Significance
Stem cells not only initiate organs, but may also contribute to organ patterning, at least in the shoot apex of flowering plants: classical microsurgical experiments imply that the shoot apical meristem promotes development of the leaf adaxial side, i.e., the upper side. In this study, we show the existence of a transient low auxin zone in the adaxial side that contributes to adaxial development. We further find that this adaxial low auxin zone results from auxin transport from leaves to the shoot apex. Thus, it is not a positive signal from stem cells, but departure of a signaling molecule from primordia to stem cells, that delivers polarity information—opposite to what is generally assumed.
Keywords: auxin, leaf polarity, meristem, stem cell
Abstract
Stem cells are responsible for organogenesis, but it is largely unknown whether and how information from stem cells acts to direct organ patterning after organ primordia are formed. It has long been proposed that the stem cells at the plant shoot apex produce a signal, which promotes leaf adaxial-abaxial (dorsoventral) patterning. Here we show the existence of a transient low auxin zone in the adaxial domain of early leaf primordia. We also demonstrate that this adaxial low auxin domain contributes to leaf adaxial-abaxial patterning. The auxin signal is mediated by the auxin-responsive transcription factor MONOPTEROS (MP), whose constitutive activation in the adaxial domain promotes abaxial cell fate. Furthermore, we show that auxin flow from emerging leaf primordia to the shoot apical meristem establishes the low auxin zone, and that this auxin flow contributes to leaf polarity. Our results provide an explanation for the hypothetical meristem-derived leaf polarity signal. Opposite to the original proposal, instead of a signal derived from the meristem, we show that a signaling molecule is departing from the primordium to the meristem to promote robustness in leaf patterning.
Organs are differentiated from stem cells, but it remains unknown whether pattern formation in organs still requires information from undifferentiated stem cells. The shoot apical meristem (SAM) of plants maintains stem cells and generates new organs, such as leaves. To form planar leaves, leaf primordia develop dorsoventral (adaxial-abaxial in plants), proximodistal, and mediolateral patterns following initiation.
Extensive molecular genetic studies of more than a decade have identified a transcriptional regulatory network containing several adaxially or abaxially expressed leaf abaxial- and adaxial-promoting genes (1–6). These genes encode transcription factors and small RNAs, and their domain-specific expression patterns are required for adaxial-abaxial asymmetric cell differentiation and lamina expansion. Regulatory genes expressed in the abaxial domain suppress those expressed in the adaxial domain and vice versa. MicroRNAs 165 and 166 (MiR165/166) and transcription factor-encoding KANADI (KAN) genes are expressed in the abaxial domain and restrict the expression of class III homeodomain leucine zipper (HD-ZIPIII) genes PHABULOSA, PHAVOLUTA, and REVOLUTA (REV) to the adaxial domain (7, 8), and KANs directly repress LOB-domain gene ASYMMETRIC LEAVES2 (AS2), another adaxial regulator (9). The trans-acting short interfering RNA3 (TAS3), another small RNA, is expressed on the adaxial side to restrict their targets, ARF-domain genes ETTIN (ETT, also known as ARF3) and ARF4, to the abaxial domain (10–12). In addition, AS2 also directly suppresses ETT expression in the adaxial domain (13). However, HD-ZIPIII directly activates the expression of HD-ZIPII genes in the adaxial domain (14, 15). It is conceivable that incipient leaf primordia may be prepatterned into adaxial and abaxial domains (16), because REV and KAN1 are restricted very early to the adaxial domain and the abaxial domain of incipient floral meristem, respectively (17, 18). These and additional mutual repression and positive regulatory interactions can confine and stabilize gene expression regions to fine-tune leaf polarity.
Classical microsurgical experiments indicated that leaf adaxial-abaxial polarity also depends on the SAM (19, 20). A “Sussex signal” has been proposed as a hypothetical molecule that passes from the SAM to the adaxial domain of an incipient leaf primordium to promote adaxial domain formation (19). Surgical experiments have demonstrated that the Sussex signal is transmitted mainly through the epidermal (L1) layer (20). The hypothetical Sussex signal is a proposed example of organ patterning directed by stem cells through long-distance coordination after organ formation. Several proposals have been made to explain the Sussex experiment. Small RNAs, including TAS3 and miR165/166, can move between the adaxial and abaxial domains to direct polarity (7, 12). A recent study found that succinic semialdehyde (SSA) inhibits abaxial domain development and proposed that a γ-aminobutyric acid (GABA) shunt metabolite, SSA or related derivatives, are potential transmissible signaling molecules (21). Nevertheless, it remains to be tested whether small RNAs or any GABA shunt metabolite can move between the meristem and leaves. Therefore, the chemical basis of this signal remains an enigma, as does its mode of transmission, and how it acts in leaf polarity (1–6).
In this study, we investigated the role of auxin distribution dynamics in leaf polarity formation in tomato and Arabidopsis. Using an auxin sensor in Arabidopsis, we show in living SAMs that there is a transient low auxin zone in the adaxial domain of early leaf primordia. Furthermore, we show that ectopic adaxial application of auxin in tomato or ectopic adaxial activation of the auxin responsive transcription factor MONOPTEROS (MP, also known as ARF5) in Arabidopsis interferes with polarity. Auxin depletion from the leaf adaxial domain is mediated by PIN-FORMED1 (PIN1), and we demonstrate that localized inhibition of auxin transport between leaf primordia and the SAM induces leaf polarity defects. We also found that auxin efflux mutants show defects in leaf adaxial-abaxial polarity. These results suggest that auxin movement between leaf primordia and the SAM establishes a transient adaxial low auxin domain, which contributes robustness to leaf polarity. The Sussex signal, or a component of it, may therefore be auxin flow out of leaves into the meristem through dynamic reorganization of the PIN1 pump network, rather than a signal from the meristem to developing leaves.
