Significance
Our findings provide insight on the molecular mechanism in which insulin regulates the dissociation of ERp44, an endoplasmic reticulum chaperon, from the serotonin (5-HT) transporter (SERT) following the completion of disulfide bond formation. Furthermore, our data show that gestational diabetes mellitus-associated defects in insulin signaling tethers SERT with ERp44, at the intracellular compartment which down-regulates 5-HT uptake rates of the placental trophoblast. All the trophoblast used in these studies were isolated and purified directly from healthy or GDM placentas in our laboratories.
Keywords: serotonin, ERp44, insulin, serotonin transporter, gestational diabetes mellitus
Abstract
Serotonin (5-HT) transporter (SERT) regulates the level of 5-HT in placenta. Initially, we found that in gestational diabetes mellitus (GDM), whereas free plasma 5-HT levels were elevated, the 5-HT uptake rates of trophoblast were significantly down-regulated, due to impairment in the translocation of SERT molecules to the cell surface. We sought to determine the factors mediating the down-regulation of SERT in GDM trophoblast. We previously reported that an endoplasmic reticulum chaperone, ERp44, binds to Cys200 and Cys209 residues of SERT to build a disulfide bond. Following this posttranslational modification, before trafficking to the plasma membrane, SERT must be dissociated from ERp44; and this process is facilitated by insulin signaling and reversed by the insulin receptor blocker AGL2263. However, the GDM-associated defect in insulin signaling hampers the dissociation of ERp44 from SERT. Furthermore, whereas ERp44 constitutively occupies Cys200/Cys209 residues, one of the SERT glycosylation sites, Asp208 located between the two Cys residues, cannot undergo proper glycosylation, which plays an important role in the uptake efficiency of SERT. Herein, we show that the decrease in 5-HT uptake rates of GDM trophoblast is the consequence of defective insulin signaling, which entraps SERT with ERp44 and impairs its glycosylation. In this regard, restoring the normal expression of SERT on the trophoblast surface may represent a novel approach to alleviating some GDM-associated complications.
Gestational diabetes mellitus (GDM) affects 3–10% of pregnancies in developed countries and continues to be a major public health problem (1). In pregnancies complicated by GDM, the signaling of insulin is impaired so that glucose uptake or production cannot be stimulated or suppressed. Like in other forms of hyperglycemia, GDM affected maternal pancreatic β-cells do not function sufficiently to provide the physiological insulin requirement resulting in decreased insulin sensitivity (increased insulin resistance) coupled with an inadequate insulin response via impairment in the insulin signaling mechanism (2–9). GDM is associated with placental pathology and various maternal and fetal complications during pregnancy, birth and later in life (2–11). The diabetic intrauterine environment results in an increased incidence of pediatric and adult complications including obesity, diabetes, and cardiovascular disease (1–7). The factors mediating these pathologies are unknown.
There is a dynamic relationship between pregnancy and serotonin (5-HT), a multifunctional signaling molecule that plays extracerebral roles during development and throughout life. As a mitogen, 5-HT promotes cell division and mitosis regulating morphogenesis, cell proliferation, migration, differentiation and acts as a developmental signal during early embryogenesis (12–21). Preclinical studies with mouse embryos lacking the gene for tryptophan hydroxylase1 (TPH1), demonstrated the importance of 5-HT in early embryonic development (15). The TPH-1 deficient embryos develop cardio-pulmonary dysfunction later in life (15), as a function of the maternal genotype (21). Clinical studies found that altered 5-HT genetics results in adult-onset mental illnesses (22). Altering the levels of free 5-HT in extracellular locations also affects the development of embryo. For example: offspring of mothers who used 5-HT transporter, SERT blocker (SSRI) in the first trimester showed approximately a twofold higher risk for cardiac abnormalities and a 1.8-fold increased risk for other congenital malformations compared with the entire national registry population (23). Furthermore, mice lacking the gene for SERT (SERT−/−) develop obesity, cardiovascular and neurological complications and their embryos show various developmental defects (21). Altogether, these studies emphasize the significance of normal 5-HT levels in development and pregnancy.
5-HT, a potent vasoconstrictor (24), plays a critical role in placentogenesis and embryogenesis (25–28). Normal zygotic implantation involves trophoblastic invasion and colonization of the uterine spiral arteries. The resultant trophoblast-mediated remodeled vessels are converted to high capacitance slow flow channels ensuring unrestricted low pressure blood flow to the developing placenta and thus the embryo (29). Local placental elevation in plasma (free) 5-HT may cause preplacental vasoconstriction elevating vascular resistance and increasing the local blood pressure to the placenta (26, 28). Furthermore, the impact of vasoconstriction and resultant increase in blood pressure can be lethal to the developing embryo (29). Pathologies of placental perfusion are associated with perinatal morbidity and mortality (28). Therefore, trophoblastic SERT plays an important role by regulating the plasma (free) 5-HT levels in uteroplacental blood during pregnancy, which may prevent vasoconstriction to the placenta thereby securing a stable blood flow to the developing embryo (25, 26, 28).
Our initial experiments show that plasma free 5-HT levels in GDM associated pregnancies are higher than their levels in normal pregnancies. Furthermore, the 5-HT uptake rates of trophoblast isolated from the placentas of GDM-associated pregnancies are significantly lower than the rates of control placentas. The biochemical analyses determine that the down-regulation of 5-HT uptake rates is a consequence of the decreased number of SERT molecules on the surface of trophoblast cells in GDM placentas. Further studies find SERT bound to ERp44, an endoplasmic reticulum (ER) protein (30–32), in GDM trophoblast and that their association keeps SERT away from the PM, retaining it in the intracellular compartment.
Like other members of the Na+- and Cl−- dependent monoamine transporter family, SERT has two sites for N-linked glycosylation (Asp208 and Asp217) (33–37) and two cysteine (Cys200 and Cys209) residues (38, 39) connected by a disulfide bond on the second extracellular loop. ERp44 binds to Cys200/Cys209 and facilitates the disulfide bridge formation (39). Interestingly, in the healthy placenta, insulin signaling assists the dissociation of SERT from ERp44 allowing the transporter proteins to be translocated to the PM. However, in GDM, due to defective insulin signaling, ERp44 cannot dissociate from Cys200/Cys209 on SERT. Consequently, in GDM trophoblast, the glycolytic enzymes cannot modify the N-glycosylation sites, Asp208, which is buried between the occupied Cys200 and Cys209 on SERT. Based on these findings, we propose that in GDM, due to defective insulin signaling, SERT cannot perform proper the posttranslational modifications neither can move to the PM of the trophoblasts.
Results
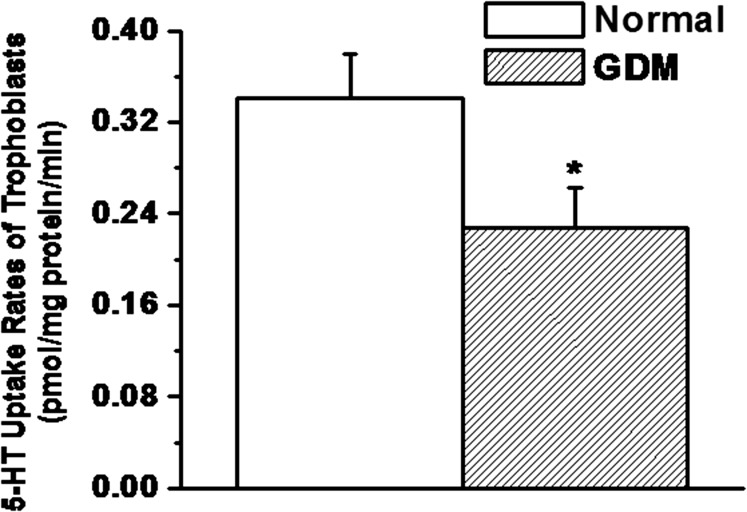
The blood glucose, free plasma 5-HT levels, along with the other parameters as listed in Table 1 were measured in GDM and normal subjects. Following published methods (40–44), trophoblast from gestational age matched normal and GDM placentas were isolated (Fig. 1) and purified (Fig. 2). 5-HT uptake rates of trophoblast were measured in 2.3 × 105 cells per group (Fig. 3). Under GDM conditions uptake rates of trophoblast were 33% lower than the trophoblast of normal placenta (P < 0.01).
Table 1.
Parameters of normal and GDM subjects
| BMI | Weight gain, lb | Blood glucose level, mg/dL | Plasma 5-HT level, ng/mL blood | |
| Normal (n = 5) | 28.9 ± 3.7 | 24 ± 5.43 | 100 ± 13.44 | 0.59 ± 0.07 |
| GDM (n = 5) | 37.5 ± 12 | 35.7 ± 6.7 | 160 ± 17.00 | 0.78 ± 0.04 |
The GDM subjects were overweight (BMI 25–29.9 kg/m2) or obese (BMI > 30 kg/m2) compared with non-GDM subjects with normal weight (BMI 18.5–24.9 kg/m2).
