Abstract
After three decades of intensive research, cytoreductive surgery remains the gold standard of treatment of malignant gliomas. Survivorship at both 1-year and 5-years has not drastically changed in the UK. Concomitant chemo- and radiotherapy has enhanced the efficiency of surgery, enabling more aggressive tumour resection whilst also preserving the surrounding healthy brain parenchyma. More accurate imaging techniques have also played a role in tumour identification, key to this has been pre- and intra-operative contrast enhancement and compounds that have a high affinity in binding to glioma cells. Intra-operative imaging has heralded the ability to give the operating surgeon continuous feedback to assess the completeness of resection. Research is shifting into investigating the complex cellular and molecular glial tumour-genesis, and has led to the development of efficacious chemotherapy agents and trial novel therapies. Oncolytic virotherapy has shown promise in clinical trials and gene therapy in-vitro studies. Surgery however remains the primary therapeutic option for the management of malignant gliomas removing the mass of proliferating malignant tumour cells and decompression of the space-occupying lesion.
Keywords: Chemotherapy, Glioma, Imaging, Neurosurgery, Radiotherapy
1. Introduction
Glial cells unlike neurons undergo mitosis and any abhorrent deviation may result in glial tumours. The initiation is thought to originate from a common pluripotent neuro-ectodermal precursor cell whose progeny retains the ability to differentiate, along the astrocytic lineage to a Grade IV glioblastoma multiforme (GBM). Primary GBMs occur de-novo [1]. Only radiation exposure and certain genetic syndromes, for example Li-Fraumeni syndrome, are well defined risk factors for malignant glioma [2].
Subtype classification is dependent on which cell type the glial tumour most closely resembles including: Astrocytoma (astrocytes), Oligodendrogliomas (oligodendrocytes) or mixed gliomas (for example oligoastrocytomas) – the three of which account for more than 70% of all brain tumours [2].
Glial tumour grading is according to WHO classification grade I to IV, I least aggressive non-anaplastic gliomas and IV most aggressive anaplastic gliomas. High-grade gliomas (HGG), WHO grade III & IV, account for approximately 70% of all gliomas and predominantly affect patients between 40 and 70 years of age [3].
2. Epidemiology & current practise
In the UK, incidence rates of central nervous cancers are 12.4 per 100,000 of the population and not changed significantly in the last three decades [4]. The prognosis and survival remains bleak despite research including advances in cytoreductive surgery, imaging technology, chemotherapy and radiotherapy. The five-year survival in the early 1970s was 8% and this has plateaued at approximately 14% since the late 1980s [4]. There is also significant morbidity with HGG, as they are commonly located in or near to eloquent brain regions, i.e. language, memory, motor and visuo-spatial centres.
The current management for HGG aims to increase survivorship and provide a good quality of life during this short reprieve, before presumed reoccurrence including distal brain metastasis [4]. As briefly mentioned this has been in part due to the combination of cytoreductive surgery, imaging technology, chemotherapy and radiotherapy. It should also be emphasised the management decisions are reached through consensus by a multidisciplinary team including the neurosurgeons, oncologists, physiotherapists and specialist nurses taking into account the individual patients pre-morbid status in particular the WHO performance status.
The Stupp protocol is the gold standard regimen for Grade IV gliomas; involving gross-total resection of tumour bulk (equal to or greater than 95%) followed by radiotherapy with concomitant and adjuvant alkylating/methylation (of tumour cell DNA) chemotherapy agent temozolomide. There was a significant increase in 2-year survival from 10.4% to 26.5% [5]. The Stupp protocol has replaced the PCV adjuvant chemotherapy regimen (procarbazine, lomustine and vincristine) traditionally given due to it failing to improve survival in prospective randomised studies, both in grade III and IV tumours [6].
The placement of intra-operative carmustine wafers (Gliadel), if the resection cavity is greater than 90%, has shown modest increases in median overall survival of approximately two-and-half months [7].
It should be noted for the over 70 years of age cohort, that radiotherapy maybe the only post-operative therapy recommended at MDT although, this alone has shown to increases median survival by 3 months without altering the quality of life compared with best supportive care.
