Abstract
AIM: To investigate whether liver steatosis reduction due to a six-month dietary intervention results in significant changes in the concentrations of fatty acids.
METHODS: A group of 35 Caucasian individuals diagnosed with different levels of steatosis were prospectively enrolled in the present study. Analysis of the fatty acid profiles was performed according to changes in liver steatosis (liver steatosis reduction by one or two degrees) after a six-month dietary intervention. The diet helped reduce body mass in obese and overweight patients, and stabilize both glycemia and dyslipidemia. Fatty acids were extracted according to the Folch method and analyzed by gas chromatography.
RESULTS: This study showed significant changes in fatty acid profiles in patients who had reduced liver steatosis by one as well as two degrees. A reduction in liver steatosis by one degree caused a significant increase in the level of the n-3 family: eicosapentaenoic acid (P < 0.055), docosapentaenoic acid-C 22:5 (P < 0.05) and docosahexaenoic acid (P < 0.05). A reduction in liver steatosis by two degrees caused a significant decrease in serum palmitoleic acid-C 16:1 (P < 0.05).
CONCLUSION: Liver steatosis reduction is associated with changes in fatty acid profiles, and these changes may reflect an alteration in fatty acid synthesis and metabolism. These findings may help better understand regression of nonalcoholic fatty liver disease.
Keywords: Nonalcoholic fatty liver disease, Fatty acids, Biochemical parameters, Gas chromatography, Sterol regulatory element binding proteins 1c, n-3 Family, n-6 Family, Diet
Core tip: A reduction in liver steatosis due to a six-month dietary intervention resulted in significant changes in the concentrations of fatty acids. These changes may reflect an alteration in fatty acid synthesis and metabolism. These results show key elements in the mechanism of the reduction of liver steatosis and allow for a better understanding of the regression of nonalcoholic fatty liver disease.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a form of chronic disease with histological changes similar to alcoholic fatty liver in individuals who do not abuse alcohol[1,2]. The main causes of NAFLD are associated with insulin resistance, metabolic syndrome and serious lipid metabolism disorders[1]. Insulin resistance causes a lack of inhibition of gluconeogenesis and promotes hyperglycemia. It stimulates hepatic fatty acid synthesis and leads to steatosis. Under the influence of oxidative stress, liver cells produce tumor necrosis factor (TNF) and interleukin, which increase insulin resistance and intensify the process of hepatic fibrosis. There is a very strong correlation between obesity and the prevalence of NAFLD[1,2]. With the increasing epidemic of obesity, the frequency of NAFLD has also increased. The treatment of NAFLD aims to eliminate pathogenic factors. The basic therapeutic recommendation is a well-balanced diet. It is desirable to limit the amount of fat consumption. The diet should also contain food with a low glycemic index, rich in fiber and antioxidants[2,3].
The composition of fatty acids present in hepatocytes is closely correlated with free fatty acids in the blood circulation. Qualitative and quantitative analyses of the free fatty acids in serum provide an indirect source of information on the synthesis of fatty acids in the liver[4]. Fatty acids associated with the metabolic activity of the liver are saturated fatty acids, such as palmitic acid and products of its conversion, monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) from the n-3 and n-6 families[1,5].
Palmitic (C 16:0) acid is a precursor of long-chain fatty acids synthesized in the liver. The enzyme catalyzing the formation of C 16:0 is fatty acid synthetase (FAS)[6]. This enzyme is regulated by a group of transcription factors that belong to a family of sterol regulatory element binding proteins (SREBP), particularly SREBP-1c. The elongation of palmitic acid leads to stearic acid (C 18:0) and its elongation product (Figure 1).
Figure 1.
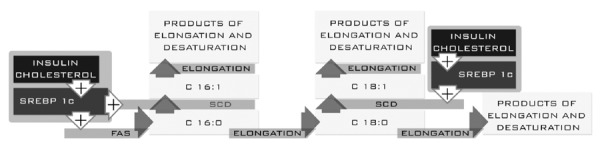
Palmitic acid plays an important structural role in cell membranes. Recent reports indicate that palmitic acid causes hepatotoxicity by induction of oxidative stress and apoptosis in liver cells[7,8]. The liver and most other tissues have the ability to synthesize MUFAs. These acids are produced by enzymatic transformations of C 16:0 and C 18:0 catalyzed by Δ9 desaturase (SCD)[9].
The fatty acid precursor of the n-3 family is α-linolenic acid (αLNA). αLNA concentration in plasma phospholipid is less than 0.5% of total fatty acids. It is a precursor of eicosapentaenoic acid (EPA 20:5 n-5) and docosahexaenoic acid (DHA, 20:6 n-3). EPA is formed by several changes catalyzed by elongase and desaturase. DHA is a product of EPA β-oxidation. Both DHA and EPA have protective effects against many illnesses. It is known that the conversion of αLNA to EPA and DHA is less than 0.2% of EPA and 0.05% of DHA[10]. EPA plays an important role in the production of a third series of eicosanoids (n = 3), which have less pro-inflammatory potential than the products of arachidonicacid[11-13] (Figure 2). EPA competes with arachidonic acid (AA) for the same enzymes: cyclooxygenase and 5-lipoxygenase. This leads to displacement of AA in the cell membranes and a reduction in the amount of strong pro-inflammatory products in exchange for EPA and DHA products[14-16] (Figure 2).
