Abstract
AIM: To compare the Helicobacter pylori (H. pylori) eradication rate of clarithromycin-based triple therapy, metronidazole-based triple therapy, sequential therapy and concomitant therapy.
METHODS: A total of 680 patients infected with H. pylori were divided into 4 groups and each group was treated with a different eradication therapy. Clarithromycin-based triple therapy was applied to the first group [rabeprazole, amoxicillin and clarithromycin (PAC) group: proton pump inhibitor (PPI), amoxicillin, clarithromycin], whereas the second group was treated with metronidazole-based triple therapy [rabeprazole, amoxicillin and metronidazole (PAM) group: PPI, amoxicillin, metronidazole]. The third group was treated with rabeprazole and amoxicillin, followed by rabeprazole, clarithromycin and metronidazole (sequential group). The final group was simultaneously treated with rabeprazole, amoxicillin clarithromycin and metronidazole (concomitant therapy group). In the case of a failure to eradicate H. pylori, second-line quadruple and third-line eradication therapies were administered.
RESULTS: The per protocol (PP) analysis was performed on 143, 139, 141 and 143 patients in the PAC, PAM, sequential and concomitant groups, respectively. We excluded patients who did not receive a C13-urea breath test (22, 20, 23 and 22 patients, respectively) and patients with less than an 80% compliance level (5, 11, 6 and 5 patients, respectively). The eradication rates were 76.2% (109/143) in the PAC group, 84.2% (117/139) in the PAM group, 84.4% (119/141) in the sequential group and 94.4% (135/143) in the concomitant group (P = 0.0002). All 14 patients who failed second-line therapy were treated with third-line eradication therapy. Among these 14 patients, 6 infections were successfully eradicated with the third-line therapy. Both PP and intention-to-treat analysis showed an eradication rate of 42.9% (6/14). In the PAC group, 3 of 4 patients were successfully cured (3/4, 75%); 2 of 2 patients in the PAM group (2/2, 100%) and 1 of 5 patients in the sequential group (1/5, 20%) were also cured. In the concomitant group, all 3 patients failed (0/3, 0%).
CONCLUSION: The eradication rate for the concomitant therapy was much higher than those of the standard triple therapy or sequential therapy (ClinicalTrials.gov number NCT01922765).
Keywords: Helicobacter pylori, Eradication, Drug resistance, Concomitant therapy, Sequential therapy
Core tip: A total 680 patients who were infected with Helicobacter pylori (H. pylori) were divided into 4 groups and each group was treated with a different eradication therapy. The eradication rates were 76.2%, 84.2%, 84.4% and 94.4% in the rabeprazole, amoxicillin and clarithromycin (PAC) group, the rabeprazole, amoxicillin and metronidazole group, the sequential group and the concomitant group. Comparing concomitant therapy and PAC therapy, the number needed to treat was 5.49. The eradication rate was 84.4% for the second-line therapy and 42.9% for the third-line therapy. The study was designed due to the high prevalence of H. pylori and gastric cancer and the recent marked increase in clarithromycin resistance.
INTRODUCTION
Helicobacter pylori (H. pylori) survives in the acidic environment of the gastric mucosa, causing gastritis, peptic ulcers, mucosa-associated lymphoid tissue lymphoma and gastric cancer[1]. Therefore, the eradication of H. pylori can markedly lower gastric and duodenal ulcer recurrence and allowing the treatment of mucosa-associated lymphoid tissue lymphoma[2].
Clarithromycin-containing triple therapy has been the gold standard first-line eradication regimen since its introduction in 1996[3]. Clarithromycin-based triple therapy is still recommended by the Maastricht IV[4], the Asia-Pacific Consensus Guidelines[5] and the Guideline for H. pylori in South Korea[6].
However, the efficacy of current conventional clarithromycin-based triple therapy has decreased to an unacceptably low level worldwide (lower than 80%)[7,8]. The main cause of this deficiency is presumed to be bacterial resistance to clarithromycin. In South Korea, the resistance rate to clarithromycin, which had been 5.9% in 1995, increased to 13.8% in 2003[9], 16.7% in 2005[10] and is currently 23.7%[11] due to the past use of antibiotics. The decrease in the H. pylori eradication rate is closely related to the resistance rate to clarithromycin.
In addition to clarithromycin-based triple therapy, metronidazole-based triple therapy, sequential therapy and concomitant therapy can be used. In conventional triple therapy, the eradication rates of clarithromycin-based and metronidazole-based therapies have been compared[12]. Sequential therapy is more effective than conventional triple therapy[13] and concomitant therapy is also more effective than conventional triple therapy[14]. The comparison between the eradication rates of sequential therapy and concomitant therapy has been controversial[15]. However, almost no studies have compared all of these therapies. Through this prospective, multicenter, randomized controlled clinical trial, we compared the eradication rate of clarithromycin-based triple therapy, metronidazole-based triple therapy, sequential therapy and concomitant therapy.
