Abstract
Distinction between Crohn’s disease of the colon-rectum and ulcerative colitis or inflammatory bowel disease (IBD) type unclassified can be of pivotal importance for a tailored clinical management, as each entity often involves specific therapeutic strategies and prognosis. Nonetheless, no gold standard is available and the uncertainty of diagnosis may frequently lead to misclassification or repeated examinations. Hence, we have performed a literature search to address the problem of differential diagnosis in IBD colitis, revised current and emerging diagnostic tools and refined disease classification strategies. Nowadays, the differential diagnosis is an untangled issue, and the proper diagnosis cannot be reached in up to 10% of patients presenting with IBD colitis. This topic is receiving emerging attention, as medical therapies, surgical approaches and leading prognostic outcomes require more and more disease-specific strategies in IBD patients. The optimization of standard diagnostic approaches based on clinical features, biomarkers, radiology, endoscopy and histopathology appears to provide only marginal benefits. Conversely, emerging diagnostic techniques in the field of gastrointestinal endoscopy, molecular pathology, genetics, epigenetics, metabolomics and proteomics have already shown promising results. Novel advanced endoscopic imaging techniques and biomarkers can shed new light for the differential diagnosis of IBD, better reflecting diverse disease behaviors based on specific pathogenic pathways.
Keywords: Crohn’s disease, Ulcerative colitis, Inflammatory bowel disease unclassified, Advanced endoscopic imaging, Biomarkers, Histopathology
Core tip: Distinction between Crohn’s disease and ulcerative colitis or inflammatory bowel disease (IBD) type unclassified can be of pivotal importance for a tailored clinical management, as each entity often involves specific therapeutic strategies and prognosis. Nonetheless, the differential diagnosis is an untangled issue, and the proper diagnosis cannot be reached in up to 10% of patients presenting with colitis. Hence, we address the problem of differential diagnosis in IBD colitis, thereby revising current and emerging diagnostic tools to refine disease classification.
INTRODUCTION
Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders of the gastrointestinal system affecting more than 1 million people in the United States and several million worldwide[1-4]. Large-bowel involvement is common, and this may ensue from either ulcerative colitis (UC) or IBD type unclassified (IBDU), as well as from the subset of non-complicated Crohn’s disease (CD) with an isolated colonic localization[5,6]. Conversely, the disease spread in other gastrointestinal segments definitively leads to the diagnosis of CD. Differential diagnosis in patients presenting with IBD colitis is nowadays of pivotal importance for a tailored clinical management, as each entity involves specific therapeutic and coping strategies[7-10]. Nevertheless, non-classical forms of either UC or Crohn’s colitis still represent a remarkable clinical matter, as no single diagnostic gold standard is available yet[6-11]. Therefore, 5% to 15% of cases do not meet strict criteria for either UC or CD[12-15] and in up to 14% of patients classified as UC and CD the diagnosis changes over time[16-21].
In the last years, basic science data have outlined a more panoramic knowledge of these disorders, suggesting novel ways to achieve the diagnosis. Recent innovations in the field of bio-medical technology have led to new serologic biomarkers and endoscopic techniques focused on polishing our traditional approach to identify and characterize patients with IBD.
This review aims to update the problem of the differential diagnosis in patients with IBD colitis, providing an overview of current and emerging diagnostic tools that might improve clinical practice within the near future.
SIZE OF THE PROBLEM
The prevalence of IBD varies according to geographic region, showing a sharp gradient between Western industrialized and developing countries, as well as when comparing urban vs rural areas[1,2]. Studies exploring temporal trends have shown that the incidence of IBD continues to increase in many areas of the world, reporting higher growth rates in those regions that have become more industrialized[1-4,22]. Accordingly, IBDs are advisedly considered as emerging global diseases[23].
In prevalence studies, UC estimates ranged greatly from 4.9 to 505 per 100000 in Europe, 4.9 to 168.3 per 100000 in Asia and the Middle East, and 37.5 to 248.6 per 100000 in North America. CD estimates ranged from 0.6 to 322 per 100000 in Europe, 0.88 to 67.9 per 100000 in Asia and the Middle East, and 16.7 to 318.5 per 100000 in North America[1-4,22,23]. The differential diagnosis is relatively easy when CD involves different gastrointestinal tract areas or shows extraluminal complications such as strictures, abscesses or fistula. However, one-third of CD patients have a pure colonic location (“L 2”) and at least two-thirds a non-stricturing, non-penetrating behaviour at the time of diagnosis, thereby sharing many behaviors with UC[15,24-30]. Disease localization appears to be quite stable a trait of CD over time, showing spreading of the disease into the small bowel in only a few cases[24,25]. By contrast, in up to one-third of cases disease behaviour is expected to change during the next 20 years, consistent with the development of strictures and/or penetrating lesions[24-26,31-33]. Within ulcerative and Crohn’s colitis with long-term follow up, disease reclassification is close to 5%-14% and mainly driven by an increased proportion of CD in adults[16-21].
IBD restricted to the colon that cannot be allocated to the CD or UC category is best termed “inflammatory bowel disease unclassified”, while the term “indeterminate colitis” is now confined to operative specimens, as originally described by Price in 1978[5,34-36]. This condition has been associated with worse prognosis than UC because of the higher frequency of relapses[37], the increased risk of colon cancer[38] and less favorable outcomes after ileal pouch-anal anastomosis, especially among those who were positive or not sorted by serological status[39-42]. To date, very few data are available on the prevalence of IBDU. In adults, studies from European countries show that the prevalence varies between 3 and 7 per 100000 inhabitants[43]. Diagnosis of IBDU accounts for 5%-15% of new cases of IBD[12-15,43] with remarkable differences between pediatric and adult cohorts (5%-30% and 5%-12%, respectively)[11,12,44-46]. A recent meta-analysis of 32 studies investigating both pediatric (n = 14) and adult (n = 18) patients showed that 13% of children and 6% of adults with IBD are classified as IBDU; this difference was statistically relevant (P < 0.0001), thereby confirming that IBDU is significantly associated with earlier onset of IBD[44]. It is not clear whether the high frequency of IBDU observed in children represents an IBD phenotype associated with childhood disease onset or reflects the difficulties in establishing a defined diagnosis[26,44,45]. Actually, for most patients, IBDU represents a provisional diagnosis, as it has been estimated that 80% of them will be reclassified to either CD or UC within 8 years[11,12,42,46]. However, in a subset of cases, IBDU remains the most accurate diagnosis as a true separate clinical entity[36,41,43-45,47].
Consistent with the above-mentioned epidemiological data, we can roughly estimate that the differential diagnosis within IBD colitis involves over 1.5 million IBD subjects in Europe (1.4 million UC plus 200000 CD-L2-B1), and almost one million in North America (830000 UC plus 140000 CD-L2-B1). In approximately 10% of them, the assessment of a distinct diagnosis is an open issue variably leading to either the diagnosis of IBDU or further reclassification of the disease, as well as to unspecific and incorrect therapeutic management.
Accordingly, the next section focuses on the potential clinical relevance of the differential diagnosis, in an attempt to show “why” patients presenting with a non-distinct entity of IBD colitis may represent an untangled matter capable of remarkable impact on both the physician’s approach and the patient’s coping strategy.
CLINICAL RELEVANCE: WHEN THE DIFFERENTIAL DIAGNOSIS AFFECTS CLINICAL MANAGEMENT
During the last fifteen years, the development of biological therapies and a widespread use of immunomodulators have radically changed treatment strategy in IBD. Consistently, prognostic indexes focusing on the assessment of mucosal inflammation have become an important treatment goal predicting long-term outcomes in terms of disease progression, complications and steroid sparing[48-54]. In this context, the issue of differential diagnosis has received renewed attention, because both medical and surgical treatments imply more and more disease-specific strategies[7-10,34,54].
Medical therapies
Since the causes of IBD are complex and only partially understood, the current medical approaches for the treatment are based on drugs exerting a direct (e.g., corticosteroids and immunomodulators) or indirect (e.g., antibiotics) effect on the inflammatory cascade of either disease[55-57]. Accordingly, most drugs are approved for both UC and CD. However, medical treatments such as certolizumab pegol and natalizumab have been approved for CD only (FDA exclusively), while golimumab and calcineurin inhibitors (cyclosporine and tacrolimus) for UC only. Furthermore, disease-specific advantages and response rates have been shown using several medical treatments. For example, aminosalicylates are recommended as a first-line option for treating and maintaining remission in UC but, besides their possible chemopreventive properties, play a much more marginal role in the management of CD[7,8,58,59]. In contrast, methotrexate has shown a higher rate of response in CD than in UC[60-64]. As a matter of fact, the results of two separate phase 3, randomized, double-blind, placebo-controlled studies of the new monoclonal antibody vedolizumab, targeting the α4β7 integrin, have shown a substantially diverse efficacy in patients with UC vs CD[65].
In recent years, growing efforts have been made to characterize and dissect the pathogenic mechanisms within the inflammatory cascade of either diseases in order to identify new targets for disease-specific therapies[51,66-71]. Besides the possible effect of a better disease control, the use of such disease-specific and targeted therapies should reduce the rate of side effects related to the use of unspecific immunomodulating treatments (i.e., malignancies, infections and life-threatening allergic reactions), therewith supporting cost-saving policies[57,72-77]. Therefore, the importance of the distinction between CD and UC will remarkably grow, as new therapies with disease-specific targets will be available in clinical practice.
Finally, yet importantly, a firm diagnostic definition is usually mandatory to fit the inclusion criteria of clinical therapeutic trials, which often represent a possible alternative to surgery in patients with refractory IBD colitis.
Surgery treatments
The differential diagnosis between UC and CD is particularly relevant for surgical therapies, especially in the case of procto-colectomy. Restorative procto-colectomy with ileal-pouch-anal-anastomosis (IPAA) is the procedure of choice in fulminant or chronic, treatment-refractory UC and UC-associated colonic neoplasia[7,78]. The vast majority of UC patients undergoing IPAA do well[79-81] and patients with a long history of severe disease recurrences may even report an ameliorated health-related quality of life[82-88]. Previous conflicting studies have reported comparable[79,89] rather than worse[90-93] long-term outcome in IBDU and indeterminate colitis. On the contrary, up to 90% of CD patients with IPAA develop complications including perianal abscess/fistula, pouchitis or anal stricture and approximately 50% require pouch revision or diversion[79-81,88,94-97]. Accordingly, patients with UC and IBDU can benefit from the option of a restorative procto-colectomy with IPAA, thereby mitigating the impact of such a radical approach on coping strategy and health-related quality of life. By contrast, a restorative surgery is not recommended in all CD patients and total procto-colectomy frequently results in a permanent ileostomy. Nevertheless, the postsurgical reclassification of UC into CD is not rare, occurring in 3.5%-12% of prospective and retrospective series of procto-colectomized patients, even in referral IBD centres[19,79,81,90,98-101].
Secondly, the differential diagnosis is crucial for the treatment of non-fistulizing perianal diseases such as hemorrhoids, skin tags, anal fissures and ulcers. Data focused on the long-term outcomes of different treatment strategies in non-fistulizing perianal disorders are still lacking in IBD, and particularly in UC and IBDU patients. A study published in 1993 designed to define the outcome of surgery for symptomatic hemorrhoids and anal fissures in patients with known CD reported a substantial incidence of complications (2/17 and 3/22, respectively)[102]. Another study by Jeffery et al[103] showed that both surgical and conservative approaches for hemorrhoids are associated with low complication rates in UC, whereas in patients with CD the complication rate is high. Consistently, when non-fistulizing perianal diseases occur in CD patients, a conservative approach is recommended and efforts should be directed to achieve healing by means of both medical and less invasive surgical therapies[104-107].
Mucosal healing
Mucosal healing is nowadays a pivotal treatment goal beyond mere symptom control. Endoscopic remission is recognized as a primary end-point in clinical trials and an important prognostic factor[6,48-54]. For UC patients, mucosal healing represents a leading clinical outcome to assess response to therapy and to drive all decision-making processes before dose escalation, drug switching and withdrawal; on the contrary, the clinical relevance of mucosal healing in CD should be weighed against signs of transmural inflammation and extraluminal complications[48,51,54,108]. In addition, there are substantial differences between CD and UC in the effect of different drugs on mucosal healing[54,109-111]. Based on all above considerations, the differential diagnosis in IBD colitis has acquired growing interest, thereby endorsing the emerging attention for all diagnostic steps capable of reducing the number of unclassified diseases.
DIFFERENTIAL DIAGNOSIS: CURRENT APPROACH
IBD colitis may present with a variety of unspecific features turning the differential diagnosis among UC, CD and IBDU into one of the greatest diagnostic challenges in gastroenterology. As stated above, no single gold standard is available for the diagnosis and the distinction of IBD. The diagnosis rests on a multidisciplinary work-up, based on clinical evaluation integrated with a combination of endoscopic, histological, radiological, and/or biochemical investigations (Figure 1)[6-11].
Figure 1.
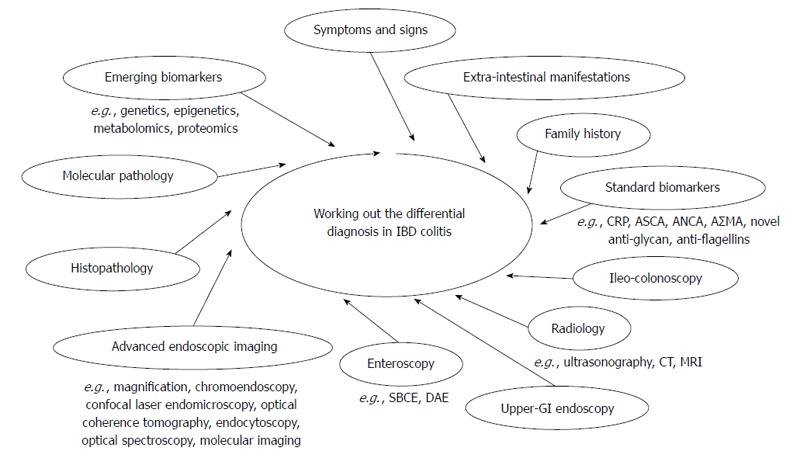
Differential diagnosis in inflammatory bowel disease colitis.
Symptoms and signs
IBD colitis-associated symptoms depend on several factors, including extent and severity of disease, as well as localization of the colonic involvement. Furthermore, superinfection with enteric pathogens and/or ongoing therapies may also alter the clinical presentation.
The main symptom of UC is visible blood in the stools, which is reported in more than 95% of active disease[112]. Rectal urgency, tenesmus and, occasionally, severe constipation represent the classical complaints of rectal involvement, while chronic diarrhea with nocturnal defecation and crampy abdominal pain are typical of left-sided or extensive UC[112].
Patients affected by Crohn’s colitis often present a UC-like clinical phenotype. Blood or muco-purulent exudates with the stool can be seen in up to 40% to 50% of patients with Crohn’s colitis, but less frequently than in UC subjects[113]. By contrast, chronic diarrhea is the most common presenting symptom in classical ileo-colonic CD, followed by abdominal pain and weight loss[34,114]. Consistent with the above clinical features, a nested, case-controlled study by Melmed et al[21] showed that UC patients with either non-bloody diarrhea or weight loss as onset symptoms have an increased likelihood of subsequent change in diagnosis to CD (100%) compared to those with none of these risk factors (16%, P < 0.001), and might thus warrant further diagnostic work-up.
Systemic symptoms such as fever, tachycardia and weight loss are usually restricted to severe and extensive disease in UC, but occur more frequently in CD, particularly in the case of severe relapse or transmural complication[34,112].
IBD colitis with persistent perianal and rectal complaints should raise the suspect of a perianal disease, which could occur in up to 10% of new onset and much more frequently during the clinical course of CD[34,105,106,115]. However, non-fistulizing, non-stricturing perianal disorders such as hemorrhoids, skin tags or anal fissures are also common in UC and IBDU, because of severe and prolonged diarrhea or as sporadic, unrelated disorders[112]. Bonheur et al[116] have recently characterized the anal skin tags of 169 consecutive IBD patients (62% CD) looking for distinctive morphological features of UC and CD. They found that 75% of anal skin tags “type 1”, previously called “elephant ears” and referred to as IBD, had CD (P = 0.005). Interestingly, this anal finding was also associated with the colonic involvement of CD (P = 0.067). By contrast, anal skin tags “type 2” showed no preference for any IBD type. This finding typically results from healed anal fissures, ulcers or hemorrhoids, and equally occurs in IBD and in non-IBD subjects[116].
Extra-intestinal manifestations
Patients with IBD may present a number of extra-intestinal manifestations (EIMs) involving most organ systems[117-121]. The probability of having EIMs increases with the duration of the intestinal disease and is generally higher in CD (24%-40%), especially Crohn’s colitis (47%), than in UC (26%-31%)[121,122]. Notably, many EIMs show disease-specific prevalence rates, thereby providing some aid in the differential diagnosis[30].
The most common EIM is represented by enteropathic arthritis. These peripheral and axial arthropathies have a lifetime prevalence of 11% in UC and 20% in CD, showing a peculiar tropism for the colonic location[28,120-122]. Peripheral arthropathies typically arise during intestinal relapses, sometimes accompanied by other EIMs such as erythema nodosum, which is similarly characterized by a higher prevalence in CD (8%-15% vs 3%-10% in UC), especially if the colon is involved[28,122-125].
Conversely, primary sclerosing cholangitis (PSC) occurs more frequently in UC (2%-4%) than in CD (1%-2%)[28,120,122,124]. This rare precancerous condition is more common in men than in women (2:1) and is unusual in non-IBD subjects (30% of cases). Most PSC patients have an associated IBD, particularly UC (77%). Notably, those with CD have colitis in 94% of cases[126].
Previous studies have also shown a slight predominance of uveitis and episcleritis in UC and Crohn’s colitis; these ocular disorders affect 3%-6% of subjects with IBD, mainly women (2:1), usually during the course of an intestinal relapse[120,122,125,127].
