Significance
Animals communicate with other members of the same species by using pheromones. Rats release a specific odor into the air when they are stressed. Because this stress-related odor increases anxiety in the other rats, it is likely that there are some anxiogenic molecules in this odor. In this study, we have tried to identify these molecules. We discovered that a combination of two molecules, 4-methylpentanal and hexanal, increased anxiety in rats. We concluded that the mixture is a pheromone in rats that can increase anxiety in conspecifics. Furthermore, we proposed that the neural circuit relating to anxiety was only activated when the two molecules were simultaneously perceived by two separate olfactory systems.
Keywords: stress-related odor, alarm pheromone, acoustic startle reflex, anxiety, rat
Abstract
Chemical communication plays an important role in the social lives of various mammalian species. Some of these chemicals are called pheromones. Rats release a specific odor into the air when stressed. This stress-related odor increases the anxiety levels of other rats; therefore, it is possible that the anxiety-causing molecules are present in the stress-related odorants. Here, we have tried to identify the responsible molecules by using the acoustic startle reflex as a bioassay system to detect anxiogenic activity. After successive fractionation of the stress-related odor, we detected 4-methylpentanal and hexanal in the final fraction that still possessed anxiogenic properties. Using synthetic molecules, we found that minute amounts of the binary mixture, but not either molecule separately, increased anxiety in rats. Furthermore, we determined that the mixture increased a specific type of anxiety and evoked anxiety-related behavioral responses in an experimental model that was different from the acoustic startle reflex. Analyses of neural mechanisms proposed that the neural circuit related to anxiety was only activated when the two molecules were simultaneously perceived by two olfactory systems. We concluded that the mixture is a pheromone that increases anxiety in rats. To our knowledge, this is the first study identifying a rat pheromone. Our results could aid further research on rat pheromones, which would enhance our understanding of chemical communication in mammals.
Chemical communication plays an important role in the social lives of various mammalian species. Some of these chemicals are called “pheromones.” Because the term pheromone was coined and defined based on findings in insects (1), there is still a debate as to whether the original definition can be applied to mammals (2). Researchers have proposed revised definitions by modifying the original definition and/or specifying additional requirements (3–6). On the basis of the original and revised definitions, we set a working definition of pheromone within this study as (i) substances that are secreted to the outside by an individual and received by a second individual of the same species, in which they cause a specific reaction; (ii) substances that are effective in minute amounts; (iii) substances that are released from living individuals; and (iv) substances that mediate communication for an evolutionarily adaptive function.
Rats release a specific odor into the air when stressed (7). This stress-related odor increases anxiety levels (8, 9) and induces a variety of anxiety-related responses depending on their situation with other rats (9–18). Rats respond to their own stress-related odor in a similar manner to odor released from the other rats, suggesting that the odor has general effects (10, 19). In addition, the stress-related odor appears to be effective in minute amounts (20, 21). Therefore, the molecules responsible for increasing anxiety levels should exist in the stress-related odor.
The responsible molecules appear to meet the definition of pheromones because they (i) were released from rats and increased anxiety in other rats and (ii) were effective in minute amounts. In addition, (iii) rats were alive while releasing the stress-related odor that included the responsible molecules. Furthermore, (iv) the communication mediated by the responsible molecules appears to have an evolutionarily adaptive function. First, the communication mediated by the responsible molecules might be evolutionarily conserved. Studies have shown that the stress-related odors are released by variety of mammalian species in addition to rats; examples include mice (22, 23), deer (24), cattle (25), swine (26), and humans (27). Second, increasing anxiety levels appear to be an adaptive response for highly developed animals. Although the odor released by mice evokes stereotypical avoidance responses, deer, cattle, and swine show cautious behavior rather than an avoidance response per se. In humans, the odor has been shown to increase anxiety levels (28). The higher the level of development of a particular organism, the more complex its life is. As a result, increased anxiety, rather than stereotypical avoidance responses, would enable developed animals to cope with a variety of dangerous stimulus appropriately, depending on the situation and the type of the stimulus, which may increase inclusive fitness. Therefore, the communication mediated by the responsible molecules can be suggested to have an evolutionarily adaptive function.
To identify the responsible molecules, the acoustic startle reflex (ASR) was used as a bioassay system for assessing the anxiogenic activity of the molecules. The ASR pertains to rapid contractions of facial and body muscles evoked by sudden and intense acoustic stimulus, which are observed in a variety of mammals, including humans (29). Earlier studies involving animals and humans have revealed that the amplitude of the ASR increases with increased level of anxiety (29). In rats, the amplitude of the ASR is expressed as a voltage change in the output of an accelerometer, which is displaced by the movement of a rat in an animal holder. Rats show an enhanced ASR when levels of anxiety are increased (8, 30, 31). Therefore, we defined molecules as positive (+) for anxiogenic activity when we observed a significant increase in the amplitude of the ASR following exposure.
In the present study, we first collected the stress-related odor by applying electrical stimulations to the perianal region of anesthetized rats (32). Because the anal glands are located immediately inside the anal verge (33) (Fig. S1), these simulations induce muscle contractions around the anus, which may squeeze the odor out of the anal glands. Then, we successively fractionated the collected odor, leading to the discovery of 4-methylpentanal and hexanal in the final small fraction that retained anxiogenic properties. Subsequent analyses with synthetic molecules revealed that the mixture of these molecules, but not either individual molecule, increased a specific type of anxiety, even in minute amounts. We further investigated the communication by behavioral and neuroscience analyses. The neural circuit related to anxiety [specifically the bed nucleus of stria terminalis (BNST)] appeared to be activated only when the two molecules were simultaneously detected, possibly via two separate nasal chemosensory systems. The mixture also evoked anxiety-related behavioral responses in a different experimental model to the ASR test.
Results
Screening for Molecules That Increase Anxiety in Rats.
