Significance
Although lungs are continuously bombarded by bacteria, pulmonary defense mechanisms normally keep them sterile. Those defenses include a complex mixture of antimicrobial peptides in the thin layer of liquid coating the airway surface. In cystic fibrosis, impaired bicarbonate secretion causes the airway surface liquid to become abnormally acidic. Here we found that an acidic pH impairs the ability of two key airway antimicrobial peptides, β-defensin-3 and LL-37, to kill bacteria. When these peptides were combined, they exhibited synergistic killing of Staphylococcus aureus, an organism that infects cystic fibrosis lungs. However, an acidic pH reduced their synergistic effect. Thus, an acidic pH impairs an important respiratory defense mechanism and may predispose the lungs of people with cystic fibrosis to bacterial infection.
Keywords: cystic fibrosis, host defense, cathelicidin, Staphylococcus aureus, Pseudomonas aeruginosa
Abstract
The pulmonary airways are continuously exposed to bacteria. As a first line of defense against infection, the airway surface liquid (ASL) contains a complex mixture of antimicrobial factors that kill inhaled and aspirated bacteria. The composition of ASL is critical for antimicrobial effectiveness. For example, in cystic fibrosis an abnormally acidic ASL inhibits antimicrobial activity. Here, we tested the effect of pH on the activity of an ASL defensin, human β-defensin-3 (hBD-3), and the cathelicidin-related peptide, LL-37. We found that reducing pH from 8.0 to 6.8 reduced the ability of both peptides to kill Staphylococcus aureus. An acidic pH also attenuated LL-37 killing of Pseudomonas aeruginosa. In addition, we discovered synergism between hBD-3 and LL-37 in killing S. aureus. LL-37 and lysozyme were also synergistic. Importantly, an acidic pH reduced the synergistic effects of combinations of ASL antibacterials. These results indicate that an acidic pH reduces the activity of individual ASL antimicrobials, impairs synergism between them, and thus may disrupt an important airway host defense mechanism.
Many organisms defend against infection by producing antimicrobials at the interface with the environment (1–4). In the mammalian respiratory system, the interface between the environment and the organism is a thin layer of airway surface liquid (ASL). To maintain sterile lungs and protect against bombardment with bacteria, mammalian lungs have evolved multiple protective mechanisms, one of which is antimicrobials in ASL that rapidly kill inhaled and aspirated bacteria (5–9).
ASL antimicrobial activity arises from a complex mixture of antimicrobial peptides, proteins, and lipids that vary in size, structure, and abundance (10, 11). Many share cationic and hydrophobic features that render them amphipathic (2, 12–17). Two effectors of innate host defense are the defensins, which have β-sheet structures and three disulfide bonds, and the cathelicidins, which have linear α-helical structures (2, 3, 15, 18). Human β-defensin-3 (hBD-3) (19, 20) and the cathelicidin LL-37 (21–23) have broad antimicrobial spectrums, including activity against Staphylococcus aureus and Pseudomonas aeruginosa. At neutral pH, they are cationic and kill bacteria by disrupting the phospholipid membrane and dissipating the electrochemical gradient (4). Both hBD-3 and LL-37 retain activity under physiological ionic strength, and they both rapidly kill bacteria (19–23).
Several previous genetic studies highlighted the potential role of defensins as antimicrobials (24). Some suggested that polymorphisms in the defensin gene cluster modulate the phenotype of cystic fibrosis (CF) airway disease, including an association with chronic P. aeruginosa infection (25–27). In addition, a genetic variant in the promoter region of a defensin gene cluster was associated with reduced hBD-1 and hBD-3 transcript levels and a higher risk of persistent S. aureus nasal colonization in a cohort of healthy individuals (28). However, two reports did not identify an association, perhaps because the populations studied had different genetic backgrounds (29, 30). Polymorphisms in defensin genes are also associated with infections outside the lung (31–33). Also consistent with an important role in defense is the observation that airway insults and disease can increase defensin gene expression (34–36).
We recently obtained additional evidence for the contribution of ASL antimicrobials to host defense. Studying a porcine model of cystic fibrosis (CF) (37, 38), we found that loss of cystic fibrosis transmembrane conductance regulator (CFTR) anion channels reduced HCO3− secretion and decreased ASL pH (39, 40), results consistent with earlier findings in human airway epithelia (41–44). Importantly, the abnormally acidic pH partially inhibited bacterial killing by ASL (40). Those findings identified a host defense defect that may predispose CF airways to bacterial infection. We also reported that an acidic pH inhibited two ASL antimicrobials, lysozyme and lactoferrin (40). However, those experiments were performed with a low ionic strength that is optimal for antimicrobial activity. Thus, although lysozyme and lactoferrin may contribute to the inhibitory effect of an acidic ASL pH, other antimicrobial factors may make a greater contribution.
With this background, we tested the hypothesis that at a more physiological ionic strength, a reduced pH would inhibit the activity of hBD-3 and LL-37. A previous study showed the effect of a low ionic strength on synergism between antimicrobials (45). Therefore, here we also tested the hypothesis that hBD-3 and LL-37 exhibit synergism in killing bacteria and that a reduced pH would inhibit synergism.
Results
hBD-3 and LL-37 Killing of S. aureus Is Concentration-Dependent and Relatively Insensitive to Ionic Strength.
We investigated antimicrobial activity using S. aureus because it is one of the first bacteria to infect CF lungs (46–48). To assay bacterial viability, we used an S. aureus strain that expresses a bacterial luciferase (S. aureus Xen29) (49); the bioluminescence is energy-dependent, and light output correlates well with counts of colony-forming units (8). To avoid confounding effects arising from bacterial growth, we used minimal bacterial growth conditions—1% tryptic soy broth (TSB) medium (8). By assaying light output, we were able to obtain real-time measurements of microbial viability in a 96-well plate format.
