SUMMARY
Norovirus, an RNA virus of the family Caliciviridae, is a human enteric pathogen that causes substantial morbidity across both health care and community settings. Several factors enhance the transmissibility of norovirus, including the small inoculum required to produce infection (<100 viral particles), prolonged viral shedding, and its ability to survive in the environment. In this review, we describe the basic virology and immunology of noroviruses, the clinical disease resulting from infection and its diagnosis and management, as well as host and pathogen factors that complicate vaccine development. Additionally, we discuss overall epidemiology, infection control strategies, and global reporting efforts aimed at controlling this worldwide cause of acute gastroenteritis. Prompt implementation of infection control measures remains the mainstay of norovirus outbreak management.
INTRODUCTION
Human norovirus, previously known as Norwalk virus, was first identified in stool specimens collected during an outbreak of gastroenteritis in Norwalk, OH, and was the first viral agent shown to cause gastroenteritis (1). Illness due to this virus was initially described in 1929 as “winter vomiting disease” due to its seasonal predilection and the frequent preponderance of patients with vomiting as a primary symptom (2).
The 1968 outbreak that led to the identification of the virus affected 50% of students at an elementary school in Norwalk and manifested primarily as nausea, vomiting, diarrhea, and low-grade fever (3). Among primary cases, 98% complained of nausea, and 92% vomited, while 58% had abdominal cramps, 52% complained of lethargy, 38% had diarrhea, and 34% had fever. The occurrence of secondary cases in 32% of family contacts allowed the estimation of a 48-h incubation period. The illness lasted ∼24 h, with complete recovery in all cases.
While no pathogen could be identified in the Norwalk outbreak, oral administration of filtrates prepared from rectal swabs from affected individuals to healthy adult male prisoners at the Maryland House of Correction in Jessup, MD, resulted in symptoms in 2 of 3 subjects (4). In the 2 subjects who became ill, the incubation period was 48 h, as was the duration of symptoms. Mild diarrhea, with 4 to 6 loose stools, lasted 24 h, while low-grade fever lasted only 8 to 12 h. Other symptoms were anorexia, moderately severe abdominal cramping, malaise, headache, and nausea (but no vomiting). A complete return to health occurred by 96 h. The filtrate of a stool sample from 1 of the 2 symptomatic experimentally infected volunteers was then administered to a further 9 subjects, 7 of whom became ill. Two of the seven subjects vomited (one subject, who vomited ∼20 times in 24 h, required parenteral fluid replacement) but failed to develop diarrhea, while two had diarrhea without vomiting, and three had both diarrhea and vomiting. Four of the seven subjects had low-grade fever that lasted 8 to 12 h, and all seven subjects complained of malaise and headache. Symptoms resolved after a mean duration of 33 h.
While the index outbreak and the human volunteer studies that followed provided an accurate picture of the manifestations of norovirus infection in previously healthy children and adults at a time when the etiologic agent was unknown, it was an incomplete one. Further observations, discussed later in this review, have provided a more complex and nuanced view of the infection. Human noroviruses are the leading cause of epidemic gastroenteritis in all age groups and have been associated with high-profile outbreaks in hospitals, nursing homes, cruise ships, and the military (5, 6). It is estimated that each year, noroviruses are responsible for 64,000 diarrheal episodes requiring hospitalization, 900,000 clinic visits among children in industrialized nations, and ∼200,000 deaths of children <5 years old in the developing world (7).
BASIC VIROLOGY AND VIRAL DIVERSITY
The Norwalk virus agent (the original prototype virus is referred to as Norwalk virus in this review) was originally visualized by using immunoelectron microscopy (1), revealing 27-nm virus-like particles (Fig. 1). Efforts to cultivate the pathogen in cell culture and to develop an animal model were unsuccessful (8); therefore, the evolving literature focused on describing the physical characteristics of this small, round-structured virus in clinical specimens and on the serologic response to infection (9, 10). Although virion morphology, as well as protein and nucleic acid composition, was similar to that of members of the Caliciviridae family (10–13), clear taxonomic classification was not achieved until the whole genome sequence was obtained and compared to sequences from other caliciviruses (14, 15).
FIG 1.
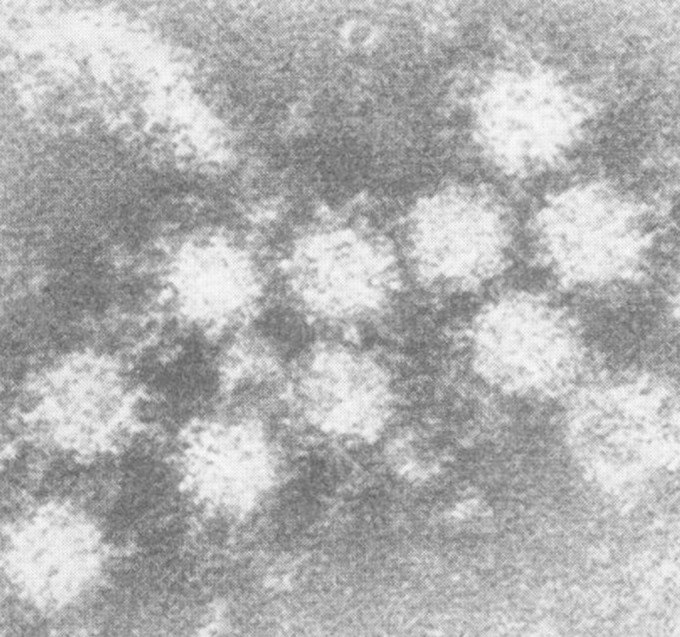
Immunoelectron microscopy identifies a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. Shown is an aggregate observed after incubation of a second-passage stool filtrate from the original Norwalk outbreak with a 1:10 dilution of postchallenge antiserum of an experimentally infected volunteer. These particles are heavily coated with antibody. (Adapted from reference 1 with permission.)
The Caliciviridae family of small, nonenveloped, positive-stranded RNA viruses is now comprised of five genera, including Norovirus, Sapovirus, Lagovirus, Nebovirus, and Vesivirus (16). The Norovirus and Sapovirus genera contain the human enteric viruses of the same names as well as a number of viruses that cause primarily enteric diseases in other animals, such as murine and canine noroviruses.
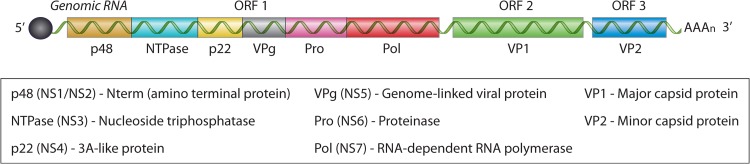
The human norovirus genome is composed of a linear, positive-sense RNA that is ∼7.6 kb in length (14). The genome is covalently linked to the viral protein genome (VPg) at the 5′ end and polyadenylated at the 3′ end (17). There are three open reading frames (ORFs), designated ORF-1, ORF-2, and ORF-3, encoding eight viral proteins (Fig. 2). ORF-2 and ORF-3 encode the structural components of the virion, viral protein 1 (VP1) and VP2, respectively. The mature virion contains 90 VP1 dimers assembled with icosahedral symmetry and arranged in such a fashion as to create hollows or cup-like structures on the virus surface. Hence, calici is derived from the Latin word calyx, or “cup” (16). ORF-1 encodes a polyprotein that is proteolytically processed into the 6 nonstructural proteins, including the norovirus protease and RNA-dependent RNA polymerase (17).
FIG 2.
The human norovirus genome. The genome is comprised of a linear, positive-sense RNA, ∼7.6 kb in length, covalently linked to the viral protein genome (VPg) (solid black circle) at the 5′ end and polyadenylated at the 3′ end. There are three open reading frames (ORFs), designated ORF-1, ORF-2, and ORF-3, encoding 8 viral proteins. ORF-1 encodes the 6 nonstructural (NS) proteins that are proteolytically processed by the virally encoded cysteine proteinase (Pro). ORF-2 and ORF-3 encode the structural components of the virion, viral protein 1 (VP1) and VP2, respectively.
Norovirus has been likened to a “shape-shifter” (18), a mythical creature that can change form or being. This description refers to its diversity, with, as determined by the VP1 amino acid sequence, at least 6 genogroups (genogroup I [GI] to GVI) and >40 genotypes (18, 19), together with its continued evolution, apparently in response to the selective pressure exerted by the human immune system (20). Human norovirus infections are caused, in decreasing order of frequency, by GII (mostly GII.4), GI, and, to a very limited extent, GIV (some genotypes of which also infect pigs) (21, 22). In addition, antibodies to GIII, a bovine norovirus, have been detected in ∼22% of humans (23), and antibodies to GVI (a genogroup that has been identified only in canines) have been detected in 22.6% of small-animal veterinarians and 5.8% of age-matched controls (24).
The genetic diversity of the human noroviruses is apparent from the observation that VP1 amino acid sequences of GII.4 strains differ by 37% to 38% from the prototypic GI.1 Norwalk virus strain and by 5% to 7% within the GII genotype alone, with as much as a 2.8% difference between strains of an individual virus (19, 25). This variability, resulting from both recombination and mutational events, is of a sufficient magnitude that it has led to the suggestion that the use of sequence difference cutoffs is no longer sufficient to classify noroviruses and that the addition of a phylogenetic approach would prove more accurate (26).
CLINICAL FEATURES OF NOROVIRUS INFECTION
Asymptomatic Infection
Fecal excretion of norovirus infection in asymptomatic individuals is common, especially in children (Table 1). Asymptomatic excretion of norovirus was detected in 19 of 163 (11.7%) children in Leon, Nicaragua (27), while in periurban Mexico City, norovirus was detected in stool samples of 31 of 63 (49.2%) asymptomatic children (28). Over a 2-year period, 37.5% of 56 children attending a day care center in central Brazil had at least one episode of asymptomatic fecal excretion of norovirus (29). Norovirus was detected as frequently in stool samples of asymptomatic as in stool samples of symptomatic children in Burkina Faso (30).
TABLE 1.
Summary of selected norovirus asymptomatic infection studies
| Reference | Location | Population | % of subjects with stool carriage (no. of subjects with stool carriage/total no. of subjects) |
|---|---|---|---|
| 27 | Nicaragua | Pediatric | 11.7 (19/163) |
| 28 | Mexico | Pediatric | 49.2 (31/63) |
| 29 | Brazil | Pediatric | 37.5 (21/56) |
| 30 | Burkina Faso | Pediatric | 24.8 (31/125)a |
| 31 | France | Pediatricb | 11.6 (5/43) |
| 32 | England | Adult/pediatric | 16.4 (361/2,205)c |
| 33 | South Korea | Food handlers | 1.0 (66/6,441) |
| 34 | South Korea | Food handlers | 3.4 (26/776) |
This percentage was 21.2% for symptomatic children.
With inherited immunodeficiency.
Age-adjusted prevalence of 12%
Asymptomatic infection is not limited to individuals residing in lesser-developed countries. Viral RNA was detected in fecal samples of 5 of 43 (11.6%) children who had a variety of inherited immunodeficiencies admitted to Necker Hospital in Paris, France, but who lacked gastrointestinal symptoms (31). More generally, norovirus was detected in the stool samples of 361 of 2,205 asymptomatic individuals in England, with the prevalence being highest in those <5 years of age, exceeding 25% through 3 years of age, but the prevalence was also >5% in those 35 years of age and older. The overall prevalence after adjusting for age was 12% (95% confidence interval [CI], 11% to 14%) (32).
Asymptomatic excretion of norovirus has diagnostic implications. Diarrhea due to another cause in an asymptomatic carrier may be misdiagnosed as being due to norovirus infection. Carriage also has epidemiological implications. A study in South Korea detected norovirus RNA in the stool samples of 66 of 6,441 (1.02%) asymptomatic food handlers (33). A smaller study found that 26 of 776 (3.4%) asymptomatic food handlers at elementary schools in Incheon, South Korea, were excreting the virus (34). A clear adverse consequence of asymptomatic carriage resulted in the transmission of norovirus by fecal microbiota transplantation using stool samples obtained from asymptomatic donors who had not been screened for the presence of the virus (35).
Symptomatic Infection
Incubation period.
The incubation period is relatively brief in most infected individuals who develop symptoms (Table 2). Examination of the onset of secondary cases in the index outbreak in 1968 led to an estimated incubation period of ∼48 h (3). In a Swedish outbreak involving children and staff at day care centers, the mean incubation period after foodborne transmission was 34 h, while that for secondary person-to-person transmission to household members was ∼52 h (36). A recent systematic review of the literature concluded that the median incubation period for genotype I and II infections is 1.2 days (95% CI, 1.1 to 2.2 days) (37). In contrast to these findings, Rockx and colleagues reported that the median duration of illness was 4 days in community-acquired cases (38). Longer estimates such as these may, however, be the result of misclassification of secondary transmissions as primary transmissions.
TABLE 2.
Summary of selected norovirus incubation time studies
| Reference | Locationc | Time to index infection (h) | Time to secondary infection (h) |
|---|---|---|---|
| 3 | USA | 48 | |
| 36 | Sweden | 34 | 52 |
| 37a | NA | 29 | |
| 38 | Netherlands | 96b |
Systematic review.
Community onset.
NA, not applicable.
Signs, symptoms, and disease course.
As seen in the index outbreak as well as in experimental passage studies, the dominant symptoms of norovirus infection are vomiting and diarrhea and are generally of a relatively short duration (Table 3). For example, in an analysis of 4 outbreaks over 3 years in an inpatient psychiatric unit in Taiwan that affected 172 patients and 7 health care workers (39), diarrhea occurred in 87.5% and vomiting occurred in 25.5% of patients, while 4.4% complained of abdominal pain and 2.2% had fever. The mean duration of symptoms was 2.1 ± 1.5 days, with a range of 1.2 to 2.8 days; 86.4% of patients had symptom resolution within 1 to 3 days.
TABLE 3.
Summary of selected norovirus symptomatic infection studies
| Reference | Location | Population | No. of patients | Symptom (% of patients)a |
|---|---|---|---|---|
| 39 | Taiwan | Psychiatric inpatients | 179 | Diarrhea (87.5) |
| Vomiting (25.5) | ||||
| Abdominal pain (4.4) | ||||
| Fever (2.2) | ||||
| 40 | USA | Military trainees | 99 | Nausea (88) |
| Vomiting (80) | ||||
| Abdominal pain (76) | ||||
| Diarrhea (67) | ||||
| Fever/chills (47) | ||||
| Headache (22) | ||||
| 41 | Afghanistan | Deployed British military personnel | 29 | Gastrointestinal symptoms (100) |
| Headache (10) | ||||
| Neck stiffness (10) | ||||
| Photophobia (10) | ||||
| Obtundation (10) | ||||
| DIC (3) | ||||
| 42 | UK | Hospital patients | 730 | Diarrhea (85) |
| Vomiting (56) | ||||
| Duration of 3 days | ||||
| 42 | UK | Hospital staff | 482 | Diarrhea (66) |
| Vomiting (73) | ||||
| Duration of 2 days | ||||
| 42 | UK | Nursing home residents | 266 | Diarrhea (73) |
| Vomiting (66) | ||||
| Duration of 2 days |
DIC, disseminated intravascular coagulation.
More severe illness may, however, occasionally occur in previously medically healthy individuals. Among 99 military trainees with norovirus infection, 88% had nausea, and 80% vomited, while 76% complained of abdominal pain, 67% had diarrhea, 47% had fever or chills, and 22% had headache (40). The temperature exceeded 100.4°F in 17% of subjects, while 17% had leukocytosis and 37% had thrombocytopenia. More dramatically, in an outbreak involving 29 British military personnel deployed to Afghanistan, the first 3 patients presented not only with gastrointestinal symptoms and fever but also with headache, neck stiffness, photophobia, and obtundation (41). One patient had disseminated intravascular coagulation, and two patients required ventilatory support. It is possible, if not likely, that an undetected second infection was present.
The illness may be more severe and prolonged in individuals with medical comorbidities. An examination of norovirus outbreaks in United Kingdom health care facilities provided an opportunity to compare the clinical pictures of presumably healthy health care providers with presumably less healthy patients (42). An analysis of a large number of confirmed cases in multiple health care facilities found that 66% of affected hospital staff had diarrhea and that 73% vomited, with these proportions being reversed in nursing home residents. Among hospital patients, diarrhea dominated, being present in 85% subjects, while only 56% vomited. While the median duration of illness in hospital staff and nursing home residents was 2 days (with 3 days being at the 75th percentile of distribution for both), it was 3 days (75th percentile, 5 days) in hospital patients, and 40% of patients ≥85 years of age were still symptomatic after 4 days.
Complications: severe disease and mortality at the extremes of age.
Infections occurring during outbreaks of GII.4 strains are associated with more severe outcomes, including mortality, than infections during outbreaks of non-GII.4 strains (43). Advanced age is a risk factor for a fatal outcome. Thus, in the Netherlands, norovirus outbreaks were significantly associated with excess mortality in individuals >85 years of age, coinciding with the emergence of new viral variants (44). Norovirus accounted for 0.5% of total deaths from 1999 to 2007 in individuals in this age group. A study of 82 patients with community-acquired norovirus infection with a median age of 77 years found an overall 30-day mortality rate of 7%, with elevated venous lactate levels at the time of hospital admission being predictive of mortality (45). Estimates indicate that 20% (95% CI, 13.3% to 26.8%) of deaths of persons >65 years of age caused by infectious intestinal disease other than Clostridium difficile in England and Wales from 2001 to 2006 were associated with norovirus infection (46). Furthermore, 13% (95% CI, 7.5% to 18.5%) of deaths caused by noninfectious intestinal disease were associated with norovirus.
Individuals at the other extreme of age are also at risk. In a study in Japan involving 71 children, the mean duration of illness was twice as long (7 days versus 3.5 days) in those <2 years of age than in those 2 to 4 years of age, and the severity of illness was greater (47). Norovirus infection in term and preterm neonates causes the full range of symptoms and signs seen in other patient groups but may also be associated with very serious complications such as necrotizing enterocolitis (48). Of 8 neonates (mean gestational age, 28 weeks) who developed norovirus-associated necrotizing enterocolitis at the age of 15 to 38 days, 2 died (49). A retrospective study found that 1 of 8 neonates with a mean gestational age of 29 weeks and with norovirus infection occurring at a postnatal age ranging from 8 to 92 days developed necrotizing enterocolitis (50). Of the other 7 neonates, all had symptoms of gastroenteritis with diarrhea and abdominal distention, 4 had apnea, 3 vomited, and 3 had blood in their stool. The mean duration of illness was 5 days (range, 2 to 11 days). While neonatal necrotizing enterocolitis involves predominantly the small bowel, Pelizzo and colleagues reported 3 premature norovirus-infected infants with ischemia of the colon without involvement of the small intestine (51). A case of an adult with norovirus gastroenteritis and ischemic colitis has also been reported (52).
Norovirus in immunocompromised hosts.
Norovirus infection-associated illness may also be more prolonged and severe in immunocompromised individuals and may be associated with remarkably persistent viral excretion in some of these individuals (Table 4).
TABLE 4.
Summary of selected studies on norovirus infection in immunocompromised hosts
| Reference | Patient population | Duration of symptoms (days) | Duration of viral excretion (days) |
|---|---|---|---|
| 53 | HSCT recipients | 6–33 | |
| 54 | HSCT recipients | 2–36 | 11–87 |
| 55 | HSCT recipients | 15–420 | 15–420 |
| 56 | Pediatric HSCT recipients | 13–263 | |
| 57 | Pediatric HSCT recipients | 60–380 | |
| 60 | Renal transplant recipients | 24–898 | 97–898 |
| 61 | Renal transplant recipients | 37–1,004 | 6–581 |
(i) Hematopoietic stem cell transplant recipients.
Norovirus diagnosis in hematopoietic stem cell transplant (HSCT) recipients is complicated by the frequent occurrence and multiple causes of diarrhea, including gastrointestinal graft-versus-host disease (GVHD), in these patients.
A small outbreak was confirmed to be caused by norovirus in 6 of 8 patients with watery diarrhea in a bone marrow transplant unit (53). In two of these patients, norovirus infection was superimposed on graft-versus-host disease involving the gastrointestinal tract. All patients were febrile. Gastrointestinal symptoms persisted for 8 to 33 days. No deaths were attributed to norovirus infection.
An outbreak of a norovirus GII.4 variant strain in a hematologic and transplantation unit affected 11 patients and 11 staff members (54). Six of the patients had been treated for lymphoma, and five had been treated for acute leukemia. Five patients had undergone hematopoietic stem cell transplantation (2 autologous and 3 allogeneic), while three had received chemotherapy and one had received only rituximab; seven were neutropenic. The median duration of symptoms was only 3 days in staff but was 7 days (range, 2 to 36 days) in patients. All 11 patients had diarrhea, while 8 of 11 had vomiting and 6 were febrile. Two patients who underwent abdominal computerized tomography had evidence of small bowel edema. To evaluate for the presence of gastrointestinal GVHD, the 3 patients who had undergone allogeneic HSCT had duodenal biopsy specimens that demonstrated villous blunting, slightly increased numbers of apoptotic crypt cells, and markedly increased numbers of intraepithelial CD8+ T lymphocytes. Three patients died, one with aspiration and two with sepsis.
Twelve allogeneic HSCT recipients with norovirus infection had onset of diarrhea for 0.25 to 6 months prior to diagnosis (55). Eleven patients were receiving immunosuppressive therapy, including two who received it for presumed GVHD (which proved to be absent). Ten patients had vomiting of a short duration. Two patients died after 4 months of diarrhea, one of these due to malnutrition and the other for unrelated reasons. In the other 10 patients, diarrhea lasted for 0.5 to 14 months (median, 3 months), with evidence of continued viral shedding.
Over a 3-year period, of 49 HSCT patients in Hong Kong <21 years of age with diarrhea, 8 (7 allogeneic HSCT recipients and 1 autologous umbilical cord cell recipient) were found to have norovirus infection (56). The cumulative incidence at 2 years was 12.9%. Two patients were infected prior to transplantation, and the infection persisted thereafter. The median age of patients was 5.2 years, and 6 were receiving immunosuppressive therapy. Four patients also had Clostridium difficile infection, but diarrhea persisted after treatment. Viral excretion persisted for 13 to 263 days (median, 145 days).
Of 61 children who received HSCT at a single center, 13 were found at some time in the course of treatment to excrete norovirus in association with diarrhea and vomiting (57). Norovirus excretion persisted for 60 to 380 days (median, 150 days), and resolution of infection correlated with time to CD3+ T cell recovery. All patients required prolonged enteral and parenteral nutritional support.
(ii) Solid organ transplant recipients.
In an analysis of 24 immunocompromised patients (mostly pediatric), most of whom were recipients of solid organ transplants, the mean duration of norovirus-associated diarrhea was ∼12 days (58). Westhoff and colleagues reported two renal transplant recipients with persistent norovirus excretion, one for >7 months and the other for 3 months (59). The first patient had resolution of symptoms after acute infection but continued to excrete the virus until spontaneous clearance occurred. The second patient had recurrent symptoms that resolved, along with norovirus excretion, with a reduction in his immunosuppressive regimen.
Over a 2-year period, 13 of 78 (16.7%) adult renal allograft recipients with prolonged or severe diarrhea were found to have norovirus infection (GII.7 and GII.17 in one patient each and GII.4 in seven patients) (60). All patients were receiving immunosuppressive therapy. Symptoms lasted for 24 to 898 days, and viral excretion lasted for 97 to 898 days. In each case, diarrhea was associated with increased serum creatinine concentrations, and 5 patients required hospitalization because of severe dehydration and allograft dysfunction. A reduction in immunosuppressive therapy was associated with symptom improvement or resolution in all cases but had no apparent effect on viral shedding, which resolved in only 3 patients.
In a retrospective study of 15 renal allograft recipients with diarrhea and norovirus infection, 14 infections were caused by GII strains, mostly GII.4 strains (61). The mean duration of diarrhea was 8.7 months. Patients lost a mean of 8.5% of their body weight. Acute renal failure occurred in four-fifths of patients, and 5 patients had graft rejection. Four patients underwent colonoscopy with biopsy; macroscopic and histological results were all normal. Immunosuppressive therapy was reduced in all patients by lowering the mycophenylate dose or discontinuing it altogether, often by substituting azathioprine.
Norovirus was detected in stool samples during 13 of 54 (36%) severe diarrhea events in 49 kidney transplant recipients, with receipt of cyclosporine together with mycophenylate being a risk factor (62). It was associated with a mean weight loss of 3.0 kg, which was significantly greater than the weight loss associated with other causes of diarrhea. Complicating interpretation is that norovirus was detected in 10% of 30 renal transplant recipients without diarrhea.
(iii) Miscellaneous immunocompromising conditions.