Figure 2.
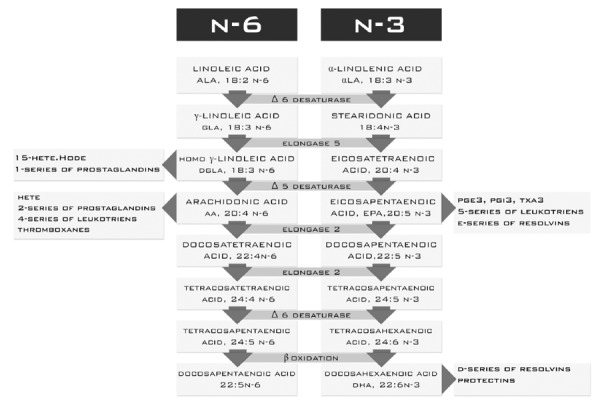
The main function of DHA is to build phospholipid membranes. The presence of this acid in the membranes accounts for approximately 5% of total fatty acids[10]. However, this amount is variable and depends on supplementation in the diet. DHA is a precursor of the anti-inflammatory resolvins (Figure 2) and lipid peroxidation products. It also affects the activity and conformation of some enzymes and indirectly affects the transcription of genes[11,17,18]. The precursor of PUFAs from the n-6 family is linoleic acid (LA). LA is converted to γ-linolenic acid (GLA) by Δ6 desaturase and elongated to dihomoγ-linolenic acid (DGLA). Enzymatic conversion of DGLA promotes pro-inflammatory mediators[19,20] (Figure 2). Another acid from the n-6 family is AA. AA is converted to prostaglandins (2 series), leukotrienes (4 series), thromboxanes, hydroxyeicosatetraenoic acids and hydroxyoctadecadienoic acids. These substances are very important and strong inflammatory mediators[21,22] (Figure 2).
MATERIALS AND METHODS
The aim of the study was to compare the fatty acid profile and biochemical parameters of patients with NAFLD, before and after a six-month dietary intervention. The fatty acid analysis was performed according to changes in liver steatosis (liver steatosis reduction by one and two degrees) following the six-month dietary intervention (Figure 3).
Figure 3.

Study plan.
Patients
A group of 35 Caucasian individuals diagnosed with NAFLD were prospectively enrolled in the study. The degree of liver steatosis was assessed by a trained physician according to the Hamaguchi score[23] using an abdominal ultrasound high-resolution B-mode scanner (Acuson X300). All NAFLD patients included in the study were negative for HBV (hepatitis B virus) and anti-HCV (hepatitis C virus) and had a negative history of alcohol intake (less than 20 g/d). After an overnight fast, venous blood was collected and placed in tubes with anticoagulant for lipid analyses. Whole blood was collected and placed in ethylenediaminetetraacetic acid tubes. Blood was immediately placed on ice or in a refrigerator, and the samples were centrifuged at 3500 rpm for 10 min at 4 °C within 2 h of collection. Plasma was then immediately stored under conditions to minimize artificial oxidation (i.e., with an antioxidant cocktail in an inert atmosphere). Standard blood biochemical analyses were carried out at the University Hospital Laboratory. Clinical and laboratory data are summarized in Tables 1 and 2. Body mass index (BMI) was calculated based on an individual’s mass and height. A signed informed consent form was obtained from each patient. The study protocol was approved by the ethics committee of Pomeranian Medical University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Table 1.
Clinical and laboratory data of patients who had reduced steatosis by one degree (n = 22)
| Parameter |
Before diet |
After diet value |
P value | ||
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Age (yr) | 53.57 ± 13.94 | 60 (20) | 53.57 ± 13.94 | 60 (20) | - |
| Body mass index (kg/m²) | 34.75 ± 6.26 | 33.41(7.2) | 32.22 ± 5.97 | 30.7 (67.21) | NS |
| Aspartatetransaminase (U/L) | 31.86 ± 19.9 | 23 (16) | 28.29 ± 20.86 | 19.5 (3) | NS |
| Alaninetransaminase (U/L) | 57.86 ± 65.6 | 33.5 (27.25) | 39.07 ± 36.28 | 23.5 (15.25) | NS |
| Gammaglutamyltrasferase (U/L) | 50.14 ± 40.2 | 31.5 (39.25) | 40.71 ± 35.25 | 26.5 (25) | NS |
| Triacylglycerols (mg/dL) | 135 ± 3.9 | 115 (41) | 126.79 ± 89 | 106 (33.75) | P < 0.05 |
| Cholesterol (mg/dL) | 206.4 ± 42.45 | 203.5 (41.3) | 195.5 ± 49.3 | 208 (54.2) | NS |
| High density lipoprotein (mg/dL) | 48.21 ± 11.4 | 49.5 (8.5) | 52.07 ± 17.22 | 47.5 (21.5) | NS |
| Low density lipoprotein (mg/dL) | 130.3 ± 42.19 | 129.5 (44.3) | 119 ± 36.5 | 128.5 (46.8) | NS |
| Glucose (mg/mL) | 109.6 ± 51.9 | 96.5 (17.8) | 115.9 ± 65.72 | 101 (14.3) | NS |
| Insulin (U/mL) | 19.6 ± 20.35 | 12.3 (16.48) | 13.1 ± 12.56 | 10.85 (8) | P < 0.055 |
| HOMA-IR | 5.42 ± 6.08 | 2.98 (2.45) | 3.65 ± 3.21 | 2.63 (3.57) | NS |
IQR: Interquartile range.
