Abstract
Purpose
To evaluate whether the levels of immunoglobulin G (IgG) antibody to Tanerella forsythia are associated with periodontal status.
Methods
Patients with a diagnosis of chronic periodontitis were considered candidates for the study; thus 80 chronic periodontitis patients and 28 healthy persons (control group) were invited to participate in this investigation. The presence of T. forsythia was detected by polymerase chain reaction (PCR) analysis using primers designed to target the respective 16S rRNA gene sequences. Peripheral blood was collected from each subject to identify the IgG1 and IgG2 serum antibodies against T. forsythia. All microbiological and immunological laboratory processes were completed blindly, without awareness of the clinical status of the study patients or of the periodontal sites tested.
Results
The bivariate analysis showed that lower mean levels of clinical attachment level (CAL) and probing depth were found in the presence of the IgG1 antibody titers against whole-cell T. forsythia; however, only the difference in CAL was statistically significant. In the presence of the IgG2 antibody titers against whole-cell T. forsythia, the periodontal parameters evaluated were higher but they did not show statistical differences, except for plaque. The unadjusted linear regression model showed that the IgG1 antibody against whole-cell T. forsythia in periodontitis patients was associated with a lower mean CAL (β=-0.654; 95% confidence interval [CI], -1.27 to -0.28; P<0.05). This statistically significant association remained after adjusting for possible confounders (β=-0.655; 95% CI, -1.28 to -0.29; P<0.05). On the other hand, smoking was a statistically significant risk factor in the model (β=0.704; 95% CI, 0.24 to 1.38; P<0.05).
Conclusions
Significantly lower mean levels of CAL were shown in the presence of the IgG1 antibody titers against whole-cell T. forsythia in periodontitis patients. Thus, the results of this study suggest that IgG1 antibody to T. forsythia may have been a protective factor from periodontitis in this sample.
Graphical Abstract
Keywords: Chronic periodontitis, Immunoglobulin G, Periodontal disease, Periodontitis
INTRODUCTION
A bacterial consortium that includes Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola is strongly linked to periodontitis [1]. Among them, T. forsythia has persisted as an under-researched microorganism due to the difficulty of growing it and difficult bacteria for genetic management [2]. Thus, few virulence factors of T. forsythia have been identified, and these features may provoke the disorder by allowing microbial progression in the periodontal pockets by removing host immune cells across the generation of apoptosis or necrosis [3]. Bacterial surface protein A (BspA) is a protein with leucine-rich repeats and bacterial Ig-like domains that favor the generation of proinflammatory cytokine expression in host cells [3,4]. A BspA equivalent in T. forsythia was found to be upregulated multifold in individuals with periodontal disease [5]. In this manner, BspA is a critical virulence element of T. forsythia, and consequently, the immune reaction in periodontitis to this antigen is likely to be vital to the pathogenicity of T. forsythia [6].
Most investigations of the humoral immune reaction to periodontopathogens and to major antigens have involved serum immunoglobulin (Ig) G antibody titers to P. gingivalis and Aggregatibacter actinomycetemcomitans [7]. Very few studies have examined the immune responses in periodontitis to the entire T. forsythia bacterium [8,9] or its constituents [4,6,10].
Besides, it is important to note that demographic and behavioral characteristics, and oral and general health status have been found to be robust elements of systemic antibody responses to periodontal pathogens in a nationally representative sample of adults in the United States [11]. Moreover, it has been reported that Hispanic individuals have a lower level of antibody titers against T. forsythia than Asian Americans and African Americans [12]; therefore, environmental and socioeconomic factors may have a higher impact on serum IgG antibody levels in the inhabitants. If risk factors for disease progress differ among ethnic/racial populations, as the above investigations have proposed, then incorrect treatments may be applied in these groups if they are not specially treated [12].
To our knowledge, few studies have investigated the relationship of IgG antibody titers to T. forsythia and periodontal status, and this association has not been adjusted for potential confounders. Thus, the objective of this study was to evaluate whether serum IgG antibody titers to T. forsythia are associated with periodontal status.
MATERIALS AND METHODS
Sample size calculation
According to Craig et al. [12], the mean serum IgG antibody levels to T. forsythia were higher in a periodontitis group when compared to a healthy group in a sample of the United States (US) Hispanic population (A difference of 2.4 EU [enzyme-linked immunosorbent assay unit] was found). Thus, a difference of 2.4 EU between groups was considered to be relevant. The sample size calculation determined that ≥21 patients per group would provide 80% power and a significance level of 0.05 (two-tailed) for detecting a true difference of 2.4 EU between groups, assuming 2.75 EU as the common standard deviation.
