Abstract
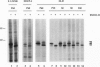
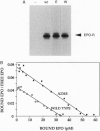
In both transfected and normal hematopoietic cells, the majority of newly made erythropoietin receptor (EPO-R) subunits are retained in the endoplasmic reticulum (ER), destined for degradation. Only a small fraction exit the ER and are competent to bind EPO, suggesting that the EPO-R folds inefficiently. The EPO-R contains a 5-amino acid motif, WSXWS, in the extracellular domain that is conserved among members of the cytokine receptor family. We describe a mutant EPO-R with a change in the middle residue of this motif, A234E, that is transported from the ER more efficiently than the wild-type (wt) receptor and is expressed in elevated numbers at the cell surface. This mutant polypeptide is processed more efficiently in the ER than its wt counterpart, suggesting that it folds better than the wt EPO-R. Inefficient folding and processing of the wt EPO-R in the ER may be one mechanism for controlling the number of plasma membrane receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M. A., Bale W. F., Spar I. L. Iodine monochloride (IC1) iodination techniques. Methods Enzymol. 1983;92:277–292. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. S., D'Andrea A. D., Haines L. L., Wong G. G. Human erythropoietin receptor: cloning, expression, and biologic characterization. Blood. 1990 Jul 1;76(1):31–35. [PubMed] [Google Scholar]
- Le A., Ferrell G. A., Dishon D. S., Le Q. Q., Sifers R. N. Soluble aggregates of the human PiZ alpha 1-antitrypsin variant are degraded within the endoplasmic reticulum by a mechanism sensitive to inhibitors of protein synthesis. J Biol Chem. 1992 Jan 15;267(2):1072–1080. [PubMed] [Google Scholar]
- Le A., Graham K. S., Sifers R. N. Intracellular degradation of the transport-impaired human PiZ alpha 1-antitrypsin variant. Biochemical mapping of the degradative event among compartments of the secretory pathway. J Biol Chem. 1990 Aug 15;265(23):14001–14007. [PubMed] [Google Scholar]
- Miyazaki T., Maruyama M., Yamada G., Hatakeyama M., Taniguchi T. The integrity of the conserved 'WS motif' common to IL-2 and other cytokine receptors is essential for ligand binding and signal transduction. EMBO J. 1991 Nov;10(11):3191–3197. doi: 10.1002/j.1460-2075.1991.tb04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Wikström L., Watowich S. S., Lodish H. F. Intermediates in degradation of the erythropoietin receptor accumulate and are degraded in lysosomes. J Biol Chem. 1993 Jun 25;268(18):13639–13649. [PubMed] [Google Scholar]
- Quelle D. E., Quelle F. W., Wojchowski D. M. Mutations in the WSAWSE and cytosolic domains of the erythropoietin receptor affect signal transduction and ligand binding and internalization. Mol Cell Biol. 1992 Oct;12(10):4553–4561. doi: 10.1128/mcb.12.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Kelly P. A. Mutational analysis of the ligand-binding domain of the prolactin receptor. J Biol Chem. 1991 Sep 5;266(25):16472–16477. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich S. S., Hilton D. J., Lodish H. F. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994 Jun;14(6):3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich S. S., Yoshimura A., Longmore G. D., Hilton D. J., Yoshimura Y., Lodish H. F. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Penny L. A., Deaven L. L., Forget B. G., Jenkins R. B. The gene for the human erythropoietin receptor: analysis of the coding sequence and assignment to chromosome 19p. Blood. 1990 Jul 1;76(1):24–30. [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Zimmers T., Neumann D., Longmore G., Yoshimura Y., Lodish H. F. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J Biol Chem. 1992 Jun 5;267(16):11619–11625. [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]