Abstract
A cancer immunotherapy strategy is described herein that combines the advantage of the well established tumor targeting capabilities of high-affinity recombinant fragments of Abs with the known efficient, specific, and potent killing ability of CD8 T lymphocytes directed against highly antigenic MHC-peptide complexes. Structurally, it consists of a previously uncharacterized class of recombinant chimerical molecules created by the genetic fusion of single-chain (sc) Fv Ab fragments, specific for tumor cell surface antigens, to monomeric scHLA-A2 complexes containing immunodominant tumor- or viral-specific peptides. The fusion protein can induce very efficiently tumor cell lysis, regardless of the expression of self peptide-MHC complexes. Moreover, these molecules exhibited very potent antitumor activity in vivo in nude mice bearing preestablished human tumor xenografts. These in vitro and in vivo results suggest that recombinant scFv-MHC-peptide fusion molecules could represent an approach to immunotherapy, bridging Ab and T lymphocyte attack on cancer cells.
Current cancer immunotherapy strategies typically employ two arms of the natural immune system: humoral and cellular. In the first, systemic injection of high-affinity mAbs directed against cell surface tumor-associated antigens has demonstrated statistically significant antitumor activities in clinical trials (1-3). Antitumor Abs that carry effector payloads such as toxins (immunotoxins) or cytokines are also potent molecules currently being tested in various clinical trials (4, 5). The second major approach for specific cancer immunotherapy consists of the potentiation of the cellular arm of the immune system, mainly through CD8+ cytotoxic T lymphocytes (CTLs). Two major strategies are currently being used: (i) active immunization of patients with antigens known to be recognized by T lymphocytes and to activate them (6-8) and (ii) adoptive transfer therapies that enable the selection and activation of highly reactive T cell subpopulations with improved antitumor activities (9). Clinical studies using MHC tetramer staining have demonstrated T lymphocyte responses against the immunizing tumor antigens in the course of vaccination. However, these promising clinical trials using active immunization have suffered from a relatively low percentage of tumor remissions and a lack of correlation between clinical and T lymphocyte responses to the vaccine (9, 10). Furthermore, in using this approach there is the potential risk of selecting tumor cell variants that have undergone HLA loss (11). The adoptive transfer approach has recently demonstrated impressive results (12, 13). Regression of metastatic melanoma was reported in patients undergoing adoptive transfer protocols with highly selected tumor-reactive T cells directed against overexpressed self-derived differentiation antigens after a nonmyeloablative conditioning regimen (12, 13). The efficiency of such T cell-based immunotherapy approaches may be limited by the absence or low expression of either MHC molecules or their associated antigenic peptides displayed by tumor cells (11). The cancer immunotherapy strategy described here combines the advantage of the well established tumor targeting properties of antitumor Abs and their recombinant fragments [such as single-chain (sc) Fv and Fab] with the known efficient, specific, and potent cytotoxic activity of CD8 T lymphocytes directed against highly antigenic MHC-peptide complexes. In this approach, scFv fragments are genetically fused to a scMHC class I molecule containing a selected antigenic peptide, to target the active MHC-peptide complex on tumor cells and induce their lysis by specific CTLs. Here we present an in vivo demonstration that the systemic injection of tumor-specific scFv-MHC-peptide recombinant fusion protein can induce appreciable regression of established tumor grafts in a model of s.c. transplanted carcinoma by the recruitment of tumor- or viral-specific human CTLs.
Materials and Methods
Plasmid Constructions. The scMHC molecule was constructed as previously described by linking human β2-microglobulin with the three extracellular domains of the HLA-A2 gene (14). The VL and VH variable domain genes of anti-Tac mAb and the SS1 Fv were constructed to form a scFv construct in which the two variable domains are connected by a 15-residue flexible linker (Gly-4-Ser)3. To generate the scHLA-A2-aTac(scFv) and scHLA-A2-SS1(scFv) expression constructs, we fused the C terminus of the scHLA-A2 molecule to the N terminus of the anti-Tac scFv or the SS1scFv genes, respectively, using a two-step PCR overlap extension reaction.