Subjects
One hundred eight subjects (79 females and 29 males), aged 33 to 82 years (with ≥18 residual teeth) who visited the dental clinics of the Universidad de Antioquia in Medellín, Colombia were invited to participate in this study between January 2009 and December 2011. Informed and written consent was obtained from each participant. The study design was approved by the Ethics Committee on Human Research of the School of Dentistry of the University of Antioquia (ID 02-2008) according to the Declaration of Helsinki on experimentation involving human subjects. Patients with a diagnosis of chronic periodontitis (the diagnostic criteria are described below), ≥18 residual teeth and ≥31 years were considered candidates for the study. Individuals with no evidence of mild, moderate, or severe periodontitis were used as a control group. Of the 108 subjects included, 28 patients belonged to the control group.
Exclusion criteria included diagnosed diabetes and autoimmune diseases. Pregnant women, intake of systemic antimicrobials with the previous six months, nonsteroidal analgesics or anti-inflammatory drugs, and earlier periodontal therapy also served as exclusion criteria.
Clinical evaluation
A medical history and clinical and radiographic examinations were performed for each patient. The diagnosis of chronic periodontitis was made on the basis of principles outlined by Eke et al. [13]; patients were categorized as having moderate periodontitis by ≥2 interproximal sites with clinical attachment level (CAL) ≥4 mm, or by ≥2 interproximal sites with probing depth (PD)≥5 mm (not at the same tooth). Severe periodontitis was defined as ≥2 interproximal sites with CAL ≥6 mm and ≥1 interproximal site with PD≥5 mm (not at the same tooth).
A trained and calibrated clinician performed all clinical examinations. The intraexaminer reproducibility was calculated before and during the study. The intraclass correlation coefficients for the mean PD and CAL were 0.91 and 0.92, respectively; the intraevaluator kappa index was in the range 0.85-0.96. The presence or absence of bleeding on probing (BOP) and plaque were registered. PD and CAL were measured at all proximal, buccal, and lingual surfaces to the nearest millimeter by a calibrated standard probe (UNC-15, Hu-Friedy, Chicago, IL, USA).
Microbial sampling
Microbial sampling on periodontitis patients was completed on pockets >5 mm. The deepest six pockets were designated for sampling. After removing supragingival plaque with a curette and isolating the area with cotton pellets, paper points (Maillefer, Ballaigues, Switzerland) were inserted into each periodontal pocket for 20 seconds. One paper point from each site was introduced into an empty 1.5-mL microfuge tube for polymerase chain reaction (PCR) analysis.
The presence of T. forsythia was detected by PCR using primers designed to target the relevant 16S rRNA gene sequences, following the approach of Ashimoto et al. [14]. Briefly, PCR mixtures (50 mL) were prepared with 5 mL of bacterial DNA (GoTaq Flexi DNA Polymerase, Promega, Madison, WI, USA), 0.5mM species-specific primers, 10 mL of 5 PCR buffer (Deoxynucleotide Triphosphates, Promega), 1.25 U of Taq DNA polymerase (Deoxynucleotide Triphosphates, Promega), 0.2 mM dNTP mix (Deoxynucleotide Triphosphates, Promega), and 1.5 mM MgCl2 (Promega). Gene-specific amplification was performed in a thermal cycler (MyCycler Termal Cycler, Bio-Rad Laboratories, Hercules, CA, USA) with the following thermal profiles: T. forsythia, initial denaturation step at 95℃ for 2 minutes, followed by 36 cycles of denaturation at 95℃ for 30 seconds, annealing at 60℃ for 1 minute and extension at 72℃ for 1 minute, and a final extension at 72℃ for 2 minutes. The PCR products were electrophoresed on 1% agarose gels and stained with 0.5-mg/mL ethidium bromide, and the presence of target bands for each bacterium was confirmed.
