Significance
As well as having potent cytotoxic activity, natural killer (NK) cells have a regulatory role and interactions between NK cells and dendritic cells (DCs) aid DC maturation and adaptive immunity. However, the mechanisms underpinning NK–DC cross-talk are poorly defined. We show that tumor cells induce rapid production of the cytokine TNF superfamily member 14 (TNFSF14) in human NK cells and that these NK cells induce DC maturation in a TNFSF14-dependent manner. The synergistic activity of NK cell activation receptors in licensed NK cells couples the release of cytotoxic granules to TNFSF14 production. Thus, NK cell activation by tumor cells is linked to the initiation of adaptive immunity via TNFSF14-mediated NK–DC cross-talk.
Keywords: natural killer cells, NK–DC interactions, TNFSF14, NK-cell licensing, tumor immunity
Abstract
Interactions between natural killer (NK) cells and dendritic cells (DCs) aid DC maturation and promote T-cell responses. Here, we have analyzed the response of human NK cells to tumor cells, and we identify a pathway by which NK–DC interactions occur. Gene expression profiling of tumor-responsive NK cells identified the very rapid induction of TNF superfamily member 14 [TNFSF14; also known as homologous to lymphotoxins, exhibits inducible expression, and competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes (LIGHT)], a cytokine implicated in the enhancement of antitumor responses. TNFSF14 protein expression was induced by three primary mechanisms of NK cell activation, namely, via the engagement of CD16, by the synergistic activity of multiple target cell-sensing NK-cell activation receptors, and by the cytokines IL-2 and IL-15. For antitumor responses, TNFSF14 was preferentially produced by the licensed NK-cell population, defined by the expression of inhibitory receptors specific for self-MHC class I molecules. In contrast, IL-2 and IL-15 treatment induced TNFSF14 production by both licensed and unlicensed NK cells, reflecting the ability of proinflammatory conditions to override the licensing mechanism. Importantly, both tumor- and cytokine-activated NK cells induced DC maturation in a TNFSF14-dependent manner. The coupling of TNFSF14 production to tumor-sensing NK-cell activation receptors links the tumor immune surveillance function of NK cells to DC maturation and adaptive immunity. Furthermore, regulation by NK cell licensing helps to safeguard against TNFSF14 production in response to healthy tissues.
Natural killer (NK) cells play an important role in protecting the host against viral infection and cancer. As well as having potent cytotoxic activity, NK cells are endowed with immunoregulatory activity (1, 2). For example, NK cell activation induces the production of chemokines, such as macrophage inflammatory protein-1α (MIP-1α) and IL-8, and proinflammatory cytokines, such as IFN-γ, GM-CSF, and TNF-α. These molecules regulate the recruitment and activity of numerous immune cell types (1, 2). Importantly, NK cells can promote development of T-cell responses via NK–dendritic cell (DC) interactions that favor both DC maturation and NK-cell activation (3–5), with NK cell-derived IFN-γ skewing T-cell differentiation toward the Th1 phenotype (6, 7).
Cytotoxic activity and cytokine production are coupled to signaling pathways downstream of a repertoire of activating and inhibitory receptors; signals from activating receptors (including NKG2D, DNAM-1, and 2B4, as well as the natural cytotoxicity receptors NKp30, NKp44, and NKp46) compete with signals from inhibitory receptors such as the killer cell immunoglobulin-like receptors (KIRs) and CD94/NKG2A heterodimers to regulate activation. In addition, NK cells express CD16, the low-affinity receptor for IgG, conferring antibody-dependent cellular cytotoxicity (8–10). Activation thus coordinates the killing of target cells, the induction of inflammation, and the promotion of adaptive immunity. This potent cytotoxicity and proinflammatory activity must be strictly controlled to minimize damage to healthy tissue. Functional competency of unstimulated NK cells is achieved via a process termed “licensing” or “education” (11–14). Licensing ensures that only those NK cells expressing inhibitory receptors for self-MHC class I can respond to target cells and NK cells that lack inhibitory receptors for self-MHC class I molecules are rendered hyporesponsive, preventing them from attacking healthy cells expressing normal levels of MHC class I molecules.
We have analyzed the consequences of human NK cell activation by tumor cells. Our results reveal induction of the TNF superfamily member 14 (TNFSF14), also known as homologous to lymphotoxins, exhibits inducible expression, and competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes (LIGHT) (15). We show that activated NK cells produce TNFSF14 in response to different stimuli, that tumor cells induce TNFSF14 production by licensed NK cells, and that TNFSF14-producing NK cells aid DC maturation during NK–DC cross-talk.
Results
Gene Expression Profiling of Tumor-Stimulated NK Cells Identifies Induction of a Putative Immunomodulatory Pathway.
Gene expression profiling has provided great insight into NK-cell function (16–18). NK cells respond very rapidly to target cells, and we reasoned that analyzing the changes in gene expression that occur during the early stages of activation by target cells might reveal new features of these responses.
