Significance
Non-small cell lung cancers (NSCLCs) harboring mutations in the epidermal growth factor receptor (EGFR) gene are often singularly reliant on EGFR activity for tumor cell survival, but the genetic basis for this dependence is not fully understood. In this study, we have performed a screen to identify a spectrum of kinase genes whose overexpression can overcome NSCLC cells’ reliance on EGFR. Using both unbiased and targeted approaches, we demonstrate that these genes commonly bypass dependence on EGFR through reactivation of downstream signaling pathways.
Keywords: epidermal growth factor receptor, non-small cell lung cancer, ORF
Abstract
Lung adenocarcinomas harboring activating mutations in the epidermal growth factor receptor (EGFR) represent a common molecular subset of non-small cell lung cancer (NSCLC) cases. EGFR mutations predict sensitivity to EGFR tyrosine kinase inhibitors (TKIs) and thus represent a dependency in NSCLCs harboring these alterations, but the genetic basis of EGFR dependence is not fully understood. Here, we applied an unbiased, ORF-based screen to identify genetic modifiers of EGFR dependence in EGFR-mutant NSCLC cells. This approach identified 18 kinase and kinase-related genes whose overexpression can substitute for EGFR in EGFR-dependent PC9 cells, and these genes include seven of nine Src family kinase genes, FGFR1, FGFR2, ITK, NTRK1, NTRK2, MOS, MST1R, and RAF1. A subset of these genes can complement loss of EGFR activity across multiple EGFR-dependent models. Unbiased gene-expression profiling of cells overexpressing EGFR bypass genes, together with targeted validation studies, reveals EGFR-independent activation of the MEK-ERK and phosphoinositide 3-kinase (PI3K)-AKT pathways. Combined inhibition of PI3K-mTOR and MEK restores EGFR dependence in cells expressing each of the 18 EGFR bypass genes. Together, these data uncover a broad spectrum of kinases capable of overcoming dependence on EGFR and underscore their convergence on the PI3K-AKT and MEK-ERK signaling axes in sustaining EGFR-independent survival.
The term “oncogene addiction” has been used to describe the phenomenon whereby tumor cells exhibit singular reliance on an oncogene or oncogenic pathway for their survival, despite the accumulation of multiple genetic lesions (1). In non-small cell lung cancer (NSCLC), this principle is perhaps best exemplified with the finding that epidermal growth factor receptor (EGFR) mutations predict response to EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib, and thus represent a dependency in the subset of tumors harboring these alterations (2–6). However, though EGFR-mutant NSCLCs typically respond dramatically to EGFR TKIs, clinical responses are not universal, even within this genetically defined cohort, with the rate of objective response estimated to be ∼71% (5, 6). Furthermore, the overwhelming majority of patients who initially respond to EGFR inhibitors ultimately develop resistance to therapy (7). A deeper understanding of the genetic underpinnings of EGFR addiction, and how EGFR-mutant cells can overcome reliance on EGFR, may improve clinical outcomes.
Here, we have applied an unbiased screening approach to identify genetic modifiers of EGFR dependence in NSCLC. Mounting evidence supports the existence of several genetic modifiers of EGFR dependence in EGFR-mutant NSCLC, which can reduce the degree to which these tumors rely on EGFR and thereby contribute to EGFR TKI resistance (8). Examples include amplification of the MET receptor tyrosine kinase (RTK) (9), activation of the NF-κB pathway (8), amplification of the HER2 (ERBB2) RTK (10), amplification of the CRKL gene (11), and activation of the AXL kinase (12). Notably, MET bypass can be reciprocally achieved via EGFR activation in MET-dependent cells (13), and analogous examples of reciprocal kinase switching have been reported in other kinase-driven cancer models (14, 15). These and other findings suggest that compensatory kinase switching may be a more general way in which oncogene-dependent cancers overcome reliance on their primary driver kinase (14, 16), but the full-range of kinases capable of mediating EGFR bypass has not been systematically studied.
Recent advances in large-scale functional genetic libraries have made it possible to query a wide range of genetic perturbations for their ability to modulate specific cellular phenotypes in mammalian systems (17, 18). Using the model of EGFR-mutant, erlotinib-sensitive NSCLC cells, we have performed a systematic ORF-based screen to identify kinase and kinase-related genes whose overexpression can complement loss of EGFR activity in an EGFR-dependent context. Our findings indicate broad potential for EGFR substitution in the setting of EGFR dependence, with compensatory mechanisms commonly conferring EGFR-independent activation of the PI3K-AKT and MEK-ERK signaling pathways. Importantly, this approach has recovered known mechanisms of erlotinib resistance as well as identified novel mediators of EGFR bypass in EGFR-mutant NSCLC. These data support the idea that the EGFR-dependent state can be redundantly driven by diverse genetic inputs that commonly converge on shared downstream signaling nodes.
Results
An ORF-Based Screen Identifies 18 Genes Whose Expression Can Substitute for EGFR in an EGFR-Dependent NSCLC Cell Line.
