Abstract
We have determined uterine glycogen content, metabolizing enzyme expression and activity in the mink, a species that exhibits obligatory embryonic diapause, resulting in delayed implantation. Gross uterine glycogen concentrations were highest in estrus, decreased 50% by diapause and 90% in pregnancy (P ≤ 0.05). Endometrial glycogen deposits, which localized primarily to glandular and luminal epithelia, decreased 99% between estrus and diapause (P ≤ 0.05) and were nearly undetectable in pregnancy. Glycogen synthase and phosphorylase proteins were most abundant in the glandular epithelia. Glycogen phosphorylase activity (total) in uterine homogenates was higher during estrus and diapause, than pregnancy. While glycogen phosphorylase protein was detected during estrus and diapause, glycogen synthase was almost undetectable after estrus, which probably contributed to a higher glycogenolysis / glycogenesis ratio during diapause. Uterine glucose-6-phosphatase 3 gene expression was greater during diapause, when compared to estrus (P ≤ 0.05) and supports the hypothesis that glucose-6-phosphate resulting from phosphorylase activity was dephosphorylated in preparation for export into the uterine lumen. The relatively high amount of hexokinase-1 protein detected in the luminal epithelia during estrus and diapause may have contributed to glucose trapping after endometrial glycogen reserves were depleted. Collectively, our findings suggest to us that endometrial glycogen reserves may be an important source of energy, supporting uterine and conceptus metabolism up to the diapausing blastocyst stage. As a result, the size of uterine glycogen reserves accumulated prior to mating may in part, determine the number of embryos that survive to the blastocyst stage, and ultimately litter size.
Keywords: Embryonic diapause, Glycogen, Mink, Uterus
Pre-embryonic growth prior to implantation is supported by nutrients in uterine glandular secretions (histotroph) that include glucose, glycogen, proteins, amino acids and fats [1,2,3]. Glucose uptake and metabolism by pre-embryos is essential for blastulation [4, 5] and hatching [6]. Moreover, endometrial decidualization is dependent upon glucose metabolism [7], and glucose is the preferred energy substrate of the myometrium [8, 9].
Although gluconeogenesis does not occur in the uterus [10, 11], glucose is stored as glycogen [12, 13]. In rodents, gross uterine glycogen concentrations peak during proestrus-estrus, then decrease during implantation and early pregnancy [12, 13]. The human uterus accumulates massive amounts of glycogen within glandular and luminal epithelia during the late-proliferative to early-secretory phases, that is mobilized during the late secretory phase [14,15,16]. While the importance of uterine glycogen reserves to a successful pregnancy remains to be determined, women with reduced fertility frequently have extremely low endometrial glycogen concentrations [17].
Storage of glucose as glycogen, begins with phosphorylation of the sugar by hexokinase (Hk), producing glucose-6-phosphate, that is isomerized to glucose-1-phosphate and converted to uridine diphosphate glucose. Glucosyl units from uridine diphosphate glucose are subsequently transferred to the non-reducing ends of glycogen molecules by glycogen synthase (Gys). Glycogen is catabolized by glycogen phosphorylase (Pyg), releasing glucose-1-phosphate, which may be isomerized to glucose-6-phosphate. Subsequently, glucose-6-phosphate may enter the glycolytic pathway as a fuel source, or be dephosphorylated by glucose-6-phosphatase (G6pc), yielding free glucose for potential export into the systemic circulation and/or uterine lumen.
Mink exhibit obligatory embryonic diapause, with as many as 17 blastocysts in a state of arrested development for as long as 50–60 days post coitum resulting in delayed implantation [18, 19]. It is likely that uterine glycogen reserves are an important source of energy for pre-embryonic growth and implantation. Murphy and James [20] detected glycogen deposits in uterine epithelia of mink during diapause but not post-implantation. However, there have been no subsequent reports on glycogen metabolism in the mink uterus, especially between estrus, embryonic diapause and pregnancy. Therefore, our objectives were to determine: (1): glycogen content of the uterine endometrium, glandular and luminal epithelia, stroma and myometrium, (2): the cellular localization of Gys, Pyg and Hk proteins and (3): Pyg activity in gross uterine homogenates during estrus, embryonic diapause and pregnancy in mink.
Materials and Methods
Animals
Mink were maintained outdoors under ranch conditions, fed a mixture of chicken and fish by-products and received water ad libitum. Initially, uteri were collected on March 4, from unmated mink (n = 3) in estrus. Additional mink (n = 6) were bred according to standard farm practices which involved mating on March 3 and 4, followed by re-mating to a different male on March 12 and 13. Subsequently, uteri were obtained from mink (n = 3) on March 23, flushed with 1.0 ml saline and examined for the presence of un-implanted blastocysts. Because implantation sites are not visible until the first week of April [21], and all recovered blastocysts had an intact zona pellucida, we concluded that embryos were in a state of diapause on March 23. Finally, uteri were collected from mink (n = 3) on April 14 with pregnancy verified by the presence of implanted embryos. Because pregnancy (implantation to parturition) in mink is approximately 30 days, with peak parturition occurring in early May [21], we judged these uteri to be from mink in mid-pregnancy, henceforth referred to as pregnant/pregnancy.
Each mink was anesthetized with ketamine hydrochloride (20 mg/kg body weight; 45–290, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA, USA) and the uterus exteriorized by mid-ventral laparotomy. Uteri were flushed with saline and/or quick-frozen on dry ice, and stored at –80.0 C. Each animal then received a lethal dose of Euthasol (011355, MWI Veterinary Supply, Boise ID, USA). For the validation of western blot analysis (WBA), uteri were collected from anestrus rats immediately after euthanasia by CO2 asphyxiation, quick frozen in liquid nitrogen and stored at –80.0 C. Animal care and research procedures were approved by the Institutional Animal Care and Use Committee of Idaho State University and complied with the Guide for the Care and Use of Laboratory Animals.
Glycogen concentrations in uterine homogenates
Glycogen concentrations were determined following a modified procedure of Good et al. [22]. Uterine tissue (50 mg) from each mink was lyophilized for 3 days, homogenized in 20 vol 30% KOH, and heated at 100 C for 30 min, to inactivate enzymes and destroy free glucose. To isolate glycogen, samples were diluted with 1.2 vol 95% ethanol, frozen at –80 C for 60 min, then thawed and centrifuged at 9,600 × g for 10 min. The supernatant was discarded and the pellets dried overnight. To breakdown glycogen to glucose, 100 μl 1.0 N HCl was added to each tube and heated at 90–100 C for 2.5 h. Glucose concentrations, as a measure of glycogen content, were detected using Glucose Auto Kit (439-90901, Wako Chemicals, Richmond, VA, USA) and quantified spectrophotometrically (λ = 505 nm), by comparing unknowns against a standard curve of increasing glucose concentrations. Intra-assay coefficient of variation was 1.3% and inter-assay coefficient of variation was 8.3%.
