1 allele (61, 64, 65). It should be noted that these different genotyping methods investigate different sections of the bacterial genomic content: the PFGE approach randomly probes the entire genome, MLST explores a tiny fraction of the core genome that encodes critical metabolic enzymes and, therefore, evolves at a slow pace, and rtxA and por sequencing analyzes putative K. kingae virulence factors which are exposed to the host's immune response and undergo frequent genetic changes (67). The remarkable linkage disequilibrium disclosed by these diverse methods thus is striking and implies that K. kingae strains comprise idiosyncratic and seemingly quite homogeneous bacterial populations. It is postulated that circulating K. kingae strains are subjected to positive selection and resist the disrupting effect of recombination because they harbor favorable genetic traits and experience clonal expansion, explaining their extensive geographic dissemination and genetic stability over time (68).
MECHANISMS OF COLONIZATION AND VIRULENCE FACTORS
Pili
To colonize the human mucosae, bacteria first have to anchor to epithelial surfaces and tissues, and many pathogens express adhesive proteinaceous appendages to avoid being washed out. The presence of fimbriae was detected in K. kingae as early as 1978 (55), and in 1992, Weir and Marrs, using electron microscopy, DNA transformation patterns, and immunoblotting, convincingly demonstrated that these fibers are type 4 pili (56). In a series of elegant studies performed in the last decade, St. Geme and coworkers showed that K. kingae's pili are essential for the adherence of the bacterium to the respiratory epithelium and synovial layer (69). The investigators disclosed a chromosomal gene cluster homologous to that found in other Gram-negative organisms, consisting of a pilA1 gene that encodes the major pilin subunit and pilA2 and fimB genes of unknown function that appear not to be necessary for pilus expression or attachment (69). As is the case for other surface-exposed virulence factors, the PilA subunit exhibits significant between-strain variation in sequence and antibody reactivity, suggesting that it is subjected to selective pressure by the immune system (70). The expression of pili in K. kingae appears to be finely regulated by three genes (the σ54 gene, pilS, and pilR) (70), and the majority of colonizing strains, as well as those isolated from individuals with bacteremia, express piliation, whereas those derived from patients with bone, joint, or endocardial infections are nonpiliated (57). This observation suggests that piliation offers a selective advantage in the colonization process and at the early mucosal and bloodstream stages of the disease but is detrimental to the bacterium for invading deep body tissues.
Additional work by the same research group identified two other genes, named pilC1 and pilC2, in physically separated chromosomal locations, that encode homologs of the Neisseria PilC proteins, which play an essential role in the adherence and piliation processes (69, 71). The Kingella kingae PilC1 and PilC2 proteins have only partial similarity to each other and contain calcium-binding sites, and at least one PilC protein is essential for pilus expression (69, 71). While the PilC1 site is required for twitching motility and adherence, the PilC2 site exerts only a minor effect on motility and has no functional role in adherence (71). Additional research indicated that a trimeric autotransporter protein called Knh is crucial for firm adherence of K. kingae organisms to the epithelium (72). The Knh protein, however, is covered by the bacterial carbohydrate capsule, which renders it inaccessible for attachment to the host cell. Based on the available data, it has been proposed that the adherence process is initiated by attachment of the long pili to their specific membrane receptor on the mucosal surface. This appears to be followed by a strong retraction of the pilus fibers that, by displacing the capsule, enables close contact between the bacterium and the host cell membrane, unmasking the Knh element, which can then anchor to the host's respiratory epithelium (72).
Polysaccharide Capsule
Synthesis of polysaccharide capsules is a convergent evolutionary strategy shared by many important human pathogens that colonize the upper respiratory tract. Capsules are lipid-anchored, outer-membrane-associated, and surface-exposed structures that confer protection from phagocytosis and complement-mediated killing. Capsules are, then, crucial virulence factors that enable bacterial survival on the mucosal surfaces by thwarting the host's defensive response. These tools, originally developed and selected to allow colonization of the respiratory epithelium, also enable survival of encapsulated organisms in the bloodstream and deep body tissues. Bacterial capsules exhibit chemical and antigenic heterogeneity within members of the same species as the result of the highly selective pressure exerted by the immune system, and this diversity is the basis for serotyping strains of organisms such as pneumococci, Haemophilus influenzae, and Neisseria meningitidis.
It has been recently demonstrated that K. kingae is also coated with a polysaccharide capsule (72). A search of the draft genome of the organism detected a locus that showed remarkable similarity to the ABC-type capsule export operon ctrABCD, as well as separate gene homologs to the ctrE/lipA and ctrF/lipB genes of the taxonomically closely related N. meningitidis species (43, 72). Wild-type K. kingae organisms stained with cationic ferritin exhibited an electron-thick perimeter on the bacterial surface, consisting of an anionic bacterial capsule, when examined by thin-section transmission electron microscopy. When the ctrA gene was insertionally inactivated, nonmucoid colonies were visualized and no capsule could be demonstrated (72). Glycoyl analysis of bacterial surface extracts of the invasive K. kingae strain 269-492 by gas chromatography under acidic conditions, mass spectrometry, and nuclear magnetic resonance (NMR) revealed that the polysaccharide capsule contains N-acetylgalactosamine (GalNAc) and 3-deoxy-d-manno-oct-3-ulosonic acid (Kdo) with a →3)-β-GalpNAc-(1→6)-β-Kdop-(2→ structure, chemically identical to the capsule synthesized by Actinobacillus pleuropneumoniae serotype 5 (the causative agent of a contagious lower respiratory tract infection in swine) (73). A second capsular polysaccharide, extracted from a different invasive K. kingae strain (PYKK181), exhibited the chemical structure →6)-α-d-GlcNAcp-(1→5)-β-d-OclAp-(2→ (74), indicating that the K. kingae capsule shows chemical and, most probably, also antigenic heterogeneity, similar to the case with other bacterial pathogens. Preliminary results indicate that all K. kingae isolates produce a polysaccharide capsule, regardless of their clinical source (asymptomatic carriage or a variety of invasive diseases) (J. W. St. Geme, personal communication). These important findings may shed light on the peculiar age-dependent epidemiological curves of K. kingae carriage and disease, because immunity to polysaccharides in humans matures between the ages of 2 and 4 years (75), explaining the increased susceptibility of young children to mucosal colonization and invasive diseases caused by encapsulated organisms in general and K. kingae in particular and the reduction in the colonization rate and incidence of invasive infections in immunologically mature older children and adults.
Exopolysaccharides and Biofilm Production
Growth as biofilms is the usual lifestyle for bacteria in most environments and particularly on human body surfaces. Many colonizing species build up biofilms consisting of large quantities of crammed bacteria contained in a polysaccharide “slime” that firmly attaches to the mucosal epithelium. Life in such enclosed and crowded conditions protects the organism from the deleterious effects of the immune response, desiccation, and antimicrobial drugs. Consequently, biofilms play an important role in bacterial adherence and establishment of colonization, as well as in the pathogenesis of persisting and difficult-to-cure infections, such as chronic osteomyelitis or lung disease in cystic fibrosis patients (74, 76). The sequence of biofilm establishment, growth, and architectural remodeling is a precisely regulated and highly dynamic process. Periodic inhibition of biofilm formation is crucial for many pathogens because it makes possible the release of trapped bacterial cells, enabling dispersion and colonization of new body niches and hosts. This complex cycle is usually modulated by secretion of exopolysaccharides that do not remain attached to the bacteria. Bendaoud et al. have shown that K. kingae strain PYKK181 synthesizes a linear galactan homopolymer with the structure →3)-β-(1→6)-Galf-(1→ that exerts potent antibiofilm activity upon other bacterial species (74), and Starr et al. found a different exopolysaccharide in strain 269-492 that contains only galactose and has the structure →5)-β-Galf-(1→ (73). Targeted mutagenesis demonstrated that production of K. kingae's capsule and exopolysaccharide involves separate genetic loci for surface location (73).
It is speculated that K. kingae exopolysaccharides facilitate colonization of the pharyngeal epithelium by inhibiting biofilm production by other organisms competing for the same niche. It is also plausible that these exopolysaccharides play a role in the regulation of the periodic release of K. kingae cells from the biofilm matrix, enabling dissemination of the bacterium by droplet transmission.
RTX Toxin
In a study carried out by Kehl-Fie and St. Geme, it was demonstrated that K. kingae organisms exert a potent and wide-spectrum cytotoxic effect, being especially harmful to macrophages, leukocytes from many animal species, synoviocytes, and, to a lesser degree, respiratory epithelial cells (77). Using mariner mutagenesis followed by screening of mutants for loss of cytotoxic activity, a locus encoding an RTX toxin system was detected in the organism's genome (77). The locus consists of 5 genes named rtxA, rtxB, rtxC, rtxD, and tolC, located in a single cluster in the chromosome, required for the manufacture and secretion of the toxin. The locus is flanked by insertion sequences and has a decreased G+C content compared to the entire K. kingae genome, suggesting that, despite the DUS barriers that limit horizontal gene transfer, it has been acquired from a donor bacterial species (77). This possibility is further supported by finding that the rtxA, rtxB, and rtxC genes encode proteins that share >70% identity with their homologs in Moraxella bovis, whereas the rtxD and tolC genes encode proteins that exhibit 81% and 64% identity, respectively, with their N. meningitidis counterparts (77). Disruption of the RTX toxin gene rtxA by transposon mutagenesis caused loss of cytotoxic activity to cultured cell lines (77). Like the RTX toxins of other bacteria, K. kingae's RTX toxin contains an amino-terminal hydrophobic domain, potential lipidation sites, and a calcium-binding motif (77). Kingella kingae RTX toxin is a 100-kDa protein that appears to be secreted in the extracellular environment in a soluble form, as well as a component of outer membrane vesicles (OMVs) that are internalized by host's cells, suggesting that K. kingae utilizes multiple mechanisms for toxin release (78).
All carried and invasive K. kingae isolates studied so far produce RTX toxin, whereas it is conspicuously absent in the less virulent K. denitrificans and K. oralis, suggesting that this bacterial constituent is universally conserved in K. kingae because it improves colonization fitness by disrupting the oropharyngeal epithelium. Incidentally, the RTX toxin may also enable survival of K. kingae in the bloodstream and invasion of the skeletal system tissues (77) and therefore have a disease-promoting effect.
The potential role of K. kingae RTX toxin has been further investigated using an animal model of infection in a recent study (79). Intraperitoneal inoculation of 7-day-old Sprague-Dawley rats with 8 × 106 CFU of a virulent K. kingae strain isolated from a child with septic arthritis (strain PYKK081) resulted in a marked reduction in the circulating white blood cells (WBCs) compared with the leukocyte count in animals injected with a toxin-deficient mutant of the same wild-type strain (designated KKNB100), suggesting that the depressed acute immune response induced by the RTX toxin may represent a strategy aimed to guarantee K. kingae's subsistence in the host's bloodstream, skeletal tissues, and endocardium (79). Animals inoculated with the wild PYKK081 strain developed a rapidly fatal illness characterized by weight loss, bacteremia, abdominal wall necrotic lesions, and damage to the pups' thymuses, spleens, liver, lungs, and bone marrow, whereas inoculation of the animals with the KKNB100 mutant did not result in clinical disease or demonstrable bacteremia, corroborating the importance of the RTX toxin as a virulence factor and its role in the pathogenesis of disease (79). Remarkably, intraperitoneal or intra-articular inoculation of 21-day-old rats with the wild-type strain did not result in observable disease, bacteremia, or septic arthritis, indicating that the age-dependent susceptibility to invasive infections observed in humans can be closely reproduced in the animal model (79).
Outer Membrane Vesicles
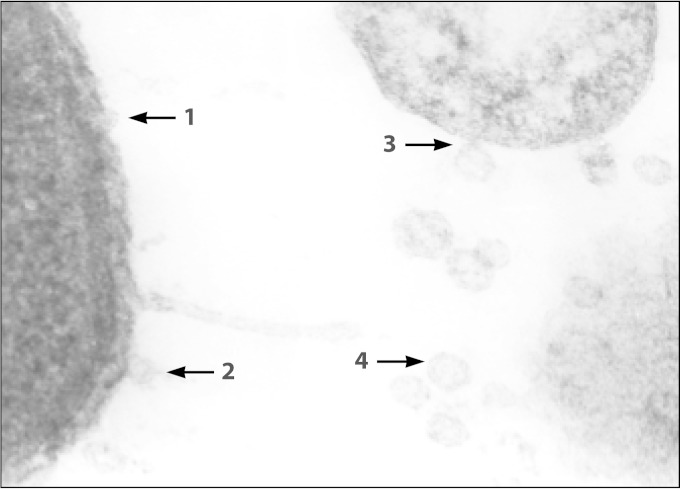
Gram-negative bacteria interact with the surroundings by releasing toxins and other proteins that exert distal effects without the need to expend energy in moving themselves. In addition, secreted material may reach sections of the environment that are inaccessible to the whole bacterium. Frequently, secretion of these bacterial products to the extracellular space is accomplished through formation of small (20- to 250-nm) spherical elements named OMVs. These structures consist of small portions of the outer membrane enclosing and entrapping periplasm proteins that bulge away from the cell, pinch off, and are then released (80) (Fig. 3). Maldonado et al. described blebbing OMVs in clinical K. kingae isolates that contain several major proteins, including the RTX toxin and the PilC2 pilus adhesin (78). These OMVs are hemolytic and leukotoxic and are internalized by human osteoblasts and synoviocytes in an in vitro model. Upon OMV acquisition, the host cells synthesize large amounts of human granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 6 (IL-6), suggesting that these cytokines may be components of the human inflammatory response to K. kingae joint and bone infections (78).
FIG 3.
Electron microscope picture of K. kingae organisms, showing sequential formation of outer membrane vesicles.
KINGELLA KINGAE CARRIAGE
Colonization Site
The human upper respiratory tract can be colonized by many potential bacterial pathogens, such as the pneumococci, Moraxella catarrhalis, or meningococci, that establish a foothold on the body's mucosal surfaces, persist, and spread from person to person. The colonized epithelial surfaces are also the gateway through which virulent bacteria enter the bloodstream, an event that may be followed by dissemination of circulating organisms to remote normally sterile body sites, such as the skeletal or the central nervous system, causing secondary focal infections.
For many years it was suspected that K. kingae could also be a member of the residing respiratory tract microbiota, and this assumption was founded on the anecdotal isolation of K. kingae from respiratory cultures of healthy individuals (1, 34, 81) and from the blood of patients with pneumonia (18, 82, 83) and the fact that many members of the Neisseriaceae family are upper respiratory tract commensals. In a pioneering study, biweekly nasopharyngeal and oropharyngeal cultures were obtained from young attendees of a child care facility over an 11-month period. A total of 109 of 624 (17.5%) oropharyngeal specimens grew K. kingae, whereas the organism was not recovered from the nasopharynx (84). These results have been confirmed in a more recent study in which 4,472 oro- and nasopharyngeal specimens were prospectively obtained from a cohort of 716 healthy children. A total of 388 (8.7%) oropharyngeal cultures but only a single nasopharyngeal culture grew K. kingae, indicating that the organism occupies a restricted upper respiratory tract niche (85).
Culture Detection of K. kingae in the Pharynx
Due to the extraordinary complexity and high concentration of the colonizing bacterial flora, culture detection of K. kingae in throat specimens poses obvious difficulties. A differential and selective medium combining blood agar with 2 μg/ml of added vancomycin (BAV medium) has been designed to facilitate identification of the organism in respiratory cultures (86). The rationale behind this formulation is to ease the identification of beta-hemolytic K. kingae colonies by inhibiting the Gram-positive flora. In a blinded study, BAV plates recovered K. kingae in 43 of 44 (97.7%) oropharyngeal cultures, compared to only 10 (22.7%) specimens seeded onto petri dishes containing plain blood agar (P < 0.001). The original BAV medium and similar media (87) have been successfully employed in epidemiological studies aimed to investigate the respiratory carriage of the organism (18, 58, 84, 85). If not plated immediately, inoculated swabs should be kept at room temperature in Amies or similar transport medium and promptly sent to the laboratory for further processing (84).
Detection by Molecular Methods
In recent years, novel molecular detection assays have enabled diagnosis of K. kingae infections in patients for whom cultures of joint exudates on routine media and in BCVs did not reveal the presence of the bacterium (88). This PCR-based strategy has been also implemented in investigations of K. kingae's carriage and the relationship between respiratory colonization and clinical disease.
Because the RTX toxin is produced by all K. kingae strains examined so far, the encoding RTX locus genes appear to be appropriate targets for detecting the organism in the blood, synovial fluid, and solid tissues, as well as in upper respiratory tract specimens (87–90). It has been shown that NAA assays targeting conserved segments of the RTX toxin-encoding genes (rtxA and/or rtxB) are able to detect as few as 30 CFU of the organism and therefore are more sensitive than PCR tests that amplify the 16S rRNA gene (88, 90, 91) or the cpn60 gene (90). The NAA assays targeting the RTX toxin genes are highly specific, can be applied to a variety of clinical specimens, and allow detection of strains exhibiting rtx locus polymorphisms (87–90, 92).
Employing a real-time PCR assay that amplifies portions of the rtxA and rtxB genes, Ceroni et al. detected K. kingae DNA sequences in the oropharynges of 8.1% of 431 young asymptomatic Swiss children and in all 27 patients with K. kingae osteoarticular infections confirmed by an NAA of the bone or joint exudates (93). More recently, the same research group found specific rtxA gene sequences in the oropharynges of all 10 children aged 6 to 48 months with a roentgenologically confirmed diagnosis of spondylodiscitis, suggesting that K. kingae was the causative agent (94). In a study comprising 123 patients younger than 4 years with skeletal system complaints, the DNA detection assay performed on an oropharyngeal specimen was positive in all 30 children with K. kingae osteoarthritis as proven by culture and/or NAA of the synovial fluid (95). In the same study, 8 oropharyngeal samples, derived from 84 patients with microbiologically unconfirmed joint or bone infections or with skeletal infections caused by other bacteria, were also positive for K. kingae DNA sequences (95), reflecting the background respiratory carriage of the organism in the young pediatric population (85). The practical implications of these results are that the sensitive NAA tests have a high negative predictive value and that failure to detect K. kingae-specific genomic sequences in a pharyngeal specimen may practically exclude the organism as the etiology of an osteoarticular infection. However, because K. kingae is carried on the pharynx by approximately 10% to 12% of children in the relevant age group (85) and by >25% of those in day care (84), the predictive value of a positive pharyngeal NAA for diagnosing an invasive infection is limited.
In a refined study, Basmaci et al. grew K. kingae in the pharyngeal cultures of 8 of 12 NAA-positive/culture-negative synovial fluid specimens of young children with arthritis (87). The researchers succeed in extracting and sequencing the rtxA gene amplicons from 6 PCR-positive synovial fluid samples and compared them with the rtxA sequences of the pharyngeal isolates. The 6 paired pharyngeal and synovial fluid amplicons were found to contain identical sequences, establishing a firm link between K. kingae organisms colonizing the pharynx and those invading the skeletal tissues (87).
More recently, a real-time PCR test that amplifies the toxin-encoding rtxB gene was employed to assess the bacterial load in asymptomatic pharyngeal carriers and children in whom the diagnosis of K. kingae joint or bone diseases was confirmed by a positive PCR test performed in either blood or skeletal system exudate (96). The number of amplification cycles required to obtain a positive result was used as a surrogate for colonization density. Contrary to what has been observed in infections induced by Streptococcus pneumoniae or H. influenzae type b, no quantitative differences in bacterial density between healthy carriers and sick children were found, suggesting that other factors are more important for the development of an invasive K. kingae infection (96). Using the same approach, it was shown that the bacterial density remains remarkably stable in colonized children aged 8 months to 4 years, despite substantial differences in the risk to develop a K. kingae infection during this wide age interval (97).
The sensitivities of cultures and NAA assays for detecting K. kingae colonization have been compared in a single study in which the yields of both approaches were assessed during the investigation of a large cluster of invasive diseases caused by the organism in a Paris day care center (98). Overall, 12 of 18 pharyngeal specimens were positive by real-time PCR, compared to 6 of 18 positive by culture on modified BAV medium (P < 0.01), suggesting that NAA assays are more sensitive than cultures to determine the true carriage rate. It should be noted, however, that when multiple strains are found to be circulating in the population, the culture approach has the advantage of enabling a comprehensive genomic comparison of recovered isolates. In addition, cultures detect living organisms, while the viability of K. kingae cells in clinical samples positive by NAA assays but negative by culture is uncertain (98). This aspect could be important when assessing the effectiveness of antibiotic prophylaxis to reduce pharyngeal K. kingae carriage.