3. Recent intra-operative advances in practise
In the last decade there have been advancements in technology to enable greater and more accurate surgical resection of the HGG, these include:
-
•
Fluorescence-guided surgery with 5-Aminolevulinic acid (5-ALA) given pre-operatively accumulates in HGG allowing for greater resection of tumour and a prolonging progression-free survival, twice as long at 6 months [8–10].
-
•
High-magnification microscopes
-
•
Intra-operative Magnetic Resonance Imaging (iMRI) – allows for real-time assessment of the tumour resection especially given intra-operative brain shift
-
•
Intra-operative ultrasound – free hand intra-operative tumour visualisation and neuronavigation
-
•
Neuronavigation with/without diffusion tensor imaging (DTI) – to intra-operatively localise and estimate tumour resection on a 2D-screen, while avoiding iatrogenic damage of eloquent structures as also DTI assists in defining the white matter tracts
A Cochrane review demonstrated that the extent of resection is significantly increased with 5-ALA iMRI and neuronavigation with DTI, however the data was insufficient to evaluate the overall benefit and effects of neuronavigation. There is no clear evidence whether 5-ALA or neuronavigation with DTI improved overall survival in patients with HGG. There is a theoretical concern that maximising the extent of resection may lead to more frequent adverse events but this was poorly reported in the included studies [11].
3.1. Robotic neurosurgery
General-purpose non-neurosurgical laparoscopy multi-manipulators such as the Da Vinci Surgical System have performed thousands of procedures [12]. The combination of robotic neurosurgery with intraoperative MRI is a logical progression for a telesurgical device, the NeuroArm. The Da Vinci Surgical System unlike the NeuroArm utilises a 3-D visual display unit to give the surgeon full stereoscopic vision. Surgical procedures performed with the NeuroArm have been just as accurate as conventional techniques in malignant glioma resection [13–15]. The integration of tele-operated robotics with surgery should reduce intra-operative error and iatrogenic trauma by decreasing tremor and improving accuracy, and precision. The NeuroArm is still experimental. The Da Vinci system is an example of a well-integrated robotic solution, however the clinical data has not supported the claim of improved patient outcomes and the procedure takes longer than traditional non-robotic surgery [14,15].
3.2. Targeted chemotherapy
Maximal surgical resection of the tumour removes all of the central core of hypoxic, proliferative cells as well as some of the migratory cells in the marginal region, providing a rapid kill of a significant number of tumour cells. Despite combination therapy, tumour reoccurrence is commonplace in most patients after initial successful treatment and consequent poor survival rates [4,5]. Gliomas can originate from neural stem cells, progenitor cells or from de-differentiated mature neural cells transformed into cancer stem cells [1,2]. Genetic sequencing has identified markers that support the diagnosis of a glioma sub-type, but more notably tumour behavioural characteristics especially to chemotherapy [16]. A chemotherapy regimen tailored to the molecular genetics would target residual glial cells and any proliferating Brain Tumour Stem Cells (BSC) responsible for proliferation-migration and differentiation of gliomas.
The molecular and cellular alterations observed in HGGs are strongly linked to signalling pathways involved in angiogenesis, cell-cycle control, cell metabolism (for example Isocitrate dehydrogenase), invasion, and signal transduction. There is also loss of chromosome-10, CDKN2A deletion and amplification of EGFR all linked to HGGs [5,16].
Isocitrate dehydrogenase 1 (IDH1; and rarely IDH2) mutations, important in cell metabolism, have been found in more than 60% of low-grade gliomas and HGGs. This mutation is favourable predictor for outcome irrespective of histological tumour type and grade [5,16].
Methyl-guanine methyl transferase (MGMT) gene is crucial in DNA damage repair and methylation of the MGMT gene promoter (epigenetic silencing) prevents the tumour cell repairing itself from chemotherapy-induced DNA damage. Temozolomide methylates DNA at the N-7 or O-6 positions of guanine residues rendering the HGG cells sensitive to DNA damage and the consequent apoptotic cascade [5,16].