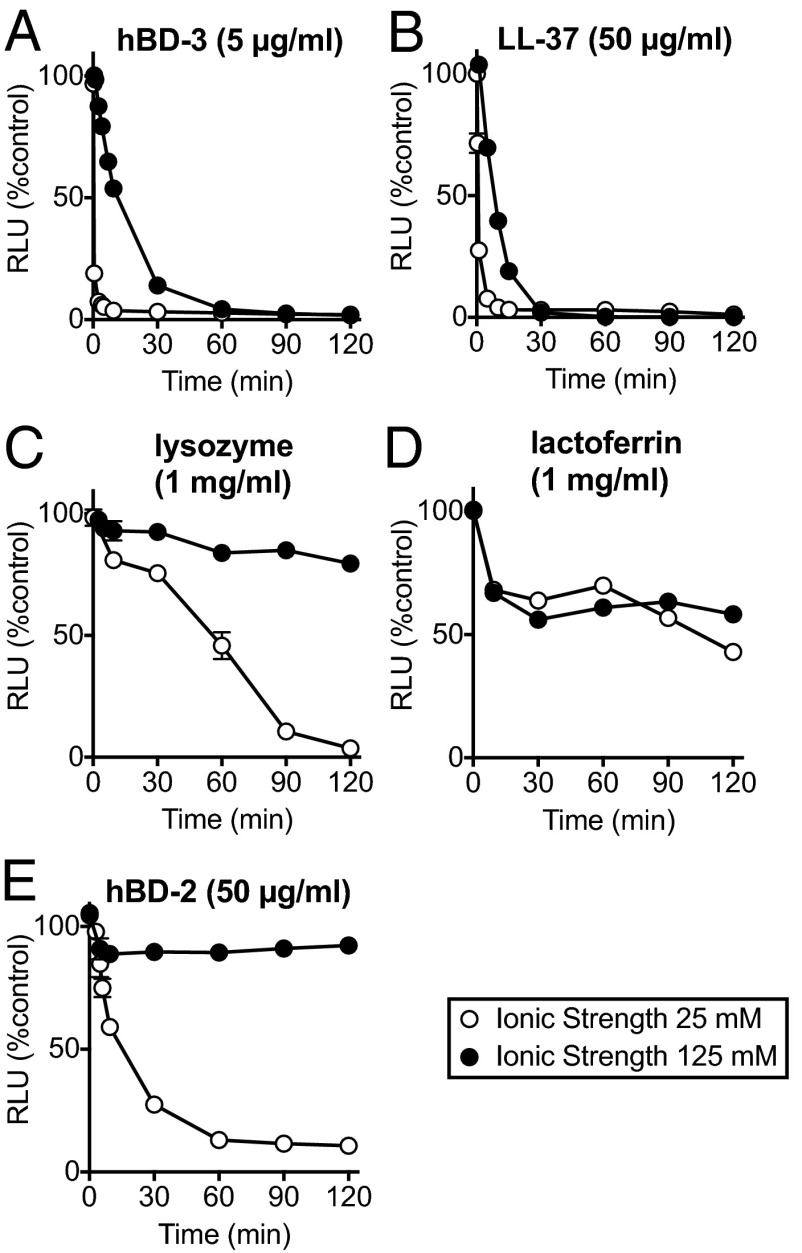
To assess the sensitivity of antibacterial activity to ionic strength, we tested hBD-3 and LL-37 in solutions with an ionic strength of either 25 or 125 mM (addition of 100 mM NaCl). We also studied lysozyme, lactoferrin, and hBD-2. At a low ionic strength, all five antimicrobials reduced S. aureus luminescence intensity (Fig. 1). At 125 mM ionic strength, hBD-3 and LL-37 retained substantial activity. However, the higher ionic strength attenuated the activity of lysozyme and hBD-2, and lactoferrin had only moderate activity at either ionic strength. Thus, in further studies, we investigated hBD-3 and LL-37 activity using the higher ionic strength.
Fig. 1.
Antimicrobial activity of ASL factors at low and high ionic strength. Data are relative luminescence (in RLU) of S. aureus (Xen-29) as a percentage of control (no added antimicrobial and same buffer conditions) at an ionic strength of 25 mM (1% TSB, 10 mM potassium phosphate buffer: open circles) and at an ionic strength of 125 mM (1% TSB, 10 mM potassium phosphate buffer and 100 mM NaCl: closed circles) both at pH 7.4. (A) hBD-3 (5 μg/mL). (B) LL-37 (50 μg/mL). (C) Lysozyme (1 mg/mL). (D) Lactoferrin (1 mg/mL). (E) hBD-2 (50 μg/mL). Data are mean ± SEM; some error bars are hidden by symbols. Results are from a single experiment in triplicate. Each experiment was repeated at least three times with similar results.
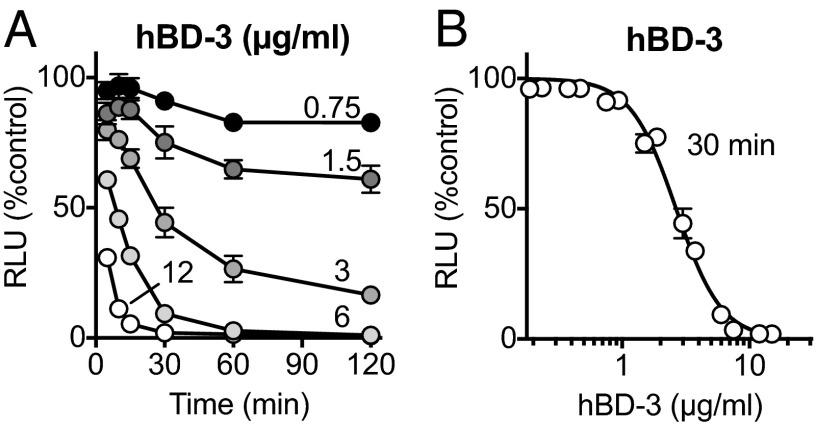
As the concentration of hBD-3 increased, it killed S. aureus more rapidly (Fig. 2A), and 30 min after addition, greater hBD-3 concentrations caused greater reductions in luminescence (Fig. 2B). The estimated IC50 for hBD-3 was 2.7 μg/mL [95% confidence interval (CI) 2.6–2.9].
Fig. 2.
Antimicrobial activity of hBD-3 is both time- and dose-dependent. (A) S. aureus luminescence as percentage of control measured over time at indicated concentrations of hBD-3 in μg/mL. (B) S. aureus luminescence with increasing concentrations of hBD-3 measured 30 min after addition. Data are mean ± SEM; some error bars are hidden by symbols. Results are from a single experiment in triplicate. Each experiment was repeated at least three times with similar results.
An Acidic pH Inhibits hBD-3 and LL-37 Activity Against S. aureus.