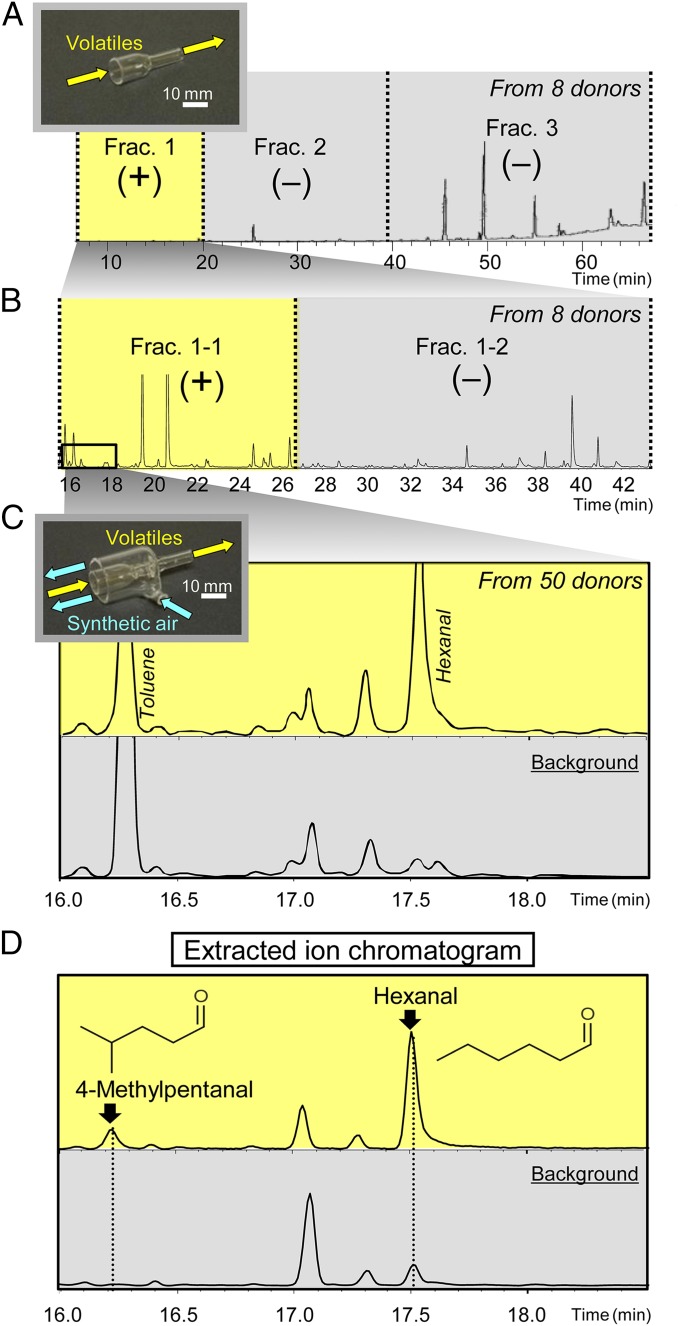
We placed an anesthetized rat as a donor in a cylindrical glass desiccator filled with activated-charcoal–filtered air and fixed a glass funnel near the perianal region (Fig. S2). The funnel was connected to a glass tube containing Tenax, an adsorbent that can trap a wide range of volatile molecules. Then, we administered electrical stimulations to the perianal region and drew air through the funnel to trap the volatile molecules in the adsorbent (300 mL/min). Because the stress-related odor from one donor was sufficient to increase anxiety in other rats (34), to obtain a large sample, we pooled the volatiles from eight donors in the adsorbent. We prepared three fractions (Frac. 1, 2, and 3) from the adsorbent and dissolved each fraction in 3 mL of purified ether. Then, 0.15 mL of ether solution was dropped onto a sheet of filter paper (50 × 50 mm) and dried with nitrogen gas. When we presented the dried filter paper 10 mm from the rat’s nose using a perforated plate, only Frac. 1 enhanced the ASR in rats (Fig. 1A and Fig. S3), suggesting that the anxiogenic molecules existed in Frac. 1. Further detailed analyses of Frac. 1 by gas chromatography and mass spectrometry (GC-MS) revealed the presence of 31 chemicals. Eleven chemicals were predominant molecules and appeared to be environmental contaminants (A−K in Fig. S4A). Therefore, we focused on the remaining 20 chemicals (1−20 in Fig. S4A). We dissolved these chemicals in ether in the same ratios as detected in Frac. 1 (total concentration was 10−5 M) and presented 0.15 mL to rats in the same way. However, these 20 chemicals did not enhance the ASR (Fig. S4B), suggesting that the responsible molecules were detected as ambiguous small peaks or that the amount of the responsible molecules pooled from the eight donors was still below the detectable threshold of the GC-MS system, even though Frac. 1 enhanced the ASR. Thus, we fractionated Frac. 1 further into two fractions, Frac. 1-1 and Frac. 1-2. When we exposed 0.15 mL of an ether solution of these fractions to rats, Frac. 1-1 enhanced their ASR (Fig. 1B and Fig. S5), suggesting that the responsible molecules exist within this small fraction. However, we did not detect any odor-specific peaks in the fraction. Therefore, we decided to refine our trapping system.
Fig. 1.
Screening for molecules that increase anxiety in rats. (A) Total ion chromatogram of the stress-related odor from eight donor rats. The volatiles were fractionated into three fractions (Frac. 1, 2, and 3). Only Frac. 1 had anxiogenic activity. +, Positive for anxiogenic activity; −, negative for anxiogenic activity. The Inset shows a glass funnel that was fixed near the perianal region (yellow arrows show the airflow). (B) Total ion chromatogram of Frac. 1. Frac. 1 was further fractionated into two fractions (Frac. 1-1 and 1-2). Frac. 1-1 had anxiogenic activity. The same glass funnel was used. (C) Magnification of total ion chromatogram of Frac. 1-1 from 50 donor rats. The amount of hexanal was increased compared with the sample prepared from the background absorbent. The Inset shows a newly developed glass funnel. We could collect stress-related odor (yellow arrows) while dispelling the other molecules in the environment by blowing synthetic air through the outer space in the funnel (blue arrows). (D) Extracted ion chromatogram (m/z 56) of Frac. 1-1 proposed two aldehydes as candidate molecules.
