Abstract
Mx proteins form a subfamily of the dynamin-like GTPases, which have well established roles in cellular trafficking. Some Mx proteins (e.g., human MxA) have antiviral activity and are tightly regulated by type I IFNs. Others (e.g., human MxB) lack antiviral activity and are thought to have normal cellular functions that remain undefined. Consistent with this hypothesis, we report that MxB is expressed without IFN treatment. MxB seems to be exclusively extranuclear and is concentrated at the cytoplasmic face of nuclear pores, suggesting a role in their regulation. We find that expression of dominant negative (GTPase-defective) MxB mutants efficiently blocks nuclear import and causes a delay in G1/S cell-cycle progression. MxB depletion using RNA interference (RNAi) leads to a similar cell-cycle defect but does not block nuclear import. MxB therefore seems not to be required for nuclear import per se but may instead regulate its efficiency and/or kinetics. These studies indicate an unexpected role for a dynamin-like protein in nucleocytoplasmic trafficking and suggest that a related function might be usurped by its antiviral relatives.
Members of the dynamin superfamily of large GTPases are known to participate in diverse cellular processes, including endocytosis (1), midzone formation during cytokinesis (2), as well as both fission (3) and fusion (4) of mitochondria. The ability of these GTPases to undergo nucleotide-regulated self-assembly seems to be critical for their physiological function. For example, dynamin undergoes cycles of assembly and disassembly (coupled to GTP binding and hydrolysis, respectively) that have an important role in regulating or driving the scission of endocytic vesicles from the plasma membrane (5, 6).
All members of the dynamin superfamily share three conserved features: a GTPase domain (≈300 aa), a middle or “assembly” region (≈220 aa), and a GTPase effector domain (≈120 aa). These domains are likely to define the in vitro enzymatic and self-assembly properties of these large GTPases, which are similar for all members of the family (7). In addition, each family member has a unique domain (or domains) thought to tailor the protein to its particular cellular function, the location of its action, or both. The Mx proteins, which form a distinct subclass of the dynamin family, have unique amino-terminal extensions of 25–90 residues that are likely to mediate specific targeting or functional interactions of these proteins. In MxB, this extension contains sequences that resemble a classical nuclear localization signal (NLS).
Humans have two Mx genes, MxA and MxB, encoding proteins of 76 and 73 kDa, respectively, that share 63% sequence identity (8). The MxA protein is a key component of the antiviral state induced by type I IFNs and is expressed only after IFN stimulation (9). Expression of MxA confers resistance to a wide range of different RNA viruses, as well as hepatitis B virus (9). The closely related MxB protein has no detectable antiviral activity, leading to the hypothesis that it may instead play a role in normal cellular physiology that has yet to be determined (7, 9). To test this hypothesis, we investigated the expression and subcellular localization of human MxB. MxB is expressed at significant levels even in the absence of IFN stimulation and seems to localize to the cytoplasmic face of nuclear pores. Using dominant-negative GTPase domain mutants and RNA interference, we also provide evidence indicating that MxB may be involved in the regulation of protein transport through nuclear pores and suggest that this role may reflect its normal cellular function.
Materials and Methods
Cell Culture. HeLa cells were maintained in DMEM supplemented with glucose, l-glutamine (Cellgro, Herndon, VA), and 10% FBS (HyClone), under 5% CO2 at 37°C.
Constructs and Cloning. The cDNA for MxB with C-terminal V5-epitope and histidine tags was obtained as a GeneStorm clone from Invitrogen (Mx2 H-M30818M). This clone encoded three amino acid mutations (L118P, R406P, and E681V). These mutations were corrected by using the Stratagene QuikChange system, and K131A and T151A mutations were introduced similarly. ΔN25 MxB was generated by mutating the starting ATG to ATC, leaving a new start codon at position 26. WT and mutated MxB cDNAs were subcloned into the BglII/KpnI sites of pEGFP-C1 (Clontech) to give fusion proteins with an N-terminal GFP moiety.
RT-PCR. HeLa cell monolayers were either left untreated or were treated with 1,000 units/ml IFN-α (PBL Biomedical Laboratories, Piscataway, NJ) for 16 hr. RNA was isolated by using RNA-Bee (Tel-Test, Friendswood, TX) according to the manufacturer's directions. One microgram of total RNA was reverse transcribed in a total of 30 μl with mouse murine leukemia virus reverse transcriptase (GIBCO/BRL) according to the manufacturer's instructions by using 0.5 mg/ml oligo(dT) 18mer as primer. This reverse cDNA was then used as template for the subsequent PCR. Primers used were: for MxB, 5′-TCTAAGGCCCACA AGCCT TG-3′ and 5′-CAGT T T TCAGCACCAGCGGACACCT-3′; for MxA, 5′-GGGCTTTCCAATCCAGCTCG-3′ and 5′-CTACCCGGGAACGGGGGC-3′; and for glyceraldehyde 3-phosphate dehydrogenase (G3PDH), 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and 5′-CATGTGGGCCATGAGGTCCACCAC-3′. PCRs were carried out for the indicated number of cycles by using an annealing temperature of 60°C and a 1.5-min elongation at 72°C. Products were electrophoresed on 2% agarose gels and visualized by ethidium bromide staining.