Glycogen content of uterine tissues
Uterine samples were fixed in 10% neutral buffered formalin, dehydrated and mounted in paraffin blocks. Transverse uterine sections (4 μm) were incubated with Periodic Acid Schiff (PAS) reagent, and counter-stained with hematoxylin. Images were captured digitally at 25, 200 and 400×, and analyzed in triplicate for each mink using ImageJ software [23]. Because PAS stains glycogen as well as other carbohydrates, a consecutive section was pre-treated with diastase (A8220, Sigma Chemical, St. Louis, MO, USA), to digest glycogen prior to PAS staining and served as a negative control. Glycogen content of each tissue was quantified by subtracting negative control values from those of sections stained with PAS without diastase and then expressed as a percentage of the area that stained positive for glycogen.
To ensure that glycogen measurements for glandular epithelia were accurate approximations of total glandular glycogen content and not affected by differences in gland number, we quantified uterine glands during estrus, diapause and pregnancy. For each mink, the total number of glands was counted in three independent cross sections at 25×, using Cell Counter in ImageJ software. To be included, a gland had to be completely separate from neighboring glands and luminal epithelium.
RNA isolation and q-PCR analysis
Total RNA was isolated from 25 to 50 mg uterine tissue from each mink using QIAGEN RNeasy Fibrous Tissue Mini Kit (74704, QIAGEN, Valencia, CA, USA) as previously described [24]. Quantitative PCR (qPCR) was performed in triplicate, using Fast Start SYBR Green Master Mix (Roche Applied Science, Indianapolis, IN, USA) containing forward and reverse primers (4 μM each) plus 5 μl of cDNA template (100–200 ng) per reaction. Each sample was subjected to 40 alternating cycles of a three-segment amplification program: (1) 15s denaturation at 95 C, (2) annealing for 1 min at 55 C (Actb, Gapdh) or 60 C (G6pc3), and (3) elongation at 72 C for 1 min. The PCR products (amplicons) were detected in real time, by measuring SYBR-Green fluorescence during the annealing stage, with the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Efficiency of amplicon doubling during each PCR cycle was, Actb: 100%, Gapdh: 99%, and G6pc3: 88%. Negative controls contained no template and amplification was never above background. Primer specificity as determined by melt-curve analyses yielded a single melting temperature for each amplicon (Table 1).
Table 1. Primers used for qPCR of mink uterine gene transcripts and amplicon characteristics.
| Genes | Forward & reverse primers | Amplicon | Melt temp. (C) | Accession No.* |
| G6pc3 | ATCAGCCTAGCCTTCAAGTGGTGT | 187 bp | 87.78 ± 0.22 | NM_138387.3 |
| AGG CTATCTTCTGGCCATTTCCCA | ||||
| Gapdh | TTGTCAGCAATGCCTCCTGTACCA | 200 bp | 85.03 ± 0.48 | AF076283.1 |
| ACCAGTGGAAGCAGGGATGATGTT | ||||
| Actb | GATGACC CAGATCATGTTCGAG | 321 bp | 86.0 ± 0.03 | AF076283.1 |
| CCATCTCCTGCTCGAAGTCC | ||||
Glucose-6-phosphatase-3 (G6pc3); Glyceraldehyde 3-phosphate dehydrogenase (Gapdh); beta-actin (Actb). *http:/www.ncbi.nlm.nih.gov.
Fold changes in gene expression were calculated by the relative standard curve method. Standard curves for each amplicon, were generated from 3 pooled uteri collected from anestrus mink in November. The cDNA from these uteri was diluted (1:10, 1:100, 1:1,000 and 1:10,000), or undiluted and used to construct standard curves (cDNA ng/ml versus quantification cycle, Cq) for each gene product (R2 = 0.98 to 0.99). We chose Gapdh with which to normalize our data, as it showed the least variation in expression (Gapdh Cq for estrus = 14.19 ± 0.03, for diapause, Cq = 14.20 ± 0.04 and for pregnancy, Cq = 14.29 ± 0.02), as compared to Beta-Actin (Actb Cq for estrus = 22.1 ± 3.2, for diapause Cq = 20 ± 1.58 and for pregnancy Cq =19.6 ± 3.3).
Western blotting analysis
As a verification of antibody specificity for immunohistochemistry, we conducted western blot analyses (WBA) for Gys and Hk1 proteins in mink and rat uterine tissues collected during anestrus. We were unable to develop WBA for the Pyg protein. Although all primary antibodies were produced against human proteins they were validated against mouse proteins by the manufacturer (Cell Signaling Technology, Danvers, MA, USA). Antibodies against Gys were not isoform specific and could therefore bind to Gys1 (muscle) and Gys2 (liver).
In brief, uterine proteins were isolated in RIPA Lysis buffer (sc-2448, Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer’s instructions. Protein concentrations were determined using Pierce BCA protein assay kit (23225, Thermo Scientific, Rockford, IL, USA). Proteins were resolved by molecular weight using SDS-PAGE and transferred onto nitrocellulose membranes, blocked for 1 h in a 5% milk buffer containing Tris Buffered Saline (TBS) + Tween 20 (BP337-100; Fisher Scientific, Pittsburgh, PA, USA) to reduce non-specific binding [26]. Membranes were incubated with primary antibodies specific for Hk1 (2024S, Cell Signaling Technology, Danvers, MA, USA 1:1000 dilution) and Gys (3886S, 1:1000 dilution) overnight (4 C) in blocking solution. Subsequently, membranes were incubated with secondary antibody (anti-rabbit IgG-HRP; 7074S; Cell Signaling Technology) at 1:1000 at room temperature for 2h. Lastly, HRP substrate (WBKLS0100, Millipore, Billerica, MA, USA) was added to the membranes and the resulting blots visualized by chemiluminescence.