Two chronic lymphocytic leukemia patients with hypogammaglobulinemia and with chronic diarrhea had persistent fecal norovirus excretion (63). Chronic diarrhea in association with profound immunocompromise due to HIV infection has also been reported. In one case, the intravenous administration of human immunoglobulin was ineffective, but diarrhea resolved after improvement in the CD4+ T cell response to antiretroviral therapy (64).
Unusual manifestations of norovirus infection.
Norovirus GII.4 was detected in 3 of 562 (0.5%) nasopharyngeal samples of children with influenza-like illness (65). In each of the 3 patients, respiratory symptoms antedated gastrointestinal illness. Norovirus has also been detected in endotracheal aspirates of 2 preterm infants with intestinal norovirus infection, raising a question about the frequent need for oxygen supplementation in these patients (66).
Hemophagocytic lymphohistiocytosis has been described for a 24-month-old boy with chronic norovirus infection who underwent matched unrelated HSCT for relapsed acute myelogenous leukemia (67). A pediatric renal allograft recipient presented with granulocytopenia and fever, which resolved together with the resolution of symptoms of norovirus infection (68). An elderly patient with norovirus gastroenteritis developed hemolytic-uremic syndrome (69). Ischemic colitis has been reported for an adult (52) and several neonates in association with norovirus infection (51). Transient hepatocellular injury manifesting as significant elevations in transaminase levels has been reported for an adult (70) and for children with norovirus infection (71). In the 4 pediatric cases reported, the serum transaminase levels peaked a mean of 13.8 days after the onset of gastroenteritis symptoms and resolved after ∼4 weeks (71).
The etiologic relationship of norovirus infection in these cases is, however, unproven. This is also true for some putative postinfectious illnesses that have been suggested to have an association with norovirus infection. Gemulla and Pessler reported 2 cases of possible postinfectious arthritis related to norovirus infection (72). Thirteen percent of patients who had been affected during a waterborne norovirus outbreak and who responded to a questionnaire reported symptoms consistent with irritable bowel syndrome 12 months after their infection (73).
A case-control study found an association of norovirus infection with exacerbations of pediatric inflammatory bowel disease (8 with ulcerative colitis and 1 with Crohn's disease) (74). However, two subsequent studies failed to identify a significant relationship between infection with this virus and exacerbations of inflammatory bowel disease (75, 76). A large case-control study of military personnel who had symptoms of acute gastroenteritis during 3 norovirus outbreaks failed to find subsequent evidence of an increased risk of irritable bowel syndrome but did detect a 1.5-fold increased incidence of constipation, dyspepsia, and symptoms of gastrointestinal reflux disease relative to controls (77).
Central nervous system manifestations have also been reported in association with norovirus infection. Encephalopathy has occurred in children and adults (78–80). While the outcome is generally benign, including in one case in which the virus was detected in cerebrospinal fluid (CSF), magnetic resonance imaging (MRI) abnormalities and permanent neurological sequelae occurred in a patient in whom the virus was detected in plasma but not cerebrospinal fluid (80). Medici and colleagues also reported finding viral RNA in the plasma of an infected child with seizures (81). Notably, a study of 39 children with uncomplicated norovirus infection detected viral RNA in the serum of 6 (15%) cases but in the CSF of none (82).
While encephalopathy has been uncommonly reported in association with norovirus infection, the occurrence of seizures appears to be more frequent. Convulsions occurred in 15 of 173 (8.7%) afebrile (≤38°C) children with norovirus gastroenteritis admitted to a hospital in Hong Kong, a frequency 5 times higher than that for children with rotavirus infection (83). In Taiwan, 19 of 64 (29.7%) children hospitalized with gastroenteritis due to norovirus developed seizures, a frequency 6 times higher than that seen in children hospitalized with rotavirus infection during the same period despite the greater severity of fever in the latter group (84). The median age of patients was 18 months (range, 15 to 21 months). Only 2 patients in the norovirus group presented with a temperature of >39°C. No subjects had neurological sequelae at follow-up in 1 year.
MANAGEMENT OF NOROVIRUS INFECTION
The treatment of norovirus gastroenteritis is supportive, involving primarily the reversal of dehydration and electrolyte abnormalities. Antiemetics and antimotility agents may play a role in some patients.
In patients with persisting symptoms, especially neonates, the elderly, and the immunocompromised, the availability of specific therapy would be of value, but no therapy has been clearly demonstrated to be effective. However, a small, blind, placebo-controlled trial of children with viral gastroenteritis found that nitazoxanide administration was associated with a reduced duration of illness, including in a subset of patients with norovirus infection (85). Siddiq and colleagues reported resolution of diarrhea in a norovirus-infected patient with refractory acute myelogenous leukemia and HSCT after administration of nitazoxanide (86).
Enteric administration of human immunoglobulin was associated with resolution of chronic diarrhea in a transplant patient (87). Florescu and colleagues performed a matched case-control study to evaluate the potential benefit of orally administered human immunoglobulin in the treatment of symptomatic norovirus infection in immunocompromised patients. Cases received 25 mg/kg of body weight of human immunoglobulin (Gamunex; Talecris Biotherapeutics, Inc., NC, USA) every 6 h for a total of 8 doses (58). Although the median age of 24 total cases plus controls was 2 years, one-third were adults, and 20 were solid organ transplant recipients. Coinfection with Clostridium difficile or rotavirus was present in 8 patients. Administration of immunoglobulin was associated with a numerical decrease in stool output at 7 days (P = 0.09). However, while this 7-day interval was counted from the time of treatment in cases, it was counted from the time of diagnosis in controls, potentially leading to bias favoring immunoglobulin administration. Despite this potential bias, there was no significant difference in the time to resolution of diarrhea between cases and controls, with the duration being ∼12 days for each group. There was a numerically greater frequency of resolution of diarrhea in cases (P = 0.078), without a statistically or clinically significant difference in the total time to resolution of diarrhea or length of hospital stay. Looking toward a potential future therapy, Chen and colleagues developed a set of norovirus-specific monoclonal antibodies (88).
A reduction of immunosuppressive therapy is indicated whenever feasible. In one case report, a reduction in immunosuppressive therapy together with a change from a calcineurin inhibitor (tacrolimus) to an inhibitor of mTOR (sirolimus) was associated with resolution of chronic diarrhea in a double-transplant recipient (HSCT and lung) (89). Similarly, Engelen and colleagues altered the immunosuppressive therapy of a heart transplant recipient with chronic norovirus-associated diarrhea by substituting everolimus for tacrolimus, with subsequent prompt resolution of diarrhea (90).
Attempts at prophylaxis have been unsuccessful to date. Prophylactic administration of bovine lactoferrin failed to prevent diarrhea, including that due to norovirus, in children (91). In this randomized, placebo-controlled trial in Peru, 544 previously weaned children 12 to 18 months of age were visited daily for 6 months, and stool samples were collected monthly and during episodes of diarrhea. Norovirus nucleic acid was detected in 34.2% of stool samples from lactoferrin recipients and in 35.5% of stool samples from placebo recipients. The prophylactic administration of “probiotic fermented” milk containing a strain of Lactobacillus casei failed to prevent norovirus infection in elderly residents of a health service facility in Japan (92).
EPIDEMIOLOGY
Norovirus is a well-described cause of epidemic gastroenteritis in both adult and pediatric populations across a wide range of geographic regions (93–97). The U.S. Centers for Disease Control and Prevention (CDC) estimates that norovirus is responsible for 60% of acute gastroenteritis cases (with a known cause), or 21 million cases, in the United States each year. With the addition of molecular methods, norovirus has also been increasingly implicated in sporadic disease (98). A systematic review of all reports of norovirus detected by reverse transcriptase PCR (RT-PCR) attributed 5 to 31% of cases of gastroenteritis in hospitalized patients and an additional 5 to 36% of cases in all patients seeking outpatient evaluation to norovirus (7). The CDC estimates that norovirus is responsible for 400,000 emergency department visits and 71,000 hospitalizations annually, although these numbers may be underestimates due to the limited number of patients who seek medical attention for viral gastroenteritis symptoms (99).
The general population is broadly vulnerable to disease across all age groups, but the majority of morbidity and mortality occurs at the extremes of age. The fecal-oral route is the main mode of transmission, although several other modalities have been described. These modalities include transmission via aerosolized viral particles in vomitus (100, 101) and through food, water, and environmental contamination (102). Some studies have associated certain norovirus genotypes with particular modes of transmission. For example, Vega and colleagues, reviewing norovirus trends in the United States from 2009 to 2013, demonstrated that GII.4 was more likely to be associated with person-to-person transmission, especially in long-term-care facilities (LTCFs) and hospital settings, whereas GI.7 and GII.12 were more frequently associated with foodborne disease (103). The environmental durability of norovirus leads to persistence of the pathogen in clinical settings and other closed-space environments, thus complicating complete disinfection and allowing for recurrent outbreaks (104, 105).
Waterborne Outbreaks
Norovirus was first reported as the causative agent of a waterborne gastrointestinal disease outbreak by Kaplan et al. in 1982. The outbreak affected ∼1,500 people in a small community in Georgia, with the highest attack rates occurring in geographic areas closest to points of interconnection between industrial and municipal water systems, where the industrial water was noted to contain coliform contamination. Evidence of norovirus was determined by increased antibody titers to Norwalk virus in patient serum (106). The diversity of waterborne sources implicated in norovirus outbreaks ranges widely, indicating the ubiquitous distribution of the virus. Outbreaks have been linked to potable water sources at camps, municipal water systems, commercial ice consumption, and recreational water exposure during rafting and swimming (107–112). While much of the water contamination is thought to come from discharge of wastewater into rivers, streams, and other bodies of water, the natural distribution of noroviruses in water systems has not been thoroughly explored. Much of the difficulty in characterizing the environmental distribution of norovirus is tied to testing limitations on diverse environmental sources (113). Bivalves, such as oysters and other shellfish, often represent the link between environmental water contamination and foodborne outbreaks, as these mollusks are thought to concentrate viruses and other microbes (113).
Water contamination with norovirus, despite sewage treatment, has been documented, with viral concentrations peaking during colder months (114). Several different methods are used to remove and reduce the burden of noroviruses in water systems, including the use of waste stabilization ponds, the application of activated sludge, or the use of submerged-membrane bioreactor treatments. These modalities have been evaluated for their ability to remove viruses from wastewater (114–117). Insufficient chlorination has been linked to some outbreaks (118).
Food, Restaurants, and Catering
Food presents two distinct models for norovirus transmission. The first is direct norovirus contamination of food at the point of production, and the second is contamination of food during preparation. In a review of several hundred reported norovirus outbreaks, foodborne transmission (362/666; 54%) and foodservice settings (294/830; 35%) were most commonly implicated (102). Fruits, vegetables, and shellfish pose a significant risk for disease transmission because they are consumed raw and may be subject to norovirus contamination from water sources. Norovirus has been recovered from a variety of food products ranging from oysters to romaine lettuce, raspberries, and other produce (119–125). Additionally, the use of contaminated water for reconstituting food products has been implicated in widespread outbreaks (e.g., custard in Bristol, United Kingdom) (126). Rebedding of shellfish and depuration with purified seawater at increased temperatures have been used to reduce the burden of norovirus from shellfish in contaminated waters (127). Various food surface decontamination strategies have been explored to enhance the produce safety and decrease viral transmission, including treatment with sodium bicarbonate and chlorine (128, 129).
Foodborne outbreaks are often linked to hygiene breaches during preparation and are often tied to ill food handlers and common source producers. Ill family members of food handlers may represent a reservoir for disease (130). Norovirus outbreaks in restaurants and canteens have been documented and have been linked to catering companies, with the extent of outbreaks varying from several isolated cases to large multistate events. In one outbreak investigated by the U.S. CDC, illnesses in 13 states among 333 people were tied to workers at a single caterer providing “boxed lunches” to series of car dealerships (131). The food preparation environment has been evaluated for reservoirs of viral persistence and methods of enhanced cleaning to reduce transmission (132).
Sporadic Disease
Caliciviruses, especially noroviruses, are important causes of sporadic gastrointestinal disease. A recent study identified norovirus as the most common causative agent in sporadic gastroenteritis as part of a multitarget PCR analysis (133). These sporadic cases may occur in individuals or within a small family cluster, and several studies documenting sporadic disease have been conducted with pediatric populations (134–138). In a study of Finnish children who developed sporadic gastroenteritis and who were being monitored as part of a rotavirus vaccine trial, human caliciviruses were isolated in 20% of placebo patients and 22% of vaccine recipients, making them the second most frequently isolated viruses in community patients with acute gastroenteritis (139). Subsequent studies in Thailand (138), Malawi (137), Chile (140), and India (134) confirmed norovirus as a common (typically first or second only to rotavirus) cause of sporadic acute gastroenteritis. A 5-year surveillance study of children <5 years old conducted in Melbourne, Australia, demonstrated that the prevalence of calicivirus infection during the study period was 9.2% (113/1,233), with 95% of the strains belonging to the norovirus genus (141).
A U.S. surveillance study of pediatric populations across three geographically distinct children's hospitals documented an 8.5% prevalence rate for caliciviruses in children with acute gastroenteritis, the vast majority of which (84%) were due to norovirus (142). These results were consistent with previous surveillance studies, although the results did not conform to seasonal trends.
OUTBREAKS
Norovirus outbreaks have been reported in a variety of settings and are uniquely suited to areas of close living quarters, shared dining facilities, and difficult environmental maintenance.
Health Care Settings
Norovirus is frequently implicated in hospital ward and nursing home outbreaks. These closed-space outbreaks can present both a logistic challenge in terms of eradicating the source and a financial burden to health care institutions. One Swiss study attributed substantial direct costs to reduced capacity due to bed closures from staff illness rather than the actual cost of outbreak investigation and expanded laboratory testing (143). Beyond the financial burden, ward closure due to norovirus outbreaks can also have a negative impact on patient care due to staff shuffling and the potential for transferring patients to units without appropriate specialized nursing care.
Within hospitals, person-to-person transmission is the primary mode of transmission and can occur between both patients and staff. In one review of reported nosocomial outbreaks, researchers estimated that more cases are involved in outbreaks tied to patient index cases than in staff-originated outbreaks and that patient-indexed outbreaks require more aggressive hygiene interventions in order to disrupt viral transmission (144).
While hospitalized patients represent one reservoir for possible transmission due to nosocomial transmission, the patients themselves are also subject to many of the same traditional risk factors as community patients. Pether and Caul described an outbreak of norovirus in patients due to chicken sandwiches served by an infected health care worker at two hospitals (145).
Schools
Schools and day care settings are frequently implicated in acute gastrointestinal outbreaks. Reports of norovirus outbreaks have been documented across the spectrum of age from day care to college settings (36, 146–148). Multiple modes of transmission, including person-to-person, foodborne, and aerosolized vomitus modes, have been described in these outbreaks, and control can be complicated by asymptomatic viral excretion (29).
Military
Norovirus is a major cause of morbidity in military training centers and fields of operation for many of the same reasons that it causes disease in civilian settings: close living quarters, the low viral inoculum required for infection, persistent viral shedding, viral resistance to disinfection, and environmental durability (41, 149). Reports of noroviral disease among military personnel have been documented in a French parachuting unit, British military forces in Afghanistan, the U.S. Air Force Academy, and the Israeli army (41, 150, 151).
Cruise Ships and Resorts
Numerous well-publicized norovirus outbreaks have been associated with cruise ships dating back several decades (152–155). The CDC Vessel Sanitation Program maintains a list of gastrointestinal outbreaks of public health significance in vessels sailing for 3 to 21 days, carrying 100 or more passengers, and on which 3% or more of passengers or crew report diarrheal symptoms to onboard medical staff during the voyage (156). Shared living and dining quarters, in addition to passenger and crew disincentives for reporting illness, have been implicated in outbreaks (101). Norovirus outbreaks have also been reported in land-based resorts (157, 158). Risk factors for disease acquisition are similar to those reported during cruise ship outbreaks, such as sharing rooms with previously affected persons and witnessing episodes of public vomiting (101, 158).
Outbreak Reporting Systems
Once norovirus was recognized as a major etiologic agent of acute gastroenteritis and a significant cause of morbidity and cost within health care systems, several countries developed robust surveillance networks to monitor norovirus activity and outbreaks. These systems range from stand-alone norovirus data collection tools to broader electronic surveillance systems for all types of infectious diseases. In terms of geographic scope, reporting systems can be either single-country electronic reporting systems, such as Germany's SurvNet@RKI, which integrates nosocomial norovirus outbreak information into a larger infectious disease surveillance system (159), to multinational molecular epidemiologic surveillance efforts such as NoroNet. Spearheaded by the National Institute for Public Health and the Environment of the Netherlands (RIVM), NoroNet is an informal network of university and public health scientists, which maintains a database of norovirus sequences and monitors outbreak activity. NoroNet is comprised of reporting members across Europe, Asia, and Australia and has been helpful in identifying the emergence of new variant strains (160). A centralized European system for monitoring foodborne outbreaks, the Food-Borne Viruses in Europe Network, or FBVE, also includes norovirus reporting (161).
In the United States, a multifaceted surveillance network was developed by the CDC for monitoring enteric pathogens. Bacterial pathogens are monitored via PulseNet and typed by using pulsed-field gel electrophoresis methodology (162), whereas enhanced surveillance for noroviruses was launched in 2009 through a network called CaliciNet. While all states had the ability to perform norovirus testing by 2008, strain typing was not uniformly performed and was typically supported through the CDC National Calicivirus Laboratory (NCL). In an effort to provide robust, epidemiologically accurate data collection and dissemination, the CDC deployed a standardized method of strain typing to certified state and local laboratory members of CaliciNet to perform strain identifications and collaborate on outbreaks. Member laboratories are required to pass a yearly proficiency test in typing methodologies (163). CaliciNet proved useful in documenting the emergence of a new strain in 2012 (164).
Beyond the identification of new strains, norovirus outbreak reporting has demonstrated the significant financial and staff resource burdens placed on hospitals as a result of norovirus outbreaks. Launched in 2009 by the United Kingdom Department of Health, the Hospital Norovirus Outbreak Reporting System (HNORS) collects direct reports from infection preventionists about suspected and confirmed norovirus outbreaks. A 2013 review of the system's first full season demonstrated that more outbreaks were documented in that period than in the previous 17 years of norovirus reporting (1,884 versus 1,817) and helped document the significant financial implications of norovirus outbreaks within the National Health Service (165). Outbreak surveillance networks dedicated specifically to foodborne sources (e.g., FoodNet [United States] and OzFoodNet [Australia]) may prove useful in identifying small-scale norovirus transmission events (102, 166, 167).
CLINICAL DIAGNOSIS AND ENVIRONMENTAL DETECTION
Rapid identification of an outbreak is the key to effective infection control. While molecular methods offer a definitive way to establish etiology, these diagnostic tests may not be available in some clinical settings due to time delays or resource limitations. Kaplan's clinical and epidemiologic criteria (Table 5), developed prior to the advent of molecular methods, can be used to rapidly identify norovirus outbreaks (168). The utility of Kaplan's criteria was reevaluated in 2006, and these criteria continued to prove useful in identifying norovirus outbreaks where molecular diagnostics were not easily accessible (169).
TABLE 5.
Kaplan's criteria
| Criterion | Description |
|---|---|
| 1 | Vomiting in more than half of symptomatic cases |
| 2 | Mean (or median) incubation period of 24 to 48 h |
| 3 | Mean (or median) duration of illness of 12 to 60 h |
| 4 | No bacterial pathogen isolated in stool culture |
Specimen Collection
Interpretation of norovirus diagnostic testing relies upon the quality of the specimens submitted for analysis and therefore requires that appropriate specimens are properly collected and handled (170). The optimal specimen for the diagnosis of norovirus infection is diarrheal stool. Specimens should be collected in a closed container within 48 to 72 h of the onset of symptoms, although norovirus may be detected in stool samples for 7 to 10 days or longer. Specimens should be refrigerated at 4°C prior to testing and frozen at −20°C or −70°C for long-term storage. Vomitus is an alternative specimen type that may be used to supplement stool sample testing during outbreak investigations. Collection and handling are the same as for stool specimens. Serum specimens are not recommended for routine diagnosis.
Norovirus Antigen Detection
Enzyme immunoassays.
A number of enzyme immunoassays (EIAs) are commercially available for the detection of norovirus GI and GII antigens in stool specimens. The most commonly performed EIAs are IDEIA Norovirus (Oxoid Ltd., Hampshire, United Kingdom) and Ridascreen Norovirus (R-Biopharm, Darmstadt, Germany). These solid-phase, sandwich-type immunoassays demonstrate a wide range of sensitivities and specificities (Table 6). For example, the sensitivities and specificities for IDEIA Norovirus range from 38.0 to 78.9% and 85.0 to 100.0%, respectively. Similarly, the sensitivities and specificities for Ridascreen Norovirus range from 31.6 to 92.0% and 65.3 to 100.0%, respectively. Factors contributing to these differences in performance include the viral load present in the stool specimen and the viral genotypes represented in the sample set, as the assay antibodies show differential genotype affinities. These characteristics in turn may be affected by the clinical context of collection (outbreak versus sporadic cases), the timing of collection relative to symptom onset, and patient demographics (pediatric versus adult). Further sources of variability include the use of different EIA kit lots and assay iterations, known as generations (at least two generations for IDEIA and three for Ridascreen), and the use of different nucleic acid amplification tests (NAATs) as reference methods.
TABLE 6.
Performance of norovirus antigen enzyme immunoassays
| Study reference | Test | Study location(s) | Case context | Population(s) | Sensitivity (%) | Specificity (%) | Reference method (reference[s]) |
|---|---|---|---|---|---|---|---|
| 363 | IDEIAa | UK | Outbreak | Not specified | 55.5 | 98.3 | Conventional RT-PCR (180, 364) |
| 365 | IDEIA | USA | Outbreak, sporadic | Pediatric, adult | 39.0 | 100.0 | Conventional RT-PCRd |
| SRSV(II)-ADb | 80.0 | 69.0 | |||||
| 193 | Ridascreenc | Germany | Outbreak, sporadic | Pediatric, adult | 34.6 | 65.3 | Nested RT-PCR (366) |
| 367 | Ridascreen | Australia | Outbreak | Not specified | 47.0 | 71.0 | Conventional RT-PCR (368) |
| 369 | IDEIA | Australia | Outbreak | Not specified | 66.0 | 85.0 | Conventional RT-PCR (368) |
| 370 | IDEIA | Netherlands | Not specified | 38.0 | 96.0 | Conventional RT-PCR (189) | |
| Ridascreen | 36.0 | 88.0 | |||||
| 371 | Ridascreen | Venezuela | Sporadic | Pediatric | 60.0 | 97.5 | Conventional RT-PCR (364) |
| 372 | IDEIA | Canada | Outbreak | Pediatric, adult | 60.6 | 100.0 | Compositee |
| Ridascreen | 80.3 | 100.0 | |||||
| 172 | IDEIA | European | Sporadic | Not specified | 46.2 | 95.7 | Conventional RT-PCRf |
| IDEIA | Multicenter | Outbreak | 65.9 | 95.7 | |||
| Ridascreen | Sporadic | 31.6 | 99.5 | ||||
| Ridascreen | Outbreak | 55.3 | 97.1 | ||||
| 373 | IDEIA | Spain | Sporadic | Pediatric | 76.9 | 85.9 | Conventional RT-PCR (189, 374) |
| Ridascreen | 59.0 | 73.1 | |||||
| 171 | IDEIA | USA, UK | Sporadic | Pediatric, adult | 59.0 | 93.3 | Compositeg |
| Outbreak | 58.7 | 88.9 | |||||
| 375 | IDEIA | Brazil | Sporadic | Pediatric | 45.0 | 100.0 | Real-time/conventional RT-PCR (184, 198) |
| Ridascreen | 63.0 | 100.0 | |||||
| 213 | IDEIA | Hungary | Sporadic | Pediatric, adult | 78.9 | 100.0 | Compositeh |
| 376 | Ridascreeni | Brazil | Sporadic | Pediatric, adult | 49.5 | 93.9 | Conventional RT-PCR (377) |
| Outbreak | 87.9 | 83.8 | |||||
| 378 | Ridascreeni | Brazil | Sporadic | Pediatric | 92.0 | 83.3 | Conventional RT-PCR (377) |
| 379 | Ridascreeni | Germany | Sporadic | Not specified | 77.0 | 96.0 | Compositej |
| 380 | Ridascreeni | Italy | Sporadic | Pediatric, adult | 40.0 | 96.0 | Real-time RT-PCR (116) |
Oxoid Ltd, Hampshire, United Kingdom.
Denka Seiken Co. Ltd., Tokyo, Japan.
R-Biopharm, Darmstadt, Germany.
RT-PCR primers were not specified.
At least 2 positive test results by RT-PCR (88), electron microscopy, and 2 enzyme immunoassays are considered a true-positive result.
