Abstract
Regional epidemiological data and resistance profiles are essential for selecting appropriate antibiotic therapy for intra-abdominal infections (IAIs). However, such information may not be readily available in many areas of Asia and current international guidelines on antibiotic therapy for IAIs are for Western countries, with the most recent guidance for the Asian region dating from 2007. Therefore, the Asian Consensus Taskforce on Complicated Intra-Abdominal Infections (ACT-cIAI) was convened to develop updated recommendations for antibiotic management of complicated IAIs (cIAIs) in Asia. This review article is based on a thorough literature review of Asian and international publications related to clinical management, epidemiology, microbiology, and bacterial resistance patterns in cIAIs, combined with the expert consensus of the Taskforce members. The microbiological profiles of IAIs in the Asian region are outlined and compared with Western data, and the latest available data on antimicrobial resistance in key pathogens causing IAIs in Asia is presented. From this information, antimicrobial therapies suitable for treating cIAIs in patients in Asian settings are proposed in the hope that guidance relevant to Asian practices will prove beneficial to local physicians managing IAIs.
Keywords: Epidemiology, Intra-abdominal infection, Antibiotics, Asia, Microbiology, Resistance
Highlights
-
•
We propose updated recommendations for antibiotic management of cIAIs in Asia.
-
•
Literature from Asian and international publications are reviewed and compared.
-
•
Antimicrobial resistance in key pathogens causing IAIs in Asia is presented.
-
•
Expert consensus of the Asian Taskforce members is also included.
1. Introduction
Management of complicated intra-abdominal infections (cIAIs) in Asia remains challenging, and differs in important aspects from practices in the West. Disease patterns differ, and available resources including operating facilities, ancillary personnel and imaging equipment vary from one region to another. Importantly, microbial resistance and access to specific antimicrobial agents varies from region to region.
Available guidelines on the management of cIAIs are predominantly written for the Western context [1,2], although Asian guidance on antimicrobial therapy for cIAIs has been previously developed [3]. The current document outlines the microbiological profiles of IAIs in the Asian region for comparison with the West, and explores antimicrobial resistance in key pathogens causing IAIs in Asia. We also provide updated guidance on antimicrobial management of cIAIs in the Asian setting.
2. Methods
The Asian Consensus Taskforce on Complicated Intra-Abdominal Infections (ACT-cIAI) met and discussed current clinical approaches to cIAI management in Asia, international guidelines on cIAI management, microbiological trends of cIAI pathogens in Asia, and the existing antibiotic options. A steering committee was formed to explore differences between Asia and the West. Where applicable, existing guidelines were consulted, including the American Infectious Diseases Society of America (IDSA)/Surgical Infection Society (SIS) guidelines, as well as published recommendations from the Asian region. Microbiology data on cIAI pathogens and data on bacterial resistance rates in Asian countries were obtained from published surveillance studies and the Tigecycline Evaluation and Surveillance Trial (TEST) database.
After a second meeting to review and refine the compiled document, the revised document was circulated to the Taskforce for final review and comments. The final document is endorsed by the International Surgical Infections Study (ISIS) Group.
3. Definitions
In accordance with the American IDSA/SIS guidelines, we are defining cIAIs as an infection that “extends beyond the hollow viscus of origin into the peritoneal space and is associated with either abscess formation or peritonitis” [1].
4. Clinical epidemiology of cIAIs in Asia
4.1. Key pathogens causing cIAIs in Asia
Chief amongst factors influencing the success of management of cIAI in Asia is the high prevalence of antimicrobial resistance, especially amongst Enterobacteriaceae. Data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) [4] provide the best available evidence for the current status of cIAI infections in Asia, but limitations include the small number of contributing sites per country and the selection process for test organisms. These sites are usually major teaching or tertiary-care centers, which potentially creates sample bias. Another limitation is that in vitro susceptibility results do not necessarily predict clinical success or failure. Furthermore, although isolates are divided into those obtained from cultures collected <48 h and ≥48 h after hospital admission to differentiate community-acquired from nosocomial infections, the time of collection may not represent the true time of acquisition of infection [5]. Thus, the SMART results may not accurately reflect the patterns of pathogen distribution in community versus healthcare settings. However, the results are useful in comparing gross trends between Asia and other global regions.
Reports from Asia–Pacific countries participating in SMART confirm the polymicrobial basis for cIAI. Enterobacteriaceae were the most frequently isolated microorganisms in patients with cIAIs, comprising 68.3%–89.5% [6–8]. Of these, Escherichia coli and Klebsiella pneumoniae were the most common, like the West, with Enterobacter spp, including Enterobacter cloacae, found at lower rates. Whilst Pseudomonas aeruginosa featured across the world as a non-Enterobacteriaceae pathogen, Acinetobacter baumannii was more prominent in the Asian setting. In some areas, including Asia, Aeromonas hydrophila and Citrobacter freundii have also been implicated in these polymicrobial infections [9]. However, the pattern of bacterial causes of cIAI infections varies across the Asia–Pacific region. Table 1 shows the most common pathogens causing IAIs in selected Asian countries, alongside data from the USA and Europe [7,9–18].