To reduce the contamination of molecules from the environment, we developed a specific glass funnel that enabled collection of the stress-related odor into an inner glass tube, while dispelling other environmental molecules by blowing synthetic air through the outer space of the funnel (Fig. S6). In addition, we changed the cylindrical glass desiccator to a glovebox that was maintained at a positive pressure and supplied with clean synthetic air. Using this refined system, we increased the number of donors used for the preparation of the adsorbent from 8 to 50. Analysis of Frac. 1-1 prepared from this adsorbent revealed a prominent increase in hexanal (Fig. 1C); therefore, we focused on hexanal and further analyzed the extracted ion chromatogram of this fraction (m/z = 56). We confirmed that the amount of hexanal was increased in the odor-trapped adsorbent compared with the background adsorbent (Fig. 1D). This analysis also enabled detection of 4-methylpentanal, which had been obscured by toluene when the total ion chromatogram was analyzed. The 4-methylpentanal appeared to be an additional and prime candidate because it was present only in the odor-trapped adsorbent and not in the background adsorbent (Fig. 1D). Together, our results indicated that 4-methylpentanal and hexanal were candidate anxiogenic molecules.
Identification of a Binary Mixture That Increases Anxiety in Rats.
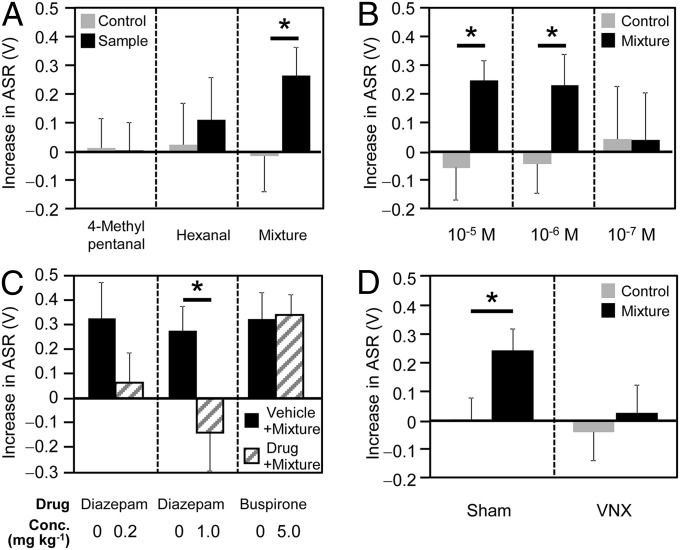
We assessed whether synthetic 4-methylpentanal and hexanal had anxiogenic properties in the ASR test. When we dissolved these chemicals in purified water and presented 0.75 mL of the aqueous solution to rats using a filter paper in the same way, neither 4-methylpentanal (1.3 × 10−6 M) nor hexanal (8.7 × 10−6 M) enhanced ASRs (Fig. 2A). However, when these two molecules were mixed in an aqueous solution at the same ratio as that detected in the adsorbent (i.e., 13:87; 4-methylpentanal, 1.3 × 10−6 M; hexanal, 8.7 × 10−6 M; total concentration of the two compounds was 10−5 M), 0.75 mL of the binary mixture enhanced the ASR, suggesting that the mixture was anxiogenic. Next, we assessed the threshold concentration for anxiogenic activity. An enhanced ASR was observed when 0.75 mL of an aqueous solution of the mixture was presented at a total concentration of 10−6 M (Fig. 2B), suggesting that the mixture was effective in minute amounts.
Fig. 2.
Characterization of the mixture of two candidate molecules. (A) The mixture, but not each molecule, enhanced the ASR (n = 14 for 4-methylpentanal and the mixture; n = 12 for hexanal). (B) The threshold concentration of the mixture in the ASR test (n = 12 for a concentration of 10−5 M and 10−6 M; n = 14 for a concentration of 10−7 M). (C) The enhancement of the ASR by the mixture was blocked by the pretreatment of a benzodiazepine, but not a serotonergic anxiolytic (diazepam 0.2 mg/kg: n = 16; diazepam 1.0 mg/kg: n = 12; and buspirone 5.0 mg/kg: n = 12). (D) The mixture enhanced the ASR of the sham rats (n = 15), but not the ASR of the vomeronasal-organ–excised rats (VNX; n = 17). Each bar represents the mean + SEM. The mixture was presented at a final concentration of 10−5 M unless mentioned otherwise. *P < 0.05 vs. the vehicle control (paired t test).
We further characterized the communication mediated by this mixture. First, to obtain information about the type of anxiety this mixture could induce, we assessed whether the anxiety was sensitive to benzodiazepines and serotonergic anxiolytics. When rats were pretreated with diazepam, but not buspirone, ASR enhancement was attenuated in a dose-dependent manner (Fig. 2C), suggesting that the mixture increased anxiety levels that were sensitive to benzodiazepines. Next, we assessed the role of the vomeronasal system (VNS) in the effects of the mixture. When we prepared vomeronasal-organ–excised (VNX) rats by surgically removing the vomeronasal organ, while preserving the function of the main olfactory system (MOS) (Fig. S7 A and B), the mixture did not result in an ASR enhancement (Fig. 2D), suggesting that the VNS is involved in the detection of the mixture.