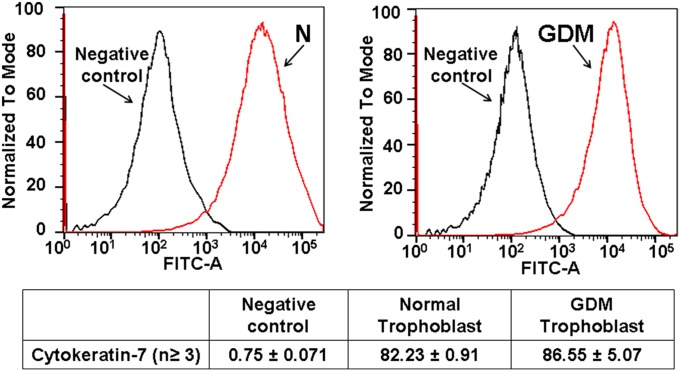
Fig. 1.
Isolation of trophoblast cells. The immunopurification trophoblast was documented by CK-7 (41) and trophoblast protein (NDGO1) (44).
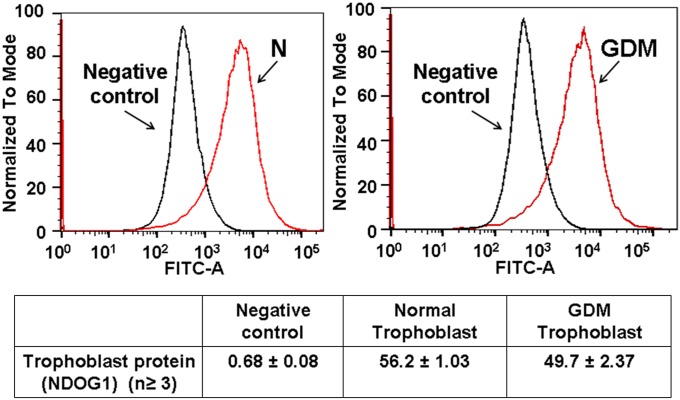
Fig. 2.
Purification of trophoblast cells. Both normal and GDM trophoblast were stained with these Abs followed by Alexa Fluor 488 anti-mouse as secondary Ab. Negative control represents trophoblast without CK-7 stain. In normal placenta the trophoblast of 82 ± 0.91% appeared as positive and in GDM placenta 86.55 ± 5.07% of trophoblast were stained with CK7. The cell lines stained with NDOG1 appeared as 56.2 ± 1.03% positive stain and 49.7 ± 2.37% pure for GDM trophoblast.
Fig. 3.
Characterization of trophoblast cells for the 5-HT uptake rates. Trophoblast cells were isolated and purified from normal and GDM placentas (all groups, n = 5). The [3H]-5HT uptake rates were measured in intact cells (2.3 × 105 per assay) (37, 39, 45). Rate of uptake is expressed as the means and SD values of triplicate determinations from three independent samples in each group. The asterisk represents the results of a two-tailed Student t test with P < 0.001 (compared with normal trophoblast uptake rates).
The 5-HT Uptake Rates of GDM Trophoblast Cells Are Lower as a Result of Reduced Surface SERT Molecules.
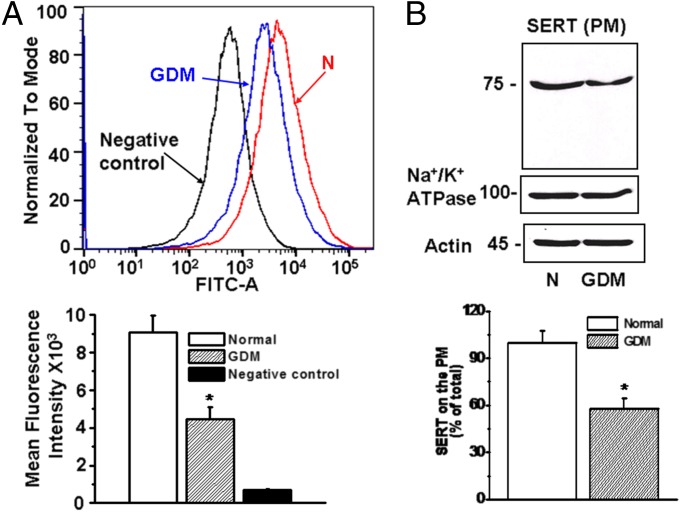
Using flow cytometry and biotinylation of surface proteins followed by WB analysis, the density of SERT molecules on the PM was determined in trophoblast cells isolated from normal and GDM placentas (Fig. 4 A and B). Flow cytometry revealed a 50% decrease in SERT density (Fig. 4A) which closely mirrored the 42% decrease determined by the surface biotinylation assay (Fig. 4B). These findings indicate that the decrease in 5-HT uptake rates of GDM trophoblast is the result of a decrease in the surface density of SERT molecules. The Na+/K+-ATPase and actin were measured at a similar level in both trophoblast cells, normal and GDM (Fig. 4).
Fig. 4.
Comparison of PM SERT expression on freshly isolated trophoblast from normal and GDM placentas. (A) Trophoblast were prepared from normal (N) and GDM placentas. PM expression of SERT was determined by flow cytometry (75, 76). Mean fluorescence intensity of SERT expression in trophoblast (5 × 104 per assay) isolated from normal placentas (red histogram) was higher than in trophoblast from GDM placentas (blue histogram), black histogram represents negative control. Flow cytometry revealed a decrease of 51% in the expression levels of SERT in trophoblast of GDM placentas. Asterisk, statistical difference between normal and GDM trophoblast. (B) For quantification of SERT on the PM, trophoblast (1.5 × 106 per biotinylation assay) cells were treated with sulfo-NHS-SS-biotin as described (37, 39, 68). The WB analysis of the biotin labeled PM proteins was performed with anti-SERT or Na+/K+-ATPase Abs (locates PM proteins). All lanes contain protein recovered from the same number of cells (1.5 × 106 per assay). The band densities were calculated as the ratio of each band to the level of actin. Averaged data from three independent experiments are presented ± SE. The values are statistically different (P < 0.001, Student t test).
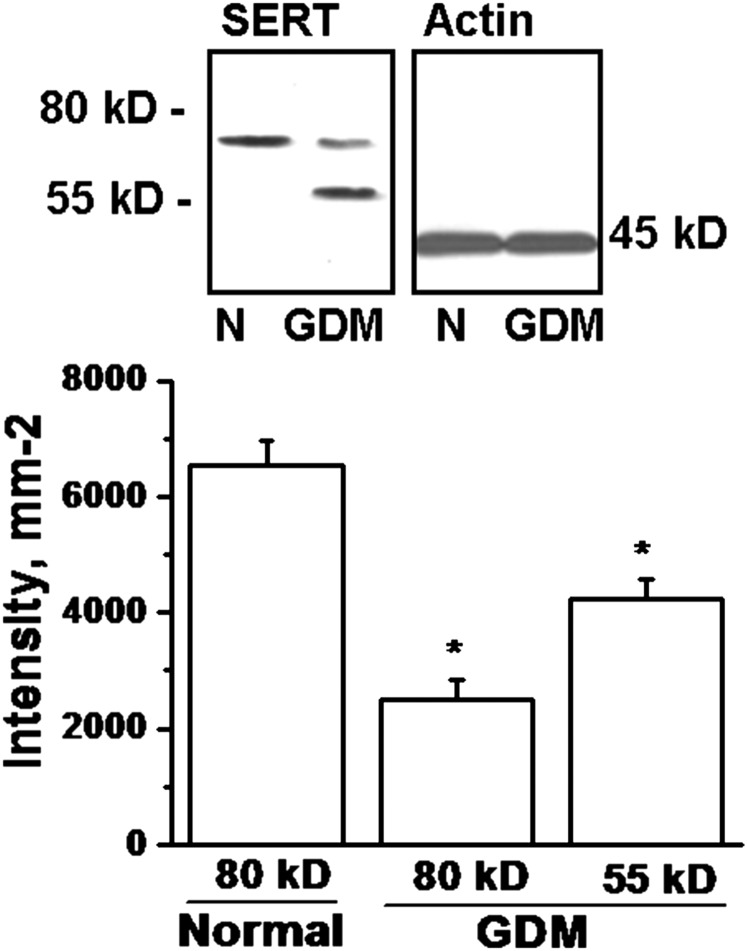
The total SERT expression in whole cells was analyzed to investigate the cause of down-regulation of SERT on the PM of GDM trophoblast. WB analyses for total trophoblastic SERT were similar between normal and GDM placentas (Fig. 5). However, the pattern of the SERT proteins on the SDS/PAGE appeared different. Normal trophoblast SERT proteins were identified as one major band at 80 kDa, whereas in GDM trophoblast they appeared as two major bands at 80 kDa and 55 kDa (Fig. 5).
Fig. 5.
WB analysis of SERT expression in whole trophoblast cells. The whole cell expression of SERT was analyzed in trophoblast cells (1.5 × 106 per assay) isolated from normal (N) and GDM (G) placentas. SERT proteins in trophoblast cells from normal placenta appeared in one major band at 80 kDa, confirming the reported studies (37, 45), whereas it appeared from GDM placenta as two bands at 80 and 55 kDa. The band densities were calculated as the ratio of each band to the level of actin. Relative SERT levels are expressed at 80 and 55 kDa as the means and SD values of triplicate determinations from four independent experiments. All lanes contain protein recovered from the same number of trophoblast (1.5 × 106 per assay). The asterisk represents the results of a two-tailed Student t test with both P < 0.001, (compared with 80-kDa band of normal trophoblast).