Other disorders positively correlated with the diagnosis of IBD such as metabolic osteopathy (22%-67%)[128-131], aphthous stomatitis (4%-30%)[28,132], asthma (7%-8%)[117], venous thromboembolism (1%-7%)[133-136], neurological disorders (3%)[137], pyoderma gangrenosum (1%-2%)[28,121,122,124], psoriasis (1%-2%)[117,138] and pulmonary disorders (< 0.5%-1%)[139] show variable or no disease-specific prevalence and cannot provide any reliable aid in the attempt to refine the differential diagnosis of IBD colitis.
Familial history
Several studies have firmly established that a family history positive for IBD is the strongest risk factor for the onset of these disorders, showing that disease pattern of relatives with a previous diagnosis of IBD may provide valuable information to predict both phenotype and course of new cases[140-145]. The lifetime risk of developing IBD has been estimated to be 5% for first-degree relatives of a CD subject and 1.6% for first-degree relatives with a UC proband, suggesting that genetic/environmental influences are stronger in CD than in UC[144].
The clinical pattern within familial cases of IBD presents high rates of concordance for both disease diagnosis (75%-83%) and localization (64%-33%), particularly in families with CD[144-146]. Consistently, a longitudinal study based on UC patients after IPAA has shown that a family history positive for CD has a significant influence on the development of CD after surgery, thereby recommending a deeper initial diagnostic work-up[101]. Thus, a familial history positive for UC, and particularly for CD, might serve as a clue for the final diagnosis in patients presenting with IBD colitis and no specific features.
Biomarkers
The main roles of biomarkers in IBD include support in making the diagnosis, prediction of further development of CD and UC in IBDU patients, determination of disease activity, risk stratification and prediction of response to therapy[52,147,148].
C-reactive protein (CRP) is a serum marker of acute phase (half-life about 19 h) produced by the liver in response to a variety of inflammatory conditions[148]. During active IBD, levels can range from 5-200 mg/L depending on disease severity, extent, and several variables affecting an individual’s capacity to produce CRP such as nutritional status, body mass index, liver function and gene polymorphisms[148,149]. CRP is used to distinguish organic from functional disorders, active from quiescent IBD, and response to therapy, but is poorly capable of differentiating CD from UC[110,150,151]. In fact, elevations in CRP are much more common (approximately 80% vs 50%) and sensitive in active CD than in UC, probably because of the deeper bowel inflammation and higher serum IL-6 levels[147,152-154]. Despite this relatively low sensitivity in UC, previous studies have shown that CRP level corresponds with both disease extent and severity, thereby showing that most UC patient with increased CRP have a moderate to severe flare exceeding the left flexure[154-156]. Rodgers et al[156] have prospectively evaluated the correlation between CRP and clinical/endoscopic activity in patients with IBD. CRP was elevated in 14%, 42%, 64%, and 83% of UC and in 54%, 70%, 75%, and 100% of CD subjects with quiescent, mild, moderate, and severe disease, respectively. A linear correlation of log(CRP) with clinical score was found for all UC extents except for proctitis, thereby confirming that CRP can broadly correlate with clinical activity and disease extent in UC[156].
Fecal biomarkers consist of proteins, leukocyte products, and leukocytes leaking from a permeable mucosa, thereby reflecting mucosal acute cellular infiltration[148]. 111Indium-labeled leukocytes, calprotectin, lactoferrin, PMN-elastase, neopterin and S100A12 proteins have been implemented in IBD to exclude functional disorders, assess the presence, the extent and the degree of gut mucosal inflammation, monitor the response to therapy and drive the timing of endoscopic controls[147,148,157-160]. Previous studies have shown that both calprotectin and lactoferrin perform better than CRP in predicting active disease at endoscopy, showing 70% to 100% sensitivity and 44% to 100% specificity depending on the cut-off value[147,157,158]. Fecal biomarker sensitivity is higher in colonic disease compared to ileal involvement[161,162] and is more influenced by endoscopic severity than by disease extent[163]. Recent data suggest that fecal calprotectin correlates more closely with endoscopic and symptom scores in UC than in CD, even excluding patients with pure ileal CD[157,158]. However, none of the available fecal biomarkers can be considered useful to refine the differential diagnosis in subjects with IBD colitis.
Various immunologic biomarkers have been used individually and as panels in attempts to improve the diagnosis of IBD, to identify homogeneous phenotypes, and to distinguish CD from UC[39,147]. Current serologic tests mainly include antibodies recognizing self-antigens or cross-reacting with several bacterial and fungal antigens. The existence of these antibodies in IBD patients often precedes the disease onset[164,165] and highlights the abnormal immune response to commensal enteric microorganisms, possibly reflecting diverse genetic predispositions and pathogenic mechanisms[40,166-170].
Perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA) were the first available and utilized in the setting of IBD. It is noteworthy that the performance of conventional ASCA IgG/IgA and the more recently studied gASCA IgG/IgA (antibodies against covalently immobilized mannan) tests[171] are comparable for CD diagnosis[39,170,172]. Therefore, in this review the term “ASCA” will refer to the conventional test, as well as to gASCA. Increased titers of ASCA identify CD patients with high specificity (85%-100%) and moderate sensitivity (approximately 39%-76%), characterizing definite disease phenotypes, such as those with ileal involvement (L1, L3), young age at onset, stricturing or penetrating behaviour and multiple bowel surgery[39,173-175]. In contrast, pANCA are more common in patients with UC (20%-85%) than in CD (2%-28%), but not nearly as sporadic in Crohn’s pancolitis[39,173,176] as in other forms of colitis, such as eosinophilic and collagenous colitis[177,178]. According to a meta-analysis of 60 studies based on 7860 IBD subjects and 3748 controls published in 2006, the ASCA+/pANCA- tests distinguished adults affected by CD from those diagnosed as UC with 55% sensitivity and 93% specificity, while the combination pANCA+/ASCA- performed particularly well only in pediatric UC patients (70% sensitivity and 93% specificity)[179]. However, when the evaluation has been restricted to patients with colonic disease, ASCA sensitivity for CD and pANCA specificity for UC decrease, resulting in a pANCA/ASCA test that discriminates less well between CD and UC[173,175,179,180]. This figure, together with a suboptimal sensitivity, hampers the contribution of such serological markers in clinical practice[39,41,181].
Addition of other serological markers to ASCA and pANCA, such as antibody to Escherichia coli outer membrane porin (anti-OmpC), bacterial flagellin (anti-CBir1) and Pseudomonas fluorescens (anti-I2), has been shown to provide only a marginal benefit[182-184]. As a matter of fact, the above-mentioned nested, case-control study by Melmed et al[21] clearly demonstrated that weight loss and non-bloody diarrhea at diagnosis but not ASCA, pANCA, anti-OmpC, anti-CBir1 and anti-I2 were predictive of a change in diagnosis from UC to CD. Accordingly, new serologic tests have been recently evaluated in the attempt to better assess the differential diagnosis in IBD[39,176]. Novel antiglycan antibodies such as anti-chitobioside carbohydrate antibodies, anti-laminaribioside carbohydrate antibodies, anti-mannobioside carbohydrate antibodies, anti-chitin IgA (Anti-C) and anti-laminarin IgA (Anti-L) have been found positive in at least one third of CD patients negative for ASCA, but their overall sensitivity in CD was low (respectively, 9%-27%, 20%-26%, 12%-27%, 10%-25% and 17%-26%), especially among those with colonic disease[170,171,176,185-187]. Similarly, anti-synthetic mannoside antibodies (AΣMA) showed lower sensitivity in CD compared to ASCA (38% vs 55%). Nonetheless, AΣMA were seen in 24% of CD patients who were negative for ASCA and were closely associated with colonic involvement (OR = 1.61, 95%CI: 1.03-2.51, P = 0.03)[175]. In a recent study by Schoepfer et al[188], serum reactivity towards two new anti-flagellins, anti-A4-Fla2 and anti-Fla-X, was found in 59% and 57% of CD patients as compared to only 6% of UC patients, thereby suggesting a possible role in distinguishing CD from UC.
The need for such serologic tests would be maximal in patients with IBDU and indeterminate colitis. One European, multicenter, follow-up study found that nearly half (48.5%) of the patients with IBD type unclassified had negative ASCA and pANCA assays and that most of them continued to have clinical characteristics precluding a final diagnosis of CD or UC over a mean disease duration of 10.7 years[41]. Positive results from ASCA or pANCA were predictive of the later development of CD and UC in 44% of patients after a mean follow-up period of 6 years. Among 26 IBDU patients with ASCA+/pANCA- result at baseline, 31% were later diagnosed as CD and 8% as UC, while ASCA-/pANCA+ corresponded to the diagnosis of CD and UC in 20% and 35% of cases out of 20 positive patients at baseline. Comparable results were also found in other studies[173,189]. Addition of tests for I2 and anti-OmpC beyond ASCA and pANCA does not appreciably increase the ability to categorize the type of IBD within unclassified colitis, with only a marginal increase in the predictive capacity and a substantial decrease in the specificity[190]. In an historical cohort of 20 patients with a well-established diagnosis of IBD type unclassified, AΣMA were found to predict development of CD with higher sensitivity (45% vs 27%) and specificity (100% vs 71%) than ASCA, thereby confirming their peculiar linkage with CD “L2” location[175].
Among IBDU and UC patients undergoing procto-colectomy with restorative IPAA, ASCA, anti-L, anti-OmpC and anti-CBir1 positivity before surgery was associated with an increased risk of CD of the pouch and post-operative fistula[39,40,101]. Conversely, high rates of chronic pouchitis have been often reported in IBDU and UC with positive serologic profile (ASCA, high pANCA titer, anti-OmpC/anti-I2), but in this case, serological positivity could reflect a more aggressive phenotype in lieu of a different diagnosis[39,190-192]. To the best of our knowledge, solid pieces of evidence on novel antiglycan antibodies and new anti-flagellin performance in IBDU/IC subjects are still lacking.
In summary, serological tests provide additional clues in differentiating CD from UC; although there is substantial heterogeneity of antibody reactivity in both diseases, this often represents a double-edged sword. A positive predictive value ≥ 85% has been suggested to be clinically relevant to differentiate between CD and UC[193]. However, the above-mentioned data show that this criterion is not always achieved using the available serologic markers and that the negative predictive value is lower. The use of large serologic panels including ASCA, pANCA and the above-mentioned novel serologic tests (e.g., AΣMA, anti-A4-Fla2 or anti-Fla-X) might enhance testing accuracy, especially among patients with UC-like phenotype, for whom the diagnostic question is most relevant and either sensitivity or specificity based on ASCA/pANCA test is lower. At the end of the day, beyond histopathological evaluation based on large surgical colorectal specimens, the serological studies are probably the most useful tool to determine the diagnosis of UC or CD from those with IBDU and indeterminate colitis, as well as to predict surgical outcome after IPAA.
Cross-sectional imaging
Cross-sectional imaging can detect and stage inflammatory, obstructive and fistulizing CD and is fundamental at first diagnosis to stage disease and to monitor follow-up[194]. Several complementary tools such as ultrasonography, computed tomography, magnetic resonance imaging and barium contrast radiology are helpful to detect small-bowel inflammation and lesions indicating the diagnosis of CD[195]. Furthermore, cross-sectional imaging generally precedes endoscopic examination in the small bowel[196]. The decision on which radiological technique should be performed depends on several factors such as patient features, nature and location of the small-bowel lesion, as well as local availability and expertise of the examiners[194]. Apart from detection of pathognomonic lesion of the small bowel, cross-sectional imaging techniques have the potential to assess the thickness of the colonic wall and its different layers to allow differentiation of CD from UC. Trans-abdominal ultrasonography is able to image inflamed and non-inflamed colonic wall with high accuracy using a total wall thickness threshold > 3 mm[197]. In addition, emerging evidence suggests that magnetic resonance colonography has a high accuracy for diagnosis of disease activity, location, and severity in IBD, as well as for penetrating and stricturing lesions, characteristic of CD[198,199]. However, no published studies based on trans-abdominal ultrasonography or magnetic resonance imaging have shown different wall layers capable of making the diagnosis between CD and UC. Very recently, a prospective endoscopic-ultrasonography study was able to distinguish between active UC and active CD using a forward-viewing radial echoendoscope in the sigmoid colon, hereby assessing the amount of thickening of each single wall layer in combination with absence/presence of paracolonic lymph nodes[200]. Even if this study could not differentiate IBD in remission from healthy controls, endoscopic-ultrasonography appears as a promising and safe technique, ready for a wider distribution in IBD clinical practice.
Ileo-colonoscopy
It has been known for a long time that ileo-colonoscopy is the exam of choice to achieve the diagnosis in patients with suspected IBD[6,11,34,45,112,201,202]. Typical forms of CD and UC have several endoscopic disease-specific features, which orient towards the proper diagnosis with high confidence in at least three quarters of cases[6,11,34,45,112]. Inspection of the terminal ileum is sufficient to confirm the diagnosis of CD in most cases. In addition, Crohn’s colitis shows a discontinuous pattern of inflammation, anal lesions and cobblestoning. Uninvolved areas frequently separate diseased segments and transition is usually abrupt (“skip lesions”). Deep linear ulcers, “absolute” rectal sparing and strictures are also typical, but not specific for CD[6,11,34,45]. By contrast, classical UC has a diffuse and continuous inflammation extending proximally from the rectum sometimes up to the ileo-cecal valve; UC endoscopic findings include granularity (sandpaper appearance to the mucosa), friability (bleeding of the mucosa to light touch), erosions and small superficial ulcers overlapped on a background of diffuse inflammation[6,45,112]. Based on these features, the accuracy of ileo-colonoscopy was found to be very high in expert hands (89%), the rate of unclassified disease low (7%) and the misdiagnosis very rare (4%) and mainly related to severe inflammatory activity (9%)[203]. Pera et al[203] noticed that the most useful endoscopic features for the differential diagnosis in IBD colitis are discontinuous involvement, anal lesions, and cobblestoning of mucosa in CD, and erosions or microulcers and granularity for UC.
However, other studies have described various degrees of heterogeneous endoscopic findings associated with both CD and UC, pointing out remarkable rates of atypical features compared to the established literature[6]. For instance, a mild and localized involvement of terminal ileum, known as “backwash ileitis” has been seen in up to 20% of extensive UC[204,205]. Similarly, slight signs of discontinuous peri-appendiceal inflammation (“cecal patch”) were reported in up to 75% of distal UC[206-209]. In the peculiar setting of primary sclerosing cholangitis, UC has been depicted without the classical distal gradient of inflammation or even with a more severe involvement of the right colon[210]. In addition, “relative” rectal sparing and ‘‘patchy colitis’’ have been reported in up to 20%-40% of untreated UC children[211-214], as well as in up to 10%-30% of adults receiving topical or systemic treatment[215-217]. Finally, tissue repairing can induce fibrotic changes within the mucosal and submucosal layers, which increase wall stiffness and mould benign luminal narrowing detectable in approximately 10% of longstanding UC[218]. Consistent with these atypical findings, 10%-20% of ileo-colonoscopy reveals an unspecific IBD pattern of colitis, thereby depicting an incomplete frame to correctly address both further histopathological analysis and clinical work-up.
Histopathology of terminal ileum and colorectal specimens
Histology based on at least two specimens separately collected from at least five sites along the colon, including the rectum, and the terminal ileum during ileo-colonoscopy and stored in separate jars is recognized as the gold standard to confirm both diagnosis and disease subclassification[6,11,34,112,219]. Colorectal mucosal histological abnormalities concerning architectural, epithelial and inflammatory changes characterized by discontinuity or variable degree in different ileo-colonic segments, focal or patchy cryptitis and lamina propria chronic inflammation with relative mucin preservation are in favor of CD. By contrast, Paneth cell metaplasia in distal colon, severe mucin depletion, heavy and widespread cell infiltration, distorted and atrophic crypts or surface erosions are more suggestive of UC[220,221].
Epithelioid well-formed, isolated, non-caseating granuloma unrelated to crypt injury (i.e., basally oriented in the mucosa and distant from crypts) is the most reliable discriminant for distinguishing CD from UC[11,219-222]. Out of the above-mentioned detailed statements, granuloma specificity decrease and other granulomatous conditions such as tuberculosis[223,224], Yersinia[225,226] acute bacterial enterocolitis[227-231], complicated diverticular disease[232-235], diversion colitis[235,236], sarcoidosis[223] and even UC[235,237-239] should be ruled out before confirming the diagnosis. We know from previous studies that colorectal granuloma is present in approximately 30% of adults and in 40% of children affected by CD[240,241] with a great variability in the literature ranging from 7% to 100% for surgical specimens[232,240]. However, in ileo-colonic biopsies, granuloma detection is quite unusual, being reported in only 13%-30% of CD[240,242]. Therefore, even when a diagnosis of IBD is made confidently, histopathological classification as UC or CD can be an ordeal because of the absence of specific microscopic features (i.e., non-cryptolytic granulomas), as well as for the presence of some exceptions occurring in UC such as discontinuous changes (i.e., cecal patch, skip lesion in appendiceal UC, uneven distribution in children and longstanding and treated UC, refractory and fulminant colitis), focal ileal inflammation (with or without macroscopic evidence of backwash ileitis) and relative rectal sparing (e.g., in children or treated UC)[11,36,219-221,230,239,243]. Additional histopathological features of CD useful for the differential diagnosis lie in the submucosal or in the transmural layer (e.g., transmural lymphoid aggregates, granulomas in the subserosa, and submucosal nerve fiber hyperplasia with ganglionitis)[11,34,220]. However, all these lesions mainly reside in the deeper layers of the bowel wall and are not reachable by endoscopic biopsies. Nevertheless, even when large colectomy specimens are available, indeterminate colitis still represents the most reliable diagnosis in between 5% and 20% of cases[36]. Therefore, one may envision that beyond conventional H&E-stained slides and additional immunohistochemistry, new molecular techniques should be used in order to refine histopathology accuracy[244]. In a recent study, Yantiss et al[245] assessed the expression of two monoclonal antibodies directed against an unknown colonic epithelial protein (Das-1) and human tropomyosin isoform-5 (CG-3) in colonic biopsy specimens from patients with colitis (25 UC, 15 CD, 15 lymphocytic, 15 collagenous, 15 ischemic) and in 10 normal controls. The results showed that suppression of Das-1 staining occurs more frequently in UC (96%) compared with CD (20%), lymphocytic (20%), collagenous (13%), and ischemic colitis (0%) cases, as well as controls (10%, P < 0.001 for all comparisons). In addition, CG-3 positivity in crypt epithelium was significantly more common in UC (52%) compared with all other groups (P ≤ 0.02 for all comparisons)[245]. These data suggest that DAS-1/CG-3 molecular staining might be useful in distinguishing UC from CD and from other colitidies. This innovation represents a possible step towards the extension of IBD histopathology into the molecular realm adding to, although not replacing, current gold standards.
