Abstract
Background and objectives
Patients receiving dialysis undergo parathyroidectomy to improve laboratory parameters in resistant hyperparathyroidism with the assumption that clinical outcomes will also improve. However, no randomized clinical trial data demonstrate the benefits of parathyroidectomy. This study aimed to evaluate clinical outcomes up to 1 year after parathyroidectomy in a nationwide sample of patients receiving hemodialysis.
Design, setting, participants, & measurements
Using data from the US Renal Data System, this study identified prevalent hemodialysis patients aged ≥18 years with Medicare as primary payers who underwent parathyroidectomy from 2007 to 2009. Baseline characteristics and comorbid conditions were assessed in the year preceding parathyroidectomy; clinical events were identified in the year preceding and the year after parathyroidectomy. After parathyroidectomy, patients were censored at death, loss of Medicare coverage, kidney transplant, change in dialysis modality, or 365 days. This study estimated cause-specific event rates for both periods and rate ratios comparing event rates in the postparathyroidectomy versus preparathyroidectomy periods.
Results
Of 4435 patients who underwent parathyroidectomy, 2.0% died during the parathyroidectomy hospitalization and the 30 days after discharge. During the 30 days after discharge, 23.8% of patients were rehospitalized; 29.3% of these patients required intensive care. In the year after parathyroidectomy, hospitalizations were higher by 39%, hospital days by 58%, intensive care unit admissions by 69%, and emergency room/observation visits requiring hypocalcemia treatment by 20-fold compared with the preceding year. Cause-specific hospitalizations were higher for acute myocardial infarction (rate ratio, 1.98; 95% confidence interval, 1.60 to 2.46) and dysrhythmia (rate ratio 1.4; 95% confidence interval1.16 to 1.78); fracture rates did not differ (rate ratio 0.82; 95% confidence interval 0.6 to 1.1).
Conclusions
Parathyroidectomy is associated with significant morbidity in the 30 days after hospital discharge and in the year after the procedure. Awareness of clinical events will assist in developing evidence-based risk/benefit determinations for the indication for parathyroidectomy.
Keywords: hemodialysis, mortality, hyperparathyroidism
Introduction
Secondary hyperparathyroidism (SHPT) is common among patients receiving dialysis (1). A major focus of therapy for these patients is directed at controlling parathyroid hormone (PTH) levels, because large, population-based observational studies have suggested an association between severely elevated PTH values and poor patient outcomes (2,3). In recent years, therapy for the management of SHPT has undergone several changes based on recommendations from the Kidney Disease Outcomes Quality Initiative (4) and the Kidney Disease Improving Global Outcomes (KDIGO) international guideline group (3). Unfortunately, the guideline working groups have had little randomized clinical trial data available to inform high-quality recommendations for care.
KDIGO guidelines currently state that patients with CKD stages 3–5D with severe hyperparathyroidism who fail to respond to medical therapy should undergo parathyroidectomy. This recommendation may have been based on observational, not-randomized clinical trial evidence describing potentially beneficial effects on short-term laboratory parameters and/or on longer-term clinical outcomes including fracture and death (5,6). Some (7–12) but not all (5) reports suggest that short-term adverse outcomes related to parathyroidectomy produce only modest adverse consequences. However, many of these reports are based on single-center experiences, and may represent highly selected and high-performing surgical units (7–12). In addition, the comparability of nonparathyroidectomized control groups in these studies is unknown.
There is a paucity of data describing the clinical outcomes that occur within the year after parathyroidectomy. Using data from the US Renal Data System (USRDS), we sought to (1) evaluate morbidity and mortality after a parathyroidectomy procedure and (2) compare event rates in the year immediately after parathyroidectomy with rates in the year immediately preceding it, using a nationwide sample of United States dialysis patients who underwent parathyroidectomy between 2007 and 2009.
Materials and Methods
Data Source, Populations, and Case Definition
This study used USRDS ESRD data, which include data from the ESRD Medical Evidence Report (US Centers for Medicare and Medicaid Services [CMS] form CMS-2728), the ESRD Death Notification (form CMS-2746), the United Network for Organ Sharing kidney transplant database, Medicare Part A institutional claims (inpatient, outpatient, skilled nursing facility, home health, and hospice), and Medicare Part B physician claims (inpatient, outpatient, and supplier).
