Significance
We show that a CUB–spacer domain interaction impedes exposure of the ADAMTS13 spacer functional exosite, preventing ADAMTS13 from interacting effectively with its complementary binding site in the VWF A2 domain. This CUB–spacer interaction is disrupted by interaction with the C-terminal domains of VWF, leading to conformational activation of ADAMTS13. Our findings also suggest that activation of ADAMTS13 reveals a cryptic epitope in the spacer domain that constitutes the autoantigenic core in patients with acquired TTP. These antibodies inhibit ADAMTS13, causing deposition of VWF and platelet-rich microthrombi in small blood vessels, resulting in organ damage. Thus, this study provides insight into the complexity of both normal haemostatic control and the pathogenesis of autoimmunity.
Keywords: ADAMTS13, VWF, TTP, autoantibodies
Abstract
A disintegrin and metalloprotease with thrombospondin motifs 13 (ADAMTS13) is a metalloprotease that regulates von Willebrand factor (VWF) function. ADAMTS13-mediated proteolysis is determined by conformational changes in VWF, but also may depend on its own conformational activation. Kinetic analysis of WT ADAMTS13 revealed ∼2.5-fold reduced activity compared with ADAMTS13 lacking its C-terminal tail (MDTCS) or its CUB1-2 domains (WTΔCUB1-2), suggesting that the CUB domains naturally limit ADAMTS13 function. Consistent with this suggestion, WT ADAMTS13 activity was enhanced ∼2.5-fold by preincubation with either an anti-CUB mAb (20E9) or VWF D4CK (the natural binding partner for the CUB domains). Furthermore, the isolated CUB1-2 domains not only bound MDTCS, but also inhibited activity by up to 2.5-fold. Interestingly, a gain-of-function (GoF) ADAMTS13 spacer domain variant (R568K/F592Y/R660K/Y661F/Y665F) was ∼2.5-fold more active than WT ADAMTS13, but could not be further activated by 20E9 mAb or VWF D4CK and was unable to bind or to be inhibited by the CUB1-2 domains, suggesting that the inhibitory effects of the CUB domains involve an interaction with the spacer domain that is disrupted in GoF ADAMTS13. Electron microscopy demonstrated a “closed” conformation of WT ADAMTS13 and suggested a more “open” conformation for GoF ADAMTS13. The cryptic spacer domain epitope revealed by conformational unfolding also represents the core antigenic target for autoantibodies in thrombotic thrombocytopenic purpura. We propose that ADAMTS13 circulates in a closed conformation, which is maintained by a CUB–spacer domain binding interaction. ADAMTS13 becomes conformationally activated on demand through interaction of its C-terminal CUB domains with VWF, making it susceptible to immune recognition.
Von Willebrand factor (VWF) is a large, multidomain glycoprotein that recognizes vascular damage by binding to exposed collagen through its A3 domain (1–3). VWF tethered to collagen responds to shear forces by adapting its conformation (4). Under conditions of low shear, it is thought to adopt a globular conformation, whereas at high shear, it unfolds and reveals its binding site for the platelet GpIbα receptor on its A1 domain, thereby facilitating platelet recruitment to the site of vascular injury.
VWF is stored before release into the plasma as multimers that can be as large as 20–40 mers (5–8). On release from the cell, the highest molecular weight multimers are the most hemostatically active. Indeed, “ultra-large” multimers present a potential hazard if their function is unregulated, because they can predispose to the formation of VWF-platelet microthrombi that can occlude small blood vessels, resulting in thrombotic thrombocytopenic purpura (TTP) (9).
The metalloprotease ADAMTS13 is able to cleave the VWF A2 domain, dramatically reducing the multimeric size of VWF and its propensity to form platelet microthrombi (10, 11). Cleavage of VWF by ADAMTS13 is a multistep process. An initial positioning interaction occurs between the D4CK domains of globular VWF and ADAMTS13 (12, 13). As unfolding occurs, exposure of the VWF scissile bond, Y1605-M1606, within the A2 domain is controlled by structural elements contained within this domain (14–17). Progressive unfolding allows distinct functional exosites within its A2 domain to be exposed and engaged by complementary binding sites on the protease, spacer, and disintegrin-like domains of ADAMTS13 (18–22). Ultimately, docking of VWF scissile bond P1′, P1, and P3 residues into subsites on the protease position the scissile bond for cleavage (23). Thus, conformational changes in VWF are essential for its efficient cleavage by ADAMTS13.
To explore the possible role of conformation on ADAMTS13 function (24, 25), we studied a recently described gain-of-function (GoF) variant of ADAMTS13 (26). Jian et al. (26) concluded that an ADAMTS13 GoF variant comprising composite spacer domain substitutions R568K/F592Y/R660K/Y661F/Y665F (hereinafter, GoF) had an approximate fourfold increased ability to cleave VWF substrates. A similar increase in activity on removal of the C-terminal domains from ADAMTS13 has been reported by others (27). Based on our investigation of the properties of ADAMTS13, the GoF variant, and their derivatives reported herein, we propose that ADAMTS13 normally adopts a globular conformation determined by interaction of its spacer and CUB domains and is unfolded during conformational activation.
Results
Increased Substrate Recognition Leads to Hyperactivity in GoF ADAMTS13.