Upper endoscopy
Esophagogastroduodenoscopy (EGD) is aimed at integrating the diagnostic work-up of IBD patients with suspected CD, upper gastrointestinal symptoms, anemia and signs of malabsorption[6,34,45,112,246]. The involvement of the upper gastrointestinal tract has been traditionally considered as a hallmark for CD[6,11,45,219,246-248]. Accordingly, classical lesions suggesting CD involvement such as stenosis, fistula, ulcers, erosions and aphthous lesions have been reported with pertinent microscopic features (e.g., focal chronic inflammation, focal crypt irregularity, irregular villous architecture, and epithelioid granulomas) in 1%-16% of symptomatic adult CD[247-252] and in 22%-53% of children[26,214,241,253,254]. Similarly, the chance to detect non-caseating, epithelioid granulomas in esophagus, stomach and proximal duodenum is quite low in adult series (0, 2%-20%)[255-258], while substantial in children (11%-43%)[26,45,259-267]. In 2%-21% of children with new onset CD, the diagnosis relies on the detection of granulomas at EGD[259-264], while no study addresses this issue in adults, in whom this percentage is expected to be much lower. Notably, even if granuloma is touted to represent the most reliable hallmark of upper CD, it is not diagnostic per se, since many other gastrointestinal disorders such as Helicobacter pylori infection, tuberculosis, gastric adenocarcinoma and sarcoidosis can lead to granulomatous lesions[219,231,257,268,269]. Random biopsies upon apparently normal mucosa should be collected from each site (e.g., duodenum, antrum, corpus, fundus, esophagus) using separate jars with the foresight to sample esophageal mucosa at least 2 cm above the Z-line to exclude inflammatory changes caused by gastro-esophageal reflux disease. Recent studies have shown that absence of specific symptoms does not preclude proximal lesions in CD populations, especially in young patients and in ileo-colonic diseases[26,34,253,258-260,262]. Conversely, “L2” locations are less likely to have an upper involvement or a stricturing disease[241].
In contrast with common beliefs, several studies have shown that in UC and also IBDU, upper gastrointestinal lesions are common and EGD findings more frequent than in healthy controls[265,270-278]. Studies based mostly on asymptomatic subjects and pediatric cohorts report a considerable prevalence of mild macroscopic gastro-duodenal lesions[214,247,254,271,277] and microscopic features pertinent with IBD (i.e., crypt distortion, focal cryptitides, crypt abscess, patchy distribution of inflammatory changes)[45,246,248,255,259,260,262,265,267,270,277,279,280] in Helicobacter pylori-negative subjects with either UC (4%-50% and 38%-70%, respectively) or CD (20%-75% and 60%-90%, respectively). However, there is no consensus concerning the definitions of what qualifies as significant involvement of IBD in the upper gastrointestinal tract and evidence of clinical correlates is lacking[246-248].
Many recent trials have directed attention to focally enhanced gastritis (FEG), a perifoveolar/periglandular mononuclear (typically CD3+ lymphocytes and CD68R+ histiocytes) and neutrophilic infiltrate around gastric crypts of the antrum and angulus in Helicobacter pylori-negative subjects, which corresponds to no or mild signs of mucosal inflammation at standard white-light endoscopy[255,270,281]. These histological features are common in both adults and child patients with CD and in those with limited colonic location (43%-76%)[219,255,258,265,270,275,282]. Further data have shown that FEG may affect also IBDU (18%-33%) and UC (8%-24%) subjects [45,258,265,273-275,282], whereby indicating that FEG may serve as a marker of IBD compared to the general population (2%-19%)[265,273-275] rather than reliably distinguishing between patients with CD and UC[274,275].
Two retrospective studies based on CD and UC subjects and negative controls have recently suggested that duodenal focal cryptitis and lymphocytic esophagitis are also common in children with IBD, and particularly in CD[265,272]. Data assessing the prevalence of duodenal focal cryptitis and lymphocytic esophagitis in adults are still lacking. Indeed, most of the macroscopic and microscopic findings observed in the upper gastrointestinal tract of asymptomatic IBD subjects are nonspecific and not helpful in discriminating CD from UC[246]. We believe that at present it is more convenient to consider every significant upper gastrointestinal involvement relating to IBD as a clue to the underlying diagnosis of CD, rather than as an atypical manifestation of UC and IBDU.
In conclusion, the value of upper endoscopy and related findings is still a topic of debate. Currently, EGD is recognized as part of the initial evaluation of children with suspected IBD and a valuable additional tool among adults presenting with either suspected CD or upper gastrointestinal symptoms[6,34,45,112,246,266]. Nevertheless, endoscopic and histopathological assessment of the upper gastrointestinal tract seems to have a limited impact for the differential diagnosis in asymptomatic adults with IBD colitis[6].
Small-bowel endoscopy
IBD patients who present symptoms and endoscopic features consistent with colitis may show small-bowel and upper GI lesions as proof of an underlying CD[196,283]. Therefore, cross-sectional imaging and small-bowel endoscopy are as useful to confirm the diagnosis in suspected CD as to exclude it in patients diagnosed with UC and IBDU. Consistent with an international consensus, cross-sectional imaging should generally precede enteroscopic examination[194,196]. Conversely, agreement on whether small-bowel capsule endoscopy (SBCE), rather than device-assisted enteroscopy, should be performed has not been completely achieved and the best choice is subject to the nature and location of the lesion, as well as to the availability of the technique and to local expertise[194-196,200]. A consensus group met in 2008 and concluded that in patients with suspected small-bowel CD with no obstructive symptoms, capsule endoscopy is an effective and smart strategy[196] given the high rate of complete small-bowel evaluations and the high negative predictive value[195,283-289]. The use of SBCE has been better defined in established CD patients with inconclusive results after conventional endoscopy and radiology[195,196,289-292].
However, SBCE has several practical limitations among subjects with either suspected or definite diagnosis of IBD. Firstly, trials focused on SBCE are mostly based on a non-validated, arbitrary score setting the finding of at least three ulcers as diagnostic of CD[6,293]. Secondly, small-bowel CD, NSAID-induced enteropathy, mycophenolate mofetil-induced enteropathy, tuberculosis, Behçet’s disease, vasculitis, ischemia and lesions of unknown clinical significance, which are reported in up to 20% of healthy subjects, may share a nonspecific appearance at capsule endoscopy, thereby requiring further enteroscopy for biopsy sampling[195,196,289,294-299]. Thirdly, emerging evidence suggests that a negative SBCE does not exclude a future diagnosis of CD[196,300,301]. In addition, a recent study based on SBCE and CD patients variably affected by abdominal pain and/or diarrhea (45%), increased number of soft stools (39%), anemia (7%), or asymptomatic (9%) has shown that jejunal involvement is less common in Crohn’s colitis (12%) compared to ileo-colonic diseases (38%)[283]. This evidence seems to lead to reconsideration of the impact of SBCE assessment in the subset of CD patients with isolated colitis, thereby shrinking the value of deep enteroscopy for those IBD patients with an unclear diagnosis.
In UC and IBDU, evidence assessing the diagnostic value of small-bowel endoscopy is still limited and mostly based on SBCE. Recent trials have revealed that minute superficial lesions (“reddish lesions and erosions”) are common among UC subjects (20%-57%). However, the findings revealed by capsule endoscopy were consistent with the diagnosis of CD only in a small group of UC (0%-16%)[302-305]. By contrast, the amount of small-bowel CD appeared substantial in IBDU (16%-43%), with the highest rates in pediatric subjects, symptomatic CD or refractory IBD colitis[300,303,306,307].
Taken together, these initial data reveal that small-bowel endoscopic assessment has a limited impact in IBD subjects with colonic involvement. Both SBCE and device-assisted enteroscopy are useful in patients with known or suspected CD but can also aid in the diagnosis of IBDU or UC in the case of crucial choices[6,195]. Currently, there is no clear evidence to recommend the use of deep enteroscopy as a standard diagnostic tool for the differential diagnosis in IBD colitis.
EMERGING AND FUTURE PERSPECTIVES
Advanced endoscopic imaging
Recent innovations in gastrointestinal endoscopy have changed our traditional approach to diagnosis in patients with IBD[195,308-310]. Emerging endoscopic imaging techniques such as high-definition[311-313], magnification[314-316] and dye-less chromoendoscopy[317,318] enable a detailed visualization of mucosal surface architecture and vascular pattern. Despite their potential in refining the differential diagnosis in subjects with suspected or established IBD, there is no evidence of clear advantages beyond standard white-light endoscopy or dye-based chromoendoscopy with either indigo carmine or methylene blue[318]. Conversely, the newly developed confocal laser endomicroscopy (CLE)[319-327] and endocytoscopy[328,329] allow for the characterization in “real time” of microscopic structures at a cellular and subcellular level, where disease specific features of both UC and CD are so far revealed only by histopathological analysis ex vivo.
Looking for confocal-aided signs of epithelial cell shedding in the terminal ileum, Liu et al[323] have shown that “gap density” is of value in distinguishing patients with IBD and healthy controls (61 vs 18 gaps/1000 cells, P < 0.001) but cannot easily differentiate patients with CD from those with UC (67 vs 61 gaps/1000 cells, P > 0.05). Recently, a study based on 79 consecutive IBD patients with a well-established diagnosis of CD (n = 40) and UC (n = 39) has shown that CLE with i.v. fluoresceine can clearly discriminate the presence of several disease-specific microscopic changes, which are conventionally used by standard histopathology to differentiate UC from CD. Based on such findings, the proposed CLE scoring system assessed with excellent accuracy (94%) the differential diagnosis blind to both clinical history and histopathological results, used as golden standards[330].
Compared with the single photon excitation performed by CLE, the newly introduced multiphoton microscopy has a superior effective resolution in thick tissue samples and an increased penetration depth without requiring exogenous fluorophores[331]. This resolution corresponds to images perfectly suited for 3D acquisition capable of reproducing the architecture of mucosal and superficial submucosal structures in detail. To the best of our knowledge, multiphoton microscopy has so far been tested in IBD tissue specimens only ex vivo[332].
Another novel advanced endoscopic technique, named “molecular imaging”, combines confocal imaging with exogenous fluorescently labeled probes to highlight in vivo specific microscopic changes on the basis of their molecular signature, thereby overcoming the limits of traditional morphological analysis to characterize either biochemical processes or molecular epitopes[333,334]. This technique has already shown “promising and immediate potential for translational science and prompt effects into clinical practice” in several fields of gastrointestinal endoscopy including IBD[333]. The possible applications of such a technique to improve the differential diagnosis in IBD colitis lies in identifying and developing proper molecular biomarkers. In the above-mentioned study by Yantiss et al[245], DAS-1/CG-3 molecular staining was found to be useful in distinguishing UC from CD and from other colitidies in histopathological colonic specimens ex vivo. Consistently, endoscopic molecular imaging has the potential to achieve comparable results in vivo combining the topical application of fluorescein-labeled DAS-1/CG-3 antibodies with CLE. Nonetheless, there is no published trial based on endoscopic molecular imaging techniques to address the matter of differential diagnosis in IBD.
Finally, several studies have recently shown the makings of another new advanced endoscopic technique based on the principle of contact light microscopy and known as endocytoscopy, for in vivo gastrointestinal microscopic imaging at a magnification up to 1390-fold[328,329,335-337]. In a pilot study, endocytoscopy was found to characterize and discriminate different inflammatory cells in the colonic mucosa of IBD patients with high accuracy, but its potential in the clinical setting is not yet established[329].
Optical coherence tomography (OCT or VLE: volumetric laser endomicroscopy) is a probe-based imaging technique, which allows for a resolution of 7-10 μm with an imaging depth of 2-3 mm[338-340]. The ability of OCT to scan multiple gastrointestinal wall layers can find utility in diagnosing the transmural inflammation of CD. In a prospective, blinded study based on 40 patients with CD and 30 with UC, Shen et al[341] described disrupted layered structure of the colonic wall, as a hallmark of transmural inflammation, with 90% sensitivity and 83% specificity for the diagnosis of CD. This study suggests that OCT may represent a valuable tool to distinguish CD from UC, especially when the differential hallmarks lie into the deepest mucosal layers and endoscopic biopsies are insufficient to assess transmural inflammation.
Optical spectroscopy is another intriguing advanced endoscopic imaging technique that includes fluorescence, reflectance and light-scattering spectroscopy and optical coherence tomography. Spectroscopy depends on the wavelength of the light source and on tissue characteristics[339]. Different light spectra backscattered between cells are specific for various diseases such as ischemia, inflammation, and malignancy. Accordingly, Raman spectroscopy has been recently proposed for distinguishing CD from UC in vitro in ex vivo tissue samples from IBD patients[342].
Taken together, current advanced endoscopic imaging techniques could offer an in vivo additional method to conventional histopathologic diagnosis. Nevertheless, further efforts are still required both in the clinical setting and in technological advancements before expanding their clinical application in differentiating UC from CD patients. There is great promise that molecular imaging will aid as a complementary tool to identify disease-specific features accurate for UC or CD at a molecular level; until then, the endoscopic diagnosis of IBD will have to rest exclusively on the morphological assessment of inflammatory changes.
Genetics
Over the last ten years, with the advent of genome-wide association studies (GWAS), a wealth of susceptibility loci have been discovered shedding some light into the knowledge of UC- and CD-specific genomic profiles[343,344]. In this respect, the recent imputation-based association analysis by Jostins and colleagues represents a milestone in the development of GWAS in IBD[345]. They have performed a meta-analysis based on 15 GWAS and ImmunoChip (Illumina, Inc, San Diego, CA) data of CD and/or UC, followed by extensive validation of significant findings, with a combined total of more than 75000 cases and controls. Seventy-one new associations were identified, expanding the number of confirmed IBD susceptibility loci to 163. These data have confirmed the existence of an important overlap in genetic risk factors between IBD and other immune-mediated disorders (70%) and that most loci contribute to both IBD phenotypes (67%)[345]. IBD genetics has highlighted a common Th17/IL23 pathway for CD and UC[345-347], in line with evidence concerning gene expression in inflamed mucosa[348], and revealed relatively fewer disease-specific involved mechanisms, such as barrier integrity for UC[349-352] and autophagy for CD[350-353]. This degree of sharing of genetic risk suggests that nearly all of the so far known susceptibility loci involved in one disease may have some role in the other[345] and tightens the implementation of genetic panels to address discrimination of UC from CD[354].
To date, efforts made to find genetic diagnostic markers in IBD have mostly focused on colonic tissue samples retrieved by endoscopic biopsy[355,356]. In the research by von Stein[357], seven candidate cDNAs differentially expressed in the colonic inflamed tissues of UC and CD patients were initially identified using the molecular subtraction process termed “subtractive suppression hybridization”. In quantitative polymerase chain reaction experiments, the differential expressions of identified genes were analyzed using a classification algorithm and the possible clinical value of these markers was overall evaluated in 301 patients in 3 stepwise studies. Accordingly, the multigene analysis based on these seven markers was able to discriminate between patients suffering from UC, CD, or IBS with an area under the receiver-operating characteristic curves ranging from 0.915 to 0.999 (P < 0.0001) using the clinical diagnosis as gold standard. Furthermore, in a subset of 20 IBDU patients, the genetic panel predicted correctly 9 out of 10 changes in clinical diagnosis made 4-12 mo later by a blinded physician[356]. More recently, this genetic panel was tested in 78 active patients with a complicated course diagnosed as most probably UC in 38, CD in 18 and IBDU in 22[358]. The tests led to the proper diagnosis in a substantial number of cases initially named as UC (11/13) or CD (3/4) and suggested the correct diagnosis in most IBDU patients (9/10) clinically reclassified as UC or CD one year later[358]. Further confirmations in larger and more heterogeneous populations are required before implementing this approach in clinical practice; yet, the era of genetic differential diagnosis in IBD colitis has begun[359].
Epigenetics
Genetics accounts for only a portion of overall disease variance, indicating that gene-environment interactions affecting gene expression with no changes in the DNA sequence, namely epigenetics, play a central role in the pathogenesis of IBD and other diseases[351,360]. Among epigenetic modifications, DNA methylation is probably the most widely studied; this process occurs when a methyl group is covalently added to cytosines that are part of cytosine-guanine dinucleotides (CpG)[351]. Methylation of “CpG islands” in promoter gene areas may lead to transcriptionally repressive activity and is associated with gene silencing[351]. DNA methylation in colonic epithelial cells normally occurs with aging but is accelerated in IBD, where it is touted to favor genetic instability and development of colorectal cancer[361]. DNA methylation change is a quantitative trait and therefore an attractive biomarker that can be found in a range of body fluids. A panel of relevant hypomethylated or hypermethylated CpGs measured in fecal and blood samples might someday be capable of distinguishing between UC and CD, enabling disease stratification, predicting treatment response, identifying patients with cancer or those who are prone to cancer development[351].
Several genome-wide expression studies are so far attracting more attention to RNA interference, providing new interesting tools to assess and characterize IBD diagnosis and phenotype[351]. MicroRNAs (miRNAs) are short (typically 22 nucleotides in length), endogenous, non-coding single-stranded RNAs that have been highly conserved throughout evolution and act in concert as master drivers of post-transcription by regulating expression of their target messenger RNAs[351]. Analysing hundreds of miRNAs present in colonic biopsy specimens, distinct profiles were identified in IBD vs controls, as well as between quiescent or active UC vs CD, and in UC patients with active or inactive disease, thereby confirming the putative role of miRNAs as contributors to IBD pathogenesis[351,362-364]. Likewise, distinctive miRNA profiles have been described in the peripheral blood of UC, CD and nonIBD subjects, suggesting their use as minimally invasive circulating biomarkers[365-367].