Results
Transient Adaxial Low Auxin Zone Contributes to Leaf Polarity.
Recent studies have shown that formation of a region of low auxin accumulation is involved in specification of valve margins in the silique, and for axillary meristem initiation (22–24), indicating that formation of local auxin minima may be a general mechanism in organ patterning.
To test whether a low auxin region exists in leaf development, we imaged the auxin signaling sensor DII-Venus signal in developing leaves of the shoot apex in Arabidopsis (25). We observed a strong DII-Venus signal (indicating lower auxin) in the adaxial side compared with the abaxial side of emerging leaf primordia, from P1, which designates the youngest primordium (with I1 designating the oldest incipient primordium), until P9 (Fig. 1 A and B and Fig. S1). During leaf maturation, the adaxial low auxin domain disappears in P10 and older leaves, but the boundary low auxin region continues to exist (22, 23), suggesting the existence of a transient adaxial low auxin zone.
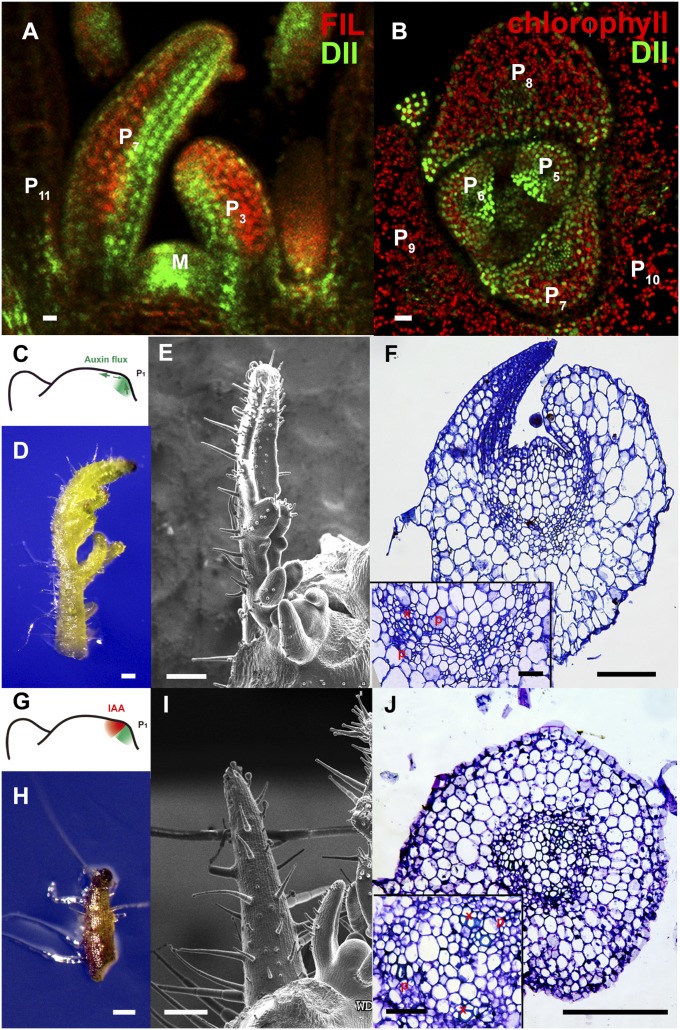
Fig. 1.
Transient adaxial low auxin domain is important for leaf polarity patterning. (A and B) Longitudinal and transverse sections through Arabidopsis SAM and leaf primordia region. DII-Venus signals are shown in green in A and B, and chlorophyll autofluorescence is in red (B). DII-Venus signal is enriched in the adaxial domain from P2 to P9. The abaxial domain in A has pFIL::DsRed-N7 (red) expression. Stronger DII-Venus signals in the boundary and adjacent adaxial domain indicated weaker auxin signaling input. Images in Fig. S1 show DII-Venus signals in leaf primordia of additional stages. (C–F) Control tomato leaf primordia, showing schematic diagram of the meristem surface auxin flux direction (C), early primordium 4–5 d after emergence (D), 7 d after emergence (E), and a transverse section through the midrib and adjacent laminal regions with close-up insertion of vascular strand (F). Note that phloem cells (p) surround the xylem (x) elements. (G–J), Tomato leaf primordia after adaxial IAA microapplication, showing site of microapplication (G), early leaf (H), and more mature (I) primordia with strong defects in adaxial-abaxial polarity, and a transverse section through the midrib and adjacent laminal regions (J). More images are shown in Figs. S5 and S6. (Scale bars: A and B, 20 μm; C–J, 200 μm; F and J Inset, 50 μm.)
To test whether the observed adaxial low auxin zone is related to polarity formation, we locally applied lanolin containing either auxin (indole-3-acetic acid; IAA), or auxin analogs (napthaleneacetic acid; NAA or 2,4-dichlorophenoxyacetic acid; 2,4-D) specifically to the adaxial side of newly formed primordia I1/P1 in tomato (Fig. S2A), which has a more accessible shoot apex than Arabidopsis. To test whether the effect of lanolin paste is restricted to the cells to which it was applied, we treated a P1 primordium with lanolin paste containing IAA on only one side. Exogenous auxin treatment is expected to convert compound leaves to simple leaves (26). We reproducibly observed conversion to simple leaves only at the treated side (Fig. S3), indicating that lanolin paste treatment had only local effects within a leaf, although phyllotaxis can be altered. Consistently, adaxial IAA treatment was able to induce a shift of auxin signaling, indicated by the synthetic pDR5::3×Venus-N7 reporter (27), to the adaxial domain in tomato (Fig. S4 A and B).
