Significance
Because of the low mass of the proton, nuclear quantum effects can dramatically alter the properties of hydrogen-bond networks, especially when short and strong hydrogen bonds occur. Here, we combine experiments and state-of-the-art simulations that include the quantum nature of both the electrons and nuclei to show that the enzyme ketosteroid isomerase contains a hydrogen-bond network in its active site that facilitates extensive quantum proton delocalization. This leads to a 10,000-fold increase in the acidity of an active-site residue compared with the limit where the nuclei are classical particles. This work opens up new avenues for understanding the interplay between quantum effects and hydrogen bonding in biological systems containing strong hydrogen bonds.
Keywords: enzyme, hydrogen bonding, nuclear quantum effects, proton delocalization, ab initio path integral molecular dynamics
Abstract
Enzymes use protein architectures to create highly specialized structural motifs that can greatly enhance the rates of complex chemical transformations. Here, we use experiments, combined with ab initio simulations that exactly include nuclear quantum effects, to show that a triad of strongly hydrogen-bonded tyrosine residues within the active site of the enzyme ketosteroid isomerase (KSI) facilitates quantum proton delocalization. This delocalization dramatically stabilizes the deprotonation of an active-site tyrosine residue, resulting in a very large isotope effect on its acidity. When an intermediate analog is docked, it is incorporated into the hydrogen-bond network, giving rise to extended quantum proton delocalization in the active site. These results shed light on the role of nuclear quantum effects in the hydrogen-bond network that stabilizes the reactive intermediate of KSI, and the behavior of protons in biological systems containing strong hydrogen bonds.
Although many biological processes can be well-described with classical mechanics, there has been much interest and debate as to the role of quantum effects in biological systems ranging from photosynthetic energy transfer, to photoinduced isomerization in the vision cycle and avian magnetoreception (1). For example, nuclear quantum effects, such as tunneling and zero-point energy (ZPE), have been observed to lead to kinetic isotope effects of greater than 100 in biological proton and proton-coupled electron transfer processes (2, 3). However, the role of nuclear quantum effects in determining the ground-state thermodynamic properties of biological systems, which manifest as equilibrium isotope effects, has gained significantly less attention (4).
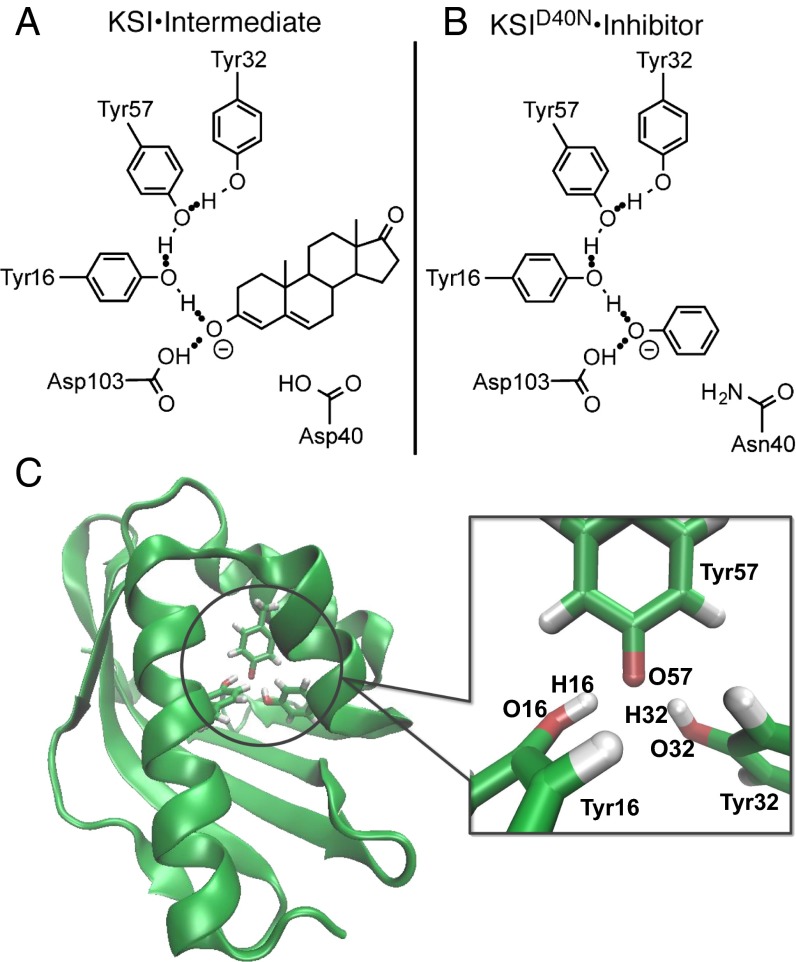
Ketosteroid isomerase (KSI) possesses one of the highest enzyme unimolecular rate constants and thus, is considered a paradigm of proton transfer catalysis in enzymology (5–11). The remarkable rate of KSI is intimately connected to the formation of a hydrogen-bond network in its active site (Fig. 1A), which acts to stabilize a charged dienolate intermediate, lowering its free energy by ∼11 kcal/mol (1 kcal = 4.18 kJ) relative to solution (Fig. S1) (6). This extended hydrogen-bond network in the active site links the substrate to Asp103 and Tyr16, with the latter further hydrogen-bonded to Tyr57 and Tyr32, which is shown in Fig. 1A.
Fig. 1.
KSI⋅intermediate and inhibitor complex. Schematic depiction of (A) the KSI⋅intermediate complex during the catalytic cycle (Fig. S1) and (B) a complex between and phenol, an inhibitor that acts as an intermediate analog. Both the intermediate and the inhibitor are stabilized by a hydrogen-bond network in the active site of KSI. (C) Image of with the tyrosine triad enlarged and the atoms O16, H16, O32, H32, and O57 labeled (shown with Tyr57 deprotonated) (16).