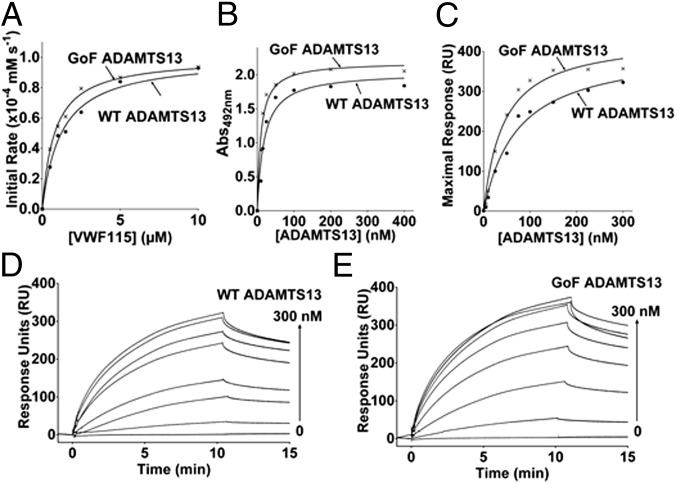
Our kinetic analysis of VWF115 cleavage suggested an approximate twofold difference between WT ADAMTS13 and the GoF variant (26) (Fig. 1A and kcat/Km values in Table 1). There was no difference in kcat values, however, with the enhanced activity instead related to an increased affinity for the substrate, as demonstrated by the Km values of 1.54 µM for WT and 0.86 µM for GoF ADAMTS13. The KD(APP) values determined by equilibrium plate binding assays suggest a twofold increased affinity of GoF ADAMTS13 for VWF (Fig. 1B). This finding was confirmed by both steady-state and global fit surface plasmon resonance (SPR) analysis (Fig. 1 C–E) and found to be related to an approximate twofold increase in association rate (Table 1).
Fig. 1.
The increased VWF115 binding affinity and proteolytic activity of GoF ADAMTS13. (A) initial rates of substrate proteolysis were used to determine kcat and Km values (listed in Table 1). (B) The increased substrate recognition suggested by the lower Km value of the GoF ADAMTS13 variant was confirmed by an equilibrium plate-binding assay. (C–E) A twofold reduction in KD(APP) was confirmed by SPR using both steady-state (C) and global fit (D and E) analyses. The results are representative of three independent experiments.
Table 1.
Activity and VWF115 binding affinity of ADAMTS13 variants
| VWF115 proteolysis | VWF115 binding | ||||||||
| Plate binding, KD(APP), nM | SPR global fitting | SPR RUmax, KD, nM | |||||||
| Variant | kcat, s−1 | Km, µM | kcat/Km, 105 M−1 s−1 | kcat/Km(Time course), 105 M−1 s−1 | ka, 104 M−1 s-1 | kd, 10−3 s−1 | KD, nM | ||
| WT ADAMTS13 | 0.71 ± 0.09 | 1.54 ± 0.20 | 4.1 ± 0.5 | 5.5 ± 0.7 | 26.4 ± 3.0 | 3.72 ± 0.16 | 2.58 ± 0.01 | 72.3 ± 1.3 | 69.8 ± 6.2 |
| GoF ADAMTS13 | 0.62 ± 0.02 | 0.86 ± 0.07* | 8.3 ± 0.7* | 10.4 ± 0.3* | 11.5 ± 1.4* | 6.82 ± 0.19* | 2.64 ± 0.01 | 38.5 ± 1.2* | 35.8 ± 2.6* |
The activities of ADAMTS13 variants were determined by kinetic analysis of the initial rates of VWF115 proteolysis. The binding between VWF115 and ADAMTS13 variants were determined by plate binding assays (KD(APP)), global fit SPR analysis (ka, kd, and KD), and steady-state SPR analysis (KD, RUmax plot). Mean values ± SEM are based on three independent experiments.
*P < 0.05, t test.
Disruption of C-Terminal Interactions Activates WT ADAMTS13.
We hypothesized that initial VWF binding causes a conformational activation of ADAMTS13 that may be unnecessary in the GoF variant. Addition of the VWF D4CK domain fragment to WT ADAMTS13 in a FRETS-VWF73 assay increased its activity (normalized against that of WT ADAMTS13) in a dose-dependent manner, resulting in a 2- to 2.5-fold increase (Fig. 2A). This hyperactivity was similar to that exhibited by GoF ADAMTS13. Furthermore, the activity of GoF ADAMTS13 was not then enhanced by the addition of VWF D4CK (Fig. 2 B and C).
Fig. 2.
WT ADAMTS13 activity can be enhanced by the addition of a VWF D4CK domain fragment or addition of an activating mAb targeting the CUB2 domain. (A–C) The effect of the VWF D4CK domain fragment on ADAMTS13 activity was determined by a FRETS-VWF73 assay. GoF ADAMTS13 activity was more than twofold greater than WT ADAMTS13 activity (P < 0.05), and was not further increased by VWF D4CK. (D–F) The effect of the activating mAb was assessed in the same manner. The antibody elicited a greater than twofold increase in activity of the WT (P < 0.05), but had no effect on the already hyperactive GoF variant. Results are presented as mean ± SEM (n = 3).
