Abstract
Cognitive side-effects such as emergence agitation (EA), postoperative delirium (POD) and postoperative cognitive dysfunction (POCD) are not infrequently complicating the postoperative care especially in elderly and fragile patients.
The aim of the present survey was to gain insight regarding concern and interest in prevention and treatment strategies for postoperative delirium and dysfunction, and the use of EEG-based depth-of-anaesthesia monitoring possibly reducing the risk for cognitive side effects among anaesthesia personnel.
Methods
A web-based validated questionnaire was sent to all Swedish anaesthesiologists and nurse anaesthetists during summer 2013. The questionnaire consisted of 3 sections, subjective preferences, routines and practices related to the perioperative handling of EA, POD, POCD.
Results
The response rate was 52%. Cardiovascular/pulmonary risks where assessed as importance by 98, 97% of responders while 69% considered the risk of neurocognitive side-effects important. When asked explicitly around cognitive side-effects 89%, 37% and 44% assessed awareness, POC and POD respectively of importance. EEG-based depth-of-anaesthesia monitors were used in 50% of hospitals. The responders were not convinced about the benefits of such monitors even in at-risk patients. Structured protocols for the management of postoperative cognitive side-effects were available only in few hospitals.
Conclusion
Swedish anaesthesia personnel are concerned about the risk of postoperative cognitive side-effects but are more concerned about cardiovascular/pulmonary risks, pain, PONV and the rare event of awareness. Most respondents were not convinced about the use of depth-of-anaesthesia monitors. There is a need to improve knowledge around risk factors, prevention and management of postoperative cognitive side effects.
Keywords: Surgery, General anaesthesia, Postoperative cognitive side effects, Emergence agitation, Postoperative delirium, Postoperative cognitive dysfunction, Depth of anaesthesia monitors, Bi-spectral index, Auditory evoked potential
Highlights
-
•
We found that routines around postoperative cognitive side effects were infrequently in place.
-
•
We found that Swedish anaesthesia personnel have a sceptic view on depth-of-anaesthesia monitors.
-
•
Depth-of-anaesthesia monitors were not commonly used even in at risk patients.
-
•
There is a need for improvement in the attitude towards postoperative cognitive side effects.
1. Introduction
Surgery and anaesthesia are nowadays safe and effective. The risk for perioperative related major morbidity and mortality is low. Today, patients expect safe and effective anaesthesia and rapid recovery with a minimum of side effects. Minor side effects such as pain, postoperative nausea, and residual “hang over” are also still not infrequently seen. Huge interest is focused on how to minimise cardiovascular and pulmonary risks, and on postoperative pain and postoperative nausea and vomiting (PONV) management [1–5]. Less attention has been paid to postoperative cognitive side effects, emergence agitation, postoperative delirium and postoperative cognitive dysfunction. Cognitive side effects have major implications on the level of care, length of hospital stay and on the patient's perceived quality of care [6]. Children and the elderly and cognitive fragile are at risk [6,7]. Postoperative cognitive impairments may arise during the early phase, such as the in most cases rather short lasting but still most distressful postoperative emergence agitation (EA). Postoperative delirium (POD) has it debut commonly day 1–2 after surgery proving sometime major concerns on the ward. The more subtle but long lasting Postoperative Cognitive Dysfunction (POCD) is generally a complication starting during the first week after surgery but may last for month. These side effects although causing major concerns for both hospital and patients have received less attention.
It has been suggested that intraoperative use of so EEG-based depth of anaesthesia (DOA) monitoring in order to fine tune, tailor anaesthetic delivery can reduce risk for postoperative cognitive side effects [8–10]. Previous surveys of anaesthetic practice in Sweden show a high degree of standardisation and that structured protocols for the perioperative management are at place [11,12]. However a survey regarding postoperative management in general showed more diverse results [13].
The aim of the present survey was to gain insight regarding routines and practice for risk assessment, diagnosis and management of postoperative cognitive side effects, and the use of EEG-based DOA monitoring among anaesthesia personnel.
2. Methods
A web-based questionnaire was sent by e-mail to anaesthesiologists (= 1326) and nurse anaesthetists (n = 1300) after approval from the Ethics Committee (Dnr 2013/163), Uppsala, Sweden, May 15th 2013 (Erik Lempert). The e-mail addresses were obtained from the Swedish Association for Anaesthesia and Intensive Care (SFAI, anaesthesiologists), and the Swedish Association of Health Professionals (Vårdförbundet). A total of 2626 questionnaires were sent including three reminders during June–August 2013.
The questionnaire consisted of 3 sections:
-
1.
Questions about subjective preferences of the respondents, for example, “what would you like…?”
-
2.
Questions about related to routines and practices.
-
3.
Questions based on case scenarios. There were 4 case scenarios, one each for POCD, POD, EA and awareness.