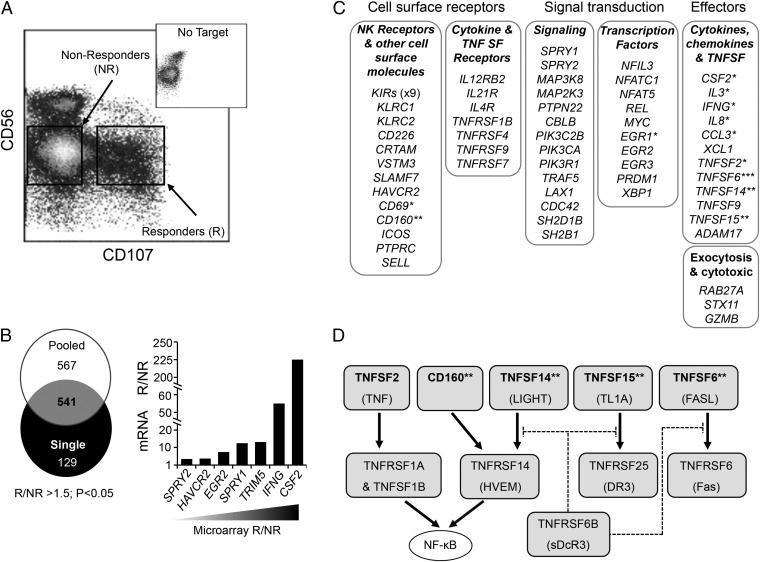
Activation of NK cells by tumor cells results in the rapid exocytosis of cytotoxic granules and the expression and release of cytokines. Granule exocytosis exposes the granule membrane protein CD107 on the NK-cell surface, allowing NK cells that have responded to a tumor target (R-NK) to be separated from nonresponding (NR-NK) cells using fluorescence-activated cell sorting (FACS). We purified human peripheral blood NK cells, cocultured them (in the absence of exogenous cytokines) for 4 h with K562 tumor cells, and sorted the R-NK and NR-NK fractions (Fig. 1A). We performed gene expression profiling on R-NK and NR-NK fractions from two sample sets, one derived from a single healthy donor and the other using fractions pooled from three additional healthy donors. Not surprisingly, given donor variability, more genes were differentially regulated in the pooled sample compared with the single donor (1,108 genes and 670 genes, respectively), but there were 541 genes that were differentially expressed by a factor of 1.5 or more (P < 0.05) in both sample sets (Fig. 1 B and C and Dataset S1). We focused our analysis on genes whose expression was up-regulated in the R-NK cells; induction of genes encoding TNF (TNFSF2), GM-CSF (CSF2), IFN-γ (IFNG), and CD69 was detected in R-NK cells as expected (Fig. 1C). Quantitative RT-PCR analysis of mRNA from R-NK and NR-NK cells confirmed induction of a selection of the genes identified by the array analysis. For example, CSF2 and IFNG were induced by some 200-fold and 50-fold, respectively, in the R-NK cells (Fig. 1B). In addition, several KIR genes and KLRC1 (encoding NKG2A) demonstrated greater expression in the R-NK fraction, consistent with the higher level of MHC class I inhibitory receptor expression in functionally responsive (educated or licensed) human NK cells (12, 19). Manual inspection of genes up-regulated in the R-NK fraction revealed many molecules associated with an immune activation phenotype, including cell surface receptors, signaling components, and transcription factors, as well as genes associated with NK-cell effector functions (Fig. 1C and Table S1). As well as TNF itself, the R-NK fraction demonstrated increased expression of several TNFSF members and TNF receptor superfamily (TNFRSF) members. In particular, we identified four molecules from the same immunoregulatory network: the Ig superfamily molecule CD160 and the TNF superfamily molecules TNFSF14 (LIGHT), TNFSF15 (also known as TNF-like ligand 1A), and TNFSF6 (Fas ligand). These genes encode cell surface molecules involved in a complex regulatory network involving both cis and trans receptor–ligand interactions that regulate immune activation events in other cell types (20, 21) (Fig. 1D). Both CD160 and TNFSF14 are ligands of TNFRSF molecule TNFRSF14, also known as HVEM (15, 22). Like other TNFRSFs, HVEM signals via TNF receptor-associated factors to induce NF-κB activity (23, 24). Furthermore, TNFSF14, TNFSF15, and TNFSF6 activity are all antagonized by TNFRSF6B (decoy receptor 3) (25). The potential that tumor cells might induce NK-cell expression of molecules within this network prompted us to explore the regulation and consequences of their expression in more detail.
Fig. 1.
Gene expression profiling of tumor-stimulated human NK cells. (A) Primary human NK cells (isolated from healthy donors) were cocultured for 4 h with K562 tumor cells, and the R-NK and NR-NK cell populations were identified by staining for cell surface expression of CD56 (as a marker of NK cells) and CD107 (as a marker of NK cell degranulation). The R-NK and NR-NK populations were purified by FACS and used as a source of mRNA for cDNA synthesis and gene expression profiling using Illumina arrays. (Inset) CD107 display (x axis) vs. CD56 expression (y axis) on NK cells in the absence of tumor targets. (B) Venn diagram of the number of genes exhibiting a significant difference in expression between the R-NK and NR-NK populations (greater than 1.5-fold change; P < 0.05) of samples from a single donor and three pooled donors. A list of the 541 genes differentially expressed in both the single donor and pooled donor datasets is provided in Dataset S1. The array data are available from the Gene Expression Omnibus (ncbi.nlm.nih.gov/geo), under accession number GSE55977. The graph shows validation (using quantitative RT-PCR) of a selection of genes exhibiting differential expression in the array analysis (in order of increasing expression in the R-NK population, as determined by the array analysis). (C) Summary of differential expression between the R-NK and NR-NK samples. Selected genes exhibiting >1.5 fold upregulation (P < 0.05) in the R-NK cell fraction are shown according to the broad function of their encoded products. Functional details along with the gene identification number and supporting references are provided in Table S1. Genes with a defined AU rich element (ARE) in the 3' untranslated region (52, 53, 65, 66) are indicated (*), as are genes encoding molecules in the HVEM regulatory axis (**); TNFSF6 (FASL) has an ARE (67) and it is linked to the HVEM axis via its binding to DcR3(***). (D) The HVEM regulatory axis. Ligands (Top) include TNF superfamily (TNFSF) molecules (TNFSF2, TNFSF14, TNFSF15 and TNFSF6) as well as the immunoglobulin superfamily molecule CD160. Alternative names are also shown. The receptor molecules (Middle) all belong to the TNF receptor superfamily (TNFRSF). Soluble decoy receptor 3 (sDcR3; also known as TNFRSF6B) interacts with TNFSF14, TNFSF15 and TNFSF6 as shown, antagonizing their action. Both TNF (TNFSF2) and TNFSF14 (LIGHT) activate the transcription factor NF-κB (circled). This panel is based on information presented in published reviews (20, 21).
NK Cells Rapidly Express Cell Surface TNFSF14 in Response to Target Cell Stimulation.