Bevacizumab is a humanised monoclonal antibody, and was the first commercially available angiogenesis inhibitor to target and inhibit vascular endothelial growth factor (VEGF) [17]. The alternative development of immunomodulatory drugs including swainsonine, (inhibiting asparagine glycosylation) has shown therapeutic potential by stimulating the body's innate immune cells to attack tumour cells [18].
3.3. Stereotactic neuroradiosurgery
The Gamma Knife is a focused array of intersecting beams of gamma radiation to treat lesions within the brain and has been a gold standard method for delivery of stereotactic neuroradiosurgery [19]. The CyberKnife system was developed with the intention of delivering radiotherapy more accurately than standard protocols by combining the radiation produced from a small linear particle accelerator and a robotic arm that allows the energy to be directed at any part of the body from any direction [19]. The Gamma Knife requires a stereotactic frame placement that does not allow movement during treatment while CyberKnife allows for mobility and the integration of real-time imaging during radiotherapy session [20,21]. There has been no direct comparison of the aforementioned radiotherapy systems. Early trials of the CyberKnife have shown acceptable toxicity and increased accuracy in radiation delivery with the potential of dose escalation and subsequent effectiveness in decreasing the tumour size [20,21].
3.4. Nanoneurotherapy
Nanotechnology has already become integrated with the everyday neurosurgical care; for example, the impregnation of external ventricular drains catheters with silver nanoparticles to reduce microbial colonisation. Recent neuronavigational technology advancements including nanoscale microelectronics have improved performance of imaging, decreased equipment size and increased the accuracy of cytoreductive surgery [22].
The prospective clinical applications of nanotechnology include a vast array of potential therapies. This will range from nanofluidic chips sensors that detect trace amounts of specific genetic material or protein products that indicate recurrence or degeneration of malignancy to self-assembling nanofibre scaffold gel containing bioactive molecules that enable tissue regeneration by activation of endogenous neuronal stems cells [22,23].
The utilisation of suspended colloids of iron oxide nanoparticles including ferumoxtran-10 has improved the sensitivity of MRI contrast studies for tumour localisation. Iron-oxide nanoparticles unlike Gadolinium is super-paramagnetic not paramagnetic. While an external magnetic field is able to magnetise both, iron oxide's magnetic susceptibility is much greater and reduces the T2 signals of absorbing tissues. T2-weighted sequences are most sensitive for pathology including glioma, and generally distinguishable from normal tissue [23]. Dual imaging utilising cross-linked iron oxide with Cyanine dye 5.5 (CLIO-Cy5.5) has shown promise in pre-clinical studies, acting both, as a preoperative contrast agent and intraoperative near-infrared fluorescence enhancement, like 5-ALA allowing tumour demarcation [9] (Fig. 1).
Fig. 1.
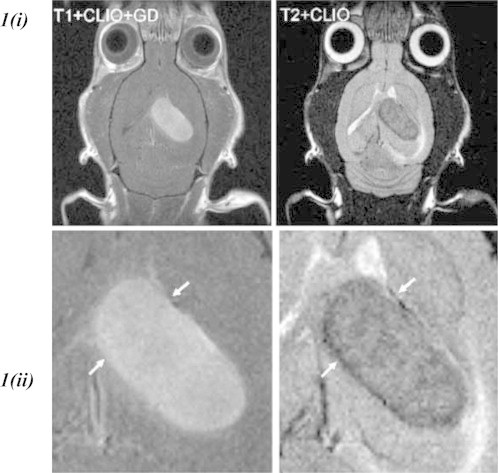
(i) Left, T1-weighted MRI of glioma-bearing rat brain after administration of gadolinium and CLIO-Cy5.5 administration. Right, T2-weighted MRI of the same rat brain after CLIO-Cy5.5 administration but before gadolinium administration. The images show good correlation in terms of tumour demarcation between the two contrast-agent modalities [22]. 1 (ii) Magnification showing the tumour region in (i). (Image adapted).
