Abstract
Autoimmune hepatitis (AIH) is an unresolving progressive liver disease of unknown etiology characterized by hypergammaglobulinemia, autoantibodies detection and interface hepatitis. Due to the absence of specific diagnostic markers and the large heterogeneity of its clinical, laboratory and histological features, AIH diagnosis may be potentially difficult. Therefore, in this in-depth review we summarize the substantial progress on etiopathogenesis, clinical, serological and histological phenotypes of AIH. AIH has a global distribution affecting any age, both sexes and all ethnic groups. Clinical manifestations vary from asymptomatic to severe or rarely fulminant hepatitis. Hypergammaglobulinemia with selective elevation of IgG is found in most cases. Autoimmune attack is perpetuated, possibly via molecular mimicry, and favored by the impaired control of T-regulatory cells. Histology (interface hepatitis, emperipolesis and hepatic rosette formation) and autoantibodies detection although not pathognomonic, are still the hallmark for a timely diagnosis. AIH remains a major diagnostic challenge. AIH should be considered in every case in the absence of viral, metabolic, genetic and toxic etiology of chronic or acute hepatitis. Laboratory personnel, hepato-pathologists and clinicians need to become more familiar with disease expressions and the interpretation of liver histology and autoimmune serology to derive maximum benefit for the patient.
Keywords: Autoimmune hepatitis, Liver autoimmunity, Liver-related autoantibodies, Non-organ specific autoantibodies, Overlap syndromes
Core tip: Autoimmune hepatitis (AIH) is a chronic liver disease of unknown etiology. In this in-depth review we summarize the substantial progress on etiopathogenesis, clinical, serological and histological phenotypes of AIH. AIH has a global distribution affecting any age, both sexes and all ethnic groups. Clinical manifestations vary from asymptomatic to severe hepatitis. Polyclononal hypergammaglobulinemia is characteristic in most cases, while histology and autoantibodies detection are still the hallmark for timely diagnosis. Laboratory personnel, hepato-pathologists and clinicians need to become more familiar with disease expressions and the interpretation of liver histology and autoimmune serology to derive maximum benefit for the patient.
INTRODUCTION
Autoimmune hepatitis (AIH) is a relatively rare progressive chronic liver disease that mainly affects women and is usually characterized by increased immunoglobulin G (IgG) levels, circulating autoantibodies, association with human leukocyte antigens (HLA) DR3 or DR4, interface hepatitis on liver histology, and a favorable response to immunosuppressive treatment[1-3].
To date, the etiology of AIH is still unknown and all the causes of chronic liver disease must be excluded in advance before diagnosing AIH (Table 1). Serological tests of high specificity for AIH diagnosis as for the diagnosis of viral hepatitis A to E or a single autoantibody with the diagnostic accuracy that antimitochondrial autoantibodies (AMA) demonstrate for primary biliary cirrhosis (PBC) are missing. In addition, the manifestations of AIH are characterized by a large heterogeneity regarding its clinical, laboratory and histological features (Table 2). It is therefore clear that AIH diagnosis may be difficult, indicating that the disease should be taken into consideration in any case of acute or chronic hepatitis, particularly when hypergammaglobulinemia is present and the patient has antecedents of other autoimmune diseases (Tables 1 and 2)[3,4].
Table 1.
Differential diagnosis of autoimmune hepatitis
| Other autoimmune liver diseases |
| Primary biliary cirrhosis |
| Primary sclerosing cholangitis (including small duct sclerosing cholangitis) |
| Variants syndromes |
| Chronic viral hepatitis |
| Chronic hepatitis B with or without hepatitis delta |
| Chronic hepatitis C |
| Chronic hepatitis non A to E |
| Cholangiopathy due to human immunodeficiency virus infection |
| Alcoholic liver disease |
| Drug-induced hepatitis |
| Granulomatous hepatitis |
| Hemochromatosis |
| Non-alcoholic steatohepatitis |
| α1-antitrypsin deficiency |
| Wilson's disease |
| Systemic lupus erythematosus |
| Celiac disease |
Table 2.
Clinical features of autoimmune hepatitis
| Characteristic | |
| Age at presentation | Any age of both sexes and all ethnic groups; bimodal distribution usually with peaks around puberty and between 4th and 6th decades although a considerable number of patients are even older (above 65 years of age) |
| Types of disease onset | Broad range from asymptomatic (‘‘en passant’’ diagnosis) to acute/severe or even fulminant hepatitis |
| Most common clinical phenotype of the disease (two thirds of patients) is characterized by an insidious onset with unspecific symptoms, such as fatigue, right upper quadrant pain, lethargy, malaise, anorexia, nausea, pruritus, fluctuating jaundice and polyarthralgia without arthritis, sometimes dating back years | |
| Acute onset of AIH does exist and contains two different clinical entities (the acute exacerbation of chronic AIH and the true acute AIH without histological findings of chronic liver disease) | |
| One third of patients at diagnosis have already developed cirrhosis irrespective of the presence of symptoms or not, suggesting a delay in diagnosis due to unfamiliar clinicians, histopathologists and/or laboratories | |
| Physical findings | Depends on the clinical status of the disease, ranging from completely normal to signs and symptoms of chronic liver disease and/or portal hypertension |
| Clinical features in special conditions | Presentation of AIH during pregnancy or more frequently after delivery |
| Development of AIH after liver transplantation for other liver diseases (de novo AIH or post-transplant plasma cell hepatitis) | |
| Development of AIH after drugs, supplements or herbals (drug-induced AIH, nitrofurantoin and minocycline implicated in 90% of cases) | |
| Specific characteristics | Frequent presence in the patient or first-degree relatives of other autoimmune or immune-mediated diseases like Hashimoto thyroiditis, Grave’s disease, vitiligo, alopecia, rheumatoid arthritis, diabetes mellitus type-1, inflammatory bowel disease, psoriasis, systemic lupus erythematosus, Sjögren’s syndrome and celiac disease |
| An unusual form of AIH has been reported in 10%-18% of patients with APECED, also known as APS-1 | |
| Complications | HCC development in AIH is less common than other liver diseases but it does exist and is associated with cirrhosis, suggesting surveillance in all cirrhotic patients with AIH |
| Drug-related complications are also significant in 10%-25% of patients; these complications are most commonly related to long-term corticosteroid use or azathioprine toxicity and/or intolerance |
AIH: Autoimmune hepatitis; HCC: Hepatocellular carcinoma; APECED: Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy; APS-1: Autoimmune polyglandular syndrome type 1.
In 1992, a panel of experts, namely the International AIH Group (IAIHG), reported the descriptive criteria[5] for the diagnosis and classification of AIH either as “definite” or “probable” and they also proposed a cumulative score which was subsequently revised in late 1998[6] and remarkably simplified in 2008[7] (Table 3). It should be noted however, that these diagnostic scores were established in order to conform the diagnostic criteria between the different centers and to give the opportunity to compare the different experiences, mainly for research purposes[5-7].
Table 3.
Simplified criteria for the diagnosis of autoimmune hepatitis
| Feature/parameter | Discriminator | Score |
| ANA or SMA + | ≥ 1:40 | +11 |
| ANA or SMA+ | ≥ 1:80 | +21 |
| or LKM + | ≥ 1:40 | +21 |
| or SLA/LP + | Any titer | +21 |
| IgG or γ-globulins level | > upper limit of normal | +1 |
| > 1.1 × upper limit | +2 | |
| Liver histology (evidence of hepatitis is a necessary condition) | Compatible with AIH | +1 |
| Typical of AIH | +2 | |
| Atypical | 0 | |
| Absence of viral hepatitis | No | 0 |
| Yes | +2 |
Addition of points achieved for all autoantibodies (maximum, 2 points). Definite autoimmune hepatitis: ≥ 7; Probable autoimmune hepatitis: ≥ 6. Typical liver histology for autoimmune hepatitis = each of the following features had to be present, namely interface hepatitis, lymphocytic/lymphoplasmocytic infiltrates in portal tracts and extending into the lobule, emperipolesis (active penetration by one cell into and through a larger cell) and hepatic rosette formation. Compatible liver histology for autoimmune hepatitis = chronic hepatitis with lymphocytic infiltration without all the features considered typical. Atypical = showing signs of another diagnosis, like steatohepatitis. ANA: Antinuclear antibodies; SMA: Smooth muscle autoantibodies; LKM: Liver/kidney microsomal; SLA/LP: Soluble liver antigen or liver pancreas; AIH: Autoimmune hepatitis.
The disease is subclassified into two major types: AIH type 1 (AIH-1) and AIH type 2 (AIH-2) (Table 4). In AIH-1, antinuclear antibodies (ANA) and/or smooth muscle autoantibodies (SMA) are detected and usually perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) are also found[1-3,5-10]. In AIH-2, specific autoantibodies, namely anti-liver/kidney microsomal antibody type 1 (anti-LKM1) or rarely anti-LKM type 3 (anti-LKM3)[1-3,5-15] and/or antibodies against liver cytosol type 1 antigen (anti-LC1), are detected[1-3,8,13,16]. Apart from differences in autoantibodies detection between AIH-1 and AIH-2, there are also other differences that are helpful to clinicians[1]. Actually, AIH-2 more frequently presents in children and young adults, has an acute or severe course and advanced histological lesions at presentation, whereas treatment failure, relapse after stopping treatment and need for long-term treatment is common compared to AIH-1[1,17].
Table 4.
Subdivision of autoimmune hepatitis according to the autoantibodies detected
| Types of AIH | Characteristic autoantibodies |
| AIH-1 | ANA, SMA, p-ANCA (p-ANNA), anti-ASGP-R, anti-SLA/LP (specific antibody; molecular target: SepSecS) |
| AIH-2 | Anti-LKM1 (molecular target: CYP2D6), anti-LKM3 (molecular target: UGT1), anti-LC1 (liver specific antibody, molecular target: FTCD), anti-ASGP-R |
| AIH as component of APECED | ANA, anti-LC (molecular target: unknown), anti-LKM (molecular target: CYP2A6, CYP1A1, CYP2B6), anti-LM (liver specific antibody; molecular target: CYP1A2) |
AIH-1: Autoimmune hepatitis type 1; AIH-2: Autoimmune hepatitis type 2; APECED: Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy; ANA: Antinuclear antibodies; SMA: Smooth muscle antibodies; p-ANCA: Perinuclear antineutrophil cytoplasmic antibodies; p-ANNA: Peripheral antineutrophil nuclear antibodies; Anti-ASGP-R: Antibodies against asialoglycoprotein receptor; Anti-SLA/LP: Antibodies against soluble liver antigen/liver pancreas; SEpSEcS: Synthase-converting O-phosphoseryl-tRNA to selenocysteinyl-tRNA; anti-LKM1: Anti-liver kidney microsomal antibodies type 1; CYP2D6: Cytochrome P450 2D6; Anti-LKM3: Anti-liver kidney microsomal antibodies type 3; UGT1: UDP-glucuronosyltransferases; Anti-LC1: Anti-liver cytosol antibodies type 1; FTCD: Formiminotransferase cyclodeaminase; CYP2A6: Cytochrome P450 2A6; CYP1A1: Cytochrome P450 1A1; CYP2B6: Cytochrome P450 2B6; Anti-LM: Antibodies against liver membrane; CYP1A2: Cytochrome P450 1A2.
The prevalence of AIH ranges from 160 to 170 cases per 1000000 inhabitants in Europe[18-22]. This prevalence appears to be similar to that of PBC. Interestingly, higher frequencies have been published in regions with quite close and stable populations. For instance, prevalence rates of 42.9 cases per 100000 and 24.5 cases per 100000 inhabitants have been reported in Alaska[23] and New Zealand[24], respectively, indicating that AIH might be underestimated or unrecognized in other populations.
The disease has a universal distribution, can affect any age, either females or males, irrespective of the ethnicity of the affected individual. However, its prevalence and manifestations seem to vary according to race and ethnicity. Actually, Alaskan natives have a high frequency of acute icteric disease at the disease onset[23], blacks carry a more progressive disease than non-blacks[25], and patients of Hispanic ancestry usually have an advanced disease at onset with a high prevalence of cirrhosis. On the other hand, individuals of Asian background demonstrate very poor survival outcomes[26]. Although most of the above mentioned studies are retrospective and have been done in tertiary centers, these observations have led to the assumption that immunogenetic factors may influence the development, clinical course and response to therapy of AIH in ethnically different patients.
ETIOLOGICAL AND PATHOGENETIC ASPECTS
The cause(s) of AIH is (are) still unknown, although over the years remarkable progress in the understanding of disease pathogenesis has been made. The prevalent assumption suggests the development of AIH in genetically predisposed individuals, after their exposure to triggering factors like microbes, viruses and xenobiotics. Afterwards, the autoimmune attack against the liver is continued, potentially through “molecular mimicry” mechanisms, and is promoted by the diminished control of regulatory T-cells[27].
Genetics of AIH
The “susceptibility” genes of AIH, acting alone or possibly in concert, interact with environmental factors whose identity is mostly unknown. The strongest association is with genes located within the HLA region on the short arm of chromosome 6, particularly those encoding the HLA class II DRB1 alleles[28]. These molecules, naturally exposed on the surface of antigen-presenting cells, are essential in the presentation of the peptide antigens to CD4 T cells.
DRB1*0301 and DRB1*0401 confer susceptibility to AIH-1 in European and North American patients[28,29] and their possession increases the score of the revised diagnostic criteria issued by the IAIHG[6]. DRB1*0405 and DRB1*0404 confer susceptibility to AIH in Japanese, Argentinean and Mexican patients[30], whereas DRB1*1301 confers susceptibility in Argentineans[31,32]. In this context, a recent genome-wide association study in the Netherlands confirmed the association of HLA-DRB1*0301 and HLA-DRB1*0401 alleles with AIH-1 and identified variants of SH2B3 and CARD10 as likely risk factors for the disease[33]. On the other hand, DRB1*0701 and DRB1*0301 confer susceptibility to AIH-2[34]. There are also some other studies concerning susceptibility to AIH, indicating an association with polymorphisms in genes located outside of the major histocompatibility complex (MHC), like the cytotoxic T lymphocyte antigen-4[35], the gene promoter of tumor necrosis factor-alpha (TNF-α)[36] and Fas[37].
Molecular mimicry and other mechanisms of liver injury in AIH
Molecular mimicry stems from the premise that self-antigens may share sequence homologies with proteins of external agents such as viruses and for this reason, after a first exposure and sensitization to foreign antigens, the immune system would react against self-proteins, perpetuating the chronic damage[11,38].
Anti-LKM1 antibodies are the best example of molecular mimicry in AIH. The major target autoantigen of anti-LKM1 antibodies in AIH-2 has been identified as the cytochrome P450 2D6 (CYP2D6)[11,39-41]. CYP2D6 shares sequence homologies with hepatitis C virus (HCV), cytomegalovirus (CMV) and herpes simplex virus type 1 (HSV-1), which could act as the initiating factors of the disease in genetically susceptible subjects[39,42]. Indeed, after testing 26 LKM-positive serum samples, Manns et al[43] have shown that most of these sera recognized a short minimal epitope of eight amino acids with the sequence DPAQPPRD. A search of electronic databases revealed a matching of the minimal epitope with immediate early protein IE 175 of HSV-1 (known also as infected cell protein 4; ICP4). Sequence identity was present for the PAQPPR sequence. This hypothesis was further supported by a case of identical twins where one sister had AIH-2 but the other was healthy. Of note, only the sister with AIH-2 was HSV positive and her serum reacted with ICP4 in lysates of HSV-infected cells[44]. In another study, Kerkar et al[42] showed a cross-reactivity between 193-212 CYP2D6 epitope and homologues of two unrelated viruses (HCV 2977-2996 and CMV 121-140). In addition, Bogdanos et al[38] reported that molecular similarities among CYP2D6, HCV and HSV may lead to LKM-1 development through a cross-reactive response in susceptible individuals[38].
A prerequisite for anti-LKM-1 production and the activation of pathogenetic mechanisms is the expression of CYP2D6 on the surface of liver cells. Although this localization is controversial, recent data indicates that CYP2D6 is exposed on the plasma membrane of hepatocytes, suggesting that either autoantibody-dependent cytotoxicity or direct lysis of liver cells due to a direct antibody-antigen binding could be operative in perpetuating the autoimmune attack against liver cells[45-47].
However, apart from molecular mimicry, epitope spreading or exposure to previously hidden autoantigens revealed because of hepatocellular injury have been suggested as alternative pathogenetic mechanisms in the development of AIH[17]. Indeed, hepatocellular damage can begin after the presentation of an autoantigen by professional antigen presenting cells via MHC and co-stimulatory molecules. Thereafter, several cytokines can drive the differentiation of uncommitted CD4 T-helper cells (Th0) to Th1-cells secreting interferon-γ (IFN-γ), pathogenic Th17-cells that secrete the proinflammatory cytokine interleukin-17 (IL-17), or Th2-cells which secrete IL-13, IL-4 and IL-10, indicating that multiple effector cells are involved in AIH pathogenesis probably because of defective immunoregulatory mechanisms (see below).