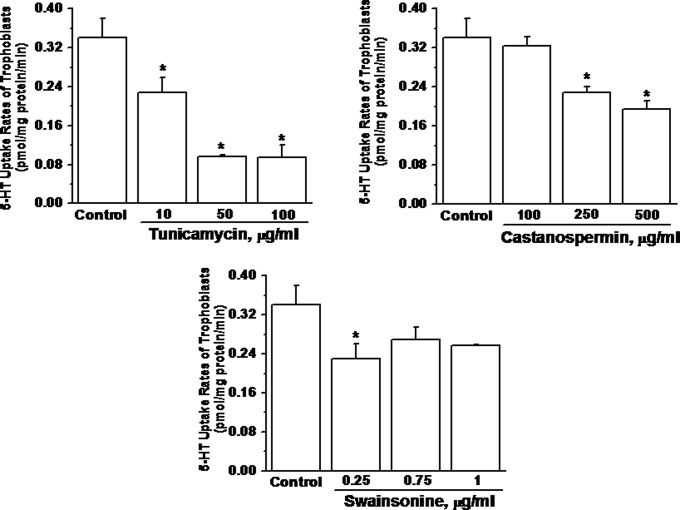
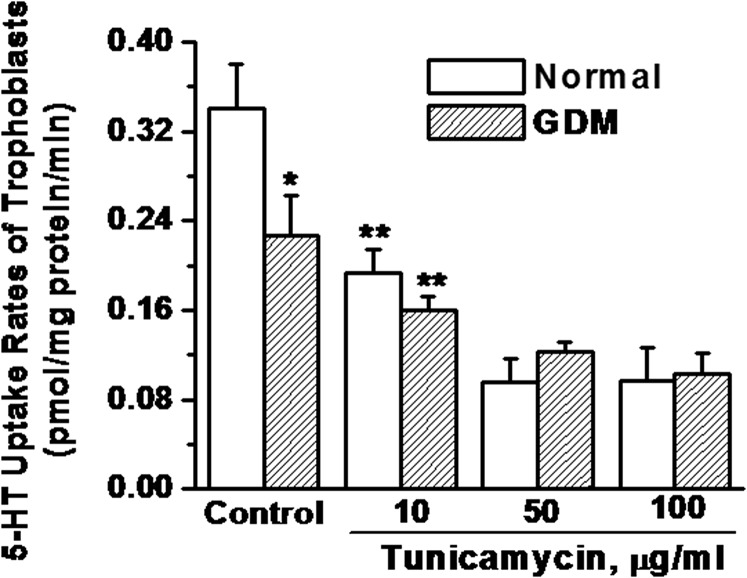
In an earlier study, glycosylation sites deleted, unglycosylated SERT protein was identified in the JAR cell line (human choriocarcinoma cells) at around 55 kDa (37, 45). Therefore, our identified lower band of SERT was analyzed to determine if it was unglycosylated or in a differentially glycosylated form. The trophoblast cells from normal and GDM placentas were pretreated with specific glycosidase inhibitors: PNGaseF, EndoH, Tunicamycin, Castanospermin and Swainsonine (Table 2). Each inhibitor acts at a different step in glycolytic pathway. The 5-HT uptake rates of the trophoblast of a normal placenta were analyzed following the treatment of these inhibitors, individually at a range of concentrations (Fig. 6). Pretreatment with Tunicamycin at 10–100 μg/mL significantly reduced the 5-HT uptake rate of trophoblast to 32–72% of the untreated group. The effects of castanospermin and swainsonine reduced the 5-HT uptake rates of trophoblast at the highest concentrations, 500 μg/mL and 1 μg/mL, respectively. The difference in the 5-HT uptake rates of GDM and normal trophoblast was 33%, which is close to the rates of healthy trophoblast cells treated with 10 μg/mL of tunicamycin. Therefore, we compared the effect of tunicamycin on the 5-HT uptake rates of trophoblast from normal and GDM placentas following tunicamycin pretreatment (Fig. 7). At 10 μg/mL tunicamycin, the 5-HT uptake rate of trophoblast from both normal and GDM placentas was down-regulated significantly; moreover the 5-HT uptake rate of tunicamycin treated trophoblast from the normal placenta was decreased by 30% nearly to the rate of untreated trophoblast from the GDM placenta. Tunicamycin prevents glycosyl modification at the initial step leaving a nascent polypeptide chain (46). Overall, these findings suggest that at least one of the two N-link glycosylation sites is not fully glycosylated in GDM trophoblast.
Table 2.
Glycosylation inhibitors
| Inhibitor and effective sites | Expected structure | Percent decrease in 5-HT uptake rates of trophoblasts |
| PNGase F cleaves between the innermost GlcNAc and asparagine residues from N-linked glycoproteins | Nascent SERT (no glycosylation) | |
| Endoglycosidase H cleaves the bond between two GlcNAc subunits, N-acetylglucosamine residue remaining on the asparagine (46). | GlcNAc- SERT | |
| Tunicamycin, a competitive inhibition of UDP-GlcNAc, prevents the glycosyl modification at initial step (46). | Nascent SERT (no glycosylation) | 35–72.3% |
| Significant (P value <0.001) | ||
| Castanospermine, α-glucosidase inhibitor, prevents removal of the glucose residues (46). | Glc3Man9GlcNAc2-SERT | 28.7% |
| Not significant (P value = 0.02) | ||
| Swainsonine inhibitor of Golgi mannosidase II (46). | Man5GlcNAc2-SERT | 7.8% |
| Not significant (P value = 1.54) |
Fig. 6.
Glycolytic enzymes inhibitors on the trophoblast cells isolated from healthy placenta. The inhibitors, Tunicamycin, catanospermin, and swainsonine (46), on the glycolytic enzyme were used individually to treat the normal trophoblast followed by measuring [3H]-5HT (2.3 × 105 intact cells per assay) (37, 39). Rate of uptake is expressed as the means and SD values of triplicate experiments. The asterisk represents the results of a two-tailed Student t test with both P < 0.001, (compared with untreated trophoblast uptake rates). The effective sites on these enzymes are listed in Table 2.
Fig. 7.
Impact of tunicamycin on the 5-HT uptake rates of trophoblast. The 5-HT uptake rates of intact trophoblast cells were measured following pretreatment with tunicamycin at various concentrations (44). [3H]-5HT uptake rates were measured in intact cells (2.3 × 105 per assay) (37, 39). Rate of uptake is expressed as the means and SD values of triplicate determinations from three independent samples in each group. Asterisks indicate statistical difference between normal and GDM trophoblast (*); treated and untreated trophoblast (**). All assays were performed in triplicate.
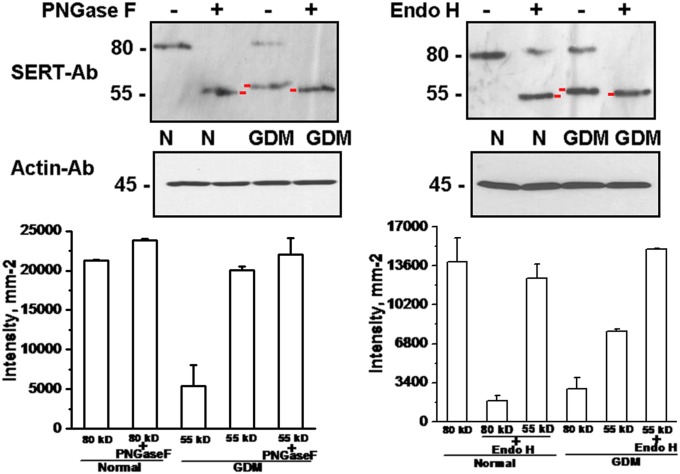
The WB analysis of inhibitor-treated trophoblast was performed with SERT Ab (Fig. 8). The higher bands in both normal and GDM trophoblast were shifted to 55KDa after inhibitor treatment (37), indicating that the formation of the lower band in GDM is relatively close to unglycosylated SERT. Overall, the results of WB analysis suggest that the lower molecular weight band of SERT in GDM trophoblast is similar to the PNGase F-treated normal trophoblast samples suggesting that in GDM, SERT does not complete the glycosyl modification Which is important for its correct folding and translocation to the PM (37, 39).
Fig. 8.
Analysis of glycosylated vs. unglycosylated SERT proteins. The source of 55-kDa band recognized by monoclonal SERT Ab in the trophoblast of GDM placentas was evaluated for differences in the N-glycosylation of the transporter protein (37, 39). Trophoblast (1.5 × 106 per assay) from normal (N) and GDM placentas were treated with PNGase F and EndoH. The active site of each inhibitor is listed in Table 2. PNGase F treatment brought the 80-kDa band in normal and GDM trophoblast cells to the 55-kDa level (37). Immunoblot analyses were done with horseradish peroxidase-conjugated streptavidin as described in Methods. The positions of molecular mass standards run on the same gel are shown in kilodaltons. Averaged data from three independent experiments are presented. Quantifications of the WB analysis results were performed by densitometric scanning. Both treatments produced bands lower than the one observed in GDM trophoblast. The difference is indicated with red markers on the blots.