Iron oxide has other potential therapeutic modalities, and its cross-linking with tumour-labelling agents could improve tumour visualisation. Chlorotoxin (CTX) has been found to preferentially bind to a variety of human malignancies [24]. CTX can be used to image gliomas due to its small size and relative ease of manipulation, and ease with cross-linking to iron oxide. It was demonstrated that CTX selectively binds to the matrix metalloproteinase-2 (MMP-2) isoform that is up-regulated in gliomas not normally expressed in brain [25] (Fig. 2).
Fig. 2.
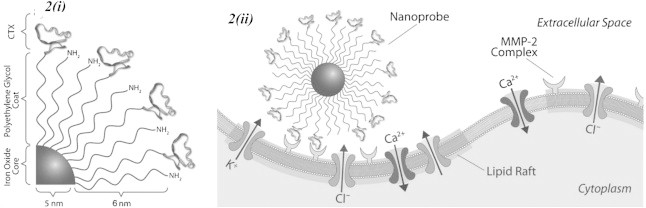
Schematic representations of Iron-oxide-CTX-nanoparticle inhibiting tumour cell invasion. (i) Surface chemistry of nanoprobe. (ii) Nanoprobe binding to lipid rafts of glioma cells containing MMP-2 and select ion channels [25].
3.5. Oncolytic virotherapy
Oncolytic viruses (OVs) have the ability to target, replicate in and lyse tumour cells without critically damaging surrounding non-cancerous tissues, important in the central nervous system. A wide range of OVs therapies have been trialled and the most widely known example is Herpes Simplex Virus (HSV), others trialled include Adenoviruses, Measles virus, Newcastle virus, Poliovirus. HSV is a large-double stranded DNA, neurotropic virus that establishes lifelong infection through latency with already available antiviral agents, and there are various mutant HSV strains available including G207 and HSV-1716 that have been developed to negate encephalitis as a complication [26–28]. HSV attenuation allows for the reduction in neurovirulence and increased tumour selectivity, for example, deletion of Gamma-one 34.5 in both mutants with the additional insertion of gene-disrupting lacZ in HSV G207 prevents the virus from replicating in non-dividing cells. A third-generation HSV, G47Δ, the ICP47 gene is deleted enhancing MHC class 1 antigen presentation therefore allowing for lymphocytic tumour infiltration and hastening the viral life cycle. This is crucial to attain high viral titres (the virus maintains potency without administration of large doses), continued replication without decreased potency and maintains a good safety profile. OVs have demonstrated potential in early clinical trials and are still undergoing investigation [28–30].
Modern recombinant DNA technology has allowed the engineering of modified viruses with improved therapeutic indices and attenuated to include therapeutic transgenes and/or increasing tumour-selective targeting through receptor targeting and tumour-specific promoters. HSV-thymidine kinase (-tk) is an example of suicide gene therapy, whereby the inactive pro-drug ganciclovir (GCV) is converted to toxic GCV-triphosphate, which is lethal to tumour cells. A phase I clinical trial demonstrated a doubling of mean survival from 7.4 to 15 months [27–29].
HGG are heterogeneous, expressing diverse epigenetic and genetic changes. Finding an appropriate therapy is difficult, targeting a common unregulated tumour pathways or using a multi-modal approach may increase treatment efficacy. A HSV mutant, G47Δ-mAngio, that expresses angiostatin and systemic administration of bevacizumab has demonstrated synergistic activity given up-regulation of VEGF in HGGs [29].
3.6. Gene therapy
Endogenous adult neurogenesis in the subventricular zone (SVZ) and subgranular zone (SGZ) generate the main cell phenotypes of the nervous system from their neural stem cell (NSC) populations. The current focus of experimental NSC therapeutics is primarily based around their use for neurodegenerative disorders and stroke. There has been recent in-vitro trials looking at modification of mesenchymal stem cells to express a single antibody against EGFR found in HGG, localising and inducing a local immune response [16,29].
Another potential therapeutic option demonstrated in rodents is inducing embryonic stem cells to express of mda-7/IL-24. The cytokines stimulate HGG cell apoptosis and autophagy while sparing healthy neural tissue. The affected HGGs have also exhibiting increased radio-sensitisation and synergic action with concomitant temozolomide therapy [30].
