Abstract
The development of more selective immunosuppressive agents to mitigate transplant rejection and autoimmune diseases requires effective strategies of blocking signaling pathways in T cells. Current immunosuppressive strategies use cyclosporin A (CsA) or FK506 to inhibit calcineurin, which dephosphorylates and promotes the nuclear import of nuclear factor of activated T cells (NFAT) transcription factors. These nuclear NFATs then transactivate cytokine genes that regulate proliferative responses of T cells. Both CsA and FK506 have debilitating side effects, including nephrotoxicity, hypertension, diabetes, and seizures, that argue for the development of alternative or complementary agents. To this end, we developed cell-based assays for monitoring NFAT dynamics in nonlymphoid cells to identify small molecules that inhibit NFAT nuclear import. Interestingly, we found that the majority of these small molecules suppress NFAT signaling by interfering with “capacitative” or “store-operated” calcium mobilization, thus raising the possibility that such mobilization processes are relevant targets in immunosuppression therapy. Further, these small molecules also show dose-dependent suppression of cytokine gene expression in T cells. Significantly, the IC50 of CsA in primary T cells was reduced by the addition of suboptimal concentrations of these compounds, suggesting the possibility that such small molecules, in combination with CsA, offer safer means of immunosuppression.
Keywords: capacitative calcium entry, store-operated calcium channels, cyclosporin A
Drugs that destroy T cells or block their antigen-dependent activation have been the mainstays of treatment of organ transplant rejection (1). The latter group is represented chiefly by the natural products cyclosporin A (CsA) and FK506, both of which act by suppressing the protein phosphatase calcineurin (2–4). Calcineurin's activity depends on calcium signaling and plays an essential role in T cell signal transduction by controlling the nuclear import of the nuclear factor of activated T cells (NFAT) family of transcription factors. The NFATs are Rel-related transcription factors encoded by four genes (NFATc1–4) that are widely expressed in cells of the immune, cardiovascular, and nervous systems (4–6). In resting cells, NFATs are localized to the cytoplasm due to a phosphorylation-dependent intramolecular masking of their nuclear location signals (NLSs) (7). During calcium signaling, calcineurin unmasks these NLSs, resulting in a rapid nuclear import of the dephosphorylated NFATs and transcriptional activation of cytokine genes (8). Besides unmasking the NLSs on the NFATs, calcineurin also masks their nuclear export signals, thereby preventing the futile cycling of these transcription factors across the nuclear envelope, thus ensuring their transcriptional functions in the nucleus (9, 10). NFAT proteins are essential for T cell activation and therefore represent potential targets for new immunosuppressive therapies. Interestingly, recent data from mouse knockout studies indicate that NFAT genes encode proteins with potentially conflicting effects in T cell activation (11–13), suggesting the utility of targeting individual NFAT family members.
We designed an image-based high-throughput screen for small organic molecules that block the calcium-triggered nuclear import of NFATs. Using HeLa cells expressing a GFP-tagged NFATc3, we have identified compounds that inhibit calcium-triggered nuclear import of this fusion protein. Remarkably, none of these compounds acted as an inhibitor of calcineurin activity in vitro, and most appeared to operate upstream of both calcineurin and NFAT. Indeed, our analyses have revealed that these compounds interfere with the calcium mobilization involving store-operated calcium (SOC) channels, suggesting their use in deciphering the mechanism of SOC currents and therapeutic modulation of the immune response. Finally, we show that these compounds not only suppress cytokine production but, at suboptimal doses, can also act together with lower doses of CsA to suppress T cell activation.
Materials and Methods
Screen for NFAT Inhibitors. Small organic molecules from a chemical library (Diverset E, ChemBridge, San Diego) were screened for their ability to inhibit the calcium-triggered nuclear import of GFP-tagged NFATc3 in HeLa cells. See Supporting Methods, which is published as supporting information on the PNAS web site, for details.
Automated Image Analysis. Fluorescence images (GFP-NFAT, Texas red phalloidin for actin and Hoechst 33258 for DNA) were collected by using an automated image acquisition system (Universal Imaging, Media, PA) (14). The DNA and actin images were first treated with a median filter and then autothresholded and binarized to generate nuclear and cell masks, respectively. The GFP-NFAT signal in each mask area was quantified, and the nucleus-to-cytoplasm ratio was expressed as percent nuclear localized NFAT (colocalization axis). The correlation coefficient was calculated by using the NFAT and DNA signals. See Supporting Methods for details.