Immunohistochemistry
Uterine samples were fixed in 10% neutral buffered formalin, dehydrated and mounted in paraffin blocks. To determine the uterine cell types that expressed Gys, Pyg and Hk1 proteins we analyzed tissue sections (4 μm) with immunohistochemistry according to Cell Signaling Technologies protocol with conditions optimized for the endometrium. In brief, uterine cross sections were incubated with sodium citrate at 90 C for 20 min to unmask antigens. Sections were permeabilized with 0.3% Triton X-100 and blocked for 1 hr in 10% donkey serum (50-230-7396, Fisher Scientific), followed by a 30 min incubation at room temperature in Levamisole (196142; Sigma Aldrich, St. Louis, MO, USA) to inactivate endogenous alkaline phosphatase. Subsequently, primary antibodies for Hk1 (rabbit anti-human; 2024S, Cell Signaling Technology), total Gys (rabbit anti-human, 3886, Cell Signaling Technology), and total Pyg (goat anti-human, sc-46347, Santa Cruz Biotechnology, CA, USA) were added to sections in 10% donkey serum, and incubated overnight at 4 C. Slides were then washed 3 times in TBS and secondary antibody (goat anti-rabbit, # T2191, Life Technologies, Grand Island, NY, USA or donkey anti-goat; sc-2020 Santa Cruz Biotechnology) added in 10% donkey serum and incubated at room temperature for 2 h. Sections were then washed in TBS, and incubated in the dark in the presence of color substrate (SK-5300, Vector Labs, Burlingame, CA, USA), until the development of a blue color was detected. While the time required for color development varied between antibodies, all sections from mink collected during estrus, diapause and pregnancy that were probed with a specific antibody were each incubated for the same duration of time, to allow for a semi-quantitative analysis of the protein. After color development, sections were washed in TBS to stop the reaction and counterstained with nuclear fast red. Sections were then dehydrated through a graded ethanol series and mounted on slides with Permount (4112; Richard Allan Scientific, Kalamozoo, MI, USA). Negative controls were similarly treated, except primary antibodies were omitted and there was no observable staining for any of the enzymes.
Glycogen phosphorylase activity
Mink uterine Pyg activity was measured according to Storey [25]. In brief, uterine samples (15–20 mg) were homogenized in 39 volumes of homogenization buffer (15 mM imidazole, 5mM EGTA, 100 mM NaF, 0.1 mM PMSF and 15 mM beta mercaptoethanol) at 30 C. Samples were centrifuged at 2,000 × g for 30 sec and 80 μl of the resulting supernatant added to the reaction buffer (final volume 1.0 ml) consisting of: 50 mM KH2PO4, 2 mg/ml oyster glycogen type 2, 0.4 mM NADP, 10 μM glucose-1-6-bisphosphate, 0.25 mM EDTA, and 15mM MgCl2 final concentrations), with phosphoglucomutase, and glucose-6-phosphate dehydrogenase (G7877-1KU and P3397-1KU; Sigma Aldrich) in amounts that exceeded Pyg activity. Because phosphoglucomutase and glucose-6-phosphate dehydrogenase were shipped in ammonium sulfate solution, that has been shown to activate glycogen phosphorylase [26], enzyme mixtures were washed three times with KH2PO4 using centrifugal filter units (UFC801024, Millipore, Billerica, MA, USA) before adding them to the reaction buffer.
To measure total Pyg (phosphorylated and non-phosphorylated) activity, we added adenosine monophosphate (AMP, 25 mM final concentration) to duplicate tubes to activate any non-phosphorylated Pyg. The resulting NADPH from the reactions was detected spectrophotometrically (λ= 340 nm every 30 sec for 10 min). Absorption values were multiplied by the extinction coefficient of NADPH (6220/M • cm) to obtain enzyme units (U), where 1 U = amount of enzyme required to metabolize 1 μM of substrate per minute. Phosphorylase activities were then normalized per milligram protein for each sample with protein concentrations determined using the Bradford assay procedure (23236, Pierce Biotechnology, Rockford, IL, USA). To validate the assay we measured total (pPyg) and unstimulated (Pyg) activities in 20, 40, 80 and 160 μl of homogenized tissue. As expected, each successive doubling in homogenate volume resulted in an approximate doubling in NADPH produced. Because glucose exerts feedback inhibition on Pyg activity [27], we added 5.0 mM glucose to duplicate reaction mixtures, which reduced total enzyme activity 65%.
Statistical analysis
Gross uterine glycogen concentrations, endometrial and myometrial glycogen content, mRNA, gland number, and Pyg activity were analyzed by one-way ANOVA, followed by Tukey’s post- hoc test using R (version 2.13.2; http://www.r-project.org). ImageJ data for stroma, glandular and luminal epithelia were analyzed by Nested ANOVA with tissue type nested within each collection period. Differences were considered significant at P ≤ 0.05.
Results
Total uterine gland number
Total gland number per uterine cross-section (mean ± SE) did not differ among estrus (344 ± 22.2), diapause (269.5 ± 34.5), and pregnancy (249.0 ± 29.0). There was a tendency toward a greater gland number during estrus, compared to diapause and pregnancy (P = 0.127).
Uterine glycogen content
For pregnant animals, we initially dissected embryos free from the uterus and measured glycogen concentrations for inter-implantation (3.31 ± 0.99 mg/g) and implantation (3.97 ± 0.49 mg/g) tissues to determine if the presence of embryos affected uterine glycogen content. Because there was no difference in glycogen concentrations between the two sites, all subsequent analyses for pregnant mink are for inter-implantation tissues.
Gross uterine glycogen concentrations were highest during estrus, decreased 50% by diapause, and 90% during pregnancy (Table 2; P ≤ 0.05). ImageJ analyses revealed that glycogen content decreased 50% in the myometrium and 80% in the endometrium between estrus and diapause (Table 2, Fig. 2; P ≤ 0.05). Glycogen content of both myometrium and endometrium during pregnancy was reduced 99% compared to estrus (P ≤ 0.05).
Table 2. Mean (± S.E.) glycogen concentrations in whole uteri, and percent glycogen content of uterine myometrium and endometrium from mink collected during estrus, diapause and pregnancy.
| Gross glycogen (mg/ g tissue) |
Myometrial glycogen (% Area PAS+) |
Endometrial glycogen (% Area PAS+) |
|
| Estrus | 25.67 ± 1.44A | 31.64 ± 1.2A | 36.07 ± 2.01A |
| Diapause | 11.51 ± 2.08B | 17.45 ± 0.60B | 7.79 ± 1.51B |
| Pregnancy | 3.31 ± 0.99C | 0.21 ± 0.08C | 0.44 ± 0.44C |
A–C Within a column, means with different superscripts differ (P ≤ 0.05).