We found that adaxial IAA treatment can induce leaf polarity defects with conversion of adaxial identity to abaxial identity (30 of 102 for IAA, 20 of 112 for NAA, and 20 of 134 for 2,4-D; Fig. 1 C–J and Fig. S5), which was confirmed by epidermal morphology and vascular tissue structure (Fig. 1J and Figs. S5 D and H and S6). By contrast, IAA application to the abaxial side, which should enhance the gradient, generally did not affect adaxial-abaxial polarity (93 of 96), as reported (28). In addition, when we applied IAA to the entire leaf primordia, we found that polarity remains normal although most treated leaves bent toward the shoot apex (69%, 76 of 111; Fig. S7). Consistent with the transient nature of this leaf auxin gradient (Fig. 1 A and B), auxin treatment of leaf primordia older than P1 no longer affect polarity. These results indicated that low auxin concentration in the adaxial side of an early leaf primordium contributes to development or maintenance of adaxial fate.
MP Mediates Auxin Signaling To Promote Adaxial-Abaxial Patterning.
The adaxial-abaxial difference in auxin concentration may affect adaxial-abaxial symmetry by inducing differential downstream gene expression. Supporting this hypothesis, we found that the transient low auxin zone in the adaxial domain contributes to suppression of transcriptional activity of MP, a component in auxin signaling (29, 30), to promote abaxial cell fate.
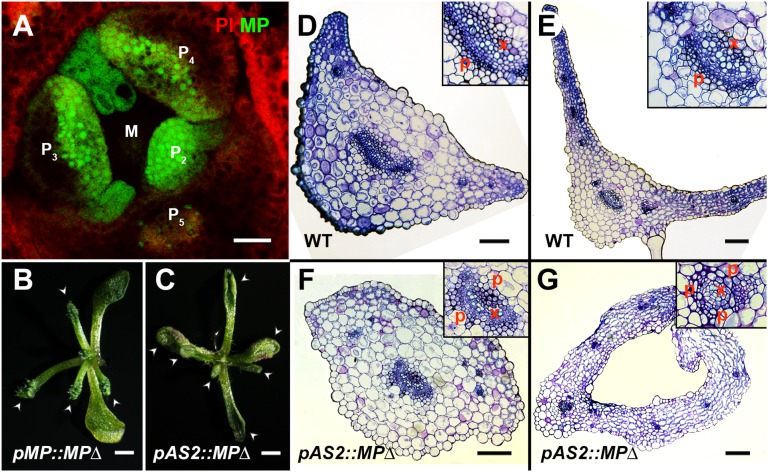
We detected MP-GFP expression in the entire young leaf primordium with adaxial enrichment in wild-type Arabidopsis plants (Fig. 2A and Fig. S8), partially overlapping with the adaxial low auxin domain (Fig. 1 A and B and Fig. S1). The distribution of MP and auxin in young leaf primordia implies that normal MP cannot activate auxin signaling in the adaxial domain, but more likely in the middle domain in between the adaxial and abaxial sides. Consistent with this hypothesis, we observed auxin signaling, reported by the DR5 reporter, in the middle domain of leaf primordia (Fig. S4A). It was recently reported that truncated alleles of MP (termed MPΔ) that escape auxin regulation and become constitutively active without auxin, but not loss-of-function alleles, cause leaf polarity defects, although the underlying mechanism was unclear (31, 32). We observed abaxial fate transition in trumpet-like leaves of transgenic pMP::MPΔ Arabidopsis plants based on vascular tissue structure and mesophyll morphology (Fig. 2B, Fig. S9, and Table S1). The truncated MPΔ is expected to activate ectopic auxin signaling in the adaxial domain, which would trigger leaf polarity defects. To determine whether the leaf polarity defect was caused by ectopic MP activity in the adaxial domain, we expressed MPΔ from the AS2 promoter in Arabidopsis. Although the AS2 promoter is activated in only the adaxial epidermis after leaf establishment (33), we could detect polarity defects in these pAS2::MPΔ plants (Fig. 2C), resembling those observed in transgenic pMP::MPΔ lines (Fig. 2B) (32). Mesophyll morphology and vascular tissue structure clearly indicated conversion of adaxial identity to abaxial identity (Fig. 2 D–G). On the contrary, expressing MPΔ in the boundary zone or in the abaxial domain did not cause polarity defects. In addition, ubiquitous MP overexpression does not lead to leaf polarity defects (34, 35). It is thus conceivable that the transient low auxin concentration in the adaxial domain and the resulting auxin gradient establishes a gradient of MP activity that acts to ensure normal establishment or maintenance of adaxial cell fate.
Fig. 2.
Ectopic adaxial MP activity induces abaxialized leaves. (A) A transverse section of meristem and primordia, stained by propidium iodide (PI, red), showing pMP::MP-GFP (green) signals in entire young leaf primordia with adaxial enrichment. (B and C) Transgenic pMP::MPΔ (B) and pAS2::MPΔ (C) plants showing disrupted leaf adaxial-abaxial polarity (arrowheads), including lacking lamina expansion and trumpet-shaped leaves. Transverse sections through the petiole regions (D and F) and blade regions (E and G) of wild-type leaves (D and E), and trumpet-like leaves from pAS2::MPΔ (F and G) indicate abaxialization in transgenic plants. (Scale bars: A, 20 μm; B and C, 1 mm; D–G, 100 μm.)
The Adaxial Low Auxin Zone Results from Polar Auxin Transport.