The results covering views about cognitive side effects POD and POCD, and the use of DOA monitoring are presented. Respondents were given 5 choices; e.g. disagree completely, disagree, no opinion, agree partly, and agree completely. For some questions (questions No 6 and 10–24) the respondents had only 3 choices; yes, no, and don't know. The questionnaire was validated before the study began by being sent to 11 anaesthesiologists and 4 anaesthetic nurses who were asked to answer and also submit their comments on them if they had any.
2.1. Statistics
Demographic data is presented as mean and numbers. Responses to the survey questions are presented as percent calculated as the numbers of a positive finding divided by the total number of responses to the question. The results are presented for all responders combining anaesthesiologists and nurse anaesthetists but also for anaesthesiologists and nurse anaesthetists separately. This is a descriptive survey study with no predefined explicit hypothesis and thus no statistical comparisons have been undertaken.
3. Results
There were 417 responses from anaesthesiologists (38%) and 669 responses from nurse anaesthetists (62%) in all 1086 responses were collected. Demographics of the responders are presented in Table 1.
Table 1.
Demographic of responders, anaesthesiologists, nurse anaesthetists and hospitals.
| Demographic of responders | Anaesthesiologist (n = 417) |
Nurse anaesthetist (n = 669) |
All (n = 1086) |
|---|---|---|---|
| Age yrs/n = male gender | 48/265 | 49/114 | 49/379 |
| Age yrs/n = female gender | 48/154 | 50/555 | 49/709 |
| University Hospitals | 301 | 400 | 701 |
| Local Hospitals | 116 | 269 | 385 |
3.1. Neurocognitive side effects
When asked about concern for the perioperative management 69% of responders considered “risk for neurocognitive side effects” of important during the preoperative assessment (Fig. 1). When asked explicitly about neurocognitive side effects, EA, POD and POCD, were of low concern while awareness with recall was considered of high importance (Fig. 2). Age, major surgery and previous stroke were considered major risk factors for the occurrence of postoperative neurocognitive side effects (Fig. 3). When asked whether they would feel a concern about postoperative cognitive side-effects if having anaesthesia themselves only 10% and 9% respectively among anaesthesiologists and nurse anaesthetists answered yes.
Fig. 1.
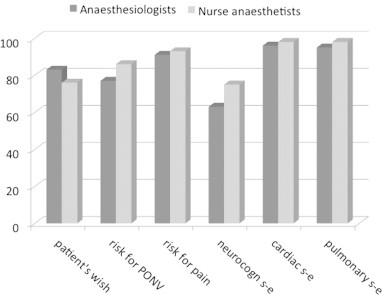
Factors of importance for anaesthesia planning preoperatively.
Fig. 2.
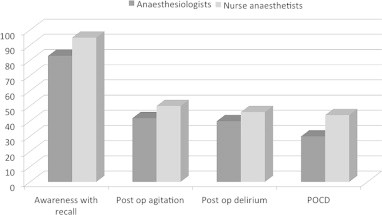
Which neurocognitive side effects are you most concerned about?
Fig. 3.
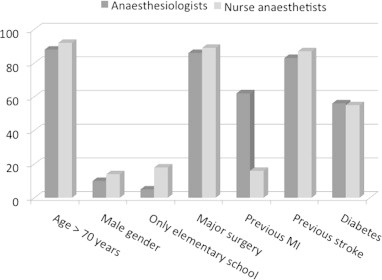
Which factors do you consider as high-risk for POCD?
3.2. Intraoperative management routines
When asked for preferred anaesthetic technique for a patient with signs and symptoms of POD requiring a hip replacement a majority preferred spinal anaesthesia (95%) followed by epidural anaesthesia (30%), TIVA (21%) and general anaesthesia based on volatile anaesthetics (15%). EEG-based DOA monitors were used in half of all departments however the frequency and indication for their use varied (Fig. 4). The trust and reliance on the EEG-based monitors were overall low (Fig. 5). When asked whether they would use an EEG-based DOA for high risk patients, 27% of anaesthesiologists and 43% of nurse anaesthetists agreed. The most commonly used device was a BIS monitor (45%) followed by entropy (24%) and AEP (3%).
Fig. 4.
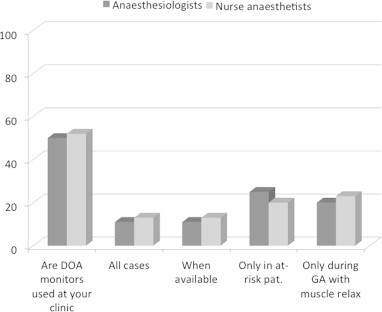
Do you use DOA monitoring?
Fig. 5.
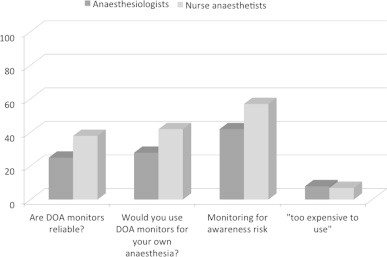
What is your opinion regarding DOA monitoring?