Table 1.
Top five pathogens causing IAIs in selected Asian and Western countries.
| Country | Rank |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Asia–Pacific [10] (N = 2189 isolates) |
E. coli (48.5%) (ESBL+ = 19.8%; ESBL− = 28.7%) |
K. pneumoniae (20.2%) (ESBL+ = 4.3%; ESBL− = 15.9%) |
P. aeruginosa (10.5%) | A. baumannii (5.0%) | E. cloacae (4.6%) |
| China [7] (N = 3420 isolates) |
E. coli (49.2%) (ESBL+ = 25.8%; ESBL− = 23.4%) |
K. pneumoniae (16.9%) (ESBL+ = 5.6%; ESBL− = 11.3%) |
P. aeruginosa (8.4%) | E. cloacae (5.8%) | A. baumannii (4.6%) |
| Indonesiaa | E. coli (20.4%) | K. pneumoniae (13.3%) | S. aureus (10.9%) | P. aeruginosa (8.4%) | S. haemolyticus (7.1%) |
| Singapore [11,12] | E. coli (20–25%) | K. pneumoniae | Pseudomonas spp | Bacteroides spp | Enterococcus spp |
| Thailand [13] (N = 1305 isolates) |
E. coli (36%) | K. pneumoniae (12%) | P. aeruginosa (8%) | A. baumannii (4%) | Enterococcus spp (3%) |
| Taiwan [14] (N = 2417 isolates) |
E. coli (38.8%) | K. pneumoniae (23.5%) | P. aeruginosa (9.3%) | E. cloacae (6.1%) | A. baumannii (3.8%) |
| India [15] (N = 542 isolates) |
E. coli (62.7%) | K. pneumoniae (16.6%) | P. aeruginosa (5.4%) | C. freundii (2.2%) | A. baumannii (2.0%) |
| Philippines [16]b (N = 77 patients) |
E. coli (35%) | K. pneumoniae (15%) | P. aeruginosa (14%) | E. cloacae (6%) | A. baumannii (5%) |
| Korea [17]b (N = 256 isolates) |
E. coli (22.1%) | P. aeruginosa (15.7%) | Enterococcus spp (15.2%) | Enterobacter spp (12.5%) | K. pneumoniae (11.4%) |
| USA [9] (N = 1522 isolates) |
E. coli (43.4%) (ESBL+ = 4.8%) |
K. pneumoniae (15.1%) (ESBL+ = 9.5%) |
P. aeruginosa (14.3%) | E. cloacae (7.4%) | P. mirabilis (3.5%) |
| Europe [9] (N = 7844 isolates) |
E. coli (51.6%) (ESBL+ = 8.0%) |
K. pneumoniae (9.7%) (ESBL+ = 16.2%) |
P. aeruginosa (8.0%) | E. cloacae (6.2%) | P. mirabilis (4.7%) |
Single-institution data from appendicitis cases in Dr Hasan Sadikin Hospital, Bandung, Indonesia (Usman N, unpublished data, 2012).
Data from single-center study of bile cultures from cholangitis cases.
4.2. Antimicrobial resistance in pathogens causing cIAIs in Asia
Isolates from Asia–Pacific countries showed the highest levels of antimicrobial resistance of the global regions included the SMART study, and a trend of increasing resistance continues year by year [5,10]. Within the region, resistance rates can vary between countries; for example, resistance in Enterobacteriaceae in China and India was higher than in the other countries of this region, especially to ampicillin–sulbactam, fluoroquinolones, and cephalosporins [8]. Updated results from ongoing monitoring continue to confirm high levels of resistance [8,18]. The high resistance rate of Enterobacteriaceae isolates to ampicillin–sulbactam supports the guideline recommendation against using ampicillin–sulbactam for patients with IAIs, especially in severe cases [1,3].
Production of β-lactamases is among the most common and clinically significant of the resistance mechanisms for Gram-negative bacilli [19]. Multiple types of β-lactamases exist (Table 2) [19,20], and bacteria usually carry several simultaneously. Clinically, the extended-spectrum β-lactamases (ESBLs) are a particular concern. [20]
Table 2.