Immunofluorescence. HeLa cells seeded on coverslips were transfected with the relevant construct by using Lipofectin (Invitrogen) according to the manufacturer's directions. Forty-eight hours after transfection, cells were washed in PBS and then fixed in 2% formaldehyde/PBS for 20 min at room temperature. Cells were permeabilized and blocked simultaneously with 1% BSA/0.1% Triton X-100/PBS for 20 min. The coverslips were incubated with appropriate primary antibodies in 1% BSA/PBS for 1 hr, washed in PBS, and then incubated with the appropriate fluorescent secondary antibody for an additional 45 min. Coverslips were then washed again in PBS and mounted on slides by using Fluoromount G. Digital images were taken with a Hamamatsu Orca charge-coupled device (CCD) camera on a Leica DM IRBE microscope. Serial Z-sections were imaged and deconvoluted by using openlab software (Improvision, Lexington, MA).
Antibodies for Microscopy. For immunofluorescence, V5 epitope-tagged MxB was visualized by using FITC-conjugated anti-V5 antibody (Invitrogen) at 1/500 dilution. For localization of nuclear pore complexes, MAb414 (Covance, Princeton) was used at a 1/1,000 dilution and visualized with an anti-mouse IgG1-Texas red conjugate (Southern Biotechnology Associates) at 1/60 dilution. DNA was detected by using Hoechst 33342 (Sigma–Aldrich). The myc-tagged reporter for nuclear transport was detected by using antibody 9E10 (Santa Cruz Biotechnology) at 1/400 dilution followed by visualization with anti-mouse IgG1-Texas red.
Immunoelectron Microscopy (ImmunoEM). Transfected HeLa cells were fixed by using a mixture of 2% (wt/vol) paraformaldehyde and 0.2% (wt/vol) glutaraldehyde in 60 mM Pipes, 25 mM Hepes, 2 mM MgCl2, and 10 mM EGTA at pH 6.9. Cell pellets were washed with 0.2 M phosphate buffer, pH 7.4, embedded in 10% (wt/vol) gelatin, and infused in 2.3 M sucrose (10). Mounted gelatin blocks were frozen in liquid nitrogen, and ultrathin sections were prepared with an Ultracut FCS ultracryomicrotome (Leica). Ultrathin cryosections were collected with 2% (vol/vol) methylcellulose and 2.3 M sucrose (11), and single or double ImmunoGold-labeled with antibodies (anti-V5 and/or MAb414) and protein A coupled to 10 or 15 nm of gold.
Nuclear Transport Assays. The construct for expression of the myc-tagged reporter for nuclear transport (a nucleoplasmin core domain-SV40 T NLS fusion in pcDNA3) was a gift from G. Dreyfuss (Howard Hughes Medical Institute, University of Pennsylvania, Philadelphia). This reporter construct was cotransfected with MxB or its mutants into HeLa cells, and cells were processed for immunofluorescence as described above. Cells were scored visually for proper (nuclear) or aberrant (cytoplasmic) localization of the myc-tagged reporter. Each data point included a minimum of 100 cells, and each experiment was repeated at least four times.
Flow Cytometry Analysis. HeLa cells were transfected with constructs encoding the appropriate GFP-fusion proteins as described above, or with small interfering RNA oligos as described below. For double thymidine block, cells were subjected to the following: a 16-hr block with 1 mM thymidine, 10-hr release, a second 16-hr block, and a final 4.5-hr release to allow cells to progress into S phase. For aphidicolin block, cells were incubated for 16 hr in the presence of 2.5 μg/ml aphidicolin (Sigma) and were then released for 4 hr to allow progression into S phase. After treatment, cells were trypsinized and washed in 2% FBS/PBS. An initial fixation with 0.2% formaldehyde/PBS for 5 min to maintain GFP fluorescence was followed by a second fixation with cold 70% ethanol on ice for 20 min. After three washes in PBS, the cells were resuspended in 0.8 ml of 2% FBS/PBS and incubated with 0.1 mg/ml propidium iodide and 0.5 mg/ml RNase for 30 min at 37°C. Flow cytometry was performed with a Becton Dickinson FACSCalibur. Cells were sorted for expression of GFP or MxB/GFP fusions in FL1 and were analyzed for propidium iodide staining by peak area in FL2. Positions of G0/G1 and G2/M peaks were defined by using unsynchronized control cells. modfit 3.0 was used to fit the propidium iodide profiles. All critical variances were <5%.