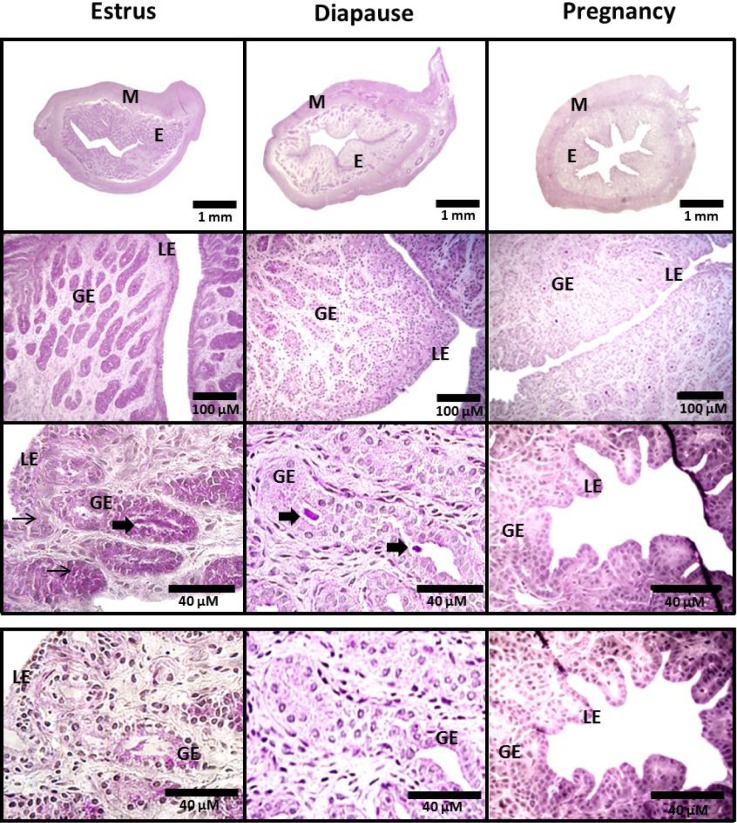
Fig. 2.
TOP PANEL: Uterine cross sectional images of mink collected during estrus, diapause and pregnancy. All sections were stained with Periodic Acid Schiff (PAS) and counterstained with hematoxylin. Images were captured at 25 × (top row), 200 × (middle row), or 400 × (bottom row). BOTTOM PANEL: Sections (400 ×) were pre-treated with diastase to digest glycogen, prior to PAS and hematoxylin staining to illustrate a negative control. Small arrows identify areas of positive PAS staining in epithelial cells. Large arrows identify positive PAS staining within the glandular lumen. M, myometrium; E, endometrium; GE, glandular epithelium; LE, luminal epithelium.
Endometrial glycogen deposits during estrus, were localized primarily to glandular (32% PAS +) and luminal (35% PAS +) epithelia, with only 1% of the stroma staining positive with PAS (Fig. 2, Table 3). Glycogen content of the stroma, glandular and luminal epithelia was over 99% lower during diapause when compared to estrus (Table 3; Fig. 2, P ≤ 0.05), with the nutrient virtually undetectable in pregnancy. Glycogen deposits were sporadically detected within the lumen of uterine glands (Fig. 2; 400×).
Table 3. Mean (± S.E.) percent glycogen content of uterine glandular epithelia, luminal epithelia, and stroma for mink collected during estrus, diapause and pregnancy.
| Glandular epithelia (% Area PAS+) |
Luminal epithelia (% Area PAS+) |
Stroma (% Area PAS+) |
|
| Estrus | 31.92 ± 6.90A,a | 34.90 ± 1.58A,a | 1.079 ± 0.21A,b |
| Diapause | 0.096 ± 0.077B,a | 0.26 ± 0.18B,a | 0.015 ± 0.015B,a |
| Pregnancy | undetectableB,a | 0.11 ± 0.06B,a | 0.003 ± 0.002B,a |
A–C Within a column, means with different superscripts differ (P ≤ 0.05). a, b Within a row, means with differ superscripts differ (P ≤ 0.05).
Uterine glycogen metabolizing enzyme expression and activity
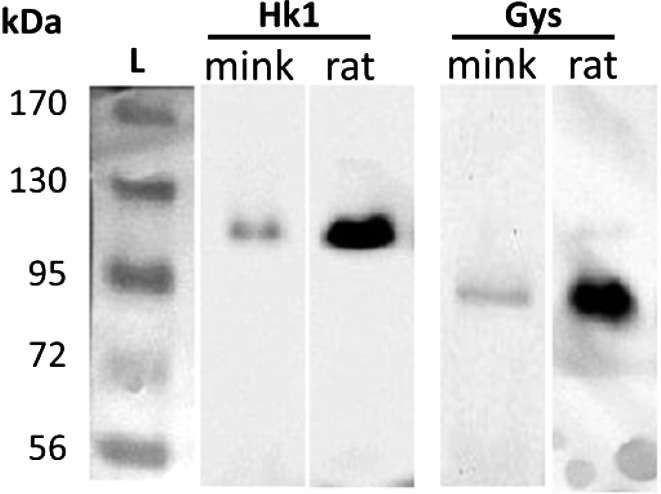
The validation of primary antibodies for Hk1 and Gys was achieved by WBA which revealed only a single band corresponding to the correct molecular weight for each protein in mink and rat uterine tissues (Fig. 1).
Fig. 1.
Western blot analyses for hexokinase 1 (Hk1) and glycogen synthase (Gys) proteins in mink and rat uterine tissues collected during anestrus. Ladder (L) with molecular weights in kilodaltons (kDa).
