Abstract
Background
Chronic obstructive pulmonary disease (COPD) is common in Germany, with a prevalence of 13.2%. The available treatments are exclusively symptomatic, except for lung transplantation, from which no more than a few patients can benefit. Over the past decade, endoscopic lung volume reduction (ELVR) has broadened the therapeutic spectrum for patients with advanced pulmonary emphysema.
Method
We review pertinent publications that were retrieved from Pubmed using the search terms “endoscopic lung volume reduction,” “endobronchial valves,” “endobronchial coil,” and “bronchoscopic thermal vapor ablation,” along with current data from the annual meeting of the German Respiratory Society (Deutsche Gesellschaft für Pneumologie).
Results
ELVR is now performed with three different techniques. Endoscopic valve implantation has been studied in three randomized controlled trials (RCTs) and several noncontrolled trials, which have shown a benefit from valve therapy particularly for patients who have only a small amount of interlobar collateral ventilation or none at all. A reduction of lobar lung volume by 56–80% was found, in association with a significant improvement of lung function (a 16–26% increase of forced expiratory volume in one second [FEV1]). The main complication of valve therapy is pneumothorax, which arises in up to 23% of cases. Coil implantation has been studied to date in only a single RCT, which revealed a significant improvement in quality of life as the primary endpoint (St. George's Respiratory Questionnaire [SGRQ]: -8 points). Bronchoscopic thermoablation has been studied only in noncontrolled intervention trials; in patients with emphysema mainly affecting the upper lobes, it has been found to reduce lobar volume by an average of 48%.
Conclusion
Endoscopic lung volume reduction has broadened the therapeutic spectrum for selected patients with advanced pulmonary emphysema. Further prospective trials are needed to evaluate the benefits, risks, and long-term effects of the available techniques for ELVR.
Chronic obstructive pulmonary disease (COPD) is a widespread disease characterized by high prevalence, morbidity, and mortality (1– 3). Across the world, 65 million people are estimated to suffer from moderate to severe COPD. Three million people died due to COPD in the year 2005. By 2020 COPD will be the third most common cause of death (1). In the USA, COPD is the sixth-ranking disease in terms of years lived with disability (3). The patient’s quality of life is affected by exercise dyspnea due to irreversible bronchial constriction and particularly to dynamic pulmonary hyperinflation (4). In the absence of causal therapy the treatment goals are—alongside symptom relief and improvement of quality of life—reduction of disease progress and mortality.
Treatment comprises strict weaning off tobacco, vaccination against influenza and pneumococci, and improvement of physical fitness by participation in a “lung sport group” or physiotherapy in the context of rehabilitation. Pharmacotherapy of COPD is based on inhaled anticholinergics and β2 sympathomimetics. Inhaled glucocorticosteroids and phosphodiesterase inhibitors can be added in patients with advanced disease and frequent exacerbations. Long-term oxygen therapy is indicated in the presence of hypoxemic respiratory insufficiency, and ventilator failure can be treated with intermittent noninvasive ventilation. Lung volume reduction surgery (LVRS), first described in 1954, can be considered in the case of advanced pulmonary emphysema (5). Originally not widely adopted owing to high perioperative mortality, LVRS was rediscovered in the 1990s (6). Surgical resection of hyperinflated portions of the lungs improves the mechanics of respiration. The National Emphysema Treatment Trial (NETT), published in 2003, showed that LVRS achieved significant improvements in exercise capacity and quality of life in patients with predominantly upper-lobe emphysema (7). However, postoperative mortality was high—7.9% after 90 days. This prompted the development of minimally invasive lung volume reduction procedures with the goal of reducing peri-interventional morbidity and mortality.
The past decade has seen the introduction of various techniques for endoscopic reduction of lung volume. What all these methods have in common is that they are used in advanced pulmonary emphysema with a forced expiratory volume (FEV1) <45% of normal and a residual volume (RV) >200% more than normal. In Germany, 0.8% of COPD patients have disease of stage III or IV according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), in which endoscopic intervention can be considered providing all conservative measures, including tobacco dehabituation, have been exhausted and pronounced emphysema is present (8). Even patients listed for lung transplantation can be helped by endoscopic lung volume reduction (ELVR).