Table 2.
Clinical and laboratory data of patients who had reduced steatosis by two degrees (n = 13)
| Parameter |
Before diet |
After diet |
P value | ||
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Age (yr) | 53.44 ± 13.65 | 55 (14) | 53.44 ± 13.65 | 55 (14) | - |
| Body mass index (kg/m²) | 33.33 ± 5.32 | 34 (6.24) | 29.32 ± 3.83 | 30.62 (5.21) | P < 0.055 |
| Aspartatetransaminase (U/L) | 33 ± 11.7 | 30 (15) | 21.67 ± 7.35 | 19 (3) | P < 0.01 |
| Alaninetransaminase (U/L) | 42.2 ± 27.75 | 33 (14) | 24.9 ± 19.74 | 20 (8) | P < 0.01 |
| Gammaglutamyltrasferase (U/L) | 60.9 ± 74.87 | 34 (32) | 42 ± 51.39 | 28 (15) | NS |
| Triacylglycerols (mg/dL) | 141.8 ± 61.33 | 151 (77) | 113.4 ± 60 | 100 (72) | NS |
| Cholesterol (mg/dL) | 209.8 ± 47 | 214 (17) | 191.6 ± 58.65 | 196 (38) | P < 0.055 |
| High density lipoprotein (mg/dL) | 52.2 ± 14.43 | 48 (20) | 55.5 ± 18.9 | 52 (21) | NS |
| Low density lipoprotein (mg/dL) | 129 ± 42.9 | 124 (27) | 113.2 ± 52.83 | 119 (39) | NS |
| Glucose (mg/mL) | 104.8 ± 10.4 | 102 (10) | 105.4 ± 14.05 | 100 (17) | NS |
| Insulin (U/mL) | 8 ± 4.65 | 8.8 (7.7) | 5.95 ± 4.35 | 4.8 (5.5) | P < 0.05 |
| HOMA-IR | 2.11 ± 1.27 | 1.93 (2.12) | 1.58 ± 1.20 | 1.28 (1.94) | P < 0.05 |
IQR: Interquartile range.
Dietary intervention
The diet was selected and matched according to the calorie needs of individual patients. The diet helped reduce body mass in obese and overweight patients, and stabilize both glycemia and dyslipidemia. In patients with normal weight, energy intake was consistent with physiological needs and guaranteed the maintenance of ideal body weight. Protein intake was 1.0 g/kg body weight per day. More than half of the protein came from dairy products and fish. The fiber in each diet varied between 25% and 30%. The contents of fruits and vegetables in the recommended diets were sufficient to ensure an appropriate level of vitamins and minerals (especially vitamins A, K, C and the B-group). Sodium intake was reduced to 5 g/24 h. The preferred type of fat was easy to digest, such as butter, cream, milk or oil. Energy from fat differed depending on the needs of the patient and ranged from 20% to 35% of the energy intake. Carbohydrate intake ranged between 50% and 65%. The amount of sugars (including fructose) was reduced to 10% of basal metabolic rate. Diet composition and energy intake were ascertained using questionnaires (24-h food diaries). Each subject was interviewed about their dietary pattern the previous day. Data from questionnaires were analyzed using food composition tables (IZZ, Poland) and DIETETYK 6.0 software (Jumar, Poland).
Isolation of fatty acids
Plasma was obtained from blood (taken at blood clots) by centrifugation for 10 min at 1200 G. Fatty acids were extracted according to the Folch method[24]. 0.5 mL of plasma was saponified with 1 mL of 2 mol/L KOH methanolic solution at 70 °C for 20 min and then methylated with 2 mL of 14% boron trifluoride in methanol under the same conditions. Then 2 mL of n-hexane and 10 mL of saturated NaCl solution were added. One milliliter of the n-hexane phase was collected for analysis.
Analysis of fatty acid methyl esters
Gas chromatography was performed using the Agilent Technologies 7890A GC System (SUPELCOWAX™ 10 Capillary GC Column (15 mm × 0.10 mm, 0.10 μm); Supelco, Bellefonte, PA, United States). Chromatographic conditions were as follows: the initial temperature was 60 °C for 0 min, increased at a rate of 40 °C/min to 160 °C (0 min), increased at a rate of 30 °C/min to 190 °C (0.5 min) and then increased at a rate of 30 °C/min to 230 °C for 2.6 min, where it was maintained for 4.9 min. The total analysis was approximately 8 min and the gas flow rate was 0.8 mL/min with hydrogen as the carrier gas. Fatty acids were identified by comparing their retention times with those of commercially available standards.
