Abstract
Chemotherapy treatment in women can frequently cause damage to the ovaries, which may lead to primary ovarian insufficiency (POI). In this study, we assessed the preventative effects of hyaluronic acid (HA) in immunosuppressive drug-induced POI-like rat models and investigated the possible mechanisms. We found that HA, which was reduced in primary and immunosuppressant-induced POI patients, could protect the immunosuppressant-induced damage to granulosa cells (GCs) in vitro. Then we found that HA blocked the tripterygium glycosides (TG) induced POI-like presentations in rats, including delayed or irregular estrous cycles, reduced 17 beta-estradiol(E2) concentration, decreased number of follicles, destruction of follicle structure, and damage of reproductive ability. Furthermore, we investigated the mechanisms of HA prevention effects on POI, which was associated with promotion of GC proliferation and PGRMC1 expression. In conclusion, HA prevents chemotherapy-induced ovarian damage by promoting PGRMC1 in GCs. This study may provide a new strategy for prevention and treatment of POI.
Autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis occur more commonly in females than in males1,2. Increasing evidence also suggests that immune-mediated processes that affect female reproductive success and cause abnormalities of the female immune system, including autoimmunity, potentially interfere at multiple levels3. Although targeted biological treatments for these autoimmune diseases have resulted in marked improvements in morbidity and mortality4, traditional therapies including nonspecific immunosuppressants, such as tripterygium glycosides (TG) and cyclophosphamide (CYC), are also commonly used in many developing countries such as China due to their favorable cost-benefit ratio5,6,7,8,9,10,11.
Autoimmune diseases most often occur in women of reproductive age, and chemotherapy treatment in premenopausal women can frequently cause ovarian damage, which may lead to primary ovarian insufficiency (POI) due to the gonadotoxicity of chemotherapy drugs12. In addition to loss of reproductive potential, POI is associated with increased risk of morbidity and mortality. Therefore, even if future pregnancy is not desired, ovarian protection during gonadotoxic therapy should be a major goal of disease management13. Although hormone replacement therapy is currently used to treat POI patients14,15,16, the treatment does not improve infertility and has an increased risk of developing breast cancer, lung cancer, heart attack and stroke17,18,19,20. Therefore, it is necessary to explore new strategies for the prevention and treatment of chemotherapy-induced ovarian damage or POI.
Granulosa cells (GCs) are closely associated with the developing oocyte in the mammalian ovary. The major functions of GCs include the production of sex steroids such as estradiol (E2) and progesterone (P4), and a myriad of growth factors thought to interact with the oocyte during its development21,22. Long-term survival of the GCs is critical for pregnancy maintenance23. Importantly, GC apoptosis is believed to trigger oocyte apoptosis, which leads to premature exhaustion of the follicle pool24. Nonspecific immunosuppressants such as TG and CYC have been reported to induce GC apoptosis and result in POI25,26,27. It was reported that CYC may act primarily on primordial follicles through induction of apoptotic changes in pre-granulosa cells which lead to follicle loss28,29. In our previous study, we found that TG inhibited the proliferation of rat GCs in vitro30. Then we constructed the TG induced POI rat model and found fewer layers of GCs in developing follicles in TG-treated ovaries as compared to normal ovaries and TG decreased reproductive outcomes in female rats30. Thus, GC survival could be prolonged by inhibiting apoptosis to reverse TG- or CYC-induced POI. More research into the cellular mechanisms of chemotherapy-induced ovarian damage could lead to the generation of treatments specifically designed to prevent POI.
Hyaluronic acid (HA) is an important matrix molecule, and is thought to inhibit the GC apoptosis24. GCs produce HA, which in turn plays important roles in ovary development31. HA is recognized as a functional ligand involved in the control of dynamic biological functions such as cell migration, proliferation, and differentiation32. Interestingly, the concentration of HA in follicular fluids is an indicator for estimation of oocyte viability for fertilization33. HA and matrix proteins (ECM)-HA encapsulated follicles looked healthy and maintained their 3-D architecture during in vitro culture34. It was previously demonstrated that cumulus expansion is the result of HA synthesis and accumulation between cumulus cells and that HA accumulation affects oocyte maturation35. The incidence of apoptotic GCs with fragmented condensed nuclei was reduced by HA36.
Therefore, we hypothesized that HA supplementation may prevent immunosuppressive agent-induced ovarian damage and help relieve POI. To investigate our hypothesis, we constructed the POI-like rat model induced by TG and evaluated HA's preventative effects in this model. We also investigated the mechanisms of HA on prevention POI with GCs.
Results
Serum HA levels are reduced in both primary and immunosuppressive agent-induced POI patients
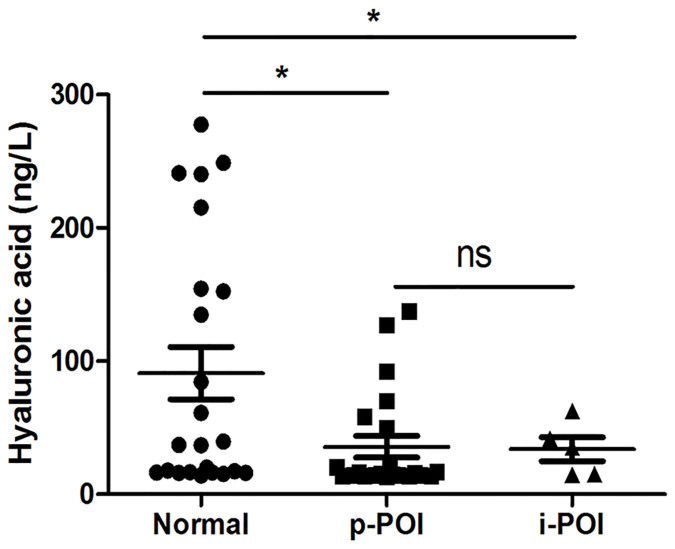
To investigate whether HA is reduced in POI patients, we collected the serum samples from 30 POI patients (including 2 CYC and 3 TG treatment-induced POI patients) and 30 healthy cycling women, and measured the HA levels. The results showed that the HA levels in the primary POI patients (p-POI) and immunosuppressive agent treatment-induced POI patients (i-POI) were 35.7 ng/L and 33.9 ng/L respectively, which was significantly lower than that in healthy women (91 ng/L; Fig. 1).
Figure 1. Quantification of HA in serum of primary ovarian insufficiency (POI) patients and healthy cycling women.
