Abstract
AIM: To analyze hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) using the tumor-node-metastasis (TNM) staging system.
METHODS: We retrospectively analyzed 372 patients with HCC who underwent hepatectomy between 1980 and 2009. We studied the outcomes of HCC patients with PVTT to evaluate the American Joint Committee on Cancer TNM staging system (7th edition) for stratifying and predicting the prognosis of a large cohort of HCC patients after hepatectomy in a single-center. Portal vein invasion (vp) 1 was defined as an invasion or tumor thrombus distal to the second branch of the portal vein, vp2 as an invasion or tumor thrombus in the second branch of the portal vein, vp3 as an invasion or tumor thrombus in the first branch of the portal vein, and vp4 as an invasion or tumor thrombus in the portal trunk or extending to a branch on the contralateral side.
RESULTS: The cumulative 5-year overall survival (5yrOS) and 5-year disease-free survival (5yrDFS) rates of the 372 patients were 58.3% and 31.3%, respectively. The 5yrDFS and 5yrOS of vp3-4 patients (n = 10) were 20.0%, and 30.0%, respectively, which was comparable with the corresponding survival rates of vp1-2 patients (P = 0.466 and 0.586, respectively). In the subgroup analysis of patients with macroscopic PVTT (vp2-4), the OS of the patients who underwent preoperative transarterial chemoembolization was comparable to that of patients who did not (P = 0.747). There was a significant difference in the DFS between patients with stage I HCC and those with stage II HCC (5yrDFS 39.2% vs 23.1%, P < 0.001); however, the DFS for stage II was similar to that for stage III (5yrDFS 23.1% vs 13.8%, P = 0.330). In the subgroup analysis of stage II-III HCC (n = 148), only alpha-fetoprotein (AFP) > 100 mg/dL was independently associated with DFS.
CONCLUSION: Hepatectomy for vp3-4 HCC results in a survival rate similar to hepatectomy for vp1-2. AFP stratified the stage II-III HCC patients according to prognosis.
Keywords: Hepatocellular carcinoma, Hepatectomy, Portal vein tumor thrombosis, Tumor-node-metastasis staging system, Alpha-fetoprotein
Core tip: Hepatectomy for selected patients with hepatocellular carcinoma (HCC) with portal vein invasion (vp) 3 or vp4 may result in a survival rate that is similar to that for hepatectomy in vp1 or vp2 patients. Alpha-fetoprotein (AFP) can stratify the stage II-III patients according to prognosis. If serum AFP is elevated in patients with stage II-III HCC, clinical trials involving neoadjuvant/adjuvant therapy should be considered.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, especially in East Asian countries[1,2]. HCC usually spreads intrahepatically via portal vein branches, and the incidence of portal vein involvement has been found to be approximately 40% in surgically resected series[3]. When tumor thrombi extend to the major portal vein, the prognosis has been reported to be poor[4]. However, hepatic resection is currently still the only therapeutic option in these patients who may have a chance for a cure, and some studies have recently reported improved survival rates after hepatectomy for selected HCC patients with thrombosis to the portal vein[5,6]. Yet, the therapeutic strategy for patients with HCC invading the portal vein remains controversial.
The tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC), which is identical to that of the Union for International Cancer Control (UICC), was revised in 2010. Some modifications have been made in the new 7th edition of the TNM staging system[7]. A recent study by Xu et al[8] reported that the current T classification is unnecessarily complex and fails to stratify patients adequately without prognosis. Several clinical staging systems are currently available for predicting the prognosis of HCC patients after hepatectomy, such as the Cancer of the Liver Italian Program scoring system[9], Japan Integrated Staging score[10], Tokyo score[11], or Okuda staging system[12]. The TNM staging system is currently the most widely used system worldwide, but a global consensus has not been reached on how to satisfactorily predict the prognosis of HCC patients after surgery[13].
The aim of this study was to analyze the results of HCC patients with portal vein tumor thrombosis (PVTT) and to evaluate the AJCC TNM staging system (7th edition) for stratifying and predicting the prognosis of a large cohort of HCC patients after hepatectomy in a single-center.