3.3. Postoperative management routines
Only 11% of responders stated that they had written protocols regarding anxiolytics for patients with symptoms of POD, while 44% reported that protocols for analgesic management for patients with signs and symptoms of cognitive side effects were in place. Eighty-nine percent of the responders would provide analgesia, 33% anxiolytics and 54% would give both drugs for the management of POD. The most preferred anxiolytic medication was an alfa-2-agonist (60%) followed by neuroleptic compound phenothiazine such as haloperidol (43%) and benzodiazepine e.g. midazolam (35%). There was rather huge difference in drug choice between the anaesthesiologists and nurse anaesthetists. The large majority of respondents would keep a patient with symptoms of POD in the PACU (anaesthesiologists 89% and nurse anaesthetists 78%). However there were few responders that were aware of any specific procedure for monitoring or assessment of postoperative delirium, 9% of anaesthesiologists and 6% of nurse anaesthetists. Only 3% responders were familiar to the use of Confusion Assessment Method (CAM) for scoring of delirium in PACU. There were likewise rarely structured procedures for the management of a patient showing signs and symptoms of POCD on follow-up (2%) and 23% of anaesthesiologists believed that “such a patient” would be referred to a neurologist and 10% believed the patient would be seen by a geriatrician. Overall 18% of anaesthesiologists and 6% of nurse anaesthetists were aware of a case from their own practice that developed signs and/or symptoms of POCD. Still 65% of responders (70% of anaesthesiologists and 60% of nurse anaesthetists) considered postoperative cognitive side-effects being a neglected area in anaesthesiology.
4. Discussion
We found that Swedish anaesthesia personnel viewed risk assessment, prevention and handling of postoperative delirium and postoperative cognitive dysfunction of less importance as compared to cardiovascular events, pain, and PONV. Protocol and/or standardised routines were only rarely implemented. No major difference could be seen between anaesthesiologists and nurse anaesthetists in the attitude around postoperative disturbances. There was a far higher concern for awareness than for postoperative cognitive side effects. The reliance in and willingness to use EEG-based depth of anaesthesia monitors, in order to possible reduce the risk/occurrence of postoperative cognitive side-effects, was also low. The view on EEG based monitors, such as the BIS-monitor, for fine-tuning anaesthetic delivery and thus potentially improve recovery and reduce risk for delayed or complicated neurocognitive recovery was also rather negative, only about one in five of respondents would use such devices in high-risk patients.
It is hard to say whether our findings are surprising or merely to be expected. Postoperative side effects: EA, POD and POCD have obvious impact not only on quality of care but may jeopardise safety and delay the recovery process. Postoperative delirium and POCD obviously delay rehabilitation, and are associated with increases in morbidity and mortality among elderly surgical patients [14]. The incidents of POCD have in some studies to be as high as 26% [15], POD have been shown up-to 25% [16] and some form of emergence agitation in up-to 50% [7]. These side effects are thus far more frequent than the risk for awareness with a prevalence of 1–2/1000 anaesthetics [17,18]. The high concern about awareness may be related to the general perception of a general failure, providing inadequate anaesthesia. It may also be related to the risk for publicity associated with recall of intraoperative pain and/or paralysis. Postoperative agitation and confusion cause less publicity. These side effects have however major impact on quality of care and are consuming huge amount if health care resources [19]. Cognitive dysfunction is more anonymous and the impact on patient well-being and health care resources are less well defined. Surgery and anaesthesia exert comparatively greater adverse effects on the elderly [14] and there is an obvious need for a more pro-active attitude towards postoperative cognitive side-effects especially in the growing elderly patient population. Age and preoperative cognitive capacity are without doubt factors of huge importance. Simple MMSE testing prior to surgery may help identify patients at risk.
There are multiple potential benefits associated to the use of DOA monitoring. The reduced needs of anaesthetic agent and improved, more rapid early recovery have been demonstrated in several studies [8,9]. There are studies suggesting that the use of DOA monitoring reduces the risk for PONV [9,20]. There are also reports suggesting a reduction in early cognitive adverse effects [21]. We found in two earlier studies that DOA monitoring reduced the risk for early cognitive impairment [8,22]. Chan et al. have shown BIS-guided anaesthesia to reduced anaesthetic exposure and decreased the risk of POCD at 3 months after surgery. They concluded that for every 1000 elderly patients undergoing major surgery, anaesthetic delivery titrated to a range of BIS between 40 and 60 years of age would prevent 23 patients from POCD and 83 patients from delirium [23]. The role of DOA monitoring in reducing the risk of awareness is less clear, the debate regarding whether DOA monitoring decreases the risks of awareness is still on going [17]. Shepherd et al. published recently a systematic review of clinical effectiveness and cost-effectiveness of DOA monitoring [24]. They concluded that the available evidence on the impact of the technologies on reducing the likelihood of awareness is limited. However, it confirmed the benefits; reductions in general anaesthetic consumption and anaesthetic recovery times. The overall attitude to the EEG-based DOA monitors was in our study critical. The sceptic attitude to DOA monitoring found may be due to the Swedish Council on Health Technology Assessment (SBU) [25] being rather negative to these devices. The conclusion of this report is in contrast to the NICE guidelines from UK, which support the use of DOA monitoring in at-risk (http://www.nice.org.uk/nicemedia/live/13965/61547/61547.pdf). There are at present no national guidelines or recommendations around their use. The use of EEG-based anaesthesia monitoring during TIVA and in patients at risk for awareness was more positive among nurse anaesthetists than the anaesthesiologists which may be a sign of that they feel that the depth of anaesthesia monitor indeed provide supportive information. We did not include questions around the use of near infrared spectroscopy (NIRS) a technique that may help reducing cerebral hypoperfusion and/or hypoxia and thus have an impact on postoperative cognitive performance.