ERp44 Enhances its Coupling with SERT in GDM at the Intracellular Level.
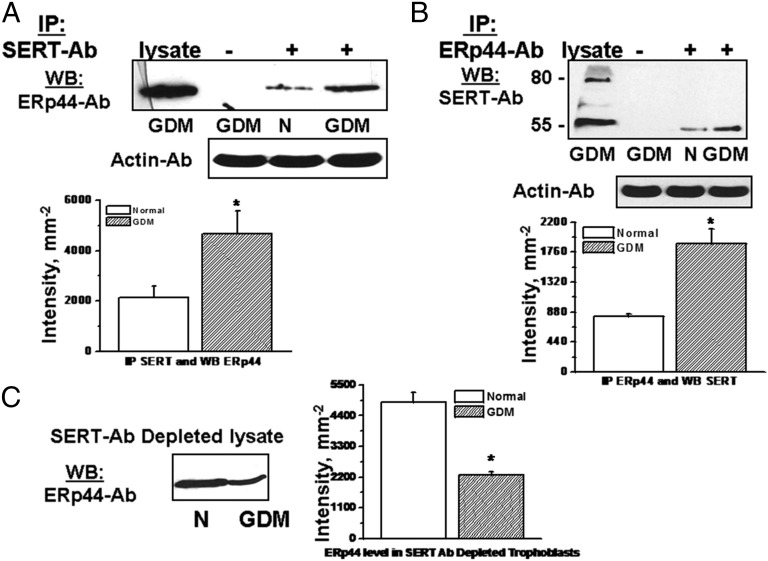
Recently, we reported that ERp44 binds to SERT on Cys200 and Cys209 (39) to build a disulfide bond between these two Cys residues (38). ERp44 works as a quality control check point for the immature proteins leaving from the ER (30–32). In co-IP assays, the level of association between ERp44 and SERT was tested in trophoblast from normal and GDM placentas. Interestingly, in GDM trophoblast, the amount of SERT precipitated with ERp44-Ab was 55% higher than the trophoblast from the normal placenta (Fig. 9). A similar percent level of precipitation was found when the cellular proteins were precipitated on protein A Sepharose beads coated with SERT Ab and the proteins on the beads were analyzed by WB with ERp44 Ab (Fig. 9A); or vice versa (Fig. 9B).
Fig. 9.
The physical association between SERT and ERp44 in trophoblast. The lysates of trophoblast (1.5 × 106 per assay) were prepared and subjected to IP in the presence (+Ab) or absence (−Ab) of monoclonal SERT Ab (A) or polyclonal ERp44 Ab (39) (B). The blots show the level of association between SERT-ERp44 elevated in GDM trophoblast. To verify these findings SERT Ab depleted lysates were analyzed for the level of unbound ERp44 in both groups (C). The band densities were calculated as the ratio of each band to the level of actin and the SERT levels are expressed as the means and SD values of triplicate determinations from three independent experiments; all groups, n = 5. Averaged data from three independent experiments are presented ± SE. The values are statistically different (P < 0.001, Student t test).
Furthermore, the SERT Ab-depleted cell lysate was analyzed for the expression levels of ERp44 in normal and GDM trophoblast (Fig. 9C). The level of ERp44 appeared 53% higher in SERT Ab-depleted lysate of normal trophoblast cells than GDM trophoblast. This finding, in particular, completes the results of the IP assays in Fig. 5 A and B, and confirms that the level of ERp44 in depleted cell lysate is higher in trophoblasts of normal placenta, than in GDM placental cells because the majority of ERp44 was depleted by SERT Ab. Thus, SERT and ERp44 coupling is enhanced in GDM trophoblast compared with normal trophoblast. Because ERp44 is highly regulated via insulin signaling, we wanted to first verify whether insulin signaling was damaged in GDM trophoblast.
Insulin Signaling Is Required for the Dissociation of ERp44 from SERT.
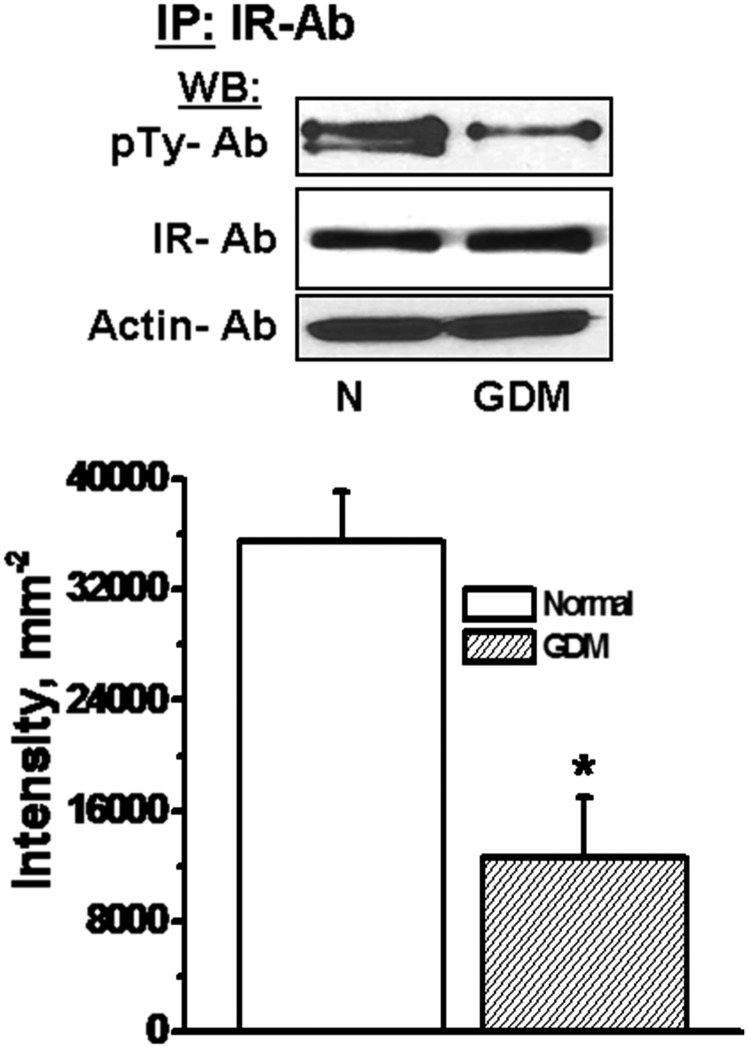
The phosphorylation level of insulin receptor (IR) is related to its signaling ability (47–50). The trophoblast from normal and GDM placentas were evaluated by WB analysis with monoclonal phospho-tyrosine as primary Ab (Fig. 10). Although the protein expression levels of IR appeared similar in both groups, the level of phospho-tyrosine Ab binding was significantly lower in GDM placental trophoblast than the normal placental trophoblast. These findings are consistent with reported studies that show lower insulin signaling in muscle cells of GDM (47) and in the choriocarcinoma JAR cell line (45).
Fig. 10.
The phosphorylation level of IR in trophoblast. Trophoblast (1.5 × 106 per assay) were isolated from normal (N) or GDM placentas. The cell lysates were either analyzed by WB with IR or actin Abs, or prepared for IP with IR Ab coated protein A beads. The following WB analysis of IR pulled down proteins with monoclonal phosphotyrosin (pTy) Ab showed a decrease in the level of phophorylated IR and the other phosphoproteins pulled down by receptor Ab. The band densities were calculated as the ratio of each band to the level of actin. Averaged data from three independent experiments are presented ± SE. The values are statistically different (P < 0.001, Student t test).
Insulin Signaling Elevates 5-HT Uptake via Releasing SERT from ERp44 to the PM.
As reported previously, insulin signaling regulates the ERp44-mediated maturation of adiponectin in adipocytes (51). The impact of insulin on the dissociation of ERp44 from SERT was tested in the trophoblast from normal placentas by treating them with various concentrations of insulin.
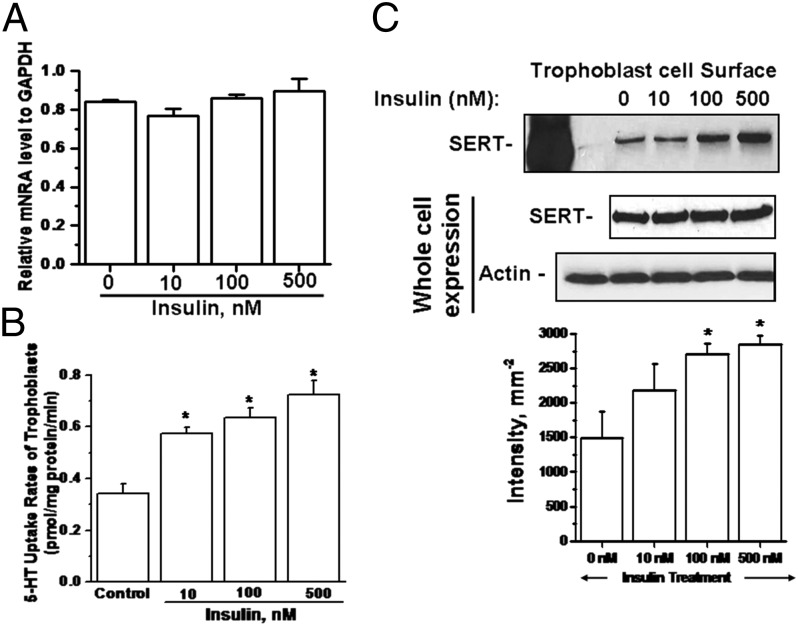
First, the experimental system for insulin treatment on trophoblast was optimized by measuring the mRNA level of SERT, the 5-HT uptake rates, and the level of SERT on the PM and in trophoblast, pretreated with various amounts of insulin (0–500 nM) for 24-hr (Fig. 11A). We found that insulin pretreatment, at any level, does not change total SERT expression at the mRNA level in trophoblast cells prepared from normal placentas (n = 5).