Secondary Screens. Secondary screens were performed in baby hamster kidney (BHK) cells transfected with a Myc-tagged NFATc3 plasmid (8). For reversibility assays, cells treated with compounds were rinsed extensively with fresh media lacking drug and then incubated with ionomycin for an additional 30 min. To test for specificity, BHK cells expressing the rat glucocorticoid receptor-β-galactosidase (GR-β-gal) fusion protein (15) were treated with compounds 10 min before addition of 100 nM dexamethasone. After 30 min, cells were fixed and stained with an antibody to β-gal (Promega). The in cell calcineurin assays were performed by using BHK cells cotransfected with myc-NFATc3, the constitutively active calcineurin catalytic subunit (ΔCnA), and the regulatory subunit, CnB. For dephosphorylation assays, BHK cells expressing myc-NFATc3 were treated with ionomycin alone or with 50 μM each compound for 30 min and then lysed in SDS sample buffer. For IL-2 assays, murine Do11 T cells were incubated with compounds at indicated concentrations for 10 min before stimulation with 1 μM ionomycin and 200 nM phorbol myristate acetate and IL-2 measured 12 h later. For primary activation, CD4+ cells purified from spleen and lymph nodes were activated by plate-bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (2 μg/ml) and IL-2 measured in supernatants 24 h later. After a 72-h activation, cells were washed, Ficoll purified, and rested in IL-2-supplemented media for 2 days before restimulation with plate-bound anti-CD3 and anti-CD28 with different concentrations of CsA, either alone or in the presence of 2 μM of selected compounds. Cytotoxicity was assayed by using the CellTiter-Glo luminescent cell viability assay reagent (Promega).
Measurement of Intracellular Calcium. To assess the inhibitory effects on ionomycin-induced calcium flux, BHK cells labeled with fluo-3 (Molecular Probes) were treated with compounds for 20 min and then with 1 μM ionomycin. Fluorescence intensities were monitored in real time for 10 min. Changes in intracellular calcium concentrations were expressed as a ratio of fluorescent intensities at time (t) and time 0 (Ft/F0).
Intracellular calcium measurements in cell populations were monitored by using a Delta Scan-1 microfluorimeter (Photon Technology International, Lawrenceville, NJ) set at 340 and 380 nm for excitation and 510 nm for emission. BHK cells labeled with fura-2/AM were pretreated with test compounds in calcium-free Hepes-buffered saline for 1 min, before depletion of intracellular stores with 1 μM thapsigargin (Tg; Sigma). After 3 min, calcium influx was evoked by addition of CaCl2 (2 mM final). See Supporting Methods for details.
Results
Cell-Based Screens for Inhibitors of NFAT Nuclear Import. NFATc3 expression has been demonstrated in T cells, myocytes, and neurons (4–6), and yet these cell types present technical complications for high-throughput screening. However, GFP-NFATc3 is also cytoplasmic when expressed in adherent non-lymphoid cell lines and enters the nucleus after calcium mobilization (7). HeLa cells where chosen for their robust growth and adherence properties, and GFP-NFATc3 expression was achieved by infecting these cells with recombinant adenoviral vectors. Nuclear import of GFP-NFATc3 was complete in HeLa cells within 1 h of ionomycin treatment (Fig. 1 A and B) and, as in T cells, was inhibited by CsA (Fig. 1B) (6, 7). In the automated screen, HeLa cells stably expressing GFP-NFATc3 were plated in 384-well plates, treated with compounds from the library, activated with ionomycin, and subsequently processed for imaging. Fluorescent images were captured sequentially by using a microscope equipped with automated stage, focusing, and image-storing capacity. Masking algorithms were used to examine the coherence between the GFP-NFATc3 signal and that of Hoechst 33258 (Fig. 1C). In brief, images of GFP-NFATc3, Texas red phalloidin-stained actin cytoskeleton, and Hoechst 33258 staining of nuclei were taken by a digital camera from identical fields of a given well using filter sets specific for each dye. Masks corresponding to the cytoplasm and nucleus were generated by digitizing the actin staining and Hoechst staining, respectively. The degree of nuclear import was determined by the ratio of the GFP-NFAT in the nuclear mask versus the GFP-NFAT in the cytoplasmic mask. Drugs that prevent the calcium-triggered nuclear import of GFP-NFATc3 would therefore yield a lower nuclear NFAT signal, hence lower colocalization values than cells with uninhibited import. This analysis was tested on a 384-well plate in which random positions were incubated with 200 nM CsA before ionomycin treatment as a positive control for inhibitory molecules. Automated image analysis of this plate revealed that each CsA-treated well was correctly identified as having a significantly lower nuclear NFAT (blue dots, Fig. 1C) than wells treated with ionomycin alone (red dots, Fig. 1C). In addition, the number of false positives from this automated analysis was low, on the order of 1:100. All untreated wells showed lower colocalization values as expected (Fig. 1C, yellow dots).
Fig. 1.