The mutant preserves the structure of the wild-type (WT) enzyme while mimicking the protonation state of residue 40 in the intermediate complex (Fig. 1B), therefore permitting experimental investigation of an intermediate-like state of the enzyme (6, 12–14). Experiments have identified that, in the absence of an inhibitor, one of the residues in the active site of is deprotonated (12). Although one might expect the carboxylic acid of Asp103 to be deprotonated, the combination of recent 13C NMR and ultraviolet visible spectroscopy (UV-Vis) experiments has shown that the ionization resides primarily on the hydroxyl group of Tyr57, which possesses an anomalously low of 6.3 ± 0.1 (12). Such a large tyrosine acidity is often associated with specific stabilizing electrostatic interactions (such as a metal ion or cationic residue in close proximity), which is not the case here, suggesting that an additional stabilization mechanism is at play (15).
One possible explanation is suggested by the close proximity of the oxygen (O) atoms on the side chains of the adjacent residues Tyr16 (O16) and Tyr32 (O32) to the deprotonated O on Tyr57 (O57) (Fig. 1C) (16). In several high-resolution crystal structures, these distances are found to be around 2.6 Å (14, 16, 17), which is much shorter than those observed in hydrogen-bonded liquids such as water, where O–O distances are typically around 2.85 Å. Such short heavy-atom distances are only slightly larger than those typically associated with low-barrier hydrogen bonds (18–20), where extensive proton sharing is expected to occur between the atoms. In addition, at these short distances, the proton’s position uncertainty (de Broglie wavelength) becomes comparable with the O–O distance, indicating that nuclear quantum effects could play an important role in stabilizing the deprotonated residue (Fig. 1C). In this work, we show how nuclear quantum effects determine the properties of protons in the active-site hydrogen-bond network of in the absence and presence of an intermediate analog by combining ab initio path integral simulations and isotope effect experiments.
Isotope Substitution Experiments Reveal Large Isotope Effect on Acidity
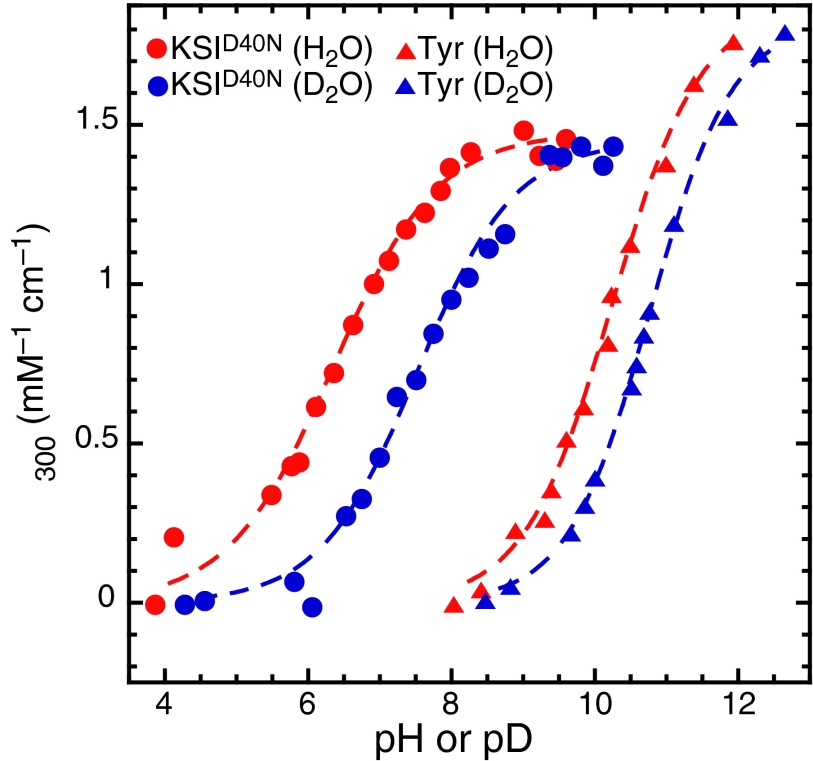
To assess the impact of nuclear quantum effects on the anomalous acidity of Tyr57, we measured the isotope effect on the acid dissociation constant on substituting hydrogens (H) in the hydrogen-bond network with deuterium (D). Because tyrosinate absorbs light at 300 nm more intensely than tyrosine, titration curves were generated by recording UV spectra of at different values (where L is H or D) (15). These experiments (Fig. 2 and Table S1) reveal a change in on H/D substitution of 1.1 ± 0.14 for tyrosine in the active site. This isotope effect is much larger than that observed for tyrosine in solution ( = 0.53 ± 0.08), and it is also, to the best of our knowledge, the largest recorded isotope effect (21). Changes in static equilibrium properties, such as the , on isotope substitution arise entirely from the quantum mechanical nature of nuclei. Such a large excess isotope effect, defined as , of 0.57 ± 0.16 thus indicates that the tyrosine triad in the active site of exhibits much larger nuclear quantum effects than those observed for tyrosine in aqueous solution.
Fig. 2.
Experimental UV-Vis titration curves of (circles) and tyrosine (triangles) in buffered H2O (red) and D2O (blue). Fractional ionization of the phenolic moiety is monitored by measuring the change in absorption at 300 nm. All datasets were well-fit to a sigmoid function, admitting four values and two values (the acidity isotope effect).
Given the possible role of nuclear quantum effects in , can one estimate how much the quantum nature of protons changes the acidity of Tyr57 compared with a situation in which all of the nuclei in the enzyme active site were classical? In the quasiharmonic limit, one can show that the varies as the inverse square root of the particle mass (m) (22, 23). By using this relation and the experimental values, we can extrapolate to the classical limit, which yields that the of Tyr57 in would be 10.1 ± 0.5 if the hydrogens were classical particles (Fig. S2). Relative to the observed of 6.3 ± 0.1, this value implies that nuclear quantum effects lower the of Tyr57 by 3.8 ± 0.5 units: an almost four orders of magnitude change in the acid dissociation constant.
