Abstract
Background and objectives
Stroke is common in patients undergoing long-term dialysis, but the implications for mortality after stroke in these patients are not fully understood.
Design, setting, participants, & measurements
A large cohort of dually-eligible (Medicare and Medicaid) patients initiating dialysis from 2000 to 2005 and surviving the first 90 days was constructed. Medicare claims were used to ascertain ischemic and hemorrhagic strokes occurring after 90-day survival. A semi-Markov model with additive hazard extension was generated to estimate the association between stroke and mortality, to calculate years of life lost after a stroke, and to determine whether race was associated with differential survival after stroke.
Results
The cohort consisted of 69,371 individuals representing >112,000 person-years of follow-up. Mean age±SD was 60.8±15.5 years. There were 21.1 (99% confidence interval [99% CI], 20.0 to 22.3) ischemic strokes and 4.7 (99% CI, 4.2 to 5.3) hemorrhagic strokes after cohort entry per 1000 patient-years. At 30 days, mortality was 17.9% for ischemic stroke and 53.4% for hemorrhagic stroke. The adjusted hazard ratio (AHR) depended on time since entry into the cohort; for patients who experienced a stroke at 1 year after cohort entry, for example, the AHR of hemorrhagic stroke for mortality was 25.4 (99% CI, 22.4 to 28.4) at 1 week, 9.9 (99% CI, 8.4 to 11.6) at 3 months, 5.9 (99% CI, 5.0 to 7.0) at 6 months, and 1.8 (99% CI, 1.5 to 2.1) at 24 months. The corresponding AHRs for ischemic stroke were 11.7 (99% CI, 10.2 to 13.1) at 1 week, 6.6 (99% CI, 6.4 to 6.7) at 3 months, and 4.7 (99% CI, 4.5 to 4.9) at 6 months, remaining significantly >1.0 even at 48 months. Median months of life lost were 40.7 for hemorrhagic stroke and 34.6 for ischemic stroke. For both stroke types, mortality did not differ by race.
Conclusions
Dialysis recipients have high mortality after a stroke with corresponding decrements in remaining years of life. Poststroke mortality does not differ by race.
Keywords: cardiovascular disease, dialysis, end-stage renal disease, epidemiology and, outcomes
Introduction
Stroke, a catastrophic event and a leading cause of disability in the general population, is particularly common in patients undergoing long-term dialysis (1–7). Stroke confers a major burden on the affected individual (8) as well as on society as a whole (9). However, how stroke is associated with mortality in dialysis recipients has not been well quantified.
We constructed a large cohort of incident dialysis patients using data from the US Renal Data System, Medicare, and Medicaid to investigate the magnitude of the association between stroke (both ischemic and hemorrhagic) and mortality, how mortality risk varies over time in the poststroke setting, how stroke might contribute to years of life lost, and whether poststroke survival varies by race. Examination of these issues might permit quantification of the collective burden of stroke in the long-term dialysis population at an epidemiologic level and provide prognostic value for patients who have sustained a stroke and for their families and providers.
Materials and Methods
Study Design and Data Sources for Analysis
We performed a retrospective cohort analysis of incident long-term dialysis recipients who were eligible for Medicare and Medicaid (dually eligible). The strategy used for linking Medicaid, through which drug prescribing information can be used to help determine the presence of permanent atrial fibrillation (AF), with Medicare has been previously described (10,11) and is provided in Supplemental Appendix 1. For purposes of sensitivity analyses, we performed additional analyses in a cohort of individuals with Medicare (i.e., we did not require them to be dually eligible); these are described in Supplemental Appendix 2.
Study Cohort and Rationale for Analytic Approach
The cohort consisted of individuals >18 years of age who initiated long-term dialysis on or after January 1, 2000, through October 2, 2005; were continuously enrolled in both Medicare and Medicaid; and had a minimum of 90 days of follow-up before December 31, 2005. Patients enrolled in managed care plans or in the Department of Veterans Affairs health system were excluded. To assure that we were studying long-term dialysis patients and to help establish the presence of comorbidities, patients were required to survive 90 days; follow-up for new strokes therefore began on day 91. Individuals were censored upon loss of Medicare or Medicaid coverage, receipt of a kidney transplant, or death on or before December 31, 2005. Because we also wanted to examine, as a covariate, the effect of permanent, nonvalvular AF, we also eliminated individuals who may have had transient, postoperative, or valvular AF (11–13).
Covariates and Descriptive Variables
Demographic and clinical variables were drawn from the Centers for Medicare & Medicaid Services (CMS) 2728 Medical Evidence Form, supplemented with a modified form (14) of the Liu Comorbidity Index (15), as described in Supplemental Appendix 1. Because permanent, non-valvular AF is a unique risk factor for stroke, we treated AF as a time-dependent covariate (11); details of the approach are described in Supplemental Appendix 1.
Stroke Identification
We used recent information on the sensitivity and specificity of stroke-related International Classification of Diseases, Ninth Revision, claims (16) to identify ischemic and hemorrhagic strokes from Medicare data, as has been done previously (17) (described in Supplemental Appendix 1). In all cases, we counted only the first stroke after the initial 90-day survival period.