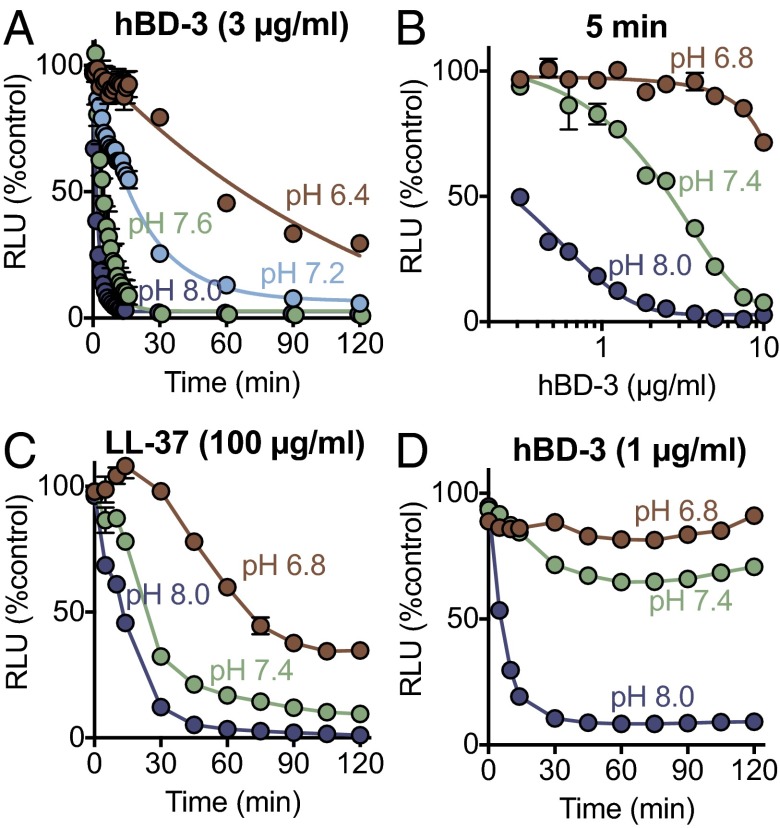
We tested the effect of pH on hBD-3 killing of S. aureus. At pH 8.0, hBD-3 rapidly killed the bacteria, so that by 1.5 min, luminescence fell by ∼95% (CI 1.44–1.62) (Fig. 3A). Lowering pH to more acidic values progressively slowed hBD-3 killing; at pH 6.4, ∼50% of the S. aureus bacteria were still viable 60 min after adding hBD-3.
Fig. 3.
Effect of pH on antimicrobial activity of hBD-3 and LL-37 against S. aureus. (A) Effect of 3 μg/mL hBD-3 on S. aureus (Xen-29) luminescence measured over time at indicated pH. (B) Effect of hBD-3 concentration on S. aureus luminescence at three different pH values; measurements were made 5 min after addition. At pH 7.4, the IC50 for hBD-3 was 2.7 μg/mL, 95% CI (2.4–2.9). (C) Effect of 100 μg/mL LL-37 on S. aureus luminescence. (D) Effect of 1 μg/mL hBD-3 on S. aureus luminescence. Data are relative luminescence (in RLU) as a percentage of control S. aureus (Xen-29) that had no added antimicrobial but had same pH buffer conditions. Data are mean ± SEM; some error bars are hidden by symbols. Results are from a single experiment in triplicate. Each experiment was repeated at least three times with similar results.
To test the effect of pH on the potency of hBD-3 against S. aureus, we measured the reduction in luminescence with increasing hBD-3 concentrations at three different pH values. At pH 8.0, ∼0.2 μg/mL hBD-3 reduced luminescence intensity by 50% (IC50) (Fig. 3B). At pH 7.4, the IC50 for hBD-3 was ∼2.9 μg/mL. As pH was reduced to 6.8, the potency of hBD-3 fell further. The relative potency of hBD-3 against S. aureus at pH 6.8, 7.4, and 8.0 was ∼1:5:75.
We also tested the hypothesis that a reduced pH would inhibit LL-37 killing of S. aureus. Fig. 3C shows that reducing the pH from 8.0 to 6.8 decreased the speed and extent of killing. However, in comparison with hBD-3, LL-37 activity was affected to a lesser extent as pH fell from 8.0 to 7.4 and 6.8 (Fig. 3 C and D).
An Acidic pH Has Little Effect on hBD-3 Activity Against P. aeruginosa.
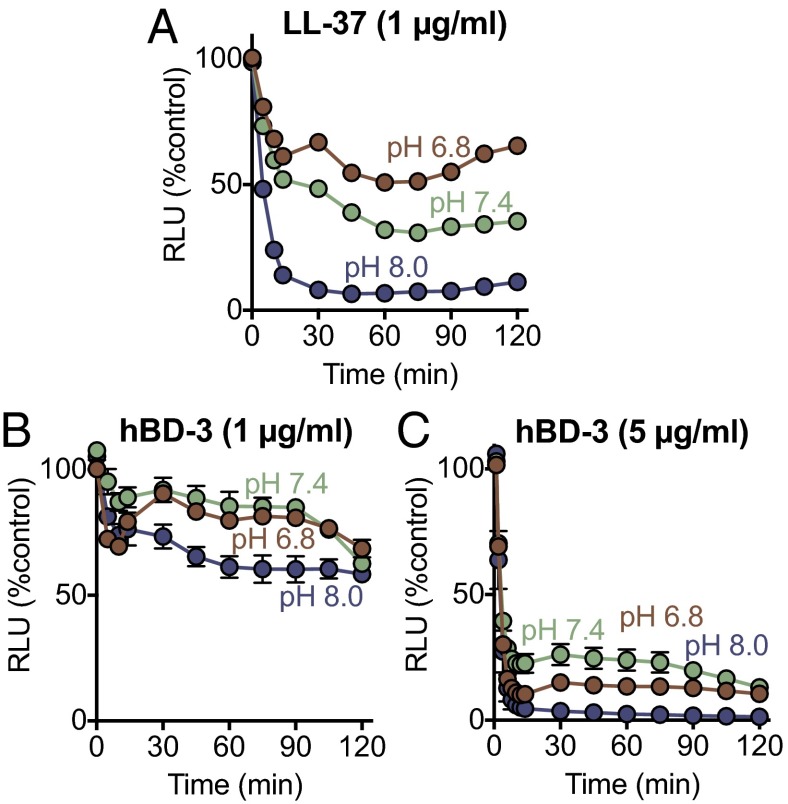
Although reducing the pH decreased the anti-staphylococcal activity of both hBD-3 and LL-37, individual antimicrobials can have distinct abilities to kill various bacteria. Therefore, we tested the effect of these two antimicrobial peptides on P. aeruginosa, an important pathogen as CF airway disease progresses (47, 50). LL-37 was much more potent at killing P. aeruginosa than S. aureus (1 μg/mL in Fig. 4A vs. 100 μg/mL for S. aureus in Fig. 3C). As with S. aureus, an acidic pH attenuated LL-37 killing of P. aeruginosa (Fig. 4A). hBD-3 was less potent at killing P. aeruginosa than S. aureus (1 and 5 μg/mL in Fig. 4 B and C vs. 1 μg/mL in Fig. 3D). However, in contrast to S. aureus, an acidic pH had minimal effects on hBD-3 anti-pseudomonal activity (Fig. 4 B and C).