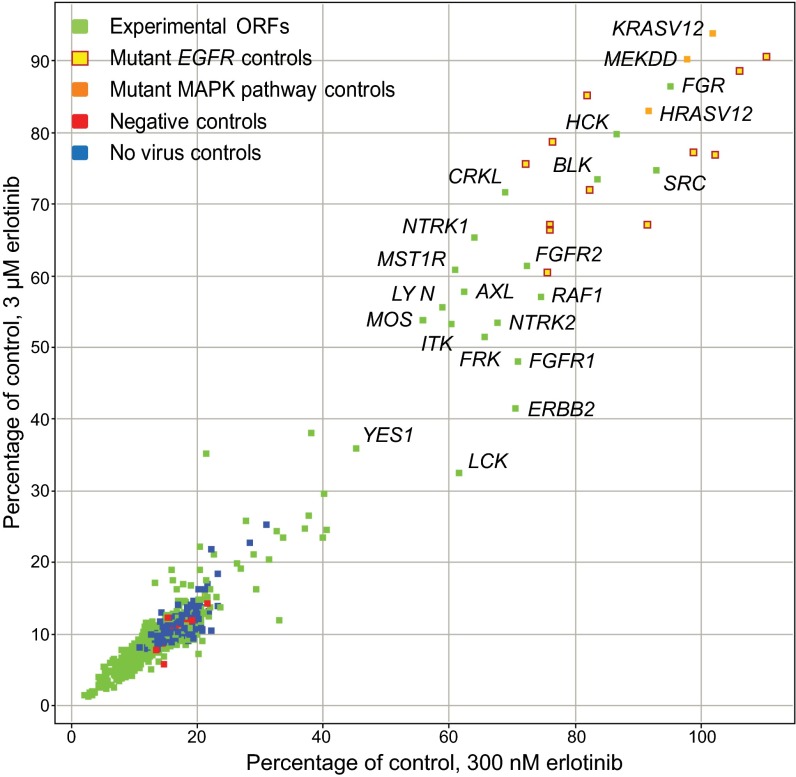
To identify genes that complement loss of EGFR activity in the setting of EGFR dependence, we performed an ORF overexpression screen in an EGFR-dependent NSCLC cell line in the presence of erlotinib. A library of 589 ORFs encoding human kinases and kinase-related proteins [Center for Cancer Systems Biology (CCSB)/Broad Institute Kinase ORF Collection] (17, 18) was expressed via lentiviral transduction in the EGFR-mutant NSCLC cell line, PC9, which is sensitive to EGFR TKIs with an IC50 of ∼30 nM (19). ORF-expressing PC9 cells were treated with 300 nM erlotinib, 3 µM erlotinib, or DMSO for 72 h before being assayed for cell viability. Experimental ORFs were screened alongside positive control EGFR ORFs encoding the T790M “gatekeeper” mutation, which can promote resistance to EGFR TKIs (20), in cis with a canonical EGFR activating mutation [EGFR-Δ(E746-A750)-T790M and EGFR-L858R-T790M] (21). Additional positive controls included ORFs encoding activating alleles of several MAPK family members.
Cell viability under screening conditions was very low; the median relative viability for all experimental ORFs was 12% at the 300-nM dose and 8% at the 3-µM dose (Fig. 1). Overexpression of 19 ORFs, of the 589 tested, led to a significant increase in viability of erlotinib-treated PC9 cells, with viability of at least 39% in 300 nM erlotinib and at least 31% in 3 µM erlotinib (Fig. 1). The genes whose ectopic expression led to greater viability in the presence of erlotinib include known modifiers of EGFR dependence (AXL, ERBB2, CRKL) as well as genes and gene families that are newly identified candidate complementation partners of EGFR in EGFR-mutant lung cancer cells, including eight of the nine Src family kinase members, as well as FGFR1 and FGFR2; ITK; MST1R; MOS; NTRK1 and NTRK2; and RAF1. The 19 genes whose expression led to apparent EGFR substitution in the primary screen were selected for validation and functional characterization.
Fig. 1.
An ORF-based screen identifies 18 kinase and kinase-related modifiers of EGFR dependence. Screening results for PC9 cells transduced with a library of kinase ORFs, treated with 300 nM or 3 μM erlotinib or vehicle, then assayed for cell viability after 72 h using CellTiter-Glo. Experimental ORFs (green points) were screened alongside EGFR-ex19del-T790M and EGFR-L858R-T790M positive controls (yellow points); activating alleles of the MAPK family (orange points); inert gene controls (red points); and no virus controls (blue points). Data are expressed as percent viability relative to vehicle. Nineteen candidate modifiers of EGFR dependence were selected for validation.