Western Blots. Cells were lysed in RIPA buffer (50 mM Tris/150 mM NaCl/10% glycerol/1% Nonidet P-40/0.5% deoxycholate/0.1% SDS/10 mM NaF/0.4 mM EDTA, pH 8.0, with leupeptin and aprotinin) and centrifuged at 10,000 × g for 10 min. Supernatant containing 10 μg of protein was run on a 10% SDS/PAGE gel and transferred to nitrocellulose. For endogenous MxB, immunoblotting was performed with a 1/500 dilution of anti-MxB guinea pig anti-sera (kind gift of I. Julkunen and K. Melen, National Public Health Institute, Helsinki), followed by anti-guinea pig horseradish peroxidase (HRP) conjugate (Southern Biotechnology Associates). Endogenous dynamin was detected in the same lysates with a 1/1,000 dilution of anti-dynamin II (Santa Cruz Biotechnology) and anti-goat HRP conjugate (Santa Cruz Biotechnology). Western blots were developed by using ECL reagents (Amersham Pharmacia) and imaged with a Kodak Imagestation.
RNA Interference (RNAi). RNA sense (ACGUCUCGCCAACCAGAUCtt) and antisense (GAUCUGGUUGGCGAGACGUtt) oligonucleotides to MxB, as well as control sense (CUGGACUUCCAGAAGAACAtt) and antisense (UGUUCUUCUGGAAGUCCAGtt) oligonucleotides to Lamin A/C, were synthesized and PAGE-purified by Ambion (Austin, TX). Each sense/antisense pair was annealed according to the manufacturer's directions in the provided buffer as 20 μM stocks. RNA oligonucleotides to t-Golgin were a kind gift from M. Marks (University of Pennsylvania, Philadelphia). Oligonucleotide duplexes were transfected into HeLa cells (at 40% confluence) by using Oligofectamine (Invitrogen) according to the manufacturer's directions. For nuclear transport assays, HeLa cells were subjected to two rounds of transfection (separated by 48 hr), the second while adhered to coverslips. Twenty-four hours after the second oligonucleotide duplex transfection, cells were transfected with the myc-tagged nuclear transport reporter described above. After an additional 24 hr, cells were processed for immunofluorescence. Protein levels were determined by Western blot after two serial transfections as for nuclear transport assays.
Results
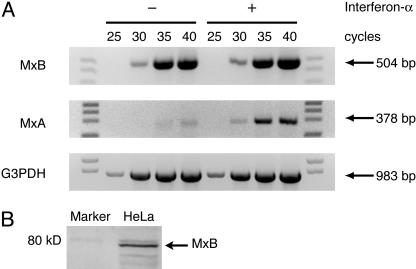
MxB Expression Does Not Require IFN Treatment. If MxB has a normal cellular function, its expression should be detectable regardless of IFN treatment, by contrast with the strict IFN dependence seen for antiviral Mx proteins. We therefore used RT-PCR to analyze MxB and MxA transcript levels in HeLa cells (Fig. 1A) and several other cell lines (Fig. 6, which is published as supporting information on the PNAS web site). MxB transcripts were readily detectable regardless of whether cells were treated with IFN-α whereas MxA transcripts were almost undetectable without IFN-stimulation. MxB transcripts were also detected across a tissue array cDNA panel (data not shown). IFN-independent expression of MxB protein could also be detected by Western blotting in HeLa cells (Fig. 1B), as previously reported for several cell lines including peripheral blood monocytes (12). IFN-α treatment did increase levels of MxB expression in several of our experiments and in those of Melen et al. (12). However, the fact that significant basal levels of MxB expression were found in most cases is consistent with a role for this protein in a normal cellular process.
Fig. 1.
Expression of MxB in the absence of IFN-α stimulation. (A) RNA extracted from HeLa cells that were untreated (–) or treated (+) with 1,000 units/ml IFN-α was reverse transcribed with oligo(dT) as primer. The resulting reverse-strand cDNA was used as template for RT-PCR with appropriate MxB and MxA primers for the indicated number of cycles. Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) is included as a control for template levels. Primer sequences are listed in Materials and Methods. (B) Endogenous MxB protein is present in HeLa cells without IFN treatment. Shown is a Western blot of endogenous MxB detected with guinea pig anti-MxB antisera previously characterized by Melen et al. (12).
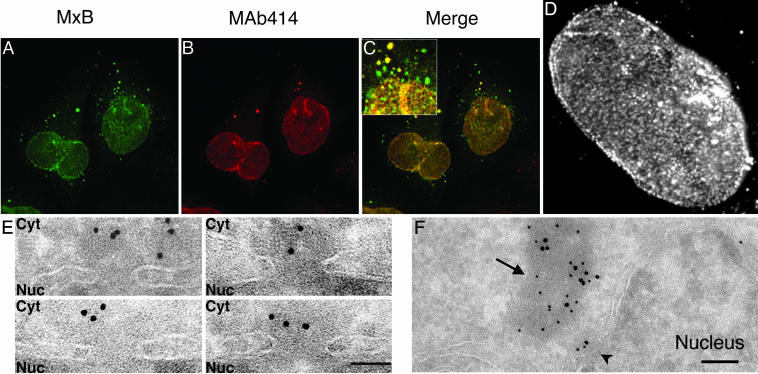
MxB Localizes to the Cytoplasmic Face of Nuclear Pores. As a first step in investigating the normal cellular function of MxB, we next investigated its subcellular localization. V5 epitope-tagged MxB was found to be enriched at the nuclear envelope and also in a punctate cytoplasmic pool in transfected cells (Fig. 2A). Similar localization was seen for transfected and endogenous MxB protein by using anti-MxB antibodies (12) although background nuclear staining by the rabbit anti-guinea pig secondary antibodies made this observation difficult to interpret. We therefore performed all subsequent experiments with epitope-tagged MxB.