Fig. 4.
Effect of pH on antimicrobial activity of hBD-3 and LL-37 against P. aeruginosa. Data are P. aeruginosa luminescence (in RLU). (A) LL-37 (1 μg/mL). (B) hBD-3 (1 μg/mL). (C) hBD-3 (5 μg/mL). Data are mean ± SEM; some error bars are hidden by symbols. Results are from a single experiment in triplicate. Each experiment was repeated at least three times with similar results.
An Acidic pH Attenuates the Synergistic Activity of hBD-3, LL-37, and Lysozyme Against S. aureus.
Although overlapping activities of multiple different ASL antimicrobials could defend the airways, synergism between antimicrobials could further enhance killing. We previously showed that a low ionic strength induced synergism between lysozyme, lactoferrin, and secretory leukocyte peptidase inhibitor in killing Escherichia coli (45). Therefore, we tested for synergistic interactions between antimicrobials at a higher ionic strength and examined the effect of pH.
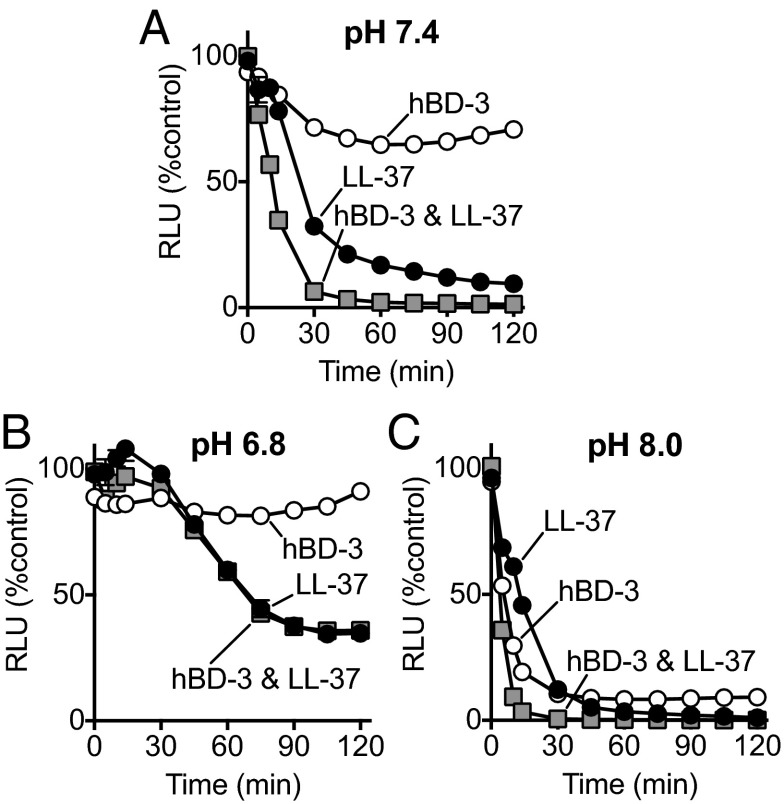
We asked whether pH affected the speed of killing by hBD-3 and LL-37 when they were combined. Compared with hBD-3 and LL-37 used alone at pH 8.0, the combination of half the concentrations of hBD-3 and LL-37 reduced S. aureus luminescence more rapidly (Fig. 5 A–C). At pH 7.4, the difference in speed of killing between the combination and the individual peptides was more marked, but at pH 6.8 the time-dependent advantage of the combination was minimal.
Fig. 5.
Effect of pH on antimicrobial activity of hBD-3 and LL-37 in combination. Data are S. aureus (Xen-29) luminescence. Combination of 50 μg/mL of LL-37 and 0.5 μg/mL of hBD-3 at (A) pH 7.4, (B) pH 6.8, and (C) pH 8.0. Data are mean ± SEM; some error bars are hidden by symbols. Results are from a single experiment in triplicate. Each experiment was repeated at least three times with similar results. Graphs corresponding to the effect of individual factors in Fig. 4 A and B are replotted in A, B, and C for comparison.
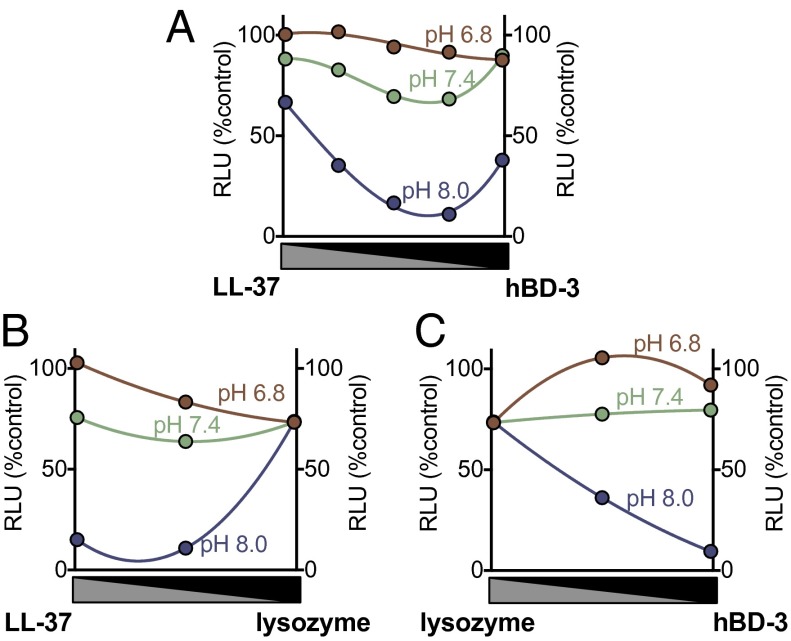
We also varied the ratio of two antimicrobial peptides/proteins, measured S. aureus luminescence, and plotted luminescence as a function of the fraction of each peptide in the mixture. A concave-down curve indicates synergism, a straight line indicates additivity, and a concave-up curve indicates antagonism (51, 52). Fig. 6A shows that LL-37 and hBD-3 had a synergistic effect on S. aureus killing at pH 8.0 and 7.4 that was eliminated at pH 6.8. We observed similar behavior with the combination of LL-37 and lysozyme (Fig. 6B). Although lysozyme and hBD-3 did not exhibit synergistic interactions, their combined effect was additive in killing S. aureus at pH 8.0 and 7.4. At pH 6.8, lysozyme and hBD-3 showed an antagonistic interaction (Fig. 6C).
