Abstract
New and known arylidene-hydrazinyl-thiazole derivatives have been synthesized by a convenient Hantzsch condensation. All compounds were evaluated for their in vitro cytotoxicity on two carcinoma cell lines, MDA-MB231 and HeLa. Significant antiproliferative activity for 2-(2-benzyliden-hydrazinyl)-4-methylthiazole on both MDA-MB-231 (IC50: 3.92 µg/mL) and HeLa (IC50: 11.4 µg/mL) cell lines, and for 2-[2-(4-methoxybenzylidene) hydrazinyl]-4-phenylthiazole on HeLa (IC50: 11.1 µg/mL) cell line is reported. Electrophoresis experiments showed no plasmid DNA (pTZ57R) cleavage in the presence of the investigated thiazoles.
Keywords: thiazole, hydrazine, cytotoxicity, DNA interaction
1. Introduction
Recent studies have shown a constant interest in thiazole compounds due to a wide spectra of biologic activities, such as the antimalarial activity of hydrazinyl-thiazoles [1], antiproliferative activity of steroidal[17,16-d]thiazole against gastric carcinoma cells [2], antitumor activity of thiazol-1H-pyrrolo-[2,3-b]pyridine in peritoneal mesothelioma experimental models [3], antiproliferative activity of thiazol-1H-indoles and thiazol-1H-7-azaindoles in MiaPaCa-2 cell line [4], CDK-1 inhibitory activity of thiazol-1H-pyrrolo[3,2-b]pyridine [5], antimicrobial activity of thiazole-oxadiazole derivatives [6], or anti-inflammatory properties of hydrazono-thiazole derivatives [7]. The thiazole rings can be found in a variety of pharmaceutical drugs, such as Ritonavir (anti-HIV) [8], Bleomycin [9] and Tiazofurin (antineoplastics) [10], Fanetizole and Meloxicam (anti-inflammatories) [11], which explains the interest in the development of new compounds containing this heterocyclic unit.
Regarding the synthesis of hydrazinyl-thiazoles, two procedures have been highlighted in the literature: the classical condensation of a carbonyl group with thiosemicarbazide followed by the cyclization of thiosemicarbazones with α-halocarbonyl derivatives [12,13,14,15], and a more recently reported one-step multi-component synthetic protocol [16,17].
The main goal of this work was to identify new possible chemotherapeutic agents based on organic heterocyclic derivatives, which are less harmful for the human body than the well-known platinum derivatives. In this paper, we present a two-step protocol for the synthesis of seven new arylidene-hydrazinyl-thiazoles 2c, 2f, 2h, 2j, 2l, 2m, 2p and nine previously reported thiazoles 2a, 2b, 2d, 2e, 2g, 2i, 2k, 2n, 2o, followed by the in vitro evaluation of the antiproliferative activity on two carcinoma cell lines, MDA-MB231 and HeLa. To identify a possible correlation between DNA damage and cytotoxicity, the interaction of the thiazole derivatives 2a, 2e, 2h, 2i with DNA was evaluated by electrophoresis.
2. Results and Discussion
2.1. Synthesis of Arylidene-Hydrazinyl-Thiazole Derivatives 2a–p
A series of arylidene-hydrazinyl-thiazole derivatives 2a–p were synthesized in two steps: the condensation of aromatic aldehydes with hydrazinecarbothioamide, followed by the cyclization of aryliden-thiosemicarbazones 1a–e with α-halocarbonyl derivatives (Scheme 1, Table 1). Both the condensation and cyclization reactions were performed in good yield by the Hantzsch protocol. Derivatives 2a, 2b, 2d, 2e, 2g, 2i, 2k, 2n and 2o have been previously prepared by other groups [17,18,19,20].
Scheme 1.
Synthesis of arylidene-hydrazinyl-thiazoles 2a–p.
Table 1.
Functional groups of the hydrazinyl-thiazole derivatives 2a–p.
| Compound 1 | a | b | c | d | e | |||
| Ar | C6H5 | C6H3Cl2(2,4) | C6H4OH(4) | C6H4OCH3(4) | C6H4Cl(3) | |||
| Compodund 2 | a | b | c | d | e | f | g | h |
| Ar | C6H5 | C6H5 | C6H5 | C6H4OCH3(4) | C6H4OCH3(4) | C6H4OCH3(4) | C6H4OCH3(4) | C6H4OH(4) |
| R1 | CH3 | C6H5 | CH3 | CH3 | C6H5 | CH3 | CH3 | CH3 |
| R2 | H | H | COCH3 | H | H | COCH3 | COOC2H5 | H |
| Compound 2 | i | j | k | l | m | n | o | p |
| Ar | C6H4OH(4) | C6H4Cl(3) | C6H4Cl(3) | C6H4Cl(3) | C6H4Cl(3) | C6H3Cl2(2,4) | C6H3Cl2(2,4) | C6H3Cl2(2,4) |
| R1 | C6H5 | CH3 | C6H5 | CH3 | CH3 | CH3 | CH3 | CH2COOC2H5 |
| R2 | H | H | H | COCH3 | COOC2H5 | COCH3 | COOC2H5 | H |
NMR and MS spectra were recorded for all the arylidene-hydrazinyl-thiazoles 2a–p. The 1H-NMR spectra of arylidene-hydrazinyl-thiazoles 2a–p present a similar pattern for the hydrazone unit. The most downfield singlet, around 12 ppm, corresponds to the hydrazinyl moiety (N-NH), which is only present in DMSO-d6 solutions, and otherwise missing due to the deuterium exchange. The singlet around 8.4~7.8 ppm is assigned to the azomethine proton (CH=N). The expected molecular ion (M+) is found in the mass spectra of all arylidene-hydrazinyl-thiazoles 2a–p. Moreover, the fragmentation pathway involved the cleavage of the nitrogen-nitrogen bond from the hydrazinyl unit. For example, in the MS spectra of thiazole 2a, this fragmentation generates a peak at m/z 113 for the aza-thiazole ion, while for the thiazole 2b the corresponding aza-thiazole ion peak is observed at m/z 175, in accordance with the substitution of the thiazole heterocycle.