Cytotoxicity Assays. Target cells were cultured in 96-well plates (2-5 × 103 cells per well) in RMPI medium 1640 plus 10% FCS. The cells were washed and incubated with methionine and serum-free medium for 4 h and then incubated overnight with 15 μCi/ml (1 Ci = 37 GBq) [35S]methionine (NEN). After3hofincubation with scHLA-A2/scFv fusion molecules (10-20 μg/ml or the indicated concentration at 37°C), effector CTL cells were added at a target:effector ratio as indicated and incubated for 8-12 h at 37°C. After incubation, [35S]methionine release from target cells was measured in a 50-μl sample of the culture supernatant. All assays were performed in triplicate.
In Vivo Antitumor Assays. BALB/c Nude mice (five to seven per group) were injected s.c. with ATAC 4 or A431K5 cells (3 × 106). When tumors had developed (≈50 mm3), mice (five to seven per group) were injected i.v. with 100-150 μg of scHLA-A2/scFv fusion molecule as indicated and 6 h later with 3 × 106 of the appropriate CTLs i.v. or i.t. The injection protocol was repeated three times (every other day). Tumor size was measured every second day and compared with mice without treatment or with mice that had been injected with 3 × 106 CTLs i.v. or i.t. alone without the scHLA-A2/scFv fusion.
Results
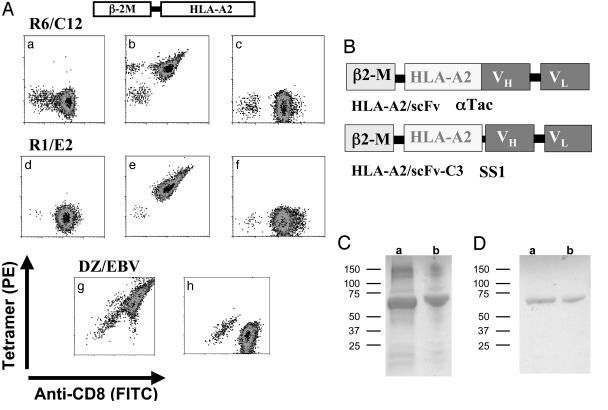
Construction and Characterization of the scHLA-A2/scFv Fusion Molecule. To generate genetic fusions between tumor-specific recombinant Ab fragments and class I MHC-peptide complexes, we used a functional scHLA-A2 construct in which the human β2-microglobulin gene is covalently linked to the three extracellular domains (α1,α2, and α3) of the HLA-A2 heavy chain gene (amino acids 1-275) through a 15-aa flexible linker (14, 15). Most importantly, this scHLA-A2 was able to bind, stain, and activate tumor- or viral-specific CTL lines or clones. As shown in Fig. 1A, scHLAA2-peptide complexes generated in the form of tetramers with melanoma differentiation antigen gp100 (16) or an Epstein-Barr virus (EBV)-derived peptide epitope (17) could stain specifically and with high avidity the corresponding T cells (Fig. 1A). The scHLA-A2-peptide tetramers could also induce T cell activation, as monitored by the peptide-specific release of IFN γ from these T cell clones/lines (data not shown). These data demonstrate that the engineered scHLA-A2-peptide complex is functional and can bind T cells as efficiently as the native cell surface-expressed molecule.
Fig. 1.
Design, expression, and purification of scHLA-A2/scFv fusion molecules. (A) Binding of in vitro refolded scHLA-A2-peptide complexes to CTLs. Melanoma differentiation antigen gp100-specific CTL clones R6C12 and R1E2 or the EBV-specific CTL line DZ/EBV were reacted with in vitro refolded purified scHLA-A2-peptide tetramers containing the G9-209M epitope recognized by R6C12, the G9-280V peptide recognized by R1E2, or the EBV-derived BMLF1 protein epitope GLC280-288 recognized by DZ/EBV CTLs. CTLs were stained with FITC-anti-CD8 (a and d), with phycoerythrin-labeled scHLA-A2-G9-209M tetramers (b, f, and h), with scHLA A2-G9-280V tetramers (c and e), or with scHLA-A2-EBV tetramers (g). R6C12, R1E2, and DZ/EBV CTLs were stained with the specific G9-209M, G9-280V, or EBV tetramers, respectively, but not with the control tetramer. (B) Design of scHLA-A2/scFv fusion. (C) SDS/PAGE analysis of inclusion bodies of scHLA-A2/aTac(scFv) (a) or scHLA A2/SS1(scFv) (b). (D) SDS/PAGE analysis of scHLA-A2/aTac(scFv) (a) or scHLA-A2/SS1(scFv) (b) folded around the G9-209M peptide after ion-exchange purification.