Cell-based screen for inhibitors of NFAT nuclear import. (A) Schematic of screen showing HeLa cells growing in 384-well plates infected with a defective adenovirus driving expression of a GFP-NFATc3 fusion protein. (B) GFP-NFATc3 is nuclear in cells treated with ionomycin (control) but arrested in the cytoplasm in cells treated with ionomycin and CsA. (C) Validation of imaging analysis data from sample 384-well plates. Cells were either left untreated (yellow dots, no ionomycin) or were treated with ionomycin alone (red dots) or together with CsA (blue dots) (see Materials and Methods for details). Image fields with cell numbers <40 are depicted as smaller dots and fields with >300 cells are shown as larger dots. (D) Specificity of NFAT inhibitors. (Upper) GFP-NFAT is cytoplasmic in untreated cells (Left). Ionomycin promotes nuclear import (Center), which is blocked by compound 2 (Right). (Lower) Cytoplasmic localization of GR-β-gal fusion protein revealed with an anti-β-gal mAb (Left), nuclear localization of GR-β-gal fusion protein after 15-min treatment with 100 nM dexamethasone (Dex) (Center), Dex-induced nuclear localization of GR-β-gal fusion protein despite the presence of 50 μM compound 2 (Right).
Individual compounds from a chemical library of 16,000 organic compounds were used in the import assay at a final concentration of 50–100 μM. Of these compounds, 74 scored positive in our initial screen. Secondary screens performed on BHK cells expressing GFP-NFATc3 (not shown) indicated that 14 of these 74 molecules showed robust inhibition of NFAT nuclear import (Table 1; Fig. 7, which is published as supporting information on the PNAS web site). The remaining compounds showed marginal effects, appeared toxic to cells, or proved to be autofluorescent.
Table 1. Properties of NFAT inhibitors.
| Code | ChemB* | Nc3† | ID-Phos‡ | GCR§ | CN¶ | IC50∥ |
|---|---|---|---|---|---|---|
| 1 | 114006 | C | Phos. | C/N | Neg. | <25 |
| 2 | 115555 | C | Phos | N | Neg. | 10 |
| 3 | 115805 | C | Phos | N | Neg. | <40 |
| 4 | 143072 | C | Phos | N | Neg. | <25 |
| 5 | 149521 | C | Phos | N | Neg. | <40 |
| 6 | 190027 | C | De-Phos | N | Neg. | <10 |
| 7 | 202034 | C | Phos | N | Neg. | 14 |
| 8 | 205327 | C | De-phos | N | Neg. | 40 |
| 9 | 211950 | C | Phos | N | Neg. | <20 |
| 10 | 219735 | C | Phos | N | Neg. | 15 |
| 11 | 228195 | C | De-phos | N | Neg. | 22 |
| 12 | 232755 | C | De-phos | N | Neg. | 32 |
| 13 | 156381 | C | Phos | N | Neg. | 15 |
| 14 | 103885 | C | Phos | N | Neg. | <10 |
Chembridge Corporation code for compounds.
Effect on NFATc3 localization in the presence of calcium ionophore (C, cytoplasmic, N, nuclear).
Effect on NFATc3 phosphorylation status in the presence of ionomycin.
Effect on the dexamethasone-induced translocation of the glucocorticoid receptor to the nucleus (C, cytoplasmic; N, nuclear).
Effect on calcineurin in vivo. Neg., negative.
Apparent concentration of inhibitor at which 50% of NFATc3 fails to enter the nucleus.
We further determined whether the effects of the compounds were reversible and specific for GFP-NFATc3 nuclear import. The inhibitory effects of all 14 compounds on NFAT nuclear import proved to be completely reversible after 30-min incubation with fresh media (not shown). To analyze the specificity of these compounds for GFP-NFATc3 nuclear import, we asked whether they would affect the conditional nuclear import of other transcription factors not related to the NFATs. We therefore assayed the 14 compounds for their ability to inhibit the dexamethasone-induced nuclear import of a GR-β-gal fusion protein (15) in BHK cells. Only one of these compounds (1) showed an effect on the trafficking of the glucocorticoid receptor (Table 1 and Fig. 1D).
