Abstract
The study of human ovarian tissue transplantation and cryopreservation has advanced significantly. Autotransplantation of human pre-antral follicles isolated from cryopreserved cortical tissue is a promising option for the preservation of fertility in young cancer patients. The purpose of the present study was to reveal the effect of vitrification after low-temperature transportation of human pre-antral follicles by using the oxygen consumption rate (OCR). Cortical tissues from 9 ovaries of female-to-male transsexuals were vitrified after transportation (6 or 18 h). The follicles were enzymatically isolated from nonvitrified tissue (group I, 18 h of transportation), vitrified-warmed tissue (group II, 6 and 18 h of transportation) and vitrified-warmed tissue that had been incubated for 24 h (group III, 6 and 18 h of transportation). OCR measurement and the LIVE/DEAD viability assay were performed. Despite the ischemic condition, the isolated pre-antral follicles in group I consumed oxygen, and the mean OCRs increased with developmental stage. Neither the transportation time nor patient age seemed to affect the OCR in this group. Meanwhile, the mean OCR was significantly lower (P < 0.05) in group II but was comparable to that of group I after 24 h of incubation. The integrity of vitrified-warmed primordial and primary follicles was clearly corroborated by the LIVE/DEAD viability assay. These results demonstrate that the OCR can be used to directly estimate the effect of vitrification on the viability of primordial and primary follicles and to select the viable primordial and primary follicles from vitrified-warmed follicles.
Keywords: Human ovarian cortical tissue, Isolation, Oxygen consumption rate, Pre-antral follicle, Vitrification
The development of chemotherapeutic agents and radiotherapy has increased the survival rate of young cancer patients [1, 2]. However, serious adverse effects such as premature ovarian failure (POF) and loss of fertility occur after treatment in young female patients [3]. Thus, improving the patient’s quality of life after treatment is of increasing importance [4].
Currently, cryopreservation of oocytes and early embryos or cryopreservation of ovarian cortical tissue is an available method for preserving fertility in young cancer patients. In addition, cryopreservation of pre-antral follicles has been evaluated as a potential option for fertility preservation [5, 6] and as an alternative to cortical fragment transplantation in patients at risk of ovarian metastasis [7].
Slow freezing of human ovarian tissue [8,9,10,11] and autotransplantation of the frozen-thawed cortical tissue have led to the birth of 24 healthy infants since 2004 [12]. Compared with the slow-freezing method, vitrification is a simple and rapid procedure for the cryopreservation of various types of cells in a high-concentration cryoprotectant without ice crystal formation. The procedure has been reported to have promising results in various mammalian species, including humans [13], monkeys [14, 15] and others [16,17,18,19]. In previous studies in humans, a variety of different vitrification protocols were used in which parameters such as tissue size, freezing rate, composition of and duration of exposure to the vitrification solution and carrier devices were varied [13, 19,20,21,22,23,24,25,26]. However, they lacked applicable approaches for directly assessing the effect of vitrification on ovarian tissue. Therefore, current studies on human ovarian tissue vitrification are focused on novel analytical approaches to simply and directly estimate the viability of ovarian tissue.
Early pre-antral follicles, especially primordial and primary follicles, are cryoresistant because they have a relatively inactive metabolic rate, a small and simple tissue structure [8] and oocytes with no zona pellucida and metaphase spindle [27, 28].
Human pre-antral follicles can be enzymatically isolated from fresh and frozen ovarian cortical tissue [29,30,31,32], and they can be cultured in situ [29, 33, 34] and in vitro [29, 35, 36]. These procedures allow monitoring and assessment of follicle quality on a morphological basis, as well as monitoring and assessment of their developmental competence [36]. However, the culture system used for human pre-antral follicles has been perceived to be problematic because of the requirement for a prolonged period of follicle development.
The LIVE/DEAD viability assay has been reported to be a relatively simple method that can be used to evaluate the viability of isolated follicles [24, 35]. However, it necessitates estimating the viability of follicles, preincubation of the follicles in 2 vital fluorescence dyes and exposure of the follicles to ultraviolet rays.
On the other hand, measurement of the oxygen consumption rate (OCR) using a scanning electrochemical microscope (SECM) has been reported to be a noninvasive method for simple and direct evaluation of the quality of oocytes and early embryos for embryo transfer in various mammalian species [37,38,39].
The purpose of the present study was to demonstrate the OCR as a potential biomarker of the quality of human early pre-antral follicles isolated from vitrified ovarian cortical tissue after long-term transportation and its applicability in selecting viable early pre-antral follicles from vitrified-warmed follicles.
Materials and Methods
Tissue donors (specimens)
From December 2010 to October 2011, 18 ovaries were removed from 9 women with gender identity disorder at the Osaka New ART Clinic. Ovaries removed from androgen-treated female-to-male transsexuals are suitable for use as research materials for ovarian cryopreservation [40]. The women were between the ages of 25 and 45 years (average: 33.0 ± 6.3 years, mean ± SD) and were administered ENARMON DEPOT (ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) or TESTRON DEPOT (Fuji Pharma Co., Ltd., Tokyo, Japan) at 12–84 months before oophorectomy. Nine of the 18 ovaries were contributed to this study. All patients were informed about this ongoing project and signed an informed consent form. The research protocol was approved by the institutional review boards of the Kyono ART Clinic and the Osaka New ART Clinic.