Statistical analysis
Statistica 7.1 software was used for the statistical analyses and all results are expressed as median and interquartile range, and mean ± standard deviation. As the distribution in most cases deviated from normal (Shapiro-Wilk test), non-parametric Mann-Whitney test was used for comparisons between groups, and P < 0.05 was considered statistically significant.
RESULTS
Changes in anthropometry and plasma findings in patients who had reduced liver steatosis by one degree
The baseline laboratory and anthropometry findings are shown in Table 1. In terms of biochemical parameters, we found a significant difference in the plasma concentration of triacylglycerols (135 ± 3.9 before the diet and 126.79 ± 89 after the diet). Insulin was on the border of statistical significance (19.6 ± 20.35 before the diet and 13.1 ± 12.56 after the diet, P < 0.055) (Table 1).
The analysis of fatty acid profiles showed statistically significant differences in the concentrations of the following acids: docosapentaenoic acid (0.0142 ± 0.0083 before the diet and 0.0215 ± 0.015 after the diet), DHA (0.0462 ± 0.0253 before the diet and 0.0923 ± 0.0953 after the diet). Eicosapentaenic acid was on the border of statistical significance (0.028 ± 0.0013 before the diet and 0.032 ± 0.018 after the diet, P < 0.055) (Table 3).
Table 3.
Fatty acid profiles in patients who had reduced steatosis by one degree (n = 22)
| Name of fatty acid (μg/mL) |
Before diet |
After diet |
P value | ||
| Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | ||
| C12:0 (lauricacid) | 0.0029 (0.0194) | 0.003 ± 0.00175 | 0.004 (0.002) | 0.0028 ± 0.0016 | NS |
| C14:0 (myristicacid) | 0.0315 (0.01038) | 0.02727 ± 0.0152 | 0.0329 (0.0174) | 0.0329 ± 0.0174 | NS |
| C14:1 (palmitoleicacid) | 0.0015 (0.0008) | 0.0016 ± 0.0019 | 0.0017 (0.0014) | 0.0018 ± 0.0013 | NS |
| C15:0 (pentadecanoate acid) | 0.008 (0.0035) | 0.0076 ± 0.004 | 0.0072 (0.0035) | 0.008 ± 0.0044 | NS |
| C16:0 (palmiticacid) | 0.7 (0.182) | 0.6658 ± 0.3366 | 0.8514 (0.3692) | 0.8056 ± 0.42 | NS |
| C16:1 (palmitoleicacid) | 0.0523 (0.0206) | 0.0576 ± 0.0392 | 0.0834 (0.0782) | 0.0762 ± 0.0524 | NS |
| C17:0 (heptadecanoicacid) | 0.015 (0.2529) | 0.1534 ± 0.4572 | 0.0129 (0.0059) | 0.1535 ± 0.3732 | NS |
| C18:0 (stearicacid) | 0.2222 (0.059) | 0.2262 ± 0.1122 | 0.2712 (0.1128) | 0.2654 ± 0.1277 | NS |
| C18:1w9 (oleic acid) | 0.6661 (0.1658) | 0.5915 ± 0.3156 | 0.8 (0.3768) | 0.7157 ± 0.3939 | NS |
| C18:1 (vaccenicacid) | 0.0573 (0.0103) | 0.0543 ± 0.0289 | 0.0755 (0.0277) | 0.063 ± 0.0302 | NS |
| C18:2n6 (linoleicacid) | 0.5825 (0.1895) | 0.5398 ± 0.3929 | 0.8198 (0.3670) | 0.7482 ± 0.3672 | NS |
| C18:3n-6 (gamma linolenic acid) | 0.0051 (0.0032) | 0.0061 ± 0.0045 | 0.0096 (0.0074) | 0.0096 ± 0.007 | NS |
| C18:3n-3 (linolenicacid) | 0.0244 (0.0056) | 0.03 ± 0.0144 | 0.0291 (0.0055) | 0.025 ± 0.127 | NS |
| C18:4 (stearidonicacid) | 0.0051 (0.003) | 0.0045 ± 0.0021 | 0.0054 (0.013) | 0.005 ± 0.0024 | NS |
| C20:4 (arachidonicacid) | 0.1596 (0.0431) | 0.1650 ± 0.0841 | 0.1540 (0.0716) | 0.1675 ± 0.1069 | NS |
| C20:5 (eicosapentaenicacid) | 0.022 (0.0021) | 0.028 ± 0.0013 | 0.029 (0.0174) | 0.032 ± 0.018 | P < 0.055 |
| C22:0 (behenicacid) | 0.0117 (0.0043) | 0.0099 ± 0.0051 | 0.0013 (0.0037) | 0.0093 ± 0.0044 | NS |
| C22:1n13 (erucate acid) | 0.0029 (0.001) | 0.006 ± 0.0062 | 0.0056 (0.0252) | 0.0242 ± 0.0211 | NS |
| C23:0 (tricosanoicacid) | 0.0021 (0.0014) | 0.0024 ± 0.0015 | 0.0025 (0.0009) | 0.00217 ± 0.0011 | NS |
| C22:4n6 (docosatetraenoicacid) | 0.005 (0.002) | 0.0045 ± 0.0025 | 0.0045 (0.0028) | 0.0043 ± 0.0026 | NS |
| C22:5w3 (docosapentaenoicacid) | 0.0137 (0.0001) | 0.0142 ± 0.0083 | 0.0242 (0.0072) | 0.0215 ± 0.015 | P < 0.05 |
| C22:6w3 (docosahexaenoicacid) | 0.0522 (0.0027) | 0.0462 ± 0.0253 | 0.0639 (0.0277) | 0.0923 ± 0.0953 | P < 0.05 |
| C24:1 (nervonicacid) | 0.0023 (0.0029) | 0.003 ± 0.0023 | 0.0028 (0.0013) | 0.0031 ± 0.0026 | NS |
| C26:0 (cerolicacid) | 0.0072 (0.0112) | 0.0117 ± 0.0098 | 0.0119 (0.0134) | 0.0124 ± 0.0234 | NS |
IQR: Interquartile range.
