Abstract
Motivated by recent global reductions in biodiversity, empirical and theoretical research suggests that more species-rich systems exhibit enhanced productivity, nutrient cycling, or resistance to disturbance or invasion relative to systems with fewer species. In contrast, few data are available to assess the potential ecosystem-level importance of genetic diversity within species known to play a major functional role. Using a manipulative field experiment, we show that increasing genotypic diversity in a habitat-forming species (the seagrass Zostera marina) enhances community resistance to disturbance by grazing geese. The time required for recovery to near predisturbance densities also decreases with increasing eelgrass genotypic diversity. However, there is no effect of diversity on resilience, measured as the rate of shoot recovery after the disturbance, suggesting that more rapid recovery in diverse plots is due solely to differences in disturbance resistance. Genotypic diversity did not affect ecosystem processes in the absence of disturbance. Thus, our results suggest that genetic diversity, like species diversity, may be most important for enhancing the consistency and reliability of ecosystems by providing biological insurance against environmental change.
There is growing recognition that humans are highly dependent on natural ecosystems for a variety of goods and services (1). Maintaining the provision of these goods and services in the face of natural and anthropogenic disturbances is critical to achieving both conservation and economic goals. Motivated by accelerating rates of worldwide decline in biodiversity (2), considerable research has focused on the consequences of local species loss for goods and services provided by ecosystems (2-8). Much of this work focuses on the effects of declining species richness on short-term processes such as production, community respiration, and nutrient cycling (2). Although the results are far from unequivocal and subject to varying interpretation (e.g., ref. 9), it does appear that, in some systems, reductions in local species diversity contribute to a decline in ecosystem properties such as productivity and resistance to disturbance (see review in ref. 2).
Nevertheless, many important ecosystems, such as kelp forests, cattail marshes, and fir forests, are dominated by, and dependent on, one or a few key plant species (10). Furthermore, individual predator and herbivore species often play a disproportionate role in determining ecosystem processes, overwhelming any effect of species diversity (11). Dominant, numerically abundant species are unlikely to go extinct as a result of human activities, but habitat fragmentation and population decline are expected to reduce the genetic diversity within populations of these species through increased genetic drift and inbreeding, along with reduced gene flow between populations (12). Recent studies suggest that genetic diversity within these dominant species may have community- or ecosystem-level consequences (13-15). Although few data are available to assess this hypothesis, genetic diversity of key species may play an analogous role to species diversity in systems with a more even distribution of species.
In this study, we assessed the relationship between genetic diversity and various community and ecosystem responses by creating field plots of the marine angiosperm Zostera marina (eelgrass). Previous research on the effects of species diversity on ecosystem functioning suggests that increasing functional diversity has a larger effect on the magnitude of ecosystem processes (e.g., refs. 16 and 17), whereas diversity within groups of functionally similar species affects the consistency or reliability of these systems (e.g., refs. 7, 18, and 19). Because genetic diversity within key species can be considered analogous to species diversity within a functional group, genetic diversity may be more likely to affect the resistance of ecosystems to perturbation than to affect the magnitude of ecosystem processes under “normal” conditions. The occurrence of both human-created and natural disturbances during our experiment allowed us to evaluate whether diversity more strongly influences ecosystem processes in the presence or absence of major disturbance/stress events.
Materials and Methods
Z. marina is a widely distributed seagrass that forms vast monospecific stands in shallow temperate estuaries worldwide. In addition to enhancing estuarine primary productivity, Zostera provides habitat for numerous fishes and invertebrates and plays a role in nutrient cycling and sediment stabilization (20). Habitat fragmentation resulting from human activities and subsequent restoration practices have led to local-scale declines in seagrass genetic diversity, but the consequences of these declines for the ecosystem services provided by eelgrass remain unclear (21).
