Significance
TH17 cells are a subset of CD4+ T helper cells that secrete the cytokine IL-17 and play a role in autoimmunity. RORγt is identified as a key transcription factor driving the TH17 differentiation. Sequence analysis indicated that transcription factor contains several conserved DNA-binding domain and isotype-specific domain that we termed transcription modulation domain (TMD). We designed a novel therapeutics, tRORγt-TMD, to deliver RORγt-TMD efficiently into the nucleus of the cells that regulates TH17 cell functions and TH17-mediated autoimmune diseases. With the same concept, tTbet-TMD also can regulate TH1 functions. In conclusion, tRORγt-TMD/tTbet-TMD can be novel and highly specific therapeutics for the treatment of TH17/TH1-mediated inflammatory disease and further allows us to discover new function of RORγt/Tbet in animals without genetic alteration.
Keywords: autoimmunity, transcription factor, RORγt, TH17, TMD
Abstract
The nuclear hormone receptor retinoic acid-related orphan receptor gamma t (RORγt) is a transcription factor (TF) specific to TH17 cells that produce interleukin (IL)-17 and have been implicated in a wide range of autoimmunity. Here, we developed a novel therapeutic strategy to modulate the functions of RORγt using cell-transducible form of transcription modulation domain of RORγt (tRORγt-TMD), which can be delivered effectively into the nucleus of cells and into the central nerve system (CNS). tRORγt-TMD specifically inhibited TH17-related cytokines induced by RORγt, thereby suppressing the differentiation of naïve T cells into TH17, but not into TH1, TH2, or Treg cells. tRORγt-TMD injected into experimental autoimmune encephalomyelitis (EAE) animal model can be delivered effectively in the splenic CD4+ T cells and spinal cord-infiltrating CD4+ T cells, and suppress the functions of TH17 cells. The clinical severity and incidence of EAE were ameliorated by tRORγt-TMD in preventive and therapeutic manner, and significant reduction of both infiltrating CD4+ IL-17+ T cells and inflammatory cells into the CNS was observed. As a result, the number of spinal cord demyelination was also reduced after tRORγt-TMD treatment. With the same proof of concept, tTbet-TMD specifically blocking TH1 differentiation improved the clinical incidence of rheumatoid arthritis (RA). Therefore, tRORγt-TMD and tTbet-TMD can be novel therapeutic reagents with the natural specificity for the treatment of inflammatory diseases associated with TH17 or TH1. This strategy can be applied to treat various diseases where a specific transcription factor has a key role in pathogenesis.
Naïve CD4+ T cells initiate a process of differentiation into effector CD4+ T cells upon stimulation with specific antigens. Infectious diseases were found to elicit preferentially a TH1 response, whereas parasitic infections provoke an expansion of TH2. TH1 differentiation requires a specific transcription factor Tbet and expresses IFN-γ, whereas TH2 needs GATA-3 and secretes IL-4, IL-5, and IL-13 (1). Regulatory T-cell is essential for the maintenance of peripheral tolerance and to control immune response. Foxp3 is a key transcription factor and expresses IL-10 to suppress or modulate the immune balance (2).
TH17 cells, a subset of T helper cells that secrete IL-17, provide host defense against bacterial and fungal infections. More importantly, TH17 cells are involved in the development of various autoimmune and inflammatory diseases when they remain active after clearance of the pathogens or the immunological balance among T-cell subsets is disrupted (3, 4). The nuclear hormone receptor retinoic acid-related orphan receptor gamma t (RORγt) has been identified as the TH17-specific transcription factor (5). IL-6 synergizes with transforming growth factor (TGF)-β to promote the expression of RORγt in favor of TH17 differentiation, and continuous RORγt expression is required to maintain the functions of TH17 cells in vivo (6, 7). In addition, IL-23 is important for enhancing the survival, proliferation, and pathological function of TH17 cells via induction of RORγt expression, and IL-21 is another cytokine that promotes the differentiation of TH17 cells in an autocrine manner and inhibits the induction of Foxp3 in Treg cells (8, 9).
TH1 cells cause the joint damage in rheumatoid arthritis (RA), a chronic autoimmune disease characterized by inflammation in the synovium leading to cartilage destruction, bone erosion, and joint deformities, mainly through IFN-γ–driven inflammatory mechanisms. However, mouse studies have demonstrated that the development of autoimmune disease does not require IFN-γ, suggesting that inhibition of expression or activity of Tbet can be better treatment strategies for autoimmunity associated with TH1 cells (10).
Targeting RORγt in TH17 cells or Tbet in TH1 cells could be therapeutically beneficial in the treatment of inflammatory autoimmune diseases. However, because transcription factors are known to be one of the protein classes that are difficult to target, a therapeutic agent aimed for specifically modulating the functions of RORγt or Tbet has yet to be discovered (11). A systematic understanding of the genomic targets of RORγt and the transcriptional network that controls differentiation of TH17 cells is beginning to emerge, and such knowledge will provides a unique opportunity to elucidate the functions of TH17 cells. Indeed, several small molecules such as digoxin (12), SR1001 (13), and TMP778/TMP920/GSK805 (14) that can inhibit the function of RORγt have been identified. Although these small molecules are effective in inhibition of TH17-mediated autoimmunity in vitro and in vivo, their functional specificity and cellular toxicity need to be thoroughly examined.
