SUMMARY
Bacterial gastroenteritis is a disease that is pervasive in both the developing and developed worlds. While for the most part bacterial gastroenteritis is self-limiting, identification of an etiological agent by bacterial stool culture is required for the management of patients with severe or prolonged diarrhea, symptoms consistent with invasive disease, or a history that may predict a complicated course of disease. Importantly, characterization of bacterial enteropathogens from stool cultures in clinical laboratories is one of the primary means by which public health officials identify and track outbreaks of bacterial gastroenteritis. This article provides guidance for clinical microbiology laboratories that perform stool cultures. The general characteristics, epidemiology, and clinical manifestations of key bacterial enteropathogens are summarized. Information regarding optimal specimen collection, transport, and processing and current diagnostic tests and testing algorithms is provided. This article is an update of Cumitech 12A (P. H. Gilligan, J. M. Janda, M. A. Karmali, and J. M. Miller, Cumitech 12A, Laboratory diagnosis of bacterial diarrhea, 1992).
INTRODUCTION
Over 1.7 billion global cases of diarrheal disease are reported annually (http://www.who.int/mediacentre/factsheets/fs330/en/index.html) and are associated with an estimated 2.2 million deaths. The burden of diarrheal disease is most critical in developing countries, facilitated by unsafe water supplies, poor sanitation, and nutritional deficiencies. Diarrheal disease in children aged <5 years in these countries is devastating, where repeated diarrheal episodes contribute to malnutrition, which in turn puts these children at heightened risk of acquiring infectious diarrhea and is associated with stunting and impaired cognitive development (1, 2). While less common in high-income countries, diarrheal diseases remain a significant health concern. There are an estimated 211 to 375 million episodes of diarrheal illnesses each year in the United States, with 1.8 million hospitalizations and 3,100 deaths (3). Many of these cases are foodborne. The Food-borne Diseases Active Surveillance Network (FoodNet) at the Centers for Disease Control and Prevention (CDC) reported 1,000 foodborne outbreaks that resulted in 48 million illnesses, 128,000 hospitalizations, and 3,000 deaths from 10 sites in the United States over a 15-year period (4). It is important to note that many cases of foodborne diarrheal illness are not part of a recognized outbreak and thus are not captured by the FoodNet data (5).
Diarrhea is defined by the Infectious Diseases Society of America (IDSA) and the American College of Gastroenterology (ACG) as the passage of three or more loose or liquid stools per day. It can be further be classified by the duration of symptoms (3, 6). Patients with acute diarrhea have symptoms lasting less than 14 days. Those with diarrhea for >14 days, but <1 month are said to have persistent diarrhea. Those experiencing diarrhea for longer than 30 days are said to have chronic diarrhea. Diarrhea may be infectious, i.e., caused by bacteria, viruses, or parasites, but with increasing frequency in high-income nations, the etiology of diarrhea is noninfectious. In these cases, diarrhea is caused by food intolerances, reactions to medication, intestinal disorders such as irritable bowel syndrome, or intestinal diseases, including Crohn's disease, ulcerative colitis, and celiac disease. In these instances, laboratory tests for infectious etiologies, including a bacterial stool culture, are useful for diagnosis by either ruling out or ruling in a common infectious process (3).
The primary mechanisms for bacterial gastroenteritis are (i) excessive secretion of fluids in the proximal small intestine induced by the action of luminal toxins expressed by enteropathogens or by minimally invasive bacteria, (ii) inflammatory or cytotoxic damage of the ileal or colonic mucosa which may produce blood and pus, or (iii) penetration of the bacterium through the mucosa to the reticuloendothelial system, as is the case with typhoid fever. Classic examples of bacteria that cause these various syndromes are presented in Table 1. Regardless of mechanism, most cases of bacterial gastroenteritis are self-limiting, and, with a few exceptions, neither empirical antimicrobial therapy nor bacterial stool culture is indicated (3). For most patients who present with acute diarrhea, symptoms have resolved by the time bacterial culture results are available, and these generally do not change patient management (7). Rather, the primary goal for the patient with acute diarrhea is symptomatic relief, rehydration (or prevention of dehydration), and potentially preventing transmission of the infection. In contrast, a bacterial stool culture is indicated for patients with severe or prolonged diarrhea, those with symptoms consistent with invasive disease, or those with a medical history predictive of complications associated with their gastrointestinal disease (3, 6, 8 – 10). For example, the American College of Gastroenterology recommends a routine stool culture for a patient who presents with any of the following symptoms: severe or persistent diarrhea, temperature of >38.5°C, bloody diarrhea, or the presence of stool leukocytes, lactoferrin, or occult blood (6). The IDSA similarly recommends that stool cultures be performed for a patient with diarrhea for >1 day, fever, dehydration, systemic illness, bloody stools, or a clinical history that would include bacterial pathogens in the differential diagnosis (3).
TABLE 1.
Types of bacterial gastroenteritis a
| Parameter | Secretory gastroenteritis | Inflammatory gastroenteritis | Invasive gastroenteritis |
|---|---|---|---|
| Location | Proximal small intestine | Colon | Distal small intestine |
| Type of illness | Watery diarrhea | Dysentery | Enteric fever |
| Stool examination | No fecal leukocytes | Fecal polymorphonuclear leukocytes | Fecal mononuclear leukocytes (if patient has diarrhea) |
| Mechanism | Enterotoxin or bacterial adherence/invasion causes a shift in water and electrolyte excretion/adsorption | Bacterial invasion or cytotoxins cause mucosal damage that leads to inflammation | Bacteria penetrate the mucosa and invade the reticuloendothelial system |
| Classic pathogens | Vibrio cholerae, ETEC, Clostridium perfringens, Bacillus cereus, Staphylococcus aureus | Shigella, STEC, Salmonella (not Salmonella Typhi/Paratyphi), Vibrio parahaemolyticus, Clostridium difficile, Campylobacter | Salmonella Typhi/Paratyphi, Yersinia enterocolitica |
Adapted from reference 321 with permission of the publisher.
In addition to the value for patient care, a bacterial stool culture is an important tool for public health. Isolates recovered from stool cultures performed by clinical laboratories are used to identify and track outbreaks at the local, national, and international levels. The dilemma with some of the newer test methods, including molecular assays, is the lack of organism recovery, which is currently needed for public health investigations.
The objectives for this practical guideline are to discuss the more common bacterial organisms associated with diarrheal disease, briefly describe emerging bacterial pathogens associated with diarrheal disease, describe stool specimen collection, transport, and processing, and discuss test methods used to identify these bacterial agents and antimicrobial susceptibility testing.
BACTERIAL PATHOGENS
Aeromonas Species
Over 26 different species of Aeromonas have been described to date, but the vast majority of these are of limited clinical or public health significance. Aeromonas spp. are ubiquitous in aquatic habitats, and concentrations peak when water temperatures rise substantially during the summer months. Consumable products such as poultry, lamb, veal, pork, and ground beef can harbor Aeromonas spp. Consumption of contaminated foods or potable water or accidental ingestion of untreated water during recreation are the most common sources of infection. In humans, Aeromonas spp. are not considered to be normal gastrointestinal flora, and the estimated human intestinal carrier/colonization rate is extremely low in healthy persons.
Most authoritative documents list Aeromonas spp. as accepted enteropathogens, although there still are no bona fide outbreaks of gastroenteritis attributable to this genus (11, 12). The incidence of Aeromonas-associated gastroenteritis on a global basis varies dramatically in association with geographic and socioeconomic factors. In developing countries where sanitary conditions are substandard, the reported incidence of Aeromonas diarrhea can be high, ranging from approximately 4% to 22% (13 – 16). In industrialized countries, regardless of patient population and sample size, Aeromonas-associated gastroenteritis has been reported at frequencies of 0% to 10% (17, 18).
Aeromonas diarrhea presents as either an acute watery diarrhea (enteritis) or as a more invasive bloody form resembling dysentery or enterocolitis (17). The secretory form is much more common than the dysenteric variety. A third, extremely rare variation of Aeromonas gastroenteritis presents as a cholera-like illness with profound watery diarrhea. Most intestinal infections associated with Aeromonas spp. are self-limiting, although chronic diarrhea exceeding for 1 year has been described (19, 20).
Several potential serious complications can result secondary to Aeromonas gastroenteritis, including ulcerative colitis, pan colitis, segmental colitis, or inflammatory bowel disease (17). In a few instances, cases of hemolytic-uremic syndrome (HUS) associated with Aeromonas hydrophila or Aeromonas veronii biovar sobria have been reported in infants and adults (21). Some Aeromonas spp. have been shown to carry the Shiga toxin (Stx) genes 1 and 2 (22), and development of HUS in patients infected with Aeromonas spp. may be attributable to this virulence factor. The most serious complication of Aeromonas gastroenteritis is translocation from the gut into the circulatory system, producing frank septicemia (23). This situation typically exists in persons with underlying conditions, including hepatic cirrhosis or malignancies of the circulatory systems. Attributable fatality rates due to Aeromonas sepsis range from 32% to 45% (17).
Bacillus cereus
B. cereus is ubiquitous in the environment, being found in decaying organic matter, soil, freshwater and salt water, vegetables, and the intestinal tracts of invertebrates (24). The spores are resistant to heat, freezing, and drying and can survive gamma radiation and pasteurization processes (25). Hydrophobic in nature, the spores can adhere to cooking and food surfaces (26, 27). B. cereus spores can germinate in foods that are not promptly cooled and refrigerated after meals or in food heated for prolonged periods at temperatures below 60°C. Outbreak surveillance data from 2009 and 2010 documented 427 illnesses associated with 25 outbreaks in the United States due to B. cereus (28).
There are two distinct syndromes associated with Bacillus cereus food poisoning: an emetic syndrome and a diarrheal syndrome. The emetic syndrome is due to intoxication by a preformed toxin ingested in food. The emetic toxin, called cereulide, is a plasmid-encoded peptide that is resistant to heat, proteolysis, and acid. As such, the toxin is not destroyed by gastric acids or proteolytic enzymes in the intestinal tract or by food reheating (29). Cereulide is responsible for symptoms of nausea and vomiting (25, 29, 30), which appear within 1/2 to 6 h after ingestion (30). These symptoms are similar to those seen with Staphylococcus aureus enterotoxins. Symptoms usually resolve within 6 to 24 h, but rare case reports have documented fulminant hepatic failure and death associated with emetic B. cereus (31 – 34). The emetic toxin is most often found in starchy foods, such as fried rice, pastry, and noodles (35).
The diarrheal syndrome is characterized by abdominal cramps, pain, and watery diarrhea within 8 to 16 h of ingestion of food that contains viable vegetative cells or spores of B. cereus. The symptoms of this diarrheal illness are similar to those seen with Clostridium perfringens food poisoning (35). Symptoms typically resolve with 12 to 24 h (35). Although rare, fatalities have occurred with B. cereus diarrheal disease (29). In the diarrhea syndrome, 3 pore-forming enterotoxins are expressed by the vegetative cells in the small intestine, which damage the ileal epithelial cell membranes. The 3 enterotoxins are hemolysin BL (HBL), nonhemolytic enterotoxin (NHE), and cytotoxin K (25, 35).
Individuals at increased risk of B. cereus diarrheal disease include those with lowered stomach acidity, such as is seen in patients with achlorydria or the elderly (36). B. cereus has been isolated from the stools of 0 to 43% healthy children and adults, at various concentrations. However, these cases represent transient colonization, most likely obtained from low-level exposure from the environment (24, 37, 38).
Campylobacter Species
Campylobacter is one of the leading causes of bacterial diarrhea worldwide (39). FoodNet estimates that 1.3 million persons in the United States are affected each year by Campylobacter infections (40). The true incidence may be up to 35 times higher due to undiagnosed or unreported cases (41). Geographic variation in rates of campylobacteriosis has been consistently observed in the United States between 1996 and 2006, with the mean annual rate of culture-confirmed campylobacteriosis being 5-fold higher in California (34 cases per 100,000 population) than in other states (42). The reason for this difference is unclear, but does not appear to be associated with increased physician visits, laboratory test ordering, or exposure to risk factors among patients in California compared to other states.
Campylobacter inhabits the intestinal tracts of food animals, such as poultry, cattle, swine, and sheep, and domestic pets, including cats and dogs. The organism rarely causes disease in animals but is shed in the feces. Meat typically becomes contaminated with animal feces harboring Campylobacter spp. during slaughtering. Transmission of the organism is typically foodborne, by ingestion of undercooked contaminated meat and meat products or contaminated dairy products. In addition, waterborne infections occur, via consumption of contaminated water and ice. Contact with infected animals, particularly cats and puppies, has also been shown to be a route of transmission. The typical incubation period for Campylobacter is 2 to 5 days, but it may be up to 10 days (43). Most cases of Campylobacter enteritis are sporadic, but the incidence increases starting in March and throughout the summer months. Outbreaks associated with Campylobacter have been due to consumption of raw milk or well water contaminated with effluent from livestock operations (44 – 46). Higher rates of Campylobacter enteritis are seen in those <4 years of age and 15 to 44 years of age (47). Travelers to developing countries are also at increased risk of Campylobacter enteritis.
Campylobacter jejuni subsp. jejuni and Campylobacter coli are the most common Campylobacter species associated with diarrheal illness. C. jejuni is responsible for >90% of cases (43, 48). Campylobacter upsaliensis, which was first isolated from dogs with diarrhea, has also been shown to cause human disease. The incidence of C. upsaliensis among patients with diarrhea may be underappreciated, as the organism cannot grow on the selective media typically used to recover Campylobacter in clinical laboratories (49 – 51). Other Campylobacter spp. associated with gastroenteritis include Campylobacter fetus subsp. fetus, Campylobacter lari, Campylobacter concisus, Campylobacter jejuni subsp. doylei, and Campylobacter hyointestinalis (48, 52).
C. jejuni and C. coli cause indistinguishable infections (48). Before the onset of diarrhea, a febrile period with malaise, abdominal pain, and myalgia occurs in about 50% of symptomatic patients (43). Diarrhea is characterized by loose watery stools, with or without blood. Blood and fecal white cells may be present. Abdominal cramping can mimic pain associated with acute appendicitis. In most cases, the diarrhea is self-limited, resolving within a week without antimicrobial therapy. However, relapse occurs in 5 to 10% of untreated patients (43). Extraintestinal Campylobacter infections such as bacteremia, urinary tract infections, cholecystitis, hepatitis, pancreatitis, nephritis, meningitis, abortion, and neonatal sepsis have also been reported (53). Campylobacter bacteremia is typically uncommon, but it occurs more frequently in patients with HIV infection, malignancy, and liver disease (54). Bacteremia and extraintestinal infections are also more common in neonates and the elderly (55).
Autoimmune complications, such as reactive arthritis and Guillain-Barré syndrome (GBS), can occur post-Campylobacter infection (56). Reactive arthritis affects 2 to 4% of patients postcampylobacteriosis and is characterized by pain and joint swelling that lasts for several weeks to a year (48). In 5% of cases, arthritis is chronic or relapsing (57). Symptoms typically begin 3 to 40 days postdiarrhea and most commonly affect the knees (58). GBS is an acute paralytic disease of the peripheral nervous system and is seen in approximately 0.1% of Campylobacter cases. Lipooligosaccharides of C. jejuni, which mimic human ganglioside, elicit autoantibodies that then react with peripheral nerve targets (56). The onset of GBS usually occurs within 2 to 21 days of the diarrheal illness (59).
Clostridium difficile
Clostridium difficile is an obligately anaerobic, spore-forming Gram-positive rod. The spores of C. difficile are resistant to stomach acid, heat, and many commercial disinfectants used in hospitals (60). Following ingestion, exposure of the spores to bile salts in the small intestine triggers germination (61). Pathogenic strains of C. difficile harbor a pathogenicity locus (PaLoc) that encodes the organism's two main virulence factors: toxin A, an enterotoxin (encoded by tcdA), and toxin B, a highly potent cytotoxin (encoded by tcdB) (62). The individual role of these two toxins in disease are controversial. Clinical isolates of C. difficile that do not express toxin A have been isolated from symptomatic patients (63, 64), albeit rarely, whereas toxin B-deficient strains have not. Both toxin A- and toxin B-deficient mutants remain capable of causing disease in hamsters, although both are attenuated compared to the wild-type strain (65).
C. difficile can readily be found in soil and the intestinal tracts of animals and humans. C. difficile colonization rates are as high as 50% in healthy infants and children <1 year of age (66, 67), whereas 3% to 5% of healthy adults are colonized (67). Much higher rates of colonization, 10 to 50%, are seen in high-risk populations, such as hospitalized patients and long-term-care facility residents. Previous antimicrobial use and previous C. difficile infection (CDI) are predictors of colonization in these populations (68 – 70). C. difficile is acquired through the ingestion of spores via the fecal-oral route or through exposure to spores in the environment. A recent study demonstrated that only a third of CDI cases could be linked by whole-genome sequencing of isolates to a symptomatic patient, whereas the remainder of cases were attributed to exposure from the environment or asymptomatic carriers (71).
C. difficile is the primary pathogen associated with antibiotic-associated colitis (72, 73). In the United States, the rate of CDI increased 4-fold between 1993 and 2009 but leveled off at 110 per 100,000 hospital stays in 2009 (74). By far the highest rate of CDI is among patients aged 65 and older, with over 1,000 cases per 100,000 hospitalizations in 2009 reported for this age group (74).
In 2005, the NAP1/027/B1 strain emerged in Canada, Europe, and the United States, concomitant with a significant rise in morbidity and mortality associated with CDI over those in previous years (75, 76). At the time, this change in severity of CDI was attributed to the “hypervirulent” nature of the NAP1/027/B1 strain. NAP1/027/B1 has since become the predominant strain in many locations, and it continues to be associated with high mortality and relapse rates (77). Early studies pointed to heightened toxin expression (78), more efficient sporulation (79, 80), expression of the binary toxin, and fluoroquinolone resistance (75) as reasons for the epidemiological success of this strain. However, some studies questioned the relevance of the NAP1/027/B1 strain type in disease severity (81, 82), and it has since been confirmed that not all NAP1/027/B1 strains express larger quantities of toxin than historical strains (83).
The range of symptoms associated with infection with toxigenic C. difficile includes asymptomatic carriage, mild to moderate diarrhea, and pseudomembranous colitis (PMC). Patients may present with a brief, self-limiting diarrhea or with profuse watery diarrhea similar to that in cholera (84). Fever, abdominal cramping, and leukocytosis can be seen in individuals with more severe diarrhea. Persons with PMC present with abdominal pain, fever, marked leukocytosis, and severe diarrhea that may be bloody. Poor prognostic indicators include a rapid increase in the peripheral white blood count with an increase in band forms and a sudden absence of diarrhea (85).
The most common conditions associated with CDI are dehydration and electrolyte disorders, which may affect up to 92% of patients. Less frequent conditions associated with CDI include septicemia, hypoalbuminemia, renal failure, septic shock, ascites, and peritonitis. The more severe complications of CDI include intestinal perforation and toxic megacolon. While these severe complications are only observed in 0.1% to 3% of all CDI cases (74, 86, 87), the mortality associated with toxic megacolon is high, ranging from 38% to 80% (86, 88).
Recurrence of CDI is seen in 10% to 20% of cases after initial symptom resolution (89). Recurrent infections are attributable to both relapse (i.e., spores that are not killed by antimicrobial therapy, which can then germinate once therapy is completed) and reinfection with a new strain (90 – 93). However, it is important to note that patients who are asymptomatically colonized with C. difficile are at decreased risk for CDI, although the reason for this remains unclear (94).
Exposure to antimicrobial agents and exposure to health care facilities are hallmark risk factors for CDI. While almost all antimicrobial agents have been associated with CDI, the most common are penicillins, second- and third-generation cephalosporins, clindamycin, and fluoroquinolones (84, 95). As stated previously, advanced aged (>65 years) is also an important risk factor for CDI; this age group has over 10-fold the number of CDI hospitalizations than the general population in the United States (74). Other, less well-defined risk factors for CDI include use of gastric acid suppressors, stool softeners, laxatives, and/or enemas, chemotherapy, and gastrointestinal surgery (96).
Clostridium perfringens
Clostridium perfringens is ubiquitous in the environment and can be found in the feces of humans and animals. Food poisoning with C. perfringens requires ingestion of a high burden of vegetative cells, usually 108. The typical mechanism for this is food contaminated with C. perfringens that is improperly cooked, stored, and reheated. Spores that survived the initial heating processes germinate and proliferate during a slow cooling of food or when the food is insufficiently reheated. Following ingestion, the organism sporulates upon entry into the small intestine, which is concomitant with expression of an enterotoxin that is responsible for patient symptoms. C. perfringens serotype A is the most common serotype associated with food poisoning and diarrhea (97, 98).
From 2009 to 2010, there were 60 confirmed C. perfringens foodborne outbreaks and 3,225 reported illnesses, making C. perfringens the second most common cause of bacterial foodborne disease in the United States in this time period (28). Symptoms most often associated with C. perfringens food poisoning are watery diarrhea, severe abdominal cramping and pain, and vomiting. The onset of symptoms ranges from 8 to 24 h after the ingestion of contaminated food. The illness is self-limiting, and symptoms resolve within 24 h.
A rare type of food poisoning called enteritis necroticans or “pig-bel” is associated with the ingestion of food, usually pork, heavily contaminated with C. perfringens serotype C. This organism produces a beta toxin that causes intestinal wall necrosis. Pig-bel has a mortality rate of 40% and primarily affects malnourished persons, especially children (99). C. perfringens has also been linked to antibiotic-associated diarrhea that does not cause pseudomembranous colitis (73, 100).
Escherichia coli
Escherichia coli was initially considered to only be a commensal residing in the gastrointestinal tract. However, several pathogenic variants (pathotypes) are now recognized and associated with diarrheal diseases. Although E. coli is easy to identify to species level, it is extremely difficult to recognize strains belonging to different pathotypes of diarrheagenic E. coli, as these are defined by the expression of one or more group-specific virulence factors. The six major diarrheagenic pathotypes described to date are enteropathogenic E. coli, Shiga toxin-producing E. coli (STEC), enteroinvasive E. coli (EIEC), enterotoxigenic E. coli, enteroaggregative E. coli, and adherent invasive E. coli (101). Of these, only STEC is routinely identified by most clinical and public health laboratories, and it will be the focus of the discussion here. STEC is defined by the presence of a Shiga toxin 1 (Stx1) and/or Shiga toxin 2 (Stx2) gene. Historically, these isolates were called enterohemorrhagic E. coli (EHEC) or verocytotoxin-producing E. coli (VTEC). STEC includes both O157 and non-O157 serotypes of E. coli.
Ruminants, such as cattle, are the major reservoir for STEC. Poor sanitation, fecal runoff into rivers and streams, and inadequate control measures in the meat and food processing industries have all led to the recovery of STEC from virtually any consumable product. Infection with STEC occurs following consumption of these contaminated products. Infections occur predominantly in the summer months but can be observed year round (102).