Statistical Analyses
We generated descriptive statistics to illustrate how individuals who experienced ischemic strokes at some point during follow-up differed from those who did not. Bivariate analyses comparing each of the explanatory variables by ever-stroke versus no-stroke were performed using Pearson chi-squared test or t test, as appropriate.
We modeled survival after a first ischemic stroke and, separately, a first hemorrhagic stroke. In both models, we began observation after cohort entry (i.e., after surviving the initial 90 days on dialysis) and used death from any cause as the outcome while controlling for demographic, comorbidity, anthropometric, and dialysis-modality factors. To quantify residual longevity between patients who experienced a stroke compared with those who did not, it was required to model changes in hazard of death upon the occurrence of each type of stroke. We used a semi-Markov model (18,19) with additive hazard extension (20) to incorporate the major changes in the survival functions due to the occurrence of a stroke by use of additive hazard extensions while still using the fully parametric model of survival from cohort entry until death. This enabled us to determine hazard ratios for all-cause mortality following first ischemic or, separately, first hemorrhagic stroke after cohort entry, and to quantify residual longevity between those who experienced a stroke compared with those who did not. Our approach is detailed extensively in the Supplemental Appendix.
For all analyses, P<0.01 was considered to represent statistically significant differences given the large size of the data set and the desire to minimize the risk of reporting false positive associations. The 99% confidence intervals (99% CIs) were obtained using Wald formulas. Calculation of 99% CIs for the stroke incidence rates was done by means of a Poisson rate confidence interval using the chi-squared approximation for rare events. All statistical analyses used SAS software, version 9.2 (SAS Institute, Inc., www.sas.com).
Compliance and Protection of Human Research Participants
The institutional review board at the University of Kansas Medical Center (KUMC). Data use agreements between KUMC and the US Renal Data Service and CMS were in place.
Results
Cohort Characteristics
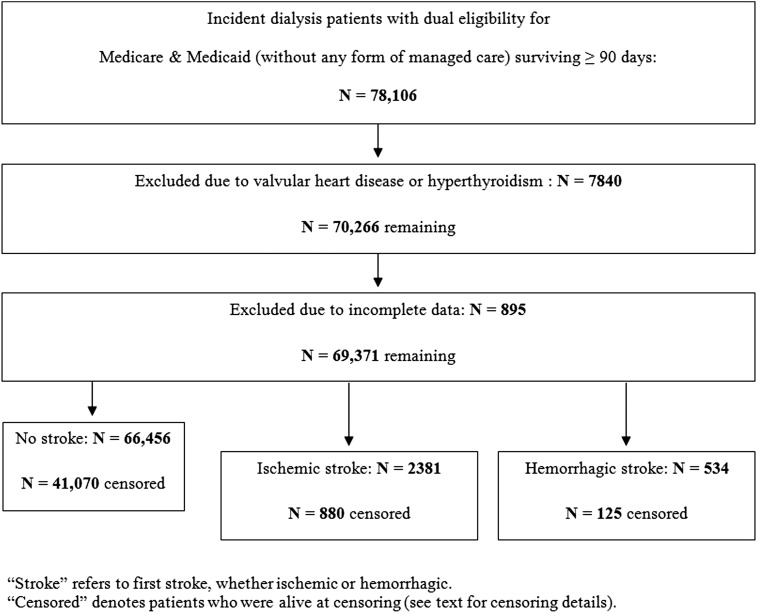
Figure 1 shows the construction of the dually-eligible cohort. There were a total of 78,106 individuals who initiated dialysis and who survived at least 90 days before our final date of December 31, 2005. After exclusions, 69,371 individuals remained.
Figure 1.
Flowchart demonstrates the creation of the study cohort.
Persons were divided into those who experienced no stroke after cohort entry, those whose first stroke after cohort entry was ischemic, and those whose first stroke after cohort entry was hemorrhagic (Table 1). In general, individuals who experienced an ischemic stroke were significantly older than those who did not, but this was not the case for hemorrhagic stroke. Men were relatively less likely than women to experience an ischemic stroke; there was no difference for hemorrhagic stroke. African Americans were over-represented in both types of stroke.
Table 1.
