Abstract
Background and objectives
Hypophosphatemia is a frequent complication during continuous renal replacement therapy (CRRT), a dialytic technique used to treat AKI in critically ill patients. This study sought to confirm that phosphate depletion during CRRT may decrease red blood cell (RBC) concentration of 2,3-diphosphoglycerate (2,3-DPG), a crucial allosteric effector of hemoglobin’s (Hgb’s) affinity for oxygen, thereby leading to impaired oxygen delivery to peripheral tissues.
Design, setting, participants, & measurements
Phosphate mass balance studies were performed in 20 patients with severe AKI through collection of CRRT effluent. RBC concentrations of 2,3-DPG, venous blood gas pH, and oxygen partial pressure required for 50% hemoglobin saturation (P50) were measured at CRRT initiation and days 2, 4, and 7. Similar measurements were obtained on days 0 and 2 in a reference group of 10 postsurgical patients, most of whom did not have AKI. Associations of 2,3-DPG with laboratory parameters and clinical outcomes were examined using mixed-effects and Cox regression models.
Results
Mean 2,3-DPG levels decreased from a mean (±SD) of 13.4±3.4 µmol/g Hgb to 11.0±3.1 µmol/g Hgb after 2 days of CRRT (P<0.001). Mean hemoglobin saturation P50 levels decreased from 29.7±4.4 mmHg to 26.7±4.0 mmHg (P<0.001). No significant change was seen in the reference group. 2,3-DPG levels after 2 days of CRRT were not significantly lower than those in the reference group on day 2. Among patients receiving CRRT, 2,3-DPG decreased by 0.53 µmol/g Hgb per 1 g phosphate removed (95% confidence interval 0.38 to 0.68 µmol/g Hgb; P<0.001). Greater reductions in 2,3-DPG were associated with higher risk for death (hazard ratio, 1.43; 95% confidence interval, 1.09 to 1.88; P=0.01).
Conclusions
CRRT-induced phosphate depletion is associated with measurable reductions in RBC 2,3-DPG concentration and a shift in the O2:Hgb affinity curve even in the absence of overt hypophosphatemia. 2,3-DPG reductions may be associated with higher risk for in-hospital death and represent a potentially avoidable complication of CRRT.
Keywords: acute renal failure, mortality, dialysis
Introduction
AKI is an increasingly common complication in critically ill patients and is associated with significant morbidity, mortality, and cost (1). The management of severe AKI requiring dialysis has evolved over the past several decades to include continuous RRT (CRRT), which was introduced to enable dialytic treatment of hemodynamically unstable patients for whom intermittent hemodialysis could be difficult to administer (2,3). CRRT may offer advantages over conventional intermittent hemodialysis, such as allowing the administration of large volumes of parenteral nutrition and other obligate fluids. It also enables slow, steady removal of uremic toxins and fluid, which may be beneficial in hemodynamically unstable patients. However, CRRT has potentially harmful unintended effects, such as extended duration of anticoagulation; patient immobilization; the unknown effects of prolonged dialyzer use; and unintended elimination of amino acids, small hormones, and electrolytes (4,5).
One such potential adverse effect of CRRT is hypophosphatemia, a complication reported in >10% of patients undergoing CRRT (6–9). We have shown that CRRT leads to net negative phosphate balance even with maintenance of normal serum phosphate levels (10). Normally, 80%–95% of filtered phosphate is reabsorbed by the proximal tubule, such that normal phosphate renal clearance is approximately 5–10 ml/min. In CRRT, small-solute clearance approximates the effluent rate, which typically exceeds 15 ml/min. Phosphate clearance is therefore higher with CRRT than by the normal kidney. This fact, along with the continuous nature of CRRT, accounts for excessive phosphate losses. Phosphate is the most abundant intracellular anion and is essential for multiple biologic functions, including nucleic acid synthesis, energy exchange, membrane transport, membrane composition, and intracellular signal transduction. The clinical manifestations of severe hypophosphatemia include decreased cardiac output, respiratory muscle weakness, and granulocyte dysfunction with impaired immune system function (11).