In this study, we demonstrated that tRORγt-TMD or tTbet-TMD, which can delivered into the nucleus in vitro and in vivo, and directly targets the endogenous RORγt or Tbet in interactomic inhibitory manner, effectively suppresses differentiation of naïve T cells into TH17 or TH1 cells, respectively, and their functions via inhibition of RORγt- or Tbet-mediated gene expression without affecting the differentiation of other T-cell subsets. tRORγt-TMD or tTbet-TMD markedly alleviated autoimmunity associated with TH17 or TH1 cells in preventive and therapeutic way. Therefore, intranucleusly transducible form of transcription factor (TF)-TMD (tTF-TMD) may be a fundamental and therapeutic strategy to modulate the functions of transcription factor specifically associated with various diseases, which can become a novel protein drug candidate for the treatment of these diseases.
Results
tRORγt-TMD Can Be Delivered Into the Nucleus of the Cells Effectively.
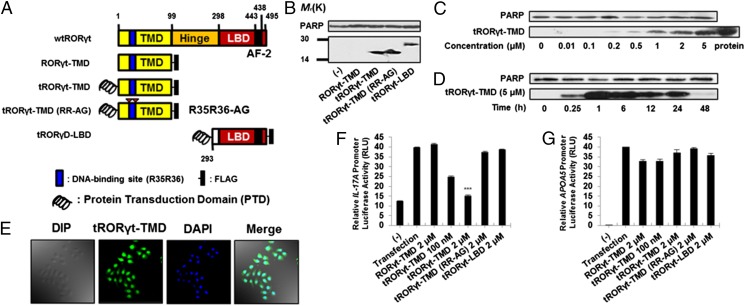
The N terminus of RORγt has a transcription modulation domain (TMD) comprising DNA-binding amino acid residues and isotype-specific sequences that may play key roles in the functional specificity of RORγt (15). Thus, we designed a novel therapeutics, tRORγt-TMD, to deliver RORγt-TMD efficiently into the nucleus of the cells in vitro and in vivo, and thereby, the delivered tRORγt-TMD competitively interferes with the transcriptional activity of endogenous RORγt at the promoter of RORγt-target genes. tRORγt-TMD was generated by fusing Hph-1-PTD (protein transduction domain) with the TMD of RORγt (16, 17). Nontransducible RORγt-TMD (RORγt-TMD), tRORγt-TMD without DNA-binding capacity [tRORγt-TMD (RR-AG)], and transducible RORγt-LBD (ligand-bindng domain, tRORγt-LBD) were generated for experimental controls (Fig. 1 A and B and Fig. S1A). Neither tRORγt-TMD nor the control proteins resulted in cytotoxicity in mouse CD4+ T cells (Fig. S1B). The level of endotoxin or bacterial DNA in each of the purified proteins was not within the range of functional influence. As shown in Fig. 1, tRORγt-TMD was transduced into mouse primary CD4+ T cells effectively in a dose- and time-dependent manner (Fig. 1 C and D). The delivered tRORγt-TMD remained inside the cells up to 48 h after transduction. Following delivery of tRORγt-TMD to HeLa cells, the majority of tRORγt-TMD was detected in the nucleus as early as 1 h after transduction, which was analyzed by confocal microscopy (Fig. 1E).
Fig. 1.
Generation of tRORγt-TMD, a transducible form of interactomic inhibitor of RORγt. (A) Structure of tRORγt-TMD and its derivatives: nontransducible RORγt-TMD, tRORγt-TMD, a mutant form of tRORγt-TMD without DNA-binding capacity [tRORγt-TMD (RR-AG)], and tRORγt-LBD. (B) Intranuclear transduction efficiency of RORγt-TMD, tRORγt-TMD, tRORγt-TMD (RR-AG), or tRORγt-LBD was examined by Western blot using with anti-FLAG antibody in mouse primary CD4+ T cells after 1-h transduction. (C and D) Dose-dependent (1 h) (C) and time-dependent (D) intranuclear transduction kinetics of tRORγt-TMD was analyzed with nuclear fraction of the cells by Western blot using with anti-FLAG antibody in mouse primary CD4+ T cells. (E) Intranuclear localization of tRORγt-TMD after transduction analyzed by confocal microscopy. (F and G) Functional specificity of tRORγt-TMD was examined in HEK293 cells cotransfected with the vectors expressing wild-type RORγt and luciferase driven by Il17-promoter (F) or with those expressing wild-type RORα1 and luciferase driven by apolipoprotein A5-promoter (G). After 24 h, luciferase activity was analyzed and the value was normalized by Renilla acrivity. Data are representative of at least five (B–D) and three (E–G) independent experiments. Error bars denote SEM. ***P < 0.001.