Ab Initio Path Integral Simulations of
To provide insights into the molecular origins of the nuclear and electronic quantum effects that stabilize the deprotonated Tyr57 residue, we performed simulations of . To treat the electronic structure in the active site, we performed ab initio molecular dynamics (AIMD) simulations using a quantum mechanical/molecular mechanical (QM/MM) approach (24–27), in which the QM region was treated by density functional theory at the B3LYP-D3 level (28, 29) (Materials and Methods). These simulations allow for bond breakage and formation as dictated by the instantaneous electronic structure rather than predefined bonding rules.
AIMD simulations are typically performed treating the nuclei as classical particles. However, a classical treatment of the nuclei would predict that the would not change on isotope substitution. Nuclear quantum effects can be exactly included in the static equilibrium properties for a given description of the electronic structure using the path integral formalism of quantum mechanics, which exploits the exact mapping of a system of quantum mechanical particles onto a classical system of ring polymers (30–33). We combined this formalism with on-the-fly electronic structure calculations and performed ab initio path integral molecular dynamics (AI-PIMD) simulations of . These simulations treat both the nuclear and electronic degrees of freedom quantum mechanically in the active-site QM region and also incorporate the fluctuations of the protein and solvent environment in the MM region. The simulations consisted of between 47 and 68 QM atoms and more than 52,000 MM atoms describing the rest of the protein and solvent (Table S2). These simulations, which until recently would have been computationally prohibitive, were made possible by accelerating the path integral molecular dynamics convergence using a generalized Langevin equation (34), using new methods to accelerate the extraction of isotope effects (22), and exploiting graphical processing units (GPUs) to perform efficient electronic structure theory evaluations through an interface to the TeraChem code (35, 36). Such a combination yielded almost three orders of magnitude speedup compared with existing AI-PIMD approaches, allowing 1.1 ps/day simulation to be obtained using six NVIDIA Titan GPUs. We have recently shown that AI-PIMD simulations using the B3LYP-D3 functional give excellent predictions of isotope effects in water, validating such a combination for the simulation of isotope effects in hydrogen-bonded systems (37).
Quantum Delocalization of Protons in
The excess isotope effect, , obtained from our simulations (SI Materials and Methods, section C) was 0.50 ± 0.03, which is in excellent agreement with the experimental value of 0.57 ± 0.16. The average distances between O57 and the adjacent O16 and O32 atoms obtained in our simulations were 2.56 and 2.57 Å, with standard deviations in both cases of 0.09 Å. The distribution of distances between O16 and O57 explored in the simulation is shown in Fig. 4A, Inset. These average O–O distances are slightly smaller than (and within the margin of error of) those in the starting crystal structure (∼2.6 Å) (16). As we will discuss below, the close proximity of the neighboring O16 and O32 groups plays a crucial role in the origins of the observed isotope effect.
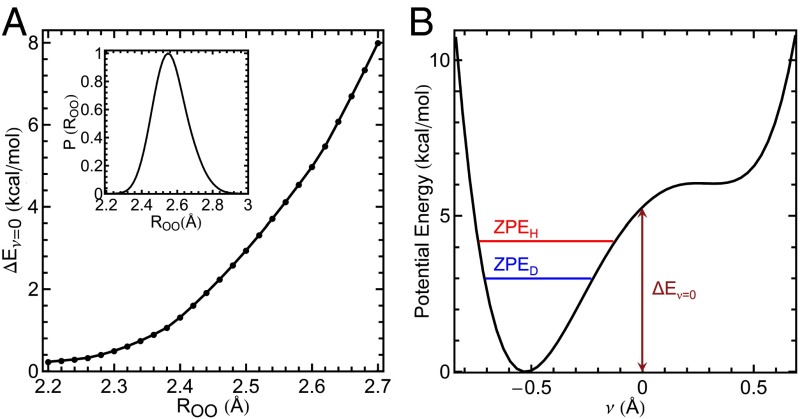
Fig. 4.
Comparison of the energy required to share a proton between residues as a function of the hydrogen-bond donor–acceptor O–O distance compared with the ZPE. (A) as a function of the O–O distance between O16 and O57 using the tyrosine triad geometry from a crystal structure (details in SI Materials and Methods, section D). A, Inset shows the probability distribution of obtained from the AI-PIMD simulation of with ionized Tyr57. The probability is normalized by the maximum value. (B) Potential energy as a function of the proton transfer coordinate ν for = 2.6 Å, indicating values for H and D (O–H and O–D) ZPEs ( and , respectively). The position of Tyr32 is fixed as the proton H16 is scanned along .
Fig. 3 A–C shows snapshots from AIMD simulations, in which the nuclei are treated classically (Fig. 3A) or quantum mechanically using the path integral formalism (AI-PIMD) (Fig. 3 B and C), whereas Movies S1–S3 show the simulation trajectories. For the quantum simulations, the H16 and H32 protons are shown as their full ring polymers, which arise from the path integral quantum mechanics formalism. The spread of the ring polymer representing each proton is related to its de Broglie wavelength (quantum mechanical position uncertainty) (38, 39). The uncertainty principle dictates that localization of a quantum mechanical particle increases its quantum kinetic energy. The protons will thus attempt to delocalize (i.e., spread their ring polymers) to reduce this energetic penalty. The resulting proton positions in Fig. 3B arise from the interplay between the chemical environment, such as the covalent O–H bond, which acts to localize the proton and the quantum kinetic energy penalty that must be paid to confine a quantum particle. Inclusion of nuclear quantum effects, thus, allows the protons to delocalize between the hydroxyl oxygens to mitigate the quantum kinetic energy penalty (Fig. 3B), which is not observed classically (Fig. 3A). Confinement of D, which because of its larger mass has a smaller position uncertainty, leads to a much less severe quantum kinetic energy penalty and hence less delocalization (Fig. 3C).