Phosphate also plays a key role in red blood cell (RBC) function and oxygen transport by influencing the intracellular concentration of 2,3-diphosphoglycerate (2,3-DPG), an allosteric effector of hemoglobin (Hgb). Reductions in 2,3-DPG concentration lead to increased affinity of hemoglobin for O2 and a leftward shift of the O2-hemoglobin dissociation curve as measured by oxygen partial pressure required for 50% hemoglobin saturation (P50), thereby limiting oxygen unloading in peripheral tissues (12). We conducted this study to test whether phosphate depletion during CRRT leads to measureable changes in RBC 2,3-DPG concentrations and therefore may limit peripheral tissue oxygen delivery in critically ill patients.
Materials and Methods
Study Population
We identified critically ill patients (age ≥18 years) with AKI requiring CRRT between November 2011 and December 2012. Exclusion criteria included pregnancy, institutionalized status, ESRD requiring maintenance dialysis, RBC enzyme defects, hyperbilirubinemia (a bilirubin level >40 mg/d interferes with standard colorimetric phosphate assay), treatment with oral phosphosoda, and diarrhea with stool loss >1 L daily. We excluded patients with more than five packed RBC transfusions over a 24-hour period (because of interference with 2,3-DPG assay). Individuals or their surrogates provided written informed consent, and the institutional review board approved the study. The attending nephrologist, independently of the investigators, determined the indications for CRRT administration. All patients received continuous veno-venous hemofiltration (CVVH) using the Nxstage System One machines with biocompatible polyethersulfone membranes, bicarbonate or citrate replacement solution delivered prefilter (rate, 1600–4000 ml/hr; blood flow rate, 200–250 ml/min). Intravenous phosphate was given for patients with a serum phosphate level ≤2.5 mg/dl according to the following algorithm: 155 mg, 310 mg, and 465 mg of sodium phosphate intravenously for levels of 2–2.5, 1.5–1.9, and <1.5 mg/dl, respectively. We recorded serum phosphate measurements (Roche/Hitachi Cobas C system), which were obtained at least daily as part of routine clinical care. A reference group of intensive care unit (ICU) patients (n=10) was enrolled after they provided informed consent. These patients had undergone cardiac or vascular/thoracic surgery, were not receiving CRRT, and had the same exclusion criteria. Serum phosphate was not routinely checked in this group.
Timed Effluent and Urine Collections
We devised a fractional effluent collection device to divert approximately 1% of effluent continuously to a sterile cooled collection bag (10). CRRT effluent was sampled from the collection bag every 24 hours for a maximum of 7 days. Samples were then divided into aliquots daily and stored at −80°C. Within 6 months of collection, samples were thawed, vortexed, centrifuged at 3200 revolutions per minute, and assayed for phosphate. The concentration of phosphate in the fractional effluent collection was multiplied by the total 24-hour effluent volume (recorded by the Nxstage CVVH machine) to obtain an estimate of phosphate removal in 24 hours.
Blood Collections
We collected venous blood in ice-cooled heparinized tubes for blood gas measurement using a Rapid Point 405 analyzer with an integral co-oximeter (Siemens Diagnostics). This instrument calculates P50 using measured oxygen saturation and PO2. To prepare samples for 2,3-DPG measurement, we collected venous blood in EDTA tubes, placed them immediately on ice, deproteinized them with 0.6 M perchloric acid to lyse erythrocytes, and neutralized them with 2.5 M potassium carbonate. We stored supernatants at −80°C and measured 2,3-DPG levels using the Roche diagnostic kit. Laboratory personnel were blinded to clinical details. The Roche 2,3-DPG assay is based on enzymatic cleavage of 2,3-DPG and oxidation of nicotinamide adenine dinucleotide recorded by spectrophotometry. 2,3-DPG assays were performed in three batches, and intra-/interassay coefficients of variation were <10%. Each batch contained multiple consecutive samples from the same participant. Control samples were evenly spread over the last two of three batches. 2,3-DPG levels were normalized to the corresponding Hgb concentration from the same sample.
From patients receiving CRRT, we collected blood samples on day 0 (before CRRT initiation [baseline]) and then days 2, 4, and 7. Not all patients survived to provide collections at all scheduled time points. In the reference cohort, blood samples were drawn postoperatively on days 0 and 2.
