Abstract
Background and objectives
Despite advances in therapy, HIV-infected individuals remain at higher risk for kidney dysfunction than uninfected individuals. It was hypothesized that urine levels of α1-microglobulin, a biomarker of proximal tubular dysfunction, would predict kidney function decline and mortality risk in HIV-infected and uninfected women.
Design, setting, participants, & measurements
In the Women’s Interagency HIV Study, urine α1-microglobulin and creatinine concentrations were measured in 903 HIV-infected and 287 uninfected women using stored urine from 1999 to 2000, when prevalence of tenofovir use was <1%. Participants were categorized into three categories by level of α1-microglobulin–to-creatinine ratio, and associations with kidney decline and all-cause mortality over 8 years were evaluated.
Results
Urine α1-microglobulin was detectable in 60% of HIV-infected and 40% of uninfected women (P<0.001). Among HIV-infected women, there were 177 (22%), 61 (7%), and 128 (14%) patients with incident CKD, with 10% annual eGFR decline, and who died, respectively. Compared with HIV-infected women in the lowest α1-microglobulin category, HIV-infected women in the highest α1-microglobulin category had a 2.1-fold risk of incident CKD (95% confidence interval, 1.3 to 3.4), 2.7-fold risk of 10% annual eGFR decline (95% confidence interval, 1.2 to 5.9), and 1.6-fold mortality risk (95% confidence interval, 1.0 to 2.6) in models adjusting for kidney risk factors, baseline eGFR, and albuminuria. Among uninfected women, the highest α1-microglobulin category was associated with 3% (relative risk, 2.2; 95% confidence interval, 1.4 to 3.5) and 5% (relative risk, 2.2; 95% confidence interval, 1.1 to 4.3) annual eGFR decline relative to the lowest α1-microglobulin category.
Conclusions
Proximal tubular dysfunction, indicated by urine α1-microglobulin, was independently associated with kidney function decline in HIV-infected and uninfected women and mortality risk among HIV-infected women.
Keywords: HIV nephropathy, CKD, proximal tubule
Introduction
Kidney disease is an established complication of HIV infection. HIV-infected persons are at higher risk for proteinuria, CKD, and ESRD compared with uninfected individuals, even after controlling for traditional kidney disease risk factors (1–6). However, standard clinical measures of kidney disease, such as serum creatinine and dipstick proteinuria, are inadequate in their ability to detect early kidney damage (7). There is a strong clinical need for biomarkers that can identify kidney dysfunction at earlier stages and localize pathology within the nephron to provide prognostic and therapeutic guidance in HIV-infected individuals. Although serum cystatin C may be more sensitive than serum creatinine for the estimation of GFR in HIV-infected individuals (8,9), we hypothesize that tubular injury and dysfunction may occur earlier than clinically apparent reductions in glomerular filtration function.
We previously showed that two tubular injury markers, IL-18 and kidney injury molecule-1 (KIM-1), are present in higher levels in the urine of HIV-infected women compared with uninfected controls and that higher urine IL-18 and KIM-1 predict longitudinal eGFR decline and mortality, independent of traditional kidney disease risk factors and albuminuria (10–12). In contrast to IL-18 and KIM-1, which are believed to be produced by proximal tubular epithelial cells in the setting of injury (13,14), α1-microglobulin (α1m) is a 26-kD lipocalin that is filtered at the glomerulus but fully reabsorbed by proximal tubular epithelial cells, where it is degraded (15). The presence of α1m in the urine is, therefore, indicative of proximal tubular dysfunction (16). A recent proteomic profiling study identified α1m as one of the earliest biomarkers detectable in urine after ischemic injury (17), suggesting that urine α1m may capture proximal tubular dysfunction at the earliest stages.
In this study of HIV-infected and uninfected women enrolled in the Women’s Interagency HIV Study (WIHS), we tested the hypotheses that HIV infection is associated with a higher prevalence of detectable urine α1m, that urine α1m predicts kidney function decline in HIV-infected and uninfected women, and that urine α1m predicts mortality risk among HIV-infected women, independent of albuminuria and three previously studied tubular injury markers: IL-18, KIM-1, and neutrophil gelatinase-associated lipocalin (NGAL).
Materials and Methods
Study Population
The WIHS is a multicenter, prospective cohort study that enrolled 3067 HIV-infected and 1070 uninfected women from six United States locations: Bronx, Brooklyn, Chicago, Los Angeles, San Francisco, and Washington DC in 1994–1995, 2001–2002, and 2011–2012. Details of study design, data collection methods, and baseline characteristics are published elsewhere (18,19). Briefly, HIV-infected participants were recruited to be representative of the population of HIV-infected women in the community. Recruitment of uninfected participants targeted women who engaged in high-risk behaviors (defined by self-reported injection drug use or high-risk sexual behavior within the year before enrollment). Participants underwent semiannual visits that included an interviewer-administered questionnaire, a physical examination, and collection of laboratory specimens.
The WIHS Kidney Aging Study was designed as a nested cohort study to investigate the onset of kidney disease in the setting of HIV infection using stored urine and serum specimens. Baseline urine and serum samples were collected between October of 1999 and March of 2000. Serum was also collected at two visits occurring at approximately 4 and 8 years after the baseline of this ancillary study. For this study, we included all 903 HIV-infected and 287 uninfected women who had stored urine available and at least one follow-up serum cystatin C measurement. The institutional review boards of the participating institutions approved the study protocol at all WIHS study sites, and informed consent was obtained from all study participants. This study of kidney injury was also approved by the University of California, San Francisco, the San Francisco Veterans Affairs Medical Center, and the Yale Committees on Human Research.
Exposure Variables
Urine α1m was measured at the Cincinnati Children's Hospital Medical Center Biomarker Laboratory by a commercially available assay (Siemens BNII nephelometer; Siemens, Munich, Germany). The detectable limit of the α1m assay was 0.6 mg/dl. Intra- and interassay coefficients of variation (CVs) were 5.2% and 13.2%, respectively. Urine creatinine was measured by colorimetric enzyme assay using a Siemens Dimension Xp and plus HM clinical analyzer (Siemens). All urine specimens were in continuous storage at −80°C until biomarker measurement without prior freeze-thaw; mean urine storage time was 11 years. Laboratory personnel performing the biomarker assays were blinded to clinical information regarding WIHS participants, including their HIV status, and the samples were evaluated in random order. The associations of urine α1m with kidney function decline were additionally compared with prior analyses of urine albumin-to-creatinine ratio (ACR), IL-18, KIM-1, and NGAL (10).
