SUMMARY
Despite significant improvements in leprosy (Hansen's disease) treatment and outlook for patients since the introduction of multidrug therapy (MDT) 3 decades ago, the global incidence remains high, and patients often have long-term complications associated with the disease. In this article, we discuss recent findings related to genetics, susceptibility, and disease reservoirs and the implications of these findings for Hansen's disease control and health outcomes for patients. We describe the continued difficulties associated with treatment of inflammatory episodes known as “leprosy reactions,” which cause much of the disability associated with the disease and can affect people for many years after MDT is complete. We also discuss some of the contemporary challenges for physicians and patients, including international and internal migration of people affected by the disease. We suggest some important areas of focus for future Hansen's disease research.
INTRODUCTION
Leprosy, or Hansen's disease (HD), is an ancient bacterial disease that, although curable, continues to be a significant health problem in many parts of the world. HD results from infection with the Mycobacterium leprae bacillus, which produces a chronic infection in humans that affects mainly peripheral nerves and skin but may also affect sites such as the eyes, mucous membranes, bones, and testes and produces a spectrum of clinical phenotypes (1–3). In the skin, M. leprae has an affinity for keratinocytes, macrophages, and histiocytes (3, 4). In peripheral nerves, M. leprae can be found in Schwann cells (2). Keratinocytes seem to play a key role in the release of the antimicrobial peptide β-defensin in response to M. leprae antigens (4). Once inside the host cell, M. leprae interacts with the host cell lipid metabolism to foster bacterial intracellular survival (5). The predilection for the Schwann cell initiates after its attachment to α2-laminin and adhesins located in the basal lamina and to α-dystroglycan and ErbB2 receptors on its cell surface (6). The entry of M. leprae bacilli into Schwann cells triggers cells to dedifferentiate into immature cells through the activation of signaling of the Erk1/2 pathway. This transformation creates a suitable environment for the bacteria to proliferate (7). More recently, it has been shown that further dedifferentiation leads to the reprogramming the Schwann cell to a “stem cell-like” cell with a plethora of new capabilities, such as redifferentiation into mesenchymal cells with the ability to spread infection or attracting macrophages to develop granulomas that could then serve as a Trojan horse for systemic dissemination of M. leprae (3, 8). The presence of bacilli in the skin produces the dermatological manifestations of the disease, and nerve infection produces axonal dysfunction and demyelination, leading to sensory loss and its consequences of disability and deformity (2, 9). In this sense, the degenerative changes associated with infection of the peripheral sensory nerves are considered a crucial event in the natural history of HD (7, 8). Once the infection is established, the occurrence of leprosy reactions, because of their inflammatory impact on peripheral nerves, remain an important contributor to sensory loss and dysfunction (9–12).
In the last few decades, particularly with the advent of multidrug therapy (MDT) and the use of anti-inflammatory therapies, there have been substantial improvements in long-term health outcomes for individuals diagnosed with HD. Although the worldwide prevalence of this disease has significantly decreased, HD is still a poorly understood illness, and often, the statistics do not capture the disability and dysfunction that remain after MDT is complete. A 1991 World Health Assembly resolution for HD “elimination” (reducing the prevalence to 1 case of HD per 10,000 people) by the year 2000 was achieved at the global level (13). However, in many regions where HD is endemic, this goal is still far from being met, and the incidence of the disease has not decreased significantly over the past decade. Among the millions of people who have been cured with multidrug therapy, there are a large number who still suffer from long-term complications of the disease, including temporary and permanent disability, deformity, and social stigma.
There were 219,075 new cases of HD reported in 2011, underscoring the persistent transmission of the disease despite an overall decrease in its prevalence (14). Under the current WHO-recommended treatment scheme, many people complete the treatment in less than a year, so the global prevalence of leprosy is often lower than the incidence; the total number of registered cases at the beginning of 2012 was 181,941 (14). India and Brazil account for the majority of these cases. In this article, after a brief discussion of the history of HD and its treatment, we touch on some of the contemporary challenges associated with HD treatment in nations where the disease is and is not endemic as well as some of the more recent findings that may have a positive impact on HD control and patient well-being in the future.
HD PAST AND PRESENT: A BRIEF SUMMARY
Skeletal and genetic evidence of the antiquity of HD provides some clues to understanding the disease in the present context. While there is only speculative evidence that the disease that we understand today to be a result of infection by M. leprae bacilli was among the skin afflictions described in ancient texts from the Old World, concrete skeletal evidence of the disease's existence in the past has been found, including a find dated to 4,000 years ago in India (15). Sequencing of the HD genome and discoveries of genetic material in human skeletal material from previous centuries also give us more information about the movement of the disease as well as about human migration. For example, Schuenemann et al. (16) compared genome sequences of M. leprae from skeletons dated to ∼1,000 years ago in Europe to contemporary strains, indicating that contemporary M. leprae strains share an ancestor dating back to <4,000 years ago.
In many (but not all) parts of the world, the deformities and disabilities caused by HD, as well as religious and social meanings associated with the disease or the physical changes that might result from it, have resulted in and continue to generate stigmatizing attitudes toward and negative beliefs about people affected by the disease. In 1943, Guy Faget, working at Carville Hospital in Louisiana, demonstrated that sulfone drugs were effective in killing M. leprae bacilli (17), but confinement policies in many countries were in place for many years after this. In Japan, mandatory confinement continued until the mid-1990s. As noted by Sato and Narita, the scientific understanding of HD as a disease with low transmissibility did not always dictate policy decisions, which were more influenced by societal stigma coupled with dilemmas associated with providing services for reintegration of those people who had been isolated (18).
In 1982, multidrug therapy was introduced, which remedied many of the problems associated with monotherapy (17); previously, most patients had to take daily doses of sulfone monotherapy for life, and if there was an interruption in taking the medication, drug resistance could develop. Many patients worldwide complete MDT, which consists of a combination of the medications rifampin, clofazimine, and dapsone, in a year or less. Although patients are considered “cured” after the completion of MDT, many experience complications after MDT is completed, including a lifelong stigma associated with having had the disease, leprosy reactions, permanent disability, and occasional relapse/reinfection. Complications associated with leprosy reactions are significant causes of disability and create particular challenges for patients and physicians over the long term (2).
THE HD BACILLUS AND ITS IMPACT
HD results from infection with Mycobacterium leprae, an intracellular acid-fast bacillus. Most people (an estimated 95% of the world's population) are not genetically susceptible to the disease (11), but there seems to be variation among population groups that may be related to both genetic factors and ancestral exposure to the bacillus. The traditional model of interaction of an infectious agent that includes host, pathogen, and environment is a unique one in the case of HD. Overall, there seems to be little pathogen variability and virulence to explain the different clinical forms, with the possible exception of the recently discovered species Mycobacterium lepromatosis in patients with HD who had diffuse leprosy of Lucio and Latapí (3, 19, 20). Confirmation of this as a new species that could cause HD requires further research, as defined by Gillis et al. (21). However, most of the clinical phenotypes may be due to genetic variability determined by different biological pathways modulated by M. leprae and reprogramming of adult Schwann cells and interactions of innate and adaptive immunity (3, 8).
Some population groups exhibit higher prevalence rates of HD than others. For example, among populations of the Western Pacific, particularly Micronesia, the prevalence (number of cases per 10,000 people) is among the highest in the world (22). This could have to do with greater genetic susceptibility, but it could also be related to the recent introduction of HD to this part of the world. Among susceptible individuals, there is great variety in terms of the individual immune response to the bacilli in the body, such that physicians who work with the disease for many years see the value in looking at each person's disease as unique.
The classification of leprosy should be determined by clinical prognosis and to distinguish which cases may be potentially infectious (1, 23, 24). The World Health Organization suggests a simple scheme for distinguishing different types of HD, which is used as the basis of the current treatment model; in this model, HD is classified based on visible symptoms and (ideally) the presence or absence of bacilli in slit-skin smears from cooler regions of the body (generally from earlobes, elbows, and/or knees) where bacilli proliferate: those patients with just 1 to 5 diagnostic skin patches and no apparent bacilli in slit-skin smears are classified as having “paucibacillary” disease, and those with >5 skin patches and bacilli visible by microscopic analyses of skin smears are classified as having “multibacillary” disease. In areas without access to slit-skin smears, the criterion for diagnosis is the number of visible lesions. Gupta et al. (25) found that this fairly arbitrary model based on the number of lesions that are identifiable can result in both over- and underdiagnosis of HD; they suggested adding additional criteria that take into account the size of the lesions and accompanying nerve enlargement. Prasad and Kaviarisan (26) note that the WHO classification system contains no treatment protocol for cases of neuritic HD in which no skin lesions or changes are present. A more nuanced system of classification recognizes a spectrum of cell-mediated responses to the disease, with five categories of the disease (23, 24); these include, from the least to the most severe (or the greatest to the least immune response), tuberculoid (TT) (Fig. 1), borderline tuberculoid (BT) (Fig. 2), borderline borderline (BB) (Fig. 3), borderline lepromatous (BL) (Fig. 4), and lepromatous (LL) (Fig. 5) (23, 24). The lepromatous leprosy (LL) group was redefined to include the subpolar and polar components of the original description (24). In the United States, the National Hansen's Disease Program uses this system (Ridley-Jopling classification system) (23, 24). This classification correlates with the immune response to M. leprae infection and thus can be used to identify patients who are considered “immunologically unstable” and at risk of developing leprosy reactions. By using this framework, those patients with borderline forms of the disease can suffer either upgrading or downgrading reactions (1). Upgrading reactions are associated with increased cell-mediated immune responses (which tend to occur when patients are receiving MDT), while downgrading reactions take place when patients develop lesions in the lepromatous pole of the spectrum after beginning with borderline forms (usually prior to initiation of MDT) (1, 8, 24).
FIG 1.