Statistical Analyses
We measured daily phosphate balance in study participants using the following equation:
 |
All study participants were anuric, and no urine phosphate was measured. We calculated for each participant the difference in 2,3-DPG concentrations and P50 from baseline to the last sample collected during CRRT. Statistical analyses were performed with SAS software, version 9.2 (SAS Institute Inc., Cary, NC) and MedCalc for Windows, version 12.1.4.0 (MedCalc Software, Mariakerke, Belgium). Data are reported as means±SDs or medians with ranges, as appropriate. To assess the univariate relationships of serum phosphate, phosphate balance, pH, and P50 with 2,3-DPG levels, we examined scatter plots and Pearson correlations. We used mixed-effects models to account for within-participant variation across days of measurement. We fit mixed effects models that assume a linear relationship between 2,3-DPG (or ∆2,3-DPG) and laboratory values:
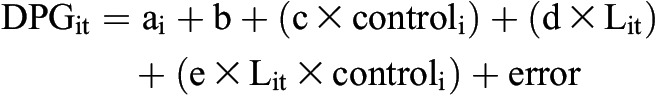 |
where i indexes participant, t is time, ai is the random effect for participant i, b is the population intercept, L is the laboratory value of interest, and controli is 1 if participant i is in the reference cohort and 0 if i is in the CRRT cohort. We first tested whether e=0 (i.e., association is the same for CRRT versus reference). If so, we refit the model and tested whether d=0 (i.e., no association between DPG at time t and variable L at time t).
To assess the association of 2,3-DPG and ∆2,3-DPG with mortality, we fit Cox regression models (adjusting for age and serum albumin) for the endpoint of time to death, with the starting point being each day of measurement and adjustment for within-participant correlation to account for repeated measures using the Wei–Lin–Weissfeld robust variance.
Results
Clinical Characteristics of CRRT Cohort
We collected blood and CRRT effluent samples from 20 patients with AKI (Table 1, Supplemental Table 1). Blood was collected at CRRT initiation and days 2, 4, and 7 during CRRT. Mean age was 58±13.4 years, and 25% of patients were women. Reasons for ICU admission were sepsis (45%), advanced heart failure (30%), cardiothoracic surgery (15%), and respiratory failure (10%). Tubular necrosis accounted for most causes of AKI (65%). CRRT was initiated a median of 3 days (range, 1–33 days) after ICU admission. Fifty percent of patients had CKD, defined as baseline eGFR<60 ml/min per 1.73 m2. All participants had blood collections until day 2 of CRRT, 9 participants had collections until day 4, and 3 participants had collections until day 7. The median number of 24-hour effluent collections per participant was 3 (range, 1–7). Mean prescribed CRRT dose was 27.7±6.2 ml/kg/hour. Daily phosphate removal through CRRT ranged from 0.9 to 2.9 g (median, 1.5 g). Median daily phosphate balance was −1.4 g (range, −0.5 to −2.6 g).
Table 1.
Demographic and clinical characteristics of study and reference cohorts
| Characteristic | CRRT Cohort (n=20) | Reference Cohort (n=10) |
|---|---|---|
| Age (yr) | 58±13.4 | 72±11.3 |
| Men (%) | 75 | 60 |
| White patients (%) | 90 | 100 |
| BMI (kg/m2) | 29.2±8.7 | 26±2.7 |
| Median duration of ICU stay (range) (d) | 9 (5–30) | 3 (1–10) |
| Mortality (%) | 50 | 0 |
| Enrollment creatinine (mg/dl) | 4.7±1.9 | 1.2±0.2 |
| Enrollment serum phosphate (mg/dl) | 5.7±1.2 | 3.1±0.4a |
| Enrollment serum calcium (mg/dl) | 7.9±0.7 | 8±0.5 |
| Enrollment P50 (mmHg) | 29.7±4.4 | 27.5±1.9 |
| Enrollment 2,3-DPG (µmol/gm Hgb) | 13.4±3.4 | 11.6±1.9 |
| Day 2 2,3-DPG (µmol/g Hgb) | 11±3.1 | 11.8±2 |
Values for continuous variables given as mean±SD or median (range), as appropriate. BMI, body mass index; ICU, intensive care unit; P50, oxygen partial pressure required for 50% hemoglobin saturation; 2,3-DPG, 2,3-diphosphoglycerate; Hgb, hemoglobin; CRRT, continous RRT.
Missing data (n=6).