However, when interpreting results from epigenetic studies, a potential pitfall lingers in determining causality, that is, whether a particular epigenetic profile is the cause or consequence of disease. The above-mentioned epigenetic studies in IBD patients were mostly performed with whole tissues, such as blood or colon biopsy samples, where epigenetic features of each cell can be masked by those of heterogeneous cell groups, making the results difficult to compare and interpret[351]. Accordingly, the development of miRNA profile-based diagnostic tools for IBD needs further studies to confirm the ability of circulating and colonic miRNAs in distinguishing IBD subtypes and their specificity in less selected populations.
Metabolomics and proteomics
The first full genome sequences were established in the mid-1990s. Shortly thereafter, genome-scale metabolic network reconstructions appeared to provide a repository of all human metabolites[368]. Metabolomics performs an analytical description of complex biological samples and aims to characterize and quantify all the small molecules involved, thereby revealing the unique chemical fingerprints that specific cellular processes leave behind[369]. Because the global cellular function depends upon the protein network environment as a whole, multivariate analysis of metabolic profiling of biofluids dovetails beautifully with IBD to screen for new molecular players involved in disease pathogenesis, measure and characterize disease activity and differentiate between disease bio-phenotypes. Several recent studies have highlighted how metabolic profiling of biofluids from IBD patients may represent a powerful approach to collect a huge amount of information on amino acids and related metabolites, on Krebs cycle intermediates, and on molecules involved in fatty acid and purine metabolism[370,371].
Similarly, proteomic and subproteomic (i.e., cellular compartments, organelles and biological fluids) array profiles have shown promise with regard to identifying active disease, providing insight into disease pathogenesis and differentiating between CD and UC[147,370]. Metabolic and proteomic profiles of serum and plasma have provided a distinctive characterization between IBD and healthy controls, as well as between UC and CD patients irrespective of the analytical technique used[370-372]. Encouraging data have been also described by protein profile analysis on peripheral blood mononuclear cells[373] and colonic tissue specimens[373-377]. By contrast, metabolomic patterns from urinary and fecal samples have often led to more conflicting results in differentiating UC from CD, likely due to the possible influence of environmental variation in urinary metabolomics, disturbances from gut microbiota, and other confounding factors such as ongoing anti-TNFα therapy or bowel resections[371].
Undeniably, current technology supporting metabolomics and proteomics analysis is sophisticated, complex and expensive[378]. Nonetheless, considering the potential breakthroughs in understanding inflammation processes, identifying new molecular targets for therapy, mapping individual gut microbiota and helping in differential diagnosis, metabolomic and proteomic techniques appear like an expanding constellation into the IBD universe.
CONCLUSION
Current classification of large-bowel involvement in IBD relies on clinical grounds and acknowledges three distinct phenotypes, UC, CD and IBDU[5]. Such a distinction is of paramount value for optimized clinical management, since surgery, modern therapies and emerging prognostic indices nearly always work on disease-specific strategies. Established clinical diagnostic methods usually prove successful in pointing at proper and defined diagnoses. However, the substantial group of IBD patients with ambiguous colonic diseases still represents an unresolved problem bearing the names of “misdiagnosis”, “disease reclassification over time” and “IBD unclassified (pro tempore)”. This implies less successful treatment strategies and can lead to repeated diagnostic tests, thereby producing a considerable overexploitation of resources, time and patients’ compliance.
The use of serologic panels including ASCA and pANCA with the addition of more recent antibodies featuring complementary profiles could partially enhance testing accuracy in IBD colitis, refine the classification of patients diagnosed as IBDU and predict surgical outcomes after IPAA.
Relatively recent innovations in gastrointestinal endoscopy have made the small bowel more accessible and have clearly improved the quality in endoscopic imaging. However, benefits in terms of differential diagnosis seem to be marginal and both specificity and clinical correlates are poor.
In the attempt to make a forecast, hereby distinguishing between emerging and future perspectives to improve the differential diagnosis in IBD, molecular pathology, optical spectroscopy and confocal laser endomicroscopy appear as the most promising tools ready for distribution in the next years (Figure 1). On the other hand, the encouraging results coming from genetics, epigenetics, metabolomics and proteomics are simultaneously shedding light on IBD biologic systems and generating non-invasive tests, which may both aid the diagnosis and contribute to further reclassifying IBD into new and more homogeneous subgroups[167]. The success of this strategy relies on the development of multiple, complimentary biomarkers relating to a molecular classification of IBD colitis, which can better reflect diverse disease behaviors from the pathogenic standpoint.
To date, the differential diagnosis in IBD colitis still relies on a multidisciplinary approach based on clinical evaluation, standard biomarkers, lower and upper endoscopy, histopathology and radiology[6,11,45,112]. Additional investigations such as deep enteroscopy, novel serologic tests and advanced endoscopic imaging techniques can help in specific situations but should not routinely be advocated. Clinical trials are now warranted to confirm the potential role of experimental laboratory tools assessing the genetic and/or metabolomic fingerprints of IBD in daily clinical practice.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 16, 2014
First decision: August 15, 2014
Article in press: November 11, 2014
P- Reviewer: Dore MP, Li SD, Zhang ZM S- Editor: Qi Y L- Editor: Logan S E- Editor: Wang CH
References
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, O’Morain C, Moum B, Colombel JF. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 3.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 6.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, Ordás I, Repici A, Rosa B, Sebastian S, Kucharzik T, Eliakim R; European Crohn’s and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn‘s disease in adults. Am J Gastroenterol. 2009;104:465–483; quiz 464, 484. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 10.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 11.Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–851. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Meucci G, Bortoli A, Riccioli FA, Girelli CM, Radaelli F, Rivolta R, Tatarella M. Frequency and clinical evolution of indeterminate colitis: a retrospective multi-centre study in northern Italy. GSMII (Gruppo di Studio per le Malattie Infiammatorie Intestinali) Eur J Gastroenterol Hepatol. 1999;11:909–913. [PubMed] [Google Scholar]
- 13.Nuij VJ, Zelinkova Z, Rijk MC, Beukers R, Ouwendijk RJ, Quispel R, van Tilburg AJ, Tang TJ, Smalbraak H, Bruin KF, et al. Phenotype of inflammatory bowel disease at diagnosis in the Netherlands: a population-based inception cohort study (the Delta Cohort) Inflamm Bowel Dis. 2013;19:2215–2222. doi: 10.1097/MIB.0b013e3182961626. [DOI] [PubMed] [Google Scholar]
- 14.Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588–597. doi: 10.1136/gutjnl-2013-304636. [DOI] [PubMed] [Google Scholar]
- 15.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MN, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJ, Chan FK; Asia-Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group. Asia-Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Moum B, Ekbom A, Vatn MH, Aadland E, Sauar J, Lygren I, Schulz T, Stray N, Fausa O. Inflammatory bowel disease: re-evaluation of the diagnosis in a prospective population based study in south eastern Norway. Gut. 1997;40:328–332. doi: 10.1136/gut.40.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myren J, Bouchier IA, Watkinson G, Softley A, Clamp SE, de Dombal FT. The OMGE multinational inflammatory bowel disease survey 1976-1986. A further report on 3175 cases. Scand J Gastroenterol Suppl. 1988;144:11–19. [PubMed] [Google Scholar]
- 18.Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. 2012;46:581–589. doi: 10.1097/MCG.0b013e318247c32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcello PW, Schoetz DJ, Roberts PL, Murray JJ, Coller JA, Rusin LC, Veidenheimer MC. Evolutionary changes in the pathologic diagnosis after the ileoanal pouch procedure. Dis Colon Rectum. 1997;40:263–269. doi: 10.1007/BF02050413. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen M, Jahnsen J, Lygren I, Sauar J, Schulz T, Stray N, Vatn MH, Moum B. Change of diagnosis during the first five years after onset of inflammatory bowel disease: results of a prospective follow-up study (the IBSEN Study) Scand J Gastroenterol. 2006;41:1037–1043. doi: 10.1080/00365520600554527. [DOI] [PubMed] [Google Scholar]
- 21.Melmed GY, Elashoff R, Chen GC, Nastaskin I, Papadakis KA, Vasiliauskas EA, Liu W, Landers C, Ippoliti AF, Targan SR. Predicting a change in diagnosis from ulcerative colitis to Crohn’s disease: a nested, case-control study. Clin Gastroenterol Hepatol. 2007;5:602–608; quiz 525. doi: 10.1016/j.cgh.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn‘s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013;29:357–362. doi: 10.1097/MOG.0b013e32836229fb. [DOI] [PubMed] [Google Scholar]
- 24.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 26.Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 27.Sjöberg D, Holmström T, Larsson M, Nielsen AL, Holmquist L, Ekbom A, Rönnblom A. Incidence and clinical course of Crohn’s disease during the first year - results from the IBD Cohort of the Uppsala Region (ICURE) of Sweden 2005-2009. J Crohns Colitis. 2014;8:215–222. doi: 10.1016/j.crohns.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34. doi: 10.1097/00004836-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Freeman HJ. Natural history and clinical behavior of Crohn’s disease extending beyond two decades. J Clin Gastroenterol. 2003;37:216–219. doi: 10.1097/00004836-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Louis E, Michel V, Hugot JP, Reenaers C, Fontaine F, Delforge M, El Yafi F, Colombel JF, Belaiche J. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003;52:552–557. doi: 10.1136/gut.52.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith BR, Arnott ID, Drummond HE, Nimmo ER, Satsangi J. Disease location, anti-Saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn’s disease. Inflamm Bowel Dis. 2004;10:521–528. doi: 10.1097/00054725-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Price AB. Overlap in the spectrum of non-specific inflammatory bowel disease--’colitis indeterminate’. J Clin Pathol. 1978;31:567–577. doi: 10.1136/jcp.31.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martland GT, Shepherd NA. Indeterminate colitis: definition, diagnosis, implications and a plea for nosological sanity. Histopathology. 2007;50:83–96. doi: 10.1111/j.1365-2559.2006.02545.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewénius J, Adnerhill I, Ekelund GR, Florén CH, Fork FT, Janzon L, Lindström C, Ogren M. Risk of relapse in new cases of ulcerative colitis and indeterminate colitis. Dis Colon Rectum. 1996;39:1019–1025. doi: 10.1007/BF02054693. [DOI] [PubMed] [Google Scholar]
- 38.Stewénius J, Adnerhill I, Anderson H, Ekelund GR, Florén CH, Fork FT, Janzon L, Lindström C, Ogren M. Incidence of colorectal cancer and all cause mortality in non-selected patients with ulcerative colitis and indeterminate colitis in Malmö, Sweden. Int J Colorectal Dis. 1995;10:117–122. doi: 10.1007/BF00341210. [DOI] [PubMed] [Google Scholar]
- 39.Prideaux L, De Cruz P, Ng SC, Kamm MA. Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2012;18:1340–1355. doi: 10.1002/ibd.21903. [DOI] [PubMed] [Google Scholar]
- 40.Tyler AD, Milgrom R, Stempak JM, Xu W, Brumell JH, Muise AM, Sehgal R, Cohen Z, Koltun W, Shen B, et al. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut. 2013;62:1433–1439. doi: 10.1136/gutjnl-2011-301957. [DOI] [PubMed] [Google Scholar]
- 41.Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M, Geboes K, Bossuyt X, Vandewalle P, Oberhuber G, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002;122:1242–1247. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 42.Tremaine WJ. Is indeterminate colitis determinable? Curr Gastroenterol Rep. 2012;14:162–165. doi: 10.1007/s11894-012-0244-x. [DOI] [PubMed] [Google Scholar]
- 43.Meucci G. What is the incidence, prevalence, and natural history of indeterminate colitis? Inflamm Bowel Dis. 2008;14 Suppl 2:S159–S160. doi: 10.1002/ibd.20594. [DOI] [PubMed] [Google Scholar]
- 44.Prenzel F, Uhlig HH. Frequency of indeterminate colitis in children and adults with IBD - a metaanalysis. J Crohns Colitis. 2009;3:277–281. doi: 10.1016/j.crohns.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, Griffiths AM, Jevon GP, Higuchi LM, Hyams JS, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C. Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis. 2006;12:258–262. doi: 10.1097/01.MIB.0000215093.62245.b9. [DOI] [PubMed] [Google Scholar]
- 47.Tremaine WJ. Review article: Indeterminate colitis--definition, diagnosis and management. Aliment Pharmacol Ther. 2007;25:13–17. doi: 10.1111/j.1365-2036.2006.03159.x. [DOI] [PubMed] [Google Scholar]
- 48.Orlando A, Guglielmi FW, Cottone M, Orlando E, Romano C, Sinagra E. Clinical implications of mucosal healing in the management of patients with inflammatory bowel disease. Dig Liver Dis. 2013;45:986–991. doi: 10.1016/j.dld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, Strid H, Ardizzone S, Veereman-Wauters G, Chevaux JB, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011;5:477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111.e2. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 51.Allen PB, Peyrin-Biroulet L. Moving towards disease modification in inflammatory bowel disease therapy. Curr Opin Gastroenterol. 2013;29:397–404. doi: 10.1097/MOG.0b013e3283622914. [DOI] [PubMed] [Google Scholar]
- 52.Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, Hanauer SB, Sandborn WJ. Treat to Target: A Proposed New Paradigm for the Management of Crohn’s Disease. Clin Gastroenterol Hepatol. 2013:Epub ahead of print. doi: 10.1016/j.cgh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Tontini GE, Bisschops R, Neumann H. Endoscopic scoring systems for inflammatory bowel disease: pros and cons. Expert Rev Gastroenterol Hepatol. 2014;8:543–554. doi: 10.1586/17474124.2014.899899. [DOI] [PubMed] [Google Scholar]
- 54.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 55.Scribano ML, Prantera C. Antibiotics and inflammatory bowel diseases. Dig Dis. 2013;31:379–384. doi: 10.1159/000354704. [DOI] [PubMed] [Google Scholar]
- 56.Lissner D, Siegmund B. Ulcerative colitis: current and future treatment strategies. Dig Dis. 2013;31:91–94. doi: 10.1159/000347194. [DOI] [PubMed] [Google Scholar]
- 57.Vermeire S, Ferrante M, Rutgeerts P. Recent advances: Personalised use of current Crohn’s disease therapeutic options. Gut. 2013;62:1511–1515. doi: 10.1136/gutjnl-2012-303958. [DOI] [PubMed] [Google Scholar]
- 58.Gisbert JP, Chaparro M, Gomollón F. Common misconceptions about 5-aminosalicylates and thiopurines in inflammatory bowel disease. World J Gastroenterol. 2011;17:3467–3478. doi: 10.3748/wjg.v17.i30.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2010;(12):CD008870. doi: 10.1002/14651858.CD008870. [DOI] [PubMed] [Google Scholar]
- 60.Saibeni S, Bollani S, Losco A, Michielan A, Sostegni R, Devani M, Lupinacci G, Pirola L, Cucino C, Meucci G, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123–127. doi: 10.1016/j.dld.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Willot S, Noble A, Deslandres C. Methotrexate in the treatment of inflammatory bowel disease: an 8-year retrospective study in a Canadian pediatric IBD center. Inflamm Bowel Dis. 2011;17:2521–2526. doi: 10.1002/ibd.21653. [DOI] [PubMed] [Google Scholar]
- 62.McDonald JW, Tsoulis DJ, Macdonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2012;12:CD003459. doi: 10.1002/14651858.CD003459.pub3. [DOI] [PubMed] [Google Scholar]
- 63.Laharie D, Reffet A, Belleannée G, Chabrun E, Subtil C, Razaire S, Capdepont M, de Lédinghen V. Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther. 2011;33:714–721. doi: 10.1111/j.1365-2036.2010.04569.x. [DOI] [PubMed] [Google Scholar]
- 64.Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 65.Cominelli F. Inhibition of leukocyte trafficking in inflammatory bowel disease. N Engl J Med. 2013;369:775–776. doi: 10.1056/NEJMe1307415. [DOI] [PubMed] [Google Scholar]