Epidemiology of K. kingae Carriage
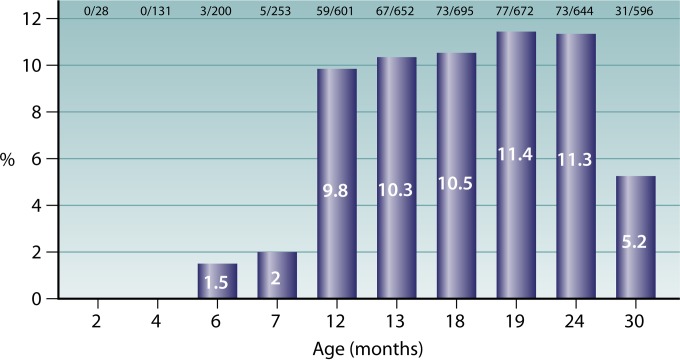
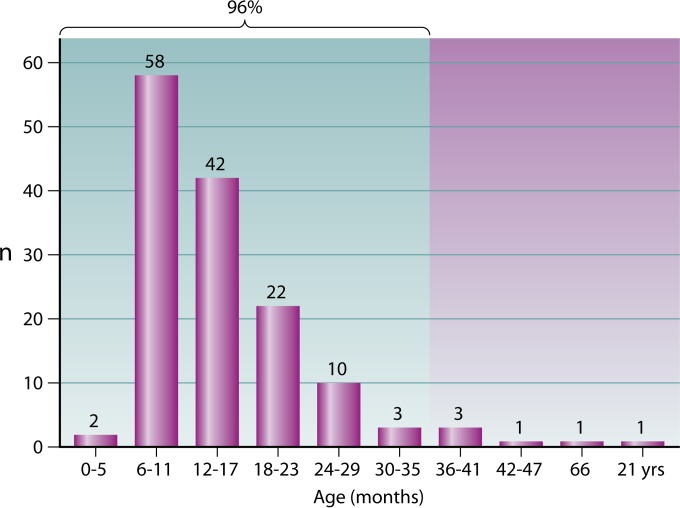
Because bacterial colonization of the mucosal surfaces is established at the interface between humans and their surroundings, the forces that shape the composition, acquisition, and elimination of residing organisms reflect a complex array of intermingling host, microbial, environmental, and socioeconomic determinants. Factors such as sampling season, the population's age and health status, crowding of living quarters, number of young children at home, day care attendance, antibiotic consumption, and smoke exposure may profoundly influence the results of prevalence studies (99). Monitoring the presence of individual components of the flora is also strongly dependent on technical and methodological aspects, such as sampling site, specimen collection technique, quality of swabs, time for transport to the laboratory, use of selective media, and number of bacterial colonies examined (99). Not surprisingly, results of epidemiological investigations on K. kingae carriage have found discrepancies in the prevalence rate, although the overall picture indicates that the pharyngeal colonization of the bacterium is strongly age dependent (85). In an early study carried out in southern Israel, K. kingae was not recovered in oropharyngeal cultures obtained from healthy infants aged <6 months, the prevalence of the organism was 10.0% in children aged 6 months to 4 years, and the prevalence decreased to 6.0% in older children (84). In a later study, oropharyngeal specimens submitted to a clinical microbiology laboratory for isolation of Streptococcus pyogenes were also plated onto BAV medium (100). The prevalence of K. kingae decreased significantly with increasing age: the organism was detected in 22 of 694 (3.2%) samples obtained from children younger than 4 years, in 10 of 679 (1.5%) of those derived from patients aged 4 to 17 years, and in 5 of 671 (0.8%) cultures from adults (P <0.001 for trend) (100). In a longitudinal study in which the younger age group was targeted, 716 healthy children living in southern Israel were sequentially cultured between the ages of 2 and 30 months (85). Kingella kingae was not isolated before the age of 6 months, and the colonization rate was low at 6 months, increased in 12-month-old children, remained relatively stable between 12 and 24 months of age, and decreased significantly at 30 months (P < 0.001) (85) (Fig. 4). Remarkably, very similar carriage rates were found in a study in which 431 children aged 6 to 48 months living in Geneva, Switzerland, were screened for K. kingae colonization by employing a NAA test, showing that the epidemiological features of K. kingae colonization are consistent among diverse pediatric populations and can be generalized (93). In a recent investigation carried out to identify risk factors for K. kingae carriage, multivariate analysis demonstrated that being 6 to 29 months old carries a strong and independent statistical association with oropharyngeal colonization (101), encompassing the age interval with the highest incidence of culture-proven infections (18, 102).
FIG 4.
Prevalence of pharyngeal K. kingae colonization among children aged 0 to 36 months.
Living conditions are known to affect the transmission of respiratory pathogens and associated morbidity. Populations in the developing world, as well as economically disadvantaged individuals living in Western countries, have high rates of colonization with respiratory organisms such as pneumococci and suffer from an increased burden of clinical disease (103). Similar to populations in the developing world, the indigent Bedouin residents of southern Israel, who live in crowded households and have large families, exhibit early acquisition and high respiratory pathogen colonization rates, as well as increased morbidity and hospitalization rates for infectious diseases (104). In a recently completed study in which the effect of living conditions on the acquisition of K. kingae was prospectively investigated, Bedouin children demonstrated significantly earlier carriage of the organisms than Jewish children who enjoy better socioeconomic status, and by the age of 13 months, 71 of 316 (22.5%) Bedouin children but only 47 of 363 (13.0%) Jewish children had already experienced colonization by K. kingae organisms (P = 0.002) (P. Yagupsky, unpublished data).
In a study aimed to determine the temporal pattern of K. kingae carriage, throat cultures were obtained between February and May, to represent the time of the year when invasive K. kingae infections are less common, and from October to December, coinciding with the peak attack rate for clinical disease (100). Overall, 21 of 1,020 (2.1%) specimens cultured between February and May and 16 of 1,024 (1.6%) of those studied from October to December grew the organism (P > 0.4). The lack of a seasonal pattern in K. kingae carriage was confirmed in an investigation of potential risk factors for pharyngeal colonization, which showed no significant association between month of the year and prevalence rate (101). It appears, then, that in addition to pharyngeal carriage, other, still-unidentified cofactors, perhaps seasonal viral respiratory infections, are important in the pathogenesis of invasive disease.
Carriage Dynamics and Turnover of Colonizing Strains
The dynamics of carriage of respiratory bacteria can be studied in longitudinal surveys in which the target population is repeatedly sampled over a prolonged period of time and isolates are analyzed using highly discriminative typing methods. In a cohort study, pharyngeal swabs were obtained from attendees in a day care facility in southern Israel over an 11-month period and cultured on BAV medium (84). Kingella kingae isolates thus detected were genotyped by employing a combination of PFGE and ribotyping with multiple restriction enzymes and immunoblotting with rabbit immune serum (105). To conclusively demonstrate strain identity and consequently person-to-person transmission of individual strains, a stringent criterion (complete identity by all three methods) was used for the data analysis. Overall, sporadic, intermittent, or continuous patterns of strain carriage were observed (105). Two distinct strains, characterized by a distinct combination of typing profiles, represented 28.0% and 46.0% of the studied isolates and, once they took root in the day care center, disseminated effectively, displacing a variety of older strains (105). A few children were found to carry a strain whose PFGE fingerprint differed from the prevalent profile by a single DNA band, suggesting gradual genetic divergence and microevolution of K. kingae in the course of prolonged carriage and repeated transmission events (105).
In a prospective investigation in which 716 healthy children were serially cultured between 2 and 30 months of age, the genomic similarity of recovered isolates was assessed by PFGE in the cohort members in whom K. kingae organisms were isolated on >1 occasion (85). Kingella kingae was detected in 283 (39.5%) children, of whom 64 had two positive cultures for the organism, 13 had three, and 3 had four. The study showed that the genotypic similarity of isolates was lost over time, and sequential colonization by organisms belonging to as many as four different PFGE genotypes was observed. Based on these data, carriage of K. kingae appears to be a dynamic process characterized by repeated acquisition and carriage of antigenically diverse strains, whereas simultaneous carriage of multiple strains is uncommon. After having been carried continuously or intermittently for a few weeks up to 8 months, colonizing strains are replaced by newly acquired strains, similar to the strain turnover noted in other bacterial pathogens that inhabit the respiratory mucosae (85). It should be pointed out, however, that in these two studies (85, 105) a single colony per positive culture was analyzed and, therefore, colonization by >1 K. kingae clone and/or persistence of previously carried strains at a reduced level, instead of strain extinction and substitution, cannot be ruled out. Recurrent detection of a strain that was isolated earlier but lost was infrequent (85, 105). This observation is consistent with eradication or at least a quantitative decrease in the presence of individual strains after a prolonged colonization period. It is suggested that sustained carriage of a given K. kingae strain induces a specific immune response that eliminates the colonizing organism but does not prevent acquisition of a novel strain characterized by a different array of antigenic determinants (106). This concept is backed by the strain-to-strain diversity of immunogenic and surface-exposed bacterial components that contribute to the colonization of the oropharyngeal mucosa, such as outer membrane proteins (OMPs) (107), the PilA1 pilus subunit (57), the RTX toxin (62, 90), and the K. kingae capsule (J. W. St. Geme, personal communication).
Immunity to K. kingae Carriage and Disease
The dynamics of K. kingae carriage and infection constitute a sequential phenomenon, characterized by almost complete lack of colonization and invasive infections in early life, followed by high colonization rates and increased morbidity in the 6- to 36-month age interval and a sharp decline of both carriage and invasive disease rates in children over 4 years of age and adults. This observation, coupled with the increased susceptibility of immunocompromised individuals to invasive K. kingae infections, indicates that an acquired immune response is required to shield from pharyngeal colonization and prevent clinical disease (18, 107). It should be noted, however, that whereas the rate of carriage of K. kingae is highest and remains stable between 12 and 24 months of age (Fig. 4), the epidemiological curve of children with clinical disease is markedly skewed to the left, and over 75% of patients are younger than 18 months (Fig. 5), indicating that acquisition of resistance to infection precedes the development of effective mucosal immunity to respiratory colonization.
FIG 5.
Age distribution of 143 patients with culture-proven K. kingae infections detected in southern Israel between 1988 and 2013.
Because the OMPs of many bacterial species play important functions in the establishment of mucosal colonization and, as such, elicit production of protective antibodies against mucosal and invasive infections, it was postulated that the antibody response to these antigens could serve as a tool to study the immune response to K. kingae carriage and disease. In a cohort investigation, serum samples were obtained in the acute and convalescent periods from children with culture-proven K. kingae endocarditis, occult bacteremia, and skeletal system infections and studied with an enzyme-linked immunosorbent assay (ELISA) employing the organism's OMPs as the coating antigen (106). IgG concentrations significantly increased in the convalescent period, whereas serum IgA antibodies decreased between the acute and convalescent phases, suggesting that the IgA immune response is short lived (106). When OMPs of the infecting isolate and those of heterologous strains were used as coating antigens, higher IgG antibody levels against the infecting strain were measured, indicating a more specific and probably a more effective immune response (106). When sera from pediatric patients with different K. kingae infections were reacted with OMP extracts of invasive strains, antigens bands of 108, 43, and 41 kDa were usually recognized, whereas reaction with other proteins showed variability depending on the strains and patient serum source (106). These experiments suggest that some immunogenic K. kingae OMPs are highly conserved, while others are inconstant and/or the exposed epitopes are polymorphic, translating to marked differences in the affinity of the antibody response.
In a longitudinal investigation in which the prevalence and fluctuations of levels of antibodies to K. kingae OMPs in a healthy population were studied, levels of IgG and IgA antibodies were measured in the sera of children at different ages with no previous clinical disease caused by the organism (107). Because IgA does not traverse the placenta, IgA- and IgG-type antibody levels were measured and compared to establish whether antibodies found in early childhood denote actual exposure to K. kingae antigens or represent maternally derived immunity. The mean IgG level was high at the age of 2 months, slowly declined thereafter (reaching an all-time nadir at 6 to 7 months), persisted at a low level until the age of 18 months, and peaked in children older than 24 months. Serum IgA was almost undetectable at 2 months, slowly increased between 4 and 7 months of age, and exhibited a further increment in children over 24 months of age (107). The scarcity of colonization and disease in early life, combined with absent serum IgA and high IgG levels, suggests that defense against mucosal carriage and infection is provided by vertically acquired immunity, because on the one hand, social contacts and potential sources of contagion multiply and maternal antibodies wane, and on the other, children older than 6 months experience increased rates of K. kingae carriage and disease. Low prevalence of respiratory colonization rates coupled with decreasing incidence of disease and rising antibody levels in older children most likely denote acquisition of immunological maturity and development of a protective immune response through reiterated exposure to the organism (107).
Transmission
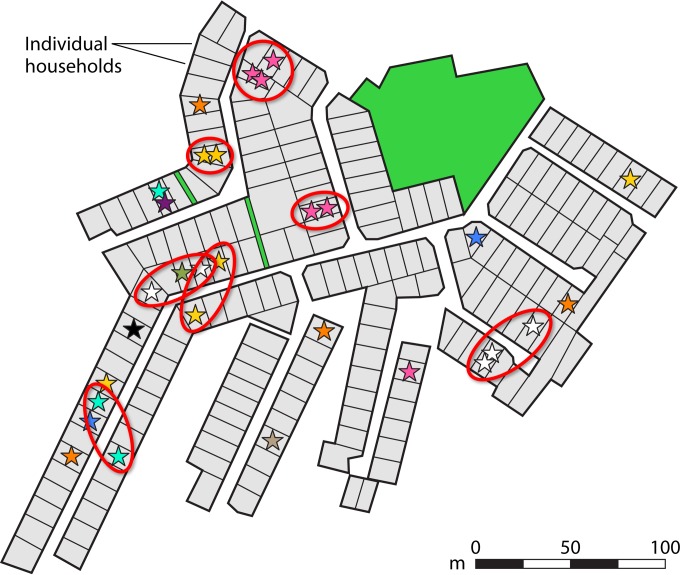
The persistence and dissemination of respiratory organisms in the human milieu are dependent on the successful establishment of endless chains of person-to-person transmission. In the last few decades, the development of sensitive molecular typing methods made it possible for the first time to discriminate between genetically different strains, enabling deep understanding of the dynamics of transmission of individual bacterial lineages in the human population across time, space, and social networks. The propagation of K. kingae was prospectively investigated in a large cohort of asymptomatic Israeli Jewish and Bedouin children living in separate cities and towns (58). All K. kingae isolates recovered from pharyngeal cultures during a 12-month period were analyzed by PFGE, employing the restriction enzyme EagI (58). Organisms derived from Bedouin children tended to differ from those isolated from Jewish children, corroborating the geographic isolation and relative lack of personal interaction between the two ethnic groups (58). Significant spatial clustering of clones was detected in Bedouin towns, quarters, and households, indicating transmission between family members and other close contacts (58) (Fig. 6). Because the traditionally nomadic Bedouin population has recently settled in towns keeping the ancestral tribal divisions, household location closely follows family ties and social mingling. Bedouin children do not usually receive out-of-home care services, and most of their social intercourse takes place within their extended families and clans, explaining the genomic similarity of K. kingae isolates detected in neighboring households. No association between place of residency and distribution of individual K. kingae clones, however, was noted among Jewish carriers, who live in Westernized conditions and attend day care centers since an early age. It is postulated that Jewish children are connected by multiple and complex social networks through which respiratory bacteria circulate, obscuring the connection between house location and spatial scattering of K. kingae strains (58).
FIG 6.
Spatial distribution of K. kingae clones carried in a Bedouin neighborhood as determined by PFGE with restriction enzyme EagI. Each star represents a positive pharyngeal culture, while the different colors represent distinct clones. Red ovals depict geographic clusters of identical strains.
Using a sensitive NAA assay, Kampouroglu et al. studied the intrafamilial transmission of the organism in a Swiss pediatric population. The research found that 55.0% of children with a PCR-documented invasive K. kingae infection and 40.0% of asymptomatic carriers had siblings with a positive pharyngeal PCR assay, suggesting dissemination of the organism within susceptible family members (108).
PATHOGENESIS OF K. KINGAE DISEASE
From Colonization to Clinical Disease
The link between K. kingae colonization of the oropharyngeal mucosa and the development of an invasive infection was convincingly demonstrated in three bacteremic children in whom a genomically identical organism was also recovered from a pharyngeal culture (109). This finding was later confirmed by the results of two studies employing NAA technology. In a survey comprising 27 young children with PCR-proven K. kingae osteoarthritis, a PCR-based assay performed on the pharyngeal swab was positive for rtxB gene sequences in all cases (95). In a second and more conclusive study, young children with arthritis and culture-negative/PCR-positive synovial fluid samples had K. kingae isolated from the pharynx. The rtxA gene sequences of the synovial exudate amplicons and those of the pharyngeal isolate were indistinguishable, suggesting that the oropharynx is probably the gateway through which invasive K. kingae organisms enter into the bloodstream, disperse, and invade the skeletal system and endocardium, for which the bacterium shows a striking affinity (87).
Strain Virulence and Tissue Tropism
Similar to observations made with other bacterial species, K. kingae's genomic heterogeneity is especially noticeable among carried organisms, whereas a large fraction of invasive diseases are induced by a few distinct strains, suggesting that K. kingae isolates differ in their virulence and some strains that colonize the pharyngeal epithelium have diminished invasive capability (64). Using an animal model, Basmaci et al. have recently demonstrated wide strain-to-strain variation in terms of virulence among K. kingae isolates (64). When 5-day-old albino Sprague-Dawley rats were inoculated intraperitoneally with 107 CFU of K. kingae, the ATCC type strain 23330 (a respiratory isolate) proved to be nonvirulent, whereas two invasive strains, derived from children with bacteremia and osteoarthritis, caused disease but showed significant differences in terms of animal survival (64).
In an investigation to characterize the K. kingae strains causing invasive infections in Israel in the period from 1991 through 2012 and the possible association, at the population level, of certain genotypes with specific clinical diseases, 181 invasive isolates from patients with bacteremia, arthritis, osteomyelitis, or endocarditis were typed by PFGE (110). A total of 32 genotypically distinct clones could be recognized, of which five (B, H, K, N, and P) were responsible for 72.9% of all cases and were detected countrywide for over 2 decades. Clone K showed a significant positive association with occult bacteremia, clone N with pediatric arthritis or osteomyelitis, and clone P with endocarditis. Other clones, which are frequently isolated from asymptomatic children in Israel (58), are rarely detected in patients with invasive diseases (110). This discrepancy suggests a compromise between transmissibility and virulence, because diseased persons are isolated from other individuals, administered antibiotic drugs, and may even have died from the disease, interrupting the chain of interpersonal transmission and causing the death of the pathogen. Clone N, which was a frequent cause of clinical disease among Israeli patients in the decade of the 1990s and exceptionally colonizes the pharynges of healthy children (58), was found to be significantly linked to skeletal system infections (110). These results imply that strains associated with deep tissue invasiveness may be rapidly eliminated from the pharyngeal mucosal surface and removed from the bloodstream, indicating that bacterial survival in different body sites requires a specific biological specialization. Interestingly, clone K organisms appear to present an ideal equilibrium between transmissibility and virulence. Organisms belonging to this clone predominated among attendees at a day care center in southern Israel as early as 1993, persisting in the oropharynx for up to 4 months (105), and this was the second most common pharyngeal clone in healthy Jewish children between 2006 and 2007 (58). On the other hand, clone K organisms exhibit enhanced invasive capabilities, representing 41.7% of all southern Israel isolates from patients over the last 2 decades, and were accountable for the excess of K. kingae infections detected among children in the region (104). Remarkably, all clone K isolates harbor multiple copies of a 33-bp fragment of the rtxA gene, which is rarely found in other K. kingae organisms (64). The possibility that the repetition of the toxin-encoding gene adds to the invasiveness and epidemic success of clone K is intriguing but remains, as yet, unproven.
The PFGE results indicating association of Israeli clones of K. kingae with particular infections have been recently corroborated in an international study in which 324 strains derived from unrelated individuals were typed by MLST and results were correlated with the patient's clinical syndrome (65). ST-35, ST-14, and ST-25 (which correspond to PFGE clones N, H, and Sp, respectively) were significantly associated with pediatric arthritis or osteomyelitis, and ST-24 (PFGE clone P) was associated with endocarditis (65).
In two recent reports, K. kingae organisms belonging to ST-25 were found to be strongly associated with childhood osteomyelitis, causing a cluster of 4 bone infections among 5 affected day care attendees (98) and later an additional sporadic case (111), and an ST-6 strain caused a cluster of 3 cases of osteomyelitis in an Israeli day care center (112). These observations imply that carriage of particular strains entails increased risk for clinical disease and invasion of specific body tissues.
Viral Infections as Cofactors in K. kingae Disease
Observations made at the time of patients' presentation to the hospital and before K. kingae disease is microbiologically confirmed frequently reveal symptoms and signs consistent with a concurrent nonspecific upper respiratory infection, hand-foot-mouth disease (113), or varicella virus (114, 115)-, or herpes simplex virus (18, 114, 116)-induced buccal ulcers. In a study aimed to assess the effect of acyclovir administration on the duration of symptoms and viral shedding in children with culture-proven herpetic gingivostomatitis, 29 affected patients also underwent bacteriological blood cultures. Four (13.8%) of these children were bacteremic, and K. kingae was isolated in all cases (114). Because the peak incidence of infections with primary herpes simplex virus and many respiratory viruses coincides with the age of K. kingae carriage, it seems plausible that damage to the mucosal layer caused by a previous or concomitant viral disease facilitates the entry of colonizing K. kingae organisms into the bloodstream and dissemination to distant sites (18, 100, 109). In a recent report, Basmaci et al. strengthened the link between invasive K. kingae disease and viral upper respiratory tract comorbidity by detecting rhinoviruses in two children with concomitant PCR-proven K. kingae infections of bone and soft tissues (111).
EPIDEMIOLOGY OF INVASIVE K. KINGAE DISEASE
As occurs with other bacteria that colonize the upper respiratory tract mucosal surfaces, at any given time, the number of asymptomatic children that carry K. kingae is far in excess of those with clinical disease. For instance, the calculated risk of young Swiss carriers to develop a K. kingae infection of the skeletal system was found to be <1% per year (93). Because the entire pediatric population of southern Israel receives health services in a single hospital (the Soroka University Medical Center [SUMC]) in which the BCV technique has been regularly employed for over 2 decades, the morbidity caused by culture-proven K. kingae infections could be accurately computed in this captive population. Despite a carriage rate of 5% to 12% in young children living in the region (85), the annual incidence of invasive K. kingae disease was only 9.4 per 100,000 children aged <5 years (102). It should be pointed out, however, that because the culture detection of K. kingae remains suboptimal, even when the BCV laboratory method is used, this figure can be considered only a minimal estimate.
