Abstract
Plant-herbivore interactions provide well studied examples of coevolution, but little is known about how such interactions are influenced by the third trophic level. Here we show that larvae of the specialized lepidopteran herbivore Heliothis subflexa reduce their vulnerability to natural enemies through adaptation to a remarkable and previously unknown feature of their host plant, Physalis angulata: The fruits of this plant lack linolenic acid (LA), which is required for the development of most insects. By overcoming this nutritional deficiency, H. subflexa larvae achieve numerous advantages. First, they gain near-exclusive access to a food resource: we demonstrate that closely related Heliothis virescens larvae cannot develop on P. angulata fruit unless the fruit are treated with LA. Second, they reduce their vulnerability to enemies: LA is a key component of volicitin, an elicitor of plant-volatile-signaling defenses. We demonstrate that volicitin is absent in the oral secretions of fruit-feeding caterpillars, that the volatile profiles of plants induced by fruit feeding differ from those induced by leaf feeding or by feeding on LA-treated fruit, and that the former are far less attractive to female Cardiochiles nigriceps parasitoids. Finally, they render themselves nutritionally unsuitable as hosts for enemies that require LA for their own development: we show that C. nigriceps larvae fail to develop within the bodies of fruit-feeding caterpillars but do develop in caterpillars feeding on LA-treated fruit. Thus, H. subflexa larvae not only overcome a serious dietary deficiency but also reduce their vulnerability to natural enemies through a form of “biochemical crypsis.”
The vulnerability of insect herbivores to attack by predators and parasitoids is often mediated by interactions with the host plant on which the herbivore feeds (1-3). Specialist herbivores that have overcome certain plant defenses may subsequently co-opt those defenses, for example, by sequestering chemical toxins produced by the plant within their bodies as a defense against their own enemies (2, 4-6). Plants, in turn, often respond to herbivory by releasing volatile chemical compounds that are attractive to predators and parasitoids that are natural enemies of the herbivores (7, 8). Thus, the particular physical and physiological characteristics of the host plant are thought to be major features influencing the vulnerability of insect herbivores to attack by predators and parasitoids, and adaptation to those specific characteristics may be expected to play an important role in avoiding attack by natural enemies (9-11). Lill et al. (3) demonstrated a strong effect of host-plant identity on parasitism rates and suggested that these differences might be influenced by features of the host plant including plant-volatile-related differences in parasitoid attraction and retention but did not address the specific mechanisms that might underlie such differences.
In this article we describe behavioral and physiological adaptations of the specialist lepidopteran herbivore Heliothis subflexa to features of its solanaceous host plant, Physalis angulata, that confer a number of advantages on the caterpillar, including reduced production of herbivore-induced volatile signals that are attractive to natural enemies. H. subflexa larvae feed exclusively on the fruits of Physalis spp., which are physically encased within an inflated calyx of the flower and defended by the presence of secondary compounds (e.g., withanolides and flavonol glycosides) (12, 13). We have discovered an additional feature of these fruits that might be expected to deter insect herbivory: P. angulata fruits lack an important fatty acid, linolenic acid (LA), which is required for the normal development and metamorphosis of most insect larvae (14-16).
The series of experiments reported below explored the implications of this remarkable feature of the plant's biology on tritrophic (plant-herbivore-natural enemy) interactions. We show that H. subflexa larvae, by overcoming the nutritional barrier posed by the lack of LA in P. angulata fruit, gain access to a food resource that is unavailable to most of their competitors. Moreover, by feeding exclusively on the fruits of P. angulata (after boring through the protective calyx in which they are enclosed), the larvae are able to co-opt the physical and chemical (17) defenses of the plant and also render themselves nutritionally unattractive to parasitic wasps and other potential natural enemies that require LA for their own development. Most remarkably, H. subflexa larvae avoid triggering certain induced plant-signaling responses, because LA is an essential component of the chemical compound volicitin, which has been demonstrated (18) to be a critical elicitor of these responses.