The study population included all prevalent patients aged ≥18 years receiving hemodialysis who underwent parathyroidectomy between January 1, 2007, and December 31, 2009. Patients were required to have Medicare as the primary insurance payer for both Part A and Part B and to have been receiving hemodialysis for at least 1 year before undergoing parathyroidectomy. Parathyroidectomy was identified from Medicare inpatient claims using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes 06.8x and 06.95 (13). The date of the parathyroidectomy was considered the index date, and the hospitalization during which parathyroidectomy occurred was considered the index hospitalization.
Patient Characteristics and Outcomes
Patient characteristics were derived from the ESRD Medical Evidence Report and Medicare claims. Characteristics assessed as of the index date included age, race (white, black, other), sex, primary ESRD cause (diabetes, hypertension, glomerulonephritis, other), body mass index, dialysis duration, geographic region (18 US Renal Networks), and comorbid conditions (congestive heart failure, cerebrovascular accident/transient ischemic attack [CVA/TIA], atherosclerotic heart disease, peripheral vascular disease, dysrhythmia, other cardiovascular diseases, and diabetes). Comorbid conditions were defined by the presence of ICD-9-CM claims (typically two outpatient claims or one inpatient claim) in the year preceding parathyroidectomy. These are methods previously used by the USRDS (14).
Characterization of Outcomes
The postparathyroidectomy assessment period timeframe was broken into three distinct periods: (1) the index hospitalization associated with the parathyroidectomy procedure, (2) the 30-day postdischarge period (to assess acute morbidity and mortality associated with the parathyroidectomy procedure), and (3) the 1-year postdischarge period (to assess longer-term outcomes). The clinical outcomes of interest were all-cause mortality and hospitalization events including total number of hospitalizations, specific causes, intensive care unit (ICU) stays, total number of hospital days, outpatient visits including emergency department visits, hospital observation stays, and outpatient physician visits with hypocalcemia. We also ascertained the total number of fractures. The process for identifying fractures, which used a combination of ICD-9-CM and Current Procedural Terminology codes and place of service, and a complete listing of the codes themselves are detailed in Supplemental Appendix 1. For hospitalizations and outpatient visits, we determined whether hypocalcemia requiring treatment was associated with the event. Treatment for hypocalcemia was defined by ICD-9-CM diagnosis code 275.41, 275.49, or 275.5 with drug code J0610 or J0620. Hospitalization with hypocalcemia was defined as any hospitalization with ICD-9-CM diagnosis code 275.41, 275.49, or 275.5. Cause-specific hospitalizations were defined using the following primary diagnosis codes: CVA/TIA 430–438; congestive heart failure 398.91; acute myocardial infarction 402.x1, 422.xx, 425.xx, 428.xx, 404.x1, 408.x3, v421, or 410.xx; and dysrhythmia 426–427, v450, or v533.
Statistical Analyses
The characteristics of hemodialysis patients undergoing parathyroidectomy were evaluated using descriptive statistics means and standard deviations or medians and 25th/75th percentiles for continuous variables, and counts and percentages for categorical variables. The study is a pre-post comparison of patients who underwent parathyroidectomy, in which events occurring in the 1 year after parathyroidectomy were compared with the same events occurring in the 1 year before parathyroidectomy. The hospitalization for the parathyroidectomy procedure was not included as an outcome in either the preparathyroidectomy or the postparathyroidectomy timeframe, but mortality during that hospitalization was included. Event rates were calculated for each outcome as the number of events divided by follow-up time years. Rate ratios (RRs) and 95% confidence intervals (95% CIs) were used to compare the occurrence of clinical events in the 1-year periods after versus immediately preceding parathyroidectomy. We present results for the population overall and within strata of important baseline characteristics including demographics, dialysis characteristics, and comorbid conditions. Differences of RRs among strata were tested in the Poisson model with interactions between outcomes in the postparathyroidectomy period, the preparathyroidectomy period, and the corresponding patient characteristic. Because this was a pre-post comparison, the correlation can be artificially increased. The generalized estimating equation method was used to manage this possible correlation. All analyses were conducted using SAS software (version 9.2; SAS, Cary, NC).