Fig. 2.
Localization of MxB to the cytoplasmic face of nuclear pores. (A) V5 epitope-tagged MxB was visualized with anti-V5 antibodies. (B) Nucleoporins were visualized by using MAb414. (C) MxB and nucleoporin localization signals merged. (D) Immunofluorescence micrograph of V5-tagged MxB at the nuclear envelope. (E) Localization of MxB to the cytoplasmic face (Cyt) but not the nuclear face (Nuc) of nuclear pores identified morphologically by immunoEM. Sections are shown in which nuclear pores are morphologically obvious. V5-MxB was stained by anti-V5 antibody and 10 nm of protein A–gold conjugate. (F) Double-labeling of MxB with 10 nm of protein A–gold and MAb414 with 15 nm of protein A–gold. An MxB-positive nuclear pore is marked with an arrowhead, and a cytoplasmic electron-dense assembly containing both MxB and MAb414 antigens is marked with an arrow. (Bars = 100 nm in E and 200 nm in F.)
At high magnification, staining of V5-tagged MxB at the nuclear surface seemed “granular” (Fig. 2D), highly reminiscent of the known distribution of nuclear pores (13). By coimmunofluorescence staining with MAb414, an antibody to nuclear pore components, we found that transiently expressed WT MxB colocalizes significantly with nucleoporins at the nuclear envelope (Fig. 2 A–C). This colocalization extends beyond the nucleus to a subset of MxB-positive punctae in the cytoplasm that also contain nucleoporins. These cytoplasmic punctae may represent annulate lamellae (AL), cytoplasmic structures of poorly understood function that consist of membrane cisternae fenestrated with densely packed assembled nuclear pore complexes (14). MxB overexpression did not affect either the distribution or number of AL (data not shown).
To examine the relationship between MxB and nuclear pores more closely, we next used immunoEM. Epitope-tagged MxB was found at the nuclear envelope and in cytoplasmic assemblies of up to 200 nm in diameter but could not be detected in the nucleoplasm (Fig. 7A, which is published as supporting information on the PNAS web site). The nuclear envelope pool of epitope-tagged MxB (visualized as 10-nm gold particles) was associated primarily with the cytoplasmic aspect of nuclear pores identified in ultrathin sections bisecting the plane of the nuclear envelope (Fig. 2E). MxB was typically 50 to 100 nm from the center of the pore, in the region where the cytoplasmic filaments terminate (15). ImmunoEM studies also showed colocalization of MxB (labeled with 10 nm gold) with the MAb414 epitope (labeled with 15 nm gold) at the cytoplasmic face of nuclear pores and in cytoplasmic assemblies (Fig. 2F). We suspect that the cytoplasmic assemblies containing MxB and nucleoporins represent annulate lamellae although they lack the characteristic morphology, probably because of the sample preparation methods required for immunoEM (16). Although ImmunoGold staining showed MAb414 on both sides of the nuclear envelope, MxB could be detected only in the cytoplasm. These observations are consistent with previous (but less detailed) immunofluorescence studies of MxB localization (8, 17) but contradict one report of intranuclear MxB localization by using guinea pig anti-MxB antibodies (12). We found that MxB present at the nuclear envelope was equally accessible to antibodies under conditions of limited digitonin permeabilization (where antibodies cannot enter the nucleus) and complete permeabilization with Triton X-100 (data not shown), consistent with MxB localization only to the cytoplasmic face of the nuclear envelope. Moreover, treatment of MxB-expressing cells with leptomycin B (an inhibitor of Crm1-mediated nuclear export) did not promote MxB accumulation in the nucleus (data not shown).
Localization of MxB to Nuclear Pores Requires GTP Binding. We next asked whether the presence of MxB at the cytoplasmic face of nuclear pores depends on its GTP binding or GTPase activity. We made mutations in the MxB GTPase domain analogous to those in well studied dominant-negative dynamin mutants that either cannot bind GTP, or can bind but do not hydrolyze GTP (18, 19). An MxB mutant defective in GTP binding was generated by substituting K131 (in the “P loop” of the GTPase domain; equivalent to K44 in dynamin) with alanine. MxB defective in GTP hydrolysis and GTP-dependent conformational changes was produced by mutating T151 (in the GTPase “switch 1” region, equivalent to T65 in dynamin) to alanine. We confirmed that these MxB mutations (K131A and T151A) have the anticipated effects on GTPγS binding in vitro (Fig. 8, which is published as supporting information on the PNAS web site) and do not significantly impair expression.