Materials and Methods
Absence of LA in P. angulata Fruit and of Volicitin in Fruit-Feeding Caterpillars. Experimental design and objectives. This series of experiments explored the chemical composition of the regurgitant of H. subflexa and Heliothis virescens caterpillars feeding on various P. angulata tissues and of the plant tissues themselves. Second-instar larvae of both species were placed in diet cups and fed either P. angulata fruits, fruits sprayed with LA, or leaves. The larvae were allowed to feed until the molt to fourth instar when oral secretions were collected. Regurgitant was analyzed for the presence of fatty acids and fatty acid conjugates. Plant tissues from undamaged fruit, calyx, and leaves then were analyzed for the presence of fatty acids.
Insects and plants. H. subflexa larvae were obtained from the F. L. Gould laboratory (North Carolina State University, Raleigh) and reared on P. angulata fruit or an artificial corn/soy meal diet. H. virescens larvae were obtained from W. J. Lewis (U.S. Department of Agriculture) and reared on P. angulata fruit or an artificial pinto-bean diet. All larvae were maintained under a 14-h/10-h light/dark cycle at 60% relative humidity and 25°C. H. subflexa utilizes several species of Physalis, but larvae are most commonly found on P. angulata (19, 20). P. angulata plants were grown from seed obtained from the F. L. Gould laboratory. Oral secretions. Caterpillar oral secretions were collected following the procedure described by Turlings et al. (21). Each sample of pooled regurgitant (≈30 larvae) was immediately stored at -80°C. Samples were centrifuged at 16,000 × g for 10 min, and the supernatant was filtered consecutively through 0.45-μm (Millex-HV, Millipore) and 0.22-μm (Millex-GX, Millipore) sterile Millipore filters to remove bacteria.
Plant extractions. Plant materials (undamaged fruits, calyxes, or leaves) were weighed (2.5 g), ground in liquid nitrogen in a precooled mortar and pestle, diluted in a 50-ml solution of chloroform/methanol (2:1), and left in the dark for 24 h at 20°C. After the sample was centrifuged at 3,000 × g, the supernatant was saved, and the tissue pellet was reextracted with a solution of chloroform/methanol/water (1:10:10). Lipids were recovered in the chloroform phase, dried under N2, and redissolved in methanol/acetic anhydride (MeOH/Ac2O) (method described below). After methanolysis, the fatty acid methyl esters were quantified with GC/MS as described below.
HPLC analysis. Caterpillar oral secretions and plant extracts were analyzed by HPLC with UV detection at 200 nm (constaMetric 4100 pump, SpectroMonitor 3200 detector, Spectra System AS 3500 autosampler, Thermo Separation Products, Riviera Beach, FL). A reverse-phase column (YMC-Pack ODS-AMQ, 250 × 4.6-mm i.d.; YMC, Kyoto) was eluted (1 ml/min) with a solvent (High Purity Solvent, gradient 20-95% CH3CN, Burdick and Jackson) containing 0.8% acetic acid, in water (Milli-Q UV Plus system, Millipore) containing 0.5% acetic acid, over 40 min and then returned to initial conditions at 45 min. The column was maintained at 60°C. For quantitative analyses, 5 μl of N-palmitoleoyl-l-glutamine solution (1 μg/μl) in CH3CN/H2O (8:2, μvol/vol) was added to each sample (50 μl) as an internal standard, and 10 μl of each sample was injected for HPLC analysis.
Acid methanolysis and chemical analysis. HPLC-purified compounds were vacuum-dried and treated with MeOH/Ac2O following the procedure described by Mori et al. (22). The samples were analyzed by GC/MS using both chemical ionization and electron ionization (6890 gas chromatograph with a 30-m × 0.25-mm i.d., 0.25-μm-film-thickness HP-5 capillary column, interfaced to a Hewlett-Packard 5973 mass selective detector). The column was held at 40°C for 1 min after injection and then heated 10°C/min to 180°C. The carrier gas was helium at a velocity of 30 cm/sec. Isobutane was used as the reagent gas for chemical ionization, and the ion source temperature was set to 250°C. Compound identifications were confirmed by comparing chromatographic retention times and mass spectra for authentic standards.
Composition of fatty acid conjugates. Caterpillar oral secretions and plant extracts were heated to 95°C for 20 min and centrifuged at 16,000 × g for 10 min. Internal standard (N-palmitoleoyl-l-glutamine) was added, and each sample was analyzed by HPLC. The results were calculated as nanograms of each component per microliter.