Results
We identified a total of 7707 patients who underwent parathyroidectomy between 2007 and 2009. After exclusion criteria were applied, 4435 patients were available for analysis (Figure 1). Table 1 describes the baseline characteristics of the final cohort. Most patients (88.4%) were aged <65 years; 57.5% were black and 66.0% had been on dialysis for longer than 5 years.
Figure 1.
Patient flow chart.
Table 1.
Baseline characteristics of parathyroidectomy patients
| Characteristic | All Patients, n (%) |
|---|---|
| Total | 4435 (100.0) |
| Age (yr) | |
| 19–44 | 1764 (39.8) |
| 45–64 | 2154 (48.6) |
| 65–74 | 410 (9.2) |
| ≥75 | 107 (2.4) |
| Race | |
| White | 1685 (38.0) |
| Black | 2551 (57.5) |
| Other | 199 (4.5) |
| Sex | |
| Men | 2298 (51.8) |
| Women | 2137 (48.2) |
| Primary cause of ESRD | |
| Diabetes | 1013 (22.8) |
| Hypertension | 1462 (33.0) |
| Glomerulonephritis | 934 (21.1) |
| Other/unknown/missing | 1026 (23.1) |
| BMI (kg/m2) | |
| <18 | 151 (3.4) |
| 18 to <25 | 1217 (27.4) |
| 25 to <30 | 1026 (23.1) |
| 30 to <35 | 772 (17.4) |
| 35 to <40 | 517 (11.7) |
| ≥40 | 536 (12.1) |
| Missing | 216 (4.9) |
| Dialysis duration (yr) | |
| 1 to < 3 | 538 (12.1) |
| 3 to < 5 | 970 (21.9) |
| ≥5 | 2927 (66.0) |
| Comorbid conditions (%)a | |
| Congestive heart failure | 1973 (44.5) |
| CVA/TIA | 522 (11.8) |
| ASHD | 1542 (34.8) |
| Peripheral vascular disease | 1389 (31.3) |
| Dysrhythmia | 1057 (23.8) |
| Cardiac disease, other | 1623 (36.6) |
| Diabetes | 1954 (44.1) |
BMI, body mass index; CVA/TIA; cerebrovascular accident/transient ischemic attack; ASHD, atherosclerotic heart disease.
Comorbid conditions were identified from the Medical Evidence Report and medical claims during 12-month period before parathyroidectomy.
Short-Term Outcomes
Immediately after parathyroidectomy, 41 patients (0.9%) died during the index hospitalization and another 48 (1.1%) died within 30 days after discharge, an overall procedure-related mortality of 2.0%.
The median duration of the index hospitalization for the parathyroidectomy procedure was 4 days (25th/75th percentile, 3–7 days), and 24.5% of patients required ICU admission. Within 30 days of discharge, 23.8% of patients were rehospitalized, and 29.3% of these required an ICU stay (Table 2).
Table 2.
Events before and after parathyroidectomy
| Outcome | Before Parathyroidectomy (n=4435) | After Parathyroidectomy (n=4435) | Rate Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients with Event, n | Events, n | Percent (95% CI) | Rate per 100 Patient-Years (95% CI) | Patients with Event, n | Events, n | Median Follow-Up, yr | Percent (95% CI) | Rate per 100 Patient-Years (95% CI) | After versus Before (95% CI) | |
| 30-Day outcomes | ||||||||||
| Hospitalization | 1057 | 1251 | 0.08 | 23.8 (22.6 to 25.1) | 344.4 (325.2 to 363.6) | |||||
| ICU stay | 367 | 367 | 0.08 | 8.3 (7.5 to 9.1) | 105.8 (93.6 to 115.2) | |||||
| Hospital days | 1057 | 6259 | 0.08 | 23.8 (22.6 to 25.1) | 1724.4 (1681 to 1768) | |||||