Outcomes
The primary outcomes of this study were longitudinal kidney function decline and all-cause mortality. Kidney function was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation for serum cystatin C (eGFRCys) (20,21). We chose a priori to estimate GFR by cystatin C rather than creatinine, because it is less susceptible to bias by muscle mass and health status (8). Cystatin C was measured centrally at the University of California at Los Angeles Clinical Immunology Research Laboratory using a particle-enhanced immunoturbidimetric assay (Gentian, Moss, Norway), which has been calibrated against the new World Standard Reference material ERM-DA471/IFCC (20). The assay was run concurrently from all three clinic visits, minimizing concerns for drift in the assay over time. Intra-assay CVs, on the basis of 10 replicates, were <2% at serum concentrations of 0.7 and 1.1 mg/L. Interassay CVs were 4.4% and 3.9% at serum concentrations of 0.8 and 2.2 mg/L. As in our prior work, eGFR was capped at 120 ml/min per 1.73 m2, because the equation has not been validated at higher values (22).
We analyzed eGFRCys as a continuous outcome expressed as change in eGFRCys in milliliters per minute per 1.73 m2 per year over approximately 8 years of follow-up. Among HIV-infected women, two dichotomized kidney outcomes were analyzed: (1) incident CKD (defined as eGFRCys<60 ml/min per 1.73 m2 at either of two follow-up visits among HIV-infected women with baseline eGFRCys≥60 ml/min per 1.73 m2) and (2) rapid decline (defined as 10% or greater annual decline in eGFRCys). Rapid decline was calculated as the relative change in eGFRCys from baseline to each follow-up visit for each participant. As a sensitivity analysis, we required an eGFRCys decline of ≥1 ml/min per 1.73 m2 per year of follow-up for all patients with incident CKD and found that this approach eliminated only one of 177 patients. Because of the small numbers of patients with incident CKD (n=16) and rapid decline (n=8) among uninfected women, we evaluated 3% and 5% annual eGFRCys declines as dichotomous outcomes in these participants.
Vital status and date of death were determined using the National Death Index and data from medical records and providers. Detailed methods have been described in prior studies (23–25). Deaths were ascertained over 8 years of follow-up in parallel with the kidney decline analyses. As a sensitivity analysis, we updated vital status through April of 2013, the most recent possible update at the time of manuscript submission. Because there were only 13 deaths among the uninfected participants, we studied the associations of α1m with mortality risk only in HIV-infected women.
Covariates
The following characteristics were tested as candidate covariates in all multivariate models: age and race/ethnicity, systolic and diastolic BP, antihypertensive use, diabetes (defined using confirmatory criteria for fasting glucose ≥126 mg/dl, self-reported diabetes, self-reported diabetes medication use, or hemoglobin A1c≥6.5%), cigarette smoking status (current, former, or never), menopause status, LDL and HDL cholesterol, triglycerides, serum albumin, body mass index, waist circumference, hepatitis C virus (HCV) infection (confirmed by detectable HCV RNA after a positive HCV antibody result), and current heroin use. Candidate HIV-related characteristics included current CD4 lymphocyte count, nadir CD4 lymphocyte count, history of AIDS diagnosis, current HIV viral load, current highly active antiretroviral therapy use, current nucleoside reverse transcription inhibitor use, current non-nucleoside reverse transcription inhibitor use, and current protease inhibitor use. During the study period, the prevalence of tenofovir use increased from <1% at baseline to 49% at the end of the 8-year follow-up period. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates with five imputations to yield approximately 95% relative efficiency (26). The percentage of missing observations for each covariate ranged from <1% to 15%.
Statistical Analyses
Forty-five percent of participants had undetectable urine α1m, and the distribution among those with detectable α1m was right-skewed. Because of the left-censored nature of the data, we analyzed α1m using three approaches: a dichotomized variable (detectable or undetectable), a log-transformed continuous variable using models that accommodate left-censored data, and an ordinal variable with three categories of urine α1m–to-creatinine ratio (α1m/cr). For analyses of HIV-infected and uninfected women modeling α1m as an ordinal variable, category 1 of α1m/cr included all participants with undetectable urine α1m. The remaining participants were divided at the median value of urine α1m/cr into categories 2 and 3 for HIV-infected and uninfected participants separately.
We compared baseline characteristics of HIV-infected women across the three categories of urine α1m/cr using chi-squared and Kruskal–Wallis tests for categorical and continuous variables, respectively; these comparisons were replicated among the uninfected participants. We then used Poisson regression with a robust variance estimator (27) to assess the cross-sectional association of HIV infection with detectable urine α1m and identify factors associated with detectable urine α1m among HIV-infected and uninfected women. As an alternate approach, we used multivariable generalized γ-regression models to identify factors associated with higher urine α1m concentration in HIV-infected and uninfected women separately. Similar to the Tobit regression method, generalized γ-regression models accommodate left-censored data but also allow log transformation of urine α1m to normalize its right-skewed distribution. Results were back-transformed to produce estimated percentage differences in urine α1m attributable to each factor.
Next, we used linear mixed models to evaluate the associations of urine α1m/cr categories with the continuous outcome of eGFRCys with random intercepts and slopes across time to account for the correlation between repeated measures at baseline, year 4, and year 8. Multivariate models sequentially adjusted for demographics, traditional risk factors for kidney disease progression, baseline eGFRCys, ACR, and the previously studied biomarkers urine IL-18/cr, KIM-1/cr, and NGAL/cr. As a sensitivity analysis, we additionally adjusted for tenofovir use during follow-up. To compare the effect size of α1m/cr with those of the previously studied biomarkers, we modified our previously published WIHS analyses (10) assessing the associations of ACR, IL-18/cr, KIM-1/cr, and NGAL/cr with eGFRCys by incorporating α1m/cr into the final multivariate-adjusted models for each biomarker.
We then used Poisson regression with a robust variance estimator to assess the associations of urine α1m/cr with incident CKD and 10% annual eGFRCys decline among HIV-infected individuals and with 3% and 5% annual eGFRCys declines among uninfected individuals. We calculated risk ratios for each outcome using category 1 as the reference category. Women with eGFRCys<60 ml/min per 1.73 m2 at baseline were excluded from the incident CKD analysis. To evaluate for possible effect modification by tenofovir use during follow-up, analyses of HIV-infected participants were stratified by tenofovir use during follow-up (ever used versus never used), and we evaluated an interaction term (α1m×tenofovir) for statistical significance in the overall model.
To account for losses to follow-up in all kidney function decline analyses, we adjusted estimates using an inverse probability weighting approach by modeling the participant’s probability of having a nonmissing outcome, with separate weights calculated at each visit (28). The inverse of this probability was then used as a weight applied to persons with known outcomes in the multivariable regression analyses of kidney decline.
Finally, we used unadjusted generalized additive models to produce a spline plot depicting the probability of mortality over the detectable range of α1m/cr in HIV-infected participants. We obtained P values for the association of α1m/cr with mortality and the test of nonlinearity. We also evaluated the associations of urine α1m/cr categories with all-cause mortality using multivariable Cox proportional hazards and the same series of adjusted models as for the kidney function decline outcomes.