The 20E9 mAb recognizes the CUB2 domain of ADAMTS13. Enhanced activity was observed on addition of the 20E9 mAb to WT ADAMTS13 (Fig. 2D), reaching a maximum 2.5-fold enhanced activity. GoF ADAMTS13 was unaffected by the antibody (Fig. 2 E and F), suggesting that the CUB domains may play an important role in ADAMTS13 activation.
Regulation of ADAMTS13 Activity by the CUB Domains.
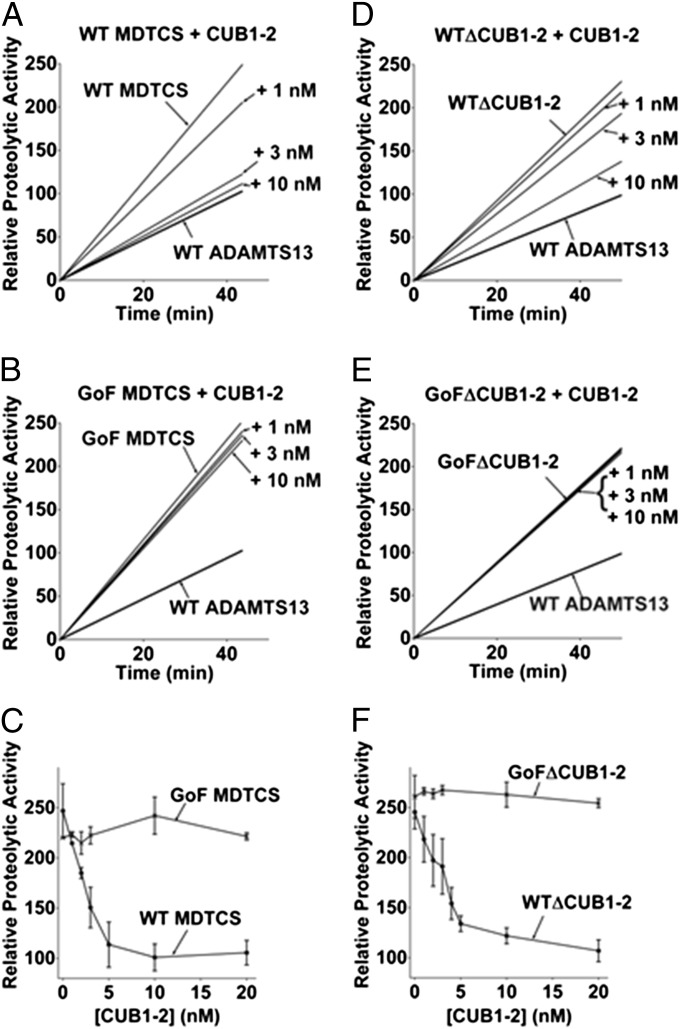
We hypothesized that the C-terminal CUB domains interact with the spacer domain in the native WT ADAMTS13. We predicted that removal of the C-terminal domains (in the truncated variant MDTCS) would produce a similar activity enhancement as that observed with VWF D4CK and 20E9 mAb. Indeed, both the WT and GoF MDTCS variants were fully hyperactive, with activity profiles indistinguishable from GoF ADAMTS13 (Fig. 3 A–C). Moreover, truncation of ADAMTS13 after the TSP2-8 domains (WT∆CUB1-2 variant) produced an enhancement similar to that seen in WT MDTCS (Fig. 3 D and F). Furthermore, reintroduction of the isolated CUB1-2 domain fragment had a dose-dependent inhibitory effect on both WT MDTCS and WT∆CUB1-2, reducing activity similar to that of WT ADAMTS13 (Fig. 3 A and D). In contrast, C-terminal truncation of GoF ADAMTS13 had no influence on its activity, and addition of the CUB1-2 domain fragment was ineffective even at high concentrations (Figs. 3 B and E).
Fig. 3.
WT ADAMTS13 hyperactivity resulting from C-terminal truncation can be inhibited by the CUB1-2 domains. (A–C) The activity of WT and GoF MDTCS variants was determined by a FRETS-VWF73 assay. Both enzymes exhibited hyperactivity compared with WT ADAMTS13 (P < 0.05). WT MDTCS activity can be inhibited by the addition of CUB1-2 domain fragment; however, the fragment had no significant effect on GoF MDTCS. (D–F) Specific removal of the CUB-domains from both WT and GoF ADAMTS13 also resulted in a greater than twofold increase in activity (P < 0.05). The CUB1-2 domain fragment exhibited inhibitory activity when added to WT∆CUB1-2 (P < 0.05). Results are presented as mean ± SEM (n = 3).
A CUB–Spacer Domain Binding Interaction.
We developed a reciprocal coimmunoprecipitation (co-IP) experiment to directly analyze any CUB–spacer domain interaction. WT MDTCS and the CUB1-2 domain fragment bound in solution and remained in complex when either fragment was pulled down specifically by the mAb. As a result, both fragments were detected in immunoprecipitation (IP) eluates and depleted from solution (Fig. 4A). In contrast, when GoF MDTCS and CUB1-2 were incubated, only the fragment being pulled down by its corresponding mAb was detected in the eluate, with the other fragment remaining in solution. Given that GoF MDTCS differs from WT MDTCS only in terms of spacer domain substitutions, this finding strongly suggests the spacer domain as the site of CUB domain binding to MDTCS.
