Abstract
This study compares in two experiments the responses of lactating dairy cows to four different progesterone-based protocols for fixed-time artificial insemination (FTAI) in terms of their effects on follicular/luteal dynamics and fertility. The protocols consisted of a progesterone intravaginal device fitted for five days, along with the administration of different combinations of gonadotropin releasing hormone, equine chorionic gonadotropin and a single or double dose (24 h apart) of prostaglandin F2α. In Experiment I, the data were derived from 232 lactating cows. Binary logistic regression identified no effects of treatment on ovulation failure or multiple ovulation 10 days post artificial insemination (AI). Based on the odds ratio, the likelihood of ovulation failure was lower (by a factor of 0.1) in cows showing at least one corpus luteum (CL) upon treatment than in cows lacking a CL; repeat breeders (> 3 AI) and cows with multiple CLs at treatment showed lower (by a factor of 0.44) and higher (by a factor of 9.0) risks of multiple ovulation, respectively, than the remaining animals. In Experiment II, the data were derived from 5173 AIs. The independent variable treatment failed to affect the conception rate 28–34 days post AI, twin pregnancy or early fetal loss 58–64 days post AI. The results of this study demonstrate the efficacy of 5-day progesterone-based protocols for FTAI. All four protocols examined were able to induce ovulation in both cyclic and non-cyclic animals so that FTAI returned a similar pregnancy rate to spontaneous estrus. Our results suggest that the ovarian response and fertility resulting from each treatment are due more to the effect of ovarian structures at treatment than to the different combinations of hormones investigated.
Keywords: Bovine, eCG, Hormone treatment, Synchronization protocols, Twin pregnancy
Despite improved scientific knowledge in the field of reproductive physiology and developments in genetics and nutrition, the reproductive efficiency of high-producing dairy cows continues to decline in parallel with increasing milk production [1, 2]. Ovarian disorders have been identified as major contributors to fertilization failure [3, 4], and the presence of anestrous or non-cyclic cows after the waiting period has been described as a major problem in dairy herds. Such disorders are induced by factors such as metabolic stress, environmental stress (e.g., heat stress) and inadequate management practices [5,6,7,8].
Additionally, the duration of estrous behavior in lactating dairy cows seems to be getting shorter, probably due to the metabolic clearance of steroid hormones related to high milk production [9]. Thus, in recent studies, the most accurate external sign of estrus, i.e., standing to be mounted, was only detected in around 60% of estrous periods [10, 11]. These latter two situations further contribute to missed detection of estrus by producers [12]. Finally, as production systems have become more intensive, the increased use of AI has meant a reduced need for bulls. As a result, cows are often falsely identified as being in estrus and inseminated when conception cannot occur [12,13,14]. The above are cogent reasons why breeding synchronization protocols for fixed-time artificial insemination (FTAI) have become standard components of the current breeding management of lactating cows and heifers.
Progesterone-based protocols for synchronizing estrus or inducing ovulation, in combination with gonadotropin releasing hormone (GnRH) or an analogue and prostaglandin F2α (PGF2α) or an analogue, allow for effective management of FTAI in lactating dairy cows, regardless of whether they are cyclic or non-cyclic (anestrous cows) and without the need to detect estrus [15, 16, 17, 18]. In effect, progesterone (P4) suppresses estrus, improves estrous expression following treatment [19, 20] and promotes subsequent ovulation [21]. Intravaginal inserts impregnated with progesterone are usually applied for 7–9 days. However, several recently developed five-day P4-based protocols for FTAI have provided results that compare favorably with those observed for longer protocols [22, 23, 24]. These five-day P4-based protocols make use of different combinations of hormones. As a result, Lima et al. [22] questioned the administration of GnRH on the first day of the protocol when animals received a single dose of prostaglandin F2α (PGF2α) 5 days later. Ribeiro et al. [23] increased the number of pregnancies per AI by administering twice the luteolytic dose of PGF2α given 24 h apart at P4-device removal in presynchronized lactating cows. Finally, Garcia-Ispierto et al. [24] improved fertility compared with spontaneous estrus by adding eCG at P4-device removal in high producing dairy cows under heat stress. The advantages of 5-day progesterone-based protocols over conventional 7 to 9-day protocols could be related to induction of ovulation of younger and healthier follicles in 5-day protocols, whereas the role of each hormonal drug and intervals between the drugs used in each protocol could depend on the cyclicity status of the cow. Therefore, in order to clarify the effectiveness of different five-day P4-based protocols, this study was designed to assess the following in cycling and non-cycling lactating dairy cows: follicular and luteal dynamics (Experiment I) and fertility (Experiment II) in response to four five-day P4-based protocols, including different combinations of GnRH (on the first day of the protocol) and eCG (at P4-device removal). The effects of a single or double dose (given 24 h apart) of PGF2α upon P4-device removal were also examined.
Materials and Methods
Cattle and herd management
The present study was performed on four commercial dairy herds in northeastern Spain. Briefly, herd management entailed housing in free stalls with concrete slatted floors and cubicles, the use of fans and water sprinklers in the warm season (May to September), rigorous postpartum checks, confirmation of estrus at the time of artificial insemination (AI) by palpation per rectum, and performance of most AIs (over 95%) by veterinarians.
The mean annual culling rate was 28%. The mean annual milk production for the herds during the study period was 11,255 kg per cow. The cows were grouped according to age (primiparous vs.multiparous), milked three times daily and fed complete rations. Dry cows were kept in a separate group and transferred to a “parturition group” 7–25 days before parturition depending on their body condition score [25, 26] and if they carried twins [27]. An early postpartum, or “fresh cow”, group was established for postpartum daily checks and nutrition controls, and 7- to 20-day postpartum, primiparous and multiparous lactating cows were transferred to separate groups. All cows were artificially inseminated. The voluntary waiting period for the herds was 50 days.
