Abstract
As the molecular top–down causality emerging through comparative genomics is combined with the bottom–up dynamic chemical networks of biochemistry, the molecular symbiotic relationships driving growth of the tree of life becomes strikingly apparent. These symbioses can be mutualistic or parasitic across many levels, but most foundational is the complex and intricate mutualism of nucleic acids and proteins known as the central dogma of biological information flow. This unification of digital and analog molecular information within a common chemical network enables processing of the vast amounts of information necessary for cellular life. Here we consider the molecular information pathways of these dynamic biopolymer networks from the perspective of their evolution and use that perspective to inform and constrain pathways for the construction of mutualistic polymers.
Keywords: dynamic chemical networks, digital and analog information, prions, amyloid, chemical and biomolecular evolution, molecular mutualisms
1. From Up and Down, and Still Somehow
Life may best be understood as information on a nanoscale [1]. The sequences of DNA and the vast repertoire of catalytic and structural forms of proteins constitute a dynamic evolving network easily seen in the top–down causality of the tree of life. Most remarkable is the information flow achieved with two biopolymers, so universal as to be termed the Central Dogma of biology [2], locked in mutualistic synergy. Hydrogen-bond donor/acceptor pairs bias the folding pathways of nucleic acids to optimally store “digital-like” information in the genome. In contrast, protein folding is context dependent, coupling “analog-like” conformational distributions with inputs from the changing environments that drive evolution. Coordinating these analog and digital forms of information has been heralded as the Darwinian Threshold of cellular life [3], and this entire issue of Life strives to review our understanding of how such mutualistic networks could have emerged as a process for biogenesis.
Early experiments [4] showed that amino acids and peptides are readily accessible from simple gaseous precursors, providing a source for the building blocks of biogenesis. The discovery that nucleic acids can serve as catalysts, ribozymes [5,6] and deoxyribozymes [7], proved that these polymers could carry both analog and digital functions, and gave substance to an RNA World hypothesis for early life [8]. Genome sequencing from across all three domains of life and the resulting top–down understanding of the progressive growth of molecular information over time was traced back to the critical threshold for cellular life as the emergence of the ribosome [3]. This organelle, the digital-to-analog converter of the Central Dogma, is itself a supramolecular RNA/protein co-assembly [9] composed of integrated biopolymers that emerged simultaneously [10,11]. Today messenger RNA, the intermediate carrier of genetic information, serves in mutualistic synergy with proteins at every stage of the process. From mRNA-binding proteins during transcription to nuclear membrane transport, from being spliced, capped and polyadenylated by ribonucleoprotein complexes (RNPs) to the mRNA-protein complexes necessary for translation in the ribosome, this positive reciprocal relationship between nucleic acids and proteins within dynamic networks provides the foundation for genomic biological information flow [12].
The origins of this protein/nucleic acid biopolymer mutualism can now be reconsidered in light of the discovery of protein-only infectious agents known as prions [13]. Prion proteins undergo Darwinian-like selection and propagation from a clonal population of diverse assembled phases existing within a cellular matrix. While the basis for such selection in disease remains unknown, the process achieves gain-of-function phenotypes from folding and assembly of cross-β forms [14,15,16] (Figure 1). These scaffolds also serve as adhesives in bacterial biofilms [17,18], as heritable elements in fungi [19], and in one, the Het-s amyloid, the assembly mediates self/non-self recognition to control incompatible fusions [20]. Increasingly beneficial functions of amyloids are being found in multicellular organisms, including the protective egg envelope of Austrofundulus Limnaeus embryos [21], barnacle adhesives [22], weevil cocoons [23], and Crysopa flava (lace-wing fly) silks [15]. Orb2 amyloid oligomers contribute to long-term memory of fruit flies [24] and the Pmel17 amyloid fibers appear to play a role in directing the polymerization of the skin pigment polymer melanin [25]. These current functions of amyloid highlight the evolutionary potential of assembled protein phases and underscore the possibility of independently evolving nucleic acid and protein biopolymers, all of which could enrich the obligate mutualisms seen in biological information networks.
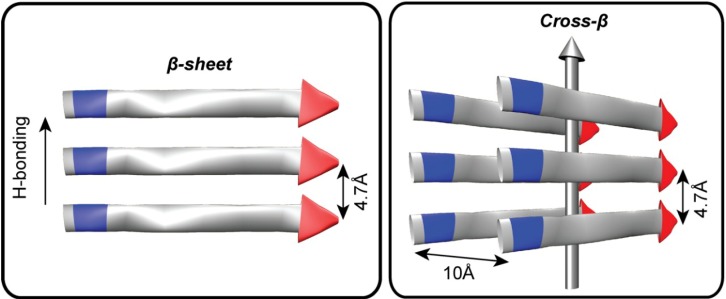
Figure 1.
The architectural core of prions and amyloids. Fibers are defined both by the length of the H-bonded β-strands and β-sheet stacking, or lamination. The distance between two H-bonded peptides in a β-sheet is 4.7Å and the approximate distance between laminated β-sheets is 10Å. The amino (N) terminus is colored blue and the carboxy (C) terminus is colored red. The vertical grey arrow indicates the H-bonding direction and the typical direction of the amyloid fiber long axis.
As Joni Mitchell sang over 40 years ago, “I have looked at life from both sides now…, and still somehow…, I don’t know life at all”. Since that time, the structural and functional analysis of both sides of biological information flow have revealed the richly dynamic far-from-equilibrium networks of nucleic acids and peptides that enable biopolymer evolution. Here we review some of this growth in the context of chemical evolution, looking at life from the above and below that is today informing the potential for the sustained emergence of function in a wider array of dynamic chemical networks.
2. Biopolymer Diversity
Discrete geometries of hydrogen-bonding donor and acceptor pairs direct genomic information, achieving “digital-like” coding fidelity. In this case, functional diversity is accumulated through mutations within the nucleic acid polymer sequence that is expressed in phenotypic variations mapped to the tree of life. The iconic right-handed B-DNA double helix [26,27] can adopt other antiparallel double helix conformations, including A-DNA, Z-DNA and A-RNA, and their biological roles are still being explored [28,29,30]. Bulges, hairpins, and cruciform conformations further contribute to the structural diversity and extend the possible functions of this scaffold [31,32]. The ability of nucleic acids to fold into polymorphic non-canonical structures in response to metal coordination (G-quadruplexes) and pH (i-motif) [33,34,35], and the growing evidence for these structures existing in vivo [36,37], have continued to expand the functional possibilities [38,39]. The extension to new nucleic acid catalysts [5,6,7] through in vitro directed evolution is reviewed in this issue of Life (Muller) and elsewhere [40,41,42,43]. Clearly the nucleic acid scaffold has remarkable functional potential for both information storage and processing.