MATERIALS AND METHODS
Subjects
From July 2013 to March 2014, this multicenter study was undertaken at 5 hospitals (Yeouido St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, Incheon St. Mary’s Hospital, St. Vincent’s Hospital and St. Paul’s Hospital) affiliated with the Catholic University of Korea. The study subjects were patients with gastric symptoms and confirmed gastritis, with gastric and duodenal ulcers on esophago-gastro-duodenoscopy. In cases of patients with coexisting gastric and duodenal ulcers, we categorized the patient based on the larger ulcer. The subjects were between 20 and 75 years of age. Women who were pregnant or lactating, patients previously treated with H. pylori eradication therapy, patients who previously underwent gastric surgery, patients with malignant neoplasms and patients with other severe concomitant diseases were all excluded.
Study design
A total of 680 subjects underwent esophago-gastro-duodenoscopy, were biopsied and had H. pylori infection confirmed with Warthin-Starry stain. This study sample size was determined as follow. We compared the eradication rate of the concomitant group and the other three groups using the χ2 test and the three tests were Bonferroni-corrected. We presumed that the eradication rate of the concomitant therapy to be 90% and that the eradication rate of conventional triple therapy was 75%. By setting the significance level to 0.05/3, the statistical power to 90% and the drop-out rate to 10%, we calculated a need for 170 patients in each group.
The subjects were randomly divided into four groups using a table of random numbers. The first group was treated with rabeprazole, amoxicillin and clarithromycin (the PAC triple group) and rabeprazole, amoxicillin and metronidazole (the PAM triple group) were administered to the second group. The third group was treated with rabeprazole and amoxicillin followed by rabeprazole, clarithromycin and metronidazole (the sequential group). The last group was treated with rabeprazole, amoxicillin, clarithromycin and metronidazole (the concomitant group). Six weeks after the treatment period and at least 2 weeks with no administration of proton pump inhibitors (PPI), we confirmed H. pylori eradication using C13-urea breath tests. If the first-line eradication therapy failed, second-line quadruple therapy, including rabeprazole, tetracycline, bismuth and metronidazole/clarithromycin, was administrated. The third-line eradication therapy, including rabeprazole, amoxicillin and levofloxacin, was utilized when we failed to eradicate the infection using the second-line therapy. To confirm patient compliance, we asked the patients to bring their remaining medication and counted the rest of their pills. Patients with a compliance of less than 80% were excluded from the study per-protocol (PP) analysis (Figure 1). This study was approved by the institutional review board at the Catholic University (SC12OISV0219) and is registered at ClinicalTrials.gov (NCT01922765).
Figure 1.
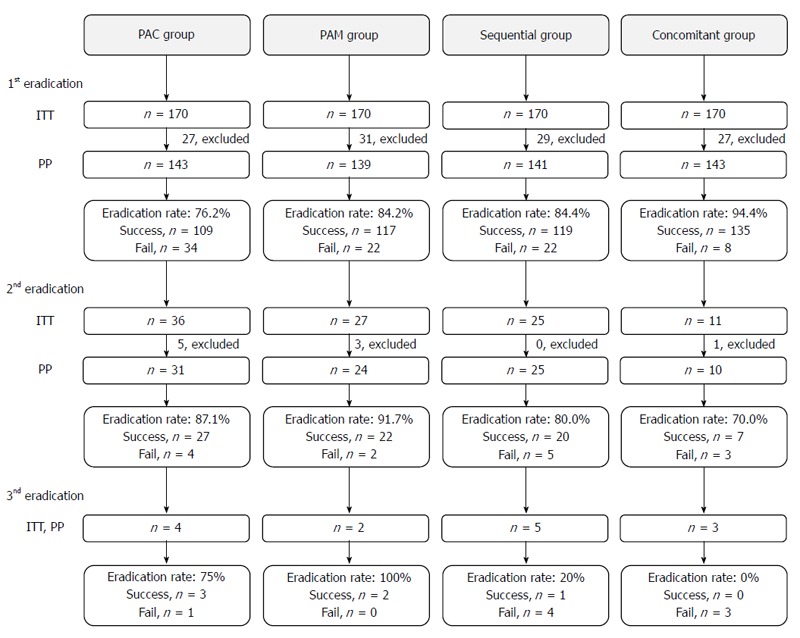
Flow diagram of participants through each eradication stage of the study. PAC: Rabeprazole, amoxicillin and clarithromycin; PAM: Rabeprazole, amoxicillin and metronidazole; ITT: Intention-to-treat; PP: Per protocol.
H. pylori eradication
The 170 patients in the PAC group were treated with rabeprazole 40 mg, amoxicillin 2.0 g and clarithromycin 1.0 g for 7 d. Patients in the PAM group received rabeprazole 40 mg, amoxicillin 2.0 g and metronidazole 1.5 g for 7 d. Patients in the sequential group were treated with rabeprazole 40 mg and amoxicillin 2.0 g for 5 d, then rabeprazole 40 mg, clarithromycin 1.0 g and metronidazole 1.5 g for an additional 5 d. Patients in the concomitant group were treated with rabeprazole 40 mg, amoxicillin 2.0 g, clarithromycin 1.0 g and metronidazole 1.5 g for 7 d.