HA concentration in serum from 25 primary POI patients (p-POI), 5 immunosuppressive agent-treatment induced POI patients (i-POI), and 30 healthy cycling women (Normal) was assessed using ELISA. The data are shown as mean ± SEM. *P < 0.05 compared with normal.
HA protects the immunosuppressive agent-induced damage to GCs in vitro
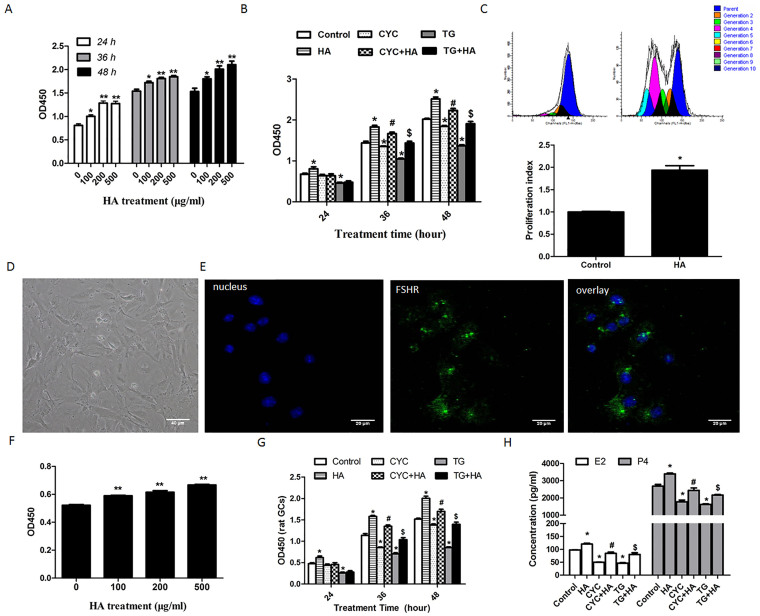
Since HA levels in the serum of POI patients were reduced (Fig. 1), we investigated whether HA supplementation may prevent the occurrence of POI induced by treatment with immunosuppressive agents. To test this hypothesis, we first investigated whether HA reversed the damage that immunosuppressive agents caused to GCs in vitro. We treated KGN cells, a human ovarian GC line, with 0, 100, 200, and 500 μg/ml HA for 24 h, 36 h, and 48 h. Cell viability assay showed that the viability of HA-treated KGN cells was significantly increased compared with that of the cells not treated with HA, even in the cells treated for only 24 h (Fig. 2a). KGN cells were then treated with 50 nM TG or 100 μM CYC combined with or without 200 μg/ml HA for 24 h, 36 h and 48 h. The results showed that the cell viability was inhibited by TG and CYC at 24 h, 36 h and 48 h (Fig. 2b). However, when the cells were treated with TG or CYC combined with HA, the cell viability showed high levels, similar to normal controls (Fig. 2b). In addition, cell proliferation testing showed that HA-treated cells had more generations than normal control cells and that the proliferation index of HA-treated cells was nearly twice that of the cells not treated with HA (Fig. 2c).
Figure 2. HA protects the immunosuppressive agent-induced damage to GCs in vitro.
(a) After treatment with HA (0, 100, 200 and 500 μg/ml) for 24 h, 36 h, and 48 h, cell viability of KGN was tested using a CCK8-kit. *P < 0.05, **P < 0.01 compared with the cells not treated with HA. (b) KGN cells were treated with CYC (100 μM) or TG (50 nM) in the presence or absence of HA (200 μg/ml) for 24 h, 36 h, and 48 h respectively. The cell proliferation was analyzed using a CCK8-kit. *P < 0.05 vs. control; #P < 0.05 CYC+HA vs. CYC; &P < 0.05 TG+HA vs. TG. (c) The proliferation of HA-treated KGN cells was analyzed using the CFSE staining method (upper panel). The data were normalized to the proliferative index (PI) of control cells (lower panel). *P < 0.05 compared with control. (d) Primary GCs were obtained from rat ovary and an optical microscope image was taken (×60). (e) Rat GCs cultured on coverslips were fixed and immunostained with specific antibodies for rat FSHR (×60). (f) After treatment with the indicated concentration of HA for 48 h, rat GC viability was analyzed using CCK8 kit. **P < 0.01vs control. (g) Rat primary GCs were treated with CYC (100 μM) or TG (50 nM) in the presence or absence of HA (200 μg/ml) for 24 h, 36 h, and 48 h respectively. The cell viability was analyzed using a CCK8-kit. *P < 0.05 vs. control; #P < 0.05 CYC+HA vs. CYC; &P < 0.05 TG+HA vs. TG. (h) Primary GCs were treated with CYC (100 μM) or TG (50 nM) in the presence or absence of HA (200 μg/ml) for 48 h. The concentration of E2 and P4 was assessed using ELISA. #P < 0.05 CYC+HA vs. CYC; $P < 0.05 TG+HA vs. TG. Average results from three independent experiments are shown.
We also isolated GCs from rat ovaries, and cultured them in the presence of HA to provide further support for the above results. The identity of rat GCs was defined by morphology and FSHR expression (Fig. 2d and 2e). The results showed that HA promoted cell proliferation of rat GCs in vitro (Fig. 2f). Similar with KGN cells, 50 nM TG or 100 μM CYC inhibited cell viability of primary rat GCs at 24 h, 36 h, and 48 h. When the primary rat GCs were treated with TG or CYC combined with HA, this reduction could be partially rescued by HA (Fig. 2g). Moreover, we investigated the preventative effects of HA on the TG- or CYC-induced decrease in estrogen production. After treatment with 50 nM TG or 100 μM CYC, we found that the E2 and P4 concentration was reduced (Fig. 2h). However, when the cells were treated with TG or CYC combined with HA, E2 and P4 concentration showed high levels, similar to normal controls (Fig. 2h).