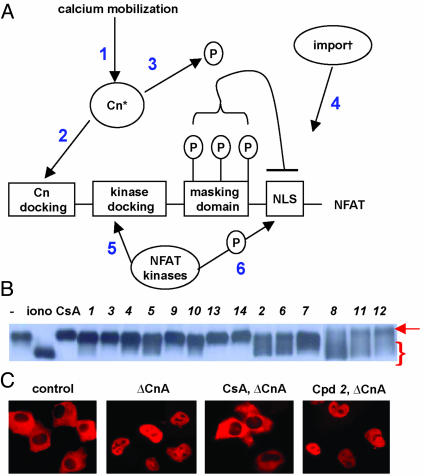
Mechanistic Studies of Inhibition. The NFATc3 inhibitors described here could be acting through one of at least six different mechanisms. Such mechanisms would include blocking calcium mobilization or calmodulin activation, calcineurin inhibition, preventing calcineurin from docking onto NFAT, activating NFAT kinases, or interfering with aspects of the nuclear import process itself (Fig. 2A) (7–10, 16–19). To narrow down the level of actions of these compounds, we asked whether they, like CsA, blocked the calcineurin-dependent dephosphorylation of NFATc3 that occurs during calcium signaling. The dephosphorylation of NFATc3 was monitored by examining the electrophoretic mobility shifts of the NFAT protein in SDS gels (7). Significantly, 9 of the 14 inhibitors of NFATc3 nuclear import prevented the normal mobility shift of NFATc3 during calcium signaling (defined as “class A”), suggesting they block dephosphorylation by inhibiting calcineurin or some event required to activate calcineurin (Fig. 2B). Five compounds appeared to block NFAT import despite at least partial NFAT dephosphorylation (defined as “class B”), suggesting the possibility that they are affecting steps downstream of calcineurin activation (Fig. 2B, Table 1). However, two of these latter five compounds proved to be either weak inhibitors (12) of NFAT translocation or complete suppressors of NFAT dephosphorylation at higher concentrations (2), whereas the other three compounds, 6, 8, and 11, proved to be effective inhibitors of nuclear import while allowing NFAT dephosphorylation. These latter compounds are the subjects of ongoing analyses. Because calcineurin is the common target of CsA and FK506, we asked whether any of the nine compounds that blocked NFATc3 dephosphorylation also inhibited calcineurin in cells. To determine the ability of these compounds to inhibit calcineurin activity, we took advantage of the observation that ΔCnA is localized to the nucleus, and that CsA drives it to the cytoplasm (ΔCnA; Fig. 2C) (7). In contrast to CsA, none of the compounds redirected ΔCnA to the cytoplasm, suggesting that calcineurin activity was not their direct target in vivo.
Fig. 2.
Small molecule inhibitors act upstream of calcineurin to block NFAT import. (A) A schematic of NFATc3 depicting the functional domains of the N terminus that regulate calcium-activated nuclear import and important regulatory sites. 1 refers to calcium mobilization as well as calmodulin activation, 2 to calcineurin docking the N terminus of NFAT, 3 to the phosphatase activity of calcineurin, 4 to the nuclear import proteins that recognize nuclear location signals, and 5 and 6 to the docking and activation of NFAT kinases that oppose calcineurin. (B) Western blot of NFATc3 from BHK cells treated for 45 min with carrier alone, ionomycin, or ionomycin together with one of the NFATc3 nuclear import inhibitors. The control cells show NFATc3 with the lowest mobility (arrow), whereas cells treated with ionomycin show the highest mobility (brackets). All compounds were used at concentrations that block all detectable nuclear import. (C) Effect of NFAT inhibitors on intracellular calcineurin activity. (Left to right) NFATc3 cytoplasmic localization in control BHK cells, nuclear location of NFATc3 in cells coexpressing ΔCnA, cytoplasmic localization of NFATc3 in cells coexpressing ΔCnA and treated with 1 μM CsA, nuclear localization of NFATc3 in cells coexpressing ΔCnA and treated with compound 2.
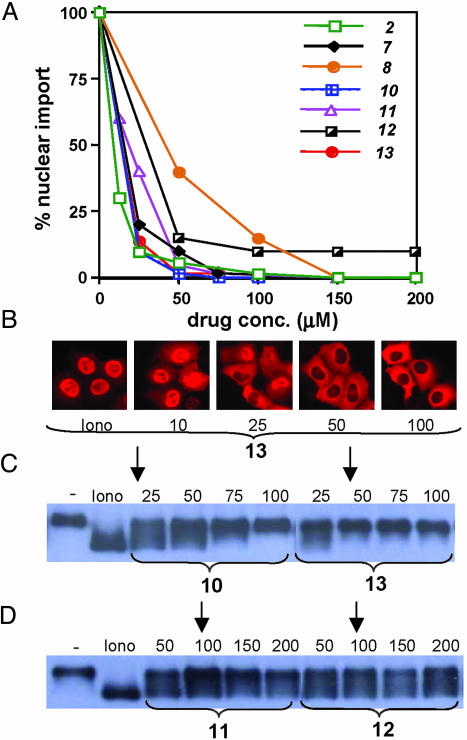
To estimate approximate inhibitory concentrations for these compounds, parallel assays were performed to titrate representative compounds on NFAT nuclear import and NFAT dephosphorylation. Based on nuclear transport assays, compounds 2, 7, 8, 10–13 were shown to have IC50s ranging from 10 to 40 μM (Fig. 3 A and B). Corresponding analyses of NFAT phosphorylation status revealed that the majority of compounds significantly suppressed NFAT dephosphorylation at concentrations where 95% of nuclear import was inhibited (Fig. 3 C and D, arrows).