Between 1988 and 2013, a total of 143 culture-confirmed cases of invasive K. kingae infections were identified at the SUMC, including 141 children younger than 4 years, of whom only two were aged <6 months. A male-to-female ratio of 1.3 has been found in a large nationwide Israeli study comprising over 300 patients (102). In Israel (117) and Sweden (6), invasive K. kingae diseases occur throughout the year, peaking during the fall and early winter months and being far less common between February and April (102, 117).
Child Care Facilities, Colonization, and Disease
During the last decades, a growing proportion of children living in countries in the developed world countries have attended out-of-home care facilities. This phenomenon has important public health significance because the risk of transmission of pediatric pathogens and the occurrence of mucosal and invasive infections are significantly increased among day care center attendees (118, 119). Respiratory organisms readily spread by direct contact or through fomites among youngsters who share objects coated with respiratory secretions or saliva (119). Day care center classes are attended by children of similar age, immunological immaturity, and susceptibility to bacterial pathogens. Therefore, once a virulent strain has been introduced in a crowded facility, rapid propagation of the organism and outbreaks of disease may occur.
In a survey comprising 1,277 children younger than 5 years referred to a pediatric emergency department in southern Israel, out-of-home care was strongly and independently associated with K. kingae colonization (odds ratio, 9.66 [95% confidence interval, 2.99 to 31.15]; P < 0.001) (101). In a prospective cohort investigation, 35 of 48 (72.9%) children attending a day care center who were cultured fortnightly over an 11-month period showed oropharyngeal K. kingae colonization at least once (84). At any given time, an average of 27.5% attendees carried the bacterium, a much larger fraction than that found in the general pediatric population of comparable age (84). Genotyping of the colonizing isolates demonstrated that identical strains were simultaneously or successively carried by multiple attendees, indicating child-to-child spread (105). In an ongoing study, the prevalence of K. kingae among children attending elementary schools was less than 3%, but PFGE typing of the recovered isolates showed temporal clustering of clones among classmates, suggesting that transmission of the organism continues well beyond preschool age (Yagupsky, unpublished data).
Outbreaks of invasive K. kingae infections, including almost the entire spectrum of the disease (septic arthritis, osteomyelitis, spondylodiscitis, endocarditis, and meningitis), have been reported from French (98), American (113, 120), and Israeli (112, 121) day care facilities in the recent decade. The epidemiological investigation of these events revealed high disease attack rates among attendees, ranging from 14% (120) to 21% (98, 121). Clustering of cases within a 1-month period was noted, as well as an elevated prevalence of K. kingae carriage among asymptomatic young contacts of the index patients (up to 45% in a U.S. cluster, as determined by culture on selective medium [120], and 85% in the French outbreak, as demonstrated by an NAA method [98]). The age range of the colonized and infected day care center attendees was 8 to 25 months, coinciding with the period in life of highest K. kingae carriage (85, 100) and susceptibility to invasive disease (102). Clinical isolates from infected children were genotypically identical to those detected in the oropharynges of their respective classmates (113, 121, 122).
The strain implicated in the day care outbreak of bone infections that occurred in 2005 in an Israeli facility belonged to the aforementioned highly virulent clone K and to MLST ST-6 (112), and the organism that caused the North Carolina cluster (113) was identical to a strain isolated in 11.3% of healthy carriers (58) and in 11.6% of patients with invasive infections in Israel (110). The global circulation of these organisms shows that outbreaks of infection usually originate in bacterial populations characterized by superb colonization capability, enhanced transmissibility, and remarkable invasiveness (64, 65).
CLINICAL FEATURES OF K. KINGAE DISEASE
Invasive K. kingae disease usually affects previously healthy children aged less than 4 years (102), whereas older children and adults frequently have predisposing conditions such as failure to thrive (102), congenital heart disease (102), prolonged corticosteroid therapy (102), primary immunodeficiency (123, 124), hematological malignancies (6, 125), liver cirrhosis (126, 127), end-stage renal disease (128), sickle cell anemia (126), diabetes mellitus (21, 128), cardiac valve pathology (9, 129, 130), systemic lupus erythematosus (131–134), rheumatoid arthritis (135), renal transplants (9), solid tumors (82), or AIDS (22, 128, 136, 137).
Children with K. kingae infections other than endocarditis commonly present with mild symptomatology, requiring an elevated index of clinical acumen. Many patients are afebrile or have a mildly elevated body temperature and are in good general condition, and the yield of blood cultures is low, suggesting that the bacteremic stage of the disease is transient (18, 102, 117). Recent or concomitant rhinorrhea, pharyngitis, stomatitis, or diarrhea is noted in the majority of cases (102). With the exception of patients with endocardial involvement who develop embolic phenomena (21, 132, 138), a single body system is usually affected.
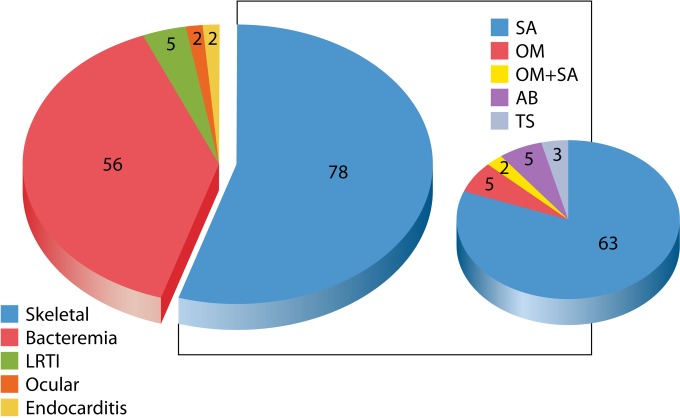
The vast majority of medical reports on K. kingae consist of descriptions of single cases of disease or small patient series in which rare conditions are, most likely, overrepresented. The large body of information gathered at the SUMC provides a more accurate and unbiased picture of clinical K. kingae morbidity (Fig. 7). Between 1988 and 2013, septic arthritis, osteomyelitis, or tenosynovitis represented the prime manifestation of K. kingae disease and was detected in 78 of 143 (54.5%) individuals, followed by occult bacteremia in 56 (39.2%), bacteremic lower respiratory infection (laryngotracheobronchitis and pneumonia) in 5 (3.5%), ocular infections (corneal abscess and periorbital cellulitis) in 2 (1.4%), and bacterial endocarditis in 2 (1.4%), including the oldest child (aged 66 months), who suffered from supravalvular aortic stenosis due to Williams' syndrome, and the only adult patient in the series, who also had systemic lupus erythematosus. Other, more rare syndromes, including spondylodiscitis, urinary tract infection (24), meningitis (139), peritonitis (140), and pericarditis (141), were not recorded in this series.
FIG 7.
Clinical syndrome in 143 consecutive patients with K. kingae infections diagnosed in southern Israel between 1988 and 2013. LRTI, lower respiratory tract infection; SA, septic arthritis; OM, osteomyelitis; AB, abortive; TS, tenosynovitis.
Skeletal System Infections
The role of K. kingae as a pathogen of the skeletal system is mostly limited to the young age group (18, 142, 143), whereas occurrence of joint or bone disease in older children or adults is uncommon (6, 25, 127, 135, 144–150). In a study in which conventional cultures, BacT/Alert BCVs, and real-time PCR with universal and pathogen-specific primers targeting the cpn60 gene were employed, K. kingae was implicated in 35 of 46 (76.1%) children younger than 4 years with septic arthritis and in 9 of 17 (52.9%) with osteomyelitis with or without associated joint invasion, and the organism was not detected in any of 20 older children with skeletal system infections (151). In a recent large study, 11 of 15 (73.3%) children with joint or bone infections were positive for K. kingae DNA sequences by broad-range PCR and later confirmed with species-specific primers, whereas the organism was not detected in any of 126 PCR-positive specimens from adult patients (152).
Suppurative arthritis constitutes the most common K. kingae infection of the skeletal system in childhood. Sixty-three of the 78 (79.5%) pediatric patients with skeletal system involvement diagnosed at the SUMC since 1988 had joint infections, 5 (6.4%) had osteomyelitis, 2 (2.6%) had both, 3 (3.8%) had tenosynovitis, and 5 (6.4%) presented with K. kingae bacteremia and transient skeletal system symptoms, suggesting an abortive infection. In a recent report comprising all 230 children with culture and/or NAA assay-confirmed K. kingae infections of the skeletal system diagnosed over a 4-year period in two large European pediatric orthopedic units, 178 (77.4%) had arthritis, 40 (17.4%) had osteomyelitis, and 12 (5.2%) had spondylodiscitis (153).
In an Israeli nationwide study comprising 169 pediatric patients with skeletal system infections, the organism was isolated from a synovial fluid sample in 109 (64.5%), from a bone exudate in 12 (7.1%), and from the blood in 48 (28.4%), emphasizing the importance of the blood culture as a diagnostic tool (102).
Septic arthritis.
Since the adoption of conjugate vaccination programs, a rapid drop in the incidence of H. influenzae type b arthritis has occurred (154, 155), whereas K. kingae has emerged as the most common etiology of joint infections in children 6 months to 3 years of age (13, 14, 16). Although the mutual relationships (competition, symbiosis, etc.) between the different components of the resident upper respiratory tract are complex and the potential antagonism between carried K. kingae and H. influenzae type b organisms has not been investigated, the increasing importance of K. kingae as a skeletal system pathogen does not appear to have resulted from the introduction of the conjugate vaccine, because colonization of H. influenzae type b in the prevaccine era was uncommon and the rate was usually lower than 5% (156). Rather, it seems that K. kingae is being increasingly implicated in the causation of pediatric septic arthritis because of the use of novel and more sensitive microbiological methods in recent years.
The disease generally involves the large weight-bearing knee, ankle, or hip joints in over 80% of cases (15, 102, 157–160), followed by the wrist, shoulder, and elbow (102, 160–169). However, the metacarpophalangeal, sternoclavicular, tarsal, and sacro-iliac joints (148, 170–173), which are rarely affected by other bacterial species, are involved with unusual frequency in K. kingae arthritis (18). Involvement of two joints was observed in 8 of 140 (5.7%) children with K. kingae suppurative arthritis (102), a proportion comparable to that found in infections induced by traditional pathogens (174). Inflammation markers measured in the blood, such as the white blood cell count, erythrocyte sedimentation rate, and C-reactive protein (CRP) levels, are frequently normal, while the joint fluid shows <50.0 × 109 WBC/liter (171) in more than 20% of patients, meeting the laboratory criterion used to exclude bacterial arthritis. Microscopic examination of the exudate rarely reveals the presence of K. kingae (18, 102, 160), probably because of the low bacterial concentration (from 11 CFU/ml to 300 CFU/ml) (175) and the difficulty in visualizing the Gram-negative bacilli against the pink-stained fibrin background. Because of the mild clinical presentation of K. kingae arthritis, when the disease involves the hip joint, patients may be mistakenly diagnosed as having transient synovitis (176), a benign inflammatory condition that is managed without antibiotics. Employing a diagnostic predictive algorithm to differentiate between the two conditions (177), 20 of 28 (71.4%) Israeli and Spanish children with culture-proven K. kingae of the hip joint would have been considered to have a ≤40% probability of infectious arthritis, and the true nature of the disease was revealed only by BCVs seeded with blood or synovial fluid specimens (178–180). It should be pointed out that this predictive model was developed many years ago when K. kingae's role in pediatric septic arthritis was still unrecognized, and none of the patients included in the original and subsequent validation studies had a proven infection caused by this organism.
Although children with K. kingae arthritis show a milder symptomatology than patients with joint infections caused by other bacteria, it is disputed whether the patients' clinical features and/or laboratory test results are specific enough to accurately predict the etiology of the disease. In a retrospective study, Basmaci et al. compared the clinical and biological characteristics of 64 French pediatric patients with NAA assays positive for K. kingae arthritis and 26 children with Staphylococcus aureus infections (181, 182). Patients with K. kingae infections were younger than those with staphylococcal joint disease (median age, 1.4 years versus 7.9 years; P < 0.001), and their hospital stay was shorter and characterized by fewer complications, yet the two populations did not significantly differ in terms of duration of symptoms, body temperature, white blood cell counts, and concentrations of a variety of acute-phase reactants (181, 182). In a Swiss study, Ceroni et al. compared 30 children with K. kingae joint infections and 30 children with arthritis caused by S. aureus, S. pyogenes, Streptococcus agalactiae, and H. influenzae type b (183). A clinical and laboratory score combining body temperature of <38°C, serum C-reactive protein (CRP) level of <55 mg/liter, leukocyte count of <14.0 × 109/liter, and <150 band forms/mm3 was built, and the data for the two patient groups at presentation were compared. Whereas 29 of 30 (96.7%) patients with K. kingae infections displayed fewer than 2 predictors, 27 of 30 (90.0%) children with arthritis caused by other bacteria presented with 3 or 4 predictors. Consistent with the concept that K. kingae behaves like a low-grade-virulence pathogen when invading the skeletal system (160), magnetic resonance imaging (MRI) of 21 patients with osteoarticular infections caused by the organism showed bone and soft tissue reactions less frequently than that of 10 patients with skeletal system infections caused by Gram-positive cocci, whereas epyphyseal cartilage abscesses were present in K. kingae disease only (184). These favorable experiences suggest that MRI may enable early differentiation (before culture or NAA tests results are known) between K. kingae infections and those caused by traditional pathogens, but this needs independent confirmation. Until the question is definitely settled, it seems prudent to administer wide antibiotic coverage to all children with suspected joint infections, pending definitive bacteriological test results.
Osteomyelitis.
Kingella kingae affects the tubular tibia, femur, humerus, radius, or ulnar bone in more than half of the cases (102, 185–192), yet involvement of the pelvis (193, 194), calcaneus (102, 195–197), talus (172, 198, 199), sternum (16, 130, 200), or clavicle (201) is also common (18, 102, 153, 172). Epiphysial invasion, which rarely occurs in osteomyelitis of other etiology, is frequently observed in K. kingae infections (152, 164, 188, 202, 203).
Kingella kingae osteomyelitis habitually has an insidious onset, and the vast majority of patients are diagnosed after more than 1 week of evolution (102, 130, 170, 172, 203, 204). The unossified epiphysis of infants receive blood supply from a metaphyseal capillary network that is obliterated before the age of 12 months. Therefore, bone infections can easily progress from the growth plate to the epiphysis and articular space, and in a substantial fraction of cases, osteomyelitic foci of the femur or humerus rupture into the adjacent hip or shoulder articulations, causing concomitant arthritis (102). The high-contrast resolution and multiplanar imaging offered by MRI have resulted in detection of large intraosseous (“Brodie”) abscesses involving the epiphyses, metaphyses, and cartilage that fistulize through the contiguous joint in 9 of 40 (22.5%) children with K. kingae osteomyelitis (153, 204). In two pediatric cases of K. kingae osteomyelitis, ascertained by either culture isolation or NAA, examination of the osteolytic lesion revealed a typical Langerhans cell histiocytosis picture, indicating that a systematic search for the organism should be carried out when this rare diagnosis is entertained in young children (189, 205). Of note, despite the frequent diagnostic delay and severe radiological pictures in some cases of osteomyelitis, no evolution to chronicity or significant residual orthopedic disabilities have been reported (18, 152).
Spondylodiscitis.
Historically, tubercular invasion of the intervertebral space from a contiguous bony focus was the most common etiology of spondylodiscitis. The declining incidence of pediatric tuberculosis in Western countries in recent years and increasing use of sensitive laboratory methods have resulted in the detection of K. kingae in more than one-quarter of children younger than 5 years with hematogenous spondylodiscitis (94, 206–208), whereas isolation of the organism in older children (209, 210) and adults (150, 211) is rare. It is presumed that the bacterium penetrates into the rich vascular network that traverses the vertebral endplates cartilage and enters the annulus in young children during a bacteremic K. kingae episode. Because intervertebral disk biopsies and aspirations are rarely performed in children (212, 213) and by the time patients present with spinal symptoms, the bacteremic phase has already passed, it is presumed that many cases of K. kingae spondylodiscitis remain undiagnosed. In a recent study, all 10 children with MRI-proven spondylodiscitis had a positive K. kingae real-time PCR assay in an oropharyngeal sample, whereas only two blood specimens were positive for the test, and all blood cultures remained sterile (94). Despite the already discussed limitations of this strategy, detection of pharyngeal carriage of the organism by culture or NAA assays may provide reasonable suspicion that K. kingae is responsible for the infection, whereas a negative NAA test could practically exclude the organism as the culprit.
Kingella kingae spondylodiscitis generally involves a single intervertebral disk (213), usually at the lumbar spine level and, with decreasing frequency, the thoracolumbar, thoracic, lumbosacral, and cervical spaces (7, 20, 207, 209, 214–218). Patients with this syndrome show gait disturbances, refuse to sit or walk, or complain of back pain. In more advanced or neglected cases, physical examination may reveal neurological signs. Roentgenological and MRI studies show narrowing of the intervertebral space (191, 207). Although intravertebral and spinal subdural abscesses have been detected by MRI (153), if it is adequately treated with antibiotics and paraspinal purulent collections and prompt surgical drainage are performed, K. kingae spondylodiscitis runs a benign clinical course. Residual narrowing of the intervertebral space is uncommon, and the disease leaves no permanent neurological deficits (191, 207).
Abortive skeletal infections.
Although bacterial infections of the skeletal system follow an unrelenting progress unless treated with antibiotics, self-limited involvement of joints and/or bones in the course of a K. kingae bacteremic episode has been reported, suggesting an abortive clinical course (168, 178, 187, 219–222). Patients with this unusual syndrome present with symptomatology suggestive of joint or bone disease, such as localized pain or limited limb motion, but lack objective signs of osteoarthritis, and therefore, antibiotic therapy is not initially provided (219). By the time K. kingae is recovered from a blood culture, the febrile condition, as well as the subjective complaints, have mostly subsided. Although a few children made an uncomplicated and full recovery without receiving antibiotic therapy (220, 221), it should be kept in mind that circulating K. kingae organisms may invade the cardiac tissues with severe consequences, and therefore, adequate antibiotics should be administered without delay whenever the bacterium is identified in a blood culture (187).
Soft Tissue Infections
A diversity of soft tissue infections, including cellulitis (185), tenosynovitis and dactilytis, (171, 223, 224), bursitis (225), and subcutaneous (6, 225), presternal (199, 200, 226, 227), and intramuscular (111) abscesses, has been described. Detection of the causative organism has been performed by culture of the aspirated exudate (171, 199, 200, 215, 225, 227), by a blood culture (171, 185, 223), or by an NAA assay (111, 200, 224).
Occult Bacteremia
Isolation of Kingella kingae from the blood without evidence of endocarditis or another nidus of infection has been reported repeatedly in children (11, 12, 220, 222, 225, 228–231) and exceptionally in adults (6, 126, 129, 232), and this syndrome represents the second most frequent expression of K. kingae disease in pediatric patients (18). A disseminated maculopapular rash, resembling that described in systemic meningococcal or gonococcal infections (125, 126, 130, 222, 233), or frank Henoch-Schonlein purpura (234) has been described in a few patients.
Mild to moderate fever is usually recorded, and the mean leukocyte count frequently is less than 15 × 10.09/liter (12, 102). Because of the seemingly benign clinical presentation, the current decision algorithms for managing febrile children aged 6 to 36 months with no apparent clinical source, which are based on the height of body temperature and presence of marked leukocytosis for drawing blood cultures (235), frequently fail to detect occult K. kingae bacteremia, and many events probably are undiagnosed. In a survey carried out in a pediatric emergency room in which blood cultures were obtained from febrile children without regard to the height of the fever or the blood leukocyte count result, K. kingae represented a common agent of bacteremia in the pediatric population of southern Israel (11).
Endocarditis
The genus Kingella is represented by the “K” in the acronym HACEK, which comprises a group of fastidious oral Gram-negative organisms responsible for 6% of all cases of endocarditis in the general population (236–239). Kingella kingae appears to cause a higher burden of endocardial infections in children and was the etiological agent of 4 of 51 (7.8%) episodes in a tertiary care pediatric hospital in Israel (240) and of 6 of 85 (7.1%) cases among New Zealand children (241).
Contrary to observations made with other K. kingae infections, only three-quarters of all pediatric patients with endocarditis caused by K. kingae are younger than 3 years (18, 242). Antecedent poor dental hygiene (243) or dental extractions (244) have been reported in adolescents and adult patients. A native valve is affected in 95% of children (138, 168, 245–252), whereas native (133, 245, 252–255) and prosthetic (21, 23, 243, 256–259) valve involvement have been reported with approximately similar frequency in adult patients. The mitral valve is affected in over 90% of cases (113, 124, 130, 133, 168, 244, 245, 247, 248, 250, 252, 255, 257, 260–266), followed by the aortic valve (6, 23, 243, 246, 249, 253–256, 259, 267, 268), while tricuspid (269) or pulmonary (130) valve endocarditis is exceptional. Involvement of multiple valves has been also described (255, 259). A wide variety of cardiac malformations (9, 124, 130, 133, 252, 262, 269) and rheumatic fever (21, 133, 259) are the usual predisposing factors, but many pediatric patients have no antecedent valvular disease (113, 130, 138, 242, 244, 247–251, 263, 266, 267, 270). A single case of endocarditis in a 4-year-old child occurring a few months after the percutaneous correction of a ventricular septal defect using a transcatheter device has been reported (271). The blood leukocyte count and other markers of inflammation, such as the erythrocyte sedimentation rate and CRP levels, are significantly increased in patients with K. kingae endocarditis compared to those recorded in patients with other infections. However, no laboratory result reliably diagnoses or excludes endocardial invasion by the organism (6, 102, 242).