Reproductive health management
In the postpartum daily checks, the following puerperal diseases were treated until resolved or until culling: signs of injury to the genital area (i.e., vaginal or recto-vulvar lacerations), metabolic diseases such as hypocalcemia and ketosis (for the latter, diagnosed during the first or second week postpartum), retained placenta (fetal membranes retained longer than 12 h after parturition), or puerperal metritis (diagnosed during the first or second week postpartum in cows not suffering placenta retention).
The herds were maintained on a weekly reproductive health program. This involved examining the reproductive tract of each animal by ultrasound from 15 to 21 days postpartum to check for normal uterine involution and ovarian structures. Reproductive disorders diagnosed at this time such as endometritis or ovarian cysts were treated until resolved. Detectable cloudy intrauterine fluid was interpreted as endometritis [28]. An ovarian cyst was diagnosed when a follicular structure larger than 20 mm in diameter (external diameter including the wall) was detected in either or both ovaries in the absence of a CL and uterine tone [29]. A second exam was performed to check uterine and ovarian structures at the end of the voluntary waiting period on days 40–46 postpartum. Possible endometritis and/or ovarian cysts were also recorded and treated at this time. In the latter exam, a cow was recorded as suffering follicular anovulation when a follicular structure of at least 8–15 mm was detected in two consecutive examinations in the absence of a corpus luteum (CL) or cyst, and no estrous signs were noted during the 7-day period between the exams [30].
Since a retained placenta or puerperal metritis were previously shown to be related to subsequent pregnancy loss in cows [31], both disorders were always treated by introducing oxytetracycline boluses into the uterus plus cefquinome sulphate i.m. and PGF2α at the end of treatment. Prostaglandin F2α or a synthetic analogue was also used to treat pyometra and ovarian cysts. In the latter case, treatment was subsequent to manual rupture of the cystic structure per rectum [32]. Cows suffering follicular anovulation received a progesterone-based treatment [7].
Insemination, pregnancy diagnosis and pregnancy loss
All cows were artificially inseminated using semen from bulls of proven fertility. Spontaneous estrus was confirmed by palpation per rectum [33, 34] in cows deemed to be in estrus using a pedometer system, and the animals inseminated at this time. Only cows showing estrous signs with strong uterine contractility (determined by uterine tone) and copious transparent vaginal fluid were inseminated [12, 13]. If a cow returned to estrus, its status was confirmed by examination per rectum, and the animal was recorded as nonpregnant. In the remaining cows, pregnancy diagnosis was performed by ultrasound 28–34 days post AI and confirmed 58–64 days post AI. Since management and cow-related factors of a noninfectious nature have been extensively linked to late embryonic or early fetal loss in our geographical area [14, 27], pregnancy loss was recorded when the 58- to 64-day diagnosis proved negative.
Experimental design
All procedures were approved by the Ethics Committee on Animal Experimentation of the University of Lleida (license numbers CEEA.09–01/12 and CEEA.09–01/13).
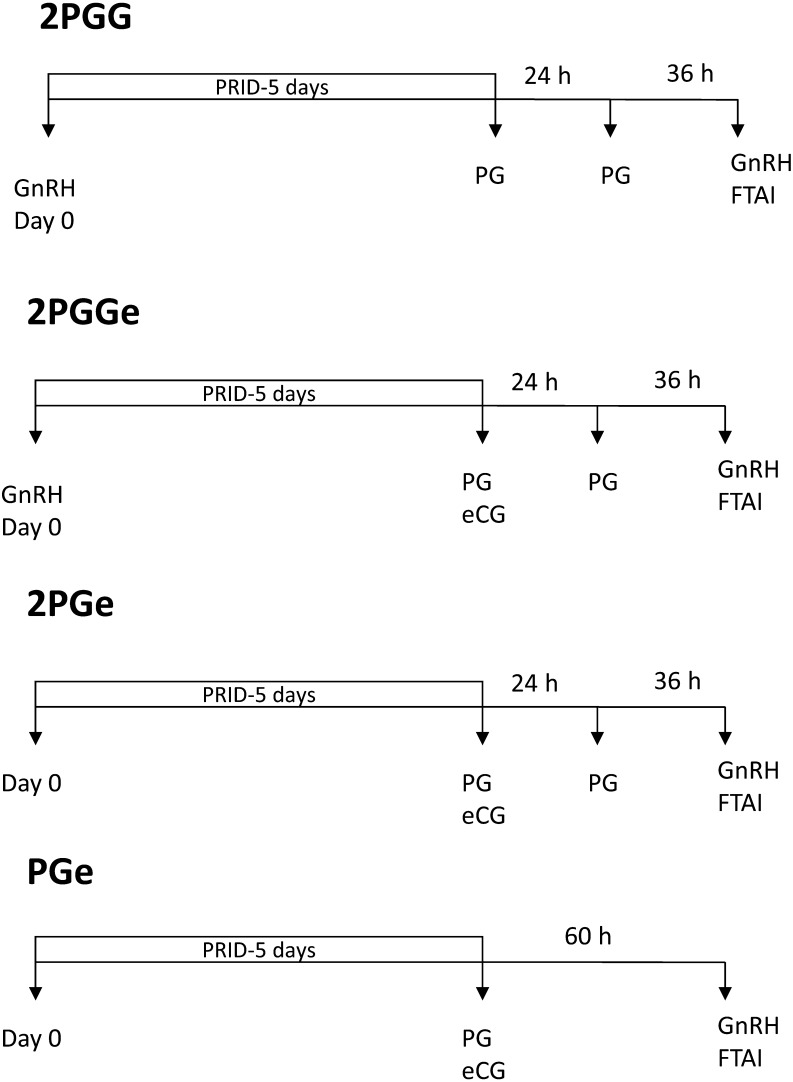
During the weekly reproductive visit, open cows with more of 50 days in milk and with no reproductive disorders such as ovarian cysts and endometritis detected by ultrasound were randomly assigned to one of the following groups: 2PGG, 2PGGe, 2PGe and PGe (Fig. 1). Cows in the 2PGG group were treated with a progesterone-releasing intravaginal device (PRID) (PRID-DELTA, containing 1.55 g of progesterone; CEVA Salud Animal, Barcelona, Spain) plus GnRH (100 μg i.m.; Cystoreline, CEVA Santé Animale, Libourne, France) at PRID insertion. The PRID was left for 5 days, and these animals were also given PGF2α (25 mg dinoprost i.m.; Enzaprost, CEVA Santé Animale, Libourne, France) at PRID removal. Twenty-four hours later, the cows received a second PGF2α dose, and they were inseminated and received a second GnRH dose 36 hours after receiving the second PGF2α dose. The remaining groups were treated with the same P4-based protocol but with the following differences: cows in the 2PGGe group received 500 IU of eCG i.m. (Syncostim, CEVA Santé Animale, Libourne, France) at PRID removal; cows in the 2PGe received eCG at PRID removal and no GnRH was given at PRID insertion; and cows in the PGe group received eCG at PRID removal and no GnRH at PRID insertion nor the second dose of PGF2α. In this latter group, cows were fixed-time inseminated 60 h after PRID removal. Only healthy cows with no signs of mastitis, lameness or digestive disorders were included in the study. Two experiments were performed to investigate effects of treatments on follicular/luteal dynamics (Experiment I) and fertility (Experiment II).