Changes in anthropometry and plasma findings in patients who had reduced liver steatosis by two degrees
The baseline laboratory and anthropometry findings are shown in Table 2. In terms of biochemical parameters, we found significant differences in the plasma concentrations of aspartate transaminase (33 ± 11.7 before the diet and 21.67 ± 7.35 after the diet), alanine transaminase (42.2 ± 27.75 before the diet and 24.9 ± 19.74 after the diet), insulin (8 ± 4.65 before the diet and 5.95 ± 4.35 after the diet), and HOMA-IR (1.93 (2.12) before the diet and 1.28 (1.94) after the diet). All of these parameters were significantly higher after the six-month dietary intervention. One of the anthropometric parameters (BMI) was on the border of statistical significance (2.11 ± 1.27 before the diet and 1.58 ± 1.20 after the diet, P < 0.055) (Table 2).
Analysis of the fatty acid profiles showed a statistically significant difference in palmitoleic acid (0.0843 ± 0.0299 before the diet and 0.0609 ± 0.0424 after the diet) (Table 4).
Table 4.
Fatty acid profiles in patients who had reduced steatosis by two degrees (n = 13)
| Name of fatty acid (μg/mL) |
Before diet |
After diet |
P value | ||
| Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | ||
| C12:0 (lauricacid) | 0.0037 (0.009) | 0.004 ± 0.00251 | 0.0023 (0.0012) | 0.0047 ± 0.002 | NS |
| C14:0 (myristicacid) | 0.0323 (0.026) | 0.034 ± 0.016) | 0.0199 (0.0055) | 0.0337 ± 0.021 | NS |
| C14:1 (palmitoleicacid) | 0.0017 (0.0009) | 0.0022 ± 0.0012 | 0.0011 (0.0006) | 0.0021 ± 0.0024 | NS |
| C15:0 (pentadecanoate acid) | 0.0105 (0.0095) | 0.0021 ± 0.007 | 0.0058 (0.0045) | 0.0075 ± 0.001 | NS |
| C16:0 (palmiticacid) | 0.9432 (0.3451) | 0.8542 ± 0.2357 | 0.623 (0.3112) | 0.7332 ± 0.3912 | NS |
| C16:1 (palmitoleicacid) | 0.0919 (0.0343) | 0.0843 ± 0.0299 | 0.0444 (0.0157) | 0.0609 ± 0.0424 | P < 0.05 |
| C17:0 (heptadecanoicacid) | 0.0129 (0.079) | 0.1105 ± 0.0898 | 0.0111 (0.0076) | 0.1014 ± 0.0732 | NS |
| C18:0 (stearicacid) | 0.2653 (0.089) | 0.2712 ± 0.1792 | 0.2476 (0.1354) | 0.2412 ± 0.1277 | NS |
| C18:1n-9 (oleic acid) | 0.7595 (0.3613) | 0.7568 ± 0.4556 | 0.4984 (0.2611) | 0.6265 ± 0.2341 | NS |
| C18:1 (vaccenicacid) | 0.076 (0.0236) | 0.0665 ± 0.036 | 0.0553 (0.0217) | 0.0549 ± 0.04 | NS |
| C18:2n6 (linoleicacid) | 0.8 (0.2755) | 0.7514 ± 0.356 | 0.5315 (0.19) | 0.6677 ± 0.3183 | NS |
| C18:3n-6 (gamma linolenic acid) | 0.0094 (0.0052) | 0.0106 ± 0.0095 | 0.0073 (0.0026) | 0.0094 ± 0.0051 | NS |
| C18:3n-3 (linolenicacid) | 0.0243 (0.0088) | 0.0236 ± 0.0137 | 0.0195 (0.0078) | 0.0225 ± 0.1647 | NS |
| C18:4 (stearidonicacid) | 0.0056 (0.003) | 0.0052 ± 0.0032 | 0.0034 (0.041) | 0.0042 ± 0.0014 | NS |
| C20:4 (arachidonicacid) | 0.1899 (0.0822) | 0.1720 ± 0.0924 | 0.1465 (0.0825) | 0.1564 ± 0.089 | NS |
| C20:5 (eicosapentaenicacid) | 0.0284 (0.0109) | 0.0315 ± 0.009 | 0.0233 (0.0124) | 0.0249 ± 0.018 | NS |