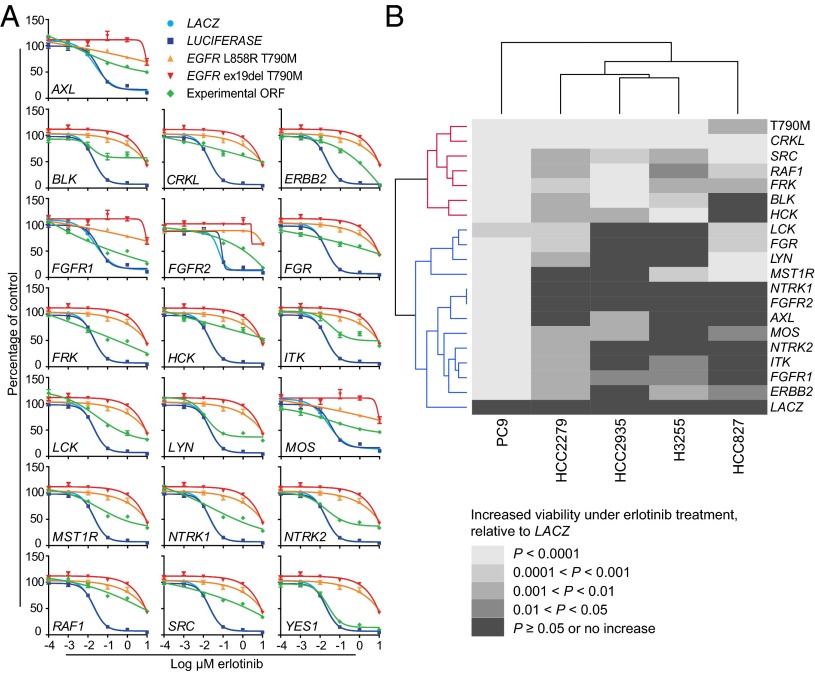
cDNA vectors corresponding to the 19 candidate EGFR bypass genes were sequence-verified and confirmed to express protein (Fig. S1A). PC9 cells overexpressing each of these ORFs were assayed for erlotinib sensitivity across multiple drug doses using 72-h growth inhibition assays (Fig. 2A). For comparison, the erlotinib-resistant EGFR-mutant positive controls EGFR-Δ(E746-A750)-T790M (henceforth EGFR-ex19del-T790M) and EGFR-L858R-T790M were tested in parallel. Ectopic expression of 18 of the 19 candidate bypass ORFs was capable of reducing erlotinib sensitivity relative to cells transduced with an inert ORF (Fig. 2A). This reduced sensitivity corresponded to a >twofold to >400-fold shift in IC50 values (Table S1). One primary screening hit, YES1, failed to bypass EGFR dependence in our validation studies, leaving seven of nine Src family kinase genes confirmed to confer the complementation phenotype (Fig. 2A and Table S1); and notably, this gene scored nearest to the cutoff we used to select ORFs for validation (Fig. 1). We noted that the known EGFR bypass gene MET did not score in our primary screen, likely due to failure of the MET expression vector to express MET protein (Fig. S1B). Overall, we estimate that the vast majority of screened ORFs express protein in PC9 cells (Fig. S1 C and D).
Fig. 2.
Validation of the primary screen. (A) PC9 cells expressing candidate EGFR bypass ORFs or controls were treated with erlotinib and assayed for cell viability after 72 h using CellTiter-Glo. Data are expressed as percent viability relative to vehicle-treated cells and represent the mean ± SD of four replicates. Graphs with identical control curves reflect experiments performed in parallel on the same day. (B) Heat map summarizing effects of bypass ORFs validated in A across five EGFR-mutant cell line models. The 18 EGFR bypass genes validated in PC9 cells were used for validation studies in HCC2279, HCC2935, H3255, and HCC827 cells (Fig. S2). Grayscale values represent increasingly significant levels of ORF-induced erlotinib resistance relative to LACZ controls (black, 0; white, +4). Data were generated from cells treated with 1 μM (PC9) or 3 μM erlotinib (HCC2279, HCC2935, H3255, HCC827) (A and Fig. S2). Dendrograms in the row and column margins represent groupings of ORFs and cell lines, respectively. ORFs segregate according to whether their EGFR bypass-promoting effects are universal or nearly universal (red cluster) or variable (blue cluster) across the cell lines tested. T790M denotes EGFR-ex19del-T790M.
The 18 genes capable of bypassing EGFR dependence in PC9 cells were used to transduce four additional EGFR-mutant NSCLC cell line models: the EGFR TKI-sensitive models HCC827, HCC2935, and H3255 (22), and the partially sensitive model HCC2279 (22, 23). Transduced cells were treated with 3 µM erlotinib and assayed for cell viability after 72 h (Fig. S2 A–E and Fig. 2B), and the effects of the 18 ORFs across all five models tested were grouped by gene and cell line (Fig. 2B). Interestingly, two classes of EGFR modifiers emerged from this analysis: one set of genes (CRKL, SRC, RAF1, FRK, BLK, and HCK, in addition to the erlotinib-resistant EGFR-ex19del-T790M mutant) whose overexpression could universally or nearly universally reduce EGFR dependence across the models tested (Fig. 2B, red cluster), and a second set of genes whose effects varied across different cell line models (Fig. 2B, blue cluster). Overexpression of variable-effect cluster members LCK, FGR, LYN, and MST1R, and ITK, for example, each leads to increased cell viability in the presence of erlotinib in three of the five models tested (Fig. 2B, blue cluster). It is possible that the differing effects of the genes in the variable-effect cluster on EGFR dependence can be attributed to the transcriptional heterogeneity of these cell lines, which fall in distinct clusters when comparing expression profiles among lung cancer cell lines (Fig. S3), as well as to varying strengths of gene-specific effects. Importantly, the clinically validated EGFR TKI resistance-mediating genes AXL and ERBB2 each modify EGFR dependence in only a subset of the models tested, suggesting that the effects of clinically relevant modifiers of EGFR dependence can also vary across different EGFR-mutant models. Together, these data identify 18 modifiers of EGFR dependence in PC9 cells, of which a subset can universally complement loss of EGFR activity across multiple EGFR-dependent contexts.
Interestingly, in investigating whether EGFR bypass ORFs are specific to EGFR TKIs, we observed that overexpression of several EGFR bypass genes, including AXL, CRKL, ERBB2, MST1R, RAF1, and several Src family kinases, can overcome sensitivity to the anaplastic lymphoma kinase (ALK) inhibitor TAE684 in EML4-ALK-positive H3122 NSCLC cells (Fig. S4A). In contrast, none of the EGFR bypass genes caused appreciable changes in cellular response to the chemotherapy drug cisplatin in PC9 cells (Fig. S4B). These findings suggest that the kinases identified in this screen do not universally mediate drug resistance, but they may have a more general role in modifying oncogene dependence in NSCLC.
