Abstract
Background and objectives
Elevated parathyroid hormone levels may be associated with adverse clinical outcomes in patients on dialysis. After the introduction of practice guidelines suggesting higher parathyroid hormone targets than those previously recommended, changes in parathyroid hormone levels and treatment regimens over time have not been well documented.
Design, setting, participants, & measurements
Using data from the international Dialysis Outcomes and Practice Patterns Study, trends in parathyroid hormone levels and secondary hyperparathyroidism therapies over the past 15 years and the associations between parathyroid hormone and clinical outcomes are reported; 35,655 participants from the Dialysis Outcomes and Practice Patterns Study phases 1–4 (1996–2011) were included.
Results
Median parathyroid hormone increased from phase 1 to phase 4 in all regions except for Japan, where it remained stable. Prescriptions of intravenous vitamin D analogs and cinacalcet increased and parathyroidectomy rates decreased in all regions over time. Compared with 150–300 pg/ml, in adjusted models, all-cause mortality risk was higher for parathyroid hormone=301–450 (hazard ratio, 1.09; 95% confidence interval, 1.01 to 1.18) and >600 pg/ml (hazard ratio, 1.23; 95% confidence interval, 1.12 to 1.34). Parathyroid hormone >600 pg/ml was also associated with higher risk of cardiovascular mortality as well as all-cause and cardiovascular hospitalizations. In a subgroup analysis of 5387 patients not receiving vitamin D analogs or cinacalcet and with no prior parathyroidectomy, very low parathyroid hormone (<50 pg/ml) was associated with mortality (hazard ratio, 1.25; 95% confidence interval, 1.04 to 1.51).
Conclusions
In a large international sample of patients on hemodialysis, parathyroid hormone levels increased in most countries, and secondary hyperparathyroidism treatments changed over time. Very low and very high parathyroid hormone levels were associated with adverse outcomes. In the absence of definitive evidence in support of a specific parathyroid hormone target, there is an urgent need for additional research to inform clinical practice.
Keywords: CKD, parathyroid hormone, hyperparathyroidism, ESRD, dialysis
Introduction
Morbidity and mortality of patients on maintenance hemodialysis remain excessively high (1). Dysregulation in mineral and bone metabolism may contribute to the development of vascular calcification, cardiovascular disease, and adverse clinical outcomes in this population (2). A cardinal manifestation of these metabolic abnormalities is the increase in parathyroid hormone (PTH) levels (secondary hyperparathyroidism [SHPT]) that starts in the early stages of CKD. By the time of dialysis start, most patients have hyperplasia of the parathyroid glands (3) and markedly elevated PTH levels (4), which tend to become higher with longer duration of RRT (5,6). Several observational studies indicate that high PTH levels may be associated with increased mortality in this population (7–14), although this association was not found in a recent meta-analysis (15). Recent clinical practice guidelines (16,17) indicate a PTH target level that is higher than what was previously recommended (18) (Table 1), suggesting continued uncertainty. The extent to which PTH levels and treatment regimens for SHPT have changed over time since introduction of these guidelines has not been well documented.
Table 1.
Parathyroid hormone targets indicated by professional organizations guidelines
| Professional Organization | Year Published | PTH Target Level (pg/ml) |
|---|---|---|
| Europe | ||
| European Renal Association–European Dialysis and Transplant Association (42) | 2000 | 85–170 |
| United Kingdom Renal Association (43) | 2002 | <4 times upper normal rangea |
| North America | ||
| National Kidney Foundation (18) | 2003 | 150–300 |
| Canadian Society of Nephrology (44) | 2006 | 100–500 |
| Kidney Disease Outcomes Quality Initiative United States commentary (17) | 2010 | 130–600 |
| Australia/New Zealand | ||
| Australian and New Zealand Society of Nephrology (45,46) | 2006 | 1–3 times upper normal rangeb |
| Japan | ||
| Japanese Society for Dialysis Therapy (35) | 2008 | 60–180 |
| Japanese Society for Dialysis Therapy (36) | 2012 | 60–240 |
| Worldwide | ||
| Kidney Disease Improving Global Outcomes (16) | 2009 | 2–9 times upper normal rangec |
For example, <260 pg/ml on the basis of an upper limit of the normal range of 65 pg/ml.
For example, this could be approximated to 65–195 pg/ml on the basis of an upper limit of the normal range of 65 pg/ml.
For example, this could be approximated to 130–585 pg/ml on the basis of an upper limit of the normal range of 65 pg/ml.
On the basis of data from the international Dialysis Outcomes and Practice Patterns Study (DOPPS), we report trends in PTH levels and SHPT therapies over the past 15 years and the associations of PTH with mortality and hospitalization.
Materials and Methods
Patients and Data Collection
The DOPPS is an international prospective cohort study of patients on in-center hemodialysis who are ≥18 years of age, which is currently in its fifth study phase (DOPPS 5) (19,20). At each phase, a new random sample of chronic hemodialysis facilities was selected, and within each participating facility, an updated census of patients on prevalent in-center hemodialysis was used to select at random 20–40 patients. Study approval was obtained by a central institutional review board and local ethics committees as required. Demographics and comorbid conditions were obtained at study entry. Data on monthly laboratory values, medication prescription, hospitalization, and death were abstracted from patient records at baseline and every 4 months. For the majority of the study period, intact PTH was the only assay available for clinical practice; <10% of DOPPS facilities reported using biointact PTH assays.
The primary analysis included DOPPS participants who had been on dialysis for >90 days, with at least one PTH value, and no history of parathyroidectomy. Given the limited follow-up time available for participants in this study phase, we only present DOPPS 5 data when describing trends in PTH levels and SHPT treatments that were assessed at the start of each study phase (Figures 1–3). A subgroup analysis was restricted to patients who did not receive active vitamin D analogs or cinacalcet and did not undergo parathyroidectomy for 12 months after study entry (untreated patients; n=5387).
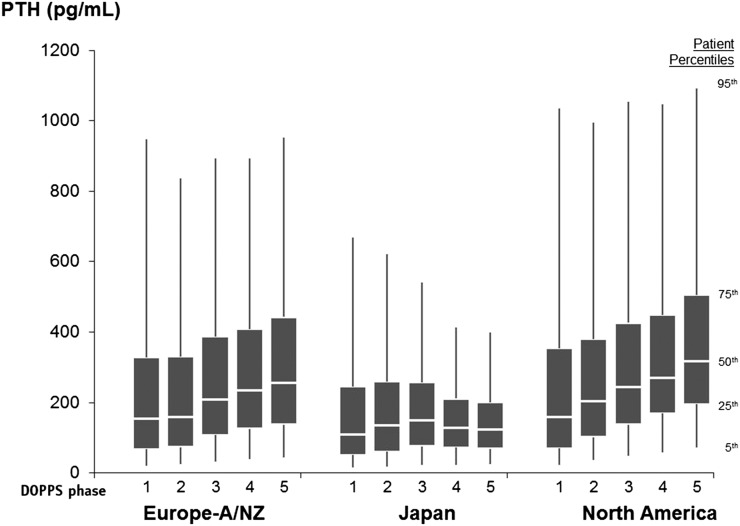
Figure 1.