A viral vector can instead be utilised to allow for cytokine expression, directly on the HGG or NSC. Adenovirus has been commonly used as the appropriate vector, unlike HSV, it is non-neurotropic and the virus is replication incompetent; it will not continue to replicate or have affinity for only neuronal cells negating any theoretical harm to healthy neurones. The mechanism of action involves the delivery of immunostimulatory genes to the HGG with consequent expression of IL-2, -4, -12, interferon-beta and -gamma, thereby the HGG becomes the target of endogenous antigen targeting cells [30]. NSC can instead be induced to express IL-12, and this NSC-IL-12 can target both local and disseminating glioma cells, and in the animal studies, the rodents were noted to have a significantly increased survivorship compared to the controls [31]. The studies both add to further potential mechanisms by which to target HGGs.
The bioengineering and direction of such a treatment would need to under strict control otherwise the result of which may be the further generation of BTSC and significant damage healthy non-tumour cells of the central nervous system.
4. Conclusion
Three decades of intensive research and a variety of chemotherapy regimes, radiotherapy and surgical approaches have been trialled and investigated, however the prognosis for patients with HGGs has not changed significantly. Cytoreductive surgery for the management of malignant glioma therapy improves survival and may enhance the efficiency of adjunct and adjuvant therapy. Reoccurrence does occur and will present with distal metastasis in the cranial vault. Novel therapies are being derived from a better understanding of cellular and molecular glial tumour-genesis, and interactions with the microenvironment, and slowly yielding a variety of possibly viable alternate therapies.
Conflict of interest
No.
Funding
None.
Consent
None.
References
- 1.Diamandis P., Sacher A.G., Tyers M., Dirks P.B. New drugs for brain tumors? Insights from chemical probing of neural stem cells. Med Hypotheses. 2009 Jun;72(6):683–687. doi: 10.1016/j.mehy.2008.10.034. Epub 2009 Mar 3. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H., Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005 Jan;109(1):93–108. doi: 10.1007/s00401-005-0991-y. Epub 2005 Feb 1. Review. [DOI] [PubMed] [Google Scholar]
- 3.MB1 No 37–cancer registration statistics. 2006. http://www.statistics.gov.uk/statbase/Product.asp?vlnk=8843 [accessed 10.10.09] [Google Scholar]
- 4.UK brain and CNS tumour statistics http://info.cancerresearchuk.org/cancerstats/types/brain/?a=5441 [accessed 10.03.14].
- 5.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R., Tonn J.C., Brada M., Pentheroudakis G. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl. 5):v190–v193. doi: 10.1093/annonc/mdq187. [DOI] [PubMed] [Google Scholar]
- 7.Hart M.G., Grant R., Garside R., Rogers G., Somerville M., Stein K. Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev. 2011 Mar 16;3:CD007294. doi: 10.1002/14651858.CD007294.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi H.A., Kosztowski T., DiMeco F., Quiñones-Hinojosa A. Origins and clinical implications of the brain tumor stem cell hypothesis. J Neurooncol. 2009 May;93(1):49–60. doi: 10.1007/s11060-009-9856-x. Epub 2009 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potapova A.A., Usachev D.J., Loshakov V.A., Cherekaev V.A., Kornienko V.N., Pronina I.N. First experience in 5-ALA fluorescence-guided and endoscopically assisted microsurgery of brain tumors. Med Laser Appl. 2008;23(2008):202–208. [Google Scholar]
- 10.Stummer W., Pichlmeier U., Meinel T., Wiestler O.D., Zanella F., Reulen H.J., ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006 May;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 11.Barone D.G., Lawrie T.A., Hart M.G. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD009685.pub2. Issue 1. Art. No.: CD009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBeth P.B., Louw D.F., Rizun P.R., Sutherland G.R. Robotics in neurosurgery. Am J Surg. 2004;188(Suppl. to October 2004):68S–75S. doi: 10.1016/j.amjsurg.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland GR, Latour I, Greer AD. Integrating an image-guided robot with intraoperative MRI – a review of the design & construction of neuroArm; 2008. [DOI] [PubMed]