Fig. 6.
Effect of pH on antimicrobial activity of combination of lysozyme, HBD-3, and LL-37. S. aureus (Xen-29) luminescence (in RLU) with varying ratios of two antimicrobial peptides. The x axis shows a ratio of antimicrobial peptides; from left to right there is an increasing ratio of one antimicrobial and a decreasing ratio of the other. (A) LL-37 and hBD-3 in combination (8 min incubation). (B) LL-37 and lysozyme in combination (30-min incubation). (C) Lysozyme and hBD-3 in combination (30-min incubation). Maximal combination (1.0) was 1 μg/mL for hBD-3, 100 μg/mL for LL-37, and 1 mg/mL for lysozyme. Data are mean ± SEM; some error bars are hidden by symbols. Results are from a single experiment in triplicate. Each experiment was repeated at least three times with similar results.
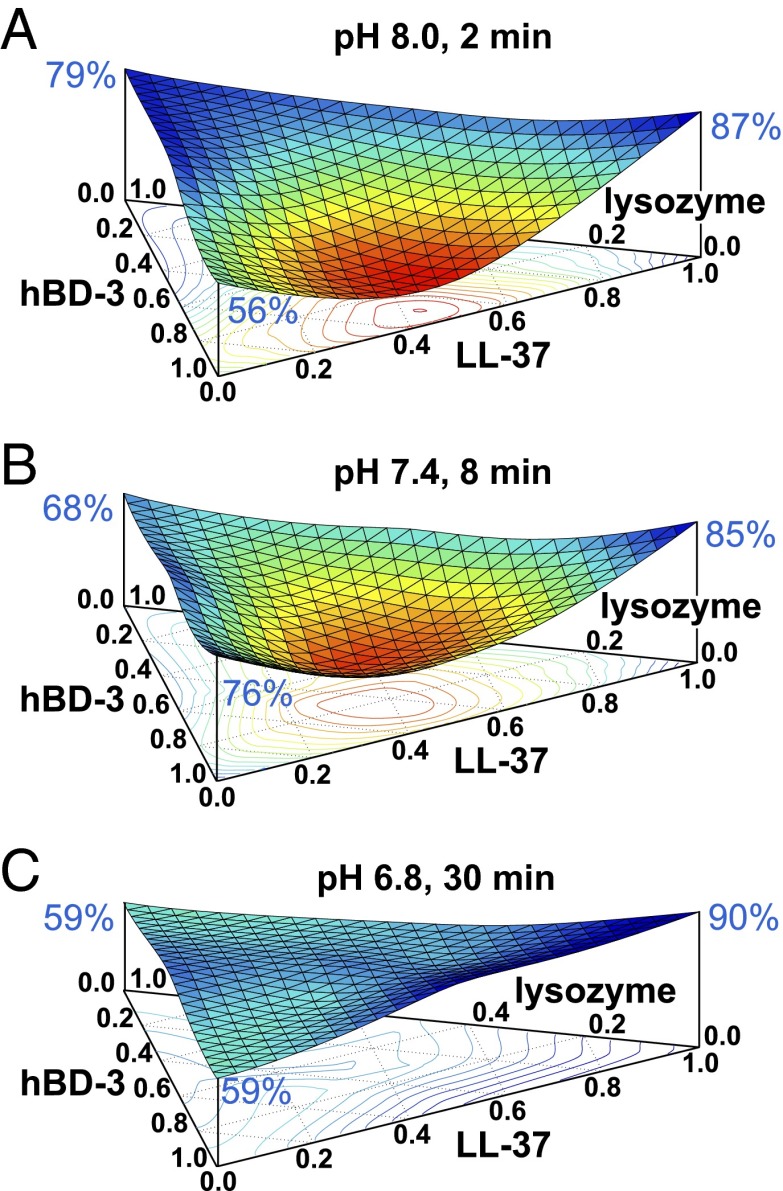
We also examined a triple combination of LL-37, hBD-3, and lysozyme. At pH 8.0, there was a strong synergistic interaction (Fig. 7A). However, as pH fell, the synergistic interactions were attenuated (Fig. 7 B and C). We also tested this triple combination with higher concentrations of each factor and found that reducing pH attenuated synergism, suggesting that inhibition does not depend on the maximal concentrations of each factor (Fig. S1).
Fig. 7.
Ternary surface plot of the effect of pH antimicrobial activity on the combination of lysozyme, hBD-3, and LL-37. Data are S. aureus (Xen-29) luminescence (vertical axis, in RLU). The surface plot color and curvature correspond to changes in relative luminescence. Red and concave indicate less luminescence and low bacteria viability; blue and flat indicate more luminescence and high bacteria viability. Relative luminescence with each antimicrobial factor alone is indicated at top of each vertical axis in blue font. (Horizontal triangular axis indicates ratio of antimicrobial factors in triple combination). Maximal concentrations (1.0 on the axes) were 1 μg/mL for hBD-3, 100 μg/mL for LL-37, and 1 mg/mL for lysozyme. Data are shown at different times for each pH. (A) pH 8.0 after 2-min incubation. (B) pH 7.4 after 8-min incubation. (C) pH 6.8 after 30-min incubation. Results are an average of three experiments, each done in triplicate. Similar results were obtained with higher concentrations of antimicrobials (Fig. S1).
Discussion
Our results suggest that hBD-3 and LL-37 have antibacterial activity as individual peptides and have an even greater effect through their synergistic interactions. The data also indicate that a reduced pH inhibits their individual and synergistic actions and could therefore impair airway defense.
The concentrations of hBD-3 and LL-37 that were effective against S. aureus (∼2 μg/mL for hBD-3 and ∼100 μg/mL for LL-37) and P. aeruginosa (∼5 μg/mL for hBD-3 and ∼1 μg/mL for LL-37) are in the range of their expected ASL concentrations (0.5–5 μg/mL for hBD-3 and 25–160 μg/mL for LL-37) (10, 19, 53–56). However, our results suggest that synergistic interactions may be even more important than activity of individual agents. Thus, peptides and proteins that only kill bacteria at concentrations greater than those in ASL might still make important synergistic contributions to antibacterial defense. Likewise, agents that show little antibacterial activity at relatively physiological ionic strength might contribute to host defense when combined with other antimicrobials. An example is lysozyme, which has a relatively high ASL concentration (250–500 μg/mL) (10), but which our data and earlier work (8) show has minimal anti-S. aureus activity as ionic strength increases toward physiological values. Nevertheless, here we found that lysozyme had synergistic effects when combined with LL-37 or with LL-37 plus hBD-3 at high ionic strength.