Descriptive characteristics of the dually-eligible cohort, by stroke status
| Characteristic | No Stroke | Hemorrhagic Stroke | Pa | Ischemic Stroke | Pb |
|---|---|---|---|---|---|
| Patients | 66,456 (95.8) | 534 (0.8) | 2381 (3.4) | ||
| Age (yr) | 60.6±15.6 | 59.2±14.6 | 0.032 | 65.9±12.7 | <0.001 |
| Men | 29,848 (44.9) | 239 (44.8) | 0.94 | 734 (30.8) | <0.001 |
| Race/ethnicity | <0.001 | <0.001 | |||
| African American | 27,339 (41.1) | 240 (44.9) | 1071 (45.0) | ||
| White | 23,243 (35.0) | 125 (23.4) | 777 (32.6) | ||
| Hispanic | 12,123 (18.2) | 127 (23.8) | 430 (18.1) | ||
| Other | 3751 (5.6) | 42 (7.9) | 103 (4.3) | ||
| BMI category | <0.001 | 0.75 | |||
| <20 kg/m2 | 6857 (10.3) | 86 (16.1) | 232 (9.7) | ||
| 20–24.9 kg/m2 | 19,682 (29.6) | 182 (34.1) | 697 (29.3) | ||
| 25–29.9 kg/m2 | 17,719 (26.7) | 140 (26.2) | 676 (28.4) | ||
| ≥30 kg/m2 | 22,198 (33.4) | 126 (23.6) | 776 (32.6) | ||
| Smoker | 4310 (6.5) | 45 (8.4) | 0.070 | 125 (5.3) | 0.016 |
| Substance abuser | 2025 (3.1) | 33 (6.2) | <0.001 | 40 (1.7) | 0.001 |
| Unemployed | 64,654 (97.3) | 523 (97.9) | 0.36 | 2357 (99.0) | <0.001 |
| Unable to ambulate | 3808 (5.7) | 14 (2.6) | 0.002 | 170 (7.1) | 0.004 |
| Unable to transfer | 1419 (2.1) | 3 (0.6) | 0.012 | 64 (2.7) | 0.068 |
| In-center HDc | 63,189 (95.1) | 516 (96.6) | 0.10 | 2266 (95.2) | 0.85 |
| Hemoglobin < 11.0 g/dl | 45,815 (75.1) | 397 (81.4) | 0.001 | 1653 (76.6) | 0.12 |
| Comorbidities | |||||
| HTN | 56,289 (84.7) | 468 (86.9) | 0.16 | 2083 (87.5) | 0.002 |
| DM | 38,529 (58.0) | 321 (60.1) | 0.32 | 1686 (70.8) | <0.001 |
| Congestive heart failure | 21,685 (32.6) | 171 (32.0) | 0.77 | 927 (38.9) | <0.001 |
| Coronary artery disease | 15,956 (24.0) | 97 (18.2) | 0.002 | 678 (28.5) | <0.001 |
| Peripheral vascular disease | 9611 (14.5) | 58 (10.9) | 0.018 | 397 (16.7) | 0.003 |
| Prior cerebrovascular accident | 7173 (10.8) | 73 (13.7) | 0.033 | 426 (17.9) | <0.001 |
| Permanent atrial fibrillation | 6707 (10.1) | 50 (9.4) | 0.58 | 401 (16.8) | <0.001 |
| Comorbidity scored | 5.0±2.8 | 4.9±2.6 | 0.45 | 5.7±2.7 | <0.001 |
| Cause of ESRD | 0.12 | <0.001 | |||
| DM | 35,039 (52.7) | 301 (56.4) | 1539 (64.7) | ||
| HTN | 16,880 (25.4) | 139 (26.0) | 561 (23.6) | ||
| GN | 6004 (9.0) | 38 (7.1) | 92 (3.9) | ||
| Other | 8525 (12.8) | 56 (10.5) | 188 (7.9) |
Characteristics are reported as n (%), except for continuous variables, which are shown as mean±1 SD. BMI, body mass index; HD, hemodialysis; HTN, hypertension; DM, diabetes mellitus.
Compares individuals with hemorrhagic stroke to those with no stroke.
Compares individuals with ischemic stroke to those with no stroke.
In-center hemodialysis is contrasted to self-care dialysis, which consists of home hemodialysis plus peritoneal dialysis.
Comorbidity score is derived from an adapted form of the Liu Comorbidity Index.
Stroke Events
The cohort had a total of 112,801 person-years of observation over a median follow-up time of 1.24 years (25th percentile, 0.52 years; 75th percentile, 2.44 years). A total of 2381 individuals (3.4%) had an ischemic stroke during follow-up, for 21.1 ischemic strokes per 1000 patient-years (99% CIs, 20.0 to 22.3); 62.9% of individuals with an ischemic stroke died, compared with 38.2% of those without any stroke (P<0.001). A total of 534 individuals (0.8%) had a hemorrhagic stroke during follow-up, for 4.7 (99% CIs, 4.2 to 5.3) hemorrhagic strokes per 1000 patient-years. Of those with a hemorrhagic stroke, 76.6% died (P<0.001 compared with no stroke).
Survival Following a Stroke
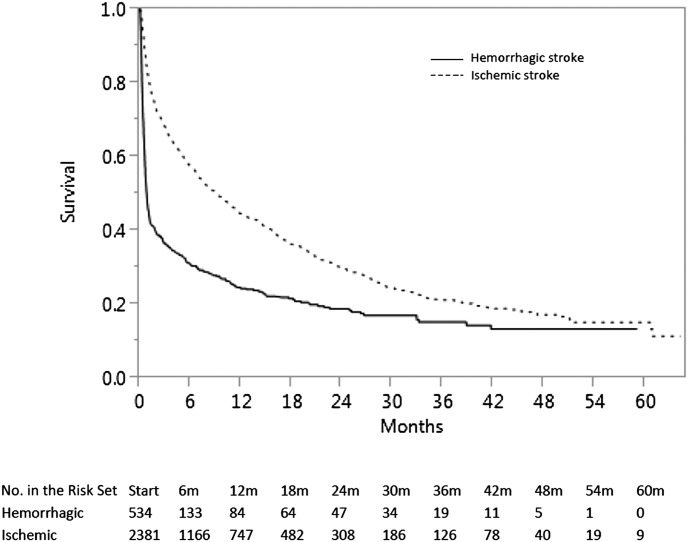
Figure 2 demonstrates unadjusted Kaplan–Meier survival curves. “Time 0” is therefore the time of the stroke. At 30 days, mortality was 17.9% for individuals with ischemic stroke and 53.4% for individuals with hemorrhagic strokes. Among individuals who had a hemorrhagic stroke, for example, those who survive approximately 18 months after cohort entry had an attenuated decline in mortality thereafter, although their survival was still substantially worse than for an ischemic stroke.