Fig. 11.
Effect of insulin on 5-HT system in trophoblast cells. Tropphoblast were treated with insulin at various concentrations (0–500 nM) for 24 hr. (A) RT-PCR analyses were performed on insulin-treated trophoblast cells (2.3 × 105 per assay). SERT mRNA levels from trophoblast cells were not altered by insulin treatment. (B) 5-HT uptake rates of trophoblast (2.3 × 105 per assay) were measured as a function of insulin treatment. (C) The level of SERT on the PM of trophoblast (1.5 × 106 per assay) isolated from normal placentas was determined by surface biotinylation technique as described in Methods. WB analysis of the biotin labeled PM proteins was performed with anti-SERT. All lanes contain protein recovered from the same number of trophoblast cells (1.5 × 106 per assay). The band densities were calculated as the ratio of each band to the level of actin. Averaged data from three independent experiments are presented ± SE. The values are statistically different (P < 0.001, Student t test).
Next, the 5-HT uptake rates of insulin-pretreated trophoblast cells were determined and we found a significant (P < 0.001) step-wise elevation in the rates compared with untreated cells (Fig. 11B). These findings were confirmed with the measurement of SERT levels on the PM of trophoblast cells following insulin treatment (Fig. 11C). The uptake rates and the surface biotinylation assays showed the most prominent effect of insulin on trophoblast’ 5-HT system at 100 nM as around twofold compared with the untreated group of cells.
Finally, the impact of insulin at 100 nM on the surface SERT expression and 5-HT uptake rates of trophoblast was studied to determine if the effect was due to the insulin treatment or through the dissociation of SERT from ERp44, and whether it could also be shown in the trophoblast of GDM placentas.
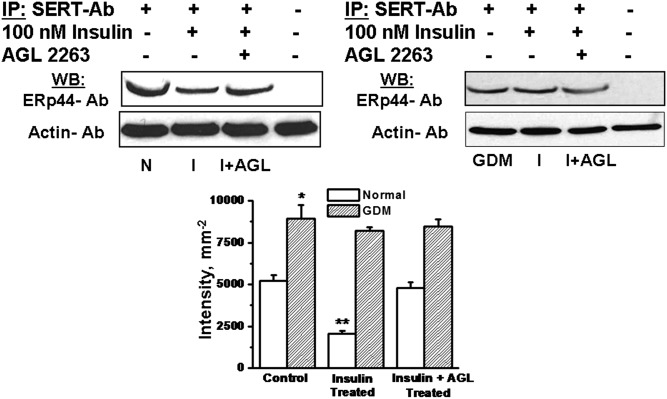
Trophoblast cells were prepared from normal or GDM placentas (Fig. 12). They were incubated in the presence of stimulants, insulin (100 nM) or both insulin and AGL2263 (AGL, 5 µM) IR blocker (52), together for 24 hr. At the end of the incubation time, the cells were prepared for co-IP assays. The soluble cellular proteins were precipitated on SERT Ab and then eluted to analyze via WB assay using ERp44 Ab (Fig. 12). The densities of the bands were normalized with the corresponding levels of actin and plotted in a bar graph. Insulin treatment decreased the level of ERp44 on SERT-Ab in Insulin-treated normal trophoblast by 45%, whereas no coupling difference was observed under GDM. In the meantime, blocking partially IR reversed insulin-mediated SERT release in normal but not GDM, suggesting the insulin signaling-dependent dissociation of ERp44 from SERT.
Fig. 12.
Insulin signaling mediated coupling between ERp44 and SERT. The impact of insulin signaling on dissociation of SERT from ERp44 was determined in trophoblast (1.5 × 106 per assay). The trophoblast of normal or GDM placentas were treated with 100 nM insulin (I) in the absence or presence of 5 μM AGL2263 (52). At the end of 24 hr incubation, the cells were lysed and the detergent soluble cellular proteins were IP on monoclonal SERT Ab coated protein A Sepharose beads. The SERT Ab bound proteins were analyzed by WB with polyclonal ERp44 Ab. First, the level of actin in each corresponding blot was normalized then the band densities were and calculated as a ratio to the level of actin. The SERT levels are expressed as the means and SD values of triplicate determinations from three independent experiments; all groups, n = 5. Averaged data from three independent experiments are presented ± SE. The values are statistically different (P < 0.001, Student t test). Asterisks indicate statistical difference between normal and GDM trophoblast (*); insulin pretreated and untreated normal trophoblast (**).
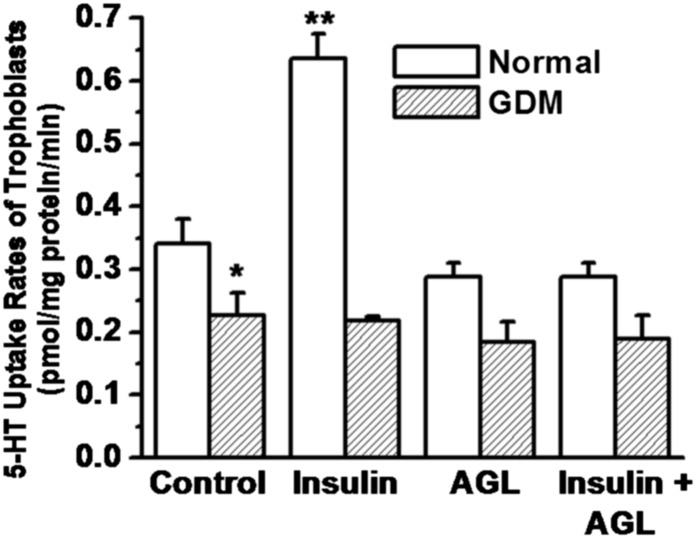
The co-IP data shows that insulin signaling elevates the dissociation rate of SERT from ERp44 in trophoblast cells of normal placentas. We confirmed that insulin treatment up-regulates 5-HT uptake rates of trophoblast by reversing the increased uptake with an IR blocker (Fig. 13). Furthermore, insulin treatment of GDM trophoblast does not facilitate the dissociation of ERp44 from SERT nor does it elevate the PM level of SERT.
Fig. 13.
Insulin signaling up-regulates 5-HT uptake rates of trophoblast of normal placentas. Insulin treatment on ERp44-SERT dissociation was followed by determining the 5-HT uptake rates of trophoblast following a pretreatment with either 100 nM insulin or 5 μM AGL2263 or with both insulin (100 nM) and AGL2263 (5 μM). Trophoblast cells were isolated and purified from normal and GDM placentas (all groups, n = 5). Then the [3H]-5HT uptake rates were measured in intact cells (2.3 × 105 per assay). Rate of uptake is expressed as the means and SD values of triplicate determinations from three independent samples in each group. The values are statistically different (P < 0.001, Student t test). Asterisks indicate statistical difference between normal and GDM trophoblast (*); insulin pretreated and untreated normal trophoblast (**).
Discussion
Peripheral 5-HT is synthesized by the intestinal enterochromaffin cells and secreted into blood (53), where the free plasma level is tightly regulated by a saturable reuptake mechanism of SERT on the PM of platelets and several tissues. SERT cDNA’s have been cloned and sequenced from a number of sources, including human placenta (54–56), platelets (57, 58), brain (59), pulmonary endothelial cells (60), enterocytes (61), and liver (62). SERT is encoded by a single copy gene (SLC6A4) for all tissues with tissue specific alternative promoters (63). We investigate the control of trophoblastic SERT on the PM of the maternal facing brush border (54, 55), isolated from GDM and normal placentas and how it may regulate free 5-HT in the placental blood.