Fig. 3.
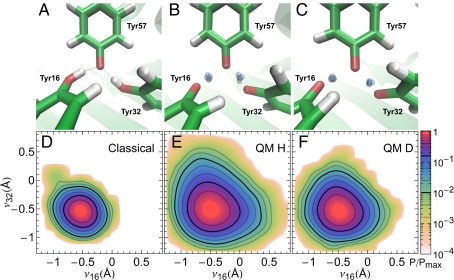
Delocalized protons in the active site of from AIMD and AI-PIMD simulations. Snapshots of (A–C) the active site of and (D–F) probability distribution along the proton-sharing coordinates and when the nuclei are treated classically (Classical) or quantum mechanically for H (QM H) and D (QM D). In A–C, green, red, and white represent C, O, and H atoms, respectively. The blue-gray spheres in the QM snapshots show uncertainty in the delocalized protons positions. For clarity, all other particles are represented by their centroids. In D–F, probabilities are shown on a log scale and normalized by their maximum values.
To characterize the degree of proton delocalization, we define a proton-sharing coordinate , where is the distance of proton HX from oxygen atom OX, and X = 16 or 32. Hence, corresponds to a proton that is equidistant between the oxygen atoms of TyrX and Tyr57, whereas a positive value indicates proton transfer to Tyr57 from TyrX. Fig. 3 D–F show the probability distribution along the proton-sharing coordinates and for classical and quantum nuclei for H and D, respectively. The free energies along and are provided in Fig. S3. In the classical AIMD simulation, H16 and H32 remain bound to their respective oxygens throughout the simulation ( and are negative), with Tyr57 ionized 99.96% of the time (Fig. 3D). However, on including nuclear quantum effects (AI-PIMD simulations), there is a dramatic increase in the range of values that and can explore (Fig. 3E). In particular, the probability that Tyr57 is protonated increases by about 150-fold for H after including quantum effects (Fig. 3E), with the proton hole equally shifted onto the adjacent Tyr16 or Tyr32 residues. Proton transfers between the residues are observed frequently (Movies S2 and S3), with site lifetimes on the order of 60 and 200 fs in the H and D simulations, respectively. Although path integral molecular dynamics simulations exactly include nuclear quantum effects for calculating static properties, they do not allow rigorous extraction of time-dependent properties; nevertheless, they offer a crude way to assess the timescale of the proton motion. The frequent transfers observed are also consistent with Fig. 3 E and F, which shows a monotonic decrease in the probability along both and [i.e., although the proton-transferred state is lower in probability, the proton-transfer process along each of the proton-sharing coordinates contains no free-energy barrier (Fig. S3) and thus, is kinetically fast].
As an experimental counterpart, we used chemical shifts of 13Cζ-Tyr–labeled as a measure of fractional ionization of each Tyr residue (SI Materials and Methods, section B and Fig. S4) (14). This analysis yielded values of 79% for the Tyr57 ionization for H and 86% for D (Table S3) compared with simulated values of 94.2% and 98.3% (±0.3%), respectively. This comparison represents good quantitative agreement, because the population difference amounts to a difference in the relative free energy between experiment and theory of 0.7 kcal/mol—an error that is within the expected accuracy of the electronic structure approach used. In addition, the change in the ionization of Tyr57 obtained experimentally on exchanging H for D (7%) is in good agreement with the value predicted from our simulations (4.1%). The underprediction of the isotope effect on fractional ionization from our simulations is in line with the slightly low value of the simulated excess isotope effect, which is consistent with recent observations that the B3LYP-D3 density functional slightly underestimates the degree of proton sharing and hence, isotope effects in hydrogen-bonded systems after including nuclear quantum effects (37).
The large degree of proton sharing with the deprotonated Tyr57 residue upon including nuclear quantum effects can be elucidated by considering the potential energy required, , to move a proton in the KSI tyrosine triad from its energetic minimum to a perfectly shared position between the two tyrosine groups . depends strongly on the positions of the residues comprising the triad and in particular, the separation between the proton donor and acceptor oxygen atoms. Fig. 4A shows computed as a function of the distance between O16 and O57, , for the tyrosine triad in the absence of the protein environment. Removing the protein environment allows us to examine how changes in the triad distances from their positions in the enzyme affect the proton delocalization behavior without introducing steric overlaps with other active-site residues (SI Materials and Methods, section D).
Fig. 4A shows that, for the range of oxygen distances observed in the tyrosine triad ( = 2.50–2.65 Å), for H16 is 3–6 kcal/mol. This energy is 6–12 times the thermal energy (T) available at 300 K, leading to a very low thermal probability of the proton-shared state (lower than ). However, upon including nuclear quantum effects, the system possesses ZPE, which in this system is ∼4 kcal/mol. The ZPE closely matches and thus floods the potential energy wells along the proton-sharing coordinate (Fig. 4B), allowing facile proton sharing (Fig. 3) (i.e., inducing a transition to a low-barrier hydrogen bond-type regime where the protons are quantum mechanically delocalized between the hydrogen-bonded heavy atoms) (18–20). This energy match leads to qualitatively different behavior of the protons in the active site of : from classical hydrogen bonding to quantum delocalization. The proton delocalization between the residues allows for ionization to be shared among three tyrosines to stabilize the deprotonation of Tyr57, leading to the large observed shift relative to the value in the classical limit (Fig. S2). This change in proton behavior gives rise to the large excess isotope effect, because an O–D stretch possesses a ZPE of ∼3 kcal/mol, which is no longer sufficient to fully flood the potential energy well in the proton-sharing coordinate ν. As the O–O separation is decreased below the values observed in the tyrosine triad of KSI, becomes negligible compared with the thermal energy (∼0.6 kcal/mol at 300 K). Hence, at very short distances (<2.3 Å), thermal fluctuations alone permit extensive proton sharing between the residues, and the ZPE plays a negligible role in determining the protons positions. Thus, one would expect a small isotope effect. However, at bond lengths in excess of 2.7 Å, becomes so large (>8 kcal/mol) (Fig. 4A) that the ZPE is not sufficient to flood the barriers, also resulting in a small expected isotope effect (40). The large excess isotope effect in , thus, arises from the close matching of the ZPE and the depth of the energetic well , which is highly sensitive to the O–O distance. Hence, although proton delocalization can occur classically at short O–O distances (<2.3 Å), nuclear quantum effects allow this to occur for a much wider range of O–O distances (up to ∼2.6 Å), making delocalization feasible without incurring the steep steric costs that would be associated with bringing oxygen atoms any closer. The distances in the active-site triad motif of KSI, thus, maximize quantum proton delocalization, which acts to stabilize the deprotonated residue.