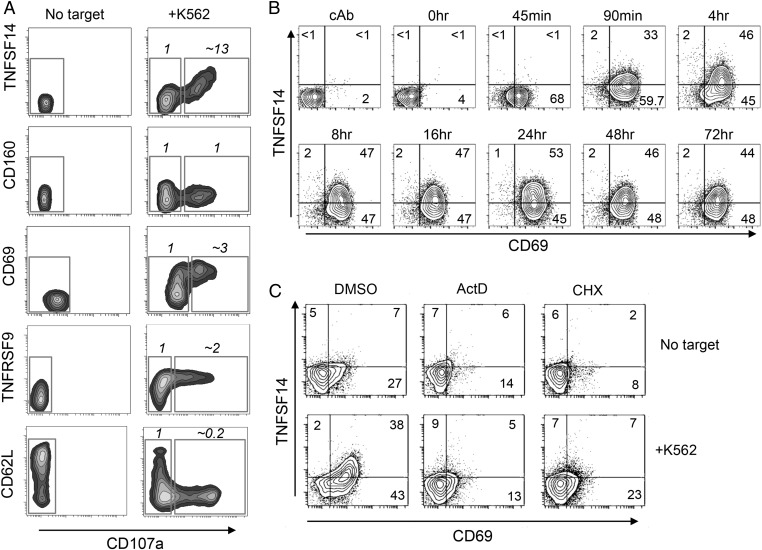
We analyzed whether tumor-mediated induction of gene expression was mirrored by expression of the corresponding proteins. The K562 tumor cell line induced the expression of CD69, TNFRSF9 (CD137), and TNFSF14 protein at the NK-cell surface (Fig. 2A). TNFSF14 was induced several fold higher on the R-NK (CD107+) cells compared with their nonresponding counterparts (CD107neg, NR-NK cells; Fig. 2A), in agreement with the microarray data. In contrast, little induction of CD160 protein was observed. Increased SELL gene expression (encoding L-selectin, CD62L) was detected in the R-NK population (Fig. 1). However, CD62L was rapidly and completely removed from the cell surface of the R-NK cells (Fig. 2A), consistent with previous observations demonstrating protease-mediated CD62L shedding upon NK-cell activation (26). The K562 cell line was not unique in its ability to induce TNFSF14 expression in NK cells, and several other human cell lines representing both hematopoietic and solid tumors preferentially induced the expression of TNFSF14 on tumor-responding (CD107+) NK cells (Fig. S1).
Fig. 2.
Tumor cell stimulation of NK cells induces production of TNFSF14. (A) Expression of cell surface molecules detected by gene expression profiling vs. CD107 display on unstimulated NK cells (No target) and NK cells stimulated with tumor cells (+K562). For the latter, the cell surface expression of molecules analyzed on the y axis is expressed as R/NR, the ratio of expression in the tumor-responding (CD107+) vs. tumor-nonresponding (CD107neg) populations (based on mean fluorescence intensity on the y axis; the corresponding gates are shown). These data are representative of three separate experiments performed. (B) Sustained expression of TNFSF14 (and CD69) in the continued presence of tumor cells. NK cells and K562 cells were cocultured for the times indicated, and cell surface TNFSF14 and CD69 were analyzed by flow cytometry. The values indicate the percentage of cells in each quadrant. cAb is an isotype control antibody for the anti-CD69 and anti-TNFSF14 staining. This experiment is representative of three separate experiments performed. (C) Induction of TNFSF14 expression requires de novo gene expression. NK cells alone (no target) or NK cells cocultured with K562 cells (+K562) were cultured for 5 h in the presence of 5 μM actinomycin D (ActD) to inhibit transcription or 50 μM cycloheximide (CHX) to inhibit translation (or with DMSO, the solvent for these compounds), and the cell surface expression of TNFSF14 and CD69 was analyzed by flow cytometry. The values indicate the percentage of cells in each quadrant. These data are representative of two separate experiments performed.
Analysis of the kinetics of TNFSF14 protein expression revealed that like CD69, TNFSF14 was detectable at the cell surface within 1.5 h of tumor cell contact. Furthermore, both CD69 and TNFSF14 remained at the cell surface in response to continual stimulation (Fig. 2B). Although we detected TNFSF14 induction using gene expression profiling, we considered the possibility that the very rapid induction of TNFSF14 at the cell surface might result from the mobilization of an intracellular pool of preexisting protein. However, stimulation of NK cells with K562 in the presence of actinomycin D or cycloheximide revealed that the induction of TNFSF14 required de novo gene expression and protein synthesis (Fig. 2C).
TNFSF14 Expression Is Induced by CD16 and by Synergism of Multiple NK-Cell Activation Receptors.
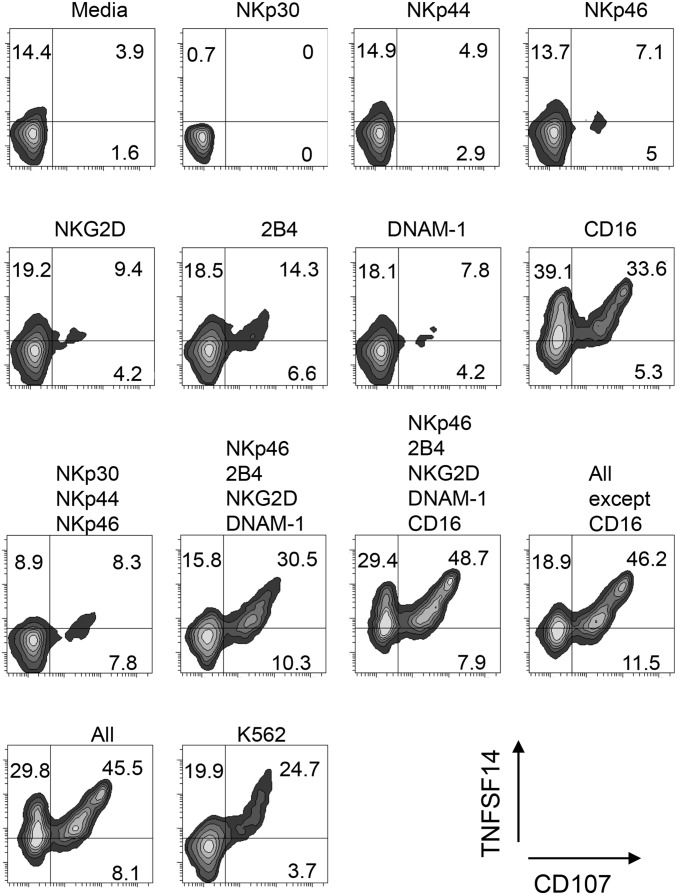
The rapid induction of TNFSF14 expression in NK cells in response to tumor cell lines suggested that the ligation of tumor-sensing NK-cell activation receptors was coupled to TNFSF14 production. We analyzed whether cross-linking of individual NK-cell activation receptors, or combinations thereof, led to TNFSF14 expression. We loaded mouse P815 cells (bearing Fc receptors) with receptor-specific antibodies, cocultured these antibody-displaying cells with human NK cells for 5 h, and then analyzed NK-cell granule exocytosis and TNFSF14 expression (Fig. 3). A low level of induction of degranulation and TNFSF14 expression was observed via cross-linking of 2B4 and NKG2D. However, the level of degranulation and TNFSF14 expression was greatly increased as combinations of activation receptors were ligated. The exception was CD16, where ligation was sufficient to induce TNFSF14 expression on the majority of NK cells. These results indicate that TNFSF14 induction was coupled to the principal activation pathways of NK cells, allowing them to produce this immunomodulatory cytokine when encountering tumor cells or infected cells, either by natural killing mechanisms or via the engagement of CD16.