Ovarian tissue treatment
The ovaries were placed in sterile 50-ml Falcon tubes (Becton, Dickinson and Company, Bedford, MA, USA) containing 40 ml of ice-cold Leibovitz’s L-15 medium (Invitrogen, San Diego, CA, USA) supplemented with sodium pyruvate (2 mM), glutamine (2 mM), 10% (v/v) synthetic serum substitute (SSS; Irvine Scientific, Santa Ana, CA, USA), penicillin G (75 µg/ml), streptomycin (50 µg/ml) and ascorbic acid (50 µg/ml); they were then transported on ice to the laboratory at the Kyono ART Clinic within 6 h (6-h transportation group). The transfer medium was removed from the surface of the ovaries by using sterilized filter paper. The ovaries placed on filter paper soaked with fresh transfer medium were overlaid on an ice-cold 150-mm culture dish (Corning, Coming, NY, USA), and then cortical tissue fragments were stripped from the ovaries. A tissue slicer was used to guarantee a 1-mm thickness of the cortical tissue specimens [24]. Recently, the first live birth from ovarian tissue autotransplantation, using ovaries transported overnight (>20 h) before cryopreservation, was reported [41]. To clarify the effect of low temperature on the OCR, the cortical tissue fragments were assigned to the vitrification (6-h transportation) group; alternatively, whole ovaries were refrigerated for an additional 12 h at 4 C and were assigned to the vitrification and viability assay (18-h transportation) groups.
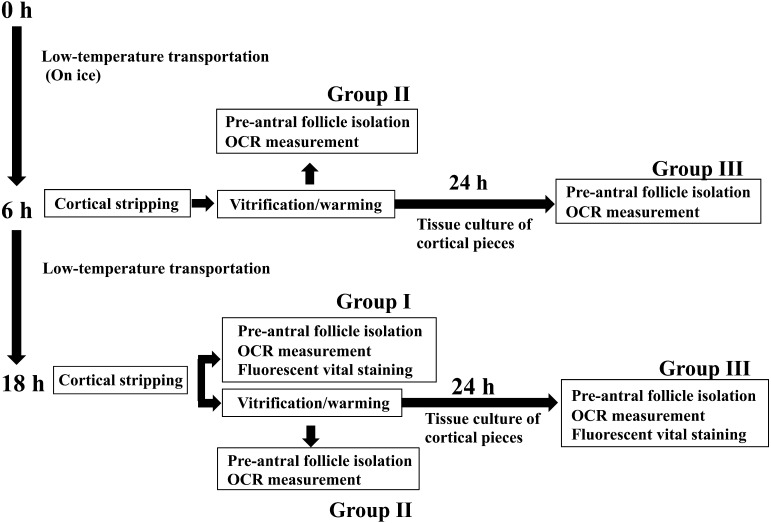
Nonvitrified cortical tissue fragments (1 × 10 × 10 mm) were stripped only from ovaries with 18 h of transportation (group I), and vitrified-warmed cortical tissue fragments (1 × 10 × 10 mm) were obtained from ovaries after either 6 or 18 h of transportation (group II). The nonvitrified cortical tissue fragments were divided into 4 pieces. Two of the 4 small cortical pieces (1 × 5 × 5 mm) were assigned to histological analysis, whereas the remaining 2 pieces were subjected to OCR measurement and dual fluorescence vital staining of pre-antral follicles. Meanwhile, for the vitrified-warmed cortical fragments, quartered small cortical pieces (1 × 5 × 5 mm) were assigned to histological analysis, tissue culture, dual fluorescence vital staining or OCR measurement of pre-antral follicles. Figure 1 shows a diagram of the experimental groups.
Fig. 1.
Schematic illustration of experimental groups.
Vitrification and warming
Following the procedure described by Kagawa et al. (2009) [24], the Cryotissue method was applied to large portions of ovarian tissue (1 x 10 x 10 mm) with a modified concentration of cryoprotectant agent (CPA) and extension of the soaking time. Ovarian tissues were initially equilibrated in 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) in HEPES-buffered TCM-199 medium supplemented with 20% (v/v) SSS (modified TCM-199 medium; mTCM-199) for 25 minutes, followed by a second equilibration in 20% EG and 20% DMSO with 0.5 M sucrose for 15 min. Subsequently, ovarian tissues were placed in a minimum volume of solution on a thin metal strip; they were then inserted into a protective container and placed in liquid nitrogen. For warming, the protective cover was removed, and the Cryotissue metal strip was immersed directly into 40 ml of 37 C mTCM-199 and 1.0 M sucrose for 1 min. Then, the ovarian tissues were transferred into 15 ml of 0.5 M sucrose in mTCM-199 for 5 min at room temperature and washed twice in mTCM-199 for 10 min.
Vitrified-warmed cortical tissue culture
The vitrified-warmed ovarian cortical fragments were then cut into 4 small pieces (5 × 5 × 1 mm) in 2 ml prewarmed Leibovitz’s medium. One of the 4 pieces was further cut into smaller pieces (1–0.5 mm3) with a scalpel, which were then individually placed in 24-well cell culture plates (Corning B.V. Life Sciences Europe, Amsterdam, Netherlands) containing 300 µl of McCoy’s 5a medium with bicarbonate supplemented with HEPES (20 mM), BSA (0.1%), glutamine (3 mM), penicillin G (0.1 mg/ml), streptomycin (0.1 mg/ml), transferrin (2.5 µg/ml), selenium (4 ng/ml), insulin (10 ng/ml) and ascorbic acid (50 µg/ml) (all were obtained from Sigma-Aldrich, St. Louis, MO, USA); they were incubated for 24 h at 37 C in 6% CO2, 5% O2 and 89% N2. After 24 h of incubation, early pre-antral follicles were isolated from the small pieces, and the OCR was measured.
