Abstract
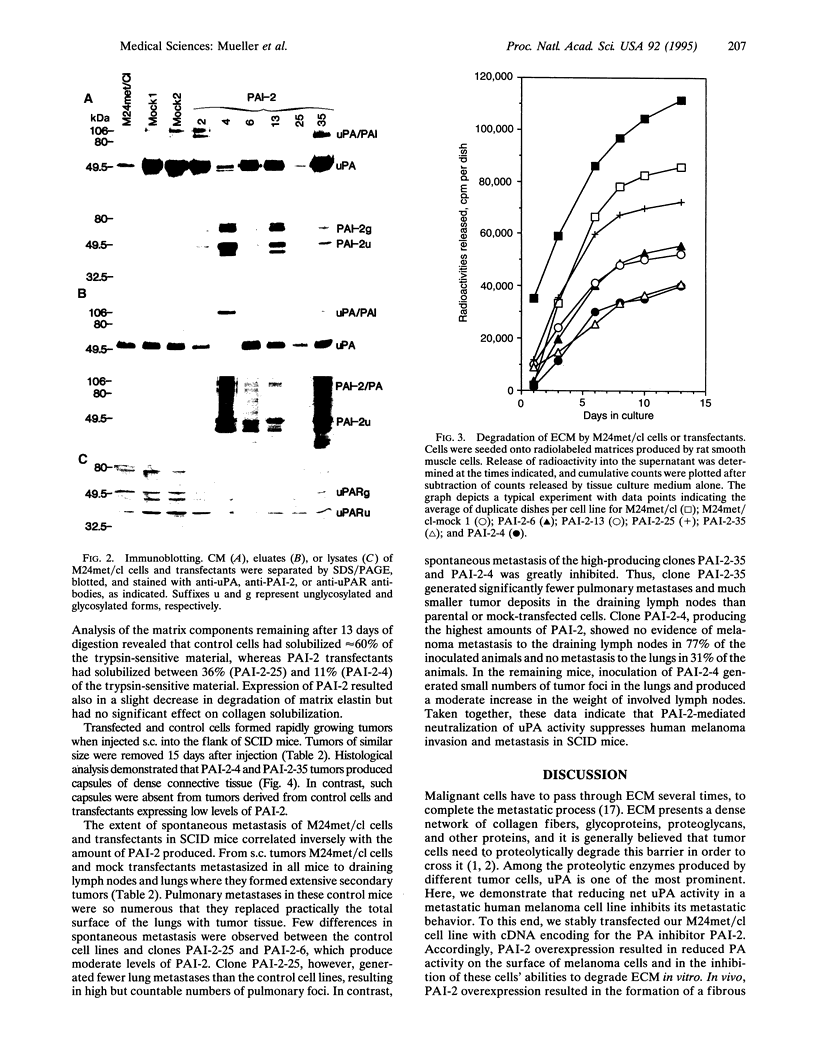
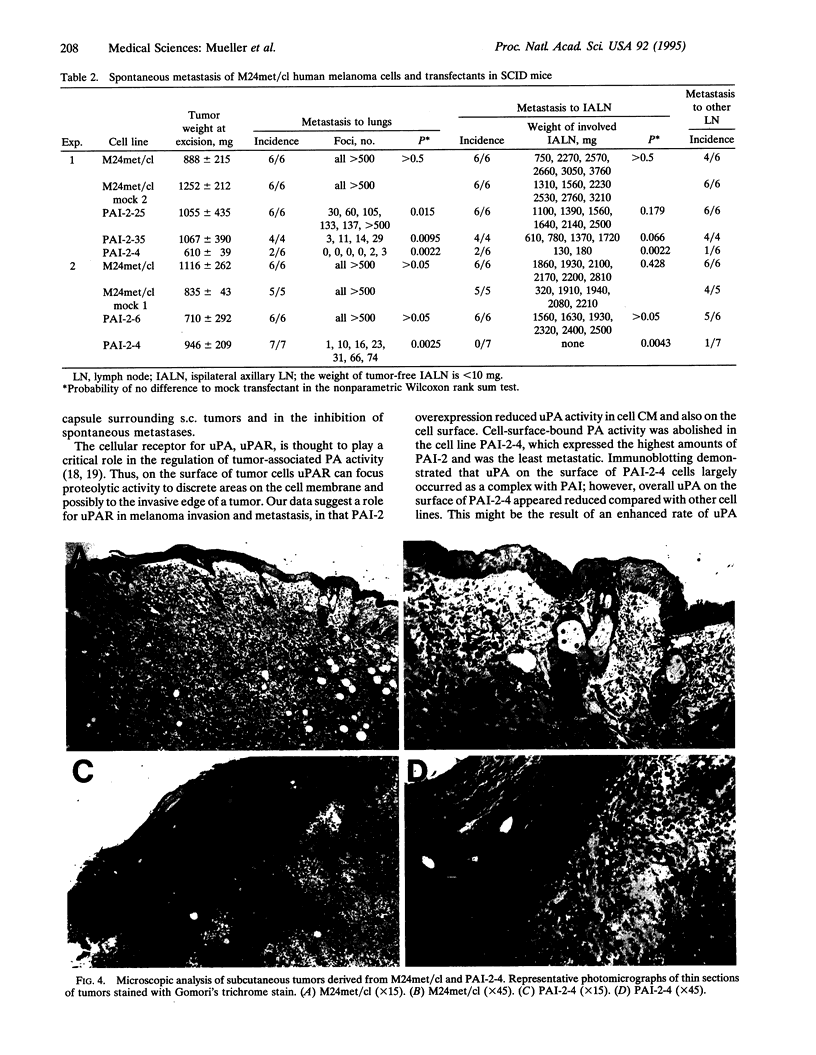
A metastatic human melanoma cell line that produces urokinase-type plasminogen activator was stably transfected with cDNA encoding human plasminogen activator inhibitor 2 (PAI-2). Transfected clones expressed PAI-2 at levels two to nine times higher than both the parental cell line and mock transfectants, as detected by ELISA of cell lysates and conditioned medium. The clone with the highest PAI-2 expression exhibited complete inhibition of soluble and cell-surface-bound plasminogen activator activity. The level of PAI-2 overexpression in these clonal cell lines correlated positively with the inhibition of their ability to degrade extracellular matrix in vitro. Parental, mock-transfected, and PAI-2-transfected cell lines produced rapidly growing tumors when injected s.c. into the skin of mice with severe combined immunodeficiency. The tumors producing the highest levels of PAI-2 were surrounded by a dense tumor capsule. Both parental cells and mock-transfected cells invariably metastasized from s.c. tumors to lymph nodes and lungs of mice. PAI-2-transfected cell lines produced significantly less or no metastases. Taken together, these data indicate a critical role for plasminogen activator activity in melanoma invasion and metastasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. H., Reich R., Miskin R. Expression of human recombinant plasminogen activators enhances invasion and experimental metastasis of H-ras-transformed NIH 3T3 cells. Mol Cell Biol. 1989 May;9(5):2133–2141. doi: 10.1128/mcb.9.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. S., Bleakley P., Woodrow G. C., Doe W. F. Inhibition of cancer cell urokinase plasminogen activator by its specific inhibitor PAI-2 and subsequent effects on extracellular matrix degradation. Cancer Res. 1990 Aug 1;50(15):4676–4684. [PubMed] [Google Scholar]