Proteins, with an even greater diversity in side chain functionality, are acutely sensitive to amino acid substitutions and are environmentally responsive in their folding dynamics. The polyamide backbone can access α-helices, β-sheets, and turns directed by non-covalent interactions ranging from van der Waals, hydrophobic effects, aromatic stacking, π-cation interactions, hydrogen-bonding and electrostatic interactions. All these factors influence secondary and tertiary peptide conformations in context-dependent ways [44,45] and provide remarkable diversity that still defies 3° and 4° structural predictions [44,46].
The growing recognition of the prevalence of protein misfolding diseases and prion infections has focused greater attention on defining higher order 4° structures and protein phases [47,48,49]. A central feature of all known prion and amyloid fibers is the range of accessible paracrystalline cross-β architectures [50,51]. Amyloid fiber cross-section is defined both by the length of the H-bonded β-strand and side-chain interactions that stabilize sheet stacking, or lamination (Figure 1). These fiber ends template the addition of individual peptides, reducing the diverse conformational space any peptide can sample to a single state. Unlike information storage in nucleic acid duplexes, the template cross-β protein strand transmits conformational information to the incoming strand, which then serves as the template for further propagation to the next incoming strand. In prions, specific morphological forms of the peptide cross-β architecture are selected for and propagated from clonal ensembles of diverse amyloid templates [52,53,54,55].
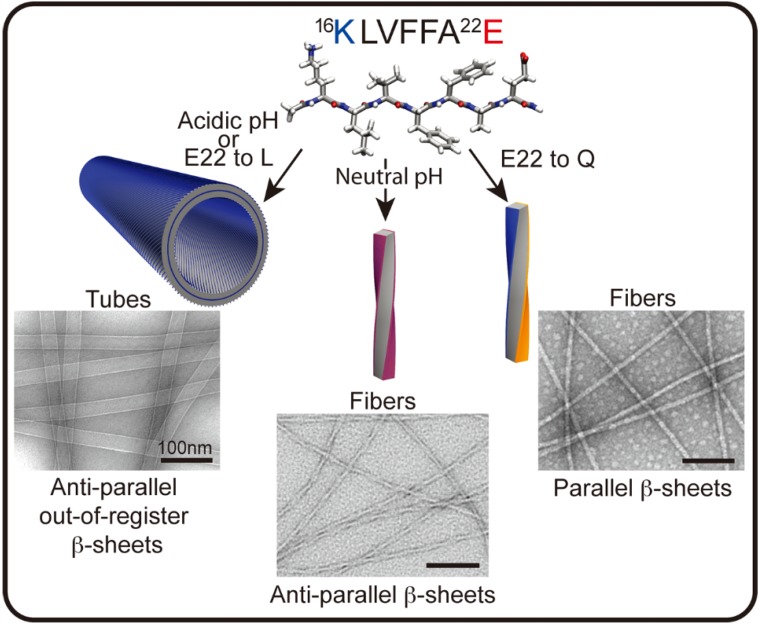
The diversity of conformational forms accessible to these peptide templates may be most easily explored in studies with simple peptides. The nucleating core of the Aβ peptide associated with Alzheimer’s disease, 17LVFF21A, can access a range of morphological phases, each responsive to pH, media dialectic, surfaces, solvent composition, and various salts [56,57,58,59]. Despite this diversity, specific conditions have been found that allow for the growth of phases sufficiently homogeneous for structural characterization. At neutral pH, anti-parallel β-sheet fibrils predominate with Aβ [16,17,18,19,20,21,22], Ac-16KLVFFA22E-NH2 (Figure 2). Cross-strand pairing between the positively charged lysine and negatively charged glutamate residues of neighboring H-bonded strands defines an in-register arrangement [59,60]. Protonation of the glutamate at low pH favors a shift of the strands to out-of-register, creating more complementary β-sheet faces that allow the number of sheets to grow (laminate) into ribbons and nanotubes [59,61,62,63]. The Aβ [16,17,18,19,20,21,22] E22L congener, Ac-KLVFFAL-NH2, removes this ionic pairing constraint completely and assembles independent of pH as nanotubes with the same out-of-register β-strands [57,59,63,64].
Figure 2.
Aβ [16,17,18,19,20,21,22] peptide assembles into distinct morphologies depending on sequence and conditions. In the cartoons above, blue represents the positively charged lysine (K), red the negatively charged glutamate (E), and orange the uncharged glutamine (Q). Two of the four faces of the fiber in the anti-parallel β-sheets present lysine and glutamate residues on the surface (purple), whereas the fibers with parallel β-sheets have a lysine surface and a glutamine surface. (Unpublished EM images from members of Lynn Lab.)
Single molecule experiments have been used to map the phase transitions [65,66] and have begun to reveal intermediate nucleation events during conformational progression [67,68,69]. The simple change of one O for an NH in Ac-KLVFFAQ-NH2 initially assembles as antiparallel β-strands, which arise as a kinetic intermediate controlled by charge repulsion in the initial particle phase. A secondary conformational mutation stabilized by glutamine side chain cross-strand pairing directs the nucleation and propagation of the thermodynamically more stable parallel β-strand registry [68]. This clear demonstration of kinetic intermediates expands the diversity of accessible informational forms that may propagate under different environmental conditions, and highlights the progressive nature of peptide nucleation and propagation as a mechanism for the selection of functional information.
The diversity of these peptide assemblies is derived not only from primary sequence, but also from the varied arrangements accessible to the ordered phases. In the simplest forms, even single aromatic amino acids [70] and dipeptides, containing aromatic [71,72,73,74] and aliphatic residues [75,76], can assemble as distinct phases. Aliphatic dipeptides (AI, IA, VV, VI, AV, VA, and IV) assemble into hexagonal prisms to form microporous crystalline materials, zeolites [75,76], and various other complex assemblies [77,78]. Diphenylalanine nanotubes (FF) can react with 2-iminothiolane, in which the subsequent thiolation of the N-terminal primary amine induces a morphological shift from nanotubes to spherical closed cages [74]. The opportunity to exploit even the simplest of peptide-assembly surfaces as templates for further chemistry with subsequent feedback control of the assembly certainly expands the potential functional diversity. As the structural understanding of these assemblies continues to grow and new surfaces are designed as templates for post-assembly modification, the functional diversity of these forms will continue to extend their informational potential.
3. Functional Assemblies
Genomic information present in the one-dimensional nucleic acid templates, as managed by proteins, produces the functional biopolymers of living dynamic cellular networks. Quite possibly the simplest functional form of this molecular information processing is found in viroids. These single stranded RNAs of only a few hundred nucleotides direct pathogenesis in many plant diseases [79]. Viroids are non-protein coding circular structures that assemble as long rod-like forms with a central conserved region and loops containing complex hydrogen-bonding patterns [80,81,82]. One class of viroids contains a hammerhead ribozyme for self-cleavage during replication cycles [80,83,84], allowing many of their functions to be self-contained and hailed as vestiges of an RNA World [85]. However, viroids are informational templates and depend on the host’s cellular network for functional replication.