Multiple RT-PCR assays were utilized in this study (76).
Utilizes a diagnostic algorithm (171) that takes into account electron microscopy, conventional RT-PCR/bidirectional sequencing (131, 184, 211, 381), and real-time RT-PCR (202).
At least 1 positive real-time RT-PCR test result by Argene Calici/Astrovirus Consensus (bioMérieux, Marcy l'Etoile, France) and SmartNorovirus (Cepheid, Sunnyvale, CA) is considered a true-positive result.
The third-generation Ridascreen Norovirus assay was used in this study.
At least 2 positive real-time RT-PCR test results by SmartNorovirus (Cepheid, Sunnyvale, CA), Ridagene Norovirus LC (R-Biopharm, Darmstadt, Germany), and an assay developed at the Robert Koch Institute (195) are considered a true-positive result.
Several studies that have evaluated the performance of EIA for detection of norovirus in outbreak investigations compared to sporadic gastroenteritis cases have shown that these assays are more sensitive in the outbreak setting, particularly if multiple samples are collected (171, 172). For example, in a large European multicenter study reported by Gray et al. (172), IDEIA had a 33.3% sensitivity and Ridascreen had a 44.4% sensitivity when two specimens per outbreak were tested. Sensitivity increased to 80.0% for both methods if >7 specimens per outbreak were tested. Similarly, Costantini et al. (171) reported that IDEIA had a 44.1% sensitivity when three specimens per outbreak were tested and a 77.8% sensitivity when >5 specimens per outbreak were tested. Based on statistical modeling, it has been estimated that at least six samples must be tested by EIA to achieve a 90% probability of detecting a norovirus outbreak (173). This minimum threshold of 6 outbreak samples has been adopted by the U.S. CDC and is recommended for outbreak management (174).
In 2011, the Ridascreen Norovirus third-generation test received Food and Drug Administration (FDA) approval for use in norovirus outbreak investigations. Notably, the intended use does not include the diagnosis of sporadic norovirus gastroenteritis cases. The package insert also acknowledges the relative insensitivity of EIA testing of limited sample numbers in outbreak settings. Compared to nucleic acid amplification tests, EIAs are generally simple to perform, do not require special molecular diagnostic laboratory facilities, and typically have a short turnaround time. For these reasons, the EIA is an attractive method for outbreak investigations, particularly in laboratories that lack molecular diagnostic capabilities. However, as nucleic acid amplification testing becomes commonplace in diagnostic and public health laboratories worldwide, real-time RT-PCR and other NAATs may replace the EIA entirely for outbreak investigation. This transition may be further hastened by the development of near-care and point-of-care molecular platforms capable of rapid sample-to-answer detection of norovirus RNA.
Rapid immunochromatographic assays.
Rapid norovirus antigen detection via lateral-flow immunochromatographic assays may provide an alternative to standard EIAs for stool screening in near-care or point-of-care settings. Several commercial rapid antigen assays are available for the rapid detection of GI and GII noroviruses, including Ridaquick Norovirus (R-Biopharm, Darmstadt, Germany) and SD Bioline Norovirus (Standard Diagnostics, Inc., Kyonggi-do, South Korea). Similar to the EIA literature, there is a wide range of reported sensitivities (Table 7), and the assays and study designs are subject to the same sources of variability. Ridaquick sensitivities range from 17.0 to 83.0%, and SD Bioline sensitivities range from 23.0 to 92.0%. Both of these tests show high specificity: 87.5 to 100.0% for Ridaquick and 99.7 to 100.0% for SD Bioline. Given these performance characteristics, positive test results are reliable, although negative test results may require follow-up with a more sensitive NAAT.
TABLE 7.
Performance of norovirus rapid antigen immunochromatographic assays
| Study reference | Test | Study location | Case context | Population(s) | Sensitivity (%) | Specificity (%) | Reference method (reference[s]) |
|---|---|---|---|---|---|---|---|
| 382 | Laboratory developed | Japan | Sporadic | Pediatric | 69.8 | 93.7 | Conventional RT-PCR (383) |
| 384 | Ridaquicka | Australia | Not specified | Not specified | 82.0 | 100.0 | Real-time RT-PCR (198) |
| 385 | Ridaquick | Netherlands | Not specified | Not specified | 57.1 | 99.1 | Real-time RT-PCR (195) |
| 375 | Ridaquick | Brazil | Sporadic | Pediatric | 69.0 | 98.0 | Real-time/conventional RT-PCR (284, 298) |
| 386 | Ridaquick | Australia | Sporadic, outbreak | Not specified | 83.0 | 100.0 | Compositeb |
| 387 | Ridaquick | USA | Not specified | Not specified | 61.4 | 100.0 | Real-time RT-PCR (387) |
| 379 | Ridaquick | Germany | Sporadic | Not specified | 69.0 | 97.0 | Compositec |
| 388 | SD Biolined | South Korea | Not specified | Pediatric, adult | 90.2 | 100.0 | Real-time RT-PCRe |
| 389 | SD Bioline | South Korea | Sporadic | Pediatric, adult | 76.5 | 99.7 | Real-time RT-PCRf |
| 390 | Ridaquick | Thailand | Sporadic | Not specified | 48.2 | 87.5 | Nested/real-time RT-PCRg |
| 391 | Ridaquick | France | Not specified | Not specified | 17.0 | 100.0 | Conventional RT-PCR (392) |
| ImmunoCardSTAT!h | 26.0 | 100.0 | |||||
| Norotopi | 52.0 | 100.0 | |||||
| SD Bioline | 23.0 | 100.0 |
R-Biopharm, Darmstadt, Germany.
Utilizes a diagnostic algorithm that takes into account electron microscopy and six conventional RT-PCR assays (386).
At least 2 positive real-time RT-PCR test results by SmartNorovirus (Cepheid, Sunnyvale, CA), Ridagene Norovirus LC (R-Biopharm, Darmstadt, Germany), and an assay developed at the Robert Koch Institute (195) are considered a true-positive result.
Standard Diagnostics, Inc., Kyonggi-do, South Korea.
Ridagene Norovirus (R-Biopharm, Darmstadt, Germany) and AccuPower Norovirus real-time RT-PCR kit (Bioneer, Daejeon, South Korea).
AccuPower Norovirus real-time RT-PCR kit (Bioneer, Daejeon, South Korea).
Norovirus real-time RT-PCR kit (Shanghai ZJ Bio-Tech, Shanghai, China) and nested RT-PCR (393).
Meridian Bioscience Europe, Nice, France.
All.Diag SA, Strasbourg, France.
Molecular Diagnostic Tests
Conventional RT-PCR.
RT-PCR is the gold standard for the detection and typing of norovirus, and numerous conventional and real-time norovirus RT-PCR assays have been developed. The first-generation norovirus assays utilized a variety of primers based solely on the first described Norwalk virus genome and required RT in a separate tube prior to PCR (12, 175–178). These assays underestimated norovirus genetic diversity and therefore did not perform well when applied to clinical specimens. The second-generation assays took advantage of sequences from additional norovirus strains and for the most part used primers directed at conserved regions of the viral polymerase (179–182). Importantly, these assays required post-PCR analysis via hybridization probes or sequencing to improve sensitivity and specificity. The difficulty in designing broadly reactive primers to accommodate norovirus diversity was illustrated in a study comparing a set of five additional second-generation, conventional RT-PCR assays tested against a panel of stool specimens selected to cover a range of norovirus genogroups/genotypes (183). Although 84% of the specimens were detected by at least one assay, the sensitivity of individual assays ranged from just 52 to 73%. These conventional assays were optimized in a variety of different ways to improve detection (184–189); however, there remained issues of assay complexity and postamplification specimen handling.
Real-time RT-PCR.
These limitations were addressed with the development of real-time RT-PCR for norovirus diagnostics. These assays used numerous detection methods, including SYBR green (190–193), hydrolysis (TaqMan) (194–202), and hybridization probes (203, 204). While many of these assays were directed at the viral polymerase gene, further sequence analysis revealed that a conserved region at the ORF1-ORF2 polymerase-capsid junction could also be used as an effective target for detection. Kageyama et al. described the first of these junction-targeting assays, which used two reactions to detect both GI and GII noroviruses (198). Similarly, Hohne and Schreier designed a two-reaction, real-time assay for GI and GII viruses using their own ORF1-ORF2 primer-probe sets (196). Importantly, this assay did not require a separate RT reaction.
To further minimize the reaction setup time and the potential for carryover contamination, GI and GII ORF1-ORF2 primer-probe sets were optimized for use in a single, multiplex TaqMan reaction (195, 197). In these assays, the probes were differentially fluorescently labeled to allow simultaneous detection and genogrouping. This multiplex, multiprobe approach directed at ORF1-ORF2 has also been used with GII/GIV TaqMan probes (199), GI/GII/GIII TaqMan probes (205), and GI/GII hybridization probes (204). It remains unclear, however, whether immediate norovirus genogrouping is important for outbreak control or clinical management. Simple, broadly reactive assays (177, 206) may be more important in the acute setting. Alternatively, the routine use of assays that detect and type norovirus may simultaneously allow laboratories to more rapidly monitor epidemiological patterns and highlight geographic regions or communities requiring further investigation (207).
Given the numerous real-time RT-PCR assays available for norovirus detection and genotyping, well-controlled, comparative studies similar to earlier work by Vinje et al. (183) are required to accurately determine the relative performance characteristics of these assays. Vainio and Myrmel (208) provided the first of these studies, by looking at two assays described above (192, 196) as well as two assays initially designed for screening of shellfish (209, 210). After a detailed analysis, the duplex, GI/GII, real-time TaqMan RT-PCR assay designed by Jothikumar et al. (209) was selected for in-house use. Compared to a conventional nested approach (208, 211), this assay, which employs modifications of the primer-probe sets reported by Kageyama et al. (198), had a clinical sensitivity of 91%. These results were superior to those of the SYBR green assay reported by Richards et al. (80%) and slightly inferior to those of the TaqMan assay reported by Hohne and Schreier (93%) but with the advantage of detection of GI and GII noroviruses in a single reaction. This study was performed on specimens from norovirus outbreaks in Norway, so additional work will be required to account for norovirus diversity in different populations. Furthermore, this assay was selected based on both clinical and practical considerations, yet it was the only assay evaluated that was capable of single-tube identification of multiple genogroups. Future work is required to provide a more comprehensive analysis of the numerous real-time norovirus assays now available with these characteristics.
Commercial RT-PCR assays.
Several commercial RT-PCR reagents are available for norovirus RNA detection (Table 8), although comparisons with one another or the large number of laboratory-developed norovirus RT-PCRs are not widely available. The Argene Calicivirus/Astrovirus consensus test (bioMérieux, Marcy l'Etoile, France) is a second-generation RT-PCR assay that requires a detection step via microplate hybridization using biotinylated probes. This test can identify caliciviruses and astroviruses but cannot distinguish between noroviruses and sapoviruses (212). Kele et al. used selected samples from sporadic cases of gastroenteritis in Hungary to evaluate the performance of Argene Calicivirus/Astrovirus Consensus and SmartNorovirus (Cepheid, Sunnyvale, CA), a set of primers and differentially labeled probes that allow real-time PCR detection and differentiation of GI and GII noroviruses by real-time RT-PCR (213). When true positives were defined as at least one positive RT-PCR result, the sensitivities of Argene Consensus and SmartNorovirus were 92.8% and 91.2%, respectively.
TABLE 8.
Commercial norovirus RT-PCR assays
| Assay | Company | Method |
|---|---|---|
| Argene Calici/Astrovirus Consensus | bioMérieux, Marcy l'Etoile, France | RT-PCR ELOSAa |
| SmartNorovirus | Cepheid, Sunnyvale, CA | Real-time RT-PCR |
| RealStar Norovirus | Altona Diagnostics, Hamburg, Germany | Real-time RT-PCR |
| Ridagene Norovirus | R-Biopharm, Darmstadt, Germany | Real-time RT-PCR |
| AccuPower Norovirus | Bioneer, Daejeon, South Korea | Real-time RT-PCR |
| Norovirus real-time RT-PCR | Shanghai ZJ Bio-Tech, Shanghai, China | Real-time RT-PCR |
ELOSA, enzyme-linked oligosorbent assay.
R-Biopharm (Darmstadt, Germany) offers two internally controlled, norovirus real-time PCR assays, Ridagene Norovirus, for qualitative detection of GI and GII noroviruses, and Ridagene Norovirus I&II, which both detects and differentiates GI and GII noroviruses in a single reaction. When the AccuPower Norovirus real-time PCR assay (Bioneer Co., Daejeon, South Korea), another internally controlled assay for detection of G1 and GII noroviruses, was compared to Ridagene Norovirus, there was 99.0% (96/97) positive agreement and 95.1% (175/184) negative agreement (214). Similarly, comparison of the Ridagene Norovirus I&II assay with conventional RT-PCR using stool specimens from distinct outbreaks in Victoria, Australia, in 2012 and 2013 revealed 98% sensitivity (85% [11/13] for GI and 100% [87/87] for GII) and 98% (98/100) specificity (215).
Future comparative studies with large, globally distributed sets of stool samples from sporadic gastroenteritis cases and outbreak settings will be required to better define the performance characteristics of these commercial reagents.
Multiplex PCR/RT-PCR tests for diarrheal pathogens.
The development and widespread use of commercial, highly multiplexed molecular diagnostic technologies have revolutionized testing for infectious diseases. Although initial efforts were dedicated to the design of respiratory virus panels, the focus has now shifted to gastrointestinal pathogens, with a number of manufacturers developing multiplex panels for diarrheal disease. The first of these panels is the xTAG Gastrointestinal Pathogen Panel (GPP) (Luminex, Austin, TX). In addition to norovirus genogroups I and II, this assay detects rotavirus A, adenovirus 40/41, Giardia, Cryptosporidium, Entamoeba histolytica, Campylobacter, C. difficile toxin A/B, Salmonella, Shigella, Vibrio cholerae, Escherichia coli O157:H7, as well as enterotoxigenic and Shiga-like toxin-producing E. coli. This method utilizes multiplex RT-PCR followed by target-specific primer extension for the addition of oligonucleotide hybridization tags. The tagged amplicons are then specifically hybridized to a set of microspheres with unique spectral signatures and coupled to capture sequences complementary to the tag sequence. Finally, the captured amplicons are labeled with a detection reagent, and bead-bound amplicons are counted via flow cytometry. This liquid-based array approach allows a significant level of multiplexing; however, it is laborious, it has a long turnaround time, and, because several steps require the handling of amplicons, there is a high risk of contamination.
A number of studies utilizing the xTAG GPP have been carried out, and the performance characteristics of the norovirus component of the assay are summarized in Table 9. Claas et al. demonstrated good sensitivity (GI, 100% [9/9]; GII, 92.5% [62/67]) and specificity (GI, 100% [642/642]; GII, 97.6% [570/584]) compared to real-time RT-PCR, although it is important to note that all xTAG GPP testing in this study was performed by the manufacturer (216). Wessels et al. also showed good sensitivity (94.4% [17/18]) and specificity (100% [375/375]) compared to real-time RT-PCR (217, 218). Studies by Mengelle et al. and Navidad et al. are difficult to interpret, as very few norovirus reference method-positive specimens (six total) were identified (219, 220). Finally, although Kahlau et al. had a large number of xTAG GPP norovirus-positive specimens, confirmatory RT-PCR testing was performed on only a subset of positive specimens, and none of the xTAG GPP-negative specimens were tested with the reference method (221).
TABLE 9.
Norovirus detection using multiplex gastrointestinal pathogen panels
| Study reference | Test | Study location(s) | Case context(s) | Population(s) | Genogroup | Sensitivityc (%) | Specificity (%) | Reference method(s) (reference) |
|---|---|---|---|---|---|---|---|---|
| 216 | xTAG GPPa | International | Sporadic | Pediatric, adult | I | 100.0 | 100.0 | Real-time RT-PCR (218) |
| Multisite | II | 92.5 | 97.6 | |||||
| 219 | xTAG GPP | France | Sporadic | Pediatric, adult | I or II | 0.0 | 93.6 | ImmunoCardSTAT!b |
| 220 | xTAG GPP | United States | Sporadic, outbreak | Pediatric, adult | I | NA | 100.0 | Real-time RT-PCR (202) |
| II | 100.0 | 100.0 | ||||||
| 217 | xTAG GPP | Netherlands | Sporadic | Not specified | I | NA | 100.0 | Real-time RT-PCR (218) |
| II | 94.4 | 100.0 | ||||||
| 222 | TaqMan Array | Tanzania, Bangladesh | Not specified | Pediatric | II | 100.0 | 96.2 | RT-PCR Luminex (223) |
| 224 | BioFire FilmArray GI Panele | United States | Not specified | Not specified | I or II | 96.2d | 99.8 | xTAG GPP,f real-time RT-PCR (202) |
Luminex, Austin, TX.
Meridian Bioscience Europe, Nice, France.
NA, not applicable; genogroup I-positive samples were not detected by the reference method.
The composite reference requires at least two positive tests to be considered positive. Sensitivity and specificity were calculated by using both prospective and retrospective samples.
bioMérieux, Marcy l'Etoile, France.
Utilized stool specimens collected in Carey-Blair medium rather than the manufacturer-recommended raw stool specimens.
Another multiplexing approach involves the use of TaqMan Low Density arrays (Life Technologies, Grand Island, NY), microfluidic cards comprised of 384 wells divided into 8 zones of 48 wells that are preloaded with a panel of singleplex TaqMan assay mixtures. Ports on the array allow extracted nucleic acids from 8 samples to be delivered to each zone for testing. Liu et al. (222) developed a gastrointestinal TaqMan array that detects 19 enteropathogens and includes the norovirus GII primer-probe set reported by Kageyama et al. (198). Compared to a laboratory-developed RT-PCR Luminex assay that also utilizes the GII primer and probe sequences of Kageyama et al. (223), the TaqMan array demonstrated 100% (31/31) sensitivity and 96.2% (75/78) specificity for norovirus GII.
While the xTAG GPP and TaqMan array methods are of high complexity and require separate nucleic acid extraction prior to amplification and detection, the BioFire FilmArray (bioMérieux, Marcy l'Etoile, France) is a moderate-complexity, 60-min sample-to-answer system that performs sample preparation, amplification, and detection in a single disposable pouch. The FilmArray GI panel detects 22 bacterial, protozoan, and viral targets, including norovirus GI/GII, although like the xTAG GPP, the FilmArray assay does not distinguish between genogroups. Khare et al. evaluated the FilmArray GI panel and showed 96.2% (52/56) sensitivity and 99.8% (441/442) specificity for norovirus GI/GII compared to a composite reference that included the xTAG GPP (224).
Additional large independent studies will be required to further characterize the norovirus component of these panels as well as other multiplex gastrointestinal panels currently in development. An important preliminary finding in these initial studies is the identification of infections with multiple pathogens. The evaluation of the clinical consequences of these coinfections will be an important area of future investigation.
Isothermal amplification.
In addition to the wide variety of RT-PCR-based amplification assays, isothermal PCR alternatives have also been developed for norovirus detection. Several groups have designed nucleic acid sequence-based amplification (NASBA) strategies by using previously reported primer pairs modified for NASBA compatibility (225–228). A small study by Houde et al. (226) determined that NASBA and RT-PCR using the GII primer sets described by Kageyama et al. (198) showed equivalent analytical sensitivities but that the NASBA assay provided less consistent signals. Many NASBA formats, including those described above, require an additional product detection step. However, Patterson et al. (229) designed a NASBA assay using a molecular beacon probe that allowed real-time detection. This assay was 88% sensitive compared to conventional RT-PCR, suggesting that the assay requires further optimization. A norovirus NASBA assay, Swiftgene Norovirus GI/GII, is commercially available in Japan (Kainos Laboratories, Tokyo, Japan).
The final, real-time, non-PCR, nucleic acid-based approach for norovirus diagnosis is RT–loop-mediated isothermal amplification (RT-LAMP) (230–232). Fukuda et al. (230) described a two-reaction, GI- and GII-specific RT-LAMP assay also using primers directed at the ORF1-ORF2 junction. Compared to conventional RT-PCR (184), the RT-LAMP assay had 100% clinical sensitivity. Based on this work, a commercial assay, Loopamp Norovirus GI and GII, was developed (Eiken Chemical, Tokyo, Japan) (232). One advantage of this approach is that detection is performed via inexpensive, real-time turbidimetry or simple, endpoint, visual examination. Future work will be required to compare the performance characteristics of RT-LAMP to those of real-time RT-PCR.
NOROVIRUS PREVENTION AND CONTROL
Several studies, with various degrees of evidence quality, on infection prevention and control practices to interrupt the transmission of norovirus in health care settings have been reported. Many of these results are summarized in the HICPAC (Healthcare Infection Control Practices Advisory Committee) guidelines for the prevention and control of norovirus gastroenteritis outbreaks in health care settings published in 2011 and in a recent review of norovirus infection control measures (233, 234). The three main strategic areas included staff and patient policy development, hand hygiene, and proper environmental disinfection. Despite the breadth of existing literature on norovirus outbreaks and mitigation strategies, a recent survey of infection preventionists showed clear room for improvement in their knowledge of both prevention and control practices (235).
Staff and Patient Policies
An efficient response to a suspected norovirus outbreak is predicated on preexisting staff training and the rapid initiation of outbreak management policies, although evidence in support of these practices is minimal and based substantially on descriptive studies and single-institution reports. Lynn and colleagues described two norovirus outbreaks in elderly care wards occurring 18 months apart (236). Lessons learned from a review of the first outbreak were used to develop policies for staff absenteeism, financial compensation, and patient movement, among other outbreak response behaviors, in advance of the second outbreak. Applications of these polices resulted in a reduced duration of hospital ward closure (from 11 to 6 days) and a reduced staff attack rate (from 41% to 18%). In a Hong Kong health system of seven hospitals with an already low incidence of nosocomial norovirus transmission, investigators demonstrated that targeted infection control measures, coupled with rapid accurate surveillance screening with PCR testing, were able to further reduce the incidence of norovirus compared to control health care systems (237). These measures included additional staff training sessions on infection control practices, which reached 91.6% of the professional staff over the study period. In order to disrupt a norovirus outbreak in a Canadian tertiary-care facility, one Alberta hospital established an outbreak management committee to reinforce routine infection prevention practices and implement infection control strategies. This was coupled with a comprehensive communication strategy for informing staff, patients, and visitors of up-to-date outbreak information (238). No studies have definitely demonstrated which education policies and practices are most effective in sustaining good infection control practice, although the HICPAC recommends staff, patient, and visitor education on symptoms, transmission, and prevention strategies during suspected or confirmed norovirus outbreaks (233).
Ward closure is another oft-employed control strategy with limited supporting evidence. It became the mainstay of infection prevention strategies in the United Kingdom in the early 2000s following publication of Health Protection Agency (HPA) guidelines which stipulated that wards should be closed for at least 72 h after the last case of norovirus and only after a thorough terminal cleaning has been performed (239). The goal of ward closure is to prevent the transfer of new susceptible patients into an affected ward and the transfer of potentially ill patients out to other unaffected wards. This costly infection control intervention is thought to be necessary to control viral gastroenteritis outbreaks because of the ease of transmissibility (240). The majority of evidence in favor of ward restriction or closure has been reported in descriptive studies (241–243). While many of these studies reported early interruption of transmission, it was at substantial cost to the affected health care systems.
The decision to implement aggressive norovirus control strategies, such as restricting ward admissions, has been evaluated from an economic standpoint in a few studies. Estimates of the cost of single-institution nosocomial norovirus outbreaks reported in the literature range from US$40,000 to more than US$650,000 (143, 244). An Austrian hospital norovirus outbreak from December 2006 to February 2007 involved 3 separate ward closures, 90 patients, and an estimated cost of €80,138 (245). Lopman and colleagues estimated the direct cost, due to bed closure and staff absences, of nosocomial gastroenteritis outbreaks to the National Health Service in the United Kingdom to be approximately US$1 million per 1,000 hospital bed-days, and >60% of viral gastroenteritis outbreaks were attributed to norovirus (241).
Evidence that complete ward closure may be an overly conservative reaction to nosocomial norovirus outbreaks at too high an opportunity cost is beginning to emerge. Beginning in 2007 to 2008, several investigators evaluated partial ward closure as a means of restricting nosocomial spread and limiting service disruptions. Cohorting by bay, as opposed to total-ward closure, resulted in decreased total closure time and improved the outbreak management efficiency in one study (246). Additionally, in a before-and-after-program implementation study conducted by the Lancashire Teaching Hospitals, the modified bay closure strategy resulted in a significant decrease in the ratio of hospital to community outbreaks, with the number of confirmed outbreaks in hospitals decreasing from 42 to 25 during the 2 study points, while the number of community outbreaks increased from 46 to 81 (r = 0.317; P = 0.025). The number of days of restricted admissions on hospital wards per outbreak also decreased from 8 days to 6 days (r = 0.742; P = 0.041). Finally, the number of hospital bed-days lost per outbreak decreased from 29 to 5 between the two norovirus seasons (r = 0.344; P < 0.001) (247). That study did not show any significant change in the number of patients or staff affected during each outbreak, although fewer lost bed-days resulted in cost savings. Currently, ward closure and limiting transfers of symptomatic patients are category II HICPAC recommendations (233).