The distribution of auxin in the SAM depends largely on auxin transport, especially auxin efflux, which is directed by the polar subcellular localization of PIN1 auxin efflux transporter in the plasma membrane in the epidermal layer cells (17, 36). PIN1 mediated auxin transport in the SAM from all directions toward an incipient primordium acts to trigger the formation of a new primordium (17, 36). However, PIN1 polarity reverses and directs back toward the meristem center from early developing leaves, although the exact stage at which this reversal occurs is not known (17, 22, 23, 37).
To better resolve PIN1 dynamics during leaf initiation, we used live imaging of PIN1 polarization in tomato vegetative SAMs. In tomato plants expressing a pAtPIN1::AtPIN1-GFP transgene (37), we found that the auxin flux reversal is tightly associated with leaf initiation (Fig. 3A and Fig. S10A) (17). At the I1 stage, PIN1-GFP polarity is directed toward this site from the surrounding area. When an incipient primordium reaches the P1 stage, epidermal PIN1-GFP adaxial to the primordium reverses polarity from being directed toward the primordium to being directed back toward the meristem and the next incipient primordium (Fig. 3A and Fig. S10B). The PIN1-GFP reversal is maintained afterward. We thus concluded that the auxin flux reversal starts around the same time when leaves initiate.
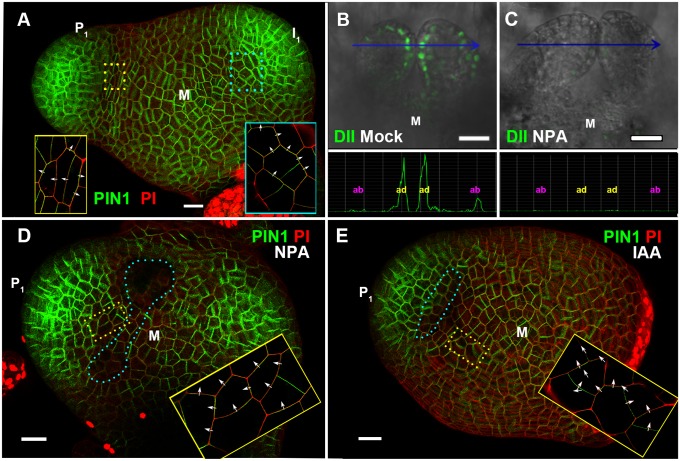
Fig. 3.
Spatial distribution of PIN1 expression and polarity patterns, and effects of NPA and IAA on auxin gradient and PIN1 localization in early leaf primordia. (A) View of vegetative tomato meristem stained by PI (red) from above showing a 3D volume rendering of pAtPIN1::AtPIN1-GFP expression and localization (green). The earliest primordium is marked as P1 and the oldest incipient primordium is labeled as I1. Regions in colored dotted line boxes were imaged at enhanced resolution and are shown in corresponding boxes after denoising and contrast enhancement to highlight polar localization of AtPIN1-GFP in each cell (images without denoising and contrast enhancement are shown in Fig. S10 A and B). Cells adaxial to I1 show AtPIN1-GFP polarity (arrows) toward the primordium, and cells adaxial to P1 show reversed AtPIN1-GFP polarity (arrows) toward the meristem center. (B and C) Signal of DII-Venus in the first pair of true leaves of Arabidopsis seedlings after mock NPA treatment (B) and after NPA treatment (C) for 5 h. Maximum intensity projections from the YFP channel (green) were overlaid with maximum intensity projections from the Nomarski channel. Quantifications of YFP channel intensities along blue lines in B and C are shown below corresponding images. (D and E) show maximum intensity projections of tomato pAtPIN1::AtPIN1-GFP (green) expressing meristems stained by PI (red) view from above. Regions in yellow dotted line boxes were imaged at enhanced resolution (and a slightly different angle) and are shown in yellow boxes within each image after denoising and contrast enhancement to highlight polar localization of AtPIN1-GFP (images without denoising and contrast enhancement are shown in Fig. S10 C and D. Tomato meristems were treated with NPA-containing lanolin paste outside I1 (D), or with lanolin paste containing IAA (E), with treated area highlighted by blue dotted line circles. Images (D and E) were acquired 24 h and 12 h, respectively, after treatment. (Scale bars: 20 μm.)
We and others have recently shown that PIN1-mediated auxin transport induces an auxin minimum at the leaf axil (22, 23, 25). We reasoned that the adaxial side reversal of PIN1 polarities may also lead to the adaxial low auxin domain. To test this hypothesis, we monitored DII-Venus signals (indicating lower auxin) before and after auxin polar transport inhibitor 1-N-naphthylphthalamic acid (NPA) treatment. As shown by live imaging, the boundary and adaxially enriched DII-Venus signal was evident in the first pair of true leaves 60 h after germination in Arabidopsis (Fig. 3B). The enriched DII-Venus signal mostly disappeared after a 5-h NPA treatment (Fig. 3C). Thus, auxin efflux is required for the transient adaxial low auxin domain formation, in addition to the boundary auxin minimum.
Inhibition of Auxin Transport Between Leaf Primordia and the SAM Induces Leaf Polarity Defects.
To test whether PIN1-directed auxin flux from leaf primordia toward the meristem center affects polarity, we locally manipulated auxin efflux in tomato, similarly to our adaxial application of auxin experiment. In the tomato shoot apex, we disturbed the auxin flux by drawing a thin line (∼20 μm or ∼2 cells in width) of lanolin paste containing NPA or the vesicle trafficking inhibitor brefeldin A (BFA), which also blocks auxin efflux, between I1 and the remainder of the meristem (Fig. 3D and Figs. S4C and S11). We found that such NPA or BFA separation of I1 from the SAM altered the auxin flux. Similar to untreated plants (Fig. 3A and Figs. S10 A and B), we inferred auxin flow from the boundary toward the SAM in mock-treated pAtPIN1::AtPIN1-GFP tomato plants 24 h after treatment (Fig. S11). In contrast, we observed cells between the lanolin line and the boundary transport auxin toward the leaf after NPA treatment, based on PIN1-GFP localization (Fig. 3D and Fig. S10C). Similarly, adaxial-specific IAA treatment partially altered the auxin flux (Fig. 3E and Fig. S10D), suggesting that local NPA and BFA treatment can alter auxin flux in the tomato shoot apex.