The paracrystalline cross-β peptide assemblies of the infectious prions are quite similar [50]. Like viroids, assembly stabilizes the structure against biotic and abiotic destruction and transmissibility is based on their ability to function as templates. These peptide templates also bind generic and functionally diverse small molecules, much like nucleic acids bind histochemical dyes [86,87,88,89]. The prototypical dye Congo Red (CR) [88] binds end-to-end along the laminate groove of Ac-KLVFFAE-NH2 nanotube surfaces [64], and binds in a similar site-specific way to the HET-s prion of the filamentous fungus Podospora anserine [90,91]. These amyloid assemblies also bind oligoelectrolytes and poly(thiophene acetic acid) [92,93], much like RNA binding proteins [94], and other biological surfaces [95,96,97,98,99,100,101]. Experimental [102,103] and computational [104] evidence indicate that β-sheets can serve as templates for homochiral polymerization of N-carboxyanhydride activated amino acid monomers [103] and in situ activated α-amino acids [102]. Model self-replicating systems of β-sheet [105,106,107] peptides have been designed and the ability of β-sheets to template the polymerization of homochiral oligopeptides up to 30 residues long has been reviewed [108].
These activities may represent only a small fraction of the functions that provide the basis for the various amyloid diseases, and these capabilities have led to speculation about an amyloid world built on the potential of these surfaces to store and transfer information [109,110,111]. As impressive as these activities are, neither RNA nor amyloid alone achieve the functional diversity necessary for even the simplest cellular networks. The mutualism so evident in every step of information transmission in a cellular network suggests that life required both sides.
4. From Both Sides Now
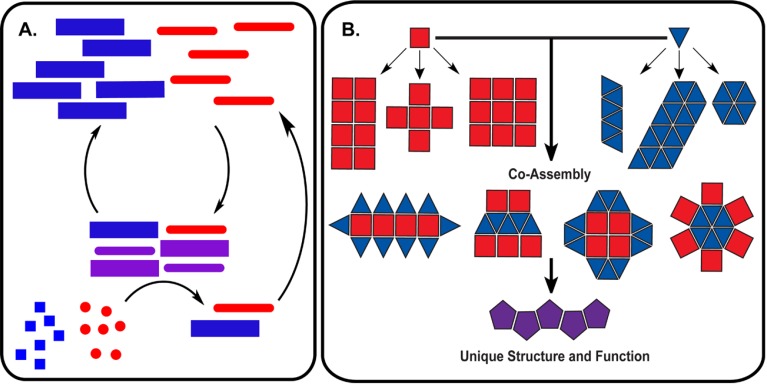
Proteins and nucleic acids both store and process chemical information in cellular networks, but they do so interdependently; proteins are made from nucleic acid templates, and proteins read the templates. To the extent that there is a code or set of rules for such a mutualism may be revealed in the specific recognition of nucleic acids by RNA- and DNA-binding proteins and their elaborate structure–function relationship [112,113,114]. As mentioned above, the pinnacle may be the nucleic acid/peptide (NA/P) associations in the ribosome [9,115], but small nuclear ribonucleoprotein (snRNP) complexes and nucleases, including Ribonuclease P (RNaseP), provide simpler examples [5]. The woven intricacies of such co-assemblies are increasingly being defined [116,117] and have been reconstituted [9,118]. As rules for co-assembly emerge, co-evolutionary strategies become possible. Figure 3 outlines minimal functional capabilities that might be achieved with the co-assemblies. The nucleation of polymers (red and blue) might propagate as a template (purple) able to catalyze the independent production of more polymers (red and blue) in Figure 3A. Mutations in such a feedback system could allow for a minimal system capable of chemical evolution. This functional capability is of course dependent on the ability of these co-assemblies to transition to some unique functional form (mixture of squares and triangles, purple), as outlined in Figure 3B. While extant biology is a sophisticated network of efficiently functioning molecular partners, the many varied functions necessary for the existence of life, relies on the emergence of such co-assembling information networks capable of sustained growth in molecular order.
Figure 3.
Autocatalysis and emergent functions from co-assemblies. (A) Co-assemblies formed from nucleic acid and peptide polymers catalyze the polymerization of additional polymers from monomer building blocks; (B) Co-assemblies derived from individual nucleic acid or peptide assemblies can significantly increase the structural diversity leading to emergent structures and functions.
Data now exists for prion infectivity being altered by oligonucleotides [119,120,121,122,123], but little structural information is available. Similarly, in vitro guanine (dG)16 and cytosine (dC)16 hexadecamers, as well as their duplexes, associate with amphiphilic self-assembling peptides. The binding is pH dependent, occurs on a much faster timescale than for assembly of the neat peptide, and indeed gives rise to novel nucleic acid/peptide co-assemblies [124]. Hybridization of oligonucleotides containing sticky-ends can be catalyzed in the presence of self-assembling peptides, mimicking at least one integral step of replication [125]. And a five-nucleotide ribozyme produces multiple translational products, including the dipeptide FF [126]. In principle [127,128], when FF reaches the critical concentration for peptide self-assembly, the resulting peptide nanotube could selectively bind the ribozyme to create a dynamic system under feedback control.
Progress in this growing understanding of peptides and nucleic acids co-assembly may well depend on careful selection of systems, and the rich diversity of functional co-assemblies with existing biopolymers provides an exciting opportunity to define the basic mutualistic codes. In the simple assemblies outlined in Figure 3, the thermodynamic process of assembly would be physically coupled to polymerization of additional polymers from monomer building blocks, with the thermodynamic/kinetic tension manifested as feedback control. Elucidating the mechanisms of association, and the structural and functional diversity of the resulting complexes, will be necessary for a more general understanding of the chemical thresholds for early evolution of molecular mutualism. Simpler synthetic [129,130] and altered biopolymer [11,131,132] networks could then be extended to the dynamic processes of minimal native biopolymer co-assemblies [133,134,135,136].
5. Conclusions: Towards Molecular Mutualisms
The cooperative functioning of nucleic acids and peptides was recognized early with the designation of a central dogma decades ago, and it may well be that our attempts to simplify the system into metabolism-first, RNA-first, or amyloid-first has limited a more extensive exploration of mutualistic networks. Single biopolymer networks have contributed significantly to systems chemistry, but sidestepped the interdependence of metabolism and replication, analog and digital information processing, and the mutualism, so apparent in living systems, that is essential for the emergence of new functions [137]. The recognition of the importance of diverse, structurally complex, non-covalent assemblies in early evolution is certainly highlighted by their prevalance in biology [138,139,140,141], and routes to creating an ecology of biomolecules with sufficient functional dynamics to evolve chemically are now emerging. We may have reached the point where the creation of a mutualistic chemical ecology [142,143] can be used to inform the progressive growth of molecular information on Earth and understand the limits on chemical evolution broadly in our Universe [144,145,146]. As technology and methodologies to analyze complex networks improve, the next scientific discoveries may come from understanding harsh and extreme environments or even new “worlds” with fundamentally different systems of molecular networks [11,147,148]. Certainly we should now be able to define the limits on building to the complex networks we might call living.