| C22:0 (behenicacid) | 0.0119 (0.0055) | 0.0147 ± 0.0083 | 0.0113 (0.0056) | 0.0107 ± 0.007 | NS |
| C22:1n13 (erucate acid) | 0.0162 (0.0004) | 0.007 ± 0.002 | 0.0143 (0.0242) | 0.0102 ± 0.0032 | NS |
| C23:0 (tricosanoicacid) | 0.0035 (0.0005) | 0.0324 ± 0.0017 | 0.0035 (0.0008) | 0.0033 ± 0.0016 | NS |
| C22:4n6 (docosatetraenoate acid) | 0.0058 (0.0022) | 0.0056 ± 0.0038 | 0.0044 (0.0029) | 0.005 ± 0.0034 | NS |
| C22:5w3 (docosapentaenate acid) | 0.0169 (0.0087) | 0.0172 ± 0.005 | 0.013 (0.0077) | 0.01615 ± 0.01 | NS |
| C22: 6w3 (docosahexanoate acid) | 0.07 (0.038) | 0.0679 ± 0.0541 | 0.0695 (0.047) | 0.0585 ± 0.0222 | NS |
| C24:1 (nervonicacid) | 0.003 (0.0029) | 0.0031 ± 0.0071 | 0.002 (0.0016) | 0.0024 ± 0.0011 | NS |
| C26:0 (cerolicanid acid) | 0.0093 (0.0013) | 0.0091 ± 0.0042 | 0.0078 (0.005) | 0.0082 ± 0.0067 | NS |
IQR: Interquartile range.
DISCUSSION
It is not known whether a reduction in fatty liver in patients with NAFLD is associated with a change in the composition of fatty acids in the blood[4], or if the extent of the reduction in steatosis influences circulating fatty acid profiles. In this study, we found significant changes in plasma lipids in patients who had reduced liver steatosis by one or two degrees. Patients who had reduced liver steatosis by one degree showed a significant improvement associated with an increase in the level of the n-3 fatty acid family: eicosapentaenoic acid (P < 0.05), docosapentaenoic acid (P < 0.05) and DHA (P < 0.05). To provide a mechanism for the changes in patients with a slight reduction in steatosis, the results of biochemical parameters should be considered. These changes were associated with decreased concentrations of TG (P < 0.05) and a decreased trend in insulin concentration (P < 0.055). Significant changes in the concentrations of the n-3 family during the decrease in TG level were observed. Fatty acids of the n-3 family, particularly EPA and DHA, have a protective effect on the liver by decreasing insulin resistance, reducing inflammation and inhibiting apoptosis in hepatocytes. These acids act at many different levels, including an influence on gene transcription, activation of enzymes and the production of cytokines[25-27]. The positive benefits of PUFAs of the n-3 family have been noted in the treatment of many diseases, including nonalcoholic fatty liver. Changing the fatty acid profile has an impact on the profile of their bioactive products. n-3 and n-6 fatty acids compete for the same enzymes needed for elongation and desaturation of carbon chains, as well as the production of their active inflammatory mediators (Figure 2). The increased presence of EPA and its conversion products (docosapentaenoic acid and DHA) resulted in severe elongation and desaturation of fatty acids of the n-3 family (Figure 4).
Figure 4.
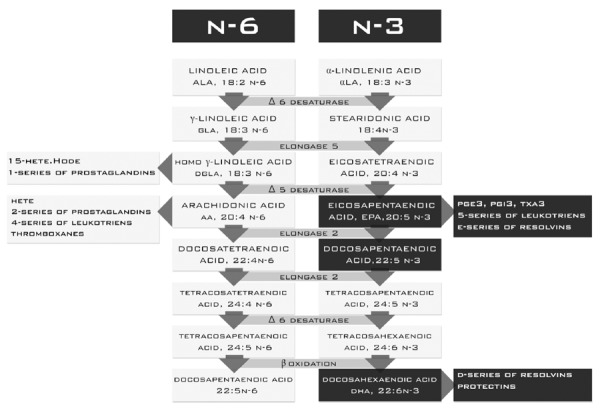
Synthesis of the n-3 and n-6 families in patients who had reduced liver steatosis by one degree.