Only a few departments had written protocols for the management of POD and the knowledge regarding routines differed between anaesthesiologists and nurse anaesthetists. Drugs used for management of delirium also differed between anaesthesiologists and nurse anaesthetists. Alfa-2-agonists (clonidine) and phenothiazine's (haloperidol) were commonly used by anaesthesiologists while benzodiazepines (midazolam) were most commonly used by nurse anaesthetists for sedation in POD. Current evidence suggests that alfa-2-agonists and phenothiazine's are the most appropriate drugs and benzodiazepines should be avoided [26,27]. The alfa-2-agonists may have a protective property but further studies are needed [26]. There is some support for the use of preoperative phenothiazine in patients at risk [28]. Benzodiazepines may not be an optimal drug class in the elderly patient for the management of POD or agitation [29].
Our results are based on voluntary responses gained from a web-based questionnaire survey sent to anaesthesiologists and nurse anaesthetists by support from the Society for Anaesthesiology and Intensive Care (SFAI) and the Swedish Association of Health Professionals in Sweden (Vårdförbundet). No incentive was provided but reminders were sent out on 3 occasions, the initial survey was sent out during summer period. We had an overall fifty-two percent response rate and it is not possible to state whether or not the results fully represent the views of the anaesthesia community practice in Sweden. The response rate among anaesthesiologists is low. It should be acknowledged that the survey was sent to all registered in the National Swedish Anaesthesia and Intensive Care trade union register. We cannot discriminate the number of active colleagues actually taking part in clinical practice. Still more than one thousand responses were compiled and analysed and thus the profile should provide a reasonable profile of current practice. We had predefined response alternatives, which may have had a limiting effect; some responders may not have been fully comfortable with any of the alternatives given. We have also in our analysis categories the answers and focused the presentation on positive findings. It should also be acknowledged that we did not include personnel involved in the care in the recovery area, nor at the general ward or the surgeons that are responsible for the more protracted management. The important role a structured and intense collaboration between anaesthesiologists, surgeons, nurses, and physiotherapists in order to facilitating postoperative recovery by provision of minimally-invasive anaesthesia and pain relief is of importance [30]. Enhanced recovery pathways have become increasingly adopted. Early mobilisation and shorten time in hospital has been found reducing the risk for delirium. It is without doubt of importance that surgeons, anaesthesia and ward personnel has a common understanding and goal. Kehlet et al. have shown that a fast-track set-up with multimodal opioid-sparing analgesia was associated with lack of POD after elective hip and knee replacement surgery in elderly patients [31]. Implementing structured preoperative risk assessment, and the use of depth of anaesthesia monitoring in order to optimise anaesthesia delivery and facilitate the recovery process has obvious potentials in improving quality of perioperative care.
In conclusion, Swedish anaesthesia personnel are less concerned about the risk of postoperative cognitive side effects as compared to the risk of cardiovascular, pulmonary, pain and PONV risks and the rare event of awareness. Most respondents were not convinced about the use of depth of anaesthesia monitors and they were sceptic to the potential benefits even in high-risk patients in contrast to some national guidelines e.g. NICE-UK. There is a need to improve their knowledge around risk factors, prevention and management of postoperative cognitive dysfunction.
Conflicts of interest
None.
Acknowledgement
The authors would like to thank the Swedish Society of Anaesthesiologists (SFAI) and the Swedish Association of Health Professionals (Vårdförbundet) for providing access to email addresses for anaesthesiologists and nurse anaesthetists respectively.
Appendix. Questionnaire and case study scenarios.
Table 1.