Fig. 4.
The CUB1-2 domain fragment binds to WT MDTCS through the spacer domain. (A) MDTCS (filled arrowhead) and CUB1-2 (open arrowheads; present also as a partially cleaved, faster mobility fragment) were immunoprecipitated using protein G beads coupled to the indicated mAb (3H9, directed to the protease domain, or 20E9, directed to the CUB2 domain). The MDTCS and CUB fragments present in solution pre-IP and post-IP, along with bound proteins after elution, were detected by polyclonal anti-ADAMTS13 Western blot analysis. Co-IP of WT MDTCS with CUB1-2 was evident under both experimental conditions, but binding of GoF MDTCS was not apparent. MDTCS and CUB1-2 were also incubated with an isotype control IgG (15D7 mAb). (B) Affinity between the CUB and spacer domains was determined by IP using magnetic beads coated with 20E9 mAb complexed to the CUB1-2 domain fragment.
MDTCS-CUB co-IP performed over a range of MDTCS concentrations yielded KD(APP) values of ∼40 nM for the binding of CUB1-2 and WT MDTCS (Fig. 4B). Minimal binding was observed between CUB1-2 and GoF MDTCS.
ADAMTS13 Can Exist in a Closed or More Open Conformation.
We hypothesized that unfolding would result in an observable change in the overall conformation of the full-length protein, and investigated this through transmission electron microscopy (TEM) of negatively stained proteins (Fig. 5). Analysis of the WT ADAMTS13 sample produced various monodisperse orientational particles measuring between 12 and 16 nm, consistent with a protein ∼190 kDa in size. The main projection view had a clear compact, globular conformation with an additional side “bulge” of projection density (Fig. 5A, panel 3); we assigned this as the closed ADAMTS13 conformation. Examination of a similar number of GoF ADAMTS13 particles revealed similar-sized particles, but the projection averages produced were somewhat more linear (Fig. 5B, panel 3), and the projection view with the side bulge was absent. In addition, the projections were not as sharply delineated as the ADAMTS13 samples; this feature, along with a different population of particle projections, suggests that the complex has adopted a more open organization with some local region of protein in a flexible/unstructured conformation (Fig. 5, end panel).
Fig. 5.
TEM analysis of WT ADAMTS13 (A) and GoF ADAMTS13 (B). Shown are representative examples of negatively stained TEM fields of view, individual particles selected for analysis, and the results of multistatistical reference-free class averaging. (Scale bars: 50 nm.) (C) Schematic representation of the proposed conformational change on activation, consistent with the existence of an interaction between the CUB domains (C1 and C2) and spacer domain (S), shielding the VWF A2 domain-binding exosites.
WT ADAMTS13 Conformational Activation Reveals Its Cryptic TTP Autoantibody Binding Epitopes.
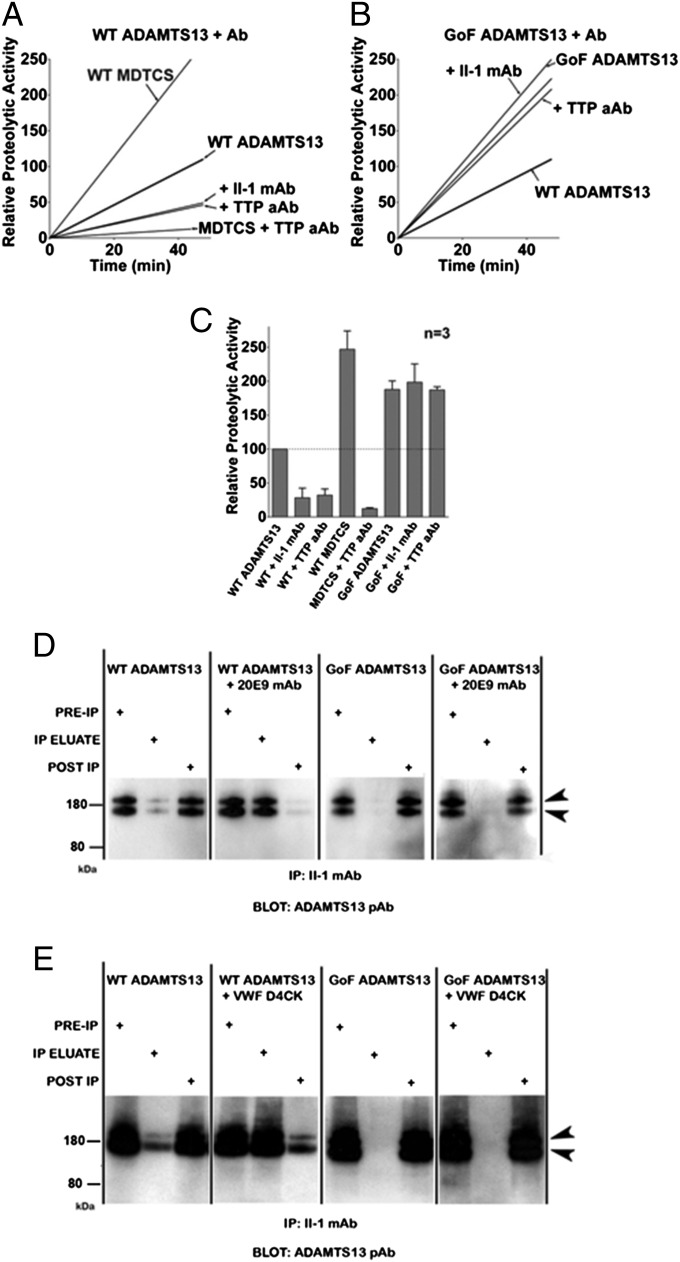
WT ADAMTS13 was inhibited by TTP patient autoantibodies and the single TTP patient-derived II-1 mAb (28) in FRETS-VWF73 assays (Fig. 6A); however, despite the addition of a large excess of these antibodies to activity assays, ADAMTS13 was inhibited by only 60%, compared with 95% inhibition of MDTCS by the same TTP aAb (Fig. 6C). This finding suggests that in its closed and relatively inactive state, WT ADAMTS13 might not be readily recognized by these antibodies. This was confirmed by IP of WT ADAMTS13 by the II-1 mAb covalently linked to magnetic beads. When converted to its open conformation using the activating 20E9 mAb (Fig. 6D) or VWF D4CK fragment (Fig. 6E), recognition of WT ADAMTS13 increased dramatically. The spacer domain mutations in GoF ADAMTS13 have been shown to abolish this antigenicity (Fig. 6B); this was confirmed in this study by both FRET and IP assays (Fig. 6 D and E).