Clinical Characteristics of Reference Cohort
We collected blood samples from 10 patients who underwent major cardiovascular surgery (Table 1, Supplemental Table 2). Nine of these patients did not have AKI. Blood was collected on day 0 (upon ICU arrival) and day 2 after surgery. Mean age was 72±11.3 years, and 40% of patients were women. Four patients underwent coronary artery bypass grafting, three patients underwent valve repair or replacement, one patient underwent combined coronary artery bypass grafting and valve repair, and two patients underwent thoracotomy for pulmonary nodule resection or trauma. Twenty percent had CKD, defined as baseline eGFR<60 ml/min per 1.73 m2.
2,3-DPG Levels on Days 0 and 2
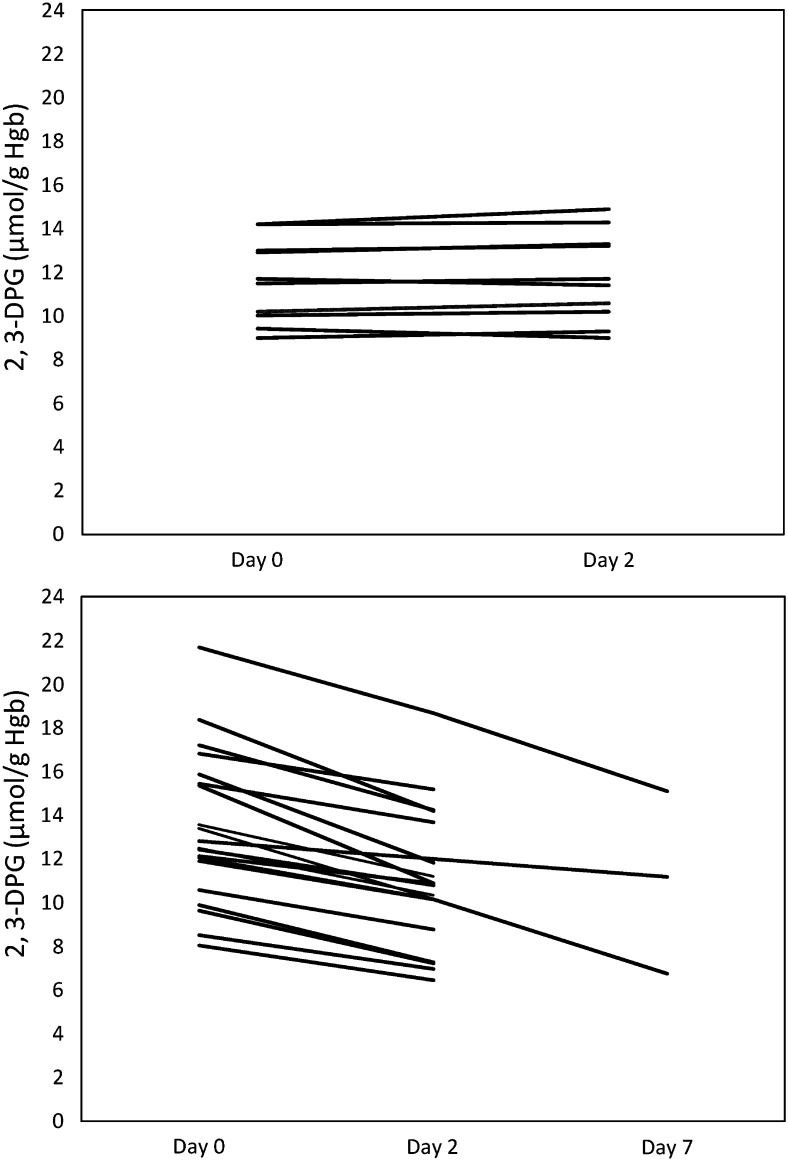
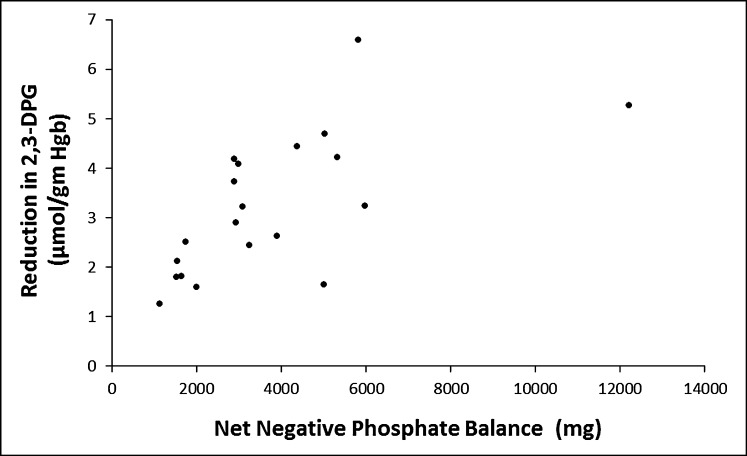
The baseline mean 2,3-DPG levels were 13.4±3.4 µmol/g Hgb in the CRRT cohort and 11.6±1.9 µmol/g Hgb in the reference group. After 2 days, 2,3-DPG levels decreased in the CRRT cohort to 11.0±3.1 µmol/g Hgb (P<0.001) but did not significantly change in the reference cohort (11.8±3.1 µmol/g Hgb) (P=0.63) (Figure 1) . P50 levels decreased in the CRRT cohort (from 29.7±4.4 mmHg to 26.7±4.0 mmHg; P<0.001) but did not significantly change in the reference cohort (P=0.31). We fit mixed-effects models, with random effects for participants, to investigate the associations between relevant laboratory values and 2,3-DPG levels in the two cohorts across measurement days. We found a positive association between serum phosphate and 2,3-DPG but no association with pH (P=0.81). 2,3-DPG increased by 0.96 µmol/g Hgb per 1-mg/dl increase in serum phosphate (95% confidence interval [95% CI], 0.76 to 1.16; P<0.001). We found an inverse association between P50 reductions and pH changes (ΔP50 decreased by 2.5 mmHg per 0.1-unit increase in ΔpH; 95% CI, 0.5 to 4.6; P=0.03). The association between P50 and 2,3-DPG was significant in the CRRT cohort but not in the reference cohort. 