The incidence of STEC infections in the United States is monitored by FoodNet. In 2012, the incidence of O157 STEC was 1.12 per 100,000 population, and the incidence of non-O157 STEC was 1.16 per 100,000 (103). Among the non-O157 STEC strains, O26, O103, O111, O121, O45, and O145 are the most common serotypes isolated in the United States (104). The incidence of STEC in other developed countries varies; it is as low as 0.4 per 100,000 in Australia (105) and as high as 5.33 per 100,000 in Ireland (106). The incidence of STEC is much higher in developing countries such as Argentina and India, but formal surveillance data are not available for these countries.
STEC disease presents as enteritis that may quickly progress to hemorrhagic colitis (107). The chief symptoms included bloody diarrhea, abdominal pain, nausea, and vomiting (108). Importantly, not all STEC infections are associated with bloody diarrhea (109, 110), and so laboratory algorithms that only test bloody specimens for STEC are no longer considered standard of care. The most common and serious complication of STEC infection is the development of HUS, which typically presents 5 to 13 days after the onset of diarrhea (11). HUS is life-threatening and consists of the triad of renal failure, microangiopathic hemolytic anemia, and thrombocytopenia. The mortality rate connected with HUS is 3% to 5% (111). It has been estimated that 61% of all HUS cases are related to STEC infection (111). HUS has been observed more frequently in O157 (11% of cases) versus non-O157 (1% of cases) STEC infections (104). Approximately 15% of children <10 years of age develop HUS following STEC infection. However, in the recent outbreak of O104 STEC in Germany, 22% of children developed HUS (112 – 114). It should be noted that this outbreak was caused by an atypical STEC strain that harbored enteroaggregative E. coli virulence factors in addition to the Shiga toxins. HUS occurs much less frequently among adults and is associated predominantly with advanced age (>75 years) (115). Increased rates of HUS have been more frequently associated with Stx2-expressing STEC strains. Exposure to antibiotics also increases the risk of HUS in children (114). However, recent data demonstrated that treatment with ciprofloxacin reduced the risk of HUS in patients infected with the 2011 German O104 STEC strain (116). These data are supported by a recent meta-analysis of studies between 1980 and 2011 (117). Despite this, the decision to treat a patient with STEC infection with antimicrobials remains controversial. In addition, use of antimotility agents has been associated with longer duration of bloody diarrhea, as well as progression to HUS (118).
Listeria monocytogenes
The genus Listeria is composed of six species, of which Listeria monocytogenes is the common human pathogen, causing intestinal as well as extraintestinal infections. L. monocytogenes is a common environmental inhabitant of soil, vegetation, and animals (119). Because Listeria spp. can survive under acidic and salt-enhanced conditions in foods and can grow at refrigeration temperatures (4°C), they have the capacity to survive and multiply in large numbers in a variety of refrigerated foods (119, 120). A high percentage (32%) of foods recalled by the FDA involve L. monocytogenes (121). The major risk factor associated with L. monocytogenes gastroenteritis is the consumption of foods heavily contaminated (107 to 109 CFU/g or ml) with L. monocytogenes (122).
The incidence of L. monocytogenes gastroenteritis is unknown. Surveillance data from the CDC and other sources, including FoodNet, have focused on invasive listeriosis (bacteremia and central nervous system infection) as a consequence of foodborne infection. In 2011, the incidence of invasive listeriosis was 0.31 per 100,000 population. Many patients with invasive listeriosis have a history of gastrointestinal symptoms that consist of diarrhea, nausea, vomiting, and fever. This, coupled with reports of L. monocytogenes outbreaks of gastroenteritis (122, 123), suggests that L. monocytogenes may be an infrequent cause of gastroenteritis in patients with negative bacterial stool cultures. One 2005 Canadian study found the maximum incidence of L. monocytogenes-associated diarrhea to vary from 0.2% to 0.5%, depending upon the population studied (123). On rare occasions, Listeria ivanovii has been reported to cause diarrhea in severely immunosuppressed individuals (124).
The typical incubation period for gastrointestinal infection is 24 h; however, it can range from 6 h to as long as 10 days (120). Once symptoms begin, diarrhea lasts for 1 to 3 days (122). In a study of cases of gastroenteritis linked to outbreaks, attack rates ranged from 50% to 90% and the median number of stools/day was 12 (range, 3 to 50) (122). The syndrome is typically characterized by a febrile illness with diarrhea, headache, and arthralgia/myalgia. Other, less frequently encountered complications include abdominal pain, nausea, vomiting, dizziness, lymphadenopathy, and presence of a rash (12, 122). Fever, which occurs in 60% to 100% of infected persons, is a cardinal feature associated with L. monocytogenes diarrhea.
The most serious complication of listeriosis is invasive disease, including septicemia and meningitis. L. monocytogenes has tropism for the brain and as a result can cause encephalitis, rhombencephalitis (brain stem encephalitis), and brain abscess. The case fatality rate for most cases of listeriosis with comorbidities has been reported to be between 20% and 40% (125).
Reputed risk factors associated with acquiring L. monocytogenes gastroenteritis include gastric acidity, use of antacids, use of H2 receptor antagonists, and use of laxatives (119, 122, 126). In addition, those with inflammatory bowel disease (IBD) and Crohn's disease may have a more frequent incidence of Listeria diarrhea (as opposed to Campylobacter or Salmonella) (123, 126).
Plesiomonas shigelloides
Plesiomonas shigelloides is the sole oxidase-positive member of the Enterobacteriaceae family. While P. shigelloides has been associated with diarrheal disease in numerous reports, a definitive causal relationship with P. shigelloides has yet to be established through volunteer or animal studies (127).
P. shigelloides is found in aquatic environments and has been isolated from both cold-blooded and warm-blooded animals. In humans, there has been a reported prevalence rate of 0.01% to 5.5% in asymptomatic individuals (128, 129). Transmission occurs primarily through the consumption of seafood, such as oysters and shellfish, or water that has been contaminated with sewage. Most cases of P. shigelloides diarrheal illness are sporadic; however, there have been reported outbreaks associated with the organism (130 – 132). Coinfection with P. shigelloides and other enteropathogens has been reported (132, 133), and some evidence suggests that P. shigelloides causes diarrhea only as a coinfecting pathogen, rather than on its own (133). Both secretory and dysentery-type diarrhea have been reported with P. shigelloides infections (130, 134). Most infections are characterized by self-limiting diarrhea with blood or mucus, abdominal cramps, vomiting, and fever (130). While most diarrheal episodes are described as acute, there have been reported chronic cases lasting over 2 weeks (135).
Salmonella Species
Salmonella, a member of the family Enterobacteriaceae, is a facultatively anaerobic Gram-negative rod. Salmonella taxonomy is a complicated matter, with two species in the genus: Salmonella enterica and Salmonella bongori. Salmonella enterica has six subspecies (S. enterica subsp. enterica, S. enterica subsp. salamae, S. enterica subsp. arizonae, S. enterica subsp. diarizonae, S. enterica subsp. indica, and S. enterica subsp. houtenae) that can be further serotyped using the Kauffmann-White-Le Minor scheme, based on the properties of their somatic (O), flagellar (H), and capsular polysaccharide (Vi) antigens (136, 137). There are over 2,500 serotypes of S. enterica (136, 137). Because of the diversity of the genus, several isolates may be difficult to identify due to atypical biochemical reactions.
Salmonella colonizes the intestinal tracts of vertebrates. Some serotypes, including Salmonella enterica subsp. enterica serotype Typhi (Salmonella Typhi), are only found in human hosts. The majority of Salmonella cases occur as the result of ingesting contaminated food or water. Salmonella can also be acquired by contact with domestic animals and their food products, farm animals or animals in petting zoo, and exotic pets like turtles, hedgehogs, and iguanas (138 – 142). Salmonella can also be transmitted from person to person via the oral-fecal route.
The incidence of Salmonella infections in the United States in 2011 was 1,645 per 100,000 population (143), with higher rates in late summer and early fall. Worldwide, there are an estimated 94 million cases of nontyphoidal Salmonella gastroenteritis and about 155,000 deaths (144). In developing countries, and the Indian subcontinent in particular, typhoidal isolates cause the majority of disease and are associated with an estimated 21.6 million annual cases and 216,500 deaths (145). In sub-Saharan Africa, nontyphoidal Salmonella, predominantly the Salmonella Typhimurium ST313 strain, are a significant cause of bloodstream infections in both children and adults (146, 147). In the United States, the most common serotypes reported are Salmonella Enteritidis, Salmonella Typhimurium, and Salmonella Newport (143).
Nontyphoidal salmonellosis consists of diarrhea, nausea, headache, and abdominal cramps, which last for 4 to 7 days. Fever may be present and usually resolves in 24 to 48 h. The disease is typically limited to the lamina propria of the small intestine, and antimicrobial therapy is not indicated. Extraintestinal manifestations, such as bacteremia, septic arthritis, urinary tract infections, and osteomyelitis, are seen in 5% of cases (148 – 153). Some individuals may become asymptomatic carriers of the organism, and shedding occurs for several weeks to a few months.
Typhoid fever is caused by Salmonella Typhi, and a similar syndrome is caused by Salmonella Paratyphi A, Salmonella Paratyphi C, and tartrate-negative variants of Salmonella Paratyphi B. In typhoid, the organism disseminates from the lamina propria to the reticuloendothelial system in infected phagocytes via lymphatic and hematogenous routes. Fever, malaise, anorexia, headaches, and vomiting are common symptoms of typhoid and typically start 1 to 3 weeks after infection. Patients may have diarrhea following ingestion of the organism, but many do not. Rose spots, which are blanching maculopapular lesions 2 to 4 mm in diameter, are seen in 5 to 30% of cases. A complication of untreated typhoid fever is the erosion of the blood vessels in the Peyer's patches, which can lead to intestinal hemorrhage (145). The organism persists in the mesenteric lymph nodes, gallbladder, and bone marrow for years. Five to 10 percent of patients will have a relapse of infection, typically 2 to 3 weeks following resolution of symptoms (154). Up to 10% of asymptomatic patients will become carriers, and 1 to 4% of these will shed for more than 1 year (154).
The severity of Salmonella disease depends on the inoculating dose (155), infecting serotype (151), and predisposing host factors. Children under 1 year of age have the highest incidence of Salmonella in the United States (143). Because Salmonella must survive the gastric acid barrier in order to gain access to the small intestine where it causes disease, patients with decreased gastric acid production, from advanced age, gastrectomy, or H2 receptor antagonists, are at increased risk of infection. Individuals with impaired cellular immunity (e.g., AIDS) or altered phagocyte function (e.g., sickle cell anemia) are at increased risk for both invasive nontyphoid Salmonella infections and typhoid (156, 157). However, these individuals do not appear to have more severe typhoid infections should they become infected (158, 159). In the United States, nearly all cases of typhoid and paratyphoid fever are in returning travelers and immigrants (160).
Shigella Species
Shigella species are host adapted to humans but have been documented in rare instances from dogs and primates (161). They can be acquired from ingestion of a variety of foods or water contaminated with human feces, sexually during oral-anal sex, or by laboratory workers. The four species of Shigella are Shigella dysenteriae, Shigella flexneri, Shigella boydii, and Shigella sonnei. Transmission by person-to-person contact is common for Shigella spp. because of a low infectious dose of 10 to 100 organisms (161). Between 2009 and 2010, Shigella accounted for 508/8,523 (2%) of reported illnesses associated with foodborne outbreaks (28). The incidence of Shigella infections reported by FoodNet in the United States in 2011 was 3.24 per 100,000 and ranged from 0.99 to 6.78 per 100,000, depending on the region (143).
Shigellosis and dysentery are diseases associated primarily with poor hygiene and lack of access to medical care. Approximately 150 million cases are reported annually in developing countries, in contrast to 1.5 million cases in industrialized nations. Of importance, one multicenter study found that half of patients with culture-negative, bloody stools were positive by PCR for Shigella, suggesting that the actual incidence of Shigella is grossly underestimated (162). Shigellosis symptoms range from watery diarrhea to mucoid and/or bloody stools, which can be accompanied by fever, malaise, and abdominal pain. In one study of 1,114 culture-confirmed patients followed for 14 days or longer, 29% (241) reported diarrhea persisting for ≥14 days (162). Factors associated with persistence were age, fever, mucoid diarrhea, vomiting, and abdominal pain. Headache and nuchal rigidity are common, with 95% and 39% of patients reporting these symptoms, respectively (161). S. dysenteriae type 1 is responsible for classic dysentery, which is manifested by fever, abdominal cramping, and bloody stool. Sepsis occurs primarily in malnourished pediatric patients in developing countries and is most commonly caused by S. flexneri (163). Long-term carriage (>1 year) occurs but is rare (164).
Meningitis, pneumonia, and urinary tract infections (UTIs) are rare complications of shigellosis and are most commonly seen with S. flexneri and S. sonnei (165 – 167). Notably, 40% of UTIs are asymptomatic and 35% are culture negative (167). Reactive arthritis has been reported in 1 to 3% of cases from outbreak data (161). The onset of reactive arthritis occurs within 3 weeks of gastrointestinal symptoms, with the duration of symptoms ranging from a few days to a few months; only S. flexneri has been associated with reactive arthritis.
HUS is the most serious complication of shigellosis. HUS occurs in ∼13% of cases of S. dysenteriae type 1 shigellosis and is attributable to the expression of Stx1 by this organism (168). However, in rare cases, non-S. dysenteriae species of Shigella have been isolated from children with HUS (168, 169). S. dysenteriae type 1 HUS is seen mainly in children <5 years old in Asia and Africa.
Staphylococcus aureus
S. aureus food poisoning is an intoxication caused by the ingestion of preformed, heat-stable enterotoxin. There are 21 known staphylococcal enterotoxins, but phage-encoded staphylococcal enterotoxin A is the most frequently reported cause of S. aureus food poisoning worldwide (170 – 172). Coagulase-negative staphylococci (CoNS) can also acquire enterotoxins, but the reported cases or outbreaks of CoNS food poisoning have been limited (173, 174).
S. aureus is ubiquitous in the environment and colonizes the skin and mucous membranes of many mammals and birds (175). In humans, the anterior nares is the most commonly colonized site, and the organism is shed on to healthy skin (176). The rate of persistent carriage of S. aureus is reported to be 10 to 35%, and the rate of intermittent colonization ranges from 20 to 75% (176, 177). For those individuals harboring S. aureus, the organism can be transferred from their hands while preparing food. S. aureus is most commonly found in foods such as cream-filled pastries, cream pies, and sandwich fillings. However, food products involved in S. aureus food poisoning differ widely from one country to another (175). The CDC estimates that there are approximately 241,000 cases of foodborne illnesses in the United States caused by S. aureus annually.
A rapid onset of symptoms is characteristic of S. aureus food poisoning. General malaise, nausea, vomiting, stomach cramps, and diarrhea can occur within 30 min of ingestion of the contaminated food. The typical incubation period is 2 to 7 h, with symptoms resolving in about 12 h (11). Patients with staphylococcal food poisoning are not febrile. In most cases, medical treatment is not required. However, hospitalization for the severity of symptoms may be seen in 10% of those with S. aureus food poisoning (178). Severe dehydration may be seen in young children and elderly patients (178).
S. aureus food poisoning requires consumption of food or beverages harboring the staphylococcal enterotoxins. Unsafe food handling practices, including neglecting to wash hands prior to handling food and to promptly refrigerate prepared foods, are the primary reason for intoxication.
Vibrio and Vibrio-Like Species
The genus Vibrio is currently comprised of over 60 species. A number of other species traditionally associated with this genus have been recently reclassified into phylogenetically related neighboring clades, including Grimontia hollisae (Vibrio hollisae). Of the more than 60 Vibrio or Vibrio-like species that have been described, only a few these taxa have been consistently associated with bacterial gastroenteritis, with the two major species being Vibrio cholerae and Vibrio parahaemolyticus. Less frequent, but still of concern, are Vibrio mimicus, Vibrio fluvialis, Vibrio vulnificus, and G. hollisae.
Vibrio and vibrio-related bacteria are widely distributed in saltwater environments with salt concentrations of 17 to 37 ppt. Freshwater habitats with low salt concentrations (<0.5 ppt) can harbor nonhalophilic Vibrio spp. such as V. cholerae and V. mimicus. Because of their intimate association with the marine environment, Vibrio spp. can be found in many inhabitants of this macroecosystem, including shellfish such as oysters, clams, shrimp, and scallops.
The preeminent pathogen of this group is V. cholerae, which can cause sporadic, epidemic, and pandemic cholera. The WHO estimates that over 1.4 billion persons worldwide are at risk of developing cholera each year, with an estimated 2.8 million cases occurring annually and with over 130,000 deaths (179, 180). Today, the highest incidence of cholera is found in Africa and the southern regions of Asia. Two serogroups of V. cholerae, O1 (El Tor biotype) and O139, are responsible for the ongoing pandemic of cholera disease.
Cholera is not common in the United States, but the incidence of vibriosis (V. parahaemolyticus, V. vulnificus, and V. alginolyticus) is increasing. There are an estimated 80,000 illnesses with 500 hospitalizations and 100 deaths each year due to Vibrio illnesses in the United States, based upon data submitted through the Cholera and Other Vibrio Illness Surveillance (COVIS) system and FoodNet (181, 182). These cases include not only patients with diarrhea but also those with primary septicemia, wound infections, and otitis externa caused by Vibrio spp. The annual incidence of vibriosis in the United States has increased from 0.09 to 0.15 per 100,000 population in 1996 to 0.28 to 0.42 per 100,000 in 2010, with the highest incidence in coastal areas (181).
V. parahaemolyticus is responsible for many outbreaks of food-associated gastroenteritis worldwide. In Japan, it has been one of the most important causes of foodborne diarrhea since the 1960s (183). This species has also been responsible for the global spread of a pandemic clone, O3:K6, causing gastroenteritis in such diverse locales as North, Central, and South America, the Indian subcontinent, parts of Africa and Europe, and Indonesia from 1996 through 2004 (184). Other clonal strains, such as O4:K12, have caused more restricted outbreaks of disease, such as on the west coast of the United States (185). V. mimicus has been reported to cause at least two outbreaks of diarrheal disease (186, 187). The number of studies and case reports worldwide describing gastrointestinal infections cause by V. fluvialis seems to be increasing as well (188, 189). In the United States, V. fluvialis is typically the third most common Vibrio species associated with gastroenteritis, following V. parahaemolyticus and non-O1, non-O139 V. cholerae.
The chief clinical features of cholera are an afebrile, painless, watery diarrhea associated with V. cholerae O1 El Tor infection, accompanied by multiple bowel movements over a short period of time. Incubation periods for cholera typically span from 18 h to 5 days (190). Asymptomatic colonization is relatively common in areas of endemicity due to constant exposure to the infecting agent under unsanitary conditions. For symptomatic persons, clinical presentations of cholera range from a mild to moderate diarrhea to a more fulminant form termed cholera gravis (190). Cholera gravis is characterized by the release of large volumes of water (500 to 1,000 ml/h), which rapidly leads to severe dehydration, shock, and death over a short period of time if left untreated. The more severe forms of cholera are associated with pandemic strains bearing the O1 serogroup that carry a series of virulence genes, the two most important of which are those for cholera toxin and toxin-coregulated pilus (191). Cholera toxin is typically only found in O1 El Tor or the epidemic O139 Bengal strains, although other serogroups (O75 and O141) occasionally harbor these elements as well and produce cholera-like disease.
Gastroenteritis caused by non-O1, non-O139 serogroups of V. cholerae is typically milder and self-limiting, since they normally lack the cholera toxin gene. These non-O1, non-O139 isolates nevertheless cause the vast majority of V. cholerae gastrointestinal infections in the United States. While disease caused by these isolates is typically mild, fatal cases of non-O1, non-O139 V. cholerae can occur (192).
V. parahaemolyticus is the most common cause of Vibrio-associated diarrhea in the United States. The most frequent symptoms linked to V. parahaemolyticus enteritis include diarrhea with abdominal cramps, with approximately half of all infected individuals having a febrile illness (193). Two prominent symptoms, nausea (76%) and vomiting (55%), help to distinguish diarrhea caused by this species from other vibriosis or other enteritides associated with bacteria.
Unlike with many other enteric pathogens, secondary complications due to Vibrio gastroenteritis are rare. The principle complication that can arise from enteric infection is secondary spread to the bloodstream, producing septicemia. In the case of V. cholerae, virtually all such bacteremias are caused by non-O1, non-O139 isolates (194). Other, infrequently encountered Vibrio species that have been demonstrated to cause septicemia subsequent to primary gastrointestinal infections include V. fluvialis and G. hollisae (195, 196).
In the case of cholera, most infections arise in areas of endemicity through contaminated water and nonhygienic conditions which perpetuate persistence of O1. However, persons can also develop cholera through ingestion of contaminated shellfish or seafood products containing high concentrations of V. cholerae. For other Vibrio and Vibrio-like infections, the two major risk factors for acquiring disease are consumption of contaminated seafood and foreign travel. Vibrio spp. have naturally been recovered from many different types of seafood, including oysters, mussels, clams, shrimp, and tilapia (197). A large number of seafood vehicles have been implicated in vibriosis outbreaks associated with non-V. cholerae vibrios (186, 193).
Yersinia enterocolitica and Yersinia pseudotuberculosis
There are currently 18 species within the genus Yersinia, nine of which are isolated from humans. Yersinia enterocolitica, the most well-established enteropathogen of the genera, has two subspecies described, Y. enterocolitica subsp. enterocolitica and Y. enterocolitica subsp. paleartica, which can be distinguished by sequencing of the 16S rRNA gene (198). Y. enterocolitica subsp. paleartica O:3/4 is the dominant serotype worldwide (199). Yersinia pseudotuberculosis is also enteropathogenic but is more commonly associated with sepsis. Y. frederiksenii, Y. kristensenii, Y. intermedia, Y. mollarettii, Y. bercovieri, and Y. rohdei can be isolated from humans (including patients with diarrhea), but they are not believed to be pathogenic except in rare cases in individuals with underlying disorders (161, 200). Pathogenic strains of Y. enterocolitica are determined by the biotype and serotype.
Y. enterocolitica and Y. pseudotuberculosis can be isolated from a host of animals, birds, foods, and environmental sources (201). Animal sources of human infections include hares, rodents, cats (Y. pseudotuberculosis), and dogs (Y. enterocolitica). Environmental sources include soil, water, and sewage (161). Pigs are a major reservoir for both Y. enterocolitica and Y. pseudotuberculosis infections worldwide (201 – 203).
