Abstract
Introduction
Acute colonic pseudo-obstruction (ACPO) is an uncommon condition that occasionally develops in hospitalized patients with serious underlying ailments. Its early recognition is essential to reduce life-threatening complications. Few low-powered randomized clinical trials (RCTs) have confirmed the effectiveness of neostigmine for treatment.
Aim
To analyse the effectiveness and main side effects of neostigmine in the treatment of ACPO.
Experimental
A literature search was performed for all published RCTs, reporting on neostigmine as treatment for ACPO.
Results
Four studies fulfilled the inclusion criteria, evaluating 127 patients: treatment group = 65, control group = 62. Neostigmine effectiveness to resolve ACPO with only one dose was 89.2% versus 14.65% (P < 0.001, NNT = 1 [95% CI 1–2]).
Conclusions
Neostigmine is a safe and effective option for patients with ACPO who failed to respond to conservative management.
Keywords: Neostigmine, Acute colonic pseudo-obstruction, Side effects
1. Background
Acute colonic pseudo-obstruction (ACPO), also known as Ogilvie syndrome, is the gross dilatation of the colon without mechanical obstruction [1–6]. It mostly occurs in elderly patients with associated medical or surgical conditions [4–8], like cardiovascular, neurological, obstetric, infectious or inflammatory, metabolic, respiratory, post-traumatic, post-surgical and pharmacological insults [3–5,7,9]. It is a rare condition, occurring in 0.046% of patients after coronary bypass grafting [8]; in 0.29% of burn patients [10], and in 0.29–1.3% % of patients after hip, knee arthroplasty or spinal surgery [11,12].
Its aetiology is still unknown [3,5]. The accurate diagnosis remains difficult and it is based upon clinical and radiographic findings [1,3–5]. It is early recognition and management are essential to reduce life-threatening complications, like colonic ischaemia and perforation, and to improve outcome [1–5,7,9]. Once diagnosed, the traditional management is conservative, including gastric decompression, fluid and electrolyte balance correction, rectal tube decompression, limiting offending drugs and the diligent treatment of any underlying condition [3–7,13], which is usually given for 48–72 h [3,5,7] if there is not right iliac fossa tenderness and/or grossly dilatation of the caecum [3]. Nonetheless, many anecdotal reports confirmed the effectiveness of neostigmine for treatment of ACPO, which promoted the interest in the pharmacological treatment of this ailment [3,6]. Furthermore, there are few low-powered randomized clinical trials (RCTs) that have analysed its usefulness [2,14]. To our understanding, only two systematic reviews have evaluated the effectiveness of neostigmine for the treatment of ACPO [2,9], however, none of them performed a meta-analytic approach to fully provide consistent evidence. Our aim was to analyse the effectiveness and main side effects of neostigmine in the treatment of ACPO through a meta-analytic approach of available RCTs.
2. Methodology
A literature search was performed using Embase, Medline, Cochrane, and Pubmed databases, using Boolean logic and the keywords “neostigmine”, “acute colonic pseudo-obstruction” and “Ogilvie syndrome”. Only RTCs were searched, without imposing language, publication date or publication status. Two reviewers independently extracted the data and any discrepancies about inclusion of studies and/or interpretation of data were resolved by arbitration and consensus. Further information was retrieved through manual search of references from recent reviews or published original studies. We performed the same approach directly on Google (search engine), following the same lineaments. The demographic information extracted from each study was first author, publication year, demographics, study design, and number of subjects (Table 1), clinical response in each group and complication rates were measured and tabulated (Table 2). The quality of each trial was assessed using the Jadad score [15] and the CONSORT Statement [16]. The statistical analysis was only done for clinical response and main side effects. For categorical variables the Peto odds ratio was used as the summary statistic. Statistical analysis was done with Comprehensive Meta-Analysist (ver 2.2.0). Results were translated into clinical outcome benefits by calculating the number needed to treat (NNT), which were calculated as the inverse of the absolute risk reduction (the difference between the control event rate minus the experimental event rate) for each study, rounding up to the nearest whole number. The same procedure was used to calculate the number needed to harm (NNH).
Table 1.