Inhibition of Bypass Kinases Restores Erlotinib Sensitivity, and Coinhibition Is Associated with Phospho-AKT Attenuation for a Subset of EGFR Bypass Genes.
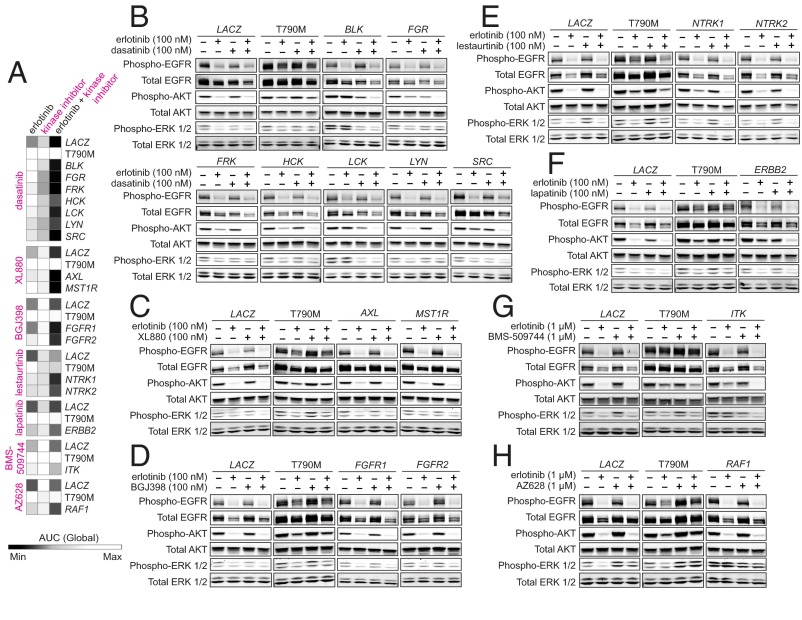
To enable the most inclusive analysis of EGFR bypass genes, all 18 EGFR modifiers identified in PC9 cells were carried forward for further study. First, because the modifier genes were predominantly kinases, we sought to determine whether the observed bypass phenotypes were kinase-dependent, as well as to verify the specificity of ORF activity, by testing whether enzymatic inhibition of bypass-promoting kinases could restore EGFR dependence. PC9 cells transduced with kinase ORFs were treated with erlotinib alone, the relevant kinase inhibitor (where available) alone, or their combination for 72 h, then assayed for cell viability (Fig. 3A and Fig. S5 A–G). Inhibitors tested included dasatinib (targeting the Src family kinases); XL880 (targeting AXL and MST1R); NVP-BGJ398 (BGJ398; targeting FGFR1 and FGFR2); lestaurtinib (targeting NTRK1 and NTRK2); lapatinib (targeting ERBB2); BMS-509744 (targeting ITK); and AZ628 (targeting RAF1). The on-target activity of these inhibitors was confirmed for a select number of ORFs (Fig. S5 H–M).
Fig. 3.
Kinase inhibition of bypass-promoting kinases restores erlotinib sensitivity, and coinhibition is associated with phospho-AKT attenuation for a subset of ORFs. (A) Inhibitor sensitivity of PC9 cells expressing indicated EGFR bypass-associated kinases and controls following treatment with erlotinib, the relevant kinase inhibitor, or their combination for 72 h. Cell viability was assayed with CellTiter-Glo, and the resultant dose–response curves (Fig. S5) were used to generate area under curve (AUC) values, plotted. Darker/lighter squares represent smaller/larger AUC values. (B–H) Immunoblot analysis of PC9 cells expressing bypass-associated kinases and controls under combination drug treatment. Transduced cells were treated with indicated doses of erlotinib, the relevant kinase inhibitor, or a combination for 6 h. Cells were incubated with 0.5% serum media 18 h before and during drug/DMSO treatment. Kinase inhibitors tested included (B) dasatinib, (C) XL880, (D) BGJ398, (E) lestaurtinib, (F) lapatinib, (G) BMS-509744, and (H) AZ628. T790M denotes EGFR-ex19del-T790M.
Cells expressing Src family kinase genes could indeed be resensitized to erlotinib when cotreated with dasatinib, whereas these cells were not similarly sensitive to either agent alone (Fig. 3A and Fig. S5A). In contrast, resensitization to combined erlotinib/dasatinib treatment was not achieved in cells expressing EGFR-ex19del-T790M. Gene-specific rescue of erlotinib sensitivity was similarly observed for AXL; MST1R; FGFR1 and FGFR2; NTRK1 and NTRK2; ERBB2; ITK; and RAF1 upon cotreatment with their respective inhibitors (Fig. 3A and Fig. S5 B–G). Combination treatment was also associated with increased levels of apoptosis in cells expressing bypass genes, compared with treatment with either inhibitor alone (Fig. S6). Together, these data suggest that the EGFR bypass phenotype induced by these ORFs is specific to the encoded gene and requires the kinase activity of the expressed protein.
A few kinase inhibitors showed somewhat different patterns of growth inhibition or rescue. Lestaurtinib alone has some activity in LACZ- and EGFR-ex19del-T790M-transduced cells (Fig. 3A and Fig. S5D), although only NTRK1- and NTRK2-expressing cells exhibit enhanced sensitivity under combination treatment. We also observed that AZ628 treatment only modestly resensitizes RAF1-transduced cells to erlotinib (Fig. 3A and Fig. S5G), consistent with the finding that RAF1 overexpression is itself a mechanism of resistance to RAF inhibition (18).