Between 1996 and 1999, FoodNet determined an annual incidence of Y. enterocolitica in the United States of 1.0/100,000 persons, with the greatest rates of infection in blacks and Asians (203). Between 1996 and 2009, FoodNet active surveillance noted a decline in the overall annual incidence (0.5/100,000 persons) of Y. enterocolitica, with rates in blacks also declining from 3.9 to 0.4 per 100,000 by 2009 (203). The overall rate of Y. enterocolitica reported by FoodNet in 2011 was 0.34 per 100,000 (143). The high infection rate in blacks has been associated with homemade chitterlings (pork intestines), and educational efforts have been cited as a possible explanation for the decrease in infections in this ethnic group. Infection rates are highest in children (201). In the United States, 32% of cases occurred in children <1 year old and 47% in children <5 years old (203). Similar epidemiology is seen outside the United States; in China, 44% of cases are reported in children <3 years of age (202). Y. enterocolitica infections are classically documented to occur in the autumn and winter; however, a study of yersiniosis in Europe conducted over a 3-year period found no clear seasonal pattern (201, 202), and winter trends in yersiniosis in high-risk populations have also diminished in the United States (203).
Y. pseudotuberculosis most commonly causes mesenteric adenitis, which manifests as an appendicitis-like syndrome with fever and right lower quadrant abdominal pain. Y. pseudotuberculosis can also cause severe septicemia (161). Symptoms associated with sepsis include fever, diarrhea, abdominal pain or tenderness, anorexia, nausea, vomiting, and malaise. Mortality rates range from 28% to 100% in treated and untreated cases, respectively (161).
Y. enterocolitica gastrointestinal disease ranges from self-limiting enteritis with diarrhea, low-grade fever, and abdominal pain to severe disease such as terminal ileitis and mesenteric lymphadenitis which also mimics appendicitis (203 – 205). Onset is generally 24 to 48 h following ingestion, with illness lasting between 7 and 14 days, but symptoms may persist for up to 2 to 12 months (201, 205). Bloody stools occur in 20 to 46% of cases, and host susceptibility, number of ingested organisms, and serotype are determining factors for severity of disease (201). Severe cases may require hospitalization due to dehydration; in one study, 27% of 571 patients were hospitalized (205).
Sepsis is uncommon and is often associated with cardiovascular, dermal, or pulmonary conditions and abscesses. Pharyngitis, with sore throat and fever as the predominant symptoms, is not unusual in yersiniosis; in one multistate outbreak, 14 of 172 (8%) patients reported pharyngitis. Fulminant symptoms, including difficulty swallowing and breathing, may occur and require immediate medical attention (161). In these cases, Y. enterocolitica can be isolated from throat cultures.
The two most common sequelae of Y. enterocolitica infection are reactive arthritis and erythema nodosum, an immunologically mediated disease resulting in inflammation of subcutaneous adipose tissue with eruption of painful nodular lesions (205). In one large study of 571 patients, 7% and 3% of 571 patients reported reactive arthritis or erythema nodosum, respectively (205). The onset of reactive arthritis generally occurs <3 weeks after enteritis, and the longer the duration of gastrointestinal symptoms, the greater the likelihood that reactive arthritis will develop (161). Joint inflammation generally subsides spontaneously after 1 to 12 months, but 10% of patients will develop chronic arthritis (206). Approximately 80% of patients developing reactive arthritis carry the HLA-B27 allele (206). Septic arthritis is less commonly encountered and is not associated with HLA-B27 (161).
Because some Y. enterocolitica serotypes are unable to synthesize siderophores (compounds that sequester iron from the host), patients with iron overload disease are more susceptible to infection (161, 201). Y. enterocolitica can be acquired from blood transfusions, as the organism readily grows at lower temperatures used to store blood products. The development and severity of disease are dependent on the species of Yersinia (other than Y. enterocolitica) and the Y. enterocolitica bioserotype acquired (200, 204).
Emerging Enteropathogens
This guideline provides technical information on enteropathogens most commonly encountered in clinical practice; however, there are many additional bacteria that have been associated with gastroenteritis. Limited information is available for the majority of these, and they are reviewed elsewhere (207). Several of these agents have enough clinical importance and high enough frequency to mention here. It should be stressed, however, that testing for these organisms is not part of routine bacterial stool cultures in the clinical microbiology laboratory at this point, due to the difficulty in differentiating these organisms from resident flora in stool.
Bacteroides fragilis.
Strains of B. fragilis carrying an ∼6-kb pathogenicity island produce a zinc metalloprotease enterotoxin that has been known by several different names, including B. fragilis toxin and fragilysin (208, 209). These enterotoxigenic B. fragilis strains (ETBF) not only have been implicated as a cause of diarrheal disease in children under 5 years of age but more recently have been associated with inflammatory diarrhea in children and adults (210). A meta-analysis of 17 studies that evaluated the association of ETBF with diarrheal disease found that 12 (71%) of the studies demonstrated a higher frequency of ETBF in patients with diarrhea than in controls (211). In contrast, a recent Indian study found no difference in the rate of isolation of ETBF as a sole pathogen from children with and without diarrhea (212). This suggests that other, mitigating factors may play a role in the infective process for ETBF.
Currently, there is no easy method to detect ETBF. Potential B. fragilis isolates can be recovered from stool on Bacteroides bile esculin agar (Becton Dickinson, Sparks, MD) and then tested for enterotoxigenicity in vitro using PCR for the Bacteroides fragilis toxin gene (bft) (212). Alternatively, the cytopathic effect (CPE) produced by fragilysin on HT29/C1 (human colon) cell lines can be evaluated (211). Both methods are employed only for research purposes at this time.
Edwardsiella tarda.
E. tarda is one of four species currently residing in the genus Edwardsiella of the family Enterobacteriaceae and is the only species considered pathogenic for humans. A common inhabitant of fish, reptiles, marine animals, and aquatic birds (213, 214), E. tarda can also be recovered from water. Approximately 80% of reported human illnesses attributed to E. tarda involve infections of the gastrointestinal tract (213). Data from a number of studies suggest that E. tarda is associated with 0.3% to 1.0% cases of gastroenteritis (161, 213). Asymptomatic carriage of E. tarda has been reported (12).
E. tarda-associated diarrhea can present in one of several forms, the most common of which is watery diarrhea. Other diarrheal syndromes linked to E. tarda include dysentery, chronic diarrhea, and enteric fever (213, 215). Risk factors for acquiring E. tarda diarrhea include consumption of contaminated fish or seafood, accidental ingestion of contaminated water, exposure to water from ornamental aquariums, and handling pet turtles (216 – 221). Person-to-person transmission has also been postulated but currently remains unsubstantiated (222). Two populations thought to be particularly susceptible to E. tarda infection are persons >50 years of age and young children <5 years of age (213, 223).
Escherichia albertii.
E. albertii was described as a new species in the genus Escherichia in 2003 (224). Most of the initial strains were misidentified as Hafnia alvei prior to the establishment of E. albertii as a species and were isolated from fecal samples from Bangladeshi children experiencing diarrheal illnesses. Subsequent evidence suggests that E. albertii is isolated fairly frequently from patients with diarrheal disease (225, 226). The organism harbors known enteropathogenic virulence factors (227, 228) and has been associated with a major outbreak of gastroenteritis involving 48 persons (229).
E. albertii grows well on routine enteric agars, is frequently misidentified biochemically as Hafnia, Salmonella, Citrobacter, or inactive E. coli strains (230), and may not be included in the databases of commercial identification systems. The important phenotypic features distinguishing E. albertii from E. coli include a negative indole reaction and inability to ferment lactose, d-sorbitol, and d-xylose (224). Phylogenetic studies indicate that Shigella boydii type 13, already known not to belong to the true shigellae, is, in fact, a member of the species E. albertii (227). In the 10th edition of the Manual of Clinical Microbiology, the species is broken down into two biogroups. Biogroup 1 represents the original E. albertii strains and biogroup 2 represents isolates formerly referred to as S. boydii 13. E. albertii can be identified by 16S rRNA gene sequencing and by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS).
Klebsiella oxytoca.
Since the late 1970s and early 1980s, K. oxytoca has been sporadically linked to cases of antibiotic-associated hemorrhagic colitis in Japan and other locations around the world (231). In 2006, in an elegant series of clinical observations and histopathological studies on six patients with antibiotic-associated hemorrhagic colitis (AAHC) convincingly established K. oxytoca as the etiological agent in persons negative for Clostridium difficile (232). C. difficile-negative patients who are at higher risk of developing K. oxytoca colitis include those previously receiving penicillins or on nonsteroidal anti-inflammatory drugs (232). At present, confirmation of K. oxytoca colitis in C. difficile-negative patients requires detection of the species-specific K. oxytoca cytotoxin by detection of CPE on HEp-2, CHO, or HeLa cells (232 – 234). In a recent study of 5,581 stool specimens submitted for C. difficile testing at an acute-care health system in China, 2.1% of specimens harbored K. oxytoca, but only 29.1% of these strains were cytotoxin producing (235).
A second highly suggested, but unproven, syndrome attributed to K. oxytoca is diarrhea. Although one study found no correlation between the presence of K. oxytoca and diarrhea (236), a later study found a high percentage of cytotoxin-positive K. oxytoca isolated from patients with health care-associated diarrhea that did not develop into AAHC (235). In the latter study, a specific selective medium termed SCITB (Simmons citrate-inositol-tryptophan-bile salts) was developed to recover K. oxytoca from stools. This medium has been shown to improve the recovery of K. oxytoca over that with MacConkey (MAC) agar by 30% (235). This medium could greatly aid in determining the significance of K. oxytoca from mild to moderate cases of diarrhea.
Providencia alcalifaciens.
A British survey of travelers to Mediterranean countries between 1987 and 1988 found a significant association between the recovery of P. alcalifaciens and diarrheal disease (237). These initial findings have been subsequently supported by other studies describing individual cases of P. alcalifaciens-associated diarrhea and at least three outbreaks of gastrointestinal disease, including one large outbreak involving >270 children in Japan (238 – 240). P. alcalifaciens strains implicated in diarrheal disease are invasive in HEp-2 cell monolayers, although the type of diarrhea that they produce is secretory (239, 241); some strains additionally produce a cytolethal distending toxin (242). Persons most at risk of developing P. alcalifaciens diarrhea are those who are involved in foreign travel or have consumed contaminated foods containing the organisms (237, 243).
Most isolates of P. alcalifaciens recovered from diarrheal stools have been isolated in pure culture, as predominant flora, or without any other recognizable enteropathogens being detected (237, 240, 243). A selective medium, termed PAM (Providencia alcalifaciens medium), has been described for the recovery of this species from feces (244). This medium has subsequently been modified as PMXMP (polymyxin-mannitol-xylitol medium for Providencia) and used with success (242, 243).
GENERAL LABORATORY TESTING CONSIDERATIONS
At this time, diagnosis of bacterial gastroenteritis is by the routine stool culture. Two key exceptions to this are the use of antigen and/or nucleic acid amplification tests for the detection of (i) C. difficile and, to a lesser extent, (ii) STEC and (iii) Campylobacter. In addition, food poisoning caused by B. cereus, C. perfringens, and S. aureus is infrequently diagnosed by clinical laboratory testing, as few patients seek medical intervention for their symptoms, which are short-lived. In cases of outbreak investigations for these organisms, feces, food, and/or vomitus is collected by, or sent to, local or state public health officials, and testing is performed at public health laboratories. Laboratory diagnosis of listeriosis associated with human diarrhea is also extremely difficult to make at this time. Diagnosis can be made either via epidemiological linkage to other cases of diarrhea in which L. monocytogenes has been isolated or by isolation and identification of L. monocytogenes from stool in persons with gastrointestinal symptoms (120). Currently there is no standard protocol for detection of listeriae from human stools, and it is unclear what methods or procedures are likely to yield maximum recovery rates. Because of these limitations, recovery of L. monocytogenes from stool should not be attempted routinely in clinical microbiology laboratories. This testing is better suited to reference and/or public health laboratories.
Specimen Collection
Feces collected in the acute phase of a diarrheal disease is the specimen of choice when bacterial gastroenteritis is suspected. If liquid or soft, approximately 5 ml should be collected (245), and if formed, 0.5 to 2 g is adequate for culture (245, 246). Clear instructions should be given for proper specimen collection. Feces should be collected in a clean, dry container with a tight lid and should not be contaminated with urine, barium, or toilet paper (which may contain barium salts). Specimen containers or collection devices should be labeled with the patient's full name and two additional patient identifiers, such as medical record number and date of birth.
Rectal swabs are generally considered less sensitive than stool for culture, but there are certain patient populations for which a properly collected rectal swab may be acceptable. For instance, a rectal swab is a useful specimen to collect from infants and young children, or when trying to recover Shigella (247 – 249). Rectal swabs must be inserted deep enough into the rectum, approximately 1 in. beyond the anal sphincter, and carefully rotated, so that feces can be collected and visible on the swab. The swab should then be placed in all-purpose transport medium and sent to the laboratory. In addition to feces, blood, bone marrow, and/or urine samples may be collected for patients presenting with symptoms consistent with typhoid fever. Duodenal contents may also be acceptable for these cases.
In cases of suspected extraintestinal Salmonella infections, blood and urine specimens should be collected in addition to stool. In suspected cases of typhoid fever, blood and/or bone marrow specimens should be collected in the first week of fever and stool and urine in subsequent weeks. The yield of Salmonella Typhi is best from bone marrow, in particular after antimicrobials have been started (250).
While in up to 94% of cases, the etiological agent is recovered from the first specimen submitted, collection of a second fecal specimens may be needed to rule out a bacterial cause of infection, especially in instances where patient symptoms persist (246). Many have documented that the yield of fecal culture for patients hospitalized for more than 3 days is poor, excluding C. difficile (8, 251 – 257). For this reason, laboratories should restrict testing to outpatients and those hospitalized for less than 3 days. Exceptions to this rule may include patients with HIV infection, severe neutropenia, suspected nondiarrheal manifestations of enteric infections (such as erythema nodosum, polyarthritis, etc.), a suspected nosocomial outbreak, or in some instances pediatric patients, from whom collection of a specimen may be difficult in the first 3 days of hospitalization.
Similarly, laboratories should develop and enforce strict specimen rejection criteria for C. difficile testing. Testing should not be performed on asymptomatic patients (i.e., those with formed stools), given the high rates of colonization among some patient populations. The exception to this policy is in cases where ileus is suspected (89), although this occurrence is rare and should involve physician consultation with the laboratory prior to testing. Repeat testing of negative specimens should also be discouraged or altogether prohibited. Several studies have shown the lack of value for repeat testing, regardless of method, for CDI (258 – 260). In addition, many laboratories have developed rejection criteria for specimens submitted within a defined interval of time following a positive result, as many patients will remain colonized with C. difficile following successful treatment (70). Finally, C. difficile testing should not be performed on infants (i.e., patients <1 year of age) due to their high colonization rate.
Transport Media and Storage Conditions
Transport media used for fecal specimens include Stuart's, Aimes, or Cary-Blair (261, 262) medium. Fresh stool specimens should be transported to the laboratory and processed within 2 h of collection (263); this is in particular critical to the survival of Shigella and Campylobacter (264). If the specimen cannot be processed within 2 h, it should be placed in Cary-Blair transport medium; refrigeration of the specimen in Cary-Blair medium at 4°C prior to processing will best conserve bacterial enteropathogens, with the exception of Shigella (265, 266).
Specimen Processing
Fecal specimens, if submitted fresh and not in preservative, should be macroscopically observed for areas that contain blood and/or mucus, as these will contain the highest number of enteric pathogens and should be used for culture. Gram staining is not typically useful when performed on stool, with the key exception of campylobacteriosis. In these cases, again with fresh, unpreserved stool specimens, the characteristic seagull-shaped campylobacters can be visualized in stool when carbol-fuchsin is used as a counterstain, with a sensitivity ranging from 66 to 94% in patients with acute enteritis (48). However, Gram staining on stool specimens is not routinely performed as part of stool culture testing and should be performed only on special request.
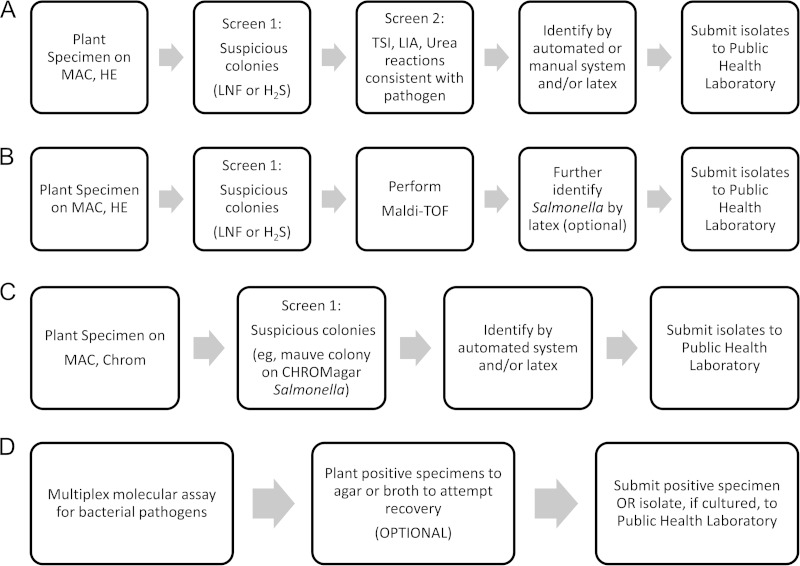
The battery of primary plating media used for routine bacterial fecal cultures will vary from laboratory to laboratory, depending on the patient population and organisms routinely isolated. Medium selection is also driven by test requisition. At a minimum, routine fecal culture setup should be designed to optimize the recovery of Salmonella, Shigella, Campylobacter, and STEC. Thus, fecal specimens received for culture should be planted to 4 media: (i) MacConkey (MAC) agar, (ii) a selective/differential medium designed for the recovery of Salmonella and Shigella, (iii) a medium designed for the recovery of Campylobacter, and (iv) a medium designed for the recovery of STEC O157 and/or enrichment broth for testing for the presence of Shiga toxins. Many laboratories also include a blood agar plate (BAP), in order to aid with the recovery of Aeromonas spp., Plesiomonas spp., and Vibrio spp., whereas other add this on request only. Table 2 presents an overview of some of the more common media used for fecal cultures, their intended use, and growth characteristics of some of the more common enteropathogenic bacteria when isolated on these media.
TABLE 2.
Commonly used media for recovery of pathogenic bacteria from stool samples
| Medium | Intended use and notes |
|---|---|
| All-purpose broths | |
| Gram-negative (GN) broth | Selective enrichment for Gram-negative rods, specifically Salmonella and Shigella (subculture after 6–8 h of incubation, not part of routine setup unless for STEC EIA), can be used for STEC EIA |
| Selenite F broth | Selective enrichment for Gram-negative rods, specifically Salmonella and Shigella (subculture after 18–24 h of incubation, may inhibit growth of some Shigella species) (not part of routine setup) |
| Organism-specific broths | |
| Alkaline peptone water | Selective enrichment broth for Vibrio, when requested (subculture to TCBS after 24 h of incubation) |
| MAC broth | Can be used for STEC EIA, enrichment for Y. enterocolitica if incubated at 25°C (not part of routine setup) |
| All-purpose agars | |
| Hektoen enteric (HE) | Selective medium for Gram-negative rods, differentiates lactose fermenters (yellow-orange) from nonfermenters (blue or green), H2S production can be detected (black precipitate) |
| MacConkey (MAC) | Selective medium for Gram-negative rods, differentiates lactose fermenters (pink) from nonfermenters (colorless) |
| Salmonella-shigella (SS) | Selective medium for Gram-negative rods, differentiates lactose fermenters (pink/red) from nonfermenters (colorless), H2S production can be detected (black precipitate) |
| Xylose-lysine-deoxycholate (XLD) | Selective medium for Gram-negative rods, differentiates lactose fermenters (yellow) from nonfermenters (red), H2S production can be detected (black precipitate) |
| Highly selective/differential agars | |
| Bismuth sulfite | Isolation of Salmonella, including Salmonella Typhi (black on this medium) |
| Brilliant green | Isolation of Salmonella (red, pink, or white with red halo on this medium), inhibits Salmonella Typhi and Salmonella Paratyphi |
| Blood agar with ampicillin | Isolation of Aeromonas (not part of routine setup unless specifically requested) |
| Campy Blood | Isolation of Campylobacter |
| Campy CVA | Isolation of Campylobacter |
| Campylosel | Isolation of Campylobacter |
| Cefsulodin-Irgasan-novobiocin (CIN) | Isolation of Yersinia enterocolitica or Aeromonas (deep red center and transparent margin [bull's eye appearance] on this medium) (not part of routine setup) |
| Charcoal selective | Isolation of Campylobacter |
| Charcoal-cefoperazone-deoxycholate agar (CCDA) | Isolation of Campylobacter |
| CHROMagar Salmonella | Isolation of Salmonella (mauve-rose on this medium) |
| CHROMagar O157 | Isolation of O157 STEC (mauve on this medium) |
| CHROMagar STEC | Isolation of 6 most common STEC serogroups (mauve on this medium) |
| Cycloserine-cefoxitin-egg yolk/cycloserine-cefositin-fructose | Isolation of Clostridium difficile (not part of routine setup unless requested) |
| HardyChrom SS | Isolation of Salmonella (black on this medium) and Shigella (teal on this medium) |
| Inositol-brilliant green-bile salt | Isolation of P. shigelloides (white to pink on this medium) (not part of routine setup unless requested) |
| MacConkey agar with sorbitol (SMAC) or cefixime-tellurite SMAC (CT-SMAC) | Isolation of E. coli O157 (colorless on this medium) |
| Thiosulfate-citrate-bile salts-sucrose (TCBS) | Isolation of Vibrio (not part of routine setup unless requested), V. cholerae is yellow on this medium, V. parahaemolyticus is green on this medium, some vibrios are inhibited |
Selective media appropriate for fecal cultures include xylose-lysine-deoxycholate (XLD), salmonella-shigella (SS), Hektoen enteric (HE), brilliant green (BG), or bismuth sulfite (BS) medium or a chromogenic medium designed for the recovery and detection of specific enteropathogens. The value of chromogenic media has been demonstrated in a number of studies that reported improved sensitivity and specificity over traditional selective/differential media for the recovery of Salmonella, Shigella, STEC, Vibrio, and Yersinia (267 – 271). However, these media are more expensive than traditional media and are not available for in vitro diagnostic use for Vibrio and Yersinia in the United States at this time.
Campylobacter selective media include the blood-free charcoal-cefoperazone-deoxycholate agar (CCDA) and charcoal-based selective agar and the blood-based Campy-CVA (cefoperazone, vancomycin, and amphotericin) and Skirrow medium. While Campy-CVA is most widely used in the United States, the use of a combination of media, including one that is charcoal based, increases the yield of Campylobacter by 10 to 15% (49). Campylobacter cultures must be incubated in a microaerobic atmosphere, typically 5% O2, 10% CO2, and 85% N2, and at 42°C (48) to optimize recovery and prevent overgrowth of enteric organisms. Campylobacter media should be incubated for a minimum of 48 h and examined at 24 and 48 h.