Three techniques currently available for endoscopic reduction of lung volume (Table 1) differ in terms of:
Table 1. Characteristics of the methods for endoscopic lung volume reduction.
| Technique | Indication | Dependent on collateral ventilation | Reversibility | Mechanism of action | Principal complications |
|---|---|---|---|---|---|
| Valve implantation | Predominantly upper- or lower-lobe emphysema | Yes | Fully reversible | Occlusion of the pulmonary lobe destroyed by emphysema | Pneumothorax |
| Coil implantation | Predominantly upper- or lower-lobe emphysema | No | Partially reversible within 4 weeks | Torquing of the bronchi | Hemoptysis. COPD exacerbations |
| Bronchoscopic thermal vapor ablation | Predominantly upper-lobe emphysema | No | Irreversible | Inflammatory reaction | Local and systemic inflammatory reaction |
COPD. chronic obstructive pulmonary disease
Indication spectrum
Mechanism of action
Reversibility
Complications.
Endoscopic valve implantation and coil implantation are CE-certified and so their funding by providers in Germany is not restricted to clinical studies. This has led to widespread use of these techniques not only in large lung centers, despite the low evidence of efficacy. To date, only a few randomized controlled trials (RCTs) with small numbers of patients have been published (Table 2). The efficacy of valve implantation has been evaluated in only three RCTs (9– 11) with a total of 362 treated patients, and for coil implantation one single RCT with 23 treated patients has been published (12). Bronchoscopic thermal vapor ablation has so far been described only in noncontrolled intervention studies; it is licensed only for clinical trials and is the subject of an ongoing multicenter RCT.
Table 2. Overview of the principal ELVR studies*.
| Study | Study design | Patient population | Time point | ∆FEV1 | ∆6-MWT | ∆SGRQ | ∆TLVR | |
|---|---|---|---|---|---|---|---|---|
| Valves | Sciurba et al. 2010:„VENT“ (9) | Randomized. controlled | Treatment group (n = 214) | 6 months | 4.3% | 9.3m | −2.8pts | – |
| Herth et al. 2012: „Euro-VENT” (10) | Randomized. controlled | Treatment group (n = 111) | 6 months | 7±20% | 15±91m | −5±14pts | – | |
| Subgroup with complete fissure. complete lobar occlusion (n = 20) | 26±24% | 22±38% | −10±15pts | 80% | ||||
| Herth et al. 2013: „Chartis-Study” (20) | Prospective. noncontrolled | CV negative (n = 51) | 1 month | 16±22% | 24±57m | −10±13pts | 56% | |
| CV positive (n = 29) | 1±15% | 10±57m | −5±15pts | 6% | ||||
| Eberhardt et al. 2012:„Complete unilateral vs. partial bilateral“ (23) | Prospective. randomized. noncontrolled | Complete unilateral occlusion (n = 11) | 3 months | 21±11% | 49±53m | −12±11pts | – | |
| Partial bilateral occlusion (n = 11) | −3±15% | −52±81 m | 2±9pts | – | ||||
| Ninane et al. 2012: „Multicenter European study“ (11) | Randomized. controlled | Partial occlusion (n = 37) | 3 months | −90mL | 7m | −4pts | 7% | |
| Coils | Slebos et al. 2012:„Bronchoscopic lung volume reduction coil treatment“ (26) | Prospective. noncontrolled | Treatment group (n = 16) | 6 months | 15±17% | 84±73m | −15±12pts | – |
| Shah et al. 2013:„RESET“ (12) | Randomized. controlled | Treatment group (n = 23) | 3 months | 14% | 52m | −8pts | – | |
| BTVA | Snell et al. 2012: „Bronchoscopic thermal vapor ablation therapy“ (29) | Prospective. noncontrolled | Treatment group (n = 44) | 6 months | 17% | 47m | −14pts | 48% |
*The inclusion and exclusion criteria of the individual studies differ only slightly. Alongside lung function parameters. exercise tolerance and distribution of emphysema are relevant for study inclusion. With regard to lung function. the preconditions for inclusion were as follows: FEV1 <45% and RV >150% (9–11, 29 ); FEV1 < 50% (20); FEV1 <40% and RV >150% (23); FEV1 <45% and TLC >100% (12,26 ). ELVR. endoscopic lung volume reduction; FEV1. forced expiratory volume in 1 s; 6-MWT. 6-minute walking test; SGRQ. St. George’s Respiratory Questionnaire; TLVR. target lobe volume reduction; CV. collateral ventilation; RV. residual volume; TLC. total lung capacity
Valve implantation
Endoscopic valve implantation was first described in 2003 (13, 14). In this procedure airways are blocked by insertion of one-way valves in the lobe of the lung most severely damaged by emphysema. These valves prevent inspiration but allow air to escape during expiration, decreasing the volume of the lobe concerned. The maximal effect is achieved with the occurrence of complete lobar atelectasis, the goals of which are amelioration of pulmonary hyperinflation, enhancement of diaphragmatic mobility, and ultimately improvement in respiratory mechanics.