Adaptation of H. subflexa Larvae to LA-Deficient P. angulata Fruit. Experimental design and objectives. These experiments explored the ability of other herbivore species to survive and develop on P. angulata fruit, which lack LA. Larvae of the closely related species H. virescens (three repetitions of 30 larvae each per treatment) were subjected to three diet treatments: LA-sprayed fruits, fruits sprayed with a control solution, or an artificial pinto-bean diet. Survival of the larvae was assessed daily, and pupae were classified as healthy or defective.
Insects and plants. H. virescens larvae and P. angulata plants were maintained according to the protocols described for the previous experiment.
LA-treated fruit. Mature fruits (marble-sized) were collected from P. angulata plants and sprayed with either a solution of LA and ethyl alcohol (1:9) or a control solution (ethyl alcohol) by using a microspray device that creates a fine, uniform spray (23). Each fruit's calyx was cut and temporarily opened by pulling it apart at the distal end to allow the fruit to be sprayed and then reclosed. (Despite the opening created by this process, caterpillars almost invariably persisted in boring through the reclosed calyx to reach the fruit.) Once dry, the fruits were placed in diet cups and changed daily. A G test (log-likelihood ratio) was used to analyze differences between treatments (SAS Institute, Cary, NC).
Vulnerability to Natural Enemies. Experimental design and objectives. These experiments explored the effects of adaptation by H. subflexa to a diet of LA-deficient P. angulata fruit on the vulnerability of the larvae to natural enemies. We examined volatile profiles from plants induced by feeding on different P. angulata tissues (leaves, fruits, or fruits treated with LA) and the relative attractiveness of each of these profiles to female Cardiochiles nigriceps parasitoids. We then measured the survival and viability of C. nigriceps larva developing on H. virescens and H. subflexa caterpillars that were fed different diets (P. angulata fruits, fruit sprayed with LA, or artificial diet).
Insects and plants. H. virescens larvae and P. angulata plants were maintained according to the protocols described above. C. nigriceps were reared on H. virescens larvae according to the procedure of Lewis and Burton (24). Parasitoids were held at 25°C, 14-h/10-h light/dark, and 70% relative humidity until used for experiments.
Volatile collection and analysis. Five third-instar H. subflexa larvae were placed on 9-week-old greenhouse-grown P. angulata plants, which then were enclosed in a volatile collection chamber. Caterpillars were either allowed to move freely within the chamber (in which case they fed exclusively on fruits) or confined to feeding exclusively on the leaves. Volatile collections (at 6-h intervals) started when the caterpillars were placed in the chambers and continued for 7 days. Volatile collections and analyses followed the method described by De Moraes et al. (8) but with larger chambers (48 × 22 cm in diameter). Samples were analyzed by GC (GC/flame ionization detector) and mass spectrometry (GC/MS electron ionization and chemical ionization). The structure of the volatile components was confirmed by comparing GC retention times and mass spectral data with those of commercially available standards.
Behavioral assays. To assess parasitoid preference, dual-choice tests were performed in a wind tunnel by using (i) plants induced by leaf feeding versus plants induced by fruit feeding and (ii) plants induced by fruit feeding versus plants induced by larvae feeding on LA-treated fruits; undamaged plants with LA-treated fruits were used as a control. Twelve third-instar caterpillars were caged on the leaves (two per leaf) or fruit (one per fruit) of greenhouse-grown P. angulata plants for 48 h. The plant-caterpillar complex then was placed at the upwind end of a 60 × 60 × 180-cm wind tunnel. A wind speed of 60 ± 2 cm/sec was used at 25 ± 2°C and 40 ± 10% relative humidity and a light level of 500 W/m2. A female C. nigriceps was released at the downwind end of the tunnel. The first plant terminal on which the female landed was recorded. Fifty complete flights were conducted for each treatment. Plant positions were switched after every fifth flight. Parasitoid preferences in the dual-choice tests were analyzed by a χ2 goodness-of-fit test (SAS Institute).
Effect of LA on survival and development of parasitoids. To determine whether the absence of LA affects parasitoid (C. nigriceps) emergence and survival, we allowed female parasitoids to parasitize both H. virescens and H. subflexa larvae that were kept on the three diet treatments: LA-sprayed fruits, fruits sprayed with the control solution, or artificial diet (pinto bean for H. virescens or corn/soy meal for H. subflexa). For each treatment, three replicates with 30 caterpillars each were performed. Differences between treatments were analyzed by using ANOVA (SAS Institute).