Selected randomized controlled trials evaluating the effect of neostigmine in ACPO.
| Author | Year | N | Agea | b | c | Conditions/patients | Dosed | Jadade | Consortf |
|---|---|---|---|---|---|---|---|---|---|
| Orlando | 1994 | 40 | 16 | 24 | Cholecystectomy: 20 Emergency surgery: 20 |
24 mg, nasal puff 6% | 4 | 20 | |
| Ponec | 1999 | 21 | 65.5 | 19 | 2 | Recent surgery: 11 Medical condition: 10 |
2 mg/3–5 min | 4 | 20 |
| von der Spoel | 2001 | 24 | 69.7 | Cardiac surgery: 10 Infectious illness: 7 Cardiac illness: 4 Gastroenterological illness: 1 Gastroenterological surgery: 1 Vascular surgery 1 |
5 mg/50 mL NS @ 4 mL/h | 4 | 27 | ||
| Fanaei | 2008 | 42 | 64 | 30 | 12 | Prostatectomy: 11 patients Laparoscopic appendectomy: 10 Laparoscopic cholecystectomy: 8 Total knee replacement: 5 Hip replacement: 3 Foot amputation: 2 Lumbar laminectomy: 1 Exploratory laparotomy: 1 Open reduction/fixation of fracture: 1 |
2/5 mg in 500 mL NS/30 min | 1 | 17 |
| 127 | 65 | 38 |
Reported as mean (years).
Male.
Female.
Dose and administration route.
Ref. 15.
Ref. 16.
Table 2.
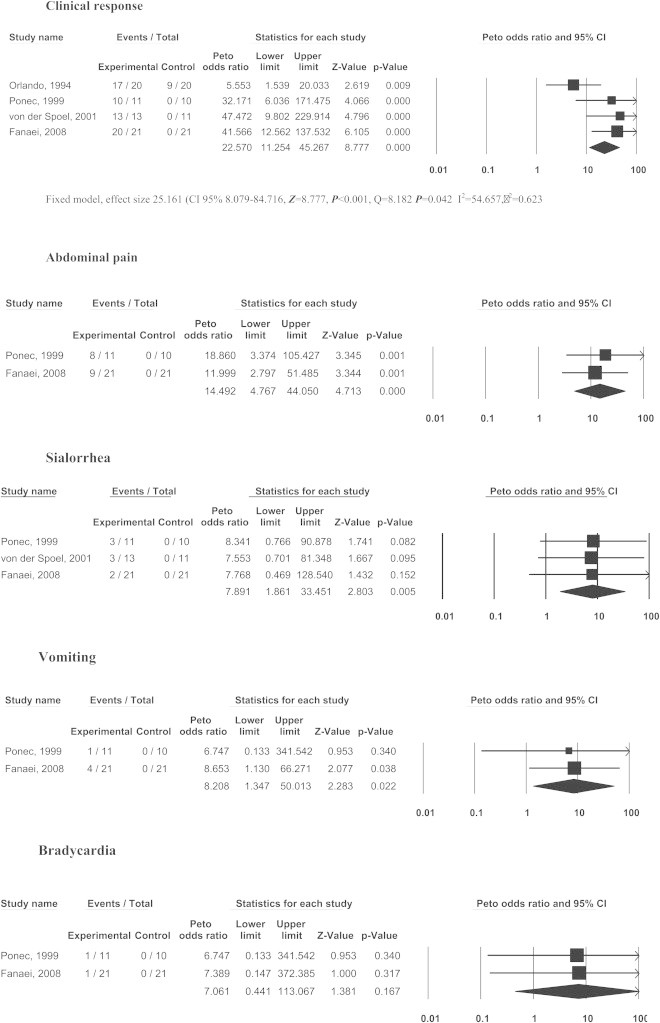
Forest plot of Clinical response and side effects of neostigmine for ACPO.
3. Results
Fig. 1 summarises the selection of studies. Four RCTs were found [17–20]. Table 1 depicts the general information of those RCTs included. The number of patients included in each one ranged from 21 to 42, totalling 127 patients, divided in two groups: 65 in the treatment group (51.2%) and 62 in the control group (48.8%). The average age ranged from 64 to 66.7 years. The male:female ratio was 1.7:1. Only three studies used the intravenous route (dose ranged from 2.0 to 5 mg) and the administration time ranged from 3 min to 12 h. Only one article used the nasal route, with a total dose of 24 mg, equivalent to 0.55 mg IV [21]. Tables 2 and 3 show the main results, as follow:
-
1)
Neostigmine effectiveness to resolve ACPO with only one dose averaged was 89.2% (ranging from 84.6 to 95.2%) versus 14.8% (from 0.0 to 45.0%) of control group (NNT = 1 [95% CI 1–2]).