Fig. 3.
Dose-dependent inhibition of NFAT import and dephosphorylation by small-molecule inhibitors. (A) Titration of various NFAT import inhibitors to determine relative IC50 values by immunofluorescence. (B) Representative analysis of titration to determine IC50 by subcellular localization of NFATc3 by immunofluorescence. (C) Titrations of representative “class A” (see text) NFATc3 inhibitors on NFATc3 dephosphorylation in the presence of ionomycin. (D) Titrations of representative “class B” (see text) NFATc3 inhibitors on ionomycin-induced NFAT dephosphorylation. In both C and D, arrows mark concentrations where at least 95% of NFAT is excluded from the nucleus.
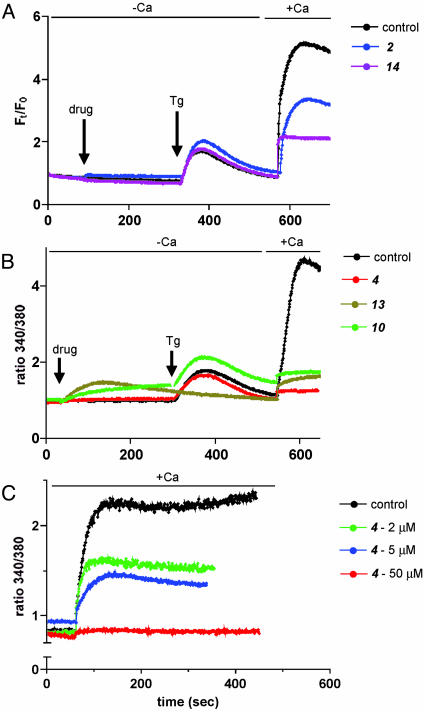
Effects on Calcium Mobilization. The bulk of our mechanistic studies indicated that the NFAT inhibitory compounds acted upstream of NFAT dephosphorylation and calcineurin activation, leaving calcium mobilization as a likely site of action. In most nonexcitable cells, ligand-induced phospholipase C activation leads to inositol trisphosphate (IP3) generation and concomitant IP3-triggered release of calcium from intracellular stores (20). This emptying of intracellular stores is thought to trigger the opening of “capacitative” or SOC channels in the plasma membrane, which results in sustained elevation of intracellular calcium. To test whether the NFAT inhibitors affected this SOC-dependent calcium mobilization influx, we followed calcium mobilization in several cell lines using calcium imaging and fluorimetry. In BHK cells treated with ionomycin, calcium imaging reveals a large spike due to store depletion and calcium entry, followed by a sustained phase of enhanced intracellular calcium levels lasting minutes (Fig. 8A, which is published as supporting information on the PNAS web site; control). This enhanced calcium level depends on extracellular calcium as the calcium chelator EGTA blocks it, whereas additional calcium restores it (Fig. 8A, EGTA). Addition of one of the NFAT inhibitors to cells before ionomycin treatment caused a marked suppression of calcium mobilization (Fig. 8 A). To further ensure that the inhibition of calcium mobilization is not due to blockade of store depletion, Do11 murine T cells were treated with compounds 3 min before addition of Tg (2 μM) in calcium-free Hepes-buffered saline. Four of the five compounds (2, 4, 10, and 14) tested did not significantly affect store depletion (Fig. 4 A and B). Compound 13 failed to evoke Tg-induced spike mainly due to premature release from internal stores rather than blocking depletion. However, on adding back calcium, all five compounds effectively inhibited the sustained calcium mobilization (Fig. 4 A and B). The inhibition was dose dependent both in BHKs and Do11 cells (Figs. 4C and 8B). We also tested the compounds after Tg treatment to rule out the blockade of store depletion by these compounds (Fig. 9, which is published as supporting information on the PNAS web site). Because calcium influx through SOCs in T cells is modulated by voltage-dependent K channels and mitochondrial homeostasis, it was essential to confirm that the inhibitory effects of the compounds on SOC-dependent calcium mobilization are not due to these factors. At least four of the five tested compounds inhibited SOC-dependent calcium mobilization in high K buffer, which clamps the membrane potential of T cells to ≈0 mV (Fig. 10A, which is published as supporting information on the PNAS web site), ruling out the possibility that these compounds work through these K channels. Similarly, the inhibitory effect of all five compounds was not affected when mitochondrial uptake was completely suppressed by carbonyl cyanide 3-chlorophenylhydrazone (CCCP), arguing against a role for mitochondria in the action of these compounds (Fig. 10B). The other compounds, 3, 5, 7, 8, 9, and 12, did not show significant inhibition of SOC (Fig. 11 A and B, which is published as supporting information on the PNAS web site). Compound 11 could not be investigated, because it strongly interfered with both fura-2 and fluo-4.