Spearman coefficients were used to evaluate correlations between α1m, IL-18, KIM-1, NGAL, ACR, and urine creatinine. Because of the moderately strong intercorrelations between all biomarkers and urine creatinine (Supplemental Table 1), primary analyses standardized the biomarkers to urine creatinine. In sensitivity analyses, we evaluated the associations of unstandardized biomarker levels with kidney decline and mortality using the methods described above.
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics of WIHS Participants
The median age was 41 years among the 903 HIV-infected women and 40 years among the 287 uninfected women included in this study. At baseline, 9% of HIV-infected and 2% of uninfected women had an eGFRCys<60 ml/min per 1.73 m2 (P<0.001); 13.8% of the participants were lost to follow-up after the second visit; these individuals provided a mean follow-up time of 3.7 years.
HIV-infected women in the highest category of α1m/cr were older, were more often African American, and had higher prevalence of smoking, diabetes mellitus, hypertension, HCV infection, and albuminuria as well as lower CD4 counts and higher HIV RNA levels (Table 1). Among uninfected participants, the highest α1m/cr category was characterized by older age and higher prevalence of smoking, diabetes mellitus, hypertension, HCV infection, heroin use, and albuminuria (Table 2).
Table 1.
Baseline characteristics of HIV-infected women in the Women’s Interagency HIV Study stratified by urine α1-microglobulin category
| Characteristic | 1 (n=358) | 2 (n=272) | 3 (n=273) | P Value |
|---|---|---|---|---|
| Range of α1m/cr (mg/g) | Below detectable | 1.2–10.2 | >10.2 | |
| Baseline age (yr) | 40 (35–44) | 40 (36–45) | 43 (38–48) | <0.001 |
| Race | ||||
| African American | 180 (50%) | 151 (56%) | 188 (69%) | <0.001 |
| Caucasian | 92 (26%) | 46 (17%) | 37 (14%) | |
| Other | 86 (24%) | 75 (28%) | 48 (18%) | |
| Cigarette smoking | ||||
| Current | 149 (42%) | 144 (53%) | 167 (61%) | <0.001 |
| Past | 100 (28%) | 72 (26%) | 52 (19%) | |
| Never | 109 (30%) | 56 (21%) | 54 (20%) | |
| Diabetes mellitus | 25 (7%) | 23 (8%) | 38 (14%) | 0.01 |
| Hypertension | 72 (20%) | 68 (25%) | 85 (31%) | <0.01 |
| Antihypertensive use | 28 (8%) | 28 (10%) | 41 (15%) | 0.02 |
| Menopause | 55 (16%) | 45 (17%) | 82 (31%) | <0.001 |
| Hepatitis C | 87 (25%) | 77 (28%) | 114 (42%) | <0.001 |
| Current heroin use | 11 (3%) | 12 (4%) | 20 (7%) | 0.04 |
| LDL (mg/dl) | 107 (85–133) | 106 (80–136) | 95 (71–125) | <0.001 |
| HDL (mg/dl) | 44 (35–55) | 45 (37–57) | 42 (33–57) | 0.16 |
| Triglycerides (mg/dl) | 135 (88–206) | 117 (89–179) | 142 (101–197) | 0.004 |
| Body mass index (kg/m2) | 26 (24–31) | 27 (24–31) | 25 (23–31) | 0.02 |
| Waist circumference (cm) | 88 (81–98) | 89 (80–100) | 87 (78–98) | 0.17 |
| Current CD4 (cells/mm3) | 448 (282–647) | 386 (234–534) | 363 (195–548) | <0.001 |
| Nadir CD4 (cells/mm3) | 235 (131–348) | 214 (114–313) | 186 (85–294) | 0.001 |
| History of AIDS | 148 (41%) | 135 (50%) | 158 (58%) | <0.001 |
| HIV viral load (copies/ml) | ||||
| ≤80 | 126 (35%) | 77 (29%) | 71 (26%) | <0.01 |
| 81–1999 | 90 (25%) | 60 (22%) | 53 (19%) | |
| 2000–9999 | 55 (15%) | 48 (18%) | 44 (16%) | |
| >10,000 | 84 (24%) | 85 (31%) | 104 (38%) | |
| Current HAART use | 221 (62%) | 153 (56%) | 155 (57%) | 0.30 |
| Current NRTI use | 254 (71%) | 176 (65%) | 172 (63%) | 0.08 |
| Current NNRTI use | 97 (27%) | 68 (25%) | 79 (29%) | 0.58 |
| Current protease inhibitor use | 158 (44%) | 109 (40%) | 110 (40%) | 0.50 |
| Albuminuriaa | 57 (16%) | 46 (17%) | 109 (40%) | <0.001 |
| eGFRCys (ml/min per 1.73 m2) | 97 (83–110) | 91 (80–105) | 76 (62–89) | <0.001 |
| eGFRCys<60 ml/min per 1.73 m2 | 15 (4%) | 10 (4%) | 59 (22%) | <0.001 |
| Serum albumin (g/dl) | 4.1 (3.8–4.4) | 4.0 (3.8–4.3) | 3.9 (3.6–4.2) | <0.001 |
Data are presented as medians (interquartile ranges) or numbers (percentages). α1-Microglobulin is standardized to urine creatinine. Category 1 comprises all participants with undetectable urine α1-microglobulin. α1m/cr, α1-Microglobulin–to-creatinine ratio; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcription inhibitor; NNRTI, non-nucleoside reverse transcription inhibitor; eGFRCys, eGFR by cystatin C.
Defined as a positive urine dipstick result (≥1+) or urine albumin-to-creatinine ratio >30 mg/g.
Table 2.