Parathyroid hormone (PTH) levels by Dialysis Outcomes and Practice Patterns Study region and phase. Eur-A/NZ, Australia, Belgium, France, Germany, Italy, New Zealand, Spain, Sweden, and the United Kingdom; North America, Canada and the United States; phase 1, 1996–2001; phase 2, 2002–2004; phase 3, 2005–2008; phase 4, 2009–2011; phase 5, 2012 to present.
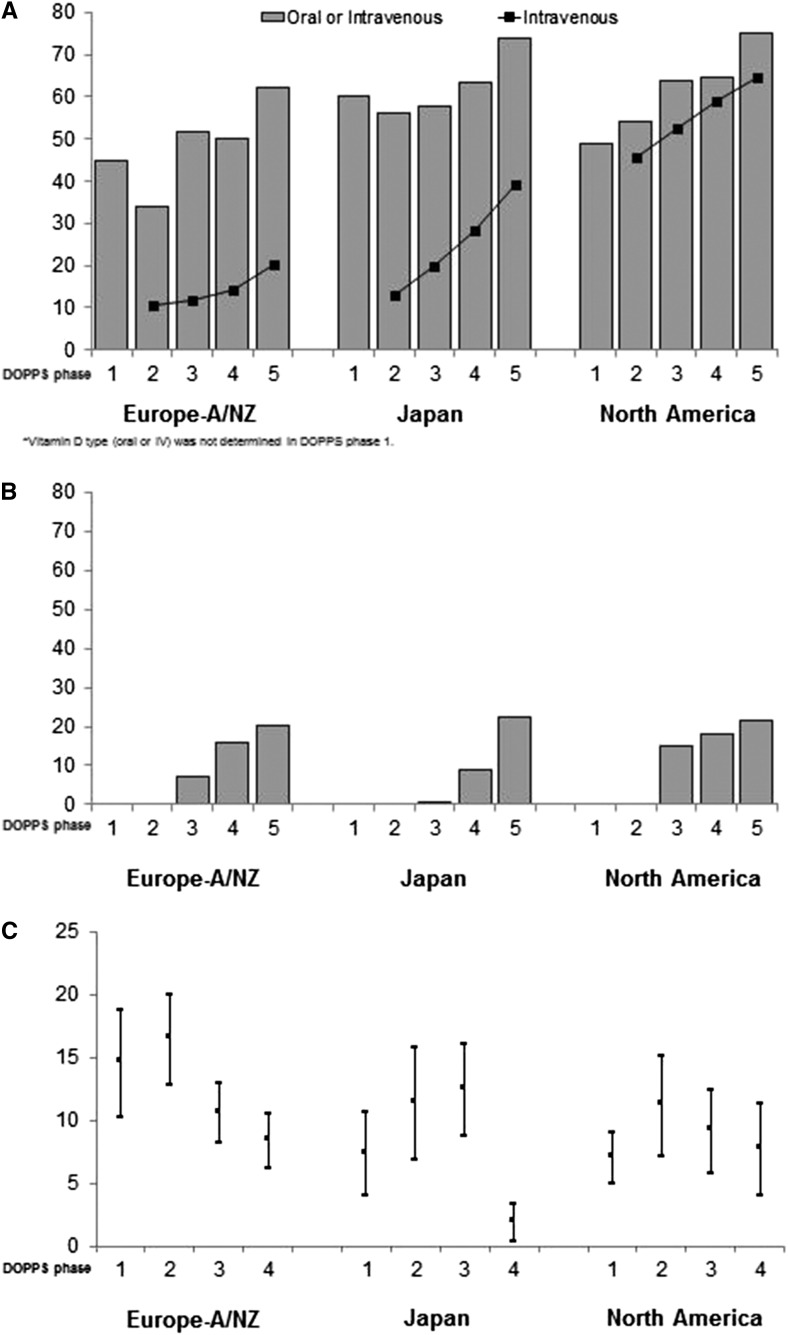
Figure 3.
Secondary hyperparathyroidism treatment regimens by Dialysis Outcomes and Practice Patterns Study (DOPPS) region and phase. Cinacalcet became available starting with DOPPS 3. Parathyroidectomy rates are on the basis of events reported during the study period and are not provided for DOPPS 5 given the limited follow-up time. Error bars correspond to 95% confidence intervals for the parathyroidectomy rates. (A) Active Vitamin D prescription (% of patients; (B) Cinacalcet prescription (% of patients); (C) Parathyroidectomy rate (per 1000 patient years). Eur-A/NZ includes Australia, Belgium, France, Germany, Italy, New Zealand, Spain, Sweden, and the United Kingdom; North America includes Canada and the United States; phase 1, 1996–2001; phase 2, 2002–2004; phase 3, 2005–2008; phase 4, 2009–2011; phase 5, 2012 to present.
Exposures and Outcomes
The main predictor was the first PTH value within 1 year of study start or after the 12-month period without SHPT treatment (untreated analysis). In sensitivity analyses, we used the median PTH during an extended baseline period of the first 3 months, 6 months, and 1 year after study start to allow for a prolonged exposure. Outcomes included all-cause and cardiovascular mortality and all-cause and cardiovascular hospitalization. Patients (n=696; 2% of the study sample) who withdrew from dialysis therapy were counted as death events. Cause of death was available for all countries and DOPPS phases, except for a subset of United States patients in phase 4.
Statistical Analyses
Standard descriptive statistics were used to describe the study cohort and patient characteristics. The association between PTH and mortality or hospitalization was assessed in Cox models stratified by country and phase accounting for facility clustering and adjusted for listed patient characteristics. The proportional hazards assumption for all model covariates was confirmed by plotting log–log survival curves.
To partially account for unmeasured confounders that may affect the relationship between PTH level and outcomes, we also conducted instrumental variable analyses using an extended two-stage residual inclusion approach, with dialysis facility as the instrument (21–23). Because facility-level confounding may occur, adjustments were made for all variables included in the standard Cox model plus four facility-level covariates (facility percentage using a catheter, percentage with albumin <3.5 g/dl, percentage with phosphorus >5.5 mg/dl, and mean hemoglobin) previously associated with mortality (Tentori et al., unpublished data, abstract presented at the American Society of Nephrology, Atlanta, Georgia, November 5–10, 2013) (12,24). We performed three separate instrumental variable analyses using different cutpoints to dichotomize PTH values: (model 1) >600 versus ≤600 pg/ml, (model 2) >450 versus ≤450 pg/ml, and (model 3) >300 versus ≤300 pg/ml. The stage 1 F statistics, which test the strength of the instrument, were 2.7, 3.3, and 3.9 for models 1–3, respectively.