Next, because EGFR TKI treatment in EGFR-mutant cells typically elicits down-regulation of the PI3K-AKT and MEK-ERK signaling pathways (2), we asked if rescue of EGFR dependence using combination treatment was associated with a distinct PI3K-AKT and/or MEK-ERK signaling profile. ORF-expressing cells were treated with erlotinib, the relevant kinase inhibitor, or their combination for 6 h, and assayed for downstream signaling activation (Fig. 3 B–H). LACZ-expressing cells undergo the expected down-regulation of phospho-EGFR, -AKT, and -ERK1/2 in the presence of erlotinib, in contrast to EGFR-ex19del-T790M-expressing cells (Fig. 3B). Cells transduced with Src family kinases, however, maintain AKT phosphorylation in the presence of 100 nM erlotinib, and this effect is reversed only upon cotreatment with dasatinib. Selective attenuation of phospho-AKT under combination treatment was similarly observed in cells transduced with ERBB2, ITK, NTRK1, NTRK2, and very modestly with FGFR1, FGFR2, AXL, and MST1R (Fig. 3 C–G).
Selective down-regulation of phospho-AKT was not observed in RAF1-expressing cells cotreated with erlotinib/AZ628 (Fig. 3H), although as mentioned above, this combination was not sufficient to fully sensitize these cells (Fig. S5G). Combined erlotinib/AZ628 treatment also fails to abolish phospho-ERK activation in these cells (Fig. 3H). Indeed, AZ628 treatment paradoxically induces phospho-RAF1 activation in RAF1-expressing cells (Fig. S5M), consistent with previous findings using RAF inhibitors in other RAFWT models (24).
EGFR Bypass Genes Commonly Induce Similar Transcriptional Effects, Which Are Anticorrelated to Those Induced by MEK and PI3K Inhibitors.
Given that a subset of kinase genes can substitute for EGFR in PC9 cells, we were interested in identifying signaling pathways that were potentially common to overexpression of these genes. A hypothesis-driven analysis described above indicated a role for activation of the AKT pathway for a subset of ORFs; however, an unbiased systematic approach might more clearly address whether EGFR bypass genes act through common or divergent pathways. We reasoned that a high-dimensional transcriptional readout would provide an unbiased approach to address this question. We assembled reagents for the 18 EGFR bypass genes (positive-phenotype genes) as well as 19 kinase genes that had failed to modify EGFR dependence in the primary screen (negative-phenotype genes). PC9 cells were transduced with these vectors and controls for 72 h, then subjected to 24-h erlotinib treatment. Gene expression profiles for each ORF were used to compute signatures as described in SI Materials and Methods.
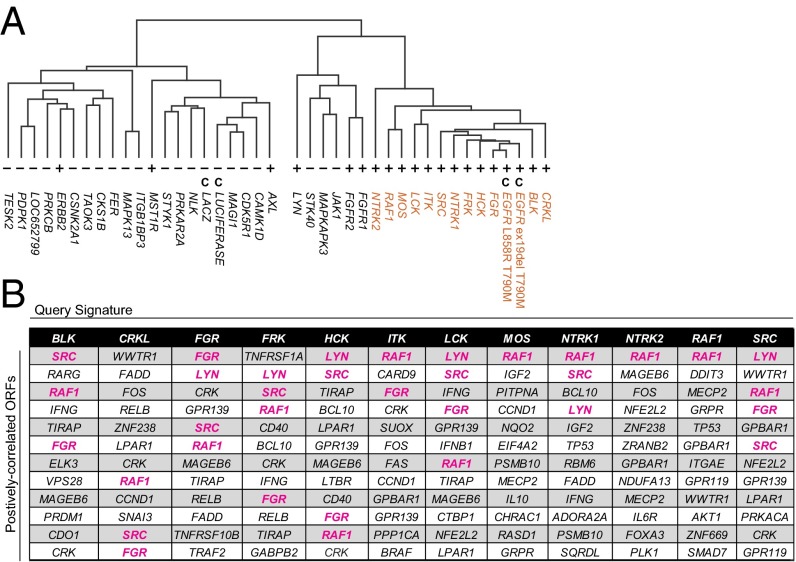
Unsupervised hierarchical clustering of these signatures yielded two distinct clusters (Fig. 4A). Intriguingly, profiles segregated largely in accordance with positive vs. negative phenotypes; one subcluster was comprised solely of 12 bypass-associated profiles and the two EGFR double-mutant controls (Fig. 4A, red). Given that this cluster contained almost all bypass-associated gene profiles and none of the negative-phenotype profiles, we chose to focus our subsequent analyses on the profiles represented in this positive-phenotype cluster.
Fig. 4.
EGFR bypass genes commonly induce similar transcriptional effects. (A) Unsupervised hierarchical clustering of PC9 cells overexpressing bypass-mediating ORFs (+); kinase ORFs unable to confer EGFR bypass (−); and controls (C). Twelve bypass-mediating ORFs displaying membership in a single cluster (positive-phenotype cluster, red) were used to query LINCS. (B) LINCS ORFs whose signatures most positively correlate with those of positive-phenotype cluster genes. Each of the 12 positive-phenotype cluster genes was used to independently query LINCS. The top ∼3% positively correlated ORFs are listed in descending order. Query ORFs commonly correlate most strongly with the same ORF and/or other EGFR bypass genes (magenta).