Fig. 4.
NFAT inhibitors block SOC-dependent calcium influx. (A) Inhibition of Tg-induced sustained calcium influx in Do11 cells. Fluo-4-labeled cells were treated with DMSO (control) or indicated compounds for (arrow) 5 min before addition of 1 μM Tg (arrow) in calcium free Hepes-buffered saline. After 5 min, calcium mobilization through SOC was induced by addition of calcium (2 mM final). Relative changes in intracellular calcium levels are represented as ratio Ft/F0, where Ft and F0 are the integrated fluorescence intensities at time t and time zero. (B) Inhibition of Tg-induced sustained calcium influx in Do11 cells. Cells were treated as in A, except that the cells were labeled with fura-2. Each graph is a representative trace of several independent experiments. (C) Dose-dependent inhibition of Tg-induced calcium influx in Do11 cells. Fura-2-labeled Do11 cells were treated with varying concentrations of compound 4 for 1 min and for 3 min with Tg in calcium-free Hepes-buffered saline before addition of calcium (2 mM final) to induce calcium influx through SOC. Fluorescence recordings 1 min before readdition of CaCl2 are shown. Each graph is a representative trace of several independent experiments.
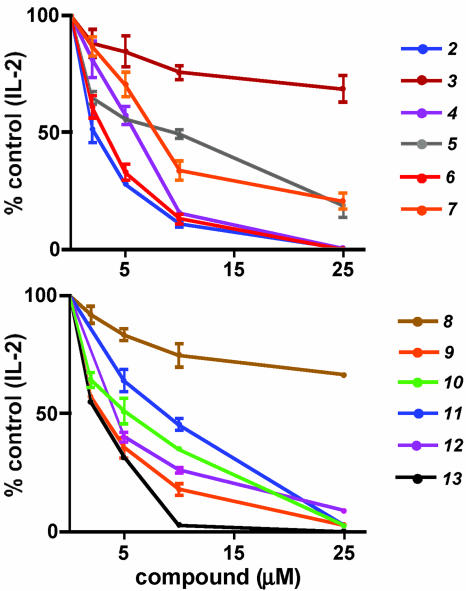
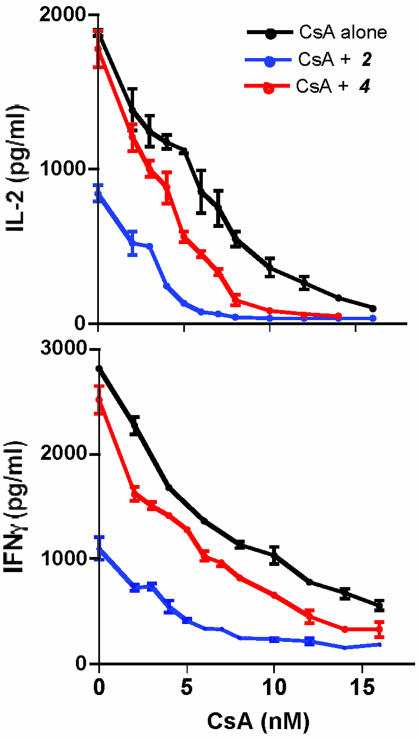
Suppression of Cytokine Production in T Cells. To assess the immunosuppressive properties of the compounds that inhibit calcium mobilization, we asked whether they could interfere with IL-2 production in the murine T cell line Do11 activated by ionomycin and the phorbol ester phorbol myristate acetate. Significantly, these compounds dramatically suppressed IL-2 production (Fig. 5) at concentrations that block NFAT translocation in BHK cells. Given their potency in down-regulating cytokine production after T cell activation, we asked whether these compounds could cooperate with CsA. Using suboptimal (2 μM) doses of compounds 2 and 4, we titrated CsA concentrations for the suppression of IL-2 and IFN-γ production in primary T cells activated with anti-CD3 and anti-CD28. Significantly, both compounds tested individually were able to reduce the apparent IC of CsA for the suppression of T cell activation (Fig. 6). Cell 50viability assays further ensured that the immunosuppressive properties of the compounds were specific to signaling events in the cells and not due to cytotoxicity (data not shown).
Fig. 5.
Suppression of IL-2 production in murine Do11 T cells by NFAT inhibitors. Do11 cells were activated for 12 h with ionomycin and phorbol myristate acetate together with various compounds at the indicated concentrations and IL-2 in the supernatant was measured by ELISA.