Involvement of regulatory T-cells
The mechanisms underlying the breaking of immune tolerance in AIH have not yet been completely clarified. The malfunction of regulatory T-cells, particularly of CD4+CD25+FOXP3+ T-cells, could be an explanation[48]. In contrast with healthy subjects, CD4+CD25+ regulatory T-cells are decreased in number and functionally impaired at diagnosis, whereas an increase is recorded during effective treatment[49,50]. However, Peiseler et al[51] found contrasting results and described normally functioning regulatory T-cells in patients with AIH. In addition, recent data have shown that intrahepatic regulatory T-cells are rather enriched than numerically deficient in untreated AIH-1 and more importantly, immunosuppression caused a disproportional loss of these cells, suggesting an association with treatment and remission and not as a causal effect[52,53].
Recently, the interaction between the IL-4 receptor, namely the CD124 molecule and circulating autoantibodies against it, has been described in AIH[54]. These autoantibodies inhibit STAT6 phosphorylation, resulting finally in a neutralizing effect on the cytokine and subsequently in uncontrolled inflammatory reactions.
Animal models of AIH
Most of the previous reported murine models of AIH were developed after a rather complex disease induction protocol and the presentation of hepatitis was often only transient, not reflecting fundamental features of AIH such as the generation of specific autoantibodies and/or T-cells and liver fibrosis[55-58]. However, recent animal models have provided brand new information on disease pathogenesis[59,60]. In addition, the identification of the autoantigens of anti-LKM1 and anti-LC1 antibodies in AIH-2, namely CYP2D6 and formiminotransferase cyclodeaminase (FTCD), has led to the development of the respective animal models[60,61].
The mice had a peak in serum aminotransferases 4-7 mo after immunization, developed periportal, portal and lobular inflammatory infiltrates with liver-infiltrating CD4+, CD8+ and B lymphocytes, including cytotoxic-specific T-cells, and produced anti-LKM1 and anti-LC1 antibodies. The genetic background is an important aspect in this animal model as mouse strains with different genes within and outside the MHC showed different susceptibilities for the disease[62]. Peripheral tolerance and expansion of regulatory T-cells, but neither sex hormones nor central tolerance, seem to underlie male resistance to experimental AIH-2[63]. In this context, the adoptive transfer of ex vivo expanded regulatory T-cells led to reinstitution of peripheral tolerance to FTCD, the inciting autoantigen, and remission of liver injury was achieved[64].
In a CYP2D6 model, the strategy was to use an adenovirus vector expressing the human CYP2D6 as a triggering molecule to break tolerance as the viral infection provides an appropriate substrate for autoimmunity by inducing strong inflammatory responses within the liver. Subsequently, aggressive lymphocytes reach the liver, molecular mimicry develops and a chronic liver disease becomes apparent as a consequence of antigen-driven, promiscuous T-cells infiltrating the liver[60,65]. Only adenovirus expressing CYP2D6 was able to induce chronic hepatitis with liver histology compatible of AIH, high titers of anti-LKM1 antibodies hepatic infiltrates with CD4+ lymphocytes, and extensive liver fibrosis[60,66].
Although the exact role of TNF-α in AIH pathogenesis has not been elucidated yet, very recently it was shown that TNF-α is essential in the induction of AIH through up-regulation of hepatic CCL20 expression, which allows migration of dysregulated splenic T-cells[67]. Therefore, anti-TNF-α treatment in AIH could have a pathophysiological basis, also taking into consideration that in AIH, TNF-α is produced in large amounts in the liver by macrophages, CD8+ T-cells and possibly Th17 cells[17].
Another animal model of AIH has been developed, inducing the loss of regulatory mechanisms such as naturally arising regulatory T-cells and programmed cell death 1 (PD-1)-mediated signaling. Mice that are not able to produce natural regulatory T-cells after neonatal thymectomy and genetically devoid of the PD-1-mediated signaling produce ANA and develop fatal hepatitis with florid CD4+ and CD8+ T-cell infiltration of the liver and massive lobular necrosis[68,69].
It should be stated, however, that we do not have enough data from animal models concerning the development of AIH-1, the most frequent phenotype of the disease (approximately 75%-80% of patients with AIH)[1,17]. Therefore, we believe that more evidence should be generated to pinpoint the immunopathogenesis of AIH, including studies on autoantibodies, auto-aggressive T-cells and effects of cytokines, in order to understand better how the chronic inflammation of the liver is induced and maintained in AIH.
CLINICAL PHENOTYPES OF THE DISEASE
Clinical features of AIH
For many years, AIH was classically related to a typical clinical phenotype of a young female patient with endocrine abnormalities and severe hepatitis. However, it is now well-established that AIH has a global distribution, can also affect males (almost 25%-30% of the patients) and can present at any age and in all ethnic groups[22,26,70-77]. The disease is usually characterized by a bimodal age pattern at onset with one peak in children and teens and a second in middle age (fourth to sixth decades and especially in women after menopause), although a considerably increasing number of patients are even older than 65-70 years[74-79].
AIH is characterized by fluctuation of disease activity and therefore its clinical spectrum ranges from no obvious signs or symptoms of liver disease to a severe, acute or even fulminant hepatitis (Table 2)[1,80]. Indeed, acute AIH presents in approximately 25% of cases with identical signs and symptoms as patients suffering from acute viral or toxic hepatitis[70,81]. However, the clinical phenotype of acute AIH at presentation may actually be due to either an exacerbation of already established AIH that has been undiagnosed or misdiagnosed or to a true acute AIH without histological lesions of chronicity in liver biopsy[70,80-82]. Of note, in some of these patients, serum IgG is normal and ANA at first screening may be negative and thus the clinician may not consider AIH, although a more appropriate autoimmune liver serology test could be contributory. Progression to acute liver failure is not frequent but in these exceptional cases, the prompt and timely diagnosis of AIH is of outmost importance as delay in diagnosis and starting of immunosuppressive treatment result in poor prognosis, while administration of therapy might avoid the need for liver transplantation[70,80-83].
Commonly, the clinical presentation is not peculiar and is characterized by several unspecific findings of various intensity (Table 2)[1,22,26,70-72,76]. Amenorrhea is also frequent but epidermal rashes and low-grade fever are rare conditions. The initial clinical evaluation is either completely normal or when frank cirrhosis has developed, typical signs and symptoms of chronic liver disease, like hepatomegaly, splenomegaly, palmar erythema and spider nevi, are present. In advanced disease, the development of ascites, esophageal varices and portal gastropathy, along with cytopenias due to hypersplenism and/or hepatic encephalopathy, are common. Approximately 12%-35% of patients are asymptomatic at diagnosis and in such cases AIH is usually documented during a random investigation for elevated transaminases which has been done for different reasons (e.g., annual check-up for insurance, investigation for other pathological entities, etc.)[22,71,75-77,84,85]. However, 30% of patients have already developed advanced disease at diagnosis, which is associated with lower overall survival and may indicate a delay in diagnosis[22,71,76,84-86]. In fact, this is a challenge for a timely and prompt diagnosis of AIH as the initiation of symptoms usually present after a subclinical course of the disease lasting back for months or years, while subclinical disease of various duration can also be observed after the first clinical expression of the disease.
Studies conducted more than 40 years ago[87-89] have shown that the disease is catastrophic without treatment as the 5 and 10 year survival rates were as low as 50% and 10% respectively, whereas a significant survival benefit has been recorded in patients treated with corticosteroids. Indeed, after immunosuppression, the 10 year overall survival rate of AIH patients has now significantly improved (80%-95%)[84,90]. For these reasons, the objective is still to spread knowledge regarding the diagnosis of AIH and adopt a more liberal attitude towards testing for autoantibodies in patients with elevated serum liver enzymes as effective treatment for the disease is available[91].
Special characteristics of AIH
The diagnosis of AIH can be done for the first time in pregnancy or more frequently soon after delivery (Table 2). Although AIH concurrent with pregnancy is a rare event, relapse of the disease may occur in patients who are in remission during pregnancy[92-97]. Therefore, this possibility should be strongly taken into account if elevation of transaminases, especially in association with hypergammaglobulinemia with selective IgG increase, is observed during pregnancy or more frequently in the postpartum period. Of note, the introduction of immunosuppression has likely enabled the occurrence of pregnancy in young females with AIH.
In susceptible individuals, the disease can present after the use of many drugs (Table 2). Reactive metabolites may act as neoantigens, triggering the immune cells to an unwanted reaction, although the precise underlying mechanisms have been elucidated only for some drugs able to induce AIH but not currently in use, such as tienilic acid and dihydralazine[8,98]. Indeed, anti-LKM type 2 antibodies (anti-LKM2) have been found in hepatitis cases induced by tienilic acid (major target autoantigen of anti-LKM2: CYP2C9)[99], whereas in dihydralazine-induced hepatitis, a typical LKM staining pattern of the liver with predominant staining of the perivenous liver cells in the absence of kidney staining was observed (liver microsomal antibodies: anti-LM)[100]. The major target autoantigen of anti-LM antibodies has been documented as the CYP1A2[100]. Of interest, anti-LM antibodies directed against the same autoantigen (CYP1A2) have also been reported in a specific and unusual form of AIH which develops in individuals with a rare autosomal recessive disease, the autoimmune polyendocrinopathy-candidiasis ectodermal dystrophy syndrome (APECED)[8,101].
Among drugs still widely used, nitrofurantoin, which is widely prescribed for urinary tract infections, and minocycline, a treatment for acne, are well defined examples of drug-induced AIH[98,102]. Of interest, these two agents are implicated in 90% of cases of drug-induced AIH worldwide[98,103]. Furthermore, recently it has been shown that patients with drug-induced AIH had similar clinical and histological characteristics compared to patients with “pure” AIH, although the latter had higher histological activity and there was a need for long-term immunosuppressive therapy[103]. Nevertheless, drug-induced AIH is an intriguing and complex disorder which could present clinically in different phenotypes across the spectrum of disease. Indeed, at least three clinical scenarios have been proposed that refer to drug-induced autoimmune liver disease, namely AIH with drug-induced liver injury, real drug induced-AIH and immune mediated drug-induced liver injury[98]. Histologically, distinguishing drug-induced liver injury from AIH remains a challenge, although a recent study has suggested that sufficient differences exist so that pathologists can use the pattern of injury to suggest the correct diagnosis[104].
Other drugs and herbal agents, like oxyphenisatin, ornidazole, methyldopa, diclofenac, IFN-α or IFN-γ, atorvastatin, liraglutide, anti-retroviral agents for human immunodeficiency virus and TNF-α blocking agents, have also been suggested in the induction of AIH development[98,105-111].
AIH has also been recorded after viral infections from hepatitis A virus, Epstein-Barr virus (EBV), human herpes virus 6 and measles[11,32,112]. In this context, the development of AIH-1 has been reported in 2/7 susceptible adults that had been previously infected by the EBV[112]. In addition, recently Cabibi[113] and Zellos et al[114] reported two more cases of AIH after EBV infection (1 with AIH-1 and for the first time 1 with AIH-2). The development of AIH-2 has been reported in some patients with HCV after treatment with IFN-α[115-118] but also rarely after acute HCV infection even after viral clearance[119]. From the clinical perspective, these findings suggest that AIH should be taken seriously into account as an alternative “emerging” diagnosis in patients diseased in the past from a viral infection if they still suffer from unexplained and prolonged hepatitis. In such conditions, liver biopsy seems mandatory in an attempt to achieve a correct and timely diagnosis of a potentially catastrophic liver disease such as AIH[1,6,7].
In some circumstances, AIH may develop after orthotopic liver transplantation which was performed for other reasons. This situation has been called de novo AIH after liver transplantation[120,121], although alternative definitions like “post-transplant immune hepatitis”, “graft dysfunction mimicking AIH” or “post-transplant plasma cell hepatitis” could be more rational[122]. Nevertheless, a rapid diagnosis of de novo AIH after liver transplantation may avoid rejection and subsequently a second liver transplantation, while improving long-term survival[121].
AIH is associated with various autoimmune diseases, either in the index patient or the first-degree relatives, commonly Hashimoto thyroiditis, Grave’s disease, vitiligo, alopecia, rheumatoid arthritis, diabetes mellitus type-1, inflammatory bowel disease, psoriasis, systemic lupus erythematosus (SLE), Sjögren’s syndrome and celiac disease (Table 2)[22,71,85,123-127]. In this context, an unusual form of AIH has been reported in approximately 10%-18% of patients with APECED, also known as autoimmune polyendocrinopathy syndrome-type 1 (APS-1; Table 2)[8,101,128]. This syndrome is characterized by chronic mucocutaneous candidiasis, ectodermal dystrophy and autoimmune destruction of several endocrine organs, leading mainly to hypoparathyroidism, adrenocortical failure and gonadal failure in females[127-131]. Mutations in the autoimmune regulator gene (AIRE) have been documented as the etiological basis of the syndrome[129,130]. As noted above, AIH as a component of APECED is characterized by the presence of anti-LM antibodies which are typically absent in those APECED patients who do not suffer from AIH (Table 3)[101]. Of interest, in AIH patients without APECED, mutations of the AIRE gene are not found, indicating that they are genetically distinct from patients with AIH as a component of APECED[132].
Rarely, AIH can concur with other frequent non-autoimmune liver disorders like chronic viral hepatitis B, C or D, non-alcoholic fatty liver disease and alcoholic liver disease[133-137]. Taken together, the above associations of AIH with other non-liver autoimmune diseases as well as non-autoimmune liver diseases may further explain the delay in diagnosis as the first physician dealing with the patient (e.g., rheumatologist, endocrinologist, etc.) may not be so familiar with the vast heterogeneity of the clinical manifestations of the disease.
Complications
As a chronic liver disease, AIH has similar complications. Indeed, at first evaluation, cirrhosis developed in almost 33% of affected subjects[71,84,85]. Unfortunately, this finding has been shown to negatively affect the 5 and 10 year survival[84]. Therefore, a timely and correct diagnosis seems mandatory in an attempt to stop the progression of chronic hepatitis to cirrhosis, decompensated disease and the development of portal hypertension and ultimately hepatocellular carcinoma (HCC). The prevalence of HCC in AIH-induced cirrhosis is lower compared to that recorded in patients with cirrhosis due to other etiologies, such as chronic viral hepatitis, alcohol or hemochromatosis[76,138,139]. On the contrary, recently a study from New Zealand reported an increased risk of either liver or extrahepatic malignancy in patients with AIH[140], while reports from the United Kingdom, Denmark, United States and Japan found male gender and cirrhosis in AIH as the most important triggering factors for the development of HCC, which finally occurs with a frequency of 1.1% per year[76,139,141-144]. Thus, although the incidence of HCC is less common than in other chronic liver diseases, the risk remains sufficient to implicate at least 6 monthly surveillance in all AIH patients with cirrhosis.
Biochemical findings
The typical serum biochemical profile shows a predominantly hepatitic pattern. Of note, the levels of bilirubin and transaminases vary from just above the normal to more than 50 times these concentrations, while the cholestatic enzymes are within normal limits or moderately increased[1,5-7]. However, it should be noted that biochemical activity does not correlate with the severity of AIH in liver biopsy. In addition, it has been shown recently that along with transaminases elevation, γ-glutamyl-transpeptidase (γ-GT) levels but not alkaline phosphatase (ALP) can also be high in AIH and moreover, it could be helpful in an independent manner for the prediction of treatment response[71,85]. In keeping with the fluctuating nature of the disease, the aminotransferases and γ-GT may spontaneously normalize, despite continuing activity at the histological level. This is another important topic that can result in delay and/or underestimation of AIH diagnosis as the second hit may become apparent after several months or years and could present even without any symptoms, explaining at least partially the presence of established cirrhosis in almost one third of patients at the time of diagnosis.
In the majority of patients, a polyclonal hypergammaglobulinemia with selective increase of IgG is observed[4-7,9,10,73,79,145]. It should be emphasized that in every day practice, IgG determination is usually not performed in the laboratory assessment of an index patient with unexplained acute or chronic elevation of aminotransferases, leading to further underestimation of the disease accompanied by significant delay in prompt diagnosis. Elevation of serum IgA suggests steatohepatitis (alcoholic or non-alcoholic) or drug-induced liver injury rather than AIH, whereas an increase in IgM levels is more characteristic of autoimmune cholestatic liver diseases. However, it should be kept in mind that the frequency of cases with increased IgG serum levels tends to decrease in children, elderly patients and those with an acute onset of the disease as almost a third of these patients may have normal IgG levels at first assessment[10,79,85]. For these reasons, AIH should never be excluded in an index patient only because IgG levels were found within normal limits. Additionally, the responsible clinician should know that low transaminases, bilirubin or IgG values do not by definition correspond to mild or inactive disease nor exclude AIH[1,4,6,7].
A marker that could have clinical significance as it might potentially contribute to AIH diagnosis, particularly in patients who present without the conventional antibodies, is complement component C4 which is characteristically low in these patients[1,5,6].
Variant forms of AIH
Within the clinical spectrum of AIH there are some patients who manifest clinical characteristics of either PBC or primary sclerosing cholangitis (PSC)[146]. Although we have long known about the existence of these conditions, there is no well-defined consensus concerning the classification of these disorders and therefore so far several terms have been used, such as “overlap syndrome”, “the hepatic form of PBC”, “autoimmune cholangitis”, “autoimmune sclerosing cholangitis” or “combined hepatitic/cholestatic syndrome”, to report patients with characteristics of both AIH and PBC or PSC[147-149]. In this context, it should be noted that in children with AIH, a specific entity has been described in almost half of patients characterized by lesions of both AIH and sclerosing cholangitis. Thus, the term “autoimmune sclerosing cholangitis” was introduced by Gregorio et al[147], also suggesting the need of an investigation of the biliary tree at least with magnetic resonance cholangiopancreatography (MRCP) in all children with a diagnosis of AIH[12,147]. So far, this variant seems to be unique for children with AIH as a prospective study in adults with AIH was negative and therefore, in the absence of cholestatic presentation, MRCP screening does not seem justified in adult-onset AIH[150].