- 66.Monteleone G, Caruso R, Pallone F. Targets for new immunomodulation strategies in inflammatory bowel disease. Autoimmun Rev. 2014;13:11–14. doi: 10.1016/j.autrev.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 68.Leiman DA, Lichtenstein GR. Therapy of inflammatory bowel disease: what to expect in the next decade. Curr Opin Gastroenterol. 2014;30:385–390. doi: 10.1097/MOG.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 69.Khanna R, Feagan BG. Ustekinumab for the treatment of Crohn’s disease. Immunotherapy. 2013;5:803–815. doi: 10.2217/imt.13.81. [DOI] [PubMed] [Google Scholar]
- 70.Lobatón T, Vermeire S, Van Assche G, Rutgeerts P. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:579–594. doi: 10.1111/apt.12639. [DOI] [PubMed] [Google Scholar]
- 71.Mozaffari S, Nikfar S, Abdolghaffari AH, Abdollahi M. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin Biol Ther. 2014;14:583–600. doi: 10.1517/14712598.2014.885945. [DOI] [PubMed] [Google Scholar]
- 72.Dave M, Purohit T, Razonable R, Loftus EV. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 2014;20:196–212. doi: 10.1097/MIB.0b013e3182a827d2. [DOI] [PubMed] [Google Scholar]
- 73.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 74.Mason M, Siegel CA. Do inflammatory bowel disease therapies cause cancer? Inflamm Bowel Dis. 2013;19:1306–1321. doi: 10.1097/MIB.0b013e3182807618. [DOI] [PubMed] [Google Scholar]
- 75.Beaugerie L. Immunosuppression-related lymphomas and cancers in IBD: how can they be prevented? Dig Dis. 2012;30:415–419. doi: 10.1159/000338144. [DOI] [PubMed] [Google Scholar]
- 76.Magro F, Peyrin-Biroulet L, Sokol H, Aldeger X, Costa A, Higgins PD, Joyce JC, Katsanos KH, Lopez A, de Xaxars TM, et al. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III) J Crohns Colitis. 2014;8:31–44. doi: 10.1016/j.crohns.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-α) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2013;108:99–105. doi: 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 78.Marcello PW, Roberts PL, Schoetz DJ, Coller JA, Murray JJ, Veidenheimer MC. Long-term results of the ileoanal pouch procedure. Arch Surg. 1993;128:500–503; discussion 503-504. doi: 10.1001/archsurg.1993.01420170030003. [DOI] [PubMed] [Google Scholar]
- 79.Murrell ZA, Melmed GY, Ippoliti A, Vasiliauskas EA, Dubinsky M, Targan SR, Fleshner PR. A prospective evaluation of the long-term outcome of ileal pouch-anal anastomosis in patients with inflammatory bowel disease-unclassified and indeterminate colitis. Dis Colon Rectum. 2009;52:872–878. doi: 10.1007/DCR.0b013e31819f5d4c. [DOI] [PubMed] [Google Scholar]
- 80.Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, Manilich E, Shen B, Martin ST. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679–685. doi: 10.1097/SLA.0b013e31827d99a2. [DOI] [PubMed] [Google Scholar]
- 81.Shen B. Crohn’s disease of the ileal pouch: reality, diagnosis, and management. Inflamm Bowel Dis. 2009;15:284–294. doi: 10.1002/ibd.20661. [DOI] [PubMed] [Google Scholar]
- 82.Gawad KA, Wenske S, von Schrenck T, Izbicki JR. Ileoanal-pouch reconstruction does not impair sphincter function or quality of life. Hepatogastroenterology. 2007;54:1477–1482. [PubMed] [Google Scholar]
- 83.Arseneau KO, Sultan S, Provenzale DT, Onken J, Bickston SJ, Foley E, Connors AF, Cominelli F. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol. 2006;4:1135–1142. doi: 10.1016/j.cgh.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Berndtsson I, Lindholm E, Oresland T, Börjesson L. Long-term outcome after ileal pouch-anal anastomosis: function and health-related quality of life. Dis Colon Rectum. 2007;50:1545–1552. doi: 10.1007/s10350-007-0278-6. [DOI] [PubMed] [Google Scholar]
- 85.Heikens JT, de Vries J, van Laarhoven CJ. Quality of life, health-related quality of life and health status in patients having restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis: a systematic review. Colorectal Dis. 2012;14:536–544. doi: 10.1111/j.1463-1318.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 86.Carmon E, Keidar A, Ravid A, Goldman G, Rabau M. The correlation between quality of life and functional outcome in ulcerative colitis patients after proctocolectomy ileal pouch anal anastomosis. Colorectal Dis. 2003;5:228–232. doi: 10.1046/j.1463-1318.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 87.Fazio VW, O’Riordain MG, Lavery IC, Church JM, Lau P, Strong SA, Hull T. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg. 1999;230:575–584; discussion 584-586. doi: 10.1097/00000658-199910000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thirlby RC, Sobrino MA, Randall JB. The long-term benefit of surgery on health-related quality of life in patients with inflammatory bowel disease. Arch Surg. 2001;136:521–527. doi: 10.1001/archsurg.136.5.521. [DOI] [PubMed] [Google Scholar]
- 89.Brown CJ, Maclean AR, Cohen Z, Macrae HM, O’Connor BI, McLeod RS. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum. 2005;48:1542–1549. doi: 10.1007/s10350-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 90.Yu CS, Pemberton JH, Larson D. Ileal pouch-anal anastomosis in patients with indeterminate colitis: long-term results. Dis Colon Rectum. 2000;43:1487–1496. doi: 10.1007/BF02236726. [DOI] [PubMed] [Google Scholar]
- 91.Stewénius I, Ekelund GR, Florén CH, Fork FT, Janzon L, Lindström C, Ogren M. Operations in unselected patients with ulcerative colitis and indeterminate colitis. A long-term follow-up study. Eur J Surg. 1996;162:131–137. [PubMed] [Google Scholar]
- 92.McIntyre PB, Pemberton JH, Wolff BG, Dozois RR, Beart RW. Indeterminate colitis. Long-term outcome in patients after ileal pouch-anal anastomosis. Dis Colon Rectum. 1995;38:51–54. doi: 10.1007/BF02053857. [DOI] [PubMed] [Google Scholar]
- 93.Atkinson KG, Owen DA, Wankling G. Restorative proctocolectomy and indeterminate colitis. Am J Surg. 1994;167:516–518. doi: 10.1016/0002-9610(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 94.Hartley JE, Fazio VW, Remzi FH, Lavery IC, Church JM, Strong SA, Hull TL, Senagore AJ, Delaney CP. Analysis of the outcome of ileal pouch-anal anastomosis in patients with Crohn’s disease. Dis Colon Rectum. 2004;47:1808–1815. doi: 10.1007/s10350-004-0644-6. [DOI] [PubMed] [Google Scholar]
- 95.Braveman JM, Schoetz DJ, Marcello PW, Roberts PL, Coller JA, Murray JJ, Rusin LC. The fate of the ileal pouch in patients developing Crohn’s disease. Dis Colon Rectum. 2004;47:1613–1619. doi: 10.1007/s10350-004-0645-5. [DOI] [PubMed] [Google Scholar]
- 96.Tekkis PP, Heriot AG, Smith O, Smith JJ, Windsor AC, Nicholls RJ. Long-term outcomes of restorative proctocolectomy for Crohn’s disease and indeterminate colitis. Colorectal Dis. 2005;7:218–223. doi: 10.1111/j.1463-1318.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 97.Connelly TM, Koltun WA. The surgical treatment of inflammatory bowel disease-associated dysplasia. Expert Rev Gastroenterol Hepatol. 2013;7:307–321; quiz 322. doi: 10.1586/egh.13.17. [DOI] [PubMed] [Google Scholar]
- 98.Deutsch AA, McLeod RS, Cullen J, Cohen Z. Results of the pelvic-pouch procedure in patients with Crohn’s disease. Dis Colon Rectum. 1991;34:475–477. doi: 10.1007/BF02049932. [DOI] [PubMed] [Google Scholar]
- 99.Hyman NH, Fazio VW, Tuckson WB, Lavery IC. Consequences of ileal pouch-anal anastomosis for Crohn’s colitis. Dis Colon Rectum. 1991;34:653–657. doi: 10.1007/BF02050345. [DOI] [PubMed] [Google Scholar]
- 100.Moss AC, Cheifetz AS. How often is a diagnosis of ulcerative colitis changed to Crohn’s disease and vice versa? Inflamm Bowel Dis. 2008;14 Suppl 2:S155–S156. doi: 10.1002/ibd.20593. [DOI] [PubMed] [Google Scholar]
- 101.Melmed GY, Fleshner PR, Bardakcioglu O, Ippoliti A, Vasiliauskas EA, Papadakis KA, Dubinsky M, Landers C, Rotter JI, Targan SR. Family history and serology predict Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2008;51:100–108. doi: 10.1007/s10350-007-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolkomir AF, Luchtefeld MA. Surgery for symptomatic hemorrhoids and anal fissures in Crohn’s disease. Dis Colon Rectum. 1993;36:545–547. doi: 10.1007/BF02049859. [DOI] [PubMed] [Google Scholar]
- 103.Jeffery PJ, Parks AG, Ritchie JK. Treatment of haemorrhoids in patients with inflammatory bowel disease. Lancet. 1977;1:1084–1085. doi: 10.1016/s0140-6736(77)92337-6. [DOI] [PubMed] [Google Scholar]
- 104.D’Ugo S, Franceschilli L, Cadeddu F, Leccesi L, Blanco Gdel V, Calabrese E, Milito G, Di Lorenzo N, Gaspari AL, Sileri P. Medical and surgical treatment of haemorrhoids and anal fissure in Crohn’s disease: a critical appraisal. BMC Gastroenterol. 2013;13:47. doi: 10.1186/1471-230X-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peyrin-Biroulet L, Loftus EV, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Perianal Crohn’s disease findings other than fistulas in a population-based cohort. Inflamm Bowel Dis. 2012;18:43–48. doi: 10.1002/ibd.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bouguen G, Siproudhis L, Bretagne JF, Bigard MA, Peyrin-Biroulet L. Nonfistulizing perianal Crohn’s disease: clinical features, epidemiology, and treatment. Inflamm Bowel Dis. 2010;16:1431–1442. doi: 10.1002/ibd.21261. [DOI] [PubMed] [Google Scholar]
- 107.American Gastroenterological Association Clinical Practice Committee. American Gastroenterological Association medical position statement: perianal Crohn’s disease. Gastroenterology. 2003;125:1503–1507. doi: 10.1016/j.gastro.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 108.Daperno M, Castiglione F, de Ridder L, Dotan I, Färkkilä M, Florholmen J, Fraser G, Fries W, Hebuterne X, Lakatos PL, et al. Results of the 2nd part Scientific Workshop of the ECCO. II: Measures and markers of prediction to achieve, detect, and monitor intestinal healing in inflammatory bowel disease. J Crohns Colitis. 2011;5:484–498. doi: 10.1016/j.crohns.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Armuzzi A, Van Assche G, Reinisch W, Pineton de Chambrun G, Griffiths A, Sladek M, Preiss JC, Lukas M, D’Haens G. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. J Crohns Colitis. 2012;6:492–502. doi: 10.1016/j.crohns.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 110.Papi C, Fascì-Spurio F, Rogai F, Settesoldi A, Margagnoni G, Annese V. Mucosal healing in inflammatory bowel disease: treatment efficacy and predictive factors. Dig Liver Dis. 2013;45:978–985. doi: 10.1016/j.dld.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 111.Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969–977. doi: 10.1016/j.dld.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 112.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 113.Lennard-Jones JE, Shivananda S. Clinical uniformity of inflammatory bowel disease a presentation and during the first year of disease in the north and south of Europe. EC-IBD Study Group. Eur J Gastroenterol Hepatol. 1997;9:353–359. doi: 10.1097/00042737-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 114.Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–1532. doi: 10.1053/j.gastro.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz DA, Loftus EV, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 116.Bonheur JL, Braunstein J, Korelitz BI, Panagopoulos G. Anal skin tags in inflammatory bowel disease: new observations and a clinical review. Inflamm Bowel Dis. 2008;14:1236–1239. doi: 10.1002/ibd.20458. [DOI] [PubMed] [Google Scholar]
- 117.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 118.Williams H, Walker D, Orchard TR. Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2008;10:597–605. doi: 10.1007/s11894-008-0108-6. [DOI] [PubMed] [Google Scholar]
- 119.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011;7:235–241. [PMC free article] [PubMed] [Google Scholar]
- 120.Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- 121.Aghazadeh R, Zali MR, Bahari A, Amin K, Ghahghaie F, Firouzi F. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005;20:1691–1695. doi: 10.1111/j.1440-1746.2005.03905.x. [DOI] [PubMed] [Google Scholar]
- 122.Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300–2307. doi: 10.3748/wjg.v9.i10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tavarela Veloso F. Review article: skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:50–53. doi: 10.1111/j.1365-2036.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- 124.Vavricka SR, Brun L, Ballabeni P, Pittet V, Prinz Vavricka BM, Zeitz J, Rogler G, Schoepfer AM. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 125.Orchard TR, Chua CN, Ahmad T, Cheng H, Welsh KI, Jewell DP. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology. 2002;123:714–718. doi: 10.1053/gast.2002.35396. [DOI] [PubMed] [Google Scholar]
- 126.Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 127.Mintz R, Feller ER, Bahr RL, Shah SA. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:135–139. doi: 10.1097/00054725-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 128.Targownik LE, Bernstein CN, Nugent Z, Leslie WD. Inflammatory bowel disease has a small effect on bone mineral density and risk for osteoporosis. Clin Gastroenterol Hepatol. 2013;11:278–285. doi: 10.1016/j.cgh.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 129.Walldorf J, Krummenerl A, Engler K, Busch J, Dollinger MM, Seufferlein T, Albert JG. Health care for osteoporosis in inflammatory bowel disease: unmet needs in care of male patients? J Crohns Colitis. 2013;7:901–907. doi: 10.1016/j.crohns.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 130.Loftus EV, Crowson CS, Sandborn WJ, Tremaine WJ, O’Fallon WM, Melton LJ. Long-term fracture risk in patients with Crohn’s disease: a population-based study in Olmsted County, Minnesota. Gastroenterology. 2002;123:468–475. doi: 10.1053/gast.2002.34779. [DOI] [PubMed] [Google Scholar]
- 131.Loftus EV, Achenbach SJ, Sandborn WJ, Tremaine WJ, Oberg AL, Melton LJ. Risk of fracture in ulcerative colitis: a population-based study from Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2003;1:465–473. doi: 10.1016/s1542-3565(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 132.Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol. 2013;19:8571–8579. doi: 10.3748/wjg.v19.i46.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cattaneo M, Vecchi M. Inflammatory bowel disease and the risk of thrombosis. Gastroenterology. 1999;117:280–281. doi: 10.1016/s0016-5085(99)70589-5. [DOI] [PubMed] [Google Scholar]
- 134.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272–2280. doi: 10.1111/j.1572-0241.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 135.Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779–787, 787.e1. doi: 10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 136.Spina L, Saibeni S, Battaglioli T, Peyvandi F, de Franchis R, Vecchi M. Thrombosis in inflammatory bowel diseases: role of inherited thrombophilia. Am J Gastroenterol. 2005;100:2036–2041. doi: 10.1111/j.1572-0241.2005.42029.x. [DOI] [PubMed] [Google Scholar]
- 137.Casella G, Tontini GE, Bassotti G, Pastorelli L, Villanacci V, Spina L, Baldini V, Vecchi M. Neurological disorders and inflammatory bowel diseases. World J Gastroenterol. 2014;20:8764–8782. doi: 10.3748/wjg.v20.i27.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Agarwal A, Andrews JM. Systematic review: IBD-associated pyoderma gangrenosum in the biologic era, the response to therapy. Aliment Pharmacol Ther. 2013;38:563–572. doi: 10.1111/apt.12431. [DOI] [PubMed] [Google Scholar]
- 139.Lu DG, Ji XQ, Liu X, Li HJ, Zhang CQ. Pulmonary manifestations of Crohn’s disease. World J Gastroenterol. 2014;20:133–141. doi: 10.3748/wjg.v20.i1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lashner BA, Evans AA, Kirsner JB, Hanauer SB. Prevalence and incidence of inflammatory bowel disease in family members. Gastroenterology. 1986;91:1396–1400. doi: 10.1016/0016-5085(86)90193-9. [DOI] [PubMed] [Google Scholar]
- 141.Sachar DB. Crohn’s disease: a family affair. Gastroenterology. 1996;111:813–815. doi: 10.1053/gast.1996.v111.agast961110813. [DOI] [PubMed] [Google Scholar]
- 142.Reynisdottir I, Gudbjartsson DF, Johannsson JH, Manolescu I, Kristjansson K, Stefansson K, Gulcher J, Bjornsson S. A genetic contribution to inflammatory bowel disease in Iceland: a genealogic approach. Clin Gastroenterol Hepatol. 2004;2:806–812. doi: 10.1016/s1542-3565(04)00351-9. [DOI] [PubMed] [Google Scholar]
- 143.Russell RK, Satsangi J. IBD: a family affair. Best Pract Res Clin Gastroenterol. 2004;18:525–539. doi: 10.1016/j.bpg.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 144.Annese V, Andreoli A, Astegiano M, Campieri M, Caprilli R, Cucchiara S, D’Incà R, Giaccari S, Iaquinto G, Lombardi G, et al. Clinical features in familial cases of Crohn’s disease and ulcerative colitis in Italy: a GISC study. Italian Study Group for the Disease of Colon and Rectum. Am J Gastroenterol. 2001;96:2939–2945. doi: 10.1111/j.1572-0241.2001.04685.x. [DOI] [PubMed] [Google Scholar]
- 145.Meucci G, Vecchi M, Torgano G, Arrigoni M, Prada A, Rocca F, Curzio M, Pera A, de Franchis R. Familial aggregation of inflammatory bowel disease in northern Italy: a multicenter study. The Gruppo di Studio per le Malattie Infiammatorie Intestinali (IBD Study Group) Gastroenterology. 1992;103:514–519. doi: 10.1016/0016-5085(92)90841-l. [DOI] [PubMed] [Google Scholar]