RORγt-Mediated Transcription Is Specifically Inhibited by tRORγt-TMD.
To examine the inhibitory effect of tRORγt-TMD on the induced expression of IL-17, which is the prominent cytokine induced by RORγt, HEK293 cells were cotransfected with plasmids expressing wild-type RORγt and luciferase driven by the IL-17A promoter (18). The transfected cells were then incubated with tRORγt-TMD, and the luciferase activity was measured. The tRORγt-TMD significantly reduced the RORγt-mediated luciferase activity in a dose-dependent manner, whereas neither the nontransducible RORγt-TMD nor the tRORγt-LBD affected this activity. Interestingly, tRORγt-TMD (RR-AG), which cannot bind to the IL-17A promoter, failed to attenuate the luciferase activity (Fig. 1F). To demonstrate the functional specificity of tRORγt-TMD, a similar experiment was performed by using two plasmids expressing wild-type RORα1 instead of RORγt and luciferase driven by the apolipoprotein A5 (APOA5) promoter (19). Inhibition of RORα1-mediated luciferase activity was not observed by transduction of tRORγt-TMD (Fig. 1G). Therefore, tRORγt-TMD can specifically inhibit the transcriptional activity of endogenous RORγt on its target genes.
tRORγt-TMD Specifically Inhibits IL-17 Cytokine Production in T-Cell Activation.
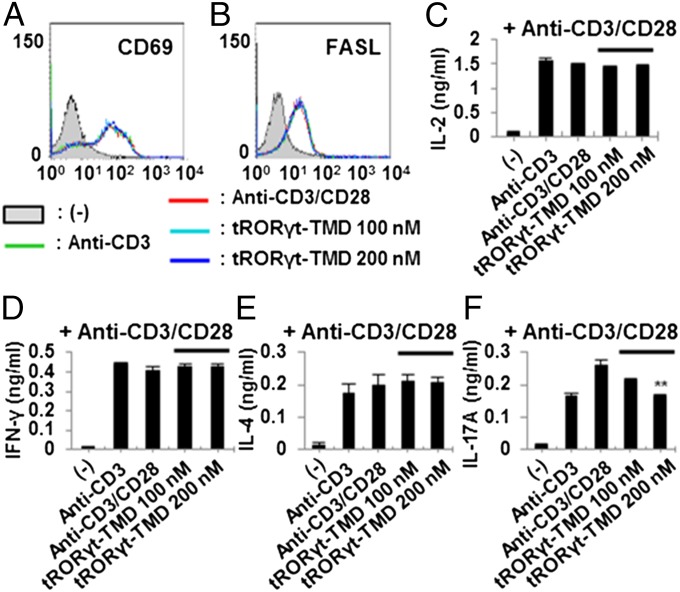
To investigate whether tRORγt-TMD affects T-cell activation or TcR-induced cytokine secretion from various T-cell subsets, total splenocytes from C57BL/6 mice were incubated with tRORγt-TMD for 1 h. The cells were washed and stimulated with plate-bound anti-CD3 antibody and soluble anti-CD28 antibody, and then the level of CD69 or FasL induction on the surface and the secretion of cytokines specific to different T-cell subsets were analyzed. Incubation with tRORγt-TMD did not inhibit the production of IL-2 or the induction of CD69 and FasL on the surface upon T-cell activation (Fig. 2 A–C). In addition, tRORγt-TMD substantially and specifically inhibited IL-17A secretion but did not influence the level of IFN-γ and IL-4 secretion from total splenocytes (Fig. 2 D–F). Thus, tRORγt-TMD can specifically down-regulate RORγt-mediated gene expression in TH17 cells by binding to its promoter without affecting the common T-cell activation signals and transcription of cytokines specific to TH1 or TH2 cells.
Fig. 2.
Specific inhibition of IL-17A secretion from TcR-stimulated splenocytes by tRORγt-TMD. (A–C) tRORγt-TMD did not affect the induced expression of CD69 (A) and FasL (B) on mouse splenocytes, and IL-2 secretion (C) from the splenocytes activated with plate-bound anti-CD3 and soluble anti-CD28 antibodies. The cells were stained with anti-CD69 or anti-FasL mAb and analyzed by FACS, and the level of IL-2 in the culture media was analyzed by ELISA. (D–F) tRORγt-TMD did not affect cytokine production from either TH1 (IFN-γ) (D) or TH2 (IL-4) (E) cells. (F) Dose-dependent inhibition of IL-17A production from TcR-stimulated splenocytes by tRORγt-TMD. The levels of IFN-γ, IL-4, or IL-17A in the culture media were analyzed by ELISA. Data are representative of at least three independent experiments. Error bars denote SEM. **P < 0.01.
tRORγt-TMD Prevents TH17 Differentiation and Functions Without Affecting Those of Other T-Cell Subsets.