Fig. 6.
WT ADAMTS13 recognition and inhibition by TTP autoantibodies is enhanced on activation by the anti-CUB 20E9 mAb. (A) Proteolysis of the FRETS-VWF73 substrate by WT ADAMTS13 was markedly decreased (P < 0.05) when the enzyme was preincubated with either TTP patient total IgG or TTP patient-derived II-1 mAb. (B and C) GoF ADAMTS13 activity in FRETS-VWF73 assays (B) was increased twofold compared with WT ADAMTS13 activity (C); however, it was not impaired by either antibody. IP of ADAMTS13 in solution using the II-1 mAb covalently coupled to magnetic beads suggested that WT ADAMTS13 is not efficiently recognized. (D and E) Pretreatment of WT ADAMTS13 with the activating anti-CUB 20E9 mAb (D) or the VWF D4CK domain fragment (E) greatly increased its capture by the beads.
Discussion
The concept of conformational activation of ADAMTS13 has been alluded to previously as a possible explanation for the suggested conflicting roles of the CUB domains (24, 29). Here we present results indicating that ADAMTS13 naturally adopts a folded or closed conformation, with folding mediated through an interaction between the C-terminal CUB domains and the more central spacer domain. The importance of this folding is suggested by the known functional importance of the spacer domain, which serves as a critical exosite that interacts with a cryptic binding site revealed on the VWF A2 domain as it unfolds (30). This provides an essential localizing mechanism that helps orientate the ADAMTS13 protease domain within reach of the VWF scissile bond. A consequence of the folded conformation of ADAMTS13 is that the important spacer domain exosite is only partially available and requires full exposure to enable efficient proteolysis of VWF.
It is now established that ADAMTS13 can interact with globular VWF through recognition of its surface-exposed C-terminal domain, D4CK, by the TSP-CUB domain region of ADAMTS13 (12, 13). This binding interaction has been considered a positioning one, enabling a small amount of ADAMTS13 to associate reversibly with VWF in its globular conformation, pending unfolding of VWF (13); however, we now present evidence that the VWF D4CK fragment can increase the activity of ADAMTS13 in a FRETS-VWF73 assay. Thus, we suggest that on binding to the VWF D4CK domains, ADAMTS13 unfolds to fully expose its cryptic spacer domain exosite. Once unfolded, the spacer domain can directly contact its VWF A2 complementary interaction site and enhance the cleavage process.
An interaction between the spacer and CUB domains of ADAMTS13 is supported by the results of addition of activating mAb, as well as activity and binding assays of C-terminal truncated WT ADAMTS13 in the presence of isolated CUB fragments. Regarding the activating mAb, we have shown that 20E9 mAb, an antibody that recognizes the CUB2 domain region of ADAMTS13, can increase the activity of WT ADAMTS13. In FRETS-VWF73 assays, isolated CUB fragments inhibited the proteolytic activities of both WT MDTCS and WT∆CUB1-2 truncation variants. Furthermore, in solution binding pulldown experiments, we found that CUB fragments can directly bind to these derivatives, with an estimated affinity of ∼40 nM. Moreover, the isolated CUB domain fragments inhibited WT∆CUB1-2, which retains its TSP domains, suggesting that the CUB-binding site on the spacer domain was not introduced by complete deletion of these C-terminal domains.