Acknowledgments
The authors acknowledge Joni Mitchell and Emory University for inspiration, National Science Foundation through the award IOS # 1423862, the NASA Astrobiology Program, under the National Science Foundation Center for Chemical Innovation (CHE-1004570 and NSF-CBC-0739189) and the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-ER15377.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mann S. Life as a nanoscale phenomenon. Angew. Chem. Int. Ed. 2008;47:5306–5320. doi: 10.1002/anie.200705538. [DOI] [PubMed] [Google Scholar]
- 2.Crick F.H.C. The biological replication of macromolecules. Sym. Soc. Exp. Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- 3.Woese C.R. On the evolution of cells. Proc. Natl. Acad. Sci. USA. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller S.L. A production of amino acids under possible primitive earth conditions. Science. 1953;117:528–529. doi: 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- 5.Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of Ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 6.Kruger K., Grabowski P.J., Zaug A.J., Sands J., Gottschling D.E., Cech T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 7.Breaker R.R., Joyce G.F. A DNA enzyme that cleaves RNA. Chem. Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 8.Gesteland R.F., Cech T.R., Atkins J.F. The RNA World. 2nd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 1999. [Google Scholar]
- 9.Fromont-Racine M., Senger B., Saveanu C., Fasiolo F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. doi: 10.1016/S0378-1119(03)00629-2. [DOI] [PubMed] [Google Scholar]
- 10.Harish A., Caetano-Anolles G. Ribosomal history reveals origins of modern protein synthesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin J.T., Mehta A.K., Lynn D.G. Digital and analog chemical evolution. Acc. Chem. Res. 2012;45:2189–2199. doi: 10.1021/ar300214w. [DOI] [PubMed] [Google Scholar]
- 12.Moore M.J. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 13.Bolton D.C., McKinley M.P., Prusiner S.B. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 14.Astbury W.T., Dickinson S., Bailey K. The X-ray interpretation of denaturation and the structure of the seed globulins. Biochem. J. 1935;29:2351–2360. doi: 10.1042/bj0292351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker K.D., Rudall K.M. Structure of the silk of chrysopa egg-stalks. Nature. 1957;179:905–906. doi: 10.1038/179905a0. [DOI] [Google Scholar]
- 16.Geddes A.J., Parker K.D., Atkins E.D.T., Beighton E. “Cross-β” conformation in proteins. J. Mol. Biol. 1968;32:343–358. doi: 10.1016/0022-2836(68)90014-4. [DOI] [PubMed] [Google Scholar]
- 17.Hammer N.D., Schmidt J.C., Chapman M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. USA. 2007;104:12494–12499. doi: 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero D., Aguilar C., Losick R., Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tessier P.M., Lindquist S. Unraveling infectious structures, strain variants and species barriers for the yeast prion [PSI+] Nat. Struct. Mol. Biol. 2009;16:598–605. doi: 10.1038/nsmb.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inge-Vechtomov S.G., Zhouravleva G.A., Chernoff Y.O. Biological roles of prion domains. Prion. 2007;1:228–235. doi: 10.4161/pri.1.4.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iconomidou V.A., Hamodrakas S.J. Natural protective amyloids. Curr. Protein Pept. Sci. 2008;9:291–309. doi: 10.2174/138920308784534041. [DOI] [PubMed] [Google Scholar]
- 22.Barlow D.E., Dickinson G.H., Orihuela B., Kulp J.L., Rittschof D., Wahl K.J. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: Amyloid-like nanofibrils are a major component. Langmuir. 2010;26:6549–6556. doi: 10.1021/la9041309. [DOI] [PubMed] [Google Scholar]
- 23.Kenchington W. The larval silk of Hypera spp. (Coleoptera: Curculionidae). A new example of the cross-β protein conformation in an insect silk. J. Insect Physiol. 1983;29:355–361. doi: 10.1016/0022-1910(83)90037-9. [DOI] [Google Scholar]
- 24.Majumdar A., Cesario W.C., White-Grindley E., Jiang H., Ren F., Khan M.R., Li L., Choi E.M., Kannan K., Guo F., et al. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell. 2012;148:515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Fowler D.M., Koulov A.V., Alory-Jost C., Marks M.S., Balch W.E., Kelly J.W. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp M. The Mona Lisa of modern science. Nature. 2003;421:416–420. doi: 10.1038/nature01403. [DOI] [PubMed] [Google Scholar]
- 27.Watson J.D., Crick F.H.C. Molecular structures of nucleic acids. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 28.Rich A., Zhang S. Z-DNA: The long road to biological function. Nat. Rev. Genet. 2003;4:566–572. doi: 10.1038/nrg1115. [DOI] [PubMed] [Google Scholar]
- 29.Hermann T., Westhof E. Non-Watson-Crick base pairs in RNA-protein recognition. Chem. Biol. 1999;6:R335–R343. doi: 10.1016/S1074-5521(00)80003-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J., Bacolla A., Wang G., Vasquez K.M. Non-B DNA structure-induced genetic instability and evolution. Cell. Mol. Life Sci. 2010;67:43–62. doi: 10.1007/s00018-009-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi J., Majima T. Conformational changes of non-B DNA. Chem. Soc. Rev. 2011;40:5893–5909. doi: 10.1039/c1cs15153c. [DOI] [PubMed] [Google Scholar]
- 32.Saini N., Zhang Y., Usdin K., Lobachev K.S. When secondary comes first—The importance of non-canonical DNA structures. Biochimie. 2013;95:117–123. doi: 10.1016/j.biochi.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collie G.W., Parkinson G.N. The application of DNA and RNA G-quadruplexes to therapeutic medicines. Chem. Soc. Rev. 2011;40:5867–5892. doi: 10.1039/c1cs15067g. [DOI] [PubMed] [Google Scholar]