The reported absolute values of ASL pH depend on several factors, including the method of assay; whether it is measured in vivo, ex vivo, in submucosal gland secretions, or in cultured epithelia; whether it is assayed in newborns or inflamed airways; and perhaps the host species (40, 42–44, 57, 58). There is also considerable within-study variation. The reported range of pH in non-CF is from ∼6.8 to 7.8. However, and importantly, pH of CF ASL is consistently reported as lower than pH of non-CF ASL. Thus, the pH values that we tested are in the reported ranges for ASL.
The antimicrobial activity of cationic amphipathic peptides such as hBD-3 and LL-37 depends on electrostatic interactions between cationic domains of the peptide and the negatively charged head groups of phospholipids in the bacterial plasma membrane. The cationic peptides insert into and disrupt the bacterial plasma membrane (59, 60). Insight into how a more alkaline pH might increase activity comes from NMR experiments with synthetic, histidine-rich peptides and lipid bilayers (61, 62). Those studies indicate that the peptide assumes a position parallel to the lipid bilayer, weakening the membrane before insertion and then inserting and disrupting the membrane. At alkaline pH, the peptide carries a lower positive charge and thus inserts into the membrane more quickly. ASL cationic peptides may interact with the bacterial membrane in a similar fashion with corresponding effects of alkaline pH. Synergistic effects may occur when one peptide in a conformation parallel to the bacterial membrane facilitates insertion of other peptides (63). We speculate that such an effect might account for synergistic activity between hBD-3 and LL-37 as well as synergism for the combination of LL-37 and lysozyme; in addition to its enzymatic activity, lysozyme has nonenzymatic antibacterial activity dependent on its cationic and hydrophobic domains that disrupt bacterial plasma membranes (64–66). A possibility that we cannot exclude is that variations in pH might render S. aureus and P. aeruginosa more or less susceptible to antimicrobial factors. In addition, it is possible that pH might modulate the inhibitory effect of hBD-3 on S. aureus cell-wall biosynthesis. Previous studies found that the bactericidal activity of several defensins involves perturbation of cell-wall biosynthesis by binding to lipid II (67–71).
Our study has several advantages: we studied more than one ASL peptide/protein, the antimicrobials were used at concentrations similar to those found in ASL, we used pH values in the physiological range, the buffers had a high ionic strength, and S. aureus and P. aeruginosa have relevance to CF lung disease. Our study also has limitations. Bacterial inocula in vivo will usually be smaller than we used, and thus we may have underestimated the potency of antimicrobials (72, 73). Unidentified factors in ASL might enhance or attenuate the activity of antimicrobials; those would have been missing from our preparations. ASL antimicrobials may bind to mucin or other factors that alter their antibacterial activity. ASL contains more peptide, protein, and lipid antimicrobials than we studied, and thus our preparation does not reproduce the complex mixture of individual factors and their synergism.
In cultured human and porcine airway epithelia, in vitro studies of airways, and in vivo studies in newborn piglets and neonatal humans, CF ASL is abnormally acidic (40, 42–44), consistent with the loss of CFTR-dependent HCO3− transport (39, 41). However, in older children and adults with CF, the effect of genotype in ASL pH is more variable (44, 58, 74). Whether inflammation, remodeling, infection, and/or age are factors that might circumvent the loss of CFTR anion channels and increase ASL pH in CF is unknown. However, we speculate that changes in ASL pH might contribute, in part, to variations in the species of bacteria that infect CF airways. For example, S. aureus is commonly cultured early and P. aeruginosa is cultured later in the course of CF airway disease in humans and pigs (46, 47, 75). Interestingly, a reduced pH impaired hBD-3 killing of S. aureus, but had minor effects on killing of P. aeruginosa. Although a reduced ASL pH might be important for initiating S. aureus infection at the onset of CF lung disease, the variety of ASL antimicrobials, their varied properties, and interactions between them leave much uncertainty.
In addition to CF, ASL pH may be abnormally reduced in asthma (76), chronic obstructive pulmonary disease (77), and acute respiratory distress syndrome (78). Acidic conditions can also be found at sites of infection outside airways, including in peritoneal fluid, pleural fluid, cerebral spinal fluid, and abscesses. In addition to inhibiting antimicrobial peptides and proteins, an acidic pH can reduce the efficacy of pharmaceutical antibiotics (79, 80). These observations suggest that an acidic pH might impair the effectiveness of antimicrobial defenses and antibiotics under several conditions.
Thus, perhaps raising pH might aid prevention and treatment of bacterial infection at multiple sites in addition to CF airways.
Materials and Methods
Materials and Bacteria.
ASL antimicrobial factors included recombinant human lysozyme (Sigma-Aldrich), iron-unsaturated human milk lactoferrin (Sigma-Aldrich), recombinant human β-defensin-2 hBD-2 (Peprotech), recombinant human β-defensin-3 hBD-3 (Peprotech), and human LL-37 (Anaspec). Lysozyme and lactoferrin were dissolved in double-deionized water. Cationic peptides were dissolved in acidified water (0.01% acetic acid) that contained 0.1% BSA.
We used S. aureus Xen-29 (Caliper LifeSciences Bioware) and P. aeruginosa PA103. S. aureus Xen-29 was derived from S. aureus 12600, a pleural fluid isolate, which is also designated as NCTC8532. S. aureus Xen-29 possesses a stable copy of the modified Photorhabdus luminescens luxABCDE operon at a single integration site on the bacterial chromosome. For maintenance of luminescence, the bacteria were grown in TSB in the presence of kanamycin (10 μg/mL). We also used P. aeruginosa, modified to express the lux operon on a pUCP19lux plasmid and grown in Tryptic Soy Broth in the presence of carbenicillin (40 μg/mL) to maintain selection.
Antimicrobial peptide activity was tested in a buffer composed of TSB [casein peptone 17 g/L, soya peptone 3 g/L, NaCl 5 g/L, K2HPO4 2.5 g/L, glucose 2.5 g/L; 1% for S. aureus and 5% (vol/vol) for P. aeruginosa] and supplemented with 10 mM potassium phosphate buffer with pH adjusted by varying the ratio of monobasic to dibasic phosphate. The ionic strength of this solution is calculated at 25 mM (8). Where indicated, 100 mM NaCl was added to the final buffer to achieve an ionic strength of 125 mM.
Luminescence Antibacterial Assay.