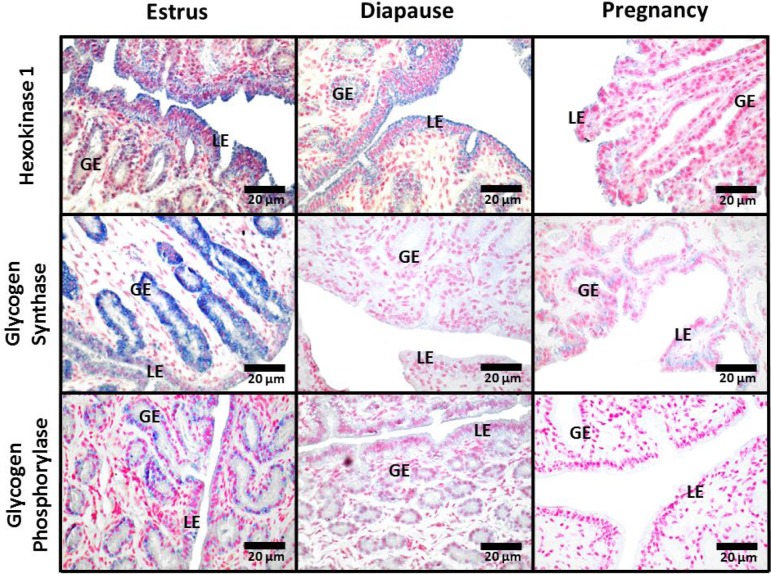
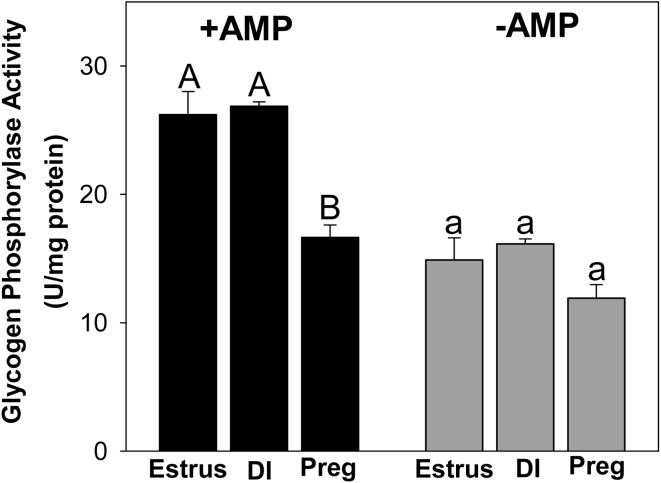
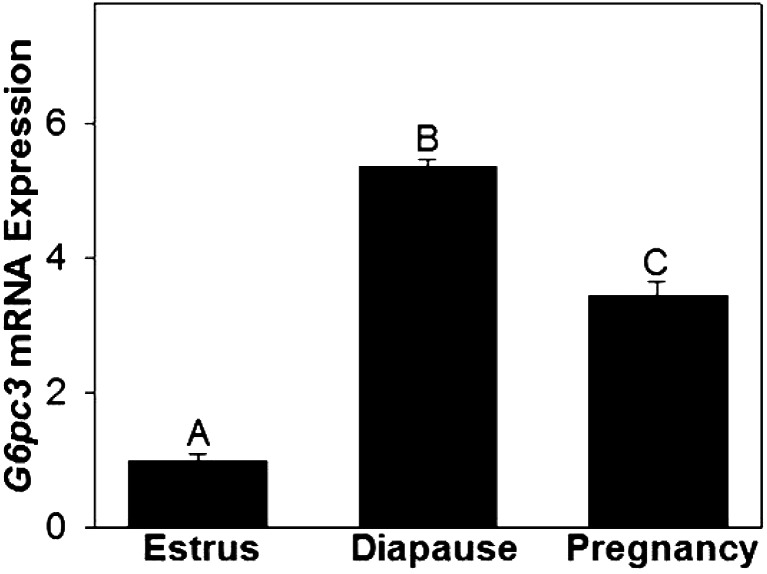
During estrus, total Gys staining by immunohistochemistry was strong in the glandular epithelia and moderate in luminal epithelia (Fig. 3). Signal strength for Gys protein was very low in both epithelia during diapause and pregnancy. No Gys staining was observed in endometrial stroma during estrus, diapause or pregnancy. A strong signal for Pyg protein was detected in glandular epithelia during estrus that was reduced during diapause and undetectable in pregnancy (Fig. 3). We could detect no Pyg staining in endometrial stroma during estrus, diapause or pregnancy. Total Pyg activity (+AMP), was highest during estrus and diapause when compared to pregnancy (P ≤ 0.05; Fig. 4). There was no difference in activity of unstimulated Pyg (–AMP) among estrus, diapause and pregnancy, although activity tended to be reduced in pregnancy when compared to diapause (P = 0.10). Immunohistochemical staining for Hk1 protein was strongest in luminal epithelia and weaker in glandular epithelia during estrus and diapause and was undetectable in both cell types during pregnancy (Fig. 3). No Hk1 protein staining was observed in endometrial stroma during estrus, diapause or pregnancy. Although we were unable to detect G6pc3 by immunohistochemistry, uterine G6pc3 mRNA expression was 5-fold higher during diapause and 3.5 fold higher during pregnancy, compared to estrus (Fig. 5, P ≤ 0.05).
Fig. 3.
Immunohistochemical localization of hexokinase-1 (Hk1), glycogen synthase (Gys) and glycogen phosphorylase (Pyg) proteins in uterine cross sections from mink collected during estrus, diapause and pregnancy. Images were captured at 400 ×. GE, glandular epithelium; LE, luminal epithelium.
Fig. 4.
Total (+AMP) and un-stimulated (–AMP) glycogen phosphorylase activities in mink uterine homogenates from animals collected during estrus, diapause (DI) and pregnancy (Preg). A,B; a,b Groups without a common superscript differ (P ≤ 0.05).
Fig. 5.
Relative (mean ± S.E.) mRNA expression levels for glucose-6-phosphatase 3 (G6pc3) by the uteri of mink collected during estrus, diapause and pregnancy. a–c For each enzyme, groups without a common letter differ (P ≤ 0.05).
Discussion
Gross and endometrial glycogen
Glycogen content (gross, endometrial, myometrial, epithelial and stromal) decreased from estrus, through diapause (P < 0.05) and pregnancy (P < 0.05; Tables 2 and 3; Fig. 2). These findings agree with those for animals that do not exhibit obligatory embryonic diapause such as rodents [12], cats [28], ferrets [29], and sheep [30], illustrating that uterine glycogen reserves are a potential source of energy for uterine and pre-embryonic growth through implantation and early pregnancy.
Endometrial glycogen deposits localized almost exclusively to glandular and luminal epithelia, supporting the hypothesis that glycogen is a source of glucose for uterine histotroph. Interestingly, glycogen reserves in both epithelia were almost completely mobilized by diapause. In support of this conclusion, it requires 8–11 days, after mating induced ovulation, for the mink conceptus to develop to the dormant blastocyst stage as it enters the uterine lumen [18, 19]. This suggests to us that the blastocysts recovered from mink on March 23, as a result of matings on March 12 & 13, had resided in the uterine lumen for only 2–3 days, with perhaps some still in the oviducts. While blastocysts from the first matings (March 3 & 4) probably were in the uterine lumen for a longer duration of time, all of them still had an intact zona pellucida. Desmarias et al. [31] showed that it required 10 days after blastocyst re-activation in late March to early April, before implantation occurred in mink. Moreover, circulating progesterone concentrations in mink are low until approximately March 22, then rapidly increase with implantation taking place 5–10 days later [32, 33]. Collectively, these findings strongly suggest to us that mink endometrial glycogen reserves were an important source of energy for uterine and conceptus growth through the dormant blastocyst stage. As further evidence, Brown and Whittingham [34] showed that 1-cell mouse conceptuses in vitro failed to develop to the blastocyst stage unless glucose was added to the incubation media. This is consistent with the finding that as morulas develop into blastocysts, they switch from pyruvate and lactate, to glucose as the preferred energy substrate [35]. Although blastulation occurs in the oviducts, Enders [18] showed that the utero-tubal junction in mink did not present a barrier to the movement of fluids between oviductal and uterine compartments.