Questions 1–3 response rates are shown as percentage.
| Questions 1–3, presented as 3 results, 1. disagree completely/disagree, 2. no opinion, 3. agree partly/agree completely | Anaesthesiologist (%) | Nurse anaesthetist (%) | All (%) |
|---|---|---|---|
| 1. If you were to have surgery with general anaesthesia, would you feel anxiety about possible postoperative cognitive loss? | 76/14/10 n = 417 |
79/12/09 n = 647 |
78/12/10 n = 1062 |
| 2. If you could choose anaesthetic regimen prior to surgery, for inguinal hernia, which method would you prefer? Regional anaesthesia/TIVA/inhalation-based anaesthesia/local anaesthesia and sedation | 43/22/10/25 n = 417 |
33/32/10/25 n = 651 |
38/27/10/25 n = 1068 |
| 3. At the time of preoperative assessment, which factors do you think should influence the choice of anaesthetic? | |||
| a Patient's wish | 5/12/83 n = 414 |
7/17/76 n = 614 |
6/14/80 n = 1055 |
| b Risk of postoperative nausea | 8/15/77 n = 414 |
4/10/86 n = 636 |
6/12/82 n = 1050 |
| c Risk of post-operative pain | 3/6/91 n = 414 |
3/4/93 n = 638 |
3/6/91 n = 1062 |
| d Risk of postoperative neurocognitive effects | 13/24/63 n = 412 |
8/17/75 n = 635 |
10/21/69 n = 1047 |
| e Risk of cardiac events | 1/3/96 n = 409 |
1/1/98 n = 641 |
1/2/97 n = 1050 |
| f Risk of lung effects | 2/3/95 n = 414 |
1/1/98n = 640 | 2/98 n = 1054 |
Table 2.
Questions 4–5 response rates are shown as percentage.
| Questions 4–5, presented as 3 results, 1. disagree completely/disagree, 2. no opinion, 3. agree partly/agree completely | Anaesthesiologist (%) | Nurse anaesthetist (%) | All (%) |
|---|---|---|---|
| 4. During preoperative assessment which of the following cognitive states do you take into account? | |||
| a Postoperative delirium (POD) | 27/33/40 n = 412 |
22/22/46 n =614 |
26/30/44 n = 1029 |
| b Postoperative cognitive dysfunction (POCD) | 35/35/30 n = 412 |
21/35/44 n = 611 |
28/35/37 n = 1023 |
| c Emergence agitation (EA) | 26/32/42 n = 411 |
17/33/50 n = 613 |
22/32/46 n = 1024 |
| d Awareness | 7/10/83 n = 414 |
3/2/95 n = 623 |
5/6/89 n = 1037 |
| 5. How important do you consider the following risk factors for postoperative neurocognitive impairment to be? | |||
| a Age > 70 years | 2/10/88 n = 413 |
2/6/92 n = 592 |
2/8/90 n = 1005 |
| b Male gender | 26/64/10 n = 410 |
24/62/14 n = 578 |
26/63/11 n = 988 |
| c Only elementary school education | 47/48/5n = 411 | 30/52/18n = 580 | 38/50/12n = 991 |
| d Extensive surgery | 2/12/86 n = 412 |
2/9/89 n = 588 |
3/10/87 n = 1000 |
| e Previous myocardial infarction | 4/36/60 n = 411 |
12/42/46 n = 582 |
8/38/54n = 993 |
| f Previous stroke | 2/15/83 n = 414 |
3/10/87 n = 585 |
3/12/85 n = 999 |
| g Diabetes | 10/34/56 n = 409 |
11/34/55 n = 580 |
10/34/56 n = 989 |
Table 3.
Questions 6–9 response rates are shown as percentage.
| Questions 6–9, presented as 3 results, 1. disagree completely/disagree, 2. no opinion, 3. agree partly/agree completely | Anaesthesiologist (%) | Nurse anaesthetist (%) | All (%) |
|---|---|---|---|
| 6a. Is anaesthetic depth measurement used at your clinic? This question has only, yes or no alternatives (yes/no) | 50/50 n = 409 |
52/48 n = 605 |
51/49 n = 1014 |
| 6b. If yes to question 6a. Following choices: “Always/when the device is available/only risk patients/only during general anaesthesia with muscle relaxants/rarely” |
11/11/25/20/33 n = 202 |
13/13/20/23/24 n = 303 |
12/12/22/22/22 n = 505 |
| 7a. If you undergo surgery yourself, would you use DOA monitoring? | 43/29/28 n = 408 |
28/30/42 n = 579 |
35/30/35 n = 987 |
| 7b. Would you use DOA monitor to reduce the risk of awareness? | 38/20/42 n = 405 |
21/22/57 n = 584 |
30/21/49 n = 989 |
| 7c. Do you think that DOA monitoring is reliable method for controlling the anaesthetic depth? | 44/31/25 n = 401 |
29/32/38 n = 578 |
36/32/32 n = 979 |
| 7d. Do you think that DOA monitoring is too expensive to be used? | 51/41/8 n = 401 |
53/40/7 n = 578 |
52/40/8 n = 979 |
| 8. In the U.S., anaesthetic depth measurement is very common with general anaesthesia. In UK, the National Institute for Clinical Excellence guidance (NICE) (Nov 2012) recommended anaesthetic depth measurement as a possible choice for general anaesthesia in patients at risk and with TIVA. Do you think it should be applied in Sweden too? | 19/40/41 n = 413 |
13/28/59 n = 588 |
16/34/50 n = 1001 |
| 9. Today, we routinely assess patients regarding for example cardiac status. Do you think it would be useful to also preoperatively evaluate neurocognitive function with for instance Mini Mental Test (MMT), or similar, to detect preoperative cognitive impairment that may increase the risk of postoperative delirium or POCD? | 21/26/53 n = 413 |
14/33/60 n = 614 |
15/29/56 n = 1027 |
Table 4.