Field Experiment. We tested the hypothesis that declining genotypic diversity will alter ecosystem properties by creating 1-m2 experimental plots of equivalent transplant density and one of four diversity treatments: one, two, four, or eight genotypes. We established three blocks of nine plots (each plot separated by 2 m) within an eelgrass bed in Bodega Bay, CA. Treatments were interspersed within and among blocks (see Table 2, which is published as supporting information on the PNAS web site). All preexisting eelgrass was removed. Initial measurements indicated no differences among plots or blocks in sediment organic content, sediment density, or particle size (P ≥ 0.39). In June 2002, we marked 256 Z. marina terminal shoots from eight areas in Bodega Bay with a numbered cable tag around the base of the shoot. To account for complications in the tagging process (e.g., lost tags, shoot mortality) and to ensure that we identified enough unique genotypes for our diverse treatments, we tagged more shoots than were actually used in the experiment. A small tissue sample was collected from each tagged shoot and stored on ice for transport to the laboratory. All samples were frozen at -80°C before extraction and genotyping (see below).
Once genotyping was complete, we located and collected each tagged terminal shoot along with one to two subterminal shoots attached by rhizomes (i.e., one transplant unit) from the field. These physiologically integrated clusters of shoots were used to minimize the effects of transplantation. Equal numbers of transplant units (eight transplants per m2, corresponding to 13-15 shoots per m2) were planted in experimental plots assigned to one of four genetic diversity treatments: one genotype (n = 6 plots), two genotypes (n = 4 plots), four genotypes (n = 5 plots), and eight genotypes (n = 9 plots). This unbalanced design was necessary because we were limited in the number of clones with sufficient numbers of transplant units to produce more two- and four-genotype plots. Treatments reflect natural levels of Zostera genetic diversity in Bodega Bay, which range from 1 to 12 genotypes per m2, with a mean of 3.04 per m2 (A.R.H., unpublished data).
To avoid confounding the potential effects of genotypic diversity with those of multilocus heterozygosity on plant performance (e.g., ref. 21), genotypes were assigned to treatments such that average multilocus heterozygosity did not vary with genotypic richness (R2 = 0.13, P = 0.62). In addition, when possible, multigenotype treatments consisted of mixtures of clones that were also grown in monoculture to control for the possibility that any increase in ecosystem function with diversity was simply due to the increasing probability of including a genotype with strong ecosystem effects as genotypic richness rises (i.e., the sampling effect; refs. 9 and 22). One plot in each block received no transplants to control for natural recruitment. Zero-density controls were not considered in statistical analyses because initial genetic diversity was not applicable.
We quantified the number of shoots per plot at biweekly intervals for the first 4 months and at monthly intervals for the remainder of the experiment. We chose shoot density as a measure of ecosystem function, because it is commonly used to approximate seagrass above-ground biomass and restoration success (20, 21), but it does not involve destructive sampling, which could have affected the outcome of the experiment. After ≈5 months (December), brant geese (Branta bernicla subsp. nigricans) migrated into our study site and consumed a significant amount of the eelgrass in our plots (see Results). At the end of the fourth month (just before grazing by the geese) and the end of the 10th month (after the plots had recovered to predisturbance shoot density), we sampled sediment porewater ammonium concentration (23, 24) and epiphyte biomass (25), following standard procedures. Ammonium concentrations were used as a measure of resource availability, because ammonium can be a limiting nutrient for Zostera growth (25). Epiphyte biomass was measured as an index of ecosystem condition because overgrowth by epiphytes contributes to seagrass decline (20, 25). In addition, we quantified invertebrate abundance and diversity on individually collected shoots by sorting all invertebrates to the lowest taxonomic level possible by using a dissecting microscope. These estimates measure relatively sedentary invertebrate species that are closely associated with Zostera, but they do not include more mobile species (e.g., crabs and fish).
Immediately after the grazing event (month 6) we again measured porewater ammonium concentrations. Epiphyte and invertebrate measurements were not taken at this time because they involve destructive sampling of individual shoots, and we wanted to avoid adding even minor disturbance that might influence the recovery process. In addition, we did not sample above- or below-ground biomass destructively during the experiment because of the large disturbance caused by taking such samples. The results of such destructive sampling from the end of the experiment are not presented here because they were performed only after the plots had recovered from grazing and thus provide no information about the effects of diversity on ecosystem variables under predisturbance, disturbance, or recovery conditions.