Results and Discussion
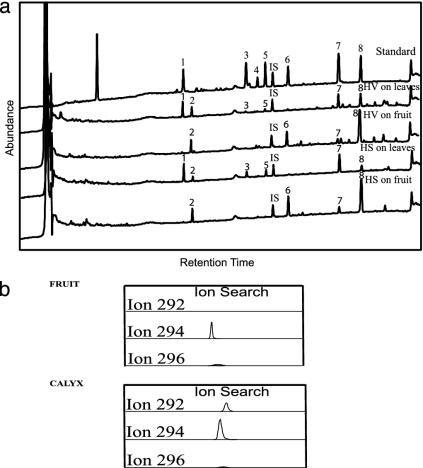
Absence of LA in P. angulata Fruit and of Volicitin in Fruit-Feeding Caterpillars. Under natural conditions, H. subflexa larvae feed exclusively on the fruits of Physalis spp. Chemical analyses (HPLC-UV) of the regurgitant of H. subflexa caterpillars fed on P. angulata fruit revealed the absence of volicitin [N-(17-hydroxylinolenoyl)-l-glutamine], an important elicitor of induced plant-volatile responses (Fig. 1a). This result was surprising because volicitin has been found in the regurgitant of the closely related species H. virescens and Helicoverpa zea (22) as well as in seven additional noctuid species (25).
Fig. 1.
(a) Typical chromatograms of oral secretions from H. virescens (HV) and H. subflexa (HS) fed on P. angulata. Chromatograms (HPLC-UV analysis at 200 nm) show the compounds detected in the supernatant of oral secretions from third-instar H. virescens and H. subflexa fed on P. angulata leaves or fruit for 48 h. Represented are: 1, N-(17-hydroxylinolenoyl)-l-glutamine (volicitin); 2, N-(17-hydroxylinoleoyl) glutamine; 3, 17-hydroxylinolenic acid; 4, 17-hydroxylinoleic acid; 5, N-linolenoyl-l-glutamine; 6, N-linoleoyl glutamine; 7, LA; 8, linoleic acid; internal standard (IS), N-palmitoleoyl-l-glutamine. It is significant that compound 1, volicitin, is not detectable in the oral secretions when either species fed on fruit. The very small amount of LA detected is likely caused by the presence of LA in the protective calyx, through which the caterpillar must bore to reach the fruit. (b) Analyses of fatty acid compounds (GC/MS electron ionization) in the fruit and calyx of P. angulata. Represented are: 1, linoleic acid (ion 294); 2, LA (ion 292); 3, oleic acid (ion 296). An ion search was performed to make sure that LA was not present in the fruits.
We repeated our chemical analyses by using regurgitant from larvae that had been fed P. angulata leaves rather than fruits and from larvae that had been fed on tobacco and found that volicitin was present in both cases. These findings suggested that the absence of volicitin in fruit-fed caterpillars was caused by some property of the fruits. To confirm this, we repeated our analyses with a second caterpillar species, H. virescens, and obtained similar results (Fig. 1a): volicitin was absent in the regurgitant of H. virescens larvae fed on P. angulata fruit but present in the regurgitant of caterpillars fed on P. angulata leaves or on tobacco.
Because volicitin is synthesized within the caterpillar by addition of a hydroxyl group and glutamine to LA obtained from the diet (26), we hypothesized that the absence of volicitin in fruit-feeding caterpillars might be explained by an absence of LA in P. angulata fruit. Chemical analyses (HPLC/GC/MS) of fruit extracts confirmed the absence of LA (Fig. 1b). A search of mass spectra for ions representing fatty acid compounds in the fruit and calyx of P. angulata revealed that LA (ion 292) was present only in the latter. We then sprayed P. angulata fruits with a synthetic solution containing LA to manipulate the presence of LA without affecting other factors such as the presence of secondary compounds. We found that volicitin was synthesized by H. subflexa caterpillars feeding on sprayed fruit, which confirmed that the absence of volicitin in the regurgitant of P. angulata fruit-fed caterpillars is attributable to the absence of LA in these fruits.