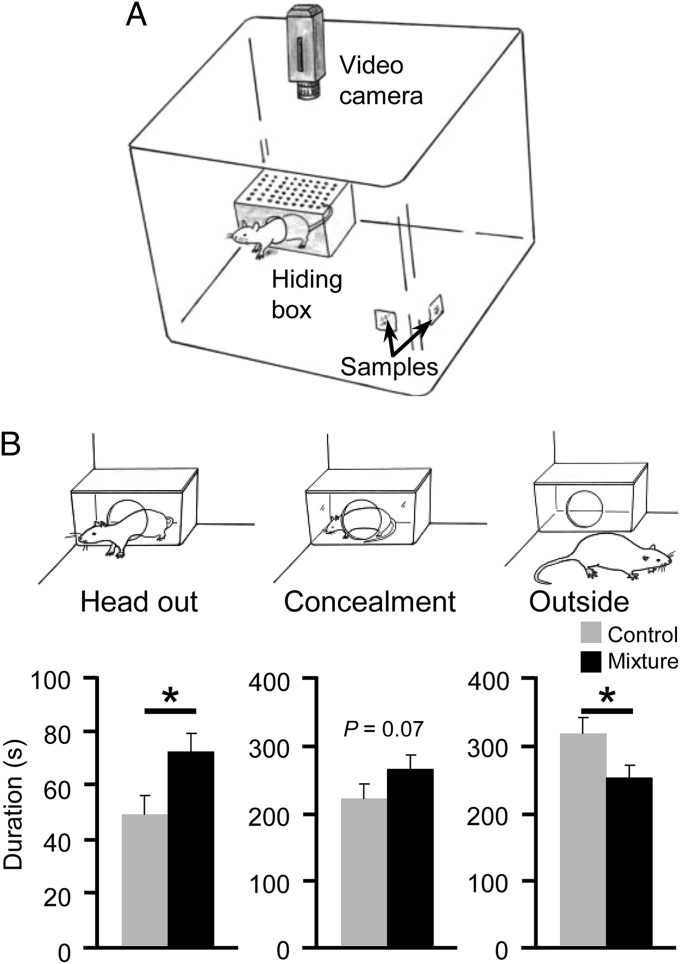
Because we had assessed the anxiogenic properties of the mixture only using the ASR test, we lastly assessed whether the mixture evoked anxiety-related responses other than enhancement of the ASR. We observed the anxiogenic effects of the mixture in the modified open-field test (9) that allowed rats to choose between being in an open arena in the presence of a test substance or hiding in a small safety box located at the corner of the apparatus, opposite the test substance (Fig. 3A). Rats show typical “head-out” behavior and search outside while keeping their hind paws in the safety box when their anxiety levels are increased (9, 35, 36). The presence of the mixture increased the time spent showing head-out behavior and decreased the time spent in the open arena (Fig. 3B). The time spent fully inside the small safety box also tended to increase, suggesting that the anxiogenic activity of the mixture was not specific to the ASR test.
Fig. 3.
The effects of the mixture in the modified open-field test. (A) A schematic diagram of the apparatus. (B) The mixture increased the duration of head-out and decreased the duration of outside behaviors (n = 12 in both groups). *P < 0.05 vs. the vehicle control (Student t test). Each bar represents the mean + SEM. The mixture was presented at a final concentration of 10−5 M.
The Neural Mechanism Underlying the Effects of the Binary Mixture.
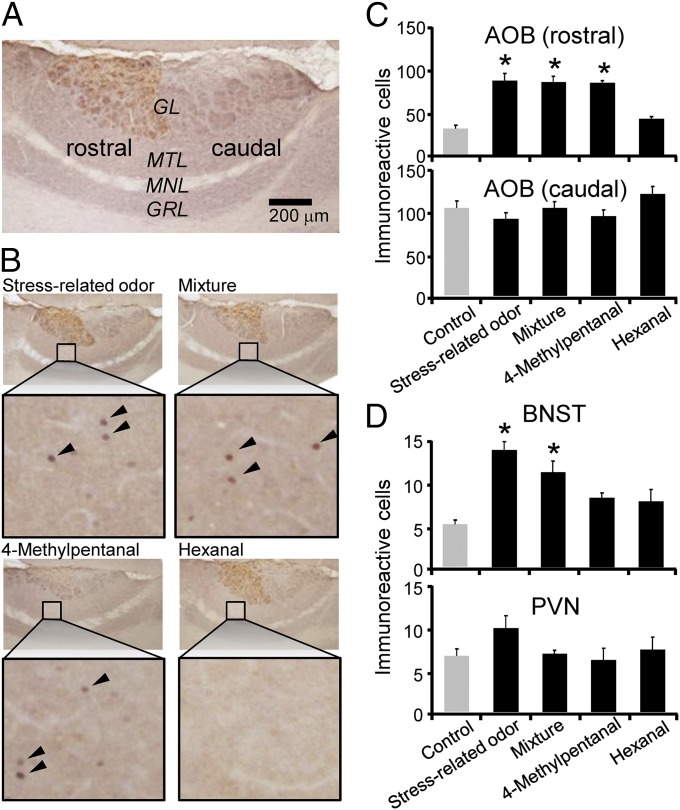
Although the VNS is involved in the detection of the mixture, the mixture is composed of both 4-methylpentanal and hexanal. Therefore, it remains unclear which molecule is, or if both molecules are, detected by the VNS. In the VNS, chemicals are detected by vomeronasal receptors that comprise three families: V1R, V2R, and formyl peptide receptor (FPR) families (37, 38). Vomeronasal neurons expressing V1Rs and V2Rs project their axons to the rostral and caudal accessory olfactory bulb (AOB), respectively (38). Coexpressing G proteins has suggested that vomeronasal neurons expressing four FPRs and one FPR also transmit their information to the rostral and caudal AOB, respectively (37). To assess the role of the VNS in the detection of each molecule and obtain information about possible receptors for the molecules, we divided the AOB into rostral and caudal regions based on their Gαi2 expression (38) and monitored the expression of c-Fos in the mitral/tufted cell layer of each region when rats detected the molecules (Fig. 4A). We observed that both the stress-related odor and the binary mixture activated the rostral, but not the caudal, AOB (Fig. 4 B and C), suggesting that one or more of the receptors expressed by sensory neuron types projecting to rostral AOB (V1Rs and four of the FPRs) were involved in detection. We further found that the 4-methylpentanal activated the rostral, but not the caudal, AOB, suggesting that 4-methylpentanal was detected by some combination of these receptors in the VNS. Conversely, hexanal activated neither the rostral nor the caudal AOB. One possibility might be that hexanal was detected by another olfactory system.