Next, to generate the hybrid molecule between the scHLA-A2 and tumor-specific scFvs that target the scMHC molecule to cells through an Ab Fv fragment, we fused at the C terminus of the HLA-A2 gene, by PCR overlap extension reaction, a scFv gene that encodes the heavy and light chain variable domains that are linked by a flexible peptide linker (Fig. 1B).
We used two scFv Ab fragments as a model system: one representing a target antigen for hematological malignancies that is derived from the humanized anti-CD25 (also known as Tac, p55, and IL-2 receptor α-subunit) mAb anti-Tac (affinity of the anti-Tac scFv is 1 nM) (18). The second is a scFv Ab fragment, termed SS1, which recognizes mesothelin, a tumor-specific antigen overexpressed on ovarian cancer cells [affinity of the SS1 scFv is 0.7 nM (19)], and which can be used as a model system with solid tumors.
The scHLA-A2/αTac(scFv) and scHLA-A2/SS1(scFv) fusion proteins were expressed in Escherichia coli BL21 cells and, upon induction with isopropyl β-D-thiogalactoside, large amounts of recombinant protein accumulated in intracellular inclusion bodies. SDS/PAGE analysis of isolated and purified inclusion bodies revealed that recombinant fusion proteins, with the correct size, constituted 80-90% of the total protein in the inclusion bodies (Fig. 1C). The inclusion bodies were isolated, solubilized, reduced, and refolded in vitro in the presence of HLA-A2-restricted peptides derived from the melanoma differentiation antigen gp100 T cell epitopes G9-209M and G9-280V (16) or the EBV-derived peptide GLC280-288 (GLCTLVAML) derived from the EBV BMLF1 protein (17, 20). ScHLA-A2/scFv fusion molecules, containing the specific peptide used to stabilize the HLA-A2 complex, were purified from the refolding solution by ion-exchange chromatography by using Q-Sepharose columns. As shown in Fig. 1D, non-reducing SDS/PAGE analysis of peak fractions revealed the presence of monomeric scHLA-A2/scFv molecules with the correct molecular mass of ≈67 kDa.
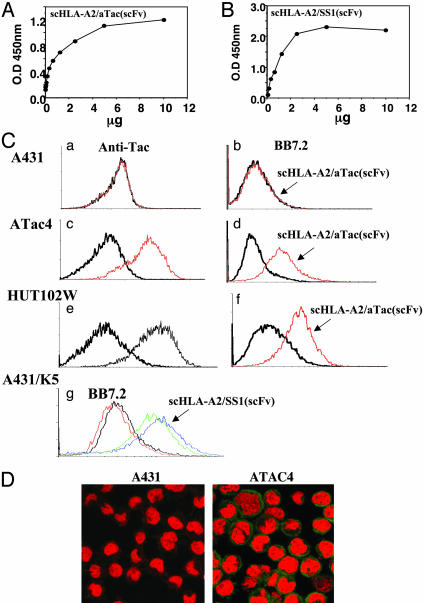
Binding of scHLA-A2/scFv to Target Cells. To first confirm the correct folding and functional binding of the fusion protein, we tested the ability of the scHLA-A2/scFv to bind its target antigen, namely the α-subunit of the IL-2 receptor (p55) and mesothelin for the scHLA-A2/aTac(scFv) or scHLA-A2/SS1(scFv), respectively. To monitor this binding, we used the mAb W6/32, which recognizes HLA molecules only when folded correctly and which contains peptide. As shown in Fig. 2, scHLA-A2/aTac(scFv) (Fig. 2A) and scHLA-A2/SS1(scFv) (Fig. 2B) bound in a dose-dependent and saturable manner to p55 and mesothelin, respectively, which suggests that the two functional domains of the molecule, the scHLA-A2 effector domain and the Ab scFv targeting domain, are folded correctly.
Fig. 2.