MATERIALS AND METHODS
Patients
In total, 372 patients underwent macroscopic curative hepatectomy for the treatment of HCC between 1980 and 2009 in the Department of Surgery, Division of Digestive Surgery at the Kyoto Prefectural University of Medicine. All of these patients were analyzed in this study. Curative resection is defined here as the complete removal of a macroscopic tumor that is not exposed on the cut surface.
There were 295 men and 77 women in the patient cohort. The mean ± SD age was 61.5 ± 9.7 years. Underlying liver diseases included cirrhosis in 203 patients (54.6%) and non-cirrhosis in 169 patients (45.4%). According to Child’s classification, modified by Pugh et al[14], 361 patients (97.0%) belonged to group A, nine (2.4%) to group B, and two (0.5%) to group C. The mean ± SD tumor diameter was 4.1 ± 3.0 cm. Hepatectomy and tumor location were defined according to Couinaud’s definition[15] of liver segmentation.
Treatment
Preoperative transarterial chemoembolization (TACE) was performed in 151 patients. The indications for hepatectomy and the types of operative procedures were usually determined based on each patient’s liver function, which was primarily assessed by the Makuuchi Criteria, which includes preoperative measurements of ascites, serum bilirubin levels, and indocyanine green retention rate at 15 min (ICGR15)[16]. Preoperative portal vein embolization was performed in four patients to prevent postoperative liver insufficiency. In total, 294 patients underwent anatomical resection, and 78 underwent non-anatomical resection.
Pathological examination
All resected liver specimens were cut at a thickness of approximately 5 mm, and the microscopic sections were viewed after staining with hematoxylin and eosin. The pathological diagnosis and classification of resected HCC tissues were performed according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Tumors were staged using the definition of TNM classification provided by the International Hepato-Pancreato-Biliary Association and the UICC[7]. Portal vein invasion (vp) was classified into four groups according to the classification system of the Liver Cancer Study Group of Japan[17]. The vp stages were defined as follows: vp1 as an invasion or tumor thrombus distal to the second branch of the portal vein; vp2 as an invasion or tumor thrombus in the second branch of the portal vein; vp3 as an invasion or tumor thrombus in the first branch of the portal vein; vp4 as an invasion or tumor thrombus in the portal trunk or extending to a branch on the contralateral side. In this study, PVTTs infiltrating the second branch or beyond the portal vein were defined as macroscopic PVTT.
Follow-up
The patients were followed up with hepatic ultrasonography, computed tomography, and the assessment of serum alpha-fetoprotein (AFP) levels and serum protein induced by vitamin K absence II levels every 3-6 mo. Disease-free survival (DFS) was defined as the interval between surgery and the date of diagnosis of the first recurrence or the date of the last follow-up. Overall survival (OS) was defined as the interval between surgery and the date of death caused by HCC recurrence, or the date of the last follow-up. The median follow-up duration was 50.3 mo.
Treatment for the hepatic recurrence of HCC
Local treatment for the initial hepatic recurrence of HCC consisted of local ablation therapy and repeat hepatectomy. TACE was performed using the Seldinger technique[18], with iodized oil or gelatin sponge cubes used as embolus material and adriamycin (10-30 mg) and mitomycin C (10-20 mg) used as anticancer drugs.
Statistical analysis
We performed univariate analyses of the clinical and pathological factors that were potentially associated with DFS. Survival was calculated using the Kaplan-Meier method and was compared between groups using the log-rank test. A multivariate analysis using the Cox proportional hazards model was performed to identify independent predictors of survival. All factors determined to be significant by the univariate analysis were entered into a multivariate regression analysis to identify independent factors. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS for Windows 11.5 software program (SPSS Inc., Chicago, IL, United States).
RESULTS
Clinicopathologic features
A test for the presence of serum hepatitis B surface antigens was positive in 85 patients, and serum anti-hepatitis C antibodies were present in 164 patients. One patient died within 30 d of the operation because of acute renal failure. The cumulative 5-year OS (5yrOS) and 5-year DFS (5yrDFS) rates of all 372 patients were 58.3% and 31.3%, respectively.