We determined treatment success or failure using a C13-urea breath test and in cases of failure, a second-line eradication therapy was administered. Patients treated with the clarithromycin-containing triple therapy who experienced failure received metronidazole 1.5 g, rabeprazole 40 mg, tetracycline 2.0 g and bismuth subcitrate 1.2 g for 14 d and patients treated with the metronidazole-containing triple therapy who experienced failure were treated with clarithromycin 1.0 g, rabeprazole 40 mg, tetracycline 2.0 g and bismuth subcitrate 1.2 g for 14 d. In cases of second-line eradication therapy failure, rabeprazole 40 mg, amoxicillin 2.0 g and levofloxacin 500 mg were applied for 14 d as a third-line eradication therapy.
Factors affecting H. pylori eradication
The patients were examined to obtain their age, gender, endoscopic diagnosis (gastritis, gastric ulcer and duodenal ulcer), eradication therapy medicine type, alcohol intake, smoking status, body mass index, total cholesterol level and LDL cholesterol level to determine the effects of H. pylori eradication.
Compliance and adverse drug reaction
We interviewed patients to investigate compliance and the adverse effects of the drugs, including abdominal bloating, abdominal pain, bitter taste, constipation, dizziness, dyspepsia, epigastric pain, general weakness, halitosis, headache, loose stool, loss of appetites, nausea, oral ulcer, skin eruption, sleeping tendency and vomiting.
Statistical analysis
The results of this study were analyzed in an intention-to-treat population and a per-protocol population. SAS, version 9.2 for Windows, was used for the statistical analyses, in which the eradication rate was analyzed by the χ2 test and the factors affecting eradication were analyzed by multivariable logistic regression. P values of < 0.05 were deemed statistically significant.
RESULTS
Patient characteristics
There was no difference (P = 0.103) between the average ages of the groups, which were 55.6 ± 13.6, 57.4 ± 12.8, 58.3 ± 12.3 and 55.8 ± 12.6 years in the PAC, PAM, sequential and concomitant groups, respectively. The male ratios were 60.0%, 62.9%, 64.7% and 62.4% (P = 0.060), respectively. Gastritis, gastric ulcers and duodenal ulcers were, respectively, confirmed in 31.2%, 47.7% and 21.2% of the PAC group patients; in 38.2%, 45.3% and 16.5% of the PAM group patients; in 30.6%, 50.6% and 18.8% of the sequential group patients; and in 38.2%, 45.9% and 15.9% of the concomitant group patients. There was no significant difference among the three groups (P = 0.553) (Table 1).
Table 1.
Characteristics of patients n (%)
| PAC group | PAM group | Sequential | Concomitant | P value | |
| (n = 170) | (n = 170) | (n = 170) | (n = 170) | ||
| Age, yr | 55.6 ± 13.6 | 57.4 ± 12.8 | 58.3 ± 12.3 | 55.8 ± 12.6 | 0.1031 |
| Gender | 0.0602 | ||||
| Male | 102 (60.0) | 107 (62.9) | 110 (64.7) | 106 (62.4) | |
| Female | 68 (40.0) | 63 (37.1) | 60 (35.3) | 64 (37.7) | |
| Diagnosis | 0.5532 | ||||
| Gastric ulcer | 81 (47.7) | 77 (45.3) | 86 (50.6) | 78 (45.9) | |
| Duodenal ulcer | 36 (21.2) | 28 (16.5) | 32 (18.8) | 27 (15.9) | |
| Gastritis | 53 (31.2) | 65 (38.2) | 52 (30.6) | 65 (38.2) |
1-way ANOVA test;
χ2 test. PAC: Rabeprazole, amoxicillin and clarithromycin; PAM: Rabeprazole, amoxicillin and metronidazole.
Eradication rate for first-line treatment
The PP analysis was performed on 143, 139, 141 and 143 patients in the PAC, PAM, sequential and concomitant groups, respectively. We excluded patients who did not receive a C13-urea breath test (22, 20, 23 and 22 patients, respectively) and patients with less than an 80% compliance level (5, 11, 6 and 5 patients, respectively). In the PP analysis, the successful eradication rate in the PAC group was 76.2% (109/143) and the eradication rate in the PAM group was 84.2% (117/139). In the sequential group, 84.4% (119/141) of patients experienced successful eradication and in the concomitant group, 94.4% experienced eradication (135/143) (Table 2). In the intention to treat (ITT) analysis, the eradication rates were 64.1%, 68.8%, 70.7% and 79.4% in the PAC, PAM, sequential and concomitant groups, respectively (Table 2).
Table 2.