We cannot entirely rule out the possibility that small amounts of LA are present in the fruits but undetectable by our methods: LA is present in plant tissues in both free and membrane-bound forms (e.g., glycolipids, phospholipids, etc.); although our methods should extract both forms, it remains possible that some fatty acids, particularly those bound to membranes, were not completely extracted. Nevertheless, the undetectability of LA in the fruits compared with the high levels detected in other plant tissues, together with the strong effects obtained by adding supplemental LA, strongly indicate that a deficiency of LA in the fruits of P. angulata explains the absence of volicitin in fruit-fed caterpillars and the other phenotypic effects described below.
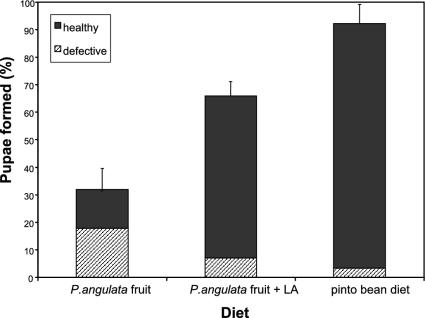
Adaptation of H. subflexa Larvae to LA-Free P. angulata Fruit. LA is essential for the normal development and metamorphosis of most insect larvae (15), especially Lepidoptera and Hymenoptera (16), which suggests that the ability of H. subflexa larvae to develop on the LA-deficient fruit of P. angulata may provide them with access to a food resource that is unavailable to lepidopteran herbivores lacking this adaptation. To explore this possibility, we examined the development of larvae of the closely related species H. virescens. We found that H. virescens larvae rarely survived on a diet of P. angulata fruit, and those larvae that did survive developed slowly and frequently displayed morphological defects (Fig. 2). To confirm that the inability of H. virescens larvae to develop on P. angulata fruit was attributable to the absence of LA, we reared larvae on P. angulata fruits that had been sprayed with a solution containing LA: survival, development, and pupal eclosion were all greatly enhanced (each of these replicates differed significantly from expectation: G > 31.0, df = 2, P < 0.0001) (Fig. 2).
Fig. 2.
Suitability of P. angulata fruit for H. virescens. Survival and pupation rates (for healthy and defective pupae) on P. angulata were assessed. Second-instar larvae were individually placed on fresh fruit in small plastic cups. Fruits were replaced daily. Three treatments were tested: (i) P. angulata fruit sprayed with ethyl alcohol; (ii) fruit sprayed with a solution of ethyl alcohol and LA (9:1); and (iii) artificial pinto-bean diet. Each replicate differed significantly from expectation (G > 31.0, df = 2, P < 0.0001).
The ability of H. subflexa larvae to develop in the absence of LA seems to provide them with almost exclusive access to P. angulata fruit. Over 2 years of fieldwork in Georgia and Florida, during which we collected hundreds of fruits from dozens of P. angulata plants, the only other insect observed was a single pyralid moth pupae. Their ability to develop on P. angulata fruits also makes it possible for H. subflexa larvae to exploit the protection from enemies provided by the fruit's protective calyx (27). In their natural habitat, H. subflexa larvae experience much lower parasitism rates than do closely related species such as H. virescens. Reported rates of parasitism for H. virescens on tobacco vary from 25% (28) to 72% (29), and rates as high as 96% have been observed on cotton (30). In contrast, Roach (31) found no parasitism of H. subflexa on P. angulata, whereas Lewis et al. (32) reported 2% parasitism and Sisterson and Gould (27) found 7% parasitism. It has been suggested that these low rates of parasitism are below expectations based on the protection afforded by the calyx alone (33), indicating that other factors must be involved.
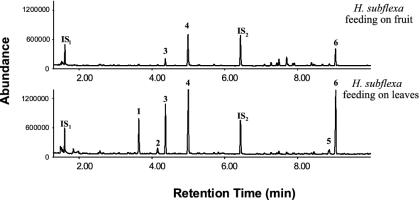
Vulnerability to Natural Enemies. One factor that might account for the low parasitism rates observed for H. subflexa relative to closely related species is a reduction in plant-signaling defenses resulting from the absence of volicitin, an important elicitor of such defenses, in the regurgitant of H. subflexa larvae, which feed exclusively on the LA-deficient fruits of P. angulata. Gas chromatographic analyses revealed significant differences between the volatile profiles of P. angulata plants on which H. subflexa fed exclusively on fruits (as they do in nature) and those on which they were forced to feed exclusively on leaves (Fig. 3), with several major compounds present only in the profiles of leaf-damaged plants. Although some parasitoid-attracting volatiles were released by fruit-damaged plants, those typically elicited by volicitin were absent.