Baseline characteristics of HIV-uninfected women in the Women’s Interagency HIV Study stratified by urine α1-microglobulin category
| Characteristic | 1 (n=173) | 2 (n=57) | 3 (n=57) | P Value |
|---|---|---|---|---|
| Range of α1m/cr (mg/g) | Below detectable | 1.3–7.5 | >7.5 | |
| Baseline age (yr) | 38 (32–43) | 42 (36–46) | 42 (38–48) | <0.001 |
| Race | ||||
| African American | 99 (57%) | 41 (72%) | 37 (65%) | 0.31 |
| Caucasian | 20 (12%) | 6 (11%) | 6 (11%) | |
| Other | 54 (31%) | 10 (18%) | 14 (25%) | |
| Cigarette smoking | ||||
| Current | 84 (49%) | 39 (68%) | 46 (81%) | <0.001 |
| Past | 49 (28%) | 7 (12%) | 6 (11%) | |
| Never | 40 (23%) | 11 (19%) | 5 (9%) | |
| Diabetes mellitus | 11 (6%) | 5 (9%) | 10 (18%) | 0.04 |
| Hypertension | 39 (23%) | 11 (19%) | 30 (53%) | <0.001 |
| Antihypertensive use | 18 (10%) | 6 (11%) | 11 (19%) | 0.19 |
| Menopause | 20 (12%) | 6 (11%) | 14 (25%) | 0.03 |
| Hepatitis C | 23 (14%) | 16 (28%) | 23 (40%) | <0.001 |
| Current heroin use | 9 (5%) | 2 (4%) | 12 (21%) | <0.001 |
| LDL (mg/dl) | 107 (89–131) | 104 (84–128) | 100 (84–132) | 0.71 |
| HDL (mg/dl) | 52 (44–63) | 49 (41–61) | 48 (39–59) | 0.29 |
| Triglycerides (mg/dl) | 94 (71–141) | 107 (69–154) | 119 (87–156) | 0.08 |
| Body mass index (kg/m2) | 29 (24–35) | 30 (26–34) | 29 (25–32) | 0.67 |
| Waist circumference (cm) | 92 (80–103) | 95 (83–107) | 94 (81–99) | 0.55 |
| Albuminuriaa | 15 (9%) | 6 (11%) | 12 (21%) | 0.04 |
| eGFRCys (ml/min per 1.73 m2) | 106 (96–117) | 97 (87–112) | 97 (79–112) | <0.001 |
| eGFRCys<60 ml/min per 1.73 m2 | 1 (2%) | 5 (9%) | 0 | <0.001 |
| Serum albumin (g/dl) | 4.1 (3.9–4.3) | 4.0 (3.8–4.2) | 4.0 (3.8–4.3) | 0.08 |
Data are presented as medians (interquartile ranges) or numbers (percentages). α1-Microglobulin is standardized to urine creatinine. Category 1 comprises all participants with undetectable urine α1-microglobulin.
Defined as a positive urine dipstick result (≥1+) or urine albumin-to-creatinine ratio>30 mg/g.
Prevalence of Detectable Urine α1m in HIV-Infected and Uninfected Women
Urine α1m was detectable in 545 (60%) HIV-infected and 114 (40%) uninfected women (P<0.001). HIV infection remained associated with a 51% higher prevalence of detectable α1m after multivariable adjustment for age, race, and traditional kidney disease risk factors (Table 3). The association was slightly attenuated with additional adjustment for ACR and eGFRCys but remained statistically significant.
Table 3.
Association of HIV infection with detectable urine α1-microglobulin in HIV-infected (n=903) versus uninfected (n=287) women at baseline visit
| Model | Prevalence Ratioa (95% Confidence Interval) | P Value |
|---|---|---|
| Demographic adjustedb | 1.52 (1.24 to 1.86) | <0.001 |
| Multivariate adjustedc | 1.51 (1.22 to 1.85) | <0.001 |
| Multivariate adjustedc+ACR | 1.46 (1.19 to 1.81) | <0.001 |
| Multivariate adjustedc+ACR+eGFRCys | 1.34 (1.08 to 1.67) | <0.01 |
ACR, albumin-to-creatinine ratio.
Adjusted prevalence ratios of detectable α1-microglobulin in HIV-infected versus uninfected women calculated using multivariable Poisson regression models.
Adjusted for age and race.
Adjusted for age, race, hypertension, diabetes mellitus, hepatitis C virus infection, smoking, LDL, triglycerides, and body mass index.
Factors Associated with Urinary α1m in HIV-Infected and Uninfected Women
Among HIV-infected participants, African-American race (relative risk [RR], 1.32; 95% confidence interval [95% CI], 1.04 to 1.69; P=0.02), current smoking (RR, 1.24; 95% CI, 1.04 to 1.47; P=0.01), and CD4 lymphocyte count <200 cells/mm3 (RR, 1.27; 95% CI, 1.04 to 1.56; P=0.02) were independently associated with detectable α1m. When we modeled urine α1m concentration as a continuous outcome, African-American race was associated with 52% higher α1m (95% CI, 22% to 90%; P<0.001), HCV infection was associated with 22% higher α1m (95% CI, 2% to 46%; P=0.03), hypertension was associated with 29% higher α1m (95% CI, 7% to 57%; P=0.01), and menopause was associated with 39% higher α1m (95% CI, 10% to 77%; P<0.01) in adjusted analyses. HIV-related factors independently associated with higher urine α1m levels included CD4 lymphocyte count <200 cells/mm3 (65%; 95% CI, 34% to 103%; P<0.001) and history of AIDS (30%; 95% CI, 10% to 53%; P=0.002).
Among uninfected women, factors associated with higher levels of α1m included diabetes mellitus (66%; 95% CI, 7% to 157%; P=0.02), heroin use (79%; 95% CI, 11 to 190; P=0.02), HCV infection (55%; 95% CI, 11 to 117; P=0.01), and current smoking (82%; 95% CI, 36% to 145%; P<0.001).
Association of Urine α1m with Kidney Function Decline in HIV-Infected and Uninfected Women
Over the approximately 8-year follow-up period, the rate of annual eGFRCys decline was −1.18 (95% CI, −1.29 to −1.06) ml/min per 1.73 m2 in HIV-infected women and −0.97 (95% CI, −1.16 to −0.79) ml/min per 1.73 m2 in uninfected women.
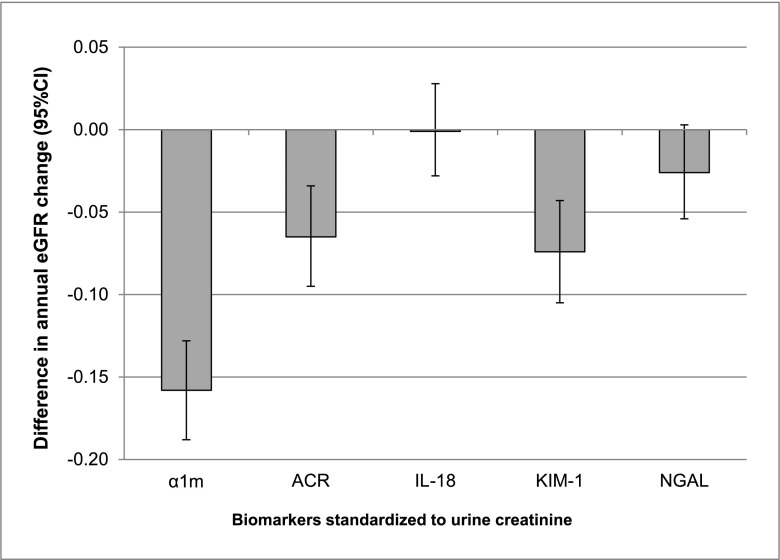
Compared to HIV-infected women with undetectable α1m, HIV-infected women in the highest category of α1m/cr experienced faster eGFRCys decline over the follow-up period. The adjusted difference in annual eGFRCys change between categories 3 and 1 was −0.19 (95% CI, −0.22 to −0.16) ml/min per 1.73 m2 per year in multivariate analyses and −0.16 (95% CI, −0.19 to −0.13) ml/min per 1.73 m2 per year after additional adjustment for ACR, IL-18/cr, KIM-1/cr, and NGAL/cr. Adjustment for tenofovir use during follow-up did not alter the association of α1m/cr with annual eGFR change. Compared with ACR, IL-18, KIM-1, and NGAL, urine α1m was associated with the largest annual change in eGFRCys in multivariate models adjusting for all four biomarkers in creatinine-standardized (Figure 1) and unstandardized analyses (Supplemental Figure 1).