Overall, percentages of missing data were low (<10% for most covariates; <15% for all); missing data were imputed using IVEware (25). All analyses used SAS 9.2 (SAS Institute, Cary, NC).
Results
Study Sample
The primary analysis included 35,655 DOPPS participants in phases 1–4 (1996–2011). The median length of follow-up was 1.61 (interquartile range=0.83–2.28) years; 20,651 (58%) patients were censored because of study end, 8137 (23%) patients died, 333 (1%) patients switched modality, 4119 (12%) patients transferred to another facility, 2073 (6%) patients received a kidney transplant, and the rest (<1%) were censored because of other reasons. 8164 patients from phase 5 were included in the trend analysis of PTH and SHPT treatments assessed at baseline in addition to patients from phases 1–4, but they were not included in mortality analyses because of the limited follow-up information available at this time.
Patient demographics and clinical characteristics over DOPPS phases (Table 2) and baseline PTH levels (Table 3) are shown.
Table 2.
Patient demographics and clinical characteristics by Dialysis Outcomes and Practice Patterns Study phase
| Patient Characteristics | DOPPS Phase | |||
|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | |
| Number of patients | 7615 | 7142 | 8559 | 12,339 |
| Age, yr | 59.7±14.9 | 62.6±14.5 | 63.9±14.2 | 64.1±14.7 |
| Men | 57.0 | 57.7 | 58.9 | 59.1 |
| Black | 20.8 | 10.0 | 7.9 | 14.2 |
| Body mass index, kg/m2 | 23.9±5.3 | 24.6±5.6 | 25.0±5.8 | 26.6±6.5 |
| Time on dialysis, yra | 2.8 [1.2–5.8] | 2.9[1.3–5.6] | 2.5 [0.9–5.8] | 2.6 [0.9–5.5] |
| Fistula | 52.6 | 62.5 | 65.7 | 62.5 |
| Graft | 31.0 | 16.1 | 10.4 | 10.7 |
| Catheter | 11.2 | 16.1 | 18.9 | 23.0 |
| Diabetes | 29.9 | 31.3 | 35.4 | 41.8 |
| Coronary arterial disease | 38.0 | 46.1 | 52.5 | 36.5 |
| Congestive heart failure | 32.1 | 29.8 | 40.1 | 28.6 |
| Cerebrovascular disease | 15.4 | 17.5 | 17.9 | 14.4 |
| Peripheral arterial disease | 22.3 | 26.8 | 28.8 | 26.1 |
| Hypertension | 76.3 | 78.8 | 81.2 | 81.5 |
| Other cardiovascular disease | 33.4 | 35.6 | 37.2 | 26.5 |
| Recurrent cellulitis | 7.8 | 8.2 | 8.5 | 9.9 |
| Cancer (other than skin) | 8.1 | 11.7 | 13.0 | 12.2 |
| Gastrointestinal bleeding | 7.2 | 6.0 | 5.4 | 4.4 |
| Lung disease | 9.7 | 11.2 | 12.9 | 12.5 |
| Neurologic disease | 8.3 | 11.4 | 11.7 | 10.2 |
| Psychiatric disorder | 20.7 | 19.7 | 11.9 | 15.9 |
| Hemoglobin, g/dl | 10.6±1.6 | 11.3±1.6 | 11.5±1.5 | 11.3±1.4 |
| Serum albumin, g/dl | 3.8±0.5 | 3.7±0.5 | 3.8±0.5 | 3.7±0.5 |
| Serum creatinine, mg/dl | 10.0±3.2 | 9.2±3.0 | 8.8±3.0 | 8.4±3.0 |
| Serum calcium, mg/dl | 9.4±0.9 | 9.4±0.9 | 9.1±0.8 | 9.0±0.8 |
| Serum phosphorus, mg/dl | 5.8±1.8 | 5.6±1.8 | 5.3±1.7 | 5.2±1.6 |
| Parathyroid hormone, pg/mla | 149 [64–324] | 169 [78–333] | 203 [106–366] | 236 [131–401] |
| Phosphate binder (any)b | 84.8 | 86.2 | 85.1 | 80.3 |
| Phosphate binder (calcium-based) | NA | 67.5 | 57.3 | 47.5 |
| Phosphate binder (noncalcium-based) | NA | 17.7 | 27.3 | 49.4 |
| Untreated patients | 23.2 | 30.1 | 16.8 | 13.0 |
Data are shown as mean±SD or percentage. Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 1: 1996–2001; phase 2: 2002–2004; phase 3: 2005–2008; phase 4: 2009–2011. NA, not applicable.
Median [interquartile range].
Includes calcium-based, noncalcium-based, or both. Type of phosphate binder (calcium-based or noncalcium-based) was not available in DOPPS phase 1.
Table 3.