Figure 2.
Unadjusted Kaplan-Meier curve illustrating survival after a first ischemic or hemorrhagic stroke after surviving 90 days of dialysis.
After multivariable adjustment, including for the time-dependent effect of the strokes, factors associated with mortality are shown as adjusted hazard ratios (AHRs) in Table 2; a stroke at 12 months after cohort entry was selected for illustrative purposes. Because the AHR for stroke is not constant over time since the stroke occurred, the AHRs for both stroke types are reported for representative time points.
Table 2.
Adjusted hazard ratios for death for patient-level factors, when stroke occurs at 12 months after cohort entry
| Variable | AHR (99% CI) | P |
|---|---|---|
| Hemorrhagic stroke | <0.001 | |
| 7 d after stroke | 25.4 (22.4 to 28.4) | |
| 1 mo | 18.1 (15.5 to 20.9) | |
| 3 mo | 9.9 (8.4 to 11.6) | |
| 6 mo | 5.9 (5.0 to 7.0) | |
| 12 mo | 3.3 (2.8 to 3.9) | |
| 24 mo | 1.8 (1.5 to 2.1) | |
| 36 mo | 1.3 (1.1 to 1.5) | |
| Ischemic stroke | <0.001 | |
| 7 d after stroke | 11.7 (10.2 to 13.1) | |
| 1 mo | 9.8 (9.5 to 10.0) | |
| 3 mo | 6.6 (6.4 to 6.7) | |
| 6 mo | 4.7 (4.5 to 4.9) | |
| 12 mo | 3.3 (3.1 to 3.4) | |
| 24 mo | 2.2 (2.0 to 2.3) | |
| 36 mo | 1.7 (1.6 to 1.8) | |
| Age per year | 1.03 (1.03 to 1.03) | <0.001 |
| Male sex | 1.04 (1.01 to 1.08) | <0.001 |
| Race/ethnicity | ||
| White | 1.0 (referent) | – |
| African American | 0.78 (0.76 to 0.81) | <0.001 |
| Hispanic | 0.68 (0.65 to 0.71) | <0.001 |
| Other | 0.59 (0.54 to 0.63) | <0.001 |
| BMI category | ||
| <20 kg/m2 | 1.30 (1.24 to 1.37) | <0.001 |
| 20–24.9 kg/m2 | 1.0 (referent) | – |
| 25–29.9 kg/m2 | 0.85 (0.81 to 0.88) | <0.001 |
| ≥30 kg/m2 | 0.77 (0.74 to 0.80) | <0.001 |
| Smoker | 1.09 (1.02 to 1.16) | 0.001 |
| Substance abuser | 1.23 (1.11 to 1.35) | <0.001 |
| Unemployed | 1.62 (1.37 to 1.92) | <0.001 |
| Inability to ambulate | 1.43 (1.33 to 1.53) | <0.001 |
| Inability to transfer | 1.24 (1.12 to 1.38) | <0.001 |
| In-center HDa | 0.95 (0.88 to 1.03) | 0.11 |
| Comorbidities | ||
| HTN | 0.76 (0.73 to 0.80) | <0.001 |
| DM | 1.04 (0.99 to 1.09) | 0.021 |
| Congestive heart failure | 1.14 (1.08 to 1.20) | <0.001 |
| Coronary artery disease | 1.04 (1.00 to 1.08) | 0.009 |
| Peripheral vascular disease | 1.10 (1.05 to 1.15) | <0.001 |
| Prior cerebrovascular accident | 1.17 (1.11 to 1.22) | <0.001 |
| Permanent AFb | 1.26 (1.16 to 1.36) | <0.001 |
| Permanent AF×timec | 1.01 (1.00 to 1.01) | <0.001 |
| Comorbidity scored | ||
| 0–3 | 1.0 (referent) | – |
| 4–6 | 1.16 (1.10 to 1.22) | <0.001 |
| ≥7 | 1.22 (1.12 to 1.32) | <0.001 |
As described in Materials and Methods, the cohort consisted of incident dialysis patients who survived at least 90 days after dialysis initiation. Thus, stroke 12 months after cohort entry represents individuals who had survived 15 months on dialysis. Adjusted hazard ratios for both stroke types at subsequent time points (e.g., 24 and 36 months) were generally quite similar; see text of Results for details. BMI, body mass index; HD, hemodialysis; HTN, hypertension; DM, diabetes mellitus; AF, atrial fibrillation; AHR, adjusted hazard ratio; 99% CI, 99% confidence interval.
In-center hemodialysis is contrasted to self-care dialysis, which consists of home hemodialysis plus peritoneal dialysis.
Because atrial fibrillation is treated as a time-dependent variable, this represents the association when atrial fibrillation occurs 12 months after cohort entry. Values were similar when other timepoints were selected.