HA prevents TG-induced ovarian damage and improves ovarian function in rats
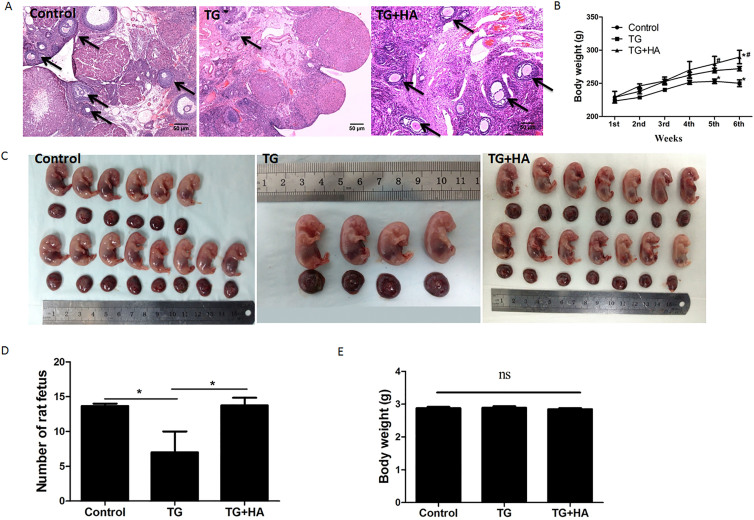
To evaluate the preventative effects of HA on immunosuppressive agent-induced POI, we constructed a POI-like rat model, as described in the Materials and Methods. After TG was intragastrically administered for 45 days, 3 of the 7 rats (43%) had delayed estrous cycles and 4 others (57%) had irregular estrous cycles (Table 2). The E2 levels in TG-treated rats were decreased to 29.623 pg/ml while the normal concentration was 47.685 pg/ml (Table 2). Ovarian and uterine indices in the TG-treated rats were decreased by 43.7% and 24.5%, respectively (Table 2). The average number of secondary and antral follicles in each TG-treated rat was decreased to 298 (normal number is 400, F = 10.25, p < 0.05) and 88 (normal number is 176, F = 10.63, p < 0.01), respectively (Table 2). Histological analysis showed that the density of follicles in ovaries of TG-treated rats was significantly reduced (Fig. 3a) and the follicle structure was destructed (Suppl. Fig. 1). Compared with the control rats, the rats treated with TG had lower body weight. After 6 weeks' treatment with TG alone, the rat body weight was decreased (Fig. 3b). These results are similar to the clinical phenomena in POI patients.
Table 1. Clinical variables.
| Variables | |
|---|---|
| POI | |
| Age (years) median (range) | 29.9 (19–37) |
| POI family history | |
| None | 20 (66.7%) |
| Have | 8 (26.7%) |
| Undetermined | 2 (6.7%) |
| Etiology | |
| Idiopathic | 25 (83.3%) |
| Immunosuppressive agent induced | 5 (16.7%) |
| Uterus size (mm × mm × mm) | |
| Average | 41.1 × 37.7 × 29.7 |
| Left ovary size (mm × mm × mm) | |
| Average | 15.9 × 14.2 × 12.9 |
| Right ovary size (mm × mm × mm) | |
| Average | 15.6 × 14.6 × 13 |
| Blood hormone levels | |
| Serum FSH (mIU/ml) (obtained at least 1 month apart) | 66.785 |
| E2 (pg/ml) | 39.605 |
| P4 (nmol/L) | 0.705 |
| Healthy cycling women | |
| Age (years) median (range) | 28.7 (20–35) |
| Blood hormone levels | |
| Serum FSH (mIU/ml) | 5.163 |
| E2 (pg/ml) | 47.827 |
| P4 (nmol/L) | 1.471 |
Table 2. POI-related index in each group.
| Groups | N | Estrous cycle | Numbers of follicles | Ovarian index | Uterine index | E2 concentration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| normal | delayed | irregular | primordial | primary | secondary | antral | |||||
| Control | 7 | 7 | 0 | 0 | 2149 ± 200.1 | 914 ± 61.75 | 400 ± 37.7 | 176 ± 12.7 | 0.5039 ± 0.07390 | 1.7352 ± 0.5105 | 47.685 ± 7.3 |
| Model | 7 | 0 | 3 | 4 | 2165 ± 137.9 | 799 ± 55.7 | 298 ± 27.5* | 88 ± 6.4* | 0.2837 ± 0.05620* | 1.3102 ± 0.2129* | 29.623 ± 1.22* |
| HA | 7 | 7 | 0 | 0 | 1920 ± 108.2 | 743 ± 35.1 | 445 ± 52.7# | 114 ± 14.1# | 0.4665 ± 0.08582# | 1.9767 ± 0.4006# | 62.785 ± 13.58# |
Note: Ovarian index = Bilateral ovarian wet weight (mg)/Body weight (g) × 100%, Uterine index = uterus wet weight (mg)/Body weight (g) × 100%.
E2 concentration: pg/ml.
*P < 0.05 vs. control group;
#P < 0.05 vs. model group.
Figure 3. Ovary function evaluation in rats treated with TG and HA.
(a) The density and structure of follicles in rat ovaries were analyzed using hematoxylin and eosin staining. The arrows indicate the follicles. Bar: 50 μm; (b) The body weight of each rat in control, TG, and TG + HA groups was measured each week. *P < 0.05 vs. control; #P < 0.05 TG + HA vs. TG. (c) At day 19 of gestation, the uteri of anesthetized rats were celiotomized and pictures of the embryos were taken. (d) The number of embryos from each rat was counted.*P < 0.05, **P < 0.01 compared with control. (e) At day 19 of gestation, the uteri of anesthetized rats were celiotomized and the weight of each embryo was measured.
Since HA levels in the serum of POI patients were reduced (Fig. 1), we investigated whether HA may prevent the occurrence of POI-like presentations in TG-treated rats. As shown in Table 2, the estrous cycles of seven rats treated with a mixture of TG and HA were all normal and the concentration of E2 in them was maintained at a normal level. The ovarian and uterine indices (0.4665 and 1.9767, respectively) in the rats treated with the mixture of TG and HA were higher than those in the rats treated with TG alone (0.2837, p = 0.0367; and 1.3102, 0.05, respectively; Table 2). Additionally, as shown in Table 2, TG mainly decreased the number of secondary (298 ± 27.5 vs 400 ± 37.7, F = 10.25, p < 0.05) and antral follicles (88 ± 6.4 vs 176 ± 12.7, F = 10.63, p < 0.01), while HA reversed the decrease to almost normal level (445 ± 52.7 and 114 ± 14.1, respectively). HE staining of ovarian tissues showed that TG treatment damaged almost all secondary and antral follicles structures, while the TG plus HA-treated group had normal follicle structure (Fig. 3a). The average body weight of the rats treated with TG increased slowly, while that of the rats treated with HA and TG increased normally, even higher than normal control after six weeks (Fig. 3b).
To investigate whether HA could protect the fertility of rats from the damage induced by TG, 21 rats were administered TG to measure their reproductive ability, as described in the Materials and Methods. As shown in Fig. 3c and 3d, the average number of offspring for each TG-treated rat was 4.67, while that of each control rat was 13.33 (F = 6.297, p < 0.05). Interestingly, each rat treated with HA and TG had 13.75 offspring on average. There was no significant difference in the body weight of each offspring among three groups (Fig. 3e).