To address whether the expression profiles of cells transduced with bypass-associated ORFs could yield insights into their biological effects, we used a large expression-profiling resource created by the Library of Integrated Network-Based Cellular Signatures (LINCS) program (www.lincscloud.org). This catalog was generated from diverse human cell lines treated with a large number of genetic and chemical perturbagens. Gene expression signatures from cells expressing each of the 12 genes in the positive-phenotype cluster (excluding controls) were independently used to query LINCS.
We first sought to identify LINCS ORFs whose transcriptional effects positively correlate with query ORFs, to potentially address whether EGFR bypass genes act through shared or distinct pathways. When considering the top ∼3% of positively correlated ORFs (Fig. 4B), query ORF signatures commonly correlate with LINCS signatures generated using the same ORF or from other EGFR bypass ORFs (Fig. 4B, magenta cells). Notably, none of the cell lines profiled in the LINCS dataset is EGFR mutant, and most are not of a lung lineage. Together with the hierarchical clustering, these data suggest that a major subset of EGFR bypass genes induce similar transcriptional effects, which do not appear to be restricted to an EGFR-mutant cellular context.
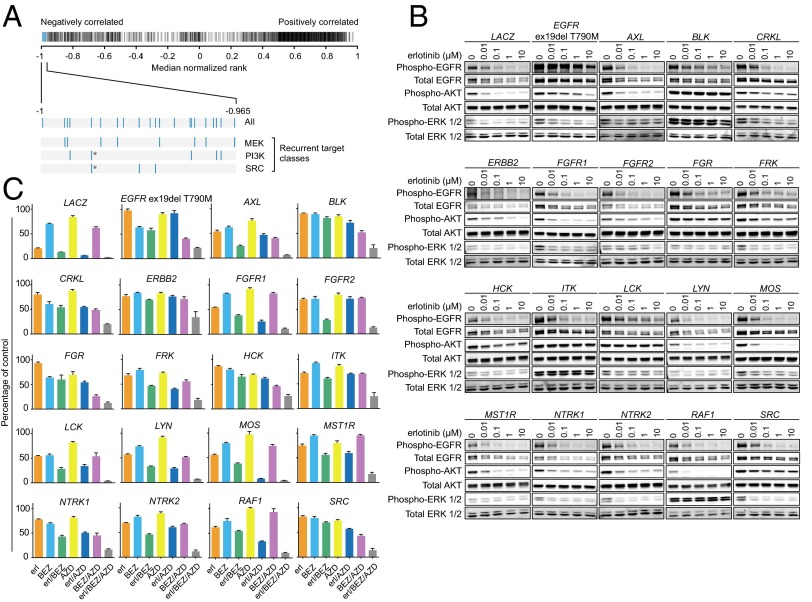
The LINCS database also contains over 34,000 gene expression profiles from cells treated with 3,103 compounds. We queried LINCS for chemical perturbations whose transcriptional effects were anticorrelated with those of query genes, hypothesizing that such perturbations could represent compound classes with potential to reverse ORF-mediated effects. Among the top 0.7% anticorrelated compounds (Fig. 5A, blue bars), 13 of 21 (>61%) could be classified as either MEK or PI3K inhibitors (Fig. 5A, Lower Left). Along with the third-most abundant target, SRC, these were the only recurrent drug targets represented at the top of this list (Fig. S7A). These findings indicated that the transcriptional states induced by our panel of query genes were opposed to those induced by PI3K or MEK inhibition, and suggested that either or both of these pathways could be reactivated in ORF-expressing cells.
Fig. 5.
EGFR bypass genes commonly reactivate AKT and/or ERK1/2 signaling, and PI3K-mTOR and MEK coinhibition restores EGFR dependence. (A) Barcode plot displaying LINCS compounds ranked by the correlations of their signatures to those of positive-phenotype cluster ORFs. The most positively/negatively correlated compounds approach ranks of 1 and −1, respectively. The 0.7% most negatively correlated compounds (blue portion of barcode plot) include several inhibitors targeting MEK, PI3K, and SRC (Fig. S7A). Each bar represents a chemical reagent. A single reagent targeting both PI3K and SRC is denoted with an asterisk. (B) Immunoblot analysis of PC9 cells overexpressing the indicated ORFs and treated with erlotinib for 6 h. Cells were incubated with 0.5% serum media 18 h before and during drug/DMSO treatment. (C) Cell viability of PC9 cells overexpressing the indicated ORFs following treatment with 100 nM erlotinib (erl), 500 nM of BEZ235 (BEZ), 2.5 µM of AZD6244 (AZD), or their combinations for 72 h. Cell viability was assayed with CellTiter-Glo. Data are expressed as percent viability relative to vehicle-treated cells and represent the mean ± SD of four replicates.
EGFR Modifier Genes Commonly Reactivate AKT and/or ERK1/2 Signaling, and PI3K-mTOR and MEK Coinhibition Restores EGFR Dependence.