2,3-DPG increased by 0.56 µmol/g Hgb per 1-mmHg increase in P50 in the CRRT cohort (95% CI, 0.48 to 0.57; P<0.001). Among patients receiving CRRT, 2,3-DPG decreased by 0.53 µmol/g Hgb per 1-g phosphate removed (95% CI, 0.38 to 0.68; P<0.001) (Figure 2). Mean 2,3-DPG levels declined by 1.8, 3.6, and 6.6 µmol/g Hgb, respectively, in three patients after 7 days of CRRT. Only one of these three patients had overt hypophosphatemia (serum phosphate level, 1.9 mg/dl).
Figure 1.
Change in 2,3-diphosphoglycerate (2,3-DPG) over time. Top panel shows group not receiving continuous RRT (CRRT ); bottom panel shows CRRT group. Hgb, hemoglobin.
Figure 2.
Relationship between net phosphate balance and 2,3-diphosphoglycerate (2,3-DPG) reduction in patients receiving continuous RRT. Hgb, hemoglobin.
2,3-DPG Levels and Mortality in CRRT
We tested the associations between 2,3-DPG and in-hospital mortality using Cox regression models adjusting for age and serum albumin, and accounting for within-participant correlation, which was induced through consideration of several time origins for the analysis. Although there is no censoring, this modeling approach is robust because it does not require parametric specification of the baseline hazard function. We found no association between absolute levels of 2,3-DPG and mortality (hazard ratio, 0.99; P=0.36) but did find an association between greater reductions in 2,3-DPG and increased risk for death (hazard ratio, 1.43; 95% CI, 1.09 to 1.88; P=0.01).
Discussion
In this study of 20 patients receiving CRRT, we show that net negative phosphate balance—even in the absence of overt hypophosphatemia—was associated with a measurable reduction in 2,3-DPG. This reduction may lead to inadequate tissue oxygen delivery in critically ill patients receiving CRRT. We also demonstrate that the reduction in 2,3-DPG is associated with higher risk for in-hospital death. Our findings suggest that CRRT-induced phosphate removal may be an iatrogenic complication in the management of patients with severe AKI.