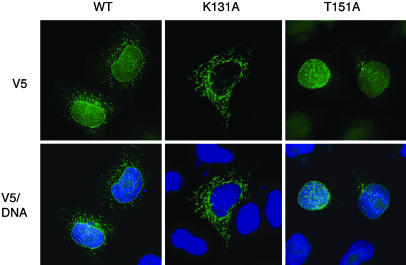
Abolishing GTP binding (in K131A MxB) almost completely prevented MxB targeting to the nuclear envelope (Fig. 3). Even at low expression levels, K131A MxB was instead found primarily in a detergent-soluble reticular cytoplasmic compartment but did not colocalize with markers for mitochondria, nucleoporins, or the endoplasmic reticulum (ER) protein BiP [although it did partly colocalize with a dapoxyl dye that is targeted to the ER (ER-Tracker)]. This phenotype may represent aberrant K131A MxB-dependent expansion of a BiP-negative ER-like compartment, as was also observed in cells overexpressing the equivalent MxA GTP binding-deficient mutant (20). Most importantly, the failure of K131A MxB to localize to the nuclear envelope argues that its NLS-like sequence (in the amino-terminal extension of MxB) is not sufficient for targeting to the nuclear envelope although it is necessary for nuclear localization of the WT protein (ref. 12 and data not shown). Association of WT MxB with the cytoplasmic face of nuclear pores therefore seems to require both its GTP binding and its amino-terminal NLS-like sequence.
Fig. 3.
Localization of MxB to the nuclear envelope requires GTP binding. Shown is immunofluorescence localization of V5 epitope-tagged WT MxB, K131A MxB, and T151A MxB in transfected HeLa cells, detected with an FITC-conjugated anti-V5 antibody and merged with DNA detected by staining with Hoechst 33342.
By contrast with the failure of nucleotide-free (K131A) MxB to be targeted to the nucleus, we found that the GTP hydrolysis-defective MxB mutant (T151A) is localized almost exclusively to the nuclear envelope (Fig. 3). The equivalent dynamin mutant (T65A) seems to become trapped at its site of action (the plasma membrane) in endocytic intermediates (18) so the behavior of T151A MxB is consistent with the hypothesis that MxB normally functions at the nuclear envelope. At moderate expression levels, T151A MxB showed the same nuclear pore-like distribution as WT MxB and colocalized with nucleoporins by immunofluorescence microscopy (Fig. 7B). ImmunoEM analysis further revealed that nearly 100% of T151A MxB accumulates at the nuclear envelope (compared with ≈16% for WT MxB) and is therefore almost completely depleted from the cytoplasmic assemblies and/or annulate lamellae (data not shown). Thus, we find that a form of MxB that cannot bind GTP fails to localize to the nuclear envelope whereas an MxB mutant that can bind, but does not hydrolyze, GTP seems to become trapped at the nuclear envelope. We did not detect any significant effect of expressing MxB mutants on the distribution or apparent number of nuclear pores or on the structure of the nuclear membrane, arguing against a role for MxB in nuclear pore complex assembly.
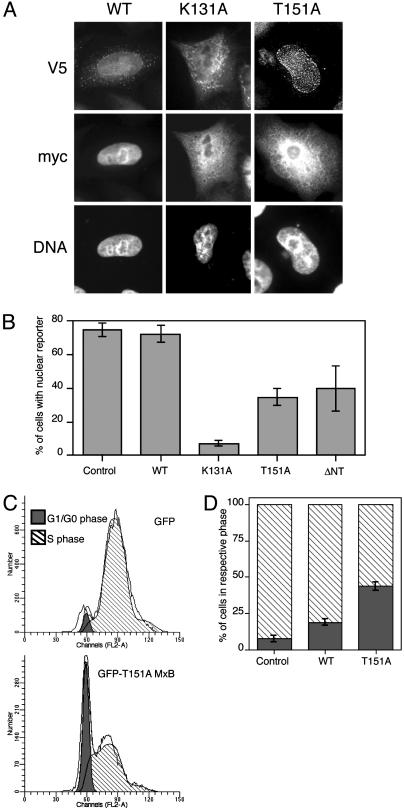
Expression of MxB Mutants Disrupts Nuclear Import. The localization of MxB to the cytoplasmic face of nuclear pores, apparently influenced by its GTP binding and hydrolysis abilities, suggested that MxB might function in regulating nuclear import. We therefore investigated whether expression of the K131A and T151A MxB mutants influences nucleocytoplasmic trafficking. Steady-state nuclear localization of a nonshuttling reporter for classical NLS-mediated nuclear import (a c-myc-nucleoplasmin core-SV40 T NLS fusion) was inhibited by overexpression of either K131A or T151A MxB, but not WT MxB (Fig. 4 A and B). An MxB mutant that lacks the N-terminal NLS and that is primarily cytoplasmically localized (ΔN25 MxB) also disrupted nuclear import of the SV40 T NLS reporter when expressed at high levels. Because the reporter used in these studies is retained within the nucleus after its import and does not shuttle, we are quite confident that the effects we observe arise from inhibition of nuclear import. Similar results were also obtained with a reporter harboring the M9 signal sequence from hnRNP A1 (Fig. 9, which is published as supporting information on the PNAS web site). Interestingly, the relative abilities of K131A and T151A MxB to inhibit nuclear import mirror the potencies of the equivalent dynamin mutants (K44A and T65F) as inhibitors of receptor-mediated endocytosis (21). We consider it unlikely that K131A and T151A MxB exert their inhibitory effects on nuclear import simply by saturating the nuclear transport machinery with an NLS-like sequence (22). Indeed, WT MxB has no effect even when expressed at high levels. Moreover, ΔN25 MxB significantly inhibits nuclear import despite completely lacking the NLS-like sequence.