2.2. In Vitro Cytotoxicity Assay
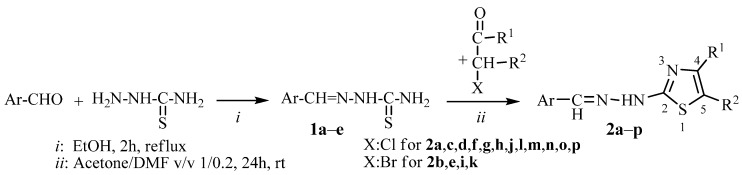
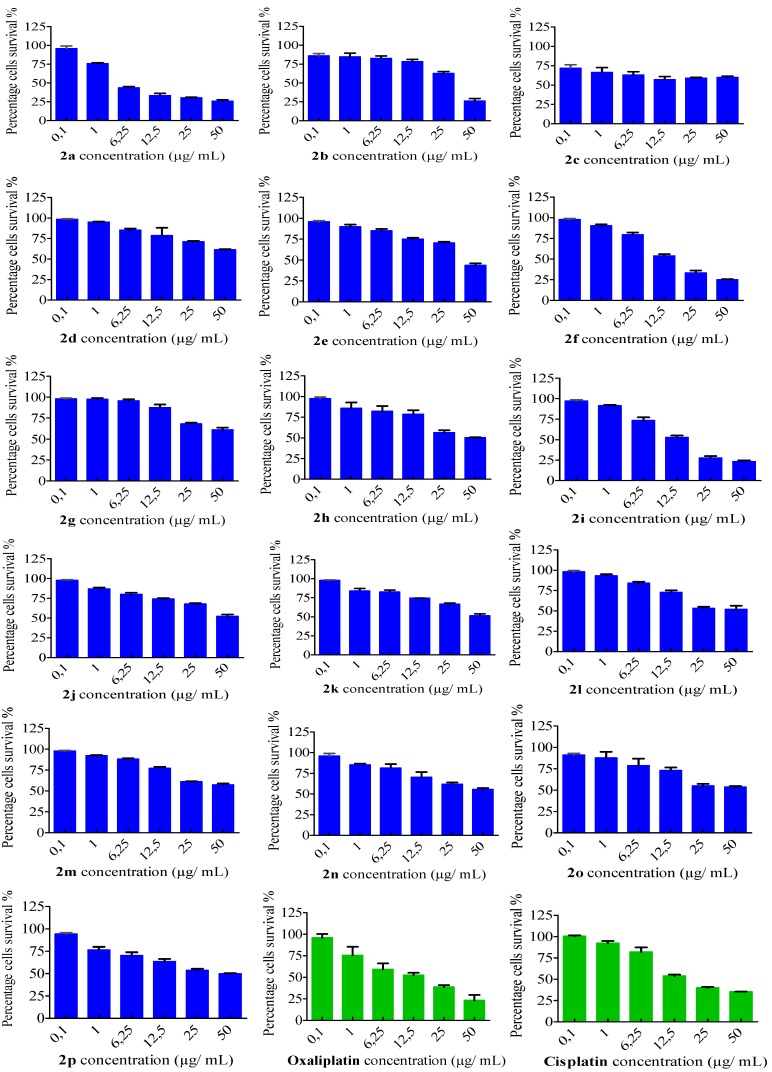
The anti-proliferative activity of the sixteen arylidene-hydrazinyl-thiazole derivatives against two human carcinoma MDA-MB231 and HeLa cell lines was evaluated using MTT assays [14,21] after 24 h of treatment. According to the IC50 data (Table 2), five thiazole derivatives, 2a, 2e, 2f, 2h and 2i, have shown significant inhibition on both MDA-MB231 and HeLa cancer cell lines. Their activity is comparable or even better than that of the platinum drugs cisplatin and oxaliplatin, which were used as controls.
Table 2.