EPA and DHA compete with AA and γ-linolenic acid for cycloxygenases and lipoxygenases. The increased concentration of these acids may result in replacement of some of the AA metabolites and GDLA with metabolites of EPA and DHA. Bioactive products of EPA may have pro- and anti-inflammatory properties. It should be noted that the pro-inflammatory metabolites of EPA have 100-fold less activity than the biological activity of AA metabolites. In addition, EPA is a substrate for a series of E resolvins, and the activity of this series is mainly due to inhibition of migration, phagocytosis of neutrophils and reduced secretion of pro-inflammatory cytokines[10-13]. DHA is a precursor of highly anti-inflammatory metabolites, such as the D series of resolvins, and protectins. These substances interact with many immune cells and platelets. The most important effects are related to reduced migration of neutrophils and T cells at the center of inflammation and decreased secretion of TNF-α, IL-12, and IFN-γ[11]. Both EPA and DHA have an impact on the regulation of transcription factors related to genes involved in lipid metabolism in the liver and cause deactivation of SREBP-1, PPRα and PPRγ[28]. To date, three types of proteins in the SPEBP family have been described: SREBP-1a, SREBP-1c, and SREBP-2. The main activities of the first two factors are found in the liver and adipose tissue. The expression of genes coding proteins of the SREBP family is achieved by changes in insulin and cholesterol concentration. High concentrations of insulin and cholesterol indirectly activate SREBP proteins, particularly SREBP-1c. Expression and activation of the SREBP family increase expression of FAS and palmitic acid synthesis[29,30] (Figure 1). Lower insulin level and increased concentrations of EPA and DHA may indicate a decrease in the expression of SREBP, which results in decreased expression of FAS and consequently the synthesis of palmitic acid. We did not observe this dependence in our patients. The reason for this may be due to multifactorial regulation based on insulin and cholesterol. Regulation based on changes in the concentration of the n-3 family seems to be less important, compared to changes in insulin and cholesterol. The n-3 family also has an impact on the regulation of other transcription factors, including PPRα and PPRγ, which regulate genes involved in fatty acid oxidation. Furthermore, PPRα inhibits the expression of genes responsible for the formation of TNF-α and IL-6[31]. PPRγ is involved with genes associated with insulin sensitivity[28]. Analysis of the fatty acid profiles in patients who had reduced liver steatosis by two degrees showed a significantly lower level of palmitoleic acid. This acid is produced by the direct conversion (SCD) of palmitic acid (Figure 5). A reduced level of palmitoleic acid may cause a decrease in SCD activity and a decrease in the formation of transcriptions factors (SREBP-1c) regulating the expression of SCD. This hypothesis seems to be confirmed by the results of the biochemical parameters. These changes were associated with a general improvement in liver function, which was manifested by significantly lower concentrations of liver enzymes (AST, P < 0.01, ALT, P < 0.01), insulin levels (P < 0.05) and insulin resistance (HOMA P < 0.05). A significantly lower level of insulin and a slightly decreased trend in cholesterol level (P < 0.055) may have a significant impact on the decreased expression of SREBP-1c (Figure 5). In this case, a reduction in palmitic acid synthesis would be observed[29]. Analysis of fatty acid profiles did not show a reduction in palmitic acid synthesis. Palmitic acid can be converted to stearic acid or desaturated to palmitoleic acid (Figure 5). Decreased concentration of palmitoleic acid without significant changes in the concentration of stearic acid may indicate decreased synthesis of palmitic acid. No significant changes in the concentration of palmitic acid could be the result of accumulation due to lack of conversion to its enzymatic products (Figure 5).
Figure 5.
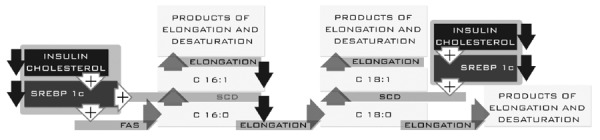
Regulation of palmitic acid formation in patients who had reduced liver steatosis by two degrees.
Fatty acid profiles help us to better understand regression of NAFLD. Liver steatosis reduction is associated with changes in fatty acid profiles, and these changes may reflect an alteration in fatty acid synthesis and metabolism. Changes in patients with a slight reduction in liver steatosis showed elevated levels of the n-3 family fatty acids. This may decrease the amount of inflammatory mediators in the liver and indirectly result in a reduction in SREBP-1c. We also noted that the main factors influencing the reduction in steatosis and reduced synthesis of fatty acids in the liver were insulin and cholesterol. According to the changes in fatty acid profiles, this was also confirmed in patients who had reduced steatosis by two degrees.
COMMENTS
Background
Nonalcoholic fatty liver disease (NAFLD) is associated with insulin resistance, metabolic syndrome and serious lipid metabolism disorders. NAFLD includes a wide spectrum of liver pathologies, ranging from pure steatosis (usually a benign and non-progressive condition) to nonalcoholic steatohepatitis, which may progress to liver cirrhosis with complications such as portal hypertension and hepatocellular carcinoma.
Research frontiers
With the increasing epidemic of obesity, the frequency of NAFLD has also increased. The main research fields related to NAFLD are associated with prevention, treatment and clarification of the mechanism of the disease.
Innovations and breakthroughs
Nonalcoholic fatty liver disease is one of the most common chronic liver diseases, with histological changes similar to alcoholic fatty liver. Diagnosis of NAFLD can usually be achieved by imaging studies. However, the liver biopsy is the golden standard in the assessment of liver fibrosis. Pathophysiology of NAFLD is still subject to intensive research. The main causes of NAFLD are related to metabolic syndrome, insulin resistance and lipid metabolic disorders. The treatment of NAFLD aims to eliminate pathogenic factors. The basic therapeutic recommendation is a well-balanced diet and lifestyle change.