Additional evidence for conformational activation of ADAMTS13 comes from our characterization of GoF ADAMTS13. This variant was found to have an enhanced association rate with VWF fragments, leading to an ∼2- to 2.5-fold overall enhanced cleavage of the scissile bond. The properties of this variant might arise in part because of an increased molecular recognition of its substrate induced by the five substitution mutations introduced in the spacer domain. Alternatively, the enhanced activities also could arise from disruption of the CUB–spacer domain interaction in this variant by the introduced spacer domain substitution mutations. We propose that this variant adopts a native unfolded conformation and thus is essentially preactivated. Support for this idea is provided by its enhanced association rate with VWF115 and by failure of 20E9 activating antibodies to enhance activity of the variant. It is again interesting that pulldown experiments with GoF MDTCS showed no binding of the isolated CUB1-2 domain fragment. Moreover, the lack of inhibition in FRETS-VWF73 assay by the CUB domain fragment of GoF MDTCS and GoF∆CUB1-2 truncation variants provides further support for the lack of a masking interaction by the CUB domains and hence a preactivation state. Based on our TEM experiments, we visualize WT ADAMTS13 as a globular molecule, with GoF ADAMTS13 suggested to be less compact.
A potential advantage of conformational activation of ADAMTS13 is that localization of the activated form of the protease will occur on the surface of its substrate, VWF. Conceivably, this might offer a protective mechanism, particularly given that ADAMTS13 is known to be susceptible to cleavage by several coagulation proteases (31, 32). Conformational activation also can have untoward consequences, however. Autoantibodies against ADAMTS13 are known to cause or predispose to acquired TTP (33). The trigger factors that initiate the autoimmune response have yet to be fully delineated. It is interesting that the majority of patients with TTP have autoantibodies directed to the ADAMTS13 spacer domain (19, 34, 35); we propose that this is because this domain is naturally cryptic, shielded by the CUB domains. During the process of ADAMTS13 activation, the cryptic epitopes are revealed and might be recognized as foreign during a concurrent immune activation. Support for this concept is provided by the increased recognition of WT ADAMTS13 spacer domain by the patient-derived II-1 mAb in the presence of the activating 20E9 antibody or VWF D4CK fragment. Consequently, our results on the conformational activation of ADAMTS13 provide insights into both the proteolytic process by which ADAMTS13 recognizes VWF and the mechanism of autoantibody formation in TTP.
Experimental Procedures
Production of Recombinant ADAMTS13 and VWF Fragments.
Recombinant human ADAMTS13 with a C-terminal Myc/His6 tag in pCDNA3.1 (31) was used to generate GoF ADAMTS13 (R568K/F592Y/R660K/Y661F/Y665F) by sequential site-directed mutagenesis. The same mutagenesis primers were also used to introduce the GoF mutations into WT MDTCS-Myc/His6. The truncated variants WT∆CUB1-2 and GoF∆CUB1-2 (ADAMTS13 residues 1–1190) and the CUB1-2 domain fragment (ADAMTS13 residues 1191–1427) were generated by inverse PCR using the full-length ADAMTS13 constructs. All ADAMTS13 variants and fragments were expressed in HEK293S cells, purified, and quantified using in-house ELISA as described previously (36). For electron microscopy, ADAMTS13 was further purified to homogeneity by gel filtration using a Superose 12 10/300 GL column (GE Healthcare).
The VWF A2 domain fragment VWF115 (VWF residues 1554–1668) was expressed and purified as described previously (31). The VWF D4CK domain fragment (VWF residues 1874–2813) in the vector pcDNA 3.1/His was transiently expressed in HEK293S, purified by FPLC, and quantified by ELISA (12).
Production and Characterization of Anti-ADAMTS13 mAb 20E9.
The 20E9 mAb was one of 30 anti-ADAMTS13 mAbs obtained after immunization of WT BALB/c mice with recombinant WT ADAMTS13, followed by fusion of their spleen cells with Sp2/0 myeloma cells. The epitope of mAb 20E9 is situated in the CUB2 domain as determined by epitope mapping using a series of ADAMTS13 variants in which all domains were individually deleted.
TTP Patient IgG Preparation.
Total IgG was isolated from the presentation plasma sample of a TTP patient with high-titer anti-ADAMTS13 IgG directed against the spacer domain, using melon gel spin columns (Thermo Scientific). Ethical approval for the use of the sample was obtained from the local Research Ethics Committee (reference 08/H0716/72).
ADAMTS13 Activity Assays.
Recombinant VWF A2 domain fragments VWF115 (VWF residues 1554–1668) and FRETS-VWF73 (VWF residues 1596–1668), which span the ADAMTS13 cleavage site and spacer domain-binding exosites, were used as proteolytic substrates to determine the activity of ADAMTS13 and its variants. Cleavage of VWF115 by ADAMTS13 was carried out as described previously (18). For FRETS-VWF73 assays, ADAMTS13, MDTCS, and ∆CUB1-2 variants (WT and GoF) were diluted to 0.3 nM in reaction buffer (5 mM Bis-Tris pH 6.0, 25 mM CaCl2, and 0.005% Tween-20) in white 96-well plates (Nunc). Purified CUB1-2 domain fragment, VWF D4CK, 20E9 mAb, II-1 mAb, or TTP patient IgG was added, followed by preincubation for 45 min at 37 °C. The reaction was initiated by the addition of an equal volume of 4 µM FRETS-VWF73 substrate (Peptanova). Fluorescence (excitation, 340 nm; emission, 460 nm) was measured at 30 °C at 1-min intervals for 1 h using a Fluostar Omega plate reader (BMG Labtech). Results were normalized to WT ADAMTS13 activity to allow cross-comparison of any changes.