Results for hepatectomy in patients with portal vein involvement
vp1 was found in 63 patients, while 10 patients had vp2, six had vp3, and four had vp4. Figure 1A shows the surgical procedures used for PVTT. Five patients with vp3 underwent only ligation of the portal veins. One of these patients underwent a closure of the portal vein stump with a running suture as it was impossible to ligate the portal vein with an adequate margin. There were no patients in whom the thrombus adhered to the portal vein wall, which would have led to combined resections. Four patients with vp4 underwent a thrombectomy and closure of the stump by a running suture of the portal vein. Table 1 shows a comparison of patient characteristics between the vp1-2 and the vp3-4 groups. There were no significant differences in any of the host-related factors, treatment-related factors, or tumor-related factors with the exception of serosal invasion between the vp1-2 and vp3-4 groups. 5yrDFS and 5yrOS of vp3-4 patients (n = 10) were 20.0% and 30.0%, respectively, which were comparable with the corresponding rates in vp1-2 patients (P = 0.466 and 0.586) (Figure 1B and C). In the subgroup analysis of the patients with macroscopic PVTT (vp2-4), the OS of the patients who underwent preoperative TACE was comparable with that of patients who did not undergo preoperative TACE (5yrOS: 29.3% vs 11.3%, P = 0.747) (Figure 1D).
Figure 1.
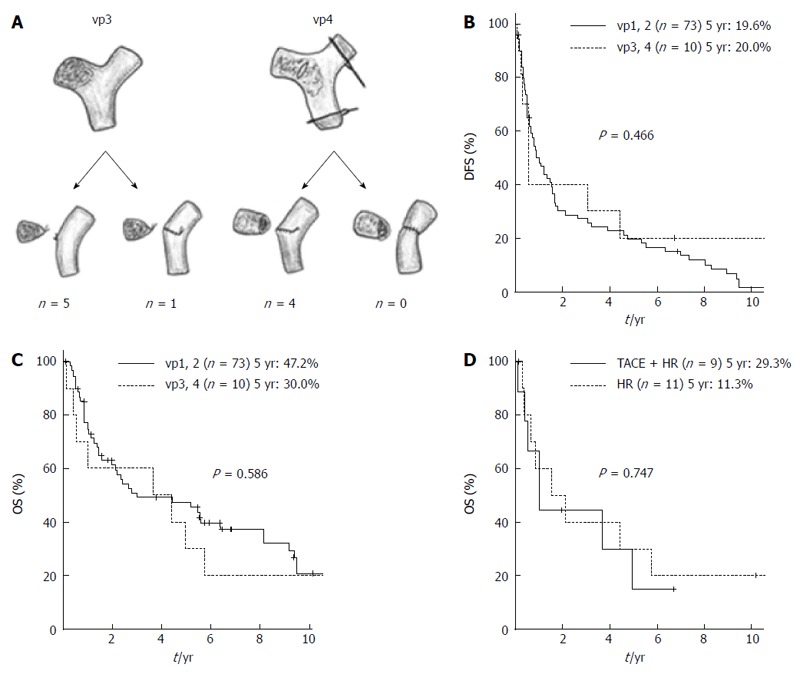
Results in patients with portal vein tumor thrombosis. A: Surgical procedures for portal vein tumor thrombosis; B: Comparison of the disease free survival rates between patients with vp1-2 HCC and those with vp3-4 HCC; C: Comparison of the overall survival rates between patients with vp1-2 HCC and those with vp3-4 HCC; D: Comparison of the overall survival rates between patients who underwent only hepatectomy and those who underwent hepatectomy combined with preoperative transarterial chemoembolization in the subgroup of HCC patients with macroscopic portal vein tumor thrombosis (vp2-4). HCC: Hepatocellular carcinoma; DFS: Disease free survival; OS: Overall survival; TACE + HR: Hepatectomy combined with preoperative transarterial chemoembolization; HR: Hepatectomy; vp: Portal vein invasion.
Table 1.