In platelets, the role of the SERT is to take up 5-HT from the circulation and accumulate it inside; from there, 5-HT is taken up by the dense granule-located vesicular monoamine transporter (VMAT) and packed in the dense granule. This effect is systemic. In contrast, the role of SERT in the trophoblast has not yet been established, despite the fact that this tissue expresses very high levels of the transporter (54). We suggest that local control of 5-HT levels in the placental vascular bed is critical during pregnancy and that trophoblastic uptake of 5-HT by SERT is the critical mechanism of its local (placental) regulation. 5-HT is a potent vasoconstrictor and the placenta requires high capacitance, low pressure perfusion. SERT regulation of local plasma 5-HT levels in placental vessels has a protective role preventing 5-HT driven vasoconstriction in the preplacental vascular bed, thereby securing a stable blood flow to the fetus. Trophoblast line the uterine spiral arteries in early implantation, remodeling the vessels to high capacitance slow flow channels. This suggests that local control of preplacental blood flow is important. Evidence that 5-HT levels play a role in regulation of maternal blood flow to the placenta is found in the pathologic condition of preeclampsia. In preeclampsia, altered placental blood flow (local hypertension) results in complications including fetal growth restriction due to significant flow related placental pathology (infarcts, distal villous hypoplasia, and abruption) (26–28) Elevation in free/unbound 5-HT in blood plasma causes preplacental vasoconstriction elevating vascular resistance and exacerbating the local blood pressure to the placenta. Indeed 5-HT concentration in preeclamptic pregnancy is significantly higher than in normal pregnant women (28) suggesting that 5-HT regulation is altered in this pregnancy specific pathology. Therefore, trophoblastic SERT clearance of 5-HT may be a critical player in the maintenance of uteroplacental blood flow during pregnancy (25). The fate of 5-HT after uptake by the trophoblast cells is not well established. However, in neuronal cells and platelets, free/unbound 5-HT in cytosol either binds to the proteins (57), or is degraded by the monoaminooxidase (MAO) system (53), or is stored and then released to the fetal circulation to provide the embryo with 5-HT needed in early embryogenesis (12, 64, 65).
There is a dynamic relationship between pregnancy, 5-HT, and glucose metabolism (18–20, 66). Clinical studies show that the free 5-HT concentration in blood is significantly higher in type 2 diabetes than healthy/control groups (20) and is elevated by 15.6% in pregnancy (67). In an in vitro model of diabetes, extracellular glucose levels were correlated with the 5-HT uptake rates of the JAR cells (45). Our data showed an elevation in the blood plasma, free 5-HT level in GDM.
Following the successful isolation and purification of trophoblast cells from healthy (normal) and GDM-associated placentas, the 5-HT uptake rates of trophoblast we show herein to be 33% lower than in normal placentas. This finding was correlated with lower SERT density on the PM of GDM trophoblast: FACS analysis together with surface biotinylation followed by WB analysis showed that the density of SERT was 42% less on the surface of GDM trophoblast than normal trophoblast. These data imply that SERT molecules are held at intracellular compartments in GDM trophoblast more than in normal trophoblast. These findings suggest that SERT is arrested in the ER of GDM trophoblast. Earlier studies identified the association of SERT with an ER protein, ERp44, during the disulfide bond formation between Cys200 and Cys209. In testing the binding ability between SERT and ERp44, our co-IP data indicated an enhanced association in GDM trophoblast. Other studies have reported a role for insulin signaling in ERp44 dissociation (51). Interestingly, surface SERT levels and 5-HT uptake rates by trophoblast cells from normal placentas significantly rose as plasma insulin levels increased. However, insulin signaling, as represented by the level of IR phosphorylation, was fourfold lower in GDM than normal trophoblast.
In general, proper posttranslational modifications are essential regulatory factors for membrane trafficking and the neurotransmitter uptake functions of SERT (37, 39, 68), NET (69) and DAT (70, 71). A modification such as N-glycosylation has an important role in the quality control pathway that ensures correct folding and processing of membrane proteins (71, 72). Defects in the glycosylation (37), oligomerization (68), or disulfide bond formation (39) processes retain SERT in the ER, similarly to other proteins (30–32, 73, 74). Despite a wealth of knowledge on the protein mediators and quality control checkpoints in SERT maturation there is limited information connecting this to human diseases.
Our studies showed that free thiol at the second external loop in SERT protein structure is sufficient for the intracellular retention of SERT, but SERT mutants without Cys residues on the second extracellular loop are able to reach the PM despite the lack of a disulfide bond (38). These studies suggest a quality control mechanism involved in SERT maturation, which recognizes exposed Cys in SERT molecules and retains them intracellularly. The ability of Cys mutants of SERT to reach the PM further implies the quality control mechanism does not recognize nonnative structures such as hydrophobic patches or immature glycans, but rather, the retention of Cys mutants of SERT is entirely thiol-dependent. SERT has two N-glycosylation sites, Asn208 and Asn217 but ERp44 binds to Cys200 and Cys209. One of the glycosylation sites on SERT is between the two Cys residues where ERp44 binds. Based on these findings, we propose that the differential glycosylation of SERT in GDM trophoblast could be a result of ERp44-retained process, whereas the two Cys residues are occupied by ERp44 the Asn208 site cannot be modified by the glycolytic enzymes. ERp44-SERT coupling affects the glycosylation pattern of SERT.
Therefore, the glycan patterns of SERT in GDM and normal trophoblast were found to be significantly different; where in GDM 37% of the expressed SERT is fully glycosylated and 63% has immature glycans. These findings parallel the 5-HT uptake rates and the surface density of SERT in GDM trophoblast. Furthermore, as reported earlier, in JAR cells the immature glycosylated form of SERT appeared at the same level with the lower molecular weight band of SERT in GDM trophoblast. SERT in GDM trophoblast was modified with immature glycans, and could not dissociate from ERp44. Thus, we hypothesize that the maturation of SERT proteins and in turn the 5-HT reuptake/efflux function is hindered by impaired insulin signaling conditions such as GDM-associated pregnancy. In fact, as reported in in vitro system, if insulin was supplemented, the PM level and the 5-HT uptake rates could be restored in JAR cells pretreated with glucose at diabetic-like concentrations (45). The new findings with normal and GDM trophoblast nicely complete the earlier data by showing that insulin signaling plays a key role in regulating the chaperone activity of ERp44 and its dissociation from SERT; although, insulin signaling does not increase total transcription or translation of SERT.
Methods
Subjects.
Placentas from subjects 18 y old or older were recruited for this study (Table 1). Our study was carried out after approval from University of Arkansas for Medical Sciences (UAMS) Institutional Review Board, which included these procedures, and for which subjects had previously provided written informed consent. The health conditions of subjects were followed by their physicians (Table 1). Inclusion and exclusion criteria were evaluated by review of medical history, interviewing the subject, and/or results of routine tests performed for the purpose of clinical care. We recruited term placentas from euglycemic (normal) (n = 5) or GDM (n = 5) affected pregnancies.
Quantitative Measurement of 5HT Levels by ELISA.
Using competitive ELISA, by following the manufacturer's instructions (IBLImmuno-Biological Laboratories) (77). Samples are detected at 405 nM absorbance by using ELISA plate reader (Molecular Devices). The 5-HT (free) levels were measured in the plasma of maternal blood drawn from healthy and GDM subjects (each group n = 5) (Table 1).
Isolation and Purification of Trophoblast Cells.
The trophoblast cells from the placentas were isolated and then purified by following the published methods (40–44). Placentas were placed in sterile trays, maternal side facing up. One cotyledon at a time was dissected using sharp, fine point scissors and blunt forceps. First the basal plate tissue was removed, and 30–40 g of villous tissue collected, avoiding fibrous tissue and vessels. After rinsing the tissue several times with sterile 0.9% NaCl supplemented with 100 units/mL penicillin, and 100 μg/mL streptomycin; all of the blood clots were removed and the tissue was minced finely with scissors.
Next, using buffers containing DNase, Dispase and Trypsin with the Pen-Strp-Neomycin antibiotics in CMF Hank’s (Ca-Mg free Hank’s with 25mM Hepes, Sigma 14185) the cells were dissociated in 3 stages. Following dissociation, cells were purified on Percoll gradients 70–5% with centrifugation at 1,200 × g for 20 min. The layer of trophoblast cells appears at 40–50% gradient in 25–10 mL volume with a density of 1.050–1.060 g/mL Tropohoblast collections were incubated with FCS to avoid cell damage. The cell viability was determined by trypan blue dye exclusion. Our average yield was between 1.5 and 3 × 108 cells per 40 g of tissue at greater that 80% viability.
Next, for the immunopurification the cells were suspended in buffer containing human HLA class I ABC antibody (Ab) W6/32 incubated with Dynabeads previously coated with goat anti-mouse IgG. At the end of incubation, the supernatant containing purified trophoblast was transferred to tubes with supplemental cytotrophoblast culture medium and centrifuged. The pellets were resuspended in the same medium. Purity of villous trophoblast was determined by cytokeratin-7 (CK-7) Ab (Fig. 1) (43) and trophoblast protein (NDGO1) (Fig. 2) (44).
Insulin and AGL2263 Blocker Treatment.
Human insulin solution is supplied by Sigma 19278 (10 mg/mL stock). Insulin concentration used in this study ranged from 10 nM to 500 nM, as published for JAR cells (45). IR blocker AGL2263 obtained from Santa Cruz Biotechnology was used at a concentration of 5 µM as recommended (52).
5-HT Uptake Assay.
Trophoblast (2.3 × 105 cells per transport assay) were initially washed with PBS solution containing 0.1 mM CaCl2 and 1mM MgCl2. The intact cells were quickly incubated with 14.6 nM 3H-5-HT at room temperature (RT) for 10 min. Whatman GF/B filters collected the cells after incubation, and excess solution was filtrated through a funnel. The uptake assay was stopped by washing twice with ice-cold PBS solution. The sample containing filters were placed into scintillation vials for counting. 2β-carbomethoxy-3 trophane (β-CIT) (Chemical Synthesis Service, National Institute of Mental Health) was used as negative control background (37).