Equilibrium Plate-Binding Assay and Surface Plasmon Resonance.
The binding of VWF115 to ADAMTS13 was determined by an equilibrium plate-binding assay (12, 18) and by SPR using a BIAcore T100 protein interaction analysis system, as described previously (12).
Co-IP Analysis of the CUB–MDTCS Interaction.
Samples containing 40 nM MDTCS (WT or GoF) in the absence and presence of 40 nM CUB1-2 fragment were immunoprecipitated using protein G Dynabeads (Invitrogen) coupled to the 3H9 mAb against the ADAMTS13 metalloprotease domain (32, 37), the 20E9 mAb directed against the ADAMTS13 CUB domains, or the IgG isotype control 15D7 mAb (38). Beads were washed, and bound protein was eluted in 1× LDS loading buffer at 70 °C for 10 min. Inputs (pre-IP), flow-through (post-IP), and bound protein in eluates were analyzed by SDS/PAGE and anti-ADAMTS13 Western blot analysis.
For CUB-MDTCS affinity determination, blocked and 20E9 mAb-coated Dynabeads were preincubated with 100 nM CUB1-2 domain fragment and used to immunoprecipitate MDTCS (WT or GoF) at increasing concentrations (5–200 nM). Bound MDTCS was eluted in 20 mM Tris pH 7.4 and 1 M NaCl. Bound and free MDTCS concentrations were determined by in-house ELISA, and KD values were determined by fitting data to a one-site binding hyperbola using GraphPad Prism 4 software.
TTP Autoantibody Binding.
The TTP patient-derived II-1 mAb (28) (kindly provided by Prof J. Voorberg and Dr. N. Sorvillo) was covalently coupled to M280 tosylactivated Dynabeads (Invitrogen) following the manufacturer’s instructions. The coupled beads were added to 40 nM ADAMTS13 (WT or GoF) and preincubated for 1 h at 37 °C in the absence and presence of 4 µg of 20E9 mAb. After a 4-h incubation, the beads were washed, and bound protein was eluted in LDS loading buffer. Inputs (pre-IP), flow through (post-IP), and eluates were analyzed by SDS/PAGE and anti-ADAMTS13 Western blot analysis.
TEM.
Fully purified samples of ADAMTS13 variants (10 µL of ∼10 µg/mL) in 20 mM Tris pH 7.8, 150 mM NaCl, and 5 mM CaCl2 were applied to glow-discharged continuous carbon-coated 400-mesh copper grids (TAAB Laboratories) for 1 min. Excess sample was blotted using Whatman paper, and the protein was negatively stained by the addition of 10 µL of 5% (wt/vol) uranyl acetate for 1 min. Images were acquired at a nominal magnification of 39,000× using a Philips Tecnai30 Polara FEG electron microscope operating at 200 kV low-dose mode, and recorded on a Gatan 4K charge-coupled device camera.
Image analysis of the recorded TEM DM3 data files was performed using EMAN2 (blake.bcm.edu/emanwiki/EMAN2). Equivalent datasets of ∼6,000 particles were boxed out of images using semiautomated swarm picking; any aggregated or overlapping data were discarded. Data were CTF-corrected, and the dataset underwent multistatistical reference-free 2D classification. Data were automasked, a maximum shift of five pixels was applied, and the cross-correlation coefficient served as the main comparator. Projection averages of each particle orientations were selected, and a low-pass Gaussian filter (20 Å) was applied.
Acknowledgments
We thank Professor Jan Voorberg and Dr. Nicoletta Sorvillo (Sanquin-AMC Landsteiner Laboratory) for providing the II-1 mAb, and Dr. Marie Scully (University College London) for providing the TTP patient plasma sample. This work was funded by a grant from the British Heart Foundation (PG/12/55/29740, to D.A.L., J.T.B.C. and B.M.L.), a grant from the Fonds voor Wetenschappelijk Onderzoek–Flanders (G.0584.11, to K.V.), and a PhD grant from the Agency for Innovation by Science and Technology (to L.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Mohri H, Yoshioka A, Zimmerman TS, Ruggeri ZM. Isolation of the von Willebrand factor domain interacting with platelet glycoprotein Ib, heparin, and collagen and characterization of its three distinct functional sites. J Biol Chem. 1989;264(29):17361–17367. [PubMed] [Google Scholar]
- 3.Roth GJ, Titani K, Hoyer LW, Hickey MJ. Localization of binding sites within human von Willebrand factor for monomeric type III collagen. Biochemistry. 1986;25(26):8357–8361. doi: 10.1021/bi00374a004. [DOI] [PubMed] [Google Scholar]
- 4.Siedlecki CA, et al. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88(8):2939–2950. [PubMed] [Google Scholar]
- 5.Mayadas TN, Wagner DD. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc Natl Acad Sci USA. 1992;89(8):3531–3535. doi: 10.1073/pnas.89.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise RJ, Pittman DD, Handin RI, Kaufman RJ, Orkin SH. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- 7.Schneider SW, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 2007;104(19):7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer LW, Shainoff JR. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980;55(6):1056–1059. [PubMed] [Google Scholar]
- 9.Moake JL, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307(23):1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 10.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87(10):4235–4244. [PubMed] [Google Scholar]
- 11.Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–4234. [PubMed] [Google Scholar]