Characteristics of patients who underwent hepatectomy for the treatment of hepatocellular carcinoma by portal vein invasion
| vp1-2 (n = 73) | vp3-4 (n = 10) | P value | |
| Host-related factors | |||
| Age (yr) | 60.1 ± 9.7 | 59.0 ± 10.5 | 0.790 |
| Gender (Male/Female) | 67/6 | 8/2 | 0.236 |
| Albumin (g/dL) | 3.88 ± 0.52 | 3.81 ± 0.45 | 0.698 |
| Indocyanine green retention rate at 15 min (%) | 17.1 ± 11.6 | 17.5 ± 11.3 | 0.977 |
| Liver cirrhosis | 31 | 6 | 0.295 |
| Treatment-related factors | |||
| Preoperative transarterial chemoembolization | 35 | 7 | 0.191 |
| Method of resection (Anatomical/Non-anatomical) | 65/8 | 9/1 | 0.927 |
| Operation time (min) | 296 ± 240 | 343 ± 387 | 0.735 |
| Blood loss (mL) | 2623 ± 1740 | 3886 ± 3390 | 0.538 |
| Blood transfusion | 29 | 4 | 0.941 |
| Positive surgical margin | 10 | 1 | 0.746 |
| Tumor-related factors | |||
| Alpha-fetoprotein (ng/mL) | 9006 ± 34819 | 11211 ± 27261 | 0.802 |
| Tumor size (mm) | 54.7 ± 33.8 | 62.6 ± 30.0 | 0.234 |
| Number of tumors (Single/Multiple) | 41/32 | 5/5 | 0.713 |
| Capsule (Present/Absent) | 57/16 | 8/2 | 0.890 |
| Bile duct invasion | 11 | 3 | 0.237 |
| Serosal invasion | 11 | 6 | 0.001 |
| Stage (UICC) | < 0.001 | ||
| I | 0 | 0 | |
| II | 59 | 0 | |
| III | 13 | 9 | |
| IV | 1 | 1 |
vp: Portal vein invasion; UICC: Union for International Cancer Control (seventh-edition criteria).
Evaluation of the AJCC TNM staging system for stratifying and predicting prognosis
There was a significant difference in DFS between patients with stage I and stage II HCC (5yrDFS: 39.2% vs 23.1%, P < 0.001) (Figure 2A). However, there was no significant difference in DFS between stage II and stage III patients (5yrDFS: 23.1% vs 13.8%, P = 0.330). In the subgroup analysis of stage II-III HCC patients (n = 148), the Cox proportional hazards model revealed that AFP ≥ 100 mg/dL was the only factor independently associated with DFS (Table 2). For the subgroup with stage II-III HCC, the patients with AFP < 100 mg/dL had a significantly better prognosis with regard to DFS than those with AFP ≥ 100 mg/dL (5yrOS: 32.2% vs 10.8%, P < 0.001) (Figure 2B); however, this trend was not observed in the subgroup with stage I HCC (5yrOS: 42.4% vs 29.4%, P = 0.111) (Figure 2C).
Figure 2.