However, as criteria for the definition of ‘‘overlaps’’ do not exist, their diagnosis is problematic, while because of lack of standardization and instability of the study populations, the characteristics of “overlap syndromes” vary among trials. Recently, it has been reported that in documented clinical cases of variant forms of AIH, the available scoring systems carry low sensitivity for the diagnosis of AIH[151]. These findings are in accordance with the results of previous reports[133,135]. Their findings of low utility of the simplified scoring for the diagnosis of AIH in variant forms of AIH are in contrast with the conclusion of another study[152]. Indeed, after the use of the latest score in 368 patients with PBC, only 6% could be classified as having AIH-PBC “overlaps’’[152], compared to 12% found by using the revised score[6], indicating by this how the prevalence of “overlap or variant conditions” is dependent on the definitions of these syndromes.
As the etiopathogenesis of AIH, PBC and PSC is still unknown, definition of criteria for these “variant forms” of AIH seems difficult and arbitrary and for these reasons, the IAIHG do not support the concept of “overlap or variant syndromes” as new and distinct disorders[151]. However, recently it has been reported[153] that the Chazouillères et al[154] criteria had higher sensitivity (92%) and specificity (97%) for identifying patients with AIH-PBC ‘‘overlap syndrome’’ compared to the revised[6] and the simplified scores[7]. Nevertheless, again these criteria do not have international consensus.
From the laboratory perspective, the concurrent detection of AMA and anti-dsDNA is associated with the presence of AIH-PBC “overlap syndrome”[155]. Additionally, either HLA-DR7 or IgG or IgM plasma cells in liver biopsies have been considered as surrogate markers for AIH-PBC “variants”[156-158]. However, neither IgG nor IgM staining pattern of plasmacytic infiltrates was specific for AIH-PBC “overlap cases”, although an IgG/IgM ratio of less than 1 was present only in PBC, with all “overlap patients” having a respective ratio above 1[157,158].
Taking into account the several autoimmune or immune-mediated diseases that have been associated with IgG4[159], a potential involvement of this IgG subclass in autoimmune liver diseases and in particular in AIH was investigated as well[160-164]. Indeed, it was found that IgG4-related AIH is present in almost a third of AIH patients and furthermore, this AIH variant is characterized, apart from the high IgG4 levels, by intense periportal infiltrate and a more favorable response to corticosteroid administration compared to IgG4-negative AIH patients[163,164]. As this IgG subclass express poor binding, activity to complement its involvement in cell-mediated lysis is obscure and therefore its pathogenetic connection to the liver damage in AIH patients seems unlikely. IgG4 is probably the final result and not the cause of a response to abnormal immunological environments that underlie the pathogenesis of the liver damage seen in AIH[163,164]. Nevertheless, it is obvious that we need more data in order to confirm, extend and define more precisely these findings along with their potential clinical significance in AIH cases.
In summary, we think that the IAIHG is right to emphasize that, due to the low frequency of “overlap syndromes or variants of AIH”, patients should be categorized as AIH, PBC and PSC based on the predominant disorder and those with “overlapping features” should not be considered as having new distinct diseases[151]. In addition, the IAIHG scores should not be used in patients with “overlapping features”. However, specific management may be required in PBC or PSC patients who have also features of AIH[165].
CLASSIFICATION OF AIH
AIH-1
AIH-1 accounts for about 80% of all cases with AIH. The detection of ANA and/or SMA is almost exclusively requisite for an AIH-1 diagnosis. In most instances, the staining pattern of ANA by indirect immunofluorescence (IIF) on tissue sections or isolated immobilized cells like HEp2 cells show a homogenous diffuse pattern, but speckled patterns are not rare[1-3,5-8,13]. ANA are directed against single or double stranded DNA, tRNA, SSA-Ro, snRNPs, laminins A and C, cyclin A or histones[2,3,8,13]. So far however, a liver-specific nuclear antigen has not been identified in AIH-1, whereas different staining patterns of ANA appear to carry limited clinical implications and diagnostic relevance in routine clinical practice and therefore, the use of HEp2 cells in the diagnostic work-up of AIH is not recommended[1,13].
SMA are detected by IIF on rodent liver, kidney and stomach sections; they are directed against cytoskeleton structures like filamentous actin (F-actin, the predominant autoantigen of SMA in AIH-1[166]), troponin, tubulin, vimentin and tropomyosin[1-3,5-8,13]. However, reliance only on anti-actin antibodies for AIH-1 diagnosis could lead to approximately a 20% decline of diagnosed patients as F-actin is a likely but not exclusive target autoantigen of SMA[13,167].
Titers of at least > 1:20 in adults and > 1:10 in children should be considered positive[6,7,12,13]. However, titration of antibody positive sera in AIH can be helpful as a very high titer of homogenously reactive ANA or anti F-actin is far more meaningful than a low albeit positive titer of ANA and/or SMA that may usually be detected in patients with hepatitis B or C[1-3,8,168]. In the majority of AIH-1 patients, disappearance of ANA and/or SMA is observed during immunosuppression[169]. However, autoantibody status is not related to the outcome of patients after withdrawal of corticosteroids. In addition, neither autoantibody titers at diagnosis nor autoantibody behavior in the course of the disease are prognostic markers for AIH-1[1-3,8,169]. Moreover, pre-transplant ANA and SMA levels in AIH patients do not seem to affect recurrence or outcome following liver transplantation[170]. These findings indicate that detection of ANA and SMA is more of diagnostic than prognostic value[1-3,8,13,169].
Of interest, 15%-30% of patients with AIH-1 have autoantibodies directed against soluble liver antigen or liver pancreas (anti-SLA/LP)[171,172]. This autoantibody is the most specific antibody ever identified in AIH-1[172-177], is associated with a more severe disease course and has a global distribution[178]. A recent meta-analysis showed that the diagnostic accuracy of anti-SLA/LP in AIH was very high[179]. Therefore, from the clinical point of view, anti-SLA/LP can be used as a significant surrogate marker for the diagnosis of AIH-1, while it may also lead to a considerable decline of cases with cryptogenic hepatitis or autoantibody-negative AIH[180]. Anti-SLA/LP antibodies target a synthase (S) converting O-phosphoseryl-tRNA (Sep) to selenocysteinyl-tRNA (Sec), giving a label of SepSecS[181,182]. Subsequently, molecular based assays like ELISAs, immunoblot and radioligand assays have been developed for the detection of these antibodies[173-177].
The reason for anti-SLA/LP association with severe disease, protracted treatment and relapse after cessation of therapy in AIH patients is not known but we were the first to report that antibodies against ribonucleoprotein/Sjögren’s syndrome A antigen (anti-Ro/SSA) and particularly to Ro52 antigen (anti-Ro52) are detected in 98% of patients with AIH-1 who have concurrent detection of anti-SLA/LP[183]. This concomitant detection of both autoantibodies was not because of cross-reactivity and was reported later in 77% of European and 96% of North American anti-SLA/LP positive patients[184,185]. Of note, anti-Ro52 antibodies either alone or in combination with anti-SLA/LP were associated with a worst outcome of patients, as attested by an increased frequency of progression to cirrhosis and liver-related deaths[185]. Accordingly, it was suggested that the associations previously described for anti-SLA/LP antibodies may be due to their concurrence with anti-Ro52 antibodies.
However, contrary to the generally accepted assumption that anti-SLA/LP either alone or in combination with anti-Ro52 antibodies are indicators of worse prognosis and treatment outcome, a very recent study from Greece demonstrated that anti-SLA/LP positivity was not associated with the clinical, laboratory or histological characteristics of AIH patients[186]. In addition, in that study, treatment response, corticosteroid withdrawal, relapse after stopping treatment and outcome were not associated with the presence of anti-SLA/LP, anti-Ro52 or double reactivity to both autoantibodies[186]. Moreover, Ro52 epitope mapping for the first time revealed new epitopes unique for AIH (other than those reported in Sjogren’s syndrome) and independent from anti-SLA/LP positivity[186]. Of course these novel findings need further investigation and external validation.
AIH-2
Less than 10%-15% of AIH cases in Europe and North America have the AIH-2 subtype[71,78,85,187,188]. Anti-LKM1 autoantibodies show a diffuse cytoplasmic staining of liver lobules (Figure 1A) and exclusively of the P3 portion of the proximal renal tubules (Figure 1B)[1-3,8,13,33]. Therefore, anti-LKM1 can be easily distinguished from AMA, which stain the proximal and distal renal tubules (Figure 2)[1-3,8,13,33]. Anti-LKM1 autoantibodies mainly target several epitopes of drug metabolizing enzymes of phase 1, namely CYP2D6 (molecular weight of 50 kDa)[33,42,43,189-191]. Of note, 0-10% of HCV patients independently of the genotype of HCV develop anti-LKM1 autoantibodies[1-3,8,13,33,168,192-195] which are directed against the same autoantigen recognized by anti-LKM1 in AIH-2, indicating underlying cross reactivity mechanisms[1-3,33,42,189-191,196,197], although the autoantibody response to immunodominant epitopes differs[33,42,43,115,188-193,197-199]. In Italian patients with chronic hepatitis C, a genetic predisposition like HLA DR7 positivity has been suggested as a triggering factor for the development of anti-LKM1[200]. From the clinical perspective, investigation for anti-LKM is recommended by the IAIHG in HCV patients under IFN-α-based therapies and, in cases of positive results, careful monitoring should be performed because occasionally IFN-α may unmask or induce AIH[1,6,7,13,115-118,133].
Figure 1.
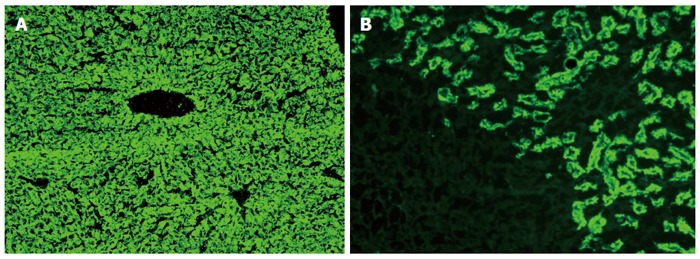
Typical diffuse staining of the cytoplasm of the entire liver lobule on rat liver sections in a case of autoimmune hepatitis type 2 positive for antibodies against liver-kidney microsomes type 1 (A); Antibodies against liver-kidney microsomes type 1 react to the proximal tubules of the rat kidney and the absence of reactivity against the distal tubules of the rat kidney (see also Figure 2) and parietal cells of the rat stomach distinguishes anti-LKM1 autoantibodies from antimitochondrial antibodies (B). Original magnification × 40.
Figure 2.
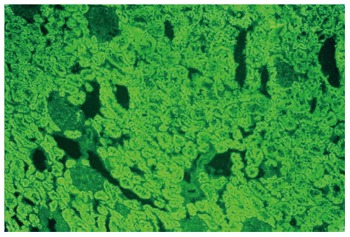
Contrary to antibodies against liver-kidney microsomes type 1 which react to the proximal tubules, antimitochondrial antibodies react to the proximal and also the distal tubules of the rat kidney (original magnification × 40). In these cases there is also reactivity to the parietal cells of the rat stomach.
Anti-LKM3 autoantibodies, either alone or in conjunction with anti-LKM1, are also detected in approximately 5%-10% of patients with AIH-2[15,199]. In addition to signals obtained from liver and kidney tissues, anti-LKM3 may present with fluorescence signals from the pancreas, adrenal gland, thyroid and stomach. Anti-LKM3 autoantibodies were first reported in 13%-15% of patients with chronic hepatitis D[201] and only occasionally in HCV patients, supporting further the concept of HCV-induced autoimmunity[193,202,203]. The main autoantigen of anti-LKM3 has been identified as the family 1 of UDP-glucuronosyl-transferases (UGT1, molecular weight of 55 kDa) both in AIH-2 and in chronic hepatitis D[199].
In approximately a third of patients with AIH-2, anti-LC1 autoantibodies are detected[1-3,8,13,16,204], in half of whom anti-LKM1 reactivity is also present[205,206]. This autoantibody is organ-specific but not species-specific and is characterized by a cytoplasmic staining of the periportal hepatocytes. It is of interest that no staining is found around the central veins[16,204]. In 10% of AIH patients this autoantibody is detected as the only one autoantibody[16,188]. It recognizes FTCD, a metabolic enzyme involved in metabolism of folate (molecular weight of 58-62 kDa)[207]. Multiple regions of FTCD trigger the LC1 autoimmune response and LC1 reactivity is predominantly directed to the FT region of FTCD[208]. Additional techniques like the Ouchterlony double diffusion, ELISA, immunoblot or counter-immunoelectrophoresis are usually required for anti-LC1 detection as its common concurrence with anti-LKM1 makes anti-LC1 detection by IIF difficult[1-3,8,16,204-206]. Titers of > 1:20 in adults and > 1:5 in children are considered positive for both anti-LKM and anti-LC1[1-3,6,7,12,13].
Detection of autoantibodies in AIH as a component of APECED (APS-1)
Hepatitis in APECED is associated with autoantibodies directed against the CYP450 complex. Indeed, CYP1A1, CYP1A2, CYP2A6 and CYP2B6 have been identified as autoantigens in APECED patients (Table 4)[101,209-211]. CYP1A1, CYP2A6 and CYP2B6 are expressed in liver and kidney, giving rise to LKM staining, while CYP1A2 is expressed only in kidney, leading to a LM staining[100].
The highest prevalence of anti-CYP2A6 antibodies was found in a Finnish group of APECED patients (15.6%), whereas anti-CYP1A2 were detected in only 6.3%[101]. Of interest, anti-CYP2A6 detection in this group of patients was not associated with the presence of hepatitis, whereas anti-CYP1A2 were found only in APECED patients with hepatitis[101]. Therefore, anti-CYP1A2 could be used as a specific marker for AIH as part of APECED, albeit with its low sensitivity[101,209]. Anti-CYP2A6 might be used as a surrogate marker for APECED if it is detected in a patient with AIH. In addition, using IIF, anti-LKM/LM antibodies were found in about 50% of patients with AIH as a component of APECED and in only 11% of APECED patients without hepatitis[101].
ANA are detected in almost a quarter of APECED patients, irrespective of the presence or absence of hepatitis. For this reason, ANA are not useful laboratory indicators for AIH diagnosis in APECED[101]. So far, anti-SLA/LP, anti-LKM1 or anti-LC1 autoantibodies have not been reported in patients with AIH as part of APECED. In addition, CYP1A2 and CYP2A6 have not been identified as hepatic autoantigens in AIH patients or in patients with other autoimmune diseases[101]. These findings suggest that AIH-1 or AIH-2 and AIH in APECED are characterized by different molecular targets of autoimmunity which do not overlap. In this context, AIH and hepatitis as an APECED component may be distinguished on the basis of a different autoantibody profile (Tables 4 and 5).
Table 5.
Differential diagnosis of liver diseases according to autoantibodies against molecularly defined autoantigens of cytochrome P450
| Anti-CYP2D6 | Anti-CYP1A2 | Anti-CYP2A6 | Chronic liver disease |
| Pos | Neg | Neg | AIH-2 (94%-100%); Hepatitis C (0%-10%) |
| Neg | Neg | Pos | Hepatitis C; APECED with or without hepatitis |
| Neg | Pos | Neg | AIH in APECED |
| dihydralazine-induced hepatitis | |||
| Pos | Neg | Pos | Hepatitis C (0%-7%) |
| Neg | Pos | Pos | AIH in APECED |
Pos: Positive; Neg: Negative; APECED; Autoimmune polyendocrinopathy-candidiasis ectodermal dystrophy syndrome.
It is not known whether in APECED patients a close monitoring of anti-LM may lead to early, or even prophylactic, treatment of AIH as a new part of the disease. Evidence that autoantibodies may be detected before the clinical manifestation of a new disease component in APECED comes from adrenal and ovarian insufficiencies, where the respective autoantibodies are detected 2-3 years before the clinical presentation of the autoimmune components[212]. The aromatic-L-amino acid decarboxylase (AADC) is another hepatic autoantigen in APECED which is expressed in the liver cytosol and was originally described as a β-cell autoantigen[211]. Of note, the prevalence of anti-AADC autoantibodies is significantly increased in APECED patients with vitiligo (88%) and hepatitis (92%)[128].
Difficulties and unmet needs in autoantibody testing
In 2004, the serology subcommittee of the IAIHG published detailed guidelines on how to test for autoantibodies relevant to AIH, including the preparation of substrates, application of serum samples, optimal dilution, fluorochrome-labeled revealing agents, selection of controls and diagnostically relevant staining patterns[13]. Ideally, the preferred first-line screening for ANA, SMA, LMK1, LKM3, LC1 and AMA should be the IIF on fresh frozen sections (4-8 wk stored at -20 °C) of a multi-organ substrate (liver, kidney and stomach), especially from rats[1-3,8,13]. The use of HEp2 cells only for ANA, SMA and AMA detection should be avoided because of an increased frequency of false-positive results.