-
2)
Size effects:
-
a)
Abdominal pain was the most common side effect, occurring in 53.1% (42.9–72.7%, OR = 17.4 [IC 95% 5.3–57.2], NNH = 2 [95% CI 1–3]).
-
b)
Sialorrhoea was the second most common side effect, occurring in 31.1% (23.1–38.1%, OR = 9.4 [IC 95% 3.0–29.2], NNH = 3 [95% CI 2–6]).
-
c)
Vomiting was the next most common side effect, seen in 15.6% (9.1–19.1%, OR = 7.5 [IC 95% 1.2–48.8], NNH = 5 [95% CI 3–375]).
-
d)
Bradycardia appeared in 6.3% (4.8–9.1%, OR = 6.3 [IC 95% 0.3–102], NNH = 16 [95% CI 6–25]). A consideration is that, even though the OR > 1, the lower limit of the confidence interval spanned below 1, therefore, this is not significant, because of the very few cases included.
Fig. 1.
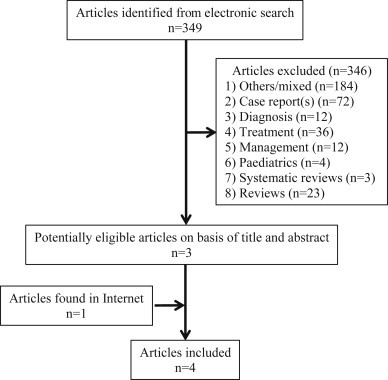
Process of study selection.
Table 3.
Clinical significance of neostigmine for ACPO.
| Author | Experimental group |
Control group |
||||||
|---|---|---|---|---|---|---|---|---|
| Year | Event | Total | Event | Total | NNT | 95% CI | ||
| Clinical response | ||||||||
| Orlando | 1994 | 17 | 20 | 9 | 20 | 3 | 1 | 8 |
| Ponec | 1999 | 10 | 11 | 0 | 10 | 1 | 1 | 2 |
| von der Spoel | 2001 | 11 | 13 | 0 | 11 | 1 | 1 | 2 |
| Fanaei | 2008 | 20 | 21 | 0 | 21 | 2 | 1 | 2 |
| Total | 58 | 65 | 9 | 62 | 1 | 1 | 2 | |
| Abdominal pain | ||||||||
| Ponec | 1999 | 8 | 11 | 0 | 10 | 1 | 1 | 2 |
| Fanaei | 2008 | 9 | 21 | 0 | 21 | 2 | 2 | 5 |
| Total | 17 | 32 | 0 | 31 | 2 | 1 | 3 | |
| Sialorrhoea | ||||||||
| Ponec | 1999 | 3 | 11 | 0 | 10 | 4 | 2 | 12 |
| von der Spoel | 2001 | 3 | 13 | 0 | 11 | 5 | 2 | 22 |
| Fanaei | 2008 | 8 | 21 | 0 | 21 | 3 | 2 | 8 |
| Total | 14 | 45 | 0 | 62 | 3 | 2 | 6 | |
| Vomiting | ||||||||
| Ponec | 1999 | 1 | 11 | 0 | 10 | 13 | 3 | 5 |
| Fanaei | 2008 | 4 | 21 | 0 | 21 | 6 | 3 | 54 |
| Total | 5 | 32 | 0 | 31 | 7 | 3 | 375 | |
| Bradycardia | ||||||||
| Ponec | 1999 | 1 | 11 | 0 | 10 | 13 | 3 | 5 |
| Fanaei | 2008 | 1 | 21 | 0 | 21 | 23 | 4 | 9 |
| Total | 2 | 32 | 0 | 31 | 16 | 6 | 25 | |
NNT=Number needed to treat; NNH=Number needed to harm.
There was substantial heterogeneity in the included studies (I2 index = 63.34%), probably because the differences were more related to study design (small number of RCTs or high response in the control arm of the study of Orlando) rather than chance. When the study of Orlando was removed from the analysis, the effectiveness of neostigmine for ACPO resolution was 95.6% versus 0.00% in the control group, (NNT = 1 [95% CI 1–1], Z = 8.757, P < 0.001, Q = 0.113, I2 = 0.000, τ2 = 0.000). But it is important to state that a funnel plot representation with only four studies is not appropriate, because the power of the test is too low to able to discriminate chance from real asymmetry.