A 23-year-old female with the tuberculoid leprosy form, manifesting as a single well-defined hypopigmented macular lesion associated with anesthesia.
FIG 2.
A 42-year-old male with borderline tuberculoid leprosy, manifesting as multiple (>5) polymorphic, partially raised, confluent, hypopigmented macules associated with anesthesia. The patient also had irregular enlargement of several large nerves in an asymmetrical pattern.
FIG 3.
A 53-year-old male with the borderline borderline form of leprosy, manifesting as multiple infiltrated plaques with punched-out centers associated with anesthesia. The patient had many nerves involved in a symmetrical pattern.
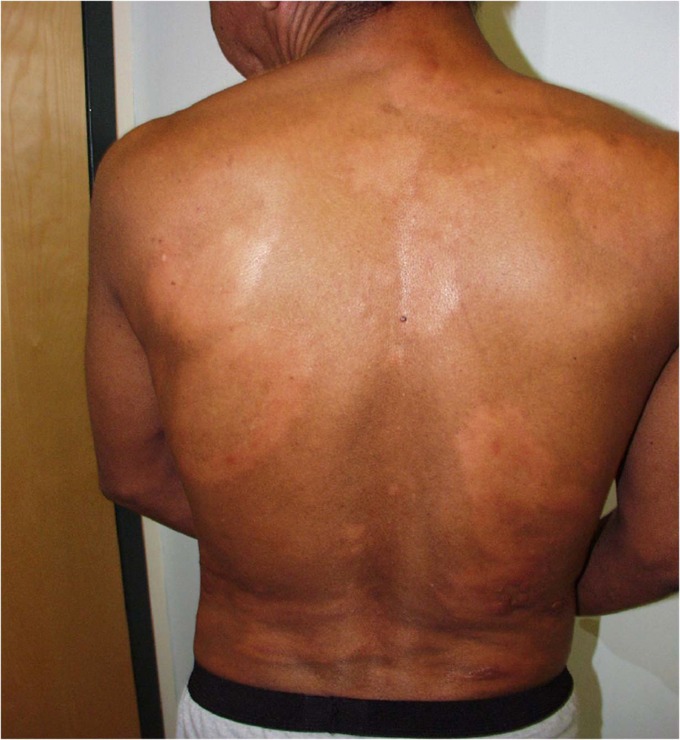
FIG 4.
A 29-year-old male with the borderline lepromatous form of leprosy, presenting with diffuse thickening of the skin with associated anesthesia and paresis.
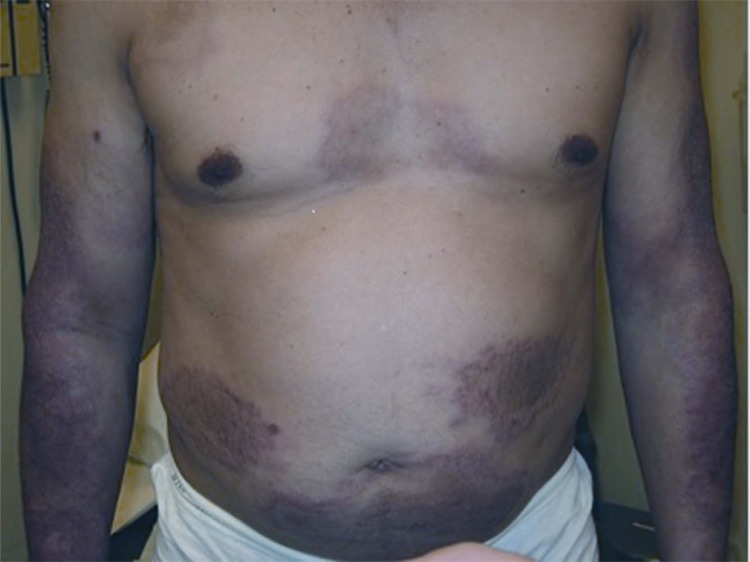
FIG 5.
A 17-year-old male with the lepromatous leprosy form of leprosy, manifesting as diffuse thickening with innumerable discrete as well as confluent nodules.
HD in its early stages is mild, often manifesting as a reaction that has prompted the patient to seek medical attention. These symptoms often do not manifest for many years after transmission because of an often extended incubation period. Although there are reported cases of infants and young children with HD (27, 28), suggesting a brief period between transmission and manifestation of symptoms in these cases, the World Health Organization states that the incubation period is generally close to 5 years, although it can be up to 20 years (29). The lack of severity of HD when it begins to appear on the body can result in delays in patients seeking diagnosis as well as delays in receiving a correct diagnosis from a health care professional: HD is often mistaken for other conditions that manifest on the skin (such as allergic reactions, autoimmune diseases such as midline granuloma, fungal infections, vitiligo, other mycobacterial infections, mucocutaneous leishmaniasis, and syphilis) or for conditions with nerve involvement (such as diabetes and rheumatoid arthritis) (29, 30). Diagnosis is particularly problematic for health care professionals in countries where HD is not endemic (31). Depending on the individual immune response, HD can remain a mild disease with no apparent physical changes and even (rarely) spontaneous cure, or, at the other end of the spectrum of immunity, it can progress so that the proliferation of M. leprae bacilli in the body causes extensive peripheral nerve damage, which in turn can result in changes in physical appearance and physical mobility.
Peripheral nerve sensorimotor dysfunction in patients with HD is a result of primary infection with M. leprae and is frequently exacerbated by episodes of leprosy reactions (9, 10). Reactions affect 30 to 50% of patients with leprosy; they are inflammatory conditions that often appear at the initiation of treatment. However, they may occur at any time during the course of the disease. Reactions may cause permanent damage to nerves and can affect skin and other organs as well (1, 32). Reactions have been categorized into two primary types: type 1 reactions, also known as reversal reactions (RRs), and type 2 reactions, or erythema nodosum leprosum (ENL) reactions (Table 1). In order to diagnose a leprosy reaction, physicians should do a physical examination and collect information on a patient's medical history (1). The types of reactions are associated with different forms of HD. Generally, patients with type 1 reactions are those who have been diagnosed with a form that falls into the spectrum of borderline types of HD (BT, BB, and BL). Generally, patients with the BL or LL form of HD experience type 2 reactions (1, 32, 33). Patients who exhibit high levels of skin infiltration with HD bacilli run a higher risk of developing type 2 reactions, since these reactions occur in patients with polar lepromatous phenotypes (34). The greatest risk factor for the occurrence of type 1 reactions seems to be the initiation of MDT (32). Another potential type of leprosy reaction is the Lucio phenomenon, associated with a form of lepromatous leprosy known as diffuse leprosy of Lucio and Latapí. This form of HD is associated with endothelial cell injury; it causes diffuse necrotizing lesions that result from nonocclusive or occlusive vasculopathy associated with direct M. leprae or M. lepromatosis endothelial cell injury (20). Corticosteroids, which serve to reduce inflammatory reactions in skin and nerves that can cause severe nerve damage, are the primary treatment recommended by the World Health Organization for leprosy reactions (9, 32, 33).
TABLE 1.
Overview of clinical manifestations and management of leprosy reactions
| Characteristic | Description for reaction typea: |
|
|---|---|---|
| 1 (RR) | 2 (ENL) | |
| Clinical phenotype | Occurs mainly in borderline disease (BT, BB, BL); it may also occur with TT and also within pure neural leprosy | Occurs in BL and LL |
| Skin manifestations | Acute onset of redness and swelling in previously existing skin lesions, and lesions may sometimes ulcerate; marked edema of the hands, feet, and face may occur; no new lesions appear | New painful and tender red papules or nodules, which occur in crops in limbs or on trunk and face; ulceration of nodules may occur; edema of the hands, feet, or face may occur; original leprosy skin patches remain as they were |
| Nerve signs and symptoms | Pain or tenderness in one or more nerves, with or without loss of nerve function; new nerve damage manifesting as numbness or muscle weakness in the hands, feet, or face | New nerve damage manifesting as numbness or muscle weakness in the hands, feet, or face; pain or tenderness in one or more nerves, with or without loss of nerve function |
| Systemic manifestations | Unusual | Fever, malaise, lymphadenitis, uveitis, neuritis, arthritis, dactylitis, orchitis |
| Diagnosis | Clinicalb | Clinicalc |
| Treatment | Corticosteroidsd | Corticosteroids/thalidomide |
BT, borderline tuberculoid; BB, borderline borderline; BL, borderline lepromatous; LL, lepromatous.
Key features in skin biopsy specimens include dermal edema, granuloma edema, and the presence of giant cells and plasma cells.
Skin biopsy specimens demonstrate a mixed dermal infiltrate of neutrophils and lymphocytes and fragmented bacilli in macrophages.
For mild reactions, nonsteroidal anti-inflammatory drugs maybe sufficient.
Type 1 Reaction (Reversal Reaction)
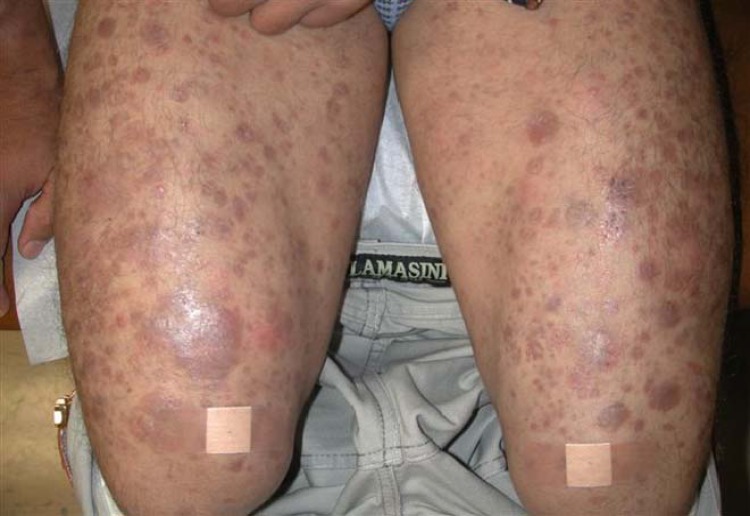
Type 1 reactions occur in borderline leprosy, and they are understood to be an intensification in the cell-mediated immune response to HD bacilli (1, 12, 32, 35–37). Patients with type 1 reactions generally have fewer HD bacilli and lower levels of antibodies to HD than patients with the lepromatous form of HD (38, 39). Type 1 reactions typically result in inflammation and pain in preexisting lesions, which may also ulcerate (Fig. 6). These reactions may also produce increased neuritis manifested as tenderness and nerve damage (10, 11, 40). Damage that occurs to nerves in the face can cause facial paralysis, including loss of the ability to close the eyelids (lagophthalmos). Associated inflammatory eye conditions, including iritis and scleritis, can lead to blindness. In addition, nerve injury to feet can result in the sudden onset of foot drop, which can lead to further permanent disability (1, 9, 39).