However, the development of locally validated sections for IIF is not feasible under real life conditions. Furthermore, sections of commercial origin are of variable quality as they are usually treated with fixatives to lengthen shelf-life, which may result in enhanced background staining and potentially to several difficulties in the interpretation of IIF patterns[9,13]. Therefore, some centers, especially in the US, for antibody testing use assays based on recombinant or purified antigens like ELISAs or immunoblot, particularly for ANA, SMA (F-actin), anti-LKM1, anti-LC1, AMA and anti-SLA/LP detection[9]. However, from the diagnostic point of view, this approach is very questionable for the index patient with unexplained elevation of transaminases and potential underestimation is not unusual[1,13].
Regarding the levels of autoantibody titers, it should be noted that they may vary and therefore it is clear that low titers do not exclude AIH diagnosis, nor do high titers establish the diagnosis[1-3,8,9,13,213]. Furthermore, repeated tests may be necessary to allow autoantibody detection and a correct diagnosis. A significant level of positivity would start at 1/40 dilution. However, for patients up to 18 years, any level of autoantibody reactivity is not frequent and therefore seropositivity at 1/20 dilution for ANA and SMA and even 1/10 for anti-LKM and anti-LC1 may be clinically important[5,12,13,145]. Thus, the laboratory should give any level of positivity from 1/10 and then the interpretation of the results should be done within the clinical context and patient’s age. Unfortunately, several laboratories ignore the recommended cut-off points and by using their own (1/80 or even 1/160) expand the proportion of “negatives”, thus contributing further to the potential underestimation of the disease.
As autoantibody detection is very important for AIH diagnosis, both laboratory personnel and clinicians need to become more familiar with AIH manifestations and interpretation of liver autoimmune serology in order to derive maximum benefit for the affected patient. In this context, the end-user, the clinician, must order tests advisedly with good clinical data and interpret these in the light of the clinical information to make wise evidence-based decisions in an attempt to minimize the problem of underestimation of AIH diagnosis.
Non-conventional autoantibodies in AIH
The detection of several autoantibodies with limited or obscure clinical importance has been published in patients with AIH[1-3]. These include antibodies to single and double-stranded DNA[1-3,214], phospholipids[215,216], histones[217], cyclic citrullinated peptide[218,219], asialoglycoprotein receptor (anti-ASGP-R)[1-3,9,180,220], chromatin[221], centromere[1-3,180], Ro52[183-185], alpha-actinin (α-actinin)[214,222-224], Saccharomyces cerevisiae[225], celiac disease-related autoantibodies[125,180,226-228], AMA[180,229-234], lactoferrin[235] and p53 protein[236].
From this repertoire of autoantibodies, we shall discuss three briefly, namely AMA, antibodies to α-actinin and anti-ASGP-R antibodies, as they appear to have some significance in patients with AIH[1-3]. Although AMA remain the serological hallmark for PBC diagnosis[1-3,231], they can also be detected in otherwise typical cases of AIH[180,229-234]. Indeed, frequencies between 3.6% to as high as 34% have been found for AMA presence in AIH cases[229,231-233,237]. The latter highest frequency was reported in Japanese patients[229]. At present, most researchers agree that the presence of AMA in AIH does not identify a subgroup of patients requiring different therapeutic options or that leads quickly into PBC development[232]. In addition, a long-term Canadian trial has shown that corticosteroid administration in patients with classical AIH who were AMA-positive over a follow-up of up to 27 years had neither clinical nor histological indices of PBC during that period[233]. In contrast, a small case-study recently reported three AMA-positive AIH patients in whom specific PBC manifestations overlapped in time, indicating the potential need of longer follow-up in an attempt to unmask late PBC development in these patients[234]. Taking together the above mentioned data, we believe that in order to define whether or not the presence of AMA in AIH is an incidental finding due to collateral bile duct injury or conceals subclinical autoimmune cholestatic liver disease and therefore can predict the future development of cholestatic pathology and the clinical onset of PBC needs to be determined in future multicenter prospective studies.
α-actinin is a ubiquitous cytoskeletal protein which belongs to the superfamily of F-actin crosslinking proteins, together with spectrin, dystrophin and their homologues and isoforms[223]. This fundamental cell molecule has recently gained attention as a dominant autoantigen in autoimmune diseases, like SLE and AIH-1. Indeed, it has been shown in murine models as well as in humans that anti-dsDNA antibodies may contribute to the pathogenesis of SLE-related glomerulonephritis by cross-reacting with α-actinin[238,239]. Furthermore, anti-α-actinin antibodies in combination with anti-F-actin antibodies have been detected in the sera of AIH patients, identifying a subset of patients with a clinically and histologically severe form of the disease[214,222]. This double reactivity against F-actin and α-actinin was not due to cross-reactivity and it was highly specific only for AIH-1 patients[214,222]. In addition, we showed recently that the baseline detection of anti-α-actinin antibodies could predict treatment response in a large cohort of AIH-1 patients and for these reasons, these autoantibodies can be used as reliable markers for monitoring treatment outcome of patients[224]. Of interest, anti-F-actin antibodies target an epitope located at positions 350-375 of the C terminus of human F-actin which actually corresponds to the α-actinin binding domain[223]. All these findings make the hypothesis of α-actinin involvement into AIH pathogenesis very attractive and indicate the need for considerable attention and further investigations[223].
The ASGP-R is a liver-specific glycoprotein of the cell membrane. The internalization of asialoglycoproteins by binding a terminal galactose residue to coated pits is the predominant function of this receptor. Of interest, ASGP-R is expressed mainly on the surface of hepatocytes at the periportal areas where interface hepatitis is found as a marker of severe inflammatory activity in AIH patients[240]. Therefore, a possible implication of anti-ASGP-R autoantibodies in AIH pathogenesis has been suggested[1,17]. The general presumption is that the target of potentially tissue-damaging auto-reactions in AIH must be liver-specific and available to the immune system in vivo. So far, ASGP-R is the only autoantigen that fulfills these criteria[1,17,240]. Additional support to this revealed from the common detection of anti-ASGPR autoantibodies in AIH patients (detection in 88% of patients), which was associated with the inflammatory activity of the disease and also by the fact that anti-ASGP-R titers decreased significantly during remission, while they reappear in disease exacerbations[241]. Therefore, anti-ASGP-R autoantibodies may be diagnostically helpful when AIH is suspected but the conventional autoantibodies are not detected[180,227,242]. However, they are frequently detected in PBC[180,243], alcoholic cirrhosis[244] and chronic hepatitis B or C[245], resulting in low disease specificity although the specificity of the respective assay for their determination has recently been improved because of the characterization of the major epitopes of ASGP-R[220]. Nevertheless, routine determination of these antibodies is not generally recommended since standardized and easily accessible assays are still awaited.
LIVER HISTOLOGY IN AIH
In all patients with suspected AIH, a liver biopsy should be performed, including those with acute/severe or even fulminant hepatitis[1,6,7,9,10,80]. In fact, liver histology is a prerequisite for the diagnosis of AIH, as has been suggested by both diagnostic criteria of the IAIHG[6,7,133,135]. It should be stated however, that although certain histological changes are characteristic, no findings are specific for AIH diagnosis[1,5-7]. Therefore, a different view of the importance of liver histology in the diagnosis of AIH has recently been reported[246]. In this report, the authors concluded that most patients with multiple features of AIH based on biochemical analyses and autoantibody testing did not need a biopsy as patients with atypical (5%) or compatible (95%) liver histology for AIH had similar biochemical features of the disease[246]. We agree with aspects of the investigators, that it is possible to initiate immunosuppression in patients with the typical serological and biochemical profile of AIH[246]. However, we also believe that histological confirmation of the diagnosis of AIH prior to therapy will facilitate treatment decisions and that liver biopsy should be performed whenever possible. This is supported by all international liver authorities, including IAIHG[6,7,9,10,12]. Nevertheless, we need further multicenter studies in order to validate these findings because liver biopsy is not performed only for diagnosis but also for the definition of grade and stage of the disease and therefore the prognosis of AIH[22,71,76,79,84,247].
A typical feature of AIH is the presence of interface hepatitis, also called piecemeal necrosis, which denotes inflammation of hepatocytes at the junction of the portal tract and hepatic parenchyma (Figure 3A). In general, the inflammation spares the biliary system, consists of lymphocytes and “clustered” plasma cells (Figure 3B) and usually extends into the lobules (progression to lobular hepatitis). However, a small subset of patients may show small duct injury but lack PBC features and respond similarly to corticosteroid therapy as patients with classical AIH do[248].
Figure 3.
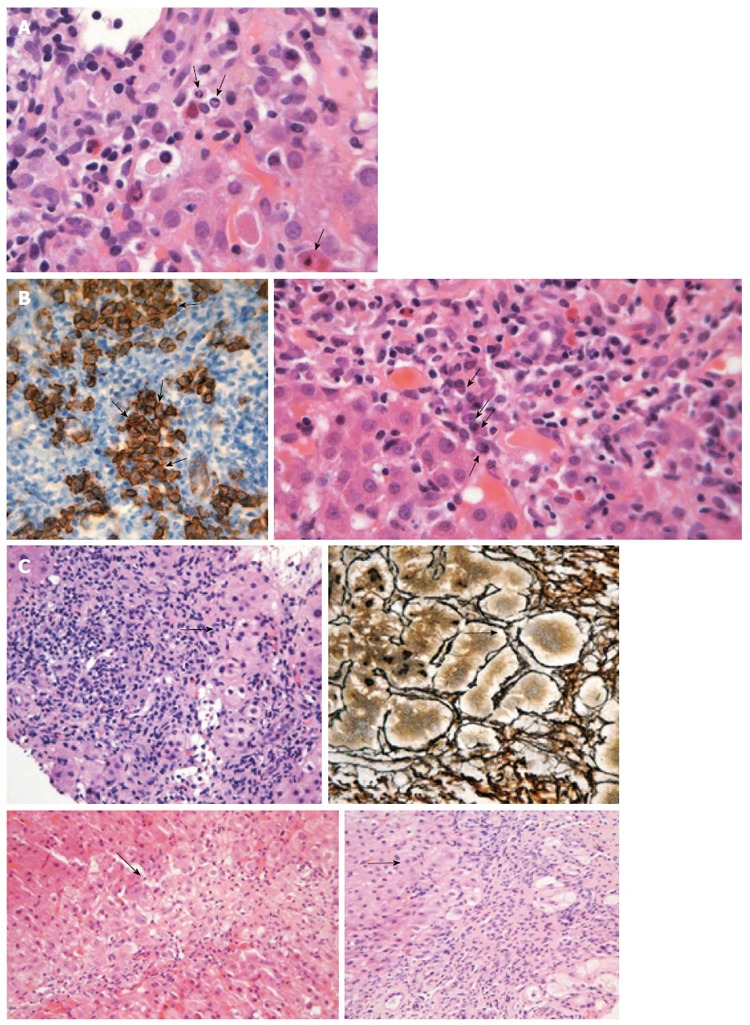
Liver histology in autoimmune hepatitis. A: Interface hepatitis with genuine apoptotic bodies (arrows) in a case with autoimmune hepatitis-type 1 (hematoxylin and eosin staining). See also the typical rim of condensed chromatin at the nuclear membrane and ordinary acidophilic bodies, at various stages of development; B: Intense portal plasmacytosis in autoimmune hepatitis (arrows in the right panel; hematoxylin and eosin staining). The plasma cells are in clusters, better seen after immunostaining with CD138 (arrows in dark brown staining of the left panel). The intensity of plasmacytosis can be useful in discriminating autoimmune hepatitis from viral hepatitis whereas there is also possible prognostic information as portal plasma cell infiltrates while on immunosuppression are associated with relapse upon drug cessation; C: Various examples of rosette formation (hematoxylin and eosin staining; periportal, periseptal in a previous collapse and with reticulin staining seen in the upper right panel; see arrows).
The degree of plasmacytosis can be helpful in discriminating AIH from viral hepatitis cases. Indeed, there are rare cases of HBV infection with comparable portal plasmacytosis, while intense plasmacytosis can also be seen in hepatitis A. In addition, plasmacytosis of the portal tract might have a prognostic implication as its presence while on immunosuppression is associated with relapse after drug withdrawal. However, about 33% of AIH patients have few or no plasma cells in the portal tract and, for this reason, the absence of plasma cell infiltration cannot exclude AIH diagnosis by itself[10,249]. According to the simplified criteria for AIH diagnosis, emperipolesis and hepatocellular rosette formation are also “typical” histological characteristics of AIH[7,250] (Figure 3C). Of note, the origin of “emperipolesis” is derived from two Greek words (en meaning inside and peripolos meaning patrol) describing by this way the active penetration by one cell into and through a larger cell. Eosinophils can be found in AIH (Figure 4A), making the differential diagnosis between AIH and drug-induced AIH more problematic. Parenchymal collapse, also known as multiacinar necrosis, in the appropriate clinical and serological setting could also be useful in supporting AIH diagnosis (Figure 4B)[1,7,10,249]. Apart from the the mildest or earliest cases of AIH, fibrosis is present in almost all patients. In untreated disease, the fibrosis may be extensive with typical cirrhotic findings. It should be noted that the necroinflammatory activity and severity of AIH at the histological level are not in accordance with the biochemical activity of the disease[1,6,7,9,10]. Therefore, it is clear that liver biopsy provides invaluable information on outcome because almost one-third of patients have cirrhosis or bridging necrosis at presentation, carrying a poorer prognosis than those without[22,71,76,79,84,247].
Figure 4.
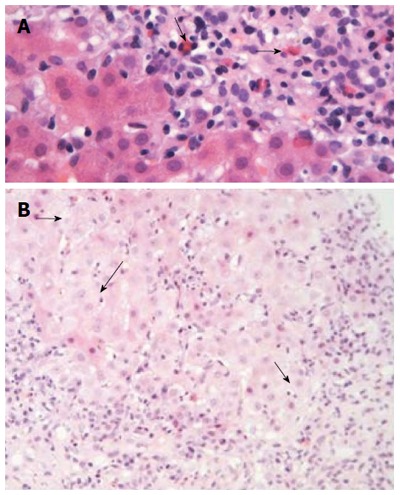
Liver histology in autoimmune hepatitis (continued). A: In some cases of autoimmune hepatitis, “numerous” eosinophils or eosinophils as a minor component of the inflammatory infiltrate can be seen (arrows; hematoxylin and eosin staining). This is an interesting and useful observation, since this finding is evaluated falsely out of the histopathological context of autoimmune hepatitis, and therefore, could make the differential diagnosis from drug-induced hepatitis more problematic; B: Interface hepatitis with hydropic change of involved hepatocytes (arrows; hematoxylin and eosin staining). Note the presence of adjacent parenchymal collapse, alternatively multiacinar necrosis, which has been considered as one of the most distinguishing morphological features of autoimmune hepatitis since its presence in the appropriate clinicopathological context is supportive of the diagnosis.
Liver histology in patients with acute to fulminant AIH is different compared to that found in AIH patients with an insidious onset[251,252]. In addition, recently the US NIH Acute Liver Failure Study Group suggested a set of criteria for autoimmune acute liver failure[80]. As in the revised and simplified criteria for AIH diagnosis[6,7], liver biopsy is also mandatory for the diagnostic criteria of autoimmune acute liver failure. In particular, the following features suggestive of an autoimmune pathogenesis were found. There are two distinctive patterns of massive hepatic necrosis. The first consists of a severe form of the so-called centrilobular form of AIH with panlobular necrosis, while in the second, a typical AIH with massive hepatic necrosis accompanied in some circumstances with centrilobular involvement is found. Additional characteristics in cases of acute liver failure due to AIH include portal lymphoid follicles, a plasma cell-enriched infiltrate and central perivenulitis[80,83,253,254].
CONCLUSION
AIH is a chronic liver disease of unknown etiology that preferentially affects females and is characterized by interface hepatitis on liver histology, hypergammaglobulinemia, circulating autoantibodies and a favorable response to immunosuppression. Not rarely, AIH is underestimated or unrecognized because of the variability of its genetic, clinical, laboratory, histological and serological characteristics. It should be clear that AIH can develop at any age in both sexes and in all ethnic groups worldwide. For these reasons, AIH should be considered seriously in every patient with unexplained acute or chronic hepatitis and/or cirrhosis.
The dominant pathogenetic hypothesis postulates that the disease develops in genetically susceptible individuals who are exposed to several triggers. Then the attack against the liver is perpetuated, possibly through mechanisms of “molecular mimicry”, and is favored by the decreased function of regulatory T-cells.
The clinical presentation of the disease ranges from completely asymptomatic (almost a third of patients) to severe acute hepatitis; approximately a third of patients already have established cirrhosis at diagnosis. AIH may first develop in pregnancy or more frequently after delivery as a subsequent event of viral infections or after the use of some drugs, including biological agents as well as de novo after orthotopic liver transplantation done for other reasons. A specific but also common clinical characteristic is its association with many other autoimmune diseases in the index patient or in first-degree relatives.
Liver biochemistry is not characteristic, with bilirubin and transaminases ranging from just above the upper normal to more than 50 times these levels, usually with normal or mildly increased cholestatic enzymes; the biochemical findings do not correlate with AIH severity at the histological level. Biochemistry may even spontaneously normalize despite continuing activity on histology; this is a very important topic as it may result in a delayed diagnosis. In the vast majority of patients, a polyclonal hypergammaglobulinemia with particular increase of serum IgG is observed; however, it should be kept in mind that 15%-25% of patients, especially in children, the elderly and in acute cases, have IgG within normal limits and therefore, AIH diagnosis should never be excluded only because of a normal IgG testing.