Indirect immunoassay (enzyme-linked immunosorbent assay) for the determination of serum antibodies IgG1 and IgG2 against T. forsythia
Peripheral blood was collected from each patient to determine serum antibodies IgG1 and IgG2 against T. forsythia. Enzyme-linked immunosorbent assay (ELISA) was performed as follows: A 96-well plate (Immulux HB, Dynex Technologies, Chantilly, VA, USA) was covered with 50 µL in a 10-µg/mL concentration of sonicate of T. forsythia. (ATCC43037) of carbonate buffer (pH 9.6) incubated overnight. The plates were blocked with 150 µL of solution of phosphate buffer (PBS; pH 7,2) with 1% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, Mo, USA), for 1 hour and 1% milk and avidin (Avidin/Biotin Blocking Kit, Vector Laboratories Inc., Burlingame, CA, USA). The sera were diluted 1/100 in PBS-Tween-BSA (1%)-biotin solution (Avidin/Biotin Blocking Kit) and were incubated at 37℃ for hour. Anti-IgG1 dilution by 1:5,000 (mouse antihuman IgG1, Invitrogen, Grand Island, NY, USA) or anti-IgG2 dilution 1:10,000 (mouse antihuman IgG2, Sigma-Aldrich) in PBS-Tween-BSA (1%) solution, were incubated for 1 hour at 37℃. Streptavidin peroxidase (Invitrogen) in a 1/1,500 dilution was incubated for 1 hour at room temperature. Between each incubation period, three washes were carried out with 250-µL PBS-tween (0.05%) solution and 2 minutes of stirring (Multiwasher-MW2001, SUMA, Immunoassay Center, Havana, Cuba). Finally, 50-µL phosphate/citrate buffer solution (0.5 M, pH 5) was added with o-phenylenediamine (Opd-Pierce), and a concentration of 1 mg/mL was added and activated with H2O2 for 5 minutes at room temperature. The reaction was stopped with a solution of sulphuric acid (2.5 M). Absorbance values were read at 490 nm (Lector Elisa stat fax 2100, Awareness Technology Inc., Palm City, FL, USA). The concentration of IgG1 and IgG2 in the samples for T. forsythia was calculated by linear regression analysis with a known concentration curve (5 µg/mL and 0.152 µg/mL, dilution 1:2) of human immunoglobulin IgG1 o IgG2 (Sigma-Aldrich). Every sample was measured in triplicate.
Antibody titers were calculated as follows: An arbitrary serum confirmed to have a moderate antibody level judged by western immunoblotting was used as a standard and taken as one EU and then serially diluted on every plate to generate a standard curve. As defined by Bishop et al. [15], each patient's serum was compared to the standard curve, and relative titer values were calculated appropriately.