HA promotes the proliferation and function of GCs via PGRMC1
P4 receptor membrane component 1 (PGRMC1) plays an important role in GC cell growth. It is reported that PGRMC1 regulates cell viability37 and sexual steroid hormone synthesis38,39. Moreover, PGRMC1 can prevent GC apoptosis in the presence or absence of P440,41,42,43.
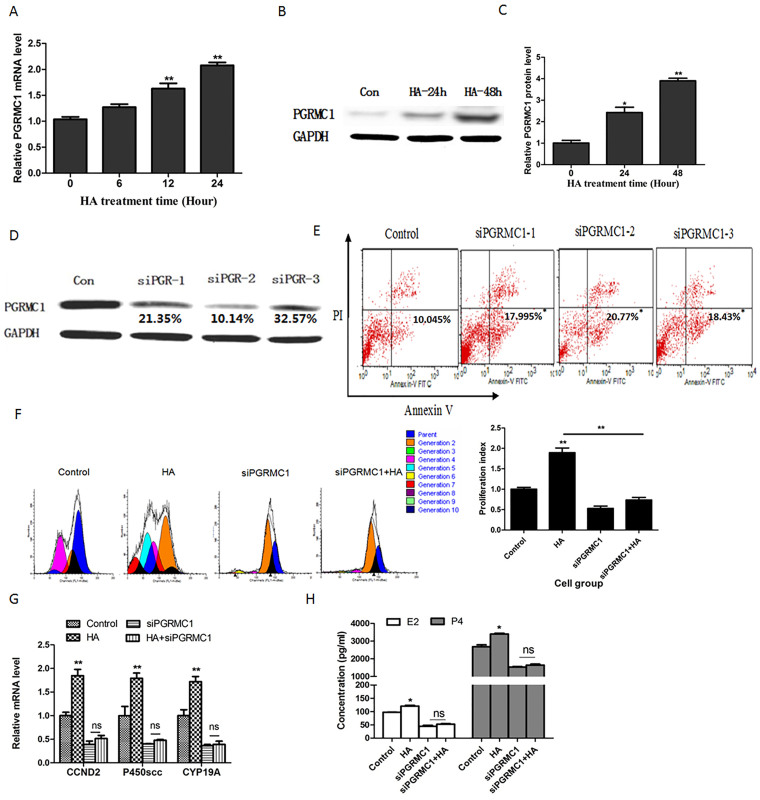
It was reported that PAIRBP1, a HA binding protein44, interacts with PGRMC1 to maintain the rat GC viability45. Therefore, we speculate that PGRMC1 may be involved in HA's proliferation promoting effects on GCs. After treatment with HA for the indicated time, we assessed the PGRMC1 mRNA and protein levels in HA-treated KGN cells. The results showed that the PGRMC1 mRNA (Fig. 4a) and protein levels (Fig. 4b and Fig. 4c) were significantly up-regulated.
Figure 4. The roles of PGRMC1 in promotion effects of HA on GCs proliferation and estrogen production.
(a) PGRMC1 mRNA level was detected using real-time RT-PCR in KGN cells treated with HA for 0 h, 6 h, 12 h, and 24 h. **P < 0.01 compared with 0 h group. (b) Western blots of PGRMC1 protein in KGN cells treated with HA for 24 h and 48 h respectively. The gels were run under the same experimental conditions as detailed in the Methods section, and full-length blots were cropped for final display (suppl. Figure 2). (c) Relative expression of PGRMC1 to GAPDH was calculated. Three replicate western blots were performed at one time and average results are from three independent experiments. **P < 0.01 compared with 0 h group. (d)Western blots of PGRMC1 and GAPDH in KGN cells transfected with control siRNA or siRNA targeting PGRMC1 for 48 h. GAPDH was used as an internal reference. The relative expression of PGRMC1 to GAPDH was calculated. (e) Apoptosis (annexin V+ PI−) was assessed using the annexin V-PI method after the KGN cells were transfection with control siRNA or siRNA targeting PGRMC1 for 48 h. *P < 0.05, **P < 0.01 compared with control. (f) KGN cells were transfected with siRNA negative sequence or siRNA specific to PGRMC1 before treatment with 200 μg/ml HA. After treatment with HA for 72 h, cell proliferation was assessed by CFSE method (left panel). The CFSE data were normalized to the PI of control (right panel). **P < 0.01 compared with control. (g) KGN cells were transfected with siRNA negative sequence or siRNA specific to PGRMC1 before treatment with 200 μg/ml HA. After treatment with HA for 24 h, CCND2, p450scc, and CYP19A mRNA levels were assessed using real-time RT-PCR. GAPDH was used as an internal reference. **P < 0.01 compared with control. (h) Primary GCs were transfected with siRNA negative sequence or siRNA specific to PGRMC1 before treatment with 200 μg/ml HA. After treatment with HA for 48 h, the concentration of E2 and P4 was assessed using ELISA. *P < 0.05 compared with control. Average results from three independent experiments are shown.
Before investigating the roles of PGRMC1 in HA's promoting effects, siRNAs specific to PGRMC1 (siPGRMC1) were synthesized to downregulate PGRMC1 expression. The results showed that three interference sequences, named siPGRMC1-1, siPGRMC1-2, andsiPGRMC1-3 inhibit PGRMC1 protein expression by 21.35%, 10.14%, and 32.57%, respectively (Fig. 4d), and these three siPGRMC1 sequences increased the percentage of apoptotic KGN cells to 18.0%, 20.8%, and 18.4%, respectively (F = 6.812, p < 0.05; Fig. 4e). Cell proliferation was then assessed after siPGRMC1 pre-transfected KGN cells were treated with or without 200 μg/ml HA for 72 hours. The results showed that there were more generations of the KGN cells treated with HA alone than those of the normal control cells (Fig. 4f). However, the promotion effect of HA on GC proliferation was attenuated after down-regulation of PGRMC1 expression using siPGRMC1 (Fig. 4g). Additionally, expression of cyclin D2 (CCND2), a marker of GC proliferation, was up-regulated after treatment with 200 μg/ml HA for 24 hours. However, HA had no effect on CCND2 expression after PGRMC1 was blocked with siPGRMC1 in GCs (Fig. 4g).