Binding of scHLA-A2/scFv fusion molecules to target antigens. (A and B) scHLA-A2/aTac(scFv) or scHLA-A2/SS1(scFv) folded around the G9-209M or EBV peptide, respectively, was tested for dose-dependent binding to recombinant purified p55 (Tac, IL-2 receptor α-subunit) (A) or mesothelin (B). Binding was monitored with anti-HLA mAb W6/32. (C) Flow cytometry analysis of the binding of scHLA-A2/aTac(scFv) folded around the G9-209M peptide to antigen-positive HLA-A2-negative cells. The binding of human anti-Tac mAb to A431 (a), ATAC4 (c), and HUT102W leukemic (e) cells monitored the expression of p55 (Tac). ATAC4 are A431 cells stably transfected with the Tac/p55 IL-2 receptor α-subunit. Control cells with secondary Ab are shown; HLA-A2 expression on these cells was monitored before and after incubation with the scHLA-A2/aTac(scFv) fusion (b, d, and f). The conversion of these targets from HLA-A2 negative to positive due to the binding of the fusion molecule is shown. In g, mesothelin-expressing A431/K5 cells were tested for binding of BB7.2 before and after incubation with the scHLA-A2/SS1(scFv) fusion refolded around the EBV peptide. Two versions of the fusion with or without a 5-aa connector between the scHLA-A2 and the scFv gene (see Fig. 1B) are shown. The scHLA-A2/SS1(scFv) with the connector was slightly but significantly better in binding and thus was selected for further analysis. (D) Confocal microscopy detection of the binding of scHLA-A2/aTac(scFv) fusion folded around the G9-209M peptide to p55-positive ATAC4 but not to Tac-negative A431 cells. Detection was with anti-HLA-A2 mAb BB7.2 and FITC-labeled secondary Ab.
To test the ability of the scHLA-A2/scFv fusion molecules to coat and target HLA-A2-peptide complexes on tumor cells, using flow cytometry we tested their binding to cells that express the specific target antigen. As a model we used target cells that are HLA-A2-negative so that the binding could be monitored easily by the use of anti-HLA-A2 Ab. In such case we were able to simulate the extreme case in which a target tumor cell lost its HLA-A2 expression, thus rendering it unsusceptible to HLA-A2-restricted CTL killing. For the HLA-A2/aTac(scFv) molecule, we first used A431 human epidermoid carcinoma cells that were stably transfected with the p55 gene (ATAC4 cells), and then we compared the staining of transfected versus nontransfected parental cells. The binding of the HLA-A2/aTac(scFv) molecule to the cells was monitored by using an anti-HLA-A2 mAb BB7.2 and FITC-labeled secondary Ab. The expression of the p55 target antigen was detected by the whole anti-Tac mAb from which the scFv fragment was derived. As shown in Fig. 2Ca, A431 cells do not express p55; however, the p55-transfected ATAC4 cells express high levels of the antigen (Fig. 2Cc). Neither cell line was HLA-A2-positive (Fig. 2C b and d). However, when ATAC4 but not A431 cells were preincubated with the scHLA-A2/aTac(scFv) molecule refolded around a gp100-derived peptide, they produced positive anti-HLA-A2 staining, indicating that they were coated with HLA-A2-peptide complexes through scFv Ab-mediated targeting (Fig. 2C b and d). Next, we tested the binding of scHLA-A2/aTac(scFv) to the HUT102W leukemic cells, which, as shown, express the p55 antigen, as detected by anti-Tac (Fig. 2Ce) but lack HLA-A2 expression (Fig. 2Cf). As shown in Fig. 2Cf, the adult T cell leukemia HUT102W cells expressing p55 produced positive anti-HLA-A2 staining only when preincubated with the HLA-A2/aTac(scFv) molecule. Similar results were observed with adult T cell leukemia p55-positive, HLA-A2-negative CRII-2 cells (data not shown). Similar assays were performed on HLA-A2-negative A431 cells that were stably transfected with mesothelin (termed A431/K5). As shown in Fig. 2Cg, positive staining with anti-HLA-A2 mAb BB7.2 was observed when these cells were preincubated with scHLA-A2/SS1(scFv) molecules containing either gp100- or EBV-derived peptides.
The binding of scHLA-A2/aTac(scFv) to CD25+ ATAC4 cells was also monitored by confocal microscopy. As shown in Fig. 2D, these cells, but not parental A431, were intensely stained with BB7.2, indicating that they were coated with HLA-A2 complexes by means of the anti-Tac scFv targeting moiety of the HLA-A2/scFv fusion molecule. These results demonstrate that the scHLA-A2/scFv fusion molecules can be used to coat HLA-A2-negative cells in a manner that depended on the specificity of the tumor-targeting Ab fragment, rendering them HLA-A2-positive cells.