- Cajot J. F., Bamat J., Bergonzelli G. E., Kruithof E. K., Medcalf R. L., Testuz J., Sordat B. Plasminogen-activator inhibitor type 1 is a potent natural inhibitor of extracellular matrix degradation by fibrosarcoma and colon carcinoma cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6939–6943. doi: 10.1073/pnas.87.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C. W., Cohen R. L., Lucas B. K., Liu G., Shuman M. A., Levinson A. D. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClerck Y. A., Perez N., Shimada H., Boone T. C., Langley K. E., Taylor S. M. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992 Feb 1;52(3):701–708. [PubMed] [Google Scholar]
- Duffy M. J. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992 May;10(3):145–155. doi: 10.1007/BF00132746. [DOI] [PubMed] [Google Scholar]
- Ellis V., Pyke C., Eriksen J., Solberg H., Danø K. The urokinase receptor: involvement in cell surface proteolysis and cancer invasion. Ann N Y Acad Sci. 1992 Dec 4;667:13–31. doi: 10.1111/j.1749-6632.1992.tb51591.x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Foekens J. A., Schmitt M., van Putten W. L., Peters H. A., Bontenbal M., Jänicke F., Klijn J. G. Prognostic value of urokinase-type plasminogen activator in 671 primary breast cancer patients. Cancer Res. 1992 Nov 1;52(21):6101–6105. [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Hollas W., Boyd D. Urokinase-dependent proteolysis in cultured colon cancer is directed by its receptor. Semin Thromb Hemost. 1991 Jul;17(3):225–230. doi: 10.1055/s-2007-1002613. [DOI] [PubMed] [Google Scholar]
- Kwaan H. C. The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev. 1992 Nov;11(3-4):291–311. doi: 10.1007/BF01307184. [DOI] [PubMed] [Google Scholar]
- Laug W. E., Cao X. R., Yu Y. B., Shimada H., Kruithof E. K. Inhibition of invasion of HT1080 sarcoma cells expressing recombinant plasminogen activator inhibitor 2. Cancer Res. 1993 Dec 15;53(24):6051–6057. [PubMed] [Google Scholar]
- Laug W. E., Wang K., Mundi R., Rideout W., 3rd, Kruithof E. K., Bogenmann E. Clonal variation of expression of the genes coding for plasminogen activators, their inhibitors and the urokinase receptor in HT1080 sarcoma cells. Int J Cancer. 1992 Sep 9;52(2):298–304. doi: 10.1002/ijc.2910520224. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery A. M., De Clerck Y. A., Langley K. E., Reisfeld R. A., Mueller B. M. Melanoma-mediated dissolution of extracellular matrix: contribution of urokinase-dependent and metalloproteinase-dependent proteolytic pathways. Cancer Res. 1993 Feb 1;53(3):693–700. [PubMed] [Google Scholar]