Patient demographics and clinical characteristics by parathyroid hormone level at study entry
| Patient Characteristics | Parathyroid Hormone at DOPPS enrollment (pg/ml)a | |||||
|---|---|---|---|---|---|---|
| <50 | 50–149 | 150–300 | 301–450 | 451–600 | >600 | |
| Patients, n (%) | 4111 (12) | 9770 (27) | 10,340 (29) | 4849 (14) | 2545 (7) | 4040 (11) |
| Age, yr | 64.1±14.1 | 64.7±14.0 | 64.0±14.3 | 62.0±14.9 | 60.5±14.9 | 56.2±15.7 |
| Men | 58.8 | 59.7 | 59.0 | 57.9 | 55.5 | 55.4 |
| Black | 6.3 | 8.2 | 13.4 | 16.7 | 18.3 | 25.2 |
| Body mass index, kg/m2 | 23.6±5.2 | 24.5±5.6 | 25.5±6.0 | 26.2±6.4 | 26.3±6.3 | 26.5±6.7 |
| Time on dialysis, yrb | 2.3 [1.0–4.9] | 2.4 [1.0–5.1] | 2.5 [1.0–5.3] | 2.7 [1.1–5.9] | 3.2 [1.2–6.6] | 4.0 [1.6–7.5] |
| Fistula | 64.8 | 65.0 | 64.8 | 63.9 | 61.9 | 58.4 |
| Graft | 17.6 | 16.5 | 16.0 | 16.8 | 16.3 | 18.7 |
| Catheter | 16.9 | 17.9 | 18.6 | 19.0 | 21.4 | 22.2 |
| Diabetes | 39.2 | 41.6 | 43.9 | 42.9 | 38.7 | 32.6 |
| Coronary artery disease | 35.0 | 36.0 | 37.8 | 36.8 | 33.0 | 28.0 |
| Congestive heart failure | 32.2 | 32.9 | 32.4 | 32.1 | 33.9 | 30.9 |
| Cerebrovascular disease | 18.7 | 18.1 | 15.9 | 15.0 | 14.4 | 11.7 |
| Peripheral arterial disease | 27.5 | 27.5 | 27.1 | 25.4 | 25.3 | 20.1 |
| Hypertension | 78.0 | 79.6 | 80.8 | 80.3 | 80.5 | 79.5 |
| Other cardiovascular disease | 34.9 | 34.5 | 32.2 | 30.3 | 30.9 | 27.6 |
| Recurrent cellulitis | 8.8 | 9.0 | 9.0 | 9.1 | 9.7 | 7.3 |
| Cancer (other than skin) | 11.4 | 12.8 | 11.9 | 10.5 | 10.5 | 8.6 |
| Gastrointestinal bleeding | 6.9 | 6.2 | 5.0 | 5.5 | 5.6 | 4.3 |
| Lung disease | 10.7 | 12.3 | 12.1 | 11.9 | 11.8 | 10.7 |
| Neurologic disease | 10.9 | 10.9 | 10.2 | 9.7 | 9.8 | 10.2 |
| Psychiatric disorder | 16.3 | 16.9 | 15.6 | 16.9 | 18.3 | 18.3 |
| Hemoglobin, g/dl | 10.9±1.5 | 11.2±1.5 | 11.3±1.5 | 11.3±1.5 | 11.3±1.5 | 11.3±1.6 |
| Serum albumin, g/dl | 3.7±0.5 | 3.7±0.5 | 3.8±0.5 | 3.8±0.4 | 3.8±0.5 | 3.8±0.5 |
| Serum creatinine, mg/dl | 8.9±3.1 | 8.7±3.1 | 8.7±3.1 | 9.0±3.1 | 9.3±3.0 | 9.9±3.3 |
| Serum calcium, mg/dl | 9.5±0.9 | 9.2±0.8 | 9.1±0.8 | 9.1±0.9 | 9.1±0.9 | 9.2±0.9 |
| Serum phosphorus, mg/dl | 5.2±1.7 | 5.2±1.6 | 5.3±1.6 | 5.6±1.7 | 5.8±1.7 | 6.2±1.9 |
| Phosphate binder (any)c | 84.6 | 82.6 | 83.2 | 84.4 | 85.0 | 85.7 |
| Phosphate binder (calcium-based) | 68.5 | 61.3 | 54.5 | 51.6 | 48.6 | 45.9 |
| Phosphate binder (noncalcium-based) | 19.0 | 25.9 | 34.7 | 40.0 | 43.9 | 49.1 |
| Vitamin D analog (i.v. or oral)d | 40.1 | 42.8 | 56.1 | 64.4 | 66.4 | 63.4 |
| Vitamin D analog (i.v.)d | 7.9 | 17.1 | 33.3 | 41.8 | 42.4 | 41.5 |
| Vitamin D analog (oral)d | 30.2 | 26.4 | 25.2 | 25.5 | 28.4 | 24.3 |
| Cinacalcete | 1.4 | 3.7 | 6.5 | 10.2 | 12.9 | 18.7 |
| Untreated patients | 34.1 | 28.2 | 17.2 | 11.2 | 9.1 | 7.7 |
Data are shown as mean±SD or percentage. All patient characteristics as reported at entry into the Dialysis Outcomes and Practice Patterns Study (DOPPS).
Parathyroid hormone values were on the basis of the first nonmissing values within 1 year since baseline.
Median [interquartile range].
Includes calcium-based, noncalcium-based, or both. Type of phosphate binder (calcium-based or noncalcium-based) was not available in DOPPS phase 1.
Includes calcitriol, doxercalciferol, paricalcitol, maxacalcitol, alphacalcidol, and falecalsitol. Type of vitamin D (i.v. or oral) was not available in DOPPS phase 1.
Cinacalcet was available in clinical practice in DOPPS phases 3 and 4 only.
Trends in PTH Levels and SHPT Treatments
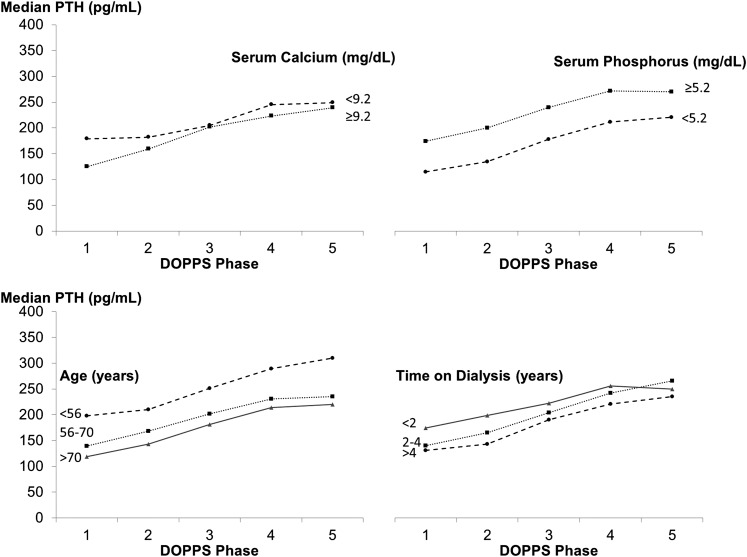
PTH levels varied within each region and study phase (Figure 1). Median PTH increased from DOPPS 1 to DOPPS 5 in Europe and Australia/New Zealand (Eur-A/NZ; from 153 to 252 pg/ml; P value for trend <0.001) and North America (from 160 to 318 pg/ml; P value for trend <0.001). In Japan, median PTH was stable over the study period. A similar rise in PTH was observed among patients with lower/higher serum calcium and phosphorus and across categories of age and time on dialysis (Figure 2).
Figure 2.
Parathyroid hormone (PTH) levels by Dialysis Outcomes and Practice Patterns Study (DOPPS) phase and selected patient characteristics. Serum calcium and phosphorus categories indicate levels below and above the median. phase 1, 1996–2001; phase 2, 2002–2004; phase 3, 2005–2008; phase 4, 2009–2011; phase 5, 2012 to present.
SHPT treatments also changed over time (Figure 3). In general, prescription of vitamin D analogs, especially intravenous analogs (P<0.001), and cinacalcet (available starting with DOPPS 3; P<0.001) increased, whereas parathyroidectomies declined over time (P<0.001). PTH target levels reported by medical directors are shown in Table 4.