Fig. 6.
NFAT inhibitors lower the apparent IC50 of CsA. Purified CD4+ cells were restimulated with plate-bound anti-CD3 and soluble anti-CD28 in the presence of indicated doses of CsA and NFAT inhibitors. After 12 h, supernatants were assayed for production of IL-2 and IFN-γ by ELISA.
Discussion
This study demonstrates the feasibility of using cell- and image-based screening to identify small molecules that suppress NFAT signaling. Remarkably, the majority of NFAT inhibitors identified in this screen targeted calcium mobilization rather than the calcium-activated phosphatase calcineurin, the target of the major immunosuppressants in clinical use. These findings underscore the importance of the calcium influx processes depending on SOC channels in calcineurin- and NFAT-dependent transcriptional events and raise the intriguing possibility that such calcium signaling processes could be amenable to therapeutic intervention for immunosuppression and other indications.
The 14 NFAT nuclear import inhibitors identified in this study appear very diverse in structure and are likely therefore to operate in functionally distinct ways. Although a majority of these compounds inhibit calcium mobilization dependent on store depletion similar to the recently reported compound 3,5-bistrifluoromethyl pyrazole, BTP2 (20), they are structurally different from the BTP compounds and hence are likely to act by different mechanisms.
The molecular nature of the calcium release-activated calcium channels or SOC channels has been the subject of intense investigation, and evidence implicates one or more of the ≈25 mammalian homologs of the transient receptor potential channels first described in the Drosophila visual system (21–23). Genetic screens for mutations that block NFAT activation have yielded mutants that show defective SOC influx in response to T cell receptor (TCR) ligation (24, 25). Additionally, a small number of patients with hereditary immunodeficiency syndromes display compromised calcium influx associated with defects in calcium release-activated calcium channels or SOC channels (26). Together with extensive physiological studies, these mutations affecting SOC currents provide strong evidence for a central role played by prolonged calcium signaling in transducing TCR activation. Our chemical screens provide additional support for the notion that SOC-dependent calcium influx represents a key step in the TCR signal transduction pathway and one that is vulnerable to disruption.
Besides the physical identity of SOCs, we know almost nothing about the signal transduction events that communicate the emptying of internal stores of calcium to store-operated channels. Although several steps in this gating process have been proposed (21–23), definitive pathways have remained elusive. Natural products and synthetic molecules that block biological processes have made important contributions to our understanding of the processes of signal transduction, protein degradation, and chromatin remodeling, to name a few. The subgroup of NFAT inhibitors described here acting upstream of NFAT dephosphorylation and calcineurin are candidates for molecules that interfere with various steps of the SOC-dependent calcium mobilization process and therefore might aid in unraveling of the complex biochemistry underlying the regulation of SOC channels.
Conclusion
We are encouraged that several of the small molecules identified in our screen of NFAT translocation inhibitors in nonlymphoid cells indeed block cytokine responses in primary T cells. That these compounds, used at subinhibitory concentrations, can shift the dose curve of CsA indeed suggests they affect the same T cell activation pathway interrupted by the clinically relevant immunosuppressants. It could be argued that inhibitors working upstream of calcineurin in fact are likely to be less specific than calcineurin and therefore subject to some of the same side effects as the calcineurin inhibitors. However, there is evidence that the SOCs may have some degree of cell and tissue specificity and therefore the potential for selective interruption. This evidence includes the large number of isoforms seen in mammalian transient receptor potential family and other calcium channels, as well as the phenotype of patients with defective SOC current, which, in addition to immunodeficiency, included only muscle hypotension and ectodermal dysplasia (27). We anticipate that the small molecules or strategies described herein will be useful leads for new immunosuppressive agents to combat organ transplant rejection and autoimmune diseases and may provide insights into the biochemistry of the enigmatic store-operated channels.
Supplementary Material
Acknowledgments
We thank Sandra Ryeom, Scott Rodig, and Shidong Jia for reading the manuscript and for providing critical suggestions. We also thank Dr. Stephen Quinn (Brigham and Women's Hospital, Boston) for instruction and use of the Delta Scan spectrofluorimeter. This work was supported by grants from the National Institutes of Health (to D.G., T.J.M., and F.M.) and from Philip Morris (to F.M.).
Abbreviations: NFAT, nuclear factor of activated T cells; Cn, calcineurin; CsA, cyclosporin A; SOC, store-operated calcium; GR-β-gal, rat glucocorticoid receptor-β-galactosidase; ΔCnA, constitutively active calcineurin; Tg, thapsigargin; BHK, baby hamster kidney.