Hypophosphatemia has been found to associate with lower RBC levels of 2,3-DPG in previous studies (13,14). Extracellular phosphate and RBC intracellular phosphate concentrations are linked through at least three phosphate transporters in RBC membranes: band 3, Na/phos co-transporter, and sodium-independent transport (15). Activation of phosphate influx by these transport mechanisms depends on extracellular phosphate (15). During CRRT, phosphate is removed from the extracellular compartment and also from other compartments, as the entire extracellular mass of phosphate can be removed in 24 or 48 hours of CRRT using typical prescriptions (10). In this study, we found indirect evidence that at least some of the phosphate is removed from intracellular stores, as measured by changes in 2,3-DPG. In the absence of significant changes in pH and other blood gas parameters, the decrease in 2,3-DPG levels probably reflect reduced erythrocyte phosphate stores. Another potential cause of RBC 2,3-DPG depletion during extracorporeal blood purification treatments is erythrocyte trauma secondary to the mechanical stress during the procedure itself, as found during the first hour of hemodialysis by Nielsen et al. (16).
CRRT-induced phosphate depletion may also lower intracellular ATP concentrations and adversely affect bioenergetics in multiple organ systems. We did not measure ATP levels in RBC or other tissues because doing so was not feasible. Phosphate removal from bone during extended duration of CRRT may also lead to demineralization and warrants further investigation in AKI survivors treated with prolonged CRRT.
Baseline 2,3-DPG levels in the reference cohort were lower than those in the CRRT cohort, probably because of hyperphosphatemia in 75% of patients at CRRT initiation. However, after 2 days the mean 2,3-DPG level in the CRRT group was lower than that in the reference group, but the difference was not statistically significant. Our documentation of no change in 2,3-DPG levels in the reference cohort suggests that the reduction in the CRRT cohort may be a direct effect of the CRRT procedure. Indeed, we found a correlation between the magnitude of phosphate depletion and the change in 2,3-DPG. We also demonstrated a significant reduction in P50 during CRRT (P=0.02), with left shifting of the O2-Hgb curve possibly resulting in tissue hypoxia. This alteration is similar to the P50 shift that has been recorded in expedition members climbing Mt. Everest (17). The magnitude of the change in 2,3-DPG levels in our study may be physiologically significant: Farber et al. pharmacologically increased 2,3-DPG concentrations in healthy volunteers by infusing fructose-phosphate solutions. With a <2 µmol/g Hgb increase in RBC concentration of 2,3-DPG and ATP, they observed an increase in P50 of 2.2 mmHg along with an improvement in myocardial energy utilization, as assessed by cardiac output during exercise (18). The reductions in 2,3-DPG that we observed after 2 days of CRRT are also similar in magnitude to the differences in 2,3-DPG between 111 critically ill patients and 34 healthy volunteers studied by Ibrahim et al. (19). While the changes in P50 may be most easily explained by the changes in 2,3-DPG, a reduction in intracellular phosphate also increases erythrocyte chloride concentration, which by itself blunts the allosteric effects of both 2,3-DPG and pH (20).
Among patients who were studied while receiving CRRT for 7 days, we found a mean 29% decrease in 2,3-DPG level from baseline and a median net negative phosphate balance of −5.8 g (range, −5.0 to −12.2 g). Our results underscore the potential harmful effects of prolonged CRRT. Although we found an association between CRRT duration and changes in 2,3-DPG and P50, we cannot be certain that CRRT-induced 2,3-DPG reduction led to inadequate peripheral tissue oxygen delivery. We did not measure peripheral tissue oxygen content or other markers of oxygen delivery such as lactate. Nevertheless, our finding of an association between the magnitude of change in 2,3-DPG and in hospital mortality provides preliminary evidence that phosphate balance may be clinically important in CRRT.
Previous studies in intensive care patients have shown an association between incident hypophosphatemia and mortality (21,22). Demirjian et al. reported an association between serum phosphate decline during CVVHD and incidence of prolonged respiratory failure requiring tracheostomy (23). Shiffl et al. observed a 57% in-hospital mortality in hypophosphatemic patients not receiving intravenous phosphate supplementation compared with 46% of those receiving supplementation (8). While prior studies have shown an association between overt hypophosphatemia and adverse clinical outcomes, our findings demonstrate a plausible physiologic signal from iatrogenic, subclinical phosphate depletion. Many other factors, including nutritional status and preexisting CKD, may also affect intracellular phosphate levels.