FIG 6.
A 38-year-old male with a type 1 reaction with acute inflammation of existing plaques. (Adapted from reference 32.)
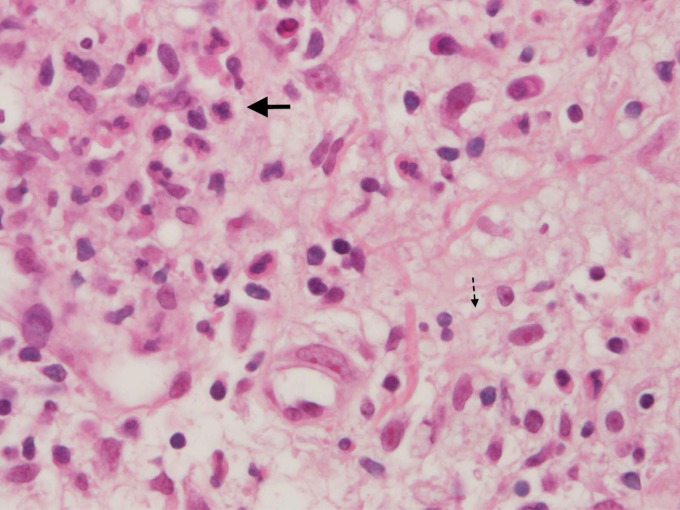
Type I reactions typically occur within the first 6 months after the start of MDT, although they can happen at any stage of the disease process (including after MDT is completed) (2, 32, 37). Edema or erythema in existing lesions and stimulus-independent (spontaneous) nerve pains are clinical signs of a type I reaction (39). Verification of the diagnosis can be accomplished by skin biopsy (Fig. 7). The presence of granuloma edema, dermal edema, plasma cells, and giant cells in the biopsy specimen is a good indicator that a type I reaction is taking place (1, 9, 38).
FIG 7.
Histopathological progression of cutaneous lesions in type 1 reactions. (A) The initial skin biopsy specimen revealed features of borderline lepromatous leprosy, with a disorganized inflammatory infiltrate in which vacuolated macrophages predominated. Occasional multinucleated giant cells were also noted. Fite stains revealed moderate numbers of bacilli (inset and thin bold arrow). (B) A biopsy specimen 1 month later revealed greater organization of the inflammatory infiltrate and increased lymphocytic clustering, with more numerous multinucleated giant cells, consistent with a type 1 leprosy reaction (thick dotted arrow) (original magnification for photographs of hematoxylin- and eosin-stained sections, ×250; original magnification for photographs of Fite-stained sections, ×1,000). (Adapted from reference 100 by permission of Oxford University Press and the Infectious Diseases Society of America.)
Type 2 Reaction (Erythema Nodosum Leprosum)
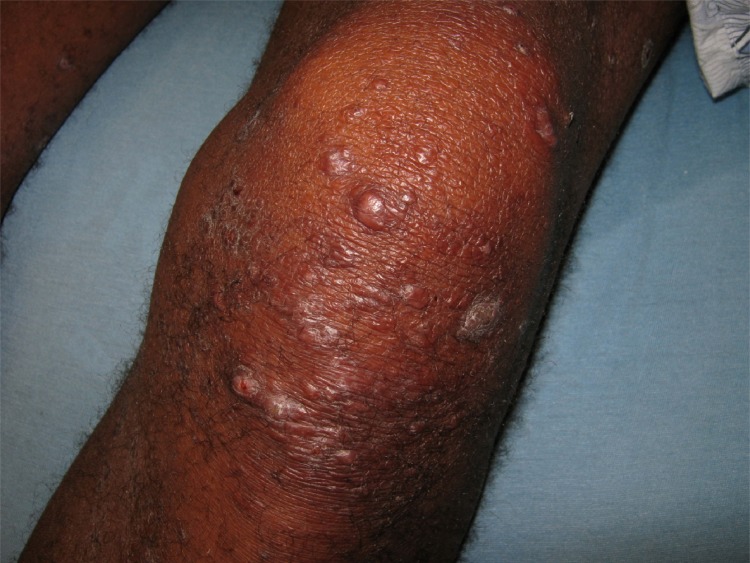
ENL results in cellular dysfunction as well as in antigen-antibody complexes being deposited directly into tissues (40). This can cause acute nerve and skin inflammation (Fig. 8) (10, 12). With type 2 reactions, there is an inflammatory infiltration of polymorphonuclear cells (neutrophils) accompanied by vasculitis and/or inflammation of the subcutaneous layer of fatty tissue (panniculitis) (Fig. 9) (12, 40). ENL is also associated with increased proinflammatory cytokine levels (12).
FIG 8.
A 51-year-old male with erythema nodosum leprosum, manifesting as newly appearing red, painful, tender red papules and nodules in crops in the extensor surface of the arm.
FIG 9.
Erythema nodosum leprosum. Several polymorphonuclear cells are seen (top left, thick bold arrow) against a background of foamy histiocytes (seen best at the bottom right [thin dotted arrow]) (hematoxylin and eosin staining; original magnification, ×400).
Type 2 reactions can be diagnosed based on a variety of symptoms in patients with borderline lepromatous or lepromatous leprosy. Patients may develop new subcutaneous nodules that are painful (40). These nodules may be accompanied by fever and malaise as well as inflammation of nerves, lymph nodes, eyes, and extremities. (Fig. 8) (1, 9, 32). As with type 1 reactions, ENL can come about during different points in the natural history of patients with the BL or LL form of the disease, although these reactions often emerge when patients are taking MDT. Some patients continue to experience episodes of ENL reactions for many years after they have been declared “cured” of HD, sometimes resulting in confusion for patients about the definition of “cure” (1, 9, 34).
In skin biopsy specimens from patients experiencing an ENL reaction, dead (fragmented) bacilli, neutrophils, and lymphocytes may be apparent within macrophages (2, 9, 40). Nerve involvement is common with these reactions (10, 11). Some patients, with the exception of those with borderline lepromatous HD, experience both type 1 and 2 reactions (2, 32, 37). Type 2 reactions can also take different forms. While some patients experience a single acute episode, others have multiple/recurring acute episodes or experience chronic ENL, the latter being most common (32, 34, 37).
High-risk factors for developing type 2 reactions include having the lepromatous form of leprosy, certain life changes in women (including puberty, pregnancy, and lactation, which result in hormonal changes), and a high bacteriological index (3 or more) (34, 37). Emotional and psychological stress, and the corresponding immunological and hormonal changes, may also be a factor in triggering reactions, although this has not been verified by large-scale, longitudinal studies (2, 32, 34, 37). Type 2 reactions tend to be more complicated to treat than type 1 reactions, because of their systemic nature and the likelihood of recurrent episodes (34, 37).
Genetics and HD
Research focusing on the relationship among human genetics, the HD genome, and HD susceptibility is useful toward developing a greater understanding of how and why people develop the disease (41–44). In a Clinical Microbiology Reviews article from 2006, Scollard et al. (12) provided a comprehensive summary of the part that genetics plays in HD susceptibility. The presence of the PARK2/PACRG gene, located on chromosome 6q25-q27, and the presence of the NRAMP1 gene (on chromosome 2q35) are associated with leprosy susceptibility (12). The chromosome 10p13 locus, the TAP1 and TAP2 (transporter associated with antigen processing) genes (located on chromosome 6p21), TNF-α (tumor necrosis factor alpha) (located on chromosome 6p21), and the VDR (vitamin D receptor) gene (on chromosome 12q12) have been found to be associated with susceptibility to HD, as have a variety of factors associated with innate and adaptive immunity (42–45). Over the last few years, there have been increasing numbers of reports that demonstrate a key role of the innate immune response in determining susceptibility to leprosy and its reactional states. Genetic regulation of the innate immune response, as shown by different polymorphisms of the NOD2 gene, has been linked with increased susceptibility to leprosy and also to the development of leprosy reactions (3, 46, 47). A key role of the innate immune system in a dysregulated inflammatory response during leprosy reactions is demonstrated by genetic variability in polymorphisms associated with Toll-like receptors (3). A particular human polymorphism in Toll-like receptor 1 (T1805G variant), which is associated with impaired mycobacterial intracellular signaling, has been shown to provide protection against the occurrence of type 1 reactions (44).
On a genomic scale, studies have also confirmed a correlation among specific aspects of individual genetics and immunity with HD susceptibility. A large-scale GWAS (genome-wide association study) was conducted with 706 patients and 1,225 controls in China (45). The researchers of that study identified six genes involved in the innate immune response to be associated with HD susceptibility (listed with their respective chromosomes): CCDC122 (13q14), C13orf31 (13q14), NOD2 (16q12), TNFSF15 (9q32), HLA-DR (6p21), and RIPK2 (8q21). That study confirmed that variants of genes in the NOD2-mediated signaling pathway are indeed associated with susceptibility to M. leprae infection (45–47). Subsequent studies have further elucidated that the differential expression of some of these genes is associated with different forms of HD. In this sense, the expression of NOD2 is associated with the tuberculoid form by promoting monocyte differentiation into dendritic cells. The expression of galectin-3 is associated with differentiation of monocytes into macrophages in the case of lepromatous leprosy (3).