Genetic Methods. DNA was extracted from ≈50 mg of frozen tissue by using the cetyltrimethylammonium bromide method (26). Each sample was genotyped at five microsatellite loci isolated from Z. marina (European Molecular Biology Laboratory loci/accession numbers: ZosmarCT-12/AJ249303, ZosmarCT-19/AJ249304, ZosmarCT-3/AJ009898, ZosmarGA-2/AJ009900, and ZosmarGA-3/AJ009901) (27, 28). Primers for three of the five loci (ZosmarCT-3, ZosmarGA-2, and ZosmarGA-3) were redesigned by using primer 3.0 computer software to yield larger products (see Table 3, which is published as supporting information on the PNAS web site, for primer sequences). For loci ZosmarCT-12 and ZosmarCT-19, we used previously published primer sequences (28).
Approximately 5 ng of DNA was used to seed a 10-μl PCR and amplified by using a Perkin-Elmer PCR System 9700. Amplification conditions were as follows: 2-min denaturation at 94°C, followed by 35-36 cycles of 30-s annealing (at 55-65°C), 45-s extension at 72°C, and 10- to 15-s denaturation at 94°C, followed by a terminal extension step of 2 min. Products were checked on 2% agarose gels before being run on polyacrylamide sequencing gels. PCR products were resolved by 6% polyacrylamide gel electrophoresis, visualized by using silver nitrate staining (Promega Silver Sequence, catalog no. Q4132), and manually scored against a pUC/M13 sequence ladder. We calculated the expected probability of each five-locus genotype, Pgen. Based on these data, all clones used in the experiment were genetically distinct with P < 0.0001.
Data Analysis. In addition to the previously mentioned grazing event, some shoots were lost because of stress associated with transplantation. This phenomenon (transplant shock) is a common source of shoot mortality during transplantation and can be a major hindrance to restoration efforts in plants (29). Thus, for analysis, we partitioned our experiment into several periods: transplantation, undisturbed growth, grazed, and postherbivory recovery. We first assessed the effect of genotypic diversity on all response variables measured in each of these periods by using a multilinear regression to protect against inflation of the type I error rate (30). Where multivariate analysis indicated a significant effect at P ≤ 0.05, univariate analyses were performed on each response variable (30). Only shoot density was measured in the transplantation phase, so a univariate analysis was sufficient. For all analyses, the full model consisted of the following independent variables: a block effect, a genotypic diversity effect, and a genotype by block interaction term. We also used shoot density from the prior sampling date as a covariate in an analysis of covariance (ANCOVA) when shoot density was significantly correlated with the response variable.
Results and Discussion
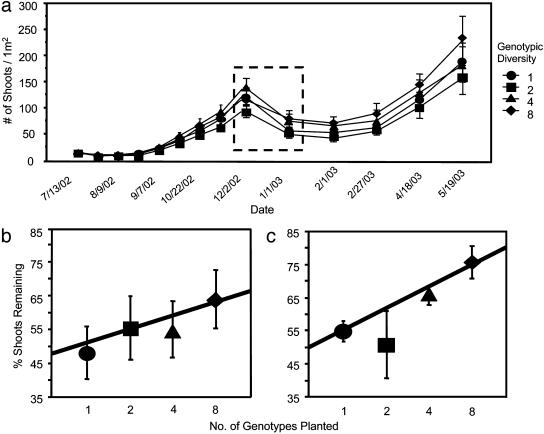
After an initial increase in eelgrass shoot density during the undisturbed growth phase as transplants spread vegetatively, there was a dramatic loss of shoots (up to 76%, Fig. 1a) 5 months into the experiment because of grazing by migratory geese. We directly observed geese grazing on eelgrass in our plots and in the surrounding natural eelgrass beds. In addition, remaining shoots in all plots were noticeably reduced in length and exhibited browsing marks, leading us to conclude that grazing was responsible for the reduction in shoot densities. During the period of seagrass expansion before this extensive herbivory, there was no detectable effect of eelgrass genetic diversity on ecosystem response (multilinear regression, P = 0.57; Table 1).Univariate analyses were performed because of a significant block effect, but even in these analyses there was no effect of genotypic diversity on any response variable (P ≥ 0.29; Table 1).
Fig. 1.