Quantum Delocalization Stabilizes an Intermediate Analog by Sharing Ionization
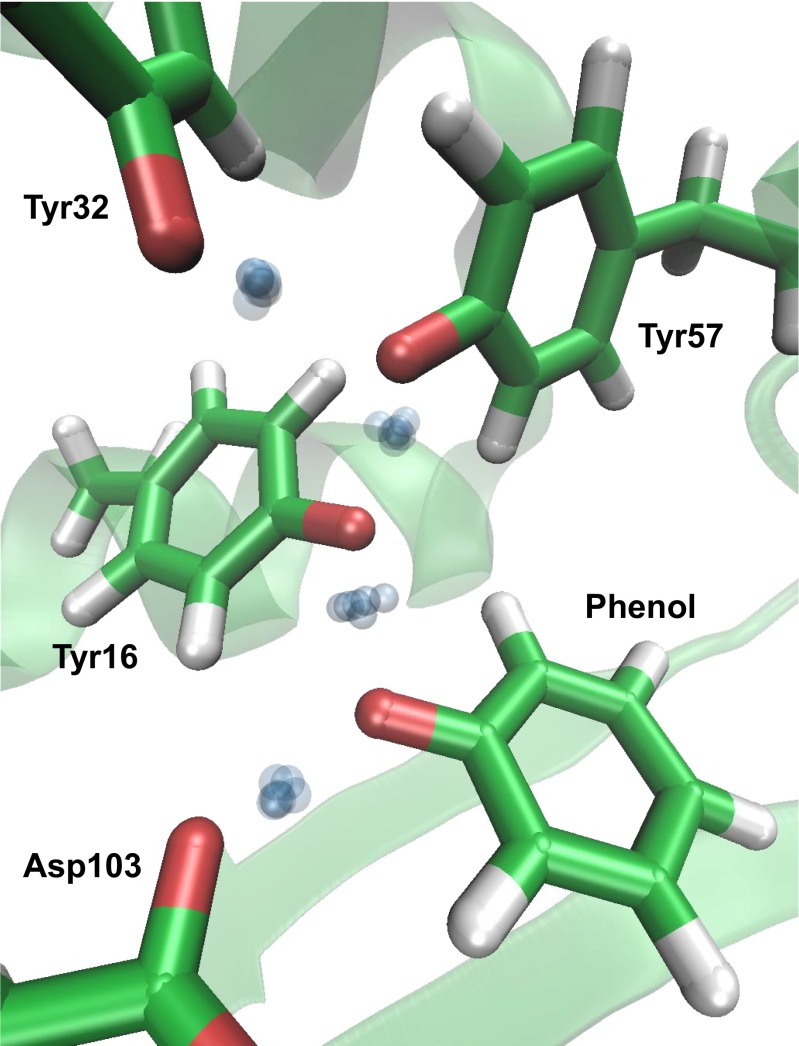
Finally, we considered the role of nuclear quantum effects when an intermediate analog participates in the active-site hydrogen-bond network. Recent experiments have investigated how the binding of intermediate analogs to affects the sharing of ionization along the extended hydrogen-bond network that is formed (Fig. 1B) (14). These experiments identified that ionization sharing is maximized when phenol, whose solution of 10 equals that of the actual intermediate of KSI (Fig. 1A), is bound (14, 41, 42). We thus performed AI-PIMD simulations of the phenol complex. The protons were observed to be delocalized across the network (Fig. 5), with partial ionizations of Tyr57, Tyr16, and phenol calculated to be 18.5%, 56.7%, and 22.5% (±0.7%), compared with estimates from previous experiments using 13C NMR of 40%, 40%, and 20% (14). Hence, simulation and experiment are in good agreement that the ionization is shared almost equally among the three residues [i.e., that there is almost no difference (<2T) in the free energy after shifting the ionization among any of the three groups]. Therefore, the ability of the protons to delocalize within the tyrosine triad, initially found in the enzyme in the absence of the intermediate analog and manifested as a strongly perturbed , extends on incorporation of the intermediate analog, which shifts the center of the ionization along the network from Tyr57 to Tyr16. In both cases, proton delocalization acts to share the ionization of a negatively charged group, which suggests that KSI could use quantum delocalization in its active-site hydrogen-bond network to distribute the ionization arising in its intermediate complex (Fig. 1A) to provide energetic stabilization.
Fig. 5.
Simulation snapshot of the active site with the bound intermediate analog (phenol) that gives rise to an extended delocalized proton network. Green, red, and white represent C, O, and H atoms, respectively. The blue spheres represent uncertainty in the delocalized proton position in the hydrogen-bond network. For clarity, all other particles are represented by their centroids.