Represents the interaction of permanent atrial fibrillation with time.
Comorbidity score is derived from an adapted form of the Liu Comorbidity Index.
In general, the AHRs were extremely high immediately after the event and remained persistently >1.0 for several years. Compared with a dialysis patient who did not experience a stroke at an identical time after cohort entry, the AHR for a hemorrhagic stroke was approximately 25 at 1 week after stroke, 18 at 1 month, 10 at 3 months, and 3 at 12 months; values at other time points are shown in Table 2. The AHR for an ischemic stroke was nearly 12 at 1 week after stroke, nearly 10 at 1 month, over 6 at 3 months, and over 3 at 12 months.
Permanent AF, treated as a time-dependent covariate with an interaction with time, conferred an AHR of 1.26 (P<0.001) when it was diagnosed at 1 year after being in the cohort; AHRs were similar when other time points were selected (data not shown) but increased slightly with time as the interaction term yielded an AHR estimate of 1.01.
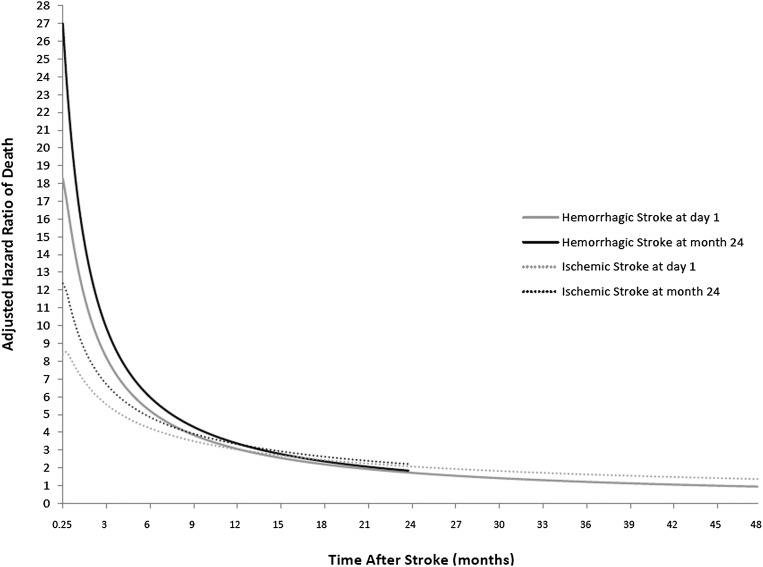
Figure 3 demonstrates the AHR for mortality after a stroke during follow-up. Two illustrative time points were selected: stroke immediately upon cohort entry (i.e., after having met the 90-day survival requirement) and stroke after having been in the cohort for 2 years. Here the AHRs for mortality after stroke are calculated compared with the mortality of the dialysis patients as a whole. (Of note, the AHR curves for each type of stroke differ depending on time in the cohort at which the stroke occurred because the baseline hazard for the no-stroke group changes over time in the cohort, as indicated by the Weibull distribution identified for this component of our survival model.) Figure 3 also shows that the AHR for mortality after stroke was generally consistent across dialysis vintage. For example, if a hemorrhagic stroke occurred 24 months after cohort entry, the AHRs were similar to those associated with stroke occurring at 12 months after entry: 19.1 at 1 month, 10.4 at 3 months, 6.2 at 6 months, and 3.4 at 12 months. If an ischemic stroke occurred at 24 months after entry, the AHRs were 10.3, 6.9, 5.0, and 3.4, respectively; the similarities in these values can be compared with those in Table 2 (which models stroke occurring at 12 months after cohort entry).
Figure 3.
Adjusted hazard ratios for death following a first ischemic or hemorrhagic stroke after cohort entry.
Years of Life Lost Following Stroke
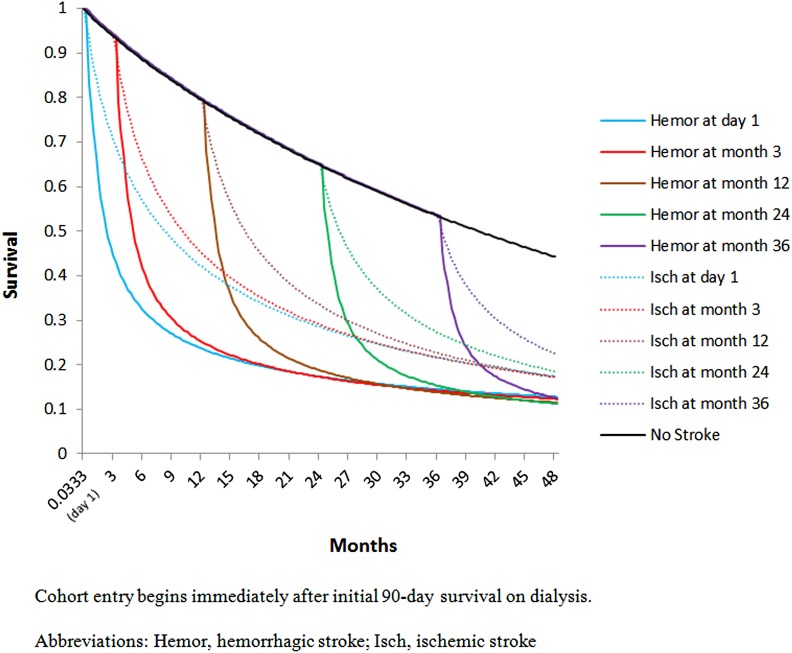
Figure 4 demonstrates adjusted survival after ischemic and hemorrhagic strokes during follow-up. The top curve represents survival among individuals who remained stroke-free. Differences in survival following strokes at day 1 of cohort entry and at months 3, 12, 24, and 36 after cohort entry are shown as departures from this curve at the associated time points. Calculated median years of life lost was therefore 40.7 months (3.4 years) for a hemorrhagic stroke in an incident, dually-eligible dialysis patient; for an ischemic stroke, the number is 34.6 months (2.9 years).