Effect of genetic diversity on Z. marina shoot density. Data are presented as mean ± SE. (a) Number of shoots per experimental plot over the course of the experiment. The dashed box highlights the loss of shoots to geese. (b) Percentage of shoots remaining in each treatment in January compared with December. Data are shown as percentages to account for variation in shoot numbers among plots before grazing by geese, but statistical analysis is by ANCOVA, not percentages. (c) Percentage of shoots remaining in each treatment at week 2 of the experiment relative to initial densities increased with increasing genotypic diversity.
Table 1. Results of multi- and univariate linear regression analyses of the effect of plot genotypic diversity on ecosystem function.
| Diversity (df = 1)
|
Block (df = 2)
|
|||||
|---|---|---|---|---|---|---|
| Sampling period | Response variable | R2 | F | P | F | P |
| Transplantation | Shoot density* | 0.47 | 11.53 | 0.003 | 1.48 | 0.26 |
| Undisturbed growth, November | Multilinear regression | 0.80 | 0.57 | 7.93 | <0.0001 | |
| Shoot density* | 0.33 | 1.10 | 0.31 | 0.38 | 0.69 | |
| Epiphyte:eelgrass biomass†‡ | 0.66 | 0.02 | 0.89 | 17.55 | <0.0001 | |
| Invertebrate abundance‡ | 0.34 | 0.29 | 0.59 | 1.69 | 0.21 | |
| Invertebrate diversity (H′)‡ | 0.36 | 1.20 | 0.29 | 3.38 | 0.06 | |
| Porewater [NH+4] | 0.10 | 0.06 | 0.81 | 0.21 | 0.81 | |
| Grazed, January | Multilinear regression | 3.39 | 0.05 | 3.01 | 0.03 | |
| Shoot density* | 0.64 | 4.87 | 0.04 | 5.38 | 0.01 | |
| Porewater [NH+4]† | 0.18 | 3.95 | 0.05 | 2.71 | 0.08 | |
| Postherbivory recovery, May | Multilinear regression | 4.02 | 0.02 | 4.02 | 0.002 | |
| Shoot density* | 0.54 | 0.25 | 0.62 | 2.74 | 0.09 | |
| Epiphyte:eelgrass biomass†‡ | 0.47 | 0.53 | 0.48 | 6.23 | 0.008 | |
| Invertebrate abundance†‡ | 0.76 | 4.27 | 0.05 | 17.84 | <0.0001 | |
| Invertebrate diversity (H′)‡ | 0.59 | 0.60 | 0.45 | 8.27 | 0.003 | |
| Porewater [NH+4]§ | 0.42 | 6.76 | 0.01 | 11.30 | <0.001 | |
Multivariate analyses (multilinear regression) included all possible response variables for a given sampling date. When independent variables in the multivariate model explained significant variance at P ≤ 0.05, univariate analyses were run on the individual response variables (ref. 30; see Data Analysis in Materials and Methods). The block by diversity interaction was never significant, so it is not presented. df, degrees of freedom.
Shoot density from the previous sampling period used as covariate in the analysis.
Log-transformed data to meet assumptions of ANOVA.
Measured on a per-shoot basis.
Samples taken in June.
In contrast, multivariate analyses of response variables in the month after the grazing event and in the recovery period did show an effect of diversity (multilinear regression, P < 0.05; Table 1). The number of shoots remaining after grazing by the geese rose with increasing plot genotypic diversity (ANCOVA, R2 = 0.64, P = 0.04), indicating that more diverse plots exhibited greater resistance to the grazing disturbance (Fig. 1b). Total shoot density did differ among blocks (P = 0.01; Table 1), potentially because of variation in water flow or tidal exposure, but the lack of a block by diversity interaction (P = 0.93) indicates that the effect of diversity on disturbance resistance was consistent across different baseline environmental conditions. The relationship between sediment nutrients and genotypic diversity was also affected by the grazing event: porewater ammonium concentration decreased with increasing genotypic diversity (P = 0.05; Table 1). This effect was not simply the result of differences in shoot density (see below). Although P values for the effect of genotypic diversity on shoot density and ammonium concentration are close to 0.05, because the multivariate analysis and both of the response variables measured during the grazing event are significant, it seems unlikely that these results are due to chance.