| 1-Year outcomes | ||||||||||
| Hospitalization | 2477 | 6147 | 55.9 (54.4 to 57.3) | 138.6 (135.1 to 142.1) | 2832 | 7571 | 1.0 | 63.9 (62.4 to 65.3) | 192.0 (187.7 to 196.3) | 1.39 (1.34 to 1.44) |
| ICU stay required | 997 | 1680 | 22.5 (21.3 to 23.7) | 37.9 (36.1 to 39.7) | 1415 | 2524 | 1.0 | 31.9 (30.5 to 33.3) | 64.0 (61.5 to 66.5) | 1.69 (1.59 to 1.80) |
| Total hospital days | 2477 | 35,004 | 789.3 (781.0 to 797.5) | 2832 | 49,096 | 1.0 | 1245.0 (1234 to 1256) | 1.58 (1.56 to 1.60) | ||
| Cause-specific hospitalization | ||||||||||
| CHF | 391 | 568 | 8.8 (8.0 to 9.7) | 12.8 (11.8 to 13.9) | 307 | 453 | 1.0 | 6.9 (6.2 to 7.7) | 11.5 (10.4 to 12.5) | 0.90 (0.80 to 1.02) |
| CVA/TIA | 67 | 76 | 1.5 (1.2 to 1.9) | 1.7 (1.3 to 2.1) | 101 | 124 | 1.0 | 2.3 (1.8 to 2.7) | 3.1 (2.6 to 3.7) | 1.83 (1.38 to 2.43) |
| Dysrhythmia | 114 | 150 | 2.6 (2.1 to 3.0) | 3.4 (2.8 to 3.9) | 154 | 192 | 1.0 | 3.5 (2.9 to 4.0) | 4.9 (4.2 to 5.6) | 1.44 (1.16 to 1.78) |
| AMI | 102 | 130 | 2.3 (1.9 to 2.7) | 2.9 (2.4 to 3.4) | 174 | 229 | 1.0 | 3.9 (3.4 to 4.5) | 5.8 (5.1 to 6.6) | 1.98 (1.60 to 2.46) |
| With hypocalcemia | 83 | 98 | 1.9 (1.5 to 2.3) | 2.2 (1.8 to 2.6) | 942 | 1486 | 1.0 | 21.2 (20.0 to 22.4) | 37.7 (35.8 to 39.6) | 17.05 (13.90 to 20.92) |
| Total outpatient visits | ||||||||||
| Total ED/observation | 3309 | 13,941 | 74.6 (73.3 to 75.9) | 314.3 (309.1 to 319.6) | 3408 | 14,916 | 1.0 | 76.8 (75.6 to 78.1) | 378.3 (372.2 to 384.3) | 1.20 (1.17 to 1.23) |
| ED/observation with hypocalcemia | 84 | 106 | 1.9 (1.5 to 2.3) | 2.4 (1.9 to 2.8) | 1072 | 1926 | 1.0 | 24.2 (22.8 to 25.4) | 48.8 (46.7 to 51.0) | 20.44 (16.81 to 24.85) |
| Outpatient/physician with hypocalcemia | 30 | 31 | 0.7 (0.4 to 0.9) | 0.7 (0.5 to 0.9) | 234 | 469 | 1.0 | 5.3 (4.6 to 5.9) | 11.9 (10.8 to 13.0) | 17.02 (11.83 to 24.48) |
ICU, intensive care unit; CHF, congestive heart failur; AMI, acute myocardial infarction; ED, emergency department; 95% CI, 95% confidence interval.
One-Year Outcomes
One-year mortality among patients undergoing parathyroidectomy was 9.8% (rate 10.99 per 100 patient-years). A total of 7571 hospitalizations occurred among 2832 unique individuals in the year after parathyroidectomy, an average of 2.7 hospitalizations per person among those hospitalized (Table 2). Compared with the preceding year, all-cause hospitalizations were higher by 39% (192.0 versus 138.6 per 100 patient-years; RR, 1.39; 95% CI, 1.34 to 1.44; Figure 2A). Overall, the total number of hospital days was higher by 58% (1245.0 versus 789.3 per 100 patient-years; RR, 1.58; 95% CI, 1.56 to 1.60) and ICU use by 69% (64.0 versus 37.9 per 100 patient-years; RR, 1.69; 95% CI, 1.59 to 1.80). Finally, hospitalizations with hypocalcemia were higher by 17-fold (37.7 versus 2.2 per 100 patient-years; RR, 17.1; 95% CI, 13.9 to 20.9; Figure 2B). The attributable risk associated with parathyroidectomy is described in Supplemental Figure 1.
Figure 2.