References
- 1.Kahan, B. D. (2003) Nat. Rev. Immunol. 3, 831–838. [DOI] [PubMed] [Google Scholar]
- 2.McKeon, F. (1991) Cell 66, 823–826. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree, G. R. (2001) J. Biol. Chem. 276, 2313–2316. [DOI] [PubMed] [Google Scholar]
- 4.Rao, A., Luo, C. & Hogan, P. G. (1997) Annu. Rev. Immunol. 15, 707–747. [DOI] [PubMed] [Google Scholar]
- 5.Hogan, P. G., Chen, L., Nardone, J. & Rao, A. (2003) Genes Dev. 17, 2205–2232. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree, G. R. & Olson, E. N. (2002) Cell 109, S67–S79. [DOI] [PubMed] [Google Scholar]
- 7.Shibasaki, F., Price, E. R., Milan, D & McKeon, F. (1996) Nature 382, 370–373. [DOI] [PubMed] [Google Scholar]
- 8.Zhu, J., Shibasaki, F., Price, R., Guillemot, J. C., Yano, T., Dotsch, V., Wagner, G., Ferrara, P. & McKeon, F. (1998) Cell 93, 851–861. [DOI] [PubMed] [Google Scholar]
- 9.Zhu, J. & McKeon, F. (1999) Nature 398, 256–260. [DOI] [PubMed] [Google Scholar]
- 10.Zhu, J & McKeon, F. (2000) Cell Mol. Life Sci. 57, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranger, A. M., Oukka, M., Rengarajan, J. & Glimcher, L. H. (1998) Immunity 9, 627–635. [DOI] [PubMed] [Google Scholar]
- 12.Oukka, M., Ho, I. C., de la Brousse, F. C., Hoey, T., Grusby, M. J. & Glimcher, L. H. (1998) Immunity 9, 295–304. [DOI] [PubMed] [Google Scholar]
- 13.Masuda, E. S., Imamura, R., Amasaki, Y., Arai, K. & Arai N. (1998) Cell Signal. 10, 599–611. [DOI] [PubMed] [Google Scholar]
- 14.Mayer, T. U., Kapoor, T. M., Haggarty, S. J., King, R. W., Schreiber. S. L. & Mitchison, T. (1999) Science 286, 971–974. [DOI] [PubMed] [Google Scholar]
- 15.Picard, D. & Yamamoto, K. R. (1987) EMBO J. 6, 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beals, C. R., Sheridan, C. M., Turck, C. W., Gardner, P. & Crabtree, G. R. (1997) Science 275, 1930–1934. [DOI] [PubMed] [Google Scholar]
- 17.Wesselborg, S., Fruman, D. A., Sagoo, J. K., Bierer, B. E. & Burakoff, S. J. (1996) J. Biol. Chem. 271, 1274–1277. [DOI] [PubMed] [Google Scholar]
- 18.Chow, C. W., Dong C, Flavell, R. A. & Davis, R. J. (2000) Mol. Cell. Biol. 20, 5227–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komeili, A. & O'Shea, E. K. (2000) Curr. Opin. Cell Biol. 12, 355–360. [DOI] [PubMed] [Google Scholar]
- 20.Zitt, C., Strauss, B., Schwarz, E. C., Spaeth, N., Rast, G., Hatzelmann, G. & Hoth, M. (2004) J. Biol. Chem. 279, 12427–12437. [DOI] [PubMed] [Google Scholar]
- 21.Putney, J. W., Jr. (1999) Proc. Natl. Acad. Sci. USA 96, 14669–146671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatachalam, K., van Rossum, D. B., Patterson, R. L., Ma, H. T. & Gill, D. L. (2002) Nat. Cell. Biol. 4, E263–E272. [DOI] [PubMed] [Google Scholar]
- 23.Clapham, D. E., Runnels, L. W. & Strubing, C. (2001) Nat. Rev. Neurosci. 2, 387–396. [DOI] [PubMed] [Google Scholar]
- 24.Fanger, C. M., Hoth, M., Crabtree, G. R. & Lewis, R. S. (1995) J. Cell. Biol. 131, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serafini, A. T., Lewis, R. S., Clipstone, N. A., Bram, R. J., Fanger, C., Fiering, S., Herzenberg, L. A. & Crabtree, G. R. (1995) Immunity 3, 239–250. [DOI] [PubMed] [Google Scholar]
- 26.Feske, S., Giltnane, J., Dolmestsch, R., Staudt, L. M. & Rao, A. (2001) Nat. Immunol. 2, 316–324. [DOI] [PubMed] [Google Scholar]
- 27.Feske, S., Okamura, H., Hogan, P. G. & Rao, A. (2003) Biochem. Biophys. Res. Commun. 311, 1117–1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.