The isolation and identification of STEC by culture methods is problematic, with the exception of O157 strains, most of which express a delayed or negative d-sorbitol reaction. Media to detect sorbitol-negative E. coli O157 strains include sorbitol-MacConkey (SMAC) agar and a modification on SMAC agar which contains cefixime and tellurite (CT-SMAC agar). Sorbitol-negative E. coli appears as colorless colonies on both of these media, but CT-SMAC agar has the advantage of eliminating many enteric flora that grow on SMAC agar, including commensal E. coli. CHROMagar O157 detects O157 serogroups, and CHROMagar STEC detects both O157 and the six most common STEC serogroups other than O157 isolated in the United States (O26, O45, O103, O111, O121, and O145). This product is labeled for research use only in the United States, but a recent study demonstrated that CHROMagar STEC had a sensitivity of 89.1% and specificity of 86.7% (272). In contrast, a second study found the specificity of CHROMagar STEC to be 98.9% (273). If a laboratory attempts to detect STEC, an enrichment broth, typically GN broth, should also be inoculated and incubated at 37°C overnight for detection of Stx1 and Stx2 by immunoassay.
Several enteric pathogens require highly selective media and will not be optimally recovered by the selection of the 4 or 5 media described above for routine fecal cultures. These include aeromonads, vibrios, yersiniae, and a number of the emerging enteropathogens. The cost-effectiveness of routinely culturing for these organisms will depend on the patient population served by an individual laboratory and potentially on the peak time of the year that these pathogens are active. For example, laboratories in the Gulf States may consider routine use of media to optimize the recovery of vibrios in addition to the battery of primary stool culture media. A variety of specialized media have been designed and are commercially available for the recovery of these pathogens, but clearly not all can be stocked by routine clinical laboratories. Two that might be considered for areas these pathogens are more endemic include cefsulodin-Irgasan-novobiocin (CIN) agar and thiosulfate-citrate-bile salts-sucrose (TCBS) agar. CIN agar is used to recover both Aeromonas and Yersinia enterocolitica, whereas TCBS is used for the recovery of Vibrio (http://www.cdc.gov/cholera/pdf/laboratory-methods-for-the-diagnosis-of-vibrio-cholerae-chapter-4.pdf). Of note, TCBS agar has a short shelf life and should be made only as needed (it requires no autoclaving). While it is excellent for the recovery of V. cholerae and V. parahaemolyticus, not all pathogenic vibrio species will grow on this medium. For instance, Grimontia hollisae grows poorly if at all on TCBS agar. These species may be detected on blood agar, which can be screened for oxidase-positive or hemolytic colonies for further evaluation.
An enrichment broth was traditionally inoculated as part of routine fecal cultures; this practice was originally designed to aid in the recovery of low levels of Salmonella, Shigella, and Campylobacter. However, many laboratories have discontinued this practice, as the yield does not justify the cost (274). Historic data from individual laboratories should be used to help determine if subcultures from enrichment broth should be used or discontinued. A key exception to this is the use of alkaline peptone water, pH 8.5 to 8.6, enrichment for a minimum of 6 to 8 h at 35°C to 37°C for the recovery of V. cholerae if necessary. Failure to use enrichment culture in addition to direct plating will result in a significant percentage of V. cholerae-containing stools being reported out as negative (275).
Stool Culture Workup
Bacterial fecal culture workup consists of examining culture media for colonies that display phenotypic properties consistent with those of enteric pathogens (e.g., lactose negative, H2S producers, etc.). These colonies are then traditionally further screened using select biochemical tests and, if yielding reactions consistent with an enteropathogen, identified by further biochemical and/or antigenic testing by a variety of algorithms. Ultimately, these traditional methods yield a precise identification, but they are time-consuming and labor-intensive. Further, the biochemical properties of certain bacterial isolates may differ from the common phenotype of the species, hampering unambiguous identification. The classic example of this is an inert E. coli strain masquerading as Shigella. Resolving these anomalous bacteria can be difficult for even specialized reference laboratories. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) is a newer proteomic technology that has the potential to revolutionize the traditional algorithms used for the identification of enteric pathogens (276), and it can be a more cost-effective alternative to colony screening and biochemical testing.
Traditional algorithms for the identification of enteric pathogens.
The traditional approach to fecal stool culture workup (excluding Campylobacter and STEC, which are described further below) is shown in Fig. 1A. The basis for this process is to minimize the cost associated with differentiating the plethora of nonpathogenic Enterobacteriaceae in a fecal specimen from the rare Salmonella and Shigella, which are isolated in only 3 to 5% of fecal specimens submitted for culture. Typically this is accomplished by selecting lactose nonfermenters and/or H2S producers, isolated on the primary plates, and submitting these to a secondary screening test. This second screen may not be required if laboratories are using a chromogenic medium as part of the primary setup (Fig. 1C) or if the volume of fecal cultures is low enough that going directly to an identification kit is cost-effective. Regardless, the second screening method used by most laboratories consists of subculturing suspicious colonies to one or more tubed/slanted media. The classic “three-tube” system consists of a triple sugar iron (TSI) agar, a lysine iron agar (LIA), and a Christensen urea agar. Alternative tubed media for secondary screening include motility-indole-ornithine (MIO), motility-indole-lysine (MIL), and motility-indole-lysine-sulfide (MILS) (277 – 280). The most common biochemical reactions for enteric pathogens on TSI agar, LIA, and urea agar (negative unless otherwise noted) are listed in Table 3.
FIG 1.
Strategies for the detection of Salmonella and Shigella on routine stool cultures. Where Hektoen enteric (HE) agar is specified, any agar listed in the text is appropriate. (A) Traditional approach to fecal stool culture workup for Salmonella and Shigella. See Table 3 for reactions of enteric pathogens. (B) Approach to identification of Salmonella and Shigella when using MALDI-TOF MS. See Table 4 for reactions of enteric pathogens. (C) Approach to identification of Salmonella and Shigella if chromogenic medium is used as part of the primary setup. (D) Approach to use of a multiplex molecular assay for the detection of Salmonella and Shigella.
TABLE 3.
Summary of identification characteristics and diagnostic test methods available in the United States a
| Organism(s) | Growth characteristics | Key biochemical reactions | Clinical laboratory diagnostic test methods |
Clinical symptoms used for diagnosis | Public health laboratory referral | |||
|---|---|---|---|---|---|---|---|---|
| Culture | Chromogenic agar | Antigen detection | NAAT | |||||
| Aeromonas spp. b | BA, beta-hemolytic | Oxidase positive; TSI, K/A, gas; LIA, K/A or A/A | ✓ | |||||
| Bacillus cereus b | BA, beta-hemolytic | ✓ | Food and stool cultured for outbreak investigations | |||||
| Campylobacter spp. | Campylobacter medium, gray-white colonies | Oxidase positive, catalase positive, sodium hippurate positive for C. jejuni | ✓ | ✓ | ✓ | |||
| Clostridium difficile b | NA | NA | ✓ | ✓ | ||||
| Clostridium perfringens b | NA | NA | ✓ | Food and stool cultured for outbreak investigations | ||||
| Edwardsiella tarda b | Lactose nonfermenter with H2S production on HE, MAC, XLD, SS | TSI, K/A, H2S, ± gas; LIA, K/K or K/NC, H2S | ✓ | |||||
| Escherichia coli O157 | SMAC, sorbitol nonfermenter | TSI, K/A or A/A, gas; LIA, K/A or A/A | ✓ | ✓ | ✓ | |||
| Escherichia coli, STEC | SMAC, sorbitol fermenter (unless O157) | TSI, K/A or A/A, gas; LIA, K/A or A/A | ✓ | ✓ | ✓ | |||
| Listeria monocytogenes b | BA, beta-hemolytic | ✓ | Food and stool cultured for outbreak investigations | |||||
| Salmonella spp. | Lactose nonfermenter with H2S production on HE, MAC, XLD, SS | TSI, K/A, H2S, ± gas; LIA, K/K or A/A or K/NC (rare), H2S | ✓ | ✓ | ✓ | ✓ | ||
| Shigella spp. | Lactose nonfermenter on HE, MAC, XLD, SS | TSI, K/A; LIA, K/A or A/A | ✓ | ✓ | ✓ | |||
| Staphylococcus aureus b | NA | NA | ✓ | Food and stool cultured for outbreak investigations | ||||
| Yersinia enterocolitica b | CIN, pink bull's eye; lactose nonfermenter on MAC; ferments sucrose on HE | TSI, A/A or K/A; LIA, K/A or A/A; urea positive | ✓ | |||||
| Vibrio spp. b | TCBS, sucrose fermentation seen with V. cholerae; no sucrose fermentation for V. parahaemolyticus; V. vulnificus is sucrose fermentation variable | Oxidase positive; TSI, A/A or K/A; LIA, K/A or A/A | ✓ | |||||
Abbreviations: BA, blood agar; HE Hektoen enteric agar; MAC, MacConkey agar; XLD, xylose-lysine-deoxycholate agar; SS, salmonella-shigella agar; CIN, cefsulodin-Irgasan-novobiocin agar; TCBS, thiosulfate-citrate-bile salts-sucrose agar; SMAC, MAC with sorbitol; TSI, triple sugar iron agar; LIA, lysine iron agar; A, acid; K, alkaline; NC, no change; NA, not applicable.
The organism is not part of the routine stool culture, and culture should be performed only on request.
If not specifically requested, identification of aeromonads, vibrios, and P. shigelloides may be overlooked by the above approach. In particular, some strains of Aeromonas and Vibrio are lactose positive on MAC or sucrose-positive on HE and XLD media. If necessary, these organisms can be identified by screening the BAP for oxidase-positive or beta-hemolytic colonies. Caution must be exercised, however, as not all aeromonads are hemolytic, in particular some isolates of Aeromonas caviae. Oxidase-positive isolates are then further screened by evaluating the reactions on TSI agar and LIA and through the use of O/129 disks and salt tolerance for vibrios.
If Yersinia culture is requested, it may be difficult to isolate from feces using routine stool culture protocols, as at 35°C the organism grows more slowly than other flora. It is occasionally recovered as pinpoint colonies on MAC or SS agar at 24 h. These colonies should be reincubated at 25°C if possible, and if the colonies have grown after 24 h (to 1 mm), they should be examined for Yersinia; colonies that remain pinpoint are most likely fecal streptococci. If CIN agar is used, yersiniae are identified as bull's-eye colonies with pink centers on this medium, due to fermentation of d-mannitol. Aeromonas and some Citrobacter, Serratia, Proteus, and Morganella strains may mimic Y. enterocolitica colony morphology on this medium, and so these isolates require final identification. One strategy is to perform Voges-Proskauer and motility tests at 25°C and 35°C; Y. enterocolitica and Y. pseudotuberculosis should be positive only for these at 25°C.
Isolates that yield reactions consistent with enteropathogens on these secondary screening media are further identified using either a manual identification system such as API 20E or an automated identification system such as MicroScan, Vitek2, Phoenix, MALDI-TOF MS, etc. These systems perform well at identifying some enteropathogens but fall short for others. For instance, commercial systems are notoriously poor at the correct identification of Vibrio species to both the genus and species levels (281, 282). This is due in part to the salt requirement of halophilic species such as V. parahaemolyticus and V. fluvialis and to the fact that some species are phenotypically similar. A prime example of this situation is the phenotypic similarity between V. fluvialis and certain Aeromonas spp. For such situations, only a limited number of tests are available to separate these species, such as growth in 0% and 6% salt, O/129 susceptibility, and gas production from d-glucose, tests normally not available on commercial panels or in most diagnostic laboratories. Similarly, most systems are unable to identify Aeromonas, Salmonella, Shigella, or Yersinia beyond the genus level; however, in most instances, this level of identification is sufficient. Similarly, identification of Salmonella to the genus level when isolated from stool is generally sufficient, and clinical laboratories should forward isolates to regional or state public health laboratories for further characterization. However, laboratories may make a concerted effort to rule out typhoidal Salmonella serotypes, if possible, given the clinical significance of these organisms or if there will be a delay getting the isolate to the public health laboratory. Salmonella Typhi can be differentiated from other serotypes by the characteristic weak H2S reaction on a TSI slant and a negative ornithine decarboxylase reaction. Salmonella Paratyphi A can be differentiated from other salmonellae by negative H2S, lysine, and citrate reactions.
Antigenic testing, for example, by latex agglutination for somatic antigen, can be used to identify Shigella to the species level, as many commercial systems may misidentify these strains as E. coli. Antigen testing for the somatic, flagellar, and capsular antigens of Salmonella may also be used to supplement commercial test system identifications. Caution must be employed, as some Citrobacter and E. coli strains may posses the Salmonella Typhi Vi capsular antigen.
Campylobacter species can be identified based on characteristic Gram stain morphology, growth on selective media at 42°C, and positive catalase and oxidase reactions alone. Campylobacter appears as gray, flat colonies on plated media and often spreads along the streak lines. Campylobacter latex agglutination kits are available for culture confirmation to genus level, but sensitivity and specificity vary depending on the manufacturer (283). For the most part, routine reporting of species-level identification of Campylobacter when isolated from stool is unnecessary. Campylobacter jejuni can be readily differentiated from other thermotolerant Campylobacter spp. by the hydrolysis of sodium hippurate, but as some C. jejuni strains fail to hydrolyze hippurate, hippuricase-negative isolates should be reported as Campylobacter spp.
Sorbitol-nonfermenting E. coli isolated on SMAC or CT-SMAC agar should be tested in O157-specific antiserum or latex reagents in order to confirm O157 STEC. Caution must be used with these reagents, as other species of bacteria may cross-react with O157 antiserum, requiring biochemical confirmation of E. coli prior to reporting.
MALDI-TOF MS.
Because of the low cost associated with performing a MALDI-TOF identification, many laboratories have begun to identify bacterial isolates phenotypically consistent with enteric pathogens directly off the primary plates (Fig. 1B), rather than performing additional screening tests. MALDI-TOF MS can be performed within the FDA-approved package insert on colonies picked from the primary BAP and, in the case of the Vitek MS, from the MAC plate. Alternatively, testing can be run as a laboratory-developed test on colonies picked from other selective media routinely used for stool cultures (XLD, SS, HE, etc.) (284). Some have shown that SS and HE agars yield only genus-level identifications (284). For Campylobacter, colonies isolated on Campylosel and Columbia blood agar are FDA cleared on the Vitek MS and perform well for species-level identification (285 – 288). In contrast, spectra are not generated when isolates are taken directly from modified CCDA; colonies isolated on this medium require subculture on a less selective medium prior to MALDI-TOF analysis (286).
MALDI-TOF MS performs well for the identification of Salmonella (to the genus level), and Campylobacter. However, the current IVD and RUO systems and databases cannot differentiate E. coli from Shigella or E. coli from STEC or other E. coli pathotypes and cannot identify Salmonella to the serotype (276). The technology is rapidly approaching these targets, through special extraction protocols and selection of stable, pathogen-specific biomarkers (289 – 292), which can be internally verified by individual laboratories. In addition, one or both of the IVD MALDI-TOF systems can be used to identify the other enteric pathogens described in this article, with the exceptions of B. cereus, P. shigelloides, E. albertii, and P. alcalifaciens. The performance of MALDI-TOF MS is detailed in Table 4.
TABLE 4.
MALDI-TOF for the identification of enteric pathogens
| Organism | Performance of MALDI-TOF a | FDA-cleared organisms b | Notes | Reference(s) |
|---|---|---|---|---|
| Aeromonas | Present in IVD and commercial RUO databases; performance not well documented | Biotyper, genus only; Vitek MS, genus only | Preliminary data promising | 322 |
| Campylobacter | Excellent for genus and species identification; many species in RUO databases | Vitek MS, C. jejuni, C. coli | FDA cleared for colonies grown on Campylosel and brucella blood agar; cannot perform directly from mCCC | 285 – 288 |
| E. tarda | Present in IVD and commercial RUO databases; performance not well documented | Vitek MS only | ||
| P. shigelloides | Present in commercial RUO databases; performance not well documented | No | ||
| Salmonella | Good identification to genus | Biotyper, genus only; Vitek MS, genus only | Performance better with MAC vs SS or HE agar; serotype-specific biomarkers have been identified | 291, 323 |
| Shigella | Cannot distinguish from E. coli by use of IVD or commercial RUO databases | No | Shigella-specific biomarkers have been identified | 292 |
| STEC | Cannot distinguish STEC (or other pathotypes) from E. coli by use of IVD or commercial RUO databases | No | E. coli pathotype-specific biomarkers have been identified | 290 |
| Vibrio | Present in IVD and commercial RUO databases; performance not well documented | Vitek MS, V. cholerae, V. parahaemolyticus, V. vulnificus | ||
| Yersinia | Present in IVD and commercial RUO databases; performance not well documented | Biotyper, Y. enterocolitica, Y. pseudotuberculosis; Vitek MS, Y. enterocolitica, Y. pseudotuberculosis, Y. frederikenii, Y. intermedia, Y. kristensenii |
IVD, in vitro diagnostic device; RUO, research use only.
FDA clearance as of 7 March 2014. Check with manufacturer or FDA.org for most up-to-date information.
Susceptibility Testing
Antimicrobials are not routinely indicated for the treatment of otherwise healthy patients with bacterial gastroenteritis, as infections typically resolve spontaneously. In some cases, the use of antimicrobials can be detrimental to the host, such as the use of antimicrobials for STEC infection, which puts the patient at increased risk for HUS, or for Salmonella, which may increase the rate of clinical relapse and prolong carriage (3). Routine susceptibility testing is therefore also not indicated for bacteria isolated from stool cultures (293, 294). Exceptions to this generalization may be bacteria isolated from infants <6 months of age, the elderly or immunocompromised, or patients with prolonged disease. In these cases, communication with the physician will aid in determining the need for susceptibility testing and the antimicrobial agents to be tested. If the laboratory identifies Salmonella Typhi or Salmonella Paratyphi A from stool, consideration should be given to susceptibility testing. Additionally, the isolation of any of the organisms described here from an extraintestinal site warrants susceptibility testing.
Current standards for susceptibility testing conditions and interpretive criteria for members of the Enterobacteriaceae (E. coli, E. tarda, Salmonella, Shigella, and Yersinia spp.) are available from the Clinical and Laboratory Standards Institute (CLSI) M100 S24 standard (294). Recommendations for Aeromonas, Campylobacter, and Vibrio are provided by the CLSI in the M45 A2 document (293). In the United States, the CDC monitors antimicrobial resistance among Campylobacter, Salmonella, Shigella, E. coli O157, and Vibrio species other than V. cholerae as part of the National Antimicrobial Resistance Monitoring System (NARMS). Primary agents used to treat Campylobacter include ciprofloxacin and erythromycin. Fluoroquinolone resistance has been reported with increasing frequency in the United States among both human and animal isolates of C. coli and C. jejuni. In 1990, NARMS noted no resistance to ciprofloxacin, whereas in 2011, 24.2% of Campylobacter isolates tested were resistant to ciprofloxacin (295). This trend of increasing resistance may be related to the historical use of veterinary fluoroquinolones in animal feed as growth promoters (296). In contrast, resistance to erythromycin remains low, at 1.8% as reported in 2011. Because of increasing resistance, testing of Campylobacter isolates from individuals with severe illness or prolonged symptoms may be warranted. Susceptibility testing is performed by broth microdilution in cation-adjusted Muller-Hinton broth (CA-MHB) and incubation in a microaerobic atmosphere at either 35°C or 42°C. Disk diffusion for ciprofloxacin and erythromycin can be performed; no zone of inhibition indicates resistance, but the appearance of any zone of inhibition requires confirmation by an MIC method (293).
Resistance documented by NARMS for nontyphoidal Salmonella isolates to the antimicrobial agents commonly used to treat severe Salmonella infections was 2.5% to ceftriaxone, 1.2% to trimethoprim-sulfamethoxazole, and <1% to ciprofloxacin (295). In contrast, fluoroquinolone resistance was much higher in typhoidal isolates, with 7.3% of Salmonella Typhi and 2.1% of Salmonella Paratyphi A isolates documented as resistant to ciprofloxacin. An additional 64.2% and 95.1% of these isolates, respectively, had ciprofloxacin MICs in the intermediate range (0.12 to 0.5 μg/ml). Azithromycin is an alternative agent recommended for the treatment of invasive salmonellosis by the World Health Organization and the American Academy for Pediatrics. However, no CLSI breakpoints exist at present for Salmonella for azithromycin. An epidemiological cutoff (ECOFF) (i.e., MIC cutoff defining the upper end of the wild-type population) of ≥32 μg/ml has been used to indicate resistance, and based on this ECOFF, 0.2% of nontyphoidal Salmonella isolates were considered resistant to azithromycin by the CDC 2011 NARMS data (295).
Among Shigella isolates tested by NARMS in 2011, 2.4% were resistant to ciprofloxacin and 3.1% to azithromycin, again using an ECOFF breakpoint of ≥32 μg/ml. Notably, resistance to azithromycin was species dependent, with 10% of Shigella flexneri isolates (n = 58) testing resistant. Trimethoprim-sulfamethoxazole resistance among Shigella isolates in 2011 was high (66.9%), whereas only 1.7% resistance to ceftriaxone was documented. If laboratories perform susceptibility testing on Salmonella or Shigella, it is important to remember that first- and second-generation cephalosporins, cephamycins, and aminoglycosides may to be appear active in vitro but are not effective clinically, and the isolates should not be reported as susceptible (294).
In 2009, the CDC started annually tracking antimicrobial resistance in Vibrio spp other than V. cholerae as part of NARMS. In 2011, 95.1% of V. alginolyticus isolates were resistant to ampicillin, whereas 40.3% of V. parahaemolyticus and 4.8% of V. vulnificus isolates were resistant (295). No isolates in 2011 tested resistant to the fluoroquinolones or tetracycline, and 0.3% were resistant to trimethoprim-sulfamethoxazole. Antimicrobial therapy is not required to manage cholera but may shorten the duration and reduce severity of disease. Similarly, otherwise healthy individuals with diarrhea caused by non-V. cholerae Vibrio spp. usually recover spontaneously. When indicated, vibrios can be tested for susceptibility by a method similar to that used for the Enterobacteriaceae. For testing halophilic species, preparation of the inoculum in 0.85% NaCl solution for both disk diffusion and broth microdilution testing is recommended by the CLSI (293).
Susceptibility testing is not routinely indicated for C. difficile, as most isolates are susceptible to both metronidazole and vancomycin, agents commonly used to treat CDI. Almost all cases are currently identified by nonculture methods.