First-line eradication rates of Helicobacter pylori
| Achieved/analyzed patients (n) | Eradication rate, (95%CI) | P value1 | P value2 | |
| Per protocol | 0.001 | |||
| PAC (n = 143) | 109/143 | 76.2% (69.3-83.2) | < 0.001 | |
| PAM (n = 139) | 117/139 | 84.2% (78.1-90.2) | 0.016 | |
| Sequential (n = 141) | 119/141 | 84.4% (78.4-90.4) | 0.018 | |
| Concomitant (n = 143) | 135/143 | 94.4% (90.6-98.2) | - | |
| Intent-to-treat | 0.001 | |||
| PAC (n = 170) | 109/170 | 64.1% (58.8-73.0) | 0.007 | |
| PAM (n = 170) | 117/170 | 68.8% (66.3-79.2) | 0.285 | |
| Sequential (n = 170) | 119/170 | 70.7% (65.0-78.5) | 0.169 | |
| Concomitant (n = 170) | 135/170 | 79.4% (74.6-86.5) | - |
P-value of χ2 test;
Bonferroni adjusted P-value of χ2 test on 2 × 2 subtables. PAC: Rabeprazole, amoxicillin and clarithromycin; PAM: Rabeprazole, amoxicillin and metronidazole.
Eradication rate for second-line treatment
A total of 99 patients were treated with second-line eradication therapy, including 86 patient who experienced first-line eradication failure and 13 patients who were excluded from the PP analysis because of low drug compliance. Nine of these 99 patients were lost during the follow-up period and the other 90 patients were analyzed as per protocol. Seventy-six patients were successfully treated with the second-line eradication therapy. The eradication rate was 84.4% (76/90) in the PP analysis and 76.8% (76/99) in the ITT analysis.
In the PP analysis, 27 of 31 PAC group patients (27/31, 87.1%), 22 of 24 PAM group patients (22/24, 91.7%) and 20 of 25 sequential group patients (20/25, 80.0%) were successfully treated. In the concomitant group, 7 of 10 patients were successfully treated (7/10, 70%) (Table 3). In the ITT analysis, 27 of 36 PAC group patients (27/36, 75.0%), 22 of 27 PAM group patients (22/27, 81.5%) and 20 of 25 sequential group patients (20/25, 80.0%) were successfully treated. In the concomitant group, 7 of 11 patients were successfully treated (7/11, 63.6%) (Table 3).
Table 3.
Second-line eradication rates of Helicobacter pylori
| Achieved/analyzed patients (n) | Eradication rate (95%CI) | P value1 | |
| Per protocol | 0.351 | ||
| PAC (n = 31) | 27/31 | 87.1% (75.3-98.9) | |
| PAM (n = 24) | 22/24 | 91.7% (80.6-100) | |
| Sequential (n = 25) | 20/25 | 80.0% (64.3-95.9) | |
| Concomitant (n = 10) | 7/10 | 70.0% (34.8-93.3) | |
| Intent-to-treat | 0.657 | ||
| PAC (n = 36) | 27/36 | 75.0% (60.9-89.1) | |
| PAM (n = 27) | 22/27 | 81.5% (66.8-96.1) | |
| Sequential (n = 25) | 20/25 | 80.0% (64.3-95.9) | |
| Concomitant (n = 11) | 7/11 | 63.6% (35.2-92.1) |
P-value of χ2 test. PAC: Rabeprazole, amoxicillin and clarithromycin; PAM: Rabeprazole, amoxicillin and metronidazole.
Eradication rate for third-line treatment
All 14 patients who failed second-line therapy were treated with third-line eradication therapy. Among these 14 patients, 6 infections were successfully eradicated with the third-line therapy. Both PP and ITT analysis showed an eradication rate of 42.9% (6/14). In the PAC group, 3 of 4 patients were successfully cured (3/4, 75%); 2 of 2 patients in the PAM group (2/2, 100%) and 1 of 5 patients in the sequential group (1/5, 20%) were also cured. In the concomitant group, all 3 patients failed (0/3, 0%) (Table 4).
Table 4.
Third-line eradication rates of Helicobacter pylori
| Achieved/analyzed patients (n) | Eradication rate (95%CI) | P value1 | |
| Treatment group | 0.052 | ||
| PAC (n = 4) | 3/4 | 75.0 (19.4-99.4) | |
| PAM (n = 2) | 2/2 | 100.0 (15.8-100) | |
| Sequential (n = 5) | 1/5 | 20.0 (0.5-71.6) | |
| Concomitant (n = 3) | 0/3 | 0.0 (0.0-70.8) |
P-value of χ2 test. PAC: Rabeprazole, amoxicillin and clarithromycin; PAM: Rabeprazole, amoxicillin and metronidazole.
Factors affecting H. pylori eradication
When comparing the age, gender, diagnosis, alcohol use, smoking status, body mass index and total and LDL cholesterol levels of the successfully eradicated and failed patients, these parameters did not affect the eradication therapy. Only the type of medication combination affected the results of eradication therapy (Table 5).
Table 5.