Isolation of pre-antral follicles from nonvitrified and vitrified-warmed cortical tissues
Nonvitrified cortical fragments (5 × 5 × 1 mm) were stripped from the ovary after 18 h of transportation and placed in a 60-mm culture dish (Becton, Dickinson and Company); they were then further cut into smaller cortical pieces with a scalpel. The fragments were then rinsed in Dulbecco’s PBS (Invitrogen, Carlsbad, CA, USA) twice, supplemented with 1 mg/ml collagenase type I (Sigma-Aldrich) and 0.05 IU/µl DNase I (Sigma-Aldrich) and incubated at 37 C for 40–60 min. The incubated cortical pieces were transferred to 60-mm culture dishes containing 2 ml of Leibovitz’s medium to terminate the enzymatic reaction. Subsequently, pre-antral follicles were mechanically isolated from cortical pieces using 30-gauge needles (Dentronics, Tokyo, Japan) (group I). For the vitrified-warmed cortical tissue, one of the small cortical pieces (5 × 5 × 1 mm) was subjected to follicular isolation immediately after warming (group II) in a manner similar to group I; however, the other piece was subjected to follicular isolation after 24 h of incubation in McCoy’s 5a medium (group III).
Classification of pre-antral follicles
All isolated follicles with a visible oocyte, an intact basement membrane and no antral cavity were selected for further analysis. The diameter of pre-antral follicles was measured under an inverted microscope with a ZILOS-tk system (ZILOS, Beverly, MA, USA) and was assigned to one of 3 groups according to diameter: ≤ 39, ≥ 40–55 and > 55 µm. Furthermore, the pre-antral follicular stages were classified according to Gougeon and Chainy (1987) [42]: primordial follicle (a single layer of flattened granulosa cells (GCs) surrounding the oocyte), intermediary follicle (a single layer of a flattened and cuboidal GC mixture surrounding the oocyte), primary follicle (a single layer of cuboidal GCs surrounding the growing oocyte) and secondary follicle (2 layers of GCs surround a growing oocyte).
Estimation of oxygen consumption
The OCR was measured for fresh and vitrified-warmed pre-antral follicles according to the method of Shiku et al. [43] using an embryo respirometer (CRAS-1.0; CLINO, Sendai, Japan) in a laboratory at Kyono ART Clinic, which was evaluated with a SECM capable of measuring the OCR of an oocyte, embryo or relatively small clump of cells. Briefly, a pre-antral follicle was placed into modified Human Tubal Fluid (mHTF; Irvine Scientific) on the flat bottom of a cone-shaped microwell in the plate (CMP-1; CLINO, Sendai, Japan). A platinum microelectrode (CME-0002; CLINO) was placed close to the follicles; a voltage of –0.6 V was then applied to reduce the oxygen concentration in the solution surrounding the follicle, and the current generated was measured. The oxygen concentration gradients in the solution surrounding the follicle were measured by scanning the z-axis at a speed of 31.0 μm/sec. The average of three measurements was considered to be the OCR of the follicle. The OCR was estimated only in morphologically normal isolated follicles.
LIVE/DEAD viability assay
The viability of isolated pre-antral follicles was also estimated using a LIVE/DEAD Viability/Cytotoxicity Kit (L-3224; Molecular Probes, Leiden, Netherlands). This vital staining kit provides a two-color fluorescence cell viability assay that is based on simultaneous determination of living and dead cells. The method has been described by Van den Hurk et al. [44]. Briefly, in living cells, the virtually nonfluorescent calcein produces an intense green fluorescence by intracellular esterase activity (excitation, 495 nm; emission, 517 nm). In dead cells, the damaged plasma membrane permits ethidium homodimer I to enter; upon binding to nucleic acid, it undergoes a 40-fold enhancement of fluorescence, producing a bright red fluorescence (excitation, 495 nm; emission, 635 nm).
Viable follicles were defined as the isolated follicles that were composed of an oocyte with over 90% of the GCs producing intense green fluorescence. Sixty-five nonvitrified isolated follicles (20 primordial follicles, 30 primary follicles and 15 secondary follicles) consuming oxygen (OCR ≥ 0.02 × 1015 fmol/sec) were evaluated under a confocal laser scanning microscope (MRC-1024; Bio-Rad, Hercules, CA) using the LIVE/DEAD Viability/Cytotoxicity Kit. In the 18-h transportation group of group III, 29 of 32 isolated follicles (9 primordial follicles, 13 primary follicles and 7 secondary follicles) were estimated in a similar fashion, and the remaining 3 follicles were lost during the assay. Only morphologically normal follicles were evaluated with the LIVE/DEAD Viability/Cytotoxicity Kit.
Histological analysis of ovarian tissue
To evaluate the follicular morphology of the ovarian cortical tissues, small cortical pieces were removed from fresh control tissues (fixed at the time of removal), 6-h transported tissues, 18-h transported tissues and vitrified-warmed tissues after 18 h of transportation from 2 patients. These pieces were fixed in 10% formalin for 6 h to allow histological analysis. The pieces were then dehydrated and embedded in paraffin wax. Subsequently, they were cut into 5-μm-thick sections and stained with hematoxylin-eosin. Follicular quality evaluation was based on the structure of the surrounding stromal tissue, GC alignment and oocyte integrity. In a random manner, 90 early pre-antral follicles (50 primordial, 30 primary and 10 secondary) were investigated in each comparison group.