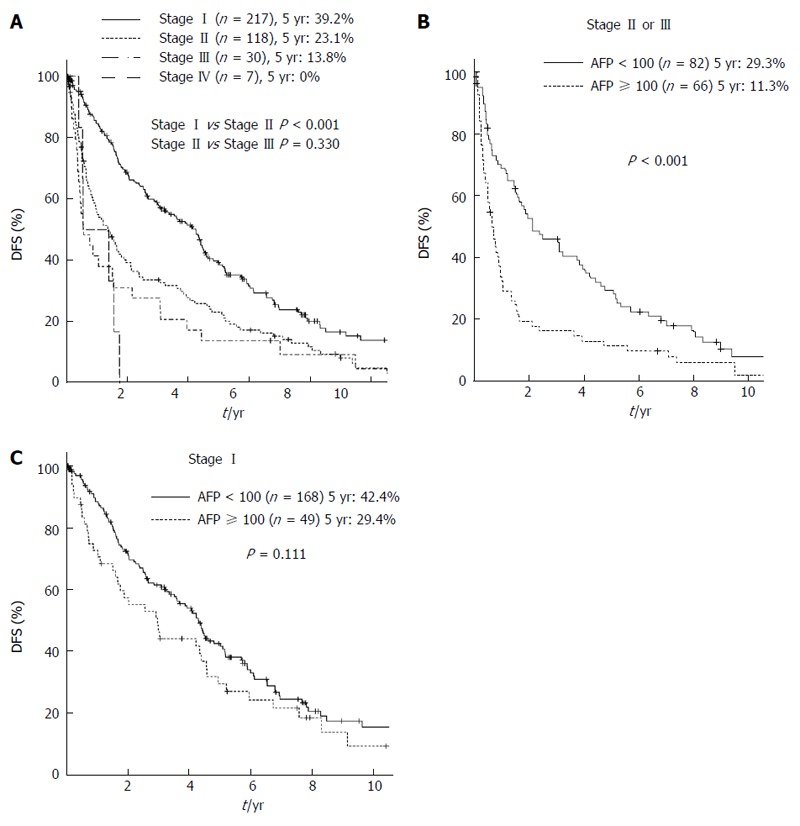
Survival curves according to the stage classifications of the 7th edition tumor-node-metastasis staging system. A: Comparison of survival curves according to the stage classifications of the 7th edition TNM staging system; B: Comparison of the DFS rates between the HCC patients with AFP < 100 mg/dL and those with AFP ≥ 100 mg/dL in the subgroup of stage II or III HCC; C: Comparison of the DFS rates between the HCC patients with AFP < 100 mg/dL and those with AFP ≥ 100 mg/dL in the subgroup of stage I HCC. HCC: Hepatocellular carcinoma; DFS: Disease free survival; AFP: Alpha-fetoprotein; TNM: Tumor-node-metastasis.
Table 2.
Results of the univariate and multivariate analyses of the prognostic factors associated with disease-free survival in patients who underwent hepatectomy for the treatment of stage II or III hepatocellular carcinoma
| No. | 5yrDFS (%) | Median (mo) | Univariate analysis P |
Multivariate analysis |
||
| Hazard ratio (95%CI) | P value | |||||
| Age (yr) | 0.060 | |||||
| < 60 | 76 | 16.7 | 8.4 | |||
| ≥ 60 | 72 | 25.8 | 19.7 | |||
| Gender | 0.898 | |||||
| Male | 123 | 22.1 | 13.8 | |||
| Female | 25 | 15.5 | 16.3 | |||
| Albumin (g/dL) | 0.080 | |||||
| < 4.0 | 78 | 20.8 | 12.7 | |||
| ≥ 4.0 | 50 | 24.1 | 22.5 | |||
| Platelet count (× 104/mm3) | 0.627 | |||||
| < 10 | 30 | 12.7 | 20.0 | |||
| ≥ 10 | 118 | 14.4 | 21.5 | |||
| Indocyanine green retention rate at 15 min (%) | 0.709 | |||||
| < 15 | 71 | 22.2 | 18.7 | |||
| ≥ 15 | 77 | 20.2 | 11.7 | |||
| Alpha-fetoprotein (mg/dL) | < 0.001 | < 0.001 | ||||
| < 100 | 82 | 29.3 | 25.8 | 1 | ||
| ≥ 100 | 66 | 11.3 | 7.7 | 1.950 (1.369 - 2.776) | ||
| Preoperative TACE | 0.452 | |||||
| Performed | 79 | 18.9 | 12.7 | |||
| Not performed | 69 | 23.7 | 18.4 | |||
| Number of tumors | 0.390 | |||||
| Single | 66 | 25.4 | 11.5 | |||
| Multiple | 82 | 17.6 | 16.3 | |||
| Growth pattern | 0.688 | |||||
| Expanding growth | 125 | 22.3 | 16.3 | |||
| Infiltrating growth | 23 | 19.0 | 8.4 | |||
| Capsule | 0.677 | |||||
| Absent | 31 | 27.8 | 10.8 | |||
| Present | 117 | 19.5 | 14.4 | |||
| Serosal invasion | 0.854 | |||||
| Negative | 128 | 22.0 | 16.3 | |||
| Positive | 20 | 15.8 | 8.4 | |||
| Portal vein invasion | 0.417 | |||||
| Absent | 68 | 21.7 | 18.1 | |||
| Present | 80 | 20.5 | 10.4 | |||
| Surgical margin | 0.799 | |||||
| Negative | 135 | 20.7 | 14.4 | |||
| Positive | 13 | 27.3 | 6.1 | |||
| Underlying liver disease | 0.266 | |||||
| Others | 71 | 23.9 | 19.7 | |||
| Cirrhosis | 77 | 18.6 | 11.4 | |||
| Tumor size (mm) | 0.025 | |||||
| < 30 | 47 | 32.6 | 25.4 | |||
| ≥ 30 | 101 | 16.2 | 10.1 | |||
| Bile duct tumor thrombosis | 0.515 | |||||
| Absent | 132 | 20.4 | 14.4 | |||
| Present | 16 | 26.7 | 8.6 | |||