All microbiological and immunological laboratory processes were completed blindly, without awareness of the clinical status of the study patients or of the periodontal sites tested.
Statistical analysis
Differences in continuous and categorical variables were examined by independent t-test (data were distributed normally) and chi-square test, respectively. IgG1/IgG2 antibody titers against whole-cell T. forsythia in the periodontitis group were related to mean levels of CAL, PD, presence of plaque, and BOP using an independent t-test. Linear regression analysis was applied stepwise in order to analyze whether the IgG1 antibody against whole-cell T. forsythia is a protective factor for CAL; the model was adjusted for age (years), gender (male/female), smoking status (smoker/nonsmoker) and socioeconomic status (amount of monthly personal income≥750 USD; yes/no). Multicollinearity was not found during model fitting. Values of P<0.05 were considered statistically significant. All analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
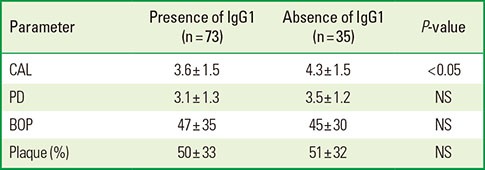
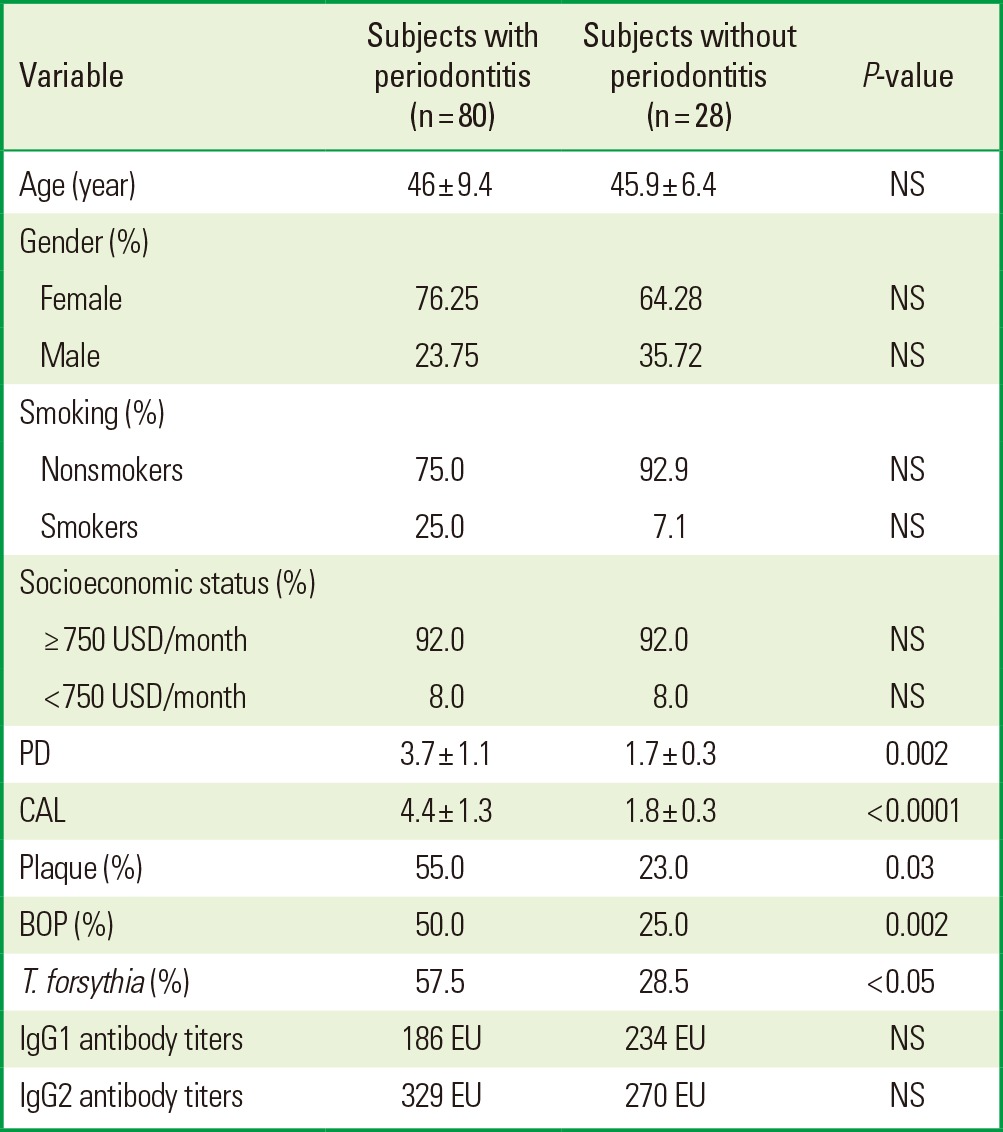
A total of 61 women and 19 men with chronic periodontitis, and 18 women and 10 men without periodontitis were studied. Table 1 describes the demographic characteristics, periodontal parameters and IgG1/IgG2 antibody titers against whole-cell T. forsythia of the patients with and without periodontitis. There was a significant difference between both groups with respect to PD (P=0.002), CAL (P<0.0001), plaque (P=0.03), and BOP (P=0.002); however, the results did not show statistically significant differences in the mean anti-whole-cell titers between the periodontal disease and healthy groups. Moreover, the prevalence of T. forsythia was higher in the group of periodontitis patients (Table 1).
Table 1.
Demographic characteristics, periodontal parameters, proportion of occurrence of Tannerella forsythia, and IgG1/IgG2 antibody titers against whole-cell T. forsythia of the patients with and without periodontitis.
Values are presented as mean±standard deviation unless otherwise indicated.
NS: not statistically significant, USD: United States Dollars, PD: probing depth, CAL: clinical attachment level, BOP: bleeding on probing, IgG: immunoglobulin G, EU: enzyme-linked immunosorbent assay units.