Nonculture Detection Methods
Recovery of C. difficile by anaerobic culture is possible but should not be used as a primary diagnostic test for C. difficile infection because of long turnaround times. Rather, three test methods are commonly used detect the presence of toxigenic C. difficile in stool specimens. These methods are glutamate dehydrogenase (GDH) enzyme immunoassays (EIAs), toxin A/B enzyme immunoassays, and toxin A/B gene nucleic acid amplification tests (NAATs). GDH is a cell wall enzyme that is abundant in C. difficile, and while EIAs for this antigen are a sensitive screen for the presence of C. difficile, they suffer from poor specificity, as they do not distinguish toxigenic from nontoxigenic strains (297). The presence of a toxigenic strain must therefore be confirmed on all GDH-positive specimens before a positive C. difficile result is reported. This can be accomplished in a number of ways, including cell culture cytotoxicity neutralization assay (CCCN), but most laboratories perform either a toxin A/B EIA or a toxin gene NAAT on GDH-positive specimens. Commercial toxin A/B EIAs suffer from poor sensitivity compared to both cytotoxigenic culture and CCCN. Sensitivity for the presence of toxigenic C. difficile is as low as 40 to 50% in some studies (297, 298). For this reason, detection of C. difficile by toxin EIA alone is no longer considered adequate to rule out CDI. Toxin gene NAATs offer the highest sensitivity and specificity among the different test options but are significantly more expensive than the EIA-based methods. Furthermore, because toxin A and/or B is required for CDI, the clinical significance of specimens that test negative for their presence by CCCN or EIA but are positive by NAAT (or culture) has been brought into question. Some have suggested that such cases represent colonization, rather than infection, by C. difficile (297, 299, 300). However, because of the poor sensitivity of the antigen tests for toxins A/B compared to cytotoxicity assays, performing toxin A/B detection tests alone will miss clinically significant cases (298).
Many have proposed testing algorithms, using one or more of the three test methods, aimed at optimizing C. difficile diagnostic performance and minimizing cost (301 – 306). Three of these testing algorithms are outlined in Table 5: (i) performing a GDH EIA with follow-up confirmatory toxin NAAT on GDH-positive specimens, (ii) performing a combination GDH and toxin A/B EIA and confirming specimens for which the two test results do not match by toxin NAAT, or (iii) performing NAAT alone on all specimens.
TABLE 5.
C. difficile diagnostic algorithms
| Algorithm, test(s) a | Result | Action |
|---|---|---|
| GDH with reflex, GDH antigen | Negative | Report as “no C. difficile present” |
| Positive | Reflex to NAAT; reporting based on NAAT results | |
| GDH/toxin lateral-flow assay, GDH antigen and toxin A/B | Both negative | Report as “no C. difficile present” |
| Both positive | Report as “C. difficile toxin present” | |
| Negative/positive or positive/negative | Reflex to NAAT | |
| Toxin NAAT, NAAT | Negative | Report as “no C. difficile present” |
| Positive | Report as “C. difficile toxin present” |
GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification test.
In addition to C. difficile, STEC infections are increasingly being diagnosed by culture-independent methods, as these methods allow for the detection of both O157 and non-O157 STEC infections (102). In 2009, the CDC published recommendations that stool samples submitted from patients with acute community-acquired diarrhea and patients with possible HUS be simultaneously cultured using selective and differential media for E. coli O157 and assayed for the detection of Stx1 and Stx2 (102). The Joint Commission issued a change to the hospital laboratory Element of Performance Standard for QSA.04.06.01 in 2013, which is consistent with the CDC recommendations. If laboratories are unable to perform a non-culture-based method for the detection of non-O157 STEC, this testing should be sent to a reference laboratory. Laboratory testing options are shown in Table 6. Stx immunoassays are performed on enrichment broths, typically GN broth, that have been incubated at 37°C overnight, and they detect (and in some cases differentiate) Stx1 and Stx2. Some assays are also FDA cleared for use directly on stool, with no broth enrichment, but performance varies among manufacturers. Several studies have demonstrated improved sensitivity for the detection of STEC over culture through the use of these immunoassays (307, 308), for both O157 and non-O157 STEC. Any broths that test positive for Stx should be forwarded to the local or state public health laboratory for isolate recovery and further characterization. The immunoassays can be performed off stool directly, but with much lower sensitivity (e.g., roughly 70%).
TABLE 6.
Testing options for STEC
| Option | Procedure a | Advantage(s) | Disadvantage(s) |
|---|---|---|---|
| Culture for O157 | (i) Plant specimen to chromogenic or selective medium (SMAC, CT-SMAC), (ii) perform latex agglutination for O157 or MUG test to identify sorbitol-nonfermenting colonies of E. coli, (iii) refer isolate to public health laboratory | Generates isolate for further public health study | Does not detect non-O157 STEC, does not detect sorbitol-fermenting O157 STEC, misses non-O157 STEC |
| Toxin testing | (i) Inoculate specimen to GN broth and incubate overnight, (ii) perform antigen detection test for Shiga toxins I and II; (iii) refer positive broths to public health laboratory | Detects all STEC, may differentiate Stx1 and Stx2 | Requires additional work on part of public health lab to find STEC from GN broth, antigen tests are expensive, may only be 50% as sensitive as NAAT |
| Culture and toxin testing | Perform both culture and toxin testing in parallel | Covers all STEC, rapid recovery of most frequent serotype (O157) | Cost and labor of additional testing to laboratory |
| Nucleic acid amplification test for stx 1 and stx 2 | (i) Perform NAAT (laboratory developed or IVD) that detects the Shiga toxin genes directly from stool, (ii) attempt to recover positive isolates, inoculate to GN broth to forward to public health laboratory, or forward positive specimens to public health laboratory | Most sensitive method, multiplex assays allow detection of many enteric pathogens | Not well validated to date, does not yield isolate for public health laboratory |
SMAC, sorbitol MacConkey agar; CT-SMAC, cefixime-tellurite SMAC; MUG, 4-methylumbelliferyl-β-d-glucuronide.
Finally, several antigen detection assays have been developed for the detection of Campylobacter from stool specimens. These enzyme immunoassays (EIAs) detect a surface antigen, called the Campylobacter-specific antigen, by use of either a microplate or lateral-flow immunochromatographic format. The antigen detection assay does not differentiate between C. jejuni and C. coli. Several studies have reported that while these antigen tests are more rapid and convenient than culture, up to 50% of positive results cannot be confirmed by other methods (309 – 313). Regardless, due to the low positive predictive value of these tests, laboratories may elect to confirm antigen-positive specimens by culture (310). Possible algorithms for the detection of Campylobacter are outlined in Table 7.
TABLE 7.
Testing options for Campylobacter
| Option (time for result) | Procedure a | Advantages | Disadvantages |
|---|---|---|---|
| Culture (2–4 days) | (i) Plant specimen to Campylobacter selective medium and incubate at 42°C under microaerobic conditions (report negative cultures after 48–72 h), (ii) Campylobacter identified by Gram stain and positive catalase and oxidase reactions (or MALDI-TOF), (iii) Campylobacter latex or sodium hippurate can be used to differentiate C. jejuni | Yields an isolate (for public health or susceptibility testing), most specific method | Requires special equipment (42°C incubator, ability to generate microaerobic atmosphere), Campylobacter is fastidious and many may not grow in culture unless conditions are ideal, takes 2–4 days for result |
| Antigen detection test (same day) | Perform antigen detection test following manufacturer's instructions | Rapid, easily batched | Concerns regarding specificity (may need to confirm positive results by culture), does not yield an isolate for further testing |
| NAAT | Perform NAAT (LDT or IVD) that detects Campylobacter directly from stool (may include extraction of nucleic acids) | Most sensitive method, multiplex assays allow detection of many enteric pathogens | Not well validated to date, does not yield isolate for further testing |
NAAT, nucleic acid amplification test; LDT, laboratory-developed test; IVD, in vitro diagnostic device.
Molecular Testing by Syndromic Panels
In recent years, syndromic panels for infectious diseases have gained popularity, in particular for screening patients who present with influenza-like illness for respiratory viruses. As of the writing of this article, five multiplex nucleic acid tests were FDA approved for the detection of organisms associated with gastroenteritis. The Prodesse ProGastro SSCS assay (Hologic Gen-Probe, San Diego, CA) detects Salmonella, Shigella, Campylobacter, and STEC. The Luminex xTAG GPP detects the organisms listed above as well as C. difficile toxin A/B, adenovirus 40/41, rotavirus A, norovirus, Giardia lamblia, Cryptosporidium, and Entamoeba histolytica. The BD MAX enteric bacterial panel (BD, Sparks, MD) detects C. jejuni and C. coli, Salmonella spp., Shigella spp./enteroinvasive E. coli (EIEC), and the stx 1 and stx 2 genes. The Nanosphere Verigene enteric pathogen test detects Campylobacter group, Salmonella spp., Vibrio group, Y. enterocolitica, and stx 1 and stx 2. The BioFire GI Panel detects C. jejuni, C. coli, C. upsaliensis, C. difficile, P. shigelloides, Salmonella, Y. enterocolitica, V. parahaemolyticus, V. vulnificus, V. cholerae, and the five diarrheagenic E. coli pathotypes (enteroaggregative, enteropathogenic, enterotoxigenic, Shiga toxin-producing, and enteroinvasive E. coli), along with Shigella. In addition to the bacterial targets, the Biofire GI Panel detects Cryptosporidium, Entamoeba histolytica, Cyclospora cayetanensis, Giardia lamblia, adenovirus 40/41, astrovirus, rotavirus A, sapovirus, and norovirus. While not FDA cleared, the Seeplex Diarrhea-V ACE (Seegene, Seoul, South Korea) has received CE marking for use in Europe. Numerous laboratory-developed multiplexed molecular assays that target enteric bacterial pathogens directly from fecal specimens have been described in the literature (314, 315).
Use of these tests to replace conventional cultures remains controversial. While it is clear that molecular assays offer improved sensitivity over culture (314, 316), there are concerns regarding specificity issues for the highly multiplexed panels (317). These molecular tests will detect organisms currently identified by routine stool cultures. However, it should be kept in mind that while routine fecal cultures may detect less frequent enteropathogens, they are designed to optimize recovery of Salmonella, Shigella, Campylobacter, and STEC. Perhaps the most pressing concern regarding the uptake of this new technology is that testing does not yield an isolate that could be used for surveillance for antimicrobial resistance or for subtyping to support the identification and investigation of outbreaks. Thus, laboratories that opt to use molecular assays should consult with their local or state public health laboratory to determine whether specimens positive for Salmonella, Shigella, Campylobacter, or STEC should be forwarded to the local or state public health laboratory for epidemiological studies (Fig. 1D).
Reporting Results
The laboratory report should reflect results for each organism routinely included in screening. For example, a negative routine fecal culture should be reported as “no Salmonella, Shigella, Campylobacter, or STEC isolated.” If the laboratory routinely adds a TCBS for the detection of Vibrio, a negative result would be indicated by “no Salmonella, Shigella, Campylobacter, STEC, or Vibrio isolated.” In contrast, recovery of any enteric pathogen, including those not specifically screened for, should be reported. The following organisms, for which the clinical significance of a stool isolate is less clear, should be reported with an indication of the number of colonies recovered on the primary plates: Aeromonas, P. shigelloides, E. tarda, and any of the emerging pathogens described in this article, as the clinical relevance of these may be less clear. For routine stool cultures, there is no need to report the presence of B. cereus, as this organism can be a transient member of the intestinal flora. However, heavy or pure growth of B. cereus may be reported as an unusual finding.
Antigen detection assays for an organism (e.g., Campylobacter spp.) or for toxins (e.g., Shiga toxin I and II or C. difficile toxin A/B) should be reported as positive or negative for the antigen/toxin tested. When molecular assays are used, the report should reflect the analyte(s) tested and a positive or negative result along with the test method used. For laboratory-developed tests (i.e., not FDA approved or cleared), a comment must be made regarding the laboratory's verification of the analytical performance of the assay. Additional comments about referral to a public health laboratory or reference laboratory for further identification may be used as warranted. However, testing by outside laboratories should not delay adding the result of a preliminary finding to the patient report.
Early parenteral volume expansion has been shown to significantly improve patient outcome for STEC infections (318). Further, because progression to HUS is associated with antimicrobial treatment in children (114), prompt reporting of results is critical for STEC infections. Therefore, laboratories should report probable and confirmed E. coli O157 isolates and Stx-positive specimens as soon as they are identified. Consideration should be given to a laboratory policy for calling positive results or the use of automated alerts via electronic medical records to notify the clinician, due to the critical nature of these infections.
CONCLUDING REMARKS
The potential number of bacteria associated with gastroenteritis is now estimated to approach or exceed 40 individual species (207). In contrast to this, both routine traditional and more contemporary molecular methods may screen for only 4 or 5 organisms (3, 246, 319). The number and type of agents routinely sought by the microbiology laboratory should be driven by geographic locale and patient history (foreign travel, disease symptomatology and underlying preexisting medical conditions). The importance of laboratory-clinician communication cannot be overemphasized for complicated cases, as patient-tailored testing is rarely feasible with the limited patient information that accompanies specimens received by the laboratory. Similarly, laboratories should ensure that physicians understand what organisms are screened for by the routine bacterial stool culture, as this is infrequently apparent to the general practitioner or even to many specialists. This being said, all clinical laboratories that perform stool cultures can provide useful information for patients with Salmonella, Shigella, STEC, and Campylobacter. The future role of bacterial stool cultures may be diminished by the emergence of nonculture diagnostic test methods for enteric pathogens. However, several pressing issues remain regarding these new technologies, including the performance of these tests and the issue of not providing an isolate to the public health laboratory as has been the practice in the past. These concerns are yet to be resolved (320). Nonetheless, these technologies have the potential for routine detection of enteropathogens that have eluded most clinical microbiology laboratories to date.
Biographies

Romney M. Humphries, Ph.D., D(ABMM), is an Assistant Professor in the Department of Pathology and Laboratory Medicine in the David Geffen School of Medicine at the University of California, Los Angeles. She is also the Section Chief for Clinical Microbiology at the UCLA Health System and program codirector for UCLA's Committee on Postgraduate Education Programs (CPEP) fellowship program. Dr. Humphries received her undergraduate degree in biochemistry from the University of Lethbridge, Canada. She completed her Ph.D. in bacterial pathogenesis in the laboratory of Dr. Glen Armstrong at the University of Calgary, Canada. There, she investigated novel anti-infective strategies for the prevention of bacterial gastroenteritis caused by enteropathogenic Escherichia coli. She subsequently completed a CPEP fellowship in medical and public health microbiology at the University of California, Los Angeles, under the direction of Dr. Michael Lewinski. At UCLA, under the guidance of Ms. Janet Hindler, Dr. Humphries developed a passion for antimicrobial susceptibility testing in the clinical laboratory. Her research focuses include the detection and characterization of pathogens that cause gastrointestinal diseases and the development of novel methods for detection and characterization of emerging antimicrobial resistance.

Andrea J. Linscott, Ph.D., D(ABMM), is the clinical microbiologist in the Department of Pathology at Ochsner Health System, New Orleans, LA. She received her Ph.D. at the University of North Texas under the direction of the late Dr. Gerard A. O'Donovan. Dr. Linscott completed an ASM Committee on Postgraduate Education Programs (CPEP) fellowship in medical and public health microbiology at the University of California, Los Angeles. She has served as committee member for both CPEP and ABMM. Her main passion is making clinical microbiology approachable for all levels of expertise through either written words or lecture.
Footnotes
[This article was published on 7 January 2015 with the title “Laboratory Diagnosis of Bacterial Gastroenteritis.” The title was updated in the current version, posted on 20 July 2020.]
REFERENCES
- 1. Fischer Walker CL, Lamberti L, Adair L, Guerrant RL, Lescano AG, Martorell R, Pinkerton RC, Black RE. 2012. Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PLoS One 7:e47908. doi: 10.1371/journal.pone.0047908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Molbak K, Valentiner-Branth P, Lanata CF, Black RE, Childhood M, Infection N. 2008. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, Tarr P, Neill M, Nachamkin I, Reller LB, Osterholm MT, Bennish ML, Pickering LK, Infectious Diseases Society of, A. 2001. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. 2011. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food–foodborne diseases active surveillance network, 10 U.S. sites, 1996-2010. MMWR Morb Mortal Wkly Rep 60:749–755. [PubMed] [Google Scholar]
- 5. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DuPont HL. 1997. Guidelines on acute infectious diarrhea in adults. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 92:1962–1975. [PubMed] [Google Scholar]
- 7. Chan SS, Ng KC, Lyon DJ, Cheung WL, Cheng AF, Rainer TH. 2003. Acute bacterial gastroenteritis: a study of adult patients with positive stool cultures treated in the emergency department. Emerg Med J 20:335–338. doi: 10.1136/emj.20.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koplan JP, Fineberg HV, Ferraro MJ, Rosenberg ML. 1980. Value of stool cultures. Lancet ii:413–416. [DOI] [PubMed] [Google Scholar]
- 9. Choi SW, Park CH, Silva TM, Zaenker EI, Guerrant RL. 1996. To culture or not to culture: fecal lactoferrin screening for inflammatory bacterial diarrhea. J Clin Microbiol 34:928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris AJ, Murray PR, Reller LB. 1996. Contemporary testing for enteric pathogens: the potential for cost, time, and health care savings. J Clin Microbiol 34:1776–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DuPont HL. 2009. Clinical practice. Bacterial diarrhea. N Engl J Med 361:1560–1569. doi: 10.1056/NEJMcp0904162. [DOI] [PubMed] [Google Scholar]
- 12. Schlenker C, Surawicz CM. 2009. Emerging infections of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 23:89–99. doi: 10.1016/j.bpg.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13. Rahouma A, Klena JD, Krema Z, Abobker AA, Treesh K, Franka E, Abusnena O, Shaheen HI, El Mohammady H, Abudher A, Ghenghesh KS. 2011. Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am J Trop Med Hyg 84:886–891. doi: 10.4269/ajtmh.2011.11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qu M, Deng Y, Zhang X, Liu G, Huang Y, Lin C, Li J, Yan H, Li X, Jia L, Kan B, Huang F, Wang Q. 2012. Etiology of acute diarrhea due to enteropathogenic bacteria in Beijing, China. J Infect 65:214–222. doi: 10.1016/j.jinf.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 15. Ghenghesh KS, Ahmed SF, El-Khalek RA, Al-Gendy A, Klena J. 2008. Aeromonas-associated infections in developing countries. J Infect Dev Ctries 2:81–98. doi: 10.3855/T2.2.81. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed D, Hoque A, Elahi MS, Endtz HP, Hossain MA. 2012. Bacterial aetiology of diarrhoeal diseases and antimicrobial resistance in Dhaka, Bangladesh, 2005-2008. Epidemiol Infect 140:1678–1684. doi: 10.1017/S0950268811002135. [DOI] [PubMed] [Google Scholar]
- 17. Janda JM, Abbott SL. 2010. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denno DM, Shaikh N, Stapp JR, Qin X, Hutter CM, Hoffman V, Mooney JC, Wood KM, Stevens HJ, Jones R, Tarr PI, Klein EJ. 2012. Diarrhea etiology in a pediatric emergency department: a case control study. Clin Infect Dis 55:897–904. doi: 10.1093/cid/cis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Del Val A, Moles JR, Garrigues V. 1990. Very prolonged diarrhea associated with Aeromonas hydrophila. Am J Gastroenterol 85:1535–1535. [PubMed] [Google Scholar]
- 20. Rautelin H, Hanninen ML, Sivonen A, Turunen U, Valtonen V. 1995. Chronic diarrhea due to a single strain of Aeromonas caviae. Eur J Clin Microbiol Infect Dis 14:51–53. doi: 10.1007/BF02112620. [DOI] [PubMed] [Google Scholar]
- 21. Figueras MJ, Aldea MJ, Fernandez N, Aspiroz C, Alperi A, Guarro J. 2007. Aeromonas hemolytic uremic syndrome. A case and a review of the literature. Diagn Microbiol Infect Dis 58:231–234. doi: 10.1016/j.diagmicrobio.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 22. Alperi A, Figueras MJ. 2010. Human isolates of Aeromonas possess Shiga toxin genes (stx1 and stx2) highly similar to the most virulent gene variants of Escherichia coli. Clin Microbiol Infect 16:1563–1567. doi: 10.1111/j.1469-0691.2010.03203.x. [DOI] [PubMed] [Google Scholar]