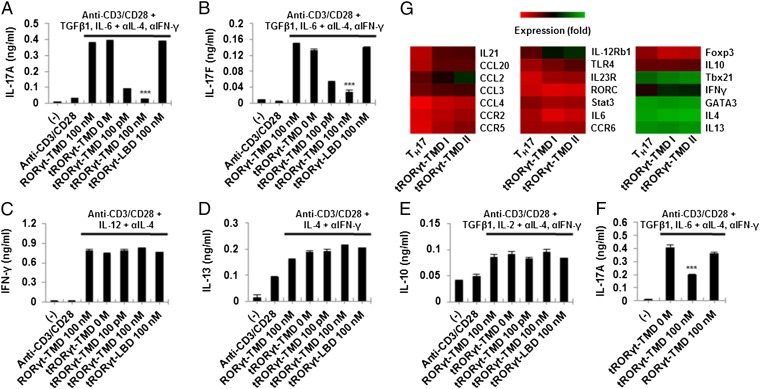
To determine whether tRORγt-TMD can specifically inhibit TH17 differentiation, naïve CD4+CD25−CD62Lhigh T cells were purified, incubated with RORγt-TMD, tRORγt-TMD, or tRORγt-LBD, and then activated with plate-bound anti-CD3 and soluble anti-CD28 antibodies in TH1-, TH2-, TH17-, or Treg-polarizing conditions. The levels of IL-17A and IL-17F were significantly decreased by tRORγt-TMD, but not by nontransducible RORγt-TMD or tRORγt-LBD under TH17-polarizing condition in dose-dependent manner (Fig. 3 A and B). Secretion of IL-17A from TH17 cells that were already differentiated from naïve T cells under TH17-polarizing condition (in vitro differentiated TH17) and CD4+ CCR6+ cells (in vivo differentiated TH17) were also inhibited by tRORγt-TMD, suggesting that not only differentiation induction from naïve T cells into TH17 cells but also the functions of TH17 cells were effectively blocked by tRORγt-TMD (Fig. S2). However, secretion of IFN-γ, IL-13, or IL-10 production under TH1-, TH2-, or Treg-polarizing condition was not affected by tRORγt-TMD (Fig. 3 C–E). When Treg cells were purified and stimulated with anti-CD3 and anti-CD28 mAb under TH17-polarizing condition in the presence of tRORγt-TMD, functional conversion of Treg cells into TH17 cells was also prevented (Fig. 3F) (20). These results confirm the inhibitory function and specificity of tRORγt-TMD on TH17 differentiation and its functions. In agreement with these results, microarray analysis of TH17 cells treated with tRORγt-TMD also demonstrated that TH17-specific molecules, including IL-21, CCL-2, CCL-20, IL-12Rβ1, and TLR-4, were down-regulated (Fig. 3G) (21, 22).
Fig. 3.
Differentiation of mouse primary naïve CD4+CD25−CD62Lhigh T cells into TH17 cells was specifically inhibited by tRORγt-TMD. (A–E) The mouse primary naïve CD4+CD25−CD62Lhigh T cells were incubated with 100 pM or 100 nM of RORγt-TMD, tRORγt-TMD, or tRORγt-LBD for 1 h, and then cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 72 h under TH17- (A and B), TH1- (C), TH2- (D), or Treg-polarizing conditions (E). (F) Treg cells were purified and then treated with tRORγt-TMD or RORγt-TMD under TH17-polarizing condition. The levels of IL-17A, IL-17F, IFN-γ, IL-13, or IL-10 in the culture media were analyzed by ELISA. (G) Microarray analysis of genes expressed in mouse primary naïve CD4+CD25−CD62Lhigh T cells untreated (TH17) or treated (tRORγt-TMD I, II) with 100 pM of tRORγt-TMD under TH17-polaring condition. Data are representative of at least three (A–F) independent experiments. Error bars denote SEM. ***P < 0.001.
tRORγt-TMD Suppresses the Progression of Experimental Autoimmune Encephalomyelitis in Preventive and Therapeutic Manner.