4. Discussion
ACPO is still a poorly understood entity with an aetiology attributed to an autonomic imbalance between the sympathetic and parasympathetic innervation of the colon [5,22], which leads to inhibition of peristalsis [22], due to either sympathetic stimulation or parasympathetic suppression [4,5,9]. Trevisani concluded that the most plausible aetiology of ACPO was because of parasympathetic suppression, and not by sympathetic over-activity [23] and this seems to be the current pathophysiological explanation [4,7].
Neostigmine, a parasympathomimetic agent that reversibly inhibits acetylcholine hydrolysis by competing with acetylcholinesterase at sites where cholinergic transmission occurs [3–5,7,19,24], exerting its effect through two mechanisms: increasing the amount of available acetylcholine and indirectly stimulating nicotinic and muscarinic receptors [5,24]. In humans, the digestive system has muscarinic receptors M1 expressed in salivary glands and stomach (promoting sialorrhoea, gastric secretion and vomiting) [25], but the most abundant muscarinic receptors both in mucosa and in smooth muscle are M2 and M3 subtypes that, when stimulated, produce muscle contraction [25,26]. Besides, neostigmine administered intravenously has a rapid onset of action (<20 min) and short duration (<2 h) [5].
The first open label trial of the use of neostigmine for ACPO was done by Hutchinson in 1992 [27]. After him, other open label trials have been conducted. Considering the clinical effectiveness of neostigmine showed in multiple previous case reports of patients with ACPO, some low powered RCTs were conducted, which likewise proved its effectiveness. Therefore, it was logical to consider that when functional paralysis of the colon exists, once mechanical obstruction has been ruled out, a pharmacological approach be used. To our knowledge, there are two systematic reviews about the effectiveness of neostigmine for ACPO [2,9]: one by Saunders in 2005 (5 open label trials, 2 retrospective analysis and only 1 RCT) and one by Elsner in 2012 (8 prospective trials, 3 retrospective observational studies, 9 case reports and only 1 RCT). For both authors, the study of Ponec was the only RCT included. Ours is the first meta-analytic approach that provides consistent evidence of its clinical usefulness assessing four RCTs with placebo as comparator. We found that neostigmine is very effective resolving ACPO, with an efficacy of ∼90% versus <15% with conservative management. Time of resolution was not analysed because such information was only available in one source, which reported a mean response time of 4 min [18]. Besides, the optimal dose of neostigmine still remains in debate [7].
It is true that other treatment options have been used in the treatment of ACPO after conservative management has failed. In 2001, Ramirez published a case report of a patient with Guillain–Barré syndrome complicated by ACPO successfully treated with tegaserod (a 5-hydroxytryptamine type 4 [5-HT4 receptor] agonist) [28]. In the same year, De Giorgio published a review about the pharmacological treatment of ACPO, and mentioned the use of other 5-HT4 receptor agonist and motilin receptor agonists [29]. Nonetheless, there are not controlled trials formally assessing their use in ACPO [29]. Furthermore, some authors have proposed other pharmacological options to prevent the reoccurrence of ACPO. For example, Sgouros found that the use of an electrolyte balanced polyethylene glycol solution after the initial resolution of ACOP increased the chances of continual response rate once initial therapeutic intervention was achieved (either with neostigmine or colonoscopy decompression with tube placement) [30].
Others favour the initial use of endoscopic means for ACPO. In his study, Tsirline concluded that colonoscopy was superior to neostigmine for ACPO (75.0% vs. 55.6%, P = 0.044) and even proposed it as first-line therapy [31]. Nevertheless, his study presented important limitations: the most important that it was retrospective; that the majority of patients had multiple interventions (neogstimine and colonoscopy) and that some patients had crossover treatments. Moreover, he did not compare the electrolyte levels among the groups as a possible cause for success/failure and finally, that the doses of neostigmine, given as bolus, ranged from 0.5 to 2 mg [31].
We do not know whether some certain causes of ACPO are more sensitive to neostigmine treatment. Even though the four found RCTs delivered information about the aetiology of ACPO, none provided specific information about the distribution of causes of ACPO on the experimental and control arms. Thus, such subgroup analysis would be unmanageable to perform. So far, we found that neostigmine is useful for medical and surgical causes of ACPO. Notwithstanding, there is clinical evidence that the same medication works in paediatric haematologic malignancies (at doses of 0.01 mg/kg/dose) with an 80% success rate [32].