Fig. 3.
Chromatographic profiles of volatiles from P. angulata during a 6-h interval after 48 h of feeding by H. subflexa on leaves or fruit. Represented are: 1, (E)-β-ocimene; 2, linalool; 3, (E)-4,8-dimethyl-1,3,7-nonatriene; 4, methyl salicylate; 5, an unidentified sesquiterpene; 6, (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene; internal standards, n-octane (IS1) and nonyl acetate (IS2).
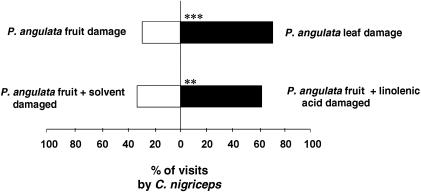
We directly tested the attractiveness of plants subjected to these two treatments, fruit feeding versus leaf feeding, to the parasitoid wasp C. nigriceps through behavioral assays (Fig. 4). C. nigriceps females were significantly more attracted to volatiles from P. angulata plants induced by H. subflexa feeding on leaves than to those from plants induced by fruit feeding (χ2 = 8.00, df = 1, P < 0.005). Furthermore, C. nigriceps females were significantly more attracted to volatiles from plants on which H. subflexa larvae fed on LA-treated fruits than to those from plants on which caterpillars fed on untreated fruits (χ2 = 4.52, df = 1, P < 0.03), strongly supporting the hypothesis that the relatively low attractiveness of volatiles induced by fruit feeding results from the absence of compounds elicited by volicitin, which is absent in the regurgitant of H. subflexa caterpillars feeding exclusively on the LA-deficient fruits of P. angulata.
Fig. 4.
Flight response of C. nigriceps to P. angulata plants subjected to damage by H. subflexa. Fruits were untreated or treated with LA in ethyl alcohol. Fruits treated with solvent alone were used as a control. Bars indicate the percentage of complete flights (n = 50). Asterisks indicate a significant difference within the choice test (χ2 test: **, P < 0.05; ***, P < 0.005). Additional controls testing parasitoid response to filter paper treated with solvent and LA and to solvent alone resulted in no complete flights.
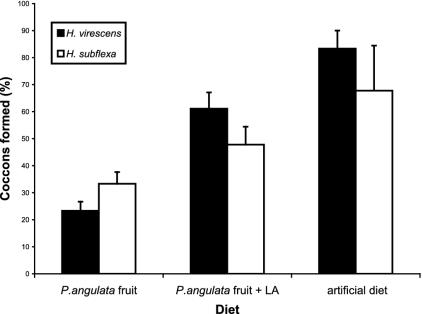
Discrimination of these alternative volatile profiles may be adaptive for the parasitoids if LA-free caterpillars are unsuitable hosts. To determine the importance of LA for parasitoid survival, we allowed C. nigriceps females to parasitize H. subflexa and H. virescens larvae that were fed on fruit, fruit treated with LA, or an artificial diet. C. nigriceps larvae formed cocoons on only 23% of parasitized H. virescens and 33% of parasitized H. subflexa caterpillars fed on untreated fruit (Fig. 5). Of the parasitoids that did emerge from fruit-fed caterpillars, many (65% on H. virescens and 57% on H. subflexa) exhibited defects in their cuticles and wings. In contrast, 83% of wasp larvae from H. virescens and 67% from H. subflexa caterpillars fed on artificial diet formed cocoons (Fig. 5). When caterpillars were allowed to feed on fruits that had been sprayed with LA, 61% of wasp larvae from H. virescens and 47% from H. subflexa larvae produced cocoons (Fig. 5). ANOVA showed no caterpillar species effect but a highly significant diet effect [ANOVA: caterpillar species, F(1,17) = 2.34, P = 0.149; diet, F(2,17) = 44.0, P < 0.00001]. Morphological defects were rarely observed in parasitoids that emerged from H. virescens or H. subflexa larvae fed on fruit sprayed with LA and were not observed at all in parasitoids emerging from larvae fed on an artificial diet. The very low survival rate for C. nigriceps larvae developing in caterpillars fed on untreated fruits indicates that LA is needed for successful development in the caterpillar host. Thus, the ability of H. subflexa larvae to develop on LA-deficient P. angulata fruit seems to make them unsuitable hosts for natural enemies that require LA.