Statistical analysis
Statistical analyses were carried out using the Statcel 3 program for Microsoft Excel. Using the Steel-Dwass method, the following comparisons were made: (1) comparisons of the OCRs of pre-antral follicles in three age groups; (2) comparisons of OCRs among follicular developmental stages and (3) comparison of the OCRs of vitrified-warmed follicles after 24 hours of incubation. A P-value < 0.05 was considered significant. Comparisons between the mean OCRs of vitrified-warmed pre-antral follicles in the 6- or 18-h transportation groups were analyzed by repeated measure ANOVA. A P value of < 0.05 was considered significant.
Results
Histological analysis of ovarian tissue
The follicles in each comparison group showed similar morphologies. The numbers of intact follicles observed were 84 (47 primordial, 28 primary and 9 secondary), 82 (46 primordial, 28 primary and 8 secondary), 84 (47 primordial, 28 primary and 9 secondary) and 83 (48 primordial, 27 primary and 8 secondary) in fresh control tissue, 6-h transported tissue, 18-h transported tissue and vitrified-warmed tissue after 18 h of transportation, respectively. In the comparison between nonvitrified and vitrified-warmed cortical tissue after 18 h of transportation, no alterations in the morphology of intact follicles were observed. GCs were well organized, and the oocyte was morphologically normal, without any signs of degeneration or retraction in both groups of cortical tissue samples (Fig. 2).
Fig. 2.
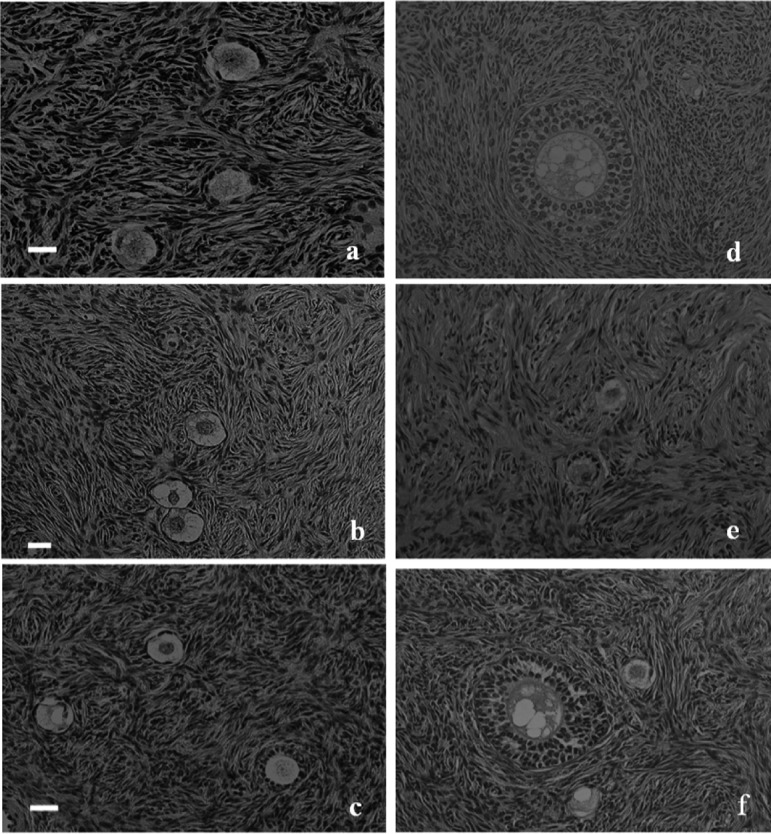
Histological analysis of fresh, nonvitrified and vitrified-warmed pre-antral follicles. (a) Primordial and primary follicles in fresh human ovarian cortical tissue. (b) Primordial and primary follicles in nonvitrified human ovarian cortical tissue after 6 h of transportation. (c) Primordial and primary follicles in nonvitrified human ovarian cortical tissue after 18 h of transportation. (d) Secondary follicles in nonvitrified human ovarian cortical tissue after 18 h of transportation. (e) Primary follicles in vitrified-warmed human ovarian cortical tissue after 18 h of transportation. (f) Primordial to secondary follicles in vitrified-warmed human ovarian cortical tissue after 18 h of transportation. (× 200; H-E staining). The scale bars represent 20 μm.
Isolation of pre-antral follicles
In group I, a total of 170 morphologically normal pre-antral follicles (71 primordial, 68 primary and 31 secondary) were isolated from nonvitrified cortical pieces of 9 patients in the 18-h transportation group. In group II, a total of 22 pre-antral follicles (10 primordial, 10 primary and 2 secondary) were isolated from vitrified-warmed cortical fragments from 2 patients in the 6-h transportation group, and 41 pre-antral follicles (19 primordial, 18 primary and 4 secondary) were isolated from vitrified-warmed fragments from 4 patients in the 18-h transportation group (Fig. 3). In group III, a total of 47 pre-antral follicles (23 primordial, 22 primary and 2 secondary) were isolated from warmed fragments that were obtained from 2 patients in the 6-h transportation group and cultivated for 24 h. Moreover, a total of 32 pre-antral follicles (10 primordial, 15 primary and 7 secondary) were isolated from 3 patients in the 18-h transportation group (Fig. 1, Table 1). In the present study, we measured the OCR of all isolated follicles and attempted to isolate and measure the OCR in approximately the same numbers of primordial and primary follicles in each experimental group. Secondary follicles were isolated to the extent possible.
Fig. 3.