Major groups of β-lactamases in Gram-negative bacteria that threaten the role of β-lactam antibiotics.
| Functional group | Common name | Examples | β-Lactams to which resistance is conferred |
|---|---|---|---|
| 1 | Cephalosporinase | AmpC | Penicillins, cephalosporins |
| 2b | Broad-spectrum penicillinase | TEM-1, SHV-1 | Penicillins, early cephalosporins |
| 2be | Extended-spectrum β-lactamases | TEM-10, SHV-2, CTX-M-type, VEB | Penicillins, cephalosporins, monobactams, β-lactamase inhibitor combinations |
| 2d | Oxacillin hydrolyzing/Cloxacillinase | OXA-1 to 11, PSE-2 | Penicillins, including oxacillin and cloxacillin |
| 2df | Carbapenemase | Carbapenems and other β-lactams | |
| 2f | Carbapenemase | KPC-1,2, SME-1 | All current β-lactams |
| 3 | Metallo-β-lactamases | IMP-1, VIM-1, NDM-1 | All β-lactams except monobactams |
Adapted from Refs. [19,20].
4.3. ESBLs in Asia and ESBL-producing pathogens causing IAIs
ESBLs almost certainly emerged de novo in Asia, with early resistance studies detecting elevated levels of ESBL phenotypes, particularly among Klebsiella isolates and particularly in China, South Korea, Japan and India [21].
Data from the SMART study for 2005–2010 show that the Asia–Pacific region has consistently had the highest ESBL positivity rates for E. coli, K. pneumoniae and Klebsiella oxytoca isolated from IAIs (Fig. 1) [22]. As of 2012, ESBL production amongst targeted Enterobacteriaceae isolates in the Asia–Pacific region was higher than other geographic areas, at nearly 40% [23]. Amongst isolates from ICU patients in Vietnam and the Philippines, the prevalence of ESBL-producers was as high as 81.0% and 58.8%, respectively [23]. However, this trend is not uniform across Asia. In Taiwan, the rates of ESBL-producing E. coli and K. pneumoniae were 7.5% and 7.0%, respectively, among 610 Enterobacteriaceae isolates [8]. ESBL positivity rates were lower among isolates recovered from patients with community-acquired IAIs than those with hospital-acquired infections (3.6% vs. 13.9% for E. coli and 2.4% vs. 13.3% for K. pneumoniae isolates, respectively) [8]. Fig. 2 illustrates the marked variability across the Asia–Pacific region in the prevalence of ESBL-producing E. coli and K. pneumoniae isolates from IAIs [18].
Fig. 1.
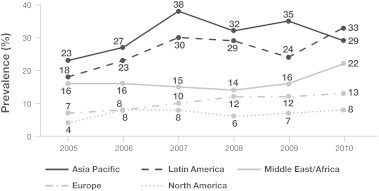
Global trends in ESBL-producing Enterobacteriaceae*: SMART study 2005–2010 [22]. * Includes ESBL-positive E. coli, K. pneumoniae and K. oxytoca
Fig. 2.
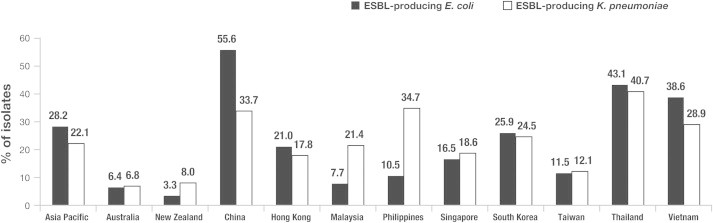
Prevalence of ESBL producers among E. coli and K. pneumoniae isolates from IAIs in 11 Asia–Pacific countries in 2010 [18]. Reprinted from Int J Antimicrob Agents, Volume 40 Supplement, Huang CC, et al., Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia–Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002–2010, S4–S10, 2012, with permission from Elsevier.
Although not as prevalent as enterobacteria, multidrug-resistant A. baumannii and P. aeruginosa are also implicated in cIAIs, particularly post-operative infections. Their rates of resistance are far higher: 29.8% of P. aeruginosa and 73.0% of A. baumannii isolates were not susceptible to at least one carbapenem in 2010 data from the Comparative Activity of Carbapenem Testing (COMPACT) II study [23]. A separate study of P. aeruginosa isolates from ICU patients in 19 Chinese hospitals reported a marked decrease in susceptibility to imipenem (from 81% to 62%) between 1996 and 2002 [24].
The advent of New Delhi metallo-β-lactamase-1 (NDM-1) K. pneumoniae heralded the emergence of carbapenamases amongst Enterobacteriaceae in Asia. Medical tourism within the region has compounded the problem, facilitating geographical spread of NDM-1 Klebsiella spp [25].
5. Recommended antimicrobial regimens
Whilst antibiotics have an important role, source control must be addressed in all cases of cIAIs to avoid prolonging morbidity and begetting resistance.
Initiation of antimicrobial therapy should occur as soon as an IAI is diagnosed or considered likely [1] (i.e., within 4–6 h). For patients with septic shock, antibiotics should be administered as soon as possible [1] (i.e., within 1 h).
Appropriate choices of antibiotics for cIAIs are best guided by local antibiograms and resistance patterns. However, if such information is not readily available, general principles governing the judicious use of antibiotics should be applied.