The differences in shoot density among treatments created by this grazing event lingered for several months (Fig. 1a). By the May 2003 sampling date, there was no longer any effect of genotypic diversity on shoot density (P = 0.62; Table 1). The time to recovery to near predisturbance densities decreased with increasing eelgrass genetic diversity (R2 = 0.69, P = 0.02), indicating that more diverse plots recovered faster. However, there was no effect of diversity on resilience, measured as the rate of shoot recovery (4, 31), 1, 2, 3, or 4 months after peak grazing (ANCOVA, F ≤ 0.99, P ≥ 0.33). This result suggests that more rapid recovery in diverse plots was due solely to differences in disturbance resistance (Fig. 1b) rather than an increase in the rate of return to equilibrium (i.e., resilience to disturbance). Because 1 month elapsed between sampling events and it is unknown exactly when during this month the grazing event took place, it is possible that our results reflect resilience of high-diversity plots over time scales <1 month. However, slow seagrass expansion rates at this time of year (Fig. 1a; ref. 25) likely preclude rapid resilience as a mechanism. Our results also suggest that the underlying mechanism is not simply an extreme form of the sampling effect where one or two “resistant” genotypes come to dominate formerly diverse plots. Resampling of the genotypic composition of our high-diversity plots 3 months after the grazing event showed no sign of dominance by a single or even a few genotypes (of 20 shoots genotyped, eight genotype plots had a mean richness of 6.44 and mean evenness of 0.89 of a maximum of 1.0).
Stress associated with transplantation (i.e., transplant shock; ref. 29) caused a loss of shoots in all plots during the first 2 weeks of the experiment, but the degree of shoot loss was not uniform across treatments. The number of shoots remaining at the end of the first 2 weeks increased with increasing plot genotypic diversity (ANCOVA, R2 = 0.47, P = 0.003, Fig. 1c). This effect was short in duration, as there was no effect of diversity on shoot density at the end of the first month of the experiment. The positive effect of genotypic diversity on shoot loss from the physiological stress of transplantation is similar to that from intense grazing. This effect can also be attributed to increased resistance (rather than resilience) among more diverse plots because there was no significant shoot growth during the initial 2-week period. Thus, genotypic diversity strongly buffered the system against the effects of both artificial and natural disturbances in this experiment.
Instead of the ANCOVA that we performed to test for disturbance resistance, previous studies (e.g., refs. 3 and 4) have suggested using the loge of the ratio of the response variable at the peak of the disturbance to that just before the disturbance. We presented the data in a similar way in Fig. 1 b and c but did not analyze these ratios statistically because this analysis would tend to overemphasize variation in plots with lower predisturbance shoot densities (32). We similarly analyzed resilience with an ANCOVA of the effect of genotypic diversity on the extent of return to pregrazing densities, with the magnitude of the disturbance (i.e., overall loss in density to grazing) as the covariate, rather than the ratio of these numbers as detailed in previous studies (3, 4). For both the resistance and resilience analyses, the results using the ratio methods were qualitatively similar to the results from the ANCOVAs.
The effect of genotypic diversity on shoot density likely cascades to the many species of epiphytic plants and invertebrates that rely on seagrass for habitat. In our experiment, abundances of associated organisms did not differ as a function of genotypic diversity on a per-shoot basis before the grazing event (Table 1). However, total animal abundance per plot should increase with eelgrass genotypic diversity during the grazing period, simply because plots with more genotypes had more shoots. More interestingly, the per-shoot abundance of invertebrates did increase with genotypic diversity in the post-grazing recovery period (P = 0.05; Table 1), suggesting that greater numbers of shoots in more diverse plots benefit epifaunal organisms. Because increasing shoot density can provide enhanced refuge against predators (20), lower predation rates in more diverse plots during the grazing and recovery period may have contributed to the observed increase in animal density. Thus, there are positive effects of genotypic diversity on animal abundance that are not a simple consequence of the increased numbers of shoots in more diverse plots.