Fig. 4.
The activation of the brain in response to the binary mixture. (A) Representative photomicrographs showing Fos expression in mitral/tufted cell layer (MTL) of the AOB, which was divided into rostral and caudal regions based on the Gαi2 expression in the glomerulus layer (GL). MNL, myelinated nerve layer; GRL, granule cell layer. (B) Representative photomicrographs of the rats that were exposed to the stress-related odor, binary mixture, 4-methylpentanal, or hexanal in their home cage. Arrowheads in the magnified photographs indicate Fos-immunoreactive cells. (C) The number of Fos-immunoreactive cells in the rostral (Upper) and caudal (Lower) AOB. The number was different between the groups in the rostral AOB [F(4, 35) = 11.0, P < 0.01 by ANOVA], but not the caudal AOB [F(4, 35) = 2.11, P = 0.101 by ANOVA]. (D) The number of Fos-immunoreactive cells in the BNST (Upper) and PVN (Lower). The number was different between the groups in the BNST [F(4, 35) = 26.3, P < 0.01 by ANOVA], but not in the PVN [F(4, 35) = 1.58, P = 0.202 by ANOVA]. Each bar represents the mean + SEM (n = 8 in all groups). *P < 0.05 vs. the control (Dunnett’s test).
We further assessed the impact of the binary mixture on the brain. We assessed the BNST, because it plays a crucial role in anxiety (39) (Fig. S8). We found that both the stress-related odor and binary mixture activated the BNST (Fig. 4D). However, 4-methylpentanal and hexanal did not activate the BNST when they were presented individually. Because 4-methylpentanal alone activated the AOB, these results indicated that detection of 4-methylpentanal in the VNS requires simultaneous detection of hexanal to activate the neural circuit for anxiety. These findings are consistent with the phenomenon that the binary mixture did not enhance the ASR in VNX rats. The neural circuit for anxiety of VNX rats was not activated because VNX rats could not detect 4-methylpentanal, even when they have detected hexanal. We also assessed the paraventricular nucleus of the hypothalamus (PVN), because it plays a pivotal role in the hypothalamic–pituitary–adrenal axis activity (Fig. S9). We found that neither the binary mixture, 4-methylpentanal, nor hexanal activated the PVN (Fig. 4D), suggesting that the mixture itself does not serve as a stressor. In addition, the stress-related odor did not affect the PVN. Because the stress-related odor can enhance the activation of the PVN to stressors (40, 41), it is possible that the stress-related odor, and probably the binary mixture, serve as modulators of stress responses by increasing anxiety.
Discussion
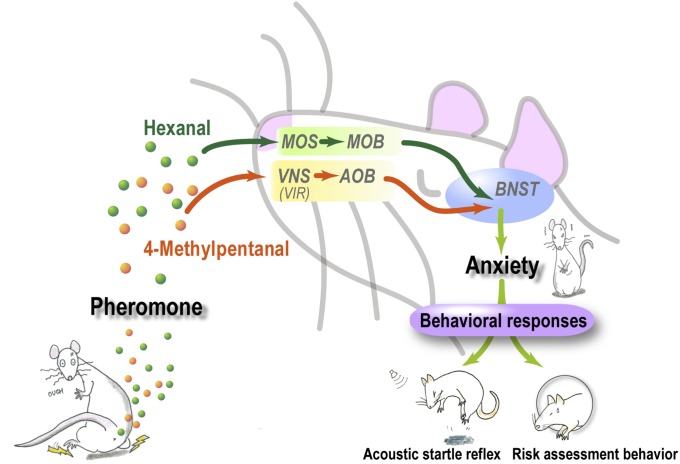
In the present study, we identified a pheromone that increases anxiety in rats. We have suggested the following pheromonal communication between rats. When rats are stressed, they release 4-methylpentanal and hexanal from the perianal region. These molecules activate the BNST, part of the neural circuit for anxiety, only when they are presented simultaneously, and they evoke a variety of anxiety-related responses in other rats. We propose that they are detected by two separate nasal chemosensory systems (Fig. 5).
Fig. 5.
Possible communication mediated by the mixture. Stressed rats release 4-methylpentanal and hexanal into the air from their perianal region. The 4-methylpentanal is perceived by V1Rs in the VNS, whereas hexanal is perceived by the MOS. The information from two separate olfactory systems activates the neural circuit related to anxiety, including the BNST, and evokes context-dependent behavioral responses.
Based on the characteristics of 4-methylpentanal and hexanal, it is possible that 4-methylpentanal plays a greater anxiogenic role in the mixture. In this study, we have demonstrated that 4-methylpentanal activated the BNST and increased anxiety through the VNS, in collaboration with detection of hexanal from another olfactory system. To the best of our knowledge, this is the first study that has revealed the bioactivity of 4-methylpentanal in organisms. Little is known regarding 4-methylpentanal, other than that it is a product of the side-chain cleavage of cholesterol. Conversely, hexanal is a common molecule and is known as a decomposition product of linoleic acid (42, 43). In addition, hexanal has been reported as the alarm pheromone in western conifer seed bugs (44), leaf-footed bugs (45), and weaver ants (46, 47). Thus, it is possible that 4-methylpentanal transmits species-specific information, whereas hexanal supports the accuracy of the information, because it is rare for these two particular molecules to be present simultaneously by chance.