Firstly, patients at risk for ESBL acquisition or multidrug-resistant P. aeruginosa or A. baumannii should be identified. The prevalence of ESBL-producing organisms implicated in cIAIs in Asia makes it imperative that moderate to severely ill patients with cIAIs with such risks are treated correctly at the outset to mitigate morbidity and mortality. Secondly, broad-spectrum antibiotic use should be avoided when the risk of infection with ESBL-producing pathogens is low or absent; for example, in community-acquired infections particularly where the community prevalence of ESBL organisms is <20%. Furthermore, a recent Indian study concluded that broad-spectrum agents are not required for mild infections even in areas with high prevailing ESBL rates [26]. Finally, antibiotic stewardship should be adopted across Asia as a pivotal strategy to stem the tide of ‘delinquent’ antibiotic use in hospitals and in the community.
Antibiotic recommendations are based on community versus hospital acquisition and clinical severity of illness, which define relative risks for acquiring resistant flora. Table 3 outlines suggested antimicrobial regimens for treating IAIs in the Asian region. This information is based on our review of the existing data and the consensus of this group, the ACT-cIAI.
Table 3.
Suggested antibiotic regimens for treating community- and hospital-acquired IAIs in the Asian region.
| Type of therapy |
||||
|---|---|---|---|---|
| Monotherapy |
Combination therapy |
|||
| Drug of choice | Alternativea | Drugs of choice | Alternativea | |
| Community-acquired cIAI | ||||
| Mild to moderate | Amoxicillin/clavulanate | Moxifloxacinb | Cefazolin, cefuroxime, ceftriaxone or cefotaxime + metronidazole | Levofloxacin + metronidazole Ciprofloxacin + metronidazoleb |
| Severe | Amoxicillin/clavulanate | Moxifloxacinb Ertapenem or tigecyclinec Meropenem, imipeneme/cilastin, doripenem, piperacillin/tazobactamd |
Cefazolin, cefuroxime, ceftriaxone, ceftazidime, cefepime or cefotaxime + metronidazole | Levofloxacin + metronidazole Ciprofloxacin + metronidazoleb |
| Healthcare-associated cIAI | ||||
| Mild to moderate | Meropenem, imipenem, imipenem/cilastin, doripenem or piperacillin/tazobactam [8] | Tigecycline, moxifloxacin, ertapenemf | Cefepime or levofloxacin/ciprofloxacing + metronidazole | |
| Severej | Meropenem, imipenem/cilastin or doripenem | Meropenem, imipenem/cilastin or doripenem + vancomycin or linezolidh Tigecycline + aztreonam/ciprofloxacin/levofloxacin |
Tigecycline + levofloxacin or ciprofloxacinh Carbapenem, tigecycline, polymixin B or colistin ± aminoglycosidei Carbapenem + tigecycline, polymixin B or colistink |
|
Alternative therapy includes the following considerations: allergy, pharmacology/pharmacokinetics, compliance, costs, and local resistance profiles.
Use in cases of β-lactam allergy.
Alternative to cephalosporin or quinolone monotherapy, if the prevalence of community-acquired ESBL+ or quinolone-resistant E. coli is >20%.
Reserve these antipseudomonal regimens for use in patients with neutropenia, septic shock or who are critically ill.
Use imipenem with caution where there is a high prevalence of increased imipenem MICs in Enterobacteriaceae.
Risk groups for P. aeruginosa include patients with neutropenia, septic shock or an indwelling central venous catheter [28].
If hospital-acquired ESBL prevalence is >20%, use the carbapenems preferentially over piperacillin/tazobactam, cefepime, ceftazidime or levofloxacin.
Use if both hospital-acquired ESBL and MRSA prevalence rates are >20%.
Use for suspected pseudomonal or Acinetobacter infections with reduced susceptibility (tigecycline only for Acinetobacter).
Antifungal therapy may be required in selected circumstances as advised in current IDSA guidelines.
Use if carbapenemases (e.g., NDM and KPC) with extended resistance to currently available antibiotics are suspected; also seek expert advice.
Cost is an important factor in Asia, and must be taken into consideration in any discussion of medical practice patterns. The direct cost of the medication can influence choice of therapy, particularly if patients must pay for medicines as an out-of-pocket expense. In addition, inappropriate initial antibiotic use (i.e., use of an agent the causative pathogen is not susceptible to) has cost implications; it has been shown to significantly raise patient care costs [27].
6. Tropical infections
A unique aspect of management in Asia is the existence of several tropical infections that can produce cIAI. These infections are more common in rural than urban centers.
Clinicians should be aware of the differential diagnosis of intra-abdominal sepsis, including tropical infectious disease conditions such as abdominal tuberculosis, salmonellosis, ascariasis, and amebiasis. Dengue hemorrhagic fever and malaria may also present symptoms overlapping with cIAI. Table 4 presents several tropical conditions, their symptoms and typical treatment.
Table 4.