The detection of several autoantibodies is still the hallmark of disease diagnosis in the absence of viral, metabolic, genetic and toxic etiology of chronic or acute liver disease. Detailed guidelines on how to test for autoantibodies relevant to AIH has been published by the IAIHG. Both laboratory personnel and clinicians should become more familiar with the disease manifestations and the interpretation of liver autoimmune serology in order to obtain maximum benefit for the patient.
Although no histological findings are specific for AIH diagnosis, liver biopsy should be performed in all suspected cases, not only for diagnosis but also for the evaluation of disease severity. Typical findings include interface hepatitis consisting of lymphocytes and abundant plasma cells, although one-third of AIH patients have few or no plasma cell infiltrates, indicating that the absence of plasma cell infiltration in the portal tracts does not preclude diagnosis. Other typical findings are the presence of emperipolesis and hepatic rosette formation. The histological findings in patients with autoimmune acute liver failure differs because the lesions predominate in the centrilobular zone, including distinctive patterns of massive liver necrosis, presence of lymphoid follicles, a plasma cell-enriched inflammatory infiltrate and central zonal necrosis/perivenulitis.
As the prevalence of “overlap syndromes or variants of AIH” is low and the current knowledge regarding etiopathogenesis of autoimmune liver diseases is limited, criteria for “overlap conditions” appear to be difficult to define. Therefore, patients with “overlapping features” should be categorized as AIH, PBC or PSC according to the predominant disease and not as suffering from a distinct disease entity.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 14, 2014
First decision: September 15, 2014
Article in press: November 19, 2014
P- Reviewer: Hu HD, Shih KN S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Wang CH
References
- 1.Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A, Dalekos GN, Muratori L. Review article: autoimmune hepatitis -- current management and challenges. Aliment Pharmacol Ther. 2013;38:887–913. doi: 10.1111/apt.12470. [DOI] [PubMed] [Google Scholar]
- 2.Dalekos GN, Zachou K, Liaskos C, Gatselis N. Autoantibodies and defined target autoantigens in autoimmune hepatitis: an overview. Eur J Intern Med. 2002;13:293–303. doi: 10.1016/s0953-6205(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 3.Czaja AJ. Autoantibodies in autoimmune liver disease. Adv Clin Chem. 2005;40:127–164. doi: 10.1016/s0065-2423(05)40004-9. [DOI] [PubMed] [Google Scholar]
- 4.Krawitt EL. Can you recognize autoimmune hepatitis? Postgrad Med. 1998;104:145–149, 152. doi: 10.3810/pgm.1998.08.563. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 7.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 8.Zachou K, Rigopoulou E, Dalekos GN. Autoantibodies and autoantigens in autoimmune hepatitis: important tools in clinical practice and to study pathogenesis of the disease. J Autoimmune Dis. 2004;1:2. doi: 10.1186/1740-2557-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 10.Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 11.Bogdanos DP, Dalekos GN. Enzymes as target antigens of liver-specific autoimmunity: the case of cytochromes P450s. Curr Med Chem. 2008;15:2285–2292. doi: 10.2174/092986708785747508. [DOI] [PubMed] [Google Scholar]
- 12.Mieli-Vergani G, Heller S, Jara P, Vergani D, Chang MH, Fujisawa T, González-Peralta RP, Kelly D, Mohan N, Shah U, et al. Autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2009;49:158–164. doi: 10.1097/MPG.0b013e3181a1c265. [DOI] [PubMed] [Google Scholar]
- 13.Vergani D, Alvarez F, Bianchi FB, Cançado EL, Mackay IR, Manns MP, Nishioka M, Penner E. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677–683. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Homberg JC, Abuaf N, Bernard O, Islam S, Alvarez F, Khalil SH, Poupon R, Darnis F, Lévy VG, Grippon P. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of “autoimmune” hepatitis. Hepatology. 1987;7:1333–1339. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- 15.Fabien N, Desbos A, Bienvenu J, Magdalou J. Autoantibodies directed against the UDP-glucuronosyltransferases in human autoimmune hepatitis. Autoimmun Rev. 2004;3:1–9. doi: 10.1016/S1568-9972(03)00051-X. [DOI] [PubMed] [Google Scholar]
- 16.Martini E, Abuaf N, Cavalli F, Durand V, Johanet C, Homberg JC. Antibody to liver cytosol (anti-LC1) in patients with autoimmune chronic active hepatitis type 2. Hepatology. 1988;8:1662–1666. doi: 10.1002/hep.1840080632. [DOI] [PubMed] [Google Scholar]
- 17.Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126–139. doi: 10.1016/j.jaut.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 19.Berdal JE, Ebbesen J, Rydning A. [Incidence and prevalence of autoimmune liver diseases] Tidsskr Nor Laegeforen. 1998;118:4517–4519. [PubMed] [Google Scholar]
- 20.Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635–647. doi: 10.1016/s1089-3261(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 21.Feld JJ, Heathcote EJ. Epidemiology of autoimmune liver disease. J Gastroenterol Hepatol. 2003;18:1118–1128. doi: 10.1046/j.1440-1746.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- 22.Werner M, Prytz H, Ohlsson B, Almer S, Björnsson E, Bergquist A, Wallerstedt S, Sandberg-Gertzén H, Hultcrantz R, Sangfelt P, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43:1232–1240. doi: 10.1080/00365520802130183. [DOI] [PubMed] [Google Scholar]
- 23.Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97:2402–2407. doi: 10.1111/j.1572-0241.2002.06019.x. [DOI] [PubMed] [Google Scholar]
- 24.Ngu JH, Bechly K, Chapman BA, Burt MJ, Barclay ML, Gearry RB, Stedman CA. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol. 2010;25:1681–1686. doi: 10.1111/j.1440-1746.2010.06384.x. [DOI] [PubMed] [Google Scholar]
- 25.Lim KN, Casanova RL, Boyer TD, Bruno CJ. Autoimmune hepatitis in African Americans: presenting features and response to therapy. Am J Gastroenterol. 2001;96:3390–3394. doi: 10.1111/j.1572-0241.2001.05272.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong RJ, Gish R, Frederick T, Bzowej N, Frenette C. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol. 2012;46:155–161. doi: 10.1097/MCG.0b013e318228b781. [DOI] [PubMed] [Google Scholar]
- 27.Muratori L, Longhi MS. The interplay between regulatory and effector T cells in autoimmune hepatitis: Implications for innovative treatment strategies. J Autoimmun. 2013;46:74–80. doi: 10.1016/j.jaut.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Czaja AJ, Doherty DG, Donaldson PT. Genetic bases of autoimmune hepatitis. Dig Dis Sci. 2002;47:2139–2150. doi: 10.1023/a:1020166605016. [DOI] [PubMed] [Google Scholar]
- 29.Muratori P, Czaja AJ, Muratori L, Pappas G, Maccariello S, Cassani F, Granito A, Ferrari R, Mantovani V, Lenzi M, et al. Genetic distinctions between autoimmune hepatitis in Italy and North America. World J Gastroenterol. 2005;11:1862–1866. doi: 10.3748/wjg.v11.i12.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czaja AJ, Donaldson PT. Genetic susceptibilities for immune expression and liver cell injury in autoimmune hepatitis. Immunol Rev. 2000;174:250–259. doi: 10.1034/j.1600-0528.2002.017401.x. [DOI] [PubMed] [Google Scholar]
- 31.Pando M, Larriba J, Fernandez GC, Fainboim H, Ciocca M, Ramonet M, Badia I, Daruich J, Findor J, Tanno H, et al. Pediatric and adult forms of type I autoimmune hepatitis in Argentina: evidence for differential genetic predisposition. Hepatology. 1999;30:1374–1380. doi: 10.1002/hep.510300611. [DOI] [PubMed] [Google Scholar]
- 32.Czaja AJ, Souto EO, Bittencourt PL, Cancado EL, Porta G, Goldberg AC, Donaldson PT. Clinical distinctions and pathogenic implications of type 1 autoimmune hepatitis in Brazil and the United States. J Hepatol. 2002;37:302–308. doi: 10.1016/s0168-8278(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 33.de Boer YS, van Gerven NM, Zwiers A, Verwer BJ, van Hoek B, van Erpecum KJ, Beuers U, van Buuren HR, Drenth JP, den Ouden JW, et al. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology. 2014;147:443–452.e5. doi: 10.1053/j.gastro.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Bogdanos DP, Hussain MJ, Underhill J, Bansal S, Longhi MS, Cheeseman P, Mieli-Vergani G, Vergani D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–882. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal K, Czaja AJ, Jones DE, Donaldson PT. Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphisms and susceptibility to type 1 autoimmune hepatitis. Hepatology. 2000;31:49–53. doi: 10.1002/hep.510310110. [DOI] [PubMed] [Google Scholar]
- 36.Cookson S, Constantini PK, Clare M, Underhill JA, Bernal W, Czaja AJ, Donaldson PT. Frequency and nature of cytokine gene polymorphisms in type 1 autoimmune hepatitis. Hepatology. 1999;30:851–856. doi: 10.1002/hep.510300412. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal K, Czaja AJ, Donaldson PT. A functional Fas promoter polymorphism is associated with a severe phenotype in type 1 autoimmune hepatitis characterized by early development of cirrhosis. Tissue Antigens. 2007;69:227–235. doi: 10.1111/j.1399-0039.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 38.Bogdanos DP, Lenzi M, Okamoto M, Rigopoulou EI, Muratori P, Ma Y, Muratori L, Tsantoulas D, Mieli- Vergani G, Bianchi FB, et al. Multiple viral/self immunological cross-reactivity in liver kidney microsomal antibody positive hepatitis C virus infected patients is associated with the possession of HLA B51. Int J Immunopathol Pharmacol. 2004;17:83–92. doi: 10.1177/039463200401700112. [DOI] [PubMed] [Google Scholar]
- 39.Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J Clin Invest. 1989;83:1066–1072. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gueguen M, Yamamoto AM, Bernard O, Alvarez F. Anti-liver-kidney microsome antibody type 1 recognizes human cytochrome P450 db1. Biochem Biophys Res Commun. 1989;159:542–547. doi: 10.1016/0006-291x(89)90027-2. [DOI] [PubMed] [Google Scholar]
- 41.Zanger UM, Hauri HP, Loeper J, Homberg JC, Meyer UA. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci USA. 1988;85:8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerkar N, Choudhuri K, Ma Y, Mahmoud A, Bogdanos DP, Muratori L, Bianchi F, Williams R, Mieli-Vergani G, Vergani D. Cytochrome P4502D6(193-212): a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. J Immunol. 2003;170:1481–1489. doi: 10.4049/jimmunol.170.3.1481. [DOI] [PubMed] [Google Scholar]
- 43.Manns MP, Griffin KJ, Sullivan KF, Johnson EF. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. J Clin Invest. 1991;88:1370–1378. doi: 10.1172/JCI115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manns MP, Jentzsch M, Mergener K. Discordant manifestation of LKM-1 antibody positive autoimmune hepatitis in identical twins. Hepatology. 1990;12:840 [abstract]. [Google Scholar]
- 45.Ma Y, Thomas MG, Okamoto M, Bogdanos DP, Nagl S, Kerkar N, Lopes AR, Muratori L, Lenzi M, Bianchi FB, et al. Key residues of a major cytochrome P4502D6 epitope are located on the surface of the molecule. J Immunol. 2002;169:277–285. doi: 10.4049/jimmunol.169.1.277. [DOI] [PubMed] [Google Scholar]
- 46.Muratori L, Parola M, Ripalti A, Robino G, Muratori P, Bellomo G, Carini R, Lenzi M, Landini MP, Albano E, et al. Liver/kidney microsomal antibody type 1 targets CYP2D6 on hepatocyte plasma membrane. Gut. 2000;46:553–561. doi: 10.1136/gut.46.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeper J, Louérat-Oriou B, Duport C, Pompon D. Yeast expressed cytochrome P450 2D6 (CYP2D6) exposed on the external face of plasma membrane is functionally competent. Mol Pharmacol. 1998;54:8–13. doi: 10.1124/mol.54.1.8. [DOI] [PubMed] [Google Scholar]
- 48.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 51.Peiseler M, Sebode M, Franke B, Wortmann F, Schwinge D, Quaas A, Baron U, Olek S, Wiegard C, Lohse AW, et al. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol. 2012;57:125–132. doi: 10.1016/j.jhep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 52.Taubert R, Hardtke-Wolenski M, Noyan F, Wilms A, Baumann AK, Schlue J, Olek S, Falk CS, Manns MP, Jaeckel E. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol. 2014;61:1106–1114. doi: 10.1016/j.jhep.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 53.Sebode M, Peiseler M, Weiler-Normann C, Schramm C, Lohse AW, Herkel J. Phenotypic alterations of regulatory T cells in autoimmune hepatitis: causal or associated with treatment and remission? Hepatology. 2014:Epub ahead of print. doi: 10.1002/hep.27301. [DOI] [PubMed] [Google Scholar]
- 54.Zingaretti C, Arigò M, Cardaci A, Moro M, Crosti M, Sinisi A, Sugliano E, Cheroni C, Marabita F, Nogarotto R, et al. Identification of new autoantigens by protein array indicates a role for IL4 neutralization in autoimmune hepatitis. Mol Cell Proteomics. 2012;11:1885–1897. doi: 10.1074/mcp.M112.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohse AW, Manns M, Dienes HP, Meyer zum Büschenfelde KH, Cohen IR. Experimental autoimmune hepatitis: disease induction, time course and T-cell reactivity. Hepatology. 1990;11:24–30. doi: 10.1002/hep.1840110106. [DOI] [PubMed] [Google Scholar]
- 56.Voehringer D, Blaser C, Grawitz AB, Chisari FV, Buerki K, Pircher H. Break of T cell ignorance to a viral antigen in the liver induces hepatitis. J Immunol. 2000;165:2415–2422. doi: 10.4049/jimmunol.165.5.2415. [DOI] [PubMed] [Google Scholar]
- 57.Djilali-Saiah I, Lapierre P, Vittozi S, Alvarez F. DNA vaccination breaks tolerance for a neo-self antigen in liver: a transgenic murine model of autoimmune hepatitis. J Immunol. 2002;169:4889–4896. doi: 10.4049/jimmunol.169.9.4889. [DOI] [PubMed] [Google Scholar]
- 58.Derkow K, Loddenkemper C, Mintern J, Kruse N, Klugewitz K, Berg T, Wiedenmann B, Ploegh HL, Schott E. Differential priming of CD8 and CD4 T-cells in animal models of autoimmune hepatitis and cholangitis. Hepatology. 2007;46:1155–1165. doi: 10.1002/hep.21796. [DOI] [PubMed] [Google Scholar]
- 59.Zierden M, Kühnen E, Odenthal M, Dienes HP. Effects and regulation of autoreactive CD8+ T cells in a transgenic mouse model of autoimmune hepatitis. Gastroenterology. 2010;139:975–986, 986.e1-3. doi: 10.1053/j.gastro.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 60.Hintermann E, Ehser J, Christen U. The CYP2D6 animal model: how to induce autoimmune hepatitis in mice. J Vis Exp. 2012;(60):3644. doi: 10.3791/3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lapierre P, Djilali-Saiah I, Vitozzi S, Alvarez F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology. 2004;39:1066–1074. doi: 10.1002/hep.20109. [DOI] [PubMed] [Google Scholar]
- 62.Lapierre P, Béland K, Djilali-Saiah I, Alvarez F. Type 2 autoimmune hepatitis murine model: the influence of genetic background in disease development. J Autoimmun. 2006;26:82–89. doi: 10.1016/j.jaut.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Lapierre P, Béland K, Martin C, Alvarez F, Alvarez F. Forkhead box p3+ regulatory T cell underlies male resistance to experimental type 2 autoimmune hepatitis. Hepatology. 2010;51:1789–1798. doi: 10.1002/hep.23536. [DOI] [PubMed] [Google Scholar]
- 64.Lapierre P, Béland K, Yang R, Alvarez F. Adoptive transfer of ex vivo expanded regulatory T cells in an autoimmune hepatitis murine model restores peripheral tolerance. Hepatology. 2013;57:217–227. doi: 10.1002/hep.26023. [DOI] [PubMed] [Google Scholar]