Table 4.
Upper limit of parathyroid hormone target levels as reported by dialysis unit medical directors
| Phase and Region | N facility | Upper Limit of Parathyroid Hormone Target Level | |||||
|---|---|---|---|---|---|---|---|
| Median | 50–199 (%) | 200–299 (%) | 300–399 (%) | 400–599 (%) | 600+ (%) | ||
| EUR-A/NZ | |||||||
| 2 | 122 | 233 | 26.2 | 41.8 | 15.6 | 13.1 | 3.3 |
| 3 | 139 | 300 | 11.5 | 24.5 | 53.2 | 5.0 | 5.8 |
| 4 | 142 | 300 | 5.6 | 13.4 | 43.0 | 23.9 | 14.1 |
| 5 | 108 | 450 | 4.6 | 6.5 | 28.7 | 36.1 | 24.1 |
| Japan | |||||||
| 2 | 54 | 300 | 5.6 | 42.6 | 35.2 | 14.8 | 1.9 |
| 3 | 60 | 300 | 21.7 | 23.3 | 43.3 | 6.7 | 5.0 |
| 4 | 56 | 180 | 60.7 | 21.4 | 8.9 | 1.8 | 7.1 |
| 5 | 57 | 300 | 3.5 | 43.9 | 43.9 | 8.8 | 0 |
| North America | |||||||
| 1a | 77 | 250 | 6.5 | 50.6 | 39.0 | 1.3 | 2.6 |
| 2 | 75 | 250 | 30.7 | 32.0 | 28.0 | 8.0 | 1.3 |
| 3 | 77 | 300 | 24.7 | 14.3 | 58.4 | 2.6 | 0 |
| 4 | 130 | 300 | 1.5 | 4.6 | 73.8 | 8.5 | 11.5 |
| 5 | 84 | 500 | 1.2 | 0 | 33.3 | 22.6 | 42.9 |
Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 1: 1996–2001; phase 2: 2002–2004; phase 3: 2005–2008; phase 4: 2009–2011. Eur-A/NZ, Australia, Belgium, France, Germany, Italy, New Zealand, Spain, Sweden, and the United Kingdom; North America, Canada and the United States.
Data on parathyroid hormone targets in DOPPS 1 were collected in the United States only.
Association of PTH with Adverse Clinical Outcomes
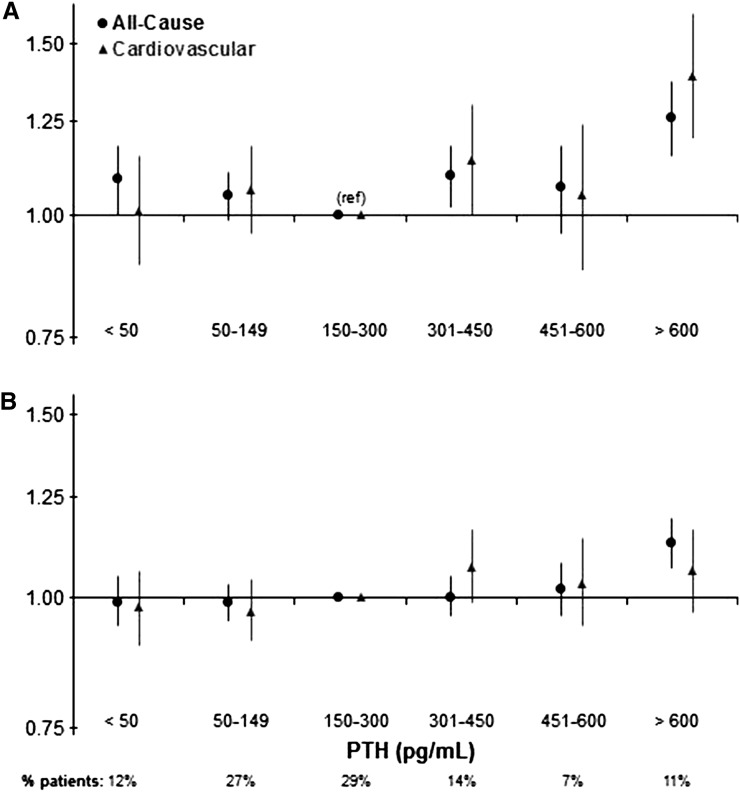
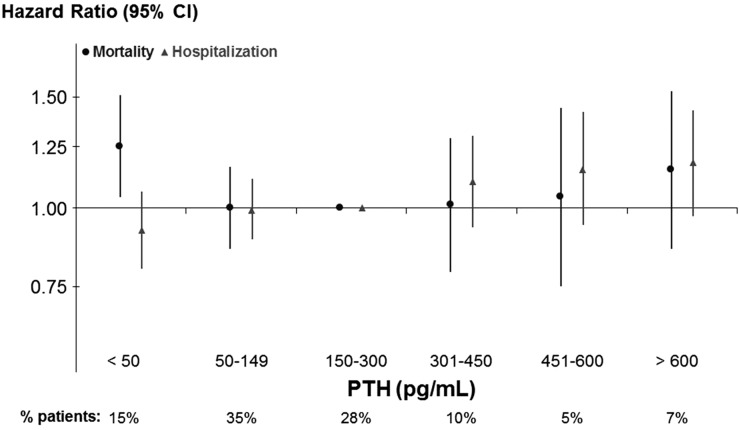
Mortality risk was higher for patients with PTH=301–450 (hazard ratio [HR], 1.09; 95% confidence interval [95% CI)], 1.01 to 1.18; P=0.02) and >600 pg/ml (HR, 1.23; 95% CI, 1.12 to 1.34; P<0.001) compared with the 150–300 pg/ml reference group. A similar pattern was observed for cardiovascular mortality. PTH>600 pg/ml was also associated with higher risk of hospitalization (Figure 4).
Figure 4.
Associations of parathyroid hormone (PTH) levels with mortality and hospitalizations among all Dialysis Outcomes and Practice Patterns Study participants. Models are adjusted for age, sex, body mass index, time on dialysis, catheter use, comorbidities, albumin, hemoglobin, creatinine, calcium, phosphorus, phosphate binder, vitamin D analog, and cinacalcet prescription. (A) mortality: all cause, n=35,601; deaths, n=8112; cardiovascular, n=31,739; deaths, n=2750. (B) hospitalization: all cause, n=35,585; hospital admissions, n=19,149; cardiovascular, n=31,724; hospital admissions, n=7348. 95% CI, 95% confidence interval; ref, reference.