Several studies describe visitor-specific policies that were implemented during outbreaks in an effort to reduce the number of transmissions among patients and staff. In 2004, 24 norovirus outbreaks were reported in the city of Baltimore, MD. Infection control staff at Johns Hopkins Hospital reported their efforts to thwart the epidemic at their institution with several enhanced infection control policies. These policies included screening of all visitors for gastrointestinal symptoms by nurse managers. Any visitors who screened positive were blocked from visiting patients in the units for 72 h (244). Ultimately, visitors were banned entirely due to the persistence of the outbreak. In another acute-care setting, visitors were registered for 14 days during an outbreak and administered a standard sign-and-symptom questionnaire to uncover possible cases of gastroenteritis among visitors to a pediatric ward (248). The total number of visitors per inpatient was also limited to 2.
Staff and patient cohorting is one of the most frequently reported control actions during outbreaks (249). This is achieved by several means, as reported in descriptive studies of nosocomial norovirus outbreaks (236, 243, 244, 248, 250). In one setting, nurses were divided into teams, with one team being assigned to symptomatic patients and an alternate team caring for asymptomatic patients only (244). In another example, staff who had worked in affected areas were restricted to those areas and not assigned to unaffected patient units (248).
In essentially all reported outbreaks in both acute-care and long-term-care settings, ill health care workers have been placed on mandatory leave if they exhibit symptoms of gastroenteritis. Staff members are typically prohibited from returning to work for 48 to 72 h after the resolution of symptoms. In one report, a New Zealand hospital instituted additional paid sick leave during a norovirus outbreak to increase compliance with the mandatory 48-h work restriction (236).
Patient activity restrictions are another common infection control strategy used to limit norovirus outbreaks (243). In the literature, this has primarily involved prioritizing diagnostic studies and therapy sessions to only the most urgent cases during outbreaks. For example, in an outbreak at the Johns Hopkins Hospital affecting an inpatient psychiatric unit, group therapy sessions were suspended during the norovirus outbreak, and this was informally tied to therapeutic delays for some patients (244).
Long-Term-Care and Other Facilities
The policies employed in acute-care settings, such as patient cohorting, activity restrictions, and work restrictions for ill and exposed staff members, have been adapted to long-term-care facilities (251). Additionally, some institutions may offer delayed admission to prospective long-term-care residents during ongoing outbreaks (252). In a prospective study of 49 Dutch nursing homes, investigators demonstrated that infection control measures were most effective when initiated within 3 days of the identification of an index case (253). Visitor restrictions have also been utilized in a number of reports of outbreaks in long-term-care or other extended-care settings. These restrictions have been centered primarily on restricting visiting privileges to immediate family only and prohibiting or discouraging children from visiting during outbreaks (243, 251).
Hand Hygiene
One of the primary recommended control strategies to interrupt norovirus transmission during outbreaks is appropriate hand hygiene, although this is based primarily on descriptive data (254). Use of soap and running water for a minimum of 20 s is recommended after patient contact with confirmed or suspected cases at a category IB level (strong recommendation and low-quality evidence) (233). Evaluation of disinfectants has proven challenging due to the limited ability to cultivate human norovirus in cell culture. Several viral proxies have been evaluated, including feline calicivirus (FCV) and murine noroviruses (MNVs), to assess the effectiveness of various disinfectants in hand contamination models (255). Researches in Germany, using FCV as a surrogate for human noroviruses, measured the virus-inhibitory effect of three types of alcohol (ethanol, 1-propanol, and 2-propanol) in vitro and in vivo with artificially contaminated fingertips. This study showed that ethanol and 1-propanol had higher log10 viral reduction values than did 2-propanol, which was not considered adequate by those authors (256). This study also demonstrated declining log10 viral reductions with increased concentrations of alcohol in each type of solution, which the authors theorized was related to the minimum amount of water necessary to achieve viral inactivation. Additionally, an FCV fecal hand contamination model supported the use of higher-concentration ethanol-based hand rubs rather than propan-1-ol-containing products (257). In one study of experimental human norovirus hand contamination, liquid soap wash and water rinse were superior to ethanol-based sanitizers based on viral recovery through quantitative RT-PCR (258). Triclosan-containing soaps and several other alcohol based-hand rubs showed inadequate levels of virus reduction (259). Overall, additional research on specific human noroviral inactivation by various common cleaning and sanitizing products is needed (233).
Isolation and Personal Protective Equipment Procedures
Symptomatic patients with vomiting and/or diarrhea should be placed in contact isolation (single room, gowns, and gloves) pending results of testing. After confirmation of norovirus infection, several descriptive studies support the use of continued contact precautions until some time period after the resolution of diarrhea, typically 48 h (236, 250, 251, 260). Enforcement of standard precautions throughout health care settings experiencing a nosocomial norovirus outbreak is thought to reduce transmission (244). The data in favor of gown and glove use for the prevention of norovirus transmission are derived primarily from observational or descriptive studies. Mask use is recommended only for staff who anticipate exposure to vomitus (143, 244).
Environmental Disinfection
Environmental persistence of norovirus has been reported in several settings, and its role in propagating outbreaks has been described in settings ranging from health care environments to food products and preparation sites to a concert hall (261–264). A large proportion of the evidence on environmental disinfection is derived from studies using FCV or other surrogate viruses, and direct correlation with success in disinfecting human noroviruses is unknown (233, 265). In general, hypochlorite (bleach) solutions of at least 1,000 ppm are the preferred disinfectants for contaminated surfaces and objects and must be used for an appropriate contact time (233, 266, 267). Quaternary ammonium compounds are also under investigation but have been somewhat less effective than bleach solutions (234, 267). Both the type of sanitizer and method of application can have an impact on virucidal success (268). In health care and non-health care settings, studies have focused on cleaning of high-touch surfaces, such as patient bathrooms, tables, chairs, computers, and commodes, in addition to floors and carpets (146, 243, 244, 261). Descriptive evidence supports rapid attention to and remediation of contaminated floors and patient care items and steam cleaning of carpets, although these data are of limited generalizability (233, 236, 244, 248). Additional steps, such as discarding all unused patient care items after discharge of a norovirus patient, disposing of curtains, and changing mop heads and cleaning solution after every three rooms, have been described and may be performed, but these steps are without direct correlation to a shortening of the duration of nosocomial outbreaks (233, 236, 243, 244, 265).
NOROVIRUS IMMUNOLOGY
An inability to cultivate norovirus in vitro until very recently and the absence of an animal model that closely resembles human disease, together with viral and human host diversity, have represented major obstacles to our understanding of the pathogenesis of and immune response to norovirus infection. As a consequence, much of our knowledge of the immunology of this highly species-specific virus revolves around studies of MNV and the use of virus-like particles (VLPs) of human norovirus. VLPs are the product of self-assembling viral structures derived from the expression of recombinant VP1. They are structurally and antigenically similar to their corresponding wild virions but lack genomic material.
Animal Models
Although no animal model to date has been entirely satisfactory, it has been demonstrated that chimpanzees can be successfully infected with GI.1 norovirus (269), while gnotobiotic pigs (270) and gnotobiotic calves (271) can be successfully infected with GII.4 norovirus. The development of a norovirus replicon that expresses nonstructural viral proteins in human hepatoma cells (272) and a reverse-genetics system driven by human elongation factor 1 alpha that produces progeny virus containing infectious viral RNA (273) may provide insights into virus-host interactions at the cellular level. Furthermore, the development of a humanized immunodeficient murine model of human norovirus infection (274) may also prove useful although perhaps only to a limited extent. For example, Taube and colleagues reconstituted Rag1−/− yc−/− BALB/c mice (yc is the γ chain of receptors for interleukin-2 [IL-2], IL-4, IL-7, IL-9, and IL-15) with human CD34+ human hematopoietic stem cells after neonatal irradiation (275). Despite this manipulation, infection by the oral route was entirely unsuccessful, and intraperitoneal inoculation with a strain of human norovirus GII.4 was required but with no evidence of associated illness.
Some insights into norovirus infection may, however, be obtained from studies of murine models utilizing MNVs. MNVs are widespread in laboratory mice, and in contrast to human noroviruses, they can be propagated in tissue culture (276). Although MNV produces little or no outward signs of infection in wild-type laboratory mice (277), knockout studies indicate that both innate and adaptive immunity play critical roles in the control of MNV infection. Particularly important are type 1 and type 2 interferons (278–282), dendritic cell functions (283–285), and the production of neutralizing antibody (286–288). As such, MNVs may provide some important understanding of human norovirus infection that would not otherwise be possible to obtain (286).
Human Host Factors in Susceptibility and Resistance to Norovirus Infection
Almost 4 decades ago, Parrino and colleagues challenged and then rechallenged, after an interval of 27 to 42 months, 12 volunteers with stool filtrates containing the norovirus (GI.1) from the index outbreak that had been serially passaged through other human volunteers (289). Six subjects developed symptoms of gastroenteritis after the initial challenge, and each of these subjects also became ill upon rechallenge. In contrast, none of the 6 subjects who remained asymptomatic upon initial challenge became ill after rechallenge. Four of the six patients who developed symptoms were challenged a third time 4 to 8 weeks after the second challenge, and only one patient became ill. The investigators concluded that they had demonstrated the presence of short-term but the absence of long-term immunity to Norwalk virus infection. This conclusion does not, however, address the 6 subjects who failed to become ill after either the first or second challenge, a finding that suggested the possibility that some individuals are intrinsically resistant to Norwalk virus infection. Five years later, the discovery of familial clustering of infection, with some related groups having apparent resistance, was also observed in a large community outbreak traced to a swimming pool (111). These observations suggested the possibility that some individuals possess inherited protection against norovirus infection. Subsequent investigations determined that the reason for this inherited resistance lies in the natural variability of histo-blood group antigens (HBGAs) and their expression on the mucosal epithelial surface of the gastrointestinal tract (290).
HBGAs are oligosaccharide epitopes whose diversity results from the sequential addition of monosaccharides to glycan precursors (291, 292). The A and B red cell antigens are synthesized from a common intermediate molecule, the H substance, of which there are 3 types, but with the final fucosylated product having the same terminal disaccharide. There is no “O antigen”: group O erythrocytes express the H antigen; hence, the ABO system is better labeled the ABO(H) system.
The fucosyltransferases FUT1 and FUT2 each add a fucose to the precursor disaccharides of the H substance. While the FUT1 gene is expressed in erythroid progenitor cells, FUT2 is expressed mostly in mucosal epithelial cells from which the enzymatic gene product is secreted into surrounding secretions such as saliva and breast milk. While also produced by FUT2, Lewis (Le) antigens are predominantly the result of fucosylation by a third enzyme, FUT3. Approximately 5% of the white population are homozygous for inactive FUT3 alleles and, as a consequence, lack Lea and Leb and are termed Lewis negative (291). Lewis antigens are also present on mucosal surfaces and are secreted into the surrounding milieu.
The presence of H, Leb, A, or B antigens in saliva and other secretions operationally defines the secretor phenotype and is evidence of an active FUT2, or “secretor,” gene. Approximately 20% of individuals of European descent have, however, inherited two null FUT2 alleles as a result, in 99% of cases, of a G428A mutation that introduces a stop codon (293). The resultant absence of HBGAs at mucosal surfaces and in surrounding secretions that characterize the nonsecretor phenotype plays a key role in protection against infection by most noroviruses, as demonstrated in challenge studies (294–296) and analyses of outbreaks, as reviewed by Rydell and colleagues (297).
Norwalk virus-derived VLPs bind to H antigen in vitro (298) and also hemagglutinate type A, AB, O, and, much more weakly, B red blood cells (295). They also bind to epithelial cells of the gastroduodenal mucosa of individuals who are secretors but not to those of nonsecretors (290). Thus, it appears that norovirus is among organisms, such as Helicobacter pylori (299), that utilize HBGAs expressed in the gastrointestinal tract as cell surface receptors (300).
Given the multiplicity of viral strains and of HBGAs, it is not surprising that there are diverse patterns of individual strain associations with individual HBGAs. In a study involving 14 norovirus strains, Huang et al. reported having identified 7 different patterns that could be classified into 2 groupings: one group of viruses recognized A and/or B and H antigens, while the second group reacted only with Lewis and/or H antigens (301). While there was some clustering of binding patterns depending on the phylogenetic relatedness of the viruses, both patterns could be found among both GI and GII viruses. GII.4 VLPs bind strongly to saliva of secretor-positive individuals regardless of the ABO(H) blood group (302), while strong binding of GI.1 VLPs is observed only with secretions of type A, AB, or O secretors (22, 294, 301, 303–306).
While initial studies indicated that a nonsecretor status seemed to provide total resistance to norovirus-associated illness, the degree of protection has now been demonstrated to be less than absolute. Examples of infection in nonsecretors include ones due to norovirus GII.2 Snow Mountain (307), GII.4 (308, 309), and GI.3 (310, 311). In fact, combining the data from two GI.3 outbreaks, it can be calculated that 11 of 22 (50%) nonsecretors and 46 of 90 (51%) secretors were infected (310, 311). In addition, binding to human Caco-2 intestinal cells by GII.6 norovirus strain VLPs is independent of HBGAs and is instead associated with cell maturity (312), as is that by the GII.4 Desert Shield strain (313). The receptors for these viruses remain unknown. In addition, inherited factors other than HBGAs may affect susceptibility to norovirus infection. For example, travelers to Mexico who have the lactoferrin T/T genotype may be protected against norovirus infection despite being at an increased risk of all-cause traveler's diarrhea (314).
In addition to resistance to infection based on secretor status, norovirus infection results in adaptive immunity to homotypic viral challenge, albeit of relatively short duration, as was first reported by Parrino and colleagues in 1977 (289) and subsequently confirmed (294). For instance, a multiple-challenge experiment with Norwalk virus found evidence of protection for ≥6 months (315), and it is commonly stated that immunity lasts as long as 2 years (316). However, the relevance of many challenge experiments has been questioned because the inocula used in this and many other studies were likely several orders of magnitude higher (317) than that required for infection, and as a consequence, the duration of immunity in the natural setting may be significantly longer than that estimated from these experiments. Thus, Teunis and colleagues estimated the 50% infectious dose (ID50) to be between 18 and 1,015 genomic equivalents (317). Atmar and colleagues found that the Norwalk virus ID50s were estimated to be ≈1,320 genomic equivalents (≈3.3 RT-PCR units) in subjects who proved to be secretor-positive blood group O or A persons and ≈2,800 (≈7.9 RT-PCR units) for all secretor-positive persons (318, 319). On the other hand, the potential for natural exposure to much larger numbers of viral particles is significant. Thus, 1 ml of virus-containing vomitus from symptomatically infected subjects contained a median of 41,000 genomic equivalents (319). The norovirus load in feces is much higher and appears to be genogroup dependent, with reported medians of 8.4 × 105 cDNA copies per g in individuals with GI infection and 3.0 × 108 cDNA copies per g in individuals with GII infection (320). It is therefore possible that estimates from observational studies of natural infection may provide the best estimates of the duration of immunity. Thus, mathematical modeling taking into account observational data describing the age-specific incidence of norovirus disease, seasonality, and population immunity led to an estimated duration of immunity of 4.1 years (95% CI, 3.2 to 5.1 years) to 8.7 years (95% CI, 6.8 to 11.3 years) (316).
Complicating the determination of immunity duration by examination of naturally acquired infections is the fact that immunity may be strain specific, and any analysis is potentially confounded by the multiple genogroups, genotypes, and strains of virus, together with the continuing evolution of the virus. Thus, administration of stool filtrates from two distinct outbreaks to prison volunteers led to a lack of cross-protection between the Norwalk and Hawaii strains (321), now known to be prototypes of GI and GII noroviruses, respectively. A prospective study of diarrhea in infants in the first weeks of life (the median age at recruitment was 19 days) to the age of 2 years found evidence of protection that was genotype specific: 97% of repeat infections were due to a genotype that differed from that of a previous infection (322).
Although IgG antibody directed against norovirus antigens is present in the serum of >90% of individuals by the time they reach adulthood, and they nonetheless remain at risk of repeat norovirus infection (323), evidence indicates that blocking antibody plays a role in immunity to reinfection. While the inability to culture the virus in vitro precludes the ability to detect neutralizing antibody by classical methods, the prevention of binding of norovirus-derived VLPs or P particles to HBGA (blocking antibody) is believed to be an accurate surrogate of neutralization (324, 325).
In a volunteer study, preexisting antibody did not protect against disease resulting from an initial challenge, but a correlation with protection emerged after subsequent rechallenges (315). However, others have reported that preexisting antibody provides at least partial protection against naturally acquired infection (326, 327). The appearance of IgA antibody against norovirus in saliva by day 5 after challenge of genetically susceptible individuals correlated with protection (294), as did an early Th1 lymphocyte response (307). High prechallenge titers of blocking antibodies also correlate with protection against experimental challenge (325). Volunteers challenged with a GII.2 strain (Snow Mountain) elicited serum IgG antibody responses that were cross-reactive with another GII.1 viral strain (Hawaii), but lesser cross-reactivity was noted with salivary IgA, and neither antibody type was cross-reactive with GI.1 (307). A similar pattern of cross-reactivity was seen with T cells exposed to Snow Mountain virus, which elicited a predominantly Th1 response. Passive protection from a maternal source occurs, as demonstrated by the observation that exclusive breastfeeding of infants from the ages of 3 to 5 months was associated with protection, and infections that did occur in the first 6 months of life were more likely to be asymptomatic than were those that occurred at 6 to 11 months of age (55% versus 36%; P < 0.001) (322).
The IgG antibody response to challenge with different GI strains results in variable heterotypic responses that are likely determined by an individual's history of natural exposure to noroviruses and deceptive imprinting (e.g., by development of “decoy epitopes”) (328).
Antibodies to antigens other than those of the viral capsid also result from viral challenge. Three-fourths of volunteers challenged with GI.1 virus had an antibody response to viral protease from either the homologous virus or GII.4 virus (Houston) (329), but these antibodies are not believed to be protective.
Immune Selection and Development of Viral Diversity
The viral capsid protein VP1 has 3 structural domains, with the shell (S) being the core from which 2 other domains, P1 and P2, protrude (330). P2 is the most exposed portion and is likely the major point of contact with HBGA ligands and with neutralizing antibody (22, 88, 331–333). In addition, mutations and recombination events involving P2 can significantly affect antigen properties and interactions with HBGAs (334). GII.4 strains, in particular, are undergoing rapid evolution that affects receptor binding by changes in surface-exposed P2 and antigenic expression, resulting in the emergence of new epidemic strains of the virus (25, 335–340). In contrast, there has been only limited evolution of GI viruses over the last 4 decades (334).
GII.4, although first recognized as a major epidemic strain in the middle of the last decade of the 20th century, has been circulating since at least 1974 (25). GII.4 undergoes epochal evolution characterized by periods of stasis followed by the emergence of a new epidemic strain. There have been 7 different GII.4 variants associated with global epidemics since the 1990s, which occurred in 1996, 2002, 2004, 2007 to 2008 (2 variant strains), 2009 to 2012, and 2012 onward (the Sydney strain) (341). Thus, on average, new variants of GII.4 have appeared every 2 to 3 years.
Evidence indicates that new variants emerge under positive selection (25), likely as a result of the pressure exerted by the development of herd immunity as larger portions of the population experience infection, with resultant viral antigenic drift (20, 342–345). GII.4 2012 Sydney, which has, to a large extent, replaced previously circulating GII.4 variants, had undergone changes in at least 2 epitopes recognized by blocking antibodies (344).
While norovirus evolution is believed to result from the selective pressure of the immune system, Schorn and colleagues demonstrated amino acid changes in P2 and P1-2 during individual chronic infections due to GII.4 in renal transplant recipients and due to GII.7 and GII.17 in an additional patient each (60). Others evaluating two hematopoietic stem cell transplant recipients and one small bowel transplant recipient chronically infected with GII.4 or GII.7 found evidence of positive selection, with accumulation of an average of 5 to 9 mostly nonsynonymous mutations per 100 days in the hypervariable region of the P2 domain of the VP1 capsid gene (346).
The immune selection of norovirus variants has important implications for vaccine development.
NOROVIRUS VACCINE
There are strong public health and economic arguments in support of the potential benefits of the development of an effective norovirus vaccine. Using a simulation model, it was concluded that a vaccine with 50% protective efficacy for 12 months and costing US$50 would save US$1,000 to US$2,000 per case averted in the United States (347). Thus, despite the biological obstacles to vaccine development, which require dealing with the complex matrix of inherited human traits and variability among noroviruses (Table 10), the potential benefits would appear to be well worth the effort. Table 11 summarizes clinical studies on norovirus vaccines.
TABLE 10.
Obstacles to norovirus vaccine development
| Obstacle to norovirus vaccine development |
|---|
| Inability to cultivate norovirus in vitro |
| Inability to directly measure neutralizing antibody |
| Inherited host variability, especially with regard to HBGA |
| Multiple viral genogroups and genotypes and their continued evolution |
| Limited heterotypic immunity |
| Likely need for continuing vaccine reformulation |
| Uncertain duration of immunity |
| Incomplete understanding of the role of cellular immunity |
| Possible acceleration of viral evolution in response to a vaccinated population |
| Uncertain efficacy in most vulnerable individuals, including young children, the elderly, and the immunocompromised |
TABLE 11.
Summary of selected norovirus vaccine studies
| Reference | VLP genotype | Adjuvant(s)a | Route | Dose (μg) | Regimen | Challenge dose (RT-PCR units) |
|---|---|---|---|---|---|---|
| 353 | GI.1 | None | Oral | 100 or 250 | 2 doses 21 days apart | None |
| 354 | GI.1 | None | Oral | 250, 500, or 2,000 | 2 doses 21 days apart | None |
| 355 | GI.1 | MLA, chitosan | Intranasal | 5, 15, 50, or 100 | 2 doses 21 days apart | None |
| 357 | GI.1 | MLA, chitosan | Intranasal | 100 | 2 doses 21 days apart | 48 (GI.1 virus) |
| 358 | GI.1/GII.4 | MLA, alum | Intramuscular | 2 doses 28 days apart | 4,000 (GII.4 virus) |
MLA, monophosphoryl lipid A.
Most efforts at vaccine development are now focusing on the use of VLPs as the immunogen (348). GII.4 P particles are immunogenic in mice (284, 349), but evidence indicates that GII.4 VLPs are superior to homologous P particles (350). Furthermore, VLP vaccines have a successful history in the prevention of hepatitis B virus and of human papillomavirus infection (351).
Intravenous administration of Norwalk virus to chimpanzees led to the appearance of virus in feces (along with liver and duodenal and jejunal tissues), but all animals remained asymptomatic (25). Animals were resistant to rechallenge with Norwalk virus ≥4 months later, and this appeared to correlate with the antibody response to the virus. Intramuscular inoculation of other chimpanzees with VLPs derived from Norwalk virus protected against intravenous challenge with the same virus 6 and 18 months later, together with the development of blockade antibodies. These data provide evidence of homologous but not heterologous resistance and indicate that norovirus-derived VLP inoculation may produce homologous immunity.
Despite all the potential obstacles to the development of an effective vaccine, the interest is high, and by 2010, Herbst-Kralovetz and colleagues were able to list 12 phase 1 trials that had reported the immunogenicity of plant or insect cell-derived norovirus VLPs after mucosal delivery (352). Both oral and intranasal administrations have been shown to elicit antibody responses (353–355). El-Kamary and colleagues reported the results of two of these trials in that same year (355). These investigators administered GI.1-derived VLPs with the Toll-like receptor 4 (TLR4) agonist monophosphoryl lipid A (MLA) and mucoadherent chitosan formulated as a dry powder by the intranasal route and found evidence of safety and dose-dependent immunogenicity, both mucosal and systemic (355). The majority of circulating IgA norovirus antigen-specific cells carried markers indicative of homing to mucosal tissues alone or to mucosal and lymphoid tissues. Furthermore, subjects developed significant norovirus IgA and IgG memory B-cell responses (356).