Conclusion
In conclusion, exhibits a large equilibrium isotope effect in the acidity of its active-site tyrosine residues arising from a highly specialized triad motif consisting of several short O–O distances, whose positions that enhance quantum delocalization of protons within the active-site hydrogen-bond network. This delocalization manifests in a very large isotope effect and substantial acidity shift. Our simulations, which include electronic quantum effects and exactly treat the quantum nature of the nuclei, show qualitatively and quantitatively different proton behavior compared with conventional simulations in which the nuclei are treated classically, and provide good agreement with experiment. The ability to perform such simulations thus offers the opportunity to investigate in unprecedented detail the plethora of systems in which short-strong hydrogen bonds occur, where incorporating both nuclear and electronic quantum effects is crucial to understand their functions.
Materials and Methods
Expression and Purification of KSI.
WT and KSID40N from Pseudomonas putida were overexpressed in BL-21 A1 cells (Invitrogen), isolated by affinity chromatography using a custom-designed deoxycholate-bound column resin, and purified by gel filtration chromatography (GE Healthcare) as described previously (43). For 13C NMR experiments, 13Cζ-tyrosine was incorporated into KSI according to the methods described previously (14).
UV-Vis Titration Experiments.
A series of buffers was prepared with a pL between 4 and 10 by weighing portions of a weak acid and its sodium-conjugate base salt and adding the appropriate form of distilled deionized water [Millipore H2O and Spectra stable isotopes sterile-filtered D2O (>99% 2H)]. Buffers were prepared at 40 mM. Tyr57 is solvent-accessible, and therefore, the tyrosine residues in the active-site network are expected to be fully deuterated in D2O solution.
The following buffer systems were used for the following pL ranges: acetic acid/sodium acetate, 4–5.25; sodium monobasic phosphate/dibasic phosphate, 5.5–8.25; and sodium bicarbonate/sodium carbonate, 8.5–10. Buffers were stored at room temperature with caps firmly sealed.
After preparation of buffers, pL was recorded using an Orion2 Star glass electrode (Thermo) immediately after calibration with standard buffers at pH 4, pH 7, and pH 10. In H2O, the pH of the buffer was taken as the reading on the electrode. In D2O, the pD of the buffer was calculated by adding 0.41 to the operational pH* from the electrode reading (44). A series of samples for titration was prepared by combining 60 μL protein (100 μM stock in buffer-free L2O), buffer (150 μL 40 mM stock), and extra L2O. The final samples were 600 μL, 10 μM protein, and 10 mM buffer.
UV-Vis measurements were carried out on the samples on a Lambda 25 Spectrophotometer (Perkin-Elmer) that acquired data from 400 to 200 nm with a 1.0-nm data interval, a 960-nm/min scan rate, and a 1.00-nm slit width. For each measurement, a background was taken to the pure buffer of a given pL before acquiring on the protein-containing sample. Spectra were recorded in duplicate to control for random detector error.
The spectra were baselined by setting the absorption at 320 nm to zero, and the change in absorption at 300 nm was followed at varying pL values using a previously established method (15) to determine the fractional ionization of a tyrosine–tyrosinate pair. The error in A300 from comparing duplicate spectra after baselining was generally between 0% and 2%. For each pL, the average A300 was calculated and converted to an extinction coefficient . The titration experiment was repeated on two independently prepared buffer stocks to control for error in buffer preparation.
Simulations.
AI-PIMD and AIMD simulations were performed using a QM/MM approach of with Tyr57 protonated, with Tyr57 ionized, with the intermediate analog bound, and tyrosine in aqueous solution. The simulations were carried out in the NVT ensemble at 300 K with a time step of 0.5 fs. The path integral-generalized Langevin equation approach was used, which allowed results within the statistical error bars to be obtained using only six path integral beads to represent each particle (34). The electronic structure in the QM region (Fig. S5) was evaluated using the B3LYP functional (28) with dispersion corrections (29). The 6–31G* basis set was used, because we found it to produce proton transfer potential energy profiles with a mean absolute error of less than 0.4 kcal/mol compared to using larger basis sets for this system (Fig. S6). Energies and forces in the QM region and the electrostatic interactions between the QM and MM regions were obtained using an MPI interface to the GPU-accelerated TeraChem package (35, 36). Atoms in the MM region were described using the AMBER03 force field (45) and the TIP3P water model (46). The simulations were performed using periodic boundary conditions with Ewald summation to treat long-range electrostatic interactions. The energies and forces within the MM region and the Lennard–Jones interactions between the QM and MM regions were calculated by MPI calls to the LAMMPS molecular dynamics package (47). The QM region of contained the p-methylene phenol side chains of residues Tyr16, Tyr32, and Tyr57 (Fig. S5A). For with the intermediate analog, residue Asp103 and the bound intermediate analog were also included in the QM region (Fig. S5B). The QM region of tyrosine in solution contained the side chain of the tyrosine residue and the 41 water molecules within 6.5 Å of the side-chain O–H group. All bonds across the QM/MM interface were capped with hydrogen link atoms in the QM region (26). These capping atoms were constrained to be along the bisected bonds and do not interact with the MM region.
The initial configuration of was obtained from a crystal structure (16) (Protein Data Bank ID code 1OGX). For with the intermediate analog, a crystal structure (14) (Protein Data Bank ID code 3VGN) was used with the ligand changed to phenol. The crystal structures were solvated in TIP3P water, and the energy was minimized before performing AI-PIMD simulations. The initial configuration for tyrosine in aqueous solution was obtained by solvating the amino acid in TIP3P (46) water using the AMBER03 force field (45) and equilibrating for 5 ns in the NPT ensemble at a temperature of 300 K and pressure of 1 bar. Each system was then equilibrated for 10 ps followed by production runs of 30 ps.