NPA/BFA treatment is expected to trap some of the auxin that would have been transported away in the primordium on the adaxial side to activate ectopic adaxial auxin signaling. Indeed, we observed strong auxin signaling shift toward the adaxial domain after NPA treatment (Fig. S4C) which is again similar to adaxial-specific IAA treatments. Because ectopic adaxial IAA application can alter auxin flux in the shoot apex, these results also suggest feedback between auxin distribution and transport during early leaf primordial development.
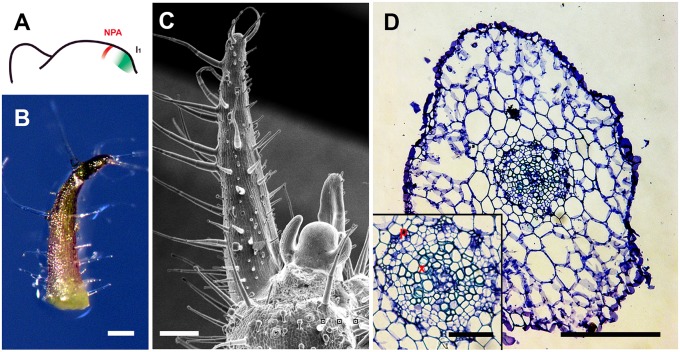
We observed defects in adaxial-abaxial polarity in primordia ∼7 d after NPA (34 of 128) or BFA (33 of 108) local treatment in tomato (Fig. 4 and Fig. S12). Both epidermal morphology and vascular tissue structure of the radial leaves represent a conversion of adaxial identity to abaxial identity in these radially symmetric leaves (Fig. 4D and Figs. S6 and S12) (20). In addition to polarity defects, we found distorted phyllotactic patterns and loss of lateral leaflets at a high frequency (Fig. S13), which were also found when IAA or auxin analogs were applied to the adaxial domain. These results support our hypothesis that auxin flux from leaf primordia toward the meristem center leads to reduced auxin accumulation in the adaxial domain, which further affects polarity.
Fig. 4.
Polar auxin transport inhibitors induced abaxialized radially symmetric leaves. Tomato leaf primordia after local NPA microapplication, showing site of microapplication (A), early leaf (B), and more mature (C) primordia with strong defects in adaxial-abaxial polarity, and a transverse section through the midrib and adjacent laminal regions (D). More images are shown in Figs. S6 and S12. (Scale bars: 200 μm, except D Inset, 50 μm.)
Based on our hypothesis, if we move NPA treatment closer to the primordium at the boundary zone, we would expect a reduced amount of auxin trapped in the primordium adaxial domain. We tested this hypothesis and found that auxin signaling was not shifted toward the adaxial domain (Fig. S4D). Consistently, we did not observe patterning defects after NPA treatment at the boundary zone (143 of 144) (Fig. S12F). In addition, absence of ectopic adaxial auxin signaling and absence of patterning defects (77 of 79) were observed after NPA-containing lanolin paste was applied to the center of leaf primordia (Fig. S4E and S12E), implying that meristem auxin flow, rather than auxin flow within a leaf primordium, is acting in adaxial domain development.
Auxin Transport Deficiency Enhances Leaf Polarity Defects.
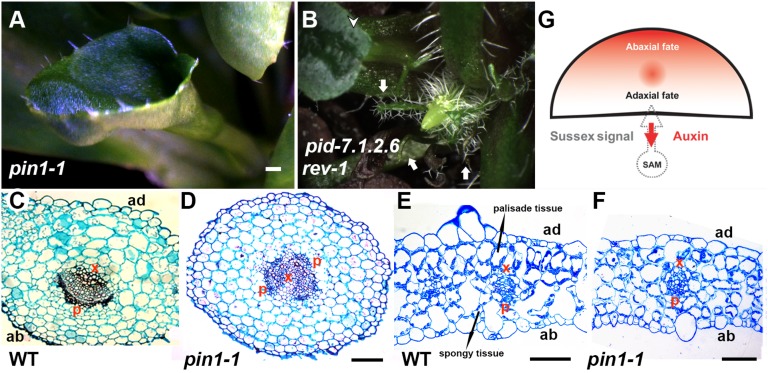
To further understand whether auxin efflux contributes to leaf polarity, we examined leaf patterning in auxin efflux mutants. Consistent with previous reports that treatment by auxin polar transport inhibitors induces lateral organ adaxial-abaxial polarity defects in several dicot species (38, 39), we found the formation of trumpet-like and rod-like leaves with polarity defects in Arabidopsis pin1 mutants (Fig. 5A, Fig. S14 A and B, and Table S1). In the strong pin1-1 allele, approximately one-quarter of all leaves have such polarity defects, and such leaves have abaxialized vasculature (Fig. 5 C and D). In the remaining pin1-1 leaves with normal shape, we observed internal anatomy difference of mesophylls (Fig. 5 E and F). In wild-type plants segregated from pin1-1/+, there is a steep contrast of adaxial and abaxial mesophyll morphology in cross-sections of the leaf blade: Cells in the adaxial two layers are larger, round, and densely packed, whereas cells in the abaxial tow layers are star-shaped and are separated by spacious air spaces. Adaxial mesophyll cells also have higher chloroplast density than abaxial ones (Fig. 5E). In pin1-1, the adaxial-abaxial gradient in cell density, cell shape, cell size, and chloroplast density is less pronounced (Fig. 5F; 7 of 7).