IC50 values for thiazoles 2a–p on the MDA-MB231 and HeLa cell lines.
| Compound | IC50 (µg/mL) | |
|---|---|---|
| MDA-MB-231 | HeLa | |
| 2a | 3.92 ± 0.015 | 11.4 ± 0.005 |
| 2b | 35.5 ± 0.003 | >100 |
| 2c | >100 | 64.87 ± 0.005 |
| 2d | >100 | >100 |
| 2e | 46.11 ± 0.009 | 11.1 ± 0.009 |
| 2f | 16.25 ± 0.008 | >100 |
| 2g | >100 | >100 |
| 2h | 48.44 ± 0.017 | 25.59 ± 0.010 |
| 2i | 18.54 ± 0.008 | 20.04 ± 0.019 |
| 2j | >100 | 57.53 ± 0.011 |
| 2k | 81.02 ± 0.001 | >100 |
| 2l | 75.50 ± 0.009 | >100 |
| 2m | >100 | >100 |
| 2n | >100 | >100 |
| 2o | >100 | >100 |
| 2p | 64.95 ± 0.009 | >100 |
| Cisplatin | 17.28 ± 0.002 | 26.12 ± 0.010 |
| Oxaliplatin | 14.09 ± 0.001 | 23.17± 0.011 |
Having the IC50 values for thiazoles 2a–p, we tried to establish a correlation between the cytotoxic activity and the molecular structure, by looking at the nature of the functional groups and their position on the arylidene-hydrazinyl-thiazole backbone. The presence of a methyl or phenyl group in position 4 (see Scheme 1) and a hydrogen or acetyl in position 5 on the thiazole ring, combined with phenyl, p-OH-phenyl or p-MeO-phenyl as the aromatic group attached to the hydrazinyl unit, led to compounds 2a, 2e, 2f, 2h and 2i, which exhibited the highest antiproliferative activity. On the other hand, the presence of chlorine atoms at the phenyl hydrazinyl unit and ethyl carboxylate group in position 5 on the thiazole ring 2m–o decreased the antiproliferative efficiency (Table 2).
The viability of the breast cancer MDA-MB-231 cells and cervical cancer HeLa cells decreased with an increase in the concentration of the thiazole derivatives 2a–p (Figure 1 and Figure 2). The profiles of the MDA-MB-231 cells survival viability, correlated to the thiazole doses, revealed a common trend for thiazoles 2a, 2f, 2i, as well as cisplatin and oxaliplatin (Figure 1). For the HeLa cell line, the same correlation is observed between compounds 2a, 2e, 2h, 2i and the chemotherapeutic drugs cisplatin and oxaliplatin (Figure 2).
Figure 1.
Viability effects of arylidene-hydrazinyl-thiazoles 2a–p on MDA-MB-231 cells using the MTT assay.
Figure 2.
Viability effects of arylidene-hydrazinyl-thiazoles 2a–p on HeLa cells using the MTT assay.
Due to its significant antiproliferative activity on both MDA-MB-231 (IC50: 3.92 µg/mL) and HeLa (IC50: 11.4 µg/mL) cell lines, the 2-(2-benzyliden-hydrazinyl)-4-methylthiazole derivative 2a was studied further and used as a starting point for the development of new arylidene-hydrazinyl-thiazole compounds for the treatment of cancer. Additionally, 2-[2-(4-methoxybenzylidene) hydrazinyl]-4-phenylthiazole (2e), with an IC50 value of 11.1 µg/mL, was also considered as a potentially useful cytotoxic compound against the HeLa cell line.
2.3. DNA Intercalation Study
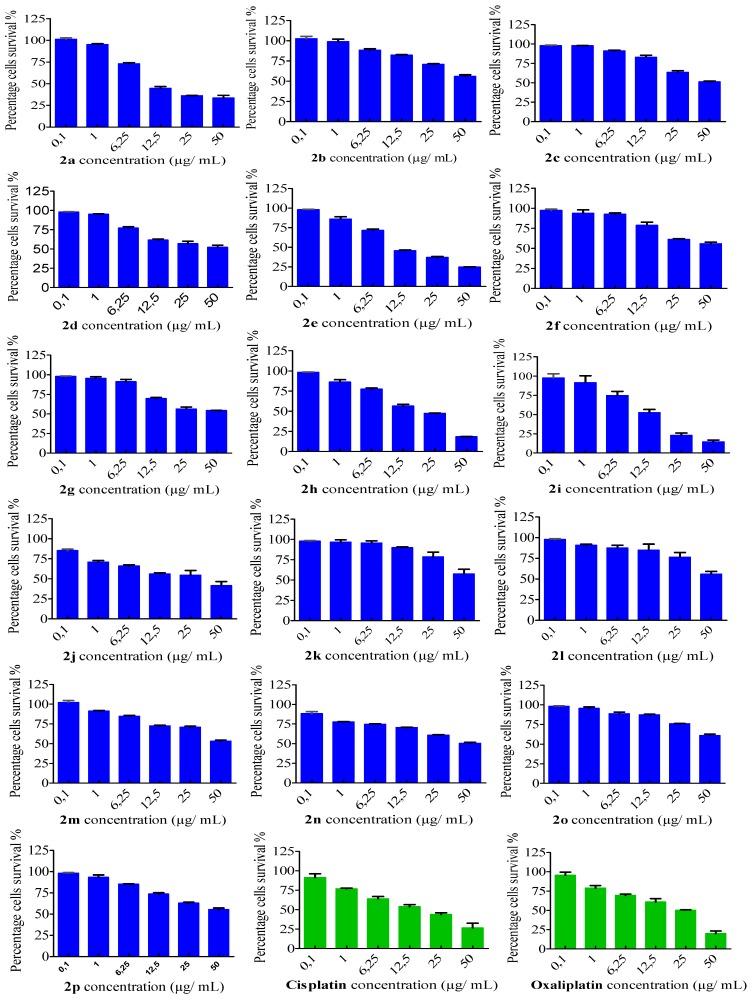
For the best candidates, the arylidene-hydrazine-thiazole derivatives 2a, 2e, 2h and 2i, their interactions with plasmid DNA (pTZ57R) were investigated. Gel electrophoresis experiments investigating the plasmid migration in agarose gel after incubation with thiazoles 2a, 2e, 2h and 2i did not reveal any ability of these compounds to generate changes in the electrophoretic mobility of supercoiled DNA. These electrophoresis experiments showed that DNA cleavage does not occur in the presence of thiazoles 2a, 2e, 2h and 2i, even at elevated concentrations of the selected compounds (Figure 3).