With regard to identifying a genetic signature associated with leprosy reactions, a recent report demonstrated a 44-gene set that regulates three different functional components of the inflammatory response, associated with type 1 reactions: regulators of the arachidonic acid metabolism mediators and anti-inflammatory and proinflammatory regulators (47). The key finding from that report is that among individuals with type 1 reactions, there seems to be a defect in the regulation of the inflammatory response against M. leprae antigens, since both components of the innate immune response (anti-inflammatory and proinflammatory) are involved. These findings may contribute to the understanding of the pathogenesis of the disease and to the development of new early diagnostic markers to identify and predict which patients will be at a higher risk of developing leprosy reactions (45–47).
Genome Sequencing and Recent Research on Armadillos in the Americas
It was only recently confirmed that strains of M. leprae present in armadillos in the U.S. South are of a single clonotype that has been matched, through whole-genome sequencing, to the clonotype found in the majority of U.S.-born residents in these regions with the disease and who have consumed armadillo meat or otherwise been in contact with armadillos (48). This form of infection with M. leprae may account for many of the cases in areas where the disease is endemic, in which people born in the United States who have had no known contact with people affected by the disease are diagnosed. The correlation between HD infection in humans and contact with armadillos has been demonstrated previously in studies in the United States and Brazil (49–52).
In regions of the Americas where there is a high prevalence of the disease (principally Brazil), person-to-person contact is still the most probable mode of HD transmission. Even among people affected by the disease who have had armadillo contact but who report no known contact with people who have the disease, their regular exposure to HD-affected people who have no visible symptoms is still a likely cause of the disease. However, Deps et al. (51) found that direct exposure (through hunting/meat consumption) to armadillos was associated with a significant increase in the HD incidence among a studied population in the state of Vitória in southern Brazil. In several regions of Brazil where HD is still a public health problem, consumption of armadillo meat is a common practice, although it is unclear if meat consumption transmits the disease; it is possible that transmission may take place through human contact with armadillos that are captured and kept in pens before consumption. In a case-control study in the state of Espirito Santo in southern Brazil, Schmitt et al. (52) did not find an association between the consumption of armadillo meat and HD, although the prevalence of HD among armadillos in this area has not yet been mapped. Generally, the presence of M. leprae among armadillos in the Americas should be considered in HD control efforts in the future, as transmission between humans and armadillos may provide a continued reservoir for the disease, potentially complicating attempts to eliminate the disease in the Americas.
Management of HD
For adults diagnosed with multibacillary HD, the World Health Organization currently recommends a year of treatment with a combination of 600 mg of rifampin once per month, 300 mg of clofazimine once per month, and daily doses of clofazimine (50 mg) and dapsone (100 mg) for a total of 12 months. The recommendation for paucibacillary patients is a 600-mg dose of rifampin once per month and daily 100-mg doses of dapsone for 6 months. For those patients with paucibacillary HD who have only one skin patch, a single-dose “ROM” treatment (which includes rifampin, ofloxacin, and minocycline) is recommended (53). Although, according to the WHO, side effects of MDT are “mild” and serious side effects are rare (53), some common side effects are troubling for patients. While not physically serious or permanent, the side effects of clofazimine (which include darkening of the skin and dry skin/scaliness of the skin [ichthyosis]) can have social and emotional consequences for patients. Changes in skin tone from clofazimine can alert others to the fact that patients are being treated for HD (in areas where this side effect is recognized or known), alert others to a general problem that patients are having (which can also generate stigma), affect patients' self-perception or self-esteem, and/or, in some cases, change a person's racial category (as reported for patients in the United States and Brazil, for example), which can have consequences in terms of how these patients are treated by others and could potentially affect employment status and social relationships, among other things (54, 55).
The World Health Organization recommends treating HD reactions with analgesics, clofazimine, thalidomide, and/or corticosteroids. While the drug thalidomide is an important drug in HD reactions, it is unavailable in most countries today because of the risk of severe birth defects in children of mothers who take the drug during pregnancy. Before 1998, the duration of MDT was 2 years for multibacillary patients and 1 year for paucibacillary patients. The reduction in the recommended treatment duration and the introduction of the ROM treatment scheme are controversial, primarily because of concerns of incomplete treatment and relapse with this shorter regimen. The move to ROM treatment for patients with single lesions is motivated in part by a lack of funds to support HD treatment in many countries (12). The reduction in treatment time (and, thus, a reduction in the amount of time that patients are registered as active cases of HD) has been misinterpreted by some to represent an attempt to demonstrate dramatic changes in global prevalence (56, 57).
Management of reactions is crucial in preventing sensorimotor dysfunction stemming from sensory loss (1, 9, 32). In treating patients with reactions, treatment should be geared toward controlling pain and inflammation and reversing nerve damage (2). In this regard, corticosteroids are generally recommended (2, 32), but MDT should be continued along with antireaction drugs for both kinds of reactions. For mild reactions, nonsteroidal anti-inflammatory medications can be used (11, 32). Although the WHO suggests tapered doses of corticosteroids over 12 weeks (39), controlling type 1 reactions requires tapered steroid doses for an extended period of time (up to 20 weeks). Clofazimine is sometimes used at higher doses in conjunction with corticosteroids to achieve control of ENL, but its effect is usually seen after many weeks. The use of other immune-suppressive drugs such as cyclosporine or methotrexate has been shown to be helpful in lieu of steroids for patients who develop an intolerance to steroids or who experience severe side effects from steroids (32).
Prolonged use of corticosteroids may lead to side effects (diabetes, weight gain, facial swelling, and anxiety) with emotional, social, and physical consequences (2, 32). Particular challenges arise when patients experience an HD reaction, and physicians have to craft a treatment plan that both effectively addresses the reaction and does not cause significant side effects in patients. Although corticosteroids are standard in treating reactions, thalidomide has shown important anti-inflammatory effects in type 2 reactions as well (1, 58), but its teratogenic effects necessitate extreme caution in prescribing it not only to women of reproductive age but also to all patients, who should be given special instructions never to share this medication. The generation of the cytokines interleukin-2 (IL-2) and gamma interferon by activated CD4+ and CD8+ lymphocytes may explain the effectiveness of thalidomide (35). Based on the inflammatory pattern of cytokine production (59, 60), tumor necrosis factor inhibitors may have an effect on treatment of type 2 reactions. Some success has been reported for the use of the drug azathioprine in patients with frequent ENL recurrences (32).
The loss of peripheral nerve function for 6 months or longer is classified as permanent nerve damage, and its management focuses on patient counseling and harm reduction. These interventions include preventing complications in the eyes when lagophthalmos is present, preventing damage to the upper extremities, and preventing ulcerations of the lower extremities. Home-based self-care is a central preventive strategy that includes daily inspections of hands, feet, and eyes as well as the regular use of artificial tears. It is also important to encourage patients to blink frequently and wear sunglasses and other forms of eye protection, to keep hands and feet moisturized, to use sterile cloths to cover any wounds, to perform exercises to avoid contractures and deformations of muscles and tendons, and, finally, to find ways (such as staying off their feet as much as possible and wearing proper shoes) to decrease pressure in the case of ulcers of the lower limbs. Additionally, referral of patients to specialists and physicians close to their homes is also considered part of the adequate management of patients with sensorineural loss and its complications.
The availability of MDT in providing a microbiological cure is not enough to prevent nerve damage and sequelae associated with leprosy reactions (32, 35). Antimicrobial drug-resistant M. leprae has been documented mostly with the use of dapsone in some areas of endemicity where dapsone has been used as monotherapy (61, 62). Fortunately, there have been only a limited number of multidrug-resistant cases identified through representative mutations in more than two genes reported in the literature (62, 63). Therefore, in comparison to the increasing concern of multidrug-resistant Mycobacterium tuberculosis, MDT in patients with M. leprae infection continues to preserve its overall efficacy. The killing of M. leprae by the use of MDT does not reverse existing nerve damage, and reactions occurring after completion of MDT may produce further nerve degeneration. The precise mechanisms leading to severe nerve damage during reactions remain to be fully elucidated (10–12). For decades, scientists have focused on attributing a cell-mediated T-cell response to type 1 reactions and, in the case of type 2 reactions, to the production and deposition of immune complexes.
From a clinical perspective, it is clear that there is a need for further research to address the prevention of leprosy reactions and reduce their impact. One important consideration is the potential role of the microbiome in modulating the inflammatory response among patients with leprosy reactions (64, 65). For example, herpesviruses are considered part of the human microbiome through persistent latent infection, with periodic reactivation, interacting with the human genome in complex pathways influencing inflammatory responses and, thus, the occurrence of some diseases (65–67). It is tempting to speculate that during the homeostatic process of autophagy, where cells digest or break down their component parts, herpesviruses may predisopose some individuals with HD to the occurrence of either type 1 or type 2 reactions (66, 68). While this is a relatively new area of inquiry, there is ample evidence in other clinical scenarios to illustrate that herpesviruses modulate inflammatory responses during particular pathological conditions, including Epstein-Barr virus (EBV) causing an exanthem associated with the use of penicillin-based antibiotics, cytomegalovirus (CMV) infection in transplant recipients, human herpesvirus 6 (HHV-6) causing the occurrence of severe drug reactions such as DRESS (drug reaction with eosinophilia and systemic symptoms) (69), or herpes simplex virus infections, in our susceptibility to bacterial infections (70). Immune modulation by herpesviruses leading to dysregulation of autophagy is an area of research that may provide important insight into the pathogenesis of leprosy reactions (66, 67). We believe that an innovative approach should be undertaken to improve our understanding of leprosy reactions and the potential relationship with herpesviruses and their influence in autophagy.