During the disturbance and recovery process, we found a negative relationship between genotypic diversity and the concentration of ammonium in sediment porewater (P ≤ 0.05; Table 1). Although increased shoot density should enhance nutrient uptake, the relationship between genotypic diversity and ammonium concentration was not simply the result of differences in shoot density among treatments, as ammonium concentration and shoot density were uncorrelated at both sampling dates (R2 ≤ 0.06, P ≥ 0.10). Although these results might indicate that systems with greater genotypic diversity more completely use available resources, decreased standing stock of ammonium during the grazing and recovery period could also be due to other factors (e.g., lower regeneration rates). Because we could not quantify below-ground processes during the experiment, it is difficult to assess the mechanism underlying this pattern, yet the similarity to results from species diversity manipulations (e.g., ref. 7) is intriguing.
Our results complement findings that species diversity contributes to the resistance of communities to various disturbances (3, 4, 7, 8, 33). Specifically, our results parallel those of refs. 3 and 4 in that diversity appears to affect the resistance, but not the resilience, of the ecosystem to disturbance. Although the design of our experiment does not permit an unequivocal test of the sampling effect or other potential underlying mechanisms, the similarities with species-richness responses suggest the underlying mechanisms may be similar. Species richness is proposed to provide “biological insurance” against fluctuations in ecosystem processes, because species differ in the manner in which they respond to changing conditions and/or in the time scales over which these responses occur (4, 7, 19, 34). Analogously, differences among genotypes in their resistance to various stresses such as grazing or transplant shock may underlie the effects of genotypic diversity on the resistance of seagrass ecosystems to disturbance. In other words, our results could be due to trade-offs among genotypes, such that “good” genotypes from the perspective of disturbance resistance may be “bad” from the perspective of rates of growth under “normal” conditions, and vice versa (19, 34).
Human disturbances are leading to demonstrable, and in some cases dramatic, reductions in the genetic diversity within species (12, 21). Understanding the ecosystem consequences of this loss of genetic variation is critically important, particularly for conservation and restoration efforts. Restored seagrass beds often exhibit reduced levels of diversity compared with those of natural populations (21). Our findings, combined with those of related experiments (21, 35), suggest mitigation efforts that involve planting seagrass meadows or restoring other foundation species need to include a diversity of genotypes to enhance the likelihood of long-term persistence in the face of changing conditions. The importance of genetic diversity of key species for maintaining ecosystem functioning may become even more important as stressors such as eutrophication, habitat fragmentation, and global climate change intensify.
Supplementary Material
Acknowledgments
We thank J. A. Hughes, D. L. Kimbro, and the Grosberg, Stachowicz, and Grosholz labs for their assistance in data collection. E. Grosholz, S. L. Williams, and R. K. Grosberg, and three anonymous reviewers provided valuable support and comments. This work was supported by National Science Foundation Biological Oceanography Grant OCE-0082049, an Environmental Protection Agency Science to Achieve Results fellowship, the University of California Coastal Environmental Quality Initiative Program, University of California Davis Bodega Marine Laboratories, and University of California Davis Center for Population Biology.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: ANCOVA, analysis of covariance.
References
- 1.Daily, G. C. (1997) Nature's Services (Island, Washington, DC).