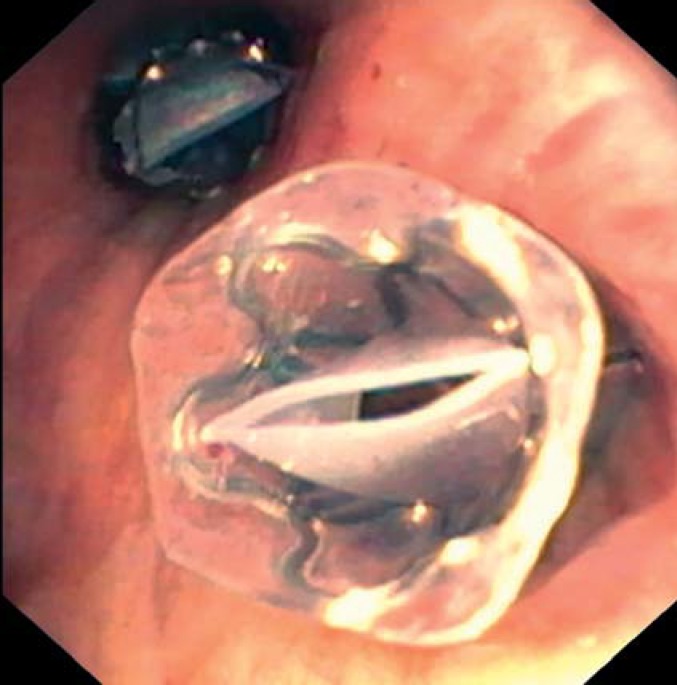
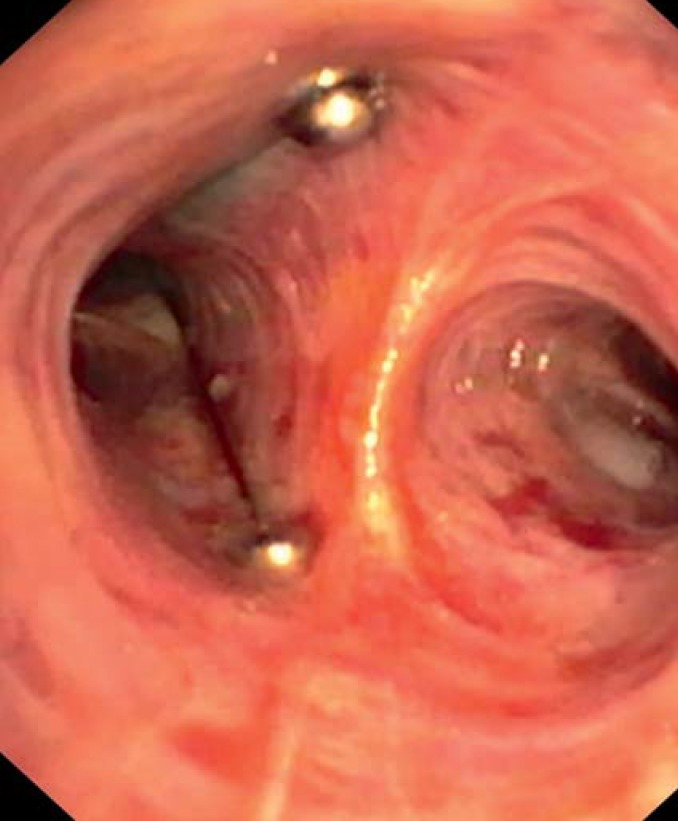
The valves are implanted by means of a catheter system via a flexible bronchoscope. Two types of valves are available, endobronchial (EBV) (Figure 1) and intrabronchial (IBV) (Figure 2); they differ in form but not in function. They can always be removed, even after a long period in situ.