Factors affecting eradication of Helicobacter pylori n (%)
| Factors | Eradicated | Not | Crude OR (95%CI) | P valuea | Adjust OR (95%CI) | P value1 |
| Age | 56.8 ± 12.9 | 57.6 ± 11.9 | 0.996 | 0.632 | ||
| (0.978-1.014) | ||||||
| Gender | ||||||
| Male | 294 | 34 | - | - | - | - |
| (61.25) | (39.53) | |||||
| Female | 186 | 52 | 0.414 | 0.080 | ||
| (38.75) | (60.47) | (0.259-0.662) | ||||
| Diagnosis | ||||||
| Gastric ulcer | 225 | 38 | 1.121 | 0.663 | ||
| (46.88) | (44.19) | (0.673-1.869) | ||||
| Duodenal ulcer | 86 | 16 | 1.018 | 0.896 | ||
| (17.92) | (18.60) | (0.529-1.957) | ||||
| Gastritis | 169 | 32 | - | - | ||
| (35.21) | (37.21) | |||||
| Treatment Group | ||||||
| PAC | 109 | 34 | - | - | - | - |
| (22.71) | (39.53) | |||||
| PAM | 117 | 22 | 1.659 | 0.430 | 1.738 | 0.559 |
| (24.38) | (25.58) | (0.914-3.012) | (0.948-3.187) | |||
| Sequential | 119 | 22 | 1.687 | 0.478 | 1.636 | 0.386 |
| (24.79) | (25.58) | (0.930-3.062) | (0.894-2.997) | |||
| Concomitant | 135 | 8 | 5.264 | 0.001 | 5.287 | 0.001 |
| (28.13) | (9.3) | (2.341-11.838) | (2.335-11.973) | |||
| Alcohol Intake | ||||||
| Yes | 165 | 27 | 1.145 | 0.591 | ||
| (34.38) | (31.4) | (0.699-1.874) | ||||
| No | 315 | 59 | - | - | ||
| (65.63) | (68.6) | |||||
| Smoking | ||||||
| Smoker | 127 | 18 | 1.359 | 0.281 | ||
| (26.46) | (20.93) | (0.778-2.374) | ||||
| Non-smoker | 353 | 68 | - | - | ||
| (73.54) | (79.07) | |||||
| BMI | 22.7 ± 2.7 | 22.2 ± 2.9 | 1.073 | 0.107 | ||
| (0.985-1.169) | ||||||
| Total Cholesterol | 188.2 ± 39.9 | 188.8 ± 35.1 | 1.000 | 0.914 | ||
| (0.993-1.007) | ||||||
| LDL Cholesterol | 107.3 ± 32.5 | 109.8 ± 31.8 | 0.998 | 0.601 | ||
| (0.989-1.007) |
P-value of Wald test for coefficients of logistic regression. PAC: Rabeprazole, amoxicillin, clarithromycin; PAM: Rabeprazole, amoxicillin, metronidazole; BMI: Body mass index; LDL: Low density lipoprotein.
Adverse drug reaction
Adverse reactions were surveyed in 627 of 680 patients and 307 of those 627 patients reported 373 minor adverse drug reactions. The percentages of patients with adverse reactions were 56.7% in the PAC group (86/152), 52.2% in the PAM group (82/157), 43.8% in the sequential group (70/160) and 43.7% in the concomitant group (69/158). There were 101, 93, 83 and 96 minor adverse events in each group, respectively (P = 0.158).
In order of frequency, bitter taste, loose stool and nausea were the most common adverse reactions in the PAC group. Nausea, dyspepsia and bitter taste prevailed in the PAM group. Nausea, loose stool and dyspepsia were the most common events in the sequential group and epigastric pain, loose stool and bitter taste were most prevalent events in the concomitant group. However, these developments were not statistically significant and there were no major adverse reactions (Table 6).
Table 6.
Incidence of adverse drug reactions n (%)
| PAC | PAM | Sequential | Concomitant | P value1 | |
| (n = 170) | (n = 170) | (n = 170) | (n = 170) | ||
| Patients with side effect | 86 (50.6) | 82 (48.2) | 70 (41.2) | 69 (40.6) | 0.158 |
| (43.07, 58.10) | (40.72, 55.75) | (33.78, 48.57) | (33.21, 47.97) | ||
| Total side effects (n) | 101 | 93 | 83 | 96 | |
| Side effect | |||||
| Abdominal bloating | 2 (1.18) | 5 (2.94) | 0 (0.00) | 0 (0.00) | 7 (1.03) |
| Abdominal pain | 8 (4.71) | 11 (6.47) | 0 (0.00) | 0 (0.00) | 19 (2.79) |
| Bitter taste | 28 (16.47) | 10 (5.88) | 11 (6.47) | 16 (9.41) | 65 (9.56) |
| Constipation | 0 (0.00) | 6 (3.53) | 0 (0.00) | 1 (0.59) | 7 (1.03) |
| Dizziness | 4 (2.35) | 3 (1.76) | 3 (1.76) | 1 (0.59) | 11 (1.62) |
| Dyspepsia | 5 (2.94) | 15 (8.82) | 13 (7.65) | 13 (7.65) | 46 (6.76) |
| Epigastric pain | 6 (3.53) | 0 (0.00) | 11 (6.47) | 21 (12.35) | 38 (5.59) |
| General weakness | 2 (1.18) | 3 (1.76) | 2 (1.18) | 2 (1.18) | 9 (1.32) |
| Halitosis | 0 (0.00) | 0 (0.00) | 1 (0.59) | 1 (0.59) | 2 (0.29) |
| Headache | 4 (2.35) | 3 (1.76) | 3 (1.76) | 4 (2.35) | 14 (2.06) |
| Loose stool | 24 (14.12) | 2 (1.18) | 17 (10.00) | 19 (11.18) | 62 (9.12) |
| Loss of appetite | 6 (3.53) | 3 (1.76) | 1 (0.59) | 0 (0.00) | 10 (1.47) |
| Nausea | 12 (7.06) | 32 (18.82) | 20 (11.76) | 15 (8.82) | 79 (11.62) |
| Oral ulcer | 0 (0.00) | 0 (0.00) | 1 (0.59) | 0 (0.00) | 1 (0.15) |
| Skin eruption | 0 (0.00) | 0 (0.00) | 1 (0.59) | 0 (0.00) | 1 (0.15) |
| Sleeping tendency | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.59) | 1 (0.15) |
| Vomiting | 0 (0.00) | 0 (0.00) | 3 (1.76) | 3 (1.76) | 6 (0.88) |
P-value of χ2 test. PAC: Rabeprazole, amoxicillin, clarithromycin; PAM: Rabeprazole, amoxicillin, metronidazole.