Despite K. kingae's remarkable susceptibility to antimicrobial drugs, serious complications, such as embolism or mycotic aneurism to the femoral (9, 130, 251), brachial (124), or ophthalmic (9) arteries, valvular insufficiency (113, 242, 245, 247, 249, 250, 264, 269), congestive heart failure (133, 249, 272) or cardiogenic shock (21, 130, 258), pulmonary infarction (269), mitral valve rupture (244) or perforation (242, 250, 263), paravalvular abscess formation (249, 259, 265), cerebrovascular accidents (113, 168, 242, 255, 257, 261–263, 265, 266, 272, 273), meningitis (113, 138, 242, 245), brain abscess (251), and embolic arthritis (138) and cellulitis (252), are common. Emergency cardiac surgery has been prescribed for life-threatening complications that do not respond to conservative medical treatment (9, 130, 244, 248–250, 259, 263, 267, 271, 272), many patients require late valvuloplasty (269) or valve replacement (242, 247, 259), and the overall mortality rate of the disease is >10% (9, 18, 21, 113, 133, 242, 266). This unusual clinical aggressiveness is probably related, in part, to delays in the identification of the bacterium (51) rather than failure to isolate the organism because of insufficient length of incubation (273). Because of the severe implications of K. kingae endocarditis, it has been strongly advised that individuals from whom the bacterium is isolated from a normally sterile site should undergo a careful and complete echocardiographic assessment (18, 115).
Lower Respiratory Tract Infections
Kingella kingae has been recovered from blood or respiratory cultures of immunocompromised as well as otherwise healthy pediatric and adult patients with laryngotracheobronchitis (18), epiglottitis (82), pneumonia (83, 274), and pleural empyema (275).
Meningitis
Kingella kingae meningitis may result from the hematogenous seeding of the organism in the central nervous system during a bacteremic episode (276–280) or by migration of septic emboli or rupture of a mycotic aneurism in patients with endocarditis (113, 132, 138, 242, 245, 272). The disease has been diagnosed in children (113, 138, 245, 277–280), adolescents (139), and adults (132, 242, 272, 276). The clinical course of K. kingae meningitis secondary to endocardial infection is severe and may leave permanent neurological sequelae such as hemiplegia (280) or hemiparesis (272), aphasia (272), and ophthalmoplegia (139) among survivors.
Ocular Infections
Kingella kingae has been recovered from patients with different ocular infections, including palpebral abscess (34), keratitis (281, 282), corneal ulcer (283), endophthalmitis (130, 284), orbital cellulitis (285), and periorbital cellulitis (Yagupsky, unpublished data).
Other Infections
Anecdotal cases of K. kingae pericarditis (141), peritonitis (140), urinary tract infection (24), and aptho-stomatitis (286) have been also reported in adult patients.
DIAGNOSIS OF INVASIVE K. KINGAE INFECTIONS
Detection by Culture
The isolation of K. kingae on bacteriological solid media is clearly unsatisfactory (17, 18, 287, 288). The sensitivity of cultures can be significantly enhanced by inoculating synovial fluid aspirates into aerobic BCVs from commercial systems such as Bactec (17, 289), BacT/Alert (173, 290–292), Hémoline DUO (129, 293), Isolator 1.5 Microbial Tube (175), and in-house-made liquid media (294). In a pioneer study, K. kingae was detected in 25 synovial fluid specimens seeded into aerobic Bactec bottles after an average of 4 days, whereas the accompanying culture plates recovered the organism in only two cases (17). Subcultures of positive BCVs onto blood agar and chocolate agar plates grew the organism without difficulty, indicating that routine bacteriological media support K. kingae's growth requirements. This observation confirms that synovial fluid has potent antibacterial properties (295), exerting an inhibitory effect on K. kingae organisms. Inoculation of the minute exudate sample aspirated from the joint of a young child in a large volume of liquid medium contained in the BCV diluted the noxious effect of the exudate, improving the growth of fastidious K. kingae organisms (17, 18). When synovial fluid samples obtained from Israeli and French children with arthritis were inoculated into Bactec (13) or BacT/Alert (14) vials, K. kingae was detected in one-half of the patients with bacteriologically documented infections.
Despite the noticeable improvement in the isolation of K. kingae by the BCV method, the culture detection of the organism remains problematic and the question of which blood culture systems and broth media are best for recovering K. kingae remains unsolved (296). In an experiment in which BacT/Alert 3D vials were spiked with different concentrations of a variety of fastidious blood-borne pathogens, K. kingae organisms remained undetected despite prolonged incubation (297). In an in vitro study aimed to identify factors influencing the isolation of K. kingae from BacT/Alert BCVs, it was demonstrated, as expected, that the sensitivity of cultures diminishes and the time to detection increases with low bacterial concentrations (298). Strikingly, the pediatric vial was clearly inferior to the adult one in terms of overall recovery and sensitivity at low bacterial concentrations, and the time to detection was more prolonged, whereas addition of blood partially corrected these deficiencies (298).
Using simulated joint fluid cultures, Høst et al. assessed the performance of different media belonging to two widely used commercial blood culture systems (BacT/Alert and Bactec 9240) for recovering K. kingae from skeletal system infections (292). The researchers inoculated BCVs with pooled synovial fluid aspirated from adult patients with noninfectious conditions and seeded with 24 different K. kingae strains (292). The investigated liquid media showed substantial differences in terms of sensitivity: all 24 strains were recovered by the BacT/Alert Aerobic and BacT/Alert Pedi-bacT vials, compared to 21 (87.5%) detected in the BacT/Alert FAN aerobic bottles and only 15 (62.5%) detected by the Bactec Peds Plus vials (292). Detection was also significantly earlier with the BacT/Alert vials, implying that this system is possibly superior to the comparator and could potentially improve the detection of K. kingae, reducing the fraction of cases of culture-negative septic arthritis. However, these experimental results have not been validated by employing authentic specimens from pediatric patients with arthritis (289, 292).
Detection by Nucleic Acid Amplification Assays
Because of the potential risk for severe complications and long-term functional disability of septic arthritis and osteomyelitis in childhood, prompt laboratory confirmation and early administration of effective antimicrobial therapy are of paramount importance to prevent late sequelae and even death. However, a substantial fraction of pediatric skeletal infections remain bacteriologically unconfirmed, and, in any case, isolation, complete identification of the culprit organism, and antibiotic susceptibility testing require a minimum of 2 to 3 days. In recent years, the use of NAA assays has allowed identification of the etiological agents of joint and bone infections within 24 h (299, 300). This approach consists of DNA extraction from clinical specimens, followed by incubation with “universal primers” that anneal to conserved portions of the 16S rRNA gene, resulting in the amplification of the intervening species-specific sequence (186, 301). The amplification products are then sequenced and the results matched with data deposited in GenBank or other comprehensive genomic databases, or the products are hybridized with organism-specific probes. Alternatively, with clinical samples a PCR procedure may be performed by employing primers that selectively target the most probable bacteria (i.e., K. kingae in children younger than 4 years and S. aureus in older patients).
In a comparative study, Rosey et al. inoculated BCVs and seeded samples of synovial fluid onto solid medium, resulting in the isolation of K. kingae in 6 of 94 (6.4%) children, whereas a combination of conventional and real-time PCR with broad-spectrum primers disclosed 15 additional cases. These results clearly demonstrated the superiority of the molecular methods and indicated that a substantial proportion of culture-negative pediatric arthritis may be attributed to K. kingae (302).
Verdier et al. conducted a large study in which conventional cultures on solid medium and use of BCVs confirmed the diagnosis of suppurative arthritis or osteomyelitis in exudate specimens from 64 of 171 (37.4%) children, of which 9 grew K. kingae (303). The 107 culture-negative specimens were then subjected to a PCR procedure targeting the universal 16S rRNA genes and 15 samples were positive, all for K. kingae (303). Juretschko et al. compared the yield of Bact/Alert FAN or PEDs-F BCVs with that of real-time PCR in children with osteoarticular infections (158). The BCVs were positive in only 5 of the 21 (23.8%) joint fluid aspirates in which K. kingae was detected by the NAA assay (158).
In recent years, NAA tests that target K. kingae-specific DNA sequences such as cpn60 (encoding the chaperonin 60 protein) or the RTX toxin-encoding genes have been introduced into clinical practice with the aim of improving detection (15, 16, 88–90, 92, 151, 160). The relative merits of universal and K. kingae-specific primers were compared in vitro by Cherkaoui et al., who found that the PCR assay targeting the rtxA gene had a sensitivity of 30 CFU, one order of magnitude higher than that of the seminested broad-range 16S rRNA gene amplification (91). These experimental results were confirmed in an in vivo study by Ferroni et al. (151), who found that in 9 of 44 (20.5%) cases of PCR-proven K. kingae infections of the skeletal system, amplification with species-specific primers targeting the cpn60 gene succeeded, while the universal 16S rRNA gene primers failed. Because of the predominant role of K. kingae in children younger than 4 years with joint or bone infections, the authors recommended the use of the K. kingae-specific primers as the first-line test in this population segment, reserving the use of broad-spectrum primers for cases with negative species-specific PCR results.
Chometon and coworkers sequentially analyzed bone and joint specimens using the BCV method, conventional PCR with broad-spectrum primers, and real-time PCR with primers derived from the K. kingae cpn60 gene, increasing the detection rate from 29% to 41% and 45%, respectively (16). These results placed K. kingae as the prime agent of pediatric skeletal system infections, being responsible for approximately 80% of all suppurative arthritis and osteomyelitis cases in children aged less than 3 years (16).
Ilharreborde et al. enrolled 89 children with presumptive joint infections, inoculated synovial fluid aspirates into BCVs, and analyzed the specimens by real-time PCR employing primers that amplify K. kingae's cpn60 gene (15). The diagnosis of suppurative arthritis was established by culture in 36 samples (40%), of which 7 grew K. kingae, whereas the NAA test disclosed 24 additional cases of K. kingae infection among the 53 children in whom the exudate cultures were negative. Of note, the PCR assay detected K. kingae DNA sequences in all 7 culture-positive samples and was negative in all those in which other microorganisms were recovered by culture, confirming the specificity of the molecular assay. Altogether, K. kingae was detected in 31 (52%) of 60 bacteriologically proven cases (15).
In a large study conducted in a French pediatric orthopedic unit, bone and joint exudates from children with suspected skeletal system infections were inoculated into BacT/Alert BCVs and subjected to amplification with primers that amplify the species' cpn60 gene. Kingella kingae was detected by BCVs and/or NAA tests in 35 of 46 (76.1%) children aged <4 years with septic arthritis and in 9 of 17 (52.9%) children in the same age range with osteomyelitis or concurrent septic arthritis and osteomyelitis (151). The standard culture on solid medium failed to recover the organism in all 44 patients, while the BCVs were positive in only 5 specimens (including four exudates and one blood culture), and in 39 of the 44 patients (88.6%) K. kingae infection was diagnosed by NAA only (151).
A sequential approach was employed by Lévy et al. in patients with culture-negative osteoarticular infections. Initially, the skeletal system exudate was subjected to amplification of the 16S rRNA gene, and the amplicons resulting from positive tests were sequenced (152). Kingella kingae represented 8% of the organisms thus identified and was found exclusively in children. Positive results for K. kingae obtained with the broad-range PCR primers were confirmed in all cases by amplification of specific cpn60 gene sequences (152).
In a study employing a multiplex PCR assay designed for the simultaneous diagnosis of K. kingae and S. aureus infections, K. kingae's cpn60 gene was amplified from synovial fluid specimens obtained from three of six children younger than 5 years with arthritis, while cultures of the exudate on solid medium were negative in all cases (304). Of note, none of the 105 synovial fluid samples aspirated from adult patients was positive for K. kingae (304).
In a recent report, the NAA test targeting the rtxA gene performed in the synovial fluid aspirate of a child with arthritis was still positive 5 days after onset of antibiotic treatment, indicating that the diagnosis of K. kingae infection may be possible in patients with partially treated disease (305).
The available published experience comparing the performances of the culture approach and NAA methods for detecting K. kingae skeletal infections is summarized in Table 1. These results convincingly demonstrate that the isolation of K. kingae remains inadequate, even when specimens are seeded into BCVs, and that the molecular diagnostic tools increase the detection rate by 4-fold. The NAA methods substantially improve the diagnosis of K. kingae disease, reduce the frustrating fraction of unconfirmed infections, and show that the organism is the leading bacterial etiology of skeletal infections in young pediatric patients.
TABLE 1.
Performance of culture methods and nucleic acid amplification assays for detecting Kingella kingae in children with skeletal system infectionsa
| Yr | Reference | Culture method | No. with positive K. kingae culture/total | PCR method | Target gene |
No. with positive PCR/total | |
|---|---|---|---|---|---|---|---|
| C-PCR | RT-PCR | ||||||
| 1998 | 301 | C | 0/1 | C | 16S rRNA gene | NA | 1/1 |
| 2003 | 186 | C + BCV | 13/18 | C | 16S rRNA gene | NA | 18/18 |
| 2005 | 303 | C + BCV | 9/24 | C | 16S rRNA gene | NA | 24/24 |
| 2007 | 302 | C + BCV | 6/21 | C + RT | 16S rRNA gene | 16S rRNA gene | 20/21 |
| 2007 | 16 | C + BCV | 17/39 | C + RT | 16S rRNA gene | cpn60 | 39/39 |
| 2008 | 200 | BCV | 2/4 | C + RT | 16S rRNA gene | cpn60 | 4/4 |
| 2008 | 206 | C | 0/1 | C | 16S rRNA gene | NA | 1/1 |
| 2009 | 15 | C + BCV | 7/31 | RT | ND | cpn60 | 31/31 |
| 2009 | 88 | C | 0/2 | C + RT | 16S rRNA gene | rtxA + rtxB | 2/2 |
| 2009 | 158 | C + BCV | 5/18 | RT | ND | ? | 18/18 |
| 2010 | 89 | C | 0/23 | RT | ND | rtxA + rtxB | 23/23 |
| 2011 | 90 | ND | 2/20 | RT | ND | rtxA | 20/20 |
| 2013 | 151 | C + BCV | 5/44 | RT | ND | cpn60 | 43/44 |
| 2013 | 152 | C | ? | C + RT | 16S rRNA gene | cpn60 | 11/11 |
| 2014 | 160 | C + BCV | 2/24 | C + RT | ND | rtxA | 27/27 |
| 2014 | 304 | C | 0/3 | RT | ND | cpn60 | 3/3 |
| 2014 | 305 | C + BCV | 0/1 | RT | ND | cpn60 | 1/1 |
| Total | 68/274 (24.8%) | 388/390 (99.5%) | |||||
Abbreviations: C, conventional; BCV, blood culture vial; RT, real time; ND, not done; NA, not applicable.
ANTIBIOTIC SUSCEPTIBILITY
Kingella kingae has been traditionally considered highly susceptible to antibiotic drugs that are administered to patients with presumptive or proven bacterial diseases (306, 307), whereas β-lactamase production has been uncommonly reported in the species (136, 187). The enzyme, a TEM-1 β-lactamase, is usually encoded on a plasmid (308), but it has been found to be chromosomally integrated in a strain recovered from the oropharynx of a 2-year-old French child with culture-negative arthritis and from her older brother (309). The presence of β-lactamase in K. kingae isolates increases the ampicillin MIC to the range of 0.25 mg/liter to 8 mg/liter, with a MIC50 of 2 mg/liter and a MIC90 of 4 mg/liter. The enzyme does not hydrolyze cephalosporin drugs, and its activity is inhibited by clavulanic acid (309).
In a large study in which β-lactamase production and genomic clonality were investigated in a large sample of K. kingae isolates from Israeli individuals with clinical infections and asymptomatic pharyngeal carriers, the enzyme was detected in only 2 of 190 (1.1%) invasive isolates from patients with bacteremia, arthritis, osteomyelitis, or endocarditis and in 68 of 428 (15.9%) randomly chosen carriage organisms (P < 0.001), suggesting that the K. kingae strains that express β-lactamase are less capable of invading the bloodstream and deep host tissues and confirming that improved colonization ability does not necessarily imply enhanced virulence (60). These findings also imply that antibiotic resistance may confer a biological advantage to K. kingae organisms inhabiting in the pharynx in early childhood, overlapping the period in life of enhanced prevalence of oropharyngeal colonization and highest antimicrobial drug consumption (18, 85, 101, 310).
Production of β-lactamase among Israeli isolates was found to be limited to four distinct PFGE clones, and all members of these clones expressed the enzyme (60). A recent study confirmed the clonality of the trait in isolates from Reykjavik (Iceland) and Minneapolis, MN (USA) (61). Mating experiments indicated that despite the fact that the plasmid is conjugative, heterologous K. kingae strains were unable to keep it and produce β-lactamase for more than a few passages (308), meaning that additional requirements from the recipient cell are needed to retain the plasmid, thus limiting the possibility of its transfer to additional strains and explaining the strict clonality of β-lactamase production observed worldwide.
The proportion of β-lactamase-producing K. kingae isolates varies widely among countries. It is exceptionally detected in continental Europe, even in countries with high antibiotic consumption such as Spain and France, and in the Montreal area of Canada, but is widespread among carried and invasive isolates from Minneapolis, MN, and invasive isolates in Reykjavik, Iceland (61). Because data on the local prevalence of the enzyme and geographic distribution of producing clones are scarce and incomplete, routine testing of all invasive K. kingae isolates for β-lactamase production is strongly advised (61).
Strikingly, the strain responsible for β-lactamase production in Minnesota is identical by PFGE and MLST to that detected in Iceland and exhibits the same rtxA and por gene alleles (61, 308). The genotypic identity of K. kingae isolates derived from these two remote and relatively secluded geographic locations suggests person-to-person spread among the large community of Minnesota residents of Icelandic descent and the ancestral homeland population, although the direction and the time of the transoceanic strain's journey are unknown.
With rare exceptions, K. kingae is susceptible to aminoglycosides, macrolides, (191), trimethoprim-sulfamethoxazole (311), fluoroquinolones, tetracycline, and chloramphenicol (18, 130, 312–315). Kingella kingae exhibits relatively high MICs to oxacillin (MIC50, 3 μg/ml; MIC90, 6 μg/ml), 40% of invasive isolates are clindamycin nonsusceptible, and all strains are highly resistant to glycopeptide antibiotics, a serious concern in regions where joint and bone infections caused by community-associated methicillin-resistant S. aureus are prevalent and clindamycin or vancomycin is initially prescribed to children with skeletal system infections, pending culture and antibiotic susceptibility testing results (306, 316, 317).
TREATMENT
Because no specific protocols for managing invasive K. kingae disease have been formulated, patients have been empirically administered a wide array of antimicrobial regimens developed for treating infections caused by other bacterial pathogens. The empirical drug regimens for septic arthritis and osteomyelitis in young children generally combine the parenteral administration of a penicillinase-stable β-lactam antibiotic such as oxacillin and a broad-spectrum second-generation (cefuroxime) or third-generation (ceftriaxone) cephalosporin (307, 316, 317). This initial antibiotic therapy is usually swapped to intravenous ampicillin, once K. kingae is convincingly identified and β-lactamase production is ruled out, or to cefuroxime. A favorable clinical response and decrease of serum CRP levels below 20 mg/liter guide switching to oral antibiotics and help to determine the duration of therapy (317). Traditionally, patients with K. kingae arthritis have been administered antibiotics for a total of 2 to 3 weeks. Recent studies, however, suggest that a short 10-day course of sequential parenteral and oral antibiotic drugs is adequate for uncomplicated joint infections caused by Gram-positive pathogens, but the experience with this novel approach for treating K. kingae arthritis is still limited (317). Although repeated aspirations and lavage of the joint space have been performed in a few children (220), most patients can be adequately managed with antibiotics only (18).
Children with K. kingae osteomyelitis have received antibiotic therapy for 3 to 6 weeks and those with spondylodiscitis from 3 to 12 weeks (18). Patients with occult K. kingae bacteremia have generally been prescribed penicillin or cephalosporin drugs by the intravenous route, which were switched to oral antibiotics once the clinical condition stabilized and endocardial involvement was excluded. The duration of antibiotic therapy has ranged from 1 to 2 weeks with excellent results (18). Patients with K. kingae endocarditis are administered a high dose of intravenous β-lactam antibiotic as monotherapy or combined with an aminoglycoside drug for 4 to 7 weeks (18). In the past, patients with central nervous system infections caused by K. kingae were treated with a penicillin drug alone (139) or in combination with chloramphenicol (278, 280), but currently, a third-generation cephalosporin (cefotaxime or ceftriaxone) as monotherapy or in combination with an aminoglycoside for 10 days to 4 weeks is preferred (276, 277, 279).