Figure 3.
Interaction of thiazoles 2a, 2e, 2h and 2i with plasmid DNA. (A) compounds 2a (lines 2–6) and 2h (lines 8–12); (B) compounds 2i (lines 2–6) and 2e (lines 8–12). The order was the same in all cases. Lane 1: linear plasmid (plasmid DNA digested with restriction enzyme EcoRI); 2: closed circular plasmid DNA; 3 to 6: closed circular plasmid DNA with 2, 4, 8 and 10 μL of the compound; 7: GeneRuler 1 kb DNA ladder (ThermoScientific), 8: closed circular plasmid DNA; 9 to 12: closed circular plasmid DNA with 2, 4, 8 and 10 μL of the compound; 13: linear plasmid.
The gel electrophoresis results for the pTZ57R DNA incubated with thiazole derivatives 2a, 2e, 2h and 2i suggested that, despite increasing thiazole concentrations, no meaningful effect on plasmid DNA was observed. This is an indication that the cytotoxic effect of these derivatives against MDA-MB-231 and HeLa cell lines does not involve interaction with DNA.
3. Experimental Section
3.1. Materials and Methods
The starting materials and solvents were obtained from commercial sources. The reagents used for cell culture experiments (fetal calf serum (FCS), penicillin-streptomycin, glutamine and RPMI 1460 cell media) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The antineoplastic drugs cisplatin and oxaliplatin were purchased from Actavis Sindan Pharma (Bucharest, Romania). The GeneRuler 1 kb DNA ladder was purchased from Thermo Scientific (Waltham, MA, USA).
Compounds 1a–e were prepared according to the literature [14]. Melting points were measured with an Electrothermal IA 9200 apparatus (Bibby Scientific Limited (Group HQ), Stone, UK), and are uncorrected values. 1H-NMR and 13C-NMR spectra were recorded in CDCl3, acetone-d6 or DMSO-d6 (locked to Me4Si) using a 400 or 600 MHz Bruker Avance NMR spectrometer (Bruker Biospin GmbH, Rheinsberg, Germany). Elemental analysis was carried out on a Vario EL III instrument. The mass spectra were recorded with a Shimadzu QP 2010 Plus GC-MS instrument (Shimadzu Corporation, Kyoto, Japan) and a Thermo Scientific LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA).
The equipment involved in cell lines multiplication included Class II LaminAir laminar hoods, a ShelLab incubator, and an Eppendorf Centrifuge 5702R with spin-out rotor. Spectrophotometric measurements were completed with Biotek Synergy 2 Multi-Mode Microplate Reader with SQ Xenon Flash light source, using well-area colorimetric scanning.
The MTT experimental data were processed with Graph Pad Prism 5 biostatistics software (Sheldon Manufacturing, Inc., Cornelius, OR, USA).
For the cytotoxicity assessment, two highly proliferative MDA-MB-231 and HeLa tumor cells were utilized. Both cell lines used in the experiment were purchased from the European Collection of Cell Cultures (ECCAC, BioTek, Winooski, VT, USA).
3.2. General Procedure for the Synthesis of Compounds 2a–p
A mixture of arylidene-hydrazine-carbothioamide (10 mmol) and α-halogenocarbonyl derivative (10 mmol) in acetone/DMF (10 mL, v/v: 1/0.2) was stirred at room temperature for 20–24 h; the reaction progress was monitored by TLC (toluene/AcOEt 2/1 v/v; silica plates). The reaction mixture was neutralized at pH 7 with NaHCO3 aqueous solution (10%). The precipitate was filtered and recrystallized from ethanol or acetic acid. For all compounds the yield, the melting point, the EI MS, and the elemental analysis are given, while the 1H- and 13C-NMR data are only provided for the new derivatives.
2a. (E)-2-(2-Benzylidenehydrazinyl)-4-methylthiazole [18]: White crystals, yield 1.7 g, 78%, m.p. 190–191 °C, crystallized from ethanol (m.p. lit. 192–194 °C); EI m/z: 218 (M+), 113 (100%), 77; Calcd. for C11H11N3S: C, 59.09; H, 4.46; N, 20.67; Found: C, 59.11; H, 4.49; N, 20.65.
2b. (E)-2-(2-Benzylidenehydrazinyl)-4-phenylthiazole [19]: Light brown crystals, yield 2.3 g, 81%, m.p. 218–219 °C, crystallized from ethanol (m.p. lit. 220 °C); EI m/z: 279 (M+), 176 (100%), 104, 77; Calcd. for C16H13N3S: C, 68.79; H, 4.69; N, 15.04; Found: C, 68.82; H, 4.71; N, 15.02.