- 146.Satsangi J, Grootscholten C, Holt H, Jewell DP. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741. doi: 10.1136/gut.38.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826.e2. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jones J, Loftus EV, Panaccione R, Chen LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG, Harmsen SW, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 150.Solem CA, Loftus EV, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 151.Colombel JF, Solem CA, Sandborn WJ, Booya F, Loftus EV, Harmsen WS, Zinsmeister AR, Bodily KD, Fletcher JG. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561–1567. doi: 10.1136/gut.2005.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gross V, Andus T, Caesar I, Roth M, Schölmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology. 1992;102:514–519. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 153.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, Moum B. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 155.Prantera C, Davoli M, Lorenzetti R, Pallone F, Marcheggiano A, Iannoni C, Mariotti S. Clinical and laboratory indicators of extent of ulcerative colitis. Serum C-reactive protein helps the most. J Clin Gastroenterol. 1988;10:41–45. doi: 10.1097/00004836-198802000-00010. [DOI] [PubMed] [Google Scholar]
- 156.Rodgers AD, Cummins AG. CRP correlates with clinical score in ulcerative colitis but not in Crohn’s disease. Dig Dis Sci. 2007;52:2063–2068. doi: 10.1007/s10620-006-9691-2. [DOI] [PubMed] [Google Scholar]
- 157.D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 158.Nancey S, Boschetti G, Moussata D, Cotte E, Peyras J, Cuerq C, Haybrard J, Charlois AL, Mialon A, Chauvenet M, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1043–1052. doi: 10.1097/MIB.0b013e3182807577. [DOI] [PubMed] [Google Scholar]
- 159.van de Logt F, Day AS. S100A12: a noninvasive marker of inflammation in inflammatory bowel disease. J Dig Dis. 2013;14:62–67. doi: 10.1111/1751-2980.12012. [DOI] [PubMed] [Google Scholar]
- 160.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 161.Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 162.Sipponen T, Haapamäki J, Savilahti E, Alfthan H, Hämäläinen E, Rautiainen H, Koskenpato J, Nuutinen H, Färkkilä M. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn’s disease detected by wireless capsule endoscopy. Scand J Gastroenterol. 2012;47:778–784. doi: 10.3109/00365521.2012.677953. [DOI] [PubMed] [Google Scholar]
- 163.Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176–180. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 164.Israeli E, Grotto I, Gilburd B, Balicer RD, Goldin E, Wiik A, Shoenfeld Y. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut. 2005;54:1232–1236. doi: 10.1136/gut.2004.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van Schaik FD, Oldenburg B, Hart AR, Siersema PD, Lindgren S, Grip O, Teucher B, Kaaks R, Bergmann MM, Boeing H, et al. Serological markers predict inflammatory bowel disease years before the diagnosis. Gut. 2013;62:683–688. doi: 10.1136/gutjnl-2012-302717. [DOI] [PubMed] [Google Scholar]
- 166.Vecchi M, Spina L, Cavallaro F, Pastorelli L. Do antibodies have a role in IBD pathogenesis? Inflamm Bowel Dis. 2008;14 Suppl 2:S95–S96. doi: 10.1002/ibd.20699. [DOI] [PubMed] [Google Scholar]
- 167.Dotan I, Plevy SE. NOD2 and pouch complications in UC patients: old-world clinical dogmas give way to biological definitions. Gut. 2013;62:1390–1391. doi: 10.1136/gutjnl-2012-303188. [DOI] [PubMed] [Google Scholar]
- 168.De Vroey B, Gower-Rousseau C, Colombel JF. Exploring life before IBD. Gut. 2013;62:662–663. doi: 10.1136/gutjnl-2012-303245. [DOI] [PubMed] [Google Scholar]
- 169.Annese V, Andreoli A, Andriulli A, Dinca R, Gionchetti P, Latiano A, Lombardi G, Piepoli A, Poulain D, Sendid B, et al. Familial expression of anti-Saccharomyces cerevisiae Mannan antibodies in Crohn’s disease and ulcerative colitis: a GISC study. Am J Gastroenterol. 2001;96:2407–2412. doi: 10.1111/j.1572-0241.2001.04043.x. [DOI] [PubMed] [Google Scholar]
- 170.Papp M, Altorjay I, Dotan N, Palatka K, Foldi I, Tumpek J, Sipka S, Udvardy M, Dinya T, Lakatos L, et al. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol. 2008;103:665–681. doi: 10.1111/j.1572-0241.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 171.Dotan I, Fishman S, Dgani Y, Schwartz M, Karban A, Lerner A, Weishauss O, Spector L, Shtevi A, Altstock RT, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn’s disease. Gastroenterology. 2006;131:366–378. doi: 10.1053/j.gastro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 172.Vermeire S, Joossens S, Peeters M, Monsuur F, Marien G, Bossuyt X, Groenen P, Vlietinck R, Rutgeerts P. Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology. 2001;120:827–833. doi: 10.1053/gast.2001.22546. [DOI] [PubMed] [Google Scholar]
- 173.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–829. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 174.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 175.Vandewalle-El Khoury P, Colombel JF, Joossens S, Standaert-Vitse A, Collot M, Halfvarson J, Ayadi A, Landers CJ, Vermeire S, Rutgeerts P, et al. Detection of antisynthetic mannoside antibodies (ASigmaMA) reveals heterogeneity in the ASCA response of Crohn’s disease patients and contributes to differential diagnosis, stratification, and prediction. Am J Gastroenterol. 2008;103:949–957. doi: 10.1111/j.1572-0241.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- 176.Lakatos PL, Papp M, Rieder F. Serologic antiglycan antibodies in inflammatory bowel disease. Am J Gastroenterol. 2011;106:406–412. doi: 10.1038/ajg.2010.505. [DOI] [PubMed] [Google Scholar]
- 177.Bohr J, Tysk C, Yang P, Danielsson D, Järnerot G. Autoantibodies and immunoglobulins in collagenous colitis. Gut. 1996;39:73–76. doi: 10.1136/gut.39.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Duerr RH, Targan SR, Landers CJ, Sutherland LR, Shanahan F. Anti-neutrophil cytoplasmic antibodies in ulcerative colitis. Comparison with other colitides/diarrheal illnesses. Gastroenterology. 1991;100:1590–1596. doi: 10.1016/0016-5085(91)90657-7. [DOI] [PubMed] [Google Scholar]
- 179.Reese GE, Constantinides VA, Simillis C, Darzi AW, Orchard TR, Fazio VW, Tekkis PP. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–2422. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 180.Vasiliauskas EA, Plevy SE, Landers CJ, Binder SW, Ferguson DM, Yang H, Rotter JI, Vidrich A, Targan SR. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn’s disease define a clinical subgroup. Gastroenterology. 1996;110:1810–1819. doi: 10.1053/gast.1996.v110.pm8964407. [DOI] [PubMed] [Google Scholar]
- 181.Saibeni S, Folli C, de Franchis R, Borsi G, Vecchi M. Diagnostic role and clinical correlates of anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (p-ANCA) in Italian patients with inflammatory bowel diseases. Dig Liver Dis. 2003;35:862–868. doi: 10.1016/j.dld.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 182.Benor S, Russell GH, Silver M, Israel EJ, Yuan Q, Winter HS. Shortcomings of the inflammatory bowel disease Serology 7 panel. Pediatrics. 2010;125:1230–1236. doi: 10.1542/peds.2009-1936. [DOI] [PubMed] [Google Scholar]
- 183.Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99:2235–2241. doi: 10.1111/j.1572-0241.2004.40369.x. [DOI] [PubMed] [Google Scholar]
- 184.Ferrante M, Henckaerts L, Joossens M, Pierik M, Joossens S, Dotan N, Norman GL, Altstock RT, Van Steen K, Rutgeerts P, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–1403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simondi D, Mengozzi G, Betteto S, Bonardi R, Ghignone RP, Fagoonee S, Pellicano R, Sguazzini C, Pagni R, Rizzetto M, et al. Antiglycan antibodies as serological markers in the differential diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:645–651. doi: 10.1002/ibd.20368. [DOI] [PubMed] [Google Scholar]
- 186.Seow CH, Stempak JM, Xu W, Lan H, Griffiths AM, Greenberg GR, Steinhart AH, Dotan N, Silverberg MS. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am J Gastroenterol. 2009;104:1426–1434. doi: 10.1038/ajg.2009.79. [DOI] [PubMed] [Google Scholar]
- 187.Rieder F, Schleder S, Wolf A, Dirmeier A, Strauch U, Obermeier F, Lopez R, Spector L, Fire E, Yarden J, et al. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn’s disease behavior. Inflamm Bowel Dis. 2010;16:263–274. doi: 10.1002/ibd.21046. [DOI] [PubMed] [Google Scholar]
- 188.Schoepfer AM, Schaffer T, Mueller S, Flogerzi B, Vassella E, Seibold-Schmid B, Seibold F. Phenotypic associations of Crohn’s disease with antibodies to flagellins A4-Fla2 and Fla-X, ASCA, p-ANCA, PAB, and NOD2 mutations in a Swiss Cohort. Inflamm Bowel Dis. 2009;15:1358–1367. doi: 10.1002/ibd.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 190.Joossens S, Colombel JF, Landers C, Poulain D, Geboes K, Bossuyt X, Targan S, Rutgeerts P, Reinisch W. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut. 2006;55:1667–1669. doi: 10.1136/gut.2005.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vecchi M, Gionchetti P, Bianchi MB, Belluzzi A, Meucci G, Campieri M, de Franchis R. p-ANCA and development of pouchitis in ulcerative colitis patients after proctocolectomy and ileoanal pouch anastomosis. Lancet. 1994;344:886–887. doi: 10.1016/s0140-6736(94)92859-2. [DOI] [PubMed] [Google Scholar]
- 192.Sandborn WJ, Loftus EV, Colombel JF, Fleming KA, Seibold F, Homburger HA, Sendid B, Chapman RW, Tremaine WJ, Kaul DK, et al. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2001;7:192–201. doi: 10.1097/00054725-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 193.Fleshner P, Ippoliti A, Dubinsky M, Vasiliauskas E, Mei L, Papadakis KA, Rotter JI, Landers C, Targan S. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:561–568. doi: 10.1016/j.cgh.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 195.Tontini GE, Vecchi M, Neurath MF, Neumann H. Advanced endoscopic imaging techniques in Crohn’s disease. J Crohns Colitis. 2014;8:261–269. doi: 10.1016/j.crohns.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 196.Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG, et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618–637. doi: 10.1055/s-0029-1214790. [DOI] [PubMed] [Google Scholar]
- 197.Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95–101. doi: 10.1148/radiol.2361040799. [DOI] [PubMed] [Google Scholar]
- 198.Ordás I, Rimola J, García-Bosch O, Rodríguez S, Gallego M, Etchevers MJ, Pellisé M, Feu F, González-Suárez B, Ayuso C, et al. Diagnostic accuracy of magnetic resonance colonography for the evaluation of disease activity and severity in ulcerative colitis: a prospective study. Gut. 2013;62:1566–1572. doi: 10.1136/gutjnl-2012-303240. [DOI] [PubMed] [Google Scholar]
- 199.Fiorino G, Bonifacio C, Malesci A, Balzarini L, Danese S. MRI in Crohn’s disease--current and future clinical applications. Nat Rev Gastroenterol Hepatol. 2012;9:23–31. doi: 10.1038/nrgastro.2011.214. [DOI] [PubMed] [Google Scholar]
- 200.Ellrichmann M, Wietzke-Braun P, Dhar S, Nikolaus S, Arlt A, Bethge J, Kuehbacher T, Wintermeyer L, Balschun K, Klapper W, et al. Endoscopic ultrasound of the colon for the differentiation of Crohn’s disease and ulcerative colitis in comparison with healthy controls. Aliment Pharmacol Ther. 2014;39:823–833. doi: 10.1111/apt.12671. [DOI] [PubMed] [Google Scholar]
- 201.Schusselé Filliettaz S, Juillerat P, Burnand B, Arditi C, Windsor A, Beglinger C, Dubois RW, Peytremann-Bridevaux I, Pittet V, Gonvers JJ, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Chronic diarrhea and known inflammatory bowel disease. Endoscopy. 2009;41:218–226. doi: 10.1055/s-0028-1119627. [DOI] [PubMed] [Google Scholar]
- 202.Shen B, Khan K, Ikenberry SO, Anderson MA, Banerjee S, Baron T, Ben-Menachem T, Cash BD, Fanelli RD, Fisher L, et al. The role of endoscopy in the management of patients with diarrhea. Gastrointest Endosc. 2010;71:887–892. doi: 10.1016/j.gie.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 203.Pera A, Bellando P, Caldera D, Ponti V, Astegiano M, Barletti C, David E, Arrigoni A, Rocca G, Verme G. Colonoscopy in inflammatory bowel disease. Diagnostic accuracy and proposal of an endoscopic score. Gastroenterology. 1987;92:181–185. [PubMed] [Google Scholar]
- 204.Haskell H, Andrews CW, Reddy SI, Dendrinos K, Farraye FA, Stucchi AF, Becker JM, Odze RD. Pathologic features and clinical significance of “backwash” ileitis in ulcerative colitis. Am J Surg Pathol. 2005;29:1472–1481. doi: 10.1097/01.pas.0000176435.19197.88. [DOI] [PubMed] [Google Scholar]
- 205.Heuschen UA, Hinz U, Allemeyer EH, Stern J, Lucas M, Autschbach F, Herfarth C, Heuschen G. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology. 2001;120:841–847. doi: 10.1053/gast.2001.22434. [DOI] [PubMed] [Google Scholar]
- 206.Bernstein CN, Shanahan F, Anton PA, Weinstein WM. Patchiness of mucosal inflammation in treated ulcerative colitis: a prospective study. Gastrointest Endosc. 1995;42:232–237. doi: 10.1016/s0016-5107(95)70097-8. [DOI] [PubMed] [Google Scholar]
- 207.D’Haens G, Geboes K, Ponette E, Penninckx F, Rutgeerts P. Healing of severe recurrent ileitis with azathioprine therapy in patients with Crohn’s disease. Gastroenterology. 1997;112:1475–1481. doi: 10.1016/s0016-5085(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 208.Yang SK, Jung HY, Kang GH, Kim YM, Myung SJ, Shim KN, Hong WS, Min YI. Appendiceal orifice inflammation as a skip lesion in ulcerative colitis: an analysis in relation to medical therapy and disease extent. Gastrointest Endosc. 1999;49:743–747. doi: 10.1016/s0016-5107(99)70293-2. [DOI] [PubMed] [Google Scholar]
- 209.Ladefoged K, Munck LK, Jorgensen F, Engel P. Skip inflammation of the appendiceal orifice: a prospective endoscopic study. Scand J Gastroenterol. 2005;40:1192–1196. doi: 10.1080/00365520510023305. [DOI] [PubMed] [Google Scholar]
- 210.Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Glickman JN, Bousvaros A, Farraye FA, Zholudev A, Friedman S, Wang HH, Leichtner AM, Odze RD. Pediatric patients with untreated ulcerative colitis may present initially with unusual morphologic findings. Am J Surg Pathol. 2004;28:190–197. doi: 10.1097/00000478-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 212.Markowitz J, Kahn E, Grancher K, Hyams J, Treem W, Daum F. Atypical rectosigmoid histology in children with newly diagnosed ulcerative colitis. Am J Gastroenterol. 1993;88:2034–2037. [PubMed] [Google Scholar]
- 213.Washington K, Greenson JK, Montgomery E, Shyr Y, Crissinger KD, Polk DB, Barnard J, Lauwers GY. Histopathology of ulcerative colitis in initial rectal biopsy in children. Am J Surg Pathol. 2002;26:1441–1449. doi: 10.1097/00000478-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 214.Levine A, de Bie CI, Turner D, Cucchiara S, Sladek M, Murphy MS, Escher JC. Atypical disease phenotypes in pediatric ulcerative colitis: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:370–377. doi: 10.1002/ibd.23013. [DOI] [PubMed] [Google Scholar]
- 215.Joo M, Odze RD. Rectal sparing and skip lesions in ulcerative colitis: a comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am J Surg Pathol. 2010;34:689–696. doi: 10.1097/PAS.0b013e3181db84cd. [DOI] [PubMed] [Google Scholar]
- 216.Kim B, Barnett JL, Kleer CG, Appelman HD. Endoscopic and histological patchiness in treated ulcerative colitis. Am J Gastroenterol. 1999;94:3258–3262. doi: 10.1111/j.1572-0241.1999.01533.x. [DOI] [PubMed] [Google Scholar]
- 217.Kleer CG, Appelman HD. Ulcerative colitis: patterns of involvement in colorectal biopsies and changes with time. Am J Surg Pathol. 1998;22:983–989. doi: 10.1097/00000478-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 218.Yamagata M, Mikami T, Tsuruta T, Yokoyama K, Sada M, Kobayashi K, Katsumata T, Koizumi W, Saigenji K, Okayasu I. Submucosal fibrosis and basic-fibroblast growth factor-positive neutrophils correlate with colonic stenosis in cases of ulcerative colitis. Digestion. 2011;84:12–21. doi: 10.1159/000320773. [DOI] [PubMed] [Google Scholar]
- 219.Feakins RM. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66:1005–1026. doi: 10.1136/jclinpath-2013-201885. [DOI] [PubMed] [Google Scholar]
- 220.Geboes K. What histologic features best differentiate Crohn’s disease from ulcerative colitis? Inflamm Bowel Dis. 2008;14 Suppl 2:S168–S169. doi: 10.1002/ibd.20598. [DOI] [PubMed] [Google Scholar]
- 221.Feakins RM. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology. 2014;64:317–335. doi: 10.1111/his.12263. [DOI] [PubMed] [Google Scholar]
- 222.Shepherd NA. Granulomas in the diagnosis of intestinal Crohn’s disease: a myth exploded? Histopathology. 2002;41:166–168. doi: 10.1046/j.1365-2559.2002.01441.x. [DOI] [PubMed] [Google Scholar]
- 223.Shepherd NA. Pathological mimics of chronic inflammatory bowel disease. J Clin Pathol. 1991;44:726–733. doi: 10.1136/jcp.44.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pulimood AB, Amarapurkar DN, Ghoshal U, Phillip M, Pai CG, Reddy DN, Nagi B, Ramakrishna BS. Differentiation of Crohn’s disease from intestinal tuberculosis in India in 2010. World J Gastroenterol. 2011;17:433–443. doi: 10.3748/wjg.v17.i4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lamps LW. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50:55–63. doi: 10.1111/j.1365-2559.2006.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Khor TS, Fujita H, Nagata K, Shimizu M, Lauwers GY. Biopsy interpretation of colonic biopsies when inflammatory bowel disease is excluded. J Gastroenterol. 2012;47:226–248. doi: 10.1007/s00535-012-0539-6. [DOI] [PubMed] [Google Scholar]