- 34.Burge S., Parkinson G.N., Hazel P., Todd A.K., Neidle S. Quadruplex DNA: Sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyoshi D., Matsumura S., Li W., Sugimoto N. Structural polymorphism of telomeric DNA regulated by pH and divalent cation. Nucleosides Nucleotides Nucl. Acids. 2003;22:203–221. doi: 10.1081/NCN-120019528. [DOI] [PubMed] [Google Scholar]
- 36.Biffi G., Tannahill D., McCafferty J., Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipps H.J., Rhodes D. G-quadruplex structures: In vivo evidence and function. Trends Cell Biol. 2009;19:414–422. doi: 10.1016/j.tcb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Baral A., Kumar P., Pathak R., Chowdhury S. Emerging trends in G-quadruplex biology—Role in epigenetic and evolutionary events. Mol. Biosyst. 2013;9:1568–1575. doi: 10.1039/c3mb25492e. [DOI] [PubMed] [Google Scholar]
- 39.Bochman M.L., Paeschke K., Zakian V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012;13:770–780. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiller D.A., Strobel S.A. The chemical versatility of RNA. Phil. Trans. R. Soc. B. 2011;366:2929–2935. doi: 10.1098/rstb.2011.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doudna J.A., Cech T.R. The chemical repertoire of natural ribozymes. Nature. 2002;418:222–228. doi: 10.1038/418222a. [DOI] [PubMed] [Google Scholar]
- 42.Ward W.L., Plakos K., DeRose V.J. Nucleic acid catalysis: Metals, nucleobases, and other cofactors. Chem. Rev. 2014;114:4318–4342. doi: 10.1021/cr400476k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman S.K. Deoxyribozymes: DNA catalysts for bioorganic chemistry. Org. Biomol. Chem. 2004;2:2701–2706. doi: 10.1039/b411910j. [DOI] [PubMed] [Google Scholar]
- 44.Dill K.A., MacCallum J.L. The protein-folding problem, 50 years on. Science. 2012;338:1042–1046. doi: 10.1126/science.1219021. [DOI] [PubMed] [Google Scholar]
- 45.Dill K.A. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 46.Compiani M., Capriotti E. Computational and theoretical methods for protein folding. Biochemistry. 2013;52:8601–8624. doi: 10.1021/bi4001529. [DOI] [PubMed] [Google Scholar]
- 47.Vabulas R.M., Raychaudhuri S., Hayer-Hartl M., Hartl F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chien P., Weissman J.S., DePace A.H. Emerging principles of conformation-based prion inheritance. Annu. Rev. Biochem. 2004;73:617–656. doi: 10.1146/annurev.biochem.72.121801.161837. [DOI] [PubMed] [Google Scholar]
- 49.Dobson C.M. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 50.Jucker M., Walker L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawaya M.R., Sambashivan S., Nelson R., Ivanova M.I., Sievers S.A., Apostol M.I., Thompson M.J., Balbirnie M., Wiltzius J.J.W., McFarlane H.T., et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 52.Ghaemmaghami S., Watts J.C., Nguyen H.O., Hayashi S., DeArmond S.J., Prusiner S.B. Conformational transformation and selection of synthetic prion strains. J. Mol. Biol. 2011;413:527–542. doi: 10.1016/j.jmb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Browning S., Mahal S.P., Oelschlegel A.M., Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collinge J., Clarke A.R. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka M., Chien P., Naber N., Cooke R., Weissman J.S. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 56.Childers W.S., Mehta A.K., Bui T.Q., Liang Y., Lynn D.G. In: Molecular Self-Assembly—Advances and Applications. 1st ed. Li A., editor. Pan Stanford Publishing Pte Ltd.; Singapore, Singapore: 2012. pp. 1–36. [Google Scholar]
- 57.Childers W.S., Anthony N.R., Mehta A.K., Berland K.M., Lynn D.G. Phase networks of cross-β peptide assemblies. Langmuir. 2012;28:6386–6395. doi: 10.1021/la300143j. [DOI] [PubMed] [Google Scholar]
- 58.Debeljuh N., Barrow C.J., Byrne N. The impact of ionic liquids on amyloid fibrilization of Aβ16–22: Tuning the rate of fibrilization using a reverse Hofmeister strategy. Phys. Chem. Chem. Phys. 2011;13:16534–16536. doi: 10.1039/c1cp22256b. [DOI] [PubMed] [Google Scholar]
- 59.Mehta A.K., Lu K., Childers W.S., Liang Y., Dublin S.N., Gong J., Snyder J.P., Pingali S.V., Thiyagarajan P., Lynn D.G. Facial symmetry in protein self-assembly. J. Am. Chem. Soc. 2008;130:9829–9835. doi: 10.1021/ja801511n. [DOI] [PubMed] [Google Scholar]
- 60.Balbach J.J., Ishii Y., Antzutkin O.N., Leapman R.D., Rizzo N.W., Dyda F., Reed J., Tycko R. Amyloid fibril formation by Aβ16–22, a seven-residue fragment of the Alzheimer’s β-amyloid peptide, and structural characterization by solid state NMR. Biochemistry. 2000;39:13748–13759. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- 61.Lu K., Jacob J., Thiyagarajan P., Conticello V.P., Lynn D.G. Exploiting amyloid fibril lamination for nanotube self-assembly. J. Am. Chem. Soc. 2003;125:6391–6393. doi: 10.1021/ja0341642. [DOI] [PubMed] [Google Scholar]
- 62.Dong J., Lu K., Lakdawala A., Mehta A.K., Lynn D.G. Controlling amyloid growth in multiple dimensions. Amyloid. 2006;13:206–215. doi: 10.1080/13506120600960809. [DOI] [PubMed] [Google Scholar]
- 63.Liang Y., Pingali S.V., Jogalekar A.S., Snyder J.P., Thiyagarajan P., Lynn D.G. Cross-strand pairing and amyloid assembly. Biochemistry. 2008;47:10018–10026. doi: 10.1021/bi801081c. [DOI] [PubMed] [Google Scholar]
- 64.Childers W.S., Mehta A.K., Lu K., Lynn D.G. Templating molecular arrays in amyloid’s cross-β grooves. J. Am. Chem. Soc. 2009;131:10165–10172. doi: 10.1021/ja902332s. [DOI] [PubMed] [Google Scholar]
- 65.Anthony N.R., Mehta A.K., Lynn D.G., Berland K.M. Mapping amyloid-β(16–22) nucleation pathways using fluorescence lifetime imaging microscopy. Soft Matter. 2014;10:4162–4172. doi: 10.1039/c4sm00361f. [DOI] [PubMed] [Google Scholar]
- 66.Liang Y., Lynn D.G., Berland K.M. Direct observation of nucleation and growth in amyloid self-assembly. J. Am. Chem. Soc. 2010;132:6306–6308. doi: 10.1021/ja910964c. [DOI] [PubMed] [Google Scholar]
