Abstract
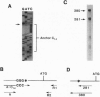
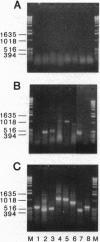
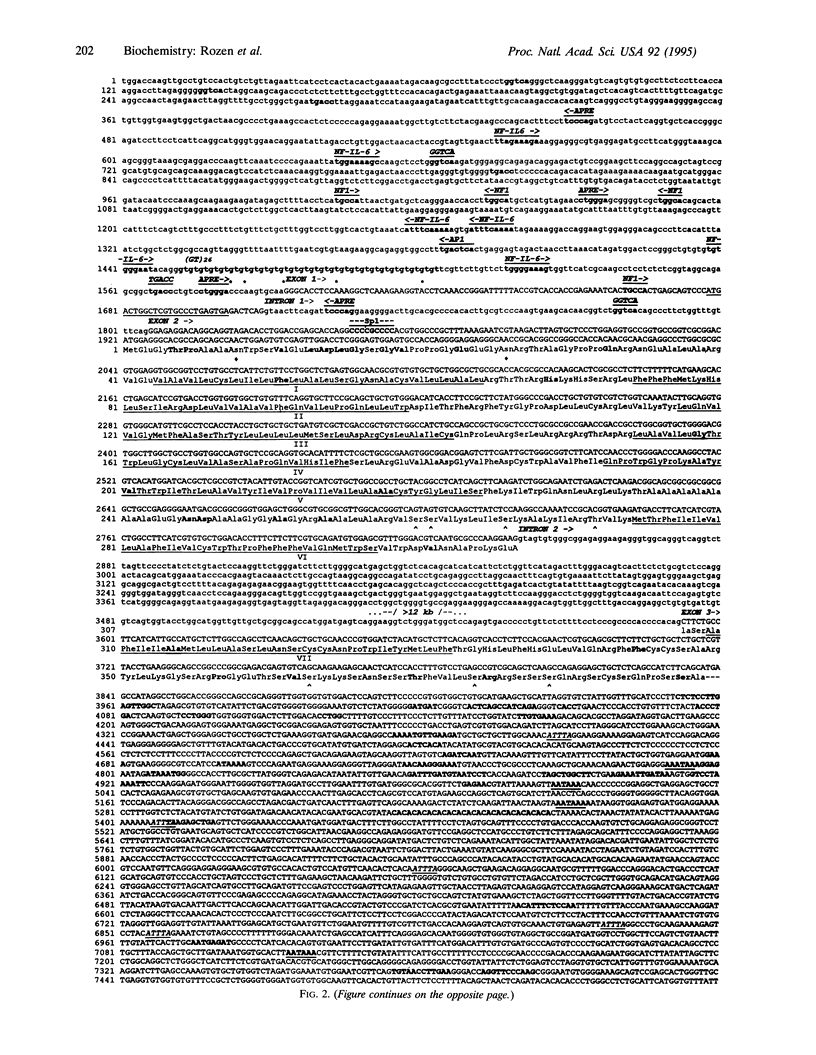
The multiple hormonal and neurotransmitter functions of the nonapeptide oxytocin are mediated by specific oxytocin receptors (OTRs). In most target tissues, the number of OTRs is strongly regulated. Specifically, in the uterus, a dramatic OTR upregulation precedes the onset of parturition. To study the molecular mechanisms underlying OTR regulation, we have isolated and characterized recombinant bacteriophage lambda EMBL3 genomic clones containing the rat OTR gene, using sequence information derived from a human myometrial OTR cDNA. The rat OTR gene spans > 20 kb and contains three exons. A 97-bp intron is in the 5' untranslated region and a > 12-kb intron interrupts the coding region between transmembrane domains 6 and 7. The promoter region lacks an apparent TATA or CCAAT box but contains multiple putative interleukin-response elements [six NF-IL6 (C/EBP beta) and four APRF (STAT3) binding motifs], supporting the notion that interleukins may mediate labor induction via transcriptional activation of the OTR gene. The predicted amino acid sequence is 93% identical to the human OTR sequence but only 48% and 38% identical to the rat V1 and V2 vasopressin receptor sequences, respectively. At parturition, the OTR gene is highly expressed in the rat uterus and gives rise to at least three transcripts (2.9, 4.8, and 6.7 kb) which differ in the length of their 3' untranslated regions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. J., Bradshaw A. D., Shiels B. R., Northemann W., Hudson G., Fey G. H. Hepatic transcription of the acute-phase alpha 1-inhibitor III gene is controlled by a novel combination of cis-acting regulatory elements. Mol Cell Biol. 1990 Jul;10(7):3483–3491. doi: 10.1128/mcb.10.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992 Jun;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M., Seibold A., Gilbert S., Ishido M., Barberis C., Antaramian A., Brabet P., Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992 May 28;357(6376):333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Dudley D. J., Trautman M. S., Araneo B. A., Edwin S. S., Mitchell M. D. Decidual cell biosynthesis of interleukin-6: regulation by inflammatory cytokines. J Clin Endocrinol Metab. 1992 Apr;74(4):884–889. doi: 10.1210/jcem.74.4.1548355. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbulev V., Büchner H., Akhundova A., Fahrenholz F. Molecular cloning and functional characterization of V2 [8-lysine] vasopressin and oxytocin receptors from a pig kidney cell line. Eur J Biochem. 1993 Jul 1;215(1):1–7. doi: 10.1111/j.1432-1033.1993.tb18000.x. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Immunologically relevant cells in the uterus. Biol Reprod. 1994 Mar;50(3):461–466. doi: 10.1095/biolreprod50.3.461. [DOI] [PubMed] [Google Scholar]