Figure 1.
Endobronchial valves (EBV) in the left upper lobe
Figure 2.
Intrabronchial valves (IBV) in the left upper lobe
The first RCT—the Endobronchial Valve for Emphysema Palliation Trial (VENT)—was published in 2010 (9). One lobe of the lung was completely occluded with EBVs in 214 patients, and 101 patients received medication alone. Six months later the patients in the treatment group showed an increase of 4.3% (34.5 mL) in FEV1 and of 2.5% (9.3 m) in the distance covered in the 6-minute walking test (6-MWT), while these parameters deteriorated in the control group. Although the difference between the groups was statistically significant, the Federal Drug Administration declined to license valve implantation for use in the USA owing to the marginal clinical advantage.
Factors predicting success of valve treatment
Subgroup analyses were planned from the outset of the VENT. Owing to the lack of existing knowledge, however, the subgroups were defined retrospectively. A group of patients could be identified who benefited from valve treatment not only statistically but also clinically. Of the patients with a complete interlobar fissure on preinterventional high-resolution computed tomography (HRCT), 42.6% showed a clinically relevant improvement of >15% in FEV1 and 20.6% increased their 6-MWT distance >15%. Other factors shown to have an influence were heterogeneous distribution of emphysema and correct occlusion of the bronchi.
The European cohort of the VENT (Euro-VENT), also an RCT, confirmed fissure integrity and lobar occlusion as predictors of successful valve treatment (10). A reduction in the volume of the treated lobe of the lung (target lobe volume reduction, TLVR) of 80% was demonstrated in patients with a complete fissure and complete lobar occlusion. In a retrospective analysis 32% of the patients with a complete fissure had TLVR >50%, compared with only 4% of patients with an incomplete fissure (15). Moreover, decreased volume of the treated lobe correlated positively with lung function, exercise tolerance, quality of life, and the BODE index (body mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease).
Thus a radiologically demonstrable uninterrupted fissure of >90% on at least one HRCT plane—axial, sagittal, or frontal—seems to be a surrogate for absent or minimal collateral ventilation. In contrast, the presence of a parenchymal bridge connecting the lobes for ≥ 10% of the fissure is assumed to indicate a high degree of collateral ventilation, leading to retrograde re-aeration of the occluded lobe and nullifying the valve effect. While the interlobar fissure in the left lung is complete in half of the cases, the fissures in the right lung are often incomplete (16). Evaluation of fissure integrity demands radiological expertise, so in addition to visual quantification dedicated software is used for fissure analysis (17, 18).
A catheter-based pulmonary assessment system enables invasive quantification of collateral ventilation. The lobe to be treated is occluded bronchoscopically by means of a balloon catheter and respiratory flow and pressure are measured. A decrease in flow speaks for only minor collateral ventilation, while unchanged flow indicates high collateral ventilation. This measurement technique has been investigated in one noncontrolled safety and feasibility study and one noncontrolled multicenter intervention study (19, 20). The decrease in volume of the targeted pulmonary lobe was correctly predicted by bronchoscopic assessment of the collateral ventilation in 75% of patients treated by insertion of valves (20). Retrospectively, the predictive power of the catheter-based pulmonary assessment system and fissure analysis were shown to be comparable (21).
Complete occlusion of a lobe is also a precondition for successful valve treatment. Studies of bilateral, incomplete occlusion with IBVs showed improvement in quality of life and volume displacement towards the untreated lobes, but there was no significant change in lung function parameters or exercise tolerance (11, 22).
Incomplete occlusion of two pulmonary lobes was compared with complete occlusion of only one lobe in a prospective randomized study of 22 patients (23). There were significant differences (p<0.05) in lung function parameters (FEV1 +267 mL, RV –546 mL), exercise tolerance on the 6-MWT (+48 m), and quality of life as measured by the St George’s Respiratory Questionnaire (SGRQ) (–12 points) in favor of unilateral complete occlusion. Partial occlusion is therefore no longer recommended.