Fig. 3.
Induction of TNFSF14 by NK-cell activation receptor cross-linking. The cell surface expression of TNFSF14 and CD107 was analyzed on NK cells after coculture with P815 cells displaying antibodies against NK-cell activation receptors. P815 cells were loaded with antibodies against a single receptor or with combinations of receptors as indicated (or without antibody, labeled “Media”). K562 tumor cells were used in place of antibody-loaded P815 cells as a positive control for TNFSF14 expression and degranulation. The values indicate the percentage of cells in each quadrant. This experiment is representative of four separate experiments performed.
Cytokine-Mediated Induction of TNFSF14 and Its Immunomodulatory Activity.
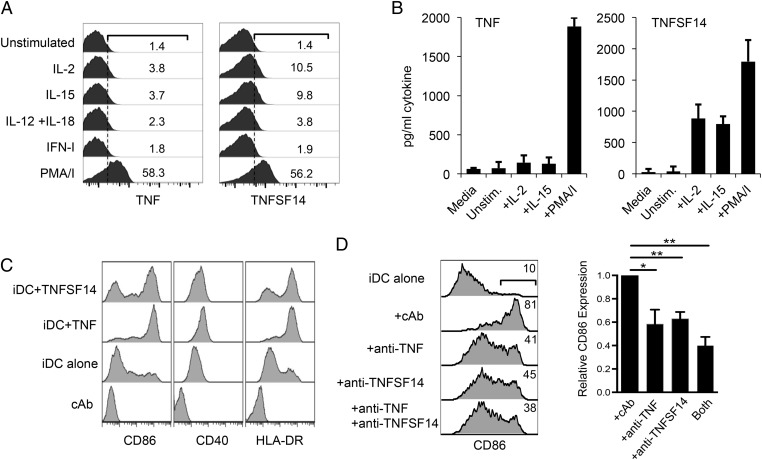
Studies on the kinetics of the immune response to infection have shown that NK-cell activation is an early event but is preceded by the synthesis of type I IFN (IFN-I) and monocyte-derived cytokines, such as IL-12 and IL-18 (27). Indeed, these cytokines activate NK cells by enhancing cytotoxic activity and IFN-γ production (28). However, neither IFN-I nor a combination of IL-12 and IL-18 was capable of inducing cell surface TNFSF14 expression (Fig. 4A). In contrast, IL-15, a cytokine produced during the early stages of innate immunity [e.g., following IFN-I stimulation of DCs (29)] did stimulate TNFSF14 expression, as did IL-2 treatment, consistent with the use of common signaling chains by the IL-2 and IL-15 receptors (Fig. 4A). Both TNF and TNFSF14 can be released from the cell surface via proteolytic cleavage (30, 31), and stimulation of NK cells with a combination of phorbol myristate acetate (PMA) and ionomycin induced shedding of both cytokines, whereas stimulation with IL-2 or IL-15 induced TNFSF14 release but little TNF (Fig. 4B).
Fig. 4.
Cytokine induction of immunomodulatory TNFSF14. (A) Expression of TNF or TNFSF14 in response to cytokine treatment or a combination of PMA and ionomycin (PMA/I), as indicated. NK cells (2.5 × 105) were cultured without stimulation (unstimulated) or in the presence of 50 U/mL IFN-I, 20 ng/mL IL-12 and IL-18, 300 U/mL IL-2, 30 ng/mL IL-15, or 50 ng/mL PMA plus 500 ng/mL ionomycin (PMA/I) for 8 h. Cells were stained for cell surface TNFSF14 expression or processed for intracellular staining for TNF. The values indicate the percentage of expressing cells. These data are representative of three donors for TNF and six donors for TNFSF14. (B) Release of soluble TNF or TNFSF14 by IL-2, IL-15, or PMA/I-treated NK cells. NK cells (2.5 × 105) were stimulated with the treatments shown in A for 24 h, and the supernatants were analyzed for TNF or TNFSF14 by ELISA (performed using at least three different donors). Media is the culture media without any added cells as a background control. Unstim., unstimulated. (C) Maturation of DCs in response to recombinant TNFSF14. The iDCs (differentiated from CD14+ monocytes) were left untreated or treated with recombinant TNF or TNFSF14 (100 ng/mL each) for 24 h and analyzed for cell surface markers of DC maturation, CD86, CD40, and HLA-DR using flow cytometry. (D, Left) Total of 1 × 105 IL-2–stimulated NK cells were applied to 3 × 105 iDCs for 15 h in the presence of antibodies against TNFSF14, TNF, or both cytokines (or cAb). The induction of CD86 on the iDCs was analyzed by flow cytometry, and the percentage of cells expressing CD86 is shown for each treatment. (D, Right) Summary of the data collected from five donors. Expression of CD86 on the DCs cocultured with IL-2–stimulated NK cells in the presence of cAb was assigned an expression level of 1. The reduction in expression of CD86 in the presence of the different anticytokine antibodies is indicated (*P < 0.05, **P < 0.005; paired Student’s t test).
Coculture of TNFSF14-transfected cells with immature DCs (iDCs) promotes DC maturation in a TNFSF14-dependent manner (32). The expression of TNFSF14 by NK cells and the importance of NK–DC interactions in the shaping of adaptive immunity (5, 33) suggested that NK cell-derived TNFSF14 might participate in this cross-talk. Stimulation of iDCs (generated in vitro from CD14+ monocytes) with purified TNF or TNFSF14 resulted in the increased expression of CD40, CD86, and HLA-DR (Fig. 4C), consistent with proinflammatory cytokine-mediated differentiation of iDCs to a more mature, antigen-presenting phenotype (32, 34). Coculture of NK cells and DCs induces CD86 expression on the DCs (3, 4). Coculture of IL-2–stimulated NK cells with iDCs induced the expression of CD86 on the iDCs, and the use of blocking antibodies demonstrated that CD86 induction was dependent upon both TNFSF14 and TNF (Fig. 4D).