SOCIAL, ECONOMIC, AND ENVIRONMENTAL FACTORS IN HD TRANSMISSION AND CONTROL
Structural Inequality
While the proximate cause of HD is infection with M. leprae bacilli among individuals with genetic susceptibility, there are many social determinants, as termed by the World Health Organization (71), that are associated with the continuation of this disease in areas of endemicity. These determinants include social and cultural factors but also the conditions of everyday life and structural inequalities that affect overall health and immunity. There is a relationship between poverty and HD, but it is important to identify what specific aspects of poverty and inequality are involved in either increasing susceptibility in individuals or providing the conditions for the spread of the disease. For example, in a study in Pará, Brazil, Barreto et al. (72) noted that numbers of reports of “starvation” (defined as having experienced full days with no meals) were higher than the average for the general population of the region and five times higher than that for the national population of Brazil. Rao and John (73) studied the relationship of nutrition in HD and found that in a comparative study of 150 people with HD and a control group of 100 people who did not have the disease, undernutrition was more common in the former group, although this undernutrition may be a result of stigma, disability, and/or depression (all of which could impact employment or access to community resources and could result in reduced income) that comes after the disease or diagnosis.
The number of people living in a household may also be a factor in HD transmission. In a study of populations on five islands of Indonesia, Bakker et al. (74) found that homes with more than seven people per household showed a significantly higher incidence of HD than those with four people or fewer. In the state of Pará in Brazil, which has a high average household population density (an average of 4.1 persons per household), Barreto et al. (72) noted that more than half of the people affected by HD surveyed lived in homes in which two or more people shared a bedroom. Pará also has an alarming incidence (20.4 new cases per 100,000 residents in 2008) of HD in people <15 years of age (72).
Several studies in Brazil have illustrated the role of internal migration and rural development trends in the spread of HD. Kerr-Pontes et al. (75) found that social inequality and “rapid, unplanned, and uncontrolled” migration correlated with high rates of HD in the state of Ceará in Brazil. Those researchers also noted that towns and cities with railroads (which were once the main form of interstate transportation) in Ceará also had higher rates of HD than did those without railroads. In another study, also in Ceará, Kerr-Pontes et al. (76) found an increased risk of HD to be associated with infrequent changing of bed linens, hunting activities in general, hunting of armadillo (a possible risk factor discussed above), and bathing in open water, such as rivers and lakes, within the 10 years prior to the study. In the state of Maranhão in Brazil, Murto et al. (77) found higher rates of HD among those who had migrated between states in the past 5 years. The mining industry in the Amazon region is responsible for the movement of people to this area from other regions where HD is hyperendemic; Penna et al. (78) note that strip miners often move between homes in different states for seasonal work, thus potentially increasing the potential for the spread of infection, particularly in regions of high endemicity. Future research that further examines the specific ways in which living conditions might be related to HD susceptibility could identify changes or improvements that will impact not only HD control efforts but also other areas of health (i.e., other neglected tropical infections) and quality of life in areas of endemicity.
International Migration
Some of the latest challenges facing HD control are not necessarily new. The movement of peoples within nations and around the globe has always been a factor in shaping the epidemiological portrait of HD. However, migration poses unique challenges to HD control in the 21st century. One such challenge created by the movement of people affected by HD to nations where there are relatively few cases of the disease is that in these host countries, services for HD treatment may be scarce (31). General practitioners and even dermatologists may be unlikely to diagnose the disease in its early stages. When they do associate a person's symptoms with HD, they may have little knowledge of where to begin with treatment. In the United States, for example, although there is a National Hansen's Disease Clinical Center and a network of regional Hansen's disease clinics around the country, many U.S. physicians are not familiar with these options.
Kuhns (79) learned of several “knowledge gaps” related to HD within general U.S. medicine. In qualitative interviews, she asked HD specialists who work at U.S. ambulatory care centers for HD as well as officials who work in HD control in the United States to identify knowledge gaps that they have observed in working with general practitioners, dermatologists, and other health care professionals who are not specialized in HD treatment. Interviewees mentioned that these knowledge gaps include the inability to recognize HD in its early stages, a lack of information about where and how to refer patients for HD treatment, and confusion about how to help undocumented immigrants who lack health insurance.
Massone et al. expressed concern over cases of HD in Europe, noting that inexperience related to diagnosing HD often results in long delays in patients beginning their treatment, which can in turn increase the chance of disability (80). Rongioletti et al. (81) described an example from Italy of the difficulty for dermatologists who are not familiar with HD to interpret possible HD symptoms. Boggild et al. (27) analyzed records of HD cases in Toronto, Canada, reported between 1979 and 2002. Those researchers found that people coming from low-prevalence regions had a greater delay between the onset of symptoms and the beginning of treatment; this may be because patients from nations where the disease is endemic are themselves more familiar with early symptoms of HD, or it could be that physicians are more likely to consider HD among people from regions or nations where the disease is highly endemic. Their general recommendation was that HD should be considered by physicians more often as a potential diagnosis when foreign-born patients present with chronic dermatitis accompanied by peripheral nerve involvement (27).
International migration is an issue not only in terms of patients receiving a diagnosis but also (or perhaps especially) in terms of the long-term, sometimes lifelong care that many people require. One of the main reasons for requiring medical care is to manage permanent neurological disability and its consequences, such as skin and soft tissue infections, chronic nonhealing ulcers associated with neuropathic (Charcot) joints secondary to peripheral nerve dysfunction, and chronic leprosy reactions, which can take place months or even years after the completion of MDT (82). If diagnosis is delayed because of problems of access to health care and unfamiliarity with HD among physicians in the host country, long-term problems associated with nerve damage are more likely.
Stigma in the context of international migration is also an important concern. In a study of former HD patients in the Netherlands, De Groot et al. (83) found that many people interviewed tended to self-stigmatize and exclude themselves from participation in social activities and said that they felt that it was difficult to find HD-related information. Many participants said that they would like to have (but could not find) access to formal or informal support groups. Stigma may also arise during the medical encounter in cases where health care workers carry their own stigmatizing ideas about the disease. In qualitative research with first-generation immigrants to north Georgia who are in treatment for HD, White (coauthor of this article) interviewed one man who recalled how the physician who first gave him his diagnosis said that there was no treatment for his disease. The physician told him he would have to be isolated in the intensive care unit (ICU), with no contact with others. Stigmatizing practices by health care professionals, which may also be compounded by xenophobic attitudes toward and preconceptions about first-generation immigrants, can be a significant problem for people seeking diagnosis and treatment of HD.
HD COMMUNITIES AND COLONIES IN THE 21st CENTURY
Providing services for people affected by HD (and their families) who are living in communities that resulted from societal stigma or forced isolation is another important challenge that has received little global attention, since it is not viewed to be a significant concern in terms of controlling the disease. Although HD is largely treated on an outpatient basis today, there are still many cured patients who are living in communities, villages, or “colonies” composed largely of other people affected by the disease and, often, their families. Many former patients continue to live in these communities because of permanent disability and stigma that they experienced (or anticipate experiencing) outside these communities. Families sometimes choose to live in these communities as well because of the lack of housing and employment opportunities on the “outside” (84). In regions of the world where the disease is endemic, societal stigma coupled with delays in treatment seeking and/or diagnosis can result in patients moving into these communities, so their numbers in some areas may be growing. There are many of these communities around the world today, including hundreds of such communities in India and 33 in Brazil.
Anthropologist James Staples (85) described the daily challenges that residents face, particularly in terms of generating sufficient income, within one such village in India. Ron Barrett (86), who also conducted ethnographic research among people affected by HD in India, noted the ways in which the stigma associated with seeking treatment for HD results in patients hiding their illness and avoiding treatment; this can result in severe disabilities and stigma, which result in people choosing or feeling compelled to move into these HD communities. Participants in a 2005 workshop in Brazil to assess the needs of residents of these communities noted that limited health care access, limited rehabilitation services, poor sanitation, building decay, lack of employment opportunities, and mental health issues were among the many problems that needed to be addressed (87). Ebenso et al. (88), in an applied study of a socioeconomic rehabilitation project among HD communities in Nigeria, identified several areas that could be improved for residents of these communities, including increased access to loans, collaboration with village heads and local governments, and inclusion of those with non-HD-related disabilities in socioeconomic rehabilitation programs. Future research may identify unmet health, social, and economic needs for these HD communities around the world, but researchers should also attempt to note the specific challenges faced by residents of these communities in different national and cultural contexts.
Restoration of citizenship to people who were forcibly isolated during the confinement era or whose rights as citizens are compromised because of their current disease status should also be a focus of efforts related to HD control in the 21st century. During the confinement era of the late 19th century and much of the 20th century, in many parts of the world (including the United States, Brazil, South Korea, Japan, and the Philippines, for example), children were isolated from parents diagnosed with the disease and were sometimes adopted by non-family members. In Brazil, nongovernmental organization (NGO) efforts have facilitated genetic testing and reunions between now-adult children and their parents and other relatives from whom they had been separated when their parents were diagnosed with HD. There have also been petitions for economic reparations (89).
FUTURE DIRECTIONS
HD continues to represent a significant global health problem and one for which we are still lacking answers for many aspects of the natural history of the disease. Generating more interest in and funding for research on HD is an important challenge for the future. Gelber and Grosset (17) suggest that as resources for HD control are diminishing, HD may “reemerge” as a significant problem. Since the incidence of HD worldwide remains high, “reemergence” is perhaps not the proper term, so globally, it is important that the WHO and other organizations that have worked with HD in the past make it known that this is still a disease of important concern, one that affects the lives of millions of people and extracts a significant social and economic toll on local communities.