- 2.Schlapfer, F. & Schmid, B. (1999) Ecol. Appl. 9, 893-912. [Google Scholar]
- 3.Tilman, D. & Downing, J. A. (1994) Nature 367, 363-365. [Google Scholar]
- 4.Tilman, D. (1996) Ecology 77, 350-363. [Google Scholar]
- 5.Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, M., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H, Giller, P. S., Good, J., et al. (1999) Science 286, 1123-1127. [DOI] [PubMed] [Google Scholar]
- 6.Loreau, M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J. P., Hector, A., Hooper, D. U., Huston, M. A., Rafaelli, D., Schmid, B., et al. (2001) Science 294, 804-808. [DOI] [PubMed] [Google Scholar]
- 7.Stachowicz, J. J., Fried, H., Osman, R. W. & Whitlatch, R. B. (2002) Ecology 83, 2575-2590. [Google Scholar]
- 8.Mulder, C. P. H., Uliassi, D. D. & Doak, D. F. (2001) Proc. Natl. Acad. Sci. USA 98, 6704-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huston, M. A. (1997) Oecologia 110, 449-460. [DOI] [PubMed] [Google Scholar]
- 10.Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. (2003) Trends Ecol. Evol. 18, 119-125. [Google Scholar]
- 11.Paine, R. T. (2002) Science 296, 736-739. [DOI] [PubMed] [Google Scholar]
- 12.Young, A., Boyle, T. & Brown, T. (1996) Trends Ecol. Evol. 11, 413-418. [DOI] [PubMed] [Google Scholar]
- 13.Madritch, M. D. & Hunter, M. D. (2003) Oecologia 136, 124-128. [DOI] [PubMed] [Google Scholar]
- 14.Neuhauser, C., Andow, D. A., Heimpel, G. E., May, G., Shaw, R. G. & Wagenius, S. (2003) Ecology 84, 545-558. [Google Scholar]
- 15.Whitham, T. G., Young, W. P., Martinsen, G. D., Gehring, C. A., Schweitzer, J. A., Shuster, S. M., Wimp, G. M., Fischer, D. G., Bailey, J. K., Lindroth, R. L., et al. (2003) Ecology 84, 559-573. [Google Scholar]
- 16.Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M. & Siemann, E. (1997) Science 277, 1300-1302. [Google Scholar]
- 17.Hooper, D. U. & Vitousek, P. M. (1997) Science 277, 1302-1305. [Google Scholar]
- 18.Naeem, S. & Li, S. (1997) Nature 390, 507-509. [Google Scholar]
- 19.Norberg, J., Swaney, D. P., Dushoff, J., Lin, J., Casagrandi, R. & Levin, S. A. (2001) Proc. Natl. Acad. Sci. USA 98, 11376-11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams, S. L. & Heck, K. L. (2001) in Marine Community Ecology, eds. Bertness, M. D., Gaines, S. D. & Hay, M. E. (Sinauer, Sunderland, MA), pp. 317-338.
- 21.Williams, S. L. (2001) Ecol. Appl. 11, 1472-1488. [Google Scholar]
- 22.Tilman, D., Lehman, C. L. & Thomson, K. T. (1997) Proc. Natl. Acad. Sci. USA 94, 1857-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg, P. & McGlathery, K. J. (2001) Limnol. Oceanogr. 46, 203-210. [Google Scholar]
- 24.Koroleff, F. (1976) in Methods of Seawater Analysis, ed. Grasshoff, K. (Verlag Chemie, Weinheim, Germany), pp. 127-133.
- 25.Williams, S. L. & Ruckelshaus, M. H. (1993) Ecology 74, 904-918. [Google Scholar]
- 26.Doyle, J. J & Doyle, J. L. (1987) Phytochem. Bull. 19, 11-15. [Google Scholar]
- 27.Reusch, T. B. H., Stam, W. T. & Olsen, J. L. (1999) Mol. Ecol. 8, 317-322. [DOI] [PubMed] [Google Scholar]
- 28.Reusch, T. B. H. (2000) Mol. Ecol. 9, 365-378.10736034 [Google Scholar]
- 29.Vilagrosa, A., Cortina, J., Gil-Pelegrin, E. & Bellot, J. (2003) Restor. Ecol. 11, 208-216. [Google Scholar]
- 30.Scheiner, S. M. & Gurevitch, J., eds. (2000) Design and Analysis of Ecological Experiments (Chapman & Hall, New York), pp. 94-112.
- 31.Pimm, S. L. (1984) Nature 307, 321-326. [Google Scholar]
- 32.Steel, R. G. D., Torrie, J. H. & Dickey, D. A., eds. (1997) Principles and Procedures of Statistics: A Biometrical Approach (McGraw-Hill, Boston), 3rd Ed., pp. 429-458.
- 33.McNaughton, S. J. (1977) Am. Nat. 111, 515-525. [Google Scholar]
- 34.Yachi, S. & Loreau, M. (1999) Proc. Natl. Acad. Sci. USA 96, 1463-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Procaccini, G. & Piazzi, L. (2001) Restor. Ecol. 9, 332-338. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.