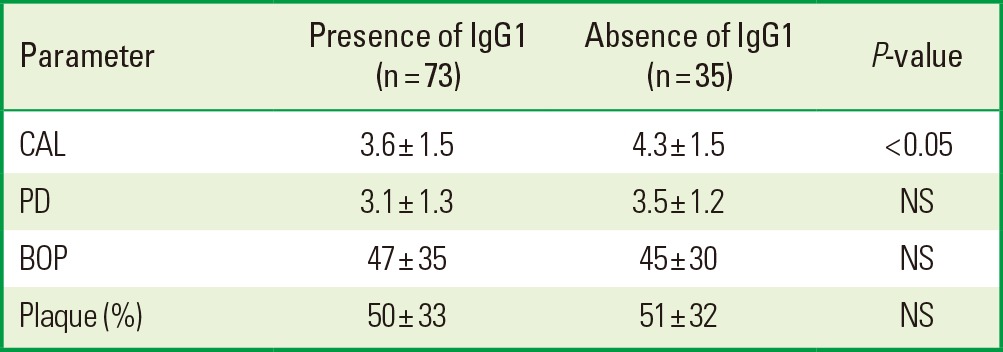
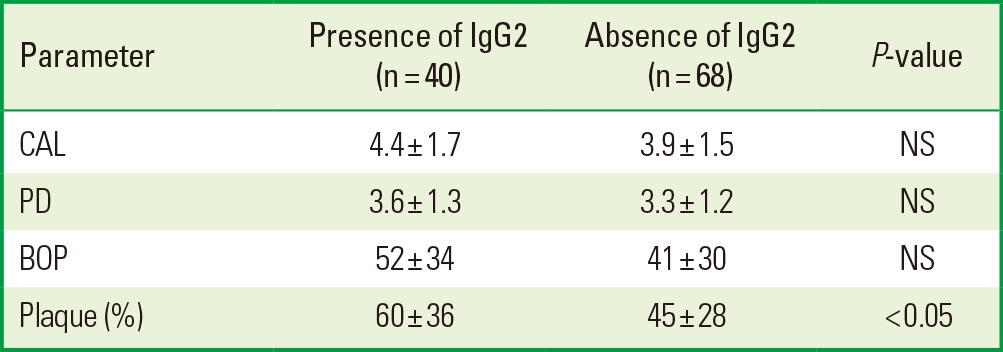
With this information, we studied whether the IgG1/IgG2 antibody titers against whole-cell T. forsythia were related to the mean levels of CAL, PD, presence of plaque, and BOP using an independent t-test. Lower mean levels of CAL and PD were shown in the presence of the IgG1 antibody titers against whole-cell T. forsythia; however, only CAL showed a statistically significant difference. Besides, the results did not present statistically significant differences in BOP and plaque (Table 2). On the other hand, in the presence of the IgG2 antibody titers against whole-cell T. forsythia, the periodontal parameters evaluated were higher but they did not show statistical differences, except for plaque (Table 3).
Table 2.
Periodontal parameters in 108 patients with presence or absence of IgG1 antibody titers against whole-cell Tannerella forsythia.
Values are presented as mean±standard deviation.
IgG: immunoglobulin G, CAL: clinical attachment level, PD: probing depth, NS: not statistically significant, BOP: bleeding on probing.
Table 3.
Periodontal parameters in 108 patients with presence or absence of IgG2 antibody titers against whole-cell Tannerella forsythia.
Values are presented as mean±standard deviation.
IgG: immunoglobulin G, CAL: clinical attachment level, PD: probing depth, NS: not statistically significant, BOP: bleeding on probing.
With these data, we analyzed whether the IgG1 antibody against whole-cell T. forsythia in the periodontitis group is a protective factor for CAL using a linear regression model adjusted for age, gender, smoking, and socioeconomic status. In the unadjusted linear model, the IgG1 antibody titers against whole-cell T. forsythia were associated with a lower mean CAL (β=-0,654; 95% confidence interval [CI], -1.27 to -0.28; P<0.05). This statistically significant association remained after adjustment for possible confounders (β=-0,655; 95% CI, -1.28 to -0.29; P<0.05) (Table 4). By use of the multivariate linear regression model, we wanted to identify explanatory factors for a difference in CAL. It is important to note that smoking was a statistically significant risk factor in the model (β=0.704; 95% CI, 0.24 to 1.38; P<0.05) (Table 4).
Table 4.
Unadjusted and adjusted linear regression models for clinical attachment level in 80 periodontitis patients.