Fig. 1.
Treatment protocols used to synchronize estrus for fixed-time AI (FTAI) in high-producing dairy cows. All cows (n=232) were fitted with a progesterone releasing intravaginal device (PRID-DELTA, containing 1.55 g of progesterone; CEVA Salud Animal, Barcelona, Spain) for 5 days (PRID-5 days).
Cows diagnosed as not pregnant received no further treatment related to the study. This meant that a cow receiving a five-day P4-based protocol was included only once in both experiments. All gynecological exams and pregnancy diagnoses were performed by the second author.
Experiment I. Effects of four different P-based protocols for FTAI on follicular/luteal dynamics
This experiment was designed to establish the possible effects of each treatment on follicular/luteal dynamics. The data examined were derived from 232 lactating Holstein-Friesian cows comprising a single dairy herd (Herd 1) for the period of December 2012 to October 2013. Ovarian follicular structures larger than 10 mm in diameter and the absence or presence of one or more CLs larger than 10 mm (as the maximum diameter) were assessed by ultrasonography immediately before treatment and AI and at 10 days after AI. In our geographical region, there are only two clearly differentiated weather periods: warm (May to September) and cool (October to April) [2, 6, 35]. Parturition and treatment dates were used to analyse the effects of season on subsequent reproductive performance.
The following data were recorded for each animal: herd, parturition and treatment dates; parity (primiparous vs.multiparous); previous retained placenta or metritis; repeat breeding syndrome (cows undergoing more than 3 AIs); treatment (2PGG, 2PGGe, 2PGe or PGe); body condition score (BCS) at treatment (from 1 to 5 units; lower than 2.5 vs. 2.5 or higher); season of treatment (cool - October to April – vs. warm - May to September); milk production at treatment (mean production during the three days before treatment) (low producers < 40 kg vs. high producers ≥ 40 kg); days in milk at treatment; cyclicity at treatment (presence of at least one CL); ovarian structures at AI (three classes: one single follicle, two or more follicles or presence of a CL); ovulation failure (absence of a CL 10 days after AI); semen-providing bull; AI technician, conception rate 28–34 days post AI; presence of twins; and pregnancy loss 58–64 days post AI.
Three binary logistic regression analyses were performed. The dependent variables considered in these three analyses, respectively, were cyclicity at the beginning of treatment (CY) (0, absence of luteal structures; 1, presence of at least one CL); ovulation failure determined 10 days after AI (OF) (0, presence of at least one CL; 1, absence of luteal structures); and multiple ovulation after AI, determined in ovulating cows 10 days after AI (MO) (0, one single CL; 1, two or more CL). Season of parturition and treatment; parity; previous retained placenta or metritis; treatment; repeat breeding syndrome; and days in milk, body condition score and milk production at treatment were considered independent variables. For the dependent variables OF and MO, CY was added respectively as a two-class (absence of luteal structures or presence of at least one CL) and three class (CL absent, one single CL or 2 or more CLs) independent variable.
Regression analyses were conducted according to the method of Hosmer and Lemeshow [36] using the logistic procedure of PASW Statistics for Windows Version 18.0 (SPSS, Chicago, IL, USA). Significance was set at P < 0.05.
Experiment II. Conception, twin pregnancy and pregnancy loss rates of fixed-time inseminated cows following four different P-based protocols compared with spontaneous estrus
Data were derived from 5173 AIs performed on 2050 lactating Holstein-Friesian cows comprising three further dairy herds (Herds 2, 3 and 4) with 160, 740 and 1150 cows, respectively, for the period of January to December 2013. The possible effects of each treatment on the variables of conception rate 28–34 days post AI, twin pregnancy and early fetal loss 58–64 days post AI were evaluated.
The data recorded for each animal were the same as those for Experiment I except for ovarian structures at AI and 10 days later, which were not recorded. Cyclicity at treatment was registered as a two classes independent variable (absence of luteal structures or presence of at least one CL). In this experiment, cows that were artificially inseminated following spontaneous estrus (n=3248 AI) during the study period were used as controls. Body condition score was not registered in control cows.