Fig. 5.
Effect of diet on the suitability of H. virescens and H. subflexa larvae as hosts for C. nigriceps. Survival and pupation rates for the parasitoid were assessed. Female wasps parasitized third-instar larvae that then were individually placed on fresh fruit in small plastic cups. Fruits were replaced daily. Three treatments were tested: (i) P. angulata fruit sprayed with ethyl alcohol; (ii) fruit sprayed with a solution of ethyl alcohol and LA (9:1); and (iii) artificial diet. ANOVA: H. virescens, F(2,8) = 101.6, P < 0.0001; H. subflexa, F(2,8) = 17.7, P < 0.005; caterpillar species, F(1,17) = 2.34, P = 0.1485; diet, F(2,17) = 44.02, P < 0.00001.
We also observed that transferring caterpillars from fruit to artificial diet after parasitism increased the rate of parasitoid emergence, which suggests that previously published parasitism rates for H. subflexa, although already quite low, may be inflated relative to what would have occurred in the field because it is a common practice in studies that measure parasitism rates to collect caterpillars in the field and then rear them on an artificial diet containing LA.
Conclusions
Through their close adaptation to particular features of the morphology, physiology, and biochemistry of P. angulata, H. subflexa larvae seem to achieve a level of protection from natural enemies exceeding that available to less-specialized herbivores. Our results indicate that H. subflexa larvae achieve multiple benefits from such narrow specialization: they gain access to a food resource that is unavailable to most of their competitors, co-opt the plants' physical and chemical deterrents for their own defense, render themselves nutritionally unattractive to parasitoids, and circumvent induced plant defenses elicited by volicitin, including the production of some volatiles attractive to natural enemies.
Investigation of plant defense mechanisms and herbivore countermeasures continues to reveal unexpected layers of complexity (8, 34, 35). The findings presented here illustrate that the vulnerability of insect herbivores to attack by natural enemies is mediated by complex interactions with the host plant and provide a documented example of a case in which adaptive specialization results in reduced vulnerability to natural enemies. This system highlights the complexity of tritrophic systems and the potential for intricate coevolutionary relationships to emerge within them.
Acknowledgments
We thank Naoki Mori for his invaluable assistance with many of the chemical techniques; Fred Gould, Sara Oppenheim, and Neil Vickers for providing H. subflexa; Ottar Bjornstat, George De Moraes, Gary Felton, Fred Gould, Bruce McPheron, Ralph Mumma, Jack Schultz, John Tooker, and Jim Tumlinson for helpful comments on the manuscript; Edward Bogus, Carolina Briceño, and Janet Saunders for technical assistance; and the Packard and Beckman foundations and U.S. Department of Agriculture-National Research Institute for financial support.
Abbreviation: LA, linolenic acid.
References
- 1.Thompson, J. N. (1994) The Coevolutionary Process (Univ. of Chicago Press, Chicago).
- 2.Bernays, E. A. & Chapman, R. F. (1994) The Behavior of Host Plant Selection by Insects (Kluwer Academic, Boston).
- 3.Lill, J. T., Marquis, R. J. & Ricklefs, R. E. (2002) Nature 417, 170-173. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich, P. R. & Raven, P. H. (1964) Evolution (Lawrence, Kans.) 18, 586-608. [Google Scholar]
- 5.Barbosa, P. & Saunders J. A. (1985) in Chemically Mediated Interactions Between Plants and Other Organisms, eds. Cooper-Driver, G. A., Swain, T. & Conn, E. E. (Plenum, New York).