Fig. 4.
Mutated MxB proteins inhibit nuclear import and prevent G1/S progression. (A) The myc-nucleoplasmin core-SV40 T NLS fusion reporter (myc) coincides completely with staining of DNA (indicating nuclear localization) in representative cells expressing WT MxB, but not in those expressing the K131A or T151A mutants. Scoring cells for nuclear (or nonnuclear) localization of the reporter gave the results presented in B. Immunofluorescence with anti-V5 antibodies was used to localize MxB mutants. (B) Quantitation of the percentage of cells positive for the given MxB protein that demonstrate exclusively nuclear localization of the NLS-reporter. Errors represent the SD of at least four experiments, each involving a minimum of 100 positive cells. (C) HeLa cells transfected with GFP alone or T151A MxB/GFP were stained with propidium iodide after double-thymidine block and release for 4.5 hr to allow cells to progress to S phase. Cells were then analyzed by flow cytometry, and data are shown only for cells sorted for GFP (or T151A MxB/GFP) expression. Western blotting was used to confirm expression of full-length GFP fusions (data not shown). (D) Quantitation of the relative percentage of cells in G0/G1 (filled bars) and S (hatched bars) phases using the program modfit. Error bars represent the SD of at least three independent experiments.
Expression of the T151A MxB Mutant Disrupts Cell-Cycle Progression. Because disruption of nuclear transport causes a block at the G1/S transition (23–25), we next tested whether our dominant-negative MxB mutants perturb cell-cycle progression. We found that overexpression of a GFP fusion protein of T151A MxB causes a significant delay in the progression of synchronized cells into S phase, as demonstrated by propidium iodide staining and flow cytometry. Almost half of the cells expressing MxB T151A/GFP remained in G0/G1 phase after 4.5-hr release from double thymidine block whereas all but 7% of the control cells had progressed into S phase (Fig. 4 C and D). Cells expressing T151A MxB do eventually complete the cell cycle (data not shown), suggesting that there is a kinetic delay in cell-cycle progression rather than a complete block, consistent with the intermediate phenotype seen for T151A MxB in the nuclear import assay (Fig. 4B). A minor delay in G1/S progression was also seen in cells overexpressing WT MxB/GFP although this delay was restricted to cells expressing particularly high levels and a stable cell line expressing endogenous levels of WT MxB/GFP was normal (data not shown). We could not assess the effect of K131A MxB in this assay because its expression was toxic over the long time course required.
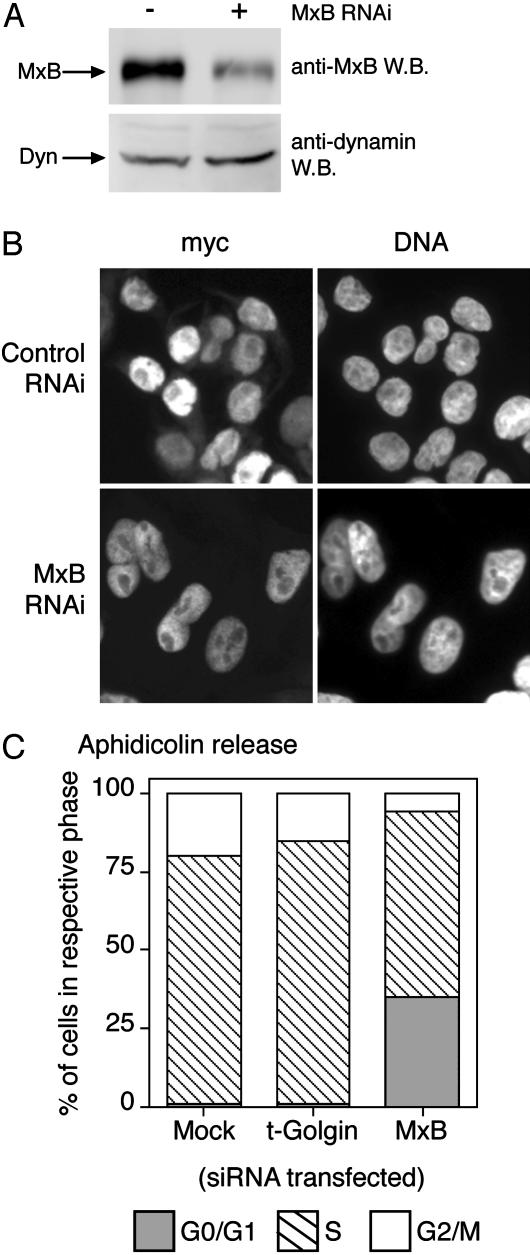
Knockdown of MxB Expression Causes Cell-Cycle Defects but Not General Inhibition of Nuclear Trafficking. We next used RNAi to determine whether MxB is required for nucleocytoplasmic trafficking. There are several reasons to expect that MxB is not required for nuclear pore function per se but at most plays a regulatory role. For example, there are no Mx proteins in yeast, yet the architecture and composition of yeast nuclear pores are quite similar to those of mammals (26). By transfecting HeLa cells with MxB-specific small interfering RNA duplexes, we could markedly reduce endogenous MxB protein levels (Fig. 5A). However, as shown in Fig. 5B, this MxB depletion had no discernible effect on steady-state nuclear import of the myctagged classical NLS reporter.