Production of E2 and P4 is the main function of GCs. We found that HA significantly enhanced the levels of the E2 synthesis enzyme, CYP19A, and the P4 synthesis enzyme, p450scc (Fig. 4g). Similar to the gene expression, E2 and P4 production was increased after treatment with 200 μg/ml HA for 48 hours in GCs (Fig. 4h). GCs were then transfected with siPGRMC1 before treatment with or without HA. The results showed that when PGRMC1 was blocked in GCs, HA was not able to promote CYP19A and p450scc expression (Fig. 4g) and E2 and P4 production (Fig. 4h). These results indicated that HA promotes both GC proliferation and their production of sex hormone biosynthesis through up-regulating PGRMC1 expression.
Discussion
TG and CYC are now widely used in clinical practice to treat inflammatory diseases, autoimmune diseases, organ transplantation and even tumors, but they have an adverse effect of reproductive toxicity46. It is necessary to protect ovarian function in this patient group. In the present study, we constructed TG-induced POI-like rats and evaluated the preventative effects of HA on POI. We found that TG induced POI-like presentations in rats, including delayed or irregular estrous cycles, reduced E2 concentration, decreased ovarian and uterine indices, decreased number of follicles, and destruction of follicle structure. However, when treated with mixture of TG and HA, the rats maintained their normal status similar to control rats. More importantly, HA-treated rats had normal fertility. We also investigated the mechanisms of HA prevention effects on POI, which was associated with promotion of GC proliferation and PGRMC1 expression. These findings have significant implications for the potential applicability of HA in the prevention of POI. Of note, we did not investigate whether HA reduced the clinical effectiveness of the immunosuppressive agents used in this study. To address this issue, both autoimmune disease and cancer models should be used to check the immunosuppressive effect and anti-tumor effect of TG or CYC in the presence of HA.
Continued survival of GCs following ovulation and their luteinization is critical for P4 production and sustaining pregnancy. However, POI patients experience accelerated atresia45. The formation of an atretic follicle is believed to be initiated by GC apoptosis24. Therefore, inhibiting apoptosis and promoting proliferation of GCs should be an important strategy for the prevention and treatment of POI. Here, we showed that HA promoted GC proliferation and blocked the TG- or CYC-induced decrease in GC viability in vitro. Moreover, we found that HA enhanced the expression of CCND2 (a marker of GC proliferation), CYP19a (an E2 synthesis enzyme) and P450scc (a P4 synthesis enzyme) in human KGN cells. In rat primary GCs, HA promoted the secretion of E2 and P4. These results indicate that HA is important for GC proliferation and function.
To further investigate the mechanism of HA's promotion effects on GC proliferation and function, we found that HA up-regulated PGRMC1 expression at both the gene and protein levels. PGRMC1, a P4 receptor, is believed to be involved in P4 signaling in the reproductive system and mediates P4's anti-apoptotic effects on GCs40,41,43. In addition, it is reported that depletion of PGRMC1 in the absence of P4 significantly increases the mRNA levels of many genes that are involved in promoting apoptosis. This suggests that PGRMC1, which is not bound to P4, regulates gene expression in a manner that would make the cells more susceptible to apoptosis41. Our results demonstrated that the promotion effects of HA on GC proliferation and sex hormone reproduction was attenuated after down-regulation of PGRMC1 expression using siPGRMC1. It was reported that HA can bind to PAIRBP1 which in turn interacts with PGRMC142. However, depleting PAIRBP1 did not alter the expression or cellular localization of PGRMC142. Therefore, PGRMC1 may be regulated by HA in other way. We found that HA could promote the expression of PGRMC1 via epigenetic silencing of miR-139-5p in GCs47. Therefore, we conclude that PGRMC1 may mediate HA's promotion effects on GC proliferation.
In conclusion, HA has a preventative effect on immunosuppressive agent-induced POI rats, which was associated with promotion of GC proliferation. We also demonstrate that HA promotes cell proliferation through up-regulation of PGRMC1 expression. These findings support the applicability and efficacy of HA in preventing POI.
Methods
Clinical POI samples and RNA isolation
EDTA blood samples were collected from 30 idiopathic POI patients (amenorrhea for over one year starting before the age of 40, and serum FSH level above 40 mIU/ml in two consecutive determinations; mean age of 29.9 ± 4.7 years) before treatment at the Nanjing Drum Tower Hospital (China) between May 2012 and January 2013. Of the 30 POI patients, 5 immunosuppressant-induced POI patients were included (2 CYC and 3 TG). In addition, we enrolled 30 healthy cycling women (mean age of 28.7 ± 4.5 years) as control group. Clinical data from these 30 patients and 30 healthy cycling women are summarized in Table 1. Informed consent was obtained from all patients and normal women. All experimental protocols were approved by Nanjing Drum Tower Hospital Ethics Committee. For analysis of HA using ELISA (Biotin linked-antibody to hyaluronan binding protein (HABP), Merck Chemicals), plasma was isolated from EDTA blood samples and stored at −80°C. The study received ethical permission from the Hospital Ethics Committee and the methods were carried out in accordance with the approved guidelines.
Cell Culture
The KGN cell line (a generous gift from Dr. Yiming Mu at the General Hospital of the People's Liberation Army, Beijing, China), derived from human ovarian granulosa cell tumor that expresses typical granulosa cell markers. KGN cells were cultured using DF-12 containing 10% FBS, and 50 mg/ml penicillin and streptomycin. Rat primary GCs were isolated from female Sprague-Dawley (SD) rats as described by Carr et al48. Briefly, SD rats were injected subcutaneously with 40 IU pregnant mares' serum gonadotropin (PMSG) (Folligon, Intervet, NSW, Australia) at 28 days of age to stimulate the development of preantral follicles. After PMSG injection for 48 h, GCs were released by puncturing the follicle. Cells were pelleted at 100 × g for 15 min, counted using trypan blue, and plated at a density of approximately 1 × l06 cells/ml on plastic dishes coated with 0.5 mg/ml human plasma fibronectin in Dulbecco's modified Eagle's medium/Ham's F-12 (DMEM/F-12, 1:1) with 15 mM HEPES, 3.15 g/L glucose containing 10% FBS, and 50 mg/ml penicillin and streptomycin. GCs were identified by FSHR antibodies (Bioworld, Louis Park, MN).