Complications
In the VENT, two patients (0.9%) of the intervention group died within 90 days of treatment. There were no deaths in the control group during this period (9). After a year, however, the mortality was comparable: 3.7% in the intervention group and 3.5% in the control group. Further complications were COPD exacerbations (9.3%), discrete hemoptysis (6.1%), valve displacement (4.7%), and pneumothorax (4.2%) (9). The incidence of postinterventional pneumothorax has risen in the years since the VENT, however, and was 23% in a retrospective single-center analysis in 2013 (see congress presentation: Gompelmann et al., Pneumothorax following endoscopic valve treatment [Pneumothorax nach endoskopischer Ventiltherapie]. Pneumologie 2014; 68/V454). This can be explained by better selection of patients: lower collateral ventilation improves the success rate but also favors occurrence of pneumothorax. However, patients with pneumothorax after valve implantation showed an above-average decrease of 65% in the volume of the treated pulmonary lobe (24). Nevertheless, pneumothorax is a complication associated with the necessity for thoracic drainage, with a longer stay in hospital, and usually with further interventions such as repeat bronchoscopy or video-assisted thoracoscopy. Moreover, tension pneumothorax is a life-threatening condition. Patients should therefore stay in hospital for observation for 48 to 72 h after valve implantation.
Implantation of coils
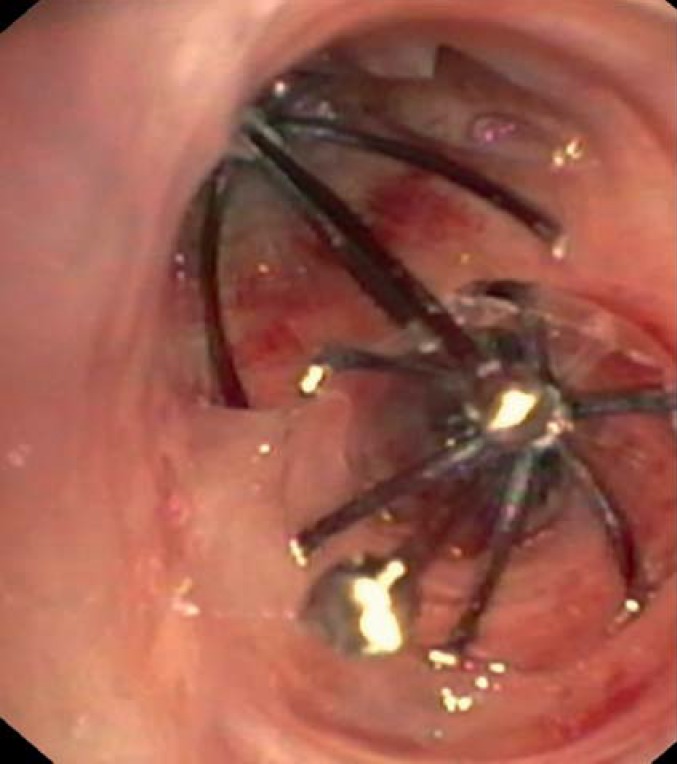
The implantation of lung volume reduction coils (LVRCs) is a nonocclusive procedure (Figure 3). Up to 10 nitinol coils are inserted into an emphysematous pulmonary lobe and achieve lung volume reduction by torquing of the bronchi. It seems that the elastic restoring forces may also be optimized. Preconditions for successful coil implantation are minimal destruction by bullous emphysema and a high RV of >225% more than normal.
Figure 3.
Coils in the left upper lobe (lung volume reduction coils, LVRC)
Coil implantation is achieved by means of a special catheter system. The coils are straightened out for introduction to the airways under radiological guidance, but resume their original spiral form when they are released and the catheter is withdrawn.
Two noncontrolled pilot studies confirmed the feasibility and safety of the procedure and showed it to be efficacious in a total of 37 patients with advanced heterogeneous emphysema (25, 26). The first RCT in which coils were used to treat emphysema (the RePneu Endobronchial Coils for the Treatment of Severe Emphysema with Hyperinflation [RESET] trial) was published in 2013 (12). Twenty-three patients with severe emphysema were treated unilaterally (n = 2) or bilaterally (n = 21) with coils and compared with 23 patients in a control arm who received conservative drug treatment. After 3 months there were significant differences in lung function (FEV1, RV), the 6-MWT, and the SGRQ in favor of the treatment group. The complications were COPD exacerbations, pulmonary infections, and pneumothorax. The two groups did not differ significantly with regard to severe complications.