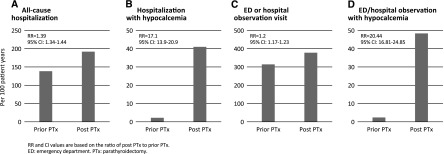
Event rates in the 1 year before and 1 year after parathyroidectomy. (A) Total hospitalizations. (B) Total hospitalizations with hypocalcemia. (C) Total emergency department or observation visits. (D) Total emergency department or observation visits with hypocalcemia. RR and 95% CI values are based on the ratio of postparathyroidectomy to prior parathyroidectomy. 95% CI, 95% confidence interval; ED, emergency department; PTx, parathyroidectomy; RR, rate ratio.
Among the cause-specific hospitalizations evaluated, we found a higher incidence of hospitalizations for CVA/TIA (RR, 1.83; 95% CI, 1.38 to 2.43), acute myocardial infarction (RR, 1.98; 95% CI, 1.60 to 2.46), and dysrhythmia (RR, 1.44; 95% CI, 1.16 to 1.78). There was a trend toward lower incidence of hospitalizations for congestive heart failure, which did not achieve statistical significance (RR, 0.90; 95% CI, 0.80 to 1.02) (Table 2).
The morbidity associated with parathyroidectomy was not treated exclusively in the inpatient setting. Patients who underwent parathyroidectomy experienced more total emergency room or hospital observation visits (378.3 versus 314.3 per 100 patient-years; RR, 1.2; 95% CI, 1.17 to 1.23; Figure 2C) and more emergency room or hospital observation stays with hypocalcemia treatment (48.8 versus 2.4 per 100 patient-years; RR, 20.4; 95% CI, 16.8 to 24.9; Figure 2D). Total outpatient visits with hypocalcemia requiring treatment were also higher (11.9 versus 0.7 per 100 patient-years; RR, 17.0; 95% CI, 11.8 to 24.5). We also evaluated fracture events. Fractures occurred infrequently before parathyroidectomy (n=95) and in the year after parathyroidectomy (n=69; RR, 0.82; 95% CI, 0.60 to 1.12).
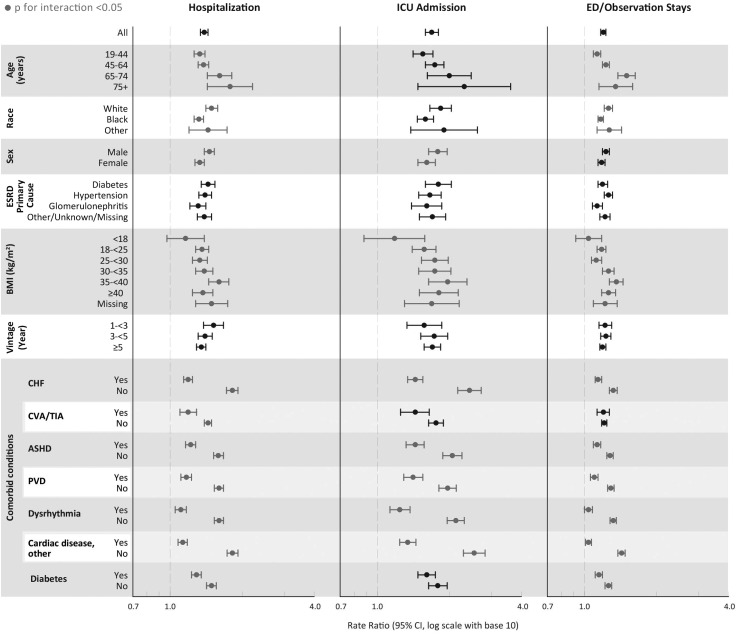
Subgroup Analyses
We performed subgroup analysis by selected patient characteristics (Figure 3). Several subgroups evaluated showed significant interactions with pre-post change in risk of the outcomes of interest (P<0.05). Among the various subgroup evaluated, those that appeared to experience greater change in risk of adverse outcomes included increasing age (hospitalization, emergency department/observation stays), white and other race (hospitalization, emergency department/observation visits), male sex (hospitalization, ICU admissions), a varying association with body mass index (all outcomes), and absence of most comorbid conditions (all outcomes). Finally, we performed a sensitivity analysis stratified by the type of procedure (total versus partial); results did not change materially and are not shown.
Figure 3.