The clinical implications of our study deserve to be highlighted, particularly in the context of recent shortages of intravenous phosphate solutions in the United States (24). Phosphate levels should be carefully monitored and maintained in the normal range by implementing phosphate repletion protocols that administer intravenous phosphate well before overt hypophosphatemia occurs. Supplementation of dialysate and replacement solutions with phosphate should also be considered (25–27). Alternative CRRT strategies, such as accelerated veno-venous hemofiltration (28), which may lead to less phosphate removal, should be tested and compared with CRRT.
Our study has several important limitations. The study was relatively small and prone to both type 1 and type 2 statistical errors. We studied only CVVH and not hemodiafiltration or continuous hemodialysis. Our findings may not be generalizable to other institutions with different CRRT prescription practices or phosphate repletion protocols. We did not have 24-hour urine collection in the reference group for measurement of phosphate mass balance. Our observation of a mortality signal associated with the normalization of 2,3-DPG at day 2 in the CRRT cohort suggests that the increased 2,3-DPG at baseline may be compensatory and physiologically relevant in these patients. We must remain cautious, however, not to overinterpret the study results given the small sample size; furthermore, we found an association between change in 2,3-DPG levels with mortality, but not between absolute levels of 2,3-DPG and mortality. Null results on the association between initial 2,3-DPG levels and mortality were also observed in the study of critically ill patients by Ibrahim et al. (19). Confirmation of our findings in a larger cohort, or in a trial designed to test phosphate replacement strategies, is warranted. Future studies may directly measure tissue perfusion and oxygenation using gastric tonometry (29,30) to determine whether the reduction of 2,3-DPG with CRRT from elevated to normal levels is detrimental for tissue oxygen delivery. This may be especially relevant for longer duration of CRRT, which in our study was associated with substantial additional decrease in 2,3-DPG.
CRRT-induced phosphate removal is associated with measureable reductions in 2,3-DPG, and reductions in 2,3-DPG may be associated with a higher risk for in-hospital death. Optimizing the delivery and safety of CRRT should be a priority in nephrology and critical care research.
Disclosures
S.S.W. reported serving as a consultant to CVS Caremark, BioTrends Research Group, Harvard Clinical Research Institute, Abbvie, and Takeda; providing expert testimony for GE Healthcare, Salix, and litigation related to mercury exposure; and receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases, Otsuka, Merck, Genzyme,Pfizer, and Satellite Healthcare.
Supplementary Material
Acknowledgments
We wish to thank Dr. Mark Kellogg for assistance with laboratory assays.
This study was funded in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32-DK007527. This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1-TR000170-05) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health. This study was supported in part by an investigator-initiated grant from NxStage Medical, Inc. Funding sources had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
Part of this work was presented at the American Society of Nephrology’s Kidney Week, November 1, 2012, San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02160214/-/DCSupplemental.
See related editorial, “Staying on Target with Continuous Dialysis,” on pages 7–8.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Davenport A, Will EJ, Davidson AM: Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med 21: 328–338, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Kramer P, Wigger W, Rieger J, Matthaei D, Scheler F: [Arteriovenous haemofiltration: a new and simple method for treatment of over-hydrated patients resistant to diuretics]. Klin Wochenschr 55: 1121–1122, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Himmelfarb J: Continuous renal replacement therapy in the treatment of acute renal failure: critical assessment is required. Clin J Am Soc Nephrol 2: 385–389, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hynote ED, McCamish MA, Depner TA, Davis PA: Amino acid losses during hemodialysis: Effects of high-solute flux and parenteral nutrition in acute renal failure. JPEN J Parenter Enteral Nutr 19: 15–21, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Ratanarat R, Brendolan A, Volker G, Bonello M, Salvatori G, Andrikos E, Yavuz A, Crepaldi C, Ronco C: Phosphate kinetics during different dialysis modalities. Blood Purif 23: 83–90, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Santiago MJ, López-Herce J, Urbano J, Bellón