This GI.1-derived VLP vaccine was subsequently further evaluated. Healthy adults with a functional FUT2 gene, as evidenced by the presence of FUT2 detected by the presence of HBGAs in saliva, were randomized to receive either placebo or the adjuvanted GI.1 VLP vaccine (357). Two doses of each vaccine were administered intranasally 3 weeks apart. Participants were then challenged orally with a dose of Norwalk virus (48 RT-PCR units) that was ∼10-fold higher than the ID50. The vaccine was well tolerated, and 70% of 47 vaccine recipients had a ≥4-fold increase in the serum concentration of Norwalk virus-specific IgA antibody levels, as measured by an enzyme immunoassay. Changes in IgG and IgM antibody levels were limited, as was the level of HBGA blocking antibody. The vaccine was modestly protective against infection after challenge, with 81% of placebo recipients showing evidence of infection compared to 62% (P = 0.05) of those who received the vaccine, and it also reduced virus-specific gastrointestinal symptoms (69% of placebo patients versus 37% of vaccine recipients; P = 0.009). A post hoc analysis found evidence that a serum HBGA blocking antibody titer of ≥1:200 was associated with a >50% relative reduction in the frequency of both viral infection and illness. In addition, antigen-specific memory T cells were generated in all subjects who received the highest dose (100 μg) of vaccine. Thus, this proof-of-concept trial demonstrated the ability of an intranasal vaccine to provide a degree of short-term protection against illness after homotypic challenge.
A systemically administered bivalent vaccine has also been evaluated in humans. Healthy adults were randomized to receive either placebo or a bivalent GI.1/GII.4-derived VLP vaccine with MLA and alum (358). The GII.4 component was a consensus product designed by alignment of the VP1 sequence of 3 GII.4 strains and was shown to be antigenically similar to GII.4 strains that have been circulating for 3 decades (359). Each vaccine was administered in 2 intramuscular doses at an interval of ≥28 days, and subjects were then challenged at least 28 days later with 4,000 RT-PCR genome equivalents of a heterologous GII.4 strain. The primary composite endpoint of reduction in any type of gastroenteritis or norovirus case ascertainment was not achieved. However, fewer of the vaccine recipients (n = 56) than placebo recipients (n = 48) reported vomiting and/or diarrhea of any severity after challenge (20% versus 41.7%; 52% reduction; P = 0.028).
There is interest in the development of norovirus vaccines to be given in combination with other immunogens. A vaccine containing human rotavirus recombinant VP6 as well as VLPs derived from GII.4, GII.12 New Orleans, and GI.3 was immunogenic for all components in BALB/c mice (360, 361). In addition, a bivalent norovirus/hepatitis E virus vaccine has been demonstrated to be immunogenic in mice (362).
Many factors will need to be effectively addressed for the development of a useful norovirus vaccine (318). Given our current understanding of the virus and the host response to it, such a vaccine will almost certainly be multivalent and, as is the case with influenza virus, may require frequent reformulations in response to viral evolution.
CONCLUSIONS
Norovirus is an important cause of morbidity due to acute gastroenteritis both within health care institutions and in the broader community. Although mortality is typically limited to the extremes of age, the disease exacts a significant toll on the health care system. Therapeutic management is usually supportive, and advances in molecular diagnostics may lead to the earlier identification of outbreaks and a reduction in person-to-person transmissions, particularly in vulnerable patient populations. Ongoing global reporting initiatives will be enhanced by improved diagnostic methods. Additionally, global control efforts will benefit from the growing knowledge of the clinical implications of various norovirus strains. Several advances into understanding the relationship among the viral strain, the host human blood group antigen type, and disease susceptibility have recently been elucidated, but this work has not yet been extended to clinical practice. The interplay of norovirus and host immunity still poses many unanswered questions. Areas of future research may overcome technical limitations, such as the inability to cultivate norovirus in vitro, and may elucidate a way to directly measure neutralizing antibodies, which could pave the way for vaccine development. The recent demonstration of infectability of B cells by the human norovirus GII.4 Sydney due to the presence of Enterobacter cloacae expressing H antigen may represent an important advance in our ability to understand norovirus replication (394). Several additional factors, such as inherited host variability, noroviral genogroup diversity, and ongoing viral evolution, will continue to complicate the process of vaccine development. Until a broadly effective, sustainable vaccine is developed, outbreak management will depend primarily on infection control efforts.
Biographies

Elizabeth Robilotti, M.D., M.P.H., is an Instructor in the Division of Infectious Diseases and Geographic Medicine at the Stanford University School of Medicine in Stanford, CA. Dr. Robilotti's research focuses on the epidemiology of health care-associated infections and the impact of antimicrobial stewardship training and practice.

Stan Deresinski, M.D., is Clinical Professor of Medicine in the Division of Infectious Diseases and Geographic Medicine at the Stanford University School of Medicine, where he is the Medical Director of the Antimicrobial Stewardship Program. He has published more than 100 peer-reviewed papers covering a broad area of Infectious Diseases and is currently Editor of Infectious Disease Alert and a Section Editor of Clinical Infectious Diseases. Dr. Deresinski is a recent past Chair of the Standards and Practice of the Infectious Diseases Society of America (IDSA) and member of the Board of Directors of the IDSA.

Benjamin A. Pinsky, M.D., Ph.D., is an Assistant Professor of Pathology and Medicine, Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, and is the Director of the Clinical Virology Laboratory at Stanford Health Care and Stanford Children's Health. Dr. Pinsky's research focuses on the development and application of molecular techniques to improve the diagnosis and management of infectious diseases.
REFERENCES
- 1.Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol 10:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahorsky J. 1929. Hyperemesis hiemis or the winter vomiting disease. Arch Pediatr 46:391–395. [Google Scholar]
- 3.Adler JL, Zickl R. 1969. Winter vomiting disease. J Infect Dis 119:668–673. doi: 10.1093/infdis/119.6.668. [DOI] [PubMed] [Google Scholar]
- 4.Dolin R, Blacklow NR, DuPont H, Formal S, Buscho RF, Kasel JA, Chames RP, Hornick R, Chanock RM. 1971. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. J Infect Dis 123:307–312. doi: 10.1093/infdis/123.3.307. [DOI] [PubMed] [Google Scholar]
- 5.Goodgame R. 2007. Norovirus gastroenteritis. Curr Infect Dis Rep 9:102–109. doi: 10.1007/s11908-007-0004-5. [DOI] [PubMed] [Google Scholar]
- 6.Widdowson M-A, Monroe SS, Glass RI. 2005. Are noroviruses emerging? Emerg Infect Dis 11:735–737. doi: 10.3201/eid1105.041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapikian AZ. 2000. The discovery of the 27-nm Norwalk virus: an historic perspective. J Infect Dis 181(Suppl 2):S295–S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cukor G, Blacklow NR. 1984. Human viral gastroenteritis. Microbiol Rev 48:157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blacklow NR, Greenberg HB. 1991. Viral gastroenteritis. N Engl J Med 325:252–264. doi: 10.1056/NEJM199107253250406. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg HB, Valdesuso JR, Kalica AR, Wyatt RG, McAuliffe VJ, Kapikian AZ, Chanock RM. 1981. Proteins of Norwalk virus. J Virol 37:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi JN, Graham DY, Wang KN, Estes MK. 1990. Norwalk virus genome cloning and characterization. Science 250:1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- 13.Matsui SM, Kim JP, Greenberg HB, Su W, Sun Q, Johnson PC, DuPont HL, Oshiro LS, Reyes GR. 1991. The isolation and characterization of a Norwalk virus-specific cDNA. J Clin Invest 87:1456–1461. doi: 10.1172/JCI115152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Wang M, Wang K, Estes MK. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 15.Lambden PR, Caul EO, Ashley CR, Clarke IN. 1993. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science 259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 16.Green KY. 2013. Caliciviridae: the noroviruses, p 582–608. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 17.Thorne LG, Goodfellow IG. 2014. Norovirus gene expression and replication. J Gen Virol 95:278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. 2010. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol 8:231–241. doi: 10.1038/nrmicro2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, Lanzavecchia A, Baric RS. 2012. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog 8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. 1999. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J Infect Dis 179:1334–1344. doi: 10.1086/314783. [DOI] [PubMed] [Google Scholar]
- 22.Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, Vinje J, Baric RS. 2008. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med 5:e31. doi: 10.1371/journal.pmed.0050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widdowson MA, Rockx B, Schepp R, van der Poel WH, Vinje J, van Duynhoven YT, Koopmans MP. 2005. Detection of serum antibodies to bovine norovirus in veterinarians and the general population in the Netherlands. J Med Virol 76:119–128. doi: 10.1002/jmv.20333. [DOI] [PubMed] [Google Scholar]
- 24.Mesquita JR, Costantini VP, Cannon JL, Lin SC, Nascimento MS, Vinje J. 2013. Presence of antibodies against genogroup VI norovirus in humans. Virol J 10:176. doi: 10.1186/1743-422X-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bok K, Abente EJ, Realpe-Quintero M, Mitra T, Sosnovtsev SV, Kapikian AZ, Green KY. 2009. Evolutionary dynamics of GII.4 noroviruses over a 34-year period. J Virol 83:11890–11901. doi: 10.1128/JVI.00864-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroneman A, Vega E, Vennema H, Vinje J, White PA, Hansman G, Green K, Martella V, Katayama K, Koopmans M. 2013. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol 158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucardo F, Nordgren J, Carlsson B, Kindberg E, Paniagua M, Mollby R, Svensson L. 2010. Asymptomatic norovirus infections in Nicaraguan children and its association with viral properties and histo-blood group antigens. Pediatr Infect Dis J 29:934–939. doi: 10.1097/INF.0b013e3181ed9f2f. [DOI] [PubMed] [Google Scholar]
- 28.Garcia C, DuPont HL, Long KZ, Santos JI, Ko G. 2006. Asymptomatic norovirus infection in Mexican children. J Clin Microbiol 44:2997–3000. doi: 10.1128/JCM.00065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques Mendanha de Oliveira D, Souza M, Souza Fiaccadori F, Cesar Pereira Santos H, das Dores de Paula Cardoso D. 2014. Monitoring of calicivirus among day-care children: evidence of asymptomatic viral excretion and first report of GI.7 norovirus and GI.3 sapovirus in Brazil. J Med Virol 86:1569–1575. doi: 10.1002/jmv.23791. [DOI] [PubMed] [Google Scholar]
- 30.Huynen P, Mauroy A, Martin C, Savadogo LG, Boreux R, Thiry E, Melin P, De Mol P. 2013. Molecular epidemiology of norovirus infections in symptomatic and asymptomatic children from Bobo Dioulasso, Burkina Faso. J Clin Virol 58:515–521. doi: 10.1016/j.jcv.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Frange P, Touzot F, Debre M, Heritier S, Leruez-Ville M, Cros G, Rouzioux C, Blanche S, Fischer A, Avettand-Fenoel V. 2012. Prevalence and clinical impact of norovirus fecal shedding in children with inherited immune deficiencies. J Infect Dis 206:1269–1274. doi: 10.1093/infdis/jis498. [DOI] [PubMed] [Google Scholar]
- 32.Phillips G, Tam CC, Rodrigues LC, Lopman B. 2010. Prevalence and characteristics of asymptomatic norovirus infection in the community in England. Epidemiol Infect 138:1454–1458. doi: 10.1017/S0950268810000439. [DOI] [PubMed] [Google Scholar]
- 33.Jeong AY, Jeong HS, Lee JS, Park YC, Lee SH, Hwang IG, Kim YJ, Kim YJ, Jo MY, Jung S, Kim K, Cheon DS. 2013. Occurrence of norovirus infections in asymptomatic food handlers in South Korea. J Clin Microbiol 51:598–600. doi: 10.1128/JCM.01856-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu JH, Kim NY, Lee EJ, Jeon IS. 2011. Norovirus infections in asymptomatic food handlers in elementary schools without norovirus outbreaks in some regions of Incheon, Korea. J Korean Med Sci 26:734–739. doi: 10.3346/jkms.2011.26.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz M, Gluck M, Koon S. 2013. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 108:1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 36.Gotz H, Ekdahl K, Lindback J, de Jong B, Hedlund KO, Giesecke J. 2001. Clinical spectrum and transmission characteristics of infection with Norwalk-like virus: findings from a large community outbreak in Sweden. Clin Infect Dis 33:622–628. doi: 10.1086/322608. [DOI] [PubMed] [Google Scholar]
- 37.Lee RM, Lessler J, Lee RA, Rudolph KE, Reich NG, Perl TM, Cummings DA. 2013. Incubation periods of viral gastroenteritis: a systematic review. BMC Infect Dis 13:446. doi: 10.1186/1471-2334-13-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockx B, De Wit M, Vennema H, Vinje J, De Bruin E, Van Duynhoven Y, Koopmans M. 2002. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis 35:246–253. doi: 10.1086/341408. [DOI] [PubMed] [Google Scholar]
- 39.Tseng CY, Chen CH, Su SC, Wu FT, Chen CC, Hsieh GY, Hung CH, Fung CP. 2011. Characteristics of norovirus gastroenteritis outbreaks in a psychiatric centre. Epidemiol Infect 139:275–285. doi: 10.1017/S0950268810000634. [DOI] [PubMed] [Google Scholar]
- 40.Arness MK, Feighner BH, Canham ML, Taylor DN, Monroe SS, Cieslak TJ, Hoedebecke EL, Polyak CS, Cuthie JC, Fankhauser RL, Humphrey CD, Barker TL, Jenkins CD, Skillman DR. 2000. Norwalk-like viral gastroenteritis outbreak in U.S. Army trainees. Emerg Infect Dis 6:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. 2002. Outbreak of acute gastroenteritis associated with Norwalk-like viruses among British military personnel—Afghanistan, May 2002. MMWR Morb Mortal Wkly Rep 51:477–479. [PubMed] [Google Scholar]
- 42.Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DW. 2004. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin Infect Dis 39:318–324. doi: 10.1086/421948. [DOI] [PubMed] [Google Scholar]
- 43.Desai R, Hembree CD, Handel A, Matthews JE, Dickey BW, McDonald S, Hall AJ, Parashar UD, Leon JS, Lopman B. 2012. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis 55:189–193. doi: 10.1093/cid/cis372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Asten L, van den Wijngaard C, van Pelt W, van de Kassteele J, Meijer A, van der Hoek W, Kretzschmar M, Koopmans M. 2012. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis 206:628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 45.Gustavsson L, Andersson LM, Brink M, Lindh M, Westin J. 2012. Venous lactate levels can be used to identify patients with poor outcome following community-onset norovirus enteritis. Scand J Infect Dis 44:782–787. doi: 10.3109/00365548.2012.686671. [DOI] [PubMed] [Google Scholar]
- 46.Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA. 2008. Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis 14:1546–1552. doi: 10.3201/eid1410.080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murata T, Katsushima N, Mizuta K, Muraki Y, Hongo S, Matsuzaki Y. 2007. Prolonged norovirus shedding in infants <or=6 months of age with gastroenteritis. Pediatr Infect Dis J 26:46–49. doi: 10.1097/01.inf.0000247102.04997.e0. [DOI] [PubMed] [Google Scholar]
- 48.Stuart RL, Tan K, Mahar JE, Kirkwood CD, Andrew Ramsden C, Andrianopoulos N, Jolley D, Bawden K, Doherty R, Kotsanas D, Bradford J, Buttery JP. 2010. An outbreak of necrotizing enterocolitis associated with norovirus genotype GII.3. Pediatr Infect Dis J 29:644–647. doi: 10.1097/INF.0b013e3181d824e1. [DOI] [PubMed] [Google Scholar]
- 49.Turcios-Ruiz RM, Axelrod P, St John K, Bullitt E, Donahue J, Robinson N, Friss HE. 2008. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr 153:339–344. doi: 10.1016/j.jpeds.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagci S, Eis-Hubinger AM, Yassin AF, Simon A, Bartmann P, Franz AR, Mueller A. 2010. Clinical characteristics of viral intestinal infection in preterm and term neonates. Eur J Clin Microbiol Infect Dis 29:1079–1084. doi: 10.1007/s10096-010-0965-4. [DOI] [PubMed] [Google Scholar]
- 51.Pelizzo G, Nakib G, Goruppi I, Fusillo M, Scorletti F, Mencherini S, Parigi GB, Stronati M, Calcaterra V. 2013. Isolated colon ischemia with norovirus infection in preterm babies: a case series. J Med Case Rep 7:108. doi: 10.1186/1752-1947-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zenda T, Kaneko S, Noriki S. 2010. Norovirus gastroenteritis accompanied by ischemic colitis: a case report. Hiroshima J Med Sci 59:83–85. [PubMed] [Google Scholar]
- 53.Doshi M, Woodwell S, Kelleher K, Mangan K, Axelrod P. 2013. An outbreak of norovirus infection in a bone marrow transplant unit. Am J Infect Control 41:820–823. doi: 10.1016/j.ajic.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, Flegel WA, Thiel E, Schneider T. 2011. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 117:5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roddie C, Paul JP, Benjamin R, Gallimore CI, Xerry J, Gray JJ, Peggs KS, Morris EC, Thomson KJ, Ward KN. 2009. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis 49:1061–1068. doi: 10.1086/605557. [DOI] [PubMed] [Google Scholar]
- 56.Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. 2012. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 18:1883–1889. doi: 10.1016/j.bbmt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, Babiker ZO, Guiver M, Turner AJ, Hughes S, Wynn RF. 2011. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant 15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 58.Florescu DF, Hermsen ED, Kwon JY, Gumeel D, Grant WJ, Mercer DF, Kalil AC. 2011. Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr Transplant 15:718–721. doi: 10.1111/j.1399-3046.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 59.Westhoff TH, Vergoulidou M, Loddenkemper C, Schwartz S, Hofmann J, Schneider T, Zidek W, van der Giet M. 2009. Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant 24:1051–1053. doi: 10.1093/ndt/gfn693. [DOI] [PubMed] [Google Scholar]
- 60.Schorn R, Hohne M, Meerbach A, Bossart W, Wuthrich RP, Schreier E, Muller NJ, Fehr T. 2010. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin Infect Dis 51:307–314. doi: 10.1086/653939. [DOI] [PubMed] [Google Scholar]
- 61.Roos-Weil D, Ambert-Balay K, Lanternier F, Mamzer-Bruneel MF, Nochy D, Pothier P, Avettand-Fenoel V, Anglicheau D, Snanoudj R, Bererhi L, Thervet E, Lecuit M, Legendre C, Lortholary O, Zuber J. 2011. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation 92:61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 62.Coste JF, Vuiblet V, Moustapha B, Bouin A, Lavaud S, Toupance O, de Rougemont A, Benejat L, Megraud F, Wolak-Thierry A, Villena I, Chemla C, Le Magrex E, de Champs C, Andreoletti L, Rieu P, Leveque N. 2013. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol 51:1841–1849. doi: 10.1128/JCM.03366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capizzi T, Makari-Judson G, Steingart R, Mertens WC. 2011. Chronic diarrhea associated with persistent norovirus excretion in patients with chronic lymphocytic leukemia: report of two cases. BMC Infect Dis 11:131. doi: 10.1186/1471-2334-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wingfield T, Gallimore CI, Xerry J, Gray JJ, Klapper P, Guiver M, Blanchard TJ. 2010. Chronic norovirus infection in an HIV-positive patient with persistent diarrhoea: a novel cause. J Clin Virol 49:219–222. doi: 10.1016/j.jcv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 65.Esposito S, Daleno C, Scala A, Senatore L, Ascolese B, Principi N. 2014. Detection of norovirus in respiratory secretions in children with respiratory tract infection. Pediatr Infect Dis J 33:314–316. doi: 10.1097/INF.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 66.Armbrust S, Kramer A, Olbertz D, Zimmermann K, Fusch C. 2009. Norovirus infections in preterm infants: wide variety of clinical courses. BMC Res Notes 2:96. doi: 10.1186/1756-0500-2-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salvador C, Meister B, Larcher H, Crazzolara R, Kropshofer G. 2014. Hemophagocytic lymphohistiocytosis after allogeneic bone marrow transplantation during chronic norovirus infection. Hematol Oncol 32:102–106. doi: 10.1002/hon.2052. [DOI] [PubMed] [Google Scholar]
- 68.Chehade H, Girardin E, Delich V, Pascual MA, Venetz JP, Cachat F. 2012. Acute norovirus-induced agranulocytosis in a pediatric kidney transplant recipient. Transpl Infect Dis 14:E27–E29. doi: 10.1111/j.1399-3062.2012.00754.x. [DOI] [PubMed] [Google Scholar]
- 69.Sugimoto T, Ogawa N, Aoyama M, Sakaguchi M, Isshiki K, Kanasaki M, Uzu T, Nishio Y, Eguchi Y, Kashiwagi A. 2007. Haemolytic uraemic syndrome complicated with norovirus-associated gastroenteritis. Nephrol Dial Transplant 22:2098–2099. doi: 10.1093/ndt/gfm104. [DOI] [PubMed] [Google Scholar]
- 70.Zenda T, Miyamoto M, Kaneko S. 2011. Norovirus gastroenteritis accompanied by marked elevation of transaminases. Hiroshima J Med Sci 60:41–43. [PubMed] [Google Scholar]
- 71.Tsuge M, Goto S, Kato F, Morishima T. 2010. Elevation of serum transaminases with norovirus infection. Clin Pediatr (Phila) 49:574–578. doi: 10.1177/0009922809353593. [DOI] [PubMed] [Google Scholar]
- 72.Gemulla G, Pessler F. 2011. Can norovirus infection lead to a postinfectious arthritis? Report of 2 possible cases. Klin Padiatr 223:43–44. doi: 10.1055/s-0030-1263143. [DOI] [PubMed] [Google Scholar]
- 73.Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, Lanzini A, San Felice del Benaco Study Investigators . 2012. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol 107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 74.Khan RR, Lawson AD, Minnich LL, Martin K, Nasir A, Emmett MK, Welch CA, Udall JN Jr. 2009. Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J Pediatr Gastroenterol Nutr 48:328–333. doi: 10.1097/MPG.0b013e31818255cc. [DOI] [PubMed] [Google Scholar]
- 75.Kolho KL, Klemola P, Simonen-Tikka ML, Ollonen ML, Roivainen M. 2012. Enteric viral pathogens in children with inflammatory bowel disease. J Med Virol 84:345–347. doi: 10.1002/jmv.23193. [DOI] [PubMed] [Google Scholar]
- 76.Masclee GM, Penders J, Pierik M, Wolffs P, Jonkers D. 2013. Enteropathogenic viruses: triggers for exacerbation in IBD? A prospective cohort study using real-time quantitative polymerase chain reaction. Inflamm Bowel Dis 19:124–131. doi: 10.1002/ibd.22976. [DOI] [PubMed] [Google Scholar]
- 77.Porter CK, Faix DJ, Shiau D, Espiritu J, Espinosa BJ, Riddle MS. 2012. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis 55:915–922. doi: 10.1093/cid/cis576. [DOI] [PubMed] [Google Scholar]
- 78.Ito S, Takeshita S, Nezu A, Aihara Y, Usuku S, Noguchi Y, Yokota S. 2006. Norovirus-associated encephalopathy. Pediatr Infect Dis J 25:651–652. doi: 10.1097/01.inf.0000225789.92512.6d. [DOI] [PubMed] [Google Scholar]
- 79.Kimura E, Goto H, Migita A, Harada S, Yamashita S, Hirano T, Uchino M. 2010. An adult norovirus-related encephalitis/encephalopathy with mild clinical manifestation. BMJ Case Rep 2010:bcr0320102784. doi: 10.1136/bcr.03.2010.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Obinata K, Okumura A, Nakazawa T, Kamata A, Niizuma T, Kinoshita K, Shimizu T. 2010. Norovirus encephalopathy in a previously healthy child. Pediatr Infect Dis J 29:1057–1059. doi: 10.1097/INF.0b013e3181e78889. [DOI] [PubMed] [Google Scholar]
- 81.Medici MC, Abelli LA, Dodi I, Dettori G, Chezzi C. 2010. Norovirus RNA in the blood of a child with gastroenteritis and convulsions—a case report. J Clin Virol 48:147–149. doi: 10.1016/j.jcv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Takanashi S, Hashira S, Matsunaga T, Yoshida A, Shiota T, Tung PG, Khamrin P, Okitsu S, Mizuguchi M, Igarashi T, Ushijima H. 2009. Detection, genetic characterization, and quantification of norovirus RNA from sera of children with gastroenteritis. J Clin Virol 44:161–163. doi: 10.1016/j.jcv.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 83.Chan CM, Chan CW, Ma CK, Chan HB. 2011. Norovirus as cause of benign convulsion associated with gastro-enteritis. J Paediatr Child Health 47:373–377. doi: 10.1111/j.1440-1754.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 84.Chen SY, Tsai CN, Lai MW, Chen CY, Lin KL, Lin TY, Chiu CH. 2009. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin Infect Dis 48:849–855. doi: 10.1086/597256. [DOI] [PubMed] [Google Scholar]