Three binary logistic regression analyses were performed for all inseminations. The dependent variables considered in these three analyses, respectively, were conception rate 28–34 days Post AI; twin pregnancy; and pregnancy loss. Herd, season of parturition and AI; parity; previous retained placenta or metritis; treatment; repeat breeding syndrome; days in milk, body condition score and milk production at AI; semen- providing bull; and AI technician were considered independent variables. For the dependent variable pregnancy loss analysis, twin pregnancy was added as an independent variable. Insemination was the experimental unit of the analyses, and the variable cow was treated as a repeated measure.
In treated animals, one single binary logistic regression analysis was also performed with conception rate 28–34 days Post AI as the dependent variable but including body condition score and cyclicity upon treatment as independent variables.
Results
Experiment I. Effects of four different P-based protocols for FTAI on follicular/luteal dynamics
The mean values for milk production, number of lactation and number of previous AIs at the time of treatment were 40.0 ± 8.4 kg, 2.5 ± 1.7 lactations and 3.5 ± 2.7 AIs, respectively (mean ± SD). Table 1 provides data on the effects of treatment for each variable. None of the 12 cows that failed to ovulate became pregnant.
Table1. Effects of the different treatments on each variable (Experiment I; N=232).
| Treatment | 2PGG | 2PGGe | 2PGe | PGe | Total |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Dependent variable* | |||||
| CY | 32/58 (55.2) | 34/57 (59.6) | 44/61 (72.1) | 38/56 (70.4) | 148/232 (63.8) |
| OF | 3/58 (5.2) | 6/57 (10.5) | 1/61 (1.6) | 2/56 (3.7) | 12/232 (5.2) |
| MO | 19/55 (34.5) | 15/51 (29.4) | 16/60 (29.4) | 12/54 (22.2) | 62/220 (28.2) |
| CR | 15/58 (25.9) | 16/57 (28.1) | 17/61 (27.9) | 18/56 (32.1) | 66/232 (28.4) |
| TW | 0/15 (0) | 3/16 (18.8) | 4/17 (23.5) | 3/18 (16.7) | 10/66 (15.2) |
| PL | 1/15 (6.7) | 1/16 (6.3) | 1/17 (5.9) | 2/18 (11.1) | 5/66 (7.6) |
* Values for each variable were not different according to Tukey-Kramer tests (P<0.05). Dependent variables: CY, cyclicity at treatment; LF, luteolysis failure at AI; OF, ovulation failure determined 10 days after AI; MO, multiple ovulation determined in ovulating cows 10 days after AI; CR, conception rate 28–34 days post AI; TW: twin pregnancy; PL: pregnancy loss 58–64 days post AI. Treatments (all cows received FTAI and a dose of GnRH 60 h after PRID removal): 2PGG: PRID for 5 days; GnRH on day 0; PGF2α at PRID removal and 24 h later. 2PGGe: PRID for 5 days; GnRH on day 0; PGF2α plus eCG at PRID removal and PGF2α 24 h later. 2PGE: PRID for 5 days; PGF2α plus eCG at PRID removal and PGF2α 24 h later. PGE: PRID for 5 days; PGF2α plus eCG at PRID removal.
Binary logistic regression analyses revealed a significant relationship (odds ratio, 2.1; 95% confidence interval, 1.14–3.74; P = 0.015) between repeat breeding syndrome and cyclicity at the beginning of the treatment. This meant that repeat breeders were 2.1 times more likely to show luteal structures (74.7%; 65/87) upon starting treatment than the remaining animals (57.3%; 83/145).
The likelihood of ovulation failure was 0.1 times lower (95% confidence interval: 0.02–0.44; P = 0.003) in cows showing luteal structures at the start of treatment (1.4%; 2/148) compared with cows lacking a CL (11.9%; 10/84).
Repeat breeders were less likely (by a factor of 0.44) to suffer multiple ovulation 10 days after AI than non-repeat breeders. Cows with multiple CLs at the time of treatment were more likely (by a factor of 9.0) to experience multiple ovulations 10 days after AI than cows lacking a CL (Table 2).
Table 2. Odds ratios of the variables included in the final logistic regression model for factors affecting the multiple ovulation rate determined in ovulating cows 10 days post AI.
| Factor | Class | n | % multiple ovulation |
Odds ratio | 95% confidence interval |
P |
| Repeat breeder syndrome | < 4 AI | 45/136 | 33.1 | Reference | ||
| ≥ 4 AI | 17/84 | 20.2 | 0.44 | 0.21–0.92 | 0.03 | |
| Luteal structures at treatment | CL absent | 23/72 | 31.9 | Reference | ||
| 1 CL | 28/134 | 20.9 | 0.8 | 0.60–0.99 | 0.05 | |
| 2 or more CLs | 11/14 | 78.6 | 9.0 | 2.2–36.70 | 0.003 |
Hosmer and Lemeshow goodness-of-fit test = 26.6; 3 df, P = 0.93. R2 Nagelkerke = 0.15.
Experiment II. Conception, twin pregnancy and pregnancy loss rates of fixed-time inseminated cows following four different P-based protocols compared with spontaneous estrus
All animals: The mean values for milk production, number of lactation and number of previous AIs for the study period were 43.9 ± 9.9 kg, 2.6 ± 1.5 lactations and 3.1 ± 2.2 AIs, respectively. The mean days in milk for control and treated cows at AI were 152 ± 135 and 159 ± 141 days, respectively. Table 3 provides data on the effects of treatment for each examined variable. Of the 5173 AIs, 1559 (30.1%) resulted in pregnancy, 288 (18.5%) of the cows had twin pregnancies and 301 (19.3%) suffered pregnancy loss.