Similar results were obtained in models adjusted for a more limited number of covariates (e.g., not adjusted for serum calcium, phosphorus, and SHPT therapies). Results were consistent in a sensitivity analysis that excluded Japan, where PTH values were much lower. We tested interactions with each of the PTH categories and potential effect modifiers. The association between PTH<50 pg/ml and mortality was even stronger among patients with diabetes and lower body mass index (P value for interaction=0.03 for both). No interaction was found with serum albumin or black race.
In analyses using median PTH values during baseline time intervals as the predictor, patients with median PTH>600 pg/ml had higher mortality compared with the 150–300 pg/ml group (median PTH at months 1–3: HR, 1.20; 95% CI, 1.09 to 1.32; months 1–6: HR, 1.14; 95% CI, 1.03 to 1.27; months 1–12: HR, 1.21; 95% CI, 1.06 to 1.37 respectively).
In instrumental variable analyses, higher mortality was observed for PTH>600 pg/ml (versus ≤600 pg/ml); the association remained positive but was somewhat weaker at lower PTH cutpoints (Table 5). Although the effect size was larger in the instrumental variable models than in the standard Cox models, the 95% CIs were wider because of reduced precision with the instrumental variable approach (26).
Table 5.
Association of high parathyroid hormone levels with mortality
| Models | i.v. Method | Standard Cox Models | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value | |
| Parathyroid hormone >300 pg/ml (versus ≤300) | ||||
| Adjustment Aa | 1.24 (0.99 to 1.55) | 0.06 | 1.12 (1.05 to 1.18) | <0.001 |
| Adjustment Bb | 1.11 (0.88 to 1.39) | 0.38 | 1.09 (1.03 to 1.15) | 0.004 |
| Parathyroid hormone >450 pg/ml (versus ≤450) | ||||
| Adjustment Aa | 1.44 (1.08 to 1.91) | 0.01 | 1.15 (1.08 to 1.23) | <0.001 |
| Adjustment Bb | 1.27 (0.95 to 1.69) | 0.10 | 1.10 (1.03 to 1.18) | 0.005 |
| Parathyroid hormone >600 pg/ml (versus ≤600) | ||||
| Adjustment Aa | 1.62 (1.12 to 2.35) | 0.02 | 1.22 (1.13 to 1.32) | <0.001 |
| Adjustment Bb | 1.44 (1.00 to 2.08) | 0.05 | 1.17 (1.08 to 1.27) | <0.001 |
Adjusted for patient age, sex, body mass index, time on dialysis, catheter use, comorbidities, albumin, hemoglobin, creatinine, facility percentage of patients with catheter use, percentage of patients having albumin <3.5 g/dl, and mean hemoglobin.
Also adjusted for additional covariates that may be intermediary in the pathway: patient calcium, phosphorus, phosphate binder, vitamin D analog, cinacalcet prescription, and facility percentage of patients having phosphorus >5.5 mg/dl.
To assess the association of PTH with outcomes independent of the potential effect of SHPT treatments, we tested the association between PTH and outcomes in untreated patients (Figure 5). Very low PTH (<50 pg/ml) was associated with mortality (HR, 1.25; 95% CI, 1.04 to 1.51); PTH>600 pg/ml also tended to be associated with mortality (HR, 1.15; 95% CI, 0.86 to 1.53), but the number of untreated patients was small (350 [7%]), limiting statistical power. A positive monotonic association between PTH and hospitalization was found in untreated patients.
Figure 5.
Associations of parathyroid hormone (PTH) levels with mortality and hospitalizations among Dialysis Outcomes and Practice Patterns Study participants not receiving secondary hyperparathyroidism treatment during the first 1 year of study observation. Adjusted for demographics, comorbidities, albumin, hemoglobin, calcium, and phosphorus. Outcomes are in relationship to the patient’s first reported PTH after 1 year of study observation without prescription for PTH-controlling medications. Mortality: 5387 patients, 1061 deaths. Hospitalization: 5381 patients, 2258 hospitalizations.
In models using linear PTH as the predictor, higher PTH was associated with hospitalization among all patients (HR, 1.02 for every 100 pg/ml; 95% CI, 1.01 to 1.02; P<0.001) and untreated patients (HR, 1.03; 95% CI, 1.01 to 1.04; P<0.01). Finally, the association of low and high PTH with mortality and hospitalization was very consistent in patients receiving SHPT treatments compared with the overall study sample (data not shown).
Discussion
Using international DOPPS data, we reported trends in PTH and SHPT treatments over the past 15 years and confirmed the association between abnormalities in PTH levels and adverse clinical outcomes.
To our knowledge, this study is the first to show a sharp increase in PTH levels across most regions. Overall, 11% of study participants had a PTH>600 pg/ml, a level that has been associated with adverse clinical outcomes in most published studies (7–14). PTH>600 pg/ml was also relatively common (7%) among patients who did not receive any SHPT treatment for 1 year, indicating that some clinicians opt not to treat patients for a prolonged period of time. This is particularly important, because when left untreated, SHPT is often a progressive disorder (3). This is supported by the fact that, like in prior analyses (12), patients with higher PTH had longer duration of dialysis.
Given the observed change in patients’ characteristics over time (Table 2), we investigated whether they may have contributed to the rise in PTH. For example, lower serum calcium levels may contribute to higher PTH levels, whereas lower serum phosphorus would have lowered PTH levels. However, PTH rose among patients with both higher and lower serum calcium and phosphorus. One could also postulate that longer duration of kidney disease in older patients may have contributed. Again, the rise in PTH was observed across all categories of age and time on dialysis. Biologically, it is not likely that observed trends in other patient characteristics (e.g., vascular access type and diabetes) drove the rise in PTH. In contrast with PTH levels in Eur-A/NZ and North America, PTH levels did not increase over time in Japan; in fact, the proportion of patients with very high PTH decreased. These findings reflect the strikingly lower PTH targets in Japan (Table 1) and are consistent with the increased achievement of PTH targets reported in the Japanese dialysis population (27).
Despite increased PTH levels, rates of parathyroidectomy declined in all regions over the study period. At each time point, parathyroidectomy rates reported in the US DOPPS sample were consistent with prior analyses of Medicare beneficiaries (28,29). Availability of intravenous vitamin D analogs (which may be more effective than the oral formulation) and the introduction of cinacalcet (30,31) likely contributed to the sharp decline in parathyroidectomy rates. New clinical practice guidelines recommending higher PTH targets and specifying indications for parathyroid surgery (16,18) may also have contributed to the parathyroidectomy decline.
The rise in cinacalcet and intravenous vitamin D analogs prescriptions is consistent with published region-specific data (32,33). Given the recent results of the Evaluation of Cinacalcet Hydrochloride Therapy to Lower CardioVascular Events Trial (34), in which cinacalcet treatment produced only an ambiguous and at most, modest effect on clinical outcomes, it will be interesting to continue to monitor potential changes in SHPT treatments.