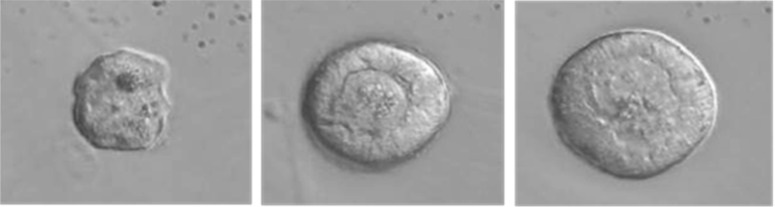
Isolated pre-antral follicles from vitrified-warmed cortical tissue from a 31-year-old woman. (a) A 37-μm-diameter follicle (primordial), (b) a 52-μm-diameter follicle (primary) and (c) a 71-μm-diameter follicle (secondary) were isolated from vitrified-warmed cortical tissue digested with collagenase I and subsequently trimmed by using 31G needles.
Table 1. Comparison of OCRs between nonvitrified and vitrified-warmed pre-antral follicles.
| Experimental group |
Transportation | Primordial follicles | Primary follicles | Secondary follicles | No. of patients |
| time (h) | OCR (n) | OCR (n) | OCR (n) | ||
| Group I | 18 | 0.11 ± 0.13a (71) | 0.28 ± 0.28d (68) | 1.08 ± 0.13g (31) | 9 |
| Group II | 6 | 0.02 ± 0.02 (10) | 0.03 ± 0.03 (10) | 0.06 ± 0.04 (2) | 2 |
| 18 | 0.02 ± 0.02b (19) | 0.03 ± 0.05e (18) | 0.05 ± 0.04h (4) | 4 | |
| Group III | 6 | 0.07 ± 0.04 (23) | 0.18 ± 0.09 (22) | 1.41 ± 0.34 (2) | 2 |
| 18 | 0.10 ± 0.08c (10) | 0.36 ± 0.17f (15) | 1.29 ± 0.83i (7) | 3 | |
Steel-Dwass method (mean ± SD) a-b, b-c, d-e, e-f, g-h, h-i, a-d, d-g, a-g, c-f, f-i, c-i. A P value of <0.05 was considered significant. OCR × 1015 fmol/sec.
Estimation of oxygen consumption from nonvitrified cortical tissue
In group I cases, the mean OCRs of the follicles in each follicular developmental stage were 0.11± 0.13 × 1015 fmol/sec (primordial follicles; n = 71), 0.28 ± 0.28 × 1015 fmol/sec (primary follicles; n = 68) and 1.08 ± 0.13 × 1015 fmol/sec (secondary follicles; n = 31) in the 18-h transportation group. The mean OCR in individual stages increased with the follicular developmental stage, and there were significant differences between each stage (Table 1).
OCRs of pre-antral follicles in relation to age
In group I, 9 patients were categorized into 3 age groups (age category I, 20–29 years; category II, 30–36 years; and category III, ≥ 37 years). Of the 9 patients, 3 were categorized into category I, 4 were categorized into category II, and 2 were categorized into category III. The mean OCRs of pre-antral follicles in relation to patient age are presented in Table 2. There was a tendency for the mean OCRs of primary and secondary follicles to increase in the younger age groups; however, no correlation was found in each patient age group.
Table 2. OCRs of nonvitrified pre-antral follicles after 18 h of transportation in relation to age.
| Patient age (years) | 25–29 | 30–36 | ≥ 37 | ||
| (n) | 3 | 4 | 2 | ||
| OCR (× 1015 fmol/sec) | Primordial | 0.12 ± 0.11 | 0.12 ± 0.16 | 0.15 ± 0.13 | NS |
| 28 | 31 | 12 | |||
| Primary | 0.34 ± 0.36 | 0.32 ± 0.28 | 0.27 ± 0.17 | NS | |
| 30 | 27 | 11 | |||
| Secondary | 1.14 ± 0.12 | 1.10 ± 0.14 | 1.00 ± 0.16 | NS | |
| 11 | 10 | 10 |
Steel-Dwass method. NS: no significance.
OCRs of follicles in relation to vitrification and 24 h of incubation
In the 18-h transportation group, the mean OCRs at each developmental stage in group II were 0.02 ± 0.02 × 1015 fmol/sec (primordial; n=19), 0.03 ± 0.05 × 1015 fmol/sec (primary; n=18) and 0.05 ± 0.04 × 1015 fmol/sec (secondary; n=4). These levels were significantly decreased compared with the mean OCRs in group I. However, after 24 h of incubation (group III), the mean OCRs at each stage were 0.10 ± 0.08 × 1015 fmol/sec (primordial; n=10), 0.36 ± 0.17 × 1015 fmol/sec (primary; n=15) and 1.29 ± 0.83 × 1015 fmol/sec (secondary; n=7). The mean OCRs in this group were elevated after 24 h of incubation; they were equivalent to those of group I and were increased with follicular developmental stage (Table 1).
In the 6-h transportation group, the mean OCRs at each stage were 0.02 ± 0.02 × 1015 fmol/sec (primordial; n=10), 0.03 ± 0.03 × 1015 fmol/sec (primary; n=10) and 0.06 ± 0.04 × 1015 fmol/sec (secondary; n=2) after warming (group II). However, in group III, the mean OCRs at each stage were 0.07± 0.04 × 1015 fmol/sec (primordial; n= 23), 0.18 ± 0.09 × 1015 fmol/sec (primary; n=22) and 1.41 ± 0.34× 1015 fmol/sec (secondary; n=2) after 24 h of incubation (Table 1). These values were significantly higher than those of group II (P < 0.05; Table 1).
Although the mean OCRs of primordial and primary follicles in 18-h transportation samples of group III had a tendency to increase relative to those in the 6-h transportation samples of group III, no significant differences were observed in the mean OCRs in group II or group III between both transportation groups.