Proinflammatory cytokines exert their effects on multiple cell types, and like TNF, TNFSF14 has previously been shown to induce the expression of intercellular adhesion molecule-1 (ICAM-1) on endothelial cells (35). Accordingly, IL-2–stimulated NK cells induced ICAM-1 expression on human umbilical vein-derived endothelial cells in a TNFSF14-dependent manner (Fig. S2).
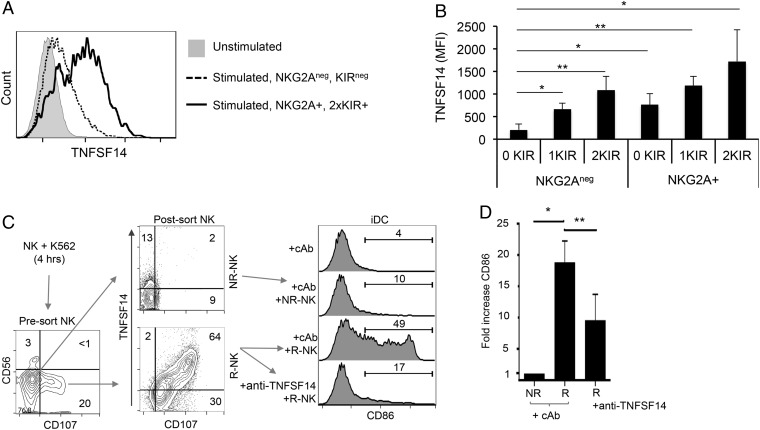
Licensed NK Cells Preferentially Produce TNFSF14 and Activate DCs in Response to Tumor Stimulation.
The induction of TNFSF14 expression was confined to the tumor-responsive NK-cell population (CD107+, R-NK; Figs. 1 and 2). This expression pattern suggested that TNFSF14 induction might be restricted to NK cells that were functionally licensed by virtue of expressing inhibitory receptors reactive with self-MHC class I molecules. We determined the KIR ligand haplotype of donor blood samples and then analyzed K562-induced TNFSF14 expression on NK cells from these donors according to whether the cells expressed KIR molecules specific for self-MHC class Ia molecules and the inhibitory receptor NKG2A (a receptor for the MHC class Ib molecule, HLA-E). Tumor-induced TNFSF14 expression was approximately 10-fold higher in the cells expressing NKG2A and in two self-reactive KIRs compared with NK cells lacking expression of these receptors (Fig. 5A and Fig. S3). Indeed, NK cells expressing either NKG2A or a single self-reactive KIR expressed significantly more TNFSF14 in response to K562 stimulation than NK cells expressing neither NKG2A nor self-reactive KIRs. Furthermore, the magnitude of TNFSF14 induction was proportional to the number of self-reactive KIRs and greater in the NKG2A+ population compared with the NKG2Aneg population (Fig. 5B). These results demonstrate that TNFSF14 expression in response to tumor target cells preferentially occurs in the licensed NK-cell population and that both NKG2A and KIRs can license TNFSF14 production. The activation of NK cells with IL-2 converts functionally hyporesponsive cells lacking inhibitory receptors for self-MHC class I molecules (i.e., unlicensed cells) into a responsive subset (11, 14). Accordingly, TNFSF14 was produced by both the licensed and unlicensed NK-cell subsets when stimulated with either IL-2 or IL-15 (Fig. S4).
Fig. 5.
TNFSF14 is produced by functionally licensed NK cells. Five donors were typed for KIR ligands and self-KIR molecules, as well as NKG2A expression, by flow cytometry. NK cells from these donors were then cocultured with K562 cells for 6 h, and cell surface TNFSF14 expression was analyzed on NK cells expressing none of these receptors, individual receptors, or combinations thereof (details of the gating strategy are provided in Fig. S3 and SI Materials and Methods). (A) Cell surface TNFSF14 expression on NK cells expressing no detectable NKG2A or self-reactive KIRs vs. NK cells expressing NKG2A and two self-reactive KIR molecules. The difference in TNFSF14 between these unlicensed and licensed NK cells is approximately 10-fold based on the mean fluorescence intensity of TNFSF14 expression. (B) Cell surface expression of TNFSF14 in response to tumor cell stimulation on NK cells expressing no (0 KIR), one (1KIR) or two (2KIR) self-reactive KIRs with or without NKG2A expression. The data show that any one of the inhibitory receptors (either NKG2A or KIR) confers a statistically significant effect on TNFSF14 expression (*P < 0.05, **P < 0.005; two-tailed Student’s t test). (C) Tumor-stimulated NK cells induce CD86 expression on iDCs in a TNFSF14-dependent manner. NK cells were cocultured with K562 tumor cells for 4 h, and the R-NK and NR-NK cells were isolated by FACS based on CD107 display alone. Samples from these sorted populations were reanalyzed for expression of cell surface TNFSF14 and CD107 molecules (Center, Post-sort NK), and the remaining cells were cocultured with iDCs (for 48 h at a ratio of 1:1) in the presence or absence of 10 μg/mL anti-TNFSF14 antibody or cAb. CD86 expression of the iDCs was analyzed by flow cytometry, and the percentage of CD86-expressing cells with the different treatments is shown. (Top) iDC analysis shows iDCs plus cAb without added NK cells. (D) Induction of cell surface CD86 expression on iDCs following coculture with NR-NK or R-NK cells in the presence of either cAb or anti-TNFSF14 antibody. These data are the fold change in mean fluorescence intensity of CD86 expression from three independent experiments (*P < 0.05, **P < 0.005**; two-tailed Student’s t test).