PREVENTION
As is the case with other pathogens of respiratory origin, the population of asymptomatic carriers at any given time is huge compared to that of diseased individuals. Despite a substantial prevalence of K. kingae carriage in children, the rate of attack of invasive disease is relatively low (93, 102), and therefore, there is no indication to eradicate the organism from the colonized mucosal surfaces of healthy individuals. However, the risk of acquisition of K. kingae with progression to a severe and even life-threatening infection appears to be greatly increased among youngsters in day care. When the available information on the 6 outbreaks of disease occurring in day cares is pooled, a total of 18 of 122 (14.8%) classmates developed a documented or presumptive K. kingae infection, including fatal endocarditis and meningitis, within a 1-month period (98, 112, 113, 120, 121). Under these circumstances, prophylactic antibiotics aimed to eradicate colonization in contacts and prevent further cases of disease were usually offered to the population at risk. The choice of rifampin relied on the exquisite susceptibility of K. kingae (98, 312), the fact that the antibiotic is secreted in saliva and respiratory secretions and therefore high antibiotic concentrations may be achieved in the oropharyngeal mucosa, and the successful experience with the drug in the suppression of respiratory colonization by H. influenzae type b and N. meningitidis and prevention of invasive infections in day care facilities (318, 319). Rifampin at a dosage of 20 mg/kg/day in two divided doses for 2 days, alone (98, 120), or in combination with amoxicillin (80 mg/kg/per day) in two divided doses for two (113) or 4 days (112), was administered. The effectiveness of these regimens, however, was usually limited. On average, eradication of pharyngeal carriage among colonized day care attendees who completed the prescribed regimen was 81.1%, ranging from only 31.2%, as determined by a sensitive NAA (98), to 100%, as established by culture (121), and renewed colonization by the original strain was subsequently observed in a few children. It should be underscored that persistence of the organism was not caused by acquisition of resistance by the infecting K. kingae strain to the administered antibiotic regimens (98, 120). Inadequate compliance and/or failure to provide antibiotic prophylaxis to young siblings could have resulted in partial elimination of the reservoir, which was followed by renewed spread of the strain in the day care population (98, 319). Because antibiotics have been relatively unsuccessful in eliminating K. kingae carriage, the mandatory administration of prophylaxis to healthy contacts in the day care setting has been disputed (93). It should be noticed, however, that after administration of antibiotic prophylaxis, no new cases of disease occurred in the index facilities, even when only incomplete suppression of the causative strain was achieved (98, 112, 113, 120, 121). It is possible that reduction of colonization by antibiotic administration, even if temporarily, was enough to prevent transmission to additional attendees. Alternatively, prolonged mucosal carriage could have elicited an effective antibody response, decreasing the individual risk of developing an invasive infection and inducing protective herd immunity.
CONCLUDING REMARKS
As the result of the adoption of sensitive detection techniques, especially the BCV and NAA methods, and increasing acquaintance of clinical microbiology laboratories with its identification, K. kingae has emerged as an important etiology of invasive pediatric infections in the last decades. Appreciation of the pathogen as a frequent cause of bacteremia and the prime cause of skeletal system infections in young children, as well as endocarditis in pediatric and adult patients, has spurred research on the pharyngeal carriage of K. kingae by the healthy population, the interpersonal transmission of the bacterium, and the role of colonization in the pathogenesis of invasive disease. Investigation of the epidemiological and molecular aspects of asymptomatic K. kingae colonization has partially elucidated the complex mechanisms involved in the persistence and dissemination of the bacterium in the population, showing striking similarities between K. kingae and traditional bacterial members of the upper respiratory mucosal microbiome. It is to be expected that further research will reveal genomic traits responsible for dissimilarities in the colonization fitness, invasiveness, and tissue tropism exhibited by different K. kingae strains, disclose host factors that promote the transition from asymptomatic carriage to invasive disease, and identify bacterial antigens able to induce a protective immune response.
Biography

Pablo Yagupsky is Professor of Pediatrics and Microbiology at the Ben-Gurion University of the Negev and Director of the Clinical Microbiology Laboratory of the Soroka University Medical Center, Beer-Sheva, Israel. Dr. Yagupsky received his M.D. from the University of Buenos Aires, Argentina, in 1973, followed by pediatric residency at the Soroka University Medical Center and a fellowship in medical and public health microbiology at the University of Rochester, NY. Between 2007 and 2011 he has served as Vice-Dean and Director of the Ben-Gurion University Medical School. Dr. Yagupsky has long-standing research interests in the epidemiology, clinical presentation, and diagnosis of Kinglla kingae infections in children and the detection of human brucellosis in areas of endemicity.
REFERENCES
- 1.Henriksen SD, Bøvre K. 1968. Moraxella kingii sp. nov. A haemolytic saccharolytic species of the genus Moraxella. J Gen Microbiol 51:377–385. [DOI] [PubMed] [Google Scholar]
- 2.Henriksen SD, Bøvre K. 1976. Transfer of Moraxella kingii Henriksen and Bøvre to the genus Kingella gen. nov. in the family Neisseriaceae. J Syst Bacteriol 6:447–450. [Google Scholar]
- 3.Davis JM, Peel MM. 1982. Osteomyelitis and septic arthritis caused by Kingella kingae. J Clin Pathol 35:219–222. doi: 10.1136/jcp.35.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolfrey BF, Lally RT, Faville RJ. 1986. Intervertebral discitis caused by Kingella kingae. Am J Clin Pathol 85:745–749. [DOI] [PubMed] [Google Scholar]
- 5.Jenny DB, Latendre PW, Iverson G. 1988. Endocarditis due to Kingella species. Rev Infect Dis 10:1065–1066. doi: 10.1093/clinids/10.5.1065. [DOI] [PubMed] [Google Scholar]
- 6.Claesson B, Falsen E, Kjellman B. 1985. Kingella kingae infections: a review and a presentation of data from 10 Swedish cases. Scand J Infect Dis 17:233–243. [DOI] [PubMed] [Google Scholar]
- 7.Clément JL, Berard J, Cahuzac P, Gaubert J. 1988. Kingella kingae osteomyelitis and osteoarthritis in children. J Pediatr Orthop 8:59–61. doi: 10.1097/01241398-198801000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Gamble JG, Rinsky LA. 1988. Kingella kingae infections in healthy children. J Pediatr Orthop 8:445–449. doi: 10.1097/01241398-198807000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Verbruggen AM, Hauglustaine D, van der Hauwert L, Rombouts JJ, Wauters W, Vanderpitte J. 1986. Infections caused by Kingella kingae: report of four cases and review. J Infect 13:133–142. doi: 10.1016/S0163-4453(86)92841-0. [DOI] [PubMed] [Google Scholar]
- 10.deGroot R, Glover D, Clausen C, Smith AL, Wilson CB. 1988. Bone and joint infections caused by Kingella kingae: six cases and review of the literature. Rev Infect Dis 10:998–1004. doi: 10.1093/clinids/10.5.998. [DOI] [PubMed] [Google Scholar]
- 11.Pavlovsky M, Press J, Peled N, Yagupsky P. 2006. Blood culture contamination in pediatric patients: young children and young doctors. Pediatr Infect Dis J 25:611–614. doi: 10.1097/01.inf.0000220228.01382.88. [DOI] [PubMed] [Google Scholar]
- 12.Yagupsky P, Dagan R. 1994. Kingella kingae bacteremia in children. Pediatr Infect Dis J 13:1148–1149. doi: 10.1097/00006454-199412000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Yagupsky P, Bar-Ziv Y, Howard CB, Dagan R. 1995. Epidemiology, etiology, and clinical features of septic arthritis in children younger than 24 months. Arch Pediatr Adolesc Med 149:537–540. doi: 10.1001/archpedi.1995.02170180067010. [DOI] [PubMed] [Google Scholar]
- 14.Moumile K, Merckx J, Glorion C, Pouliquen JC, Berche P, Ferroni A. 2005. Bacterial aetiology of acute osteoarticular infections in children. Acta Paediatr 94:419–422. doi: 10.1080/08035250410023278. [DOI] [PubMed] [Google Scholar]
- 15.Ilhaerreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Ligouri S, Vitoux C, Lefevre Y, Doit C, Fitoussi F, Pennecot G, Bingen E, Mazda K, Bonacorsi S. 2009. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol 47:1837–1841. doi: 10.1128/JCM.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Bérard J, Vandenesch F, Freydière AM. 2007. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J 26:377–381. doi: 10.1097/01.inf.0000259954.88139.f4. [DOI] [PubMed] [Google Scholar]
- 17.Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, Simu A. 1992. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol 30:1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagupsky P. 2004. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis 4:32–41, 2004. doi: 10.1016/S1473-3099(04)01046-1. [DOI] [PubMed] [Google Scholar]
- 19.Garcia PC, Irribarra LT, Ramirez VM, Cervilla VV, De la Barra RD, Montiela FA, Ortiz CM, Jacobelli JB. 2000. Rendimiento del estudio microbiológico en el diagnóstico de la infección osteoarticular. Rev Chilena Infectol 17:101–108. [Google Scholar]
- 20.Budnik I, Porte L, Arce JD, Vial S, Zamorano J. 2011. Espondilodiskitis caused by Kingella kingae in children: a case report. Rev Chilena Infectol 28:369–373. doi: 10.4067/S0716-10182011000500012. [DOI] [PubMed] [Google Scholar]
- 21.Elyès B, Mehdi G, Kamel BH, Hela Z, Imen BS. 2006. Kingella kingae septic arthritis with endocarditis in an adult. Joint Bone Spine 73:472–473. doi: 10.1016/j.jbspin.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Urs S, D'Silva BS, Jeena CP, Sona CP, Beena K, Shetty KR. 1994. Kingella kingae septicemia in associacion with HIV disease. Trop Doct 24:127. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty RN, Meigh RE, Kaye GC. 1999. Kingella kingae prosthetic valve endocarditis. Indian Heart J 51:438–439. [PubMed] [Google Scholar]
- 24.Ramana KV, Mohanty SK. 2009. An adult case of urinary tract infection with Kingella kingae: a case report. J Med Case Rep 3:7236. doi: 10.1186/1752-1947-3-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mardaneh J, Eslami G, Fallah F, Goudarzi H, Soltan Dallal MM. 2011. Septic arthritis caused by Kingella kingae: a case report. Iran Red Crescent Med J 13:899–900. [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan IJ, Hayek L. 1993. Endocarditis caused by Kingella denitrificans. J Infect 27:291–295. doi: 10.1016/0163-4453(93)92213-G. [DOI] [PubMed] [Google Scholar]
- 27.Salvo S, Mazón A, Kutz M, Inza E. 1993. Vaginitis caused by Kingella denitrificans in a 3-year-old female patient. Enferm Infecc Microbiol Clin 11:395–396. [PubMed] [Google Scholar]
- 28.Maccato M, McLean W, Riddle G, Faro S. 1991. Isolation of Kingella denitrificans from amniotic fluid in a woman with chorioamnionitis. A case report. J Reprod Med 36:685–687. [PubMed] [Google Scholar]
- 29.Minamoto GY, Sordillo EM. 1992. Kingella denitrificans as a cause of granulomatous disease in a patient with AIDS. Clin Infect Dis 15:1052. doi: 10.1093/clind/15.6.1052. [DOI] [PubMed] [Google Scholar]
- 30.Chen C. 1996. Distribution of a newly described species, Kingella oralis, in the human oral cavity Oral Microbiol Immunol 11:425–427. [DOI] [PubMed] [Google Scholar]
- 31.Dewhirst FE, Chen CK, Paster BJ, Zambon JJ. 1993. Phylogeny of species in the family Neisseriaceae isolated from human dental plaque and description of Kingella oralis sp. nov. Int J Syst Bacteriol 43:490–499. (Corrected.) doi: 10.1099/00207713-43-3-490. [DOI] [PubMed] [Google Scholar]
- 32.Lawson PA, Malnick H, Collins MD, Shah JJ, Chattaway MA, Bendall R, Hartley JW. 2005. Description of Kingella potus sp. nov., an α-hemolytic organism isolated from a wound caused the bite of an exotic mammal. J Clin Microbiol 43:3526–3529. doi: 10.1128/JCM.43.7.3526-3529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Véron M, Lenvoisé-Furet A, Coustére C, Ged C, Grimont F. 1993. Relatedness of three species of “false Neisseriae,” Neisseria caviae, Neisseria cuniculi, and Neisseria ovis, by DNA-DNA hybridization and fatty acid analysis. Int J Syst Bacteriol 43:210–212. doi: 10.1099/00207713-43-2-210. [DOI] [PubMed] [Google Scholar]
- 34.Henriksen SD. 1969. Corroding bacteria from the respiratory tract. 1. Moraxella kingae. APMIS 75:85–90. [PubMed] [Google Scholar]
- 35.Jantzen E, Bryn K, Bergan T, Bøvre K. 1974. Gas chromatography of bacterial whole cell methanolysates. V. Fatty acid composition of Neisseriae and Moraxellae. Acta Pathol Microbiol Scand B 82:767–769. [DOI] [PubMed] [Google Scholar]
- 36.Wallace PL, Hollis DG, Weaver RE, Moss W. 1988. Cellular fatty acid composition of Kingella species, Cardiobacterium hominis, and Eikenella corrodens. J Clin Microbiol 26:1592–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janda WM, Bradna JJ, Ruther P. 1989. Identification of Neisseria spp. Haemophilus spp and other fastidious gram-negative bacteria with the MicroScan Haemophilus-Neisseria identification panel. J Clin Microbiol 27:869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett SJ, Sneath PHA. 1994. A numerical phenotypic taxonomic study of the genus Neisseria. Microbiology 140:2867–2891. doi: 10.1099/00221287-140-10-2867. [DOI] [PubMed] [Google Scholar]
- 39.Heiske A, Mutters R. 1994. Differentiation of selected members of the family Neisseriaceae (Alysiella, Eikenella, Kingella, Simonsiella and CDC groups EF-4 and M-50) by carbohydrate fingerprints and selected phenotypic features Zentralbl Bakteriol 281:67–79. [DOI] [PubMed] [Google Scholar]
- 40.Rossau R, Vandenbussche G, Thielemans S, Segers P, Grosch H, Göthe E, Mannheim W, De Ley J. 1989. Ribosomal ribonucleic acid and cistron similarities and deoxyribonucleic acid homologies of Neisseria, Kingella, Eikenella, Simonsiella, Alysiella, and Centers for Diseases Control groups EF-4 and M-5 in the emended family Neisseriaceae. Int J Syst Bacteriol 39:185–198. doi: 10.1099/00207713-39-2-185. [DOI] [Google Scholar]
- 41.Rossau R, Van Landschoot A, Gillis H, De Ley J. 1991. Taxonomy of Moraxellaceae fam. nov., a new bacterial family to accommodate the genera Moraxella, Acinetobacter, and Psychrobacter and related organisms. Int J Syst Bacteriol 41:310–319. doi: 10.1099/00207713-41-2-310. [DOI] [Google Scholar]
- 42.Enright MC, Carter PE, MacLean IA, McKenzie H. 1994. Phylogenetic relationship between some members of the genera Neisseria, Acinetobacter, Moraxella, and Kingella based on partial 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol 44:387–391. doi: 10.1099/00207713-44-3-387. [DOI] [PubMed] [Google Scholar]
- 43.Adeolu M, Gupta RS. 2013. Phylogenomics and molecular signatures for the order Nesisseriales: proposal for division of the order Neissseriales into the emended family Neisseriaceae and Chromobacteriaceae fam. nov. Antonie Van Leeuwenhoek 104:1–24. doi: 10.1007/s10482-013-9920-6. [DOI] [PubMed] [Google Scholar]
- 44.Tønjun T, Bukholm G, Bøvre K. 1989. Differentiation of some species of Neisseriaceae and other bacterial groups by DNA-DNA hybridization. APMIS 97:395–405. doi: 10.1111/j.1699-0463.1989.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 45.Brujin J, Verhage J, Bosboom RW, Brus F. 2000. Septic arthritis in two young children caused by unusual Gram-negative pathogens. Ned Tijdschr Geneeskd 144:1494–1497. [PubMed] [Google Scholar]
- 46.Henriksen SD. 1976. Moraxella, Neisseria, Branhamella, and Acinetobacter. Annu Rev Microbiol 30:63–83. doi: 10.1146/annurev.mi.30.100176.000431. [DOI] [PubMed] [Google Scholar]
- 47.Ødum L, Frederiksen W. 1981. Identification and characterization of Kingella kingae. APMIS 89:311–315. [DOI] [PubMed] [Google Scholar]
- 48.Yu PK, Rolfzen MA, Johnson RA, Hopkins MK, Anhalt JP. 1991. Application of the quadFERM+ for the identification of fastidious Gram-positive and Gram-negative bacilli. Diagn Microbiol Infect Dis 14:185–187. doi: 10.1016/0732-8893(91)90057-M. [DOI] [PubMed] [Google Scholar]
- 49.Valenza G, Ruoff C, Vogel U, Frosch M, Abele-Horn M. 2007. Microbiological evaluation of the new VITEK 2 Neisseria-Haemophilus identification card. J Clin Microbiol 45:3493–3497. doi: 10.1128/JCM.00953-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell EA, Blecker-Shelly D, Montgomery S, Mortensen JE. 2013. Application of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of the fastidious pediatric pathogens Aggregatibacter, Eikenella, Haemophilus, and Kingella. J Clin Microbiol 51:3862–3864. doi: 10.1128/JCM.02233-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couturier MR, Mehinovic E, Croft AC, Fisher MA. 2011. Identification of HACEK clinical isolates by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 49:1104–1106. doi: 10.1128/JCM.01777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarridge JE. 2004. Impact of 16S rDNA sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17:840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dusch H, Zbinden R, Von Graenitz A. 1995. Growth differences of Capnocytophaga canimorsus and some other fastidious organisms on various Columbia-based blood agar media. Zentralbl Bakteriol 282:362–366. doi: 10.1016/S0934-8840(11)80705-X. [DOI] [PubMed] [Google Scholar]
- 54.Frøholm LO, Bøvre K. 1972. Fimbriation associated with the spreading-corroding colony type in Moraxella kingii. APMIS 80:641–648. [PubMed] [Google Scholar]
- 55.Frøholm LO, Bøvre K. 1978. Density gradient centrifucation in urographin of Moraxella and Kingella cells and appendages. Acta Pathol Microbiol Scand B 86:77–86. [DOI] [PubMed] [Google Scholar]
- 56.Weir S, Marrs CF. 1992. Identification of type 4 pili in Kingella denitrificans. Infect Immun 60:3437–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kehl-Fie TE, Porsch EA, Yagupsky P, Grass EA, Obert C, Benjamin DK Jr, St. Geme JW III. 2010. Examination of type IV pilus expression and pilus-associated phenotypes in Kingella kingae clinical isolates. Infect Immun 78:1692–1699. doi: 10.1128/IAI.00908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yagupsky P, Weiss-Salz I, Fluss R, Freedman L, Peled N, Trefler R, Porat N, Dagan R. 2009. Dissemination of Kingella kingae in the community and long-term persistence of invasive clones. Pediatr Infect Dis J 28:707–710. doi: 10.1097/INF.0b013e31819f1f36. [DOI] [PubMed] [Google Scholar]
- 59.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 60.Yagupsky P, Slonim A, Amit U, Porat N, Dagan R. 2013. β-Lactamase production by Kingella kingae in Israel is clonal and common in carriage organisms but rare among invasive strains. Eur J Clin Microbiol Infect Dis 32:1049–1053. doi: 10.1007/s10096-013-1849-1. [DOI] [PubMed] [Google Scholar]