Outcomes by select patient characteristics. Data points in gray represent subgroups in which a significant interaction (P<0.05) exists between the subgroup and parathyroidectomy. Data points in black have a nonsignificant P for interaction (P>0.05). ASHD, atherosclerotic heart disease; BMI, body mass index; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; ICU, intensive care unit; PVD, peripheral vascular disease.
Discussion
In this nationwide study of patients undergoing surgical parathyroidectomy, we observed significant clinical outcomes in the first 30 days and up through 1 year after the procedure. Most pronounced were high rehospitalization rates, more ICU visits, more hospital days, and 2% mortality within 30 days of hospital discharge after the procedure. We specifically evaluated both all-cause events and cause-specific events (i.e., hospitalization with hypocalcemia), because cause-specific outcomes are likely specific but lack sensitivity. The relative increase in adverse outcomes at 1 year varied significantly by selected patient characteristics. Of note, the absence of most comorbid conditions was associated with a greater relative increase in adverse outcomes. We hypothesize that this is due to the already high event rates in patients with comorbid conditions in the year preceding parathyroidectomy, such that a similar absolute increase in events postparathyroidectomy results in a smaller relative increase. These data provide important new evidence regarding adverse clinical outcomes related to surgical parathyroidectomy, which should be recognized when considering this procedure as a treatment option for patients with severe SHPT.
The treatment of uncontrolled PTH elevations remains controversial and the best approach to therapy is unknown. Given the uncertainty regarding the benefits and risks of parathyroidectomy, use of the procedure in the dialysis population has varied substantially over the past few decades, ranging from a high of 12.5 per 1000 patient-years in 1992 to a low of 5.5 per 1000 patient-years in 2005; more recently, the rate was 9 per 1000 patient-years in 2007 (15,16). Similar rates have been observed in other countries (17).
Based in part on observational studies consistently showing higher risk of adverse clinical outcomes in patients whose PTH levels are outside of the range between 2–9 times the upper limit of normal for dialysis patients, KDIGO recommends targeting PTH levels to within this range (3). However, few studies have comprehensively evaluated the risks related to parathyroidectomy. Studies that have examined the issue are reports from potentially high-performing, single surgical centers that have focused primarily on the benefits of parathyroidectomy. Almost uniformly, these studies show an improvement in laboratory parameters.
A small number of studies have evaluated parathyroidectomy using a nationwide cohort of hemodialysis patients. Kestenbaum et al. (5) evaluated the effects of parathyroidectomy on survival using USRDS data (1988–1999) and found that 30-day mortality was elevated for patients who underwent parathyroidectomy compared with those who did not (3.1% versus 1.2%); this elevated risk continued to 90 days and then inverted at 1 year. The 30-day and 1-year mortality they report are somewhat higher than we found (30-day, 3.1% versus 2.0%; 1-year, 17% versus 9.8%). These differences may represent a number of improvements, including greater surgical experience, improved surgical technique, or improved patient selection. Alternatively, they may arise from how the two cohorts were constructed; specifically, we excluded patients with <1 year of dialysis before parathyroidectomy. This exclusion may have biased our results toward a healthier population. Given our present data, we are unable to draw definitive conclusion regarding temporal changes in survival after parathyroidectomy. Finally, Rudser et al. (18) evaluated the risk of fractures after parathyroidectomy in a nationwide cohort, and demonstrated a lower risk of fractures (hip, vertebra, and distal radius-wrist) in patients who underwent parathyroidectomy compared with matched controls.
In the current analysis, we used a pre-post comparison to investigate the risks related to parathyroidectomy. This was primarily motivated by the knowledge that patients who ultimately undergo parathyroidectomy are highly selected (e.g., assessed to be able to survive the surgery), and secondly by the understanding that the direct patient outcomes should be viewed from the perspective of how the patients will contrast their care before and after the surgery. Previous studies have attempted to contrast patients who do and do not undergo parathyroidectomy and control for potential differences through individual or propensity score matching; with such an approach, substantial residual bias likely remains because all prognostic factors that physicians may assess when deciding whether to perform parathyroidectomy are unlikely to be recorded in databases, and thus the validity of these previous results seems questionable. Therefore, it is not surprising that these studies have found that survival in patients selected for parathyroidectomy is better than in a matched population. We were unable to directly assess the potential survival benefit of parathyroidectomy with our study design. We did observe a 30-day mortality of 2%, similar to other dialysis studies (5,19), but substantially higher than in studies evaluating 30-day mortality after parathyroidectomy in a mixed general population (0.11% at 30 days) (2).