We then analyzed the ability of the tumor-stimulated NK cells to mediate CD86 expression by iDCs. We cocultured NK cells with K562 tumor cells for 4 h and sorted the R-NK and NR-NK cell fractions based on display of CD107 at the cell surface (as performed for the gene expression profiling). These sorted fractions were then analyzed for TNFSF14 expression; the bulk of R-NK cells were TNFSF14+CD107+. Importantly, although some TNFSF14 expression was detected in the NR-NK fraction, the level of expression of TNFSF14 at the cell surface was much higher in the R-NK population (Fig. 5C). The sorted R-NK and NR-NK fractions were then cocultured with autologous iDCs for 48 h in the presence of anti-TNFSF14 or a control antibody. The R-NK fraction expressing TNFSF14 induced the expression of CD86 on almost half of the iDCs in a TNFSF14-dependent manner. In contrast, the NR-NK fraction produced little TNFSF14, and these NK cells did not induce CD86 expression on the cocultured iDCs (Fig. 5 C and D). Thus, licensed NK cells, endowed with the ability to respond to target cell stimulation, induce the expression of TNFSF14 in response to tumor cells, and these stimulated licensed cells induce DC expression of CD86 expression in a TNFSF14-dependent manner following NK–DC cross-talk.
Discussion
These results reveal that NK-cell activation, either by tumor cells, IL-2, or IL-15, results in the rapid production of biologically active TNFSF14, a cytokine endowed with potent and diverse immunomodulatory activity, including the ability to enhance antitumor immunity (36, 37). Human NK cells expressing TNFSF14 have previously been observed in the gut, where its expression is constitutive (38). Our results demonstrate its very rapid induction on peripheral blood-derived NK cells in response to a variety of stimuli. TNFSF14 was originally described as a molecule produced by activated T cells that binds to HVEM (TNFRSF14), the receptor for HSV; TNFSF14 competes with the HSV glycoprotein D molecule for HVEM binding, thereby blocking HSV entry (15). This study also identified lymphotoxin-α as an HVEM ligand and set the stage for subsequent studies from which a complex web of interactions between TNFSF members and both functional and decoy receptors has emerged (20, 21, 25). Several functions have since been attributed to TNFSF14, but the precise outcome of TNFSF14/HVEM interactions are dependent upon the availability of other ligands and receptors in either cis or trans conformations that compete for interaction with one another (20, 21, 25).
The ectopic expression of TNFSF14 at tumor sites enhances cytotoxic T-lymphocyte responses (36, 37). Our results reveal that NK cells can provide the TNFSF14 upon interaction with tumor cells. Ligation of tumor-sensing NK-cell activation receptors thus coordinates cytotoxic granule exocytosis (leading to tumor cell destruction) with the production of chemokines, TNF, IFN-γ, and, as shown here, the rapid expression of TNFSF14. Collectively, these molecules aid local responses, such as the recruitment of inflammatory and immune cells to the lesion, and promote the maturation of DCs and skewing toward Th1 responses. A role for TNF in NK cell-induced DC maturation has been reported previously (3, 5). Our results show that TNFSF14 also participates in this process. Whereas TNF and TNFSF14 share certain proinflammatory activities, TNFSF14 has nonredundant functions; TNFSF14-deficient mice exhibit reduced migration of cells to lymph nodes during immune responses, and TNFSF14 (from a source other than T cells) is required in these animals to initiate T-cell responses (39). TNFSF14 alone is not as potent as TNF in promoting DC maturation, and other factors are likely required (32). The requirement for TNFSF14 in promoting adaptive responses varies according to the antigenic dose, with lower doses showing an increased dependency on this cytokine (39). Our data, together with data previously reported (4), show that IL-2–stimulated NK cells make little TNF. However, IL-2–activated NK cells produce TNF following NKp30 ligation and promote DC maturation in a TNF-dependent (and NKp30-dependent) manner (5). Hence, IL-2 induces TNFSF14 directly and NKp30 ligation (by the DCs) induces TNF. Both cytokines then induce DC maturation. Resting NK cells have been previously shown to induce DC maturation in a TNF-dependent manner (3). However, these assays were performed in the absence of tumor cells and were optimal in the presence of LPS, suggesting that DC-derived TNF may be driving the maturation process in this case.
In our experiments, NK cells stimulated with tumor cells alone (without exogenous cytokines) produced TNFSF14, and NK and DC coculture induced DC maturation in a TNFSF14-dependent manner, establishing a role for TNFSF14 and the HVEM axis in NK–DC cross-talk. We cocultured both sorted R-NK and NR-NK cells with iDCs, and only the TNFSF14-producing R-NK cells promoted DC maturation. Importantly, iDCs express both HVEM and TNFSF14 (32, 40), and we cannot formally exclude a role for DC-derived TNFSF14 in the DC maturation process. However, our results with NK cells support data from Morel et al. (32) showing that the TNFSF14-transfected cells delivered TNFSF14-dependent (and HVEM-dependent) maturation signals to iDCs, as well as experiments in which TNFSF14 supplied in the trans configuration signals through HVEM to activate NF-κB (41). NK cells are also activated as a result of NK–DC cross-talk (4, 42, 43), and additional interactions between members of the HVEM regulatory network have been shown to activate NK cells (44). In addition, DC-derived IL-15 is of major importance in NK-cell activation (29, 45), and the ability of IL-15 to induce TNFSF14 production by NK cells provides a basis for the activation of both NK cells and DCs during their interaction. IL-2 is mainly produced by CD4+ cells during adaptive immune responses (46). The ability of IL-2 to induce TNFSF14 production by NK cells may simply reflect the sharing of signaling chains by the IL-2 and IL-15 receptors. However, it is now evident that NK-cell responses are not confined to the early stages of an immune response but are more durable, persisting into the adaptive phases when IL-2 is more abundant (27, 47–51).
Our gene expression profiling identified the rapid induction of a number of transcripts encoding cytokines and chemokines, including GM-CSF, IFN-γ, TNF, IL-3, IL-8, and CCL3/MIP-1α. The rapid induction of these genes (and others) can be attributed, in part, to the presence of AU (adenylate-uridylate)-rich elements (AREs) in the 3′ untranslated regions that stabilize preexisting mRNA upon cellular activation. Indeed, AREs were first identified in the CSF2 gene (52), and have subsequently been identified in many mRNAs encoding chemokines and inflammatory cytokines (53). However, the major TNFSF14 transcript has no readily discernible ARE, and its expression was dependent upon new transcription, consistent with data showing induction in T cells via the transcription factor, nuclear factor of activated T cells (NFAT) (54). With the exception of CD16, coinduction of TNFSF14 expression and granule exocytosis required the synergistic action of multiple NK-cell activation receptors. This result mirrors the findings of Bryceson et al. (55), where similar combinations were required to induce calcium flux, granule exocytosis, and production of TNF and IFN-γ, with CD16 being the only single receptor sufficient to induce these responses. Such synergy is not required for all NK-cell responses, with chemokine production requiring fewer activation signals than granule exocytosis or cytokine production (56). Thus, TNFSF14 more closely resembles TNF and IFN-γ rather than CCL3/MIP-1α production in terms of its induction via cell surface receptors. Whether these differences reflect a requirement for activation-induced gene expression for these cytokines compared with the stabilization of preexisting chemokine transcripts is currently undergoing testing.