- 23. Wu C-J, Tsai P-J, Chen P-L, Wu IC, Lin Y-T, Chen Y-H, Wang L-R, Ko W-C. 2012. Aeromonas aquariorum septicemia and enterocolitis in a cirrhotic patient. Diagn Microbiol Infect Dis 74:406–408. doi: 10.1016/j.diagmicrobio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 24. Jensen GB, Hansen BM, Eilenberg J, Mahillon J. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ Microb 5:631–640. doi: 10.1046/j.1462-2920.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 25. Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotiranta A, Haapasalo M, Kari K, Kerosuo E, Olsen I, Sorsa T, Meurman JH, Lounatmaa K. 1998. Surface structure, hydrophobicity, phagocytosis, and adherence to matrix proteins of Bacillus cereus cells with and without the crystalline surface protein layer. Infect Immun 66:4895–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ronner U, Husmark U, Henriksson A. 1990. Adhesion of bacillus spores in relation to hydrophobicity. J Appl Bacteriol 69:550–556. doi: 10.1111/j.1365-2672.1990.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease C, Prevention. 2013. Surveillance for foodborne disease outbreaks—United States, 2009-2010. MMWR Morb Mortal Wkly Rep 62:41–47. [PMC free article] [PubMed] [Google Scholar]
- 29. Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M. 1994. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol Lett 121:31–34. doi: 10.1111/j.1574-6968.1994.tb07071.x. [DOI] [PubMed] [Google Scholar]
- 30. Ehling-Schulz M, Fricker M, Scherer S. 2004. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res 48:479–487. doi: 10.1002/mnfr.200400055. [DOI] [PubMed] [Google Scholar]
- 31. Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, Hoedemaekers G, Fourie L, Heyndrickx M, Mahillon J. 2005. Fatal family outbreak of Bacillus cereus-associated food poisoning. J Clin Microbiol 43:4277–4279. doi: 10.1128/JCM.43.8.4277-4279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naranjo M, Denayer S, Botteldoorn N, Delbrassinne L, Veys J, Waegenaere J, Sirtaine N, Driesen RB, Sipido KR, Mahillon J, Dierick K. 2011. Sudden death of a young adult associated with Bacillus cereus food poisoning. J Clin Microbiol 49:4379–4381. doi: 10.1128/JCM.05129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahler H, Pasi A, Kramer JM, Schulte P, Scoging AC, Bar W, Krahenbuhl S. 1997. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med 336:1142–1148. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- 34. Takabe F, Oya M. 1976. An autopsy case of food poisoning associated with Bacillus cereus. Forensic Sci 7:97–101. doi: 10.1016/0300-9432(76)90024-8. [DOI] [PubMed] [Google Scholar]
- 35. Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606. doi: 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- 36. Clavel T, Carlin F, Lairon D, Nguyen-The C, Schmitt P. 2004. Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J Appl Microbiol 97:214–219. doi: 10.1111/j.1365-2672.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- 37. Yea CL, Lee CL, Pan TM, Horng CB. 1994. Isolation of Bacillus cereus in the feces of healthy adults in Taipei City. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 27:148–151. [PubMed] [Google Scholar]
- 38. Turnbull PC, Kramer JM. 1985. Intestinal carriage of Bacillus cereus: faecal isolation studies in three population groups. J Hyg (Lond) 95:629–638. doi: 10.1017/S0022172400060733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moore JE, Corcoran D, Dooley JSG, Fanning S, Lucey B, Matsuda M, McDowell DA, Megraud F, Millar BC, O'Mahony R, O'Riordan L, O'Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P. 2005. Campylobacter. Vet Res 36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- 40. Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis 32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 41. Centers for Disease Control and Prevention. 2010. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2009. MMWR Morb Mortal Wkly Rep 59:418–422. [PubMed] [Google Scholar]
- 42. Ailes E, Scallan E, Berkelman RL, Kleinbaum DG, Tauxe RV, Moe CL. 2012. Do differences in risk factors, medical care seeking, or medical practices explain the geographic variation in campylobacteriosis in Foodborne Diseases Active Surveillance Network (FoodNet) sites? Clin Infect Dis 54(Suppl 5):S464–S471. doi: 10.1093/cid/cis050. [DOI] [PubMed] [Google Scholar]
- 43. Blaser M, Allos BM. 2005. Campylobacter jejuni and related species, p 2548–2557. In Mandell J, Benner E, Dolin R (ed), Principles and practice of infectious disease, 6th ed Elsevier/Churchill Livingstone, New York, NY. [Google Scholar]
- 44. Clark CG, Price L, Ahmed R, Woodward DL, Melito PL, Rodgers FG, Jamieson F, Ciebin B, Li A, Ellis A. 2003. Characterization of waterborne outbreak-associated Campylobacter jejuni, Walkerton, Ontario. Emerg Infect Dis 9:1232–1241. doi: 10.3201/eid0910.020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jay-Russell MT, Mandrell RE, Yuan J, Bates A, Manalac R, Mohle-Boetani J, Kimura A, Lidgard J, Miller WG. 2013. Using major outer membrane protein typing as an epidemiological tool to investigate outbreaks caused by milk-borne Campylobacter jejuni isolates in California. J Clin Microbiol 51:195–201. doi: 10.1128/JCM.01845-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wood RC, MacDonald KL, Osterholm MT. 1992. Campylobacter enteritis outbreaks associated with drinking raw milk during youth activities. A 10-year review of outbreaks in the United States. JAMA 268:3228–3230. [PubMed] [Google Scholar]
- 47. Tauxe R. 2001. Incidence, trends and source of campylobacteriosis in developed countries, p 43. The increasing incidence of campylobacteriosis in humans. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 48. Ftizgerald C, Nachamkin I. 2011. Campylobacter and Arcobacter, p 885–889. In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (ed), Manual of clinical microbiology. ASM Press, Washington, DC: [Google Scholar]
- 49. Endtz HP, Ruijs GJ, Zwinderman AH, van der Reijden T, Biever M, Mouton RP. 1991. Comparison of six media, including a semisolid agar, for the isolation of various Campylobacter species from stool specimens. J Clin Microbiol 29:1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Labarca JA, Sturgeon J, Borenstein L, Salem N, Harvey SM, Lehnkering E, Reporter R, Mascola L. 2002. Campylobacter upsaliensis: another pathogen for consideration in the United States. Clin Infect Dis 34:59–60. doi: 10.1086/340266. [DOI] [PubMed] [Google Scholar]
- 51. Lastovica AJ, Le Roux E, Penner JL. 1989. “Campylobacter upsaliensis” isolated from blood cultures of pediatric patients. J Clin Microbiol 27:657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Butzler JP. 2004. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect 10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 53. MJB JE. 2008. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections, p 99–121. In Nachamkin I, Szymanski CM, Blaser MJ (ed), Campylobacter, 3rd ed ASM Press, Washington, DC: [Google Scholar]
- 54. Fernandez-Cruz A, Munoz P, Mohedano R, Valerio M, Marin M, Alcala L, Rodriguez-Creixems M, Cercenado E, Bouza E. 2010. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore) 89:319–330. doi: 10.1097/MD.0b013e3181f2638d. [DOI] [PubMed] [Google Scholar]
- 55. Ruiz-Contreras J, Ramos JT, Hernandez-Sampelayo T, Gurbindo MD, Garcia de Jose M, De Miguel MJ, Cilleruelo MJ, Mellado MJ. 1995. Sepsis in children with human immunodeficiency virus infection. The Madrid HIV Pediatric Infection Collaborative Study Group. Pediatr Infect Dis J 14:522–526. [DOI] [PubMed] [Google Scholar]
- 56. Nachamkin I, Allos BM, Ho T. 1998. Campylobacter species and Guillain-Barre syndrome. Clin Microbiol Rev 11:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. 2007. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum 37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schonberg-Norio D, Mattila L, Lauhio A, Katila ML, Kaukoranta SS, Koskela M, Pajarre S, Uksila J, Eerola E, Sarna S, Rautelin H. 2010. Patient-reported complications associated with Campylobacter jejuni infection. Epidemiol Infect 138:1004–1011. doi: 10.1017/S0950268809991099. [DOI] [PubMed] [Google Scholar]
- 59. Rees JH, Soudain SE, Gregson NA, Hughes RA. 1995. Campylobacter jejuni infection and Guillain-Barre syndrome. N Engl J Med 333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 60. Ali S, Moore G, Wilson AP. 2011. Spread and persistence of Clostridium difficile spores during and after cleaning with sporicidal disinfectants. J Hosp Infect 79:97–98. doi: 10.1016/j.jhin.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 61. Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hatheway CL. 1990. Toxigenic clostridia. Clin Microbiol Rev 3:66–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Elliott B, Squire MM, Thean S, Chang BJ, Brazier JS, Rupnik M, Riley TV. 2011. New types of toxin A-negative, toxin B-positive strains among clinical isolates of Clostridium difficile in Australia. J Med Microbiol 60:1108–1111. doi: 10.1099/jmm.0.031062-0. [DOI] [PubMed] [Google Scholar]
- 64. Kim H, Riley TV, Kim M, Kim CK, Yong D, Lee K, Chong Y, Park JW. 2008. Increasing prevalence of toxin A-negative, toxin B-positive isolates of Clostridium difficile in Korea: impact on laboratory diagnosis. J Clin Microbiol 46:1116–1117. doi: 10.1128/JCM.01188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 66. Hall I, O'Toole E. 1935. Intestinal flora in newborn infants with a description of a new pathogen anaerobe, Bacillus difficile. Am J Dis Child 49:390–402. doi: 10.1001/archpedi.1935.01970020105010. [DOI] [Google Scholar]
- 67. Viscidi R, Willey S, Bartlett JG. 1981. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology 81:5–9. [PubMed] [Google Scholar]
- 68. Walker KJ, Gilliland SS, Vance-Bryan K, Moody JA, Larsson AJ, Rotschafer JC, Guay DR. 1993. Clostridium difficile colonization in residents of long-term care facilities: prevalence and risk factors. J Am Geriatr Soc 41:940–946. [DOI] [PubMed] [Google Scholar]
- 69. Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. 2007. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 45:992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 70. Guerrero DM, Becker JC, Eckstein EC, Kundrapu S, Deshpande A, Sethi AK, Donskey CJ. 2013. Asymptomatic carriage of toxigenic Clostridium difficile by hospitalized patients. J Hosp Infect 85:155–158. doi: 10.1016/j.jhin.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 71. Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O'Connor L, Ip CL, Golubchik T, Batty EM, Finney JM, Wyllie DH, Didelot X, Piazza P, Bowden R, Dingle KE, Harding RM, Crook DW, Wilcox MH, Peto TE, Walker AS. 2013. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 369:1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kelly CP, Pothoulakis C, LaMont JT. 1994. Clostridium difficile colitis. N Engl J Med 330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 73. Asha NJ, Tompkins D, Wilcox MH. 2006. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J Clin Microbiol 44:2785–2791. doi: 10.1128/JCM.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lucado J, Gould C, Elixhauser A. 2012. Clostridium difficile infections (CDI) in hospital stays, 2009: statistical brief 124. Healthcare Cost and Utilization Project (HCUP) statistical briefs, Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 75. Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 76. Wilcox MH, Shetty N, Fawley WN, Shemko M, Coen P, Birtles A, Cairns M, Curran MD, Dodgson KJ, Green SM, Hardy KJ, Hawkey PM, Magee JG, Sails AD, Wren MW. 2012. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 55:1056–1063. doi: 10.1093/cid/cis614. [DOI] [PubMed] [Google Scholar]
- 77. Marsh JW, Arora R, Schlackman JL, Shutt KA, Curry SR, Harrison LH. 2012. Association of relapse of Clostridium difficile disease with BI/NAP1/027. J Clin Microbiol 50:4078–4082. doi: 10.1128/JCM.02291-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 79. Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, Wullt M, Burman LG. 2008. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol 46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, Johnson S, Gerding DN, Vedantam G. 2010. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol 192:4904–4911. doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cloud J, Noddin L, Pressman A, Hu M, Kelly C. 2009. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin Gastroenterol Hepatol 7:868–873 e862. doi: 10.1016/j.cgh.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 82. Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW, Almassalha LM, Ewing SA, Ring C, Galecki AT, Rogers MA, Washer L, Newton DW, Malani PN, Young VB, Aronoff DM. 2012. Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis 55:1661–1668. doi: 10.1093/cid/cis786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hunt JJ, Ballard JD. 2013. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol Mol Biol Rev 77:567–581. doi: 10.1128/MMBR.00017-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bartlett JG. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med 145:758–764. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 85. Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, Simmons RL. 2002. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 235:363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Earhart MM. 2008. The identification and treatment of toxic megacolon secondary to pseudomembranous colitis. Dimens Crit Care Nurs 27:249–254. doi: 10.1097/01.DCC.0000338869.70035.2b. [DOI] [PubMed] [Google Scholar]
- 87. Berman L, Carling T, Fitzgerald TN, Bell RL, Duffy AJ, Longo WE, Roberts KE. 2008. Defining surgical therapy for pseudomembranous colitis with toxic megacolon. J Clin Gastroenterol 42:476–480. doi: 10.1097/MCG.0b013e31804bbe12. [DOI] [PubMed] [Google Scholar]
- 88. Hall JF, Berger D. 2008. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg 196:384–388. doi: 10.1016/j.amjsurg.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 89. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare Epidemiology of America, Infectious Diseases Society of America. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 90. Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. 2000. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol 38:2386–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. 1989. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 159:340–343. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 92. Tang-Feldman Y, Mayo S, Silva J Jr, Cohen SH. 2003. Molecular analysis of Clostridium difficile strains isolated from 18 cases of recurrent Clostridium difficile-associated diarrhea. J Clin Microbiol 41:3413–3414. doi: 10.1128/JCM.41.7.3413-3414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wilcox MH, Fawley WN, Settle CD, Davidson A. 1998. Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection? J Hosp Infect 38:93–9100. doi: 10.1016/S0195-6701(98)90062-7. [DOI] [PubMed] [Google Scholar]
- 94. Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. 1998. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet 351:633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 95. Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. 2008. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 46(Suppl 1):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 96. Barbut F, Petit JC. 2001. Epidemiology of Clostridium difficile-associated infections. Clin Microbiol Infect 7:405–410. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 97. Borriello SP, Barclay FE, Welch AR, Stringer MF, Watson GN, Williams RK, Seal DV, Sullens K. 1985. Epidemiology of diarrhoea caused by enterotoxigenic Clostridium perfringens. J Med Microbiol 20:363–372. doi: 10.1099/00222615-20-3-363. [DOI] [PubMed] [Google Scholar]
- 98. Smedley JG III, Fisher DJ, Sayeed S, Chakrabarti G, McClane BA. 2004. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol 152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 99. Cooke RA. 1979. Pig Bel. Perspect Pediatr Pathol 5:137–152. [PubMed] [Google Scholar]
- 100. Borriello SP, Larson HE, Welch AR, Barclay F, Stringer MF, Bartholomew BA. 1984. Enterotoxigenic Clostridium perfringens: a possible cause of antibiotic-associated diarrhoea. Lancet i:305–307. [DOI] [PubMed] [Google Scholar]
- 101. Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gould LH, Bopp C, Strockbine N, Atkinson R, Baselski V, Body B, Carey R, Crandall C, Hurd S, Kaplan R, Neill M, Shea S, Somsel P, Tobin-D'Angelo M, Griffin PM, Gerner-Smidt P. 2009. Recommendations for diagnosis of Shiga toxin-producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep 58:1–14. [PubMed] [Google Scholar]
- 103. CDC. 2013. Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996-2012. MMWR Morb Mortal Wkly Rep 62:283–287. [PMC free article] [PubMed] [Google Scholar]
- 104. Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garman KN, Lathrop S, Medus C, Spina NL, Webb TH, White PL, Wymore K, Gierke RE, Mahon BE, Griffin PM. 2013. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis 10:453–460. doi: 10.1089/fpd.2012.1401. [DOI] [PubMed] [Google Scholar]
- 105. Vally H, Hall G, Dyda A, Raupach J, Knope K, Combs B, Desmarchelier P. 2012. Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000-2010. BMC Public Health 12:63. doi: 10.1186/1471-2458-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. European Food SafetyAuthority, European Centre for Disease Prevention and Control. 2011. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EEA, with special reference to the German outbreak strain STEC O104. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 107. Su C, Brandt LJ. 1995. Escherichia coli O157:H7 infection in humans. Ann Intern Med 123:698–714. doi: 10.7326/0003-4819-123-9-199511010-00009. [DOI] [PubMed] [Google Scholar]
- 108. Rohde H, Qin J, Cui Y, Li D, Loman NJ, Hentschke M, Chen W, Pu F, Peng Y, Li J, Xi F, Li S, Li Y, Zhang Z, Yang X, Zhao M, Wang P, Guan Y, Cen Z, Zhao X, Christner M, Kobbe R, Loos S, Oh J, Yang L, Danchin A, Gao GF, Song Y, Yang H, Wang J, Xu J, Pallen MJ, Aepfelbacher M, Yang R. 2011. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med 365:718–724. doi: 10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- 109. Manning SD, Madera RT, Schneider W, Dietrich SE, Khalife W, Brown W, Whittam TS, Somsel P, Rudrik JT. 2007. Surveillance for Shiga toxin-producing Escherichia coli, Michigan, 2001-2005. Emerg Infect Dis 13:318–321. doi: 10.3201/eid1302.060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. van Duynhoven YT, Friesema IH, Schuurman T, Roovers A, van Zwet AA, Sabbe LJ, van der Zwaluw WK, Notermans DW, Mulder B, van Hannen EJ, Heilmann FG, Buiting A, Jansen R, Kooistra-Smid AM. 2008. Prevalence, characterisation and clinical profiles of Shiga toxin-producing Escherichia coli in The Netherlands. Clin Microbiol Infect 14:437–445. doi: 10.1111/j.1469-0691.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 111. Walker CLF, Applegate JA, Black RE. 2012. Haemolytic-uraemic syndrome as a sequela of diarrhoeal disease. J Health Popul Nutr 30:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tarr PI, Gordon CA, Chandler WL. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 113. Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G. 2011. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 114. Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI. 2012. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin Infect Dis 55:33–41. doi: 10.1093/cid/cis299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zoufaly A, Cramer JP, Vettorazzi E, Sayk F, Bremer JP, Koop I, de Weerth A, Schmiedel S, Jordan S, Fraedrich K, Asselborn NH, Nitschke M, Neumann-Grutzeck C, Magnus T, Ruther C, Fellermann K, Stahl RK, Wegscheider K, Lohse AW. 2013. Risk factors for development of hemolytic uremic syndrome in a cohort of adult patients with STEC 0104:H4 infection. PLoS One 8:e59209. doi: 10.1371/journal.pone.0059209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Geerdes-Fenge HF, Lobermann M, Nurnberg M, Fritzsche C, Koball S, Henschel J, Hohn R, Schober HC, Mitzner S, Podbielski A, Reisinger EC. 2013. Ciprofloxacin reduces the risk of hemolytic uremic syndrome in patients with Escherichia coli O104:H4-associated diarrhea. Infection 41:669–673. doi: 10.1007/s15010-012-0387-6. [DOI] [PubMed] [Google Scholar]
- 117. Borgatta B, Kmet-Lunacek N, Rello J. 2012. E. coli O104:H4 outbreak and haemolytic-uraemic syndrome. Med Intensiva 36:576–583. doi: 10.1016/j.medin.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 118. Bell BP, Griffin PM, Lozano P, Christie DL, Kobayashi JM, Tarr PI. 1997. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics 100:E12. doi: 10.1542/peds.100.1.e12. [DOI] [PubMed] [Google Scholar]
- 119. Allerberger F, Wagner M. 2010. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect 16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 120. Goulet V, King LA, Vaillant V, de Valk H. 2013. What is the incubation period for listeriosis? BMC Infect Dis 13:11–11. doi: 10.1186/1471-2334-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Drevets DA, Bronze MS. 2008. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol 53:151–165. doi: 10.1111/j.1574-695X.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 122. Ooi ST, Lorber B. 2005. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis 40:1327–1332. doi: 10.1086/429324. [DOI] [PubMed] [Google Scholar]
- 123. Schlech WF III, Schlech WF IV, Haldane H, Mailman TL, Warhuus M, Crouse N, Haldane DJ. 2005. Does sporadic Listeria gastroenteritis exist? A 2-year population-based survey in Nova Scotia, Canada. Clin Infect Dis 41:778–784. doi: 10.1086/432724. [DOI] [PubMed] [Google Scholar]
- 124. Guillet C, Join-Lambert O, Le Monnier A, Leclercq A, Mechaï F, Mamzer-Bruneel MF, Bielecka MK, Scortti M, Disson O, Berche P, Vazquez-Boland J, Lortholary O, Lecuit M. 2010. Human listeriosis caused by Listeria ivanovii. Emerg Infect Dis 16:136–138. doi: 10.3201/eid1601.091155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Goulet V, Hebert M, Hedberg C, Laurent E, Vaillant V, De Valk H, Desenclos J-C. 2012. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clin Infect Dis 54:652–660. doi: 10.1093/cid/cir902. [DOI] [PubMed] [Google Scholar]
- 126. Hohmann EL, Kim J. 2012. Case records of the Massachusetts General Hospital. Case 8-2012. A 53-year-old man with Crohn's disease, diarrhea, fever, and bacteremia. N Engl J Med 366:1039–1045. doi: 10.1056/NEJMcpc1110054. [DOI] [PubMed] [Google Scholar]
- 127. Olsvik O, Wachsmuth K, Kay B, Birkness KA, Yi A, Sack B. 1990. Laboratory observations on Plesiomonas shigelloides strains isolated from children with diarrhea in Peru. J Clin Microbiol 28:886–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Arai T, Ikejima N, Itoh T, Sakai S, Shimada T, Sakazaki R. 1980. A survey of Plesiomonas shigelloides from aquatic environments, domestic animals, pets and humans. J Hyg (Lond) 84:203–211. doi: 10.1017/S002217240002670X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pitarangsi C, Echeverria P, Whitmire R, Tirapat C, Formal S, Dammin GJ, Tingtalapong M. 1982. Enteropathogenicity of Aeromonas hydrophila and Plesiomonas shigelloides: prevalence among individuals with and without diarrhea in Thailand. Infect Immun 35:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ. 1986. Plesiomonas enteric infections in the United States. Ann Intern Med 105:690–694. doi: 10.7326/0003-4819-105-5-690. [DOI] [PubMed] [Google Scholar]