- 61.Basmaci R, Bonacorsi S, Bidet P, Balashova NV, Lau J, Muñoz-Almagro C, Gene A, Yagupsky P. 13 June 2014. Genotyping, local prevalence, and international dissemination of β-lactamase-producing Kingella kingae strains. Clin Microbiol Infect doi: 10.1111/1469-0691.12648. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan JB, Lo G, Xie G, Johnson SL, Chain PSG, Donnelly R, Kachlany SC, Balashova N. 2012. Genome sequence of Kingella kingae septic arthritis isolate PYKK081. J Bacteriol 194:3017. doi: 10.1128/JB.00421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fournier PE, Rouli L, El Karkouri K, Nguyen TT, Yagupsky P, Raoult D. 2012. Genomic comparison of Kingella kingae strains. J Bacteriol 194:5972. doi: 10.1128/JB.01418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basmaci R, Yagupsky P, Ilharreborde B, Guyot K, Porat N, Chomton M, Thiberge JM, Mazda K, Bingen E, Bonacorsi S, Bidet P. 2012. Multilocus sequence typing and rtxA toxin gene sequencing analysis of Kingella kingae isolates demonstrates genetic diversity and international clones. PLoS One 7:e38078. doi: 10.1371/journal.pone.0038078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basmaci R, Bidet P, Yagupsky P, Muñoz-Almagro C, Balashova NV, Doit C, Bonacorsi S. 2014. Major intercontinentally distributed sequence types of Kingella kingae and development of a rapid molecular typing tool. J Clin Microbiol 52:3890–3897. doi: 10.1128/JCM.01609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frye SA, Nilsen M, Tønjun T, Ambur OA. 2013. Dialects of the DNA uptake sequence in Neisseriaceae. PLoS Genet 9:e1003458. doi: 10.1371/journal.pgen.1003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner KME, Feil EJ. 2007. The secret life of the multilocus sequence type. Int J Antimicrob Agents 29:129–135. doi: 10.1016/j.ijantimicag.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Muzzi A, Donati C. 2011. Population genetics and evolution of the pan-genome of Streptococcus pneumoniae. Int J Med Microbiol 301:619–622. doi: 10.1016/j.ijmm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Kehl-Fie TE, Miller SE, St. Geme JW III. 2008. Kingella kingae expresses type IV pili that mediate adherence to respiratory epithelial and synovial cells. J Bacteriol 190:7157–7163. doi: 10.1128/JB.00884-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kehl-Fie TE, Porsch EA, Miller SE, St. Geme JW III. 2009. Expression of Kingella kingae type IV pili is regulated by σ54, PilS, and PilR. J Bacteriol 191:4976–4986. doi: 10.1128/JB.00123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porsch EA, Johnson MDL, Broadnax AD, Garrett CK, Redinbo MR, St. Geme JW III. 2013. Calcium binding properties of the Kingella kingae PilC1 and PilC2 proteins have differential effect on type IV pilus-mediated adherence and twitching motility. J Bacteriol 195:886–895. doi: 10.1128/JB.02186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porsch EA, Kehl-Fie TE, St. Geme JW III. 2012. Modulation of Kingella kingae adherence to human epithelial cells by type IV pili, capsule, and a novel trimeric autotransporter. mBio 3:e00372–12. doi: 10.1128/mBio.00372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Starr KF, Porsch EA, Heiss C, Black I, Azadi P, St. Geme JW III. 2013. Characterization of the Kingella kingae polysaccharide capsule and exopolisaccharide. PLoS One 8:e75409. doi: 10.1371/journal.pone.0075409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol 193:3879–3886. doi: 10.1128/JB.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weintraub A. 2003. Immunology of bacterial polysaccharide antigens. Carbohydr Res 338:2539–2547. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Kaplan JB. 2010. Biofilm dispersal: mechanisms, clinical implications, potential therapeutic uses. J Dent Res 89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kehl-Fie TE, St. Geme JW III. 2007. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J Bacteriol 189:430–436. doi: 10.1128/JB.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maldonado R, Wei R, Kachlani SC, Kazi M, Balashova NV. 2011. Cytotoxic effects of Kingella kingae outer membrane vesicles on human cells. Microb Pathog 51:22–30. doi: 10.1016/j.micpath.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang D, Nudell Y, Lau J, Zakharian E, Balashova NV. 2014. RTX-toxin plays a key role in Kingella kingae virulence in an infant animal model. Infect Immun 82:2318–2328. doi: 10.1128/IAI.01636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted outer membrane vesicles. Annu Rev Microbiol 64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bøvre K, Hagen N, Berdal BP, Jantzen E. 1977. Oxidase positive rods from cases of suspected gonorrhoeae. A comparison of conventional gas chromatography and genetic methods of identification. Acta Pathol Microbiol Scand B 85B:27–37. [PubMed] [Google Scholar]
- 82.Kennedy CA, Rosen H. 1988. Kingella kingae bacteraemia and adult epiglottitis in a granulocytopenic host. Am J Med 85:701–702. doi: 10.1016/S0002-9343(88)80243-2. [DOI] [PubMed] [Google Scholar]
- 83.Gremillon DH, Crawford GE. 1981. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 71:539–542. [DOI] [PubMed] [Google Scholar]
- 84.Yagupsky P, Dagan R, Prajgrod F, Merires M. 1995. Respiratory carriage of Kingella kingae among healthy children. Pediatr Infect Dis J 14:673–678. doi: 10.1097/00006454-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 85.Amit U, Flaishmakher S, Dagan R, Porat N, Yagupsky P. 2014. Age-dependent carriage of Kingella kingae in young children and turnover of colonizing strains. J Pediatr Infect Dis Soc 3:160–162. doi: 10.1093/jpids/pit003. [DOI] [PubMed] [Google Scholar]
- 86.Yagupsky P, Merires M, Bahar J, Dagan R. 1995. Evaluation of a novel vancomycin-containing medium for primary isolation of Kingella kingae from upper respiratory tract specimens. J Clin Microbiol 33:426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basmaci R, Ilharreborde B, Bidet P, Doit C, Lorrot M, Mazda K, Bingen E, Bonacorsi S. 2012. Isolation of Kingella kingae in the oropharynx during K. kingae arthritis on children. Clin Microbiol Infect 18:e134–136. doi: 10.1111/j.1469-0691.2012.03799.x. [DOI] [PubMed] [Google Scholar]
- 88.Cherkaoui A, Ceroni D, Emonet S, Lefevre Y, Schrenzel J. 2009. Molecular diagnosis of Kingella kingae osteoarticular infections by specific real-time PCR assay. J Med Microbiol 58:65–68. doi: 10.1099/jmm.0.47707-0. [DOI] [PubMed] [Google Scholar]
- 89.Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. 2010. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop 30:301–304. doi: 10.1097/BPO.0b013e3181d4732f. [DOI] [PubMed] [Google Scholar]
- 90.Lehours P, Freydière AM, Richer O, Burucoa C, Boisset S, Lanotte F, Prère MP, Ferroni A, Lafuente C, Vandenesch F, Mégrau F, Ménard A. 2011. The rtxA toxin gene of Kingella kingae: a pertinent target for molecular diagnosis of osteoarticular infections. J Clin Microbiol 49:1245–1250. doi: 10.1128/JCM.01657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cherkaoui A, Ceroni D, Ferey S, Emonet S, Schrenzel J. 2009. Pediatric osteo-articular infections with negative culture results: what about Kingella kingae? Rev Med Suisse 5:2235–2239. (In French.) [PubMed] [Google Scholar]
- 92.Ceroni D, Cherkaoui A, Kaelin A, Schrenzel J. 2010. Kingella kingae spondylodiscitis in young children: toward a new approach for bacteriological investigations? A preliminary report. J Child Orthop 4:173–175. doi: 10.1007/s11832-009-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ceroni D, Dubois-Ferrière V, Anderson R, Combescure C, Lamah L, Cherkaoui A, Schrenzel J. 2012. Small risk of osteoarticular infections in children with asymptomatic carriage of Kingella kingae. Pediatr Infect Dis J 31:983–985. doi: 10.1097/INF.0b013e31825d3419. [DOI] [PubMed] [Google Scholar]
- 94.Ceroni D, Belaieff W, Kanavaki A, Anderson Della Llana R, Lascombes P, Dubois-Ferrière V, Dayer R. 2013. Possible association of Kingella kingae with infantile spondylodiscitis. Pediatr Infect Dis J 32:1296–1298. doi: 10.1097/INF.0b013e3182a6df50. [DOI] [PubMed] [Google Scholar]
- 95.Ceroni D, Dubois-Ferrière V, Cherkaoui A, Gesuele R, Combescure C, Lamah L, Manzano S, Hibbs J, Schrenzel J. 2013. Detection of Kingella kingae osteoarticular infections in children by oropharyngeal swab PCR. Pediatrics 131:e230–235. doi: 10.1542/peds.2012-0810. [DOI] [PubMed] [Google Scholar]
- 96.Ceroni D, Llana RA, Kherad O, Dubois-Ferrière V, Lascombes P, Renzi G, Manzano S, Cherkaoui A, Schrenzel J. 2013. Comparing the oropharyngeal colonization density of Kingella kingae between asymptomatic carriers and children with invasive osteoarticular infections. Pediatr Infect Dis J 32:412–414. doi: 10.1097/INF.0b013e3182846e8f. [DOI] [PubMed] [Google Scholar]
- 97.Ceroni D, Dubois-Ferriere V, Kherad O, Llana RA, Kherad O, Lascombes P, Renzi G, Manzano S, Cherkaoui A, Schrenzel J. 2013. Oropharyngeal colonization density of Kingella kingae. Pediatr Infect Dis 32:803–804. doi: 10.1097/INF.0b013e31828ac051. [DOI] [PubMed] [Google Scholar]
- 98.Bidet P, Collin E, Basmaci R, Courroux C, Prisse V, Dufour V, Bingen E, Grimprel E, Bonacorsi S. 2013. Investigation of an outbreak of osteoarticular infections caused by Kingella kingae in a childcare center using molecular techniques. Pediatr Infect Dis J 32:558–560. doi: 10.1097/INF.0b013e3182867f5e. [DOI] [PubMed] [Google Scholar]
- 99.García-Rodriguez JA, Fresnadillo Martinez MJ. 2002. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 50(Suppl S2):59–73. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- 100.Yagupsky P, Peled N, Katz O. 2002. Epidemiological features of invasive Kingella kingae infections and respiratory carriage of the organism. J Clin Microbiol 40:4180–4184. doi: 10.1128/JCM.40.11.4180-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amit U, Dagan R, Yagupsky P. 2013. Prevalence of pharyngeal carriage of Kingella kingae in young children and risk factors for colonization. Pediatr Infect Dis J 32:191–193. doi: 10.1097/INF.0b013e3182755779. [DOI] [PubMed] [Google Scholar]
- 102.Dubnov-Raz G, Ephros M, Garty BZ, Schlesinger Y, Maayan-Metzger A, Hasson J, Kassis I, Schwartz-Harari O, Yagupsky P. 2010. Invasive pediatric Kingella kingae infections: a nationwide collaborative study. Pediatr Infect Dis J 29:639–643. doi: 10.1097/INF.0b013e3181d57a6c. [DOI] [PubMed] [Google Scholar]
- 103.Reisman J, Rudolph K, Bruden D, Hurlburt D, Bruce MG, Hannessy T. 2014. Risk factors for pneumococcal colonization of the nasopharynx in Alaska native adults and children. J Pediatr Infect Dis Soc 3:104–111. doi: 10.1093/jpids/pit069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amit U, Dagan R, Porat N, Trefler R, Yagupsky P. 2012. Epidemiology of invasive Kingella kingae infections in two distinct pediatric populations cohabiting in one geographic area. Pediatr Infect Dis J 31:415–417. doi: 10.1097/INF.0b013e318240cf8a. [DOI] [PubMed] [Google Scholar]
- 105.Slonim A, Walker ES, Mishori E, Porat N, Dagan R, Yagupsky P. 1998. Person-to-person transmission of Kingella kingae among day care center attendees. J Infect Dis 78:1843–1846. [DOI] [PubMed] [Google Scholar]
- 106.Yagupsky P, Slonim A. 2005. Characterization and immunogenicity of Kingella kingae outer-membrane proteins. FEMS Immunol Med Microbiol 43:45–50. doi: 10.1016/j.femsim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 107.Slonim A, Steiner M, Yagupsky P. 2003. Immune response to invasive Kingella kingae infections, age-related incidence of disease, and levels of antibody to outer-membrane proteins. Clin Infect Dis 37:521–527. doi: 10.1086/376913. [DOI] [PubMed] [Google Scholar]
- 108.Kampouroglou G, Dubois-Ferriere V, De La Llana RA, Renzi G, Manzano S, Cherkaoui A, Schrenzel J, Ceroni D. 2014. A prospective study of intrafamilial oropharyngeal transmission of Kingella kingae. Pediatr Infect Dis 33:410–411. doi: 10.1097/INF.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 109.Yagupsky P, Porat N, Pinco E. 2009. Pharyngeal colonization by Kingella kingae in children with invasive disease. Pediatr Infect Dis J 28:155–157. doi: 10.1097/INF.0b013e318184dbb8. [DOI] [PubMed] [Google Scholar]
- 110.Amit U, Porat N, Basmaci R, Bidet P, Bonacorsi S, Dagan R, Yagupsky P. 2012. Genotyping of invasive Kingella kingae isolates reveals predominant clones and association with specific clinical syndromes. Clin Infect Dis 55:1074–1079. doi: 10.1093/cid/cis622. [DOI] [PubMed] [Google Scholar]
- 111.Basmaci R, Ilharreborde B, Doit C, Presedo A, Lorrot M, Alison M, Mazda K, Bidet P, Bonacorsi S. 2013. Two atypical cases of Kingella kingae invasive infection with concomitant human rhinovirus infection. J Clin Microbiol 51:3137–3139. doi: 10.1128/JCM.01134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yagupsky P, Erlich Y, Ariela S, Trefler R, Porat N. 2006. Outbreak of Kingella kingae skeletal system infections in children in daycare. Pediatr Infect Dis J 25:526–532. doi: 10.1097/01.inf.0000215243.42501.4f. [DOI] [PubMed] [Google Scholar]
- 113.Seña AC, Seed P, Nicholson B, Joyce M, Cunningham CK. 2010. Kingella kingae endocarditis and a cluster investigation among daycare attendees. Pediatr Infect Dis J 29:86–88. doi: 10.1097/INF.0b013e3181b48cc3. [DOI] [PubMed] [Google Scholar]
- 114.Amir J, Yagupsky P. 1998. Invasive Kingella kingae infection associated with stomatitis in children. Pediatr Infect Dis J 17:757–758. doi: 10.1097/00006454-199808000-00021. [DOI] [PubMed] [Google Scholar]
- 115.Dayan A, Delclaux B, Quentin R, Rabut H, Lavandier M, Goudeau A. 1989. The isolation of Kingella kingae by hemoculture must always suggest the diagnosis of endocarditis. Presse Med 18:1340–1341. [PubMed] [Google Scholar]
- 116.Yagupsky P, Press J. 2003. Arthritis following stomatitis in a sixteen-month-old child. Pediatr Infect Dis J 22:573–574; 576–577. doi: 10.1097/00006454-200306000-00021. [DOI] [PubMed] [Google Scholar]
- 117.Dubnov-Raz G, Scheuerman O, Chodick G, Finkelstein Y, Samra Z, Garty BZ. 2008. Invasive Kingella kingae infections in children: clinical and laboratory characteristics. Pediatrics 122:1305–1309. doi: 10.1542/peds.2007-3070. [DOI] [PubMed] [Google Scholar]
- 118.Nafstad P, Hagen JA, Oie L, Magnus P, Jaakola JK. 1999. Day care and respiratory health. Pediatrics 103:753–758. doi: 10.1542/peds.103.4.753. [DOI] [PubMed] [Google Scholar]
- 119.Robinson J. 2001. Infectious diseases in schools and child care facilities. Pediatr Rev 22:39–45. doi: 10.1542/pir.22-2-39. [DOI] [PubMed] [Google Scholar]
- 120.Kiang KM, Ogunmodede F, Juni BA, Boxrud DJ, Glennen A, Bartkus JM, Cebelinski EA, Harriman K, Koop S, Faville R, Danila R, Lynfield R. 2005. Outbreak of osteomyelitis/septic arthritis caused by Kingella kingae among child care center attendees. Pediatrics 116:e206–213. doi: 10.1542/peds.2004-2051. [DOI] [PubMed] [Google Scholar]
- 121.Yagupsky P. 2014. Outbreaks of Kingella kingae infections in day care facilities Emerg. Infect Dis 20:746–753. doi: 10.3201/eid2005.131633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yagupsky P. 2012. Risk for invasive Kingella kingae infections and day-care facility attendance. Pediatr Infec Dis J 31:1101. doi: 10.1097/INF.0b013e31825fb703. [DOI] [PubMed] [Google Scholar]
- 123.Staretz-Haham O, Melamed R, Lifshitz M, Porat N, Fieschi C, Casanova JL, Levi J. 2003. Interleukin-12 receptor ß-1 deficiency presenting as recurrent Salmonella infection. Clin Infect Dis 37:137–140. doi: 10.1086/375229. [DOI] [PubMed] [Google Scholar]
- 124.Ferber B, Bruckheimer E, Schlesinger Y, Berger I, Glaser J, Olsha O, Branski D. 1997. Kingella kingae endocarditis in a child with hair-cartilage hypoplasia. Pediatr Cardiol 18:445–446. doi: 10.1007/s002469900227. [DOI] [PubMed] [Google Scholar]
- 125.Redfield DC, Overturf GD. 1980. Bacteria, arthritis, and skin lesions due to Kingella kingae. Arch Dis Child 55:411–414. doi: 10.1136/adc.55.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toshniwal R, Drahi TC, Kocka FE, Kallick CA. 1986. Manifestations of Kingella kingae infections in adults: resemblance to neisserial infections. Diagn Microbiol Infect Dis 5:81–85. doi: 10.1016/0732-8893(86)90095-7. [DOI] [PubMed] [Google Scholar]
- 127.Franklin GW, Shafi NA. 1983. Spontaneous septic artritis due to Kingella kingae. Clin Microbiol Newsl 9:925–928. [Google Scholar]
- 128.Jeff L, Marques MB, Brookings ES, Waites KB. 1997. Detection of Kingella kingae bacteraemia and identification of the organism in the clinical laboratory: experience from two cases. Clin Microbiol Newsl 19:73–76. doi: 10.1016/S0196-4399(97)84532-1. [DOI] [Google Scholar]
- 129.Roiz MP, Peralta FG, Arjona R. 1997. Kingella kingae bacteremia in an immunocompetent adult host. J Clin Microbiol 35:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goutzmanis JJ, Gonis G, Gilbert GL. 1991. Kingella kingae infection in children: ten cases and review of the literature. Pediatr Infect Dis 10:677–683. doi: 10.1097/00006454-199109000-00011. [DOI] [PubMed] [Google Scholar]
- 131.Shinagawa T, Kato Y, Matsuo H, Yakii M, Fujino M. 1987. Infective endocarditis due to Kingella kingae in a patient with systemic lupus erythematosus. Kansenshogaku Zasshi 61:1285–1291. [DOI] [PubMed] [Google Scholar]
- 132.Wolak T, Abu-Shakra M, Flusser D, Liel-Cohen N, Buskila D, Sukenik S. 2000. Kingella endocarditis and meningitis in a patient with SLE and associated antiphospholipid syndrome. Lupus 9:393–396. doi: 10.1191/096120300678828389. [DOI] [PubMed] [Google Scholar]
- 133.Ødum L, Jensen KT, Slotsbjerg TD. 1984. Endocarditis due to Kingella kingae. Eur J Clin Microbiol 3:263–264. doi: 10.1007/BF02014899. [DOI] [PubMed] [Google Scholar]
- 134.Huhn P. 1973. Endocarditis in a patient with systemic lupus erythematosus. West Va Med J 69:35–36. [PubMed] [Google Scholar]
- 135.Lewis DA, Settas L. 1983. Kingella kingae causing septic arthritis in Felty's syndrome. Postgrad Med J 59:525–526. doi: 10.1136/pgmj.59.694.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sordillo EM, Rendel M, Sood R, Belinfanti J, Murray O, Brook D. 1993. Septicemia due to β-lactamase-positive Kingella kingae. Clin Infect Dis 17:818–819. doi: 10.1093/clinids/17.4.818. [DOI] [PubMed] [Google Scholar]
- 137.Kerlikowske K, Chambers HF. 1989. Kingella kingae endocarditis in a patient with the acquired immunodeficiency syndrome. West J Med 151:558–560. [PMC free article] [PubMed] [Google Scholar]
- 138.Sarda H, Ghazali D, Thibault M, Leturdu F, Adams C, Le Loc'h H. 1998. Infection multifocale invasive à Kingella kingae. Arch Pediatr 5:159–162. doi: 10.1016/S0929-693X(97)86830-3. [DOI] [PubMed] [Google Scholar]
- 139.Van Erps J, Schmedding E, Naessens A, Keymeulen B. 1992. Kingella kingae, a rare cause of bacterial meningitis. Clin Neurol Neurosurg 94:173–175. doi: 10.1016/0303-8467(92)90078-H. [DOI] [PubMed] [Google Scholar]
- 140.Bofinger JJ, Fekete T, Samuel R. 2007. Bacterial peritonitis caused by Kingella kingae. J Clin Microbiol 45:3118–3120. doi: 10.1128/JCM.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matta M, Wermert D, Podglajen I, Sanchez O, Buu-Hoï A, Gutmann L, Meyer G, Mainardi JL. 2007. Molecular diagnosis of Kingella kingae pericarditis by amplification and sequencing of the 16S rRNA gene. J Clin Microbiol 45:3133–3134. doi: 10.1128/JCM.00809-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Timsit S, Pannier S, Glorion C, Chéron G. 2005. Acute osteomyelitis and septic arthritis in children: one year experience. Arch Pediatr 12:16–22. doi: 10.1016/j.arcped.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 143.Nordal E, Olausson S, Hvidsten D, Klingenberg C. 2004. Kingella kingae and osteoarticular infections in children. Tidsskr Nor Laegeforen 124:492–493. [PubMed] [Google Scholar]