Our identification of NK-cell TNFSF14 expression was due to its induction in the R-NK population following tumor stimulation. The presence of R-NK and NR-NK populations in a single individual reflects the functional licensing of only those NK cells that express inhibitory receptors against self-MHC molecules (11–14). We were able to demonstrate that TNFSF14 protein production occurred preferentially in these licensed NK cells and that both KIR family and NKG2A/CD94 receptors were involved in this process. Previous studies have shown that self-reactive KIRs, as well as other MHC class I receptors, notably NKG2A/CD94 and ILT2, play a role in functional licensing (12, 13, 19). At high NK/DC ratios, activated NK cells kill DCs, an activity that has been suggested to allow NK cells to regulate DC function and the onset of adaptive immunity (3, 43). Restricting target cell-induced TNFSF14 production to licensed NK cells may be important in allowing the activated NK cell to kill the DC if required. Furthermore, restricting the expression of TNFSF14 to this self-MHC–regulated population of NK cells clearly provides a safeguard against the release of this proinflammatory mediator in response to healthy tissue. The emerging evidence for a role for TNFSF14 in inflammatory disease is a testament to the need for such regulation (57, 58). However, infection may require the host to maximize its responses and use unlicensed NK cells that are uninhibited by self-MHC class I molecules; proinflammatory cytokines have been shown to override the licensing mechanism and allow unlicensed cells to respond (11, 59). Accordingly, both licensed and unlicensed NK cells produced TNFSF14 when stimulated with IL-2 or IL-15. In vivo, such responses are likely to be localized to the lesion, thereby minimizing collateral damage.
In summary, we have shown that human NK-cell activation leads to the induction of TNFSF14 expression. The ability of NK cells to produce TNFSF14 confers the potential to modulate many cell types involved in immune and inflammatory responses. In particular, the ability to aid DC maturation provides a key link between innate and adaptive immunity. The broad immunomodulatory activity of TNFSF14 must be efficiently regulated, and restricting TNFSF14 expression to the licensed NK-cell population provides a safeguard by which potentially damaging activity against healthy tissues is restricted.
Materials and Methods
Primary Cell Isolation and Culture.
Samples were collected following the receipt of informed consent and ethical review by the Leeds Teaching Hospitals National Health Service Trust. Peripheral blood mononuclear cells from healthy donors were used to purify NK cells (by indirect selection) or monocytes (via CD14 selection) using reagents from Miltenyi Biotec. Monocytes were cultured for 5 d in the presence of 800 units (U)/mL GM-CSF and 400 U/mL IL-4 (R&D Systems) to generate iDCs. KIR and KIR ligand typing was performed using a KIR HLA ligand kit (from Olerup) that amplifies pertinent alleles of the HLA-C1, HLA-C2, HLA-Bw4Thr80, HLA-Bw4Ile80, and HLA-ABw4 groups to be identified.
Gene Expression Analysis.
A scaled-up NK-cell degranulation assay (60–62) provided the material for the microarray analysis. After 4 h of coculture, cells were sorted on a Beckman Coulter MoFlo cell sorter and R-NK and NR-NK cells were purified according to the CD56dimCD107neg and CD56dimCD107+ phenotypes, respectively. cDNA was synthesized from fractions pooled from three donors (collected from six experiments) and a separate sample from one donor (collected from three experiments), with each sample containing approximately 1 million cells. Array analysis was performed using Illumina Sentrix HumanRef-8 v3 Beadchips and an Illumina BeadArray reader, as described in detail in the SI Materials and Methods. The data are available from the Gene Expression Omnibus (ncbi.nlm.nih.gov/geo), under accession number GSE55977. For validation, R-NK and NR-NK fractions were sorted from repeat degranulation assays and analyzed using quantitative RT-PCR with Taqman probes (from ABI/Life Technologies). Samples were normalized to 18S rRNA and compared using the comparative cycle threshold (Ct) method also known as the 2-ΔΔCT method, as previously described (63).
TNFSF14 Expression.
TNFSF14 (and TNF) expression by responding and nonresponding NK cells was assayed using antibodies against CD56, CD3, CD107, TNF, and TNFSF14 and a BD Biosciences LSRII flow cytometer (details of antibody clones are provided in SI Materials and Methods). Analysis was performed on NK cells treated with 50 U/mL IFN-I, 20 ng/mL IL-12 plus 20 ng/mL IL-18, 300 U/mL IL-2, 30 ng/mL IL-15, or 50 ng/mL PMA plus 500 ng/mL ionomycin (Miltenyi Biotec) for 8 h before analysis. All degranulation assays used an effector/target ratio of 1:1 with either tumor target cells or antibody-loaded P815 cells. For the latter, P815 cells were loaded with combinations of anti-NK receptor antibodies (or an IgG control) at 7 μg/mL for 20 min at room temperature and washed once before NK-cell coculture. Soluble cytokine was detected by ELISA using supernatants from NK cells stimulated with IL-2, IL-15, or PMA/ionomycin (or in media alone) for 24 h. Supernatants were diluted and assayed using either a TNFSF14 ELISA (R&D Systems) or TNF ELISA (Peprotech).
NK and DC Coculture.
The iDCs were prepared from monocytes (as described above), and fresh autologous NK cells were purified on day 5 of the iDC culture. NK cells were cocultured with K562 and R-NK and NR-NK fractions collected by FACS based on CD107 expression. These sorted NK cells were cocultured with iDCs at a 1:1 ratio for 48 h in either media alone or 12 μg/mL neutralizing anti-TNFSF14 antibody (goat polyclonal; R&D Systems), anti-TNF (goat polyclonal; R&D Systems), or normal goat IgG. IL-2–activated NK cells were cocultured at an NK/DC ratio of 1:3 for 15 h. The IL-2–activated NK cells were cocultured with iDCs at lower NK/iDC ratios and for a shorter time period than when using tumor-stimulated NK cells because of the propensity of the more cytotoxic IL-2–activated NK cells to kill iDCs (64). Induction of CD86 expression was analyzed on the DCs in the coculture by gating on CD11c+ cells.