Fig. 5.
RNAi depletion of MxB does not inhibit bulk nuclear import but delays cell-cycle progression. (A) MxB protein levels are largely depleted by RNAi when detected by Western blot, with dynamin levels shown as a loading control. (B) HeLa cells subjected to two serial transfections with MxB-specific small interfering RNA oligonucleotide duplexes show normal nuclear import of a transfected myc-tagged NLS-reporter (myc). (C) MxB depletion by RNAi delays progression of cells from G0/G1 phase (filled bars) into S phase (hatched bars) after 4 hr of release from aphidicolin block. RNAi depletion of the unrelated protein t-Golgin behaves as mock-transfected cells.
MxB depletion had a much more profound effect on cell-cycle progression, however, which we anticipated would be more sensitive than the steady-state nuclear localization assay to kinetic delays in nuclear transport. As seen in Fig. 5C, MxB knockdown cells exhibited a significant delay in progression from G0/G1 into S phase, similar to that seen in cells expressing T151A MxB expression (Fig. 4). Four hours after release from aphidicolin block, 35% of the cells transfected with MxB-specific small interfering RNA remained in G0/G1 phase, compared with just 1% of mock-transfected cells or cells subjected to RNAi of the unrelated protein t-Golgin.
Discussion
Using tools and approaches similar to those used to identify the cellular functions of dynamin, we provide evidence suggesting that the related large GTPase MxB plays a role in regulating nucleocytoplasmic transport and/or cell-cycle progression. MxB is localized to the cytoplasmic face of nuclear pores in a way that seems to require both its binding to GTP and the presence of its N-terminal NLS-like sequence. Dominant-negative mutants of MxB that fail to bind or to hydrolyze GTP inhibit nuclear import, suggesting that one normal cellular function of this protein may be to regulate this vital process. The localization of WT MxB to nuclear pores, without its translocation into the nucleus, is reminiscent of the nuclear import intermediates that accumulate in ATP-depleted cells (27). One possibility is that MxB gates nuclear pores by associating with them in a way that resembles these intermediates, thus temporarily restricting nuclear transport. Overexpression of an MxB mutant that cannot hydrolyze GTP (T151A MxB) might lock the majority of nuclear pores in such a restricted state, thus causing a general inhibition of nucleocytoplasmic transport. The K131A and ΔN25 MxB mutants might instead inhibit nucleocytoplasmic transport by associating with other regulatory components of the nuclear pore complex and causing their mislocalization away from the nuclear envelope. Based upon the observations presented in this report, we hypothesize that GTP-regulated cycling of MxB (and possibly other associated regulatory components) to and from nuclear pore complexes may play an important role in modulating nucleocytoplasmic transport.
Impairing MxB function in cells by expressing a dominant-negative mutant, or by RNAi knockdown of MxB levels, leads to a significant delay in progression from G0/G1 into S phase in the cell cycle. Although other explanations cannot be excluded, this finding is consistent with the suggestion that MxB regulates the timing of nuclear import of cell-cycle regulatory proteins. Determining whether specific cargos are dependent on MxB for nuclear import or whether MxB knockdown has a general effect on the kinetics of nuclear import will be important questions to address. Meanwhile, our findings that MxB mutants inhibit nucleocytoplasmic transport and influence cell-cycle progression suggest that its relatives with antiviral properties, such as MxA, might exploit similar regulatory processes for their ability to inhibit viral replication.
Supplementary Material
Acknowledgments
We thank members of the Lemmon, Marks, and Van Duyne laboratories, as well as Y. Goldman, M. Marks, C. Burd, M. Chou, and K. Ferguson for discussions and critical reading of the manuscript; K. Melen and I. Julkunen for their generous gift of anti-MxB antisera; M. Marks for the siRNA oligos to t-Golgin; and Gideon Dreyfuss for the myc-tagged nucleoplasmin core-NLS fusion construct. This work was supported in part by U.S. Army Breast Cancer Research Program Fellowship DAMD17-02-1-0546 (to M.C.K.) and National Institutes of Health Grant GM056846 (to M.A.L.).