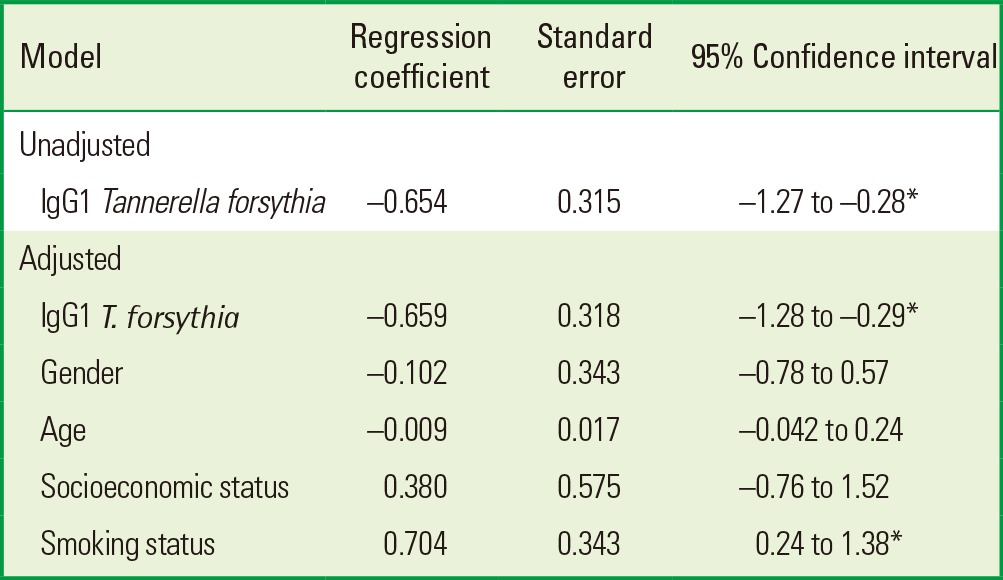
IgG1 T. forsythia: immunoglobulin G1 antibody titers against whole-cell Tanerella forsythia.
*P<0.05, statistically significance.
DISCUSSION
The complicated connection between periodontal pathogens, their products, and the host's means of defense define the outcomes of periodontitis [1]. Because periodontitis is a polymicrobial infectious disease [16], it is accepted that infection with periodontal bacteria leads to humoral immunological reactions and raises the quantity of serum IgG antibody against bacteria [17].
Among the periodontopathogen constituents that stimulate macrophages, the most broadly studied is lipopolysaccharide (LPS), a strong virulence factor of gram-negative microorganisms [18]. T. forsythia is frequently located cohabiting with P. gingivalis in subgingival dental biofilms [1], which can, in turn, affect systemic health. T. forsythia LPS might be associated with the regulation of proinflammatory cytokines, and it has been found to stimulate their creation by human macrophages, with the interleukin-8 secretion level of T. forsythia LPS being about 1.5 times that of P. gingivalis LPS [19]. Furthermore, T. forsythia and BspA (its most important surface virulence issue) augmented atherosclerotic injury development in ApoE-/- mice [20].
To our knowledge, the present study is the first to explore the relationship of the IgG antibody titers to T. forsythia and periodontal status in Latin America, and the first adjusting for potential confounders, providing additional evidence for the connection between periodontitis and IgG1 antibody titers against whole-cell T. forsythia, discernable, in this case, as a protective factor for CAL. Although our results did not show statistically significant differences in the mean anti-whole-cell titers between periodontal disease and healthy groups, the data suggested that the IgG1 antibody titers against whole-cell T. forsythia might play a protective role in chronic periodontitis by reducing the loss of attachment tissue. Hall et al. [6] reported similar results in 100 patients with cardiac disorders and periodontal disease and 73 patients who experienced myocardial infarction but were periodontally healthy. Their results showed no statistically significant differences in the mean anti-whole-cell titers between periodontitis and healthy groups, suggesting that infection by T. forsythia occurred in all individuals irrespective of their disease status, and/or T. forsythia expressed common antigens shared by other organisms. They also revealed that antibodies to the T. forsythia BspA protein were produced in individuals with periodontal disease and increased antibody levels related with reduced periodontal CAL. BspA-specific antibodies may have a protective function, likely through blocking BspA-mediated inflammatory periodontal destruction [20]. Similarly, Onishi et al. [3] demonstrated that in periodontitis patients, the IgG levels of healthy sites were significantly higher than those of diseased sites, and the levels showed negative correlations with PD and CAL, suggesting that anti-forsythia detaching factor (FDF) IgG levels in gingival crevicular fluid are associated with periodontal status. These results suggested that FDF would stimulate the creation of neutralizing antibody.
As pointed out by Gemmell et al. [21], if particular antibodies with elevated avidity and protecting IgG subclasses to immunodominant antigens are created, the infection may be dissipated and the disease will not advance; nevertheless, they documented that some periodontal bacterial antigens induce host-destructive immune responses. Califano et al. [22] speculated that T. forsythia may suppress the host humoral immune response or, indeed, could be not a genuine bacterium but rather a commensal species within the subgingival environment. In line with this, a previous report showed that higher serum IgG antibody levels to P. gingivalis, and not to other subgingival species, are related with earlier disease, consequent attachment loss and, probably, the occurrence of multiple sites of attachment loss [12]. Further investigations are required to verify this conclusion.