- 14.Haidegger T., Kovacs L., Fordos G., Benyo Z., Kazanzides P. Proceedings 20. 2008. Future trends in robotic neurosurgery. NBC 2008; pp. 229–233. [Google Scholar]
- 15.Pandya S., Motkoski J.W., Serrano-Almeida C., Greer A.D., Latour I., Sutherland G.R. Advancing neurosurgery with image-guided robotics. J Neurosurg. 2009 Apr 17 doi: 10.3171/2009.2.JNS081334. [DOI] [PubMed] [Google Scholar]
- 16.Ricard D., Idbaih A., Ducray F., Lahutte M., Hoang-Xuan K., Delattre J.Y. Primary brain tumours in adults. Lancet. 2012 doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 17.Stummer W., Kamp M.A. The importance of surgical resection in malignant glioma. Curr Opin Neurol. 2009 Sep 4 doi: 10.1097/WCO.0b013e3283320165. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Sun J.Y., Yang H., Miao S., Li J.P., Wang S.W., Zhu M.Z. Suppressive effects of swainsonine on C6 glioma cell in vitro and in vivo. Phytomedicine: Int J Phytother Phytopharm. 2009 doi: 10.1016/j.phymed.2009.02.012. PMID 19427771. [DOI] [PubMed] [Google Scholar]
- 19.Cheng W., Alder J.R., Jr. An overview of CyberKnife radiosurgery. Chin J Clin Oncol. 2006;3(4) [Google Scholar]
- 20.Hara W., Tran P., Li G., Su Z., Puataweepong P., Adler J.R., Jr. Cyberknife for brain metastases of malignant melanoma and renal cell carcinoma. Neurosurgery. 2009 Feb;64(Suppl. 2):A26–A32. doi: 10.1227/01.NEU.0000339118.55334.EA. [DOI] [PubMed] [Google Scholar]
- 21.Koong A.C., Christofferson E., Le Q.T., Goodman K.A., Ho A., Kuo T. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 01 October 2005;63(2):320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Elder J.B., Liu C.Y., Apuzzo M.L. Neurosurgery in the realm of 10(-9), part 2: applications of nanotechnology to neurosurgery–present and future. Neurosurgery. 2008 Feb;62(2):269–284. doi: 10.1227/01.neu.0000315995.73269.c3. discussion 284–5. [DOI] [PubMed] [Google Scholar]
- 23.Leary S.P., Liu C.Y., Apuzzo M.L. Toward the emergence of nanoneurosurgery: part III–nanomedicine: targeted nanotherapy, nanosurgery, and progress toward the realization of nanoneurosurgery. Neurosurgery. 2006 Jun;58(6):1009–1026. doi: 10.1227/01.NEU.0000217016.79256.16. discussion 1009–26. Review. [DOI] [PubMed] [Google Scholar]
- 24.Mamelak A.N., Jacoby D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin Drug Deliv. 2007;4(2):175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- 25.Veiseh O., Gunn J.W., Kievit F.M., Sun C., Fang C., Lee J.S.H. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Nanomedicine. 2008 Aug;3(4):495–505. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury Oncolytic viral anti-cancer therapy: a magic bullet? Lancet. February 24, 2001;357 doi: 10.1016/S0140-6736(05)71407-0. [DOI] [PubMed] [Google Scholar]
- 27.Varghese S., Rabkin S.D. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 28.Cassady K.A., Parker J.N. Herpesvirus vectors for therapy of brain tumors. Open Virol J. 2010;4:103–108. doi: 10.2174/1874357901004010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobias A., Ahmed A., Moon K.S., Lesniak M.S. The art of gene therapy for glioma: a review of the challenging road to the bedside. Neurol Neurosurg Psychiatry. 2013;84(2):213–222. doi: 10.1136/jnnp-2012-302946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Germano I.M., Emdad L., Qadeer Z.A., Uzzman M. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis, radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer Gene Ther. Sep 2010;17(9):664–674. doi: 10.1038/cgt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehtesham M., Kabos P., Kabosova A., Neuman T., Black K.L., Yu J.S. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. October 15, 2002;62:5657–5663. [PubMed] [Google Scholar]