DISCUSSION
The first-line worldwide choice for H. pylori eradication is clarithromycin-based triple therapy, which involves using a PPI and two antibiotics, amoxicillin and clarithromycin[4]. As a first-line eradication therapy, clarithromycin-based triple therapy has been recommended in most guidelines, such as the American College of Gastroenterology, Maastricht IV consensus, the Asia-Pacific Consensus Guidelines and the Guideline for H. pylori in South Korea. However, in most countries, including South Korea, the eradication rate has decreased to below 80% since 2000[16], despite a previous effective rate well over 90% before 2000[17]. The main cause of this decrease is clarithromycin resistance. Thus, there are doubts as to whether we should keep using the current standard triple therapy.
In South Korea, metronidazole is mainly used to treat enterocolitis and gynecologic diseases and resistance to metronidazole frequently occurs. The clarithromycin-based triple therapy was recommended as the gold standard triple therapy due to its high resistance rate for metronidazole. However, in the past 20 years, clarithromycin has mainly been used for pulmonary infectious diseases, whereas the usage of metronidazole has decreased due to better hygienic conditions. Therefore, in this study, the eradication rate of the metronidazole-containing triple therapy was higher than that of the clarithromycin-containing triple therapy. Therefore, we can consider the former as an alternative to clarithromycin-based triple therapy.
To overcome the low eradication rate of clarithromycin therapy, sequential therapy and concomitant therapy have been tried. In sequential therapy, amoxicillin prevents resistance to clarithromycin, which is used later[18]. Amoxicillin disrupts the bacterial cell wall of H. pylori and enables clarithromycin and metronidazole to infiltrate into the bacteria. Due to the disrupted cell wall, H. pylori is unable to form an efflux pump to export clarithromycin and the effect of the antibiotics can be maintained and is effective, even in cases of clarithromycin-resistant H. pylori[19-21].
Sequential therapy has been reported to be more effective than standard triple therapy in many Asian countries. There was a difference among the eradication rates of sequential therapy in several countries, with the highest, 95%, in Thailand[22]; the lowest, 78%, in Turkey[23]; and 89%, in China[24]. However, according to a meta-analysis, sequential therapy was more effective than the standard triple therapy[25]. Additionally, in South Korea, the eradication rate of sequential therapy was 91.8%, which was higher than that of the triple therapy, 80.2%[26]. Sequential therapy was effective for patients with clarithromycin resistance[12,27], patients with metronidazole resistance[28] and even for patients with resistance to both clarithromycin and metronidazole[12]. However, only sequential therapy and standard triple therapy were compared in these studies. The shortcoming of sequential therapy is that the medications change during treatment and patients to consider it difficult to take the medicines. There is also no standard second-line therapy in the case of eradication failure.
Concomitant therapy involves the simultaneous use of a PPI and three types of antibiotics, although it could cause antibiotic abuse and unnecessary resistance. There is no standard second-line eradication medication if the first-line therapy fails. Additionally, debate remains regarding the optimal treatment period, which could be 5, 7, or 10 d. A treatment period of less than 5 d resulted in less than a 90% eradication rate, whereas a 7 d treatment period resulted in a near 90% eradication rate. When the patients were treated for 10 d, the eradication rate was over 90%[29]. There was no difference in the effect of concomitant therapy in clarithromycin-sensitive and resistant groups[30] as well as in metronidazole-sensitive and resistant groups[31]. When H. pylori was resistant to both clarithromycin and metronidazole, there was no difference in the effectiveness of concomitant therapy and sequential therapy[15]. A meta-analysis showed that concomitant therapy was more effective than standard triple therapy[14] and there was no difference in the eradication rates of concomitant therapy and sequential therapy (93.0% vs 93.1%, respectively)[15]. Compared with sequential therapy, a benefit of concomitant therapy is that the patient considers this treatment option simpler and shorter than the other options.