2c. (E)-1-(2-(2-(Benzylidene)hydrazinyl)-4-methylthiazol-5-yl)ethanone: Yellow crystals, yield 1.9 g, 76%, m.p. 222–223 °C, crystallized from ethanol; 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.41 (s, 3H), 2.51 (s, 3H), 7.47–7.49 (m, 3H), 7.69 (dd, 2H, 3J = 7.8Hz, 4J = 1.3Hz), 8.11 (s, 1H), 12.48 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 18.1, 29.5, 122.4, 126.7 (2C), 128.8 (2C), 129.8, 134.1, 144.9, 156.6, 169.1, 188.8; EI m/z: 259 (M+), 182, 141 (100%), 77; Calcd. for C13H13N3OS: C, 60.21; H, 5.05; N, 16.20; Found: C, 60.24; H, 5.09; N, 16.19.
2d. (E)-2-(2-(4-Methoxybenzylidene)hydrazinyl)-4-methylthiazole [19]: White crystals, yield 1.6 g, 68%, m.p. 179–180 °C, crystallized from ethanol (m.p. lit. 170 °C); EI m/z: 247 (M+), 140, 134, 114 (100%), 107, 77; Calcd. for C12H13N3OS: C, 58.28; H, 5.30; N, 16.99; Found: C, 58.31; H, 5.33; N, 16.97.
2e. (E)-2-(2-(4-Methoxybenzylidene)hydrazinyl)-4-phenylthiazole [19]: Light orange crystals, yield 2.3 g, 76%, m.p. 195–196 °C, crystallized from ethanol (m.p. lit. 196 °C); EI m/z: 309 (M+), 202, 176 (100%), 133, 107, 77; Calcd. for C17H15N3OS: C, 66.00; H, 4.89; N, 13.58; Found: C, 66.04; H, 4.92; N, 13.57.
2f. (E)-1-(2-(2-(4-Methoxybenzylidene)hydrazinyl)-4-methylthiazol-5-yl)ethanone: White yellow crystals, yield 2.1 g, 73%, m.p. 214–215 °C, crystallized from ethanol; 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.41 (s, 3H), 2.49 (s, 3H), 3.80 (s, 3H), 7.01 (d, 2H, 3J = 8.6 Hz), 7.64 (d, 2H, 3J = 8.6 Hz), 8.06 (s, 1H), 12.27 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 17.5, 29.1, 55.3, 114.3 (2C), 112.8, 126.5, 128.3 (2C), 144.9, 156.7, 160.7, 169.1, 188.3; EI m/z: 289 (M+), 141 (100%), 134, 120, 107, 77; Calcd. for C14H15N3O2S: C, 58.11; H, 5.23; N, 14.52; Found: C, 58.14; H, 5.27; N, 14.53.
2g. (E)-Ethyl 2-(2-(4-methoxybenzylidene)hydrazinyl)-4-methylthiazole-5-carboxylate [20]: White crystals, yield 2.5 g, 81%, m.p. 181–182 °C, crystallized from ethanol, (m.p. lit. 180–182 °C); EI m/z: 319 (M+), 186 (100%), 134, 107, 77; Calcd. for C15H17N3O3S: C, 56.41; H, 5.37; N, 13.16; Found: C, 56.45; H, 5.40; N, 13.17.
2h. (E)-4-((2-(4-Methylthiazol-2-yl)hydrazono)methyl)phenol: Brown crystals, yield 0.9 g, 42%, m.p. 196 °C, crystallized from acetic acid; 1H-NMR (acetone-d6, 600 MHz, δ ppm): 2.16 (s, 3H), 4.28 (bb, 1H), 6.28 (s, 1H), 6.87 (d, 2H, 3J = 8.6Hz), 7.52 (d, 2H, 3J = 8.6Hz), 7.97 (s, 1H); 13C-NMR (acetone-d6, 125 MHz, δ ppm): 16.9, 102.8, 116.4 (2C), 126.9, 128.9 (2C), 143.3, 148.0, 169.9, 173.2; EI m/z: 233 (M+), 120, 114 (100%), 107, 77; Calcd. for: C11H11N3OS, C, 56.63; H, 4.75; N 18.01; Found: C, 56.65; H, 4.79; N, 18.03.
2i. (E)-4-((2-(4-Phenylthiazol-2-yl)hydrazono)methyl)phenol [22]: Light orange crystals, yield 1.4 g, 48%, m.p. 242 °C, crystallized from acetic acid, (m.p. lit. 241–243 °C); EI m/z: 295 (M+), 202, 176 (100%), 134, 120, 77; Calcd. for: C16H13N3OS; C, 65.06; H, 4.44; N, 14.23; Found: C, 65.10; H, 4.47; N, 14.20.
2j. (E)-2-(2-(3-Chlorobenzylidene)hydrazinyl)-4-methylthiazole: White crystals, yield 1.5 g, 69%, m.p. 177–178 °C, crystallized from ethanol; 1H-NMR (CDCl3, 600 MHz, δ ppm): 2.33 (s, 3H), 6.22 (s, 1H), 7.31 (d, 2H, 3J = 7.2Hz), 7.48 (t, 1H, 3J = 7.2 Hz), 7.66 (s, 1H), 7.82 (s, 1H); 13C-NMR (DMSO-d6, 125 MHz, δ ppm): 16.9, 102.5, 124.8, 125.3, 128.6, 130.6, 133.6, 136.9, 139.6, 146.7, 167.9. EI m/z: 251/253 (M+/M+2), 140, 138, 114 (100%), 111; Calcd. for: C11H10ClN3S; C, 52.48; H, 4.00; N, 16.69; Found: C, 52.52; H, 4.03; N, 16.67.