Tropical infectious diseases producing cIAI.
| Condition | Symptoms | Diagnostic options | Treatment | Comments |
|---|---|---|---|---|
| Amebic infection | Fever, pain in right upper abdomen, liver tenderness, tachycardia, hepatomegaly, nausea and vomiting, jaundice | Test for Entamoeba histolytica via IHA, ELISA, or PCR test for DNA in urine; abdominal ultrasound or CT | Metronidazole, tinidazole | Medical management is sufficient for simple amebiasis; for liver abscesses larger than 5 cm, percutaneous drainage may also be required |
| Intestinal ascariasis | Intestinal obstruction, vomiting of passing of worms in stool, symptoms of appendicitis | X-Ray of abdomen; abdominal ultrasound to confirm; gastrograffin | Albendazole, mebendazole | Ascariasis is endemic in Asia Pacific. Drug therapy should be followed by repeat stool examination to confirm eradication, as recurrence is common |
| Abdominal tuberculosis | Acute or chronic intestinal obstruction, abdominal distention, perforation, enterocutaneous or perianal fistula | Sputum for AFB smear and culture; AFB smear and culture of abdominal biopsy sample; abdominal ultrasound or CT scan | Isoniazid, rifampin, pyrazinamide, ethambutol; plus coverage for IAI | Treatment will depend on the type of abdominal complication. Resistance to drug treatment is a problem in Asia |
| Intra-abdominal salmonellosis | Nausea, vomiting, diarrhea, dyspepsia, bloating; in severe cases, perforation or abscess | Culture for Salmonella of blood, stool, rectal swab or gastric washing samples; ultrasound of abdomen | Antibiotic coverage for high-severity IAI Ceftriaxone, cefotaxime; various second-line regimens |
Drug resistance is an increasing problem in Asia High mortality |
7. Management illustration: antibiotic therapy in acute cholangitis
The presentation of acute cholangitis varies from mild biliary colic to septic shock. Patients with acute cholangitis may deteriorate rapidly and progress to a life-threatening critical condition [29], underscoring the importance of early diagnosis.
The five orders of clinical management for acute cholangitis are hemodynamic stabilization, assessment, intensive care, antimicrobial therapy, and biliary decompression with definitive therapy. Clinical judgment in the sequencing and timing of these therapeutic activities has a significant impact on clinical outcome.
7.1. Microbiology
The most frequent pathogens are the gastrointestinal flora E. coli, and Klebsiella, Enterobacter and Enterococcus spp (Table 5) [30–32]. Streptococcus and Proteus spp are less frequently involved [33]. Anaerobes, such as Clostridium and Bacteroides spp, have been isolated in 4%–20% of bile cultures from patients with acute biliary infections [34]. Empirical coverage for these organisms is advised in cases of bilioenteric anastamoses. Resistant pathogens may be encountered in patients with healthcare-associated infections and empirical coverage should be adjusted accordingly. Once bacteriology and susceptibility data are available, the most appropriate antibiotics must be selected.
Table 5.
Common pathogens involved in acute cholangitis.
| Community acquired |
Hospital acquired | ||
|---|---|---|---|
| Aerobes | Anaerobes | ||
|
Gram negativeEscherichia coli Klebsiella pneumoniae Enterobacter spp Citrobacter spp Pseudomonas spp Salmonella typhi Salmonella paratyphi |
Gram positiveStreptococcus spp Enterococcus faecalis |
Clostridium spp Bacteroides spp Proteus spp |
MDR ESBL-producing E. coli and Klebsiella spp in the community Methicillin-resistant Staphylococcus aureus Acinetobacter baumannii |
MDR, multi-drug resistant; ESBL, extended-spectrum beta-lactamase.
7.2. Antimicrobial therapy
Antimicrobial therapy should be initiated as soon as the diagnosis of biliary infection is suspected and should definitely commence before any procedure is performed [34]. For patients in septic shock, antimicrobials should be administered within 1 h of recognition; for others, up to 4 h may elapse before beginning antimicrobial therapy [34].
In general, the principles and concepts of antimicrobial choice are based on knowledge of the most likely offending microorganism, the pharmacokinetics, pharmacodynamics and potential adverse effects of the drugs, local antimicrobial susceptibility data, the severity of infection, history of antimicrobial medications, and the patient's pathophysiological and immunological status. The availability and cost of the drugs may be factors for consideration. The selection of this empiric regimen is outlined in Table 3, and varies based on the resistance profile of organisms, particularly E. coli. When culture and susceptibility data become available, therapy is revised to a more targeted narrow-spectrum therapy. Patients who are unresponsive to antibiotic therapy may require urgent or early biliary drainage or cholecystectomy.
The recommended antimicrobial therapy protocol will differ between institutions because of differences in the local susceptibility of the microorganisms and drug availability. An antibiotic stewardship program will provide a relevant guide to the choice of antimicrobial therapy.