Rats showed activation of the rostral AOB when 4-methylpentanal was detected, suggesting the possible role of V1Rs or four FPRs in the detection of 4-methylpentanal. V1Rs might bind 4-methylpentanal because V1Rs bind low-molecular-weight volatile molecules (48, 49), whereas disease/inflammation-related high-molecular-weight substances are known as ligands for FPRs (50). In contrast, hexanal did not clearly activate Fos expression in the AOB, suggesting that, to be effective, it may have to be detected by olfactory systems other than the VNS. Among the MOS, septal organ, and Grueneberg ganglion, it is likely that the MOS detects hexanal. Numerous studies have used hexanal as a stimulus to analyze the MOS of mammals, which observe the activation of the main olfactory bulb in response to hexanal (38, 51), and identified a set of odorant receptors for hexanal (52). In contrast, identified receptors in the septal organ (53, 54) and Grueneberg ganglion (55, 56), in addition to the characteristics of their ligands (22), suggest that hexanal is not detected by these olfactory systems. Taken together, 4-methylpentanal and hexanal might be detected by V1Rs in the VNS and by the MOS, respectively.
In the present study, 4-methylpentanal detection by the VNS requires simultaneous hexanal detection, most likely from the MOS, to activate the BNST. These results suggest that information from the two olfactory systems converges at a particular site between the AOB and the BNST. Although recent studies have hypothesized, based on anatomical evidence, that the medial amygdala is the candidate site for the convergence of information (57, 58), there are other sites that receive direct projections from both the main olfactory bulb and the AOB in a similar manner (59). In addition, the absence of a good experimental model has prevented further assessment of this hypothesis from a functional point of view. Therefore, the present results would provide an ideal experimental model for analyzing the convergence of two olfactory systems. Recent developments in technologies that enable the gene manipulations in rats (60) would also support a highly interesting future investigation.
In addition to its role as an anxiogenic pheromone, the mixture of 4-methylpentanal and hexanal could be a main component of the alarm pheromone in rats. The expression “alarm pheromone” originates from the “alarm substances” found in minnow that were defined as “the substances that communicate the presence of danger, provided that they are produced by members of the same species” (quoted text from ref. 61; see also ref. 62). Based on this definition, the stress-related odor has been referred to as an alarm pheromone since the term pheromone was coined (14). In the present study, we revealed that the mixture of 4-methylpentanal and hexanal shared many characteristics with the alarm pheromone reported in the literature (8, 9, 13, 63, 64), suggesting that the mixture could also be a main component of the alarm pheromone. However, the intensity of the effects observed in the ASR (34) and modified open-field test (9) seems weak when comparisons are made between the mixture and the alarm pheromone. In this study, we have identified 4-methylpentanal and hexanal by focusing on the specific mass-to-charge ratio. Therefore, further research is needed to assess whether other molecules in the stress-related odor also contribute as components of the alarm pheromone.
In conclusion, we have identified a rat pheromone that increased anxiety in conspecifics. Because stress-related odor characteristics are similar between rats and humans, analyses of this odor in rats may also shed light on the communication mediated by this odor in humans and other mammals.
Materials and Methods
Animals.
Experimentally naïve male Wistar rats were purchased at 7 wk of age (Clea). All experiments were approved by the Animal Care and Use Committee of the Faculty of Agriculture of the University of Tokyo and were based on guidelines that were adapted from the Consensus Recommendations on Effective Institutional Animal Care and Use Committees by the Scientists Center for Animal Welfare (65).
Analysis of Released Volatiles from the Perianal Region of Donor Rats.
Frac. 1, 2, and 3 were prepared by using an Agilent 7890A gas chromatograph equipped with an on-column injector and a TC-WAX column [0.53-mm inner diameter (i.d.) × 30 m; GL Sciences Co.). Frac. 1 was analyzed or was further fractionated by using an Agilent 7890 gas chromatograph combined with a 5973 mass selective detector and a flame ionization detector equipped with a TC-WAX capillary column (0.25 mm i.d. × 60 m).
ASR test.
When the subjects were 9 wk old, we conducted the ASR test as described in our previous study (34), by using startle apparatus and software (Startle Reflex System 2004; O'Hara & Co.). The holder consisted of an acrylic cylinder (length, 200 mm; diameter, 56 mm), an acrylic plate with 42 perforations (diameter, 2 mm) as the front stopper, an acrylic plate as the rear stopper, and an acrylic bottom plate to support the cylinder. Rats underwent two consecutive ASR tests. In the experimental room, each rat was placed inside an animal holder, and the holder was attached to the platform in a dark soundproof chamber (480 × 350 × 370 mm) with background noise (65 dB wideband). Following this, the ASR test, consisting of a baseline trial, sample presentation, and a test trial, was initiated. During the baseline trial, the rat was exposed to 30 auditory stimuli (105 dB, 100 ms, white noise) at an interstimulus interval of 30 s, after an initial 5-min acclimation period. The sample presentation took place immediately after the baseline trial. The door of the soundproof chamber was opened and a folded filter paper (50 × 50 mm) was inserted into a slit on the cylinder so that the filter paper was placed 10 mm away from the rat′s nose. The perforated front stopper enabled the rat to perceive the volatile odor of the samples. The test sample and vehicle of the test sample was presented in a counterbalanced order. Then, the door was closed and the test trial was conducted in the same manner as the baseline trial. The startle amplitude was defined as the maximal peak-to-peak voltage that occurred during the first 200 ms after the onset of the startle-eliciting auditory stimulus. A more detailed description is given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Y. Warita for managing chemical analyses, Y. Kodama for assistance in collecting the volatiles from 50 donors, Y. Matsuoka for conducting the VNX surgery, and N. Mori for synthesizing chemicals. This study was supported by Japan Society for the Promotion of Science KAKENHI Grants 21228006 and 23688035.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414710112/-/DCSupplemental.