Table 3. Effects of the different treatments on each variable (Experiment II; N=5173).
| Treatments | Conception rate 28–34 days post AI | Twin pregnancy | Early fetal loss 58–64 days post AI |
| N (%) | N (%) | N (%) | |
| 2PGG | 159/480 (33.1) | 25/159 (15.7) | 34/159 (21.4) |
| 2PGGe | 156/482 (32.4) | 33/156 (21.2) | 32/156 (20.5) |
| 2PGe | 149/479 (31.1) | 26/149 (17.4) | 29/149 (19.5) |
| PGe | 153/484 (31.6) | 34/153 (22.2) | 30/153 (19.6) |
| Control | 942/3248 (29) | 170/942 (18) | 176/942 (18.7) |
| Total | 1559/5173 (30.1%) | 288/1559 (18.5%) | 301/1559 (19.3%) |
Treatments (all cows received FTAI and a dose of GnRH 60 h after PRID removal): 2PGG: PRID for 5 days; GnRH on day 0; PGF2α at PRID removal and 24 h later. 2PGGe: PRID for 5 days; GnRH on day 0; PGF2α plus eCG at PRID removal and PGF2α 24 h later. 2PGE: PRID for 5 days; PGF2α plus eCG at PRID removal and PGF2α 24 h later. PGE: PRID for 5 days; PGF2α plus eCG at PRID removal. Control: cows that were inseminated following spontaneous estrus.
No significant effects were found of any of the variables examined with respect to the conception rate. Since the effects of season of AI and parity showed a tendency to be significant (P = 0.09 and P = 0.08, respectively), one single binary logistic regression analysis was also performed with conception rate 28–34 days post AI as the dependent variable for control cows.
The likelihood of twin pregnancy was 1.9 times higher (95% confidence interval: 1.3–3.6; P = 0.01) in multiparous cows (20.8%; 221/1060) compared with primiparous cows (13.4%; 67/499).
Cows carrying twin pregnancies were more likely (by a factor of 2.5; 95% confidence interval: 1.6–4.1; P < 0.0001) to suffer pregnancy loss (36.1%; 104/288) than the cows carrying single pregnancies (15.5%; 197/1271).
Control cows: Based on the odds ratio, AI during the warm period led to a 0.86-fold decrease (95% confidence interval: 0.78–0.95; P = 0.02) in the pregnancy rate (27.2%; 362/1332) compared with that during the cool period (30.3%, 580/1916). The likelihood of pregnancy was lower in multiparous cows (0.76-fold; 95% confidence interval: 0.68–0.83; P = 0.01) (27.8%, 641/2309) compared with primiparous animals (32.1%, 301/939).
Treated cows: Binary logistic regression analyses revealed a significant relationship (odds ratio, 0.5; 95% confidence interval, 0.3–0.7; P < 0.001) between cows with a BCS < 2.5 units at the time of treatment and the conception rate 28–34 days post AI. This meant that cows with a BCS < 2.5 units were 0.5 times less likely to become pregnant (22.3%; 87/398) than the remaining animals (34.5%; 530/1535).
The independent variable treatment failed to affect any of the dependent variables examined in both experiments. No interactions were found.
Discussion
To the best of our knowledge, no prior study has investigated ovarian structure dynamics and fertility in response to different short (5-day) progesterone-based synchronization protocols including combinations of GnRH, PGF2α and eCG. The results of our study indicate the similarly good performance of all these protocols in high-producing dairy cows. Points to be highlighted that cows with two or more corpora lutea at the start of treatment showed a greater risk of multiple ovulation than cows lacking luteal structures; cyclic cows showed a lower risk of ovulation failure when compared with non-cyclic cows; and cows with a BCS of 2.5 or more at the time of treatment were more likely to get pregnant than the remaining animals. Therefore, our results suggest that the ovarian response and fertility resulting from each treatment were due more to the effect of ovarian structures and BCS at treatment than to the different combinations of hormones investigated.
All four protocols led to acceptable estrous synchronization for FTAI. In effect, the mean pregnancy rate of 32.1% (617/1925) for treated cows was comparable to that of 29% (942/3248) for inseminations performed at spontaneous estrus. The good response to all protocols was probably due more to the effect of P4 plus the second GnRH dose used to induce ovulation than to the actions of the hormone combinations administered at the start or end of treatment. One reason for this statement is the fact that P4 and the second GnRH dose were the only fixed treatments for all protocols. These results reinforce the idea that short P4-based protocols may lead to improved fertility compared with the more conventional longer protocols [24]. Higher postovulatory circulating concentrations of progesterone have been noted for these 5-d protocols with a consequent improvement in fertility compared with a 7-day ovulation synchronization protocol [37, 38].
Twin pregnancies increased the risk of pregnancy loss by 2.3 times during the late embryo early fetal period, in agreement with previous results [39,40,41]. Besides early pregnancy loss, twin pregnancies also have other effects such as increasing the risks of abortion, dystocia, retained placenta, calf mortality, occurrence of freemartins, a need for postpartum therapy, longer rebreeding intervals and culling [42, 43]. Over the past two decades, the twinning rate has risen alongside milk production [42, 44]. However, several studies performed on high-producing dairy cows in our geographical area [45,46,47] have detected no effect of milk production on double ovulation. According to some authors, double ovulation may be more correlated with the previously used synchronization protocol. Andreu-Vazquez et al. [48] reported that non-cycling cows subjected to 7–9-day P4-based protocols adding eCG were more likely to suffer a twin pregnancy compared with the remaining animals in their study, including cows receiving a 5-day P4-based protocol. In our study, cows with two or more CLs at the start of the protocol carried a 9.0-fold higher risk of double ovulation after treatment compared with non-cyclic animals. No effect of adding eCG to a short-P4-based protocol was detected. These results, regardless of the synchronization protocol used, probably point to a maternal trait and are consistent with the findings of other studies that have examined twinning rates [42, 43]. Cow health and well-being should not be discarded as a factor favoring double ovulation [45]. To the best of our knowledge, this idea of a maternal predisposition to twinning has not yet been proposed for animals with double ovulation. The question that arises is what type of protocol should we use in open cows with two or more corpora lutea? Maybe a shorter protocol such as PG2α plus eCG, GnRH 48 h later followed by FTAI 24 h following GnRH treatment would be the best option for these cows. Indeed, this latter protocol was found to reduce the risk of twin pregnancy (by a factor of 0.4) in multiparous cows showing silent ovulation [47]. In the present study, the likelihood of twin pregnancy was 1.8 times higher in multiparous than in primiparous cows, reinforcing previous results [48].