Figure 4.
Modeled survival of individuals with no stroke, with ischemic (Isch) stroke, and with hemorrhagic (Hemor) stroke, when stroke occurs at various times after cohort entry.
Association of Race/Ethnicity with Poststroke Mortality
We next examined whether race/ethnicity modified the association between stroke and mortality (Table 3). The hazard ratio for mortality did not differ across groups.
Table 3.
Relative hazard ratios of hemorrhagic and ischemic stroke for mortality, by race/ethnicity
| Race/Ethnicity | HR (95% CI) | |
|---|---|---|
| Hemorrhagic Stroke | Ischemic Stroke | |
| White | 1.0 (referent) | 1.0 (referent) |
| African American | 0.97 (0.83 to 1.13) | 1.14 (0.83 to 1.57) |
| Hispanic | 1.04 (0.85 to 1.26) | 1.24 (0.86 to 1.80) |
| Other | 0.81 (0.55 to 1.19) | 1.02 (0.59 to 1.76) |
HR, hazard ratio; 95% CI, 95% confidence interval.
Sensitivity Analyses
Finally, to assess the robustness of our analysis, we performed multiple sensitivity analyses, electing to model a stroke that occurs upon cohort entry. We first eliminated the adapted (14) Liu Comorbidity Index (15). This approach yielded nearly identical AHRs for ischemic and hemorrhagic and strokes, with AHRs for ischemic strokes of 8.4 (versus 8.4 with the adapted Liu Index included) at 1 week, 4.3 (versus 4.3) at 6 months, and 2.1 (versus 2.1) at 24 months, and for hemorrhagic stroke of 18.4 (versus 18.3) at 1 week, 5.4 (versus 5.4) at 6 months, and 1.8 (versus 1.7) at 24 months. Using a more sensitive definition of ischemic stroke again resulted in nearly identical AHRs (8.1, 4.2, and 2.0, respectively), although use of the sensitive hemorrhagic stroke definition (which likely captures some events that were not hemorrhagic strokes) now became somewhat greater at 23.0, 6.7, and 2.1, respectively.
To examine the effect that a previous stroke might have on our findings, we eliminated individuals with a previous stroke (either during the predialysis phase, as indicated by history of a cerebrovascular accident on the CMS 2728 form, or during the first 90 days on dialysis). As shown in Table 4, AHRs for ischemic stroke increased slightly to 9.3 at 1 week, 4.6 at 6 months, and 2.2 at 24 months; analogous values for hemorrhagic stroke also showed modest increases to 19.7, 5.5, and 1.8, respectively. We then performed a subset analysis on only the hemodialysis patients (again, eliminating those with previous stroke to facilitate comparisons); AHRs increased somewhat to 11.1, 5.4, and 2.6 for ischemic stroke and to 24.8, 6.8, and 2.2 for hemorrhagic stroke.
Table 4.
Adjusted hazard ratios for ischemic and hemorrhagic strokes in the dually-eligible and Medicare cohorts after elimination of individuals with a previous stroke and those receiving peritoneal dialysis
| Type of Stroke by Time after Stroke | AHRs in Original Cohort | AHRs in Group with No Prior Strokea | AHRs in HD-Only Patientsb | |||
|---|---|---|---|---|---|---|
| Dually Eligible | Medicare-Eligible | Dually Eligible | Medicare-Eligible | Dually Eligible | Medicare-Eligible | |
| Ischemic strokec | ||||||
| 1 wk | 8.4 | 7.8 | 9.3 | 11.6 | 11.1 | 11.3 |
| 6 mo | 4.3 | 4.2 | 4.6 | 5.3 | 5.4 | 5.2 |
| 24 mo | 2.1 | 2.0 | 2.2 | 2.5 | 2.6 | 2.5 |
| Hemorrhagic strokec | ||||||
| 1 wk | 18.3 | 21.5 | 19.7 | 25.5 | 23.8 | 24.5 |
| 6 mo | 5.4 | 6.3 | 5.5 | 7.4 | 6.8 | 7.4 |
| 24 mo | 1.7 | 2.0 | 1.8 | 2.3 | 2.2 | 2.4 |
"Dually eligible" refers to individuals with dual eligibility for Medicare and Medicaid. "Medicare eligible" refers to patients with Medicare. aRestricts analysis to individuals without an indication of a previous stroke, either on the Centers for Medicare & Medicaid Services 2728 dialysis intake form or in Medicare claims during the first 90 days of dialysis.