POI rat model establishment
A POI-like rat model was induced using TG as previously reported30. Female SD rats aged 12 weeks and weighing 220–250 g were provided by the experimental animal center of Nanjing Medical University. All rats had been acclimatized for 5 days before exfoliative cytoscopy of the vagina, which was performed to screen the rats with normal estrous cycle. The normal rats were randomly divided into 3 groups: model group, HA group and blank control group, with 7 rats in each group. Rats in the model group were intragastrically administered TG (Catalogue number: 3253, Tocris Bioscience, Bristol, UK) at a dose of 60 mg/kg/day; those in HA group were intragastrically administered TG (60 mg/kg/day) followed by intragastrically administering HA (20 mg/kg/day) (Qufu Hantang Biotechnology Co., Ltd. PR. China); the animals in blank control group were intragastrically administered physiological saline (10 ml/kg/day) for a total of 45 days. The investigation conforms to the guidelines of the Experimental Animal Management Committee (Jiangsu Province, China). All experimental protocols were approved by the Experimental Animal Management Committee (Jiangsu Province, China).
POI rat serum estrogen analysis
After 45 days, femoral artery blood samples were collected from all 21 rats (1 ml/rat) in model group, HA group, and blank control group. Then the serum estrogen was measured using γ-radioimmunoassay with a GC-1200 γ-radioimmunoassay counter according to the radioimmunoassay kit manufacturer's instructions (Tianjin Nine Tripods Medical & Bioengineering Co., Ltd. PR China).
POI rat ovarian and uterus index measurement
Rats were sacrificed using chloral hydrate overdose and complete bilateral ovaries and uterus were carefully separated from the surrounding adipose tissue and fascia. The wet weight of the bilateral ovaries and uterus was measured using an electronic balance, and the ovarian and uterine index was calculated according to the following formula: Ovarian index = Bilateral ovarian wet weight (mg)/Body weight (g) × 100%; Uterine index = uterus wet weight (mg)/Body weight (g) × 100%.
Number of follicles
The right ovary of each rat was fixed in 4% neutral buffered paraformaldehyde, embedded in paraffin, and cut into 3–5 μm sections. The sections were then stained with hematoxylin and eosin. The number of follicles at different stages and the corpus luteum were counted, as described by Myers et al49. Briefly, the oocyte-containing follicles in the developmental stage were classified and counted in every 5th section. Follicle classification was carried out according to the following criteria: primordial follicles contained a single layer of flattened GCs, primary follicles had at least three cuboidal GCs in a single layer, secondary follicles had at least two layers without an antral space, and antral follicles were identified as containing at least two layers of GCs that displayed an antral space.
Reproductive capacity evaluation
The reproductive ability of female rats was measured by the number of offspring. The reproductive ability of an additional 21 rats was assessed. Briefly, 7 rats were chosen randomly as normal controls, while 14 others were intragastrically administered TG at 60 mg/kg/day for 45 days, 7 rats of which served as a POI model and the remaining 7 rats were treated with HA (20 mg/kg/day) as in the HA group. The female rats were then caged with sexually active male rats (female:male = 1:1). We inspected daily for the vaginal plug, and the time that the vaginal plug was discovered was regarded as pregnancy day 0. At day 19 of gestation, we performed a cesarean section on anesthetized rats and counted and weighed the fetuses.
FSHR immunocytochemistry
Primary GCs from rat ovary were grown on coverslips in DMEM/F12 media. The cells were fixed with methanol/acetone and stained with FSHR antibodies (Bioworld, Louis Park, MN, USA) and followed FITC-labeled secondary antibodies(Bioworld, Louis Park, MN, USA). Then the nuclear of cells were stained with DAPI (Sigma, USA). Fluorescent images were collected with a confocal scanning laser system (Olympus Fluoview 1000; Olympus Corp., Lake Success, NY) attached to an inverted microscope (IX81; Olympus Corp., Tokyo, Japan).
RNA isolation and qRT-PCR
RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Total RNA (1 μg) was reverse-transcribed using Revert Aid™ First Strand cDNA Synthesis Kit (Fermentas, Pittsburgh PA, USA). PGRMC1 (Forward: CCATCAACGGCAAGGTG; Reverse: GGGCAGCAGTGAGGTCAG), CCND2 (Forward: ACAGAAGTGCGAAGAAGAGGT; Reverse: ATGGAGTTGTCGGTGTAAATG), p450scc (Forward: GGAGAAGCTCGGCAACG; Reverse: AGGGCGGGATGAGGAAT), CYP19a (Forward: TGTTGAAGAGGCAATAATAAAGG; Reverse: CCAAGTCCACGACAGGCT) and GAPDH (Forward: GTGAAGCAGGCGTCGGA; Reverse: AGGTGGAGGAGTGGGTGTC) were measured using a real-time PCR kit (Roche, Basel, Switzerland). The target gene mRNA was determined in triplicate in three to six separate experiments and normalized to GAPDH. qRT-PCR Assays-on-Demand for PGRMC1, CCND2, p450scc, CYP19a and GAPDH were performed in the ABI 7500 Fast (PE Applied Biosystems) using relative quantification. Analysis and fold differences were determined using the comparative cycle threshold (CT) method. Fold change was calculated from the ΔΔCT values with the formula 2−ΔΔCT, and data are presented as relative to expression in control cells.
Small interfering RNA (siRNA) transfection
siRNA knockdown was performed in KGN cells using siRNA duplexes to silence PGRMC1 (Invitrogen, Carlsbad, CA, USA). The cells were transfected with the recommended reagent according to the manufacturer's protocol. After the cells were transfected for 24 h, their medium was replaced with DF-12 medium with 10% FBS, and 24 h later (48 h after transfection with siRNA), the cells were treated with or without 5 μM HA for 12 h, and total RNA or whole-cell extracts were harvested. The sequences of siRNA are as follows:
siPGRMC1-1, GGUGUUCGAUGUGACCAAA dTdT;
siPGRMC1-2, GCAUCUUCCUGCUCUACAA dTdT;
siPGRMC1-3, GCAUACUCAUGGCCAUCAA dTdT.
Western blot analysis
Protein (100 μg) was subjected to 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Massachusetts). The membranes were blocked for 1 h in PBST (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween-20) containing 2% nonfat dried milk, and then PGRMC1 antibody (ProteinTech Group, Inc., Chicago) and GAPDH antibody (Sigma, USA) were used. Protein bands were detected using the enhanced chemiluminescence (ECL) reaction (Millipore, Massachusetts).