- 85.Rossignol JF, El-Gohary YM. 2006. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment Pharmacol Ther 24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 86.Siddiq DM, Koo HL, Adachi JA, Viola GM. 2011. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect 63:394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chagla Z, Quirt J, Woodward K, Neary J, Rutherford C. 2013. Chronic norovirus infection in a transplant patient successfully treated with enterally administered immune globulin. J Clin Virol 58:306–308. doi: 10.1016/j.jcv.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 88.Chen Z, Sosnovtsev SV, Bok K, Parra GI, Makiya M, Agulto L, Green KY, Purcell RH. 2013. Development of Norwalk virus-specific monoclonal antibodies with therapeutic potential for the treatment of Norwalk virus gastroenteritis. J Virol 87:9547–9557. doi: 10.1128/JVI.01376-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boillat Blanco N, Kuonen R, Bellini C, Manuel O, Estrade C, Mazza-Stalder J, Aubert JD, Sahli R, Meylan P. 2011. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl Infect Dis 13:213–215. doi: 10.1111/j.1399-3062.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 90.Engelen MA, Gunia S, Stypmann J. 2011. Elimination of norovirus in a chronic carrier under immunosuppression after heart transplantation—effect of everolimus. Transpl Int 24:e102–e103. doi: 10.1111/j.1432-2277.2011.01330.x. [DOI] [PubMed] [Google Scholar]
- 91.Ochoa TJ, Chea-Woo E, Baiocchi N, Pecho I, Campos M, Prada A, Valdiviezo G, Lluque A, Lai D, Cleary TG. 2013. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J Pediatr 162:349–356. doi: 10.1016/j.jpeds.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagata S, Asahara T, Ohta T, Yamada T, Kondo S, Bian L, Wang C, Yamashiro Y, Nomoto K. 2011. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br J Nutr 106:549–556. doi: 10.1017/S000711451100064X. [DOI] [PubMed] [Google Scholar]
- 93.Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS, O'Brien SJ, IID2 Study Executive Committee. 2012. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 97.Chikhi-Brachet R, Bon F, Toubiana L, Pothier P, Nicolas JC, Flahault A, Kohli E. 2002. Virus diversity in a winter epidemic of acute diarrhea in France. J Clin Microbiol 40:4266–4272. doi: 10.1128/JCM.40.11.4266-4272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel MM, Hall AJ, Vinje J, Parashar UD. 2009. Noroviruses: a comprehensive review. J Clin Virol 44:1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 99.Rha B, Burrer S, Park S, Trivedi T, Parashar UD, Lopman BA. 2013. Emergency department visit data for rapid detection and monitoring of norovirus activity, United States. Emerg Infect Dis 19:1214–1221. doi: 10.3201/eid1908.130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirking HL, Cortes J, Burrer S, Hall AJ, Cohen NJ, Lipman H, Kim C, Daly ER, Fishbein DB. 2010. Likely transmission of norovirus on an airplane, October 2008. Clin Infect Dis 50:1216–1221. doi: 10.1086/651597. [DOI] [PubMed] [Google Scholar]
- 101.Wikswo ME, Cortes J, Hall AJ, Vaughan G, Howard C, Gregoricus N, Cramer EH. 2011. Disease transmission and passenger behaviors during a high morbidity Norovirus outbreak on a cruise ship, January 2009. Clin Infect Dis 52:1116–1122. doi: 10.1093/cid/cir144. [DOI] [PubMed] [Google Scholar]
- 102.Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, Rocks JJ, Kiel J, Montes JS, Moe CL, Eisenberg JN, Leon JS. 2012. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect 140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinje J. 2014. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol 52:147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopman B, Gastanaduy P, Park GW, Hall AJ, Parashar UD, Vinje J. 2012. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol 2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 105.Heijne JC, Teunis P, Morroy G, Wijkmans C, Oostveen S, Duizer E, Kretzschmar M, Wallinga J. 2009. Enhanced hygiene measures and norovirus transmission during an outbreak. Emerg Infect Dis 15:24–30. doi: 10.3201/eid1501.080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaplan JE, Goodman RA, Schonberger LB, Lippy EC, Gary GW. 1982. Gastroenteritis due to Norwalk virus: an outbreak associated with a municipal water system. J Infect Dis 146:190–197. doi: 10.1093/infdis/146.2.190. [DOI] [PubMed] [Google Scholar]
- 107.Cannon RO, Poliner JR, Hirschhorn RB, Rodeheaver DC, Silverman PR, Brown EA, Talbot GH, Stine SE, Monroe SS, Dennis DT, Glass RI. 1991. A multistate outbreak of Norwalk virus gastroenteritis associated with consumption of commercial ice. J Infect Dis 164:860–863. doi: 10.1093/infdis/164.5.860. [DOI] [PubMed] [Google Scholar]
- 108.Wilson R, Anderson LJ, Holman RC, Gary GW, Greenberg HB. 1982. Waterborne gastroenteritis due to the Norwalk agent: clinical and epidemiologic investigation. Am J Public Health 72:72–74. doi: 10.2105/AJPH.72.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.ter Waarbeek HL, Dukers-Muijrers NH, Vennema H, Hoebe CJ. 2010. Waterborne gastroenteritis outbreak at a scouting camp caused by two norovirus genogroups: GI and GII. J Clin Virol 47:268–272. doi: 10.1016/j.jcv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Jones EL, Gaither M, Kramer A, Gerba CP. 2009. An analysis of water quality in the Colorado River, 2003–04; an investigation into recurring outbreaks of norovirus among rafters. Wilderness Environ Med 20:6–13. doi: 10.1580/06-WEME-OR-43.1. [DOI] [PubMed] [Google Scholar]
- 111.Koopman JS, Eckert EA, Greenberg HB, Strohm BC, Isaacson RE, Monto AS. 1982. Norwalk virus enteric illness acquired by swimming exposure. Am J Epidemiol 115:173–177. [DOI] [PubMed] [Google Scholar]
- 112.Koh SJ, Cho HG, Kim BH, Choi BY. 2011. An outbreak of gastroenteritis caused by norovirus-contaminated groundwater at a waterpark in Korea. J Korean Med Sci 26:28–32. doi: 10.3346/jkms.2011.26.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gentry J, Vinje J, Guadagnoli D, Lipp EK. 2009. Norovirus distribution within an estuarine environment. Appl Environ Microbiol 75:5474–5480. doi: 10.1128/AEM.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Victoria M, Guimaraes FR, Fumian TM, Ferreira FF, Vieira CB, Shubo T, Leite JP, Miagostovich MP. 2010. One year monitoring of norovirus in a sewage treatment plant in Rio de Janeiro, Brazil. J Water Health 8:158–165. doi: 10.2166/wh.2009.012. [DOI] [PubMed] [Google Scholar]
- 115.da Silva AK, Le Guyader FS, Le Saux JC, Pommepuy M, Montgomery MA, Elimelech M. 2008. Norovirus removal and particle association in a waste stabilization pond. Environ Sci Technol 42:9151–9157. doi: 10.1021/es802787v. [DOI] [PubMed] [Google Scholar]
- 116.da Silva AK, Le Saux JC, Parnaudeau S, Pommepuy M, Elimelech M, Le Guyader FS. 2007. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl Environ Microbiol 73:7891–7897. doi: 10.1128/AEM.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sima LC, Schaeffer J, Le Saux JC, Parnaudeau S, Elimelech M, Le Guyader FS. 2011. Calicivirus removal in a membrane bioreactor wastewater treatment plant. Appl Environ Microbiol 77:5170–5177. doi: 10.1128/AEM.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kukkula M, Maunula L, Silvennoinen E, von Bonsdorff CH. 1999. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk-like viruses. J Infect Dis 180:1771–1776. doi: 10.1086/315145. [DOI] [PubMed] [Google Scholar]
- 119.Alfano-Sobsey E, Sweat D, Hall A, Breedlove F, Rodriguez R, Greene S, Pierce A, Sobsey M, Davies M, Ledford SL. 2012. Norovirus outbreak associated with undercooked oysters and secondary household transmission. Epidemiol Infect 140:276–282. doi: 10.1017/S0950268811000665. [DOI] [PubMed] [Google Scholar]
- 120.Lowther JA, Gustar NE, Powell AL, Hartnell RE, Lees DN. 2012. Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Appl Environ Microbiol 78:5812–5817. doi: 10.1128/AEM.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lowther JA, Henshilwood K, Lees DN. 2008. Determination of norovirus contamination in oysters from two commercial harvesting areas over an extended period, using semiquantitative real-time reverse transcription PCR. J Food Prot 71:1427–1433. [DOI] [PubMed] [Google Scholar]
- 122.McLeod C, Hay B, Grant C, Greening G, Day D. 2009. Localization of norovirus and poliovirus in Pacific oysters. J Appl Microbiol 106:1220–1230. doi: 10.1111/j.1365-2672.2008.04091.x. [DOI] [PubMed] [Google Scholar]
- 123.Kim HY, Kwak IS, Hwang IG, Ko G. 2008. Optimization of methods for detecting norovirus on various fruit. J Virol Methods 153:104–110. doi: 10.1016/j.jviromet.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 124.Tian P, Yang D, Mandrell R. 2011. Differences in the binding of human norovirus to and from romaine lettuce and raspberries by water and electrolyzed waters. J Food Prot 74:1364–1369. doi: 10.4315/0362-028X.JFP-10-494. [DOI] [PubMed] [Google Scholar]
- 125.Tian P, Yang D, Mandrell R. 2011. A simple method to recover norovirus from fresh produce with large sample size by using histo-blood group antigen-conjugated to magnetic beads in a recirculating affinity magnetic separation system (RCAMS). Int J Food Microbiol 147:223–227. doi: 10.1016/j.ijfoodmicro.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 126.Brugha R, Vipond IB, Evans MR, Sandifer QD, Roberts RJ, Salmon RL, Caul EO, Mukerjee AK. 1999. A community outbreak of food-borne small round-structured virus gastroenteritis caused by a contaminated water supply. Epidemiol Infect 122:145–154. doi: 10.1017/S0950268898001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dore B, Keaveney S, Flannery J, Rajko-Nenow P. 2010. Management of health risks associated with oysters harvested from a norovirus contaminated area, Ireland, February-March 2010. Euro Surveill 15(19):pii=19567 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19567. [PubMed] [Google Scholar]
- 128.Malik YS, Goyal SM. 2006. Virucidal efficacy of sodium bicarbonate on a food contact surface against feline calicivirus, a norovirus surrogate. Int J Food Microbiol 109:160–163. doi: 10.1016/j.ijfoodmicro.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 129.Kim SW, Baek SB, Ha JH, Lee MH, Choi C, Ha SD. 2012. Chlorine treatment to inactivate norovirus on food contact surfaces. J Food Prot 75:184–188. doi: 10.4315/0362-028X.JFP-11-243. [DOI] [PubMed] [Google Scholar]
- 130.Kuo HW, Schmid D, Jelovcan S, Pichler AM, Magnet E, Reichart S, Allerberger F. 2009. A foodborne outbreak due to norovirus in Austria, 2007. J Food Prot 72:193–196. [DOI] [PubMed] [Google Scholar]
- 131.Anderson AD, Garrett VD, Sobel J, Monroe SS, Fankhauser RL, Schwab KJ, Bresee JS, Mead PS, Higgins C, Campana J, Glass RI, Outbreak Investigation Team. 2001. Multistate outbreak of Norwalk-like virus gastroenteritis associated with a common caterer. Am J Epidemiol 154:1013–1019. doi: 10.1093/aje/154.11.1013. [DOI] [PubMed] [Google Scholar]
- 132.Fallahi S, Mattison K. 2011. Evaluation of murine norovirus persistence in environments relevant to food production and processing. J Food Prot 74:1847–1851. doi: 10.4315/0362-028X.JFP-11-081. [DOI] [PubMed] [Google Scholar]
- 133.Pang XL, Preiksaitis JK, Lee BE. 2014. Enhanced enteric virus detection in sporadic gastroenteritis using a multi-target real-time PCR panel: a one-year study. J Med Virol 86:1594–1601. doi: 10.1002/jmv.23851. [DOI] [PubMed] [Google Scholar]
- 134.Monica B, Ramani S, Banerjee I, Primrose B, Iturriza-Gomara M, Gallimore CI, Brown DW, Fathima M, Moses PD, Gray JJ, Kang G. 2007. Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, South India. J Med Virol 79:544–551. doi: 10.1002/jmv.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bon F, Fascia P, Dauvergne M, Tenenbaum D, Planson H, Petion AM, Pothier P, Kohli E. 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol 37:3055–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O'Ryan ML, Mamani N, Gaggero A, Avendano LF, Prieto S, Pena A, Jiang X, Matson DO. 2000. Human caliciviruses are a significant pathogen of acute sporadic diarrhea in children of Santiago, Chile. J Infect Dis 182:1519–1522. doi: 10.1086/315874. [DOI] [PubMed] [Google Scholar]
- 137.Dove W, Cunliffe NA, Gondwe JS, Broadhead RL, Molyneux ME, Nakagomi O, Hart CA. 2005. Detection and characterization of human caliciviruses in hospitalized children with acute gastroenteritis in Blantyre, Malawi. J Med Virol 77:522–527. doi: 10.1002/jmv.20488. [DOI] [PubMed] [Google Scholar]
- 138.Malasao R, Maneekarn N, Khamrin P, Pantip C, Tonusin S, Ushijima H, Peerakome S. 2008. Genetic diversity of norovirus, sapovirus, and astrovirus isolated from children hospitalized with acute gastroenteritis in Chiang Mai, Thailand. J Med Virol 80:1749–1755. doi: 10.1002/jmv.21244. [DOI] [PubMed] [Google Scholar]
- 139.Pang XL, Joensuu J, Vesikari T. 1999. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr Infect Dis J 18:420–426. doi: 10.1097/00006454-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 140.O'Ryan ML, Lucero Y, Prado V, Santolaya ME, Rabello M, Solis Y, Berrios D, O'Ryan-Soriano MA, Cortes H, Mamani N. 2009. Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr Infect Dis J 28:879–884. doi: 10.1097/INF.0b013e3181a4bb60. [DOI] [PubMed] [Google Scholar]
- 141.Kirkwood CD, Clark R, Bogdanovic-Sakran N, Bishop RF. 2005. A 5-year study of the prevalence and genetic diversity of human caliciviruses associated with sporadic cases of acute gastroenteritis in young children admitted to hospital in Melbourne, Australia (1998–2002). J Med Virol 77:96–101. doi: 10.1002/jmv.20419. [DOI] [PubMed] [Google Scholar]
- 142.Zintz C, Bok K, Parada E, Barnes-Eley M, Berke T, Staat MA, Azimi P, Jiang X, Matson DO. 2005. Prevalence and genetic characterization of caliciviruses among children hospitalized for acute gastroenteritis in the United States. Infect Genet Evol 5:281–290. doi: 10.1016/j.meegid.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 143.Zingg W, Colombo C, Jucker T, Bossart W, Ruef C. 2005. Impact of an outbreak of norovirus infection on hospital resources. Infect Control Hosp Epidemiol 26:263–267. doi: 10.1086/502537. [DOI] [PubMed] [Google Scholar]
- 144.Mattner F, Mattner L, Borck HU, Gastmeier P. 2005. Evaluation of the impact of the source (patient versus staff) on nosocomial norovirus outbreak severity. Infect Control Hosp Epidemiol 26:268–272. doi: 10.1086/502538. [DOI] [PubMed] [Google Scholar]
- 145.Pether JV, Caul EO. 1983. An outbreak of food-borne gastroenteritis in two hospitals associated with a Norwalk-like virus. J Hyg (Lond) 91:343–350. doi: 10.1017/S0022172400060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Centers for Disease Control and Prevention. 2008. Norovirus outbreak in an elementary school—District of Columbia, February 2007. MMWR Morb Mortal Wkly Rep 56:1340–1343. [PubMed] [Google Scholar]
- 147.Centers for Disease Control and Prevention. 2009. Norovirus outbreaks on three college campuses—California, Michigan, and Wisconsin, 2008. MMWR Morb Mortal Wkly Rep 58:1095–1100. [PubMed] [Google Scholar]
- 148.Marks PJ, Vipond IB, Regan FM, Wedgwood K, Fey RE, Caul EO. 2003. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol Infect 131:727–736. doi: 10.1017/S0950268803008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McCarthy M, Estes MK, Hyams KC. 2000. Norwalk-like virus infection in military forces: epidemic potential, sporadic disease, and the future direction of prevention and control efforts. J Infect Dis 181(Suppl 2):S387–S391. doi: 10.1086/315582. [DOI] [PubMed] [Google Scholar]
- 150.Mayet A, Andreo V, Bedubourg G, Victorion S, Plantec J, Soullie B, Meynard J, Dedieu J, Polveche P, Migliani R. 2011. Food-borne outbreak of norovirus infection in a French military parachuting unit, April 2011. Euro Surveill 16(30):pii=19930 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19930. [PubMed] [Google Scholar]
- 151.Chapman AS, Witkop CT, Escobar JD, Schlorman CA, DeMarcus LS, Marmer LM, Crum ME. 2011. Norovirus outbreak associated with person-to-person transmission, US Air Force Academy, July 2011. MSMR 18(11):2–5. [PubMed] [Google Scholar]
- 152.Gunn RA, Terranova WA, Greenberg HB, Yashuk J, Gary GW, Wells JG, Taylor PR, Feldman RA. 1980. Norwalk virus gastroenteritis aboard a cruise ship: an outbreak on five consecutive cruises. Am J Epidemiol 112:820–827. [DOI] [PubMed] [Google Scholar]
- 153.Addiss DG, Yashuk JC, Clapp DE, Blake PA. 1989. Outbreaks of diarrhoeal illness on passenger cruise ships, 1975–85. Epidemiol Infect 103:63–72. doi: 10.1017/S0950268800030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Centers for Disease Control and Prevention. 2002. Outbreaks of gastroenteritis associated with noroviruses on cruise ships—United States, 2002. MMWR Morb Mortal Wkly Rep 51:1112–1115. [PubMed] [Google Scholar]
- 155.Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, Charles M, Chege W, Isakbaeva E, Wright JG, Mintz E, Forney D, Massey J, Glass RI, Monroe SS. 2004. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus—United States, 2002. J Infect Dis 190:27–36. doi: 10.1086/420888. [DOI] [PubMed] [Google Scholar]
- 156.Centers for Disease Control and Prevention. 4 April 2014, posting date. Outbreak updates for international cruise ships. Vessel sanitation program. Centers for Disease Control and Prevention, Atlanta, GA; http://www.cdc.gov/nceh/vsp/surv/gilist.htm. [Google Scholar]
- 157.Domenech-Sanchez A. 2011. Gastroenteritis outbreak caused by norovirus associated with the children's club of a hotel located in Majorca, Spain. Clin Microbiol Infect 17:949–951. doi: 10.1111/j.1469-0691.2010.03342.x. [DOI] [PubMed] [Google Scholar]
- 158.Domenech-Sanchez A, Juan C, Perez JL, Berrocal CI. 2011. Unmanageable norovirus outbreak in a single resort located in the Dominican Republic. Clin Microbiol Infect 17:952–954. doi: 10.1111/j.1469-0691.2010.03411.x. [DOI] [PubMed] [Google Scholar]
- 159.Hauri AM, Westbrock HJ, Claus H, Geis S, Giernat S, Forssbohm M, Uphoff H. 2011. Electronic outbreak surveillance in Germany: a first evaluation for nosocomial norovirus outbreaks. PLoS One 6:e17341. doi: 10.1371/journal.pone.0017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, Iritani N, Kroneman A, Vennema H, Vinje J, White PA, Koopmans M. 2013. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill 18(1):pii=20345 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20345. [PubMed] [Google Scholar]
- 161.Koopmans M, Vennema H, Heersma H, van Strien E, van Duynhoven Y, Brown D, Reacher M, Lopman B, European Consortium on Foodborne Viruses. 2003. Early identification of common-source foodborne virus outbreaks in Europe. Emerg Infect Dis 9:1136–1142. doi: 10.3201/eid0909.020766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Swaminathan B, Barrett TJ, Hunter SB, Tauxe RV, CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis 7:382–389. doi: 10.3201/eid0703.017303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinje J. 2011. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis 17:1389–1395. doi: 10.3201/eid1708.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Centers for Disease Control and Prevention. 2013. Emergence of new norovirus strain GII.4 Sydney—United States, 2012. MMWR Morb Mortal Wkly Rep 62:55. [PMC free article] [PubMed] [Google Scholar]
- 165.Harris JP, Adams NL, Lopman BA, Allen DJ, Adak GK. 2014. The development of Web-based surveillance provides new insights into the burden of norovirus outbreaks in hospitals in England. Epidemiol Infect 142:1590–1598. doi: 10.1017/S0950268813002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scallan E. 2007. Activities, achievements, and lessons learned during the first 10 years of the Foodborne Diseases Active Surveillance Network: 1996-2005. Clin Infect Dis 44:718–725. doi: 10.1086/511648. [DOI] [PubMed] [Google Scholar]
- 167.Kirk MD, McKay I, Hall GV, Dalton CB, Stafford R, Unicomb L, Gregory J. 2008. Food safety: foodborne disease in Australia. The OzFoodNet experience. Clin Infect Dis 47:392–400. doi: 10.1086/589861. [DOI] [PubMed] [Google Scholar]
- 168.Kaplan JE, Feldman R, Campbell DS, Lookabaugh C, Gary GW. 1982. The frequency of a Norwalk-like pattern of illness in outbreaks of acute gastroenteritis. Am J Public Health 72:1329–1332. doi: 10.2105/AJPH.72.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Turcios RM, Widdowson MA, Sulka AC, Mead PS, Glass RI. 2006. Reevaluation of epidemiological criteria for identifying outbreaks of acute gastroenteritis due to norovirus: United States, 1998-2000. Clin Infect Dis 42:964–969. doi: 10.1086/500940. [DOI] [PubMed] [Google Scholar]
- 170.Centers for Disease Control and Prevention. 12 April 2012, posting date. Norovirus specimen collection. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/norovirus/lab-testing/collection.html. [Google Scholar]
- 171.Costantini V, Grenz L, Fritzinger A, Lewis D, Biggs C, Hale A, Vinje J. 2010. Diagnostic accuracy and analytical sensitivity of IDEIA Norovirus assay for routine screening of human norovirus. J Clin Microbiol 48:2770–2778. doi: 10.1128/JCM.00654-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gray JJ, Kohli E, Ruggeri FM, Vennema H, Sanchez-Fauquier A, Schreier E, Gallimore CI, Iturriza-Gomara M, Giraudon H, Pothier P, Di Bartolo I, Inglese N, de Bruin E, van der Veer B, Moreno S, Montero V, de Llano MC, Hohne M, Diedrich SM. 2007. European multicenter evaluation of commercial enzyme immunoassays for detecting norovirus antigen in fecal samples. Clin Vaccine Immunol 14:1349–1355. doi: 10.1128/CVI.00214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MP, Estes MK. 2004. Laboratory efforts to cultivate noroviruses. J Gen Virol 85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- 174.Centers for Disease Control and Prevention. 2011. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recommend Rep 60(RR3):1–18. [Google Scholar]
- 175.De Leon R, Matsui SM, Baric RS, Herrmann JE, Blacklow NR, Greenberg HB, Sobsey MD. 1992. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol 30:3151–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Green J, Norcott JP, Lewis D, Arnold C, Brown DW. 1993. Norwalk-like viruses: demonstration of genomic diversity by polymerase chain reaction. J Clin Microbiol 31:3007–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jiang X, Wang J, Graham DY, Estes MK. 1992. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol 30:2529–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moe CL, Gentsch J, Ando T, Grohmann G, Monroe SS, Jiang X, Wang J, Estes MK, Seto Y, Humphrey C, Stine S, Glass RI. 1994. Application of PCR to detect Norwalk virus in fecal specimens from outbreaks of gastroenteritis. J Clin Microbiol 32:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ando T, Monroe SS, Gentsch JR, Jin Q, Lewis DC, Glass RI. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J Clin Microbiol 33:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Green J, Gallimore CI, Norcott JP, Lewis D, Brown DW. 1995. Broadly reactive reverse transcriptase polymerase chain reaction for the diagnosis of SRSV-associated gastroenteritis. J Med Virol 47:392–398. doi: 10.1002/jmv.1890470416. [DOI] [PubMed] [Google Scholar]
- 181.Le Guyader F, Estes MK, Hardy ME, Neill FH, Green J, Brown DW, Atmar RL. 1996. Evaluation of a degenerate primer for the PCR detection of human caliciviruses. Arch Virol 141:2225–2235. doi: 10.1007/BF01718228. [DOI] [PubMed] [Google Scholar]
- 182.Vinje J, Koopmans MP. 1996. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J Infect Dis 174:610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- 183.Vinje J, Vennema H, Maunula L, von Bonsdorff CH, Hoehne M, Schreier E, Richards A, Green J, Brown D, Beard SS, Monroe SS, de Bruin E, Svensson L, Koopmans MP. 2003. International collaborative study to compare reverse transcriptase PCR assays for detection and genotyping of noroviruses. J Clin Microbiol 41:1423–1433. doi: 10.1128/JCM.41.4.1423-1433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, Natori K, Takeda N, Katayama K. 2002. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods 100:107–114. doi: 10.1016/S0166-0934(01)00404-9. [DOI] [PubMed] [Google Scholar]