- Mueller B. M., Reisfeld R. A., Edgington T. S., Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B. M., Romerdahl C. A., Trent J. M., Reisfeld R. A. Suppression of spontaneous melanoma metastasis in scid mice with an antibody to the epidermal growth factor receptor. Cancer Res. 1991 Apr 15;51(8):2193–2198. [PubMed] [Google Scholar]
- Olson D., Pöllänen J., Høyer-Hansen G., Rønne E., Sakaguchi K., Wun T. C., Appella E., Danø K., Blasi F. Internalization of the urokinase-plasminogen activator inhibitor type-1 complex is mediated by the urokinase receptor. J Biol Chem. 1992 May 5;267(13):9129–9133. [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Russo-Payne H., Wilson E. L. Inhibition of urokinase-type plasminogen activator by antibodies: the effect on dissemination of a human tumor in the nude mouse. Cancer Res. 1991 Jan 1;51(1):274–281. [PubMed] [Google Scholar]
- Quax P. H., van Muijen G. N., Weening-Verhoeff E. J., Lund L. R., Danø K., Ruiter D. J., Verheijen J. H. Metastatic behavior of human melanoma cell lines in nude mice correlates with urokinase-type plasminogen activator, its type-1 inhibitor, and urokinase-mediated matrix degradation. J Cell Biol. 1991 Oct;115(1):191–199. doi: 10.1083/jcb.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. R., Schultz R. M. Relationship between secreted urokinase plasminogen activator activity and metastatic potential in murine B16 cells transfected with human urokinase sense and antisense genes. Cancer Res. 1990 Dec 1;50(23):7623–7633. [PubMed] [Google Scholar]
- Zou Z., Anisowicz A., Hendrix M. J., Thor A., Neveu M., Sheng S., Rafidi K., Seftor E., Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994 Jan 28;263(5146):526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- de Vries T. J., Quax P. H., Denijn M., Verrijp K. N., Verheijen J. H., Verspaget H. W., Weidle U. H., Ruiter D. J., van Muijen G. N. Plasminogen activators, their inhibitors, and urokinase receptor emerge in late stages of melanocytic tumor progression. Am J Pathol. 1994 Jan;144(1):70–81. [PMC free article] [PubMed] [Google Scholar]