Cell viability analysis
Cell viability was assessed using a Cell Counting Kit-8 (CCK8, Dojin Laboratories, Kumamoto, Japan). Briefly, KGN and primary GC cells were plated in 96-well plates in DF-12 supplemented with 10% FBS at a density of 3 × 103 cells/well. After 6 h, the medium was changed to serum-free medium, and the cells were treated with HA (0, 100, 200, 500 μg/ml). After culture of 24 h, 10 μL of a solution containing WST-8 was added to each well. WST-8 is reduced by dehydrogenases in the cells giving an orange colored formazan, which is soluble in the tissue culture medium. The amount of the formazan dye generated by dehydrogenases in cells is directly proportional to the number of living cells. Following incubation of an additional 4 h, the absorbance was measured at 450 nm with a multi-detection microplate reader (Hynergy™ HT, BIO-TEK).
Annexin V/PI (propidium iodide) staining
After transfection with siPGRMC1 sequences for 48 h, KGN cells were collected by centrifugation, washed once with binding buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCl2), and stained with 5 μl Annexin V-FITC (BD Biosciences, San Jose, CA) at room temperature for 15 min. The cells were stained with PI and then analyzed using flow cytometry. Viable cells were negative for both PI and annexin V; apoptotic cells were positive for annexin V and negative for PI, whereas late apoptotic dead cells displayed both high annexin V and PI labeling. Non-viable cells that underwent necrosis were positive for PI and negative for annexin V.
Cell Proliferation Analysis
Cell proliferation was assessed followed dye-loading of primary granulose cells or KGN cells with carboxyfluorescein succinimidyl ester (CFSE, Life Technologies). Briefly, cells were incubated with 5 μM CFSE for 10 min followed by two washes with phosphate-buffered saline (PBS). The CFSE-labeled cells were equally divided into four experimental groups: control group (KGN alone without treatment), HA group (KGN treated with 200 μg/ml HA), siPGRMC1 group (KGN transfected siRNA specific to PGRMC1) and siPGRMC1 + HA group (siPGRMC1 transfected KGN with 200 μg/ml HA). After staining with CFSE for 48 h, the KGN cells were detached, washed with PBS, fixed with 4% paraformaldehyde, rinsed twice with PBS, and stored at 4°C until flow cytometry (FACS Calibur; BD Biosciences). The flow cytometry data were analyzed using ModFit (Verity Software House, Topsham, ME) to estimate the KGN cell proliferation. To compare the experimental variable effects, the proliferation index, a statistic generated by ModFit that relates to the number of population doublings the KGN cells had undergone following CFSE loading, was used50,51.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 5.01, San Diego, CA, USA). Data are presented as the mean ± standard error of the mean (SEM) for the number of independent experiments indicated in each Figure legend. Analysis with one-way ANOVA followed by the Student–Newman–Keuls multiple comparisons tests were used for the comparison of experimental groups. Statistical significance was defined at P < 0.05.
Author Contributions
G.F.Z., G.J.Y., Y.Y.H. and Y.L.H. conceived the idea and were principal investigators responsible for directing and conducting the work, analysis & interpretation of data, and manuscript writing. G.F.Z., J.C. and X.Z. participated in the conception, research planning and data analysis. G.F.Z., J.C. and X.Z. were responsible for all animal studies, imaging & efficacy assessment. T.F. and H.X.S. were responsible for clinical samples collection and analysis. H.X.S. contributed reagents/materials/analysis tools. All authors contributed to the writing of the manuscript.
Supplementary Material
Supplementary Material
Acknowledgments
This work was supported by the State Key Development Program of Basic Research of China Grant (973 Project No. 2010CB945104), Natural Science Funds of Jiangsu Province (BK20130091) and China Postdoctoral Science Foundation (Number: 2012M521063).
References
- Pennell L. M. et al. Sex affects immunity. J. Autoimmun. 38, J282–291 (2012). [DOI] [PubMed] [Google Scholar]
- D'Amico F., Skarmoutsou E. & Mazzarino M. C. The Sex Bias in Systemic Sclerosis: on the Possible Mechanisms Underlying the Female Disease Preponderance. Clin. Rev. Allergy Immunol. 13, 8392–8399; 10.1007/s12016-013-8392-9 (2013). [DOI] [PubMed] [Google Scholar]
- Sen A., Kushnir V. A., Barad D. H. & Gleicher N. Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol. 10, 37–50 (2013). [DOI] [PubMed] [Google Scholar]
- Murphy G., Lisnevskaia L. & Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet 382, 809–818 (2013). [DOI] [PubMed] [Google Scholar]
- Leuenroth S. J. et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl. Acad. Sci. U S A. 104, 4389–4394 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R. et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 151, 229–240 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J. & Dai S. M. A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis:mechanism, efficacy, and safty. Rheumatol. Int. 31, 1123–1129 (2011). [DOI] [PubMed] [Google Scholar]
- Cameron M., Gagnier J. & Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2, CD002948 (2011). [DOI] [PubMed] [Google Scholar]
- Han R., Rostami-Yazdi M., Gerdes S. &Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br. J. Clin. Pharmacol. 74, 424–436 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS One 7, e37693 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. J. et al. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 319, 203–213 (2012). [DOI] [PubMed] [Google Scholar]
- Morgan S., Anderson R. A., Gourley C., Wallace W. H. & Spears N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update. 18, 525–535 (2012). [DOI] [PubMed] [Google Scholar]
- Marder W., Fisseha S., Ganser M. A. & Somers E. C. Ovarian Damage During chemotherapy in Autoimmune Diseases: Broad Health Implications beyond Fertility. Clin. Med. Insights Reprod. Health. 2012, 9–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers E. C., Marder W., Christman G. M., Ognenovski V. & McCune W. J. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 52, 2761–2767 (2005). [DOI] [PubMed] [Google Scholar]
- Manger K., Wildt L., Kalden J. R. & Manger B. Prevention of gonadal toxicity and preservation of gonadal function and fertility in young women with systemic lupus erythematosus treated by cyclophosphamide: the PREGO-Study. Autoimmun. Rev. 5, 269–272 (2006). [DOI] [PubMed] [Google Scholar]