Further details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Adam Davison and Liz Straszynski (Flow Cytometry Facility, University of Leeds School of Medicine) for their assistance with the cell sorting used in this study. This work was supported by Yorkshire Cancer Research, the Leeds Teaching Hospitals National Health Service Trust, and the Bioinformatics Institute, Agency for Science, Technology and Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Microarray-based gene expression profiling data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE55977).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411072112/-/DCSupplemental.
References
- 1.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195(3):335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerosa F, et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195(3):327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale M, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106(2):566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 6.Martín-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 7.Morandi B, Bougras G, Muller WA, Ferlazzo G, Münz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36(9):2394–2400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 8.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 12.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA. 2008;105(8):3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32(8):364–372. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauri DN, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 16.Hanna J, et al. Novel insights on human NK cells’ immunological modalities revealed by gene expression profiling. J Immunol. 2004;173(11):6547–6563. doi: 10.4049/jimmunol.173.11.6547. [DOI] [PubMed] [Google Scholar]
- 17.Bezman NA, et al. Immunological Genome Project Consortium Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13(10):1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walzer T, Jaeger S, Chaix J, Vivier E. Natural killer cells: From CD3(-)NKp46(+) to post-genomics meta-analyses. Curr Opin Immunol. 2007;19(3):365–372. doi: 10.1016/j.coi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, et al. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179(9):5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev. 2011;244(1):169–187. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 22.Cai G, et al. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9(2):176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 23.Marsters SA, et al. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272(22):14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 24.Hsu H, et al. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272(21):13471–13474. doi: 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 25.Ware CF, Sedý JR. TNF Superfamily Networks: Bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14) Curr Opin Immunol. 2011;23(5):627–631. doi: 10.1016/j.coi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey M, et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161(1):400–408. [PubMed] [Google Scholar]
- 27.Welsh RM, Waggoner SN. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology. 2013;435(1):37–45. doi: 10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr Opin Virol. 2011;1(6):497–512. doi: 10.1016/j.coviro.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: A molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Morel Y, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165(8):4397–4404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 32.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167(5):2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 33.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25(1):47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Zou GM, Hu WY. LIGHT regulates CD86 expression on dendritic cells through NF-kappaB, but not JNK/AP-1 signal transduction pathway. J Cell Physiol. 2005;205(3):437–443. doi: 10.1002/jcp.20420. [DOI] [PubMed] [Google Scholar]
- 35.Madge LA, Kluger MS, Orange JS, May MJ. Lymphotoxin-alpha 1 beta 2 and LIGHT induce classical and noncanonical NF-kappa B-dependent proinflammatory gene expression in vascular endothelial cells. J Immunol. 2008;180(5):3467–3477. doi: 10.4049/jimmunol.180.5.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamada K, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6(3):283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 37.Yu P, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 38.Cohavy O, Zhou J, Ware CF, Targan SR. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol. 2005;174(2):646–653. doi: 10.4049/jimmunol.174.2.646. [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Yang Y, Wang Y, Wang Z, Fu YX. LIGHT regulates inflamed draining lymph node hypertrophy. J Immunol. 2011;186(12):7156–7163. doi: 10.4049/jimmunol.1002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamada K, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164(8):4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 41.Cheung TC, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183(11):7286–7296. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5(4):405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 43.Ferlazzo G, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195(3):343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Šedý JR, et al. CD160 activation by herpesvirus entry mediator augments inflammatory cytokine production and cytolytic function by NK cells. J Immunol. 2013;191(2):828–836. doi: 10.4049/jimmunol.1300894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koka R, et al. Cutting edge: Murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173(6):3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 46.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 47.Björkström NK, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Vergès S, et al. Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108(36):14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang PA, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109(4):1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481(7381):394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 53.Stumpo DJ, Lai WS, Blackshear PJ. Inflammation: Cytokines and RNA-based regulation. Wiley Interdiscip Rev RNA. 2010;1(1):60–80. doi: 10.1002/wrna.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellano R, et al. Mechanisms regulating expression of the tumor necrosis factor-related light gene. Role of calcium-signaling pathway in the transcriptional control. J Biol Chem. 2002;277(45):42841–42851. doi: 10.1074/jbc.M207689200. [DOI] [PubMed] [Google Scholar]
- 55.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doherty TA, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17(5):596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ware CF. Targeting the LIGHT-HVEM pathway. Adv Exp Med Biol. 2009;647:146–155. doi: 10.1007/978-0-387-89520-8_10. [DOI] [PubMed] [Google Scholar]
- 59.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11(4):321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1-2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 61.Meade JL, et al. Proteolytic activation of the cytotoxic phenotype during human NK cell development. J Immunol. 2009;183(2):803–813. doi: 10.4049/jimmunol.0713829. [DOI] [PubMed] [Google Scholar]
- 62.Holmes TD, et al. A human NK cell activation/inhibition threshold allows small changes in the target cell surface phenotype to dramatically alter susceptibility to NK cells. J Immunol. 2011;186(3):1538–1545. doi: 10.4049/jimmunol.1000951. [DOI] [PubMed] [Google Scholar]
- 63.Wilson EB, et al. Human tumour immune evasion via TGF-β blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS ONE. 2011;6(9):e22842. doi: 10.1371/journal.pone.0022842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “L’union fait la force”. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 65.Santis AG, López-Cabrera M, Sánchez-Madrid F, Proudfoot N. Expression of the early lymphocyte activation antigen CD69, a C-type lectin, is regulated by mRNA degradation associated with AU-rich sequence motifs. Eur J Immunol. 1995;25(8):2142–2146. doi: 10.1002/eji.1830250804. [DOI] [PubMed] [Google Scholar]
- 66.Mou Z, et al. HuR posttranscriptionally regulates early growth response-1 (Egr-1) expression at the early stage of T cell activation. FEBS Lett. 2012;586(24):4319–4325. doi: 10.1016/j.febslet.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 67.Drury GL, Di Marco S, Dormoy-Raclet V, Desbarats J, Gallouzi IE. FasL expression in activated T lymphocytes involves HuR-mediated stabilization. J Biol Chem. 2010;285(41):31130–31138. doi: 10.1074/jbc.M110.137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.