It is possible that mink pre-embryonic metabolism to the dormant blastocyst stage depends largely on uterine glycogen reserves as an energy source, whereas subsequent blastocyst reactivation and implantation is supported by glucose transported from the maternal circulation. Such a phenomenon could represent a distinguishing feature of the reproductive cycle of species exhibiting obligatory embryonic diapause. Because mink pre-embryos may reside in a relatively hypoxic uterine lumen for much longer durations of time than species that do not exhibit diapause, a continuous source of glucose for anaerobic metabolism could be critical to survival, and influence the number of embryos that undergo implantation and therefore litter size at birth.
As evidence of glucose transport into the uterine lumen, many types of glucose transporters (GLUTs 1, 3, 4, 8) have been detected in the endometrium of rat [36], sheep [37], and humans [38]. Das et al. [39] detected Glut1 protein in apical and basolateral membranes of sheep uterine glandular epithelial cells during late gestation. Korgun et al. [36] detected Glut3 protein in apical membranes of rat uterine luminal epithelia on days 1–6 of gestation only, which would include implantation around day 5. In mice, Glut1 protein expression by stromal cells was low on day 4 post coitus (just before implantation), increased on day 5 and was greatest on day 7 [40]. Sheep endometrial Glut1 mRNA expression increased from day 10 to 14 of pregnancy, prior to implantation (day 16), and remained elevated through day 20 [39].
Low glycogen content in the uterine stroma of mink compared to the epithelia (Table 3, Fig. 2) was not surprising. The mink endometrium is reported to not undergo decidualization in preparation for implantation [41], and may have lower energy requirements than the stroma of species that decidualize. For example, rodent endometrium, which decidualizes prior to implantation, had a stromal cell glycogen content greater than the uterine epithelia [42]. Therefore, because the uterine stroma of mink delineated with ImageJ software included a considerable amount of extracellular matrix, in addition to cells, it is possible that our estimates of stromal cell glycogen content were low.
Interestingly, we detected glycogen deposits in what appeared to be the lumen of mink uterine glands (Fig. 2), which agrees with previous findings [24]. Burton et al. [1] reported glycogen deposits in the lumen of human uterine glands as well the inter-vellus space separating maternal and fetal membranes at the end of the first trimester. It is possible that glycogen, secreted intact by uterine epithelia is taken up through phagocytosis by pre-embryos. The carnivore trophoblast becomes extremely phagocytic as implantation progresses [43]. Glycogen may also be degraded intra-luminally, as glycogen granules contain anabolic and catabolic enzymes. As evidence, glycogen phosphorylase activity in sheep uterine luminal fluids increased during pre-implantation, peaking near the expected time of implantation [30].
Myometrial glycogen
Myometrial glycogen content in mink decreased 50% between estrus and diapause (P < 0.05; Table 2). We suspect that the mobilized glycogen supported myometrial contractions facilitating sperm transport and embryo spacing. Mating induces myometrial contractions [44] and it is reasonable to predict that part of the mobilized glycogen was used to support these contractions. However, myometrial glycogen content in the rat increased the week before birth [45], presumably to support contractions during parturition, and we cannot rule out this possibility in mink.
It is possible that glycogen reserves of the myometrium contribute to endometrial and/or embryonic energy requirements, since blood vessels penetrate through the myometrium and pass into the endometrium. The immediate product of glycogen catabolism by Pyg is glucose-1-phosphate which may be converted to glucose-6-phosphate by phosphoglucomutase. This is a reversible reaction allowing for interconversion as the concentration of one or the other molecules increase. Phosphoglucomutase activity was detected in human myometrium [46]. Moreover, glucose-1-phosphatase was detected in the myometrium of the Indian desert hedgehog, and the level of enzyme activity was comparable to glandular epithelia during pro-estrus and estrus [47].
For the glucose component of glucose-6-phosphate to be exported by smooth muscle cells would require the expression of glucose-6-phosphatase. This enzyme was detected in human [46], but not goat myometrium [48]. Unfortunately, even after experimenting with a wide range of incubation times for each antibody (primary and secondary) and color development, we were unable to detect any of the enzyme proteins within the myometrium. We speculate that because the tissues had been fixed for a prolonged period of time prior to immunohistochemistry, that continued cross linking of proteins altered their conformation, reducing antigenicity. Nevertheless, our finding of increased G6pc3 gene expression in whole uterine homogenates during diapause and pregnancy, when compared to estrus (P < 0.05; Fig. 5) agrees with the rapid reduction in myometrial glycogen content observed at these times (P < 0.05; Table 2). The potential role of myometrial glycogen as a source of energy for endometrium and embryos, deserves further investigation.
Uterine glycogen metabolizing enzyme expression and activity
The large reduction in glycogen content of the glandular and luminal epithelia between estrus and diapause (Table 3) occurred in parallel with a pronounced reduction in the amount of Gys protein (Fig. 3). Uterine Pyg protein staining was strongest in the glandular epithelia during estrus, it decreased by diapause and was undetectable in pregnancy (Fig. 3). Consistent with these findings, mink uterine Pyg activity (total) was significantly greater during estrus and diapause when compared to pregnancy (Fig. 4). We interpret these findings to mean that as Gys protein levels (and presumably activity) decreased between estrus and diapause, the relatively constant level of Pyg activity resulted in uterine glycogen mobilization.
While uterine glycogen synthesis in mink appears to be predominantly under estrogenic regulation, the influence of hormones on glycogen catabolism is poorly understood. Because circulating progesterone concentrations in mink increase following mating induced ovulation [33], it is reasonable to expect that the hormone might be involved in uterine glycogen catabolism. Paul and Duttagupta [49] showed that in bilaterally ovariectomized rats, exogenous estradiol increased uterine glycogen content, whereas concomitant treatment with progesterone, significantly inhibited glycogen synthesis in a dose-dependent pattern. While progesterone might inhibit uterine glycogen synthesis in mink, perhaps by reducing Gys expression, it is also possible that the hormone promotes glycogen catabolism. Preliminary findings, from our laboratory show that in ovariectomized mink pre-treated for 3 days with estradiol, followed by progesterone for 3 days, uterine glycogen content was significantly lower than in mink treated with estradiol alone (unpublished data). This suggests to us that progesterone was glycogenolytic in the mink uterus. Because prolactin is luteotropic in mink and circulating concentrations of the hormone increase before progesterone, it is also possible that prolactin affects uterine glycogen metabolism. Prolactin receptors have been detected in the mink uterus and ovaries [50, 51], and the hormone has been shown to stimulate glycogenolysis in the rat epididymis [52].