Fig. 5.
Adaxial-abaxial polarity phenotypes associated with polar auxin transport defects. (A) A radialized trumpet-shaped leaf found in a pin1-1 plant. (B) Radialized trumpet-shaped and rod-shaped leaves found in a pid-7.1.2.6 rev-1 plant. Arrowheads indicate trumpet-shaped leaves, and arrows indicate rod-shaped leaves. Transverse section through the petiole regions of a wild-type leaf (C) and a trumpet-like leaf from pin1-1 (D) indicate abaxialization in pin1 mutants. Transverse section through a wild-type leaf (E) and a pin1-1 leaf of regular shape (F) showing the four mesophyll layers. (G) Conceptual model of the influence on polarity of polar auxin transport from the leaf axil to the SAM. p, phloem cells; x, xylem cells. (Scale bars: A and B, 500 μm; C–F, 100 μm.)
Furthermore, we found synergistic effects on adaxial-abaxial polarity when auxin transport and REV, an HD-ZIPIII transcription factor important for adaxial cell fate, are both impaired. Although we did not observe leaf defects in adaxial-abaxial polarity in single Arabidopsis mutants of rev or pinoid (pid), a regulator of PIN protein activity, we found radial leaves in pid rev double mutants (Fig. 5B and Table S1). Similarly, the polarity defect of pin1 is significantly enhanced by rev so that close to one-half of all leaves are either trumpet-like or rod-like in pin1 rev (Fig. S14 C–E and Table S1). In the rest of leaves with normal leaf shape, we found similar reduced adaxial-abaxial gradient of mesophyll cells. In addition, we found that vascular tissue in the petiole region, but not in the blade region, was abaxialized (Fig. S15; 7 of 7). In contrast, we found that vascular tissue has normal adaxial-abaxial organization in both the blade and the petiole of leaves from pin1-1/+ rev-6 siblings. These data supported a model that PIN-dependent auxin efflux plays a role in the formation of the adaxial low auxin domain, which subsequently can have effects in leaf polarity (Fig. 5G).
Discussion
Whereas stem cells are responsible for organogenesis, it is not known whether and how information from undifferentiated stem cells directs organ patterning. We have identified a transient adaxial low auxin domain and have demonstrated that this low auxin domain contributes to leaf polarity patterning. Without the asymmetry in auxin distribution, both sides can take abaxial fate, suggesting that the adaxial low auxin domain is a causal, or at least permissive, element in establishing or maintaining adaxial fate. The transient adaxial low auxin domain disappears when leaf primordia reach P9 (Fig. 1 A and B and Fig. S1), which may be due to the balance between auxin transport and auxin biosynthesis: The adaxially expressed HD-ZIPIII proteins activate auxin biosynthesis (40), which could eventually eliminate the adaxial low auxin zone. Because flower meristems occupy the majority of primordia after the floral transition, and floral meristem formation is associated with active auxin signaling (41, 42), the adaxial low auxin zone may not exist during floral meristem formation (17), but may, rather, be leaf-specific.
Our data further suggest that auxin activates MP to promote abaxial, or discourage adaxial, cell fate. Activating the transcription factor activity of MP independently of auxin control in the adaxial domain resulted in leaf polarity defects (Fig. 2 and Fig. S9). The adaxially enriched MP expression (Fig. 2A and Fig. S8) and abaxial-enriched auxin (Fig. 1 A and B and Fig. S1) would result in highest auxin signaling in the middle domain (Fig. S4A), which could act in both adaxial-abaxial and mediolateral polarity patterning (43). We speculate that ectopic MP activities may promote middle domain identity in the adaxial domain to compete with the normal adaxial domain identity.
In addition, the abaxially enriched expression and activity of two other related ARFs, ETT and ARF4, that act in adaxial-abaxial polarity (10, 11) may also stabilize the earlier cue provided by the auxin gradient along the adaxial-abaxial axis. However, ETT and ARF4 have limited interactions with auxin/indole-3-acetic acid (AUX/IAA) proteins (25), and they can function without domains for interaction with AUX/IAA proteins, which are repressors that can be directly degraded upon auxin sensing (30, 44). MP, which acts mainly as an activator of transcription, and ETT and ARF4, which act mainly as repressors of transcription, have been shown to be able to bind the same DNA motifs thought to be present in the promoter of auxin-inducible genes (29). These three transcription factors could thus act in a combinatorial fashion in translating the transient auxin gradient we observed into specific patterns of expression of genes involved in adaxial-abaxial polarity.
Furthermore, we showed that leaf to SAM auxin flow is responsible for the adaxial low auxin domain and, thus, acts as a signal influencing formation or maintenance of the leaf adaxial domain (Fig. 5G). This auxin flow can explain at least in part the proposed Sussex signal from classical microsurgical experiments (19). We obtained indistinguishable abaxialized leaves either by surgical or NPA/BFA separation of leaf primordia from the SAM at comparable frequency (Fig. 4 and Figs. S12 and S16). Both separation experiments, and adaxial auxin treatment experiment, specifically affect leaf polarity at the I1 and P1 stages. Also consistent with the L1-specific PIN1 distribution, surgical experiments have demonstrated that the proposed Sussex signal is transmitted mainly through the L1 layer (20).