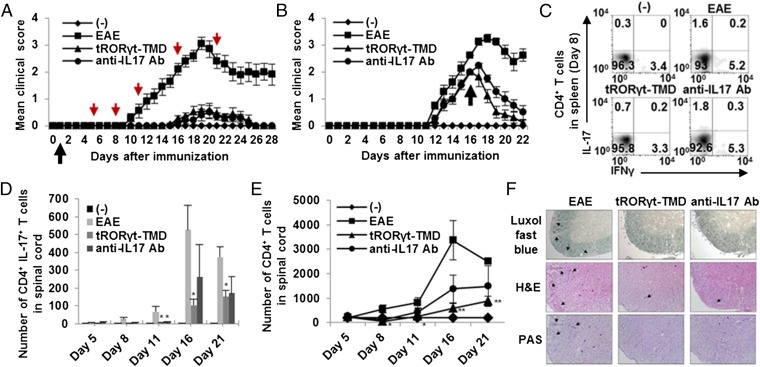
To determine whether tRORγt-TMD can prevent pathogenic progression of experimental autoimmune encephalomyelitis (EAE), disease-preventing potential of tRORγt-TMD was examined in EAE-induced mice in comparison with that of anti-IL17 mAb (23, 24). First, we found that tRORγt-TMD does not have any in vivo toxicity in mice tissues and did not affect RORγ-related thymocyte survival (Fig. S3). Then, we confirmed that tRORγt-TMD injected mice via i.p. route can be delivered into splenic CD4+ T cells (Fig. S4A). PBS-injected mice started to develop the signs of EAE around day 9 and reached peak disease manifestation (clinical score: 3.05 ± 0.23) at day 19. When EAE-induced mice were treated with tRORγt-TMD every other day from day 1, no EAE-associated symptoms was observed until day 14. After day 14, some mice developed mild EAE (clinical score: 0.55 ± 0.27), but they quickly subsided (Fig. 4A). Anti-IL17 mAb treatment every other day showed the disease-preventing efficacy similar to that of tRORγt-TMD (clinical score: 0.5 ± 0.25). To examine the therapeutic potential of tRORγt-TMD, EAE-induced mice with a clinical score above 2 were treated with tRORγt-TMD or anti-IL17 mAb from day 16. The tRORγt-TMD and anti-IL17 mAb treatment quickly and markedly suppressed EAE progression (Fig. 4B). Therefore, specific inhibition of RORγt function in TH17 cells by tRORγt-TMD has preventive and therapeutic potential for EAE.
Fig. 4.
Preventive and therapeutic potential of tRORγt-TMD in the amelioration of EAE through inhibition of TH17 differentiation and function. (A and B) Clinical assessment of EAE mice injected with PBS (EAE), tRORγt-TMD (2 mg/kg), or anti-IL17 mAb (2 mg/kg) every other day from day 1 (preventive) (A) or day 16 (therapeutic) (B) after EAE induction. The black arrows indicate the point of the first injection of tRORγt-TMD, and the red arrows indicate the analyzed day (days 5, 8, 11, 16, and 21). The (-) control is normal mice. (C and D) Splenocytes and mononuclear cells from spinal cord were reactivated with PMA/ionomycin for 4 h, and then stained for anti-CD4 and intracellular-stained with anti–IL-17A/IFN-γ mAb followed by FACS analysis. Percentages of CD4+ IL-17+/IFN-γ+ T cells in spleen (C) and absolute number of CD4+ IL-17+ T cells in spinal cord (D) were measured. (E) Inhibition of CD4+ T-cell infiltration into the spinal cord by tRORγt-TMD during the amelioration of EAE. Mononuclear cells from spinal cord were prepared at different time point. The cells were then stained for CD4 and analyzed by FACS. The total numbers of CD4+ T-cell in spinal cord were measured. (F) Spinal cord sections obtained from each mouse at day 21 after EAE induction were analyzed for the extent of demyelination and inflammation. Data are representative of more than three experiments with 10 to 40 mice per group (A and B) or one experiment with at least three to five mice per group (C–F). Error bars denote SEM. *P < 0.05, **P < 0.01.
To confirm whether the tRORγt-TMD–mediated inhibition of TH17 differentiation is responsible for its therapeutic effectiveness on EAE, the level of TH17 cells in the spleen were examined on day 8 and those in spinal cord on day 5, 8, 11, 16, and 21 after immunization, respectively. The level of TH17 cells (CD4+ IL-17A+) and TH1 cells (CD4+ IFN-γ+) were significantly reduced in the spleen/lymph node/spinal cord/brain, and thereby, the number of both populations in the spinal cord were low in tRORγt-TMD–treated mice. In anti-IL17 mAb-treated mice, the level of TH17 and TH1 cells in the spleen/lymph node was comparable to that in PBS-injected EAE mice, but a low level of TH17 cells was detected in the spinal cord/brain probably due to the blockage of IL-17A–dependent infiltration (Fig. 4C and Fig. S5). Therefore, the absolute number of CD4+ IL-17+ T cells in spinal cord was also decreased (Fig. 4D). These results were in contrast to the finding that tRORγt-TMD did not affect IFN-γ secretion under TH1-polarizing condition (Fig. 3C). Therefore, it is hypothesized that TH17 cells play an important role in the initial stages of EAE onset and progression, and generation of encephalogenic TH1 cells depend on the in vivo inflammatory microenvironment created by the TH17 cells.