Ovulation failure is considered to be a major cause of infertility in dairy cattle [8, 15, 16]. Under our experimental conditions, no effects of the synchronization protocol, parity, season of AI or milk production were observed on ovulation failure after treatment. Only cows with a CL at the treatment outset were ten times less likely to suffer ovulation failure. The absence of luteal structures in the remaining animals suggests that most were anestrous cows. Although the addition of eCG to P4-based protocols has been noted to promote ovulation in anestrous dairy cows [49], it is logical that cows suffering anestrus would be less sensitive to any type of treatment than cows with a CL, which are probably normal cycling cows. However, it should be noted that the figure of 5.2% of cows failing to ovulate here is close to the 6.5% reported for 1,917 AIs performed at spontaneous estrus [45]. In this latter study, the warm period of the year was the main factor found to promote ovulation failure. In the present study, P4 treatment probably reduced the effects of heat stress. Cows inseminated following a spontaneous estrus during the warm period suffered a decrease in the pregnancy rate compared with cows inseminated during the cool period. In contrast, season was not a factor affecting the fertility of treated cows. In the same sense, P4 protocols probably overcome the negative effect of age. Primiparous cows with spontaneous breeding had a higher conception rate than their multiparous partners, in agreement with extensive studies [14], whereas parity was not a factor affecting treated cows.
Negative energy balance (NEB) is closely related to a loss of body condition score (BCS) at calving and has been shown to affect the subsequent productive and reproductive performance of postpartum cows [25, 26]. NEB during the early postpartum period can affect fertility later in the lactation period by reducing the number of ovarian cycles [50]. Moreover, carry-over effects of NEB can result in deficiencies in oocyte, embryo and CL quality [51] and increase the risk of metabolic disorders [52, 53]. Herein, cows with a BCS < 2.5 units showed a lower conception rate irrespective of treatment. Management practices should focus on reducing this problem in herds to avoid infertility.
As anticipated, repeat breeding cows (> 4 AI) showed more luteal activity at the treatment outset than the remaining animals. Treatments failed to affect the pregnancy rate in these cyclic animals (Experiment II), but it was precisely their cyclicity that increased the likelihood of luteal activity at the start of treatment compared with the remaining cows (Experiment I). It is more difficult, however, to explain why repeat breeder cows showed a lower risk of multiple ovulations than the remaining cows. Perhaps, if repeat breeders carry a greater risk of single ovulation, this could be one of the reasons why repeat breeding syndrome reduces fertility. When the possibility exists of fertilizing not only one but two or more oocytes, the chance of pregnancy increases [20, 54]. More extensive studies are needed to assess the ovulation behavior of repeat breeder cows.
The results of this study demonstrate the efficacy of 5-day progesterone-based protocols for FTAI. All four protocols examined were able to induce ovulation in both cyclic and non-cyclic animals so that FTAI returned a similar pregnancy rate to spontaneous estrus.
Acknowledgments
The authors thank Ana Burton for assistance with the English translation. This study was funded by the University of Lleida (C-13018 with CEVA Santé Animale, France).
References
- 1.Lucy MC. Reproductive loss in high-producing dairy cattle: where will it end? J Dairy Sci 2001; 84: 1277–1293. [DOI] [PubMed] [Google Scholar]
- 2.López-Gatius F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology 2003; 60: 89–99. [DOI] [PubMed] [Google Scholar]
- 3.Opsomer G, Gröhn YT, Hertl J, Coryn M, Deluyker H, de Kruif A. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology 2000; 53: 841–857. [DOI] [PubMed] [Google Scholar]
- 4.Yániz J, López-Gatius F, Bech-Sàbat G, García-Ispierto I, Serrano B, Santolaria P. Relationships between milk production, ovarian function and fertility in high-producing dairy herds in north-eastern Spain. Reprod Domest Anim 2008; 43(Suppl 4): 38–43. [DOI] [PubMed] [Google Scholar]
- 5.García-Ispierto I, López-Gatius F, Santolaria P, Yániz JL, Nogareda C, López-Béjar M. Factors affecting the fertility of high producing dairy herds in northeastern Spain. Theriogenology 2007; 67: 632–638. [DOI] [PubMed] [Google Scholar]
- 6.García-Ispierto I, López-Gatius F, Bech-Sabat G, Santolaria P, Yániz JL, Nogareda C, De Rensis F, López-Béjar M. Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 2007; 67: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 7.López-Gatius F, Mirzaei A, Santolaria P, Bech-Sàbat G, Nogareda C, García-Ispierto I, Hanzen C, Yániz JL. Factors affecting the response to the specific treatment of several forms of clinical anestrus in high producing dairy cows. Theriogenology 2008; 69: 1095–1103. [DOI] [PubMed] [Google Scholar]