There has long been speculation that the classical microsurgical incision experiments cause inevitable tissue damage that leads to polarity defects (16). Our experiments used minimally invasive approaches to ectopically elevate auxin content, to ectopically activate auxin signaling, or to locally block PIN function, and resulted in similar leaf polarity defects as those obtained by microsurgical incision. These results suggest the existence of a signal rather than a pure artificial effect caused by tissue damage. Neither microsurgical incision nor our experiments led to polarity defects in all leaves, suggesting that this signal contributes to, but is not completely necessary for, polarity.
The auxin signal communication between the developing leaf and the meristem contributes to the robustness of leaf polarity, as does the well-studied canonical transcriptional network (1–6). It has been proposed that incipient leaf primordia acquire a prepatterned adaxial-abaxial axis (16), whereas the proposed meristem signal stabilizes and maintains this axis (5). Modeling mutual inhibitory interactions between classical adaxial and abaxial genes suggested that a regulatory network containing only these two groups of genes is likely instable (45). Auxin provides an additional signal to stabilize adaxial-abaxial patterning. Additive effects between auxin and classical polarity genes are supported by the observation of synergistic effects of double mutations in pid rev and pin1 rev on leaf polarity. Although neither strong pid nor rev alleles have leaf polarity defects, abaxialized leaves were found in pid rev double mutants, suggesting that auxin and REV act in parallel, and not in the same, pathways. In addition, the auxin signal likely reinforces the primary adaxial-abaxial field, because polarity defects were more often observed in mature leaves in auxin transport mutants (Fig. 5, Fig. S14, and Table S1), in plants treated with auxin transport inhibitors (39), and in MPΔ plants (Fig. 2, Fig. S9, and Table S1) (32). Similar to leaf polarity, a recent report found that not one, but two, independent signaling mechanisms define phyllotaxis, the spatiotemporal pattern of leaves initiation at the meristem, leading to increased precision (46).
The auxin transport-based signal identified in this work does not rule out other explanations for the Sussex experiment (19). In addition to auxin, siRNAs, miRNAs, GABA shunt metabolites, and lipids are all candidates for a meristem-derived mobile signal. It remains to be tested whether the reported polarity defects observed in the classical Sussex experiment are caused by auxin transport alone or by multiple factors.
Materials and Methods
Tomato (Solanum lycopersicum) plants were grown in MS medium and grown under controlled conditions (25 °C at 60% humidity, with 16 h of light and 8 h of dark), and Arabidopsis thaliana plants were grown in pots or in medium at 22 °C with 16 h of light and 8 h of dark unless otherwise stated. Detailed plant materials and growth conditions, construction of transgenic plants, microsurgery, chemical treatments, immunohistochemistry, confocal microscopy, optical microscopy, electron microscopy, image processing, and other procedures are fully described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. N. Ori, E. A. Schultz, and D. Weijers for seeds; Dr. C. Li for allowing us to use his tomato growth facility; and Drs. Y. Eshed, H. Huang, C. Kuhlemeier, O. Leyser, and L. Xu for discussions. Y.J. thanks Profs. S.-N. Bai and Z.-H. Xu for their encouragement. This work was supported by National Basic Research Program of China (973 Program) Grant 2014CB943500, National Natural Science Foundation of China Grant 31222033, State Key Laboratory of Plant Genomics Grant SKLPG2011B0103, US National Science Foundation 2010 Project Grant MCB-0929349, and France ANR-12-BSV6-0005 Grant (AuxiFlo). The E.M. laboratory is also supported by funds from the Howard Hughes Medical Institute and Gordon and Betty Moore Foundation Grant GBMF3406, and the Y.J. laboratory is supported by the Hundred Talents Program of the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421878112/-/DCSupplemental.
References
- 1.Barton MK. Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev Biol. 2010;341(1):95–113. doi: 10.1016/j.ydbio.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Bowman JL, Floyd SK. Patterning and polarity in seed plant shoots. Annu Rev Plant Biol. 2008;59:67–88. doi: 10.1146/annurev.arplant.57.032905.105356. [DOI] [PubMed] [Google Scholar]
- 3.Braybrook SA, Kuhlemeier C. How a plant builds leaves. Plant Cell. 2010;22(4):1006–1018. doi: 10.1105/tpc.110.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efroni I, Eshed Y, Lifschitz E. Morphogenesis of simple and compound leaves: A critical review. Plant Cell. 2010;22(4):1019–1032. doi: 10.1105/tpc.109.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husbands AY, Chitwood DH, Plavskin Y, Timmermans MC. Signals and prepatterns: New insights into organ polarity in plants. Genes Dev. 2009;23(17):1986–1997. doi: 10.1101/gad.1819909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Yang L, Huang H. Transcriptional, post-transcriptional and post-translational regulations of gene expression during leaf polarity formation. Cell Res. 2007;17(6):512–519. doi: 10.1038/cr.2007.45. [DOI] [PubMed] [Google Scholar]
- 7.Yao X, et al. Two types of cis-acting elements control the abaxial epidermis-specific transcription of the MIR165a and MIR166a genes. FEBS Lett. 2009;583(22):3711–3717. doi: 10.1016/j.febslet.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 8.Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004;131(12):2997–3006. doi: 10.1242/dev.01186. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, et al. KANADI1 regulates adaxial-abaxial polarity in Arabidopsis by directly repressing the transcription of ASYMMETRIC LEAVES2. Proc Natl Acad Sci USA. 2008;105(42):16392–16397. doi: 10.1073/pnas.0803997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pekker I, Alvarez JP, Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17(11):2899–2910. doi: 10.1105/tpc.105.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia D, Collier SA, Byrne ME, Martienssen RA. Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol. 2006;16(9):933–938. doi: 10.1016/j.cub.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 12.Chitwood DH, et al. Pattern formation via small RNA mobility. Genes Dev. 2009;23(5):549–554. doi: 10.1101/gad.1770009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki M, et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development. 2013;140(9):1958–1969. doi: 10.1242/dev.085365. [DOI] [PubMed] [Google Scholar]