Nonetheless, it should be acknowledged that the treatment of ACPO with neostigmine is indeed associated with some side effects, among them abdominal pain (>50% of patients, related to the strong smooth muscle contraction), sialorrhoea (>30% of patients) and vomiting (>15%). Bradycardia, the most fearsome side effect, occurred only in the minority of patients (∼6.5%). The main drawback of this meta-analysis is the relative few low powered RCTs assessed. However, the majority of studies presented good-regular design quality, according to the Jadad and CONSORT assessments.
5. Conclusions
Neostigmine is a safe and effective option for patients with ACPO who failed to respond to conservative management but it is associated with some important side effects. Further studies are required to specifically assess the early use of neostigmine versus conservative management, both to determine the probably role of neostigmine as a first line therapy for ACPO and to weigh the incidence and severity of such side effects. Such studies will also provide information about the success rate according with the different conditions associated with ACPO. (Sort Of Recommendation: A, Quality of Evidence: Level I (consistency of evidence across studies).
Conflict of Interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Ertberg P., Vilandt J., Bødker B. Diagnosis and treatment of acute colonic pseudoobstruction. Ugeskr Laeger. 2013 Apr 22;175(17):1176–1180. [PubMed] [Google Scholar]
- 2.Elsner J.L., Smith J.M., Ensor C.E. Intravenous neostigmine for postoperative acute colonic pseudo- obstruction. Ann Pharmacother. 2012;46:430–435. doi: 10.1345/aph.1Q515. [DOI] [PubMed] [Google Scholar]
- 3.De Giorgio R., Knowles C.H. Acute colonic pseudo- obstruction. Br J Surg. 2009 Mar;96(3):229–239. doi: 10.1002/bjs.6480. [DOI] [PubMed] [Google Scholar]
- 4.Maloney N., Vargas H. Acute intestinal pseudo-obstruction (Ogilvie's syndrome) Clin Colon Rectal Surg. 2005 May;18(2):96–101. doi: 10.1055/s-2005-870890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders M.D. Acute colonic pseudo-obstruction. Best Pract Res Clin Gastroenterol. 2007;21(4):671–687. doi: 10.1016/j.bpg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Batke M., Cappell M.S. Adynamic ileus and acute colonic pseudo-obstruction. Med Clin North Am. 2008 May;92(3):649–670. doi: 10.1016/j.mcna.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Jain A., Vargas H.H. Advances and challenges in the management of acute colonic pseudo-obstruction (Ogilvie Syndrome) Clin Colon Rectal Surg. 2012 March;25(1):37–45. doi: 10.1055/s-0032-1301758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenofsky P.L., Beamer L., Smith R.S. Ogilvie syndrome as a postoperative complication. Arch Surg. Jun 2000;135(6):682–687. doi: 10.1001/archsurg.135.6.682. [DOI] [PubMed] [Google Scholar]
- 9.Saunders1 M.D., Kimmey M.B. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther. 2005 Nov 15;22(10):917–925. doi: 10.1111/j.1365-2036.2005.02668.x. [DOI] [PubMed] [Google Scholar]
- 10.Kadesky K., Purdue G.F., Hunt J. Acute pseudo-obstruction in critically ill patients with burns. J Burn Care Rehabil. 1995 Mar-Apr;16(2 Pt 1):132–135. doi: 10.1097/00004630-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Clarke H.D., Berry D.J., Larson D.R. Acute pseudo-obstruction of the colon as a postoperative complication of hip arthroplasty. J Bone Joint Surg Am. 1997 Nov;79(11):1642–1647. doi: 10.2106/00004623-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Norwood M.G., Lykostratis H., Garcea G., Berry D.P. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. Sep 2005;7(5):496–499. doi: 10.1111/j.1463-1318.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisen G.M., Baron T.H., Dominitz J.A. Acute colonic pseudo-obstruction. Gastrointest Endosc. 2002;56(6):789–792. doi: 10.1016/s0016-5107(02)70348-9. [DOI] [PubMed] [Google Scholar]