- 131. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. 1978. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond) 80:275–280. doi: 10.1017/S0022172400053638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Centers for Disease Control and Prevention. 1998. Plesiomonas shigelloides and Salmonella serotype Hartford infections associated with a contaminated water supply—Livingston County, New York, 1996. MMWR Morb Mortal Wkly Rep 47:394–396. [PubMed] [Google Scholar]
- 133. Escobar JC, Bhavnani D, Trueba G, Ponce K, Cevallos W, Eisenberg J. 2012. Plesiomonas shigelloides infection, Ecuador, 2004-2008. Emerg Infect Dis 18:322–324. doi: 10.3201/eid1802.110562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kain KC, Kelly MT. 1989. Clinical features, epidemiology, and treatment of Plesiomonas shigelloides diarrhea. J Clin Microbiol 27:998–991001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Khan AM, Faruque ASG, Hossain MS, Sattar S, Fuchs GJ, Salam MA. 2004. Plesiomonas shigelloides-associated diarrhoea in Bangladeshi children: a hospital-based surveillance study. J Trop Pediatr 50:354–356. doi: 10.1093/tropej/50.6.354. [DOI] [PubMed] [Google Scholar]
- 136. Popoff MY, Bockemühl J, Brenner FW. 2000. Supplement 1999 (no. 43) to the Kauffmann-White scheme. Res Microbiol 151:893–896. doi: 10.1016/S0923-2508(00)01157-8. [DOI] [PubMed] [Google Scholar]
- 137. Popoff MY, Bockemühl J, Hickman-Brenner FW. 1997. Supplement 1996 (no. 40) to the Kauffmann-White scheme. Res Microbiol 148:811–814. doi: 10.1016/S0923-2508(97)82457-6. [DOI] [PubMed] [Google Scholar]
- 138. Behravesh CB, Ferraro A, Deasy M, Dato V, Moll M, Sandt C, Rea NK, Rickert R, Marriott C, Warren K, Urdaneta V, Salehi E, Villamil E, Ayers T, Hoekstra RM, Austin JL, Ostroff S, Williams IT. 2010. Human Salmonella infections linked to contaminated dry dog and cat food, 2006-2008. Pediatrics 126:477–483. doi: 10.1542/peds.2009-3273. [DOI] [PubMed] [Google Scholar]
- 139. Public Health Agency of Canada. 2006. An international outbreak of human salmonellosis associated with animal-derived pet treats—Canada and Washington state, 2005. Can Commun Dis Report 32:150–155. [PubMed] [Google Scholar]
- 140. Pickering LK, Marano N, Bocchini JA, Angulo FJ. 2008. Exposure to nontraditional pets at home and to animals in public settings: risks to children. Pediatrics 122:876–886. doi: 10.1542/peds.2008-1942. [DOI] [PubMed] [Google Scholar]
- 141. Sanyal D, Douglas T, Roberts R. 1997. Salmonella infection acquired from reptilian pets. Arch Dis Child 77:345–346. doi: 10.1136/adc.77.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Centers for Disease Control and Prevention. 2006. Human salmonellosis associated with animal-derived pet treats—United States and Canada, 2005. MMWR Morb Mortal Wkly Rep 55:702–705. [PubMed] [Google Scholar]
- 143. CDC. 2012. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet surveillance report for 2011 (final report). CDC, Atlanta, GA. [Google Scholar]
- 144. Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 145. Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 146. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Morpeth SC, Ramadhani HO, Crump JA. 2009. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49:606–611. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Black PH, Kunz LJ, Swartz MN. 1960. Salmonellosis—a review of some unusual aspects. N Engl J Med 262:921–927. doi: 10.1056/NEJM196005052621806. [DOI] [PubMed] [Google Scholar]
- 149. Blaser MJ, Feldman RA. 1981. From the centers for disease control. Salmonella bacteremia: reports to the Centers for Disease Control, 1968-1979. J Infect Dis 143:743–746. [DOI] [PubMed] [Google Scholar]
- 150. Cherubin CE, Fodor T, Denmark LI, Master CS, Fuerst HT, Winter JW. 1969. Symptoms, septicemia and death in salmonellosis. Am J Epidemiol 90:285–291. [DOI] [PubMed] [Google Scholar]
- 151. Shimoni Z, Pitlik S, Leibovici L, Samra Z, Konigsberger H, Drucker M, Agmon V, Ashkenazi S, Weinberger M. 1999. Nontyphoid Salmonella bacteremia: age-related differences in clinical presentation, bacteriology, and outcome. Clin Infect Dis 28:822–827. doi: 10.1086/515186. [DOI] [PubMed] [Google Scholar]
- 152. Sirinavin S, Jayanetra P, Lolekha S, Layangkul T. 1988. Predictors for extraintestinal infection in Salmonella enteritis in Thailand. Pediatr Infect Dis J 7:44–48. doi: 10.1097/00006454-198801000-00011. [DOI] [PubMed] [Google Scholar]
- 153. Wittler RR, Bass JW. 1989. Nontyphoidal Salmonella enteric infections and bacteremia. Pediatr Infect Dis J 8:364–367. doi: 10.1097/00006454-198906000-00008. [DOI] [PubMed] [Google Scholar]
- 154. Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N Engl J Med 347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 155. Blaser MJ, Newman LS. 1982. A review of human salmonellosis. I. Infective dose. Rev Infect Dis 4:1096–1106. [DOI] [PubMed] [Google Scholar]
- 156. Gruenewald R, Blum S, Chan J. 1994. Relationship between human immunodeficiency virus infection and salmonellosis in 20- to 59-year-old residents of New York City. Clin Infect Dis 18:358–363. doi: 10.1093/clinids/18.3.358. [DOI] [PubMed] [Google Scholar]
- 157. Thamlikitkul V, Dhiraputra C, Paisarnsinsup T, Chareandee C. 1996. Non-typhoidal Salmonella bacteraemia: clinical features and risk factors. Trop Med Int Health 1:443–448. doi: 10.1046/j.1365-3156.1996.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 158. Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, Chow SC, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Shao JF, Bartlett JA, Maro VP. 2011. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Levine MM, Farag TH. 2011. Invasive salmonella infections and HIV in Northern Tanzania. Clin Infect Dis 52:349–351. doi: 10.1093/cid/ciq109. [DOI] [PubMed] [Google Scholar]
- 160. Lynch MF, Blanton EM, Bulens S, Polyak C, Vojdani J, Stevenson J, Medalla F, Barzilay E, Joyce K, Barrett T, Mintz ED. 2009. Typhoid fever in the United States, 1999-2006. JAMA 302:859–865. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- 161. Janda JM, Abbott SL. 2006. The Enterobacteriaceae. ASM Press, Washington, DC. [Google Scholar]
- 162. von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, Canh DG, Chaicumpa W, Agtini MD, Hossain A, Bhutta ZA, Mason C, Sethabutr O, Talukder K, Nair GB, Deen JL, Kotloff K, Clemens J. 2006. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med 3:e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sansonetti PJ. 2006. Shigellosis: an old disease in new clothes? PLoS Med 3:e354. doi: 10.1371/journal.pmed.0030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Levine MM, DuPont HL, Khodabandelou M, Hornick RB. 1973. Long-term Shigella-carrier state. N Engl J Med 288:1169–1171. doi: 10.1056/NEJM197305312882207. [DOI] [PubMed] [Google Scholar]
- 165. Bennish ML, Azad AK, Yousefzadeh D. 1991. Intestinal obstruction during shigellosis: incidence, clinical features, risk factors, and outcome. Gastroenterology 101:626–634. [DOI] [PubMed] [Google Scholar]
- 166. Margolin L, Engelhard D. 2003. Bilateral pneumonia associated with Shigella sonnei dysentery. Am J Infect Control 31:445–446. doi: 10.1067/mic./2003.69. [DOI] [PubMed] [Google Scholar]
- 167. Papasian CJ, Enna-Kifer S, Garrison B. 1995. Symptomatic Shigella sonnei urinary tract infection. J Clin Microbiol 33:2222–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Butler T. 2012. Haemolytic uraemic syndrome during shigellosis. Trans R Soc Trop Med Hyg 106:395–399. doi: 10.1016/j.trstmh.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 169. Khan WA, Griffiths JK, Bennish ML. 2013. Gastrointestinal and extra-intestinal manifestations of childhood shigellosis in a region where all four species of Shigella are endemic. PLoS One 8:e64097. doi: 10.1371/journal.pone.0064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Betley MJ, Mekalanos JJ. 1985. Staphylococcal enterotoxin A is encoded by phage. Science 229:185–187. doi: 10.1126/science.3160112. [DOI] [PubMed] [Google Scholar]
- 171. Kérouanton A, Hennekinne JA, Letertre C, Petit L, Chesneau O, Brisabois A, De Buyser ML. 2007. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int J Food Microbiol 115:369–375. doi: 10.1016/j.ijfoodmicro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 172. Schelin J, Wallin-Carlquist N, Cohn MT, Lindqvist R, Barker GC, Rådström P. 2011. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence 2:580–592. doi: 10.4161/viru.2.6.18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Breckinridge JC, Bergdoll MS. 1971. Outbreak of food-borne gastroenteritis due to a coagulase-negative enterotoxin-producing staphylococcus. N Engl J Med 284:541–543. doi: 10.1056/NEJM197103112841010. [DOI] [PubMed] [Google Scholar]
- 174. Udo EE, Al-Bustan MA, Jacob LE, Chugh TD. 1999. Enterotoxin production by coagulase-negative staphylococci in restaurant workers from Kuwait City may be a potential cause of food poisoning. J Med Microbiol 48:819–823. doi: 10.1099/00222615-48-9-819. [DOI] [PubMed] [Google Scholar]
- 175. Le Loir Y, Baron F, Gautier M. 2003. Staphylococcus aureus and food poisoning. Genet Mol Res 2:63–76. [PubMed] [Google Scholar]
- 176. Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Williams RE. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev 27:56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Que Y-A, Moreillon P. 2009. Staphylococcus aureus (including staphylococcal toxic shock), p 2543–2578, Mandell, Douglas and Bennett's principles and practice of infectious diseases, 7th ed Elsevier, Philadelphia, PA. [Google Scholar]
- 179. Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. 2012. The global burden of cholera. Bull World Health Organ 90:209–218. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mahan MJ, Kubicek-Sutherland JZ, Heithoff DM. 2013. Rise of the microbes. Virulence 4:213–222. doi: 10.4161/viru.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996-2010: review of surveillance data from 2 systems. Clin Infect Dis 54(Suppl 5):S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sims JN, Isokpehi RD, Cooper GA, Bass MP, Brown SD, St John AL, Gulig PA, Cohly HHP. 2011. Visual analytics of surveillance data on foodborne vibriosis, United States, 1973-2010. Environ Health Insights 5:71–85. doi: 10.4137/EHI.S7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hara-Kudo Y, Saito S, Ohtsuka K, Yamasaki S, Yahiro S, Nishio T, Iwade Y, Otomo Y, Konuma H, Tanaka H, Nakagawa H, Sugiyama K, Sugita-Konishi Y, Kumagai S. 2012. Characteristics of a sharp decrease in Vibrio parahaemolyticus infections and seafood contamination in Japan. Int J Food Microbiol 157:95–9101. doi: 10.1016/j.ijfoodmicro.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 184. Nair GB, Ramamurthy T, Bhattacharya SK, Dutta B, Takeda Y, Sack DA. 2007. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin Microbiol Rev 20:39–48. doi: 10.1128/CMR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gonzalez-Escalona N, Strain EA, De Jesús AJ, Jones JL, Depaola A. 2011. Genome sequence of the clinical O4:K12 serotype Vibrio parahaemolyticus strain 10329. J Bacteriol 193:3405–3406. doi: 10.1128/JB.05044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Chitov T, Kirikaew P, Yungyune P, Ruengprapan N, Sontikun K. 2009. An incidence of large foodborne outbreak associated with Vibrio mimicus. Eur J Clin Microbiol Infect Dis 28:421–424. doi: 10.1007/s10096-008-0639-7. [DOI] [PubMed] [Google Scholar]
- 187. Kay MK, Cartwright EJ, Maceachern D, McCullough J, Barzilay E, Mintz E, Duchin JS, Macdonald K, Turnsek M, Tarr C, Talkington D, Newton A, Marfin AA. 2012. Vibrio mimicus infection associated with crayfish consumption, Spokane, Washington, 2010. J Food Prot 75:762–764. doi: 10.4315/0362-028X.JFP-11-410. [DOI] [PubMed] [Google Scholar]
- 188. Igbinosa EO, Okoh AI. 2010. Vibrio fluvialis: an unusual enteric pathogen of increasing public health concern. Int J Environ Res Public Health 7:3628–3643. doi: 10.3390/ijerph7103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chowdhury G, Pazhani GP, Dutta D, Guin S, Dutta S, Ghosh S, Izumiya H, Asakura M, Yamasaki S, Takeda Y, Arakawa E, Watanabe H, Mukhopadhyay AK, Bhattacharya MK, Rajendran K, Nair GB, Ramamurthy T. 2012. Vibrio fluvialis in patients with diarrhea, Kolkata, India. Emerg Infect Dis 18:1868–1871. doi: 10.3201/eid1811.120520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 191. Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Farina C, Marini F, Schiaffino E, Luzzi I, Dionisi AM, Leoni F, Ottaviani D, Bordoni S. 2010. A fatal Vibrio cholerae O37 enteritis. J Med Microbiol 59:1538–1540. doi: 10.1099/jmm.0.023093-0. [DOI] [PubMed] [Google Scholar]
- 193. Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J Infect Dis 181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 194. Tan KK, Sin KS, Ng AJ, Yahya H, Kaur P. 1994. Non-O1 Vibrio cholerae septicaemia: a case report. Singapore Med J 35:648–649. [PubMed] [Google Scholar]
- 195. Gras-Rouzet S, Donnio PY, Juguet F, Plessis P, Minet J, Avril JL. 1996. First European case of gastroenteritis and bacteremia due to Vibrio hollisae. Eur J Clin Microbiol Infect Dis 15:864–866. doi: 10.1007/BF01691217. [DOI] [PubMed] [Google Scholar]
- 196. Lai C-H, Hwang C-K, Chin C, Lin H-H, Wong W-W, Liu C-Y. 2006. Severe watery diarrhoea and bacteraemia caused by Vibrio fluvialis. J Infect 52:95–98. doi: 10.1016/j.jinf.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 197. Tusevljak N, Rajic A, Waddell L, Dutil L, Cernicchiaro N, Greig J, Wilhelm BJ, Wilkins W, Totton S, Uhland FC, Avery B, McEwen SA. 2012. Prevalence of zoonotic bacteria in wild and farmed aquatic species and seafood: a scoping study, systematic review, and meta-analysis of published research. Foodborne Pathog Dis 9:487–497. doi: 10.1089/fpd.2011.1063. [DOI] [PubMed] [Google Scholar]
- 198. Neubauer H, Aleksic S, Hensel A, Finke EJ, Meyer H. 2000. Yersinia enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups. Int J Med Microbiol 290:61–64. doi: 10.1016/S1438-4221(00)80107-1. [DOI] [PubMed] [Google Scholar]
- 199. Batzilla J, Antonenka U, Hoper D, Heesemann J, Rakin A. 2011. Yersinia enterocolitica palearctica serobiotype O:3/4—a successful group of emerging zoonotic pathogens. BMC Genomics 12:348. doi: 10.1186/1471-2164-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sulakvelidze A. 2000. Yersiniae other than Y. enterocolitica, Y pseudotuberculosis, and Y pestis: the ignored species. Microbes Infect 2:497–513. doi: 10.1016/S1286-4579(00)00311-7. [DOI] [PubMed] [Google Scholar]
- 201. Drummond N, Murphy BP, Ringwood T, Prentice MB, Buckley JF, Fanning S. 2012. Yersinia enterocolitica: a brief review of the issues relating to the zoonotic pathogen, public health challenges, and the pork production chain. Foodborne Pathog Dis 9:179–189. doi: 10.1089/fpd.2011.0938. [DOI] [PubMed] [Google Scholar]
- 202. Zheng H, Sun Y, Lin S, Mao Z, Jiang B. 2008. Yersinia enterocolitica infection in diarrheal patients. Eur J Clin Microbiol Infect Dis 27:741–752. doi: 10.1007/s10096-008-0562-y. [DOI] [PubMed] [Google Scholar]
- 203. Ong KL, Gould LH, Chen DL, Jones TF, Scheftel J, Webb TH, Mody RK, Mahon BE. 2012. Changing epidemiology of Yersinia enterocolitica infections: markedly decreased rates in young black children, Foodborne Diseases Active Surveillance Network (FoodNet), 1996-2009. Clin Infect Dis 54(Suppl 5):S385–S390. doi: 10.1093/cid/cis053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Fredriksson-Ahomaa M, Cernela N, Hächler H, Stephan R. 2012. Yersinia enterocolitica strains associated with human infections in Switzerland 2001-2010. Eur J Clin Microbiol Infect Dis 31:1543–1550. doi: 10.1007/s10096-011-1476-7. [DOI] [PubMed] [Google Scholar]
- 205. Rosner BM, Werber D, Hohle M, Stark K. 2013. Clinical aspects and self-reported symptoms of sequelae of Yersinia enterocolitica infections in a population-based study, Germany 2009-2010. BMC Infect Dis 13:236. doi: 10.1186/1471-2334-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Simonet ML. 1999. Enterobacteria in reactive arthritis: Yersinia, Shigella, and Salmonella. Rev Rhum Engl ed 66:14S–18S. (Discussion, 19S.) [PubMed] [Google Scholar]
- 207. Janda JM, Abbott SL. 2011. Revisiting bacterial gastroenteritis, part I. Issues, possible approaches, and an ever-expanding list of etiologic agents. Clin Microbiol Newsl 33:71–76. doi: 10.1016/j.clinmicnews.2011.04.002. [DOI] [Google Scholar]
- 208. Holton J. 2008. Enterotoxigenic Bacteroides fragilis. Curr Infect Dis Rep 10:99–9104. doi: 10.1007/s11908-008-0018-7. [DOI] [PubMed] [Google Scholar]
- 209. Wick EC, Sears CL. 2010. Bacteroides spp. and diarrhea. Curr Opin Infect Dis 23:470–474. doi: 10.1097/QCO.0b013e32833da1eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sears CL, Islam S, Saha A, Arjumand M, Alam NH, Faruque ASG, Salam MA, Shin J, Hecht D, Weintraub A, Sack RB, Qadri F. 2008. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin Infect Dis 47:797–803. doi: 10.1086/591130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sears CL. 2009. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev 22:349–369. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Ramamurthy D, Pazhani GP, Sarkar A, Nandy RK, Rajendran K, Sur D, Manna B, Ramamurthy T. 2013. Case-control study on the role of enterotoxigenic Bacteroides fragilis as a cause of diarrhea among children in Kolkata, India. PLoS One 8:e60622. doi: 10.1371/journal.pone.0060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Janda JM, Abbott SL. 1993. Infections associated with the genus Edwardsiella: the role of Edwardsiella tarda in human disease. Clin Infect Dis 17:742–748. doi: 10.1093/clinids/17.4.742. [DOI] [PubMed] [Google Scholar]
- 214. Nimmervoll H, Wenker C, Robert N, Albini S. 2011. Septicaemia caused by Edwardsiella tarda and Plesiomonas shigelloides in captive penguin chicks. Schweiz Arch Tierheilkd 153:117–121. doi: 10.1024/0036-7281/a000165. [DOI] [PubMed] [Google Scholar]
- 215. Clarridge JE, Musher DM, Fainstein V, Wallace RJ. 1980. Extraintestinal human infection caused by Edwardsiella tarda. J Clin Microbiol 11:511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Vandepitte J, Lemmens P, de Swert L. 1983. Human Edwardsiellosis traced to ornamental fish. J Clin Microbiol 17:165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Nagel P, Serritella A, Layden TJ. 1982. Edwardsiella tarda gastroenteritis associated with a pet turtle. Gastroenterology 82:1436–1437. [PubMed] [Google Scholar]
- 218. Marsh PK, Gorbach SL. 1982. Invasive enterocolitis caused by Edwardsiella tarda. Gastroenterology 82:336–338. [PubMed] [Google Scholar]
- 219. Spencer JD, Hastings MC, Rye AK, English BK, Ault BH. 2008. Gastroenteritis caused by Edwardsiella tarda in a pediatric renal transplant recipient. Pediatr Transplant 12:238–241. doi: 10.1111/j.1399-3046.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 220. Engel JJ, Martin TL. 2006. Edwardsiella tarda as a cause of postdysenteric ulcerative colitis. Int J Colorectal Dis 21:184–185. doi: 10.1007/s00384-004-0688-z. [DOI] [PubMed] [Google Scholar]
- 221. Tsuji A, Hirasawa K, Arakuma T, Izumi K, Kobori K, Sunohara K, Yoshizawa N, Watanabe H, Izumiya H. 2008. A 12-year-old boy with acute gastroenteritis caused by Edwardsiella tarda O4:H4. J Infect Chemother 14:433–435. doi: 10.1007/s10156-008-0638-8. [DOI] [PubMed] [Google Scholar]
- 222. Desenclos JC, Conti L, Junejo S, Klontz KC. 1990. A cluster of Edwardsiella tarda infection in a day-care center in Florida. J Infect Dis 162:782–782. doi: 10.1093/infdis/162.3.782. [DOI] [PubMed] [Google Scholar]
- 223. Bockemuhl J, Pan-Urai R, Burkhardt F. 1971. Edwardsiella tarda associated with human disease. Pathol Microbiol 37:393–401. [PubMed] [Google Scholar]
- 224. Huys G, Cnockaert M, Janda JM, Swings J. 2003. Escherichia albertii sp. nov., a diarrhoeagenic species isolated from stool specimens of Bangladeshi children. Int J Syst Evol Microbiol 53:807–810. doi: 10.1099/ijs.0.02475-0. [DOI] [PubMed] [Google Scholar]
- 225. Konno T, Yatsuyanagi J, Takahashi S, Kumagai Y, Wada E, Chiba M, Saito S. 2012. Isolation and identification of Escherichia albertii from a patient in an outbreak of gastroenteritis. Jpn J Infect Dis 65:203–207. doi: 10.7883/yoken.65.203. [DOI] [PubMed] [Google Scholar]
- 226. Stock I, Rahman M, Sherwood KJ, Wiedemann B. 2005. Natural antimicrobial susceptibility patterns and biochemical identification of Escherichia albertii and Hafnia alvei strains. Diagn Microbiol Infect Dis 51:151–163. doi: 10.1016/j.diagmicrobio.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 227. Hyma KE, Lacher DW, Nelson AM, Bumbaugh AC, Janda JM, Strockbine NA, Young VB, Whittam TS. 2005. Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J Bacteriol 187:619–628. doi: 10.1128/JB.187.2.619-628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ooka T, Seto K, Kawano K, Kobayashi H, Etoh Y, Ichihara S, Kaneko A, Isobe J, Yamaguchi K, Horikawa K, Gomes TAT, Linden A, Bardiau M, Mainil JG, Beutin L, Ogura Y, Hayashi T. 2012. Clinical significance of Escherichia albertii. Emerg Infect Dis 18:488–492. doi: 10.3201/eid1803.111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ooka T, Tokuoka E, Furukawa M, Nagamura T, Ogura Y, Arisawa K, Harada S, Hayashi T. 2013. Human gastroenteritis outbreak associated with Escherichia albertii, Japan. Emerg Infect Dis 19:144–146. doi: 10.3201/eid1901.120646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Abbott SL, O'Connor J, Robin T, Zimmer BL, Janda JM. 2003. Biochemical properties of a newly described Escherichia species, Escherichia albertii. J Clin Microbiol 41:4852–4854. doi: 10.1128/JCM.41.10.4852-4854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Beaugerie L, Metz M, Barbut F, Bellaiche G, Bouhnik Y, Raskine L, Nicolas J-C, Chatelet F-P, Lehn N, Petit J-C. 2003. Klebsiella oxytoca as an agent of antibiotic-associated hemorrhagic colitis. Clin Gastroenterol Hepatol 1:370–376. doi: 10.1053/S1542-3565(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 232. Hogenauer C, Langner C, Beubler E, Lippe IT, Schicho R, Gorkiewicz G, Krause R, Gerstgrasser N, Krejs GJ, Hinterleitner TA. 2006. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 355:2418–2426. doi: 10.1056/NEJMoa054765. [DOI] [PubMed] [Google Scholar]