| Stage | 0.389 | |||||
| II | 118 | 23.1 | 16.3 | |||
| III | 30 | 13.8 | 6.7 | |||
5yrDFS: Cumulative 5-yr disease free survival; TACE: Transarterial chemoembolization.
DISCUSSION
HCC patients with PVTT have been treated using a number of techniques, including surgical resection[5], TACE[19], hepatic arterial infusion (HAI) chemotherapy[20], and systemic chemotherapy[21]. Only a limited number of extensive studies have evaluated the prognostic factors for HCC patients with PVTT[5,22-26]. Ohkubo et al[24] reported that for patients with tumor diameters < 10 cm and no intrahepatic metastasis, curative resection can lead to a favorable prognosis in patients with HCC infiltrating the second branch of the portal vein or beyond. Ikai et al[26] reported that an index using the factors of ascites, prothrombin activity, and tumor diameter is useful for making appropriate treatment strategy decisions for HCC patients with PVTT in the major portal vein. Recently, Ban et al[5] reported that a hepatectomy and thrombectomy for vp4 may result in a survival benefit similar to that achieved with a hepatectomy for vp3. In this study, a hepatectomy for patients with vp3-4 HCC was found to provide a comparable survival benefit to the benefits achieved through a hepatectomy for vp1-2. This aggressive procedure is therefore considered to be an effective treatment method in selected patients with HCC with PVTT involving the major portal vein.
TACE is usually contraindicated for patients with portal obstruction, because of the high risk for hepatic insufficiency[27]. However, Lee et al[28] reported in 1997 that TACE is safe for the treatment of HCC with portal trunk obstruction when patients have sufficient collateral circulation around the portal trunk. Minagawa et al[22] reported better survival in HCC patients with PVTT in or beyond the second branch of the portal vein that were treated with hepatectomy combined with preoperative TACE and 42% of these patients had a 5yrOS. When the number of primary nodules is less than two, the portal trunk is not occluded by a tumor thrombus, and the ICGR15 is better than 20%, however, 60% of patients with 4.3 mo of mean survival could not undergo hepatic resection. In this study, we could not find a survival benefit with preoperative TACE for HCC patients with PVTT. Similarly, Ban et al[5] reported that the efficacy of combined preoperative TACE could not be demonstrated compared with hepatectomy alone. Recently, the use of sorafenib[29] or HAI[30] using a cisplatin-based regimen was introduced as a therapeutic regimen for the management of HCC with PVTT. Additional postoperative TACE, HAI, or systemic chemotherapy for treating patients who have HCC with PVTT should be investigated further.