To assess the degree of demyelination and inflammation of the spinal cord and brain of EAE-induced mice treated with tRORγt-TMD, a histopathological evaluation of the CNS was performed. In the PBS-injected EAE mice, profound EAE lesions were detected in the spinal cord accompanying T-cell infitration, demyelination, and inflammation. In contrast, in the tRORγt-TMD–treated mice, all of these symptoms associated with EAE were significantly diminished in the spinal cord and brain (Fig. 4 E and F, and Fig. S6). To test effective delivery of tRORγt-TMD into the spinal cord in vivo and, thereby, suppressed EAE symptoms in EAE-induced mice, the presence of tRORγt-TMD in the spinal cord was examined by immunohistochemical staining. Indeed, significant levels of tRORγt-TMD were detected in the spinal cord-infiltrating CD4+ T cells prepared at day 21 (Fig. S4B).
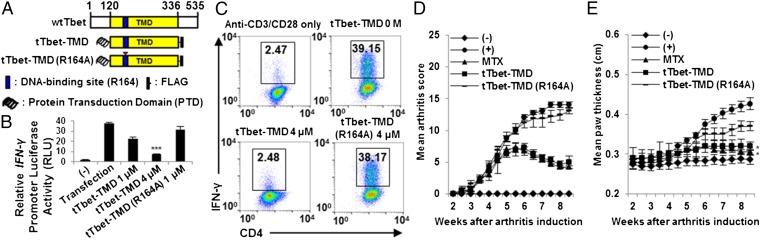
tTbet-TMD, Interatomic Inhibitor of Tbet, Suppresses TH1-Mediated Autoimmunity.
To confirm whether this strategy can be applied to TH1-specific transcription factor, Tbet, tTbet-TMD containing Hph-1-PTD and TMD of Tbet was generated and intranuclear delivery of tTbet-TMD was as effecient as that of tRORγt-TMD (Fig. 5A). tTbet-TMD can specifically inhibit the transcription activity of Tbet binding to the promoter of IFN-γ and block the differentiation of naïve T cells into TH1 cells without affecting the differentiation of other T-cell subsets (Fig. 5 B and C). However, tTbet-TMD (R164A), which cannot bind to the IFN-γ promoter, failed to attenuate the luciferase activity (25). Additionally, the clinical severity of arthritis was significantly mitigated by tTbet-TMD, and its therapeutic efficacy was comparable to that of i.p.-injected methotrexate (MTX) (Fig. 5 D and E and Fig. S7).
Fig. 5.
Preventive potential of tTbet-TMD in the alleviation of CIA. (A) Structure of tTbet-TMD. (B) Competitive inhibition of Tbet-dependent transcriptional activity by tTbet-TMD was analyzed in HEK293 cells cotransfected with the vectors expressing wild-type Tbet and luciferase driven by IFN-γ promoter. (C) The mouse primary naïve CD4+CD25−CD62Lhigh T cells were incubated with 1 or 4 μM of tTbet-TMD for 1 h, and then cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 48 h under TH1-polarizing conditions. The cells were stained for CD4 and IFN-γ, and then analyzed by FACS. (D and E) i.p. injection of MTX (35 mg/kg) and tTbet-TMD (2.5 mg/kg) were performed every other day for 4–8 wk from day 1 after primary immunization. (D) Inflammatory condition of the paws was observed before mice were killed. (E) Microscopic analysis of arthritis was assessed by paw thickness. Data are representative of at least three (B and C) independent experiments or eight mice per group (D and E). Error bars denote SEM. *P < 0.05, ***P < 0.001.
Discussion
RORγt is a major transcription factor that is essential for TH17 cell differentiation. Thus, it plays critical roles in orchestrating a TH17 cell-mediated inflammatory microenvironment including IL-17 secretion, which often leads to autoimmunity such as rheumatoid arthritis (RA) and multiple sclerosis (MS). Thereby, it have been well recognized that inhibition of TH17 cell differentiation and functions would be an important therapy for such autoimmune diseases. Biologics (ixekizumab, brodalumab, and secukinumab) that inhibit IL-17 functions have been developed, but clinical trials in various autoimmune diseases have been reported to be partially successful. It is due to their ineffective biological activity in some autoimmune diseases models and their adverse effects in a certain subset of patients. These results suggested that functional inhibition of RORγt would be more critical to modulate TH17-mediated autoimmunity rather than targeting individual TH17-specific cytokines and surface molecules.
Sequence analysis revealed that RORγt has transcription modulation domain (1–99) on N terminus, comprising isotype-specific domain and DNA-binding domain (DBD), which binds to the major groove of specific DNA helices (AGGTCA) upstream of the transcription initiation sites. Ligand-binding domain on C terminus, linked by the hinge region, contains 12 helices and is responsible for not only ligand binding but also nuclear localization and dimerization (26).