During second-line eradication therapy, patients who experienced the failure of the clarithromycin-containing triple therapy were treated with quadruple-therapy containing metronidazole and the patients who experienced the failure of the metronidazole-containing triple therapy were treated with clarithromycin-containing quadruple therapy for 14 d. The eradication rates in this case were 76.8% and 84.8% according to ITT and PP analyses, respectively. In the sub-group analysis, the concomitant group had the most effective first-line eradication rate, whereas its second-line eradication rate was the lowest, at 70.0%. In South Korea, resistance to metronidazole and tetracycline has increased, but there was no difference in the standard second-line eradication rates[32].
There are still no guidelines or standard therapies for third-line eradication therapy. A PPI, amoxicillin, which is less frequently resistant and other new antibiotics are usually applied. The new antibiotics include levofloxacin, moxifloxacin, rifabutin and quinolone, but H. pylori becomes resistant to quinolone relatively easily. Additionally, rifabutin is commonly used for Mycobacterium tuberculosis, which is already cross-resistant to rifampicin. We selected levofloxacin because the Korean guidelines recommend levofloxacin for 14 d as a third-line eradication therapy. Only 14 patients were treated with this third-line eradication therapy. The eradication rate of the standard triple therapy was 75%-100%, which was high, but the eradication rates of the sequential and concomitant therapies were very low (0%-20%).
Many studies have evaluated the factors that affect H. pylori eradication rates, such as antibiotic resistance, types of gastropathy, alcohol consumption[33], smoking[34], hyperlipidemia[35] and concomitant disease. In this study, we surveyed both the successfully eradicated and failed patients. Regarding age, sex, diagnosis, alcohol use, smoking status, body mass index, cholesterol and LDL-cholesterol, no significant differences were found. The only notable factor was the combination of medicine that the patients took.
A strength of this study was that it was set in a location with a high prevalence of H. pylori and gastric cancer. The recent increase in clarithromycin resistance has markedly increased the number of targeted, prospective, multicenter, randomized clinical trials. We compared all 4 of the therapies currently in use. Additionally, the patients were treated not only with the first-line therapy but also with the second-line and third-line therapies, in a short period of time without follow-up loss. This research was completed in a short period of time to minimize antibiotic resistance influenced by other factors and re-infection. The PPI, rabeprazole was selected because we wanted to minimize the effect of CYP2C19 in locations where the homozygous extensive metabolizer of CYP2C19 was low and the poor metabolizer was high.
A limitation of this study was that more accurate eradication rates could be obtained if we tested for H. pylori infection using two different methods and defined infection as positivity in both tests. Successful eradication would be defined as negative results for both tests, at which point a more accurate eradication rate could be assessed. To complement these data, we biopsied four in the different gastric mucosa, 2 in the gastric antrum and 2 in the gastric body and the samples were stained with Warthin-Starry. We also used the C13-urea breath test, which has a 98% accuracy rate. The eradication rate of sequential therapy and concomitant therapy could be altered by changing the duration of therapy. If we diversified the duration of the eradication therapy and compared the outcomes, a more objective result could be obtained.
The problem with concomitant therapy is that there is no second-line suitable medication in case of treatment failure with the first-line regimen. However, there was no difference in the treatment failure after the first-, second- and third-line eradication therapies among the PAC, PAM, sequential and concomitant groups, as 1, 0, 4, or 3 patients were not successfully treated, respectively, out of the 170 patients in each group. This finding indicates that there is no difference in the eradication results if we select any therapy and continue to second and third-line therapy.
ACKNOWLEDGMENTS
We thank Ka Young Kim from Cornell University, who provided great support in scientific editing and assisted with writing the manuscript.
COMMENTS
Background
Currently, the Helicobacter pylori (H. pylori) eradication rate of clarithromycin-based triple therapy has decreased to an unacceptably low level and novel therapeutic strategies are necessary.
Research frontiers
This is the first study to compare all four therapies of H. pylori currently in use, which are clarithromycin-based triple therapy, metronidazole-based triple therapy, sequential therapy and concomitant therapy in a prospective, multicenter, randomized controlled trial.
Innovations and breakthroughs
The study is designed on a location with a high prevalence of H. pylori and gastric cancer where clarithromycin resistance is markedly increasing. To minimize other factors that cause antibiotic resistance like re-infection, the patients were treated by a short time interval between the first-, second- and third-line therapies without follow-up loss.
Applications
This study offered that the concomitant therapy could be the first-line therapeutic option for the patients of H. pylori infection.
Terminology
The concomitant group was treated with proton pump inhibitor (PPI), amoxicillin, clarithromycin and metronidazole for 7 d and the sequential group was treated with PPI and amoxicillin for 5 d followed by PPI, clarithromycin and metronidazole for 5 d.
Peer review
This study compared the concomitant therapy in the eradication of H. pylori infection with three other treatment regimens. The result was that concomitant therapy achieved the highest eradication rate of 94.4%. This result is in line with current concept of therapies that has been reported in the literature.