- 227.Day DW, Mandal BK, Morson BC. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978;2:117–131. doi: 10.1111/j.1365-2559.1978.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 228.Price AB, Jewkes J, Sanderson PJ. Acute diarrhoea: Campylobacter colitis and the role of rectal biopsy. J Clin Pathol. 1979;32:990–997. doi: 10.1136/jcp.32.10.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.El-Maraghi NR, Mair NS. The histopathology of enteric infection with Yersinia pseudotuberculosis. Am J Clin Pathol. 1979;71:631–639. doi: 10.1093/ajcp/71.6.631. [DOI] [PubMed] [Google Scholar]
- 230.Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Mucosal biopsy diagnosis of colitis: acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology. 1994;107:755–763. doi: 10.1016/0016-5085(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 231.De Hertogh G, Geboes K. Crohn’s disease and infections: a complex relationship. MedGenMed. 2004;6:14. [PMC free article] [PubMed] [Google Scholar]
- 232.Shepherd NA. Diverticular disease and chronic idiopathic inflammatory bowel disease: associations and masquerades. Gut. 1996;38:801–802. doi: 10.1136/gut.38.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Makapugay LM, Dean PJ. Diverticular disease-associated chronic colitis. Am J Surg Pathol. 1996;20:94–102. doi: 10.1097/00000478-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 234.Burroughs SH, Bowrey DJ, Morris-Stiff GJ, Williams GT. Granulomatous inflammation in sigmoid diverticulitis: two diseases or one? Histopathology. 1998;33:349–353. doi: 10.1046/j.1365-2559.1998.00521.x. [DOI] [PubMed] [Google Scholar]
- 235.Warren BF, Shepherd NA, Price AB, Williams GT. Importance of cryptolytic lesions and pericryptal granulomas in inflammatory bowel disease. J Clin Pathol. 1997;50:880–881. doi: 10.1136/jcp.50.10.880-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Warren BF, Shepherd NA. Diversion proctocolitis. Histopathology. 1992;21:91–93. doi: 10.1111/j.1365-2559.1992.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 237.Mahadeva U, Martin JP, Patel NK, Price AB. Granulomatous ulcerative colitis: a re-appraisal of the mucosal granuloma in the distinction of Crohn’s disease from ulcerative colitis. Histopathology. 2002;41:50–55. doi: 10.1046/j.1365-2559.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 238.Lee FD, Maguire C, Obeidat W, Russell RI. Importance of cryptolytic lesions and pericryptal granulomas in inflammatory bowel disease. J Clin Pathol. 1997;50:148–152. doi: 10.1136/jcp.50.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bressenot A, Geboes K, Vignaud JM, Guéant JL, Peyrin-Biroulet L. Microscopic features for initial diagnosis and disease activity evaluation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1745–1752. doi: 10.1097/MIB.0b013e318281f2e8. [DOI] [PubMed] [Google Scholar]
- 240.Rubio CA, Orrego A, Nesi G, Finkel Y. Frequency of epithelioid granulomas in colonoscopic biopsy specimens from paediatric and adult patients with Crohn’s colitis. J Clin Pathol. 2007;60:1268–1272. doi: 10.1136/jcp.2006.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.de Bie CI, Paerregaard A, Kolacek S, Ruemmele FM, Koletzko S, Fell JM, Escher JC. Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:378–385. doi: 10.1002/ibd.23008. [DOI] [PubMed] [Google Scholar]
- 242.Heresbach D, Alexandre JL, Branger B, Bretagne JF, Cruchant E, Dabadie A, Dartois-Hoguin M, Girardot PM, Jouanolle H, Kerneis J, et al. Frequency and significance of granulomas in a cohort of incident cases of Crohn’s disease. Gut. 2005;54:215–222. doi: 10.1136/gut.2004.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57:1233–1244. doi: 10.1136/jcp.2003.015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Das KM, Vecchi M, Sakamaki S. A shared and unique epitope(s) on human colon, skin, and biliary epithelium detected by a monoclonal antibody. Gastroenterology. 1990;98:464–469. doi: 10.1016/0016-5085(90)90839-s. [DOI] [PubMed] [Google Scholar]
- 245.Yantiss RK, Das KM, Farraye FA, Odze RD. Alterations in the immunohistochemical expression of Das-1 and CG-3 in colonic mucosal biopsy specimens helps distinguish ulcerative colitis from Crohn disease and from other forms of colitis. Am J Surg Pathol. 2008;32:844–850. doi: 10.1097/PAS.0b013e31815b121c. [DOI] [PubMed] [Google Scholar]
- 246.Paerregaard A. What does the IBD patient hide in the upper gastrointestinal tract? Inflamm Bowel Dis. 2009;15:1101–1104. doi: 10.1002/ibd.20862. [DOI] [PubMed] [Google Scholar]
- 247.Kuriyama M, Kato J, Morimoto N, Fujimoto T, Okada H, Yamamoto K. Specific gastroduodenoscopic findings in Crohn’s disease: Comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig Liver Dis. 2008;40:468–475. doi: 10.1016/j.dld.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 248.Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618–1623. doi: 10.1007/s10620-012-2072-0. [DOI] [PubMed] [Google Scholar]
- 249.Rutgeerts P, Onette E, Vantrappen G, Geboes K, Broeckaert L, Talloen L. Crohn’s disease of the stomach and duodenum: A clinical study with emphasis on the value of endoscopy and endoscopic biopsies. Endoscopy. 1980;12:288–294. doi: 10.1055/s-2007-1021762. [DOI] [PubMed] [Google Scholar]
- 250.Nugent FW, Roy MA. Duodenal Crohn’s disease: an analysis of 89 cases. Am J Gastroenterol. 1989;84:249–254. [PubMed] [Google Scholar]
- 251.Nugent FW, Richmond M, Park SK. Crohn’s disease of the duodenum. Gut. 1977;18:115–120. doi: 10.1136/gut.18.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Clinical aspects of Crohn’s disease of the upper gastrointestinal tract: a comparison with distal Crohn’s disease. Am J Gastroenterol. 1997;92:1467–1471. [PubMed] [Google Scholar]
- 253.Crocco S, Martelossi S, Giurici N, Villanacci V, Ventura A. Upper gastrointestinal involvement in paediatric onset Crohn’s disease: prevalence and clinical implications. J Crohns Colitis. 2012;6:51–55. doi: 10.1016/j.crohns.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 254.Kovacs M, Muller KE, Arato A, Lakatos PL, Kovacs JB, Varkonyi A, Solyom E, Polgar M, Nemes E, Guthy I, et al. Diagnostic yield of upper endoscopy in paediatric patients with Crohn’s disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis. 2012;6:86–94. doi: 10.1016/j.crohns.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 255.Oberhuber G, Püspök A, Oesterreicher C, Novacek G, Zauner C, Burghuber M, Vogelsang H, Pötzi R, Stolte M, Wrba F. Focally enhanced gastritis: a frequent type of gastritis in patients with Crohn’s disease. Gastroenterology. 1997;112:698–706. doi: 10.1053/gast.1997.v112.pm9041230. [DOI] [PubMed] [Google Scholar]
- 256.Parente F, Molteni P, Bollani S, Maconi G, Vago L, Duca PG, Rembacken B, Axon AT, Bianchi Porro G. Prevalence of Helicobacter pylori infection and related upper gastrointestinal lesions in patients with inflammatory bowel diseases. A cross-sectional study with matching. Scand J Gastroenterol. 1997;32:1140–1146. doi: 10.3109/00365529709002994. [DOI] [PubMed] [Google Scholar]
- 257.Wright CL, Riddell RH. Histology of the stomach and duodenum in Crohn’s disease. Am J Surg Pathol. 1998;22:383–390. doi: 10.1097/00000478-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 258.Danelius M, Ost A, Lapidus AB. Inflammatory bowel disease-related lesions in the duodenal and gastric mucosa. Scand J Gastroenterol. 2009;44:441–445. doi: 10.1080/00365520802647426. [DOI] [PubMed] [Google Scholar]
- 259.Tobin JM, Sinha B, Ramani P, Saleh AR, Murphy MS. Upper gastrointestinal mucosal disease in pediatric Crohn disease and ulcerative colitis: a blinded, controlled study. J Pediatr Gastroenterol Nutr. 2001;32:443–448. doi: 10.1097/00005176-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 260.Abdullah BA, Gupta SK, Croffie JM, Pfefferkorn MD, Molleston JP, Corkins MR, Fitzgerald JF. The role of esophagogastroduodenoscopy in the initial evaluation of childhood inflammatory bowel disease: a 7-year study. J Pediatr Gastroenterol Nutr. 2002;35:636–640. doi: 10.1097/00005176-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 261.De Matos V, Russo PA, Cohen AB, Mamula P, Baldassano RN, Piccoli DA. Frequency and clinical correlations of granulomas in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:392–398. doi: 10.1097/MPG.0b013e31812e95e1. [DOI] [PubMed] [Google Scholar]
- 262.Castellaneta SP, Afzal NA, Greenberg M, Deere H, Davies S, Murch SH, Walker-Smith JA, Thomson M, Srivistrava A. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:257–261. doi: 10.1097/00005176-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 263.Shaoul R, Karban A, Weiss B, Reif S, Wasserman D, Pacht A, Eliakim R, Wardi J, Shirin H, Wine E, et al. NOD2/CARD15 mutations and presence of granulomas in pediatric and adult Crohn’s disease. Inflamm Bowel Dis. 2004;10:709–714. doi: 10.1097/00054725-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 264.Ravikumara M, Ashok D, Sandhu BK, Spray CH, Fell JME, Paerregaard A, Murphy MS. Upper gastrointestinal endoscopy is valuable in the diagnosis of Crohn’s disease. J Pediatr Gastroenterol Nutr. 2006;43(suppl 2):S27. [Google Scholar]
- 265.Hummel TZ, ten Kate FJ, Reitsma JB, Benninga MA, Kindermann A. Additional value of upper GI tract endoscopy in the diagnostic assessment of childhood IBD. J Pediatr Gastroenterol Nutr. 2012;54:753–757. doi: 10.1097/MPG.0b013e318243e3e3. [DOI] [PubMed] [Google Scholar]
- 266.IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1–7. doi: 10.1097/01.mpg.0000163736.30261.82. [DOI] [PubMed] [Google Scholar]
- 267.Ruuska T, Vaajalahti P, Arajärvi P, Mäki M. Prospective evaluation of upper gastrointestinal mucosal lesions in children with ulcerative colitis and Crohn’s disease. J Pediatr Gastroenterol Nutr. 1994;19:181–186. doi: 10.1097/00005176-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 268.Maeng L, Lee A, Choi K, Kang CS, Kim KM. Granulomatous gastritis: a clinicopathologic analysis of 18 biopsy cases. Am J Surg Pathol. 2004;28:941–945. doi: 10.1097/00000478-200407000-00015. [DOI] [PubMed] [Google Scholar]
- 269.Wimmer B, Plamenig D, Gilg MM, Fortunat W, Langner C. Granulomatous duodenitis mimicking Crohn’s disease caused by Capnocytophaga sp. Inflamm Bowel Dis. 2011;17:E31–E32. doi: 10.1002/ibd.21645. [DOI] [PubMed] [Google Scholar]
- 270.Parente F, Cucino C, Bollani S, Imbesi V, Maconi G, Bonetto S, Vago L, Bianchi Porro G. Focal gastric inflammatory infiltrates in inflammatory bowel diseases: prevalence, immunohistochemical characteristics, and diagnostic role. Am J Gastroenterol. 2000;95:705–711. doi: 10.1111/j.1572-0241.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 271.Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:39–44. doi: 10.1002/ibd.21356. [DOI] [PubMed] [Google Scholar]
- 272.Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn’s disease. Inflamm Bowel Dis. 2011;17:45–49. doi: 10.1002/ibd.21347. [DOI] [PubMed] [Google Scholar]
- 273.Sharif F, McDermott M, Dillon M, Drumm B, Rowland M, Imrie C, Kelleher S, Harty S, Bourke B. Focally enhanced gastritis in children with Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2002;97:1415–1420. doi: 10.1111/j.1572-0241.2002.05785.x. [DOI] [PubMed] [Google Scholar]
- 274.McHugh JB, Gopal P, Greenson JK. The clinical significance of focally enhanced gastritis in children. Am J Surg Pathol. 2013;37:295–299. doi: 10.1097/PAS.0b013e31826b2a94. [DOI] [PubMed] [Google Scholar]
- 275.Roka K, Roma E, Stefanaki K, Panayotou I, Kopsidas G, Chouliaras G. The value of focally enhanced gastritis in the diagnosis of pediatric inflammatory bowel diseases. J Crohns Colitis. 2013;7:797–802. doi: 10.1016/j.crohns.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 276.Honma J, Mitomi H, Murakami K, Igarashi M, Saigenji K, Toyama K. Nodular duodenitis involving CD8+ cell infiltration in patients with ulcerative colitis. Hepatogastroenterology. 2001;48:1604–1610. [PubMed] [Google Scholar]
- 277.Hori K, Ikeuchi H, Nakano H, Uchino M, Tomita T, Ohda Y, Hida N, Matsumoto T, Fukuda Y, Miwa H. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol. 2008;43:193–201. doi: 10.1007/s00535-007-2143-8. [DOI] [PubMed] [Google Scholar]
- 278.Lin J, McKenna BJ, Appelman HD. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study. Am J Surg Pathol. 2010;34:1672–1677. doi: 10.1097/PAS.0b013e3181f3de93. [DOI] [PubMed] [Google Scholar]
- 279.Korelitz BI, Waye JD, Kreuning J, Sommers SC, Fein HD, Beeber J, Gelberg BJ. Crohn’s disease in endoscopic biopsies of the gastric antrum and duodenum. Am J Gastroenterol. 1981;76:103–109. [PubMed] [Google Scholar]
- 280.Cameron DJ. Upper and lower gastrointestinal endoscopy in children and adolescents with Crohn’s disease: a prospective study. J Gastroenterol Hepatol. 1991;6:355–358. doi: 10.1111/j.1440-1746.1991.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 281.Srivastava A, Lauwers GY. Pathology of non-infective gastritis. Histopathology. 2007;50:15–29. doi: 10.1111/j.1365-2559.2006.02553.x. [DOI] [PubMed] [Google Scholar]
- 282.Xin W, Greenson JK. The clinical significance of focally enhanced gastritis. Am J Surg Pathol. 2004;28:1347–1351. doi: 10.1097/01.pas.0000138182.97366.b4. [DOI] [PubMed] [Google Scholar]
- 283.Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390–1396. doi: 10.1097/MIB.0b013e31828133c1. [DOI] [PubMed] [Google Scholar]
- 284.Kornbluth A, Colombel JF, Leighton JA, Loftus E. ICCE consensus for inflammatory bowel disease. Endoscopy. 2005;37:1051–1054. doi: 10.1055/s-2005-870315. [DOI] [PubMed] [Google Scholar]
- 285.Tukey M, Pleskow D, Legnani P, Cheifetz AS, Moss AC. The utility of capsule endoscopy in patients with suspected Crohn’s disease. Am J Gastroenterol. 2009;104:2734–2739. doi: 10.1038/ajg.2009.404. [DOI] [PubMed] [Google Scholar]
- 286.Rosa B, Moreira MJ, Rebelo A, Cotter J. Lewis Score: a useful clinical tool for patients with suspected Crohn’s Disease submitted to capsule endoscopy. J Crohns Colitis. 2012;6:692–697. doi: 10.1016/j.crohns.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 287.Eliakim R, Carter D. Endoscopic assessment of the small bowel. Dig Dis. 2013;31:194–198. doi: 10.1159/000353367. [DOI] [PubMed] [Google Scholar]
- 288.Girelli CM, Porta P, Malacrida V, Barzaghi F, Rocca F. Clinical outcome of patients examined by capsule endoscopy for suspected small bowel Crohn’s disease. Dig Liver Dis. 2007;39:148–154. doi: 10.1016/j.dld.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 289.Eliakim R. Video capsule endoscopy of the small bowel. Curr Opin Gastroenterol. 2013;29:133–139. doi: 10.1097/MOG.0b013e32835bdc03. [DOI] [PubMed] [Google Scholar]
- 290.Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn’s disease treatment: The impact of small bowel capsule endoscopy. J Crohns Colitis. 2014;8:1610–1615. doi: 10.1016/j.crohns.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 291.Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240–1248; quiz 1249. doi: 10.1038/ajg.2009.713. [DOI] [PubMed] [Google Scholar]
- 292.Cullen G, Donnellan F, Doherty GA, Smith M, Cheifetz AS. Evaluation of the small bowel in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2013;7:239–251. doi: 10.1586/egh.13.11. [DOI] [PubMed] [Google Scholar]
- 293.Mow WS, Lo SK, Targan SR, Dubinsky MC, Treyzon L, Abreu-Martin MT, Papadakis KA, Vasiliauskas EA. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:31–40. doi: 10.1016/s1542-3565(03)00289-1. [DOI] [PubMed] [Google Scholar]
- 294.Doherty GA, Moss AC, Cheifetz AS. Capsule endoscopy in suspected Crohn’s disease: “yield” does not equal “diagnosis”. Am J Gastroenterol. 2010;105:2111; author reply 2111–2112. doi: 10.1038/ajg.2010.203. [DOI] [PubMed] [Google Scholar]
- 295.Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007;13:6140–6149. doi: 10.3748/wjg.v13.i46.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 297.Dost D, van Leerdam ME, van Dekken H, Weimar W, Kuipers EJ, Bijl AH, van Gelder T. Crohn’s-like enterocolitis associated with mycophenolic acid treatment. Gut. 2008;57:1330. doi: 10.1136/gut.2007.139972. [DOI] [PubMed] [Google Scholar]
- 298.Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 299.Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007;66:S99–107. doi: 10.1016/j.gie.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 300.Maunoury V, Savoye G, Bourreille A, Bouhnik Y, Jarry M, Sacher-Huvelin S, Ben Soussan E, Lerebours E, Galmiche JP, Colombel JF. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified) Inflamm Bowel Dis. 2007;13:152–155. doi: 10.1002/ibd.20060. [DOI] [PubMed] [Google Scholar]
- 301.Lopes S, Figueiredo P, Portela F, Freire P, Almeida N, Lérias C, Gouveia H, Leitão MC. Capsule endoscopy in inflammatory bowel disease type unclassified and indeterminate colitis serologically negative. Inflamm Bowel Dis. 2010;16:1663–1668. doi: 10.1002/ibd.21249. [DOI] [PubMed] [Google Scholar]
- 302.Mehdizadeh S, Chen G, Enayati PJ, Cheng DW, Han NJ, Shaye OA, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Diagnostic yield of capsule endoscopy in ulcerative colitis and inflammatory bowel disease of unclassified type (IBDU) Endoscopy. 2008;40:30–35. doi: 10.1055/s-2007-995359. [DOI] [PubMed] [Google Scholar]