To calculate the excess isotope effect, (SI Materials and Methods, section C), we used the thermodynamic free-energy perturbation path integral estimator (22). Combined with an appropriate choice of the integration variable to smooth the free-energy derivatives (22), this approach allowed us to evaluate the isotope effects in the liquid phase using only a single AI-PIMD trajectory. Simulations performed with D substitution showed no change within the statistical error bars reported.
Supplementary Material
Acknowledgments
The authors thank Christine Isborn, Nathan Luehr, and Todd Martinez for their assistance in interfacing our program to the TeraChem code. L.W. acknowledges a postdoctoral fellowship from the Stanford Center for Molecular Analysis and Design. S.D.F. thanks the National Science Foundation (NSF) Predoctoral Fellowship Program and the Stanford Bio-X Interdisciplinary Graduate Fellowship for support. T.E.M. acknowledges support from a Terman Fellowship, an Alfred P. Sloan Research Fellowship, a Hellman Faculty Scholar Fund Fellowship, and Stanford University startup funds. This work was supported, in part, by National Institutes of Health Grant GM27738 (to S.G.B.). This work used the Extreme Science and Engineering Discovery Environment, which is supported by NSF Grant ACI-1053575 (Project TG-CHE140013).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417923111/-/DCSupplemental.
References
- 1.Lambert N, et al. Quantum biology. Nat Phys. 2013;9(1):10–18. [Google Scholar]
- 2.Sutcliffe MJ, Scrutton NS. A new conceptual framework for enzyme catalysis. Hydrogen tunnelling coupled to enzyme dynamics in flavoprotein and quinoprotein enzymes. Eur J Biochem. 2002;269(13):3096–3102. doi: 10.1046/j.1432-1033.2002.03020.x. [DOI] [PubMed] [Google Scholar]
- 3.Klinman JP, Kohen A. Hydrogen tunneling links protein dynamics to enzyme catalysis. Annu Rev Biochem. 2013;82:471–496. doi: 10.1146/annurev-biochem-051710-133623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez A, Tuckerman ME, Hjalmarson HP, von Lilienfeld OA. Enol tautomers of Watson-Crick base pair models are metastable because of nuclear quantum effects. J Am Chem Soc. 2010;132(33):11510–11515. doi: 10.1021/ja102004b. [DOI] [PubMed] [Google Scholar]
- 5.Feierberg I, Åqvist J. The catalytic power of ketosteroid isomerase investigated by computer simulation. Biochemistry. 2002;41(52):15728–15735. doi: 10.1021/bi026873i. [DOI] [PubMed] [Google Scholar]
- 6.Pollack RM. Enzymatic mechanisms for catalysis of enolization: Ketosteroid isomerase. Bioorg Chem. 2004;32(5):341–353. doi: 10.1016/j.bioorg.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Warshel A, Sharma PK, Chu ZT, Åqvist J. Electrostatic contributions to binding of transition state analogues can be very different from the corresponding contributions to catalysis: Phenolates binding to the oxyanion hole of ketosteroid isomerase. Biochemistry. 2007;46(6):1466–1476. doi: 10.1021/bi061752u. [DOI] [PubMed] [Google Scholar]
- 8.Chakravorty DK, Hammes-Schiffer S. Impact of mutation on proton transfer reactions in ketosteroid isomerase: Insights from molecular dynamics simulations. J Am Chem Soc. 2010;132(21):7549–7555. doi: 10.1021/ja102714u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanoian P, Sigala PA, Herschlag D, Hammes-Schiffer S. Hydrogen bonding in the active site of ketosteroid isomerase: Electronic inductive effects and hydrogen bond coupling. Biochemistry. 2010;49(48):10339–10348. doi: 10.1021/bi101428e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herschlag D, Natarajan A. Fundamental challenges in mechanistic enzymology: Progress toward understanding the rate enhancements of enzymes. Biochemistry. 2013;52(12):2050–2067. doi: 10.1021/bi4000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amyes TL, Richard JP. Specificity in transition state binding: The Pauling model revisited. Biochemistry. 2013;52(12):2021–2035. doi: 10.1021/bi301491r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fafarman AT, et al. Quantitative, directional measurement of electric field heterogeneity in the active site of ketosteroid isomerase. Proc Natl Acad Sci USA. 2012;109(6):E299–E308. doi: 10.1073/pnas.1111566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried SD, Boxer SG. Thermodynamic framework for identifying free energy inventories of enzyme catalytic cycles. Proc Natl Acad Sci USA. 2013;110(30):12271–12276. doi: 10.1073/pnas.1310964110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigala PA, et al. Quantitative dissection of hydrogen bond-mediated proton transfer in the ketosteroid isomerase active site. Proc Natl Acad Sci USA. 2013;110(28):E2552–E2561. doi: 10.1073/pnas.1302191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwans JP, Sunden F, Gonzalez A, Tsai Y, Herschlag D. Uncovering the determinants of a highly perturbed tyrosine pKa in the active site of ketosteroid isomerase. Biochemistry. 2013;52(44):7840–7855. doi: 10.1021/bi401083b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha NC, Kim MS, Lee W, Choi KY, Oh BH. Detection of large pKa perturbations of an inhibitor and a catalytic group at an enzyme active site, a mechanistic basis for catalytic power of many enzymes. J Biol Chem. 2000;275(52):41100–41106. doi: 10.1074/jbc.M007561200. [DOI] [PubMed] [Google Scholar]
- 17.Sigala PA, et al. Testing geometrical discrimination within an enzyme active site: Constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole. J Am Chem Soc. 2008;130(41):13696–13708. doi: 10.1021/ja803928m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleland WW, Kreevoy MM. Low-barrier hydrogen bonds and enzymic catalysis. Science. 1994;264(5167):1887–1890. doi: 10.1126/science.8009219. [DOI] [PubMed] [Google Scholar]