- 8.Peter AT, Vos PLAM, Ambrose DJ. Postpartum anestrus in dairy cattle. Theriogenology 2009; 71: 1333–1342. [DOI] [PubMed] [Google Scholar]
- 9.López H, Satter LD, Wiltbank MC. Relationship between level of milk production and estrous behavior of lactating dairy cows. Anim Reprod Sci 2004; 81: 209–223. [DOI] [PubMed] [Google Scholar]
- 10.Roelofs JB, van Eerdenburg FJ, Soede NM, Kemp B. Various behavioral signs of estrous and their relationship with time of ovulation in dairy cattle. Theriogenology 2005; 63: 1366–1377. [DOI] [PubMed] [Google Scholar]
- 11.Cutullic E, Delaby L, Causeur D, Michel G, Disenhaus C. Hierarchy of factors affecting behavioural signs used for oestrus detection of Holstein and Normande dairy cows in a seasonal calving system. Anim Reprod Sci 2009; 113: 22–37. [DOI] [PubMed] [Google Scholar]
- 12.Roelofs J, López-Gatius F, Hunter RHF, van Eerdenburg FJCM, Hanzen C. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010; 74: 327–344. [DOI] [PubMed] [Google Scholar]
- 13.López-Gatius F. Site of semen deposition in cattle: a review. Theriogenology 2000; 53: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 14.López-Gatius F. Factors of a noninfectious nature affecting fertility after artificial insemination in lactating dairy cows. A review. Theriogenology 2012; 77: 1029–1041. [DOI] [PubMed] [Google Scholar]
- 15.Roche JF, Crowe MA, Boland MP. Postpartum anoestrus in dairy and beef cows. Anim Reprod Sci 1992; 28: 371–378. [Google Scholar]
- 16.Rhodes FM, McDougall S, Burke CR, Verkerk GA, Macmillan KL. Invited review: Treatment of cows with an extended postpartum anestrous interval. J Dairy Sci 2003; 86: 1876–1894. [DOI] [PubMed] [Google Scholar]
- 17.Yániz JL, Murugavel K, López-Gatius F. Recent developments in oestrous synchronization of postpartum dairy cows with and without ovarian disorders. Reprod Domest Anim 2004; 39: 86–93. [DOI] [PubMed] [Google Scholar]
- 18.Macmillan KL. Recent advances in the synchronization of estrus and ovulation in dairy cows. J Reprod Dev 2010; 56(Suppl): S42–S47. [DOI] [PubMed] [Google Scholar]
- 19.Vailes LD, Washburn SP, Britt JH. Effects of various steroid milieus or physiological states on sexual behavior of Holstein cows. J Anim Sci 1992; 70: 2094–2103. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Ispierto I, López-Gatius F, Bech-Sàbat G, Yániz JL, Angulo E, Maris C, Floc’h S, Martino A. Effects of a progesterone-based oestrous synchronization protocol in 51- to 57-day postpartum high-producing dairy cows. Reprod Domest Anim 2010; 45: e168–e173. [DOI] [PubMed] [Google Scholar]
- 21.Galvão KN, Santos JE. Factors affecting synchronization and conception rate after the Ovsynch protocol in lactating Holstein cows. Reprod Domest Anim 2010; 45: 439–446. [DOI] [PubMed] [Google Scholar]
- 22.Lima FS, Ayres H, Favoreto MG, Bisinotto RS, Greco LF, Ribeiro ES, Baruselli PS, Risco CA, Thatcher WW, Santos JE. Effects of gonadotropin-releasing hormone at initiation of the 5-d timed artificial insemination (AI) program and timing of induction of ovulation relative to AI on ovarian dynamics and fertility of dairy heifers. J Dairy Sci 2011; 94: 4997–5004. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro ES, Monteiro APA, Lima FS, Ayres H, Bisinotto RS, Favoreto M, Greco LF, Marsola RS, Thatcher WW, Santos JEP. Effects of presynchronization and length of proestrus on fertility of grazing dairy cows subjected to a 5-day timed artificial insemination protocol. J Dairy Sci 2012; 95: 2513–2522. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Ispierto I, Roselló MA, De Rensis F, López-Gatius F. A five-day progesterone plus eCG-based fixed-time AI protocol improves fertility over spontaneous estrus in high-producing dairy cows under heat stress. J Reprod Dev 2013; 59: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Gatius F, Yániz J, Madriles-Helm D. Effects of body condition score and score change on the reproductive performance of dairy cows: a meta-analysis. Theriogenology 2003; 59: 801–812. [DOI] [PubMed] [Google Scholar]
- 26.Roche JR, Friggens NC, Kay JK, Fisher MW, Stafford KJ, Berry DP. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J Dairy Sci 2009; 92: 5769–5801. [DOI] [PubMed] [Google Scholar]
- 27.López-Gatius F, García-Ispierto I. Ultrasound and endocrine findings that help to assess the risk of late embryo/early foetal loss by non-infectious cause in dairy cattle. Reprod Domest Anim 2010; 45(Suppl 3): 15–24. [DOI] [PubMed] [Google Scholar]
- 28.López-Helguera I, López-Gatius F, Garcia-Ispierto I. The influence of genital tract status in postpartum period on the subsequent reproductive performance in high producing dairy cows. Theriogenology 2012; 77: 1334–1342. [DOI] [PubMed] [Google Scholar]
- 29.Hanzen CH, Bascon F, Theron L, López-Gatius F. 2007: Ovarian cysts in cattle Part 1: Definitions, symptoms and diagnostic. Ann Med Vet 2007; 151: 247–256. [Google Scholar]
- 30.López-Gatius F, Santolaria P, Yániz J, Rutllant J, López-Béjar M. Persistent ovarian follicles in dairy cows: a therapeutic approach. Theriogenology 2001; 56: 649–659. [DOI] [PubMed] [Google Scholar]