JM, del Castillo J, Carrillo A: Hypophosphatemia and phosphate supplementation during continuous renal replacement therapy in children. Kidney Int 75: 312–316, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Schiffl H, Lang SM: Severe acute hypophosphatemia during renal replacement therapy adversely affects outcome of critically ill patients with acute kidney injury. Int Urol Nephrol 45: 191–197, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Troyanov S, Cardinal J, Geadah D, Parent D, Courteau S, Caron S, Leblanc M: Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant 18: 961–966, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Waikar SS: Phosphate balance in continuous venovenous hemofiltration. Am J Kidney Dis 61: 1043–1045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knochel JP: The pathophysiology and clinical characteristics of severe hypophosphatemia. Arch Intern Med 137: 203–220, 1977 [PubMed] [Google Scholar]
- 12.Chanutin A, Hermann E: The interaction of organic and inorganic phosphates with hemoglobin. Arch Biochem Biophys 131: 180–184, 1969 [DOI] [PubMed] [Google Scholar]
- 13.Lichtman MA, Miller DR, Cohen J, Waterhouse C: Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med 74: 562–568, 1971 [DOI] [PubMed] [Google Scholar]
- 14.Travis SF, Sugerman HJ, Ruberg RL, Dudrick SJ, Delivoria-Papadopoulos M, Miller LD, Oski FA: Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N Engl J Med 285: 763–768, 1971 [DOI] [PubMed] [Google Scholar]
- 15.Shoemaker DG, Bender CA, Gunn RB: Sodium-phosphate cotransport in human red blood cells. Kinetics and role in membrane metabolism. J Gen Physiol 92: 449–474, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen AL, Andersen EM, Jørgensen LG, Jensen HA: Oxygen and 2,3 biphosphoglycerate (2,3-BPG) during haemodialysis. Scand J Clin Lab Invest 58: 459–467, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Winslow RM, Samaja M, West JB: Red cell function at extreme altitude on Mount Everest. J Appl Physiol 56: 109–116, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Farber MO, Sullivan TY, Fineberg N, Carlone S, Manfredi F: Effect of decreased O2 affinity of hemoglobin on work performance during exercise in healthy humans. J Lab Clin Med 104: 166–175, 1984 [PubMed] [Google Scholar]
- 19.Ibrahim ED, McLellan SA, Walsh TS: Red blood cell 2,3-diphosphoglycerate concentration and in vivo P50 during early critical illness. Crit Care Med 33: 2247–2252, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Duhm J, Gerlach E: On the mechanisms of the hypoxia-induced increase of 2,3-diphosphoglycerate in erythrocytes. Studies on rat erythrocytes in vivo and on human erythrocytes in vitro. Pflugers Arch 326: 254–269, 1971 [DOI] [PubMed] [Google Scholar]
- 21.Shor R, Halabe A, Rishver S, Tilis Y, Matas Z, Fux A, Boaz M, Weinstein J: Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci 36: 67–72, 2006 [PubMed] [Google Scholar]
- 22.Zazzo JF, Troché G, Ruel P, Maintenant J: High incidence of hypophosphatemia in surgical intensive care patients: Efficacy of phosphorus therapy on myocardial function. Intensive Care Med 21: 826–831, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Demirjian S, Teo BW, Guzman JA, Heyka RJ, Paganini EP, Fissell WH, Schold JD, Schreiber MJ: Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol Dial Transplant 26: 3508–3514, 2011 [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration. FDA drug shortages. Drugs 2013 9-26-2013. Available at: http://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?Al=Sodium Phosphate Injection&st=c. Accessed October 9, 2014
- 25.Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G: Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand 55: 39–45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morabito S, Pistolesi V, Tritapepe L, Zeppilli L, Polistena F, Fiaccadori E, Pierucci A: Regional citrate anticoagulation in CVVH: A new protocol combining citrate solution with a phosphate-containing replacement fluid. Hemodial Int 17: 313–320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiremath S, Slivar S, Magner P: Phosphate balance with continuous renal replacement therapy: A simple solution. Am J Kidney Dis 62: 644, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Gashti CN, Salcedo S, Robinson V, Rodby RA: Accelerated venovenous hemofiltration: Early technical and clinical experience. Am J Kidney Dis 51: 804–810, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Heard SO: Gastric tonometry: The hemodynamic monitor of choice (Pro). Chest 123[Suppl]: 469S–474S, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kipnis E, Ramsingh D, Bhargava M, Dincer E, Cannesson M, Broccard A, Vallet B, Bendjelid K, Thibault R: Monitoring in the intensive care. Crit Care Res Pract 2012: 473507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.