Given that PI3K and MEK inhibitor treatment signatures were anticorrelated with those of our positive-phenotype query genes (Fig. 5A), we asked whether EGFR bypass genes reactivate the PI3K-AKT or MEK-ERK pathways. PC9 cells overexpressing EGFR bypass genes were treated with erlotinib for 6 h and profiled for activation of EGFR, AKT, and ERK1/2 (Fig. 5B). Indeed, persistent activation of AKT and/or ERK1/2 signaling under erlotinib treatment is a common feature of the majority of EGFR bypass genes, with sustained phospho-AKT most prominently associated with BLK, FGR, FRK, HCK, LCK, SRC, ITK, and CRKL; and sustained phospho-ERK1/2 most prominently associated with RAF1, BLK, ITK, MOS, AXL, and CRKL. Other ORFs, such as those encoding FGFR family genes, appear to display very modest activation of one or both of these pathways, which may also be reflective of their more modest rescue phenotype (Fig. 2A). We also observed that cells expressing several kinases (BLK, LCK, FGR, and FRK) maintain EGFR phosphorylation in the presence of erlotinib; we found that overexpression of these and other Src family kinases in PC9 cells can also modify a specific tyrosine residue, Y845, on EGFR that is known to be the major site for c-Src-mediated phosphorylation (Fig. S8A) (25).
We similarly tested a representative subset of EGFR bypass ORFs in H3255 cells for their ability to reactivate downstream pathways (Fig. S8B). Consistent with our findings in PC9 cells, we observed varying degrees of phospho-ERK1/2 activation under erlotinib treatment among kinases that can bypass EGFR in this cell line. In contrast, we noted that several of the ORFs that fail to promote erlotinib resistance in H3255 cells do not reactivate ERK1/2, although it was impossible to exclude the possibility of ERK1/2 reactivation by LYN and NTRK2. The observed differences in phospho-ERK1/2 activation in this cell line potentially explain the sometimes varied resistance-inducing effects of ORFs in different EGFR-mutant models.
To determine whether reactivation of these signal transducers is required for ORF-induced bypass, PC9 cells transduced with EGFR bypass genes were treated with erlotinib; the dual PI3K-mTOR inhibitor NVP-BEZ235 (BEZ235); the MEK inhibitor AZD6244; or their combinations, and assayed for cell viability after 72 h (Fig. 5C). Drug doses of BEZ235 (500 nM) and AZD6244 (2.5 µM) were determined empirically to be the lowest doses capable of maximally inhibiting AKT and ERK1/2 phosphorylation (Fig. S7 B and C), inhibit the activity of their targets alone and in each of the combinations tested (Fig. S7D), and are in similar ranges to those used by other investigators in EGFR-mutant cell lines (26). Combined BEZ235/AZD6244 treatment was sufficient to restore sensitivity to erlotinib across all ORF-expressing cell lines, with sensitivity often enhanced relative to erlotinib treatment in LACZ-expressing cells. In cases where at least partial restoration of erlotinib sensitivity could be achieved using either BEZ235 or AZD6244, resensitization was always potentiated by combining these two agents. Together, these data are consistent with the concept that reactivation of AKT and ERK1/2 signaling commonly underlies ORF-mediated EGFR bypass.
Discussion
We report the use of a systematic ORF-based screening approach to identify the spectrum of kinases capable of bypassing EGFR dependence in EGFR-mutant NSCLC cells. To our knowledge, this represents the first cDNA-based genetic complementation screen for loss of EGFR in an EGFR-mutant model. This approach has identified known as well as previously unidentified drivers of EGFR bypass in EGFR-mutant NSCLC. More generally, it has revealed the breadth of kinases with compensatory potential in the setting of EGFR oncogene addiction: 18 genes for PC9 cells, including both tyrosine and serine/threonine kinases.
Among the newly identified drivers of EGFR bypass in EGFR-mutant NSCLC, Src family kinases were highly represented, comprising over one-third of screening hits. The finding that Src family kinases are sufficient to modify EGFR dependence is in line with considerable evidence linking EGFR activity with this family: c-Src itself has been characterized extensively with respect to its cooperative relationship with EGFR (27), and the introduction of dominant-active c-Src can reduce the inhibitory effects of erlotinib in head and neck squamous cell carcinoma models (28). Src family kinase activation has been observed in cetuximab-resistant colorectal adenocarcinoma and NSCLC squamous cell carcinoma in vitro models (29, 30), and it has been reported that CRIPTO1-mediated EGFR TKI resistance in NSCLC is Src-dependent (31). Furthermore, a recent report described increased expression and activation of Src, mediated by integrin activation, in EGFR TKI-resistant lung adenocarcinoma models (32). The present study extends these findings to demonstrate that overexpression of SRC is itself sufficient to bypass EGFR dependence in EGFR-dependent NSCLC, and that this function is shared by seven of the nine family members.
Similarly, we noted that fibroblast growth factor receptor (FGFR) family kinase genes FGFR1 and FGFR2, though not previously recognized as sufficient for EGFR bypass, nonetheless align with previous work describing up-regulation of FGFR1 as well as the ligand FGF2 in gefitinib-resistant NSCLC models, with concomitant dependency on the FGFR pathway (33, 34), and with other studies implicating FGF ligands in TKI resistance (15, 35).
Other genes identified, including the neurotrophic tyrosine kinase receptor (NTKR) family kinases NTRK1 and NTRK2, the Tec family kinase ITK, MST1R, and the serine/threonine kinases MOS and RAF1, have not previously been appreciated to modify EGFR dependence in EGFR-mutant lung cancer cells, and thus underscore the power of this screening approach in identifying novel mediators of bypass for a given dependency.