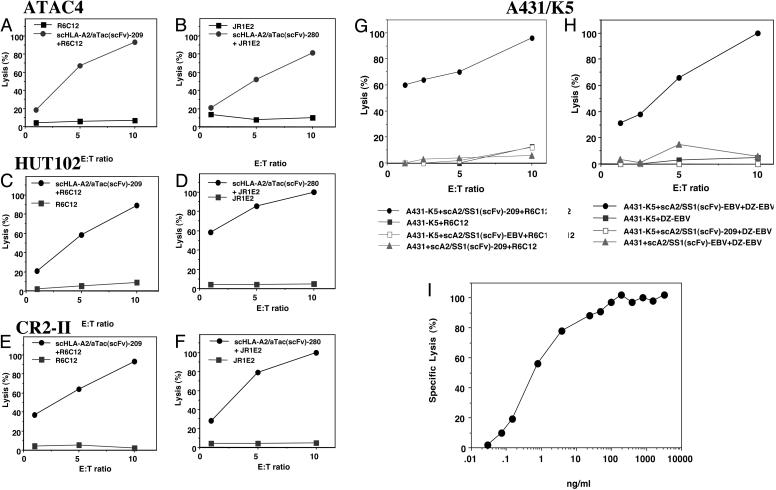
Potentiation of CTL-Mediated Killing by scHLA-A2/scFv. To test the ability of the scHLA-A2/scFv molecule to potentiate the susceptibility of HLA-A2-negative cells to CTL-mediated killing, first we incubated radiolabeled target cells with scHLA-A2/aTac(scFv) or scHLA-A2/SS1(scFv) refolded around gp100- or EBV-derived peptides, and then we tested them in a [35S]methionine-release assay in the presence of HLA-A2-restricted melanoma gp100- or EBV-peptide-specific CTLs. As shown in Fig. 3 A-F, scHLA-A2/aTac(scFv) induced efficiently CTL-mediated lysis, which reached 100% killing of CD25-positive, HLA-A2-negative ATAC4, HUT102, and CR2-II cells. When these cells were incubated with CTLs alone, without prior coating with the fusion protein, no cytotoxicity was observed (Fig. 3 A-F). A431 cells that do not express CD25 were not lysed (data not shown). To demonstrate the specificity of scHLA-A2/aTac(scFv)-mediated CTL killing for the HLA-A2-restricted antigenic peptide used in the refolding of the fusion molecule, we used two CTL clones specific for the gp100 major T cell epitopes G9-209M and G9-280V. As shown in Fig. 3 A, C, and E, CD25-positive, HLA-A2-negative ATAC4, HUT102, or CR2-II cells that were coated with scHLA-A2/aTac(scFv) folded around the G9-209M peptide were lysed by the G9-209M-specific CTL clone R6C12 but not with R6C12 CTLs alone. Similar results were observed when these cells were tested with a scHLAA2/scFv molecule folded around the G9-280V epitope and the JR1E2 CTL clone specific for the G9-280V peptide (Fig. 3 B, D, and F). These studies were expanded to include other scHLA-A2/scFv fusions folded around control tumor or viral epitopes, and a specific cytotoxicity was observed only when the correct combination of peptide and CTL specificities were used (data not shown). Similar results demonstrating the potentiation of CTL-mediated killing after coating with the scHLA-A2/scFv fusion molecule were observed on HLA-A2-positive cells (data not shown). Next, we tested in similar [35S]methionine release assays the activity of scHLA-A2/SS1(scFv) on mesothelin-positive, HLA-A2-negative A431/K5 cells. As shown in Fig. 3 G and H, this fusion molecule induced very efficient killing of target cells when folded around a tumor-derived peptide (G9-209M; see Fig. 3G) or a viral-derived peptide (EBV; see Fig. 3H).
Fig. 3.
Potentiation of CTL-mediated lysis of HLA-A2-negative tumor cells by the scHLA-A2/scFv fusion molecule. CD25-transfected ATAC4 or CD25-positive leukemic HLA-A2-negative cells coated or not coated with the scHLA-A2/aTac(scFv) fusion molecule folded around the G9-209M (A, C, and E) or G9-280V (B, D, and F) gp100-derived peptides were incubated with melanoma-reactive gp100-peptide-specific CTL clones R6C12 or JR1E2, respectively, in a [35S]methionine release assay. Mesothelin-expressing A431/K5 cells were coated or not coated with a scHLA-A2/SS1(scFv) fusion molecule folded around the gp100-derived G9-209M peptide (G) or EBV peptide (H) and incubated with the R6C12 G9-209M-specific CTL clone or EBV-specific line, respectively. A431/K5 cells incubated with scHLA-A2/SS1(scFv) folded around the EBV (G) or G9-209M (H) peptides were not killed by R6C12 G9-209M-specific CTLs or DZ/EBV EBV-specific CTLs, respectively. Insensitivity of scHLA-A2/SS1(scFv)-coated A431 cells is also shown as the control. (I) Dose-dependent activity of the scHLA-A2/SS1(scFv) molecule folded around the EBV peptide on A431/K5 cells using EBV-specific CTLs.