In the management of acute cholangitis, the choice of antimicrobial agents is guided by the severity of infection. This practice is consistent with the IDSA guidelines for IAIs [1]. For grade I acute cholangitis, the recommended antimicrobials in the Tokyo Guidelines 2013 are first- or second-generation cephalosporins (e.g., cefazolin, cefuroxime), ertapenem or fluroquinolones [34]. Coverage of anaerobic bacteria is generally not indicated unless an endobiliary stent or a biliary-enteric anastomosis is present, or for immunocompromised patients. Consistent with the North American guidelines, ampicillin/sulbactam is not recommended unless an aminoglycoside is added, because of limited antimicrobial activity against E. coli [1].
Antimicrobial recommendations for grade II and III (see Table 6) [35] acute cholangitis include wide-spectrum penicillin/tazobactam, third- or fourth-generation cephalosporins, fluoroquinolones or carbapenems (imipenem/cilastin, meropenem, doripenem), or aztreonam [34]. Additional coverage for anaerobes with metronidazole 500 mg IV given every 6–8 h is indicated for patients with bilio-enteric anastomosis or biliary stents [36]. For patients with suspected hospital-acquired multidrug-resistant organisms – especially patients with indwelling stents and malignancies – antibiotics should be selected accordingly. Vancomycin is recommended for grade III community-acquired acute cholangitis to cover Enterococcus spp and it may be added for hospital-acquired MRSA biliary infections of any severity. In the presence of vancomycin-resistant Enterococcus (VRE), linezolid, daptomycin or tigecycline are recommended.
Table 6.
Definitions of grade III and grade II acute cholangitis from TG 13 [38].
Severe (grade III) acute cholangitis is defined as acute cholangitis that is associated with the onset of dysfunction in at least one of the following organs/systems:
|
Moderate (grade II) acute cholangitis is associated with any two of the following:
|
To achieve the best clinical outcome, the drug dosage must be titrated according to the patient's renal and hepatic function, particularly for patients with septicemic shock. The recommended duration of antimicrobial treatment for grades II and III acute cholangitis ranges from 4 to 7 days. Antimicrobial therapy should not be discontinued before the clearance of obstruction or calculi in the biliary tract. To mitigate the risk of infective endocarditis, at least 2 weeks of antimicrobial therapy is recommended if Gram-positive cocci, such as Enterococcus spp and Streptococcus spp, are diagnosed [34].
7.3. Antibiotic stewardship – an essential practice
The prolonged, improper, and unregulated use of antibiotics is a key factor in the rapid rise of resistant pathogens in Asia over the past decade. Therefore, implementing antibiotic stewardship measures in Asian hospitals is particularly important to reduce selection pressure on resistant pathogens and slow their rise. Proper antibiotic use is also an important part of providing quality clinical care.
The essential components of antibiotic stewardship programs are provided in existing guidelines [37,38]. Several strategies are effective in the institutional setting: developing a multidisciplinary team, close collaboration with the infection control program, prospective audit and feedback to the prescriber, and computer-assisted audit and surveillance [38].
Educational programs on topics such as appropriate drug selection, dosing, route, and duration of antimicrobial therapy will facilitate good stewardship. Implementation of these strategies, along with regular surveys of the diversity and quantity of antimicrobials used and any changes in resistance patterns, are crucial to the success of institutional stewardship programs.
8. Conclusion
Selection of appropriate empiric antibiotic therapy is critical for preventing unnecessary morbidity and mortality from cIAIs. Consideration of regional epidemiological data and regional resistance profiles is essential for antibiotic selection [3]. The aim of this paper was to provide updated recommendations on antibiotic therapy for IAIs based on the latest microbiological and resistance data available for Asia. We have examined SMART data and presented an overview of the local microbiological profiles for cIAIs as well as the antibiotic resistance patterns for key pathogens. From this, we have proposed antimicrobial therapies suitable for treating cIAIs in patients in Asian settings, which we hope will prove beneficial to local physicians managing IAIs.
Considerable gaps remain in our knowledge of the epidemiology and antimicrobial resistance among pathogens causing IAIs in many Asian countries. Addressing this issue will help physicians confidently select antibiotic therapies for cIAIs that are appropriate and specific to their location.
Conflict of interest statement
AK has received honoraria from Pfizer and has served on advisory boards for MSD and Astra Zeneca. All other authors declare no competing interests.
Ethical approval
Not required.
Acknowledgments
Funding: Editorial and administrative support was provided by Weber Shandwick, funded by Pfizer. ACT-cIAI members retained control of the content at all stages of development.