Immunohistochemical detection of Hk1 protein was strongest in luminal epithelia during estrus and diapause and undetectable in pregnancy (Fig. 3). In rats, uterine Hk activity was higher during estrus than any other stage of the estrous cycle [54]. In pregnant rats, uterine Hk activity peaked on day 1, days 5–6 (peri-implantation) and again on day 12, during mid-gestation [13]. Spellman et al. [53] showed that Hk activity in human endometrium increased progressively throughout the menstrual cycle, being significantly higher during secretory than proliferative phases. Species differences probably account for some of these inconsistencies. Nevertheless, hexokinase activity during diapause in mink could contribute to glucose trapping by the epithelia, after glycogen stores were depleted.
In summary, our findings indicate to us that glycogen, synthesized by uterine glandular and luminal epithelia of the mink is likely to be a significant source of glucose for histotrophic secretions. Both Gys and Pyg proteins were most evident in glandular epithelia. While Pyg protein was detected during estrus and diapause, Gys protein was almost undetectable post-estrus. Uterine Pyg activity (total) was higher during estrus and diapause than pregnancy. We interpret these findings to mean that as uterine glycogen synthesis decreases between estrus and diapause, this unmasks the glycogenolytic activity of Pyg. Consistent with our hypothesis that the resulting glucose from glycogen breakdown and/or Hk1 trapping, may be exported into the uterine lumen, G6pc3 mRNA expression was 5-fold greater during diapause, compared to estrus. The near complete mobilization of glycogen from uterine luminal and glandular epithelia, by the diapausing blastocyst stage, suggests that this nutrient may in part, determine the number of embryos that survive to the peri-implantation stage and perhaps litter size at birth.
Acknowledgments
This publication was made possible by the INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences), and awards from the Mink Farmers Research Foundation (Fur Commission USA; Coronado, CA) to JR. Mink were generously provided by Messrs Lee and R Moyle, of Moyle Mink and Tannery, Heyburn, Idaho. We thank Dr K Aho for statistical advice and Ms H Rogers for immunohistochemical analyses. A preliminary report of these findings was published (Proc Soc Study Reprod 2011, 44th Ann Meet, Abstract #385).
References
- 1.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002; 87: 2954–2959. [DOI] [PubMed] [Google Scholar]
- 2.Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod 2001; 64: 1608–1613. [DOI] [PubMed] [Google Scholar]
- 3.Hempstock J, Cindrova-Davies T, Jauniaux E, Burton GJ. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol 2004; 2: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin KL, Leese HJ. Role of glucose in mouse preimplantation embryo development. Mol Reprod Dev 1995; 40: 436–443. [DOI] [PubMed] [Google Scholar]
- 5.Riley JK, Moley KH. Glucose utilization and the PI3-K pathway: mechanisms for cell survival in preimplantation embryos. Reproduction 2006; 131: 823–835. [DOI] [PubMed] [Google Scholar]
- 6.Wordinger RJ, Brinster RL. Influence of reduced glucose levels on the in vitro hatching, attachment, and trophoblast outgrowth of the mouse blastocyst. Dev Biol 1976; 53: 294–296. [DOI] [PubMed] [Google Scholar]
- 7.Frolova AI, O’Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol 2011; 25: 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo A, Angioni S, Spedicato M, Minoia G, Mutinati M, Trisolini C, Sciorsci RL. Uterine contractility is strongly influenced by steroids and glucose metabolism: an in vitro study on bovine myometrium. Gynecol Endocrinol 2011; 27: 636–640. [DOI] [PubMed] [Google Scholar]
- 9.Yochim JM, Saldarini RJ. Glucose utilization by the myometrium during early pseudopregnancy in the rat. J Reprod Fertil 1969; 20: 481–489. [DOI] [PubMed] [Google Scholar]
- 10.Yánez AJ, Nualart F, Droppelmann C, Bertinat R, Brito M, Concha II, Slebe JC. Broad expression of fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase provide evidence for gluconeogenesis in human tissues other than liver and kidney. J Cell Physiol 2003; 197: 189–197. [DOI] [PubMed] [Google Scholar]
- 11.Zimmer DB, Magnuson MA. Immunohistochemical localization of phosphoenolpyruvate carboxykinase in adult and developing mouse tissues. J Histochem Cytochem 1990; 38: 171–178. [DOI] [PubMed] [Google Scholar]
- 12.Demers LM, Yoshinaga K, Greep RO. Uterine glycogen metabolism of the rat in early pregnancy. Biol Reprod 1972; 7: 297–304. [DOI] [PubMed] [Google Scholar]
- 13.Greenstreet RA, Fotherby K. Carbohydrate metabolism in the rat uterus during early pregnancy. Steroids Lipids Res 1973; 4: 48–64. [PubMed] [Google Scholar]
- 14.Cornillie FJ, Lauweryns JM, Brosens IA. Normal human endometrium. An ultrastructural survey. Gynecol Obstet Invest 1985; 20: 113–129. [DOI] [PubMed] [Google Scholar]
- 15.Spornitz UM. The functional morphology of the human endometrium and decidua. Adv Anat Embryol Cell Biol 1992; 124: 1–99. [DOI] [PubMed] [Google Scholar]
- 16.Verma V. Ultrastructural changes in human endometrium at different phases of the menstrual cycle and their functional significance. Gynecol Obstet Invest 1983; 15: 193–212. [DOI] [PubMed] [Google Scholar]
- 17.Girish C, Naveen S, Nagarajappa A, Manjunath M. A correlative study of endometrial glycogen content and other contributory factors on female fertility. Intl J Biomel Adv Res 2012; 3: 30–35. [Google Scholar]
- 18.Enders RK. Reproduction in the mink (Mustela vison). Proc Am Philos Soc 1952; 96: 691–755. [Google Scholar]
- 19.Hansson A. The physiology of reproduction in mink with special reference to delayed implantation. Acta Zoologica 1947; 28: 1–136. [Google Scholar]
- 20.Murphy BD, James DA. Mucopolysaccharide histochemistry of the mink uterus during gestation. Can J Zool 1974; 52: 687–693. [DOI] [PubMed] [Google Scholar]
- 21.Concannon P, Pilbeam T, Travis H. Advanced implantation in mink (Mustela vison) treated with medroxyprogesterone acetate during early embryonic diapause. J Reprod Fertil 1980; 58: 1–6. [DOI] [PubMed] [Google Scholar]