- 233. Minami J, Okabe A, Shiode J, Hayashi H. 1989. Production of a unique cytotoxin by Klebsiella oxytoca. Microb Pathog 7:203–211. doi: 10.1016/0882-4010(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 234. Joainig MM, Gorkiewicz G, Leitner E, Weberhofer P, Zollner-Schwetz I, Lippe I, Feierl G, Krause R, Hinterleitner T, Zechner EL, Hogenauer C. 2010. Cytotoxic effects of Klebsiella oxytoca strains isolated from patients with antibiotic-associated hemorrhagic colitis or other diseases caused by infections and from healthy subjects. J Clin Microbiol 48:817–824. doi: 10.1128/JCM.01741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Cheng VCC, Yam W-C, Tsang L-L, Yau MCY, Siu GKH, Wong SCY, Chan JFW, To KKW, Tse H, Hung IFN, Tai JWM, Ho P-L, Yuen K-Y. 2012. Epidemiology of Klebsiella oxytoca-associated diarrhea detected by Simmons citrate agar supplemented with inositol, tryptophan, and bile salts. J Clin Microbiol 50:1571–1579. doi: 10.1128/JCM.00163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Zollner-Schwetz I, Högenauer C, Joainig M, Weberhofer P, Gorkiewicz G, Valentin T, Hinterleitner TA, Krause R. 2008. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin Infect Dis 47:74–78. doi: 10.1086/592074. [DOI] [PubMed] [Google Scholar]
- 237. Haynes J, Hawkey PM. 1989. Providencia alcalifaciens and travellers' diarrhoea. Bmj 299:94–95. doi: 10.1136/bmj.299.6691.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Murata T, Iida T, Shiomi Y, Tagomori K, Akeda Y, Yanagihara I, Mushiake S, Ishiguro F, Honda T. 2001. A large outbreak of foodborne infection attributed to Providencia alcalifaciens. J Infect Dis 184:1050–1055. doi: 10.1086/323458. [DOI] [PubMed] [Google Scholar]
- 239. Albert MJ, Alam K, Ansaruzzaman M, Islam MM, Rahman AS, Haider K, Bhuiyan NA, Nahar S, Ryan N, Montanaro J, et al. 1992. Pathogenesis of Providencia alcalifaciens-induced diarrhea. Infect Immun 60:5017–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Guth BE, Irino K, Trabulsi LR. 1999. Clonal structure of Providencia alcalifaciens strains isolated from diarrhoeal stools in Sao Paulo, Brazil. J Med Microbiol 48:205–209. doi: 10.1099/00222615-48-2-205. [DOI] [PubMed] [Google Scholar]
- 241. Janda JM, Abbott SL, Woodward D, Khashe S. 1998. Invasion of HEp-2 and other eukaryotic cell lines by Providenciae: further evidence supporting the role of Providencia alcalifaciens in bacterial gastroenteritis. Curr Microbiol 37:159–165. doi: 10.1007/s002849900357. [DOI] [PubMed] [Google Scholar]
- 242. Shima A, Hinenoya A, Asakura M, Sugimoto N, Tsukamoto T, Ito H, Nagita A, Faruque SM, Yamasaki S. 2012. Molecular characterizations of cytolethal distending toxin produced by Providencia alcalifaciens strains isolated from patients with diarrhea. Infect Immun 80:1323–1332. doi: 10.1128/IAI.05831-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Yoh M, Matsuyama J, Ohnishi M, Takagi K, Miyagi H, Mori K, Park K-S, Ono T, Honda T. 2005. Importance of Providencia species as a major cause of travellers' diarrhoea. J Med Microbiol 54:1077–1082. doi: 10.1099/jmm.0.45846-0. [DOI] [PubMed] [Google Scholar]
- 244. Senior BW. 1997. Media for the detection and recognition of the enteropathogen Providencia alcalifaciens in faeces. J Med Microbiol 46:524–527. doi: 10.1099/00222615-46-6-524. [DOI] [PubMed] [Google Scholar]
- 245. Linscott AJ. 2010. Specimen collection, transport and acceptability, p 2.0.1–2.1.26. In Garcia L. (ed), Clinical microbiology procedures handbook, 3rd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 246. Gilligan PH, Janda JM, Karmali MA, Miller JM. 1992. Cumitech 12A, Laboratory diagnosis of bacterial diarrhea. Coordinating ed, Nolte FS. American Society for Microbiology, Washington, DC. [Google Scholar]
- 247. Stuart RD. 1959. Transport medium for specimens in public health bacteriology. Public Health Rep 74:431–438. doi: 10.2307/4590473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Adkins HJ, Santiago LT. 1987. Increased recovery of enteric pathogens by use of both stool and rectal swab specimens. J Clin Microbiol 25:158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Hardy AV, Mackel D, Frazier D, Hamerick D. 1953. The relative efficacy of cultures for shigella. US Armed Forces Med J 4:393–394. [PubMed] [Google Scholar]
- 250. Gasem MH, Dolmans WM, Isbandrio BB, Wahyono H, Keuter M, Djokomoeljanto R. 1995. Culture of Salmonella typhi and Salmonella paratyphi from blood and bone marrow in suspected typhoid fever. Trop Geograp Med 47:164–167. [PubMed] [Google Scholar]
- 251. Bowman RA, Bowman JM, Arrow SA, Riley TV. 1992. Selective criteria for the microbiological examination of faecal specimens. J Clin Pathol 45:838–839. doi: 10.1136/jcp.45.9.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Fan K, Morris AJ, Reller LB. 1993. Application of rejection criteria for stool cultures for bacterial enteric pathogens. J Clin Microbiol 31:2233–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Siegel DL, Edelstein PH, Nachamkin I. 1990. Inappropriate testing for diarrheal diseases in the hospital. JAMA 263:979–982. doi: 10.1001/jama.1990.03440070067034. [DOI] [PubMed] [Google Scholar]
- 254. Yannelli B, Gurevich I, Schoch PE, Cunha BA. 1988. Yield of stool cultures, ova and parasite tests, and Clostridium difficile determinations in nosocomial diarrheas. Am J Infect Control 16:246–249. doi: 10.1016/S0196-6553(88)80003-8. [DOI] [PubMed] [Google Scholar]
- 255. Seyler L, Lalvani A, Collins L, Goddard L, Bowler IC. 2007. Safety and cost savings of an improved three-day rule for stool culture in hospitalised children and adults. J Hosp Infect 67:121–126. doi: 10.1016/j.jhin.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 256. Zaidi AK, Macone A, Goldmann AD. 1999. Impact of simple screening criteria on utilization of low-yield bacterial stool cultures in a Children's Hospital. Pediatrics 103:1189–1192. doi: 10.1542/peds.103.6.1189. [DOI] [PubMed] [Google Scholar]
- 257. Church DL, Cadrain G, Kabani A, Jadavji T, Trevenen C. 1995. Practice guidelines for ordering stool cultures in a pediatric population. Alberta Children's Hospital, Calgary, Alberta, Canada. Am J Clin Pathol 103:149–153. [DOI] [PubMed] [Google Scholar]
- 258. Aichinger E, Schleck CD, Harmsen WS, Nyre LM, Patel R. 2008. Nonutility of repeat laboratory testing for detection of Clostridium difficile by use of PCR or enzyme immunoassay. J Clin Microbiol 46:3795–3797. doi: 10.1128/JCM.00684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Deshpande A, Pasupuleti V, Patel P, Ajani G, Hall G, Hu B, Jain A, Rolston DDK. 2011. Repeat stool testing to diagnose Clostridium difficile infection using enzyme immunoassay does not increase diagnostic yield. Clin Gastroenterol Hepatol 9:665–669. doi: 10.1016/j.cgh.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 260. Khanna S, Pardi DS, Rosenblatt JE, Patel R, Kammer PP, Baddour LM. 2012. An evaluation of repeat stool testing for Clostridium difficile infection by polymerase chain reaction. J Clin Gastroenterol 46:846–849. doi: 10.1097/MCG.0b013e3182432273. [DOI] [PubMed] [Google Scholar]
- 261. Wang WL, Reller LB, Smallwood B, Luechtefeld NW, Blaser MJ. 1983. Evaluation of transport media for Campylobacter jejuni in human fecal specimens. J Clin Microbiol 18:803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. York M, Rodriguez-Wong P, Church DL. 2010. Fecal culture for aerobic pathogens of gastroenteritis, p 3.5.1.1–3.5.1.22. In Garcia L. (ed), Clinical microbiology procedures handbook, 3rd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 263. Baron EJ, Thomson R. 2010. Specimen collection, transport, and processing: bacteriology, p 228–271. In Versalovic J. (ed), Manual of clinical microbiology. ASM Press, Washington, DC. [Google Scholar]
- 264. Wells JG, Morris GK. 1981. Evaluation of transport methods for isolating Shigella spp. J Clin Microbiol 13:789–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Nataro JP, Bopp CA, Fields PI, Kaper JB, Stockbine NA. 2010. Escherichia, Shigella and Salmonella, p 603–625. In Versalovic J. (ed), Manual of clinical microbiology. ASM Press, Washington, DC. [Google Scholar]
- 266. Cary SG, Blair EB. 1964. New transport medium for shipment of clinical specimens. I. Fecal specimens. J Bacteriol 88:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Maddocks S, Olma T, Chen S. 2002. Comparison of CHROMagar Salmonella medium and xylose-lysine-desoxycholate and Salmonella-Shigella agars for isolation of Salmonella strains from stool samples. J Clin Microbiol 40:2999–3003. doi: 10.1128/JCM.40.8.2999-3003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Church DL, Emshey D, Lloyd T, Pitout J. 2010. Clinical and economic evaluation of BBL CHROMagar Salmonella (CHROMSal) versus subculture after selenite broth enrichment to CHROMSal and Hektoen enteric agars to detect enteric Salmonella in a large regional microbiology laboratory. Diagn Microbiol Infect Dis 68:13–19. doi: 10.1016/j.diagmicrobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 269. Church DL, Emshey D, Semeniuk H, Lloyd T, Pitout JD. 2007. Evaluation of BBL CHROMagar O157 versus sorbitol-MacConkey medium for routine detection of Escherichia coli O157 in a centralized regional clinical microbiology laboratory. J Clin Microbiol 45:3098–3100. doi: 10.1128/JCM.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Eddabra R, Piemont Y, Scheftel JM. 2011. Evaluation of a new chromogenic medium, chromID Vibrio, for the isolation and presumptive identification of Vibrio cholerae and Vibrio parahaemolyticus from human clinical specimens. Eur J Clin Microbiol Infect Dis 30:733–737. doi: 10.1007/s10096-010-1145-2. [DOI] [PubMed] [Google Scholar]
- 271. Renaud N, Lecci L, Courcol RJ, Simonet M, Gaillot O. 2013. CHROMagar Yersinia, a new chromogenic agar for screening of potentially pathogenic Yersinia enterocolitica isolates in stools. J Clin Microbiol 51:1184–1187. doi: 10.1128/JCM.02903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Gouali M, Ruckly C, Carle I, Lejay-Collin M, Weill F-X. 2013. Evaluation of CHROMagar STEC and STEC O104 chromogenic agar media for detection of Shiga toxin-producing Escherichia coli in stool specimens. J Clin Microbiol 51:894–900. doi: 10.1128/JCM.03121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Hirvonen JJ, Siitonen A, Kaukoranta S-S. 2012. Usability and performance of CHROMagar STEC medium in detection of Shiga toxin-producing Escherichia coli strains. J Clin Microbiol 50:3586–3590. doi: 10.1128/JCM.01754-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Lue YA. 1986. Is enrichment broth necessary for stool cultures? Clin Microbiol Newsl 8:5–6. [Google Scholar]
- 275. Lesmana M, Richie E, Subekti D, Simanjuntak C, Walz SE. 1997. Comparison of direct-plating and enrichment methods for isolation of Vibrio cholerae from diarrhea patients. J Clin Microbiol 35:1856–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. He Y, Li H, Lu X, Stratton CW, Tang YW. 2010. Mass spectrometry biotyper system identifies enteric bacterial pathogens directly from colonies grown on selective stool culture media. J Clin Microbiol 48:3888–3892. doi: 10.1128/JCM.01290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Stager CE, Erikson E, Davis JR. 1983. Rapid method for detection, identification, and susceptibility testing of enteric pathogens. J Clin Microbiol 17:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Ederer GM, Lund ME, Blazevic DJ, Reller LB, Mirrett S. 1975. Motility-indole-lysine-sulfide medium. J Clin Microbiol 2:266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Procop GW, Wallace JD, Tuohy MJ, Lasalvia MM, Addison RM, Reller LB. 2008. A single-tube screen for Salmonella and Shigella. Am J Clin Pathol 130:284–289. doi: 10.1309/MTDBAXHDPKAL6GF5. [DOI] [PubMed] [Google Scholar]
- 280. Reller LB, Mirrett S. 1975. Motility-indole-lysine medium for presumptive identification of enteric pathogens of Enterobacteriaceae. J Clin Microbiol 2:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. O'Hara CM, Sowers EG, Bopp CA, Duda SB, Strockbine NA. 2003. Accuracy of six commercially available systems for identification of members of the family Vibrionaceae. J Clin Microbiol 41:5654–5659. doi: 10.1128/JCM.41.12.5654-5659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Saini A, Kaur H, Purwar S, Kholkute SD, Roy S. 2012. Discrepancies in identification of Vibrio cholerae strains as members of Aeromonadaceae and Enterobacteriaceae by automated microbial identification system. Lett Appl Microbiol 55:22–26. doi: 10.1111/j.1472-765X.2012.03252.x. [DOI] [PubMed] [Google Scholar]
- 283. Miller RS, Speegle L, Oyarzabal OA, Lastovica AJ. 2008. Evaluation of three commercial latex agglutination tests for identification of Campylobacter spp. J Clin Microbiol 46:3546–3547. doi: 10.1128/JCM.01027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Anderson NW, Buchan BW, Riebe KM, Parsons LN, Gnacinski S, Ledeboer NA. 2012. Effects of solid-medium type on routine identification of bacterial isolates by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 50:1008–1013. doi: 10.1128/JCM.05209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Martiny D, Visscher A, Catry B, Chatellier S, Vandenberg O. 2013. Optimization of Campylobacter growth conditions for further identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). J Microbiol Methods 94:221–223. doi: 10.1016/j.mimet.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 286. Alispahic M, Hummel K, Jandreski-Cvetkovic D, Nobauer K, Razzazi-Fazeli E, Hess M, Hess C. 2010. Species-specific identification and differentiation of Arcobacter, Helicobacter and Campylobacter by full-spectral matrix-associated laser desorption/ionization time of flight mass spectrometry analysis. J Med Microbiol 59:295–301. doi: 10.1099/jmm.0.016576-0. [DOI] [PubMed] [Google Scholar]
- 287. Bessede E, Solecki O, Sifre E, Labadi L, Megraud F. 2011. Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin Microbiol Infect 17:1735–1739. doi: 10.1111/j.1469-0691.2011.03468.x. [DOI] [PubMed] [Google Scholar]
- 288. Martiny D, Dediste A, Debruyne L, Vlaes L, Haddou NB, Vandamme P, Vandenberg O. 2011. Accuracy of the API Campy system, the Vitek 2 Neisseria-Haemophilus card and matrix-assisted laser desorption ionization time-of-flight mass spectrometry for the identification of Campylobacter and related organisms. Clin Microbiol Infect 17:1001–1006. doi: 10.1111/j.1469-0691.2010.03328.x. [DOI] [PubMed] [Google Scholar]
- 289. Fagerquist CK, Garbus BR, Miller WG, Williams KE, Yee E, Bates AH, Boyle S, Harden LA, Cooley MB, Mandrell RE. 2010. Rapid identification of protein biomarkers of Escherichia coli O157:H7 by matrix-assisted laser desorption ionization-time-of-flight-time-of-flight mass spectrometry and top-down proteomics. Anal Chem 82:2717–2725. doi: 10.1021/ac902455d. [DOI] [PubMed] [Google Scholar]
- 290. Clark CG, Kruczkiewicz P, Guan C, McCorrister SJ, Chong P, Wylie J, van Caeseele P, Tabor HA, Snarr P, Gilmour MW, Taboada EN, Westmacott GR. 2013. Evaluation of MALDI-TOF mass spectroscopy methods for determination of Escherichia coli pathotypes. J Microbiol Methods 94:180–191. doi: 10.1016/j.mimet.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 291. Dieckmann R, Malorny B. 2011. Rapid screening of epidemiologically important Salmonella enterica subsp. enterica serovars by whole-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol 77:4136–4146. doi: 10.1128/AEM.02418-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Khot PD, Fisher MA. 2013. Novel approach for differentiating Shigella species and Escherichia coli by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51:3711–3716. doi: 10.1128/JCM.01526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. CLSI. 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline, 2nd ed CLSI document M45-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 294. CLSI. 2014. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement, M100 S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 295. CDC. 2013. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): human isolates, final report, 2011. CDC, Atlanta, GA. [Google Scholar]
- 296. Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis 7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Planche TD, Davies KA, Coen PG, Finney JM, Monahan IM, Morris KA, O'Connor L, Oakley SJ, Pope CF, Wren MW, Shetty NP, Crook DW, Wilcox MH. 2013. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis 13:936–945. doi: 10.1016/S1473-3099(13)70200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Humphries RM, Uslan DZ, Rubin Z. 2013. Performance of Clostridium difficile toxin enzyme immunoassay and Nucleic acid amplification tests stratified by patient disease severity. J Clin Microbiol 51:869–873. doi: 10.1128/JCM.02970-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Gerding DN, Olson MM, Peterson LR, Teasley DG, Gebhard RL, Schwartz ML, Lee JT Jr. 1986. Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch Intern Med 146:95–100. [PubMed] [Google Scholar]
- 300. Lashner BA, Todorczuk J, Sahm DF, Hanauer SB. 1986. Clostridium difficile culture-positive toxin-negative diarrhea. Am J Gastroenterol 81:940–943. [PubMed] [Google Scholar]
- 301. Culbreath K, Ager E, Nemeyer RJ, Kerr A, Gilligan PH. 2012. Evolution of testing algorithms at a university hospital for detection of Clostridium difficile infections. J Clin Microbiol 50:3073–3076. doi: 10.1128/JCM.00992-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Kvach EJ, Ferguson D, Riska PF, Landry ML. 2010. Comparison of BD GeneOhm Cdiff real-time PCR assay with a two-step algorithm and a toxin A/B enzyme-linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection. J Clin Microbiol 48:109–114. doi: 10.1128/JCM.01630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Larson AM, Fung AM, Fang FC. 2010. Evaluation of tcdB real-time PCR in a three-step diagnostic algorithm for detection of toxigenic Clostridium difficile. J Clin Microbiol 48:124–130. doi: 10.1128/JCM.00734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Ota KV, McGowan KL. 2012. Clostridium difficile testing algorithms using glutamate dehydrogenase antigen and C. difficile toxin enzyme immunoassays with C. difficile nucleic acid amplification testing increase diagnostic yield in a tertiary pediatric population. J Clin Microbiol 50:1185–1188. doi: 10.1128/JCM.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Sharp SE, Ruden LO, Pohl JC, Hatcher PA, Jayne LM, Ivie WM. 2010. Evaluation of the C. Diff Quik Chek Complete Assay, a new glutamate dehydrogenase and A/B toxin combination lateral flow assay for use in rapid, simple diagnosis of Clostridium difficile disease. J Clin Microbiol 48:2082–2086. doi: 10.1128/JCM.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Novak-Weekley SM, Marlowe EM, Miller JM, Cumpio J, Nomura JH, Vance PH, Weissfeld A. 2010. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol 48:889–893. doi: 10.1128/JCM.01801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Hermos CR, Janineh M, Han LL, McAdam AJ. 2011. Shiga toxin-producing Escherichia coli in children: diagnosis and clinical manifestations of O157:H7 and non-O157:H7 infection. J Clin Microbiol 49:955–959. doi: 10.1128/JCM.02119-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Vallieres E, Saint-Jean M, Rallu F. 2013. Comparison of three different methods for detection of Shiga toxin-producing Escherichia coli in a tertiary pediatric care center. J Clin Microbiol 51:481–486. doi: 10.1128/JCM.02219-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Granato PA, Chen L, Holiday I, Rawling RA, Novak-Weekley SM, Quinlan T, Musser KA. 2010. Comparison of premier CAMPY enzyme immunoassay (EIA), ProSpecT Campylobacter EIA, and ImmunoCard STAT! CAMPY tests with culture for laboratory diagnosis of Campylobacter enteric infections J Clin Microbiol 48:4022–4027. doi: 10.1128/JCM.00486-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Giltner CL, Saeki S, Bobenchik AM, Humphries RM. 2013. Rapid detection of Campylobacter antigen by enzyme immunoassay leads to increased positivity rates. J Clin Microbiol 51:618–620. doi: 10.1128/JCM.02565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Dediste A, Vandenberg O, Vlaes L, Ebraert A, Douat N, Bahwere P, Butzler JP. 2003. Evaluation of the ProSpecT Microplate Assay for detection of Campylobacter: a routine laboratory perspective. Clin Microbiol Infect 9:1085–1090. doi: 10.1046/j.1469-0691.2003.00705.x. [DOI] [PubMed] [Google Scholar]
- 312. Tolcin R, LaSalvia MM, Kirkley BA, Vetter EA, Cockerill FR III, Procop GW. 2000. Evaluation of the Alexon-trend ProSpecT Campylobacter microplate assay. J Clin Microbiol 38:3853–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Myers AL, Jackson MA, Selvarangan R. 2011. False-positive results of Campylobacter rapid antigen testing. Pediatr Infect Dis J 30:542. doi: 10.1097/INF.0b013e31821524db. [DOI] [PubMed] [Google Scholar]
- 314. de Boer RF, Ott A, Kesztyus B, Kooistra-Smid AM. 2010. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. J Clin Microbiol 48:4140–4146. doi: 10.1128/JCM.01124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Cunningham SA, Sloan LM, Nyre LM, Vetter EA, Mandrekar J, Patel R. 2010. Three-hour molecular detection of Campylobacter, Salmonella, Yersinia, and Shigella species in feces with accuracy as high as that of culture. J Clin Microbiol 48:2929–2933. doi: 10.1128/JCM.00339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Buchan BW, Olson WJ, Pezewski M, Marcon MJ, Novicki T, Uphoff TS, Chandramohan L, Revell P, Ledeboer NA. 2013. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and shiga toxin-producing Escherichia coli isolates in stool specimens. J Clin Microbiol 51:4001–4007. doi: 10.1128/JCM.02056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Wessels E, Rusman LG, van Bussel MJ, Claas EC. 2014. Added value of multiplex Luminex Gastrointestinal Pathogen Panel (xTAG((R)) GPP) testing in the diagnosis of infectious gastroenteritis. Clin Microbiol Infect 20:O182–O187. doi: 10.1111/1469-0691.12364. [DOI] [PubMed] [Google Scholar]
- 318. Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI. 2005. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115:e673–680. doi: 10.1542/peds.2004-2236. [DOI] [PubMed] [Google Scholar]
- 319. Hines J, Nachamkin I. 1996. Effective use of the clinical microbiology laboratory for diagnosing diarrheal diseases. Clin Infect Dis 23:1292–1301. [DOI] [PubMed] [Google Scholar]
- 320. Jones TF, Gerner-Smidt P. 2012. Nonculture diagnostic tests for enteric diseases. Emerg Infect Dis 18:513–514. doi: 10.3201/eid1803.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Steiner TS, Guerrant RL. 2010. Principles and syndromes of enteric infection, p 1335–1351. In Mandell GL, Bennett JE, Dolin R (ed), Mandell, Douglas and Bennett's principles and practices of infectious diseases, vol 1 Churchill Livingstone Elsevier, Philadelphia, PA. [Google Scholar]
- 322. Benagli C, Demarta A, Caminada A, Ziegler D, Petrini O, Tonolla M. 2012. A rapid MALDI-TOF MS identification database at genospecies level for clinical and environmental Aeromonas strains. PLoS One 7:e48441. doi: 10.1371/journal.pone.0048441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Kuhns M, Zautner AE, Rabsch W, Zimmermann O, Weig M, Bader O, Gross U. 2012. Rapid discrimination of Salmonella enterica serovar Typhi from other serovars by MALDI-TOF mass spectrometry. PLoS One 7:e40004. doi: 10.1371/journal.pone.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]