References
- 1.Solomkin J.S., Mazuski J.E., Bradley J.S., Rodvold K.A., Goldstein E.J., Baron E.J. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 2.Sartelli M., Viale P., Catena F., Ansaloni L., Moore E., Malangoni M. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3. doi: 10.1186/1749-7922-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsueh P.R., Hawkey P.M. Consensus statement on antimicrobial therapy of intra-abdominal infections in Asia. Int J Antimicrob Agents. 2007;30:129–133. doi: 10.1016/j.ijantimicag.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Chow J.W., Satishchandran V., Snyder T.A., Harvey C.M., Friedland I.R., Dinubile M.J. In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2002 Study for Monitoring Antimicrobial Resistance Trends (SMART) Surg Infect (Larchmt) 2005;6:439–448. doi: 10.1089/sur.2005.6.439. [DOI] [PubMed] [Google Scholar]
- 5.Baquero F., Hsueh P.R., Paterson D.L., Rossi F., Bochicchio G.V., Gallagher G. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2005 results from Study for Monitoring Antimicrobial Resistance Trends (SMART) Surg Infect (Larchmt) 2009;10:99–104. doi: 10.1089/sur.2008.0020. [DOI] [PubMed] [Google Scholar]
- 6.Chen W.Y., Jang T.N., Huang C.H., Hsueh P.R. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections at a medical center in Taiwan: results of the Study for Monitoring Antimicrobial Resistance Trends (SMART) 2002-2006. J Microbiol Immunol Infect. 2009;42:317–323. [PubMed] [Google Scholar]
- 7.Yang Q., Wang H., Chen M., Ni Y., Yu Y., Hu B. Surveillance of antimicrobial susceptibility of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in China: the 2002-2009 Study for Monitoring Antimicrobial Resistance Trends (SMART) Int J Antimicrob Agents. 2010;36:507–512. doi: 10.1016/j.ijantimicag.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.H., Hsueh P.R., Badal R.E., Hawser S.P., Hoban D.J., Bouchillon S.K. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J Infect. 2011;62:280–291. doi: 10.1016/j.jinf.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Hawser S.P., Bouchillon S.K., Hoban D.J., Badal R.E. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli from patients with intra-abdominal infections worldwide from 2005–2007: results from the SMART study. Int J Antimicrob Agents. 2009;34:585–588. doi: 10.1016/j.ijantimicag.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh P.R. Study for Monitoring Antimicrobial Resistance Trends (SMART) in the Asia-Pacific region, 2002-2010. Int J Antimicrob Agents. 2012;40(Suppl.):S1–S3. doi: 10.1016/S0924-8579(12)00244-0. [DOI] [PubMed] [Google Scholar]
- 11.Tan T.Y., Hsu L.Y., Koh T.H., Ng L.S., Tee N.W., Krishnan P. Antibiotic resistance in Gram-negative bacilli: a Singapore perspective. Ann Acad Med Singap. 2008;37:819–825. [PubMed] [Google Scholar]
- 12.Liew Y.X., Krishnan P., Yeo C.L., Tan T.Y., Lee S.Y., Lim W.P. Surveillance of broad-spectrum antibiotic prescription in Singaporean hospitals: a 5-year longitudinal study. PLoS One. 2011;6(12):e28751. doi: 10.1371/journal.pone.0028751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Antimicrobial Resistance Surveillance Center Thailand. Prevalence of pathogens recovered from abdominal/ascites fluid in Thailand in 2009. Available from: http://narst.dmsc.moph.go.th/a5.php. [accessed 25.06.13].
- 14.Lee Y.L., Chen Y.S., Toh H.S., Huang C.C., Liu Y.M., Ho C.M. Antimicrobial susceptibility of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2006 to 2010. Int J Antimicrob Agents. 2012;40(Suppl. 1):S29–S36. doi: 10.1016/S0924-8579(12)70007-9. [DOI] [PubMed] [Google Scholar]
- 15.Hawser S.P., Badal R.E., Bouchillon S.K., Hoban D.J., The SMART India Working Group Antibiotic susceptibility of intra-abdominal infection isolates from Indian hospitals during 2008. J Med Microbiol. 2010;59:1050–1054. doi: 10.1099/jmm.0.020784-0. [DOI] [PubMed] [Google Scholar]
- 16.Salvador V.B., Lozada M.C., Consunji R.J. Microbiology and antibiotic susceptibility of organisms in bile cultures from patients with and without cholangitis at an Asian academic medical center. Surg Infect (Larchmt) 2011;12:105–111. doi: 10.1089/sur.2010.005. [DOI] [PubMed] [Google Scholar]
- 17.Goo J.C., Seong M.H., Shim Y.K., Lee H.S., Han J.H., Shin K.S. Extended spectrum-β-lactamase or carbapenemase producing bacteria isolated from patients with acute cholangitis. Clin Endosc. 2012;45:155–160. doi: 10.5946/ce.2012.45.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C.C., Chen Y.S., Toh H.S., Lee Y.L., Liu Y.M., Ho C.M. Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002-2010. Int J Antimicrob Agents. 2012;40(Suppl.):S4–S10. doi: 10.1016/S0924-8579(12)70003-1. [DOI] [PubMed] [Google Scholar]