A431/K5 cells were not lysed when scHLA-A2/scFv fusions carrying the G9-209M epitope or the EBV epitope were used with EBV- or G9-209M-specific CTLs, respectively (Fig. 3 G and H), indicating the CTL-dependent specificity induced by the scHLAA2/scFv fusion molecule. The CTL-mediated killing was very specific and potent. As shown, a 70-100% killing of target cells was observed at a very low target:effector ratio of 1:5-1:10. These results clearly demonstrate, in vitro, the notion that the scHLAA2/scFv fusion can be used efficiently for Ab-guided, antigen-specific tumor targeting of MHC-peptide complexes on tumor cells to render them susceptible or more receptive to lysis by relevant CTLs and, thus, potentiate antitumor immune responses. We have also shown that virus-specific CTLs can be targeted to tumor cells by coating target cells with scHLA-A2/scFv fusions that are folded around a very immunogenic viral epitope. Moreover, the results presented in Fig. 3 G and H also demonstrate the specificity of the T cell response, in that only T cells specific for the MHC-peptide complex within the fusion Ab kill the target cells.
To determine the potency of the scHLA-A2/scFv molecule in mediating efficient CTL killing, we performed dose-dependent titration assays. Mesothelin-expressing A431/K5 cells were incubated with increasing concentrations of scHLA-A2/SS1(scFv) folded around the EBV peptide, and lysis with EBV-specific CTL was determined. As shown in Fig. 3I, an efficient dose-dependent lytic activity was observed, which was saturated at a concentration of 100 ng/ml. The IC50 of the scHLA-A2/SS1(scFv) molecule was 0.5 ng/ml (7 pM), which demonstrates the highly efficient and potent CTL-mediated killing induced by this targeting strategy. Similar sensitivity and activity were observed with the scHLA-A2/aTac(scFv) molecule (data not shown).
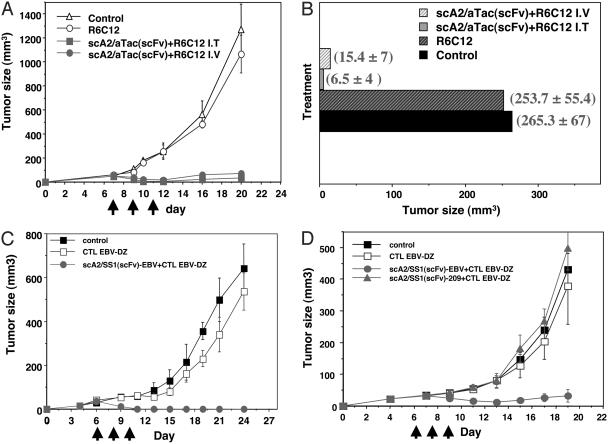
Antitumor Activity of scHLA-A2/scFv. To evaluate the in vivo activity of scHLA-A2/scFv fusion molecules in a human tumor model, we performed antitumor activity assays in nude mice bearing preestablished human tumor xenografts generated with ATAC4 and A431/K5 cells that express CD25 and mesothelin, respectively. As shown in Fig. 4 A and B, marked regressions of tumors were observed in tumor-bearing mice that were treated with the scHLAA2/scFv fusion molecule and human CTLs, compared with the controls, which received CTL alone or no treatment.
Fig. 4.
Antitumor activity of scHLA-A2/scFv fusion molecules in nude mice model of human tumor xenografts. (A and B) ATAC4 cells (3 × 106) were injected s.c. into nude mice, and 4-7 days after injection ≈40- to 50-mm3 tumors were generated. Mice were injected i.v. three times every other day with 100 μg of purified scHLA A2/aTac(scFv) folded around the gp100-derived G9-209M peptide. Five to 6 h after fusion molecule injection, gp100 G9-209M R6C12-specific CTLs (2-3 × 106) were injected i.v. or i.t. In B, the mean tumor size of treated and control groups is shown. (C and D) A431/K5 cells (3 × 106) were injected as above. Mice were injected i.v. three times every other day with 100 μg of purified scHLA-A2/SS1(scFv) folded around the EBV-derived peptide. Five to 6 h after fusion molecule injection, EBV-specific CTLs (2-3 × 106) were injected i.t. (C) or i.v. (D). Tumor size was measured every 2 days, and tumor volume was calculated. The days of treatment are indicated.