In a recent commentary article, Scollard (90) noted that the shift from confinement to outpatient treatment of HD, although unquestionably better for patients' families and social lives, has resulted in fewer opportunities for research on the disease. Throughout much of the 20th century, breakthroughs associated with HD treatment were made possible in part through the presence of a large number of people in closed communities who acted as voluntary or involuntary guinea pigs. This also means that fewer physicians specialize in the disease in general, and in countries where the disease is not endemic, very few physicians have experience with people with HD in its early stages.
The shift in many countries from a vertical treatment plan, in specialized centers for HD, to horizontalization, in which general health posts provide MDT and care for HD patients, also affects the degree of specialization among those who are working with people affected by the disease. Although greater access to MDT may be achieved through horizontalization, health posts that act primarily as pharmaceutical distribution locations cannot provide the same quality of care as centers with physicians, physical therapists, psychologists, and social workers familiar with HD and its long-term physical and social effects. In nations where the disease is endemic and in regions of high endemicity, as in parts of Brazil, the use of community health agents (local people trained in basic health education, diagnosis, and treatment follow-up for common health problems) is particularly promising in terms of case detection and patient follow-up as well as potential research assistants and fonts of information regarding HD in local communities (91).
Another potentially positive step in HD control is the recent development of a new diagnostic tool, a lateral flow test that requires a drop of blood to measure the presence of antibodies to HD bacilli before symptoms appear, specifically through the use of the M. leprae-specific phenolic glycolipid I (PGL-I) antigen (John Spencer, personal communication). The findings of a number of studies (92–95) contributed to the development of this test, which is being produced by the Infectious Disease Research Institute (IDRI) in conjunction with OrangeLife Laboratories in Rio de Janeiro, Brazil (96). Those researchers are investigating whether this test could be potentially useful with family contacts (95). If effective, such a test could potentially address the crucial need for more sensitive diagnostic tools for case detection in HD control today (35). If implemented on a large scale in areas with high prevalence rates, as a standard test conducted at primary care visits, early case detection could be improved, but it is not yet known if prophylactic treatment would be the procedure used in the case of positive tests. Consideration must be taken regarding the potential stigma that a diagnosis of HD might bring, particularly for individuals with no symptoms and (possibly) no perception that they might have a disease. There is also the possibility of underdiagnois of paucibacillary cases, since most of these cases are seronegative in response to this test (94, 95).
Funding for HD research and interest among scientists in nations where the disease is and is not endemic might be generated through the suggestion that understanding more about M. leprae, its effects on the body, and its evolution as a bacillus can give us insight into many other diseases and conditions as well as a greater understanding of population genetics. For example, a study demonstrating the ability of M. leprae bacilli to reprogram Schwann cells in the human body to become more like stem cells may have applications for stem cell research in the future (8). While this finding may not have direct applications in terms of HD control, applications in other areas of health and medicine may encourage continued research on this complex disease, which ultimately could lead to its true elimination (if not eradication). The potential use of vaccinations to control leprosy needs to be reassessed. The Mycobacterium bovis BCG (bacillus Calmette-Guérin) vaccine used to prevent disseminated forms of tuberculosis has been reported to impart protection against HD in different populations (97). However, we believe that a key public health outcome, along with decreasing further transmission (98), is to focus control efforts on preventing HD-associated disability and to foster improvements in the quality of life for those affected by HD (99).
Biographies

Cassandra White received her B.A. (1991) and M.A. (1993) from the University of Florida and her Ph.D. in Anthropology from Tulane University in 2001. She is an Associate Professor in the Department of Anthropology at Georgia State University in Atlanta, GA, where she has taught for 10 years. She is the author of An Uncertain Cure: Living with Leprosy in Brazil (Rutgers University Press, 2009) and several peer-reviewed articles about Hansen's disease in the United States and Brazil. Her current research focus is on the experience of Hansen's disease and treatment seeking for first-generation Brazilian immigrants to the United States.

Carlos Franco-Paredes received his M.D. from the La Salle University School of Medicine in México City, México, in 1995. He did his internship and residency in Internal Medicine at Emory University in Atlanta, GA, and received his M.P.H. in International Health from Emory in 2002. He is currently a staff member in the Phoebe Physician Group in southwest Georgia, where he provides consultations in infectious disease and travel medicine. He is also affiliated with the Unidad de Medicina Experimental, Facultad de Medicina, Universidad Nacional Autónoma de México, México City, México, and the Hospital Infantil de México, Federico Gómez, México City, México. He is an Associate Editor of Public Library of Sciences (PLoS) journals (PLoS Neglected Tropical Diseases), and he has 130 publications in peer-reviewed journals. Dr. Franco-Paredes' current research focuses on vaccine-preventable diseases, travel-related vaccines, social determinants of infectious diseases, and neglected tropical diseases.
REFERENCES
- 1.Walker SL, Lockwood DNJ. 2007. Leprosy. Clin Dermatol 25:165–172. doi: 10.1016/j.clindermatol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Graham A, Furlong S, Margoles LM, Owusu K, Franco-Paredes C. 2010. Clinical management of leprosy reactions. Infect Dis Clin Pract 18:235–238. doi: 10.1097/IPC.0b013e3181deba2a. [DOI] [Google Scholar]
- 3.Polycarpou A, Walker SL, Lockwood DNJ. 2013. New findings in the pathogenesis of leprosy and implications for the management of leprosy. Curr Opin Infect Dis 26:413–419. doi: 10.1097/QCO.0b013e3283638b04. [DOI] [PubMed] [Google Scholar]
- 4.Cogen AL, Walker SL, Roberts CH, Hagge DA, Neupane KD, Khadge S, Lockwood DN. 2012. Human beta-defensin 3 is up-regulated in cutaneous leprosy type 1 reactions. PLoS Negl Trop Dis 6:e1869. doi: 10.1371/journal.pntd.0001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degang Y, Akama T, Hara T, Tanigawa K, Ishido Y, Gidoh M, Makino M, Ishii N, Suzuki K. 2012. Clofazimine modulates the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages. PLoS Negl Trop Dis 6:e1936. doi: 10.1371/journal.pntd.0001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambukkana A. 2001. Molecular basis for the peripheral nerve predilection of Mycobacterium leprae. Curr Opin Microbiol 4:21–27. doi: 10.1016/S1369-5274(00)00159-4. [DOI] [PubMed] [Google Scholar]
- 7.Rambukkana A. 2010. Usage of signaling in neurodegeneration and regeneration of peripheral nerves by leprosy bacteria. Prog Neurobiol 91:102–107. doi: 10.1016/j.pneurobio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. 2013. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell 152:51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britton WJ, Lockwood DNJ. 2004. Leprosy. Lancet 363:1209–1219. doi: 10.1016/S0140-6736(04)15952-7. [DOI] [PubMed] [Google Scholar]
- 10.Scollard DM. 2008. The biology of nerve injury in leprosy. Lepr Rev 79:242–253. [PubMed] [Google Scholar]
- 11.Lockwood DN, Saunderson PR. 2012. Nerve damage in leprosy: a continuing challenge to scientists, clinicians, and service providers. Int Health 4:77–85. doi: 10.1016/j.inhe.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Scollard D, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. 2006. The continuing challenges of leprosy. Clin Microbiol Rev 19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 2003. The final push strategy to eliminate leprosy as a public health problem. Questions and answers, 2nd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.World Health Organization. 2012. Global leprosy situation 2012. Wkly Epidemiol Rec 87:317–328. [PubMed] [Google Scholar]
- 15.Robbins G, Tripathy VM, Misra VN, Mohanty RK, Shinde VS, Schug MD. 2009. Ancient skeletal evidence for leprosy in India (2000 B.C.) PLoS One 4:e5669. doi: 10.1371/journal.pone.0005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuenemann VJ, Singh P, Mendum TA, Krause-Kyora B, Jäger G, Bos KI, Herbig A, Economou C, Benjak A, Busso P, Nebel A, Boldsen JL, Kjellström A, Wu H, Stewart GR, Taylor GM, Bauer P, Lee OY, Wu HH, Minnikin DE, Besra GS, Tucker K, Roffey S, Sow SO, Cole ST, Nieselt K, Krause J. 2013. Genome-wide comparison of medieval and modern Mycobacterium leprae. Science 341:179–183. doi: 10.1126/science.1238286. [DOI] [PubMed] [Google Scholar]