In the present study, the IgG1 antibody titers against whole-cell T. forsythia in the periodontitis group were associated with a lower mean CAL. This statistically significant association remained after adjusting for probable confounders. Dye et al. [23] indicated that elevated IgG titers to P. gingivalis were most strongly related with periodontitis (odds ratio, 2.07-2.74) in unadjusted models. They recommend that adjusting these confounders may elucidate this connection, as was accomplished in the present investigation.
In this investigation, smoking was found to be a significant risk factor for CAL (β=0.704; 95% CI, 0.24-1.38; P<0.05), as has been traditionally demonstrated. Other researchers also confirmed that smokers have significantly greater amounts of P. gingivalis, A. actinomycetemcomitans, and T. forsythia than never-smokers [24]. In line with this, in this study, the multivariate model for CAL including IgG1 antibody against whole-cell T. forsythia and controlling for smoking was significant. Interestingly, among the racial groups Quinn et al. [25] studied, they were unable to detect any increase in periodontal destruction or a significant decrease in serum IgG2 levels in periodontitis subjects who were black and smoked or in their age-matched nonsmoking controls. Therefore, more studies are needed in which potential confounders are adjusted.
In the current study, IgG1 and IgG2 each show a different relevance to T. forsythia. Similarly, when Chung et al. [26] examined serum IgG concentrations in patients with different forms of periodontitis, there were no differences in the concentrations of four IgG subclasses in patients with chronic periodontitis; however in aggressive periodontitis patients younger than 35 years of age, levels of IgG2 were significantly elevated, and they also found significant differences in IgG2 levels within the control group when stratified by age. Additionally, is important to note that there have been questions about the protective function of high levels of IgG2 because of its lower avidity, which is thought to be insufficient for removing some periodontopathogens [27,28]. Further investigations are required to elucidate this matter.
Craig et al. [12] reported that serum IgG antibody to P. gingivalis was found to be higher in an African-American group, whereas serum IgG antibody to T. forsythia was lower in a US Hispanic population. For this reason, it is important to study specific populations, which allows us to elucidate the role of serum antibodies in the progression of periodontitis. The extent of the systemic antibody reactions to periodontal bacteria is determined by the complex interplay of environmental and behavioral aspects, and oral and general health-related characteristics [11].
A limitation of the present study is its cross-sectional design, which makes it difficult to determine the cause and effect relationship between the IgG1 antibody titers against whole-cell T. forsythia and CAL. This concern can be solved by longitudinal studies with a sufficient follow-up period. Nonetheless, the results of this study suggest that IgG1 antibody to T. forsythia can be considered a protective factor for periodontal disease in our sample, and this association remained after adjusting for various potential confounders.
This investigation demonstrated that IgG1 antibody titers against whole-cell T. forsythia are associated with reduced periodontal CAL, suggesting a protective function.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Institute of Science and Technology, Francisco José de Caldas-Colciencias (No. 1308-459-21661).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 2.Amano A, Chen C, Honma K, Li C, Settem RP, Sharma A. Genetic characteristics and pathogenic mechanisms of periodontal pathogens. Adv Dent Res. 2014;26:15–22. doi: 10.1177/0022034514526237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onishi H, Arakawa S, Nakajima T, Izumi Y. Levels of specific immunoglobulin G to the forsythia detaching factor of Tannerella forsythia in gingival crevicular fluid are related to the periodontal status. J Periodontal Res. 2010;45:672–680. doi: 10.1111/j.1600-0765.2010.01283.x. [DOI] [PubMed] [Google Scholar]