Fluorescence vital staining of pre-antral follicles
Dual fluorescence vital staining with the LIVE/DEAD Viability/Cytotoxicity Kit revealed intense green fluorescence (reflecting intracellular esterase activity) in 65 nonvitrified isolated follicles (20 primordial, 30 primary and 15 secondary) that consumed oxygen in the 18-h transportation samples of group I.
In the 18-h transportation samples of group III, all 9 primordial follicles and 13 primary follicles that consumed oxygen survived; however, the remaining primordial and primary follicles that did not consume oxygen showed bright red fluorescence (ethidium homodimer-I signal) in the oocyte and in >10% of the total GCs. Meanwhile, in the case of secondary follicles that consumed oxygen, intense green fluorescence was detected in oocytes of all 7 follicles; however, in 4 of the 7 secondary follicles, dead cells with bright red fluorescence were observed in varying proportions, from 24 to 45% of the total GC cells (Fig. 4, Table 3).
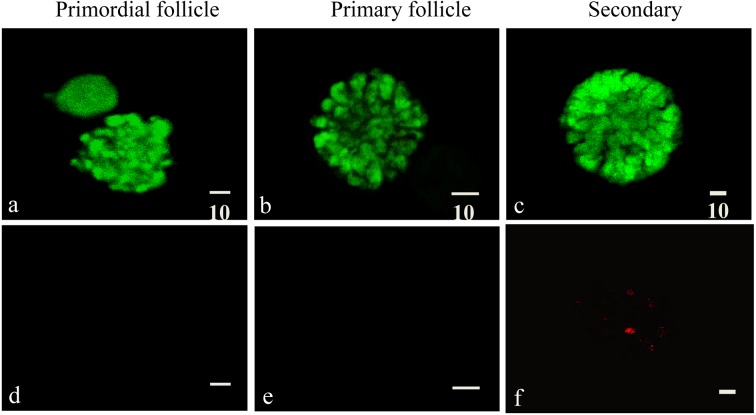
Fig. 4.
Dual fluorescence vital staining of vitrified-warmed pre-antral follicles in the 18-h transportation group. Intense green fluorescence was detected in the primordial (a), primary (b) and secondary (c) follicular granulosa cells (GCs) and oocyte. No red fluorescence was detected in the primordial (d) and primary follicles (e). (f) Red fluorescence was detected in a portion of GCs in the secondary follicles. The scale bars represent 10 μm. Calcein, 494/517 nm; ethmd-1, 494/635 nm.
Table 3. Results of dual fluorescence vital staining for nonvitrified and vitrified-warmed pre-antral follicles in the 18-h transportation group.
| No.of surviving follicles (%) |
Primordial (%) | Primary (%) | Secondary (%) | |
| Group I | Oocyte | 20/20 (100.0) | 30/30 (100.0) | 15/15 (100.0) |
| GCs | 20/20 (100.0) | 30/30 (100.0) | 15/15 (100.0) | |
| Group III | Oocyte | 9/9 (100.0) | 13/13 (100.0) | 7/7 (100.0) |
| GCs | 9/9 (100.0) | 13/13 (100.0) | 4/7 (57.1) | |
Surviving follicles: an intense green fluorescent signal (calcein) was detectable, but a red fluorescent signal (ethmd-1) was not.
Discussion
This preliminary study targeted 9 female-to-male transsexuals who were 33.0 ± 6.3 years of age (mean ± SD; range, 25–45 years) and had received androgen therapy at 12–84 months before oophorectomy. In this study, we demonstrated that human early pre-antral follicles consume oxygen. We isolated such follicles from nonvitrified and vitrified ovarian cortical tissue after long-term transportation. The OCR increased with developmental stage but did not seem to be affected by patient age or transportation duration in this confined patient group. Meanwhile, the OCR of vitrified follicles decreased immediately after warming but was comparable to that of nonvitrified follicles after 24 h of incubation. The OCRs further reflected the effect of vitrification on the viability of primordial and primary follicles.
OCR measured by using SECM has been reported to be an effective biomarker for the viability and developmental competence of vitrified human blastocysts. After warming, the OCR of a vitrified blastocyst was significantly lower than that of a nonvitrified blastocyst; however, after 6 h of incubation, the OCR recovered to the level exhibited by nonvitrified blastocysts. In parallel, mitochondrial cytochrome c oxidase activity was not observed immediately after warming but was detected after 24 h [45].
The balance of aerobic metabolism in human early pre-antral follicles is not well understood. In view of the literature, we first attempted to investigate the OCRs of human early pre-antral follicles isolated from nonvitrified cortical tissue after 18 h of transportation. These ovaries were in an ischemic state during transportation, and thus it was expected that pre-antral follicles might rely predominantly on glycolysis for ATP production owing to the Pasteur effect. However, oxygen consumption was detected in all developmental stages of early pre-antral follicles. Notably, oxygen consumption was observed in the primordial and primary follicles before the beginning of rapid follicular growth and steroidogenesis. This implies that both the tricarboxylic acid cycle and oxidative phosphorylation might be essential for the follicular growth from the primordial (transit) follicle stage to the primary follicle stage in humans.
We next investigated whether vitrification and warming influence the OCR of pre-antral follicles. Our results revealed that the mean OCRs of group II follicles in all developmental stages were low in both the 6-h and 18-h transportation groups. However, after 24 h of incubation, the mean OCRs were restored.
The viabilities of vitrified-warmed primordial and primary follicles that consumed oxygen were corroborated by the results of the LIVE/DEAD viability assay, wherein these follicles showed intense green fluorescence in both the oocytes and > 90% of the total GCs. In the case of vitrified-warmed secondary follicles that consumed oxygen, although the oocytes had a high survival rate in this assay, bright red fluorescence was detected in >10% of the total GCs in several follicles.