- 17.Gelber R, Grosset J. 2012. The chemotherapy of leprosy: an interpretive history. Lepr Rev 83:221–240. [PubMed] [Google Scholar]
- 18.Sato H, Narita M. 2003. Politics of leprosy segregation in Japan: the emergence, transformation and abolition of the patient segregation policy. Soc Sci Med 56:2529–2539. doi: 10.1016/S0277-9536(02)00285-X. [DOI] [PubMed] [Google Scholar]
- 19.Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, Li W, Nair RG. 2008. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol 130:856–864. doi: 10.1309/AJCPP72FJZZRRVMM. [DOI] [PubMed] [Google Scholar]
- 20.Vargas-Ocampo F. 2007. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev 78:248–260. [PubMed] [Google Scholar]
- 21.Gillis TP, Scollard DM, Lockwood DNJ. 2011. What is the evidence that the putative Mycobacterium lepromatosis species causes diffuse lepromatous leprosy? Lepr Rev 82:205–209. [PubMed] [Google Scholar]
- 22.Woodall P, Scollard D, Rajan L. 2011. Hansen's disease among Micronesian and Marshallese persons living in the United States. Emerg Infect Dis 17:1202. doi: 10.3201/eid1707.102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridley DS, Jopling WH. 1966. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34:255–273. [PubMed] [Google Scholar]
- 24.Ridley DS. 1974. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ 51:451–465. [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta R, Kar HK, Bharadwaj M. 2012. Revalidation of various clinical criteria for the classification of leprosy—a clinic-pathological study. Lepr Rev 83:354–362. [PubMed] [Google Scholar]
- 26.Prasad PVS, Kaviarasan PK. 2010. Leprosy therapy, past and present: can we hope to eliminate it? Indian J Dermatol 55:316–324. doi: 10.4103/0019-5154.74528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boggild AK, Keystone JS, Kain K. 2004. Leprosy: a primer for Canadian physicians. CMAJ 170:71–78. [PMC free article] [PubMed] [Google Scholar]
- 28.Brubaker ML, Meyers WM, Bourland J. 1985. Leprosy in children one year of age and under. Int J Lepr Other Mycobact Dis 53:517–523. [PubMed] [Google Scholar]
- 29.World Health Organization. 2012. Leprosy: fact sheet no. 101. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs101/en/. [Google Scholar]
- 30.White C. 2002. Sociocultural considerations in the treatment of leprosy in Rio de Janeiro, Brazil 2002. Lepr Rev 73:356–365. [PubMed] [Google Scholar]
- 31.Lockwood D, Reid AJC. 2001. The diagnosis of leprosy is delayed in the United Kingdom. QJM 94:207–212. doi: 10.1093/qjmed/94.4.207. [DOI] [PubMed] [Google Scholar]
- 32.Franco-Paredes C, Jacob JT, Stryjewska B, Yoder L. 2009. Two patients with leprosy and the sudden appearance of inflammation in the skin and new sensory loss. PLoS Negl Trop Dis 3:e425. doi: 10.1371/journal.pntd.0000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moschella S. 2004. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol 51:417–426. doi: 10.1016/j.jaad.2003.11.072. [DOI] [PubMed] [Google Scholar]
- 34.Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, Lockwood DN. 2006. Clinical course of erythema nodosum leprosum: an 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg 74:868–879. [PubMed] [Google Scholar]
- 35.Rodrigues LC, Lockwood DNJ. 2011. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis 11:464–470. doi: 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- 36.Lockwood DNJ, Lucas SB, Desikan KV, Ebenezer G, Suneetha S, Nicholls P. 2008. The histological diagnosis of leprosy type 1 reactions: identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol 61:595–600. doi: 10.1136/jcp.2007.053389. [DOI] [PubMed] [Google Scholar]
- 37.Kumar BS, Dogra S, Kaur I. 2004. Epidemiological characteristics of leprosy reactions: 15 years experience from north India. Int J Lepr Other Mycobact Dis 72:125–133. doi:. [DOI] [PubMed] [Google Scholar]
- 38.Walker SL, Lockwood DNJ. 2008. Leprosy type 1 (reversal) reactions and their management. Lepr Rev 79:372–386. [PubMed] [Google Scholar]
- 39.van Brakel WH, Nicholls PG, Das L, Barkataki P, Suneetha SK, Jadhav RS, Madali P, Lockwood DN, Wilder-Smith E, Desikan KV. 2005. The INFIR cohort study: investigating prediction, detection, and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in North India. Lepr Rev 76:14–34. [PubMed] [Google Scholar]
- 40.Kahawita IP, Lockwood DNJ. 2008. Towards understanding the pathology of erythema nodosum leprosum. Trans R Soc Trop Med Hyg 102:329–337. doi: 10.1016/j.trstmh.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Shinde V, Marcinek P, Rani DS, Sunder SR, Sunder A, Jain S, Arun S, Jain S, Nath I, Kumarasamy T, Velavan TP, Valluri V. 2013. Genetic evidence of TAP1 gene variant as a susceptibility factor in Indian leprosy patients. Hum Immunol 74:803–807. doi: 10.1016/j.humimm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Rajalingam R, Singal DP, Mehra NK. 1997. Transporter associated with antigen-processing (TAP) genes and susceptibility to tuberculoid leprosy and pulmonary tuberculosis. Tissue Antigens 49:168–172. doi: 10.1111/j.1399-0039.1997.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 43.Shaw MA, Donalson IA, Collins A, Peacock CS, Lins-Laison Z, Shaw J, Ramos F, Silveira F, Blackwell JM. 2001. Association and linkage of leprosy phenotypes with HLA class II and tumour necrosis factor genes. Genes Immun 2:196–204. doi: 10.1038/sj.gene.6363754. [DOI] [PubMed] [Google Scholar]
- 44.Misch EA, Macdonald M, Ranjit C, Sapkota BR, Wells RD, Siddiqui MR, Kaplan G, Hawn TR. 2008. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl Trop Dis 2:e231. doi: 10.1371/journal.pntd.0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Chen H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LJ, Yin G, Jiang ZX, Wang XD, Yu YP, Hu ZH, Gong CH, Liu YQ, Liu RY, Wang DM, Wei D, Liu JX, Cao WK, Cao HC, Li YP, Yan WG, Wei SY, Wang KJ, Hibberd ML, Yang S, Zhang XJ, Liu JJ. 2009. Genomewide association study of leprosy. N Engl J Med 361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 46.Berrington WR, Macdonald M, Khadge S, Sapkota BR, Janer M, Hagge DA, Kaplan G, Hawn TR. 2010. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J Infect Dis 201:1422–1435. doi: 10.1086/651559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orlova M, Cobat A, Houng NT, Ba NN, Van Thuc N, Spencer J, Nedelec Y, Barreiro L, Thai VH, Abel L, Alcais A, Schurr E. 2013. Gene set signature of reversal reaction type 1 in leprosy patients. PLoS Genet 9:e1003624. doi: 10.1371/journal.pgen.1003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truman RW, Singh P, Sharma R, Busso P, Rougemont J, Paniz-Mondolfi A, Kapopoulou A, Brisse S, Scollard DM, Gillis TP, Cole ST. 2011. Probable zoonotic leprosy in the southern United States. N Engl J Med 364:1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumpkin LR III, Cox GF, Wolf JE Jr. 1983. Leprosy in five armadillo handlers. J Am Acad Dermatol 9:899–903. doi: 10.1016/S0190-9622(83)70206-9. [DOI] [PubMed] [Google Scholar]
- 50.Abide JM, Webb RM, Jones HL, Young L. 2008. Three indigenous cases of leprosy in the Mississippi delta. South Med J 101:635–638. doi: 10.1097/SMJ.0b013e31816f8610. [DOI] [PubMed] [Google Scholar]
- 51.Deps PD, Alves BL, Gripp CG, Aragao RL, Guedes B, Filho JB, Andreatta MK, Marcari RS, Prates I, Rodrigues LC. 2008. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol 74:338–342. doi: 10.4103/0378-6323.42897. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt JV, Dechandt IT, Dopke G, Ribas ML, Cerci FB, Viesi JMZ, Marchioro HZ, Zunino MMB, Miot HA. 2010. Armadillo meat intake was not associated with leprosy in a case control study, Curitiba (Brazil). Mem Inst Oswaldo Cruz 105:857–862. doi: 10.1590/S0074-02762010000700003. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. 2013. WHO multidrug therapy (MDT). World Health Organization, Geneva, Switzerland: http://www.who.int/lep/mdt/en/. [Google Scholar]
- 54.White C. 2009. An uncertain cure: living with leprosy in Brazil. Rutgers University Press, New Brunswick, NJ. [Google Scholar]
- 55.Nations MK, Lira GV, Catrib AMF. 2009. Stigma, deforming metaphors, and patients' moral experience of multibacillary leprosy in Sobral state, Ceará, Brazil. Cad Saúde Publica 25:1215–1224. doi: 10.1590/S0102-311X2009000600004. [DOI] [PubMed] [Google Scholar]
- 56.Lockwood DN. 2002. Leprosy elimination—a virtual phenomenon or a reality? BMJ 324:1516–1518. doi: 10.1136/bmj.324.7352.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao PN, Lakshmi TSS. 2005. “Final push of leprosy” in India: what is being pushed? Lepr Rev 71:226–229. [DOI] [PubMed] [Google Scholar]
- 58.Walker SL, Waters MF, Lockwood DN. 2007. The role of thalidomide in the management of erythema nodosum leprosum. Lepr Rev 78:197–215. [PubMed] [Google Scholar]
- 59.Ramien ML, Wong A, Keystone JS. 2011. Severe refractory erythema nodosum leprosum successfully treated with the tumor necrosis factor inhibitor etanercept. Clin Infect Dis 52:e133–e135. doi: 10.1093/cid/ciq213. [DOI] [PubMed] [Google Scholar]