Immunoprecipitation and Western Blot Analysis.
Trophoblast (1.5 × 106 cells per IP assay) were lysed in immunoprecipitation (IP) buffer [55 mM triethylamine (pH 7.5), 111 mM NaCl, 2.2 mM EDTA, 0.44% SDS, 1% Triton X-100] supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor mixture (PIM) as described (37, 39). Initially, cell lysate was incubated with protein A Sepharose beads to eliminate none-specific interaction (preclear). Anti-SERT monoclonal (Mab Technology) Ab, anti-ERp44 polyclonal Ab (Cell Signaling) or anti-IR Ab (Santa Cruz Biotechnology) was conjugated to protein A bead for 2 hr before incubating together with precleared cell lysate overnight at 4 °C.
Western blot analysis was done the next day using anti-ERp44 polyclonal Ab (Cell Signaling, Danvers, MA) (diluted 1:1,000), monoclonal anti-SERT, or monoclonal Phospho-tyrosine for primary Ab (eBioscience). Horseradish peroxidase (HRP) conjugated anti-rabbit or anti-mouse was used as the secondary Ab. VersaDoc 1000 gel visualization and analysis system was applied to analysis of densitometry of individual bands.
Glycolytic Enzymes’ Inhibitors Treatment.
Trophoblast (1.5 × 106 cells for glycolytic enzyme inhibitors’ treatment) were first lysed in IP buffer supplemented with PIM/PMSF (37). Protein concentration was determined under nanodrop 2000 instrument (Thermo Scientific). Glycoproteins were denatured at 100 °C for 10 min first then combined with 10G7 buffers, Nonidet P-40, and 2,000 U of PNGase F solution (New England Biolabs) for incubation at 37 °C. The reaction mixture was separated by SDS/PAGE, and WB analysis was performed using SERT Ab following the ECL blotting system.
Cell Surface Biotinylation.
The trophoblast surface protein expression (1.5 × 106 cells per biotinylation assay) was detected after treatment of the cells with membrane-impermeant NHS-SS-biotin as described (37, 39). Briefly, upon the biotinylation reaction, the cells were treated with 100 mM glycine to quench unreacted NHS-SS-biotin and lysed in Tris buffer 1% SDS, 1% TX100, and PIM/PMSF. The biotinylated proteins were recovered with an excess of streptavidin-agarose beads during overnight incubation. Biotinylated proteins were eluted in sample buffer, resolved by SDS/PAGE and transferred to nitrocellulose, and were detected with the SERT Ab as described (37, 39).
Flow Cytometry.
The level of SERT proteins on the PM of trophoblast (5 × 104 cells per assay) was determined using a specific Ab (76) designed by our group and generated by Proteintech Group against a synthetic peptide corresponding to the second extracellular loop of SERT. This portion of the protein is not affected by the posttranslational modifications of SERT such as glycosylation, disulfide bond formation, and thus, should recognize SERT in trophoblast isolated from normal and GDM placentas.
CK7 was applied to stain the intracellular compartment of purified trophoblast cells. Briefly, cells were washed with PBS and fixed with 4% formaldehyde for 20 min, and then permeabilized with 0.1% Tx-100/PBS for 15 min at RT. After washing, cells were blocked with 0.5% BSA for 1 hr. Then, they were incubated with CK7 (Novus Biological) primary monoclonal Ab for 1 hr and Alexa Fluor 488 goat anti-mouse secondary Ab for an additional 1 hr at RT (75, 76).
Extracellular staining was performed to confirm trophoblast identity by using NDOG1 (trophoblast cell protein) (ThermoFisher Scientific). Cells were directly blocked with BSA without a permeabilizing step, then washed and incubated with NDOG1 polyclonal Ab or SERT Ab on trophoblast PM for 1 hr and then incubated with secondary Ab, FITC conjugated goat anti-rabbit IgG (41–44). All flow cytometry experiments were performed in the UAMS Flow Cytometry Core Facility.
Data Analysis.
Nonlinear regression fits of experimental and calculated data were performed with Origin, which uses the Marquardt–Levenberg nonlinear least squares curve fitting algorithm. Each figure shows a representative experiment that was performed at least three times. Data with error bars represent the means ± SD for triplicate samples. Data were analyzed by ANOVA (analysis of variance) to compare data sets and two-sided t tests based on the ANOVA mean squared error.
Acknowledgments
We gratefully acknowledge the UAMS Flow Cytometry Core and thank Ms. Amber Ward for assistance in obtaining consents from subjects and providing us the samples. L.M.’s work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Pierre et Marie Curie, and by grants from the Fondation de France, the Fondation pour la Recherche Médicale “Equipe FRM DEQ2014039529”, the French Ministry of Research (Agence Nationale pour la Recherche) ANR-12-BSV1-0015-01, and the Investissements d'Avenir program managed by the ANR under reference ANR-11-IDEX-0004-02. This work was supported by NIH Child Health and Human Development Grants HD058697 and HD053477, Heart Lung and Blood Institute Grant HL091196, American Heart Association [13GRNT17240014], the Minnie Merrill Sturgis Diabetes Research Fund, and the Sturgis Charitable Trust (F.K.). Y.L. is supported by AHA predoctoral fellowship 14PRE20500039.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1. Centers for Disease Control and Prevention (2011) National Diabetes Fact Sheet, www.cdc.gov/diabetes/pubs/estimates11.htm.
- 2.Metzger BE, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30(Suppl 2):S105–S111. doi: 10.2337/dc07-s201. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: Role in short- and long-term implications for mother and fetus. J Nutr. 2003;133(5) Suppl 2:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita H, Shao J, Friedman JE. Physiologic and molecular alterations in carbohydrate metabolism during pregnancy and gestational diabetes mellitus. Clin Obstet Gynecol. 2000;43(1):87–98. doi: 10.1097/00003081-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264(1 Pt 1):E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 8.Vrachnis N, et al. Impact of maternal diabetes on epigenetic modifications leading to diseases in the offspring. Exp Diabetes Res. 2012;2012:538474. doi: 10.1155/2012/538474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colomiere M, Permezel M, Riley C, Desoye G, Lappas M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol. 2009;160(4):567–578. doi: 10.1530/EJE-09-0031. [DOI] [PubMed] [Google Scholar]
- 10.Bentley-Lewis R, Dawson DL, Wenger JB, Thadhani RI, Roberts DJ. Placental histomorphometry in gestational diabetes mellitus: The relationship between subsequent type 2 diabetes mellitus and race/ethnicity. Am J Clin Pathol. 2014;141(4):587–592. doi: 10.1309/AJCPX81AUNFPOTLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balsells M, García-Patterson A, Gich I, Corcoy R. Major congenital malformations in women with gestational diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(3):252–257. doi: 10.1002/dmrr.1304. [DOI] [PubMed] [Google Scholar]
- 12.Liu KP, Tamir H, Hsiung S, Adlersberg M, Gershon MD. Prenatal development of serotonin binding protein in relation to other transmitter-related characteristics of central serotonergic neurons. Brain Res. 1987;429(1):31–41. doi: 10.1016/0165-3806(87)90135-0. [DOI] [PubMed] [Google Scholar]
- 13.Lauder JM, Tamir H, Sadler TW. Serotonin and morphogenesis. I. Sites of serotonin uptake and -binding protein immunoreactivity in the midgestation mouse embryo. Development. 1988;102(4):709–720. doi: 10.1242/dev.102.4.709. [DOI] [PubMed] [Google Scholar]
- 14.Lauder JM. Neurotransmitters as growth regulatory signals: Role of receptors and second messengers. Trends Neurosci. 1993;16(6):233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 15.Côté F, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA. 2003;100(23):13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubenstein JLR. Development of serotonergic neurons and their projections. Biol Psychiatry. 1998;44(3):145–150. doi: 10.1016/s0006-3223(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 17.Bonnin A, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16(7):804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulmann N, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7(10):e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barradas MA, Gill DS, Fonseca VA, Mikhailidis DP, Dandona P. Intraplatelet serotonin in patients with diabetes mellitus and peripheral vascular disease. Eur J Clin Invest. 1988;18(4):399–404. doi: 10.1111/j.1365-2362.1988.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 21.Côté F, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci USA. 2007;104(1):329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor SE, et al. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 23. FDA Drug Safety Communication (2011) Selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. Available at www.fda.gov/Drugs/DrugSafety/ucm283375.htm.