Applications
Liver steatosis reduction is associated with changes in fatty acid profiles, and these changes may reflect an alteration in fatty acid synthesis and metabolism. These findings may help better understand regression of NAFLD.
Terminology
Nonalcoholic fatty liver disease refers to lipid infiltration of the hepatic parenchymal cells, resulting in a yellow-colored liver. The abnormal lipid accumulation is usually in the form of triglycerides, either as a single large droplet or multiple small droplets. Fatty liver is caused by an imbalance in the metabolism of fatty acids. According to MESH, sterol regulatory element binding protein is a protein that regulates expression of genes involved in fatty acid metabolism and lipogenesis.
Peer review
In this study, the author detected the changes of the composition of serum fatty acids in the patients with NAFLD, and explored the relationship between the liver steatosis reduction and the changes in fatty acid profiles.
Footnotes
Supported by National Science Centre, Poland, No. NCNNrNN404150539
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 13, 2014
First decision: June 10, 2014
Article in press: July 30, 2014
P- Reviewer: Gong ZJ, Lesmana CRA, Zhu X S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Conlon BA, Beasley JM, Aebersold K, Jhangiani SS, Wylie-Rosett J. Nutritional management of insulin resistance in nonalcoholic fatty liver disease (NAFLD) Nutrients. 2013;5:4093–4114. doi: 10.3390/nu5104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanuri G, Bergheim I. In Vitro and in Vivo Models of Non-Alcoholic Fatty Liver Disease (NAFLD) Int J Mol Sci. 2013;14:11963–11980. doi: 10.3390/ijms140611963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpentier YA, Portois L, Sener A, Malaisse WJ. Correlation between liver and plasma fatty acid profile of phospholipids and triglycerides in rats. Int J Mol Med. 2008;22:255–262. [PubMed] [Google Scholar]
- 5.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan G, Unal R, Pokrovskaya I, Yao-Borengasser A, Phanavanh B, Lecka-Czernik B, Rasouli N, Kern PA. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res. 2006;47:2444–2450. doi: 10.1194/jlr.M600248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012;11:1. doi: 10.1186/1476-511X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DS, Jeong SK, Kim HR, Kim DS, Chae SW, Chae HJ. Metformin regulates palmitate-induced apoptosis and ER stress response in HepG2 liver cells. Immunopharmacol Immunotoxicol. 2010;32:251–257. doi: 10.3109/08923970903252220. [DOI] [PubMed] [Google Scholar]
- 9.Finelli C, Tarantino G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J Gastrointestin Liver Dis. 2012;21:293–302. [PubMed] [Google Scholar]
- 10.Ratnayake WM, Galli C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann Nutr Metab. 2009;55:8–43. doi: 10.1159/000228994. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–374. doi: 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 13.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman R. Ω-3 and Ω-6 essential fatty acid status in human health and disease. In: Yehuda S, Mostofsky DI, editors. Handbook of Essential Fatty Acid Biology: Biochemistry, Physiology, and Behavioral Neurology; 1997. pp. 139–182. [Google Scholar]
- 15.Lands WE, Morris A, Libelt B. Quantitative effects of dietary polyunsaturated fats on the composition of fatty acids in rat tissues. Lipids. 1990;25:505–516. doi: 10.1007/BF02537156. [DOI] [PubMed] [Google Scholar]
- 16.Mantzioris E, Cleland LG, Gibson RA, Neumann MA, Demasi M, James MJ. Biochemical effects of a diet containing foods enriched with n-3 fatty acids. Am J Clin Nutr. 2000;72:42–48. doi: 10.1093/ajcn/72.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Walczewska A, Stępień T, Bewicz-Binkowska D, Zgórzyńska E. [The role of docosahexaenoic acid in neuronal function] Postepy Hig Med Dosw (Online) 2011;65:314–327. doi: 10.5604/17322693.945763. [DOI] [PubMed] [Google Scholar]
- 18.Kajarabille N, Díaz-Castro J, Hijano S, López-Frías M, López-Aliaga I, Ochoa JJ. A new insight to bone turnover: role of ω-3 polyunsaturated fatty acids. ScientificWorldJournal. 2013;2013:589641. doi: 10.1155/2013/589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411–1414. doi: 10.1093/jn/128.9.1411. [DOI] [PubMed] [Google Scholar]
- 20.Johnson MM, Swan DD, Surette ME, Stegner J, Chilton T, Fonteh AN, Chilton FH. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997;127:1435–1444. doi: 10.1093/jn/127.8.1435. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- 22.Nowak JZ. [Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty acids] Postepy Hig Med Dosw (Online) 2010;64:115–132. [PubMed] [Google Scholar]
- 23.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 27.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 28.Di Minno MN, Russolillo A, Lupoli R, Ambrosino P, Di Minno A, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:5839–5847. doi: 10.3748/wjg.v18.i41.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azzout-Marniche D, Bécard D, Guichard C, Foretz M, Ferré P, Foufelle F. Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes. Biochem J. 2000;350 Pt 2:389–393. [PMC free article] [PubMed] [Google Scholar]
- 30.Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Müller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]