- 185.Medici MC, Martinelli M, Ruggeri FM, Abelli LA, Bosco S, Arcangeletti MC, Pinardi F, De Conto F, Calderaro A, Chezzi C, Dettori G. 2005. Broadly reactive nested reverse transcription-PCR using an internal RNA standard control for detection of noroviruses in stool samples. J Clin Microbiol 43:3772–3778. doi: 10.1128/JCM.43.8.3772-3778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.O'Neill HJ, McCaughey C, Wyatt DE, Mitchell F, Coyle PV. 2001. Gastroenteritis outbreaks associated with Norwalk-like viruses and their investigation by nested RT-PCR. BMC Microbiol 1:14. doi: 10.1186/1471-2180-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saito H, Saito S, Kamada K, Harata S, Sato H, Morita M, Miyajima Y. 1998. Application of RT-PCR designed from the sequence of the local SRSV strain to the screening in viral gastroenteritis outbreaks. Microbiol Immunol 42:439–446. doi: 10.1111/j.1348-0421.1998.tb02307.x. [DOI] [PubMed] [Google Scholar]
- 188.Schreier E, Doring F, Kunkel U. 2000. Molecular epidemiology of outbreaks of gastroenteritis associated with small round structured viruses in Germany in 1997/98. Arch Virol 145:443–453. doi: 10.1007/s007050050038. [DOI] [PubMed] [Google Scholar]
- 189.Vennema H, de Bruin E, Koopmans M. 2002. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J Clin Virol 25:233–235. doi: 10.1016/S1386-6532(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 190.Miller I, Gunson R, Carman WF. 2002. Norwalk like virus by light cycler PCR. J Clin Virol 25:231–232. doi: 10.1016/S1386-6532(02)00110-5. [DOI] [PubMed] [Google Scholar]
- 191.Pang X, Lee B, Chui L, Preiksaitis JK, Monroe SS. 2004. Evaluation and validation of real-time reverse transcription-PCR assay using the LightCycler system for detection and quantitation of norovirus. J Clin Microbiol 42:4679–4685. doi: 10.1128/JCM.42.10.4679-4685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Richards GP, Watson MA, Kingsley DH. 2004. A SYBR green, real-time RT-PCR method to detect and quantitate Norwalk virus in stools. J Virol Methods 116:63–70. doi: 10.1016/j.jviromet.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 193.Schmid M, Oehme R, Schalasta G, Brockmann S, Kimmig P, Enders G. 2004. Fast detection of noroviruses using a real-time PCR assay and automated sample preparation. BMC Infect Dis 4:15. doi: 10.1186/1471-2334-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dreier J, Stormer M, Made D, Burkhardt S, Kleesiek K. 2006. Enhanced reverse transcription-PCR assay for detection of norovirus genogroup I. J Clin Microbiol 44:2714–2720. doi: 10.1128/JCM.00443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hoehne M, Schreier E. 2006. Detection of norovirus genogroup I and II by multiplex real-time RT-PCR using a 3′-minor groove binder-DNA probe. BMC Infect Dis 6:69. doi: 10.1186/1471-2334-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hohne M, Schreier E. 2004. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J Med Virol 72:312–319. doi: 10.1002/jmv.10573. [DOI] [PubMed] [Google Scholar]
- 197.Ishida S, Yoshizumi S, Ikeda T, Miyoshi M, Okano M, Okui T. 2008. Sensitive and rapid detection of norovirus using duplex TaqMan reverse transcription-polymerase chain reaction. J Med Virol 80:913–920. doi: 10.1002/jmv.21142. [DOI] [PubMed] [Google Scholar]
- 198.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol 41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Logan C, O'Leary JJ, O'Sullivan N. 2007. Real-time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J Virol Methods 146:36–44. doi: 10.1016/j.jviromet.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 200.Rolfe KJ, Parmar S, Mururi D, Wreghitt TG, Jalal H, Zhang H, Curran MD. 2007. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J Clin Virol 39:318–321. doi: 10.1016/j.jcv.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 201.Scipioni A, Bourgot I, Mauroy A, Ziant D, Saegerman C, Daube G, Thiry E. 2008. Detection and quantification of human and bovine noroviruses by a TaqMan RT-PCR assay with a control for inhibition. Mol Cell Probes 22:215–222. doi: 10.1016/j.mcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 202.Trujillo AA, McCaustland KA, Zheng DP, Hadley LA, Vaughn G, Adams SM, Ando T, Glass RI, Monroe SS. 2006. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol 44:1405–1412. doi: 10.1128/JCM.44.4.1405-1412.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hymas W, Atkinson A, Stevenson J, Hillyard D. 2007. Use of modified oligonucleotides to compensate for sequence polymorphisms in the real-time detection of norovirus. J Virol Methods 142:10–14. doi: 10.1016/j.jviromet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 204.Mohamed N, Belak S, Hedlund KO, Blomberg J. 2006. Experience from the development of a diagnostic single tube real-time PCR for human caliciviruses, norovirus genogroups I and II. J Virol Methods 132:69–76. doi: 10.1016/j.jviromet.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 205.Wolf S, Williamson WM, Hewitt J, Rivera-Aban M, Lin S, Ball A, Scholes P, Greening GE. 2007. Sensitive multiplex real-time reverse transcription-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl Environ Microbiol 73:5464–5470. doi: 10.1128/AEM.00572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ludert JE, Alcala AC, Liprandi F. 2004. Primer pair p289-p290, designed to detect both noroviruses and sapoviruses by reverse transcription-PCR, also detects rotaviruses by cross-reactivity. J Clin Microbiol 42:835–836. doi: 10.1128/JCM.42.2.835-836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gunson RN, Carman WF. 2005. Comparison of two real-time PCR methods for diagnosis of norovirus infection in outbreak and community settings. J Clin Microbiol 43:2030–2031. doi: 10.1128/JCM.43.4.2030-2031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vainio K, Myrmel M. 2006. Molecular epidemiology of norovirus outbreaks in Norway during 2000 to 2005 and comparison of four norovirus real-time reverse transcriptase PCR assays. J Clin Microbiol 44:3695–3702. doi: 10.1128/JCM.00023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jothikumar N, Lowther JA, Henshilwood K, Lees DN, Hill VR, Vinje J. 2005. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl Environ Microbiol 71:1870–1875. doi: 10.1128/AEM.71.4.1870-1875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Loisy F, Atmar RL, Guillon P, Le Cann P, Pommepuy M, Le Guyader FS. 2005. Real-time RT-PCR for norovirus screening in shellfish. J Virol Methods 123:1–7. doi: 10.1016/j.jviromet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 211.Vinje J, Hamidjaja RA, Sobsey MD. 2004. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J Virol Methods 116:109–117. doi: 10.1016/j.jviromet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 212.Bon F, Giraudon H, Sancey C, Barranger C, Joannes M, Pothier P, Kohli E. 2004. Development and evaluation of a new commercial test allowing the simultaneous detection of noroviruses and sapoviruses by reverse transcription-PCR and microplate hybridization. J Clin Microbiol 42:2218–2220. doi: 10.1128/JCM.42.5.2218-2220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kele B, Lengyel G, Deak J. 2011. Comparison of an ELISA and two reverse transcription polymerase chain reaction methods for norovirus detection. Diagn Microbiol Infect Dis 70:475–478. doi: 10.1016/j.diagmicrobio.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 214.Hyun J, Kim HS, Kim HS, Lee KM. 2014. Evaluation of a new real-time reverse transcription polymerase chain reaction assay for detection of norovirus in fecal specimens. Diagn Microbiol Infect Dis 78:40–44. doi: 10.1016/j.diagmicrobio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 215.Dunbar NL, Bruggink LD, Marshall JA. 2014. Evaluation of the RIDAGENE real-time PCR assay for the detection of GI and GII norovirus. Diagn Microbiol Infect Dis 79:317–321. doi: 10.1016/j.diagmicrobio.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 216.Claas E, Burnham CA, Mazulli T, Templeton K, Topin F. 2013. Performance of the xTAG Gastrointestinal Pathogen Panel (GPP), a multiplex molecular assay for simultaneous detection of bacterial, viral and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol 23:1041–1045. doi: 10.4014/jmb.1212.12042. [DOI] [PubMed] [Google Scholar]
- 217.Wessels E, Rusman LG, van Bussel MJ, Claas EC. 2014. Added value of multiplex Luminex Gastrointestinal Pathogen Panel (xTAG GPP) testing in the diagnosis of infectious gastroenteritis. Clin Microbiol Infect 20:O182–O187. doi: 10.1111/1469-0691.12364. [DOI] [PubMed] [Google Scholar]
- 218.van Maarseveen NM, Wessels E, de Brouwer CS, Vossen AC, Claas EC. 2010. Diagnosis of viral gastroenteritis by simultaneous detection of adenovirus group F, astrovirus, rotavirus group A, norovirus genogroups I and II, and sapovirus in two internally controlled multiplex real-time PCR assays. J Clin Virol 49:205–210. doi: 10.1016/j.jcv.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 219.Mengelle C, Mansuy JM, Prere MF, Grouteau E, Claudet I, Kamar N, Huynh A, Plat G, Benard M, Marty N, Valentin A, Berry A, Izopet J. 2013. Simultaneous detection of gastrointestinal pathogens with a multiplex Luminex-based molecular assay in stool samples from diarrhoeic patients. Clin Microbiol Infect 19:E458–E465. doi: 10.1111/1469-0691.12255. [DOI] [PubMed] [Google Scholar]
- 220.Navidad JF, Griswold DJ, Gradus MS, Bhattacharyya S. 2013. Evaluation of Luminex xTAG gastrointestinal pathogen analyte-specific reagents for high-throughput, simultaneous detection of bacteria, viruses, and parasites of clinical and public health importance. J Clin Microbiol 51:3018–3024. doi: 10.1128/JCM.00896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kahlau P, Malecki M, Schildgen V, Schulz C, Winterfeld I, Messler S, Mattner F, Schildgen O. 2013. Utility of two novel multiplexing assays for the detection of gastrointestinal pathogens—a first experience. Springerplus 2:106. doi: 10.1186/2193-1801-2-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu J, Kibiki G, Maro V, Maro A, Kumburu H, Swai N, Taniuchi M, Gratz J, Toney D, Kang G, Houpt E. 2011. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. J Clin Virol 50:308–313. doi: 10.1016/j.jcv.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Khare R, Espy MJ, Cebelinski E, Boxrud D, Sloan LM, Cunningham SA, Pritt BS, Patel R, Binnicker MJ. 2014. Multiplex detection of gastrointestinal pathogens: a comparative evaluation of two commercial panels using clinical stool specimens. J Clin Microbiol 52:3667–3673. doi: 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Greene SR, Moe CL, Jaykus LA, Cronin M, Grosso L, Aarle P. 2003. Evaluation of the NucliSens Basic kit assay for detection of Norwalk virus RNA in stool specimens. J Virol Methods 108:123–131. doi: 10.1016/S0166-0934(02)00286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Houde A, Leblanc D, Poitras E, Ward P, Brassard J, Simard C, Trottier YL. 2006. Comparative evaluation of RT-PCR, nucleic acid sequence-based amplification (NASBA) and real-time RT-PCR for detection of noroviruses in faecal material. J Virol Methods 135:163–172. doi: 10.1016/j.jviromet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 227.Kou X, Wu Q, Zhang J, Fan H. 2006. Rapid detection of noroviruses in fecal samples and shellfish by nucleic acid sequence-based amplification. J Microbiol 44:403–408. [PubMed] [Google Scholar]
- 228.Moore C, Clark EM, Gallimore CI, Corden SA, Gray JJ, Westmoreland D. 2004. Evaluation of a broadly reactive nucleic acid sequence based amplification assay for the detection of noroviruses in faecal material. J Clin Virol 29:290–296. doi: 10.1016/S1386-6532(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 229.Patterson SS, Smith MW, Casper ET, Huffman D, Stark L, Fries D, Paul JH. 2006. A nucleic acid sequence-based amplification assay for real-time detection of norovirus genogroup II. J Appl Microbiol 101:956–963. doi: 10.1111/j.1365-2672.2006.02934.x. [DOI] [PubMed] [Google Scholar]
- 230.Fukuda S, Takao S, Kuwayama M, Shimazu Y, Miyazaki K. 2006. Rapid detection of norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol 44:1376–1381. doi: 10.1128/JCM.44.4.1376-1381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Iturriza-Gomara M, Xerry J, Gallimore CI, Dockery C, Gray J. 2008. Evaluation of the Loopamp (loop-mediated isothermal amplification) kit for detecting norovirus RNA in faecal samples. J Clin Virol 42:389–393. doi: 10.1016/j.jcv.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 232.Yoda T, Suzuki Y, Yamazaki K, Sakon N, Kanki M, Aoyama I, Tsukamoto T. 2007. Evaluation and application of reverse transcription loop-mediated isothermal amplification for detection of noroviruses. J Med Virol 79:326–334. doi: 10.1002/jmv.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.MacCannell T, Umscheid CA, Agarwal RK, Lee I, Kuntz G, Stevenson KB, Healthcare Infection Control Practices Advisory Committee. 2011. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol 32:939–969. doi: 10.1086/662025. [DOI] [PubMed] [Google Scholar]
- 234.Barclay L, Park GW, Vega E, Hall A, Parashar U, Vinje J, Lopman B. 2014. Infection control for norovirus. Clin Microbiol Infect 20:731–740. doi: 10.1111/1469-0691.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kosa KM, Cates SC, Hall AJ, Brophy JE, Frasier A. 2014. Knowledge of norovirus prevention and control among infection preventionists. Am J Infect Control 42:676–678. doi: 10.1016/j.ajic.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lynn S, Toop J, Hanger C, Millar N. 2004. Norovirus outbreaks in a hospital setting: the role of infection control. N Z Med J 117:U771. [PubMed] [Google Scholar]
- 237.Cheng VC, Wong LM, Tai JW, Chan JF, To KK, Li IW, Hung IF, Chan KH, Ho PL, Yuen KY. 2011. Prevention of nosocomial transmission of norovirus by strategic infection control measures. Infect Control Hosp Epidemiol 32:229–237. doi: 10.1086/658330. [DOI] [PubMed] [Google Scholar]
- 238.Albers MK. 2004. An unwanted visitor. Aggressive infection control strategies are needed to shorten the hospital visit of the easily spread norovirus. Can Nurse 100:21–26. [PubMed] [Google Scholar]
- 239.Chadwick PR, Beards G, Brown D, Caul EO, Cheesbrough J, Clarke I, Curry A, O'Brien S, Quigley K, Sellwood J, Westmoreland D. 2000. Management of hospital outbreaks of gastro-enteritis due to small roundstructured viruses. J Hosp Infect 45(1):1–10. doi: 10.1053/jhin.2000.0662. [DOI] [PubMed] [Google Scholar]
- 240.Hansen S, Stamm-Balderjahn S, Zuschneid I, Behnke M, Ruden H, Vonberg RP, Gastmeier P. 2007. Closure of medical departments during nosocomial outbreaks: data from a systematic analysis of the literature. J Hosp Infect 65:348–353. doi: 10.1016/j.jhin.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lopman BA, Reacher MH, Vipond IB, Hill D, Perry C, Halladay T, Brown DW, Edmunds WJ, Sarangi J. 2004. Epidemiology and cost of nosocomial gastroenteritis, Avon, England, 2002-2003. Emerg Infect Dis 10:1827–1834. doi: 10.3201/eid1010.030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Georgiadou SP, Loukeris D, Smilakou S, Daikos GL, Sipsas NV. 2011. Effective control of an acute gastroenteritis outbreak due to norovirus infection in a hospital ward in Athens, Greece, April 2011. Euro Surveill 16(28):pii=19915 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19915. [PubMed] [Google Scholar]
- 243.Russo PL, Spelman DW, Harrington GA, Jenney AW, Gunesekere IC, Wright PJ, Doultree JC, Marshall JA. 1997. Hospital outbreak of Norwalk-like virus. Infect Control Hosp Epidemiol 18:576–579. doi: 10.2307/30141270. [DOI] [PubMed] [Google Scholar]
- 244.Johnston CP, Qiu H, Ticehurst JR, Dickson C, Rosenbaum P, Lawson P, Stokes AB, Lowenstein CJ, Kaminsky M, Cosgrove SE, Green KY, Perl TM. 2007. Outbreak management and implications of a nosocomial norovirus outbreak. Clin Infect Dis 45:534–540. doi: 10.1086/520666. [DOI] [PubMed] [Google Scholar]
- 245.Fretz R, Schmid D, Jelovcan S, Tschertou R, Krassnitzer E, Schirmer M, Hell M, Allerberger F. 2009. An outbreak of norovirus gastroenteritis in an Austrian hospital, winter 2006-2007. Wien Klin Wochenschr 121:137–143. doi: 10.1007/s00508-008-1135-x. [DOI] [PubMed] [Google Scholar]
- 246.Haill CF, Newell P, Ford C, Whitley M, Cox J, Wallis M, Best R, Jenks PJ. 2012. Compartmentalization of wards to cohort symptomatic patients at the beginning and end of norovirus outbreaks. J Hosp Infect 82:30–35. doi: 10.1016/j.jhin.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 247.Illingworth E, Taborn E, Fielding D, Cheesbrough J, Diggle PJ, Orr D. 2011. Is closure of entire wards necessary to control norovirus outbreaks in hospital? Comparing the effectiveness of two infection control strategies. J Hosp Infect 79:32–37. doi: 10.1016/j.jhin.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 248.Cheng FW, Leung TF, Lai RW, Chan PK, Hon EK, Ng PC. 2006. Rapid control of norovirus gastroenteritis outbreak in an acute paediatric ward. Acta Paediatr 95:581–586. doi: 10.1080/08035250500449874. [DOI] [PubMed] [Google Scholar]
- 249.Greig JD, Lee MB. 2012. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect 140:1151–1160. doi: 10.1017/S0950268811002731. [DOI] [PubMed] [Google Scholar]
- 250.McCall J, Smithson R. 2002. Rapid response and strict control measures can contain a hospital outbreak of Norwalk-like virus. Commun Dis Public Health 5:243–246. [PubMed] [Google Scholar]
- 251.Cooper E, Blamey S. 2005. A norovirus gastroenteritis epidemic in a long-term-care facility. Infect Control Hosp Epidemiol 26:256–258. doi: 10.1086/502535. [DOI] [PubMed] [Google Scholar]
- 252.Anderson KL. 2009. Norovirus outbreak management in a resident-directed care environment. Geriatr Nurs 30:318–328. doi: 10.1016/j.gerinurse.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 253.Friesema IH, Vennema H, Heijne JC, de Jager CM, Morroy G, van den Kerkhof JH, de Coster EJ, Wolters BA, ter Waarbeek HL, Fanoy EB, Teunis PF, van der Linde R, van Duynhoven YT. 2009. Norovirus outbreaks in nursing homes: the evaluation of infection control measures. Epidemiol Infect 137:1722–1733. doi: 10.1017/S095026880900274X. [DOI] [PubMed] [Google Scholar]
- 254.Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, Society for Healthcare Epidemiology of America, Association for Professionals in Infection Control, Infectious Diseases Society of America Hand Hygiene Task Force. 2002. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol 23:S3–S40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 255.Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinje J. 2010. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot 73:2232–2238. [DOI] [PubMed] [Google Scholar]
- 256.Gehrke C, Steinmann J, Goroncy-Bermes P. 2004. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J Hosp Infect 56:49–55. doi: 10.1016/j.jhin.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 257.Kampf G, Grotheer D, Steinmann J. 2005. Efficacy of three ethanol-based hand rubs against feline calicivirus, a surrogate virus for norovirus. J Hosp Infect 60:144–149. doi: 10.1016/j.jhin.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 258.Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. 2010. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol 76:394–399. doi: 10.1128/AEM.01729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lages SL, Ramakrishnan MA, Goyal SM. 2008. In-vivo efficacy of hand sanitisers against feline calicivirus: a surrogate for norovirus. J Hosp Infect 68:159–163. doi: 10.1016/j.jhin.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 260.Weber DJ, Sickbert-Bennett EE, Vinje J, Brown VM, MacFarquhar JK, Engel JP, Rutala WA. 2005. Lessons learned from a norovirus outbreak in a locked pediatric inpatient psychiatric unit. Infect Control Hosp Epidemiol 26:841–843. doi: 10.1086/502504. [DOI] [PubMed] [Google Scholar]
- 261.Cheesbrough JS, Green J, Gallimore CI, Wright PA, Brown DW. 2000. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect 125:93–98. doi: 10.1017/S095026889900432X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Horm KM, Davidson PM, Harte FM, D'Souza DH. 2012. Survival and inactivation of human norovirus surrogates in blueberry juice by high-pressure homogenization. Foodborne Pathog Dis 9:974–979. doi: 10.1089/fpd.2012.1171. [DOI] [PubMed] [Google Scholar]
- 263.Horm KM, D'Souza DH. 2011. Survival of human norovirus surrogates in milk, orange, and pomegranate juice, and juice blends at refrigeration (4 degrees C). Food Microbiol 28:1054–1061. doi: 10.1016/j.fm.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 264.Evans MR, Meldrum R, Lane W, Gardner D, Ribeiro CD, Gallimore CI, Westmoreland D. 2002. An outbreak of viral gastroenteritis following environmental contamination at a concert hall. Epidemiol Infect 129:355–360. doi: 10.1017/S0950268802007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. 2010. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control 38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 266.Barker J, Vipond IB, Bloomfield SF. 2004. Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. J Hosp Infect 58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 267.Jimenez L, Chiang M. 2006. Virucidal activity of a quaternary ammonium compound disinfectant against feline calicivirus: a surrogate for norovirus. Am J Infect Control 34:269–273. doi: 10.1016/j.ajic.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 268.Bolton SL, Kotwal G, Harrison MA, Law SE, Harrison JA, Cannon JL. 2013. Sanitizer efficacy against murine norovirus, a surrogate for human norovirus, on stainless steel surfaces when using three application methods. Appl Environ Microbiol 79:1368–1377. doi: 10.1128/AEM.02843-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, Engle R, Yu C, Kapikian AZ, Sosnovtsev SV, Purcell RH, Green KY. 2011. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A 108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J Virol 80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Souza M, Azevedo MS, Jung K, Cheetham S, Saif LJ. 2008. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus. J Virol 82:1777–1786. doi: 10.1128/JVI.01347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 273.Katayama K, Murakami K, Sharp TM, Guix S, Oka T, Takai-Todaka R, Nakanishi A, Crawford SE, Atmar RL, Estes MK. 2014. Plasmid-based human norovirus reverse genetics system produces reporter-tagged progeny virus containing infectious genomic RNA. Proc Natl Acad Sci U S A 111:E4043–E4052. doi: 10.1073/pnas.1415096111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Taube S, Kolawole AO, Hohne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. 2013. A mouse model for human norovirus. mBio 4(4):e00450-13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Taube S, Rubin JR, Katpally U, Smith TJ, Kendall A, Stuckey JA, Wobus CE. 2010. High-resolution X-ray structure and functional analysis of the murine norovirus 1 capsid protein protruding domain. J Virol 84:5695–5705. doi: 10.1128/JVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ohsugi T, Matsuura K, Kawabe S, Nakamura N, Kumar JM, Wakamiya M, Morikawa S, Urano T. 2013. Natural infection of murine norovirus in conventional and specific pathogen-free laboratory mice. Front Microbiol 4:12. doi: 10.3389/fmicb.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173. doi: 10.1258/la.2008.008009. [DOI] [PubMed] [Google Scholar]
- 278.Thackray LB, Duan E, Lazear HM, Kambal A, Schreiber RD, Diamond MS, Virgin HW. 2012. Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication. J Virol 86:13515–13523. doi: 10.1128/JVI.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Changotra H, Jia Y, Moore TN, Liu G, Kahan SM, Sosnovtsev SV, Karst SM. 2009. Type I and type II interferons inhibit the translation of murine norovirus proteins. J Virol 83:5683–5692. doi: 10.1128/JVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol 81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 282.Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, Green KY, Lopez-Otin C, Xavier RJ, Thackray LB, Virgin HW. 2012. Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe 11:397–409. doi: 10.1016/j.chom.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Elftman MD, Gonzalez-Hernandez MB, Kamada N, Perkins C, Henderson KS, Nunez G, Wobus CE. 2013. Multiple effects of dendritic cell depletion on murine norovirus infection. J Gen Virol 94:1761–1768. doi: 10.1099/vir.0.052134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fang H, Tan M, Xia M, Wang L, Jiang X. 2013. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS One 8:e63269. doi: 10.1371/journal.pone.0063269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, Colonna M. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog 4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wobus CE, Thackray LB, Virgin HW IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. 2008. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog 4:e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.McFadden N, Bailey D, Carrara G, Benson A, Chaudhry Y, Shortland A, Heeney J, Yarovinsky F, Simmonds P, Macdonald A, Goodfellow I. 2011. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog 7:e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med 297:86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- 290.Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, Huang P, Jiang X, Le Pendu J. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoen N, Clement M, Le Pendu J. 2001. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565–573. doi: 10.1016/S0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- 292.Vicentini F, Denadai W, Gomes YM, Rose TL, Ferreira MS, Le Moullac-Vaidye B, Le Pendu J, Leite JP, Miagostovich MP, Spano LC. 2013. Molecular characterization of noroviruses and HBGA from infected Quilombola children in Espirito Santo State, Brazil. PLoS One 8:e69348. doi: 10.1371/journal.pone.0069348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. 1995. Sequence and expression of a candidate for the human secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem 270:4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 294.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat Med 9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 295.Hutson AM, Atmar RL, Marcus DM, Estes MK. 2003. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J Virol 77:405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hutson AM, Atmar RL, Estes MK. 2004. Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol 12:279–287. doi: 10.1016/j.tim.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Rydell GE, Kindberg E, Larson G, Svensson L. 2011. Susceptibility to winter vomiting disease: a sweet matter. Rev Med Virol 21:370–382. doi: 10.1002/rmv.704. [DOI] [PubMed] [Google Scholar]