Figure 1.
Magnitudes of associations with annual eGFR change across creatinine-standardized urine biomarkers. All estimates were derived from multivariate models comparing category 3 with category 1 for α1-microglobulin (α1m) and comparing highest with lowest tertile for albumin-to-creatinine ratio (ACR), IL-18, kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL). Multivariate models were adjusted for age, race, traditional kidney disease risk factors, HIV-related factors, and all displayed biomarkers. Results are reported as eGFR change in milliliters per minute per 1.73 m2 per year (95% confidence interval [95% CI]).
Among HIV-infected women, 177 (22%) and 61 (7%) cases of incident CKD and 10% annual eGFRCys decline occurred, respectively. Relative to those with undetectable α1m, the highest category of α1m/cr was associated with 2- to 3-fold risks of incident CKD and rapid decline after adjustment for demographics, baseline eGFRCys, traditional kidney disease risk factors, and HIV-related factors (Table 4). Even after additional adjustment for ACR, IL-18, KIM-1, and NGAL, the highest category of α1m/cr remained associated with a 1.9-fold risk of incident CKD and a 2.8-fold risk of rapid decline. Results were similar when urine α1m was modeled without standardization to urine creatinine (Supplemental Table 2) and when we required an eGFRCys decline of ≥1 ml/min per 1.73 m2 per year of follow-up for all patients with incident CKD. There were no statistically significant interactions with kidney decline outcomes when individuals were stratified by tenofovir use during follow-up.
Table 4.
Associations of urine α1-microglobulin with kidney function decline and mortality in HIV-infected women.
| Incident CKD | 10% Decline in eGFRCys | All-Cause Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | C3 | C1 | C2 | C3 | |
| Range of α1m/cr (mg/g) | — | 1.2–10.2 | >10.2 | — | 1.2–10.2 | >10.2 | — | 1.2–10.2 | >10.2 |
| No. at risk | 343 | 262 | 214 | 358 | 272 | 273 | 358 | 272 | 273 |
| No. events | 48 | 50 | 79 | 9 | 17 | 35 | 29 | 36 | 63 |
| Risk Ratio (95% CI) | Risk Ratio (95% CI) | Risk Ratio (95% CI) | Risk Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | ||||
| Demographic adjusteda | Reference | 1.35 (0.58 to 3.14) | 3.56 (2.16 to 5.88) | Reference | 2.53 (1.14 to 5.64) | 4.53 (2.21 to 9.28) | Reference | 1.60 (0.98 to 2.63) | 2.66 (1.70 to 4.17) |
| Multivariate adjustedb | — | 1.31 (0.62 to 2.74) | 2.36 (1.42 to 3.92) | — | 2.21 (1.01 to 4.84) | 2.99 (1.42 to 6.29) | — | 1.47 (0.89 to 2.44) | 1.69 (1.06 to 2.69) |
| Adjusted+ACRc | — | 1.34 (0.66 to 2.72) | 2.08 (1.28 to 3.38) | — | 2.38 (1.11 to 5.12) | 2.70 (1.23 to 5.92) | — | 1.50 (0.91 to 2.49) | 1.61 (1.00 to 2.59) |
| Adjusted+ACR, IL-18, KIM-1, NGALd | — | 1.13 (0.65 to 1.98) | 1.87 (1.23 to 2.84) | — | 2.68 (1.26 to 5.72) | 2.76 (1.28 to 5.98) | — | 1.65 (0.98 to 2.76) | 1.73 (1.07 to 2.82) |
Standardized to urine creatinine and stratified by categories. Category 1 comprises all participants with undetectable urine α1-microglobulin. All analyses use category 1 as the reference category. eGFRCys, cystatin C-based eGFR; C1, category 1; C2, category 2; C3, category 3; α1m/cr, α1–microglobulin-to-creatinine ratio; 95% CI, 95% confidence interval; ACR, albumin-to-creatinine ratio; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin.
Adjusted for age and race.
Adjusted for age, race, baseline eGFRCys, hypertension, diabetes mellitus, hepatitis C virus infection, HIV viral load, CD4 lymphocyte count, antiretroviral therapy use, and serum albumin.
Adjusted for all covariates listed above with the addition of ACR.
Adjusted for all covariates listed above with the addition of ACR, IL-18, KIM-1, NGAL, and liver fatty acid binding protein.
Among the uninfected participants, we examined the associations of urine α1m/cr with continuous eGFRCys decline and two dichotomous outcomes: 3% and 5% annual eGFRCys decline. Compared to individuals with undetectable α1m, the highest category of α1m/cr was associated with −0.21 (95% CI, −0.28 to −0.13; P<0.001) ml/min per 1.73 m2 annual eGFRCys decline in multivariate analyses and −0.27 (95% CI, −0.34 to −0.19; P<0.001) ml/min per 1.73 m2 annual eGFRCys decline after additional adjustment for ACR, IL-18/cr, KIM-1/cr, and NGAL/cr. Individuals in the highest α1m/cr category had approximately 2-fold adjusted risks of 3% and 5% annual eGFRCys decline relative to those with undetectable α1m (Table 5).
Table 5.