Identifying the spectrum of kinases capable of EGFR bypass is of considerable clinical interest given that patients with EGFR-mutant NSCLCs almost invariably acquire resistance to EGFR TKIs (7); a large fraction (30%) of acquired resistance cases are driven by unknown mechanism(s) (36); and because mounting evidence suggests that activation of alternative driver kinases, such as MET, represents a common route by which kinase-driven cancers acquire resistance to therapy (9, 12, 18). A systematic study of kinase-driven EGFR bypass may reveal the scope of potential kinases switches, and whether they act through common or divergent pathways in sustaining EGFR-independent survival. Our findings suggest that the diverse kinases capable of replacing EGFR in PC9 cells uniformly converge upon downstream pathways.
More generally, our observation that a large number of kinase inputs can redundantly sustain cancer cell survival is consistent with recent reports describing broad potential for growth factor-mediated inhibitor resistance in several tumor dependency models (14, 15), with the finding that coactivation of multiple RTKs in glioblastoma cells overcomes reliance on any one RTK for downstream signaling activation (16), and with the identification of nine kinase-related genes whose overexpression can overcome RAF inhibition in BRAF-mutant melanoma cells (18). Taken together, our finding that a diverse set of kinases can redundantly drive the EGFR-dependent state may thus represent a more general feature of signal transduction in oncogene-dependent cancers.
Materials and Methods
Kinase ORF Screen.
Screening was performed using a kinase ORF library of 589 ORFs (CCSB/Broad Institute Kinase ORF Collection) (17, 18), along with the controls displayed in Fig. 1. Transduced PC9 cells were treated with 3 µM erlotinib, 300 nM erlotinib, or DMSO, and cell viability was assayed 3 d later using CellTiter-Glo (Promega). Additional details are described in SI Materials and Methods.
Additional Materials and Methods.
Additional materials and methods, including cell culture and reagents; screen validation and drug sensitivity assays; immunoblotting; gene expression profiling; and LINCS analysis, are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Broad Genetic Perturbation Platform for generation of the ORF library; the Broad Connectivity Map team for generation of the Library of Integrated Network-Based Cellular Signatures (LINCS) dataset and technical assistance; V. Dancik for LINCS compound identification; and J. Chmielecki, H. Greulich, E. Stover, Z. Tothova, and X. Wu for helpful discussions. Support for this work was provided by National Cancer Institute Grants R01CA116020, R01CA109038, and P50CA090578 (to M.M.) and National Institutes of Health Common Fund Grant 5U54HG006093 (to T.R.G.).
Footnotes
Conflict of interest statement: M.M. was a consultant and received research support from Novartis, and receives research support from Bayer. M.M., L.A.G., and T.R.G. are equity holders in, and consultants for, Foundation Medicine.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412228112/-/DCSupplemental.
References
- 1.Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, et al. Spanish Lung Cancer Group Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18(1):73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Bivona TG, et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471(7339):523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 10.Takezawa K, et al. HER2 amplification: A potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2(10):922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung HW, et al. Amplification of CRKL induces transformation and epidermal growth factor receptor inhibitor resistance in human non-small cell lung cancers. Cancer Discov. 2011;1(7):608–625. doi: 10.1158/2159-8290.CD-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachleitner-Hofmann T, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008;7(11):3499–3508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 14.Harbinski F, et al. Rescue screens with secreted proteins reveal compensatory potential of receptor tyrosine kinases in driving cancer growth. Cancer Discov. 2012;2(10):948–959. doi: 10.1158/2159-8290.CD-12-0237. [DOI] [PubMed] [Google Scholar]
- 15.Wilson TR, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487(7408):505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stommel JM, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, et al. A public genome-scale lentiviral expression library of human ORFs. Nat Methods. 2011;8(8):659–661. doi: 10.1038/nmeth.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johannessen CM, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo WL, et al. Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations. J Thorac Oncol. 2010;5(7):1048–1053. doi: 10.1097/JTO.0b013e3181dd1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 21.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 22.Sos ML, et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J Clin Invest. 2009;119(6):1727–1740. doi: 10.1172/JCI37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Liang K, Li X, Fan Z. Responses of cancer cells with wild-type or tyrosine kinase domain-mutated epidermal growth factor receptor (EGFR) to EGFR-targeted therapy are linked to downregulation of hypoxia-inducible factor-1alpha. Mol Cancer. 2007;6:63. doi: 10.1186/1476-4598-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatzivassiliou G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 25.Biscardi JS, et al. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274(12):8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 26.Faber AC, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci USA. 2009;106(46):19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6(3):209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Stabile LP, et al. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clin Cancer Res. 2013;19(2):380–392. doi: 10.1158/1078-0432.CCR-12-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. 2007;67(17):8240–8247. doi: 10.1158/0008-5472.CAN-07-0589. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler DL, et al. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009;8(8):696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park KS, et al. CRIPTO1 expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor resistance. J Clin Invest. 2014;124(7):3003–3015. doi: 10.1172/JCI73048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanda R, et al. Erlotinib resistance in lung cancer cells mediated by integrinbeta1/Src/Akt-driven bypass signaling. Cancer Res. 2013;73(20):6243–6253. doi: 10.1158/0008-5472.CAN-12-4502. [DOI] [PubMed] [Google Scholar]
- 33.Ware KE, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39. doi: 10.1038/oncsis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terai H, et al. Activation of the FGF2-FGFR1 autocrine pathway: A novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11(7):759–767. doi: 10.1158/1541-7786.MCR-12-0652. [DOI] [PubMed] [Google Scholar]
- 35.Ware KE, et al. Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS ONE. 2010;5(11):e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.