Given the presumed benefits of parathyroidectomy on both laboratory parameters and clinical outcomes, a number of cost-effectiveness studies have attempted to compare surgical parathyroidectomy with other therapeutic interventions (20–22). These studies have generally overlooked a significant source of costs for the parathyroidectomy group, specifically increased outpatient utilization and higher risk of hospitalization within the first 30 days and the first year. An analysis by Belozeroff et al. (23) suggested that after accounting for a decrease in drug utilization and an increase in physician encounters, total costs after parathyroidectomy increased by $434 per month. Future analyses assessing costs of management of elevated PTH should account for the increased medical utilization after parathyroidectomy and patient quality of life, in addition to the benefits that have been attributed to parathyroidectomy.
Our study should be evaluated in light of the following limitations. First, our results included only Medicare participants undergoing in-center hemodialysis for at least 1 year before parathyroidectomy. The generalizability of our results to other populations is unknown, although it is possible that broader application of parathyroidectomy would lead to greater use in less appropriate patients, potentially worsening the risk/benefit ratio. Second, the effect of parathyroidectomy on risk of mortality is an important clinical question, but we were unable to evaluate it given our study design. Next, we were unable to determine whether the adverse outcomes observed in the postparathyroidectomy period directly resulted from the parathyroidectomy procedure, whether patients with ESRD in general fare poorly after surgery (24), or whether the outcomes were simply due to receiving maintenance dialysis for an additional year (25). Given the consistency of results across time in dialysis subgroups, the latter seems an unlikely explanation. Finally, because we followed patients for only 1 year, we were unable to evaluate the long-term outcomes after parathyroidectomy.
Our study also has a number of strengths. We used a nationwide cohort of patients receiving dialysis who underwent parathyroidectomy during a contemporary period. Because of the pre-post design, our results are not limited by selection bias. Finally, our study evaluated patient-oriented outcomes and can serve as the basis for counseling patients regarding outcomes should they elect to pursue parathyroidectomy.
Parathyroidectomy is associated with significant morbidity, including more hospitalizations, ICU stays, total hospital days, and emergency department/observation visits, both with and without hypocalcemia treatment. No change was detected in fracture rates up to 1 year after parathyroidectomy, given that fractures occurred infrequently. Mortality during the parathyroidectomy hospitalization and the 30 days immediately after discharge was substantial at 2%. Although we are unable to provide a comprehensive risk-benefit analysis of parathyroidectomy with the present data, the new information we report contributes to the understanding of the risks involved, assisting providers and patients in making informed decisions. Future work such as investigating select patient groups with particularly severe disease or suboptimal outcomes should be undertaken to better understand the risks and benefits of parathyroidectomy in dialysis patients with SHPT.
Disclosures
A.I., J.L., J.B.W., and A.J.C. are employed by the Chronic Disease Research Group, which receives research support from Amgen. K.A.L., T.D., and B.D.B. are employed by Amgen. G.A.B. is employed by Denver Nephrologists, which receives research support from Amgen.
Supplementary Material
Acknowledgments
The authors thank Chronic Disease Research Group colleagues Delaney Berrini for manuscript preparation, Edward Constantini and Susan Everson for figure design, and Nan Booth, MSW, ELS, for manuscript editing.
This study was supported by a research contract from Amgen Inc (Thousand Oaks, CA). The contract provides for the Minneapolis Medical Research Foundation authors to have final determination of manuscript content.
The data reported here were supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03520414/-/DCSupplemental.
See related editorial, “Dysphoria Induced in Dialysis Providers by Secondary Hyperparathyroidism,” on pages 9–11.