- 24.Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243–1251. [PubMed] [Google Scholar]
- 25.Ganapathy V, Leibach FH. Human placenta: A direct target for cocaine action. Placenta. 1994;15(8):785–795. doi: 10.1016/s0143-4004(05)80181-x. [DOI] [PubMed] [Google Scholar]
- 26.Filshie GM, et al. Urinary 5-hydroxyindole acetate concentration in pregnancy induced hypertension. BMJ. 1992;304(6836):1223. doi: 10.1136/bmj.304.6836.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redline RW, et al. Society for Pediatric Pathology, Perinatal Section, Maternal Vascular Perfusion Nosology Committee Maternal vascular underperfusion: Nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7(3):237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 28.Middelkoop CM, Dekker GA, Kraayenbrink AA, Popp-Snijders C. Platelet-poor plasma serotonin in normal and preeclamptic pregnancy. Clin Chem. 1993;39(8):1675–1678. [PubMed] [Google Scholar]
- 29.Red-Horse K, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114(6):744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27(2):315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anelli T, et al. Thiol-mediated protein retention in the endoplasmic reticulum: The role of ERp44. EMBO J. 2003;22(19):5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anelli T, et al. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J. 2007;26(19):4177–4188. doi: 10.1038/sj.emboj.7601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gielen W, Viehöfer B. The effect of neuraminidase on the 5-hydroxytryptamine uptake of human platelets. Experientia. 1974;30(10):1177–1178. doi: 10.1007/BF01923675. [DOI] [PubMed] [Google Scholar]
- 34.Dette GA, Wesemann W. The role of sialic acid in 5-HT binding to synaptic membranes. Experientia. 1979;35(9):1152–1153. doi: 10.1007/BF01963255. [DOI] [PubMed] [Google Scholar]
- 35.Michal F, et al. Effect of 5-hydroxytryptamine on the potassium ion exchange of human platelets enriched in sialic acid. Biochem J. 1972;129:977–978. doi: 10.1042/bj1290977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Launay JM, et al. One-step purification of the serotonin transporter located at the human platelet plasma membrane. J Biol Chem. 1992;267(16):11344–11351. [PubMed] [Google Scholar]
- 37.Ozaslan D, et al. Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter. A specific role for sialic acid residues. J Biol Chem. 2003;278(45):43991–44000. doi: 10.1074/jbc.M306360200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Chen JG, Liu-Chen S, Rudnick G. External cysteine residues in the serotonin transporter. Biochemistry. 1997;36(6):1479–1486. doi: 10.1021/bi962256g. [DOI] [PubMed] [Google Scholar]
- 39.Freyaldenhoven S, et al. The role of ERp44 in maturation of serotonin transporter protein. J Biol Chem. 2012;287(21):17801–17811. doi: 10.1074/jbc.M112.345058. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 41.Manoussaka MS, Jackson DJ, Lock RJ, Sooranna SR, Kumpel BM. Flow cytometric characterisation of cells of differing densities isolated from human term placentae and enrichment of villous trophoblast cells. Placenta. 2005;26(4):308–318. doi: 10.1016/j.placenta.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Clover LM, Coghill E, Redman CW, Sargent IL. A three-colour flow cytometry technique for measuring trophoblast intracellular antigens: The relative expression of TAP1 in human cytotrophoblast and decidual cells. Placenta. 2000;21(8):743–753. doi: 10.1053/plac.2000.0583. [DOI] [PubMed] [Google Scholar]
- 43.Maldonado-Estrada J, Menu E, Roques P, Barré-Sinoussi F, Chaouat G. Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J Immunol Methods. 2004;286(1-2):21–34. doi: 10.1016/j.jim.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Bulmer JN, Billington WD, Johnson PM. Immunohistologic identification of trophoblast populations in early human pregnancy with the use of monoclonal antibodies. Am J Obstet Gynecol. 1984;148(1):19–26. doi: 10.1016/s0002-9378(84)80026-5. [DOI] [PubMed] [Google Scholar]
- 45.Unal R, et al. At diabetes-like concentration, glucose down-regulates the placental serotonin transport system by abolishing its homo-oligomerization. J Neurochem. 2007;101:937–948. doi: 10.1111/j.1471-4159.2007.04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Elbein AD. Glycosidase inhibitors: Inhibitors of N-linked oligosaccharide processing. FASEB J. 1991;5(15):3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- 47.Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J Cell Biol. 2005;170(3):455–464. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avruch J. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem. 1998;182(1-2):31–48. [PubMed] [Google Scholar]
- 49.Barbour LA, et al. Chronically increased S6K1 and IRS1 serine phosphorylation are associated with skeletal muscle insulin resistance in GDM women with impaired glucose tolerance postpartum. J Clinical Endocrinology & Metabolism. 2011;96:1431–1441. doi: 10.1210/jc.2010-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boileau P, Caüzac M, Pereira MA, Girard J, Hauguel-De Mouzon S. Dissociation between insulin-mediated signaling pathways and biological effects in placental cells: Role of protein kinase B and MAPK phosphorylation. Endocrinology. 2001;142(9):3974–3979. doi: 10.1210/endo.142.9.8391. [DOI] [PubMed] [Google Scholar]
- 51.Wang ZV, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27(10):3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seale AP, de Jesus LA, Park MC, Kim YS. Vanadium and insulin increase adiponectin production in 3T3-L1 adipocytes. Pharmacol Res. 2006;54(1):30–38. doi: 10.1016/j.phrs.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Tamir H, Gershon MD. Serotonin-storing secretory vesicles. Ann N Y Acad Sci. 1990;600:53–66, discussion 67. doi: 10.1111/j.1749-6632.1990.tb16872.x. [DOI] [PubMed] [Google Scholar]
- 54.Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem. 1989;264(4):2195–2198. [PubMed] [Google Scholar]
- 55.Padbury JF, et al. Placental biogenic amine transporters: Cloning and expression. Brain Res Mol Brain Res. 1997;45(1):163–168. doi: 10.1016/s0169-328x(96)00309-9. [DOI] [PubMed] [Google Scholar]
- 56.Ramamoorthy S, et al. Regulation of the human serotonin transporter. Cholera toxin-induced stimulation of serotonin uptake in human placental choriocarcinoma cells is accompanied by increased serotonin transporter mRNA levels and serotonin transporter-specific ligand binding. J Biol Chem. 1993;268(29):21626–21631. [PubMed] [Google Scholar]
- 57.Tamir H, Kupsky WJ, Huang YL, Gershon MD. Serotonin-binding glycoprotein of rat platelets. J Cell Sci. 1983;62:439–458. doi: 10.1242/jcs.62.1.439. [DOI] [PubMed] [Google Scholar]
- 58.Lesch KP, Wolozin BL, Murphy DL, Reiderer P. Primary structure of the human platelet serotonin uptake site: Identity with the brain serotonin transporter. J Neurochem. 1993;60(6):2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 59.Lesch KP, Wolozin BL, Estler HC, Murphy DL, Riederer P. Isolation of a cDNA encoding the human brain serotonin transporter. J Neural Transm. 1993;91(1):67–72. doi: 10.1007/BF01244919. [DOI] [PubMed] [Google Scholar]
- 60. Bhat GB and Block ER. (1990). Hypoxia directly increases serotonin transport by porcine pulmonary artery endothelial cell plasma membrane vesicles. Am J Respir Cell Mol Biol. 4:363-7. [DOI] [PubMed]
- 61.Wade PR, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16(7):2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Margolis KJ, Gershon MD, Schwartz GJ, Sze JY. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS ONE. 2012;7(3):e32511. doi: 10.1371/journal.pone.0032511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley CC, Blakely RD. Alternative splicing of the human serotonin transporter gene. J Neurochem. 1997;69(4):1356–1367. doi: 10.1046/j.1471-4159.1997.69041356.x. [DOI] [PubMed] [Google Scholar]
- 64.Noorlander CW, et al. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS ONE. 2008;3(7):e2782. doi: 10.1371/journal.pone.0002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mekontso-Dessap A, et al. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. 2006;113(1):81–89. doi: 10.1161/CIRCULATIONAHA.105.554667. [DOI] [PubMed] [Google Scholar]
- 66.Viau M, Lafond J, Vaillancourt C. Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reprod Biomed Online. 2009;19(2):207–215. doi: 10.1016/s1472-6483(10)60074-0. [DOI] [PubMed] [Google Scholar]
- 67.Gall V, et al. Platelet serotonin concentration at term pregnancy and after birth: Physiologic values for Croatian population. Coll Antropol. 2011;35(3):715–718. [PubMed] [Google Scholar]
- 68.Kilic F, Rudnick G. Oligomerization of serotonin transporter and its functional consequences. Proc Natl Acad Sci USA. 2000;97(7):3106–3111. doi: 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nguyen TT and Amara SG. (1996). N-linked oligosaccharides are required for cell surface expression of the norepinephrine transporter but do not influence substrate or inhibitor recognition. J. Neurochem. 67: 645-655.28. [DOI] [PubMed]
- 70.Chen R, et al. Direct evidence that two cysteines in the dopamine transporter form a disulfide bond. Mol Cell Biochem. 2007;298(1-2):41–48. doi: 10.1007/s11010-006-9348-7. [DOI] [PubMed] [Google Scholar]
- 71.Torres GE, et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278(4):2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 72.Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- 73.Braakman I, Hoover-Litty H, Wagner KR, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114(3):401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klausner RD. Architectural editing: Determining the fate of newly synthesized membrane proteins. New Biol. 1989;1(1):3–8. [PubMed] [Google Scholar]
- 75.Mercado CP, et al. A serotonin-induced N-glycan switch regulates platelet aggregation. Sci Rep. 2013;3:2795. doi: 10.1038/srep02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziu E, et al. Down-regulation of the serotonin transporter in hyperreactive platelets counteracts the pro-thrombotic effect of serotonin. J Mol Cell Cardiol. 2012;52(5):1112–1121. doi: 10.1016/j.yjmcc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brenner B, et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102(1):206–215. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]