- 67.Buchanan L.E., Dunkelberger E.B., Tran H.Q., Cheng P.N., Chiu C.C., Cao P., Raleigh D.P., de Pablo J.J., Nowick J.S., Zanni M.T. Mechanism of IAPP amyloid fibril formation involves an intermediate with a transient β-sheet. Proc. Natl. Acad. Sci. USA. 2013;110:19285–19290. doi: 10.1073/pnas.1314481110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang C., Ni R., Smith J.E., Childer W.S., Mehta A.K., Lynn D. Kinetic intermediates in amyloid assembly. J. Am. Chem. Soc. 2014;136:15146–15149. doi: 10.1021/ja508621b. [DOI] [PubMed] [Google Scholar]
- 69.Lin D., Luo Y., Wu S., Ma Q., Wei G., Yang X. Investigation of the aggregation process of amyloid-β-(16–22) peptides and the dissolution of intermediate aggregates. Langmuir. 2014;30:3170–3175. doi: 10.1021/la4048165. [DOI] [PubMed] [Google Scholar]
- 70.Perween S., Chandanshive B., Kotamarthi H.C., Khushalani D. Single amino acid based self-assembled structure. Soft Matter. 2013;9:10141–10145. doi: 10.1039/c3sm51054a. [DOI] [Google Scholar]
- 71.Frederix P.W.J.M., Ulijn R.V., Hunt N.T., Tuttle T. Virtual screening for dipeptide aggregation: Toward predictive tools for peptide self-assembly. J. Phys. Chem. Lett. 2011;2:2380–2384. doi: 10.1021/jz2010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reches M., Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300:625–627. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]
- 73.De Groot N.S., Parella T., Aviles F.X., Vendrell J., Ventura S. Ile-Phe dipeptide self-assembly: Clues to amyloid formation. Biophys. J. 2007;92:1732–1741. doi: 10.1529/biophysj.106.096677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reches M., Gazit E. Formation of closed-cage nanostructures by self-assembly of aromatic dipeptides. Nano Lett. 2004;4:581–585. doi: 10.1021/nl035159z. [DOI] [Google Scholar]
- 75.Soldatov D.V., Moudrakovski I.L., Ripmeester J.A. Dipeptides as microporous materials. Angew. Chem. Int. Ed. 2004;43:6308–6311. doi: 10.1002/anie.200460952. [DOI] [PubMed] [Google Scholar]
- 76.Gorbitz C.H. Nanotubes from hydrophobic dipeptides: Pore size regulation through side chain substitution. New J. Chem. 2003;27:1789–1793. doi: 10.1039/b305984g. [DOI] [Google Scholar]
- 77.Gorbitz C.H. Structures of dipeptides: The head-to-tail story. Acta Cryst. 2010;B66:84–93. doi: 10.1107/S0108768109053257. [DOI] [PubMed] [Google Scholar]
- 78.Gorbitz C.H. Microporous organic materials from hydrophobic dipeptides. Chem. Eur. J. 2007;13:1022–1031. doi: 10.1002/chem.200601427. [DOI] [PubMed] [Google Scholar]
- 79.Flores R., Hernandez C., Martinez de Alba A.E., Daros J.A., di Serio F. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005;43:117–139. doi: 10.1146/annurev.phyto.43.040204.140243. [DOI] [PubMed] [Google Scholar]
- 80.Flores R., Serra P., Minoia S., di Serio F., Navarro B. Viroids: From genotype to phenotype just relying on RNA sequence and structural motifs. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanger H.L., Klotz G., Riesner D., Gross H.J., Kleinschmidt A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sano T., Candresse T., Hammond R.W., Diener T.O., Owens R.A. Identification of multiple structural domains regulating viroid pathogenicity. Proc. Natl. Acad. Sci. USA. 1992;89:10104–10108. doi: 10.1073/pnas.89.21.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daros J.A., Marcos J.F., Hernandez C., Flores R. Replication of avocado sunblotch viroid: Evidence for a symmetric pathway with two rolling circles and hammerhead ribozyme processing. Proc. Natl. Acad. Sci. USA. 1994;91:12813–12817. doi: 10.1073/pnas.91.26.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutchins C.J., Rathjen P.D., Forster A.C., Symons R.H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986;14:3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flores R., Gago-Zachert S., Serra P., Sanjuan R., Elena S.F. Viroids: Survivors from the RNA world? Annu. Rev. Microbiol. 2014;68:395–414. doi: 10.1146/annurev-micro-091313-103416. [DOI] [PubMed] [Google Scholar]
- 86.Buell A.K., Esbjorner E.K., Riss P.J., White D.A., Aigbirhio F.I., Toth G., Welland M.E., Dobson C.M., Knowles T.P. Probing small molecule binding to amyloid fibrils. Phys. Chem. Chem. Phys. 2011;13:20044–20052. doi: 10.1039/c1cp22283j. [DOI] [PubMed] [Google Scholar]
- 87.Levine H. Thioflavine T interactions with synthetic Alzheimers Disease β-amyloid peptides—detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Divry P., Florkin M. The optic properties of amyloid. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales. 1927;97:1808–1810. (In French) [Google Scholar]
- 89.Buxbaum J.N., Linke R.P. A molecular history of the amyloidoses. J. Mol. Biol. 2012;421:142–159. doi: 10.1016/j.jmb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 90.Schutz A.K., Soragni A., Hornemann S., Aguzzi A., Ernst M., Bockmann A., Meier B.H. The amyloid-Congo red interface at atomic resolution. Angew. Chem. Int. Ed. 2011;50:5956–5960. doi: 10.1002/anie.201008276. [DOI] [PubMed] [Google Scholar]
- 91.Dos Reis S., Coulary-Salin B., Forge V., Lascu I., Begueret J., Saupe S.J. The HET-s prion protein of the filamentous fungus Podospora anserina aggregates in vitro into amyloid-like fibrils. J. Biol. Chem. 2002;277:5703–5706. doi: 10.1074/jbc.M110183200. [DOI] [PubMed] [Google Scholar]
- 92.Bäcklund F.G., Wigenius J., Westerlund F., Inganäs O., Solin N. Amyloid fibrils as dispersing agents for oligothiophenes: Control of photophysical properties through nanoscale templating and flow induced fibril alignment. J. Mater. Chem. C. 2014;2:7811–7822. doi: 10.1039/C4TC00692E. [DOI] [Google Scholar]
- 93.Herland A., Bjork P., Hania P.R., Scheblykin I.G., Inganas O. Alignment of a conjugated polymer onto amyloid-like protein fibrils. Small. 2007;3:318–325. doi: 10.1002/smll.200600377. [DOI] [PubMed] [Google Scholar]
- 94.Lunde B.M., Moore C., Varani G. RNA-binding proteins: Modular design for efficient function. Nat. Rev. Mol. Cell Bio. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saha S., Deep S. Switch in the aggregation pathway of bovine serum albumin mediated by electrostatic interactions. J. Phys. Chem. B. 2014;118:9155–9166. doi: 10.1021/jp502435f. [DOI] [PubMed] [Google Scholar]