In this study, we developed a novel therapeutic strategy to suppress the functions of endogenous RORγt in interactomic and competitive manner by intranuclear delivery of TMD of RORγt in vitro and in vivo. tRORγt-TMD is a fusion protein between TMD of RORγt and a human origin Hph-1-PTD that can be delivered into the nucleus effectively in dose- and time-dependent manner. tRORγt-TMD, not tRORγt-TMD without DNA-binding capacity [tRORγt-TMD (RR-AG)], significantly inhibited IL-17A promoter activity mainly through the competition with endogenous tRORγt for promoter binding. Importantly, tRORγt-TMD did not affect the APOA5 promoter activity induced by RORα1, suggesting that transcriptional inhibition of tRORγt-TMD is highly isotype-specific. tRORγt-TMD suppressed the secretion of IL-17 from the splenocytes, but neither secretion of TH1- and TH2-specific cytokines from the splenocytes nor the molecules induced by TcR stimulation on their surface were affected by tRORγt-TMD. Consistent with these results, tRORγt-TMD can prevent TH17 differentiation, but not TH1, TH2, and Treg differentiation even at a level of picomolars. The gene known to be induced by RORγt such as IL-17A/F, IL-21, CCL-2, CCL-20, IL-12Rβ1, and TLR-4 were significantly suppressed by tRORγt-TMD, which was confirmed by microarray analysis.
T cells are known to be crucial for inducing EAE, animal model of MS, where the inflammatory lesions are characterized by massive infiltration of inflammatory cells, inducing T cells, B cells, and macrophages (27, 28). Previously, it has been agreed in the field that only TH1 plays a critical role in neurologic inflammatory disease, but recent reports have emphasized the pathogenic role of TH17 cells and T cells secreting IL-17/IFN-γ together rather than that of TH1 (29). Therapeutic potential of tRORγt-TMD was clearly demonstrated in EAE in a preventive and therapeutic manner. tRORγt-TMD effectively inhibited TH17 cell differentiation in the spleen. Thereby, the number of CD4+ T cells and many inflammatory cells was greatly reduced in the spinal cord, and the neuronal demyelination was significantly decreased. As expected, anti-IL17 mAb did not inhibit TH17 cell differentiation in the spleen, but prevented the migration of TH17 cells into the spinal cord. Interestingly, tRORγt-TMD also blocked the generation of IFN-γ–secreting CD4+ T cells in the spleen (Fig. 4C). These results may indicate that TH17 cells play an important role in forming the inflammatory microenvionment including IL-17 secretion at the early stage of EAE, and such inflammatory condition may involve the generation of a subpopulation of TH17 cells secreting IFN-γ, which has been reported to be pathogenic in EAE induction. The expression of GM-CSF, which is the encephalitogenic cytokine produced by TH17 cells, was also inhibited by tRORγt-TMD (Fig. S8) (30). Transduction capability and the stable presence of tRORγt-TMD in the spinal cords are synergistically important to suppress the functions of TH17 cells. All of these therapeutic elements may account for the slightly better therapeutic efficacy of tRORγt-TMD than that of anti-IL17 mAb not only in EAE but also in colitis animal model (Fig. S9).
Two previous studies showed that two small molecules targeting the ligand-binding domain of RORγt alleviated autoimmune diseases by inhibiting RORγt transcriptional activity (31). Recently, three small molecules were shown to inhibit the RORγt-dependent transcriptional network to varying extents and by divergent mechanisms. One small molecule inhibited RORγt binding to its target DNA, whereas the other two affected RORγt-mediated transcription predominantly without removing DNA binding (14, 32). However, to our surprise, our results showed that tRORγt-TMD, being as a therapeutic protein, was much more effective and specific than these small molecules in modulation of TH17-mediated autoimmunity. tRORγt-TMD showed a great therapeutic potential in EAE animal model with less concentration and less treatment frequency compared with these compounds (33).
Taking these results together, we demonstrated that interacomic modulation of RORγt functions is a novel therapeutic strategy in a variety of diseases with TH17-mediated inflammatory etiology. Functional inhibition of tRORγt-TMD on human TH17 cells function was also confirmed with human PBMCs (Fig. S10). IL-17–secreting γδ-T cells were found at high frequencies in the CNS of mice with EAE and are important for mediating autoimmune pathology (34). Inhibition of RORγt in γδ-T cells by tRORγt-TMD may offer additive effects to its therapeutic activity because γδ-T-cell–derived IL-17 is one of the earliest sources of the cytokine after infection (35).
Therapeutic proof of concept of tTF-TMD was confirmed with Tbet, master TF for TH1 cell differentiation and functions. Consistent with the results by tRORγt-TMD, tTbet-TMD inhibited the transcriptional activity of endogenous Tbet and prevented the differentiation of naïve T cells into TH1 cells, not into other T-cell subsets. Therapeutic efficacy of tTbet-TMD was comparable to that of MTX in collagen-induced arthritis (CIA)-induced animal model of RA.
In conclusion, tRORγt-TMD and tTbet-TMD can be novel and highly specific therapeutics for the treatment of TH17 and TH1-mediated inflammatory diseases, and further allows us to unravel new function of RORγt and Tbet in other immune cells or in animals without genetic alteration. Moreover, local delivery of tTF-TMD through the skin barrier has been demonstrated (36, 37). It is also notable that skin-penetrating capability of tRORγt-TMD and tTbet-TMD enables its therapeutic potency to be confined to local lesion area without systemic toxicity in a case of RA or in many skin autoimmune diseases. This novel strategy can be easily applicable to development of a novel therapeutics for the treatment of various diseases, where a specific transcription factor has a key role in pathogenesis.