- 60.Faber WR, Jensema AJ, Goldschmidt WFM. 2006. Treatment of recurrent erythema nodosum leprosum with infliximab. N Engl J Med 355:739. doi: 10.1056/NEJMc052955. [DOI] [PubMed] [Google Scholar]
- 61.Kai M, Nguyen Phuc NH, Nguyen HA, Pham TH, Nguyen KH, Miyamoto Y, Maeda Y, Fukutomi Y, Nakata N, Matsuoka M, Makino M, Nguyen TT. 2011. Analysis of drug-resistant strains of Mycobacterium leprae in an endemic area of Vietnam. Clin Infect Dis 52:e127–e132. doi: 10.1093/cid/ciq217. [DOI] [PubMed] [Google Scholar]
- 62.Williams DL, Gillis TP. 2004. Molecular detection of drug resistance in Mycobacterium leprae. Lepr Rev 75:118–130. [PubMed] [Google Scholar]
- 63.Maeda S, Matsuoka M, Nakata N, Kai M, Maeda Y, Hashimoto K, Kimura H, Kobayashi K, Kashiwabara Y. 2001. Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob Agents Chemother 45:3635–3639. doi: 10.1128/AAC.45.12.3635-3639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prober C. 2005. Sixth disease and the ubiquity of human herpesviruses. N Engl J Med 352:753–755. doi: 10.1056/NEJMp048302. [DOI] [PubMed] [Google Scholar]
- 65.Dreyfus DH. 2013. Herpes viruses and the microbiome. J Allergy Clin Immunol 132:1278–1286. doi: 10.1016/j.jaci.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 66.Choi AMK, Ryter SF, Levine B. 2013. Autophagy in human health and disease. N Engl J Med 368:651–661. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 67.Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469:323–333. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willis MS, Patterson C. 2013. Proteotoxicity and cardiac dysfunction—Alzheimer's disease of the heart? N Engl J Med 368:455–464. doi: 10.1056/NEJMra1106180. [DOI] [PubMed] [Google Scholar]
- 69.Cacoub P, Musette P, Descamps B, Meyer O, Speirs C, Finzi L, Rourjeau JC. 2011. The DRESS syndrome: a literature review. Am J Med 124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 70.Unanue ER. 2007. Viral infections and non-specific protection—good or bad? N Engl J Med 357:1345–1346. doi: 10.1056/NEJMcibr074519. [DOI] [PubMed] [Google Scholar]
- 71.World Health Organization. 2012. Social determinants of health: report by the Secretariat. World Health Organization, Geneva, Switzerland: http://www.who.int/social_determinants/B_132_14-en.pdf?ua=1. [Google Scholar]
- 72.Barreto JG, De Souza Guimarães L, Leão MRN, Ferreira DVG, Lima RA, Salgado CG. 2011. Anti-PGL-I seroepidemiology in leprosy cases: household contacts and school children from a hyperendemic municipality of the Brazilian Amazon. Lepr Rev 82:358. [PubMed] [Google Scholar]
- 73.Rao PS, John AS. 2012. Nutritional status of leprosy patients in India. Indian J Lepr 84:17–22. [PubMed] [Google Scholar]
- 74.Bakker M, Hatta M, Kwenang A, Van Mosseveld P, Faber WR, Klatser PR, Oskam L. 2006. Risk factors for developing leprosy—a population-based cohort study in Indonesia. Lepr Rev 77:48–61. [PubMed] [Google Scholar]
- 75.Kerr-Pontes LRS, Montenegro ACD, Barreto ML, Werneck GL, Feldmeier H. 2004. Inequality and leprosy in Northeast Brazil: an ecological study. Int J Epidemiol 33:262–269. doi: 10.1093/ije/dyh002. [DOI] [PubMed] [Google Scholar]
- 76.Kerr-Pontes LRS, Barreto ML, Evangelista CM, Rodrigues LC, Heukelbach J, Feldmeier H. 2006. Socioeconomic, environmental, and behavioural risk factors for leprosy in North-East Brazil: results of a case-control study. Int J Epidemiol 35:994–1000. doi: 10.1093/ije/dyl072. [DOI] [PubMed] [Google Scholar]
- 77.Murto C, Chammartin F, Schwarz K, da Costa LMM, Kaplan C, Heukelbach J. 2013. Patterns of migration and risks associated with leprosy among migrants in Maranhão, Brazil. PLoS Negl Trop Dis 7:e2422. doi: 10.1371/journal.pntd.0002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penna MLF, Wand-Del-Rey-de-Oliveira ML, Penna G. 2009. Spatial distribution of leprosy in the Amazon region of Brazil. Emerg Infect Dis 15:650–652. doi: 10.3201/eid1504.081378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhns K. 2012. What's to know? Navigating knowledge gaps of Hansen's disease in the U.S. M.A. thesis Department of Anthropology, Georgia State University, Atlanta, GA: http://scholarworks.gsu.edu/anthro_theses/66/. [Google Scholar]
- 80.Massone C, Nunzi E, Cerroni L. 2010. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol 32:417–419. doi: 10.1097/DAD.0b013e3181bb0cda. [DOI] [PubMed] [Google Scholar]
- 81.Rongioletti F, Gallo R, Cozzani E, Parodi A. 2009. Leprosy: a diagnostic trap for dermatopathologists in nonendemic area. Am J Dermatopathol 31:607–610. doi: 10.1097/DAD.0b013e3181a105a1. [DOI] [PubMed] [Google Scholar]
- 82.Jacob JT, Kozarsky P, Dismukes R, Bynoe V, Margoles L, Leonard M, Tellez I, Franco-Paredes C. 2008. Five-year experience with type 1 and type 2 reactions in Hansen disease at a US travel clinic. Am J Trop Med Hyg 79:452–454. [PubMed] [Google Scholar]
- 83.De Groot R, Van Brakel WH, De Vries HJ. 2011. Social implications of leprosy in the Netherlands—stigma among ex-leprosy patients in a non-endemic setting. Lepr Rev 82:168–177. [PubMed] [Google Scholar]
- 84.White C. 2003. Carville and Curupaiti: experiences of confinement and community. Hist Cien Saude Manguinhos 10:123–141. doi: 10.1590/S0104-59702003000400006. [DOI] [PubMed] [Google Scholar]
- 85.Staples J. 2007. Peculiar people, amazing lives: leprosy, social exclusion, and community making in South India. Orient Longman Pvt Ltd, New Delhi, India. [Google Scholar]
- 86.Barrett R. 2005. Self-mortification and the stigma of leprosy in northern India. Med Anthropol Q 19:216–230. doi: 10.1525/maq.2005.19.2.216. [DOI] [PubMed] [Google Scholar]
- 87.MORHAN. 2005. Consolidated report on the first national seminar on former Hansen's disease colonies. AIFO, Bologna, Italy: http://www.morhan.org.br/views/upload/relatorio_colonias_pdf.pdf. [Google Scholar]
- 88.Ebenso B, Fashona A, Ayuba M, Idah M, Adeyemi G, Fada S. 2007. Impact of socio-economic rehabilitation on leprosy stigma in Northern Nigeria: findings of a retrospective study. Asia Pac Disabil Rehabil J 18:98–119. [Google Scholar]
- 89.Rocha P. 14 September 2012. Os filhos do preconceito. ISTOÉ Independente 2012(2236). http://www.istoe.com.br/reportagens/237935_OS+FILHOS+DO+PRECONCEITO. [Google Scholar]
- 90.Scollard D. 2012. Chemotherapy has changed (almost) everything. Lepr Rev 83:245–246. [PubMed] [Google Scholar]
- 91.Moura MLN, Dupnik KM, Sampaio GAA, Nóbrega PFC, Jeronimo AK, do Nascimento-Filho JM, Dantas RLM, Queiroz JW, Barbosa JD, Dias G, Jeronimo SMB. 2013. Active surveillance of Hansen's disease (leprosy): importance for case finding among extra-domiciliary contacts. 2013. PLoS Negl Trop Dis 7:e2093. doi: 10.1371/journal.pntd.0002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reece ST, Ireton G, Mohamath R, Guderian J, Goto W, Gelber R, Groathouse N, Spencer J, Brennan P, Reed S. 2006. ML0405 and ML2331 are antigens of Mycobacterium leprae with potential for diagnosis of leprosy. Clin Vaccine Immunol 13:333–340. doi: 10.1128/CVI.13.3.333-340.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duthie MS, Hay MN, Morales CZ, Carter L, Mohamath R, Ito L, Oyafuso K, Manini MI, Balagon MV, Tan EV, Saunderson PR, Reed SG, Carter D. 2010. Rational design and evaluation of a multiepitope chimeric fusion protein with the potential for leprosy diagnosis. Clin Vaccine Immunol 17:298–303. doi: 10.1128/CVI.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duthie MS, Hay MN, Rada EM, Convit J, Ito L, Oyafuso LK, Manini MI, Goulart IM, Lobato J, Goulart LR, Carter D, Reed SG. 2011. Specific IgG antibody responses may be used to monitor leprosy treatment and efficacy and as recurrence prognostic markers. Eur J Clin Microbiol Infect Dis 30:1257–1265. doi: 10.1007/s10096-011-1221-2. [DOI] [PubMed] [Google Scholar]
- 95.Spencer JS, Duthie MS, Geluk A, Balagon MF, Kim HJ, Wheat WH, Chatterjee D, Jackson M, Li W, Kurihara JN, Maghanoy I, Mallari P, Saunderson P, Brennan PJ, Dockrell HM. 2012. Identification of serological biomarkers of infection, disease progression and treatment efficacy for leprosy. Mem Inst Oswaldo Cruz 107(Suppl 1):79–89. doi: 10.1590/S0074-02762012000900014. [DOI] [PubMed] [Google Scholar]
- 96.Infectious Disease Research Institute. 2013. Leprosy 2013: the problem and the solutions. http://www.idri.org/documents/IDRILeprosyGallery.pdf IDRI, Seattle, WA. [Google Scholar]
- 97.Franco-Paredes C, Rouphael N, Del Rio C, Santos-Preciado JI. 2006. Vaccination strategies to prevent tuberculosis in the new millennium: from BCG to new vaccine candidates. Int J Infect Dis 10:93–102. doi: 10.1016/j.ijid.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Smith CS, Noordeen SK, Richardus JH, Sansarricq H, Cole ST, Soares RS, Savioli L, Aertsh A, Baruaf S. 2014. A strategy to halt leprosy transmission. Lancet Infect Dis 14:96–97. doi: 10.1016/S1473-3099(13)70365-7. [DOI] [PubMed] [Google Scholar]
- 99.Lockwood DNJ, Shetty V, Oliveira Penna G. 2014. Hazards of setting targets to eliminate disease: lessons from the leprosy elimination campaign. BMJ 348:g1136. doi: 10.1136/bmj.g1136. [DOI] [PubMed] [Google Scholar]
- 100.Scollard DM, Joyce MP, Gillis TP. 2006. Development of leprosy and type 1 leprosy reactions after treatment with infliximab: a report of two cases. Clin. Infect. Dis. 43:e19–e22. doi: 10.1086/505222. [DOI] [PubMed] [Google Scholar]