The OCRs recorded in group III were found to directly reflect the cellular viability of vitrified-warmed primordial and primary follicles with a GC monolayer compared with the secondary follicles. According to the data, the OCR increased with follicular developmental stage, and the most notable elevation of the mean OCR was detected in the secondary follicle stage. This could be attributed to the increasing number of GCs surrounding the oocytes and the onset of the steroidogenesis in GCs. There could be a greater difference in the number of GCs in individual secondary follicles. The discrepancy between the results of the 2 different assays suggests the necessity for further research investigating the OCR of secondary follicles of various qualities by using the LIVE/DEAD viability assay. Alternatively, pre-antral follicles that were isolated enzymatically had a sticky surface. To avoid contamination of the platinum microelectrode probe of CRAS-1.0 respirometer, it was ensured that the probe was not brought too close to the follicles during the measurement of OCR. It is possible that the OCRs were relatively attenuated in this study, and hence, further modification of the isolation protocol could contribute to estimating the viability of secondary follicles on the basis of their OCRs.
Meanwhile, the mean OCRs did not seem to be affected by patient age or transportation time in this confined patient group. However, the mean OCRs of primordial and primary follicles in the 18-h transportation group had a tendency to increase relative to that in the 6-h transportation group, and there is a tendency for the mean OCRs of primary and secondary follicles to increase in the younger age groups. More extensive investigations might be necessary to corroborate the relation between the mean OCRs of the isolated follicles and patient age or transportation time.
Autotransplantation of isolated follicles is an emerging technology with potential application as an alternative to cortical fragment transplantation in patients at risk of ovarian metastasis [7]. Primordial and primary follicles are highly cryoresistant, and therefore, they are the primary target tissues in the vitrification of human ovarian cortex tissue [8, 27, 46]. The data in this study show that OCR measurement could be an available method for selecting viable human primordial and primary follicles after vitrification.
In conclusion, in this preliminary study, we demonstrate that the OCR can be used for simple and direct estimation of the effect of vitrification on the viability of human primary and primordial follicles and that measurement of the OCR is a method available for selecting viable follicles from vitrified-warmed follicles. Although the necessity for observing vitrified pre-antral follicles with electron microscopy still remains, our results may contribute to improvement of the methods of vitrification of human ovarian tissue, in vitro culture systems for isolated early pre-antral follicles and use of isolated early pre-antral follicles for autotransplantation in the clinical setting in the future.
Acknowledgments
The authors are grateful to Dr M Yokoo (Akita Prefectural University) and Dr H Abe (Yamagata University) for excellent technical assistance.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2.Lamar CA, DeCherney AH. Fertility preservation: state of the science and future research directions. Fertil Steril 2009; 91: 316–319. [DOI] [PubMed] [Google Scholar]
- 3.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol 2005; 6: 209–218. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update 2006; 12: 519–535. [DOI] [PubMed] [Google Scholar]
- 5.Demeestere I, Simon P, Englert Y, Delbaere A. Preliminary experience of ovarian tissue cryopreservation procedure: alternatives, perspectives and feasibility. Reprod Biomed Online 2003; 7: 572–579. [DOI] [PubMed] [Google Scholar]
- 6.Isachenko V, Montag M, Isachenko E, van der Ven K, Dorn C, Roesing B, Braun F, Sadek F, van der Ven H. Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reprod Biomed Online 2006; 13: 228–234. [DOI] [PubMed] [Google Scholar]
- 7.Kim SS, Soules MR, Battaglia DE. Follicular development, ovulation, and corpus luteum formation in cryopreserved human ovarian tissue after xenotransplantation. Fertil Steril 2002; 78: 77–82. [DOI] [PubMed] [Google Scholar]
- 8.Eyden B, Radford J, Shalet SM, Thomas N, Brison DR, Lieberman BA. Ultrastructural preservation of ovarian cortical tissue cryopreserved in dimethylsulfoxide for subsequent transplantation into young female cancer patients. Ultrastruct Pathol 2004; 28: 239–245. [DOI] [PubMed] [Google Scholar]
- 9.Fabbri R, Venturoli S, D’Errico A, Iannascoli C, Gabusi E, Valeri B, Seracchioli R, Grigioni WF. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol 2003; 89: 259–266. [DOI] [PubMed] [Google Scholar]
- 10.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, Dor J.Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril 2007; 87: 418.e7–418e.15. [DOI] [PubMed] [Google Scholar]
- 11.Newton H, Fisher J, Arnold JR, Pegg DE, Faddy MJ, Gosden RG. Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod 1998; 13: 376–380. [DOI] [PubMed] [Google Scholar]
- 12.Dolmans MM, Jadoul P, Gilliaux S, Amorim CA, Luyckx V, Squifflet J, Donnez J, Van Langendonckt A. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet 2013; 30: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013; 110: 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeoman RR, Wolf DP, Lee DM. Coculture of monkey ovarian tissue increases survival after vitrification and slow-rate freezing. Fertil Steril 2005; 83(Suppl 1): 1248–1254. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki N, Hashimoto S, Igarashi S, Takae S, Yamanaka M, Yamochi T, Takenoshita M, Hosoi Y, Morimoto Y, Ishizuka B. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod 2012; 27: 2420–2429. [DOI] [PubMed] [Google Scholar]