Questions 10–14 response rates are shown as percentage, respondents had 3 choices.
| Questions 10–14, this questions has only, yes/no/do not know alternatives |
Anaesthesiologist (%) |
Nurse anaesthetist (%) |
All (%) |
|---|---|---|---|
| Case study 1: Postoperative delirium (POD) | |||
| Case study 1: Postoperative delirium (POD) healthy patient who is 75 years old, no medications, with a hip fracture and needs to undergo acute surgery. Her pain is relieved with opioids. Saturation on air preoperatively is 88%, blood pressure 160/100 and pulse 110. The patient has a fever, is agitated and confused and has difficulties giving adequate answers. | |||
| 10a. Do you have a written protocol regarding preoperative anxiolytics agents for this type of patient (POD)? yes/no/do not know |
8/74/17 n = 408 |
13/61/26 n = 586 |
11/67/22 n = 994 |
| 10b. Is this protocol followed? yes/no/do not know |
12/38/50 n = 35 |
14/26/56 n = 76 |
12/32/53 n = 111 |
| 11a. Do you have a written procedure for preoperative analgesic agents for this patient type (POD)? yes/no/do not know |
45/38/17 n = 405 |
48/27/24 n = 576 |
44/3420 n = 981 |
| 11b. Is the written procedure followed? yes/no/do not know |
47/18/33 n = 178 |
48/14/38 n = 277 |
47/16/35 n = 455 |
| 12. Which anaesthetic method would you choose for the above described patient? | |||
| a Spinal blockade yes/no/do not know |
96/3/1 n = 405 |
94/3/3 n = 554 |
95/3/2 n = 959 |
| b Epidural block yes/no/do not know |
28/67/5 n = 298 |
33/55/13 n = 502 |
30/61/10 n = 800 |
| c Peripheral nerve blockade yes/no/do not know |
30.8/64/7/4.7 n = 395 |
34/53/13 n = 502 |
34/58/8 n = 897 |
| d Inhalation anaesthesia yes/no/do not know |
15/78/6 n = 395 |
15/78/7 n = 551 |
15/78/6 n = 946 |
| e Total intravenous anaesthesia (TIVA) yes/no/do not know |
17/77/6 n = 395 |
24/63/13 n = 550 |
21/70/9 n = 945 |
|
13. Will you use the anaesthetic monitoring equipment in case of general anaesthesia? yes/no |
27/72 n = 404 |
43/57 n = 566 |
35/64 n = 970 |
| 14. If yes, which? | |||
| Auditory evoked potential (AEP) | 4 | 1.5 | 3 |
| Bispectral-index (BIS) | 45 | 44 | 44 |
| Entropy | 23 | 25 | 24 |
| Cerebral state index monitor (CSI) | 0.7 | 0.3 | 0.5 |
| Other | 27 | 29 | 28 |
| n = 112 | n = 239 | n = 351 | |
Table 5.
Questions 15–16 response rates are shown as percentage, respondents had 2 choices.
| Questions 15–16, this question has only, yes/no/alternatives | Anaesthesiologist (%) | Nurse anaesthetist (%) | All (%) |
|---|---|---|---|
| 15. At the postoperative ward, the patient's state is worsened, and she becomes motorically agitated, pulling the hoses. It is unclear whether the patient is in pain or not, what is your first action? | |||
| a Administer pain relief | 86/14 n = 326 |
91/9 n = 402 |
89/11 n = 728 |
| b Administer anxiolytic | 26/74 n = 264 |
40/60 n = 215 |
33/67 n = 479 |
| c Both | 45/55 n = 327 |
64/36 n = 343 |
54/46 n = 670 |
| 16. If you choose to administer an anxiolytic drug which would you choose? This question has only, yes/no/do not know alternatives | |||
| a Benzodiazepine, such as midazolam | 24/68/8 n = 442 |
48/31/22 n = 508 |
35/50/15 n = 950 |
| b Alfa-2 agonists such as clonidine (Catapresan)/dexmetomedin (Dexol) | 73/20/6 n = 348 |
56/18/36 n = 588 |
60/19/21 n = 936 |
| c Neuroleptic, butyrophenones, e.g. (Haloperidol) | 58/37/5 n = 357 |
21/42/37 n = 563 |
43/36/21 n = 920 |
Table 6.