Materials and Methods
The RORγt-DBD (RORγt-TMD) and RORγt-LBD that encode amino acids 1–99 and 293–495, respectively, of the wild-type RORγt (1–495) were amplified from the RORγt plasmid (from D. R. Littman, The Kimmel Center for Biology and Medicine of the Skirball Institute, New York University School of Medicine, New York) by PCR. The tTbet-TMD that encode amino acids 120–336 of the wild-type Tbet were amplified from the Tbet plasmid (from L. H. Glimcher, Weill Cornell Medical College, New York) by PCR. Animal experimental procedures were approved by Yonsei Laboratory Animal Research Center (YLARC)-Institutional Animal Care and Use Committee guidelines (YLARC2010-0035). For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the Translational Research Center for Protein Function Control, NRF (2009-0083522), and the Brain Korea 21 (BK21) PLUS program; T.Y.P. is awarded a fellowship by BK21 PLUS program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413687112/-/DCSupplemental.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 10.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12(7):597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber K. Newsmaker: Lycera. Nat Biotechnol. 2011;29(8):679. doi: 10.1038/nbt0811-679. [DOI] [PubMed] [Google Scholar]
- 12.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472(7344):486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao S, et al. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40(4):477–489. doi: 10.1016/j.immuni.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: Critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 16.Choi JM, et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006;12(5):574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- 17.Park TY, et al. Amelioration of neurodegenerative diseases by cell death-induced cytoplasmic delivery of humanin. J Control Release. 2013;166(3):307–315. doi: 10.1016/j.jconrel.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genoux A, et al. Transcriptional regulation of apolipoprotein A5 gene expression by the nuclear receptor RORalpha. Arterioscler Thromb Vasc Biol. 2005;25(6):1186–1192. doi: 10.1161/01.ATV.0000163841.85333.83. [DOI] [PubMed] [Google Scholar]
- 20.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esplugues E, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475(7357):514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofstetter HH, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237(2):123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Sommereyns C, Théate I, Michiels T, Van Snick J. Anti-IL-17A autovaccination prevents clinical and histological manifestations of experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2007;1110:330–336. doi: 10.1196/annals.1423.035. [DOI] [PubMed] [Google Scholar]
- 25.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 26.Dzhagalov I, Zhang N, He YW. The roles of orphan nuclear receptors in the development and function of the immune system. Cell Mol Immunol. 2004;1(6):401–407. [PubMed] [Google Scholar]
- 27.Traugott U, Shevach E, Chiba J, Stone SH, Raine CS. Chronic relapsing experimental allergic encephalomyelitis: Identification and dynamics of T and B cells within the central nervous system. Cell Immunol. 1982;68(2):261–275. doi: 10.1016/0008-8749(82)90111-3. [DOI] [PubMed] [Google Scholar]
- 28.Nyland H, Mörk S, Matre R. In-situ characterization of mononuclear cell infiltrates in lesions of multiple sclerosis. Neuropathol Appl Neurobiol. 1982;8(5):403–411. doi: 10.1111/j.1365-2990.1982.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 29.Duhen R, et al. Cutting edge: The pathogenicity of IFN-γ-producing Th17 cells is independent of T-bet. J Immunol. 2013;190(9):4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 31.Jetten AM. Immunology: A helping hand against autoimmunity. Nature. 2011;472(7344):421–422. doi: 10.1038/472421a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skepner J, et al. Pharmacologic inhibition of RORγt regulates Th17 signature gene expression and suppresses cutaneous inflammation in vivo. J Immunol. 2014;192(6):2564–2575. doi: 10.4049/jimmunol.1302190. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Li ZX, Qiu CX, Wang D, Cui QH. The relationship between rational drug design and drug side effects. Brief Bioinform. 2012;13(3):377–382. doi: 10.1093/bib/bbr061. [DOI] [PubMed] [Google Scholar]
- 34.Sheridan C. Footrace to clinic heats up for T-cell nuclear receptor inhibitors. Nat Biotechnol. 2013;31(5):370. doi: 10.1038/nbt0513-370. [DOI] [PubMed] [Google Scholar]
- 35.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Choi JM, et al. Transduction of the cytoplasmic domain of CTLA-4 inhibits TcR-specific activation signals and prevents collagen-induced arthritis. Proc Natl Acad Sci USA. 2008;105(50):19875–19880. doi: 10.1073/pnas.0805198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park TY, Shin MJ, Park SD, Lee SK. Alleviation of abnormal synaptic neurotransmitter release by cell-permeable form of the truncated SNAP-25 upon transcutaneous delivery. Neurosci Lett. 2013;543:52–57. doi: 10.1016/j.neulet.2013.02.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.