- 4.Onishi H, Ro M, Hayashi J, Tatsumi J, Satomi N, Yatabe K, et al. Modification of forsythia detaching factor by gingival crevicular fluid in periodontitis. Arch Oral Biol. 2013;58:1007–1013. doi: 10.1016/j.archoralbio.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Yoo JY, Kim HC, Zhu W, Kim SM, Sabet M, Handfield M, et al. Identification of Tannerella forsythia antigens specifically expressed in patients with periodontal disease. FEMS Microbiol Lett. 2007;275:344–352. doi: 10.1111/j.1574-6968.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 6.Hall LM, Dunford RG, Genco RJ, Sharma A. Levels of serum immunoglobulin G specific to bacterial surface protein A of Tannerella forsythia are related to periodontal status. J Periodontol. 2012;83:228–234. doi: 10.1902/jop.2011.110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinane DF, Mooney J, Ebersole JL. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol 2000. 1999;20:289–340. doi: 10.1111/j.1600-0757.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 8.Haffajee AD, Socransky SS, Dzink JL, Taubman MA, Ebersole JL. Clinical, microbiological and immunological features of subjects with refractory periodontal diseases. J Clin Periodontol. 1988;15:390–398. doi: 10.1111/j.1600-051x.1988.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 9.Persson GR, Schlegel-Bregenzer B, Chung WO, Houston L, Oswald T, Roberts MC. Serum antibody titers to Bacteroides forsythus in elderly subjects with gingivitis or periodontitis. J Clin Periodontol. 2000;27:839–845. doi: 10.1034/j.1600-051x.2000.027011839.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoneda M, Hirofuji T, Motooka N, Nozoe K, Shigenaga K, Anan H, et al. Humoral immune responses to S-layer-like proteins of Bacteroides forsythus. Clin Diagn Lab Immunol. 2003;10:383–387. doi: 10.1128/CDLI.10.3.383-387.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlachojannis C, Dye BA, Herrera-Abreu M, Pikdoken L, Lerche-Sehm J, Pretzl B, et al. Determinants of serum IgG responses to periodontal bacteria in a nationally representative sample of US adults. J Clin Periodontol. 2010;37:685–696. doi: 10.1111/j.1600-051X.2010.01592.x. [DOI] [PubMed] [Google Scholar]
- 12.Craig RG, Boylan R, Yip J, Mijares D, Imam M, Socransky SS, et al. Serum IgG antibody response to periodontal pathogens in minority populations: relationship to periodontal disease status and progression. J Periodontal Res. 2002;37:132–146. doi: 10.1034/j.1600-0765.2002.00031.x. [DOI] [PubMed] [Google Scholar]
- 13.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 15.Bishop RF, Cipriani E, Lund JS, Barnes GL, Hosking CS. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol. 1984;19:447–452. doi: 10.1128/jcm.19.4.447-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker C, Sedlacek MJ. An in vitro biofilm model of subgingival plaque. Oral Microbiol Immunol. 2007;22:152–161. doi: 10.1111/j.1399-302X.2007.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murayama Y, Nagai A, Okamura K, Kurihara H, Nomura Y, Kokeguchi S, et al. Serum immunoglobulin G antibody to periodontal bacteria. Adv Dent Res. 1988;2:339–345. doi: 10.1177/08959374880020022401. [DOI] [PubMed] [Google Scholar]
- 18.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 19.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HR, Jun HK, Choi BK. Tannerella forsythia BspA increases the risk factors for atherosclerosis in ApoE(-/-) mice. Oral Dis. 2014;20:803–808. doi: 10.1111/odi.12214. [DOI] [PubMed] [Google Scholar]
- 21.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 22.Califano JV, Gunsolley JC, Schenkein HA, Tew JG. A comparison of IgG antibody reactive with Bacteroides forsythus and Porphyromonas gingivalis in adult and early-onset periodontitis. J Periodontol. 1997;68:734–738. doi: 10.1902/jop.1997.68.8.734. [DOI] [PubMed] [Google Scholar]
- 23.Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, Pretzl B, et al. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol. 2009;80:634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- 24.Guglielmetti MR, Rosa EF, Lourencao DS, Inoue G, Gomes EF, De Micheli G, et al. Detection and quantification of periodontal pathogens in smokers and never-smokers with chronic periodontitis by real-time polymerase chain reaction. J Periodontol. 2014;85:1450–1457. doi: 10.1902/jop.2014.140048. [DOI] [PubMed] [Google Scholar]
- 25.Quinn SM, Zhang JB, Gunsolley JC, Schenkein HA, Tew JG. The influence of smoking and race on adult periodontitis and serum IgG2 levels. J Periodontol. 1998;69:171–177. doi: 10.1902/jop.1998.69.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Chung HY, Lu HC, Chen WL, Lu CT, Yang YH, Tsai CC. Immunoglobulin G profiles in different forms of periodontitis. J Periodontal Res. 2003;38:471–476. doi: 10.1034/j.1600-0765.2003.00675.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen HA, Johnson BD, Sims TJ, Darveau RP, Moncla BJ, Whitney CW, et al. Humoral immune responses to Porphyromonas gingivalis before and following therapy in rapidly progressive periodontitis patients. J Periodontol. 1991;62:781–791. doi: 10.1902/jop.1991.62.12.781. [DOI] [PubMed] [Google Scholar]
- 28.Whitney C, Ant J, Moncla B, Johnson B, Page RC, Engel D. Serum immunoglobulin G antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: titer, avidity, and subclass distribution. Infect Immun. 1992;60:2194–2200. doi: 10.1128/iai.60.6.2194-2200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


