Abstract
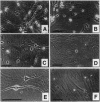
Neuronal precursor cells persist in the adult forebrain ependymal/subependymal zone (SZ) and have been found to produce neurons in cultures derived from birds, rodents, and humans. We postulated that the survival of neurons generated from these cells might be constrained in adulthood by the local absence of trophic support. To test this hypothesis, we established explant cultures of adult rat forebrain SZ and assessed the effect of defined neurotrophins on the survival of new neurons arising from these explants. We found that microtubule-associated protein 2+ neurons arose from explants derived from a wide area of the SZ, spanning the rostral 6 mm of the ventricular system. In cultures exposed to brain-derived neurotrophic factor (BDNF), > 35% of new neurons survived at 22 days in vitro (DIV), and > 25% survived at 42 DIV, concurrent with the virtually complete loss of neurons in unsupplemented controls. The surviving cells expressed trkB, the high-affinity receptor for BDNF. In contrast, neither nerve growth factor nor neurotrophic factor 3 enhanced neuronal survival. Thus, BDNF supports the survival of neurons produced by the adult rat forebrain and may act as a permissive factor for neuronal recruitment in adulthood.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Das G. D. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966 Mar;126(3):337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Bansal R., Warrington A. E., Gard A. L., Ranscht B., Pfeiffer S. E. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989 Dec;24(4):548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Barami K., Kirschenbaum B., Lemmon V., Goldman S. A. N-cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron. 1994 Sep;13(3):567–582. doi: 10.1016/0896-6273(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Bernhardt R., Huber G., Matus A. Differences in the developmental patterns of three microtubule-associated proteins in the rat cerebellum. J Neurosci. 1985 Apr;5(4):977–991. doi: 10.1523/JNEUROSCI.05-04-00977.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992 Oct;9(4):583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Corotto F. S., Henegar J. A., Maruniak J. A. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993 Jan 12;149(2):111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Carnahan J., Greenberg M. E. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994 Mar 18;263(5153):1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Yancopoulos G. D. The neurotrophins and their receptors. Trends Cell Biol. 1993 Aug;3(8):262–268. doi: 10.1016/0962-8924(93)90054-5. [DOI] [PubMed] [Google Scholar]
- Goldman S. A., Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A., Zaremba A., Niedzwiecki D. In vitro neurogenesis by neuronal precursor cells derived from the adult songbird brain. J Neurosci. 1992 Jul;12(7):2532–2541. doi: 10.1523/JNEUROSCI.12-07-02532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C., Juhasz M., Jackson C., Wright P., Ip N. Y., Lindsay R. M. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994 Jan;14(1):335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. S. Formation and turnover of neurons in young and senescent animals: an electronmicroscopic and morphometric analysis. Ann N Y Acad Sci. 1985;457:173–192. doi: 10.1111/j.1749-6632.1985.tb20805.x. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B., Nedergaard M., Preuss A., Barami K., Fraser R. A., Goldman S. A. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994 Nov-Dec;4(6):576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Lois C., Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994 May 20;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Marsh H. N., Scholz W. K., Lamballe F., Klein R., Nanduri V., Barbacid M., Palfrey H. C. Signal transduction events mediated by the BDNF receptor gp 145trkB in primary hippocampal pyramidal cell culture. J Neurosci. 1993 Oct;13(10):4281–4292. doi: 10.1523/JNEUROSCI.13-10-04281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead C. M., van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992 Jan;12(1):249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards L. J., Kilpatrick T. J., Bartlett P. F. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. P., Read J., Price J. The generation of neurons and oligodendrocytes from a common precursor cell. Neuron. 1991 Oct;7(4):685–693. doi: 10.1016/0896-6273(91)90381-9. [DOI] [PubMed] [Google Scholar]