Some modifications have been made in the new 7th edition TNM staging system[7]. Lu et al[31] also reported that the TNM staging system provides inadequate information from which to determine the prognosis of HCC patients. In this study, DFS of stage II was comparable with that of stage III. One reason for this result may be the comparable survival between HCC patients of groups vp1-2 and vp3-4. Similar to our analysis, Xu et al[8] showed no significant survival difference of between stages II and III using the 7th TNM classification. In this study, in the subgroup analysis of stage II-III HCC (n = 148), the Cox proportional hazards model revealed that only AFP ≥ 100 mg/dL was independently associated with disease-free survival. Similar to our results, Leung et al[13] mentioned that the accuracy of stratification is lost for the stage III population subgroup in the TNM classification, and an AFP value ≥ 200 ng/mL was found to be an additional important factor affecting treatment outcome. To date, there are several systematic reviews on the role of neoadjuvant/adjuvant therapy for patients with HCC treated with hepatectomy[32-35]. A clinical trial to examine the recurrence-preventative effect of sorafenib when administered after curative treatments such as resection or ablation (STORM trial) is in progress[34]. If serum AFP is elevated in patients with stage II-III HCC, clinical trials involving neoadjuvant/adjuvant therapy should be performed.
The limitations of the present study include its retrospective nature, the fact that all of the patients were treated at a single center and the different follow-up times for the early and late groups[36]. Moreover, a lead-time bias was present because of recent advances in diagnostic modalities[37]. However, we believe that the results of the study to be acceptable, although the prognosis of patients who undergo hepatectomy procedures for HCC with thrombosis to the portal vein is still unsatisfactory.
In conclusion, aggressive hepatectomy for selected HCC patients with vp3 or vp4 may provide a comparable survival benefit to that achieved via hepatectomy for vp1 or vp2. AFP can be used to stratify stage II-III patients according to prognosis. If serum AFP is elevated in patients with stage II-III HCC, clinical trials involving neoadjuvant/adjuvant therapy should be considered.
COMMENTS
Background
The therapeutic strategy for patients with advanced hepatocellular carcinoma (HCC) invading the portal vein remains controversial. The tumor-node-metastasis (TNM) staging system is currently the most widely used system worldwide; however, a global consensus has not been established on how to satisfactorily predict the prognosis of HCC patients after surgery.
Research frontiers
When tumor thrombi extend to the major portal vein, the prognosis has been reported to be poor. However, hepatic resection is still currently the only therapeutic option in these patients who may have a chance for a cure, and some studies have recently reported improved survival rates after hepatectomy for selected HCC patients with thrombosis to the portal vein.
Innovations and breakthroughs
In this study, a hepatectomy for patients with portal vein invasion (vp) 3-4 HCC was found to provide a comparable survival benefit to that achieved after hepatectomy for vp1-2. This aggressive procedure is therefore considered to be an effective treatment method in selected patients with HCC with portal vein tumor thrombosis (PVTT) for the major portal vein.
Applications
We analyze the results of HCC patients with PVTT to evaluate the American Joint Committee on Cancer TNM staging system (7th edition) for stratifying and predicting the prognosis of a large cohort of HCC patients after hepatectomy in a single-center.
Terminology
HCC usually spreads intrahepatically via portal vein branches, and the incidence of portal vein involvement has been found to be approximately 40% in surgically resected series. When tumor thrombi extend to the major portal vein, the prognosis has been reported to be poor. However, hepatic resection is currently still the only therapeutic option in these patients who may have a chance for a cure, and some studies have recently reported improved survival rates after hepatectomy for selected HCC patients with thrombosis to the portal vein. Yet, the therapeutic strategy for patients with HCC invading the portal vein remains controversial.