- 16.Haidari K, Salehnia M, Rezazadeh Valojerdi M. The effect of leukemia inhibitory factor and coculture on the in vitro maturation and ultrastructure of vitrified and nonvitrified isolated mouse preantral follicles. Fertil Steril 2008; 90: 2389–2397. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogenology 2000; 53: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 18.Courbiere B, Odagescu V, Baudot A, Massardier J, Mazoyer C, Salle B, Lornage J. Cryopreservation of the ovary by vitrification as an alternative to slow-cooling protocols. Fertil Steril 2006; 86(Suppl): 1243–1251. [DOI] [PubMed] [Google Scholar]
- 19.Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril 2006; 85(Suppl 1): 1150–1156. [DOI] [PubMed] [Google Scholar]
- 20.Isachenko V, Isachenko E, Rahimi G, Krivokharchenko A, Alabart JL, Nawroth F. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen: negative effect of disaccharides in vitrification solution. Cryo Lett 2002; 23: 333–344. [PubMed] [Google Scholar]
- 21.Isachenko E, Isachenko V, Rahimi G, Nawroth F. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Eur J Obstet Gynecol Reprod Biol 2003; 108: 186–193. [DOI] [PubMed] [Google Scholar]
- 22.Isachenko E, Isachenko V, Nawroth F, Rahimi G, Kreienberg R, Reinsberg J, Weiss J. Human ovarian tissue preservation: is vitrification acceptable method for assisted reproduction? Cryo Lett 2008; 29: 301–314. [PubMed] [Google Scholar]
- 23.Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss JM. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction 2009; 138: 319–327. [DOI] [PubMed] [Google Scholar]
- 24.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online 2009; 18: 568–577. [DOI] [PubMed] [Google Scholar]
- 25.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod 2009; 24: 1670–1683. [DOI] [PubMed] [Google Scholar]
- 26.Li YB, Zhou CQ, Yang GF, Wang Q, Dong Y. Modified vitrification method for cryopreservation of human ovarian tissues. Chin Med J (Engl) 2007; 120: 110–114. [PubMed] [Google Scholar]
- 27.Shaw JM, Cox SL, Trounson AO, Jenkin G. Evaluation of the long-term function of cryopreserved ovarian grafts in the mouse, implications for human applications. Mol Cell Endocrinol 2000; 161: 103–110. [DOI] [PubMed] [Google Scholar]
- 28.Shaw JM, Oranratnachai A, Trounson AO. Fundamental cryobiology of mammalian oocytes and ovarian tissue. Theriogenology 2000; 53: 59–72. [DOI] [PubMed] [Google Scholar]
- 29.Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril 2001; 75: 141–146. [DOI] [PubMed] [Google Scholar]
- 30.Abir R, Biron-Shental T, Orvieto R, Garor R, Krissi H, Fisch B. Transplantation of frozen-thawed late-gestational-age human fetal ovaries into immunodeficient mice. Fertil Steril 2009; 92: 770–777. [DOI] [PubMed] [Google Scholar]
- 31.Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola SA, Donnez J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod 2006; 21: 413–420. [DOI] [PubMed] [Google Scholar]
- 32.Vanacker J, Camboni A, Dath C, Van Langendonckt A, Dolmans MM, Donnez J, Amorim CA. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril 2011; 96: 379–383: e3. [DOI] [PubMed] [Google Scholar]
- 33.Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod 1999; 14: 2519–2524. [DOI] [PubMed] [Google Scholar]
- 34.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008; 23: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 35.Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod 2009; 24: 92–99. [DOI] [PubMed] [Google Scholar]
- 36.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009; 24: 2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshi H. In vitro production of bovine embryos and their application for embryo transfer. Theriogenology 2003; 59: 675–685. [DOI] [PubMed] [Google Scholar]
- 38.Preis KA, Seidel GE, Jr, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev 2007; 74: 893–903. [DOI] [PubMed] [Google Scholar]
- 39.Tejera A, Herrero J, de Los Santos MJ, Garrido N, Ramsing N, Meseguer M. Oxygen consumption is a quality marker for human oocyte competence conditioned by ovarian stimulation regimens. Fertil Steril 2011; 96: 618–623: e2. [DOI] [PubMed] [Google Scholar]
- 40.Van Den Broecke R, Van Der Elst J, Liu J, Hovatta O, Dhont M. The female-to-male transsexual patient: a source of human ovarian cortical tissue for experimental use. Hum Reprod 2001; 16: 145–147. [DOI] [PubMed] [Google Scholar]
- 41.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, van der Ven H, Montag M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril 2012; 97: 387–390. [DOI] [PubMed] [Google Scholar]
- 42.Gougeon A, Chainy GBN. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil 1987; 81: 433–442. [DOI] [PubMed] [Google Scholar]
- 43.Shiku H, Shiraishi T, Ohya H, Matsue T, Abe H, Hoshi H, Kobayashi M. Oxygen consumption of single bovine embryos probed by scanning electrochemical microscopy. Anal Chem 2001; 73: 3751–3758. [DOI] [PubMed] [Google Scholar]
- 44.van den Hurk R, Spek ER, Hage WJ, Fair T, Ralph JH, Schotanus K. Ultrastructure and viability of isolated bovine preantral follicles. Hum Reprod Update 1998; 4: 833–841. [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka M, Hashimoto S, Amo A, Ito-Sasaki T, Abe H, Morimoto Y. Developmental assessment of human vitrified-warmed blastocysts based on oxygen consumption. Hum Reprod 2011; 26: 3366–3371. [DOI] [PubMed] [Google Scholar]
- 46.Dolmans MM, Yuan WY, Camboni A, Torre A, Van Langendonckt A, Martinez-Madrid B, Donnez J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online 2008; 16: 705–711. [DOI] [PubMed] [Google Scholar]