Regarding the ATAC4 tumors (Fig. 3A), 8 of 10 mice treated with i.t.-injected CTLs had a complete regression of their tumors, and 2 had a 90% regression of their tumors. In the group treated with i.v. CTLs, 4 had complete regressions and 6 had significant regressions of >70% (in a group of 10 treated mice). Most significant are the antitumor effects observed on ATAC4 tumors on day 10 (Fig. 4B), in which the average size of the tumors in the control groups receiving CTL alone or no treatment was 253-265 mm3, whereas the treated group, with either i.v. or i.t. injection of CTLs, had an average tumor size of 6.5-15.4 mm3, an average of a 34-fold reduction in tumor size when the CTLs were injected i.v., and an 80-fold reduction when injected i.t. Similar studies were performed in nude mice with A431/K5 tumor xenografts. As shown in Fig. 4 C and D, marked regressions in tumors were observed when these mice were treated i.v. with the scHLA-A2/SS1(scFv) fusion molecule folded around the EBV epitope and when EBV-specific CTLs were used i.t. (Fig. 4C) or i.v. (Fig. 4D) to treat the mice. In the group receiving the CTLs i.t. (Fig. 4C) all mice exhibited a complete regression of their tumors, and when CTLs were injected i.v. (Fig. 4D), 6 of 10 mice treated had a complete regression, 3 had ≈50% regression of their tumors, and 1 did not respond to the treatment. The antitumor activity in mice was observed with both antitumor and antiviral CTLs when the scHLAA2/scFv fusion molecule was carrying a tumor or viral T cell epitope, respectively. Tumor xenografts generated with parental A431 cells were not affected by treatments consisting of scHLAA2/scFv fusions and the corresponding CTLs or CTL alone (data not shown). As shown in Fig. 4D, we also performed a specificity control group consisting of five mice receiving the i.v.-injected scHLA-A2/SS1(scFv) fusion molecule folded around the melanoma-derived G9-209M peptide and EBV-specific CTLs (as shown in vitro in Fig. 3G). Tumor growth in this control group was indistinguishable from the control mice that did not receive treatment or received CTLs alone (Fig. 4D). For initial toxicity tests, we injected i.v. a group of four BALB/c mice with 1 mg of scHLA-A2/SS1(scFv) folded around the EBV-derived peptide. No toxicities were observed after these injections. The results thus demonstrate that the scHLA-A2/scFv fusion molecule can induce tumor growth inhibition and regression of established human tumor xenografts in vivo in nude mice.
Discussion
In this study we have demonstrated that targeting an MHC-peptide/scFv fusion protein to tumor cells can function in vitro and most significantly in vivo in a human solid xenograft tumor model. The recombinant fusion molecule has the capability of bridging a scFv Ab fragment, specific for a defined antigen stable expressed on tumor cells, with a selected highly antigenic MHC-peptide complex recognized by CTLs. This strategy has two major advantages: First, it takes advantage of the use of recombinant Ab fragments that can localize on those malignant cells that express a tumor marker, usually associated with the transformed phenotype (such as growth factor receptors and/or differentiation antigens), with a relatively high degree of specificity. Second, this strategy has the ability to recruit a particular population of highly reactive cytotoxic T cells specific to a preselected, highly antigenic peptide epitope present in the targeted MHC-peptide complex, such as viral-specific T cell epitopes. This platform approach generates multiple molecules with many tumor-specific scFv fragments that target various tumor-specific antigens, combined with the ability to target many types of MHC-peptide complexes carrying single, preselected, and highly antigenic peptides derived from tumor, viral, or bacterial T cell epitopes.