Peer review
In this study, a hepatectomy for patients with vp3-4 HCC was found to provide a comparable survival benefit to the benefits achieved through a hepatectomy for vp1-2. This aggressive procedure is therefore considered to be an effective treatment method in selected patients with HCC with PVTT involving the major portal vein.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 21, 2014
First decision: May 13, 2014
Article in press: July 11, 2014
P- Reviewer: Sakamoto Y S- Editor: Nan J L- Editor: AmEditor E- Editor: Ma S
References
- 1.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, et al. Changing trends in long-term outcomes after hepatic resection for hepatocellular carcinoma: A 30-year, single-center experience. Anticancer Res. 2013;33:5097–5105. [PubMed] [Google Scholar]
- 3.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799; discussion 799-800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 5.Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13:1921–1928. doi: 10.1007/s11605-009-0998-0. [DOI] [PubMed] [Google Scholar]
- 6.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. Springer: New York; 2010. pp. 191–200. [Google Scholar]
- 8.Xu LB, Wang J, Liu C, Pang HW, Chen YJ, Ou QJ, Chen JS. Staging systems for predicting survival of patients with hepatocellular carcinoma after surgery. World J Gastroenterol. 2010;16:5257–5262. doi: 10.3748/wjg.v16.i41.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679–680. doi: 10.1053/jhep.2000.16475. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–425. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 14.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 15.Couinaud C. [Liver lobes and segments: notes on the anatomical architecture and surgery of the liver ] Presse Med. 1954;62:709–712. [PubMed] [Google Scholar]
- 16.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. doi: 10.1016/s0002-9610(99)80227-x. [DOI] [PubMed] [Google Scholar]
- 17.Liver Cancer study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 2nd ed. In: Tokyo: Kanehara; 2003. pp. 13–28. [Google Scholar]
- 18.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 19.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]
- 20.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 21.Okada S, Okazaki N, Nose H, Yoshimori M, Aoki K. Prognostic factors in patients with hepatocellular carcinoma receiving systemic chemotherapy. Hepatology. 1992;16:112–117. doi: 10.1002/hep.1840160119. [DOI] [PubMed] [Google Scholar]
- 22.Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379–384. doi: 10.1097/00000658-200103000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, Kianmanesh R, Ng IO, Curley SA, Yamaoka Y, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403–410. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Ohkubo T, Yamamoto J, Sugawara Y, Shimada K, Yamasaki S, Makuuchi M, Kosuge T. Surgical results for hepatocellular carcinoma with macroscopic portal vein tumor thrombosis. J Am Coll Surg. 2000;191:657–660. doi: 10.1016/s1072-7515(00)00740-7. [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Hsieh SR, Chen JT, Ho WL, Lin MC, Yeh DC, Liu TJ, P’eng FK. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273–1279. doi: 10.1001/archsurg.135.11.1273. [DOI] [PubMed] [Google Scholar]
- 26.Ikai I, Hatano E, Hasegawa S, Fujii H, Taura K, Uyama N, Shimahara Y. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg. 2006;202:431–438. doi: 10.1016/j.jamcollsurg.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Yamada R, Kishi K, Sato M, Sonomura T, Nishida N, Tanaka K, Shioyama Y, Terada M, Kimura M. Transcatheter arterial chemoembolization (TACE) in the treatment of unresectable liver cancer. World J Surg. 1995;19:795–800. doi: 10.1007/BF00299773. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Kondo M, Morimoto M, Numata K, Nozaki A, Tanaka K. Hepatic arterial infusion therapy with a fine powder formulation of cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Jpn J Clin Oncol. 2011;41:69–75. doi: 10.1093/jjco/hyq145. [DOI] [PubMed] [Google Scholar]
- 31.Lu W, Dong J, Huang Z, Guo D, Liu Y, Shi S. Comparison of four current staging systems for Chinese patients with hepatocellular carcinoma undergoing curative resection: Okuda, CLIP, TNM and CUPI. J Gastroenterol Hepatol. 2008;23:1874–1878. doi: 10.1111/j.1440-1746.2008.05527.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 33.Lau WY, Lai EC, Leung TW, Yu SC. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43–48. doi: 10.1097/SLA.0b013e3181571047. [DOI] [PubMed] [Google Scholar]
- 34.Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM) Cancer. 2009;115:4646. doi: 10.1002/cncr.24673. [DOI] [PubMed] [Google Scholar]
- 35.Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:50–55. doi: 10.1159/000333259. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, et al. Clinical analysis of anatomical resection for the treatment of hepatocellular carcinoma based on the stratification of liver function. World J Surg. 2014;38:1154–1163. doi: 10.1007/s00268-013-2369-y. [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Rosner GL, Broemeling LD. Bayesian inference for the lead time in periodic cancer screening. Biometrics. 2007;63:873–880. doi: 10.1111/j.1541-0420.2006.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