Restricts analysis to individuals receiving hemodialysis and without an indication of a previous stroke (as defined above).
Scenario modeled is a stroke occurring immediately upon cohort entry.
Generalizability
To determine whether our findings are generalizable to the larger Medicare-only population, we examined these individuals (n=265,685) in a series of analyses. This cohort had a total of 444,637 person-years of observation over a median follow-up time of 1.30 years (25th percentile, 0.51 years; 75th percentile, 2.53 years). Their characteristics are shown in Supplemental Table 1. There were 20.1 (99% CIs, 19.6–20.7) new ischemic strokes and 4.1 (99% CIs, 3.8–4.3) new hemorrhagic strokes. The 30-day mortality was similar to that in dually eligible individuals: 20.2% for ischemic stroke (versus 17.9% for the dually eligible patients) and 53.1% (versus 53.4%). These results are also shown in Table 4 and demonstrate that AHRs for both stroke types are broadly similar to those for the dually-eligible population, whether as originally modeled, when previous strokes are eliminated, or when only hemodialysis patients (without previous strokes) are analyzed.
Discussion
How major disease processes and events might alter the “life trajectory” of those affected is a question of major public health interest (9,21). In the general population, how stroke affects years of life lost has been studied (9,22,23). However, how stroke is associated with mortality in dialysis patients has not been well investigated. We found that among individuals who survived the first 90 days of dialysis, a first new stroke during follow-up was associated with large AHRs for mortality immediately after the event but that this decreased substantially over time in the modest fraction of individuals who survived. Overall, these estimates appeared to be robust to multiple sensitivity analyses, and generalizability to the larger Medicare population appeared to be high. A hemorrhagic stroke in 90-day survivors was associated with a foreshortening of median residual life by nearly 41 months and an ischemic stroke with a shortening of more than 34 months.
As would be expected given the catastrophic nature of a hemorrhagic stroke, the AHR in the immediate aftermath of the event was extremely high. The overall mortality pattern of ischemic stroke was generally similar in pattern to that of hemorrhagic stroke and, although attenuated in magnitude, was nevertheless was associated with only 50% survival at approximately 9 months.
Previous investigators have examined stroke epidemiology questions (5,7,24,25). Our overall stroke incidence rate of 25.8 new events per 1000 patient-years is in the middle of the range reported in other studies of dialysis patients, with estimates by Power et al. (14.9) (7) and Iseki et al. (24) constituting the lower end and Sozio et al. (49.0) (5) the higher. We found 82% of the total stroke events to be ischemic, similar to the 75% (7) and 76% (5) reported in other racially diverse cohorts. Lastly, unadjusted survival rates following strokes were somewhat worse than those of other studies. For example, while our 85% 1-month survival after ischemic stroke was similar to the 75%–93% reported by others (7,24,25), our 31% survival rate at 2 years was lower than the 42% (24) and 56% (7) reported by others. Similarly, our 1-month hemorrhagic stroke survival of 45% was lower than that of other studies (which ranged from 48% to 68%) (7,24,25). However, the studies used different stroke definitions, so these findings are not directly comparable.
Compared with the general population, in turn, a recent review by Feigin et al. (26) showed that in high-income countries, 30-day case fatality rates were about 13%–23% for ischemic strokes and about 25%–35% for hemorrhagic strokes. This suggests that, compared with the general population, 30-day ischemic stroke mortality in dialysis patients is similar, while hemorrhagic stroke was substantially greater. The chronically anticoagulated state of hemodialysis patients in particular might partially explain the worse outcome in hemorrhagic stroke.
The relationship between stroke and mortality did not appear to vary substantially by race. We were initially uncertain as to whether there would be an interaction between race and stroke on mortality. In the general population, minorities have not only higher event rates for most major cardiovascular events (27) but also higher unadjusted mortality rates following these events (28). In distinction, the overall survival of African Americans and Hispanics undergoing dialysis is higher than that of whites, at least in older individuals (29), which may suggest that the former groups have a relatively greater ability to survive cardiovascular events.
The manner in which stroke might affect mortality remains uncertain. Stroke is probably part of an epiphenomenon involving inflammation, nutrition, and frailty. Individuals with a substantial inflammatory burden or a high degree of frailty are at risk for both stroke and death. Alternatively, it is also plausible that a disabling stroke might adversely affect functional status and nutrition, leading to frailty and premature death. In our study, causality cannot be determined.
Our findings should be interpreted in the context of important limitations. First, our outcomes were based on claims, so specific stroke characteristics such as size or severity of the stroke are unknown. Indeed, subclinical strokes, which appear to be common (30), are not easily detected, particularly with use of claims-based data; thus, studies such as ours likely underestimate stroke burden and its implications. Second, our primary analysis examined individuals who were dually eligible for Medicare and Medicaid. However, the sensitivity analysis for the AHR for stroke conducted in a far larger cohort of individuals (n>265,000) with Medicare (irrespective of Medicaid) revealed broadly similar findings. Third, chronic AF, treated as a time-dependent covariate, was afforded special scrutiny. Because AF can be transient or paroxysmal and therefore difficult to identify from claims data, we are likely underestimating the actual effect of AF on stroke. Fourth, we do not consider the implications that use of warfarin or antiplatelet agents might have on strokes, an issue that remains of interest to the renal community. Fifth, receipt of a renal allograft constitutes a competing risk, which we did not explicitly model using our novel analytic approach. However, because generally healthier patients receive kidney transplants, inclusion of such individuals in the risk pool would have almost certainly have increased the computed years of life lost. As such, our findings probably underestimate the true years of life lost for stroke patients. Additionally, we have not investigated poststroke disability. Stroke exacts a substantial burden among those who survive it, so a study of stroke mortality alone cannot render a full accounting of the true burden of stroke in dialysis patients. Finally, we have no information on fatal strokes which occur outside of the hospital.