- 19.Frey PA, Whitt SA, Tobin JB. A low-barrier hydrogen bond in the catalytic triad of serine proteases. Science. 1994;264(5167):1927–1930. doi: 10.1126/science.7661899. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Abeygunawardana C, Talalay P, Mildvan AS. NMR evidence for the participation of a low-barrier hydrogen bond in the mechanism of delta 5-3-ketosteroid isomerase. Proc Natl Acad Sci USA. 1996;93(16):8220–8224. doi: 10.1073/pnas.93.16.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfsberg M, Van Hook W, Paneth P, Rebelo LPN. Isotope Effects: In the Chemical, Geological, and Bio Sciences. Springer; Dordrecht, The Netherlands: 2009. [Google Scholar]
- 22.Ceriotti M, Markland TE. Efficient methods and practical guidelines for simulating isotope effects. J Chem Phys. 2013;138(1):014112. doi: 10.1063/1.4772676. [DOI] [PubMed] [Google Scholar]
- 23.Marsalek O, et al. Efficient calculation of free energy differences associated with isotopic substitution using path-integral molecular dynamics. J Chem Theory Comput. 2014;10(4):1440–1453. doi: 10.1021/ct400911m. [DOI] [PubMed] [Google Scholar]
- 24.Warshel A, Levitt M. Theoretical studies of enzymic reactions: Dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- 25.Field MJ, Bash PA, Karplus M. A combined quantum mechanical and molecular mechanical potential for molecular dynamics simulations. J Comput Chem. 1990;11(6):700–733. [Google Scholar]
- 26.Eurenius KP, Chatfield DC, Brooks BR, Hodoscek M. Enzyme mechanisms with hybrid quantum and molecular mechanical potentials. I. Theoretical considerations. Int J Quantum Chem. 1996;60(6):1189–1200. [Google Scholar]
- 27.Monard G, Merz KM. Combined quantum mechanical/molecular mechanical methodologies applied to biomolecular systems. Acc Chem Res. 1999;32(10):904–911. [Google Scholar]
- 28.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98(7):5648–5652. [Google Scholar]
- 29.Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys. 2010;132(15):154104. doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- 30.Feynman RP, Hibbs AR. Quantum Mechanics and Path Integrals. McGraw-Hill; New York: 1964. [Google Scholar]
- 31.Chandler D, Wolynes PG. Exploiting the isomorphism between quantum theory and classical statistical mechanics of polyatomic fluids. J Chem Phys. 1981;74(7):4078–4095. [Google Scholar]
- 32.Berne BJ, Thirumalai D. On the simulation of quantum systems: Path integral methods. Annu Rev Phys Chem. 1986;37:401–424. [Google Scholar]
- 33.Marx D, Parrinello M. Ab initio path integral molecular dynamics: Basic ideas. J Chem Phys. 1996;104(11):4077–4082. [Google Scholar]
- 34.Ceriotti M, Manolopoulos DE. Efficient first-principles calculation of the quantum kinetic energy and momentum distribution of nuclei. Phys Rev Lett. 2012;109(10):100604. doi: 10.1103/PhysRevLett.109.100604. [DOI] [PubMed] [Google Scholar]
- 35.Ufimtsev IS, Martinez TJ. Quantum chemistry on graphical processing units. 3. Analytical energy gradients, geometry optimization, and first principles molecular dynamics. J Chem Theory Comput. 2009;5(10):2619–2628. doi: 10.1021/ct9003004. [DOI] [PubMed] [Google Scholar]
- 36.Isborn CM, Götz AW, Clark MA, Walker RC, Martínez TJ. Electronic absorption spectra from mm and ab initio QM/MM molecular dynamics: Environmental effects on the absorption spectrum of photoactive yellow protein. J Chem Theory Comput. 2012;8(12):5092–5106. doi: 10.1021/ct3006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Ceriotti M, Markland TE. Quantum fluctuations and isotope effects in ab initio descriptions of water. J Chem Phys. 2014;141(10):104502. doi: 10.1063/1.4894287. [DOI] [PubMed] [Google Scholar]
- 38.Parrinello M, Rahman A. Study of an F center in molten KCl. J Chem Phys. 1984;80(2):860–867. [Google Scholar]
- 39.Markland TE, Berne BJ. Unraveling quantum mechanical effects in water using isotopic fractionation. Proc Natl Acad Sci USA. 2012;109(21):7988–7991. doi: 10.1073/pnas.1203365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKenzie RH, Bekker C, Athokpam B, Ramesh SG. Effect of quantum nuclear motion on hydrogen bonding. J Chem Phys. 2014;140(17):174508. doi: 10.1063/1.4873352. [DOI] [PubMed] [Google Scholar]
- 41.Fried SD, Boxer SG. Evaluation of the energetics of the concerted acid-base mechanism in enzymatic catalysis: The case of ketosteroid isomerase. J Phys Chem B. 2012;116(1):690–697. doi: 10.1021/jp210544w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Childs W, Boxer SG. Proton affinity of the oxyanion hole in the active site of ketosteroid isomerase. Biochemistry. 2010;49(12):2725–2731. doi: 10.1021/bi100074s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraut DA, et al. Testing electrostatic complementarity in enzyme catalysis: Hydrogen bonding in the ketosteroid isomerase oxyanion hole. PLoS Biol. 2006;4(4):e99. doi: 10.1371/journal.pbio.0040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covington AK, Paabo M, Robinson RA, Bates RG. Use of the glass electrode in deuterium oxide and the relation between the standardized pD (paD) scale and the operational pH in heavy water. Anal Chem. 1968;40(4):700–706. [Google Scholar]
- 45.Duan Y, et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003;24(16):1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 46.Jorgensen WWL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79(2):926–935. [Google Scholar]
- 47.Plimpton S. Fast parallel algorithms for short-range molecular dynamics. J Comput Phys. 1995;117(1):1–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.