- Kokcu A. Premature ovarian failure from current perspective. Gynecol. Endocrinol. 26, 555–562 (2010). [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z. Preservation of fertility and ovarian function and minimalization of chemotherapy associated gonadotoxicity and premature ovarian failure: the role of inhibin-A and -B as markers. Mol. Cell. Endocrinol. 187, 93–105 (2002). [DOI] [PubMed] [Google Scholar]
- Heiss G. W. R. et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 5, 1036–1045 (2008). [DOI] [PubMed] [Google Scholar]
- Shelling A. N. Premature ovarian failure. Reproduction 140, 633–641 (2010). [DOI] [PubMed] [Google Scholar]
- Zhao G. et al. ERβ-Mediated Estradiol Enhances Epithelial Mesenchymal Transition of Lung Adenocarcinoma through Increasing Transcription of Midkine. Mol. Endocrinol. 26, 1304–1315 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock J. C., Rainey W. & Carr B. R. Ovarian granulosa cell lines. Mol. Cell. Endocrinol. 228, 67–78 (2004). [DOI] [PubMed] [Google Scholar]
- Salvador L. M. et al. Regulation of primordial follicle assembly and development. Hum. Reprod. Update. 11, 461–471 (2005). [DOI] [PubMed] [Google Scholar]
- Hennebold J. D. Preventing Granulosa Cell Apoptosis Through the Action of a Single MicroRNA. Biol. Reprod. 83, 165–167 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunjung W. A. et al. Effect of hyaluronan to inhibit caspase activation in porcine granulosa cells. Biochem. Biophys. Res. Commun. 382, 160–164 (2009). [DOI] [PubMed] [Google Scholar]
- Ataya K. & Moghissi K. Chemotherapy-induced premature ovarian failure: mechanisms and prevention. Steroids 6, 607–626 (1989). [DOI] [PubMed] [Google Scholar]
- Ataya K. M., Pydyn E. F. & Ramahi-Ataya A. J. The effect of “activated” cyclophosphamide on human and rat ovarian granulosa cells in vitro. Reprod. Toxicol. 4, 121–125 (1990). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Effect of triptolide on progesterone production from cultured rat granulosa cells. Arzneimittelforschung 62, 301–306 (2012). [DOI] [PubMed] [Google Scholar]
- Dann E. J. et al. Fertility and ovarian function are preserved in women treated with an intensified regimen of cyclophosphamide, adriamycin, vincristine and prednisone (Mega-CHOP) for non-Hodgkin lymphoma. Hum. Reprod. 20, 2247–2249 (2005). [DOI] [PubMed] [Google Scholar]
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol. Cell Endocrinol. 169, 123–131 (2000). [DOI] [PubMed] [Google Scholar]
- Su J. et al. Tripterygium glycosides impairs the proliferation of granulosa cells and decreases the reproductive outcomes in female rats. Birth Defects Res. B Dev. Reprod. Toxicol. 101, 283–291 (2014). [DOI] [PubMed] [Google Scholar]
- Rodgers R. J. & Irving-Rodgers H. F. The roles of the ovarian extracellular matrix in fertility. Soc. Reprod. Fertil. Suppl. 67, 217–230 (2010). [DOI] [PubMed] [Google Scholar]
- Kothapalli D. et al. Hyaluronan and CD44 antagonize mitogen-dependent cyclin D1 expression in mesenchymal cells. J. Cell. Biol. 176, 535–544 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H. et al. Hyaluronan in follicular fluids and fertilization of oocytes. Fertil. Steril. 74, 1148–1152 (2000). [DOI] [PubMed] [Google Scholar]
- Desai N., Abdelhafez F., Calabro A. & Falcone T. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod. Biol. Endocrinol. 10, 29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo M., Kimura N. & Sato E. Induction of oocyte maturation by hyaluronan-CD44 interaction in pigs. J. Reprod. Dev. 56, 15–19 (2010). [DOI] [PubMed] [Google Scholar]
- Kaneko T. et al. Hyaluronic acid inhibits apoptosis in granulosa cells via CD44. J. Assist. Reprod. Genet. 17, 162–167 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso J. J., Liu X., Gawkowska A., Lodde V. & Wu C. A. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol. Cell. Endocrinol. 320, 153–161 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M. R. et al. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum. Mol. Genet. 17, 3776–3783 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohe H. J., Ahmed I. S., Twist K. E. & Craven R. J. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol. Ther. 121, 14–19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso J. J., Pappalardo A., Losel R. & Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology 147, 3133–3140 (2006). [DOI] [PubMed] [Google Scholar]
- Peluso J. J. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids 76, 903–909 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso J. J., Yuan A., Liu X. & Lodde V. Plasminogen activator inhibitor 1 RNA-binding protein interacts with progesterone receptor membrane component 1 to regulate progesterone's ability to maintain the viability of spontaneously immortalized granulosa cells and rat granulosa cells. Biol. Reprod. 88, 20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster J., Karlsson T., Karlstrom P. O., Poromaa I. S. & Dahl N. Down-regulation of progesterone receptor membrane component 1 (PGRMC1) in peripheral nucleated blood cells associated with premature ovarian failure (POF) and polycystic ovary syndrome (PCOS). Reprod. Biol. Endocrinol. 8, 58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Grammatikakis N., Yoneda M., Banerjee S. D. & Toole B. P. Molecular characterization of a novel intracellular hyaluronan-binding protein. J. Bio. Chem. 275, 29829–29839 (2000). [DOI] [PubMed] [Google Scholar]
- Rebar R. W. Premature Ovarian Failure. Obstet. Gynecol. 113, 1355–1363 (2009). [DOI] [PubMed] [Google Scholar]
- Fu Y. et al. Therapeutic mechanisms of Tongmai Dasheng Tablet on tripterygium glycosides induced rat model for premature ovarian failure. J. Ethnopharmacol. 139, 26–33 (2012). [DOI] [PubMed] [Google Scholar]
- Zhao G. et al. Hyaluronic Acid Promotes the Expression of Progesterone Receptor Membrane Component 1 via Epigenetic Silencing of miR-139-5p in Granulosa Cells. Biol. Reprod. 114, 120295; 10.1095/biolreprod.114.120295 (2014). [DOI] [PubMed] [Google Scholar]
- Carr D. W., DeManno D., Atwood A., Hunzicker-Dunn M. & Scott J. D. Follicle-stimulating hormone regulation of A-kinase anchoring proteins in granulosa cells. J. Biol. Chem. 268, 20729–20732 (1993). [PubMed] [Google Scholar]
- Myers M., Britt K. L., Wreford N. G., Ebling F. J. & Kerr J. B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127, 569–580 (2004). [DOI] [PubMed] [Google Scholar]
- Wallace P. K. & Muirhead K. A. Cell tracking 2007: a proliferation of probes and applications. Immunol. Invest. 36, 527–561 (2007). [DOI] [PubMed] [Google Scholar]
- Munson M. E. An improved technique for calculating relative response in cellular proliferation experiments. Cytometry A. 77, 909–10 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material