Associations of urine α1-microglobulin with kidney function decline in HIV-uninfected women
| 3% Annual eGFRCys Decline | 5% Annual eGFRCys Decline | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | C3 | |
| Range of α1m/cr (mg/g) | — | 0.1–0.8 | >0.8 | — | 0.1–0.8 | >0.8 |
| No. at risk | 173 | 57 | 57 | 173 | 57 | 57 |
| No. events | 33 | 18 | 30 | 13 | 7 | 14 |
| Risk Ratio (95% CI) | Risk Ratio (95% CI) | Risk Ratio (95% CI) | Risk Ratio (95% CI) | |||
| Demographic adjusteda | Reference | 1.65 (1.06 to 2.56) | 2.23 (1.52 to 3.29) | Reference | 1.46 (0.64 to 3.32) | 2.30 (1.19 to 4.46) |
| Multivariate adjustedb | — | 1.77 (1.10 to 2.84) | 2.16 (1.37 to 3.38) | — | 1.55 (0.68 to 3.52) | 2.28 (1.16 to 4.51) |
| Adjusted+ACRc | — | 1.77 (1.10 to 2.83) | 2.21 (1.41 to 3.45) | — | 1.68 (0.73 to 3.89) | 2.19 (1.12 to 4.29) |
| Adjusted+ACR, IL-18, KIM-1, NGALd | — | 1.61 (1.02 to 2.55) | 2.13 (1.39 to 3.28) | — | 1.72 (0.76 to 3.93) | 2.28 (1.19 to 4.38) |
Standardized to urine creatinine and stratified by categories. Category 1 comprises all participants with undetectable urine α1-microglobulin. All analyses use category 1 as the reference category. eGFRCys, cystatin C-based eGFR; C1, category 1; C2, category 2; C3, category 3; α1m/cr, α1–microglobulin-to-creatinine ratio; ACR, albumin-to-creatinine ratio; KIM-1, kidney injury molecule-1.
Adjusted for age and race.
Adjusted for age, race, baseline eGFRCys, hypertension, diabetes mellitus, hepatitis C virus infection, smoking, systolic blood pressure, and serum albumin.
Adjusted for all covariates listed above with the addition of ACR.
Adjusted for all covariates listed above with the addition of ACR, IL-18, KIM-1, NGAL, and liver fatty acid binding protein.
Association of Urine α1m with Mortality in HIV-Infected Women
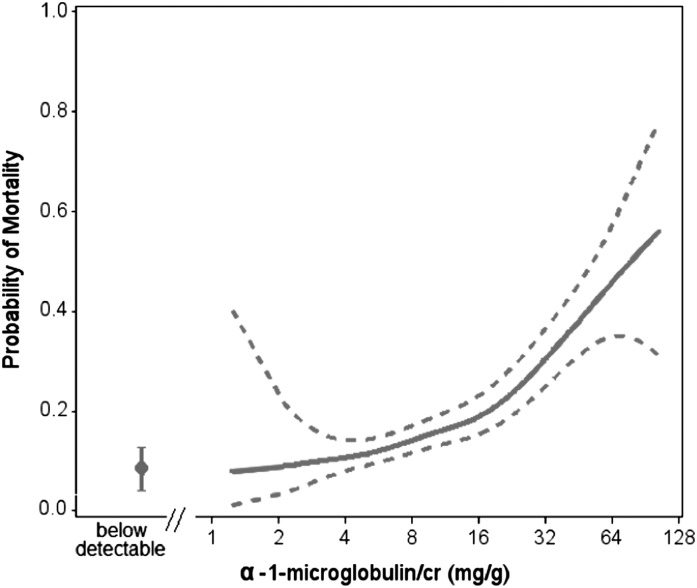
During the 8 years of follow-up in the WIHS Kidney Aging Study, there were 128 (14%) deaths among 903 HIV-infected women included in this study. When we used a spline plot to assess probability of mortality across the range of detectable urine α1m/cr (Figure 2), higher urine α1m/cr levels were incrementally associated with higher mortality (P<0.001). Although the slope appeared to be steeper at higher values of α1m/cr, the test for nonlinearity did not reach statistical significance (P=0.37).
Figure 2.
Spline plot displaying unadjusted association of urine α1-microglobulin with mortality over a median follow-up of 8 years. Solid line denotes predicted probability of mortality; dotted lines represent 95% confidence bounds. Below detectable estimate represents the proportion of deaths in individuals with undetectable α1-microglobulin. The highest 2.5% of values were truncated. P<0.001 for association of urine α1-microglobulin–to-creatinine ratio with mortality. P=0.37 for test of nonlinearity. cr, creatinine.
Compared to women with undetectable α1m, women in the highest category of α1m/cr had a 1.7-fold risk of all-cause mortality in multivariate analyses (Table 4). Results were similar when α1m was not standardized to urine creatinine (hazard ratio [HR], 1.64; 95% CI, 0.94 to 2.87) (Supplemental Table 2). Updating vital status through April of 2013 resulted in a total of 224 deaths among 903 HIV-infected participants, with a median follow-up of 13 years. The highest category of α1m/cr remained independently associated with mortality in the multivariate-adjusted model (HR, 1.49; 95% CI, 1.05 to 2.11; P=0.03), but the association was no longer statistically significant after adjustment for albuminuria and the additional biomarkers (HR, 1.42; 95% CI, 0.98 to 2.05; P=0.06).
Discussion
In this large cohort of women with predominantly preserved eGFR, HIV-infected women had a higher prevalence of detectable α1m than uninfected women. Among HIV-infected and uninfected women, urine α1m was predictive of kidney function decline independent of traditional risk factors, albuminuria, and other biomarkers of tubular injury, including IL-18, KIM-1, and NGAL. Additionally, urine α1m was independently associated with mortality risk among HIV-infected women. To our knowledge, this is the first ambulatory cohort study to investigate the ability of urine α1m to predict longitudinal outcomes in HIV-infected and uninfected women.
Formerly known as Protein HC, α1m is a size- and charge-heterogeneous lipocalin found in blood, liver, kidney, and connective tissues of a variety of vertebrate species (15). Synthesized by the liver, α1m circulates in the blood in free and protein-bound forms, with approximately 50% of plasma α1m bound to IgA. The 26-kD free form of α1m is filtered by the glomerulus and almost entirely reabsorbed by proximal tubular epithelial cells, where it is degraded by intracellular lysosomes (29,30). Active transport of α1m from the luminal space into proximal tubular epithelial cells is mediated by the endocytic receptor megalin, which also facilitates reabsorption of other low molecular mass proteins, such as retinol-binding protein and β2-microglobulin (31). Although its exact physiologic function remains unknown, α1m is hypothesized to have antioxidant properties and possible roles in humoral or cellular immune response (16).
Urine α1m was recognized as a marker of proximal tubular dysfunction over two decades ago, but its use in clinical research studies was sparse until recent years. By proteomic analysis, Devarajan et al. (17) identified α1m as one of the earliest urinary markers of AKI in children undergoing cardiopulmonary bypass surgery, with urine levels rising 3-fold within 2 hours of surgery compared with controls who did not develop AKI (P<0.01). Wu et al. (32) additionally showed that individuals with drug-induced interstitial nephritis had higher levels of urine α1m compared with age- and sex-matched controls and that urine α1m levels correlated with the degree of inflammatory infiltration, interstitial edema, and tubular atrophy on kidney biopsy. Finally, O’Seaghdha et al. (33) investigated the associations of multiple urinary biomarkers, including α1m, with incident kidney disease and related outcomes in 2948 Framingham Heart Study participants. Although urine α1m was not associated with incident CKD or albuminuria, each 1 SD of log-transformed α1m was associated with a 30% higher risk for all-cause mortality (33).