- 19.Nicasio A.M., Kuti J.L., Nicolau D.P. The current state of multidrug-resistant Gram-negative bacilli in North America. Pharmacotherapy. 2008;28:235–249. doi: 10.1592/phco.28.2.235. [DOI] [PubMed] [Google Scholar]
- 20.Bush K. Bench-to-bedside review: the role of β-lactamases in antibiotic-resistant Gram-negative infections. Crit Care. 2010;14:224. doi: 10.1186/cc8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkey P.M. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect. 2008;14(Suppl. 1):159–165. doi: 10.1111/j.1469-0691.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 22.Badal R., Lob S., Bouchillon S., Hoban D., Johnson A., Hackel M. Global trends in frequency and susceptibility of extended-spectrum beta-lactamase positive E. coli, K. pneumoniae, and K. oxytoca isolate from intra-abdominal infections since 2005 – the SMART study. Clin Microbiol Infect. 2012;18(s3) Abstract 1179. [Google Scholar]
- 23.Kiratisin P., Chongthaleong A., Tan T.Y., Lagamayo E., Roberts S., Garcia J. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: results of Asia-Pacific surveillance from the COMPACT II study. Int J Antimicrob Agents. 2012;39:311–316. doi: 10.1016/j.ijantimicag.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Chen M. Surveillance for antimicrobial resistance among clinical isolates of Gram-negative bacteria from intensive care unit patients in China, 1996 to 2002. Diagn Microbiol Infect Dis. 2005;51:201–208. doi: 10.1016/j.diagmicrobio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Nordmann P., Naas T., Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta N., Sohanlal T., Soman R., Shetty A., Rodrigues C. The relevance of ESBL producing isolates in patients surgically treated for acute appendicitis. J Assoc Physicians India. 2011;59:293–295. [PubMed] [Google Scholar]
- 27.Yang L., Xiao Y.H., Nie Y., Zheng Y.D., Wang J., Yan Q. Impact of misuse of antimicrobial therapies on inpatient costs. Beijing Da Xue Xue Bao. 2010;42:279–283. [PubMed] [Google Scholar]
- 28.Cheong H.S., Kang C.I., Wi Y.M., Kim E.S., Lee J.S., Ko K.S. Clinical significance and predictors of community-onset Pseudomonas aeruginosa bacteremia. Am J Med. 2008;121:709–714. doi: 10.1016/j.amjmed.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Welch J.P., Donaldson G.A. The urgency of diagnosis and surgical treatment of acute suppurative cholangitis. Am J Surg. 1976;131:527–532. doi: 10.1016/0002-9610(76)90003-9. [DOI] [PubMed] [Google Scholar]
- 30.Saharia P.C., Cameron J.L. Clinical management of acute cholangitis. Surg Gynecol Obstet. 1976;142:369–372. [PubMed] [Google Scholar]
- 31.Chang W.T., Lee K.T., Wang S.R., Chuang S.C., Kuo K.K., Chen J.S. Bacteriology and antimicrobial susceptibility in biliary tract disease: an audit of 10-year's experience. Kaohsiung J Med Sci. 2002;18:221–228. [PubMed] [Google Scholar]
- 32.Maluenda F., Csendes A., Burdiles P., Diaz J. Bacteriological study of choledochal bile in patients with common bile duct stones, with or without acute suppurative cholangitis. Hepatogastroenterology. 1989;36:132–135. [PubMed] [Google Scholar]
- 33.Csendes A., Mitru N., Maluenda F., Diaz J.C., Burdiles P., Csendes P. Counts of bacteria and pyocites of choledochal bile in controls and in patients with gallstones or common bile duct stones with or without acute cholangitis. Hepatogastroenterology. 1996;43:800–806. [PubMed] [Google Scholar]
- 34.Gomi H., Solomkin J.S., Takada T., Strasberg S.M., Pitt H.A., Yoshida M. TG13 antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:60–70. doi: 10.1007/s00534-012-0572-0. [DOI] [PubMed] [Google Scholar]
- 35.Kiriyama S., Takada T., Strasberg S.M., Solomkin J.S., Mayumi T., Pitt H.A. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:24–34. doi: 10.1007/s00534-012-0561-3. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen M.L., Justesen T. Anaerobic and aerobic bacteriological studies in biliary tract disease. Scand J Gastroenterol. 1976;11:437–446. [PubMed] [Google Scholar]
- 37.World Health Organization, South East Asia Regional Office . 2010. Regional strategy on prevention and containment of antimicrobial resistance, 2010-2015.http://www.searo.who.int/LinkFiles/BCT_hlm-407.pdf Available from: [accessed 20.10.12] [Google Scholar]
- 38.Dellit T.H., Owens R.C., McGowan J.E., Gerding D.N., Weinstein R.A., Burke J.P. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]