- 14.Kayani B., Spalding D.R., Jiao L.R., Habib N.A., Zacharakis E. Does neostigmine improve time to resolution of symptoms in acute colonic pseudo-obstruction? Int J Surg. 2012;10(9):453–457. doi: 10.1016/j.ijsu.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Schulz K.F., Altman D.G., Moher D., for the CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med. 2010:152. doi: 10.3736/jcim20100702. Epub 24 March. [DOI] [PubMed] [Google Scholar]
- 17.Orlando E., Finelli F., Colla M., Giotto E., Terragni P., Olivero G. A double-blind study of neostigmine versus placebo in paralytic ileus as a result of surgical interventions. Minerva Chir. 1994 May;49(5):451–455. [PubMed] [Google Scholar]
- 18.Ponec R.J., Saunders M.D., Kimmey M.B. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 19.van der Spoel J.I., Oudemans-van Straaten H.M., Stoutenbeek C.P., Bosman R.J., Zandstra D.F. Neostigmine resolves critical illness-related colonic ileus in intensive care patients with multiple organ failure–a prospective, double-blind, placebo-controlled trial. Intensive Care Med. 2001 May;27(5):822–827. doi: 10.1007/s001340100926. [DOI] [PubMed] [Google Scholar]
- 20.Fanaei S.A., Hasan Ali M., Mehran M. Neostigmine for the treatment of acute colonic pseudo-obstruction in patients with abdominal surgery. Kowsar Med J. 2008;12(4):331–359. [Google Scholar]
- 21.Broggini M., Benvenuti C., Botta V., Fossati A., Valenti M. Bioavailability of intranasal neostigmine: comparison with intravenous route. Methods Find Exp Clin Pharmacol. 1991 Apr;13(3):193–198. [PubMed] [Google Scholar]
- 22.Ponzano C., Nardi S., Carrieri P., Basili G. Diagnostic problems, pathogenetic hypothesis and therapeutic proposals in Ogilvie's syndrome. Review of 470 observations from the literature and presentation of 5 new cases. Minerva Chir. 1997 Nov;52(11):1311–1320. [PubMed] [Google Scholar]
- 23.Trevisani G.T., Hyman N.H., Church J.M. Neostigmine: safe and effective treatment for acute colonic pseudo-obstruction. Dis Colon Rectum. 2000 May;43(5):599–603. doi: 10.1007/BF02235569. [DOI] [PubMed] [Google Scholar]
- 24.Nair V.P., Hunter J.M. Anticholinesterases and anticholinergic drugs. Contin Educ Anaesth Crit Care Pain. 2004;4(5):164–168. [Google Scholar]
- 25.Tobin G., Giglio D., Lundgren O. Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol. 2009;60(1):3–21. [PubMed] [Google Scholar]
- 26.Uchiyama T., Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res. 2004;40(6):237–247. doi: 10.1540/jsmr.40.237. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson R., Griffiths C. Acute colonic pseudo-obstruction: a pharmacological approach. Ann R Coll Surg Engl. 1992;74:364–367. [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez R., Zuckerman M.J., Hejazi R.A. Treatment of acute colonic pseudo-obstruction with tegaserod. Am J Med Sci. 2010 Jun;339(6):575–576. doi: 10.1097/MAJ.0b013e3181db6b95. [DOI] [PubMed] [Google Scholar]
- 29.De Giorgio R., Barbara G., Stanghellini V. Review article: the pharmacological treatment of acute colonic pseudo-obstruction. Aliment Pharmacol Ther. 2001 Nov;15(11):1717–1727. doi: 10.1046/j.1365-2036.2001.01088.x. [DOI] [PubMed] [Google Scholar]
- 30.Sgouros S., Vlachogiannakos J., Vassiliadis K. Effect of polyethylene glycol electrolyte balanced solution on patients with acute colonic pseudo-obstruction after resolution of colonic dilation: a prospective, randomised, placebo controlled trial. Gut. 2006;55:638–642. doi: 10.1136/gut.2005.082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsirline V.B., Zemlyak A.Y., Avery M.J. Colonoscopy is superior to neostigmine in the treatment of Ogilvie's syndrome. Am J Surg. 2012 Dec;204(6):849–855. doi: 10.1016/j.amjsurg.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Jw Lee, Bang K.W., Jang P.S. Neostigmine for the treatment of acute colonic pseudo-obstruction (ACPO) in pediatric hematologic malignancies. Korean J Hematol. 2010;45:62–65. doi: 10.5045/kjh.2010.45.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]