- 65.Ehser J, Holdener M, Christen S, Bayer M, Pfeilschifter JM, Hintermann E, Bogdanos D, Christen U. Molecular mimicry rather than identity breaks T-cell tolerance in the CYP2D6 mouse model for human autoimmune hepatitis. J Autoimmun. 2013;42:39–49. doi: 10.1016/j.jaut.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter J, Manns MP, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwamoto S, Kido M, Aoki N, Nishiura H, Maruoka R, Ikeda A, Okazaki T, Chiba T, Watanabe N. TNF-α is essential in the induction of fatal autoimmune hepatitis in mice through upregulation of hepatic CCL20 expression. Clin Immunol. 2013;146:15–25. doi: 10.1016/j.clim.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Kido M, Watanabe N, Okazaki T, Akamatsu T, Tanaka J, Saga K, Nishio A, Honjo T, Chiba T. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology. 2008;135:1333–1343. doi: 10.1053/j.gastro.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 69.Aoki N, Kido M, Iwamoto S, Nishiura H, Maruoka R, Tanaka J, Watanabe T, Tanaka Y, Okazaki T, Chiba T, et al. Dysregulated generation of follicular helper T cells in the spleen triggers fatal autoimmune hepatitis in mice. Gastroenterology. 2011;140:1322–1333.e1-5. doi: 10.1053/j.gastro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Abe M, Mashiba T, Zeniya M, Yamamoto K, Onji M, Tsubouchi H. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol. 2011;46:1136–1141. doi: 10.1007/s00535-011-0421-y. [DOI] [PubMed] [Google Scholar]
- 71.Muratori P, Granito A, Quarneti C, Ferri S, Menichella R, Cassani F, Pappas G, Bianchi FB, Lenzi M, Muratori L. Autoimmune hepatitis in Italy: the Bologna experience. J Hepatol. 2009;50:1210–1218. doi: 10.1016/j.jhep.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Miyake Y, Iwasaki Y, Sakaguchi K, Shiratori Y. Clinical features of Japanese male patients with type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006;24:519–523. doi: 10.1111/j.1365-2036.2006.03013.x. [DOI] [PubMed] [Google Scholar]
- 73.Floreani A, Niro G, Rosa Rizzotto E, Antoniazzi S, Ferrara F, Carderi I, Baldo V, Premoli A, Olivero F, Morello E, et al. Type I autoimmune hepatitis: clinical course and outcome in an Italian multicentre study. Aliment Pharmacol Ther. 2006;24:1051–1057. doi: 10.1111/j.1365-2036.2006.03104.x. [DOI] [PubMed] [Google Scholar]
- 74.Schramm C, Kanzler S, zum Büschenfelde KH, Galle PR, Lohse AW. Autoimmune hepatitis in the elderly. Am J Gastroenterol. 2001;96:1587–1591. doi: 10.1111/j.1572-0241.2001.03782.x. [DOI] [PubMed] [Google Scholar]
- 75.Peng M, Li Y, Zhang M, Jiang Y, Xu Y, Tian Y, Peng F, Gong G. Clinical features in different age groups of patients with autoimmune hepatitis. Exp Ther Med. 2014;7:145–148. doi: 10.3892/etm.2013.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Eslick GD, Weltman M. Systematic review with meta-analysis: clinical manifestations and management of autoimmune hepatitis in the elderly. Aliment Pharmacol Ther. 2014;39:117–124. doi: 10.1111/apt.12563. [DOI] [PubMed] [Google Scholar]
- 78.Granito A, Muratori L, Pappas G, Muratori P, Ferri S, Cassani F, Lenzi M, Bianchi FB. Clinical features of type 1 autoimmune hepatitis in elderly Italian patients. Aliment Pharmacol Ther. 2005;21:1273–1277. doi: 10.1111/j.1365-2036.2005.02488.x. [DOI] [PubMed] [Google Scholar]
- 79.Al-Chalabi T, Boccato S, Portmann BC, McFarlane IG, Heneghan MA. Autoimmune hepatitis (AIH) in the elderly: a systematic retrospective analysis of a large group of consecutive patients with definite AIH followed at a tertiary referral centre. J Hepatol. 2006;45:575–583. doi: 10.1016/j.jhep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, Manns MP, Norman GL, Lee WM. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi H, Zeniya M. Acute presentation of autoimmune hepatitis: Does it exist? A published work review. Hepatol Res. 2011;41:498–504. doi: 10.1111/j.1872-034X.2011.00808.x. [DOI] [PubMed] [Google Scholar]
- 82.Ferrari R, Pappas G, Agostinelli D, Muratori P, Muratori L, Lenzi M, Verucchi G, Cassani F, Chiodo F, Bianchi FB. Type 1 autoimmune hepatitis: patterns of clinical presentation and differential diagnosis of the ‘acute’ type. QJM. 2004;97:407–412. doi: 10.1093/qjmed/hch072. [DOI] [PubMed] [Google Scholar]
- 83.Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Sakai N, Sakaguchi K, Shiratori Y. Clinical characteristics of fulminant-type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther. 2006;23:1347–1353. doi: 10.1111/j.1365-2036.2006.02894.x. [DOI] [PubMed] [Google Scholar]
- 84.Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42:53–62. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 85.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naïve patients. J Hepatol. 2011;55:636–646. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 86.Landeira G, Morise S, Fassio E, Ramonet M, Alvarez E, Caglio P, Longo C, Domínguez N. Effect of cirrhosis at baseline on the outcome of type 1 autoimmune hepatitis. Ann Hepatol. 2012;11:100–106. [PubMed] [Google Scholar]
- 87.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 88.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 89.Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 90.Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol. 2002;35:75–81. doi: 10.1097/00004836-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 91.Brissos J, Carrusca C, Correia M, Cabral J. Autoimmune hepatitis: trust in transaminases. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buchel E, Van Steenbergen W, Nevens F, Fevery J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am J Gastroenterol. 2002;97:3160–3165. doi: 10.1111/j.1572-0241.2002.07124.x. [DOI] [PubMed] [Google Scholar]
- 93.Samuel D, Riordan S, Strasser S, Kurtovic J, Singh-Grewel I, Koorey D. Severe autoimmune hepatitis first presenting in the early post partum period. Clin Gastroenterol Hepatol. 2004;2:622–624. doi: 10.1016/s1542-3565(04)00245-9. [DOI] [PubMed] [Google Scholar]
- 94.Efe C, Purnak T, Ozaslan E. Autoimmune hepatitis in the postpartum period. Clin Res Hepatol Gastroenterol. 2012;36:391–393. doi: 10.1016/j.clinre.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun. 2012;38:J239–J244. doi: 10.1016/j.jaut.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Heneghan MA, Norris SM, O’Grady JG, Harrison PM, McFarlane IG. Management and outcome of pregnancy in autoimmune hepatitis. Gut. 2001;48:97–102. doi: 10.1136/gut.48.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McFarlane IG, Heneghan MA. Autoimmunity and the female liver. Hepatol Res. 2004;28:171–176. doi: 10.1016/j.hepres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014;6:160–168. doi: 10.4254/wjh.v6.i4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beaune P, Dansette PM, Mansuy D, Kiffel L, Finck M, Amar C, Leroux JP, Homberg JC. Human anti-endoplasmic reticulum autoantibodies appearing in a drug-induced hepatitis are directed against a human liver cytochrome P-450 that hydroxylates the drug. Proc Natl Acad Sci USA. 1987;84:551–555. doi: 10.1073/pnas.84.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bourdi M, Larrey D, Nataf J, Bernuau J, Pessayre D, Iwasaki M, Guengerich FP, Beaune PH. Anti-liver endoplasmic reticulum autoantibodies are directed against human cytochrome P-450IA2. A specific marker of dihydralazine-induced hepatitis. J Clin Invest. 1990;85:1967–1973. doi: 10.1172/JCI114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Obermayer-Straub P, Perheentupa J, Braun S, Kayser A, Barut A, Loges S, Harms A, Dalekos G, Strassburg CP, Manns MP. Hepatic autoantigens in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Gastroenterology. 2001;121:668–677. doi: 10.1053/gast.2001.27103. [DOI] [PubMed] [Google Scholar]
- 102.Appleyard S, Saraswati R, Gorard DA. Autoimmune hepatitis triggered by nitrofurantoin: a case series. J Med Case Rep. 2010;4:311. doi: 10.1186/1752-1947-4-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, Lucena MI, Castiella A, Lindor K, Björnsson E. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lisotti A, Azzaroli F, Brillanti S, Mazzella G. Severe acute autoimmune hepatitis after natalizumab treatment. Dig Liver Dis. 2012;44:356–357. doi: 10.1016/j.dld.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Grasland A, Sterpu R, Boussoukaya S, Mahe I. Autoimmune hepatitis induced by adalimumab with successful switch to abatacept. Eur J Clin Pharmacol. 2012;68:895–898. doi: 10.1007/s00228-011-1191-4. [DOI] [PubMed] [Google Scholar]
- 107.van Casteren-Messidoro C, Prins G, van Tilburg A, Zelinkova Z, Schouten J, de Man R. Autoimmune hepatitis following treatment with infliximab for inflammatory bowel disease. J Crohns Colitis. 2012;6:630–631. doi: 10.1016/j.crohns.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 108.O’Leary JG, Zachary K, Misdraji J, Chung RT. De novo autoimmune hepatitis during immune reconstitution in an HIV-infected patient receiving highly active antiretroviral therapy. Clin Infect Dis. 2008;46:e12–e14. doi: 10.1086/524082. [DOI] [PubMed] [Google Scholar]
- 109.Saitis A, Gatselis N, Zachou K, Dalekos GN. Use of TNFα antagonists in refractory AIH: revealing the unforeseen. J Hepatol. 2013;59:197–198. doi: 10.1016/j.jhep.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 110.Borman MA, Urbanski S, Swain MG. Anti-TNF-induced autoimmune hepatitis. J Hepatol. 2014;61:169–170. doi: 10.1016/j.jhep.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 111.Kern E, VanWagner LB, Yang GY, Rinella ME. Liraglutide-induced autoimmune hepatitis. JAMA Intern Med. 2014;174:984–987. doi: 10.1001/jamainternmed.2014.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vento S, Guella L, Mirandola F, Cainelli F, Di Perri G, Solbiati M, Ferraro T, Concia E. Epstein-Barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346:608–609. doi: 10.1016/s0140-6736(95)91438-2. [DOI] [PubMed] [Google Scholar]
- 113.Cabibi D. Autoimmune hepatitis following Epstein-Barr virus infection. BMJ Case Rep. 2008;2008:bcr0620080071. doi: 10.1136/bcr.06.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zellos A, Spoulou V, Roma-Giannikou E, Karentzou O, Dalekos GN, Theodoridou M. Autoimmune hepatitis type-2 and Epstein-Barr virus infection in a toddler: art of facts or an artifact? Ann Hepatol. 2013;12:147–151. [PubMed] [Google Scholar]
- 115.Dalekos GN, Wedemeyer H, Obermayer-Straub P, Kayser A, Barut A, Frank H, Manns MP. Epitope mapping of cytochrome P4502D6 autoantigen in patients with chronic hepatitis C during alpha-interferon treatment. J Hepatol. 1999;30:366–375. doi: 10.1016/s0168-8278(99)80092-0. [DOI] [PubMed] [Google Scholar]
- 116.Muratori L, Lenzi M, Cataleta M, Giostra F, Cassani F, Ballardini G, Zauli D, Bianchi FB. Interferon therapy in liver/kidney microsomal antibody type 1-positive patients with chronic hepatitis C. J Hepatol. 1994;21:199–203. doi: 10.1016/s0168-8278(05)80395-2. [DOI] [PubMed] [Google Scholar]
- 117.Todros L, Saracco G, Durazzo M, Abate ML, Touscoz G, Scaglione L, Verme G, Rizzetto M. Efficacy and safety of interferon alfa therapy in chronic hepatitis C with autoantibodies to liver-kidney microsomes. Hepatology. 1995;22:1374–1378. [PubMed] [Google Scholar]
- 118.Cholongitas E, Samonakis D, Patch D, Senzolo M, Burroughs AK, Quaglia A, Dhillon A. Induction of autoimmune hepatitis by pegylated interferon in a liver transplant patient with recurrent hepatitis C virus. Transplantation. 2006;81:488–490. doi: 10.1097/01.tp.0000196716.07188.c4. [DOI] [PubMed] [Google Scholar]
- 119.Vento S, Cainelli F, Renzini C, Concia E. Autoimmune hepatitis type 2 induced by HCV and persisting after viral clearance. Lancet. 1997;350:1298–1299. doi: 10.1016/S0140-6736(05)62476-2. [DOI] [PubMed] [Google Scholar]
- 120.Kerkar N, Hadzić N, Davies ET, Portmann B, Donaldson PT, Rela M, Heaton ND, Vergani D, Mieli-Vergani G. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351:409–413. doi: 10.1016/S0140-6736(97)06478-7. [DOI] [PubMed] [Google Scholar]
- 121.Mieli-Vergani G, Vergani D. De novo autoimmune hepatitis after liver transplantation. J Hepatol. 2004;40:3–7. doi: 10.1016/j.jhep.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 122.Fiel MI, Schiano TD. Plasma cell hepatitis (de-novo autoimmune hepatitis) developing post liver transplantation. Curr Opin Organ Transplant. 2012;17:287–292. doi: 10.1097/MOT.0b013e3283536622. [DOI] [PubMed] [Google Scholar]
- 123.Rigopoulou EI, Dalekos G, Bogdanos DP. How common are connective tissue disorders in patients with autoimmune hepatitis? Semin Arthritis Rheum. 2007;36:332; author reply 333. doi: 10.1016/j.semarthrit.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 124.Stefanidis I, Giannopoulou M, Liakopoulos V, Dovas S, Karasavvidou F, Zachou K, Koukoulis GK, Dalekos GN. A case of membranous nephropathy associated with Sjögren syndrome, polymyositis and autoimmune hepatitis. Clin Nephrol. 2008;70:245–250. doi: 10.5414/cnp70245. [DOI] [PubMed] [Google Scholar]
- 125.Gatselis NK, Zachou K, Norman GL, Tzellas G, Speletas M, Gabeta S, Germenis A, Koukoulis GK, Dalekos GN. IgA antibodies against deamidated gliadin peptides in patients with chronic liver diseases. Clin Chim Acta. 2012;413:1683–1688. doi: 10.1016/j.cca.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 126.Fujii K, Rokutanda R, Osugi Y, Koyama Y, Ota T. Adult-onset Still’s disease complicated by autoimmune hepatitis: successful treatment with infliximab. Intern Med. 2012;51:1125–1128. doi: 10.2169/internalmedicine.51.6824. [DOI] [PubMed] [Google Scholar]
- 127.Panetta F, Nobili V, Sartorelli MR, Papa RE, Ferretti F, Alterio A, Diamanti A. Celiac disease in pediatric patients with autoimmune hepatitis: etiology, diagnosis, and management. Paediatr Drugs. 2012;14:35–41. doi: 10.2165/11593150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 128.Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune polyglandular syndrome type 1. Clin Rev Allergy Immunol. 2000;18:167–183. doi: 10.1385/CRIAI:18:2:167. [DOI] [PubMed] [Google Scholar]
- 129.Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 130.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 131.Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 132.Vogel A, Liermann H, Harms A, Strassburg CP, Manns MP, Obermayer-Straub P. Autoimmune regulator AIRE: evidence for genetic differences between autoimmune hepatitis and hepatitis as part of the autoimmune polyglandular syndrome type 1. Hepatology. 2001;33:1047–1052. doi: 10.1053/jhep.2001.24031. [DOI] [PubMed] [Google Scholar]
- 133.Papamichalis PA, Zachou K, Koukoulis GK, Veloni A, Karacosta EG, Kypri L, Mamaloudis I, Gabeta S, Rigopoulou EI, Lohse AW, et al. The revised international autoimmune hepatitis score in chronic liver diseases including autoimmune hepatitis/overlap syndromes and autoimmune hepatitis with concurrent other liver disorders. J Autoimmune Dis. 2007;4:3. doi: 10.1186/1740-2557-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Georgiadou SP, Zachou K, Liaskos C, Gabeta S, Rigopoulou EI, Dalekos GN. Occult hepatitis B virus infection in patients with autoimmune liver diseases. Liver Int. 2009;29:434–442. doi: 10.1111/j.1478-3231.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 135.Gatselis NK, Zachou K, Papamichalis P, Koukoulis GK, Gabeta S, Dalekos GN, Rigopoulou EI. Comparison of simplified score with the revised original score for the diagnosis of autoimmune hepatitis: a new or a complementary diagnostic score? Dig Liver Dis. 2010;42:807–812. doi: 10.1016/j.dld.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 136.Azhar A, Niazi MA, Tufail K, Malek AH, Balasubramanian M, Araya V. A new approach for treatment of hepatitis C in hepatitis C-autoimmune hepatitis overlap syndrome. Gastroenterol Hepatol (N Y) 2010;6:233–236. [PMC free article] [PubMed] [Google Scholar]
- 137.Rigopoulou EI, Zachou K, Gatselis N, Koukoulis GK, Dalekos GN. Autoimmune hepatitis in patients with chronic HBV and HCV infections: patterns of clinical characteristics, disease progression and outcome. Ann Hepatol. 2013;13:127–135. [PubMed] [Google Scholar]