References
- 1.Karlson P, Luscher M. ‘Pheromones’: A new term for a class of biologically active substances. Nature. 1959;183(4653):55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 2. Griffiths PR, Brennan PA (September 6, 2014) Roles for learning in mammalian chemosensory responses. Horm Behav, 10.1016/j.yhbeh.2014.08.010. [DOI] [PubMed]
- 3.Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- 4.Otte D. Effects and funtions in the evolution of signaling systems. Annu Rev Ecol Evol Syst. 1974;5:385–417. [Google Scholar]
- 5.Stern K, McClintock MK. Regulation of ovulation by human pheromones. Nature. 1998;392(6672):177–179. doi: 10.1038/32408. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt TD. Pheromones and signature mixtures: Defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196(10):685–700. doi: 10.1007/s00359-010-0564-y. [DOI] [PubMed] [Google Scholar]
- 7.Valenta JG, Rigby MK. Discrimination of the odor of stressed rats. Science. 1968;161(3841):599–601. doi: 10.1126/science.161.3841.599. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki H, Kiyokawa Y, Takeuchi Y, Mori Y. The alarm pheromone in male rats as a unique anxiety model: Psychopharmacological evidence using anxiolytics. Pharmacol Biochem Behav. 2010;94(4):575–579. doi: 10.1016/j.pbb.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Kiyokawa Y, Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromone increases defensive and risk assessment behaviors in male rats. Physiol Behav. 2006;87(2):383–387. doi: 10.1016/j.physbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Abel EL, Bilitzke PJ. A possible alarm substance in the forced swimming test. Physiol Behav. 1990;48(2):233–239. doi: 10.1016/0031-9384(90)90306-o. [DOI] [PubMed] [Google Scholar]
- 11.Courtney RJ, Reid LD, Wasden R. Suppression of running times by olfactory stimuli. Psychon Sci. 1968;12:315–316. [Google Scholar]
- 12.Dua JK, Dobson MJ. Role of olfactory cues in acquisition and extinction of avoidance. J Exp Psychol. 1974;103:461–465. [Google Scholar]
- 13.Inagaki H, Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Enhancement of the acoustic startle reflex by an alarm pheromone in male rats. Physiol Behav. 2008;93(3):606–611. doi: 10.1016/j.physbeh.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Kikusui T, Takigami S, Takeuchi Y, Mori Y. Alarm pheromone enhances stress-induced hyperthermia in rats. Physiol Behav. 2001;72(1-2):45–50. doi: 10.1016/s0031-9384(00)00370-x. [DOI] [PubMed] [Google Scholar]
- 15.King MG. Stimulus generalization of conditioned fear in rats over time: Olfactory cues and adrenal activity. J Comp Physiol Psychol. 1969;69(3):590–600. doi: 10.1037/h0028211. [DOI] [PubMed] [Google Scholar]
- 16.King MG, Pfister HP, DiGiusto EL. Differential preference for and activation by the odoriferous compartment of a shuttlebox in fear-conditioned and naive rats. Behav Biol. 1975;13(2):175–181. doi: 10.1016/s0091-6773(75)91835-0. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Kiyokawa Y, Takeuchi Y, Mori Y. Pretreatment with CP-154526 blocks the modifying effects of alarm pheromone on components of sexual behavior in male, but not in female, rats. Chem Senses. 2011;36(7):623–632. doi: 10.1093/chemse/bjr017. [DOI] [PubMed] [Google Scholar]
- 18.Mackay-Sim A, Laing DG. Discrimination of odors from stressed rats by non-stressed rats. Physiol Behav. 1980;24(4):699–704. doi: 10.1016/0031-9384(80)90400-x. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki H, Kiyokawa Y, Takeuchi Y, Mori Y. Male rats respond to their own alarm pheromone. J Vet Med Sci. 2012;74(1):79–82. doi: 10.1292/jvms.11-0225. [DOI] [PubMed] [Google Scholar]
- 20.Abel EL. Gradient of alarm substance in the forced swimming test. Physiol Behav. 1991;49(2):321–323. doi: 10.1016/0031-9384(91)90050-x. [DOI] [PubMed] [Google Scholar]
- 21.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromone that aggravates stress-induced hyperthermia is soluble in water. Chem Senses. 2005;30(6):513–519. doi: 10.1093/chemse/bji044. [DOI] [PubMed] [Google Scholar]
- 22.Brechbühl J, et al. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci USA. 2013;110(12):4762–4767. doi: 10.1073/pnas.1214249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr WJ, Martorano RD, Krames L. Responses of mice to odors associated with stress. J Comp Physiol Psychol. 1970;71(2):223–228. doi: 10.1037/h0029164. [DOI] [PubMed] [Google Scholar]
- 24.Müller-Schwarze D, Altieri R, Porter N. Alert odor from skin gland in deer. J Chem Ecol. 1984;10(12):1707–1729. doi: 10.1007/BF00987357. [DOI] [PubMed] [Google Scholar]
- 25.Boissy A, Terlouw C, Le Neindre P. Presence of cues from stressed conspecifics increases reactivity to aversive events in cattle: Evidence for the existence of alarm substances in urine. Physiol Behav. 1998;63(4):489–495. doi: 10.1016/s0031-9384(97)00466-6. [DOI] [PubMed] [Google Scholar]
- 26.Vieuille-Thomas C, Signoret JP. Pheromonal transmission of an aversive experience in domestic pig. J Chem Ecol. 1992;18(9):1551–1557. doi: 10.1007/BF00993228. [DOI] [PubMed] [Google Scholar]
- 27.Ackerl K, Atzmueller M, Grammer K. The scent of fear. Neuroendocrinol Lett. 2002;23(2):79–84. [PubMed] [Google Scholar]
- 28.Albrecht J, et al. Smelling chemosensory signals of males in anxious versus nonanxious condition increases state anxiety of female subjects. Chem Senses. 2011;36(1):19–27. doi: 10.1093/chemse/bjq087. [DOI] [PubMed] [Google Scholar]
- 29.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59(2):107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 30.Miles L, Davis M, Walker D. Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology. 2011;36(8):1563–1574. doi: 10.1038/npp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42(6):461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 32.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromones with different functions are released from different regions of the body surface of male rats. Chem Senses. 2004;29(1):35–40. doi: 10.1093/chemse/bjh004. [DOI] [PubMed] [Google Scholar]