2k. (E)-2-(2-(3-Chlorobenzylidene)hydrazinyl)-4-phenylthiazole [17]: White green crystals, yield 2.2 g, 72%, m.p. 183–184 °C, crystallized from ethanol, (m.p. lit. 163–164 °C); EI m/z: 313/315 (M+/M+2), 202, 176 (100%), 138, 111; Calcd. for: C16H12ClN3S: C, 61.24; H, 3.85; N, 13.39; Found: C, 61.28; H, 3.88; N, 13.37.
2l. (E)-1-(2-(2-(3-Chlorobenzylidene)hydrazinyl)-4-methylthiazol-5-yl)ethanone: Light yellow crystals; yield: 2 g, 71%, m.p. 239–240 °C crystallized from ethanol; 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.41 (s, 3H), 2.49 (s, 3H), 7.44–7.47 (m, 2H, 3J = 7.1Hz), 7.63 (t, 1H, 3J = 7.1Hz), 7.7 (s, 1H), 8.07 (s, 1H), 12.32 (s, 1H); 13C-RMN (DMSO-d6, 100 MHz, δ ppm): 17.9, 29.5, 121.9, 125.3, 125.9, 129.4, 130.7, 133.6, 136.2, 143.2, 169.0, 172.0, 188.0. EI m/z: 293/295 (M+/M+2), 250, 182, 156, 141 (100%), 138, 111. Calcd. for C13H12ClN3OS: C, 53.15; H, 4.12; N, 14.30; Found: C, 53.17; H, 4.15; N, 14.28.
2m. (E)-Ethyl 2-(2-(3-chlorobenzylidene)hydrazinyl)-4-methylthiazole-5-carboxylate: White crystals, yield 2.3 g, 72%, m.p. 235–236 °C, crystallized from ethanol; 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 1.27 (t, 3H, 3J = 7.1 Hz), 2.47 (s, 3H), 4.21 (q, 2H, 3J = 7.1 Hz), 7.46–7.49 (m, 2H,), 7.67–7.65 (m, 1H), 7.72 (s, 1H), 8.09 (s, 1H), 12.56 (s, 1H); 13C-RMN (acetone-d6, 100 MHz, δ ppm): 14.6, 31.1, 60.2, 125.6, 126.4, 129.8, 131.2, 133.1, 134.9, 140.0, 156.0, 165.6, 171.1. EI m/z: 323/325 (M+/M+2), 212, 186 (100%); Calcd. for C14H14ClN3O2S: C, 51.93; H, 4.36; N, 12.98; Found: C, 51.97; H, 4.39; N, 12.96.
2n. (E)-1-(2-(2-(2,4-Dichlorobenzylidene)hydrazinyl)-4-methylthiazol-5-yl)ethanone [20]: Yellow crystals, yield 2.6 g, 81%, m.p. 241–242 °C, crystallized from ethanol, (m.p. lit. 240–242 °C); EI m/z: 327/329/ (M+/M+2), 315, 156, 141 (100%), 145, 112; Calcd. for C13H11Cl2N3OS: C, 47.57; H, 3.38; N, 12.80; Found: C, 47.60; H, 3.40; N, 12.79.
2o. (E)-Ethyl 2-(2-(2,4-dichlorobenzylidene)hydrazinyl)-4-methylthiazole-5-carboxylate [20]: White crystals, yield 2.8 g, 79%, m.p. 224–225 °C, crystallized from ethanol, (m.p. lit. 223–224 °C); EI m/z: 357/359 (M+/M+2), 212, 186 (100%), 172, 112; Calcd. for C14H13Cl2N3O2S: C, 46.94; H, 3.66; N, 11.73; Found: C, 46.97; H, 3.69; N, 11.71.
2p. (E)-Ethyl 2-(2-(2-(2,4-dichlorobenzylidene)hydrazinyl)thiazol-4-yl)acetate: White yellow crystals, yield 2.8 g, 80%, m.p. 140–141 °C, crystallized from ethanol; 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 1.19 (t, 3H, 3J = 7 Hz), 3.58 (s, 2H), 4.08 (q, 2H, 3J = 7 Hz), 6.69 (s, 1H), 7.48 (dd, 1H, 3J = 8.5 Hz, 4J = 1.9 Hz), 7.65 (d, 1H, 4J = 1.9 Hz), 7.87 (d, 1H, 3J = 8.5 Hz), 8.25 (s, 1H), 12.29 (s, 1H); 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 14.1, 36.8, 60.2, 102.2, 127.3, 127.9, 129.3, 130.7, 132.7, 133.9, 136.9, 145.6, 169.9; EI m/z: 357/359 (M+/M+2), 212, 186, 170 (100%); Anal. Calcd. for C14H13Cl2N3O2S: C, 46.94; H, 3.66; N, 11.73; Found: C, 46.97; H, 3.68; N, 11.71.
3.3. In Vitro Anticancer Screening
3.3.1. Cell Cultures
Both MDA-MB-231 and HeLa cell lines were grown under sterile conditions in Cole-type culture flasks (25 cm2, Nunclon Easy Flask), using cell growth media (RPMI 1460) supplemented with 5% fetal calf serum (FCS), 0.1% penicillin–streptomycin, and 0.1% glutamine. The culture flasks were kept in an incubator at constant humidified atmosphere, temperature (37 °C), and CO2 level (5%). The cells passage was performed by enzymatic methods using Trypsin.