- 96.Gilbert J., Campanella O., Jones O.G. Electrostatic stabilization of β-lactoglobulin fibrils at increased ph with cationic polymers. Biomacromolecules. 2014;15:3119–3127. doi: 10.1021/bm500762u. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen S.B., Yde P., Giehm L., Sundbye S., Christiansen G., Mathiesen J., Jensen M.H., Jensen P.H., Otzen D.E. Multiple roles of heparin in the aggregation of p25alpha. J. Mol. Biol. 2012;421:601–615. doi: 10.1016/j.jmb.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 98.Matsuzaki K., Kato K., Yanagisawa K. Aβ polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta. 2010;1801:868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 99.Li S., Sidorov A.N., Mehta A.K., Bisignano A.J., Das D., Childers W.S., Schuler E., Jiang Z., Orlando T.M., Berland K., et al. Neurofibrillar Tangle Surrogates: Histone H1 Binding to Patterned Phosphotyrosine Peptide Nanotubes. Biochemistry. 2014;53:4225–4227. doi: 10.1021/bi500599a. [DOI] [PubMed] [Google Scholar]
- 100.Suk J.Y., Zhang F., Balch W.E., Linhardt R.J., Kelly J.W. Heparin accelerates gelsolin amyloidogenesis. Biochemistry. 2006;45:2234–2242. doi: 10.1021/bi0519295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terzi E., Holzemann G., Seelig J. Interaction of Alzheimer β-amyloid peptide (1–40) with lipid membranes. Biochemistry. 1997;36:14845–14852. doi: 10.1021/bi971843e. [DOI] [PubMed] [Google Scholar]
- 102.Illos R.A., Bisogno F.R., Clodic G., Bolbach G., Weissbuch I., Lahav M. Oligopeptides and copeptides of homochiral sequence, via β-sheets, from mixtures of racemic alpha-amino acids, in a one-pot reaction in water; relevance to biochirogenesis. J. Am. Chem. Soc. 2008;130:8651–8659. doi: 10.1021/ja709969v. [DOI] [PubMed] [Google Scholar]
- 103.Rubinstein I., Eliash R., Bolbach G., Weissbuch I., Lahav M. Racemic beta sheets in biochirogenesis. Angew. Chem. Int. Ed. 2007;46:3710–3713. doi: 10.1002/anie.200605040. [DOI] [PubMed] [Google Scholar]
- 104.Wagner N., Rubinov B., Ashkenasy G. β-Sheet-induced chirogenesis in polymerization of oligopeptides. Chemphyschem. 2011;12:2771–2780. doi: 10.1002/cphc.201100292. [DOI] [PubMed] [Google Scholar]
- 105.Rubinov B., Wagner N., Rapaport H., Ashkenasy G. Self-replicating amphiphilic β-sheet peptides. Angew. Chem. Int. Ed. 2009;48:6683–6686. doi: 10.1002/anie.200902790. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi Y., Mihara H. Construction of a chemically and conformationally self-replicating system of amyloid-like fibrils. Bioorgan. Med. Chem. 2004;12:693–699. doi: 10.1016/j.bmc.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 107.Rubinov B., Wagner N., Matmor M., Regev O., Ashkenasy N., Ashkenasy G. Transient Fibril Structures Facilitating Nonenzymatic Self-Replication. ACS Nano. 2012;6:7893–7901. doi: 10.1021/nn302223v. [DOI] [PubMed] [Google Scholar]
- 108.Weissbuch I., Illos R.A., Bolbacj G., Lahav M. Racemic β-sheets as templates of relevance to the origin of homochirality of peptides: Lessons from crystal chemistry. Acc. Chem. Res. 2009;42:1128–1140. doi: 10.1021/ar900033k. [DOI] [PubMed] [Google Scholar]
- 109.Maury C.P.J. Self-propagating β-sheet polypeptide structures as prebiotic informational molecular entities: The amyloid world. Orig. Life Evol. Biosph. 2009;39:141–150. doi: 10.1007/s11084-009-9165-6. [DOI] [PubMed] [Google Scholar]
- 110.Childers W.S., Ni R., Mehta A.K., Lynn D.G. Peptide membranes in chemical evolution. Curr. Opin. Chem Biol. 2009;13:652–659. doi: 10.1016/j.cbpa.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brack A., Orgel L.E. β Structures of alternating polypeptides and their prebiotic significance. Nature. 1975;256:383–387. doi: 10.1038/256383a0. [DOI] [PubMed] [Google Scholar]
- 112.Hoffman M.M., Khrapov M.A., Cox J.C., Yao J., Tong L., Ellington A.D. AANT: The amino acid-nucleotide interaction database. Nucleic Acids Res. 2004;32:D174–D181. doi: 10.1093/nar/gkh128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jones S., van Heyningens P., Berman H.M., Thornton J.M. Protein-DNA interactions: A structural analysis. J. Mol. Biol. 1999;287:877–896. doi: 10.1006/jmbi.1999.2659. [DOI] [PubMed] [Google Scholar]
- 114.Nadassy K., Wodak S.J., Janin J. Structural features of protein-nucleic acid recognition sites. Biochemistry. 1999;38:1999–2017. doi: 10.1021/bi982362d. [DOI] [PubMed] [Google Scholar]
- 115.Wool I.G. The structure and function of eukaryotic ribosomes. Annu. Rev. Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- 116.Esakova O., Krasilnikov A.S. Of proteins and RNA: The RNase P/MRP family. RNA. 2010;16:1725–1747. doi: 10.1261/rna.2214510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frank D.N., Pace N. Ribonuclease P: Unity and diversity in a tRNA processing ribozyme. Annu. Rev. Biochem. 1998;67:153–180. doi: 10.1146/annurev.biochem.67.1.153. [DOI] [PubMed] [Google Scholar]
- 118.Woodson S.A. RNA folding and ribosome assembly. Curr. Opin. Chem. Biol. 2008;12:667–673. doi: 10.1016/j.cbpa.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Macedo B., Millen T.A., Braga C.A., Gomes M.P., Ferreira P.S., Kraineva J., Winter R., Silva J.L., Cordeiro Y. Nonspecific prion protein-nucleic acid interactions lead to different aggregates and cytotoxic species. Biochemistry. 2012;51:5402–5413. doi: 10.1021/bi300440e. [DOI] [PubMed] [Google Scholar]
- 120.Supattapone S. Biochemistry. What makes a prion infectious? Science. 2010;327:1091–1092. doi: 10.1126/science.1187790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geoghegan J.C., Valdes P.A., Orem N.R., Deleault N.R., Williamson R.A., Harris B.T., Supattapone S. Selective incorporation of polyanionic molecules into hamster prions. J. Biol. Chem. 2007;282:36341–36353. doi: 10.1074/jbc.M704447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deleault N.R., Geoghegan J.C., Nishina K., Kascsak R., Williamson R.A., Supattapone S. Protease-resistant prion protein amplification reconstituted with partially purified substrates and synthetic polyanions. J. Biol. Chem. 2005;280:26873–26879. doi: 10.1074/jbc.M503973200. [DOI] [PubMed] [Google Scholar]