- 33.Montagna W, Noback CR. Histochemical observations on the sebaceous glands of the rat. Am J Anat. 1947;81(1):39–61. doi: 10.1002/aja.1000810103. [DOI] [PubMed] [Google Scholar]
- 34.Inagaki H, et al. The volatility of an alarm pheromone in male rats. Physiol Behav. 2009;96(4–5):749–752. doi: 10.1016/j.physbeh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(Suppl):S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- 36.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: A review. Neurosci Biobehav Rev. 2001;25(7-8):597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 37.Liberles SD, et al. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci USA. 2009;106(24):9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori K, von Campenhause H, Yoshihara Y. Zonal organization of the mammalian main and accessory olfactory systems. Philos Trans R Soc Lond B Biol Sci. 2000;355(1404):1801–1812. doi: 10.1098/rstb.2000.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Mapping the neural circuit activated by alarm pheromone perception by c-Fos immunohistochemistry. Brain Res. 2005;1043(1–2):145–154. doi: 10.1016/j.brainres.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi T, Kiyokawa Y, Arata S, Takeuchi Y, Mori Y. c-Fos expression during the modulation of sexual behavior by an alarm pheromone. Behav Brain Res. 2013;237:230–237. doi: 10.1016/j.bbr.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 42.St Angelo AJ. Lipid oxidation on foods. Crit Rev Food Sci Nutr. 1996;36(3):175–224. doi: 10.1080/10408399609527723. [DOI] [PubMed] [Google Scholar]
- 43.St Angelo AJ, Legendre MG, Dupuy HP. Identification of lipoxygenase-linoleate decomposition products by direct gas chromatography-mass spectrometry. Lipids. 1980;15(1):45–49. doi: 10.1007/BF02534117. [DOI] [PubMed] [Google Scholar]
- 44.Blatt SE, Borden JH, Pierce HD, Gries R, Gries G. Alarm pheromone system of the western conifer seed bug, Leptoglossus occidentalis. J Chem Ecol. 1998;24(6):1013–1031. [Google Scholar]
- 45.Soares Leal W, Ricardo Panizzi A, Carla Niva C. Alarm pheromone system of leaf-footed bugLeptoglossus zonatus (Heteroptera: Coreidae) J Chem Ecol. 1994;20(5):1209–1216. doi: 10.1007/BF02059755. [DOI] [PubMed] [Google Scholar]
- 46.Bradshaw JW, Baker R, Howse PE. Multicomponent alarm pheromones of the weaver ant. Nature. 1975;258(5532):230–231. doi: 10.1038/258230a0. [DOI] [PubMed] [Google Scholar]
- 47.Bradshaw JW, Baker R, Howse PE. Multicomponent alarm pheromones in the mandibular glands of major workers of the African weaver ant, Oecophylla longinoda. Physiol Entomol. 1979;4(1):15–25. [Google Scholar]
- 48.Del Punta K, et al. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419(6902):70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 49.Leinders-Zufall T, et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405(6788):792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- 50.Rivière S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459(7246):574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 51.Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: Domain organization and odorant structural features. Nat Neurosci. 2000;3(10):1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- 52.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2(60):ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaluza JF, Gussing F, Bohm S, Breer H, Strotmann J. Olfactory receptors in the mouse septal organ. J Neurosci Res. 2004;76(4):442–452. doi: 10.1002/jnr.20083. [DOI] [PubMed] [Google Scholar]
- 54.Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24(38):8383–8390. doi: 10.1523/JNEUROSCI.2222-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleischer J, Schwarzenbacher K, Besser S, Hass N, Breer H. Olfactory receptors and signalling elements in the Grueneberg ganglion. J Neurochem. 2006;98(2):543–554. doi: 10.1111/j.1471-4159.2006.03894.x. [DOI] [PubMed] [Google Scholar]
- 56.Fleischer J, Schwarzenbacher K, Breer H. Expression of trace amine-associated receptors in the Grueneberg ganglion. Chem Senses. 2007;32(6):623–631. doi: 10.1093/chemse/bjm032. [DOI] [PubMed] [Google Scholar]
- 57.Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29(3):624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 2012;520(8):1819–1830. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pro-Sistiaga P, et al. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504(4):346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- 60.Mashimo T. Gene targeting technologies in rats: Zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats. Dev Growth Differ. 2014;56(1):46–52. doi: 10.1111/dgd.12110. [DOI] [PubMed] [Google Scholar]
- 61.Pfeiffer W. Alarm substances. Experientia. 1963;19:113–123. doi: 10.1007/BF02171582. [DOI] [PubMed] [Google Scholar]
- 62.Von Frisch K. Uber einen schreckstoff der fischhaut und seine biologische bedeutung. Z Vgl Physiol. 1941;29:46–145. [Google Scholar]
- 63.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Removal of the vomeronasal organ blocks the stress-induced hyperthermia response to alarm pheromone in male rats. Chem Senses. 2007;32(1):57–64. doi: 10.1093/chemse/bjl036. [DOI] [PubMed] [Google Scholar]
- 64.Kiyokawa Y, Kodama Y, Kubota T, Takeuchi Y, Mori Y. Alarm pheromone is detected by the vomeronasal organ in male rats. Chem Senses. 2013;38(8):661–668. doi: 10.1093/chemse/bjt030. [DOI] [PubMed] [Google Scholar]
- 65. Scientists Center for Animal Welfare (1987) Consensus recommendations on effective institutional Animal Care and Use Committees. Lab Anim Sci 37(Special issue):11–13. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.