Perhaps most fundamentally, the focus of our study was limited to stroke. Other major life-changing events, such as myocardial infarction or hip fracture, doubtless have a substantial effect on years of life lost. In the absence of such information, the relative importance of stroke cannot be fully appreciated. Nonetheless, confidence in our findings is strengthened by the large sample size, the richness of the data used, and our use of multiple sensitivity analyses that generally supported the primary findings.
In conclusion, strokes in patients receiving dialysis are associated with a substantially increased risk for death in these patients and are associated with substantial years of life lost. Long-term stroke survivors have slightly lower hazard ratios than do individuals who recently had strokes after having spent several years on dialysis. Perhaps unexpectedly, race is not differentially associated with survival for ischemic or hemorrhagic strokes. The implications of stroke, such as poststroke functional status and stroke-related disability, should be further investigated.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for this study was provided by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) grants K23-DK085378 (to J.B.W.) and R01-DK080111 (to T.I.S.)
The data reported here have been supplied by the US Renal Data System (DUA#2007-10 & 2009-19) and CMS (DUA#19707). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02900314/-/DCSupplemental.
References
- 1.United States Renal Data System: USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Seliger SL, Gillen DL, Longstreth WT, Jr, Kestenbaum B, Stehman-Breen CO: Elevated risk of stroke among patients with end-stage renal disease. Kidney Int 64: 603–609, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO: Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol 14: 2623–2631, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Wiesholzer M, Harm F, Tomasec G, Barbieri G, Putz D, Balcke P: Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol 21: 35–39, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, Powe NR, Parekh RS: Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: The choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 54: 468–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Perales C, Vázquez E, García-Cortés MJ, Borrego J, Polaina M, Gutiérrez CP, Lozano C, Liébana A: Ischaemic stroke in incident dialysis patients. Nephrol Dial Transplant 25: 3343–3348, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Power A, Chan K, Singh SK, Taube D, Duncan N: Appraising stroke risk in maintenance hemodialysis patients: A large single-center cohort study. Am J Kidney Dis 59: 249–257, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Magalhaes R, Abreu P, Correia M, Whiteley W, Silva MC, Sandercock P: Functional status three months after the first ischemic stroke is associated with long-term outcome: data from a community-based cohort. Cerebrovasc Dis 38: 46–54, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM: Lifetime risks of cardiovascular disease. N Engl J Med 366: 321–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, Spertus JA, Shireman TI: Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis 58: 73–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI: The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 81: 469–476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE: Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med 131: 927–934, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE: Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370–2375, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Rigler SK, Wetmore JB, Mahnken JD, Dong L, Ellerbeck EF, Shireman TI: Impact of a modified data capture period on Liu comorbidity index scores in Medicare enrollees initiating chronic dialysis. BMC Nephrol 14: 51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH: A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 100–128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis MA, Rigler SK, Spertus JA, Zhou X, Mukhopadhyay P, Shireman TI: Stroke and the “stroke belt” in dialysis: Contribution of patient characteristics to ischemic stroke rate and its geographic variation. J Am Soc Nephrol 24: 2053–2061, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putter H, Fiocco M, Geskus RB: Tutorial in biostatistics: Competing risks and multi-state models. Stat Med 26: 2389–2430, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Dabrowska DM, Sun G, Horowitz MM: Cox regression in a Markov renewal model: An application to the analysis of bone marrow transplant data. J Am Stat Assoc 89: 867–877, 1994 [Google Scholar]
- 20.Xie X, Strickler HD, Xue X: Additive hazard regression models: An application to the natural history of human papillomavirus. Comput Math Methods Med 2013: 796270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgenstern LB, Smith WS. Setting priorities for stroke care and research. Int J Stroke 8: 445–446, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology: Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 45: 315–353, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group: Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 383: 245–254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iseki K, Fukiyama K, Okawa Dialysis Study (OKIDS) Group. The Okinawa Dialysis Study (OKIDS) Group : Clinical demographics and long-term prognosis after stroke in patients on chronic haemodialysis. Nephrol Dial Transplant 15: 1808–1813, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Toyoda K, Fujii K, Fujimi S, Kumai Y, Tsuchimochi H, Ibayashi S, Iida M: Stroke in patients on maintenance hemodialysis: A 22-year single-center study. Am J Kidney Dis 45: 1058–1066, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V: Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. The Lancet Neurology 8: 355–369, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G, Investigators R, REGARDS Investigators : Association of race and sex with risk of incident acute coronary heart disease events. JAMA 308: 1768–1774, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM: Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med 150: 314–324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, Shaffi K, Weiner DE, Sarnak MJ: Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis 61: 271–278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.