Abbreviations: NLS, nuclear localization signal; RNAi, RNA interference; immunoEM, immunoelectron microscopy.
References
- 1.van der Bliek, A. M. & Meyerowitz, E. M. (1991) Nature 351, 411–414. [DOI] [PubMed] [Google Scholar]
- 2.Thompson, H. M., Skop, A. R., Euteneuer, U., Meyer, B. J. & McNiven, M. A. (2002) Curr. Biol. 12, 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smirnova, E., Shurland, D. L., Ryazantsev, S. N. & van der Bliek, A. M. (1998) J. Cell Biol. 143, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong, E., Wagner, J., Scott, S., Okreglak, V., Holewinske, T., Cassidy-Stone, A. & Nunnari, J. (2003) J. Cell Biol. 160, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinshaw, J. E. & Schmid, S. L. (1995) Nature 374, 190–192. [DOI] [PubMed] [Google Scholar]
- 6.Sever, S., Damke, H. & Schmid, S. L. (2000) Traffic 1, 385–392. [DOI] [PubMed] [Google Scholar]
- 7.Danino, D. & Hinshaw, J. E. (2001) Curr. Opin. Cell Biol. 13, 454–460. [DOI] [PubMed] [Google Scholar]
- 8.Aebi, M., Fah, J., Hurt, N., Samuel, C. E., Thomis, D., Bazzigher, L., Pavlovic, J., Haller, O. & Staeheli, P. (1989) Mol. Cell. Biol. 9, 5062–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller, O. & Kochs, G. (2002) Traffic 3, 710–717. [DOI] [PubMed] [Google Scholar]
- 10.Raposo, G., Tenza, D., Mecheri, S., Peronet, R., Bonnerot, C. & Desaymard, C. (1997) Mol. Biol. Cell 8, 2631–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou, W., Geuze, H. J. & Slot, J. W. (1996) Histochem. Cell Biol. 106, 41–58. [DOI] [PubMed] [Google Scholar]
- 12.Melen, K., Keskinen, P., Ronni, T., Sareneva, T., Lounatmaa, K. & Julkunen, I. (1996) J. Biol. Chem. 271, 23478–23486. [DOI] [PubMed] [Google Scholar]
- 13.Davis, L. I. & Blobel, G. (1986) Cell 45, 699–709. [DOI] [PubMed] [Google Scholar]
- 14.Kessel, R. G. (1992) Int. Rev. Cytol. 133, 43–120. [DOI] [PubMed] [Google Scholar]
- 15.Suntharalingam, M. & Wente, S. (2003) Dev. Cell 4, 775–789. [DOI] [PubMed] [Google Scholar]
- 16.Ewald, A., Kossner, U., Scheer, U. & Dabauvalle, M. C. (1996) J. Cell Sci. 109, 1813–1824. [DOI] [PubMed] [Google Scholar]
- 17.Pavlovic, J., Zurcher, T., Haller, O. & Staeheli, P. (1990) J. Virol. 64, 3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks, B., Stowell, M. H., Vallis, Y., Mills, I. G., Gibson, A., Hopkins, C. R. & McMahon, H. T. (2001) Nature 410, 231–235. [DOI] [PubMed] [Google Scholar]
- 19.van der Bliek, A. M., Redelmeier, T. E., Damke, H., Tisdale, E. J., Meyerowitz, E. M. & Schmid, S. L. (1993) J. Cell Biol. 122, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Accola, M. A., Huang, B., Al Masri, A. & McNiven, M. A. (2002) J. Biol. Chem. 277, 21829–21835. [DOI] [PubMed] [Google Scholar]
- 21.Damke, H., Binns, D., Ueda, H., Schmid, S. & Baba, T. (2001) Mol. Biol. Cell 12, 2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfarb, D. S., Gariepy, J., Schoolnik, G. & Kornberg, R. D. (1986) Nature 322, 641–644. [DOI] [PubMed] [Google Scholar]
- 23.Harel, A., Orjalo, A., Vincent, T., Lachish-Zalait, A., Vasu, S., Shah, S., Zimmerman, E., Elbaum, M. & Forbes, D. (2003) Mol. Cell 11, 853–864. [DOI] [PubMed] [Google Scholar]
- 24.Nishimoto, T., Eilen, E. & Basilico, C. (1978) Cell 15, 475–483. [DOI] [PubMed] [Google Scholar]
- 25.Dasso, M., Nishitani, H., Kornbluth, S., Nishimoto, T. & Newport, J. (1992) Mol. Cell. Biol. 12, 3337–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weis, K. (2003) Cell 112, 441–451. [DOI] [PubMed] [Google Scholar]
- 27.Newmeyer, D. D. & Forbes, D. J. (1988) Cell 52, 641–653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.