- 303.Di Nardo G, Oliva S, Ferrari F, Riccioni ME, Staiano A, Lombardi G, Costamagna G, Cucchiara S, Stronati L. Usefulness of wireless capsule endoscopy in paediatric inflammatory bowel disease. Dig Liver Dis. 2011;43:220–224. doi: 10.1016/j.dld.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 304.Hisabe T, Ninomiya K, Matsui T, Karashima Y, Sato Y, Nagahama T, Takaki Y, Hirai F, Yao K, Higashi D, et al. Small bowel lesions detected with wireless capsule endoscopy in patients with active ulcerative colitis and with post-proctocolectomy. Dig Endosc. 2011;23:302–309. doi: 10.1111/j.1443-1661.2011.01149.x. [DOI] [PubMed] [Google Scholar]
- 305.Higurashi T, Endo H, Yoneda M, Hosono K, Sakai E, Takahashi H, Inamori M, Uchiyama S, Kojima T, Kawana K, et al. Capsule-endoscopic findings of ulcerative colitis patients. Digestion. 2011;84:306–314. doi: 10.1159/000333086. [DOI] [PubMed] [Google Scholar]
- 306.Kalla R, McAlindon ME, Drew K, Sidhu R. Clinical utility of capsule endoscopy in patients with Crohn’s disease and inflammatory bowel disease unclassified. Eur J Gastroenterol Hepatol. 2013;25:706–713. doi: 10.1097/MEG.0b013e32835ddb85. [DOI] [PubMed] [Google Scholar]
- 307.Murrell Z, Vasiliauskas E, Melmed G, Lo S, Targan S, Fleshner P. Preoperative wireless capsule endoscopy does not predict outcome after ileal pouch-anal anastomosis. Dis Colon Rectum. 2010;53:293–300. doi: 10.1007/DCR.0b013e3181b71a2c. [DOI] [PubMed] [Google Scholar]
- 308.Iacucci M, Panaccione R, Ghosh S. Advances in novel diagnostic endoscopic imaging techniques in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:873–880. doi: 10.1097/MIB.0b013e318280143f. [DOI] [PubMed] [Google Scholar]
- 309.Neumann H, Neurath MF. Colonoscopy, inflammatory bowel disease. Endoscopy. 2014;46:322–326. doi: 10.1055/s-0034-1365297. [DOI] [PubMed] [Google Scholar]
- 310.Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol. 2014;12:368–376.e1. doi: 10.1016/j.cgh.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 311.Sharma P, Gupta N, Kuipers EJ, Repici A, Wallace M. Advanced imaging in colonoscopy and its impact on quality. Gastrointest Endosc. 2014;79:28–36. doi: 10.1016/j.gie.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 312.Subramanian V, Ramappa V, Telakis E, Mannath J, Jawhari AU, Hawkey CJ, Ragunath K. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350–355. doi: 10.1002/ibd.23002. [DOI] [PubMed] [Google Scholar]
- 313.Gross SA, Buchner AM, Crook JE, Cangemi JR, Picco MF, Wolfsen HC, DeVault KR, Loeb DS, Raimondo M, Woodward TA, et al. A comparison of high definition-image enhanced colonoscopy and standard white-light colonoscopy for colorectal polyp detection. Endoscopy. 2011;43:1045–1051. doi: 10.1055/s-0030-1256894. [DOI] [PubMed] [Google Scholar]
- 314.Nishio Y, Ando T, Maeda O, Ishiguro K, Watanabe O, Ohmiya N, Niwa Y, Kusugami K, Goto H. Pit patterns in rectal mucosa assessed by magnifying colonoscope are predictive of relapse in patients with quiescent ulcerative colitis. Gut. 2006;55:1768–1773. doi: 10.1136/gut.2005.086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kunihiro M, Tanaka S, Sumii M, Ueno Y, Ito M, Kitadai Y, Yoshihara M, Shimamoto F, Haruma K, Chayama K. Magnifying colonoscopic features of ulcerative colitis reflect histologic inflammation. Inflamm Bowel Dis. 2004;10:737–744. doi: 10.1097/00054725-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 316.Fujiya M, Saitoh Y, Nomura M, Maemoto A, Fujiya K, Watari J, Ashida T, Ayabe T, Obara T, Kohgo Y. Minute findings by magnifying colonoscopy are useful for the evaluation of ulcerative colitis. Gastrointest Endosc. 2002;56:535–542. doi: 10.1067/mge.2002.127101. [DOI] [PubMed] [Google Scholar]
- 317.Neumann H, Vieth M, Günther C, Neufert C, Kiesslich R, Grauer M, Atreya R, Neurath MF. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: a randomized controlled study. Inflamm Bowel Dis. 2013;19:1935–1942. doi: 10.1097/MIB.0b013e318290550e. [DOI] [PubMed] [Google Scholar]
- 318.Tontini GE, Vecchi M, Neurath MF, Neumann H. Review article: newer optical and digital chromoendoscopy techniques vs. dye-based chromoendoscopy for diagnosis and surveillance in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1198–1208. doi: 10.1111/apt.12508. [DOI] [PubMed] [Google Scholar]
- 319.Watanabe O, Ando T, Maeda O, Hasegawa M, Ishikawa D, Ishiguro K, Ohmiya N, Niwa Y, Goto H. Confocal endomicroscopy in patients with ulcerative colitis. J Gastroenterol Hepatol. 2008;23 Suppl 2:S286–S290. doi: 10.1111/j.1440-1746.2008.05559.x. [DOI] [PubMed] [Google Scholar]
- 320.Trovato C, Sonzogni A, Fiori G, Ravizza D, Tamayo D, Botti F, Carrara A, Zefelippo A, Contessini-Avesani E, Crosta C. Confocal laser endomicroscopy for the detection of mucosal changes in ileal pouch after restorative proctocolectomy. Dig Liver Dis. 2009;41:578–585. doi: 10.1016/j.dld.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 321.Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388–392, 392.e1-2. doi: 10.1053/j.gastro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 322.Li CQ, Xie XJ, Yu T, Gu XM, Zuo XL, Zhou CJ, Huang WQ, Chen H, Li YQ. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol. 2010;105:1391–1396. doi: 10.1038/ajg.2009.664. [DOI] [PubMed] [Google Scholar]
- 323.Liu JJ, Wong K, Thiesen AL, Mah SJ, Dieleman LA, Claggett B, Saltzman JR, Fedorak RN. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest Endosc. 2011;73:1174–1180. doi: 10.1016/j.gie.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 324.Neumann H, Vieth M, Atreya R, Grauer M, Siebler J, Bernatik T, Neurath MF, Mudter J. Assessment of Crohn’s disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis. 2012;18:2261–2269. doi: 10.1002/ibd.22907. [DOI] [PubMed] [Google Scholar]
- 325.Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Liu J, Dlugosz A, Neumann H. Beyond white light endoscopy: the role of optical biopsy in inflammatory bowel disease. World J Gastroenterol. 2013;19:7544–7551. doi: 10.3748/wjg.v19.i43.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Buda A, Hatem G, Neumann H, D’Incà R, Mescoli C, Piselli P, Jackson J, Bruno M, Sturniolo GC. Confocal laser endomicroscopy for prediction of disease relapse in ulcerative colitis: a pilot study. J Crohns Colitis. 2014;8:304–311. doi: 10.1016/j.crohns.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 328.Neumann H, Kiesslich R. Endomicroscopy and endocytoscopy in IBD. Gastrointest Endosc Clin N Am. 2013;23:695–705. doi: 10.1016/j.giec.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 329.Neumann H, Vieth M, Neurath MF, Atreya R. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis. 2013;19:356–362. doi: 10.1002/ibd.23025. [DOI] [PubMed] [Google Scholar]
- 330.Tontini GE, Mudter J, Vieth M, Atreya R, Günther C, Zopf Y, Wildner D, Kiesslich R, Vecchi M, Neurath MF, et al. Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: a pilot study. Endoscopy. 2014 doi: 10.1055/s-0034-1391226. [DOI] [PubMed] [Google Scholar]
- 331.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 332.Schürmann S, Foersch S, Atreya R, Neumann H, Friedrich O, Neurath MF, Waldner MJ. Label-free imaging of inflammatory bowel disease using multiphoton microscopy. Gastroenterology. 2013;145:514–516. doi: 10.1053/j.gastro.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 333.Atreya R, Goetz M. Molecular imaging in gastroenterology. Nat Rev Gastroenterol Hepatol. 2013;10:704–712. doi: 10.1038/nrgastro.2013.125. [DOI] [PubMed] [Google Scholar]
- 334.Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, Willma M, App C, Münster T, Kessler H, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313–318. doi: 10.1038/nm.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kutsukawa M, Kudo SE, Ikehara N, Ogawa Y, Wakamura K, Mori Y, Ichimasa K, Misawa M, Kudo T, Wada Y, et al. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc. 2014;79:648–656. doi: 10.1016/j.gie.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 336.Cipolletta L, Bianco MA, Rotondano G, Piscopo R, Meucci C, Prisco A, Cipolletta F, de Gregorio A, Salvati A. Endocytoscopy can identify dysplasia in aberrant crypt foci of the colorectum: a prospective in vivo study. Endoscopy. 2009;41:129–132. doi: 10.1055/s-0028-1103452. [DOI] [PubMed] [Google Scholar]
- 337.Pohl H, Rösch T, Tanczos BT, Rudolph B, Schlüns K, Baumgart DC. Endocytoscopy for the detection of microstructural features in adult patients with celiac sprue: a prospective, blinded endocytoscopy-conventional histology correlation study. Gastrointest Endosc. 2009;70:933–941. doi: 10.1016/j.gie.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 338.Kirtane TS, Wagh MS. Endoscopic Optical Coherence Tomography (OCT): Advances in Gastrointestinal Imaging. Gastroenterol Res Pract. 2014;2014:376367. doi: 10.1155/2014/376367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.ASGE Technology Committee. Enhanced imaging in the GI tract: spectroscopy and optical coherence tomography. Gastrointest Endosc. 2013;78:568–573. doi: 10.1016/j.gie.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 340.Familiari L, Strangio G, Consolo P, Luigiano C, Bonica M, Barresi G, Barresi V, Familiari P, D’Arrigo G, Alibrandi A, et al. Optical coherence tomography evaluation of ulcerative colitis: the patterns and the comparison with histology. Am J Gastroenterol. 2006;101:2833–2840. doi: 10.1111/j.1572-0241.2006.00826.x. [DOI] [PubMed] [Google Scholar]
- 341.Shen B, Zuccaro G, Gramlich TL, Gladkova N, Trolli P, Kareta M, Delaney CP, Connor JT, Lashner BA, Bevins CL, et al. In vivo colonoscopic optical coherence tomography for transmural inflammation in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:1080–1087. doi: 10.1016/s1542-3565(04)00621-4. [DOI] [PubMed] [Google Scholar]
- 342.Bi X, Walsh A, Mahadevan-Jansen A, Herline A. Development of spectral markers for the discrimination of ulcerative colitis and Crohn’s disease using Raman spectroscopy. Dis Colon Rectum. 2011;54:48–53. doi: 10.1007/DCR.0b013e3181fcf68d. [DOI] [PubMed] [Google Scholar]
- 343.Ellinghaus D, Zhang H, Zeissig S, Lipinski S, Till A, Jiang T, Stade B, Bromberg Y, Ellinghaus E, Keller A, et al. Association between variants of PRDM1 and NDP52 and Crohn’s disease, based on exome sequencing and functional studies. Gastroenterology. 2013;145:339–347. doi: 10.1053/j.gastro.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Cleynen I, González JR, Figueroa C, Franke A, McGovern D, Bortlík M, Crusius BJ, Vecchi M, Artieda M, Szczypiorska M, et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62:1556–1565. doi: 10.1136/gutjnl-2011-300777. [DOI] [PubMed] [Google Scholar]
- 345.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–52. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 348.Granlund Av, Flatberg A, Østvik AE, Drozdov I, Gustafsson BI, Kidd M, Beisvag V, Torp SH, Waldum HL, Martinsen TC, et al. Whole genome gene expression meta-analysis of inflammatory bowel disease colon mucosa demonstrates lack of major differences between Crohn’s disease and ulcerative colitis. PLoS One. 2013;8:e56818. doi: 10.1371/journal.pone.0056818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kaser A, Pasaniuc B. IBD genetics: focus on (dys) regulation in immune cells and the epithelium. Gastroenterology. 2014;146:896–899. doi: 10.1053/j.gastro.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 350.Mokry M, Middendorp S, Wiegerinck CL, Witte M, Teunissen H, Meddens CA, Cuppen E, Clevers H, Nieuwenhuis EE. Many inflammatory bowel disease risk loci include regions that regulate gene expression in immune cells and the intestinal epithelium. Gastroenterology. 2014;146:1040–1047. doi: 10.1053/j.gastro.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 351.Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. doi: 10.1053/j.gastro.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Murthy A, Li Y, Peng I, Reichelt M, Katakam AK, Noubade R, Roose-Girma M, DeVoss J, Diehl L, Graham RR, et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506:456–462. doi: 10.1038/nature13044. [DOI] [PubMed] [Google Scholar]
- 354.Van Limbergen J, Radford-Smith G, Satsangi J. Advances in IBD genetics. Nat Rev Gastroenterol Hepatol. 2014;11:372–385. doi: 10.1038/nrgastro.2014.27. [DOI] [PubMed] [Google Scholar]
- 355.Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis. 2007;13:807–821. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- 356.von Stein P, Lofberg R, Kuznetsov NV, Gielen AW, Persson JO, Sundberg R, Hellstrom K, Eriksson A, Befrits R, Ost A, et al. Multigene analysis can discriminate between ulcerative colitis, Crohn’s disease, and irritable bowel syndrome. Gastroenterology. 2008;134:1869–1881; quiz 2153-2154. doi: 10.1053/j.gastro.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 357.von Stein OD. Isolation of differentially expressed genes through subtractive suppression hybridization. Methods Mol Biol. 2001;175:263–278. doi: 10.1385/1-59259-235-X:263. [DOI] [PubMed] [Google Scholar]
- 358.Janczewska I, Kapraali M, Saboonchi F, Nekzada Q, Wessulv Å, Khoshkar J, Marouf F, Gorsetman J, Risberg D, Lissing M, et al. Clinical application of the multigene analysis test in discriminating between ulcerative colitis and Crohn’s disease: a retrospective study. Scand J Gastroenterol. 2012;47:162–169. doi: 10.3109/00365521.2011.647065. [DOI] [PubMed] [Google Scholar]
- 359.Huett A, Xavier RJ. Neither hide nor hair: the difficulty of identifying useful disease biomarkers. Gastroenterology. 2008;134:2164–2168. doi: 10.1053/j.gastro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 360.Stylianou E. Epigenetics: the fine-tuner in inflammatory bowel disease? Curr Opin Gastroenterol. 2013;29:370–377. doi: 10.1097/MOG.0b013e328360bd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–3577. [PubMed] [Google Scholar]
- 362.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 363.Olsen J, Gerds TA, Seidelin JB, Csillag C, Bjerrum JT, Troelsen JT, Nielsen OH. Diagnosis of ulcerative colitis before onset of inflammation by multivariate modeling of genome-wide gene expression data. Inflamm Bowel Dis. 2009;15:1032–1038. doi: 10.1002/ibd.20879. [DOI] [PubMed] [Google Scholar]
- 364.Coskun M, Bjerrum JT, Seidelin JB, Troelsen JT, Olsen J, Nielsen OH. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J Gastroenterol. 2013;19:4289–4299. doi: 10.3748/wjg.v19.i27.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zahm AM, Thayu M, Hand NJ, Horner A, Leonard MB, Friedman JR. Circulating microRNA is a biomarker of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2011;53:26–33. doi: 10.1097/MPG.0b013e31822200cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wu F, Guo NJ, Tian H, Marohn M, Gearhart S, Bayless TM, Brant SR, Kwon JH. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2011;17:241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Paraskevi A, Theodoropoulos G, Papaconstantinou I, Mantzaris G, Nikiteas N, Gazouli M. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 368.Palsson B. Metabolic systems biology. FEBS Lett. 2009;583:3900–3904. doi: 10.1016/j.febslet.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 370.Roda G, Caponi A, Benevento M, Nanni P, Mezzanotte L, Belluzzi A, Mayer L, Roda A. New proteomic approaches for biomarker discovery in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1239–1246. doi: 10.1002/ibd.21212. [DOI] [PubMed] [Google Scholar]
- 371.Storr M, Vogel HJ, Schicho R. Metabolomics: is it useful for inflammatory bowel diseases? Curr Opin Gastroenterol. 2013;29:378–383. doi: 10.1097/MOG.0b013e328361f488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Bennike T, Birkelund S, Stensballe A, Andersen V. Biomarkers in inflammatory bowel diseases: current status and proteomics identification strategies. World J Gastroenterol. 2014;20:3231–3244. doi: 10.3748/wjg.v20.i12.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hatsugai M, Kurokawa MS, Kouro T, Nagai K, Arito M, Masuko K, Suematsu N, Okamoto K, Itoh F, Kato T. Protein profiles of peripheral blood mononuclear cells are useful for differential diagnosis of ulcerative colitis and Crohn’s disease. J Gastroenterol. 2010;45:488–500. doi: 10.1007/s00535-009-0183-y. [DOI] [PubMed] [Google Scholar]
- 374.Sharma U, Singh RR, Ahuja V, Makharia GK, Jagannathan NR. Similarity in the metabolic profile in macroscopically involved and un-involved colonic mucosa in patients with inflammatory bowel disease: an in vitro proton ((1)H) MR spectroscopy study. Magn Reson Imaging. 2010;28:1022–1029. doi: 10.1016/j.mri.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 375.Seeley EH, Washington MK, Caprioli RM, M’Koma AE. Proteomic patterns of colonic mucosal tissues delineate Crohn’s colitis and ulcerative colitis. Proteomics Clin Appl. 2013;7:541–549. doi: 10.1002/prca.201200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.M’Koma AE, Seeley EH, Washington MK, Schwartz DA, Muldoon RL, Herline AJ, Wise PE, Caprioli RM. Proteomic profiling of mucosal and submucosal colonic tissues yields protein signatures that differentiate the inflammatory colitides. Inflamm Bowel Dis. 2011;17:875–883. doi: 10.1002/ibd.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Poulsen NA, Andersen V, Møller JC, Møller HS, Jessen F, Purup S, Larsen LB. Comparative analysis of inflamed and non-inflamed colon biopsies reveals strong proteomic inflammation profile in patients with ulcerative colitis. BMC Gastroenterol. 2012;12:76. doi: 10.1186/1471-230X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Fiocchi C. Inflammatory bowel disease: evolutionary concepts in biology, epidemiology, mechanisms and therapy. Curr Opin Gastroenterol. 2013;29:347–349. doi: 10.1097/MOG.0b013e3283622b73. [DOI] [PubMed] [Google Scholar]