- Kato S., Tora L., Yamauchi J., Masushige S., Bellard M., Chambon P. A far upstream estrogen response element of the ovalbumin gene contains several half-palindromic 5'-TGACC-3' motifs acting synergistically. Cell. 1992 Feb 21;68(4):731–742. doi: 10.1016/0092-8674(92)90148-6. [DOI] [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992 Apr 9;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992 May 28;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- McCarthy M. M., Kleopoulos S. P., Mobbs C. V., Pfaff D. W. Infusion of antisense oligodeoxynucleotides to the oxytocin receptor in the ventromedial hypothalamus reduces estrogen-induced sexual receptivity and oxytocin receptor binding in the female rat. Neuroendocrinology. 1994 May;59(5):432–440. doi: 10.1159/000126689. [DOI] [PubMed] [Google Scholar]
- McCarthy M. M., Kow L. M., Pfaff D. W. Speculations concerning the physiological significance of central oxytocin in maternal behavior. Ann N Y Acad Sci. 1992 Jun 12;652:70–82. doi: 10.1111/j.1749-6632.1992.tb34347.x. [DOI] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Sepulveda W., Mazor M., Brandt F., Cotton D. B., Dinarello C. A., Mitchell M. D. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992 Oct;167(4 Pt 1):863–872. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- Romero R., Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992 Oct;167(4 Pt 1):1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Coirini H., Johnson A. E., Flanagan L. M., Frankfurt M., Pfaff D. W., McEwen B. S. The oxytocin receptor: a target for steroid hormones. Regul Pept. 1993 Apr 29;45(1-2):115–119. doi: 10.1016/0167-0115(93)90192-b. [DOI] [PubMed] [Google Scholar]
- Seibold A., Brabet P., Rosenthal W., Birnbaumer M. Structure and chromosomal localization of the human antidiuretic hormone receptor gene. Am J Hum Genet. 1992 Nov;51(5):1078–1083. [PMC free article] [PubMed] [Google Scholar]
- Soloff M. S., Alexandrova M., Fernstrom M. J. Oxytocin receptors: triggers for parturition and lactation? Science. 1979 Jun 22;204(4399):1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. Human endometrium: an active site of cytokine production and action. Endocr Rev. 1991 Aug;12(3):272–290. doi: 10.1210/edrv-12-3-272. [DOI] [PubMed] [Google Scholar]
- Vince G. S., Starkey P. M., Jackson M. C., Sargent I. L., Redman C. W. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990 Sep 14;132(2):181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Goodman R. H., Habener J. F. Developmental expression of the rat somatostatin gene. Endocrinology. 1984 Jul;115(1):90–94. doi: 10.1210/endo-115-1-90. [DOI] [PubMed] [Google Scholar]