3.3.2. Cell Treatment and Cytotoxicity Evaluation
For cytotoxicity evaluation, the stock solutions of thiazoles 2a–p in DMSO (2000 μg/mL) were used to prepare diluted samples with the following concentrations: 0.1, 1, 6.25, 12.50, 25 and 50 µg/mL using RPMI 1460 media. The cells were placed on flat bottom 96-well micro plates for tissue culture (ca. 10.000 cells/well) and cultured in complete medium as described above (200 μL).
After 24 h, the solutions of thiazoles 2a–p were added separately to each well.
After treatment (24 h) with thiazole derivatives, the culture medium was removed from the wells, without disturbing the attached cells, and 60 μL of MTT-Hanks media solution was added to each well. After incubating the plates for 2 h at 37 °C, the MTT solution was removed, and the formazan crystals were solubilized by adding DMSO (100 μL).
The 96-well plates were measured with a multimode microplate reader, by monochromator-based absorbance detection at 570 nm wavelength. The optical density, quantified by colorimetric measurements, is directly proportional with the amount of formazan crystals formed in the cells and it is an indicator of the cellular viability.
Untreated cells were used as reference for cell proliferation. For each compound, reagent blank (media and MTT) and color control (wells containing media and thiazole derivatives solution, without cells) were used. For Positive control, two antineoplastic drugs, cisplatin and oxaliplatin, were used in the same concentrations as the studied compounds. All experiments were performed in triplicate.
3.4. DNA Electrophoresis Tests
The plasmid DNA was purified from an overnight culture of Escherichia coli DH5α cells using the DNA-spin™ Plasmid DNA Purification Kit (ThermoScientific).
The DMSO solutions of thiazoles 2a, 2e, 2h (1 mM) and 2i (2 mM) were mixed in different ratios (2, 4, 8, 10 µL) with closed circular plasmid DNA pTZ57R, (1 µL, 540 ng), resulting in nucleotide/thiazoles ratios of 1:1.2, 1:2.6, 1:1.5, 1:6.5 for compounds 2a, 2h, 2e and 1:2.6; 1:5.2; 1:10; 1:13 for compound 2i. The mixtures were loaded into agarose gel 1% in TAE buffer (40 mM tris-acetate, 1 mM EDTA, pH 8.0). After migration, the gels were stained for 30 min in water containing ethidium bromide (2 μg/mL), according to standard procedures [23].
4. Conclusions
A series of arylidene-hydrazinyl-thiazole derivatives 2a–p were synthesized with good yields by the Hantzsch protocol and their structures confirmed by NMR spectroscopy and mass spectrometry. The in vitro cytotoxicity was evaluated for all thiazoles 2a–p on two carcinoma cell lines, MDA-MB231 and HELA. An excellent inhibition of cancer cells proliferation was reported for five thiazole derivatives. Among them, 2-(2-benzyliden-hydrazinyl)-4-methylthiazole 2a exhibited a significant antiproliferative activity on both MDA-MB-231 (IC50: 3.92 µg/mL) and HeLa (IC50: 11.4 µg/mL) cell lines, while 2-[2-(4-methoxybenzylidene) hydrazinyl]-4-phenylthiazole 2e showed a similar cytotoxic effect (IC50 value 11.1 µg/mL) on the HeLa cell line. It was also shown that these thiazole derivatives do not interact with plasmid DNA.
Acknowledgments
This work was supported by the Swiss Enlargement Contribution in the framework of the Romanian-Swiss Research Programme, project number IZERZO-142198/1.
Author Contributions
Adriana Grozav and Ovidiu Crisan performed the synthesis. Valentina Pileczki, Ioana Berindan-Neagoe and Adriana Grozav performed the in vitro cytotoxicity tests. Luiza Ioana Gaina contributed with the spectroscopic, spectrometric and electrophoresis data. Luminita Silaghi-Dumitrescu, Bruno Therrien and Valentin Zaharia designed the research, supervised the elaboration of the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Makam P., Thakur P.K., Kannan T. In vitro and in silico antimalarial activity of 2-(2-hydrazinyl)thiazole derivatives. Eur. J. Pharm. Sci. 2014;52:138–145. doi: 10.1016/j.ejps.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B.L., Song L.X., Li Y.F., Li Y.L., Guo Y.Z., Zhang E., Liu H.M. Synthesis and biological evaluation of dehydroepiandrosterone-fused thiazole, imidazo[2,1-b]thiazole, pyridine steroidal analogues. Steroids. 2014;80:92–101. doi: 10.1016/j.steroids.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Carbone A., Pennati M., Parrino B., Lopergolo A., Barraja P., Montalbno A., Spanò V., Sbarra S., Doldi V., de Cesare M., et al. Novel 1H-pyrrolo[2,3-b]pyridine derivatives nortopsentin analogues: synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013;56:7060–7072. doi: 10.1021/jm400842x. [DOI] [PubMed] [Google Scholar]
- 4.Diana P., Carbone A., Barraja P., Montalbano A., Parrino B., Lopergolo A., Pennati M., Zaffaroni N., Cirrincione G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem. 2011;6:1300–1309. doi: 10.1002/cmdc.201100078. [DOI] [PubMed] [Google Scholar]