Bacteria were grown overnight at 37 °C in medium described above, diluted 1:100, and grown to exponential phase. Bacteria were harvested by centrifugation and suspended in the 1% or 5% TSB buffer at the indicated pH and ionic strength. Bacteria (5 × 104 cfu) were incubated with antimicrobial factors in a 96-well plates (Optiplate; Packard Instruments) in a total volume of 120 μL. Luminescence was measured with a luminometer (Spectra Max L, Molecular Devices) and reported as relative light units (RLU). A previous study determined that reductions in luminescence have an excellent correlation with a decrease in cfu (8). All experiments included control bacteria that did not receive antimicrobials but were incubated in buffer of identical ionic strength and pH. Data are shown as relative luminescence as a percentage of control (RLU percentage of control). Exposure of bacteria to the various pH values did not cause bacterial death (Fig. S2).
Testing Combinations of Antimicrobials.
Experiments testing combinations of antimicrobials are often done using a checkerboard assay, and interactions are analyzed using isobolograms (81). In this study, we followed a more general approach (51, 52, 82) for several reasons. First, some factors (lysozyme) were completely inhibited at high ionic strength. Therefore, a full dose–response and IC50 calculations to design a checkerboard experiment were not possible. Second, preliminary studies showed that these antimicrobial factors kill bacteria at different rates. Third, we wanted to study more than two factors in combination and the effect of pH on the combination (83).
Statistical Analyses.
For dose–response curves in Figs. 2B and 3B, we used a Hill equation. For time-kill curves in Fig. 3A, we used a one-phase decay equation. For ternary surface plots in Fig. 7, we used ternplot (a MATLAB plugin by Carl Sandrock).
Supplementary Material
Acknowledgments
We thank Drs. Paul McCray, Robert Gray, Tony Fischer, and Lynda Ostedgaard for valuable discussions; Dr. Timothy Yahr and Peter Taft for technical assistance; and Theresa Mayhew for her help with the manuscript preparation. This work was funded by the National Institutes of Health (HL091842 and HL51670) and by the Roy J. Carver Charitable Trust. M.H.A.A. was funded by Cystic Fibrosis Foundation Fellowship CF0086552. M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422091112/-/DCSupplemental.
References
- 1.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12(7):503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8(9):402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 3.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 4.Brogden KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 5.Waterer GW. Airway defense mechanisms. Clin Chest Med. 2012;33(2):199–209. doi: 10.1016/j.ccm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Cole AM, et al. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169(12):6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75(1):34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 8.Travis SM, et al. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol. 1999;20(5):872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 9.Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol. 2006;306:153–182. doi: 10.1007/3-540-29916-5_6. [DOI] [PubMed] [Google Scholar]
- 10.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67(7):3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do TQ, et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J Immunol. 2008;181(6):4177–4187. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 13.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 14.Hancock RE. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001;1(3):156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 15.Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11(1):23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 16.Underwood MA, Bevins CL. Defensin-barbed innate immunity: Clinical associations in the pediatric population. Pediatrics. 2010;125(6):1237–1247. doi: 10.1542/peds.2009-3289. [DOI] [PubMed] [Google Scholar]
- 17.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 18.Antcheva N, et al. Artificial beta-defensin based on a minimal defensin template. Biochem J. 2009;421(3):435–447. doi: 10.1042/BJ20082242. [DOI] [PubMed] [Google Scholar]
- 19.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276(8):5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 20.Maisetta G, et al. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob Agents Chemother. 2006;50(2):806–809. doi: 10.1128/AAC.50.2.806-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42(9):2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travis SM, et al. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68(5):2748–2755. doi: 10.1128/iai.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noore J, Noore A, Li B. Cationic antimicrobial peptide LL-37 is effective against both extra- and intracellular Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57(3):1283–1290. doi: 10.1128/AAC.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurevic RJ, Chrisman P, Mancl L, Livingston R, Dale BA. Single-nucleotide polymorphisms and haplotype analysis in beta-defensin genes in different ethnic populations. Genet Test. 2002;6(4):261–269. doi: 10.1089/10906570260471787. [DOI] [PubMed] [Google Scholar]
- 25.Dörk T, Stuhrmann M. Polymorphisms of the human beta-defensin-1 gene. Mol Cell Probes. 1998;12(3):171–173. doi: 10.1006/mcpr.1998.0165. [DOI] [PubMed] [Google Scholar]
- 26.Crovella S, et al. A polymorphism in the 5′ UTR of the DEFB1 gene is associated with the lung phenotype in F508del homozygous Italian cystic fibrosis patients. Clin Chem Lab Med. 2011;49(1):49–54. doi: 10.1515/CCLM.2011.023. [DOI] [PubMed] [Google Scholar]
- 27.Tesse R, et al. Association of beta-defensin-1 gene polymorphisms with Pseudomonas aeruginosa airway colonization in cystic fibrosis. Genes Immun. 2008;9(1):57–60. doi: 10.1038/sj.gene.6364440. [DOI] [PubMed] [Google Scholar]
- 28.Nurjadi D, Herrmann E, Hinderberger I, Zanger P. Impaired β-defensin expression in human skin links DEFB1 promoter polymorphisms with persistent Staphylococcus aureus nasal carriage. J Infect Dis. 2013;207(4):666–674. doi: 10.1093/infdis/jis735. [DOI] [PubMed] [Google Scholar]
- 29.Segat L, et al. Analysis of DEFB1 regulatory SNPs in cystic fibrosis patients from North-Eastern Italy. Int J Immunogenet. 2010;37(3):169–175. doi: 10.1111/j.1744-313X.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 30.Vankeerberghen A, et al. Distribution of human beta-defensin polymorphisms in various control and cystic fibrosis populations. Genomics. 2005;85(5):574–581. doi: 10.1016/j.ygeno.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, et al. Bacterial colonization and beta defensins in the female genital tract in HIV infection. Curr HIV Res. 2012;10(6):504–512. doi: 10.2174/157016212802429848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehlotra RK, et al. Copy number variation within human beta-defensin gene cluster influences progression to AIDS in the multicenter AIDS cohort study. J AIDS Clin Res. 2012;3(10):1000184. doi: 10.4172/2155-6113.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesse R, et al. Association between DEFB1 gene haplotype and herpes viruses seroprevalence in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2009;26(8):573–582. doi: 10.3109/08880010903271705. [DOI] [PubMed] [Google Scholar]