- 31.López-Gatius F, Labèrnia J, Santolaria P, López-Béjar M, Rutllant J. Effect of reproductive disorders previous to conception on pregnancy attrition in dairy cows. Theriogenology 1996; 46: 643–648. [DOI] [PubMed] [Google Scholar]
- 32.Hanzen C, Bascon F, Theron L, López-Gatius F. Ovarian cysts in cattle 3. Therapeutic aspects. Ann Med Vet 2008; 152: 103–115. [Google Scholar]
- 33.López-Gatius F, Camón-Urgel J. Increase of pregnancy rate in dairy cattle after preovulatory follicle palpation and deep cornual insemination. Theriogenology 1988; 29: 1099–1103. [DOI] [PubMed] [Google Scholar]
- 34.López-Gatius F, Camón-Urgel J. Confirmation of estrus rates by palpation per rectum of genital organs in normal repeat dairy cows. Zentralbl Veterinarmed A 1991; 38: 553–556. [DOI] [PubMed] [Google Scholar]
- 35.Labèrnia J, López-Gatius F, Santolaria P, Hanzen C, Laurent Y, Houtain JY. Influence of calving season on the interactions among reproductive disorders of dairy cows. Anim Sci 1998; 67: 387–393. [Google Scholar]
- 36.Hosmer DW, Lemeshow S . Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 37.Lopez H, Sartori R, Wiltbank MC. Reproductive hormones and follicular growth during development of one or multiple dominant follicles in cattle. Biol Reprod 2005; 72: 788–795. [DOI] [PubMed] [Google Scholar]
- 38.Perry GA, Smith MF, Lucy MC, Green JA, Parks TE, MacNeil MD, Roberts AJ, Geary TW. Relationship between follicle size at insemination and pregnancy success. Proc Natl Acad Sci USA 2005; 102: 5268–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Gatius F, Santolaria P, Yániz J, Rutllant J, López-Béjar M. Factors affecting pregnancy loss from gestation Day 38 to 90 in lactating dairy cows from a single herd. Theriogenology 2002; 57: 1251–1261. [DOI] [PubMed] [Google Scholar]
- 40.López-Gatius F, Santolaria P, Yániz JL, Garbayo JM, Hunter RHF. Timing of early foetal loss for single and twin pregnancies in dairy cattle. Reprod Domest Anim 2004; 39: 429–433. [DOI] [PubMed] [Google Scholar]
- 41.López-Gatius F, Hunter RHF. Spontaneous reduction of advanced twin embryos: its occurrence and clinical relevance in dairy cattle. Theriogenology 2005; 63: 118–125. [DOI] [PubMed] [Google Scholar]
- 42.Nielen M, Schukken YH, Scholl DT, Wilbrink HJ, Brand A. Twinning in dairy cattle: A study of risk factors and effects. Theriogenology 1989; 32: 845–862. [DOI] [PubMed] [Google Scholar]
- 43.Andreu-Vázquez C, Garcia-Ispierto I, Ganau S, Fricke PM, López-Gatius F. Effects of twinning on the subsequent reproductive performance and productive lifespan of high-producing dairy cows. Theriogenology 2012; 78: 2061–2070. [DOI] [PubMed] [Google Scholar]
- 44.Kinsel ML, Marsh WE, Ruegg PL, Etherington WG. Risk factors for twinning in dairy cows. J Dairy Sci 1998; 81: 989–993. [DOI] [PubMed] [Google Scholar]
- 45.López-Gatius F, López-Béjar M, Fenech M, Hunter RHF. Ovulation failure and double ovulation in dairy cattle: risk factors and effects. Theriogenology 2005; 63: 1298–1307. [DOI] [PubMed] [Google Scholar]
- 46.López-Gatius F, Santolaria P, Martino A, Delétang F, De Rensis F. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain. Theriogenology 2006; 65: 820–830. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Ispierto I, López-Gatius F. A three-day PGF2α plus eCG-based fixed-time AI protocol improves fertility compared with spontaneous estrus in dairy cows with silent ovulation. J Reprod Dev 2013; 59: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreu-Vázquez C, Garcia-Ispierto I, López-Gatius F. Photoperiod length and the estrus synchronization protocol used before AI affect the twin pregnancy rate in dairy cattle. Theriogenology 2012; 78: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Ispierto I, López-Helguera I, Martino A, López-Gatius F. Reproductive performance of anoestrous high-producing dairy cows improved by adding equine chorionic gonadotrophin to a progesterone-based oestrous synchronizing protocol. Reprod Domest Anim 2012; 47: 752–758. [DOI] [PubMed] [Google Scholar]
- 50.Butler WR, Smith RD. Interrelationships between energy balance and postpartum reproductive function in dairy cattle. J Dairy Sci 1989; 72: 767–783. [DOI] [PubMed] [Google Scholar]
- 51.Leroy JL, Vanholder T, Van Knegsel AT, Garcia-Ispierto I, Bols PE. Nutrient prioritization in dairy cows early postpartum: mismatch between metabolism and fertility? Reprod Domest Anim 2008; 43(Suppl 2): 96–103. [DOI] [PubMed] [Google Scholar]
- 52.Collard BL, Boettcher PJ, Dekkers JC, Petitclerc D, Schaeffer LR. Relationships between energy balance and health traits of dairy cattle in early lactation. J Dairy Sci 2000; 83: 2683–2690. [DOI] [PubMed] [Google Scholar]
- 53.Ingvartsen KL, Moyes K. Nutrition, immune function and health of dairy cattle. Animal 2013; 7(Suppl 1): 112–122. [DOI] [PubMed] [Google Scholar]
- 54.Echternkamp SE, Gregory KE, Dickerson GE, Cundiff LV, Koch RM, Van Vleck LD. Twinning in cattle: II. Genetic and environmental effects on ovulation rate in puberal heifers and postpartum cows and the effects of ovulation rate on embryonic survival. J Anim Sci 1990; 68: 1877–1888. [DOI] [PubMed] [Google Scholar]