An advantage of coil implantation is the fact that it is not dependent on collateral ventilation (see congress presentation: Gompelmann D et al., Endoscopic lung volume reduction by means of coil implantation in patients with severe heterogeneous pulmonary emphysema and incomplete fissures: a retrospective analysis [Endoskopische Lungenvolumenreduktion mittels Coil-Implantation bei Patienten mit schwerem heterogenem Lungenemphysem und inkompletten Fissuren: eine retrospektive Analyse]. Pneumologie 2012; 66/P428). The lack of full reversibility, however, is a disadvantage. In a small case series (n = 3), individual coils could be removed within 4 weeks of implantation (see congress presentation: Hetzel M et al., Reversibility of implanation of RePneu coils [Reversibilität der Implantation von RePneu Coils]. Pneumologie 2013; 67/P314). Complete removal of coils seems difficult, however, and is certainly not feasible in all cases.
Bronchoscopic thermal vapor ablation
Bronchoscopic thermal vapor ablation (BTVA) induces an inflammatory reaction in the damaged pulmonary parenchyma by instillation of water vapor. After 8 to 12 weeks the local inflammatory reaction leads to fibrosis and scar formation and thus to the desired reduction in lung volume. This irreversible procedure is employed only in patients with predominantly upper lobe pulmonary emphysema, but is independent of collateral ventilation (27).
The water vapor, heated to 75 °C, is instilled via a special balloon catheter that occludes the targeted portion of the lung. This enables specific treatment of an area selected by means of HRCT. The required dose of vapor is calculated according to the volume of lung tissue to be treated.
To date only prospective, noncontrolled intervention studies of BTVA have appeared (28, 29). The first of these, published in 2009, showed improvement of quality of life in 11 patients who received BTVA in a dosage of 5 cal/g, but there were no relevant changes in lung function parameters or exercise tolerance. A heterogeneity index of >1.2 between upper and lower lobe was identified as a predictor of response to BTVA (see congress presentation: Herth FJF et al., The efficacy of bronchoscopic thermal vapor ablation in patients with upper lobe emphysema: the impact of heterogeneity of disease. ATS 2010. Abstract 5167).
In an ensuing noncontrolled study, unilateral BTVA with a vapor dosage of 10 cal/g was carried out in 44 patients with a heterogeneity index of >1.2. Six months after treatment the volume of the treated lobe had decreased by 48%, accompanied by significant improvements in lung function parameters and quality of life (29). The most frequently occurring complication was a pronounced inflammatory reaction in the first few weeks after treatment, characterized by dyspnea, cough, fever, and mild hemoptysis. Therefore all patients should receive prophylactic antibiotic and anti-inflammatory medication and be kept in hospital for observation following BTVA. The severity of the local inflammatory reaction correlates with the response to treatment (30). The worse the inflammation, the more effective BTVA seems to be. Moreover, there appears to be a correlation between the volume of the treated lobe of the lung and the scale of the inflammatory reaction. Patients with a treated lung volume of >1700 mL more frequently required re-admission to the hospital owing to pronounced local inflammation. By 6 months after BTVA, however, these were the patients who showed the greatest treatment benefit.
Summary
The three procedures for ELVR described here—endoscopic valve treatment, coil implantation, and BTVA—differ not only in their methods but also in their mechanisms of action and their spectra of complications. To date, there are no published comparisons of the various techniques for ELVR. The different procedures are seldom in competition, however, since the indications for their use differ according to the extent of collateral ventilation and the distribution of emphysema.
Valve implantation—the only reversible method—is effective in patients with advanced, heterogeneous pulmonary emphysema and low collateral ventilation. Preconditions for successful treatment are confirmation of low collateral ventilation by interlobar fissure analysis or bronchoscopy and complete occlusion of the treated lobe. Postinterventional inpatient monitoring is necessary because of the risk of pneumothorax, and valve implantation should be restricted to centers with expertise in the management of complicated pneumothorax.