- 144.Shuler TE, Riddle CD, Potts DW. 1990. Polymicrobic septic arthritis caused by Kingella kingae and Enterococcus. Orthopedics 13:254–256. [DOI] [PubMed] [Google Scholar]
- 145.Salminen I, Von Essen R, Koota K, Nissinen A. 1984. A pitfall in purulent arthritis brought out in Kingella kingae infection of the knee. Ann Rheum 43:656–657. doi: 10.1136/ard.43.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vincent A, Podewell C, Franklin GW, Korn JH. 1981. Septic arthritis due to Kingella (Moraxella) kingii: case report and review of the literature. J Rheumatol 8:501–503. [PubMed] [Google Scholar]
- 147.Holland J. 1998. Kingella kingae septic arthritis in an eight-year-old child. Clin Microbiol Newsl 20:80–81. doi: 10.1016/S0196-4399(98)80003-2. [DOI] [Google Scholar]
- 148.Le Bars H, Lamini N, Brunet JF, Duval H, Samjee I, Minet J. 2010. Sacroiliitis due to Kingella kingae: update on this pathogen. Ann Biol Clin (Paris) 68:341–345. doi: 10.1684/abc.2010.0439. [DOI] [PubMed] [Google Scholar]
- 149.Meis JF, Sauerwein RW, Gyssens IC, Horrevorts AM, van Kampen A. 1992. Kingella kingae diskitis in an adult. Clin Infect Dis 15:530–532. doi: 10.1093/clind/15.3.530. [DOI] [PubMed] [Google Scholar]
- 150.Estève V, Porcheret H, Clerc D, Dorfmann H, Le Pennec MP. 2001. Septic arthritis due to Kingella kingae in an adult. Joint Bone Spine 68:85–86. doi: 10.1016/S1297-319X(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 151.Ferroni A, Al Khoury H, Dana C, Quesne G, Berche P, Glorion C, Péjin Z. 2013. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect 19:822–828. doi: 10.1111/clm.12031. [DOI] [PubMed] [Google Scholar]
- 152.Lévy PY, Fournier PE, Fenollar F, Raoult D. 2013. Systematic PCR detection in culture-negative osteoarticular infections. Am J Med 126:1143. doi: 10.1016/j.amjmed.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 153.Mallet C, Ceroni D, Litzelmann E, Dubois-Ferriere V, Lorrot M, Bonacorsi S, Mazda K, Ilharreborde B. 2014. Unusually severe cases of Kingella kingae osteoarticular infections in children. Pediatr Infect Dis J 33:1–4. doi: 10.1097/INF.0b013e3182a22cc6. [DOI] [PubMed] [Google Scholar]
- 154.Howard AW, Viskontas D, Sabbagh C. 1999. Reduction in osteomyelitis and septic arthritis related to Haemophilus influenzae type b vaccination. J Pediatr Orthop 19:705–709. [PubMed] [Google Scholar]
- 155.Luhmann JD, Luhmann SJ. 1999. Etiology of septic arthritis in children: an update for the 1990s. Pediatr Emerg Care 15:40–42. doi: 10.1097/00006565-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 156.Granoff DM, Daum RS. 1980. Spread of Haemophilus influenzae type b: recent epidemiologic and therapeutic considerations. J Pediatr 97:854–860. doi: 10.1016/S0022-3476(80)80288-5. [DOI] [PubMed] [Google Scholar]
- 157.Clément JL, Salesse-Lavergne M, Prère MF, Chaffai M, Boulot J, Lebarbier P, Cahuzac JP, Gaubert J. 1986. Osteoarthritis due to Kingella kingae. Apropos of 4 cases. Chir Pediatr 27:359–362. [PubMed] [Google Scholar]
- 158.Juretschko S, Beavers-May T, Hemphill C, Romero J, Stovall S. 2009. Superior detection of Kingella kingae and Staphylococcus aureus in paediatric osteoarticular infections using molecular analysis, abstr O-469. Abstr 19th Eur Congr Clin Microbiol Infect Dis. [Google Scholar]
- 159.Masson AT, Gudnason T, Jonmundsson GK, Elensdottir H, Kristinsson KG, Kristjansson M, Haraldsson A. 2011. Bacterial osteomyelitis and arthritis in Icelandic children. Laeknabladid 97:91–96. [DOI] [PubMed] [Google Scholar]
- 160.Williams N, Cooper C, Cundy P. 2014. Kingella kingae septic arthritis in children: recognising an elusive pathogen. J Child Orthop; doi: 10.1007/s11832-014-0549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pons Odena M, González Pascual E, Ros Villadoms J, Gené Giralt A, May Llanas E, Hugurt Carol R. 1999. Osteoarticular infection by Kingella kingae: two case reports. An Esp Pediatr 50:491–494. [PubMed] [Google Scholar]
- 162.Gay RM, Lane TW, Keller DC. 1983. Septic arthritis caused by Kingella kingae. J Clin Microbiol 17:168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boyce F, Cassidy M, Price R. 1983. Kingella kingae septic arthritis. Clin Microbiol Newsl 5:46. doi: 10.1016/S0196-4399(83)80070-1. [DOI] [Google Scholar]
- 164.Bosworth DE. 1983. Kingella (Moraxella) kingae infections in children. Am J Dis Child 137:650–653. [DOI] [PubMed] [Google Scholar]
- 165.Patel NJ, Morre TL, Weiss TD, Zuckner J. 1983. Kingella kingae infectious arthritis: case report and review of literature of Kingella and Moraxella infections. Arthritis Rheum 26:557–559. doi: 10.1002/art.1780260417. [DOI] [PubMed] [Google Scholar]
- 166.Powell JM, Bass JW. 1983. Septic arthritis caused by Kingella kingae. Am J Dis Child 137:974–976. [DOI] [PubMed] [Google Scholar]
- 167.La Selve H, Bérard J, Barbe G, Cochat N, Cochat P. 1986. Osteoarthritis and osteomyelitis due to Kingella kingae in children. Apropos de 5 cases. Pediatrie 41:297–304. [PubMed] [Google Scholar]
- 168.Le CT. 1983. Kingella (Moraxella) kingae infections. Am J Dis Child 137:1212–1213. [DOI] [PubMed] [Google Scholar]
- 169.Rosenbaum J, Lieberman DH, Katz WA. 1980. Moraxella infectious arthritis: first report in an adut. Ann Rheum Dis 39:184–185. doi: 10.1136/ard.39.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gene A, García-García JJ, Sala P, Sierra M, Huguet R. 2004. Enhanced culture detection of Kingella kingae, a pathogen of increasing clinical importance in pediatrics. Pediatr Infect Dis J 23:886–888. doi: 10.1097/01.inf.0000137591.76624.82. [DOI] [PubMed] [Google Scholar]
- 171.Yagupsky P, Dagan R, Howard CB, Einhorn M, Kassis I, Simu A. 1993. Clinical features and epidemiology of invasive Kingella kingae infections in Southern Israel. Pediatrics 92:800–804. [PubMed] [Google Scholar]
- 172.Lundy DW, Kehl DK. 1998. Increasing prevalence of Kingella kingae in osteo-articular infections in young children. J Pediatr Orthop 18:262–267. [PubMed] [Google Scholar]
- 173.Ricard E, Martin C, Bertin P, Denes E. 2014. Arthrite septique sternoclaviculaire à Kingella kingae chez un adult. Med Mal Infect 44:79–81. doi: 10.1016/j.medmal.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 174.Trujillo M, Nelson JD. 1997. Suppurative and reactive arthritis in children. Semin Pediatr Infect Dis 8:242–249. doi: 10.1016/S1045-1870(97)80018-0. [DOI] [Google Scholar]
- 175.Yagupsky P, Press J. 1997. Use of the Isolator 1.5 Microbial Tube for culture of synovial fluid from patients with septic arthritis. J Clin Microbiol 35:2410–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vásquez MA, Palacián MP, Cruz Villuendas M, Marne C, Paz Ruiz-Echarri M, Revillo MJ. 2012. Kingella kingae pediatric septic arthritis. Arch Argent Pediatr 110:e126–e128. doi: 10.5546/aap.2012.e126. [DOI] [PubMed] [Google Scholar]
- 177.Kocher MS, Zurakowski D, Kasser JR. 1999. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg 81-A:1662–1670. [DOI] [PubMed] [Google Scholar]
- 178.Yagupsky P, Press J. 2004. Unsuspected Kingella kingae infections in afebrile children with mild skeletal symptoms: the importance of blood cultures. Eur J Pediatr 163:563–564. [DOI] [PubMed] [Google Scholar]
- 179.Yagupsky P. 2014. Another look: is there a flaw to current hip septic arthritis diagnostic algorithms? Clin Orthop Rel Res 472:383–384. doi: 10.1007/s11999-013-3340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yagupsky P, Dubnov-Raz G, Gené A, Ephros M, Israeli-Spanish Kingella kingae Research Group. 2014. Differentiating Kingella kingae septic arthritis of the hip from transient synovitis in young children. J Pediatr 165:985–989.e1. doi: 10.1016/j.jpeds.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 181.Basmaci R, Lorrot M, Bidet P, Doit C, Vitoux C, Penneçot G, Mazda K, Bingen E, Ilharreborde B, Bonacorsi S. 2011. Comparison of clinical and biologic features of Kingella kingae and Staphylococcus aureus arthritis at initial evaluation. Pediatr Infect Dis J 30:902–904. doi: 10.1097/INF.0b013e31821fe0f7. [DOI] [PubMed] [Google Scholar]
- 182.Basmaci R, Ilharreborde B, Lorrot M, Bidet P, Bingen E, Bonacorsi S. 2011. Predictive score to discriminate Kingella kingae from Staphylococcus aureus arthritis in France. Pediatr Infect Dis J 30:1121–1122. doi: 10.1097/INF.0b013e31822ce994. [DOI] [PubMed] [Google Scholar]
- 183.Ceroni D, Cherkaoui A, Combescure C, François P, Kaelin A, Schrenzel J. 2011. Differentiating osteoarticular infections caused by Kingella kingae from those due to typical pathogens in young children. Pediatr Infect Dis J 30:906–909. doi: 10.1097/INF.0b013e31821c3aee. [DOI] [PubMed] [Google Scholar]
- 184.Kanavaki A, Ceroni D, Tchernin D, Hanquinet S, Merlini L. 2012. Can early MRI distinguish between Kingella kingae and gram-positive cocci in osteoarticular infections in children? Pediatr Radiol 42:57–62. doi: 10.1007/s00247-011-2220-2. [DOI] [PubMed] [Google Scholar]
- 185.Benard JM, Jean-Baptiste A, Nelet F, Debray H, Pujol B, Goldstein F, Gutmann L, Acar JF, Enjolras M. 1989. Kingella kingae osteomyelitis. Arch Fr Pediatr 46:521–524. [PubMed] [Google Scholar]
- 186.Moumile K, Merckx J, Glorion C, Berche P, Ferroni A. 2003. Osteoarticular infections caused by Kingella kingae in children; contribution of polymerase chain reaction to the microbiologic diagnosis. Pediatr Infect Dis 22:837–839. doi: 10.1097/01.inf.0000083848.93457.e7. [DOI] [PubMed] [Google Scholar]
- 187.Birgisson H, Steingrimsson O, Gudnason T. 1997. Kingella kingae infections in paediatric patients: 5 cases of septic arthritis, osteomyelitis and bacteraemia. Scand J Infect Dis 29:495–498. doi: 10.3109/00365549709011861. [DOI] [PubMed] [Google Scholar]
- 188.Shelton MM, Nachtigal MP, Yngve DA, Herndon WA, Riley HD. 1988. Kingella kingae osteomyelitis: report of two cases involving the epiphysis. Pediatr Infect Dis J 7:421–424. doi: 10.1097/00006454-198806000-00011. [DOI] [PubMed] [Google Scholar]
- 189.Skouby SO, Knudsen FU. 1982. Kingella kingae osteomyelitis mimicking an eosinophilic granuloma. Acta Paediatr Scand 71:511–512. doi: 10.1111/j.1651-2227.1982.tb09463.x. [DOI] [PubMed] [Google Scholar]
- 190.Noftal F, Mersal A, Yaschuk Y, Wedge J, Albritton W. 1988. Osteomyelitis due to Kingella kingae infection. Can J Surg 31:21–22. [PubMed] [Google Scholar]
- 191.Chanal C, Tiget F, Chapuis P, Campagne D, Jan M, Sirot J. 1987. Spondilytis and osteomyelitis caused by Kingella kingae in children. J Clin Microbiol 25:2407–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Claesson B, Kjellman Falsen E. 1989. Kingella osteomyelitis. Pediatr Infect Dis J 8:128–129. [Google Scholar]
- 193.Klein JD, Leach KA. 2007. Pediatric pelvic osteomyelitis. Clin Pediatr 46:787–790. doi: 10.1177/0009922807303810. [DOI] [PubMed] [Google Scholar]
- 194.Wilmes D, Omoumi P, Squifflet J, Cornu O, Rodriguez-Villalobos H, Yombi JC. 2012. Osteomyelitis pubis caused by Kingella kingae in an adult patient: report of the first case. BMC Infect Dis 12:236. doi: 10.1186/1471-2334-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.La Scola B, Iorgulescu I, Bollini G. 1998. Five cases of Kingella kingae skeletal infection in a French hospital. Eur J Clin Microbiol Infect Dis 17:512–515. [DOI] [PubMed] [Google Scholar]
- 196.Yagupsky P, Howard CB, Einhorn M, Dagan R. 1993. Kingella kingae osteomyelitis of the calcaneus in young children. Pediatr Infect Dis J 12:540–541. doi: 10.1097/00006454-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 197.Kuzumoto K, Kubota N, Saito Y, Fujioka F, Yumoto K, Hidaka E, Yoshiyuki K. 2013. A case of osteomyelitis due to Kingella kingae. Kansenshogaku Zasshi 87:207–210. [DOI] [PubMed] [Google Scholar]
- 198.Gené Giralt A, Carol RH, Gonzalez Cuevas A, Ventura Gomez N, Latorre Otin C. 1998. Osteomyelitis of the astragalus caused by Kingella kingae. Enferm Infecc Microbiol Clin 16:253–254. [PubMed] [Google Scholar]
- 199.Baticle E, Courtivron B, Baty G, Holstein A, Morange V, Mereghetti L, Goudeau A, Lanotte P. 2008. Pediatric osteoarticular infections caused by Kingella kingae from 1995 to 2006 at CHRU de Tours. Ann Biol Clin 66:454–458. [DOI] [PubMed] [Google Scholar]
- 200.Luegmair M, Chaker M, Ploton C, Berard J. 2008. Kingella kingae: osteoarticular infections of the sternum in children: a report of six cases. J Child Orthop 2:443–447. doi: 10.1007/s11832-008-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rotbart HA, Gelfand WM, Glode MP. 1984. Kingella kingae osteomyelitis of the clavicle. J Pediatr Orthop 4:500–502. doi: 10.1097/01241398-198408000-00025. [DOI] [PubMed] [Google Scholar]
- 202.Lacour M, Duarte M, Beutler A, Auckenthaler R. 1991. Osteoarticular infections due to Kingella kingae in children. Eur J Pediatr 150:612–618. doi: 10.1007/BF02072618. [DOI] [PubMed] [Google Scholar]
- 203.O'Neill JM, Beavers-May T, Wheeler JG, Jacobs RF. 2003. Destructive osteomyelitis of the fibular epiphysis due to Kingella kingae. J Ark Med Soc 99:293–294. [PubMed] [Google Scholar]
- 204.Ruttan TK, Higginbotham E, Higginbotham N, Allen CH, Hauger S. 27 May 2014. Invasive Kingella kingae resulting in a Brodie abscess. J Pediatr Infect Dis Soc; doi: 10.1093/jpids/piu046. [DOI] [PubMed] [Google Scholar]
- 205.El Houmami N, Dubourg G, Minodier P, Verschuur A, Bouvier C, Jouve JL, Raoult D, Fournier PE. 20 August 2014. Kingella kingae DNA in Langerhans cell histiocytosis of bone. Pediatr Infect Dis J doi: 10.1097/INF.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 206.Fuursted K, Arpi M, Lindblad BE, Pedersen LN. 2008. Broad-range PCR as a supplement to culture for detection of bacterial pathogens in patients with a clinically diagnosed spinal infection. Scand J Infect Dis 40:772–777. doi: 10.1080/00365540802119994. [DOI] [PubMed] [Google Scholar]
- 207.Garron E, Viehweger E, Launay F, Guillaume JM, Jouve JL, Bollini G. 2002. Nontuberculous spondylodiscitis in children. J Pediatr Orthop 22:321–328. doi: 10.1097/01241398-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 208.Spiegel PG, Kengla KW, Isaacson AS, Wilson JC. 1972. Intervertebral disk-space inflammation in children. J Bone Joint Surg Am 49:1508–1520. [PubMed] [Google Scholar]
- 209.Bining HJ, Saigal G, Chankowski J, Rubin EE, Camlioglu EB. 2006. Kingella kingae spondylodiscits in a child. Br J Radiol 79:e181–e183. doi: 10.1259/bjr/35629425. [DOI] [PubMed] [Google Scholar]
- 210.Ruiz E, Pérez A, Asensi F, Otero MC, Santos M. 2000. Espondilitis por Kingella kingae en una niña de 10 años. Enferm Infecc Microbiol Clin 18:363–364. [PubMed] [Google Scholar]
- 211.Ducoulombier V, Dehecq E, Luraschi H, Prudhomme C, Bessard D, Houvenagel E. 2011. Kingella kingae spondylodiscitis in an adult. Med Mal Infect 41:110–112. doi: 10.1016/j.medmal.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 212.Amir J. 1992. Kingella kingae infection in children. Pediatr Infect Dis J 11:339. doi: 10.1097/00006454-199204000-00020. [DOI] [PubMed] [Google Scholar]
- 213.Amir J, Schockelford PG. 1991. Kingella kingae intervertebral disk infection. J Clin Microbiol 29:1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Petrus M, Rance F, Clement JL, Prere MF, Ibañez MH, Netter JC. 1990. Spondilodiscite à Kingella kingae. Ann Pediatr (Paris) 37:170–172. [PubMed] [Google Scholar]
- 215.Raymond J, Bergeret M, Bargy F, Missenard G. 1986. Isolation of two strains of Kingella kingae associated with septic arthritis. J Clin Microbiol 24:1110–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wong AS, Dyke J, Perry D, Anderson DC. 1978. Paraspinal mass associated with intervertebral disk infection secondary to Moraxella kingii. J Pediatr 92:86–88. doi: 10.1016/S0022-3476(78)80082-1. [DOI] [PubMed] [Google Scholar]
- 217.Falsen E, Brorson JE, Enger EA. 1977. Moraxella kingii: a rare cause of osteomyelitis. Z Kinderchir 22:186–189. [Google Scholar]
- 218.Donnio PY, Minet J, Guerin MN, Le Gall E, Cormier M. 1985. Spondilodiscite a Kingella kingae chez un enfant. Med Mal Infect 12:741–744. [Google Scholar]
- 219.Weiss-Salz I, Yagupsky P. 2010. Kingella kingae: from asymptomatic colonization to invasive pediatric infections. Pediatr Health 4:311–320. doi: 10.2217/phe.10.28. [DOI] [Google Scholar]
- 220.Lebel E, Rudensky B, Karasik M, Itzchaki M, Schlesinger Y. 2006. Kingella kingae infections in children. J Pediatr Orthop B 15:289–292. doi: 10.1097/01202412-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 221.Caballero Rabasco MA, González Cuevas A, Martínez Roig A. 2010. Isolated bacteremia caused by Kingella kingae. An Pediatr (Barc) 72:89–90. doi: 10.1016/j.anpedi.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 222.Dodman T, Robson J, Pincus D. 2000. Kingella kingae infections in children. J Pediatr Child Health 36:87–90, 2000. doi: 10.1046/j.1440-1754.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- 223.Chiquito PE, Elliot J, Namnyak SS. 1991. Kingella kingae dactylitis in an infant. J Infect 22:102–103. doi: 10.1016/0163-4453(91)91318-R. [DOI] [PubMed] [Google Scholar]
- 224.Ceroni D, Merlini L, Salvo D, Lascombes P, Dubois-Ferrière V. 2013. Pyogenic flexor synovitis of the finger due to Kingella kingae. Pediatr Infect Dis J 32:702–703. doi: 10.1097/INF.0b013e3182868f17. [DOI] [PubMed] [Google Scholar]
- 225.Moylett EH, Rossmann SN, Epps HR, Demmler GJ. 2000. Importance of Kingella kingae as a paediatric pathogen in the United States. Pediatr Infect Dis J 19:263–265. doi: 10.1097/00006454-200003000-00023. [DOI] [PubMed] [Google Scholar]
- 226.Bayat A, Pedersen RS. 2011. Pathognomonic presentation of Kingella kingae infection. Ugeskr Laeger 173:359–360. [PubMed] [Google Scholar]
- 227.Rolle U, Schille R, Hormann D, Friedrich T, Handrick W. 2001. Soft tissue infection caused by Kingella kingae in a child. J Pediatr Surg 36:946–947. doi: 10.1053/jpsu.2001.23997. [DOI] [PubMed] [Google Scholar]
- 228.Hansen WM, Glupczynski YG, Verstreken L. 1987. Kingella kingae septicemia. Acta Clin Belg 42:40–42. [DOI] [PubMed] [Google Scholar]
- 229.Krause I, Nimri R. 1996. Kingella kingae occult bacteremia in a toddler. Pediatr Infect Dis J 15:557–558. doi: 10.1097/00006454-199606000-00027. [DOI] [PubMed] [Google Scholar]
- 230.Piñeda Solas V, Valdesoiro Navarrete L, Fontanals Ameyrich D, Lliminana Ordas C, Anton Lopez J, Cabezas Maspoch MR. 2002. Bacteriemia oculta por Kingella kingae. An Esp Pediatr 56:191–192. doi: 10.1016/S1695-4033(02)78956-X. [DOI] [PubMed] [Google Scholar]
- 231.Graham DR, Band JD, Thornsberry C, Hollis DG, Weaver RE. 1990. Infections caused by Moraxella, Moraxella urethralis, Moraxella-like groups M-5 and M-6, and Kingella kingae in the United States, 1953-1980. Rev Infect Dis 12:423–431. doi: 10.1093/clinids/12.3.423. [DOI] [PubMed] [Google Scholar]
- 232.Förstl H, Ruckedschel G, Lang M, Eder W. 1984. Septicemia caused by Kingella kingae. Eur J Clin Microbiol 3:267–269. doi: 10.1007/BF02014900. [DOI] [PubMed] [Google Scholar]