- 6.Bernays, E.A. & Grahan, M. (1988) Ecology 69, 886-892. [Google Scholar]
- 7.Turlings, T. C. J., Tumlinson, J. H. & Lewis, W.J. (1990) Science 250, 1251-1253. [DOI] [PubMed] [Google Scholar]
- 8.De Moraes, C. M., Lewis, W. J., Paré, P. W., Alborn, H. T. & Tumlinson, J. H. (1998) Nature 393, 570-573. [Google Scholar]
- 9.Greany, P. D., Vinson, S. B. & Lewis, W. J. (1984) Bioscience 34, 690-696. [Google Scholar]
- 10.Barbosa, P. B., Segarra, A. E., Gross, P., Caldas, A., Ahlstrom, K., Carlson, R. W., Ferguson, D. C., Grissell, E. E., Hodges, R. W., Marsh, P. M., et al. (2001) Ecology 82, 698-704. [Google Scholar]
- 11.Mira, A. & Bernays, E. A. (2002) Oikos 97, 387-397. [Google Scholar]
- 12.Shingu, K., Shoji, Y., Hikaru, O. & Nohara, T. (1992) Chem. Pharm. Bull. 40, 2448-2451. [Google Scholar]
- 13.Ismail, N. & Alam, M. (2001) Fitoterapia 72, 676-679. [DOI] [PubMed] [Google Scholar]
- 14.Vanderzant, E. S. (1967) Ann. Entomol. Soc. Am. 61, 120-125. [Google Scholar]
- 15.Stanley-Samuelson, D. W., Jurenka, R. A., Cripps, C., Blomquist, G. J. & de Renobales, M. (1988) Arch. Insect Biochem. Physiol. 9, 1-33. [Google Scholar]
- 16.Canavoso, L. E., Jouni, Z. E., Karnas, K. J., Pennington, J. E. & Wells, M. A. (2001) Annu. Rev. Nutr. 21, 23-46. [DOI] [PubMed] [Google Scholar]
- 17.Geitzenauer, H. & Bernays, E. A. (1996) Ecol. Entomol. 21, 227-234. [Google Scholar]
- 18.Alborn, H. T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin J. H. & Tumlinson, J. H. (1997) Science 276, 945-949. [Google Scholar]
- 19.Laster, M. L. (1972) Environ. Entomol. 1, 682-687. [Google Scholar]
- 20.Yepez, F. F., Clavijo, J. & Romero, I. (1990) Rev. Fac. Agron. (Maracay) 16, 169-175. [Google Scholar]
- 21.Turlings, T. C. J., McCall, P. J., Alborn, H. T. & Tumlinson, J. H. (1993) J. Chem. Ecol. 19, 411-425. [DOI] [PubMed] [Google Scholar]
- 22.Mori, N., Alborn, H. T., Teal, P. E. A. & Tumlinson, J. H. (2001) J. Insect Physiol. 47, 749-757. [DOI] [PubMed] [Google Scholar]
- 23.Alborn, H. T. (1988) Dissertation (University of Göteborg, Göteborg, Sweden).
- 24.Lewis, W. J. & Burton, R. L. (1970) J. Econ. Entomol. 63, 656-658. [Google Scholar]
- 25.Pohnert, G., Jung, V., Haukioja, E., Lempka, K. & Boland, W. (1999) Tetrahedron 55, 11275-11280. [Google Scholar]
- 26.Paré, P. W., Alborn, H. T. & Tumlinson, J. H. (1998) Proc. Natl. Acad. Sci. USA 95, 13971-13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisterson, M. S. & Gould, F. L. (1999) Ecology 80, 1071-1075. [Google Scholar]
- 28.Johnson, M. T. (1997) Environ. Entomol. 26, 207-214. [Google Scholar]
- 29.Neunzig, H. H. (1969) North Carolina Exp. Station Tech. Bull. 196, 1-76. [Google Scholar]
- 30.Lewis, W. J., Sparks, A. N., Jones, R. L. & Barras, D. J. (1972) Environ. Entomol. 1, 468-471. [Google Scholar]
- 31.Roach, S. H. (1975) Environ. Entomol. 4, 725-728. [Google Scholar]
- 32.Lewis, W. J., Brazzel, J. R. & Vinson, S. B. (1967) J. Econ. Entomol. 60, 615-616. [Google Scholar]
- 33.Oppenheim, S. J. & Gould, F. (2002) Evolution (Lawrence, Kans.) 56, 679-689. [DOI] [PubMed] [Google Scholar]
- 34.De Moraes, C. M., Mescher, M. C. & Tumlinson, J. H. (2001) Nature 410, 577-580. [DOI] [PubMed] [Google Scholar]
- 35.Li, X., Schuler, M. A. & Berenbaum, M. R. (2002) Nature 419, 712-715. [DOI] [PubMed] [Google Scholar]