- 22.Good CA, Kramer H, Somogyi M. The determination of glycogen. J Biol Chem 1933; 100: 485–491. [Google Scholar]
- 23.Abramoff M, Magalhaes P, Ram S. Image processing with ImageJ. Biophotonics Intl 2004; 11: 36–42. [Google Scholar]
- 24.Rose J, Hunt J, Shelton J, Wyler S, Mecham D. The effects of estradiol and catecholestrogens on uterine glycogen metabolism in mink (Neovison vison). Theriogenology 2011; 75: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storey KB. Tissue-specific controls on carbohydrate catabolism during anoxia in goldfish. Physiol Zool 1987; 60: 601–607. [Google Scholar]
- 26.Sprang SR, Withers SG, Goldsmith EJ, Fletterick RJ, Madsen NB. Structural basis for the activation of glycogen phosphorylase b by adenosine monophosphate. Science 1991; 254: 1367–1371. [DOI] [PubMed] [Google Scholar]
- 27.Hue L, Bontemps F, Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J 1975; 152: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson AB, Kosters BA. Preimplantation changes in the uterine mucosa of the cat. Am J Anat 1944; 75: 1–37. [Google Scholar]
- 29.Buchanan GD. Reproduction in the ferret (Mustela furo). II. Changes following ovariectomy during early pregnancy. J Reprod Fertil 1969; 18: 305–316. [DOI] [PubMed] [Google Scholar]
- 30.O’Shea T, Murdoch BE. Activity of enzymes of glycogen metabolism in the reproductive tract of the ewe at mating and during early pregnancy. Aust J Biol Sci 1978; 31: 355–361. [DOI] [PubMed] [Google Scholar]
- 31.Desmarais JA, Bordignon V, Lopes FL, Smith LC, Murphy BD. The escape of the mink embryo from obligate diapause. Biol Reprod 2004; 70: 662–670. [DOI] [PubMed] [Google Scholar]
- 32.Allais C, Martinet L. Relation between daylight ratio, plasma progesterone levels and timing of nidation in mink (Mustela vison). J Reprod Fertil 1978; 54: 133–136. [DOI] [PubMed] [Google Scholar]
- 33.Lagerkvist G, Einarsson EJ, Forsberg M, Gustafsson H. Profiles of oestradiol-17 beta and progesterone and follicular development during the reproductive season in mink (Mustela vison). J Reprod Fertil 1992; 94: 11–21. [DOI] [PubMed] [Google Scholar]
- 34.Brown JJ, Whittingham DG. The roles of pyruvate, lactate and glucose during preimplantation development of embryos from F1 hybrid mice in vitro. Development 1991; 112: 99–105. [DOI] [PubMed] [Google Scholar]
- 35.Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012; 143: 417–427. [DOI] [PubMed] [Google Scholar]
- 36.Korgun ET, Demir R, Hammer A, Dohr G, Desoye G, Skofitsch G, Hahn T. Glucose transporter expression in rat embryo and uterus during decidualization, implantation, and early postimplantation. Biol Reprod 2001; 65: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 37.Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. ii. glucose transporters in the uterus and peri-implantation conceptuses. Biol Reprod 2009; 80: 94–104. [DOI] [PubMed] [Google Scholar]
- 38.von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab 2003; 88: 3885–3892. [DOI] [PubMed] [Google Scholar]
- 39.Das UG, He J, Ehrhardt RA, Hay WW, Jr, Devaskar SU. Time-dependent physiological regulation of ovine placental GLUT-3 glucose transporter protein. Am J Physiol Regul Integr Comp Physiol 2000; 279: R2252–R2261. [DOI] [PubMed] [Google Scholar]
- 40.Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 2009; 150: 1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol 2004; 265: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casimiri V, Rath NC, Parvez H, Psychoyos A. Effect of sex steroids on rat endometrial epithelium and stroma cultured separately. J Steroid Biochem 1980; 12: 293–298. [DOI] [PubMed] [Google Scholar]
- 43.Bevilacqua E, Hoshida M-S, Amarante-Paffaro A, Albieri-Borges A, Zago Gomes S. Trophoblast phagocytic program: roles in different placental systems. Int J Dev Biol 2010; 54: 495–505. [DOI] [PubMed] [Google Scholar]
- 44.Crane LH, Martin L. Postcopulatory myometrial activity in the rat as seen by video-laparoscopy. Reprod Fertil Dev 1991; 3: 685–698. [DOI] [PubMed] [Google Scholar]
- 45.Vasilenko P, 3rd, Adams WC, Frieden EH. Uterine size and glycogen content in cycling and pregnant rats: influence of relaxin. Biol Reprod 1981; 25: 162–169. [DOI] [PubMed] [Google Scholar]
- 46.Danesino V, Ricciardi I, Sallusto A. [Adenosine triphosphatase, phosphoglucomutase and glucose-6-phosphatase activity of human myometrium]. Arch Ostet Ginecol 1962; 67: 513–522 (In Italian). [PubMed] [Google Scholar]
- 47.Sharma A, Mathur RS. Cyclic changes in the uterine phosphatases during breeding and non-breeding seasons of Hemiechinus auritus collaris Gray, the Indian long-cared hedgehog. Acta Histochem 1974; 49: 64–73. [PubMed] [Google Scholar]
- 48.Bhattacharya M, Saigal RP. Histoenzymic studies on phosphatases in the uterine wall of the goat (Capra hircus). Gegenbaurs Morphol Jahrb 1984; 130: 603–608. [PubMed] [Google Scholar]
- 49.Paul PK, Duttagupta PN. Inhibition of oestrogen-induced increase in hepatic and uterine glycogen by progesterone in the rat. Acta Endocrinol (Copenh) 1973; 72: 762–770. [DOI] [PubMed] [Google Scholar]
- 50.Rose J, Oldfield JE, Stormshak F. Changes in serum prolactin concentrations and ovarian prolactin receptors during embryonic diapause in mink. Biol Reprod 1986; 34: 101–106. [DOI] [PubMed] [Google Scholar]
- 51.Rose J, Stormshak F, Adair J, Oldfield JE. Prolactin binding sites in the uterus of the mink. Mol Cell Endocrinol 1983; 31: 131–139. [DOI] [PubMed] [Google Scholar]
- 52.Reddy YD, Reddy KV, Govindappa S. Effect of prolactin and bromocriptine administration on epididymal function—a biochemical study in rats. Indian J Physiol Pharmacol 1985; 29: 234–238. [PubMed] [Google Scholar]
- 53.Spellman CM, Fottrell PF, Baynes S, O’Dwyer EM, Clinch JD. A study of some enzymes and isoenzymes of carbohydrate metabolism in human endometrium during the menstrual cycle. Clin Chim Acta 1973; 48: 259–268. [DOI] [PubMed] [Google Scholar]
- 54.Greenstreet R, Fotherby K. Carbohydrate metabolism in rat uterus during the oestrous cycle. Steroids Lipids Res 1973; 4: 341–350. [PubMed] [Google Scholar]