- 233.Shanson DC, Gazzard BG. 1984. Kingella kingae septicaemia with a clinical presentation resembling disseminated gonococcal infection. Br Med J 289:730–731. doi: 10.1136/bmj.289.6447.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gauthier M, Chevalier I, Tapiero B. 2004. Henoch-Schonlein purpura associated with Kingella kingae bacteremia. Acta Paediatr 93:717–718. doi: 10.1080/08035250410023782. [DOI] [PubMed] [Google Scholar]
- 235.Baraff LJ, Bass JW, Fleisher GR, Klein JO, McCracken GH Jr, Powell KR, Schriger DL. 1993. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 22:1198–1210. doi: 10.1016/S0196-0644(05)80991-6. [DOI] [PubMed] [Google Scholar]
- 236.Geraci JE, Wilson WR. 1982. Endocarditis due to Gram-negative bacteria. Report of 56 cases. Mayo Clin Proc 57:145–147. [PubMed] [Google Scholar]
- 237.Berbari EF, Cockrill FR III, Steckelberg JM. 1997. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc 72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- 238.Thekekara AG, Denham B, Duff DF. 1994. Eleven year review of infective endocarditis. Ir Med J 87:80–82. [PubMed] [Google Scholar]
- 239.Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, Fernández-Hidalgo N, Almirante B, Bouza E, Forno D, del Rio A, Hannan MM, Harkness J, Kanafani ZA, Lalani T, Lang S, Raymond N, Read K, Vinogradova T, Woods CW, Wray D, Corey GR, Chu VH, International Collaboration on Endocarditis Prospective Cohort Study Investigators. 2013. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One 8:63181. doi: 10.1371/journal.pone.0063181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Marom D, Levy I, Gutwein O, Birk E, Ashkenazi S. 2011. Healthcare-associated versus community-associated infective endocarditis in children. Pediatr Infect Dis J 30:585–588. doi: 10.1097/INF.0b013e31820f66c7. [DOI] [PubMed] [Google Scholar]
- 241.Webb R, Voss L, Roberts S, Hornung T, Rumball E, Lennon D. 2014. Infective endocarditis in New Zealand children 1994-2012. Pediatr Infect Dis J 33:437–442. doi: 10.1097/INF.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 242.Foster MA, Walls T. 2014. High rates of complications following Kingella kingae infective endocarditis in children. A case series and review of the literature. Pediatr Infect Dis J 33:785–786. doi: 10.1097/INF.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 243.Grant JM, Borttolussi RA, Roy DL, Wort J. 1983. Prosthetic valve bacterial endocarditis caused by Kingella kingae. Can Med Assoc J 129:406–410. [PMC free article] [PubMed] [Google Scholar]
- 244.Bagherirad M, Entesari-Tatafi D, Mirzaee S, Appelbe A, Yap C, Athan E. 2013. A case of Kingella kingae endocarditis complicated with native mitral valve rupture. Australas Med J 6:172–174. doi: 10.4066/AMJ.2013.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Martínez-Olorón P, Romero Ibarra C, Alvarez Torroba L, Pérez Ocón. 2011. Kingella kingae endocarditis. An Pediatr (Barc) 74:274–275. doi: 10.1016/j.anpedi.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 246.Rabin RL, Wong P, Noonan JA, Plumley DD. 1983. Kingella kingae endocarditis in a child with a prosthetic valve and bifurcation graft. Am J Dis Child 137:403–404. [DOI] [PubMed] [Google Scholar]
- 247.Brachlow A, Chatterjee A, Stamato T, Hoffman W. 2004. Endocarditis due to Kingella kingae: a patient report. Clin Pediatr 43:283–286. doi: 10.1177/000992280404300311. [DOI] [PubMed] [Google Scholar]
- 248.Berkun Y, Brand A, Klar A, Halperin E, Hurvitz H. 2004. Kingella kingae endocarditis and sepsis in an infant. Eur J Pediatr 163:687–688. doi: 10.1007/s00431-004-1520-z. [DOI] [PubMed] [Google Scholar]
- 249.Rotstein A, Konstantinov IE, Penny DJ. 2010. Kingella-infective endocarditis resulting in a perforated aortic root abscess and fistulous connection between the sinus of Valsalva and the left atrium in a child. Cardiol Young 20:332–333. doi: 10.1017/S1047951110000314. [DOI] [PubMed] [Google Scholar]
- 250.Youssef D, Henaine R, Di Filippo S. 2010. Subtle bacterial endocarditis due to Kingella kingae in an infant: a case report. Cardiol Young 20:448–450. doi: 10.1017/S1047951110000351. [DOI] [PubMed] [Google Scholar]
- 251.La Selve H, Bozio A, Vidil P, Gallet S, Barbe G, David M. 1985. Endocardite aigue à Kingella kingae chez un nourisson. Arch Fr Pediatr 42:35–36. [PubMed] [Google Scholar]
- 252.Adachi R, Hammerberg O, Richardson H. 1983. Infective endocarditis caused by Kingella kingae. Can Med Assoc J 128:1087–1088. [PMC free article] [PubMed] [Google Scholar]
- 253.Miridjanian A, Berrett D. 1978. Infective endocarditis caused by Moraxella kingae. West Med J 129:344–346. [PMC free article] [PubMed] [Google Scholar]
- 254.Lepori M, Bochud PY, Owlya R, Broccard A, Schaller MD. 2001. Endocarditis due to HACEK bacteria. A case report of endocarditis due to Kingella kingae. Rev Med Suisse Romande 121:47–50. [PubMed] [Google Scholar]
- 255.Lewis MB, Bamford JM. 2000. Global aphasia without hemiparesis secondary to Kingella kingae endocarditis. Arch Neurol 57:1774–1775. doi: 10.1001/archneur.57.12.1774. [DOI] [PubMed] [Google Scholar]
- 256.Zbinden R, Hay A, Luthy R, Cohen D, Heinzer I. 1998. Antibody response in six HACEK endocarditis cases under therapy. APMIS 106:547–552. doi: 10.1111/j.1699-0463.1998.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 257.Sage MJ, Maslowski AH, MacCulloch D. 1983. Bacterial endocarditis due to Kingella kingae. N Z Med J 96:795–796. [PubMed] [Google Scholar]
- 258.Ravdin JI, Brandstetter RD, Wade MJ, Roberts RB. 1982. Endocarditis resulting from Kingella kingae, presenting initially as culture-negative bacterial endocarditis. Heart Lung 11:552–554. [PubMed] [Google Scholar]
- 259.Korach A, Olshtain-Pops K, Schwartz D, Moses A. 2009. Kingella kingae prosthetic valve endocarditis complicated by a paravalvular abscess. Isr Med Assoc J 11:251–253. [PubMed] [Google Scholar]
- 260.Zeimis RT, Hanley OQ. 1985. Endocarditis caused by Kingella kingae: case report and review. Lab Med 16:547–550. [Google Scholar]
- 261.Wolff AH, Ullman RF, Strampfer MJ, Cunha BA. 1987. Kingella kingae endocarditis: report of a case and review of the literature. Heart Lung 16:579–583. [PubMed] [Google Scholar]
- 262.Lee WL, Dooling EC. 1984. Acute Kingella kingae endocarditis with recurrent emboli in a child with mitral prolapse. Ann Neurol 16:88–89. doi: 10.1002/ana.410160117. [DOI] [PubMed] [Google Scholar]
- 263.Holmes AA, Hung T, Human DG, Campbell AI. 2011. Kingella kingae endocarditis: a rare case of mitral valve perforation. Ann Pediatr Cardiol 4:210–212. doi: 10.4103/0974-2069.84664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rodríguez Bouza H, de la Fuente Aguado J, Rubianes Gonzalez M, Crespo Casal M, Sopeña Perez-Argüelles B. 2001. Endocarditis por Kingella kingae. An Med Intern 18:655–656. [PubMed] [Google Scholar]
- 265.Gelbart B, Connell TG, Konstantinov IE, Phillips R, Starr M. 2009. Kingella kingae endocardial abscess and cerebral infarction in a previously well immunocompetent child. BMJ Case Rep; doi: 10.1136/bcr.09.2009.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Waghorn DJ, Cheetham CH. 1997. Kingella kingae endocarditis following chickenpox in infancy. Eur J Clin Microbiol Infect Dis 16:944–946. doi: 10.1007/BF01700567. [DOI] [PubMed] [Google Scholar]
- 267.Birk E, Sharoni E, Dagan O, Gelber O, Georghiou GP, Vidne BA, Erez E. 2004. The Ross procedure as the surgical treatment of active aortic valve endocarditis. J Heart Valve Dis 13:73–77. [PubMed] [Google Scholar]
- 268.Giamarellou H, Galanakis N. 1987. Use of intravenous ciprofloxacin in difficult-to-treat infections. Am J Med 82(Suppl 4A):346–351. [PubMed] [Google Scholar]
- 269.Christensen CE, Emmanoulides GC. 1967. Bacterial endocarditis due to “Moraxella new species I.” N Engl J Med 277:803–804. [DOI] [PubMed] [Google Scholar]
- 270.Marom D, Ashkenazi S, Samra Z, Birk E. 2013. Infective endocarditis in previously healthy children with structurally normal hearts. Pediatr Cardiol 34:1415–1421. doi: 10.1007/s00246-013-0665-9. [DOI] [PubMed] [Google Scholar]
- 271.Kassis I, Shachor-Meyouhas Y, Khatib I, Khoury A, Le TP, Lorber A. 2012. Kingella endocarditis after closure of ventricular septal deffect with a transcatheter device. Pediatr Infect Dis J 31:105–106. doi: 10.1097/INF.0b013e31823c3ec1. [DOI] [PubMed] [Google Scholar]
- 272.Wells L, Rutter N, Donald F. 2001. Kingella kingae endocarditis in a sixteen-month-old-child. Pediatr Infect Dis J 20:454–455. doi: 10.1097/00006454-200104000-00020. [DOI] [PubMed] [Google Scholar]
- 273.Baron EJ, Scott JD, Thompkins LS. 2005. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganiss from standard automated blood cultures. Clin Infect Dis 41:1677–1680. doi: 10.1086/497595. [DOI] [PubMed] [Google Scholar]
- 274.Gómez-Garcés JL, Oteo J, Garcia G, Alos JI. 2001. Kingella kingae pneumonia: a rare pathology or a pathology rarely diagnosed? Clin Microbiol Newsl 23:192–193. doi: 10.1016/S0196-4399(01)80054-4. [DOI] [Google Scholar]
- 275.Morrison VA, Wagner KF. 1989. Clinical manifestations of Kingella kingae infections: case report and review. Rev Infect Dis 11:76–81. [DOI] [PubMed] [Google Scholar]
- 276.Reekmans A, Noppen M, Naessens A, Vincken W. 2000. A rare manifestation of Kingella kingae infection. Eur J Intern Med 11:343–344. doi: 10.1016/S0953-6205(00)00115-1. [DOI] [PubMed] [Google Scholar]
- 277.Hay F, Chellum P, Romaru A, d'Auzac P, Vidal MP, Zelinski A. 2002. Kingella kingae: une rare cause de méningite. Arch Pediatr 9:37–40. doi: 10.1016/S0929-693X(01)00692-3. [DOI] [PubMed] [Google Scholar]
- 278.Namnyak SS, Quinn RJM, Ferguson JDM. 1991. Kingella kingae meningitis in an infant. J Infect 23:104–106. doi: 10.1016/0163-4453(91)94327-G. [DOI] [PubMed] [Google Scholar]
- 279.Cantarín Extremera V, Alvarez-Coca González J, Martínez-Párez J, Sáez Nieto JA, Rubio Villanueva JL. 2007. Meningitis due to Kingella kingae. An Pediatr (Barc) 66:627–628. doi: 10.1157/13107403. [DOI] [PubMed] [Google Scholar]
- 280.Walterspiel NJ. 1983. Kingella kingae meningitis with bilateral infarction of the basal ganglia. Infection 11:307–308. doi: 10.1007/BF01641352. [DOI] [PubMed] [Google Scholar]
- 281.Muñoz-Egea MC, García-Pedrazuela M, González-Pallarés I, Martínez-Perez M, Fernández-Roblas R, Esteban J. 2013. Kingella kingae keratitis. J Clin Microbiol 51:1627–1628. doi: 10.1128/JCM.03426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Megged O, Wasim J, Rozenman Y, Elimelech M, Assous MV. 2014. Kingella kingae corneal infections in children. J Pediatr Infect Dis Soc 3:89–90. doi: 10.1093/JPIDS/pit013. [DOI] [PubMed] [Google Scholar]
- 283.Mollee T, Kelly P, Tilse M. 1992. Isolation of Kingella kingae from a corneal ulcer. J Clin Microbiol 30:2516–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Carden SM, Colville DJ, Gonis G, Gilbert GL. 1991. Kingella kingae endophtalmitis in an infant. Aust N Z J Ophtalmol 19:217–219. doi: 10.1111/j.1442-9071.1991.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 285.Connell PP, Carey B, Kollpiara D, Fenton S. 2006. Kingella kingae orbital cellulitis in a 3-year-old. Eye (Lond) 20:1086–1088. doi: 10.1038/sj.eye.6702119. [DOI] [PubMed] [Google Scholar]
- 286.Van Damme PA, van Herpen CM, Meis JF. 2004. An adult case of oral infection with Kingella kingae. Int J Oral Maxillofac Surg 33:105–107. doi: 10.1054/ijom.2002.0440. [DOI] [PubMed] [Google Scholar]
- 287.Yagupsky P. 1999. Use of blood culture sytems for isolation of Kingella kingae from synovial fluid. J Clin Microbiol 37:3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Solís Gómez B, Gallinas Victoriano F, Bernaola Iturbe E, Baranda-Areta V, Garcia Mata L, Torroba Alvarez L. 2004. Septic arthritis due to Kingella kingae: difficulties of diagnosis. An Pediatr (Barc) 61:190–191. doi: 10.1157/13064605. [DOI] [PubMed] [Google Scholar]
- 289.Pérez A, Herranz M, Padilla E, Ferres F. 2009. Usefulness of synovial fluid inoculation in blood culture bottles for diagnosisng Kingella kingae septic arthritis: state of the question. Enferm Infecc Microbiol Clin 27:605–606. doi: 10.1016/j.eimc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 290.Costers M, Wouters C, Moens P, Verhaegen J. 2003. Three cases of Kingella kingae infection in young children. Eur J Pediatr 162:530–531. doi: 10.1007/s00431-003-1220-0. [DOI] [PubMed] [Google Scholar]
- 291.Lejbkowicz F, Cohn L, Hashman N, Kassis I. 1999. Recovery of Kingella kingae from blood and synovial fluid of two pediatric patients by using the BacT-Alert system. J Clin Microbiol 37:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Høst B, Schumacher H, Prag J, Arpi M. 2000. Isolation of Kingella kingae from synovial fluids using four commercial blood culture bottles. Eur J Clin Microbiol Infect Dis 19:608–611. doi: 10.1007/s100960000324. [DOI] [PubMed] [Google Scholar]
- 293.Abuamara S, Louis JS, Guyard MF, Barbier-Frebourg N, Tocques S, Lechevallier J, Mallet E. 2000. Les infections ostéoarticulaires à Kingella kingae chez l'enfant. À propos d'une série récente de huit cas. Arch Pediatr 7:927–932. doi: 10.1016/S0929-693X(00)90005-8. [DOI] [PubMed] [Google Scholar]
- 294.Le Ho C, Matsiota-Bernard P, Espinasse F, Ucla E, Nauciel C. 1993. Un cas d'arthrite à Kingella kingae chez un nourisson. Méd Mal Infect 23:264–265. [Google Scholar]
- 295.Gruber BF, Miller BS, Onnen J, Welling R, Wojtys EM. 2008. Antibacterial properties of synovial fluid in the knee. J Knee Surg 21:180–185. doi: 10.1055/s-0030-1247816. [DOI] [PubMed] [Google Scholar]
- 296.Yagupsky P. 2008. Use of blood culture vials and nucleic acid amplification for the diagnosis of pediatric septic arthritis. Clin Infect Dis 46:1631–1632. doi: 10.1086/587754. [DOI] [PubMed] [Google Scholar]
- 297.Wilms MC, Stanzel S, Reinert RR, Burckhardt I. 2009. Effects of preincubation temperature on the detection of fastidious organisms in delayed-entry samples in the BacT/Alert 3D blood culture system. J Microbiol Methods 79:194–198. doi: 10.1016/j.mimet.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 298.Gené Giralt A, Palacín-Camacho E, Sierra Soler M, Carol RH. 2008. Kingella kingae: condiciones determinantes del crecimiento en botella de hemocultivo. Enferm Infecc Microbiol Clin 26:181–182. doi: 10.1157/13116759. [DOI] [PubMed] [Google Scholar]
- 299.Fenollar F, Lévy PY, Raoult D. 2008. Usefulness of broad-range PCR for the diagnosis of osteoarticular infections. Curr Opin Rheumatol 20:463–470. doi: 10.1097/BOR.0b013e3283032030. [DOI] [PubMed] [Google Scholar]
- 300.Ferroni A. 2007. Epidemiology and bacteriological diagnosis of paediatric acute osteoarticular infections. Arch Paediatr 14(Suppl 2):S91–S96. doi: 10.1016/S0929-693X(07)80041-8. [DOI] [PubMed] [Google Scholar]
- 301.Stähelin J, Goledenberger D, Gnehm HE, Altwegg M. 1998. Polymerase chain reaction diagnosis of Kingella kingae arthritis in a young child. Clin Infect Dis 27:1328–1329. [DOI] [PubMed] [Google Scholar]
- 302.Rosey AL, Albachin E, Quesnes G, Cadilhac C, Pejin Z, Glorion C, Berche P, Ferroni A. 2007. Development of a broad-range 16S rDNA real-time PCR for the diagnosis of septic arthritis in children. J Microbiol Methods 68:88–93. doi: 10.1016/j.mimet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 303.Verdier I, Gayet-Ageron A, Ploton C, Taylor P, Benito Y, Freydiere AM, Chotel F, Bérard J, Vanhems P, Vandenesch F. 2005. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae: description of twenty-four recent pediatric diagnoses. Pediatr Infect Dis J 24:692–696. doi: 10.1097/01.inf.0000172153.10569.dc. [DOI] [PubMed] [Google Scholar]
- 304.Haldar M, Butler M, Quinn CD, Stratton CW, Tang YW, Burnham CA. 2014. Evaluation of a real-time PCR assay for simultaneous detection of Kingella kingae and Staphylococcus aureus from synovial fluid in suspected septic arthritis. Ann Lab Med 34:313–316. doi: 10.3343/alm.2014.34.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Grivea IN, Michoula AN, Basmaci R, Dailiana ZH, Tsimitselis G, Bonacorsi S, Syrogiannopoulos GA. 7 August 2014. Kingella kingae sequence type-complex 14 arthritis in a 16-month child in Greece. Pediatr Infect Dis J doi: 10.1097/INF.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 306.Yagupsky P. 2012. Antibiotic susceptibility of Kingella kingae isolates from children with skeletal system infections. Pediatr Infect Dis J 31:212. doi: 10.1097/INF.0b013e31824041b8. [DOI] [PubMed] [Google Scholar]
- 307.Rasmont Q, Yombi JC, Van der Linden D, Docquier PL. 2008. Osteoarticular infections in Belgian children: a survey of clinical, biological, radiological and microbiological data. Acta Orthop Belg 74:374–385. [PubMed] [Google Scholar]
- 308.Banerjee A, Kaplan JB, Soherwardy A, Nudell Y, MacKenzie GA, Johnson S, Balashova NV. 24 June 2013. Characterization of a TEM-1 β-lactamase producing Kingella kingae clinical isolates. Antimicrob Agents Chemother doi: 10.1128/AAC.00318-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Basmaci R, Bidet P, Berçot B, Kwon T, Gaumetou E, Bonacorsi S. 21 July 2014. First identification of a chromosomally located penicillinase gene in Kingella kingae species isolated in continental Europe. Antimicrob Agents Chemother doi: 10.1128/AAC.03562-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rossignoli A, Clavenna A, Bonati M. 2007. Antibiotic prescription and prevalence rate in the outpatient paediatric population: analysis of surveys published during 2000-2005. Eur J Clin Pharmacol 63:1099–1106. doi: 10.1007/s00228-007-0376-3. [DOI] [PubMed] [Google Scholar]
- 311.Yagupsky P. 2008. Trimethoprim-sulfamethoxazole for osteoarthritis caused by Staphylococcus aureus or Kingella kingae. Pediatr Infect Dis J 27:1042–1043. doi: 10.1097/INF.0b013e318187a2e7. [DOI] [PubMed] [Google Scholar]
- 312.Kugler KC, Biedenbach DJ, Jones RN. 1999. Determination of the antimicrobial activity of 29 clinical important compounds tested against fastidious HACEK group organisms. Diagn Microbiol Infect Dis 34:73–76. [DOI] [PubMed] [Google Scholar]
- 313.Jensen KT, Schonheyder H, Thomsen VF. 1994. In-vitro activity of β-lactam and other antimicrobial agents against Kingella kingae. J Antimicrob Chemother 33:635–640. [DOI] [PubMed] [Google Scholar]
- 314.Prère MF, Seguy M, Vezard Y, Lareng MB. 1986. Sensibilitè aux antibiotiques de Kingella kingae. Path Biol 34:604–607. [PubMed] [Google Scholar]
- 315.Yagupsky P, Katz O, Peled N. 2001. Antibiotic susceptibility of Kingella kingae isolates from respiratory carriers and patients with invasive infections. J Antimicrob Chemother 47:191–193. doi: 10.1093/jac/47.2.191. [DOI] [PubMed] [Google Scholar]
- 316.Saphyakhajon P, Joshi AY, Huskins WC, Henry NK, Boyce TG. 2008. Empiric antibiotic therapy for acute osteoarticular infections with suspected methicillin-resistant Staphylococcus aureus or Kingella. Pediatr Infect Dis J 27:765–767. doi: 10.1097/INF.0b013e31816fc34c. [DOI] [PubMed] [Google Scholar]
- 317.Pääkkönen M, Peltola H. 2013. Treatment of acute septic arthritis. Pediatr Infect Dis J 32:684–685. doi: 10.1097/INF.0b013e31828e1721. [DOI] [PubMed] [Google Scholar]
- 318.Gardner P. 2006. Prevention of meningococcal disease. N Engl J Med 355:1466–1473. doi: 10.1056/NEJMcp063561. [DOI] [PubMed] [Google Scholar]
- 319.Murphy TV, McCracken GH Jr, Moore BS, Gulig PA, Hansen HJ. 1983. Haemophilus influenzae type b disease after rifampin prophylaxis in a day care center: possible reasons for its failure. Pediatr Infect Dis J 2:193–198. [DOI] [PubMed] [Google Scholar]