Although the magnitudes of association between α1m levels and annual eGFR decline in our study may appear small (−0.19 and −0.21 ml/min per 1.73 m2 per year in the HIV-infected and uninfected participants, respectively), the effect sizes are comparable with those of established risk factors for kidney decline in other populations. In the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study of patients with type 1 diabetes, microalbuminuria (defined as 30–299 mg/d) was associated with −0.12-ml/min per 1.73 m2 annual eGFR change compared with no albuminuria (34). In the same study, systolic BP≥130 was associated with a −0.22-ml/min per 1.73 m2 annual eGFR change compared with systolic BP<120, and hemoglobin A1c levels of 8–8.9 were associated with −0.23 ml/min per 1.73 m2 annual eGFR decline compared with hemoglobin A1c levels of <7. These observations suggest that associations of this magnitude are clinically important. Future studies must validate our findings in additional cohorts of individuals with varied risk factors for kidney disease progression.
In persons with HIV infection, proximal tubular dysfunction is an increasingly common presentation of renal toxicity (35), with one study reporting a 25% prevalence of tubular damage in individuals on antiretroviral therapy without proteinuria or significant impairment of glomerular function (36). Recent evidence also suggests that α1m and other low molecular mass proteins can detect subclinical impairments in proximal tubular function among HIV-infected individuals receiving tenofovir, a nucleotide analog reverse transcription inhibitor associated with a particular form of proximal tubular dysfunction known as Fanconi’s syndrome (37), AKI (38), and CKD (39). In a 48-week trial, Vrouenraets et al. (40) randomized 20 individuals with HIV infection to continue therapy with zidovudine/lamivudine or switch to tenofovir/emtricitabine and noted an approximately 50% rise (P=0.09) in mean urine α1m/cr in subjects randomized to tenofovir/emtricitabine compared with unchanged urine α1m/cr levels in persons continued on zidovudine/lamivudine. Similarly, Post et. al reported higher urine levels at 48 weeks of retinol-binding protein and β2-microglobulin by 50% (P<0.001) and 24% (P<0.001), respectively, in antiretroviral-naïve individuals randomized to tenofovir/emtricitabine (n=193) compared with abacavir/lamivudine (n=192) (41). Although urine α1m was not measured in that study, retinol-binding protein and β2-microglobulin undergo transport into proximal tubular cells by the same endocytic receptor as urine α1m (31), suggesting that these low molecular mass proteins may provide similar information when measured in urine. Notably, none of the subjects in the trial described above met the protocol definition for proximal tubular dysfunction, which relied on other criteria, such as hypophosphatemia, hypokalemia, nondiabetic glycosuria, metabolic acidosis, and rise in serum creatinine. This finding highlights the need for novel biomarkers that capture drug-related toxicity before the onset of clinical Fanconi’s syndrome or reduction in glomerular filtration function.
This study offers several important implications for clinical care. First, the associations of urine α1m with kidney function decline and mortality suggest that minor impairments of proximal tubular function are not benign. Additional studies should investigate the potential reversibility of proximal tubular dysfunction to determine whether tubular dysfunction is a modifiable risk factor for kidney decline. Larger studies of α1m in diverse populations may also enable the identification of a threshold α1m level beyond which kidney risk escalates. Second, our study utilized urine specimens collected prior to the widespread use of tenofovir. Although subsequent tenofovir use did not modify the observed associations of urine α1m with kidney decline outcomes, our study was not designed or powered to determine whether urinary α1m identifies a subset of individuals who are particularly susceptible to tubular toxicity from tenofovir or other drugs. Future clinical studies could specifically evaluate the potential applications of α1m in risk stratification before initiation of tenofovir and early detection of tubular toxicity during therapy. Additionally, a growing number of uninfected individuals at high risk for HIV acquisition are now receiving pre-exposure prophylaxis with tenofovir. Early recognition of tubular toxicity from tenofovir will be particularly important in this population of predominantly young individuals, in whom the risks of therapy are incompletely delineated. Third, biomarkers sensitive for the detection of tubular dysfunction and injury could revolutionize our methods for detecting nephrotoxicity in drug trials, which often fail to identify drug-related nephrotoxicity in initial stages of development because of their sole reliance on serum creatinine and proteinuria.
There are several limitations to this study. First, because we exclusively studied women, we cannot generalize these results to men. Second, biomarker measurements were made using urine samples collected over a decade ago. However, with protein degradation over time, we would expect our results to shift toward null findings. Third, although it would have been desirable to confirm the diagnosis of CKD with at least two separate eGFR measurements at the 4- and 8-year time points, this was not feasible because of the availability of only one serum sample per patient at each of these visits. Fourth, to ensure comparability with the HIV-infected women, recruitment of the uninfected WIHS cohort targeted women who engage in high-risk behaviors, such as injection drug use and high-risk sexual behavior (19). Therefore, the uninfected women in this study had a high prevalence at baseline of established risk factors for kidney disease. This may limit the generalizability of our findings to healthier, uninfected populations. Fifth, because of the low rates of incident CKD, 10% annual eGFRCys decline, and mortality in the uninfected participants, we lacked sufficient power to analyze the associations of α1m with these outcomes. Sixth, we did not have access to serum concentrations of α1m, and therefore, we cannot exclude the possibility that higher serum levels in susceptible individuals contributed to our observations. Seventh, although we adjusted for multiple potential confounders, the possibility of residual confounding exists for our associations of urine α1m with kidney function decline and mortality.
In conclusion, we found that urine α1m independently predicted kidney function decline in HIV-infected and uninfected women as well as mortality risk among HIV-infected women, highlighting the importance of proximal tubular dysfunction to longitudinal outcomes. Future research should identify temporal patterns of α1m excretion and their associations with clinical outcomes, and determine specific thresholds of risk in HIV-infected and uninfected individuals. In HIV-infected persons, studies should specifically investigate the role of urine α1m in detection of drug toxicity before the onset of overt kidney disease. Although our findings must be validated in subsequent cohorts, urine α1m seems to be a promising candidate for inclusion in a future kidney biomarker panel.
Disclosures
R.S. received an honorarium from Merck for participating in a Renal Expert Input Forum; this honorarium was donated to the Northern California Institute for Research and Education to support kidney research. C.R.P. is a coinventor on an IL-18 patent issued to the University of Colorado.