Despite the low amount of published evidence, the partially reversible coil implantation plays a leading role in the endoscopic treatment of severe pulmonary emphysema, because it is currently the only effective endoscopic procedure licensed for treatment of patients with an incomplete fissure. An ongoing prospective multicenter RCT, the RENEW study, has the goal of demonstrating the efficacy of coil implantation in a larger number of patients. For now, however, coil implantation should preferably be restricted to studies or clinical registries.
BTVA is also independent of collateral ventilation and is carried out in patients with predominantly upper-lobe emphysema. No randomized studies of BTVA have been published to date. A multicenter RCT, the StepUp study, is currently investigating a sequential approach involving treatment of one or two segments of the lung on each side with the aim of improving the safety of the procedure. The results are expected at the end of 2015.
The costs of valve and coil implantation are covered by German health insurance providers—a fact that has resulted in the use of these techniques spreading to nonspecialized centers. The sometimes insufficiently strict selection of patients and the uncritical and therefore often unsuccessful use of ELVR lead to a falsely negative impression of the efficacy of these methods. Only if durable efficacy of the various procedures can be demonstrated in the framework of studies and treatment in clinical registries will ELVR become a genuine treatment option for patients with severe pulmonary emphysema. Optimal patient selection, choice of the most suitable procedure, performance of ELVR primarily at experienced centers, and expertise in the management of the potential complications are crucial.
Key Messages.
Endoscopic lung volume reduction (ELVR) represents an option for the treatment of advanced pulmonary emphysema with FEV1 <45% and a residual volume of >200%.
The currently available methods for ELVR are valve implantation (reversible), coil implantation (partially reversible), and bronchoscopic thermal vapor ablation (BTVA; irreversible).
Only a very small number of randomized controlled trials of the ELVR techniques have yet been carried out, so treatment should continue to be restricted to studies and clinical registries.
Successful use of the various ELVR procedures depends on careful patient selection with regard to lung function parameters, the scale of emphysematous destruction, and collateral ventilation.
Despite the low invasiveness of the ELVR techniques, complications requiring careful management may arise: valve treatment is often associated with the risk of pneumothorax, coil implantation with hemoptysis and COPD exacerbations, and BTVA with an inflammatory reaction.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Dr. Gompelmann has received honoraria and travel costs from Pulmonx.
Dr. Eberhardt has received honoraria and travel costs from Olympus Europe and Pulmonx.
Prof. Herth has received third-party funding for commissioned clinical studies and for research projects performed at his own initiative from Olympus Europe, Pulmonx, Uptake Medical, and Pneum Rx.
References
- 1.Gillissen AET. Zur Lage und Zukunft der Pneumologie in Deutschland. Herne: FRISCHTEXTE Verlag; 2014. Weißbuch Lunge 2014. Herausforderungen, Zukunftsperspektiven, Forschungsansätze. [Google Scholar]
- 2.World Health Organisation. Burden of COPD. www.who.int/respiratory/copd/burden/en/ (last accessed on 9 September 2014)
- 3.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, Cote CG, Marin MJ, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan OC. The surgical treatment of pulmonary emphysema. W V Med J. 1954;5:283–285. [PubMed] [Google Scholar]
- 6.Cooper JD, Patterson GA, Sundaresan RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg. 1996;112:1319–1329. doi: 10.1016/S0022-5223(96)70147-2. [DOI] [PubMed] [Google Scholar]
- 7.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 8.Geldmacher H, Biller H, Herbst A, et al. The prevalence of chronic obstructive pulonary disease (COPD) in Germany. Results of the BOLD study. Dtsch Med Wochenschr. 2008;133:2609–2614. doi: 10.1055/s-0028-1105858. [DOI] [PubMed] [Google Scholar]