Questions 17–19 response rates are shown as percentage, respondents had 3 choices.
| Questions 17–19, this question has only, yes/no/do not know alternatives | Anaesthesiologist (%) | Nurse anaesthetist (%) | All (%) |
|---|---|---|---|
| 17. The patient's condition persists after 2 h, what do you do? | |||
| a Retain the patient at the postoperative ward until the condition stabilises | 89/9/2 n = 407 |
78/3/19 n = 534 |
84/6/10 n = 941 |
| b Send the patient to the general ward where there is a written care protocol for this condition | 13/74/13 n = 355 |
12/50/38 n = 563 |
13/62/25 n = 918 |
| c Send the patient to the ward due to shortage of beds (even when written care protocol is absent) | 16/74/10 n = 354 |
9/54/37 n = 553 |
13/64/23 n = 907 |
| 18. Are there procedures for monitoring patients who developed postoperative delirium? | |||
| a PACU | 9/74/17 n = 408 |
6/39/55 n = 561 |
7/57/36 n = 969 |
| b Surgical ward | 2/43/55 n = 402 |
3/10/87 n = 508 |
2/28/70 n = 910 |
| 19. Is a Confusion Assessment Method (CAM, CAMICU) used at the PACU in order to evaluate POD? | 5/77/18 n = 406 |
1/37/62 n = 558 |
3/57/40 n = 964 |
Table 7.
Questions 20–21 response rates are shown as percentage, respondents had 3 choices.
| Questions 20–21, this questions has only, yes/no/do not know alternatives |
Anaesthesiologist (%) |
Nurse anaesthetist (%) |
All (%) |
|---|---|---|---|
| Case study 2: Postoperative cognitive dysfunction (POCD). Patient, farmer warehouse worker, age 55, with moderate alcohol consumption and previous coronary artery surgery. The patient also had a minor stroke, but without residual impact. Recently he has undergone surgery for stomach cancer and is now returning, four weeks later, for a planned follow-up. He is upset, angry and sad about the inherent frustration of not being able to plan the day as he had previously been able to. Says memory is short, it fails, and that it takes time to figure out what he planned to do, or not do. Requires adequate treatment of the cognitive symptoms. | |||
| 20. How would a similar patient be handled at your hospital? | |||
| a Do you have a written care protocol? | 2/63/35 n = 406 |
4/20/76 n = 543 |
2/42/56 n = 949 |
| b Would the patient be evaluated for cognitive function ? | 23/25/52 n = 404 |
13/7/80 n = 543 |
18/16/66 n = 947 |
| c Is feedback from previous such patient given to the anaesthesia ward? | 11/49/40 n = 405 |
10/22/68 n = 542 |
10/36/54 n = 947 |
| d Would this patient be referred to a neurologist ? | 23/14/63 n = 402 |
9/3/88 n = 539 |
16/9/75 n = 941 |
| e Would this patient be referred to a geriatrician? | 10/28/62 n = 402 |
2/8/90 n = 539 |
6/19/75 n = 941 |
| f Are you aware of any cases of persisting cognitive impairment in your own practise? | 18/64/18 n = 403 |
6/58/36 n = 544 |
13/60/27 n = 947 |
| 21. Do you think that POCD is a neglected area within the field of anaesthesia? | 70/2/28 n = 401 |
60/1/39 n = 499 |
65/2/33 n = 900 |
References
- 1.Chandrakantan A., Glass P.S. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107(Suppl. 1):i27–40. doi: 10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]
- 2.Fleisher L.A., Beckman J.A., Brown K.A. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13–e118. doi: 10.1016/j.jacc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H., Jensen T.S., Woolf C.J. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 4.Priebe H.J. Recent advances in preoperative cardiac evaluation. Curr Pharm Des. 2012;18:6182–6194. doi: 10.2174/138161212803832317. [DOI] [PubMed] [Google Scholar]
- 5.Tusman G., Bohm S.H., Warner D.O., Sprung J. Atelectasis and perioperative pulmonary complications in high-risk patients. Curr Opin Anaesthesiol. 2012;25:1–10. doi: 10.1097/ACO.0b013e32834dd1eb. [DOI] [PubMed] [Google Scholar]
- 6.Krenk L., Rasmussen L.S., Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54:951–956. doi: 10.1111/j.1399-6576.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 7.Dahmani S., Stany I., Brasher C. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth. 2010;104:216–223. doi: 10.1093/bja/aep376. [DOI] [PubMed] [Google Scholar]
- 8.Jildenstal P.K., Hallen J.L., Rawal N., Gupta A., Berggren L. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. Eur J Anaesthesiol. 2011;28:213–219. doi: 10.1097/EJA.0b013e328340dbb9. [DOI] [PubMed] [Google Scholar]