In parallel with the decline in parathyroidectomies, we have observed increases in the use of intravenous vitamin D analogs and cinacalcet, which are effective in lowering PTH levels, that are shown by the fact that prevalence of very high PTH (e.g., >600 pg/ml) remained relatively stable over time. Hence, it is not likely that changes in therapeutic approaches per se were the main contributors to the rise in PTH. Rather, it seems that clinicians’ attitudes and overall management of SHPT may have changed. Given the lack of strong evidence to advocate for tighter control, clinicians may have grown to accept relatively higher PTH targets, and this may have resulted in the higher PTH levels. This is supported by the fact that medical directors reported adopting more liberal PTH targets (Table 4). Interestingly, the shift to higher PTH targets occurred before the publication of Kidney Disease Improving Global Outcomes (KDIGO) guidelines in 2009, thus reflecting clinicians’ attitudes and real-world practice as opposed to specific clinical practice guidelines. Along this line is also the high proportion of medical directors in the current DOPPS phase (24.1% in Eur-A/NZ and 42.9% in North America) who target an upper limit of PTH≥600 pg/ml, exceeding the current KDIGO target, which was on the basis of a low level of evidence (16). Consistent with local guidelines (35,36) in Japan, reported PTH targets declined through DOPPS 4 and increased slightly in DOPPS 5.
Considering changes in SHPT, it is interesting to report the high proportion of patients with low PTH who were prescribed SHPT therapies (43% of those with PTH<150 pg/ml and 41% of those with PTH<50 pg/ml), given the potential for excessive treatment to contribute to the development of adynamic bone disease.
The rise in PTH should be considered in the context of the possible association of higher PTH with adverse clinical outcomes. In this study, elevated PTH (starting at levels as low as 300 pg/ml) was associated with increased risk of death and hospitalization. These findings are consistent with results of prior analyses of large databases of patients with ESRD (7–14). Although mechanisms are incompletely understood, elevated PTH may exacerbate hypercalcemia and hyperphosphatemia (37), which in turn, contribute to soft tissue calcification, development of cardiovascular disease, and adverse clinical outcomes.
Results from an instrumental variable analysis (21–23) support the association between higher PTH and mortality. Because the effect sizes were larger in the instrumental variable analyses than in the standard Cox regression, the direction of bias caused by unmeasured confounders may have been toward the null, and thus, Cox models may have underestimated the effect of high PTH on mortality. Given that a randomized trial assessing the effect of different PTH levels on clinical outcomes has not been conducted, our instrumental variable analyses results provide some additional evidence within the realm of an observational study.
Consistent with prior reports (10,14,38), very low PTH (<50 pg/ml) was also associated with adverse outcomes. Such low PTH levels may contribute to overall poor health, which may result in increased morbidity and mortality. Additionally, the significant interaction between low PTH and body mass index is consistent with prior reports (39) and indicates potentially different effects in patients with different nutritional status. Furthermore, low PTH levels contribute to the development of adynamic bone disease and may increase the risk of cardiovascular calcifications through increased calcium deposition in soft tissues (40).
We note that a recent meta-analysis (15) concluded that there is not enough evidence supporting the association between PTH levels and mortality. However, Palmer et al. (15) treated PTH solely as a continuous variable (i.e., evaluating for a linear association of PTH with mortality). Results of this analysis and prior analyses (10,14,38) suggest that the relationship between PTH and adverse outcomes is not linear but rather, described by a U or J curve, with PTH thresholds below (e.g., <50 pg/ml) and above (e.g., >300 pg/ml) which risks may be increased.
Consistent with our main findings, in analyses of patients who did not receive any SHPT treatments, mortality risk was higher for PTH<50 pg/ml, with a nonsignificant trend for PTH>600 pg/ml. These estimates are useful, because they are not biased by the effect of SHPT treatments or treatment responsiveness. Additionally, a sizable percentage of patients untreated for 1 year had PTH>600 pg/ml, indicating an opportunity for improvement of clinical practice.
Although we fully endorse the notion that treatment of SHPT should be on the basis of evaluation and management of combinations of all serum markers of mineral bone disorder, including serum calcium and phosphorus (41) and possibly, fibroblast growth factor 23, our findings are particularly timely, because they show a robust association of high PTH with adverse outcomes at a time when mean PTH levels are rising internationally.
The DOPPS study design allowed us to present trends in SHPT and associations of PTH with outcomes in a cohort of real world patients, similar to what a clinician may encounter when rounding in the dialysis unit. However, we must acknowledge some important limitations. Multiple assays are available for measurement of intact PTH, and large interassay variability may have contributed to misclassification of specific patients. Assuming that this variation is random with respect to patient characteristics, this would tend to bias associations of PTH with mortality to the null. Additionally, during a portion of the study period, assays for measurement of biointact PTH were also available and may have contributed to the large variability in PTH levels. However, because the biointact assay results in lower levels compared with the intact PTH assay, this would not have contributed to the rise in PTH. Lower PTH levels measured with the biointact assay may actually have led to an underestimation of the strength of the association between PTH and clinical outcomes. Finally, this association was consistent in models restricted to dialysis units that used intact PTH assays only.
As with any observational study, the reported associations do not prove causality and may be affected by unmeasured confounders. However, on the basis of the available data, patients with high PTH were younger, were more likely to be black, and had fewer comorbidities; hence, it is not likely that differences in health status drove the association between high PTH and adverse outcomes. Furthermore, validity of this association is shown by the fact that results were consistent and robust across all models, including instrumental variable models that can partially account for unmeasured confounders (21–23).
The recent rise in PTH levels warrants attention and continued monitoring. In the absence of definitive evidence in support of a specific PTH target, the best approach to modify SHPT treatments on the basis of PTH levels remains unclear. Research to establish up-to-date, truly evidence-based targets for markers of mineral and bone disorder is urgently needed.
Disclosures
F.T. has received honoraria from Amgen, Dialysis Clinic Inc., and Renal Research Institute. S.H.J. has received honoraria from Abbott, Amgen, and Genzyme. M.F. received a research grant and speaker fees from Kyowa Hakko Kirin. D.C.M. has received speaker fees from Amgen and Sanofi. R.L.P. has received speaker fees from Amgen, Kyowa Hakko Kirin, and Vifor; served as a consultant for Pursuit Vascular; and served on an advisory panel for Merck. B.M.R. has received speaker fees for Kyowa Hakko Kirin. The remaining authors have no conflicts to report.