- 34.Andresen E, Günther G, Bullwinkel J, Lange C, Heine H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS ONE. 2011;6(7):e21898. doi: 10.1371/journal.pone.0021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallsen K, Andresen E, Heine H. Histone deacetylase (HDAC) 1 controls the expression of beta defensin 1 in human lung epithelial cells. PLoS ONE. 2012;7(11):e50000. doi: 10.1371/journal.pone.0050000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace E, et al. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS ONE. 2012;7(3):e33601. doi: 10.1371/journal.pone.0033601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2(29):29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostedgaard LS, et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3(74):74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JH, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143(6):911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pezzulo AA, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89(4):1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coakley RD, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA. 2003;100(26):16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290(3):C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 44.Abou Alaiwa MH, et al. Neonates with cystic fibrosis have a reduced nasal liquid pH: a small pilot study. J Cyst Fibros. 2014;13(4):373–377. doi: 10.1016/j.jcf.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong DS, et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 47. Cystic Fibrosis Foundation (2008) Cystic Fibrosis Foundation Patient Registry: Annual Data Report 2008 (Cystic Fibrosis Foundation, Bethesda)
- 48.Wong JK, Ranganathan SC, Hart E. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Staphylococcus aureus in early cystic fibrosis lung disease. Pediatr Pulmonol. 2013;48(12):1151–1159. doi: 10.1002/ppul.22863. [DOI] [PubMed] [Google Scholar]
- 49.Kadurugamuwa JL, et al. Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun. 2003;71(2):882–890. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 51.Peterson JJ, Novick SJ. Nonlinear blending: A useful general concept for the assessment of combination drug synergy. J Recept Signal Transduct Res. 2007;27(2-3):125–146. doi: 10.1080/10799890701417576. [DOI] [PubMed] [Google Scholar]
- 52.Cornell JA. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data. 3rd Ed Wiley; New York: 2002. [Google Scholar]
- 53.Brogan TD, Ryley HC, Neale L, Yassa J. Soluble proteins of bronchopulmonary secretions from patients with cystic fibrosis, asthma, and bronchitis. Thorax. 1975;30(1):72–79. doi: 10.1136/thx.30.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sørensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90(7):2796–2803. [PubMed] [Google Scholar]
- 55.Agerberth B, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160(1):283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- 56.Bergsson G, et al. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J Immunol. 2009;183(1):543–551. doi: 10.4049/jimmunol.0803959. [DOI] [PubMed] [Google Scholar]
- 57.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107(3):317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McShane D, et al. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21(1):37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 59.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999;1462(1-2):55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 60.Wu M, Maier E, Benz R, Hancock RE. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38(22):7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 61.Bechinger B. Towards membrane protein design: pH-sensitive topology of histidine-containing polypeptides. J Mol Biol. 1996;263(5):768–775. doi: 10.1006/jmbi.1996.0614. [DOI] [PubMed] [Google Scholar]
- 62.Aisenbrey C, Bechinger B, Gröbner G. Macromolecular crowding at membrane interfaces: Adsorption and alignment of membrane peptides. J Mol Biol. 2008;375(2):376–385. doi: 10.1016/j.jmb.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 63.Bechinger B, Salnikov ES. The membrane interactions of antimicrobial peptides revealed by solid-state NMR spectroscopy. Chem Phys Lipids. 2012;165(3):282–301. doi: 10.1016/j.chemphyslip.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Düring K, Porsch P, Mahn A, Brinkmann O, Gieffers W. The non-enzymatic microbicidal activity of lysozymes. FEBS Lett. 1999;449(2-3):93–100. doi: 10.1016/s0014-5793(99)00405-6. [DOI] [PubMed] [Google Scholar]
- 65.Ibrahim HR, Matsuzaki T, Aoki T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001;506(1):27–32. doi: 10.1016/s0014-5793(01)02872-1. [DOI] [PubMed] [Google Scholar]
- 66.Nash JA, Ballard TN, Weaver TE, Akinbi HT. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J Immunol. 2006;177(1):519–526. doi: 10.4049/jimmunol.177.1.519. [DOI] [PubMed] [Google Scholar]
- 67.Wei G, et al. Through the looking glass, mechanistic insights from enantiomeric human defensins. J Biol Chem. 2009;284(42):29180–29192. doi: 10.1074/jbc.M109.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Leeuw E, et al. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 2010;584(8):1543–1548. doi: 10.1016/j.febslet.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sass V, et al. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun. 2010;78(6):2793–2800. doi: 10.1128/IAI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitt P, et al. Insight into invertebrate defensin mechanism of action: Oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J Biol Chem. 2010;285(38):29208–29216. doi: 10.1074/jbc.M110.143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schneider T, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328(5982):1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
- 72.Malley R, et al. Development of a model of focal pneumococcal pneumonia in young rats. J Immune Based Ther Vaccines. 2004;2(1):2. doi: 10.1186/1476-8518-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eames I, Tang JW, Li Y, Wilson P. Airborne transmission of disease in hospitals. J R Soc Interface. 2009;6(Suppl 6):S697–S702. doi: 10.1098/rsif.2009.0407.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garland AL, et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci USA. 2013;110(40):15973–15978. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosorok MR, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32(4):277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 76.Hunt JF, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161(3 Pt 1):694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 77.Kostikas K, et al. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165(10):1364–1370. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 78.Gessner C, et al. Exhaled breath condensate acidification in acute lung injury. Respir Med. 2003;97(11):1188–1194. doi: 10.1016/s0954-6111(03)00225-7. [DOI] [PubMed] [Google Scholar]
- 79.Konig C, Simmen HP, Blaser J. Effect of pathological changes of pH, pO2 and pCO2 on the activity of antimicrobial agents in vitro. Eur J Clin Microbiol. 1993;12(7):519–526. doi: 10.1007/BF01970957. [DOI] [PubMed] [Google Scholar]
- 80.Falagas ME, McDermott L, Snydman DR. Effect of pH on in vitro antimicrobial susceptibility of the Bacteroides fragilis group. Antimicrob Agents Chemother. 1997;41(9):2047–2049. doi: 10.1128/aac.41.9.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peterson JJ. A review of synergy concepts of nonlinear blending and dose-reduction profiles. Front Biosci (Schol Ed) 2010;2:483–503. doi: 10.2741/s80. [DOI] [PubMed] [Google Scholar]
- 82.Cornell JA. The fitting of Scheffé-type models for estimating solubilities of multisolvent systems. J Biopharm Stat. 1991;1(2):303–329. doi: 10.1080/10543409108835025. [DOI] [PubMed] [Google Scholar]
- 83.White DB, Slocum HK, Brun Y, Wrzosek C, Greco WR. A new nonlinear mixture response surface paradigm for the study of synergism: A three drug example. Curr Drug Metab. 2003;4(5):399–409. doi: 10.2174/1389200033489316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.