References
- 1.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Gupta PK, Smith RB, Gupta H, Forse RA, Fang X, Lydiatt WM: Outcomes after thyroidectomy and parathyroidectomy. Head Neck 34: 477–484, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 5.Kestenbaum B, Andress DL, Schwartz SM, Gillen DL, Seliger SL, Jadav PR, Sherrard DJ, Stehman-Breen C: Survival following parathyroidectomy among United States dialysis patients. Kidney Int 66: 2010–2016, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Trombetti A, Stoermann C, Robert JH, Herrmann FR, Pennisi P, Martin PY, Rizzoli R: Survival after parathyroidectomy in patients with end-stage renal disease and severe hyperparathyroidism. World J Surg 31: 1014–1021, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Naranda J, Ekart R, Pečovnik-Balon B: Total parathyroidectomy with forearm autotransplantation as the treatment of choice for secondary hyperparathyroidism. J Int Med Res 39: 978–987, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Kovacevic B, Ignjatovic M, Zivaljevic V, Cuk V, Scepanovic M, Petrovic Z, Paunovic I: Parathyroidectomy for the attainment of NKF-K/DOQI™ and KDIGO recommended values for bone and mineral metabolism in dialysis patients with uncontrollable secondary hyperparathyroidism. Langenbecks Arch Surg 397: 413–420, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Gagné ER, Ureña P, Leite-Silva S, Zingraff J, Chevalier A, Sarfati E, Dubost C, Drüeke TB: Short- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patients. J Am Soc Nephrol 3: 1008–1017, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Gasparri G, Camandona M, Abbona GC, Papotti M, Jeantet A, Radice E, Mullineris B, Dei Poli M: Secondary and tertiary hyperparathyroidism: Causes of recurrent disease after 446 parathyroidectomies. Ann Surg 233: 65–69, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neonakis E, Wheeler MH, Krishnan H, Coles GA, Davies F, Woodhead JS: Results of surgical treatment of renal hyperparathyroidism. Arch Surg 130: 643–648, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Jofré R, López Gómez JM, Menárguez J, Polo JR, Guinsburg M, Villaverde T, Pérez Flores I, Carretero D, Rodríguez Benitez P, Pérez García R: Parathyroidectomy: Whom and when? Kidney Int Suppl 85: S97–S100, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Saunders BD, Wainess RM, Dimick JB, Doherty GM, Upchurch GR, Gauger PG: Who performs endocrine operations in the United States? Surgery 134: 924–931, discussion 931, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 63[Suppl]: A7, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Kestenbaum B, Seliger SL, Gillen DL, Wasse H, Young B, Sherrard DJ, Weiss NS, Stehman-Breen CO: Parathyroidectomy rates among United States dialysis patients: 1990-1999. Kidney Int 65: 282–288, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Li S, Chen YW, Peng Y, Foley RN, St Peter WL: Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. Am J Kidney Dis 57: 602–611, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Chuang CH, Wang JJ, Weng SF, Chung KM, Chen YP, Huang CC, Yang CM, Chien CC: Epidemiology and mortality among dialysis patients with parathyroidectomy: Taiwan National Cohort Study. J Nephrol 26: 1143–1150, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B: Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol 18: 2401–2407, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sharma J, Raggi P, Kutner N, Bailey J, Zhang R, Huang Y, Herzog CA, Weber C: Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg 214: 400–407, discussion 407–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayan R, Perkins RM, Berbano EP, Yuan CM, Neff RT, Sawyers ES, Yeo FE, Vidal-Trecan GM, Abbott KC: Parathyroidectomy versus cinacalcet hydrochloride-based medical therapy in the management of hyperparathyroidism in ESRD: a cost utility analysis. Am J Kidney Dis 49: 801–813, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Plosker GL: Cinacalcet: A pharmacoeconomic review of its use in secondary hyperparathyroidism in end-stage renal disease. Pharmacoeconomics 29: 807–821, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Komaba H, Moriwaki K, Goto S, Yamada S, Taniguchi M, Kakuta T, Kamae I, Fukagawa M: Cost-effectiveness of cinacalcet hydrochloride for hemodialysis patients with severe secondary hyperparathyroidism in Japan. Am J Kidney Dis 60: 262–271, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Belozeroff V, Cooper K, Hess G, Chang CL: Healthcare use and costs before and after parathyroidectomy in patients on dialysis. BMC Health Serv Res 13: 248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider CR, Cobb W, Patel S, Cull D, Anna C, Roettger R: Elective surgery in patients with end stage renal disease: what’s the risk? Am Surg 75: 790–793, discussion 793, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG: Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int 57: 1176–1181, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.