- 298.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol 76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikstrom S, Sjostrom R, Linden S, Backstrom A, Lundberg C, Arnqvist A, Mahdavi J, Nilsson UJ, Velapatino B, Gilman RH, Gerhard M, Alarcon T, Lopez-Brea M, Nakazawa T, Fox JG, Correa P, Dominguez-Bello MG, Perez-Perez GI, Blaser MJ, Normark S, Carlstedt I, Oscarson S, Teneberg S, Berg DE, Boren T. 2004. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 300.Moulds JM, Nowicki S, Moulds JJ, Nowicki BJ. 1996. Human blood groups: incidental receptors for viruses and bacteria. Transfusion 36:362–374. doi: 10.1046/j.1537-2995.1996.36496226154.x. [DOI] [PubMed] [Google Scholar]
- 301.Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, Jiang X. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol 79:6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Frenck R, Bernstein DI, Xia M, Huang P, Zhong W, Parker S, Dickey M, McNeal M, Jiang X. 2012. Predicting susceptibility to norovirus GII.4 by use of a challenge model involving humans. J Infect Dis 206:1386–1393. doi: 10.1093/infdis/jis514. [DOI] [PubMed] [Google Scholar]
- 303.Hutson AM, Atmar RL, Graham DY, Estes MK. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis 185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 304.Marionneau S, Airaud F, Bovin NV, Le Pendu J, Ruvoen-Clouet N. 2005. Influence of the combined ABO, FUT2, and FUT3 polymorphism on susceptibility to Norwalk virus attachment. J Infect Dis 192:1071–1077. doi: 10.1086/432546. [DOI] [PubMed] [Google Scholar]
- 305.Shirato H. 2012. Norovirus recognition sites on histo-blood group antigens. Front Microbiol 3:177. doi: 10.3389/fmicb.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shirato H. 2011. Norovirus and histo-blood group antigens. Jpn J Infect Dis 64:95–103. [PubMed] [Google Scholar]
- 307.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Carlsson B, Kindberg E, Buesa J, Rydell GE, Lidon MF, Montava R, Abu Mallouh R, Grahn A, Rodriguez-Diaz J, Bellido J, Arnedo A, Larson G, Svensson L. 2009. The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 norovirus infection. PLoS One 4:e5593. doi: 10.1371/journal.pone.0005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Jin M, He Y, Li H, Huang P, Zhong W, Yang H, Zhang H, Tan M, Duan ZJ. 2013. Two gastroenteritis outbreaks caused by GII noroviruses: host susceptibility and HBGA phenotypes. PLoS One 8:e58605. doi: 10.1371/journal.pone.0058605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Nordgren J, Kindberg E, Lindgren PE, Matussek A, Svensson L. 2010. Norovirus gastroenteritis outbreak with a secretor-independent susceptibility pattern, Sweden. Emerg Infect Dis 16:81–87. doi: 10.3201/eid1601.090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Rockx BH, Vennema H, Hoebe CJ, Duizer E, Koopmans MP. 2005. Association of histo-blood group antigens and susceptibility to norovirus infections. J Infect Dis 191:749–754. doi: 10.1086/427779. [DOI] [PubMed] [Google Scholar]
- 312.Murakami K, Kurihara C, Oka T, Shimoike T, Fujii Y, Takai-Todaka R, Park Y, Wakita T, Matsuda T, Hokari R, Miura S, Katayama K. 2013. Norovirus binding to intestinal epithelial cells is independent of histo-blood group antigens. PLoS One 8:e66534. doi: 10.1371/journal.pone.0066534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Harrington PR, Vinje J, Moe CL, Baric RS. 2004. Norovirus capture with histo-blood group antigens reveals novel virus-ligand interactions. J Virol 78:3035–3045. doi: 10.1128/JVI.78.6.3035-3045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mohamed JA, DuPont HL, Jiang ZD, Belkind-Gerson J, Figueroa JF, Armitige LY, Tsai A, Nair P, Martinez-Sandoval FJ, Guo DC, Hayes P, Okhuysen PC. 2007. A novel single-nucleotide polymorphism in the lactoferrin gene is associated with susceptibility to diarrhea in North American travelers to Mexico. Clin Infect Dis 44:945–952. doi: 10.1086/512199. [DOI] [PubMed] [Google Scholar]
- 315.Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis 161:18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- 316.Simmons K, Gambhir M, Leon J, Lopman B. 2013. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis 19:1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. 2008. Norwalk virus: how infectious is it? J Med Virol 80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 318.Atmar RL, Estes MK. 2012. Norovirus vaccine development: next steps. Expert Rev Vaccines 11:1023–1025. doi: 10.1586/erv.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. 2014. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chan MC, Sung JJ, Lam RK, Chan PK, Lee NL, Lai RW, Leung WK. 2006. Fecal viral load and norovirus-associated gastroenteritis. Emerg Infect Dis 12:1278–1280. doi: 10.3201/eid1208.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, Kapikian AZ, Chanock RM. 1974. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis 129:709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]
- 322.Saito M, Goel-Apaza S, Espetia S, Velasquez D, Cabrera L, Loli S, Crabtree JE, Black RE, Kosek M, Checkley W, Zimic M, Bern C, Cama V, Gilman RH, Norovirus Working Group in Peru. 2014. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin Infect Dis 58:483–491. doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Koo HL, Ajami N, Atmar RL, DuPont HL. 2010. Noroviruses: the leading cause of gastroenteritis worldwide. Discov Med 10:61–70. [PMC free article] [PubMed] [Google Scholar]
- 324.Rockx B, Baric RS, de Grijs I, Duizer E, Koopmans MP. 2005. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J Med Virol 77:439–446. doi: 10.1002/jmv.20473. [DOI] [PubMed] [Google Scholar]
- 325.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ryder RW, Singh N, Reeves WC, Kapikian AZ, Greenberg HB, Sack RB. 1985. Evidence of immunity induced by naturally acquired rotavirus and Norwalk virus infection on two remote Panamanian islands. J Infect Dis 151:99–105. doi: 10.1093/infdis/151.1.99. [DOI] [PubMed] [Google Scholar]
- 327.Kuritsky JN, Osterholm MT, Korlath JA, White KE, Kaplan JE. 1985. A statewide assessment of the role of Norwalk virus in outbreaks of food-borne gastroenteritis. J Infect Dis 151:568. doi: 10.1093/infdis/151.3.568. [DOI] [PubMed] [Google Scholar]
- 328.Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. 2010. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ajami NJ, Barry MA, Carrillo B, Muhaxhiri Z, Neill FH, Prasad BV, Opekun AR, Gilger MA, Graham DY, Atmar RL, Estes MK. 2012. Antibody responses to norovirus genogroup GI.1 and GII.4 proteases in volunteers administered Norwalk virus. Clin Vaccine Immunol 19:1980–1983. doi: 10.1128/CVI.00411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 331.Lochridge VP, Hardy ME. 2007. A single-amino-acid substitution in the P2 domain of VP1 of murine norovirus is sufficient for escape from antibody neutralization. J Virol 81:12316–12322. doi: 10.1128/JVI.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Chen Y, Tan M, Xia M, Hao N, Zhang XC, Huang P, Jiang X, Li X, Rao Z. 2011. Crystallography of a Lewis-binding norovirus, elucidation of strain-specificity to the polymorphic human histo-blood group antigens. PLoS Pathog 7:e1002152. doi: 10.1371/journal.ppat.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Chen L, Wu D, Ji L, Wu X, Xu D, Cao Z, Han J. 2013. Bioinformatics analysis of the epitope regions for norovirus capsid protein. BMC Bioinformatics 14(Suppl 4):S5. doi: 10.1186/1471-2105-14-S4-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lindesmith LC, Donaldson EF, Baric RS. 2011. Norovirus GII.4 strain antigenic variation. J Virol 85:231–242. doi: 10.1128/JVI.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bull RA, Eden JS, Rawlinson WD, White PA. 2010. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog 6:e1000831. doi: 10.1371/journal.ppat.1000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Shanker S, Choi JM, Sankaran B, Atmar RL, Estes MK, Prasad BV. 2011. Structural analysis of histo-blood group antigen binding specificity in a norovirus GII.4 epidemic variant: implications for epochal evolution. J Virol 85:8635–8645. doi: 10.1128/JVI.00848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Allen DJ, Gray JJ, Gallimore CI, Xerry J, Iturriza-Gomara M. 2008. Analysis of amino acid variation in the P2 domain of the GII-4 norovirus VP1 protein reveals putative variant-specific epitopes. PLoS One 3:e1485. doi: 10.1371/journal.pone.0001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.de Rougemont A, Ruvoen-Clouet N, Simon B, Estienney M, Elie-Caille C, Aho S, Pothier P, Le Pendu J, Boireau W, Belliot G. 2011. Qualitative and quantitative analysis of the binding of GII.4 norovirus variants onto human blood group antigens. J Virol 85:4057–4070. doi: 10.1128/JVI.02077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Iritani N, Kaida A, Kubo H, Abe N, Murakami T, Vennema H, Koopmans M, Takeda N, Ogura H, Seto Y. 2008. Epidemic of genotype GII.2 noroviruses during spring 2004 in Osaka City, Japan. J Clin Microbiol 46:2406–2409. doi: 10.1128/JCM.01993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Iritani N, Vennema H, Siebenga JJ, Siezen RJ, Renckens B, Seto Y, Kaida A, Koopmans M. 2008. Genetic analysis of the capsid gene of genotype GII.2 noroviruses. J Virol 82:7336–7345. doi: 10.1128/JVI.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ramani S, Atmar RL, Estes MK. 2014. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol 30:25–33. doi: 10.1097/MOG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Debbink K, Donaldson EF, Lindesmith LC, Baric RS. 2012. Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity. J Virol 86:1214–1226. doi: 10.1128/JVI.06189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Debbink K, Lindesmith LC, Donaldson EF, Baric RS. 2012. Norovirus immunity and the great escape. PLoS Pathog 8:e1002921. doi: 10.1371/journal.ppat.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Debbink K, Lindesmith LC, Donaldson EF, Costantini V, Beltramello M, Corti D, Swanstrom J, Lanzavecchia A, Vinje J, Baric RS. 2013. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis 208:1877–1887. doi: 10.1093/infdis/jit370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chan-It W, Thongprachum A, Okitsu S, Mizuguchi M, Ushijima H. 2014. Genetic analysis and homology modeling of capsid protein of norovirus GII.14. J Med Virol 86:329–334. doi: 10.1002/jmv.23720. [DOI] [PubMed] [Google Scholar]
- 346.Hoffmann D, Hutzenthaler M, Seebach J, Panning M, Umgelter A, Menzel H, Protzer U, Metzler D. 2012. Norovirus GII.4 and GII.7 capsid sequences undergo positive selection in chronically infected patients. Infect Genet Evol 12:461–466. doi: 10.1016/j.meegid.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 347.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. 2012. The potential economic value of a human norovirus vaccine for the United States. Vaccine 30:7097–7104. doi: 10.1016/j.vaccine.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Richardson C, Bargatze RF, Goodwin R, Mendelman PM. 2013. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev Vaccines 12:155–167. doi: 10.1586/erv.12.145. [DOI] [PubMed] [Google Scholar]
- 349.Tan M, Jiang X. 2012. The formation of P particle increased immunogenicity of norovirus P protein. Immunology 136:28–29. doi: 10.1111/j.1365-2567.2012.03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tamminen K, Huhti L, Koho T, Lappalainen S, Hytonen VP, Vesikari T, Blazevic V. 2012. A comparison of immunogenicity of norovirus GII-4 virus-like particles and P-particles. Immunology 135:89–99. doi: 10.1111/j.1365-2567.2011.03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. 2010. Virus-like particles in vaccine development. Expert Rev Vaccines 9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 352.Herbst-Kralovetz M, Mason HS, Chen Q. 2010. Norwalk virus-like particles as vaccines. Expert Rev Vaccines 9:299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 117:40–48. doi: 10.1016/S0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 354.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. 2003. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol 108:241–247. doi: 10.1016/S1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 355.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, Bargatze RF, Sztein MB, Tacket CO. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis 202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ramirez K, Wahid R, Richardson C, Bargatze RF, El-Kamary SS, Sztein MB, Pasetti MF. 2012. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin Immunol 144:98–108. doi: 10.1016/j.clim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 357.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. 2011. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinje J, Gregoricus N, Frenck RW Jr, Moe CL, Al-Ibrahim MS, Barrett J, Ferreira J, Estes MK, Graham DY, Goodwin R, Borkowski A, Clemens R, Mendelman PM. 9 September 2014. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Parra GI, Azure J, Fischer R, Bok K, Sandoval-Jaime C, Sosnovtsev SV, Sander P, Green KY. 2013. Identification of a broadly cross-reactive epitope in the inner shell of the norovirus capsid. PLoS One 8:e67592. doi: 10.1371/journal.pone.0067592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. 2011. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine 29:8126–8133. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 361.Tamminen K, Lappalainen S, Huhti L, Vesikari T, Blazevic V. 2013. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS One 8:e70409. doi: 10.1371/journal.pone.0070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wang L, Cao D, Wei C, Meng XJ, Jiang X, Tan M. 2014. A dual vaccine candidate against norovirus and hepatitis E virus. Vaccine 32:445–452. doi: 10.1016/j.vaccine.2013.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Richards AF, Lopman B, Gunn A, Curry A, Ellis D, Cotterill H, Ratcliffe S, Jenkins M, Appleton H, Gallimore CI, Gray JJ, Brown DW. 2003. Evaluation of a commercial ELISA for detecting Norwalk-like virus antigen in faeces. J Clin Virol 26:109–115. doi: 10.1016/S1386-6532(02)00267-6. [DOI] [PubMed] [Google Scholar]
- 364.Green SM, Lambden PR, Deng Y, Lowes JA, Lineham S, Bushell J, Rogers J, Caul EO, Ashley CR, Clarke IN. 1995. Polymerase chain reaction detection of small round-structured viruses from two related hospital outbreaks of gastroenteritis using inosine-containing primers. J Med Virol 45:197–202. doi: 10.1002/jmv.1890450215. [DOI] [PubMed] [Google Scholar]
- 365.Burton-MacLeod JA, Kane EM, Beard RS, Hadley LA, Glass RI, Ando T. 2004. Evaluation and comparison of two commercial enzyme-linked immunosorbent assay kits for detection of antigenically diverse human noroviruses in stool samples. J Clin Microbiol 42:2587–2595. doi: 10.1128/JCM.42.6.2587-2595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Fleischer J, Wagner Wiening C, Kimmig P. 2000. Surveillance of group illnesses with Norwalk-like viruses (NLV) in Baden-Wurttemberg. Isolation and detection of Norwalk-like viral RNA from fecal samples with RT-PCR. Gesundheitswesen 62:604–608. (In German.) doi: 10.1055/s-2000-13042. [DOI] [PubMed] [Google Scholar]
- 367.Dimitriadis A, Marshall JA. 2005. Evaluation of a commercial enzyme immunoassay for detection of norovirus in outbreak specimens. Eur J Clin Microbiol Infect Dis 24:615–618. doi: 10.1007/s10096-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 368.Yuen LK, Catton MG, Cox BJ, Wright PJ, Marshall JA. 2001. Heminested multiplex reverse transcription-PCR for detection and differentiation of Norwalk-like virus genogroups 1 and 2 in fecal samples. J Clin Microbiol 39:2690–2694. doi: 10.1128/JCM.39.7.2690-2694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Dimitriadis A, Bruggink LD, Marshall JA. 2006. Evaluation of the Dako IDEIA norovirus EIA assay for detection of norovirus using faecal specimens from Australian gastroenteritis outbreaks. Pathology 38:157–165. doi: 10.1080/00313020600559645. [DOI] [PubMed] [Google Scholar]
- 370.de Bruin E, Duizer E, Vennema H, Koopmans MP. 2006. Diagnosis of norovirus outbreaks by commercial ELISA or RT-PCR. J Virol Methods 137:259–264. doi: 10.1016/j.jviromet.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 371.Gonzalez GG, Liprandi F, Ludert JE. 2006. Evaluation of a commercial enzyme immunoassay for the detection of norovirus antigen in fecal samples from children with sporadic acute gastroenteritis. J Virol Methods 136:289–291. doi: 10.1016/j.jviromet.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 372.Castriciano S, Luinstra K, Petrich A, Smieja M, Lee C, Jang D, Portillo E, Chernesky M. 2007. Comparison of the RIDASCREEN norovirus enzyme immunoassay to IDEIA NLV GI/GII by testing stools also assayed by RT-PCR and electron microscopy. J Virol Methods 141:216–219. doi: 10.1016/j.jviromet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 373.Wilhelmi de Cal I, Revilla A, del Alamo JM, Roman E, Moreno S, Sanchez-Fauquier A. 2007. Evaluation of two commercial enzyme immunoassays for the detection of norovirus in faecal samples from hospitalised children with sporadic acute gastroenteritis. Clin Microbiol Infect 13:341–343. doi: 10.1111/j.1469-0691.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- 374.Jiang X, Huang PW, Zhong WM, Farkas T, Cubitt DW, Matson DO. 1999. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods 83:145–154. doi: 10.1016/S0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 375.Kirby A, Gurgel RQ, Dove W, Vieira SC, Cunliffe NA, Cuevas LE. 2010. An evaluation of the RIDASCREEN and IDEIA enzyme immunoassays and the RIDAQUICK immunochromatographic test for the detection of norovirus in faecal specimens. J Clin Virol 49:254–257. doi: 10.1016/j.jcv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 376.Morillo SG, Luchs A, Cilli A, Ribeiro CD, Calux SJ, Carmona RCC, Timenetsky MCST. 2011. Norovirus 3rd generation kit: an improvement for rapid diagnosis of sporadic gastroenteritis cases and valuable for outbreak detection. J Virol Methods 173:13–16. doi: 10.1016/j.jviromet.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 377.Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, Parashar UD, Ando T, Glass RI. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J Infect Dis 186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- 378.Siqueira JAM, Linhares AC, Oliveira DS, Soares LS, Lucena MS, Wanzeller AL, Mascarenhas JD, Gabbay YB. 2011. Evaluation of third-generation RIDASCREEN enzyme immunoassay for the detection of norovirus antigens in stool samples of hospitalized children in Belem, Para, Brazil. Diagn Microbiol Infect Dis 71:391–395. doi: 10.1016/j.diagmicrobio.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 379.Geginat G, Kaiser D, Schrempf S. 2012. Evaluation of third-generation ELISA and a rapid immunochromatographic assay for the detection of norovirus infection in fecal samples from inpatients of a German tertiary care hospital. Eur J Clin Microbiol Infect Dis 31:733–737. doi: 10.1007/s10096-011-1366-z. [DOI] [PubMed] [Google Scholar]
- 380.Rovida F, Campanini G, Sarasini A, Adzasehoun KM, Piralla A, Baldanti F. 2013. Comparison of immunologic and molecular assays for the diagnosis of gastrointestinal viral infections. Diagn Microbiol Infect Dis 75:110–111. doi: 10.1016/j.diagmicrobio.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 381.Mattison K, Grudeski E, Auk B, Charest H, Drews SJ, Fritzinger A, Gregoricus N, Hayward S, Houde A, Lee BE, Pang XL, Wong J, Booth TF, Vinje J. 2009. Multicenter comparison of two norovirus ORF2-based genotyping protocols. J Clin Microbiol 47:3927–3932. doi: 10.1128/JCM.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Takanashi S, Okame M, Shiota T, Takagi M, Yagyu F, Tung PG, Nishimura S, Katsumata N, Igarashi T, Okitsu S, Ushijima H. 2008. Development of a rapid immunochromatographic test for noroviruses genogroups I and II. J Virol Methods 148:1–8. doi: 10.1016/j.jviromet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 383.Yan H, Yagyu F, Okitsu S, Nishio O, Ushijima H. 2003. Detection of norovirus (GI, GII), sapovirus and astrovirus in fecal samples using reverse transcription single-round multiplex PCR. J Virol Methods 114:37–44. doi: 10.1016/j.jviromet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 384.Derrington P, Schreiber F, Day S, Curtis C, Lyon M. 2009. Norovirus Ridaquick: a new test for rapid diagnosis of norovirus. Pathology 41:687–688. doi: 10.3109/00313020903305886. [DOI] [PubMed] [Google Scholar]
- 385.Bruins MJ, Wolfhagen MJ, Schirm J, Ruijs GJ. 2010. Evaluation of a rapid immunochromatographic test for the detection of norovirus in stool samples. Eur J Clin Microbiol Infect Dis 29:741–743. doi: 10.1007/s10096-010-0911-5. [DOI] [PubMed] [Google Scholar]
- 386.Bruggink LD, Witlox KJ, Sameer R, Catton MG, Marshall JA. 2011. Evaluation of the RIDAQUICK immunochromatographic norovirus detection assay using specimens from Australian gastroenteritis incidents. J Virol Methods 173:121–126. doi: 10.1016/j.jviromet.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 387.Battaglioli G, Nazarian EJ, Lamson D, Musser KA, St George K. 2012. Evaluation of the RIDAQuick norovirus immunochromatographic test kit. J Clin Virol 53:262–264. doi: 10.1016/j.jcv.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 388.Kim HS, Hyun J, Kim JS, Song W, Kang HJ, Lee KM. 2012. Evaluation of the SD Bioline norovirus rapid immunochromatography test using fecal specimens from Korean gastroenteritis patients. J Virol Methods 186:94–98. doi: 10.1016/j.jviromet.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Park KS, Baek KA, Kim DU, Kwon KS, Bing SH, Park JS, Nam HS, Lee SH, Choi YJ. 2012. Evaluation of a new immunochromatographic assay kit for the rapid detection of norovirus in fecal specimens. Ann Lab Med 32:79–81. doi: 10.3343/alm.2012.32.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pombubpa K, Kittigul L. 2012. Assessment of a rapid immunochromatographic test for the diagnosis of norovirus gastroenteritis. Eur J Clin Microbiol Infect Dis 31:2379–2383. doi: 10.1007/s10096-012-1579-9. [DOI] [PubMed] [Google Scholar]
- 391.Ambert-Balay K, Pothier P. 2013. Evaluation of 4 immunochromatographic tests for rapid detection of norovirus in faecal samples. J Clin Virol 56:194–198. doi: 10.1016/j.jcv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 392.Sdiri-Loulizi K, Ambert-Balay K, Gharbi-Khelifi H, Sakly N, Hassine M, Chouchane S, Guediche MN, Pothier P, Aouni M. 2009. Molecular epidemiology of norovirus gastroenteritis investigated using samples collected from children in Tunisia during a four-year period: detection of the norovirus variant GGII.4 Hunter as early as January 2003. J Clin Microbiol 47:421–429. doi: 10.1128/JCM.01852-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Kittigul L, Pombubpa K, Taweekate Y, Diraphat P, Sujirarat D, Khamrin P, Ushijima H. 2010. Norovirus GII-4 2006b variant circulating in patients with acute gastroenteritis in Thailand during a 2006–2007 study. J Med Virol 82:854–860. doi: 10.1002/jmv.21746. [DOI] [PubMed] [Google Scholar]
- 394.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]