- 12.Zanardelli S, et al. A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood. 2009;114(13):2819–2828. doi: 10.1182/blood-2009-05-224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feys HB, Anderson PJ, Vanhoorelbeke K, Majerus EM, Sadler JE. Multi-step binding of ADAMTS-13 to von Willebrand factor. J Thromb Haemost. 2009;7(12):2088–2095. doi: 10.1111/j.1538-7836.2009.03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch CJ, Lane DA, Luken BM. Control of VWF A2 domain stability and ADAMTS13 access to the scissile bond of full-length VWF. Blood. 2014;123(16):2585–2592. doi: 10.1182/blood-2013-11-538173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marti T, Rösselet SJ, Titani K, Walsh KA. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, et al. A novel calcium-binding site of von Willebrand factor A2 domain regulates its cleavage by ADAMTS13. Blood. 2011;117(17):4623–4631. doi: 10.1182/blood-2010-11-321596. [DOI] [PubMed] [Google Scholar]
- 17.Luken BM, Winn LY, Emsley J, Lane DA, Crawley JT. The importance of vicinal cysteines, C1669 and C1670, for von Willebrand factor A2 domain function. Blood. 2010;115(23):4910–4913. doi: 10.1182/blood-2009-12-257949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanardelli S, et al. ADAMTS13 substrate recognition of von Willebrand factor A2 domain. J Biol Chem. 2006;281(3):1555–1563. doi: 10.1074/jbc.M508316200. [DOI] [PubMed] [Google Scholar]
- 19.Pos W, et al. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood. 2010;115(8):1640–1649. doi: 10.1182/blood-2009-06-229203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groot R, Bardhan A, Ramroop N, Lane DA, Crawley JT. Essential role of the disintegrin-like domain in ADAMTS13 function. Blood. 2009;113(22):5609–5616. doi: 10.1182/blood-2008-11-187914. [DOI] [PubMed] [Google Scholar]
- 21.de Groot R, Lane DA, Crawley JT. The ADAMTS13 metalloprotease domain: Roles of subsites in enzyme activity and specificity. Blood. 2010;116(16):3064–3072. doi: 10.1182/blood-2009-12-258780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawley JT, de Groot R, Xiang Y, Luken BM, Lane DA. Unraveling the scissile bond: How ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118(12):3212–3221. doi: 10.1182/blood-2011-02-306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang Y, de Groot R, Crawley JT, Lane DA. Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) Proc Natl Acad Sci USA. 2011;108(28):11602–11607. doi: 10.1073/pnas.1018559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao Z, et al. Recombinant CUB-1 domain polypeptide inhibits the cleavage of ULVWF strings by ADAMTS13 under flow conditions. Blood. 2005;106(13):4139–4145. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muia J, et al. Abstracts of the 60th annual meeting of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, June 23-26, 2014. J Thromb Haemost. 2014;12(Suppl 1):90. doi: 10.1111/jth.12588. [DOI] [PubMed] [Google Scholar]
- 26.Jian C, et al. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012;119(16):3836–3843. doi: 10.1182/blood-2011-12-399501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci USA. 2006;103(50):19099–19104. doi: 10.1073/pnas.0607264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pos W, et al. VH1-69 germline encoded antibodies directed towards ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7(3):421–428. doi: 10.1111/j.1538-7836.2008.03250.x. [DOI] [PubMed] [Google Scholar]
- 29.Majerus EM, Anderson PJ, Sadler JE. Binding of ADAMTS13 to von Willebrand factor. J Biol Chem. 2005;280(23):21773–21778. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 30.Gao W, Anderson PJ, Sadler JE. Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood. 2008;112(5):1713–1719. doi: 10.1182/blood-2008-04-148759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawley JT, et al. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105(3):1085–1093. doi: 10.1182/blood-2004-03-1101. [DOI] [PubMed] [Google Scholar]
- 32.Feys HB, et al. Inactivation of ADAMTS13 by plasmin as a potential cause of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8(9):2053–2062. doi: 10.1111/j.1538-7836.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 33.Furlan M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 34.Luken BM, et al. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005;93(2):267–274. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 35.Klaus C, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103(12):4514–4519. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 36.Chion CK, Doggen CJ, Crawley JT, Lane DA, Rosendaal FR. ADAMTS13 and von Willebrand factor and the risk of myocardial infarction in men. Blood. 2007;109(5):1998–2000. doi: 10.1182/blood-2006-07-038166. [DOI] [PubMed] [Google Scholar]
- 37.Feys HB, et al. Thrombotic thrombocytopenic purpura directly linked with ADAMTS13 inhibition in the baboon (Papio ursinus) Blood. 2010;116(12):2005–2010. doi: 10.1182/blood-2010-04-280479. [DOI] [PubMed] [Google Scholar]
- 38.Schoolmeester A, et al. Monoclonal antibody IAC-1 is specific for activated alpha2beta1 and binds to amino acids 199 to 201 of the integrin alpha2 I-domain. Blood. 2004;104(2):390–396. doi: 10.1182/blood-2003-12-4224. [DOI] [PubMed] [Google Scholar]