Footnotes
Supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, No. 2013R1A1A2062603
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 30, 2014
First decision: May 29, 2014
Article in press: July 25, 2014
P- Reviewer: Ding SZ, D'Elios MM S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Hentschel E, Brandstätter G, Dragosics B, Hirschl AM, Nemec H, Schütze K, Taufer M, Wurzer H. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993;328:308–312. doi: 10.1056/NEJM199302043280503. [DOI] [PubMed] [Google Scholar]
- 3.Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O’Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1:138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 5.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. [Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea] Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 8.Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim do H, Jung KW, Choi KD, Song HJ, Jung HY, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011;58:246–250. [PubMed] [Google Scholar]
- 9.Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004;48:4843–4847. doi: 10.1128/AAC.48.12.4843-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang TJ, Kim N, Kim HB, Lee BH, Nam RH, Park JH, Lee MK, Park YS, Lee DH, Jung HC, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010;44:536–543. doi: 10.1097/MCG.0b013e3181d04592. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Kim N, Kim JM, Nam RH, Chang H, Kim JY, Shin CM, Park YS, Lee DH, Jung HC. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18:206–214. doi: 10.1111/hel.12031. [DOI] [PubMed] [Google Scholar]
- 12.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556–563. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–3079; quiz 1080. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 14.Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109–118. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41.e1. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho DK, Park SY, Kee WJ, Lee JH, Ki HS, Yoon KW, Cho SB, Lee WS, Joo YE, Kim HS, et al. [The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy] Korean J Gastroenterol. 2010;55:368–375. doi: 10.4166/kjg.2010.55.6.368. [DOI] [PubMed] [Google Scholar]
- 17.Heo J, Jeon SW. [Changes in the eradication rate of conventional triple therapy for Helicobacter pylori infection in Korea] Korean J Gastroenterol. 2014;63:141–145. doi: 10.4166/kjg.2014.63.3.141. [DOI] [PubMed] [Google Scholar]
- 18.Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002;19:67–70. doi: 10.1016/s0924-8579(01)00456-3. [DOI] [PubMed] [Google Scholar]
- 19.Zullo A, Rinaldi V, Winn S, Meddi P, Lionetti R, Hassan C, Ripani C, Tomaselli G, Attili AF. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:715–718. doi: 10.1046/j.1365-2036.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 20.Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003;51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 21.De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94–100. doi: 10.7326/0003-4819-144-2-200601170-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sirimontaporn N, Thong-Ngam D, Tumwasorn S, Mahachai V. Ten-day sequential therapy of Helicobacter pylori infection in Thailand. Am J Gastroenterol. 2010;105:1071–1075. doi: 10.1038/ajg.2009.708. [DOI] [PubMed] [Google Scholar]
- 23.Nadir I, Yonem O, Ozin Y, Kilic ZM, Sezgin O. Comparison of two different treatment protocols in Helicobacter pylori eradication. South Med J. 2011;104:102–105. doi: 10.1097/SMJ.0b013e318200c209. [DOI] [PubMed] [Google Scholar]
- 24.Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol. 2010;16:4357–4362. doi: 10.3748/wjg.v16.i34.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gisbert JP, Calvet X, O’Connor A, Mégraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010;44:313–325. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- 26.Kwon JH, Lee DH, Song BJ, Lee JW, Kim JJ, Park YS, Kim N, Jeong SH, Kim JW, Lee SH, et al. Ten-day sequential therapy as first-line treatment for Helicobacter pylori infection in Korea: a retrospective study. Helicobacter. 2010;15:148–153. doi: 10.1111/j.1523-5378.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 27.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-analysis. J Clin Pharm Ther. 2009;34:41–53. doi: 10.1111/j.1365-2710.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 29.Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011;34:604–617. doi: 10.1111/j.1365-2036.2011.04770.x. [DOI] [PubMed] [Google Scholar]
- 30.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 31.Okada M, Nishimura H, Kawashima M, Okabe N, Maeda K, Seo M, Ohkuma K, Takata T. A new quadruple therapy for Helicobacter pylori: influence of resistant strains on treatment outcome. Aliment Pharmacol Ther. 1999;13:769–774. doi: 10.1046/j.1365-2036.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim SY, Jung SW. [Helicobacter pylori eradication therapy in Korea] Korean J Gastroenterol. 2011;58:67–73. doi: 10.4166/kjg.2011.58.2.67. [DOI] [PubMed] [Google Scholar]
- 33.Kuepper-Nybelen J, Thefeld W, Rothenbacher D, Brenner H. Patterns of alcohol consumption and Helicobacter pylori infection: results of a population-based study from Germany among 6545 adults. Aliment Pharmacol Ther. 2005;21:57–64. doi: 10.1111/j.1365-2036.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Sung KC, Rhee EJ, Ryu SH, Beck SH. Prevalence of Helicobacter pylori infection and its association with cardiovascular risk factors in Korean adults. Int J Cardiol. 2005;102:411–417. doi: 10.1016/j.ijcard.2004.05.040. [DOI] [PubMed] [Google Scholar]