- 138.Teufel A, Weinmann A, Centner C, Piendl A, Lohse AW, Galle PR, Kanzler S. Hepatocellular carcinoma in patients with autoimmune hepatitis. World J Gastroenterol. 2009;15:578–582. doi: 10.3748/wjg.15.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, Bomford A, O’Grady JG, Harrison PM, Heneghan MA. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for follow-up and screening. Hepatology. 2008;48:863–870. doi: 10.1002/hep.22432. [DOI] [PubMed] [Google Scholar]
- 140.Ngu JH, Gearry RB, Frampton CM, Stedman CA. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 2012;55:522–529. doi: 10.1002/hep.24743. [DOI] [PubMed] [Google Scholar]
- 141.Migita K, Watanabe Y, Jiuchi Y, Nakamura Y, Saito A, Yagura M, Ohta H, Shimada M, Mita E, Hijioka T, et al. Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese National Hospital Organization-autoimmune hepatitis prospective study) Liver Int. 2012;32:837–844. doi: 10.1111/j.1478-3231.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 142.Yamamoto K, Hiura M, Tabaru A, Honma Y, Narita R, Abe S, Shimajiri S, Okamoto K, Yamaguchi K, Harada M. Rapid progression of hepatocellular carcinoma in a patient with autoimmune hepatitis. Intern Med. 2011;50:1409–1413. doi: 10.2169/internalmedicine.50.4873. [DOI] [PubMed] [Google Scholar]
- 143.Montano-Loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol. 2008;103:1944–1951. doi: 10.1111/j.1572-0241.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 144.Hino-Arinaga T, Ide T, Kuromatsu R, Miyajima I, Ogata K, Kuwahara R, Hisamochi A, Torimura T, Sata M. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. J Gastroenterol. 2012;47:569–576. doi: 10.1007/s00535-011-0519-2. [DOI] [PubMed] [Google Scholar]
- 145.Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev. 2014;13:435–440. doi: 10.1016/j.autrev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 146.Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14:3368–3373. doi: 10.3748/wjg.14.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544–553. doi: 10.1053/jhep.2001.22131. [DOI] [PubMed] [Google Scholar]
- 148.Levy C, Naik J, Giordano C, Mandalia A, O’Brien C, Bhamidimarri KR, Schiff ER, Martin P. Hispanics with primary biliary cirrhosis are more likely to have features of autoimmune hepatitis and reduced response to ursodeoxycholic acid than non-Hispanics. Clin Gastroenterol Hepatol. 2014;12:1398–1405. doi: 10.1016/j.cgh.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 149.Rojas CP, Bodicharla R, Campuzano-Zuluaga G, Hernandez L, Rodriguez MM. Autoimmune hepatitis and primary sclerosing cholangitis in children and adolescents. Fetal Pediatr Pathol. 2014;33:202–209. doi: 10.3109/15513815.2014.898721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lewin M, Vilgrain V, Ozenne V, Lemoine M, Wendum D, Paradis V, Ziol M, Arrivé L, Beaugrand M, Poupon R, et al. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: a prospective magnetic resonance imaging and histological study. Hepatology. 2009;50:528–537. doi: 10.1002/hep.23024. [DOI] [PubMed] [Google Scholar]
- 151.Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374–385. doi: 10.1016/j.jhep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 152.Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, Lindor K. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345–353. doi: 10.1038/ajg.2009.616. [DOI] [PubMed] [Google Scholar]
- 153.Kuiper EM, Zondervan PE, van Buuren HR. Paris criteria are effective in diagnosis of primary biliary cirrhosis and autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol. 2010;8:530–534. doi: 10.1016/j.cgh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 154.Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 155.Muratori P, Granito A, Pappas G, Pendino GM, Quarneti C, Cicola R, Menichella R, Ferri S, Cassani F, Bianchi FB, et al. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am J Gastroenterol. 2009;104:1420–1425. doi: 10.1038/ajg.2009.126. [DOI] [PubMed] [Google Scholar]
- 156.Coss Adame E, Granados J, Uribe M, Torre A. Does HLA-DR7 differentiate the overlap syndrome of auto-immune hepatitis-primary biliary cirrhosis (AIH-PBC) from those with auto-immune hepatitis type 1? Ann Hepatol. 2011;10:28–32. [PubMed] [Google Scholar]
- 157.Lee H, Stapp RT, Ormsby AH, Shah VV. The usefulness of IgG and IgM immunostaining of periportal inflammatory cells (plasma cells and lymphocytes) for the distinction of autoimmune hepatitis and primary biliary cirrhosis and their staining pattern in autoimmune hepatitis-primary biliary cirrhosis overlap syndrome. Am J Clin Pathol. 2010;133:430–437. doi: 10.1309/AJCPE93GZSHUNTAI. [DOI] [PubMed] [Google Scholar]
- 158.Abe K, Takahashi A, Nozawa Y, Imaizumi H, Hayashi M, Okai K, Kanno Y, Watanabe H, Ohira H. The utility of IgG, IgM, and CD138 immunohistochemistry in the evaluation of autoimmune liver diseases. Med Mol Morphol. 2014;47:162–168. doi: 10.1007/s00795-014-0082-z. [DOI] [PubMed] [Google Scholar]
- 159.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 160.Umemura T, Zen Y, Hamano H, Ichijo T, Kawa S, Nakanuma Y, Kiyosawa K. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471–1472. doi: 10.1136/gut.2007.122283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Paik WH, Ryu JK, Park JM, Song BJ, Park JK, Kim YT, Lee K. Clinical and pathological differences between serum immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J Gastroenterol. 2013;19:4031–4038. doi: 10.3748/wjg.v19.i25.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 163.Chung H, Watanabe T, Kudo M, Maenishi O, Wakatsuki Y, Chiba T. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int. 2010;30:222–231. doi: 10.1111/j.1478-3231.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- 164.Umemura T, Zen Y, Hamano H, Joshita S, Ichijo T, Yoshizawa K, Kiyosawa K, Ota M, Kawa S, Nakanuma Y, et al. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. J Gastroenterol. 2011;46 Suppl 1:48–55. doi: 10.1007/s00535-010-0323-4. [DOI] [PubMed] [Google Scholar]
- 165.Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther. 2012;36:517–533. doi: 10.1111/j.1365-2036.2012.05223.x. [DOI] [PubMed] [Google Scholar]
- 166.Granito A, Muratori L, Muratori P, Pappas G, Guidi M, Cassani F, Volta U, Ferri A, Lenzi M, Bianchi FB. Antibodies to filamentous actin (F-actin) in type 1 autoimmune hepatitis. J Clin Pathol. 2006;59:280–284. doi: 10.1136/jcp.2005.027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liaskos C, Bogdanos DP, Davies ET, Dalekos GN. Diagnostic relevance of anti-filamentous actin antibodies in autoimmune hepatitis. J Clin Pathol. 2007;60:107–108. doi: 10.1136/jcp.2006.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cassani F, Cataleta M, Valentini P, Muratori P, Giostra F, Francesconi R, Muratori L, Lenzi M, Bianchi G, Zauli D, et al. Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology. 1997;26:561–566. doi: 10.1002/hep.510260305. [DOI] [PubMed] [Google Scholar]
- 169.Czaja AJ. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J Hepatol. 1999;30:394–401. doi: 10.1016/s0168-8278(99)80096-8. [DOI] [PubMed] [Google Scholar]
- 170.Dbouk N, Parekh S. Impact of pretransplant antinuclear antibody and antismooth muscle antibody titers on disease recurrence and graft survival following liver transplantation in autoimmune hepatitis patients. J Gastroenterol Hepatol. 2013;28:537–542. doi: 10.1111/j.1440-1746.2012.07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wies I, Brunner S, Henninger J, Herkel J, Kanzler S, Meyer zum Büschenfelde KH, Lohse AW. Identification of target antigen for SLA/LP autoantibodies in autoimmune hepatitis. Lancet. 2000;355:1510–1515. doi: 10.1016/s0140-6736(00)02166-8. [DOI] [PubMed] [Google Scholar]
- 172.Herkel J, Heidrich B, Nieraad N, Wies I, Rother M, Lohse AW. Fine specificity of autoantibodies to soluble liver antigen and liver/pancreas. Hepatology. 2002;35:403–408. doi: 10.1053/jhep.2002.30699. [DOI] [PubMed] [Google Scholar]
- 173.Kanzler S, Weidemann C, Gerken G, Löhr HF, Galle PR, Meyer zum Büschenfelde KH, Lohse AW. Clinical significance of autoantibodies to soluble liver antigen in autoimmune hepatitis. J Hepatol. 1999;31:635–640. doi: 10.1016/s0168-8278(99)80342-0. [DOI] [PubMed] [Google Scholar]
- 174.Ballot E, Homberg JC, Johanet C. Antibodies to soluble liver antigen: an additional marker in type 1 auto-immune hepatitis. J Hepatol. 2000;33:208–215. doi: 10.1016/s0168-8278(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 175.Baeres M, Herkel J, Czaja AJ, Wies I, Kanzler S, Cancado EL, Porta G, Nishioka M, Simon T, Daehnrich C, et al. Establishment of standardised SLA/LP immunoassays: specificity for autoimmune hepatitis, worldwide occurrence, and clinical characteristics. Gut. 2002;51:259–264. doi: 10.1136/gut.51.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma Y, Okamoto M, Thomas MG, Bogdanos DP, Lopes AR, Portmann B, Underhill J, Dürr R, Mieli-Vergani G, Vergani D. Antibodies to conformational epitopes of soluble liver antigen define a severe form of autoimmune liver disease. Hepatology. 2002;35:658–664. doi: 10.1053/jhep.2002.32092. [DOI] [PubMed] [Google Scholar]
- 177.Czaja AJ, Donaldson PT, Lohse AW. Antibodies to soluble liver antigen/liver pancreas and HLA risk factors for type 1 autoimmune hepatitis. Am J Gastroenterol. 2002;97:413–419. doi: 10.1111/j.1572-0241.2002.05479.x. [DOI] [PubMed] [Google Scholar]
- 178.Efe C, Ozaslan E, Wahlin S, Purnak T, Muratori L, Quarneti C, Yüksel O, Muratori P. Antibodies to soluble liver antigen in patients with various liver diseases: a multicentre study. Liver Int. 2013;33:190–196. doi: 10.1111/liv.12022. [DOI] [PubMed] [Google Scholar]
- 179.Zhang WC, Zhao FR, Chen J, Chen WX. Meta-analysis: diagnostic accuracy of antinuclear antibodies, smooth muscle antibodies and antibodies to a soluble liver antigen/liver pancreas in autoimmune hepatitis. PLoS One. 2014;9:e92267. doi: 10.1371/journal.pone.0092267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Czaja AJ. Autoantibody-negative autoimmune hepatitis. Dig Dis Sci. 2012;57:610–624. doi: 10.1007/s10620-011-2017-z. [DOI] [PubMed] [Google Scholar]
- 181.Yuan J, Palioura S, Salazar JC, Su D, O’Donoghue P, Hohn MJ, Cardoso AM, Whitman WB, Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc Natl Acad Sci USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Palioura S, Sherrer RL, Steitz TA, Söll D, Simonovic M. The human SepSecS-tRNASec complex reveals the mechanism of selenocysteine formation. Science. 2009;325:321–325. doi: 10.1126/science.1173755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liaskos C, Bogdanos DP, Rigopoulou EI, Norman GL, Shurns Z, Al-Chalabi T, Krawitt EL, Mieli-Vergani G, Czaja AJ, Vergani D, et al. Antibody responses specific for soluble liver antigen co-occur with Ro-52 autoantibodies in patients with autoimmune hepatitis. J Hepatol. 2007;46(Suppl 1):S250 (abstract). [Google Scholar]
- 184.Eyraud V, Chazouilleres O, Ballot E, Corpechot C, Poupon R, Johanet C. Significance of antibodies to soluble liver antigen/liver pancreas: a large French study. Liver Int. 2009;29:857–864. doi: 10.1111/j.1478-3231.2009.01986.x. [DOI] [PubMed] [Google Scholar]
- 185.Montano-Loza AJ, Shums Z, Norman GL, Czaja AJ. Prognostic implications of antibodies to Ro/SSA and soluble liver antigen in type 1 autoimmune hepatitis. Liver Int. 2012;32:85–92. doi: 10.1111/j.1478-3231.2011.02502.x. [DOI] [PubMed] [Google Scholar]
- 186.Zachou K, Gampeta S, Gatselis NK, Oikonomou K, Goulis J, Manoussakis MN, Renaudineau Y, Bogdanos DP, Dalekos GN. Anti-SLA/LP alone or in combination with anti-Ro52 and fine specificity of anti-Ro52 antibodies in patients with autoimmune hepatitis. Liver Int. 2014:Epub ahead of print. doi: 10.1111/liv.12658. [DOI] [PubMed] [Google Scholar]
- 187.Duchini A, McHutchison JG, Pockros PJ. LKM-positive autoimmune hepatitis in the western United States: a case series. Am J Gastroenterol. 2000;95:3238–3241. doi: 10.1111/j.1572-0241.2000.03207.x. [DOI] [PubMed] [Google Scholar]
- 188.Bridoux-Henno L, Maggiore G, Johanet C, Fabre M, Vajro P, Dommergues JP, Reinert P, Bernard O. Features and outcome of autoimmune hepatitis type 2 presenting with isolated positivity for anti-liver cytosol antibody. Clin Gastroenterol Hepatol. 2004;2:825–830. doi: 10.1016/s1542-3565(04)00354-4. [DOI] [PubMed] [Google Scholar]
- 189.Yamamoto AM, Cresteil D, Boniface O, Clerc FF, Alvarez F. Identification and analysis of cytochrome P450IID6 antigenic sites recognized by anti-liver-kidney microsome type-1 antibodies (LKM1) Eur J Immunol. 1993;23:1105–1111. doi: 10.1002/eji.1830230519. [DOI] [PubMed] [Google Scholar]
- 190.Klein R, Zanger UM, Berg T, Hopf U, Berg PA. Overlapping but distinct specificities of anti-liver-kidney microsome antibodies in autoimmune hepatitis type II and hepatitis C revealed by recombinant native CYP2D6 and novel peptide epitopes. Clin Exp Immunol. 1999;118:290–297. doi: 10.1046/j.1365-2249.1999.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Duclos-Vallée JC, Hajoui O, Yamamoto AM, Jacz-Aigrain E, Alvarez F. Conformational epitopes on CYP2D6 are recognized by liver/kidney microsomal antibodies. Gastroenterology. 1995;108:470–476. doi: 10.1016/0016-5085(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 192.Muratori L, Lenzi M, Ma Y, Cataleta M, Mieli-Vergani G, Vergani D, Bianchi FB. Heterogeneity of liver/kidney microsomal antibody type 1 in autoimmune hepatitis and hepatitis C virus related liver disease. Gut. 1995;37:406–412. doi: 10.1136/gut.37.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dalekos GN, Makri E, Loges S, Obermayer-Straub P, Zachou K, Tsikrikas T, Schmidt E, Papadamou G, Manns MP. Increased incidence of anti-LKM autoantibodies in a consecutive cohort of hepatitis C patients from central Greece. Eur J Gastroenterol Hepatol. 2002;14:35–42. doi: 10.1097/00042737-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 194.Gatselis NK, Georgiadou SP, Koukoulis GK, Tassopoulos N, Zachou K, Liaskos C, Hatzakis A, Dalekos GN. Clinical significance of organ- and non-organ-specific autoantibodies on the response to anti-viral treatment of patients with chronic hepatitis C. Aliment Pharmacol Ther. 2006;24:1563–1573. doi: 10.1111/j.1365-2036.2006.03165.x. [DOI] [PubMed] [Google Scholar]
- 195.Ferri S, Muratori L, Quarneti C, Muratori P, Menichella R, Pappas G, Granito A, Ballardini G, Bianchi FB, Lenzi M. Clinical features and effect of antiviral therapy on anti-liver/kidney microsomal antibody type 1 positive chronic hepatitis C. J Hepatol. 2009;50:1093–1101. doi: 10.1016/j.jhep.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 196.Sugimura T, Obermayer-Straub P, Kayser A, Braun S, Loges S, Alex B, Lüttig B, Johnson EF, Manns MP, Strassburg CP. A major CYP2D6 autoepitope in autoimmune hepatitis type 2 and chronic hepatitis C is a three-dimensional structure homologous to other cytochrome P450 autoantigens. Autoimmunity. 2002;35:501–513. doi: 10.1080/0891693021000069556. [DOI] [PubMed] [Google Scholar]