- 9.Sciurba FC, Ernst A, Herth FJF, et al. VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Noppen M, Valipor A, et al. International VENT Study Group. Efficacy predictors of lung volume reduction with Zephyr valves in a european cohort. Eur Respir J. 2012;39:1334–1342. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 11.Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J. 2012;39:1319–1325. doi: 10.1183/09031936.00019711. [DOI] [PubMed] [Google Scholar]
- 12.Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. The Lancet Respiratory Medicine. 2013;1:233–240. doi: 10.1016/S2213-2600(13)70047-X. [DOI] [PubMed] [Google Scholar]
- 13.Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet. 2003;361:931–933. doi: 10.1016/S0140-6736(03)12762-6. [DOI] [PubMed] [Google Scholar]
- 14.Snell GI, Holsworth L, Borrill ZL, et al. The potential for bronchoscopic lung volume reduction using bronchial prostheses: a pilot study. Chest. 2003;124:1073–1080. doi: 10.1378/chest.124.3.1073. [DOI] [PubMed] [Google Scholar]
- 15.Valipour A, Herth FJ, Burghuber OC, et al. VENT Study Group. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J. 2014;43:387–396. doi: 10.1183/09031936.00133012. [DOI] [PubMed] [Google Scholar]
- 16.Koenigkam-Santos M, de Paula WD, Owsijetitsch M, et al. Incomplete pulmonary fissures evaluated by volumetric thin-section CT: semi-quantitative evaluation for small fissure gaps identification, description of prevalence and severity of fissural defects. Eur J Radiol. 2013;82:2365–2370. doi: 10.1016/j.ejrad.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Koenigkam-Santos M, Puderbach M, Gompelmann D, et al. Incomplete fissures in severe emphysematous patients evaluated with MDCT: incidence and interobserver agreement among radiologists and pneumologists. Eur J Radiol. 2012;81:4161–4166. doi: 10.1016/j.ejrad.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 18.van Rikxoort EM, Goldin JG, Galperin-Aizenberg M, et al. A method for the automatic quantification of the completeness of pulmonary fissures: evaluation in a database of subjects with severe emphysema. Eur Radiol. 2012;22:302–309. doi: 10.1007/s00330-011-2278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gompelmann D, Eberhardt R, Michaud G, Ernst A, Herth FJF. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: A feasibility study. Respiration. 2010;80:419–425. doi: 10.1159/000319441. [DOI] [PubMed] [Google Scholar]
- 20.Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J. 2013;41:302–308. doi: 10.1183/09031936.00015312. [DOI] [PubMed] [Google Scholar]
- 21.Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis system and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology. 2014;19:524–530. doi: 10.1111/resp.12253. [DOI] [PubMed] [Google Scholar]
- 22.Sterman DH, Metha AC, Wood DE, et al. IBV Valve US Pilot Research Team. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration. 2010;79:222–233. doi: 10.1159/000259318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberhardt R, Gompelmann D, Schuhmann M, Heussel CP, Herth FJ. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest. 2012;142:900–908. doi: 10.1378/chest.11-2886. [DOI] [PubMed] [Google Scholar]
- 24.Gompelmann D, Herth FJF, Slebos DJ, et al. Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration. 2014;87:485–491. doi: 10.1159/000360641. [DOI] [PubMed] [Google Scholar]
- 25.Herth FJ, Eberhard R, Gompelmann D, Slebos DJ, Ernst A. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis. 2010;4:225–231. doi: 10.1177/1753465810368553. [DOI] [PubMed] [Google Scholar]
- 26.Slebos DJ, Klooster K, Ernst A, Herth FJ, Kerstjens HA. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest. 2012;142:574–582. doi: 10.1378/chest.11-0730. [DOI] [PubMed] [Google Scholar]
- 27.Gompelmann D, Eberhardt R, Ernst A, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation in patients with emphysema. Respiration. 2013;86:324–331. doi: 10.1159/000354175. [DOI] [PubMed] [Google Scholar]
- 28.Snell GI, Hopkins P, Westall G, Holsworth L, Carle A, Williams TJ. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg. 2009;88:1993–1998. doi: 10.1016/j.athoracsur.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapor ablation therapy in the management of heterogeneous emphysema. Eur Respir J. 2012;39:1326–1333. doi: 10.1183/09031936.00092411. [DOI] [PubMed] [Google Scholar]
- 30.Gompelmann D, Heussel CP, Eberhardt R, et al. Efficacy of bronchoscopic thermal vapor ablation and lobar fissure completeness in patients with heterogeneous emphysema. Respiration. 2012;83:400–406. doi: 10.1159/000336239. [DOI] [PubMed] [Google Scholar]