- 5.Carbone A., Pennati M., Barraja P., Montalbano A., Parrino B., Spanò V., Lopergolo A., Sbarra S., Doldi V., Zaffaroni N., et al. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]piridine, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014;21:1654–1666. doi: 10.2174/09298673113206660307. [DOI] [PubMed] [Google Scholar]
- 6.Desai N.C., Bhatt N., Somani H., Trivedi A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1,3,4-oxadiazoles. Eur. J. Med. Chem. 2013;67:54–59. doi: 10.1016/j.ejmech.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helal M.H.M., Salem M.A., El-Gaby M.S.A., Aljahdali M. Synthesis and biological evaluation of some novel thiazole compounds as potential anti-inflammatory agents. Eur. J. Med. Chem. 2013;65:517–526. doi: 10.1016/j.ejmech.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Izawa K., Onishi T. Industrial Syntheses of the Central Core Molecules of HIV Protease Inhibitors. Chem. Rev. 2006;106:2811–2827. doi: 10.1021/cr050997u. [DOI] [PubMed] [Google Scholar]
- 9.Lopes R.M., Schwartsmann G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001;1:364–369. doi: 10.1016/s1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 10.Jayarama N.H., Pillwein K., Craig R.N., Hoffman R., Webe G. Selective sensitivity to tiazofurin of human leukemic cells. Biochem. Pharmacol. 1986;35:2029–2032. doi: 10.1016/0006-2952(86)90737-9. [DOI] [PubMed] [Google Scholar]
- 11.Lednicer D., Mitscher L.A., George G.I. Organic Chemistry of Drug Synthesis. Volume 4. Wiley; New York, NY, USA: 1990. pp. 95–97. [Google Scholar]
- 12.Simiti I., Coman M. Contribution à l’étude de quelques hétérocycles VIII. Condensation de la dichloroacetone symetrique avec des thiosemicarbazones et le comportement chimiques des produits résultés. Bull. Soc. Chim. Fr. 1969;9:3276–3281. [Google Scholar]
- 13.Simiti I., Coman M. Contribution à l’étude de quelques hétérocycles XX. La condensation de quelques thiosemicarbazones avec l’ester γ-bromacétylacétique. Ann. Chim. 1972;7:31–38. [Google Scholar]
- 14.Ignat A., Lovasz T., Vasilescu M., Fischer-Fodor E., Tatomir C.B., Cristea C., Silaghi-Dumitrescu L., Zaharia V. Heterocycles 27. Microwave Assisted Synthesis and Antitumour Activity of Novel Phenothiazinyl-Thiazolyl-Hydrazine Derivatives. Arch. Pharm. Chem. Life Sci. 2012;345:574–583. doi: 10.1002/ardp.201100355. [DOI] [PubMed] [Google Scholar]
- 15.Zaharia V., Ignat A., Palibroda N., Ngameni B., Kuete V., Fokunang C.N., Moungang M.L., Ngadjui B.T. Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and hydrazino-bisthiazoles and their anticancer activity. Eur. J. Med. Chem. 2010;45:5080–5085. doi: 10.1016/j.ejmech.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Venzke D., Flores A.F.C., Quina F.H., Pizzuti L., Pereira C.M.P. Ultrasound promoted greener synthesis of 2-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazoles. Ultrason. Sonochem. 2011;18:370–374. doi: 10.1016/j.ultsonch.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D.N., Li J.T., Song Y.L., Liu H.M., Li H.Y. Efficient one-pot three-component synthesis of N-(4-arylthiazol-2-yl) hydrazones in water under ultrasound irradiation. Ultrason. Sonochem. 2012;19:475–478. doi: 10.1016/j.ultsonch.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Maccioni E., Cardia M.C., Distinto S., Bonsignore L., de Logu A. An investigation of the biological effect of structural modifications of isothiosemicarbazones and their cyclic analogues. Farmaco. 2003;58:951–959. doi: 10.1016/S0014-827X(03)00154-X. [DOI] [PubMed] [Google Scholar]
- 19.Denisova A.B., Andronnikova G.P. Bromination of 4-substitutedThiazolylhydrazones. Chem. Heterocycl. Compd. 1995;31:863–867. [Google Scholar]
- 20.Parameshwar M., Ramakrishna K., Amresh P., Tharanikkarasu K. 2-(2-Hydrazinyl)thiazole derivatives: Design, synthesis and in vitro antimycobacterial studies. Eur. J. Med. Chem. 2013;69:564–576. doi: 10.1016/j.ejmech.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 21.Ceballos-Torres J., Virag P., Cenariu M., Prashar S., Fajardo M., Fischer-Fodor E., Gómez-Ruiz S. Anti-cancer applications of titanocene-functionalised nanostructured systems: An insight into Cell Death Mechanisms. Chem. Eur. J. 2014;20:10811–10828. doi: 10.1002/chem.201400300. [DOI] [PubMed] [Google Scholar]
- 22.Alam M.S., Liu L., Lee Y.E., Lee D.U. Synthesis, antibacterial activity and quantum-chemical studies of novel 2-arylidenehydrazinyl-4-arylthiazole analogues. Chem. Pharm. Bull. 2011;59:568–573. doi: 10.1248/cpb.59.568. [DOI] [PubMed] [Google Scholar]
- 23.Ausubel F.M., Brent R., Kingston R.E., Moore D.M., Seidman J.G., Smith J.A., Struhl K. Short Protocols in Molecular Biology. 5th ed. Volume 1 Wiley; New York, NY, USA: 2002. [Google Scholar]