Supplementary Material
Acknowledgments
The WIHS Kidney Aging Study is funded by Grant 1 R01 AG034853-01A2 (Principal Investigator: M.G.S.), which was administered by the Northern California Institute for Research and Education with resources of the Veterans Affairs Medical Center (San Francisco, California). M.M.E. is supported by Grant 1K23-DK-081317. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), Grant U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), Grant U01-AI-103408; Bronx WIHS (Kathryn Anastos), Grant U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), Grant U01-AI-031834; Chicago WIHS (M.H.C.), Grant U01-AI-034993; Metropolitan Washington WIHS (M.Y.), Grant U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), Grant U01-AI-103397; UNC WIHS (Adaora Adimora), Grant U01-AI-103390; Connie Wofsy Women’s HIV Study, northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), Grant U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), Grant U01-AI-042590; and Southern California WIHS (Alexandra Levine and M.N.), Grant U01-HD-032632 (WIHS I to WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders, and the National Institutes of Health (NIH) Office of Research on Women’s Health. WIHS data collection is also supported by Grants UL1-TR000004 (to UCSF CTSA) and UL1-TR000454 (to Atlanta CTSA).
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03220314/-/DCSupplemental.
References
- 1.Gardner LI, Holmberg SD, Williamson JM, Szczech LA, Carpenter CC, Rompalo AM, Schuman P, Klein RS, HIV Epidemiology Research Study Group : Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr 32: 203–209, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Aaron KJ, Kempf MC, Christenson RH, Wilson CM, Muntner P, Shrestha S: Prevalence of proteinuria and elevated serum cystatin C among HIV-Infected Adolescents in the Reaching for Excellence in Adolescent Care and Health (REACH) study. J Acquir Immune Defic Syndr 61: 499–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt CM, Winston JA, Malvestutto CD, Fishbein DA, Barash I, Cohen AJ, Klotman ME, Klotman PE: Chronic kidney disease in HIV infection: An urban epidemic. AIDS 21: 2101–2103, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lucas GM, Mehta SH, Atta MG, Kirk GD, Galai N, Vlahov D, Moore RD: End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS 21: 2435–2443, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Eggers PW, Kimmel PL: Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol 15: 2477–2485, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Rasch MG, Helleberg M, Feldt-Rasmussen B, Kronborg G, Larsen CS, Pedersen C, Pedersen G, Gerstoft J, Obel N: Increased risk of dialysis and end-stage renal disease among HIV patients in Denmark compared with the background population. Nephrol Dial Transplant 29: 1232–1238, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Wu I, Parikh CR: Screening for kidney diseases: Older measures versus novel biomarkers. Clin J Am Soc Nephrol 3: 1895–1901, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG: Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: The FRAM study. Arch Intern Med 167: 2213–2219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driver TH, Scherzer R, Peralta CA, Tien PC, Estrella MM, Parikh CR, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Abraham A, Shlipak MG: Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS 27: 2291–2299, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jotwani V, Scherzer R, Abraham A, Estrella MM, Bennett M, Devarajan P, Anastos K, Cohen MH, Nowicki M, Sharma A, Young M, Tien PC, Grunfeld C, Parikh CR, Shlipak MG: Does HIV infection promote early kidney injury in women? Antivir Ther 19: 79–87, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak MJ, Parikh CR: Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 61: 565–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, Bennett M, Butch A, Anastos K, Cohen M, Nowicki M, Sharma A, Young M, Sarnak M, Parikh C, Shlipak M: Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med 15: 291–300, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Akerström B, Lögdberg L, Berggård T, Osmark P, Lindqvist A: alpha(1)-Microglobulin: A yellow-brown lipocalin. Biochim Biophys Acta 1482: 172–184, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Weber MH, Verwiebe R: Alpha 1-microglobulin (protein HC): Features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem 30: 683–691, 1992 [PubMed] [Google Scholar]
- 17.Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR: Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis 56: 632–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J, WIHS Collaborative Study Group : The Women’s Interagency HIV Study. Epidemiology 9: 117–125, 1998 [PubMed] [Google Scholar]
- 19.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA: The Women’s Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 12: 1013–1019, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J: Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis 58: 682–684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG: HIV viremia and changes in kidney function. AIDS 23: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyatt CM, Hoover DR, Shi Q, Tien PC, Karim R, Cohen MH, Goderre JL, Seaberg EC, Lazar J, Young MA, Klotman PE, Anastos K: Pre-existing albuminuria predicts AIDS and non-AIDS mortality in women initiating antiretroviral therapy. Antivir Ther 16: 591–596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, Minkoff H, Hessol NA: Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med 113: 91–98, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, Anastos K, Augenbraun M, Cohen MH: Trends in mortality and causes of death among women with HIV in the United States: A 10-year study. J Acquir Immune Defic Syndr 51: 399–406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilks WR, Richardson S, Spiegehalter DJ: Markov Chain Monte Carlo in Practice, London, Chapman & Hall, 1996 [Google Scholar]
- 27.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Robins JM, Finkelstein DM: Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 56: 779–788, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Christensen EI, Nielsen S: Structural and functional features of protein handling in the kidney proximal tubule. Semin Nephrol 11: 414–439, 1991 [PubMed] [Google Scholar]
- 30.Strober W, Waldmann TA: The role of the kidney in the metabolism of plasma proteins. Nephron 13: 35–66, 1974 [DOI] [PubMed] [Google Scholar]
- 31.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE: Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Yang L, Su T, Wang C, Liu G, Li XM: Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol 5: 1954–1959, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Seaghdha CM, Hwang SJ, Larson MG, Meigs JB, Vasan RS, Fox CS: Analysis of a urinary biomarker panel for incident kidney disease and clinical outcomes. J Am Soc Nephrol 24: 1880–1888, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, Steffes MW, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group : Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol 25: 810–818, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Palacio M, Romero S, Casado JL: Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev 14: 179–187, 2012 [PubMed] [Google Scholar]
- 36.Ando M, Yanagisawa N, Ajisawa A, Tsuchiya K, Nitta K: Kidney tubular damage in the absence of glomerular defects in HIV-infected patients on highly active antiretroviral therapy. Nephrol Dial Transplant 26: 3224–3229, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Hall AM, Hendry BM, Nitsch D, Connolly JO: Tenofovir-associated kidney toxicity in HIV-infected patients: A review of the evidence. Am J Kidney Dis 57: 773–780, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS: Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 78: 1171–1177, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG: Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 26: 867–875, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrouenraets SM, Fux CA, Wit FW, Garcia EF, Furrer H, Brinkman K, Hoek FJ, Abeling NG, Krediet RT, Reiss P, Prepare Study Group : Persistent decline in estimated but not measured glomerular filtration rate on tenofovir may reflect tubular rather than glomerular toxicity. AIDS 25: 2149–2155, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Post FA, Moyle GJ, Stellbrink HJ, Domingo P, Podzamczer D, Fisher M, Norden AG, Cavassini M, Rieger A, Khuong-Josses MA, Branco T, Pearce HC, Givens N, Vavro C, Lim ML: Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-Week results from the ASSERT study. J Acquir Immune Defic Syndr 55: 49–57, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.