Acknowledgments
The Dialysis Outcomes and Practice Patterns Study Program is supported by Amgen, Kyowa Hakko Kirin, AbbVie Inc., Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma, Ltd. Additional support for specific projects and countries is also provided by Amgen, BHC Medical, Janssen, Takeda, and Kidney Foundation of Canada (for logistics support) in Canada; Hexal, Deutsche Gesellschaft fur Nephrologie (DGfN), Shire, and WiNe Institute in Germany; and the Japanese Society for Peritoneal Dialysis for Peritoneal Dialysis Outcomes and Practice Patterns Study in Japan. F.T. is supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Award K01DK087762.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. All support is provided without restrictions on publications.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Dysphoria Induced in Dialysis Providers by Secondary Hyperparathyroidism,” on pages 9–11.
References
- 1.United States Renal Data System : 2012 United States Renal Data System Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 3.de Francisco AM, Ellis HA, Owen JP, Cassidy MJ, Farndon JR, Ward MK, Kerr DN: Parathyroidectomy in chronic renal failure. Q J Med 55: 289–315, 1985 [PubMed] [Google Scholar]
- 4.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Plone M, Dillon MA, Burke SK, Slatopolsky E: Hyperparathyroidism and dialysis vintage. Clin Nephrol 54: 295–300, 2000 [PubMed] [Google Scholar]
- 6.Malberti F, Marcelli D, Conte F, Limido A, Spotti D, Locatelli F: Parathyroidectomy in patients on renal replacement therapy: An epidemiologic study. J Am Soc Nephrol 12: 1242–1248, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, Akizawa T, Saito A, Asano Y, Kurokawa K, Pisoni RL, Port FK: Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int 11: 340–348, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Lacson E, Jr., Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC, ARO Investigators : Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, Sprague SM, Goldfarb S: KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 55: 773–799, 2010 [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 19.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 44[Suppl 2]: 7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57: S74–S81, 2000 [Google Scholar]
- 21.Angrist JD, Imbens GW, Rubin DB: Identification of causal effects using instrumental variables. J Am Stat Assoc 91: 444–455, 1996 [Google Scholar]
- 22.Wooldridge JM. Introductory Econometrics: A Modern Approach, Southwestern College Publishers, Mason, OH, 2008 [Google Scholar]
- 23.Terza JV, Basu A, Rathouz PJ: Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J Health Econ 27: 531–543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendelssohn DC, Pisoni RL, Arrington CJ, Yeates KE, Leblanc M, Deziel C, Akiba T, Krishnan M, Fukuhara S, Lameire N,Port FK, Wolfe RA: A practice-related risk score (PRS): A DOPPS-derived aggregate quality index for haemodialysis facilities. Nephrol Dial Transplant 23: 3227–3233, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Raghunathan TE, Solenberger PW, Van Hoewyk J: IVEware: Imputation and Variance Estimation Software, Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, MI, 2002. [Google Scholar]
- 26.Cameron AC, Trivedi PK: Microeconomics Methods and Applications, New York, Cambridge University Press, 2005, p 103 [Google Scholar]
- 27.Akizawa T, Kido R, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, Fukuhara S, Kurokawa K: Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: Associations with changing practice patterns. Clin J Am Soc Nephrol 6: 2280–2288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Chen YW, Peng Y, Foley RN, St Peter WL: Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. Am J Kidney Dis 57: 602–611, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW: Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized, double-blind, multicenter study. J Am Soc Nephrol 16: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 32.St Peter WL, Li Q, Liu J, Persky M, Nieman K, Arko C, Block GA: Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 4: 354–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukagawa M, Fukuma S, Onishi Y, Yamaguchi T, Hasegawa T, Akizawa T, Kurokawa K, Fukuhara S: Prescription patterns and mineral metabolism abnormalities in the cinacalcet era: Results from the MBD-5D study. Clin J Am Soc Nephrol 7: 1473–1480, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkovic V, Neal B: Trials in kidney disease—time to EVOLVE. N Engl J Med 367: 2541–2542, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Guideline Working Group, Japanese Society for Dialysis Therapy : Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial 12: 514–525, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T, CKD-MBD Guideline Working Group. Japanese Society for Dialysis Therapy : Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17: 247–288, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Molnar MZ, Zaritsky JJ, Sim JJ, Streja E, Kovesdy CP, Salusky I, Kalantar-Zadeh K: Correlates of parathyroid hormone concentration in hemodialysis patients. Nephrol Dial Transplant 28: 1516–1525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avram MM, Mittman N, Myint MM, Fein P: Importance of low serum intact parathyroid hormone as a predictor of mortality in hemodialysis and peritoneal dialysis patients: 14 years of prospective observation. Am J Kidney Dis 38: 1351–1357, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Drechsler C, Krane V, Grootendorst DC, Ritz E, Winkler K, März W, Dekker F, Wanner C, German Diabetes and Dialysis Study Investigators : The association between parathyroid hormone and mortality in dialysis patients is modified by wasting. Nephrol Dial Transplant 24: 3151–3157, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC: Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19: 1827–1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Block GA, Kilpatrick RD, Lowe KA, Wang W, Danese MD: CKD-mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J Am Soc Nephrol 8: 2132–2140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledebo I, Lameire N, Charra B, Locatelli F, Kooistra M, Kessler M, Jacobs C: Improving the outcome of dialysis—opinion vs scientific evidence. Report on the Dialysis Opinion Symposium at the ERA-EDTA Congress, 6 September 1999, Madrid. Nephrol Dial Transplant 15: 1310–1316, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Mactier R, Davies S, Dudley C, Harden P, Jones C, Kanagasundaram S, Lewington A, Richardson D, Taal M, Andrews P, Baker R, Breen C, Duncan N, Farrington K, Fluck R, Geddes C, Goldsmith D, Hoenich N, Holt S, Jardine A, Jenkins S, Kumwenda M, Lindley E, Macgregor M, Mikhail A, Sharples E, Shrestha B, Shrivastava R, Steddon S, Warwick G, Wilkie M, Woodrow G, Wright M: Summary of the 5th edition of the Renal Association Clinical Practice Guidelines (2009-2012). Nephron Clin Pract 118[Suppl 1]: c27–c70, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF, Canadian Society of Nephrology Committee for Clinical Practice Guidelines : Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 45.McMahon LP, MacGinley R, KHA-CARI : KHA-CARI guideline: Biochemical and haematological targets: Haemoglobin concentrations in patients using erythropoietin-stimulating agents. Nephrology (Carlton) 17: 17–19, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Elder G, Faull R, Branley P, Hawley C, Caring for Australasians with Renal Impairment (CARI) : The CARI guidelines. Management of bone disease, calcium, phosphate and parathyroid hormone. Nephrology (Carlton) 11[Suppl 1]: S230–S261, 2006 [DOI] [PubMed] [Google Scholar]