- 9.Punjasawadwong Y., Boonjeungmonkol N., Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007:CD003843. doi: 10.1002/14651858.CD003843.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Radtke F.M., Franck M., Lendner J. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110:i98–i105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 11.Segerdahl M., Warren-Stomberg M., Rawal N., Brattwall M., Jakobsson J. Clinical practice and routines for day surgery in Sweden: results from a nation-wide survey. Acta Anaesthesiol Scand. 2008;52:117–124. doi: 10.1111/j.1399-6576.2007.01472.x. [DOI] [PubMed] [Google Scholar]
- 12.Segerdahl M., Warren-Stomberg M., Rawal N., Brattwall M., Jakobsson J. Children in day surgery: clinical practice and routines. The results from a nation-wide survey. Acta Anaesthesiol Scand. 2008;52:821–828. doi: 10.1111/j.1399-6576.2008.01669.x. [DOI] [PubMed] [Google Scholar]
- 13.Brattwall M., Stomberg M.W., Jakobsson J. Drug choice for ambulatory anaesthesia: a matter of personal preferences. Acta Anaesthesiol Scand. 2013;57:810–811. doi: 10.1111/aas.12067. [DOI] [PubMed] [Google Scholar]
- 14.Strom C., Rasmussen L.S., Sieber F.E. Should general anaesthesia be avoided in the elderly? Anaesthesia. 2014;69(Suppl. 1):35–44. doi: 10.1111/anae.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moller J.T., Cluitmans P., Rasmussen L.S. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 16.Deiner S., Silverstein J.H. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl. 1):i41–46. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avidan M.S., Jacobsohn E., Glick D. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 18.Pandit J.J., Cook T.M., Jonker W.R., O'Sullivan E. A national survey of anaesthetists (NAP5 baseline) to estimate an annual incidence of accidental awareness during general anaesthesia in the UK. Br J Anaesth. 2013;110:501–509. doi: 10.1093/bja/aet016. [DOI] [PubMed] [Google Scholar]
- 19.Markar S.R., Smith I.A., Karthikesalingam A., Low D.E. The clinical and economic costs of delirium after surgical resection for esophageal malignancy. Ann Surg. 2013;258:77–81. doi: 10.1097/SLA.0b013e31828545c1. [DOI] [PubMed] [Google Scholar]
- 20.Myles P.S. Prevention of awareness during anaesthesia. Best Pract Res Clin Anaesthesiol. 2007;21:345–355. doi: 10.1016/j.bpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Monk T.G., Weldon B.C. Anesthetic depth is a predictor of mortality: it's time to take the next step. Anesthesiology. 2010;112:1070–1072. doi: 10.1097/ALN.0b013e3181d5e0eb. [DOI] [PubMed] [Google Scholar]
- 22.Jildenstal P.K., Hallén J.L., Rawal N., Berggren L. Does depth of anesthesia influence postoperative cognitive dysfunction or inflammatory response following major ENT surgery? J Anesth Clin Res. 2012;3 http://omicsonline.org/does-depth-of-anesthesia-influence-postoperative-inflammatory-response-following-major-ent-surgery-2155-6148.1000220.pdf [accessed 12.03.14] [Google Scholar]
- 23.Chan M.T., Cheng B.C., Lee T.M., Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd J., Jones J., Frampton G. Clinical effectiveness and cost-effectiveness of depth of anaesthesia monitoring (E-entropy, bispectral index and narcotrend): a systematic review and economic evaluation. Health Technol Assess. 2013;17:1–264. doi: 10.3310/hta17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Statens beredning för medicinsk utvärdering (SBU) Statens beredning för medicinsk utvärdering; Stockholm: 2008. EEG-baserad anestesidjupsmonitorering vid kirurgi. [Google Scholar]
- 26.Jacobi J., Fraser G.L., Coursin D.B. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H., Lu Y., Liu M. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17:R47. doi: 10.1186/cc12566. http://ccforum.com/content/pdf/cc12566.pdf [accessed 12.03.14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrader S.L., Wellik K.E., Demaerschalk B.M. Adjunctive haloperidol prophylaxis reduces postoperative delirium severity and duration in at-risk elderly patients. Neurologist. 2008;14:134–137. doi: 10.1097/NRL.0b013e318166b88c. [DOI] [PubMed] [Google Scholar]
- 29.Rathier M.O., Baker W.L. A review of recent clinical trials and guidelines on the prevention and management of delirium in hospitalized older patients. Hosp Pract. 1995;2011(39):96–106. doi: 10.3810/hp.2011.10.928. [DOI] [PubMed] [Google Scholar]
- 30.Kehlet H., Dahl J.B. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003 Dec 6;362(9399):1921–1928. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 31.Krenk L., Rasmussen L.S., Hansen T.B., Bogø S., Søballe K., Kehlet H. Delirium after fast-track hip and knee arthroplasty. Br J Anaesth. 2012 Apr;108(4):607–611. doi: 10.1093/bja/aer493. [DOI] [PubMed] [Google Scholar]