- 197.Dalekos GN, Obermayer-Straub P, Bartels M, Maeda T, Kayser A, Braun S, Loges S, Schmidt E, Gershwin ME, Manns MP. Cytochrome P450 2A6: a new hepatic autoantigen in patients with chronic hepatitis C virus infection. J Hepatol. 2003;39:800–806. doi: 10.1016/s0168-8278(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 198.Ma Y, Peakman M, Lobo-Yeo A, Wen L, Lenzi M, Gäken J, Farzaneh F, Mieli-Vergani G, Bianchi FB, Vergani D. Differences in immune recognition of cytochrome P4502D6 by liver kidney microsomal (LKM) antibody in autoimmune hepatitis and chronic hepatitis C virus infection. Clin Exp Immunol. 1994;97:94–99. doi: 10.1111/j.1365-2249.1994.tb06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Durazzo M, Philipp T, Van Pelt FN, Lüttig B, Borghesio E, Michel G, Schmidt E, Loges S, Rizzetto M, Manns MP. Heterogeneity of liver-kidney microsomal autoantibodies in chronic hepatitis C and D virus infection. Gastroenterology. 1995;108:455–462. doi: 10.1016/0016-5085(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 200.Muratori P, Czaja AJ, Muratori L, Granito A, Guidi M, Ferri S, Volta U, Mantovani W, Pappas G, Cassani F, et al. Evidence of a genetic basis for the different geographic occurrences of liver/kidney microsomal antibody type 1 in hepatitis C. Dig Dis Sci. 2007;52:179–184. doi: 10.1007/s10620-006-9495-4. [DOI] [PubMed] [Google Scholar]
- 201.Crivelli O, Lavarini C, Chiaberge E, Amoroso A, Farci P, Negro F, Rizzetto M. Microsomal autoantibodies in chronic infection with the HBsAg associated delta (delta) agent. Clin Exp Immunol. 1983;54:232–238. [PMC free article] [PubMed] [Google Scholar]
- 202.Csepregi A, Nemesánszky E, Luettig B, Obermayer-Straub P, Manns MP. LKM3 autoantibodies in hepatitis C cirrhosis: a further phenomenon of the HCV-induced autoimmunity. Am J Gastroenterol. 2001;96:910–911. doi: 10.1111/j.1572-0241.2001.03383.x. [DOI] [PubMed] [Google Scholar]
- 203.Bachrich T, Thalhammer T, Jäger W, Haslmayer P, Alihodzic B, Bakos S, Hitchman E, Senderowicz AM, Penner E. Characterization of autoantibodies against uridine-diphosphate glucuronosyltransferase in patients with inflammatory liver diseases. Hepatology. 2001;33:1053–1059. doi: 10.1053/jhep.2001.24101. [DOI] [PubMed] [Google Scholar]
- 204.Abuaf N, Johanet C, Chretien P, Martini E, Soulier E, Laperche S, Homberg JC. Characterization of the liver cytosol antigen type 1 reacting with autoantibodies in chronic active hepatitis. Hepatology. 1992;16:892–898. doi: 10.1002/hep.1840160407. [DOI] [PubMed] [Google Scholar]
- 205.Lenzi M, Manotti P, Muratori L, Cataleta M, Ballardini G, Cassani F, Bianchi FB. Liver cytosolic 1 antigen-antibody system in type 2 autoimmune hepatitis and hepatitis C virus infection. Gut. 1995;36:749–754. doi: 10.1136/gut.36.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Muratori L, Cataleta M, Muratori P, Lenzi M, Bianchi FB. Liver/kidney microsomal antibody type 1 and liver cytosol antibody type 1 concentrations in type 2 autoimmune hepatitis. Gut. 1998;42:721–726. doi: 10.1136/gut.42.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lapierre P, Hajoui O, Homberg JC, Alvarez F. Formiminotransferase cyclodeaminase is an organ-specific autoantigen recognized by sera of patients with autoimmune hepatitis. Gastroenterology. 1999;116:643–649. doi: 10.1016/s0016-5085(99)70186-1. [DOI] [PubMed] [Google Scholar]
- 208.Muratori L, Sztul E, Muratori P, Gao Y, Ripalti A, Ponti C, Lenzi M, Landini MP, Bianchi FB. Distinct epitopes on formiminotransferase cyclodeaminase induce autoimmune liver cytosol antibody type 1. Hepatology. 2001;34:494–501. doi: 10.1053/jhep.2001.27179. [DOI] [PubMed] [Google Scholar]
- 209.Clemente MG, Obermayer-Straub P, Meloni A, Strassburg CP, Arangino V, Tukey RH, De Virgiliis S, Manns MP. Cytochrome P450 1A2 is a hepatic autoantigen in autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1997;82:1353–1361. doi: 10.1210/jcem.82.5.3913. [DOI] [PubMed] [Google Scholar]
- 210.Clemente MG, Meloni A, Obermayer-Straub P, Frau F, Manns MP, De Virgiliis S. Two cytochromes P450 are major hepatocellular autoantigens in autoimmune polyglandular syndrome type 1. Gastroenterology. 1998;114:324–328. doi: 10.1016/s0016-5085(98)70484-6. [DOI] [PubMed] [Google Scholar]
- 211.Gebre-Medhin G, Husebye ES, Gustafsson J, Winqvist O, Goksøyr A, Rorsman F, Kämpe O. Cytochrome P450IA2 and aromatic L-amino acid decarboxylase are hepatic autoantigens in autoimmune polyendocrine syndrome type I. FEBS Lett. 1997;412:439–445. doi: 10.1016/s0014-5793(97)00797-7. [DOI] [PubMed] [Google Scholar]
- 212.Ahonen P, Miettinen A, Perheentupa J. Adrenal and steroidal cell antibodies in patients with autoimmune polyglandular disease type I and risk of adrenocortical and ovarian failure. J Clin Endocrinol Metab. 1987;64:494–500. doi: 10.1210/jcem-64-3-494. [DOI] [PubMed] [Google Scholar]
- 213.Makaritsis KP, Gatselis NK, Ioannou M, Petinaki E, Dalekos GN. Polyclonal hypergammaglobulinemia and high smooth-muscle autoantibody titers with specificity against filamentous actin: consider visceral leishmaniasis, not just autoimmune hepatitis. Int J Infect Dis. 2009;13:e157–e160. doi: 10.1016/j.ijid.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 214.Renaudineau Y, Dalekos GN, Guéguen P, Zachou K, Youinou P. Anti-alpha-actinin antibodies cross-react with anti-ssDNA antibodies in active autoimmune hepatitis. Clin Rev Allergy Immunol. 2008;34:321–325. doi: 10.1007/s12016-007-8050-1. [DOI] [PubMed] [Google Scholar]
- 215.Liaskos C, Rigopoulou E, Zachou K, Georgiadou S, Gatselis N, Papamihali R, Dalekos GN. Prevalence and clinical significance of anticardiolipin antibodies in patients with type 1 autoimmune hepatitis. J Autoimmun. 2005;24:251–260. doi: 10.1016/j.jaut.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 216.Gabeta S, Norman GL, Gatselis N, Liaskos C, Papamichalis PA, Garagounis A, Zachou K, Rigopoulou EI, Dalekos GN. IgA anti-b2GPI antibodies in patients with autoimmune liver diseases. J Clin Immunol. 2008;28:501–511. doi: 10.1007/s10875-008-9211-6. [DOI] [PubMed] [Google Scholar]
- 217.Chen M, Shirai M, Czaja AJ, Kurokohchi K, Arichi T, Arima K, Kodama T, Nishioka M. Characterization of anti-histone antibodies in patients with type 1 autoimmune hepatitis. J Gastroenterol Hepatol. 1998;13:483–489. doi: 10.1111/j.1440-1746.1998.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 218.Montano-Loza A, Czaja AJ, Carpenter HA, Piette A, Murphy D, Shums Z, Burlingame R, Norman GL. Frequency and significance of antibodies to cyclic citrullinated peptide in type 1 autoimmune hepatitis. Autoimmunity. 2006;39:341–348. doi: 10.1080/08916930600783348. [DOI] [PubMed] [Google Scholar]
- 219.Fusconi M, Vannini A, Dall’Aglio AC, Pappas G, Cassani F, Ballardini G, Frisoni M, Grassi A, Bianchi FB, Zauli D. Anti-cyclic citrullinated peptide antibodies in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2005;22:951–955. doi: 10.1111/j.1365-2036.2005.02686.x. [DOI] [PubMed] [Google Scholar]
- 220.Hausdorf G, Roggenbuck D, Feist E, Büttner T, Jungblut PR, Conrad K, Berg C, Klein R. Autoantibodies to asialoglycoprotein receptor (ASGPR) measured by a novel ELISA--revival of a disease-activity marker in autoimmune hepatitis. Clin Chim Acta. 2009;408:19–24. doi: 10.1016/j.cca.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 221.Czaja AJ, Shums Z, Binder WL, Lewis SJ, Nelson VJ, Norman GL. Frequency and significance of antibodies to chromatin in autoimmune hepatitis. Dig Dis Sci. 2003;48:1658–1664. doi: 10.1023/a:1024748714580. [DOI] [PubMed] [Google Scholar]
- 222.Guéguen P, Dalekos G, Nousbaum JB, Zachou K, Putterman C, Youinou P, Renaudineau Y. Double reactivity against actin and alpha-actinin defines a severe form of autoimmune hepatitis type 1. J Clin Immunol. 2006;26:495–505. doi: 10.1007/s10875-006-9045-z. [DOI] [PubMed] [Google Scholar]
- 223.Oikonomou KG, Zachou K, Dalekos GN. Alpha-actinin: a multidisciplinary protein with important role in B-cell driven autoimmunity. Autoimmun Rev. 2011;10:389–396. doi: 10.1016/j.autrev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 224.Zachou K, Oikonomou K, Renaudineau Y, Chauveau A, Gatselis N, Youinou P, Dalekos GN. Anti-α actinin antibodies as new predictors of response to treatment in autoimmune hepatitis type 1. Aliment Pharmacol Ther. 2012;35:116–125. doi: 10.1111/j.1365-2036.2011.04908.x. [DOI] [PubMed] [Google Scholar]
- 225.Czaja AJ, Shums Z, Donaldson PT, Norman GL. Frequency and significance of antibodies to Saccharomyces cerevisiae in autoimmune hepatitis. Dig Dis Sci. 2004;49:611–618. doi: 10.1023/b:ddas.0000026306.36511.c8. [DOI] [PubMed] [Google Scholar]
- 226.Yiannaki EE, Zintzaras E, Analatos A, Theodoridou C, Dalekos GN, Germenis AE. Evaluation of a microsphere-based flow cytometric assay for diagnosis of celiac disease. J Immunoassay Immunochem. 2004;25:345–357. doi: 10.1081/ias-200033832. [DOI] [PubMed] [Google Scholar]
- 227.Germenis AE, Yiannaki EE, Zachou K, Roka V, Barbanis S, Liaskos C, Adam K, Kapsoritakis AN, Potamianos S, Dalekos GN. Prevalence and clinical significance of immunoglobulin A antibodies against tissue transglutaminase in patients with diverse chronic liver diseases. Clin Diagn Lab Immunol. 2005;12:941–948. doi: 10.1128/CDLI.12.8.941-948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dalekos GN, Bogdanos DP, Neuberger J. Celiac disease-related autoantibodies in end-stage autoimmune liver diseases: what is the message? Liver Int. 2008;28:426–428. doi: 10.1111/j.1478-3231.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 229.Nezu S, Tanaka A, Yasui H, Imamura M, Nakajima H, Ishida H, Takahashi S. Presence of antimitochondrial autoantibodies in patients with autoimmune hepatitis. J Gastroenterol Hepatol. 2006;21:1448–1454. doi: 10.1111/j.1440-1746.2006.04434.x. [DOI] [PubMed] [Google Scholar]
- 230.Rigopoulou EI, Dalekos GN. Molecular diagnostics of primary biliary cirrhosis. Expert Opin Med Diagn. 2008;2:621–634. doi: 10.1517/17530059.2.6.621. [DOI] [PubMed] [Google Scholar]
- 231.Liaskos C, Bogdanos DP, Rigopoulou EI, Dalekos GN. Development of antimitochondrial antibodies in patients with autoimmune hepatitis: art of facts or an artifact? J Gastroenterol Hepatol. 2007;22:454–455. doi: 10.1111/j.1440-1746.2006.04751.x. [DOI] [PubMed] [Google Scholar]
- 232.Montano-Loza AJ, Carpenter HA, Czaja AJ. Frequency, behavior, and prognostic implications of antimitochondrial antibodies in type 1 autoimmune hepatitis. J Clin Gastroenterol. 2008;42:1047–1053. doi: 10.1097/MCG.0b013e3181587d18. [DOI] [PubMed] [Google Scholar]
- 233.O’Brien C, Joshi S, Feld JJ, Guindi M, Dienes HP, Heathcote EJ. Long-term follow-up of antimitochondrial antibody-positive autoimmune hepatitis. Hepatology. 2008;48:550–556. doi: 10.1002/hep.22380. [DOI] [PubMed] [Google Scholar]
- 234.Dinani AM, Fischer SE, Mosko J, Guindi M, Hirschfield GM. Patients with autoimmune hepatitis who have antimitochondrial antibodies need long-term follow-up to detect late development of primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2012;10:682–684. doi: 10.1016/j.cgh.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 235.Muratori L, Muratori P, Zauli D, Grassi A, Pappas G, Rodrigo L, Cassani F, Lenzi M, Bianchi FB. Antilactoferrin antibodies in autoimmune liver disease. Clin Exp Immunol. 2001;124:470–473. doi: 10.1046/j.1365-2249.2001.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Himoto T, Yoneyama H, Kurokohchi K, Inukai M, Masugata H, Goda F, Haba R, Watanabe S, Senda S, Masaki T. Clinical significance of autoantibodies to p53 protein in patients with autoimmune liver diseases. Can J Gastroenterol. 2012;26:125–129. doi: 10.1155/2012/890698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Muratori P, Muratori L, Gershwin ME, Czaja AJ, Pappas G, MacCariello S, Granito A, Cassani F, Loria P, Lenzi M, et al. ‘True’ antimitochondrial antibody-negative primary biliary cirrhosis, low sensitivity of the routine assays, or both? Clin Exp Immunol. 2004;135:154–158. doi: 10.1111/j.1365-2249.2004.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Renaudineau Y, Croquefer S, Jousse S, Renaudineau E, Devauchelle V, Guéguen P, Hanrotel C, Gilburd B, Saraux A, Shoenfeld Y, et al. Association of alpha-actinin-binding anti-double-stranded DNA antibodies with lupus nephritis. Arthritis Rheum. 2006;54:2523–2532. doi: 10.1002/art.22015. [DOI] [PubMed] [Google Scholar]
- 239.Renaudineau Y, Deocharan B, Jousse S, Renaudineau E, Putterman C, Youinou P. Anti-alpha-actinin antibodies: a new marker of lupus nephritis. Autoimmun Rev. 2007;6:464–468. doi: 10.1016/j.autrev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 240.McFarlane BM, Sipos J, Gove CD, McFarlane IG, Williams R. Antibodies against the hepatic asialoglycoprotein receptor perfused in situ preferentially attach to periportal liver cells in the rat. Hepatology. 1990;11:408–415. doi: 10.1002/hep.1840110312. [DOI] [PubMed] [Google Scholar]
- 241.Treichel U, Gerken G, Rossol S, Rotthauwe HW, Meyer zum Büschenfelde KH, Poralla T. Autoantibodies against the human asialoglycoprotein receptor: effects of therapy in autoimmune and virus-induced chronic active hepatitis. J Hepatol. 1993;19:55–63. doi: 10.1016/s0168-8278(05)80176-x. [DOI] [PubMed] [Google Scholar]
- 242.Czaja AJ, Pfeifer KD, Decker RH, Vallari AS. Frequency and significance of antibodies to asialoglycoprotein receptor in type 1 autoimmune hepatitis. Dig Dis Sci. 1996;41:1733–1740. doi: 10.1007/BF02088738. [DOI] [PubMed] [Google Scholar]
- 243.Yoshioka M, Mizuno M, Morisue Y, Shimada M, Hirai M, Nasu J, Okada H, Sakaguchi K, Yamamoto K, Tsuji T. Anti-asialoglycoprotein receptor autoantibodies, detected by a capture-immunoassay, are associated with autoimmune liver diseases. Acta Med Okayama. 2002;56:99–105. doi: 10.18926/AMO/31695. [DOI] [PubMed] [Google Scholar]
- 244.Hilgard P, Schreiter T, Stockert RJ, Gerken G, Treichel U. Asialoglycoprotein receptor facilitates hemolysis in patients with alcoholic liver cirrhosis. Hepatology. 2004;39:1398–1407. doi: 10.1002/hep.20172. [DOI] [PubMed] [Google Scholar]
- 245.Husa P, Chalupa P, Stroblová H, Husová L, Slesinger P, Zajíc J. Autoantibodies to asialoglycoprotein receptor in chronic hepatitis C patients. Acta Virol. 2001;45:7–11. [PubMed] [Google Scholar]
- 246.Björnsson E, Talwalkar J, Treeprasertsuk S, Neuhauser M, Lindor K. Patients with typical laboratory features of autoimmune hepatitis rarely need a liver biopsy for diagnosis. Clin Gastroenterol Hepatol. 2011;9:57–63. doi: 10.1016/j.cgh.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 247.Schiano TD, Fiel MI. To B(iopsy) or not to B(iopsy) …. Clin Gastroenterol Hepatol. 2011;9:3–4. doi: 10.1016/j.cgh.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 248.Czaja AJ, Muratori P, Muratori L, Carpenter HA, Bianchi FB. Diagnostic and therapeutic implications of bile duct injury in autoimmune hepatitis. Liver Int. 2004;24:322–329. doi: 10.1111/j.1478-3231.2004.0924.x. [DOI] [PubMed] [Google Scholar]
- 249.Dienes HP, Erberich H, Dries V, Schirmacher P, Lohse A. Autoimmune hepatitis and overlap syndromes. Clin Liver Dis. 2002;6:349–362, vi. doi: 10.1016/s1089-3261(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 250.Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG, Breen E, Allison AC, van Rooijen N, McGuffog C, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci USA. 2011;108:16735–16740. doi: 10.1073/pnas.1112251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Te HS, Koukoulis G, Ganger DR. Autoimmune hepatitis: a histological variant associated with prominent centrilobular necrosis. Gut. 1997;41:269–271. doi: 10.1136/gut.41.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fujiwara K, Fukuda Y, Yokosuka O. Precise histological evaluation of liver biopsy specimen is indispensable for diagnosis and treatment of acute-onset autoimmune hepatitis. J Gastroenterol. 2008;43:951–958. doi: 10.1007/s00535-008-2254-x. [DOI] [PubMed] [Google Scholar]
- 253.Guindi M. Histology of autoimmune hepatitis and its variants. Clin Liver Dis. 2010;14:577–590. doi: 10.1016/j.cld.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 254.Yasui S, Fujiwara K, Yonemitsu Y, Oda S, Nakano M, Yokosuka O. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol. 2011;46:378–390. doi: 10.1007/s00535-010-0316-3. [DOI] [PubMed] [Google Scholar]