- 123.Grossman A., Zeiler B., Sapirstein V. Prion protein interactions with nucleic acid: Possible models for prion disease and prion function. Neurochem. Res. 2003;28:955–963. doi: 10.1023/A:1023215207981. [DOI] [PubMed] [Google Scholar]
- 124.Wang M., Law M., Duhamel J., Chen P. Interaction of a self-assembling peptide with oligonucleotides: Complexation and aggregation. Biophys. J. 2007;93:2477–2490. doi: 10.1529/biophysj.106.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braun S., Humphreys C., Fraser E., Brancale A., Bochtler M., Dale T. Amyloid-associated nucleic acid hybridisation. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turk R.M., Chumachenko N.V., Yarus M. Multiple translational products from a five-nucleotide ribozyme. Proc. Natl. Acad. Sci. USA. 2010;107:4585–4589. doi: 10.1073/pnas.0912895107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carny O., Gazit E. A model for the role of short self-assembled peptides in the very early stages of the origin of life. FASEB J. 2005;19:1051–1055. doi: 10.1096/fj.04-3256hyp. [DOI] [PubMed] [Google Scholar]
- 128.Carny O., Gazit E. Creating prebiotic sanctuary: Self-assembling supramolecular peptide structures bind and stabilize RNA. Orig. Life Evol. Biosph. 2011;41:121–132. doi: 10.1007/s11084-010-9219-9. [DOI] [PubMed] [Google Scholar]
- 129.Otto S., Furlan R.L., Sanders J.K. Dynamic combinatorial libraries of macrocyclic disulfides in water. J. Am. Chem. Soc. 2000;122:12063–12064. doi: 10.1021/ja005507o. [DOI] [Google Scholar]
- 130.Carnell J., Waudby C.A., Belenguer A.M., Stuart M.C., Peyralans J.J.-P., Otto S. Mechanosensitive self-replication driven by self-organization. Science. 2010;327:1502–1506. doi: 10.1126/science.1182767. [DOI] [PubMed] [Google Scholar]
- 131.Ivnitski D., Amit M., Rubinov B., Cohen-Luria R., Ashkenasy N., Ashkenasy G. Introducing charge transfer functionality into prebiotically relevant β-sheet peptide fibrils. Chem. Commun. 2014;50:6733–6736. doi: 10.1039/c4cc00717d. [DOI] [PubMed] [Google Scholar]
- 132.Goodwin J.T., Lynn D.G. Template-directed synthesis: Use of a reversible reaction. J. Am. Chem. Soc. 1992;114:9197–9198. doi: 10.1021/ja00049a067. [DOI] [Google Scholar]
- 133.McCleskey S.C., Griffin M.J., Schneider S.E., McDevitt J.T., Anslyn E.V. Differential receptors create patterns diagnostic for ATP and GTP. J. Am. Chem. Soc. 2003;125:1114–1115. doi: 10.1021/ja021230b. [DOI] [PubMed] [Google Scholar]
- 134.Schneider S.E., O’Neil S.N., Anslyn E.V. Coupling rational design with libraries leads to the production of an ATP selective chemosensor. J. Am. Chem. Soc. 2000;122:542–543. doi: 10.1021/ja9935153. [DOI] [Google Scholar]
- 135.Butterfield S.M., Sweeney M.M., Waters M.L. The recognition of nucleotides with model β-hairpin receptors: Investigation of critical contacts and nucleotide selectivity. J. Org. Chem. 2005;70:1105–1114. doi: 10.1021/jo0491105. [DOI] [PubMed] [Google Scholar]
- 136.Butterfield S.M., Waters M.L. A designed β-hairpin peptide for molecular recognition of ATP in water. J. Am. Chem. Soc. 2003;125:9580–9581. doi: 10.1021/ja0359254. [DOI] [PubMed] [Google Scholar]
- 137.Norris V., Loutelier-Bourhis C., Thierry A. How did metabolism and genetic replication get married? Orig. Life Evol. Biosph. 2012;42:487–495. doi: 10.1007/s11084-012-9312-3. [DOI] [PubMed] [Google Scholar]
- 138.Root-Bernstein R. Simultaneous origin of homochirality, the genetic code and its directionality. Bioessays. 2007;29:689–698. doi: 10.1002/bies.20602. [DOI] [PubMed] [Google Scholar]
- 139.Hunding A., Kepes F., Lancet D., Minsky A., Norris V., Raine D., Sriram K., Root-Bernstein R. Compositional complementarity and prebiotic ecology in the origin of life. Bioessays. 2006;28:399–412. doi: 10.1002/bies.20389. [DOI] [PubMed] [Google Scholar]
- 140.Root-Bernstein R., Dillon P.F. Molecular Complementarity I: The complementarity theory of the origin and evolution of life. J. Theor. Biol. 1997;188:447–479. doi: 10.1006/jtbi.1997.0476. [DOI] [PubMed] [Google Scholar]
- 141.Root-Bernstein R. A modular hierarchy-based theory of the chemical origins of life based on molecular complementarity. Acc. Chem. Res. 2012;45:2169–2177. doi: 10.1021/ar200209k. [DOI] [PubMed] [Google Scholar]
- 142.Dale T. Protein and nucleic acid together: A mechanism for the emergence of biological selection. J. Theor. Biol. 2006;240:337–342. doi: 10.1016/j.jtbi.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 143.Segre D., DBen-Eil D., Lancet D. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. USA. 2000;97:4112–4117. doi: 10.1073/pnas.97.8.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Flores Martinez C.I. SETI in the light of cosmic convergent evolution. Acta Astronaut. 2014;104:341–349. doi: 10.1016/j.actaastro.2014.08.013. [DOI] [Google Scholar]
- 145.Chela-Flores J. From systems chemistry to systems astrobiology: Life in the universe as an emergent phenomenon. Int. J. Astrobiol. 2013;12:8–16. doi: 10.1017/S1473550412000262. [DOI] [Google Scholar]
- 146.Goodwin J.T., Walker S.I., Amin S., Armbrust G., Burrows C.J., Lynn D.G. Alternative Chemistries of Life: Empirical Approaches. [(accessed on 3 December 2014)]. Available online: http://chemistry.emory.edu/home/assets/alternativechem.pdf.
- 147.Russell M.J., Barge L.M., Bhartia R., Bocanegra D., Bracher P.J., Branscomb E., Kidd R., McGlynn S., Meier D.H., Nitschke W., et al. The drive to life on wet and icy worlds. Astrobiology. 2014;14:308–343. doi: 10.1089/ast.2013.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruiz-Mirazo K., Briones C., de la Escosura A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014;114:285–366. doi: 10.1021/cr2004844. [DOI] [PubMed] [Google Scholar]