- 14.Bou-Torrent J, et al. ATHB4 and HAT3, two class II HD-ZIP transcription factors, control leaf development in Arabidopsis. Plant Signal Behav. 2012;7(11):1382–1387. doi: 10.4161/psb.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turchi L, et al. Arabidopsis HD-Zip II transcription factors control apical embryo development and meristem function. Development. 2013;140(10):2118–2129. doi: 10.1242/dev.092833. [DOI] [PubMed] [Google Scholar]
- 16.Hagemann W, Gieissberg S. Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst Evol. 1996;199(3-4):121–152. [Google Scholar]
- 17.Heisler MG, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15(21):1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 18.Yadav RK, et al. Plant stem cell maintenance involves direct transcriptional repression of differentiation program. Mol Syst Biol. 2013;9:654. doi: 10.1038/msb.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sussex IM. Experiments on the cause of dorsiventrality in leaves. Nature. 1951;167(4251):651–652. doi: 10.1038/167651a0. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt D, Frenz M, Mandel T, Kuhlemeier C. Microsurgical and laser ablation analysis of leaf positioning and dorsoventral patterning in tomato. Development. 2005;132(1):15–26. doi: 10.1242/dev.01544. [DOI] [PubMed] [Google Scholar]
- 21.Toyokura K, et al. Succinic semialdehyde dehydrogenase is involved in the robust patterning of Arabidopsis leaves along the adaxial-abaxial axis. Plant Cell Physiol. 2011;52(8):1340–1353. doi: 10.1093/pcp/pcr079. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, et al. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell. 2014;26(5):2055–2067. doi: 10.1105/tpc.114.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Kohlen W, Rossmann S, Vernoux T, Theres K. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell. 2014;26(5):2068–2079. doi: 10.1105/tpc.114.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorefan K, et al. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature. 2009;459(7246):583–586. doi: 10.1038/nature07875. [DOI] [PubMed] [Google Scholar]
- 25.Vernoux T, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol. 2011;7:508. doi: 10.1038/msb.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig D, Bayer E, Kang J, Kuhlemeier C, Sinha N. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development. 2009;136(17):2997–3006. doi: 10.1242/dev.033811. [DOI] [PubMed] [Google Scholar]
- 27.Shani E, et al. Cytokinin regulates compound leaf development in tomato. Plant Cell. 2010;22(10):3206–3217. doi: 10.1105/tpc.110.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12(4):507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boer DR, et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell. 2014;156(3):577–589. doi: 10.1016/j.cell.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Hagen G, Guilfoyle T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol. 2002;49(3-4):373–385. [PubMed] [Google Scholar]
- 31.Garrett JJ, et al. A novel, semi-dominant allele of MONOPTEROS provides insight into leaf initiation and vein pattern formation. Planta. 2012;236(1):297–312. doi: 10.1007/s00425-012-1607-0. [DOI] [PubMed] [Google Scholar]
- 32.Krogan NT, Berleth T. A dominant mutation reveals asymmetry in MP/ARF5 function along the adaxial-abaxial axis of shoot lateral organs. Plant Signal Behav. 2012;7(8):940–943. doi: 10.4161/psb.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwakawa H, et al. Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. Plant J. 2007;51(2):173–184. doi: 10.1111/j.1365-313X.2007.03132.x. [DOI] [PubMed] [Google Scholar]
- 34.Krogan NT, Ckurshumova W, Marcos D, Caragea AE, Berleth T. Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol. 2012;194(2):391–401. doi: 10.1111/j.1469-8137.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 35.Hardtke CS, et al. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development. 2004;131(5):1089–1100. doi: 10.1242/dev.00925. [DOI] [PubMed] [Google Scholar]
- 36.Reinhardt D, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426(6964):255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 37.Bayer EM, et al. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev. 2009;23(3):373–384. doi: 10.1101/gad.497009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3(7):677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni DA, Wang LJ, Xu ZH, Xia ZA. Foliar modifications induced by inhibition of polar transport of auxin. Cell Res. 1999;9(1):27–35. doi: 10.1038/sj.cr.7290003. [DOI] [PubMed] [Google Scholar]
- 40.Huang T, et al. Arabidopsis KANADI1 acts as a transcriptional repressor by interacting with a specific cis-element and regulates auxin biosynthesis, transport, and signaling in opposition to HD-ZIPIII factors. Plant Cell. 2014;26(1):246–262. doi: 10.1105/tpc.113.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi N, et al. A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell. 2013;24(3):271–282. doi: 10.1016/j.devcel.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Li W, et al. LEAFY controls auxin response pathways in floral primordium formation. Sci Signal. 2013;6(270):ra23. doi: 10.1126/scisignal.2003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakata M, et al. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell. 2012;24(2):519–535. doi: 10.1105/tpc.111.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finet C, et al. Parallel structural evolution of auxin response factors in the angiosperms. Plant J. 2010;63(6):952–959. doi: 10.1111/j.1365-313X.2010.04292.x. [DOI] [PubMed] [Google Scholar]
- 45.Tameshige T, et al. Pattern dynamics in adaxial-abaxial specific gene expression are modulated by a plastid retrograde signal during Arabidopsis thaliana leaf development. PLoS Genet. 2013;9(7):e1003655. doi: 10.1371/journal.pgen.1003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Besnard F, et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature. 2014;505(7483):417–421. doi: 10.1038/nature12791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.