Tumor progression is often associated with the secretion of immune-suppressive factors and/or the down-regulation of MHC class I antigen-presentation functions (11). Even when a specific CTL response is demonstrated in patients, this response is low because the antitumor CTL population is rare, very infrequent, and in some cases not functional or anergic (10). Moreover, it is well established that the number of MHC-peptide complexes on the surface of tumor cells that present a particular tumor-associated peptide is low. Various strategies have been used to predict or count this number to be ≈100-300 complexes per cell (21, 22). Thus, in both, low frequency of a particular tumor-associated MHC-peptide complex, or down-regulation of MHC expression on tumor cells, Ab-guided, tumor-specific targeting of class I MHC-peptide complexes onto tumor cells, as shown herein, can be an effective and efficient strategy to render cells more susceptible to lysis by the relevant, preselected, desirable HLA-A2-restricted CTLs.
The tumor-targeting recombinant Ab fragments consist of the Fv variable domains, which are the smallest functional modules of Abs necessary to maintain antigen binding. Their small size makes them especially useful for clinical applications because it improves tumor penetration. Recombinant Abs have already been used to redirect T cells by using a classical approach of bispecific Abs in which one Ab arm is directed against a tumor-specific antigen and the other arm is directed against an effector cell-associated molecule such as CD3 for CTLs and CD16 for natural killer cells (23, 24). The bispecific approach (e.g., with anti-CD3) recruits T cells independently from their specificity. However, this strategy has not led to influencing in vivo therapy results on solid tumors, and problems with systemic symptoms due to peripheral activation of T cells (usually through CD3) were observed. The major advantage of our approach over the bispecific Ab approach is the activation of T cells i.t. by the ability to target to the tumor cell surface the cognate native signal for CTL activation and recognition as well as the ability to recruit a specific selected population of cytotoxic T cells that is governed by the specificity of the peptide presented in the targeted complex. A major advantage of our approach is the use of a recombinant molecule that can be produced in a homogeneous form and in large quantities. Importantly, the molecular mass of the scHLA-A2/scFv fusion molecule is ≈65 kDa. This is an optimal size with respect to the known requirements for good tumor penetration on one hand and good pharmacokinetic properties on the other hand, which are reflected by a relatively long half-life (24-48 h) and stability in the circulation of such Ab fusion proteins (25). Another manipulation that can be applied to our MHC/scFv fusion and influence binding, tumor uptake, and activity is its avidity. Thus, a bivalent construct can be made, and the various properties should be compared to the current monovalent molecule. A recent study describing the generation of Ab MHC class I tetramers was recently published in which efficient CTL-mediated killing of tumor target cells was observed by using Fab-streptavidin-MHC tetramer conjugates (26, 27). This approach was shown to inhibit the growth of tumor cells in a tumor protection assay (win assay) in severe combined immunodeficient mice (28, 29). The limitation of this approach is the large molecular mass of these molecules (≈400 kDa) and the fact that soluble MHC tetramers can induce T cell activation themselves, whereas monomeric MHC molecules cannot induce activation unless in a relatively high local concentration (30, 31). In another recent study using a murine transgenic mouse system, in vivo targeting of an antitumor Ab conjugated to antigenic murine MHC class I complexes induced specific growth inhibition of syngeneic tumor grafts (32). Our study demonstrates a recombinant human MHC/scFv chimerical fusion protein that can efficiently target viral- or tumor-specific CTLs to tumor cells and also possesses very potent activity in vitro and in vivo on preestablished human tumor xenografts in nude mice.
The coating of tumor cells that had down-regulated their own MHC expression through the use of this targeting approach potentiates the cells for CTL-mediated killing while using a target on the tumor cells that is usually involved in the transformation process; most classical examples are growth factor receptors.
This fact also supports the notion that, by using this approach, escape mutants that lose the targeted receptor are not likely to have a growth advantage because the receptor is directly involved in the key survival functions of the cancer cells.
As for the selection of the peptide epitope for the targeted MHC-peptide complex, it has been shown that CTL precursors directed against influenza, EBV, and cytomegalovirus epitopes (peptides) are maintained at high frequencies in the circulation of cancer patients and healthy individuals, and that these CTLs are usually active and possess a memory phenotype (17, 20). Thus, these CTLs would be the source of choice to be redirected to the tumor cells through the use of a MHC-peptide/scFv fusion molecule folded around such viral-derived epitopes, which was also demonstrated in this study. Another important aspect of this study, which is supported by others (26, 27), is that the coating of antigenic MHC-peptide complexes on the surface of tumor cells without transmembrane anchoring is sufficient to induce their efficient lysis by specific CTLs without knowledge about whether autologous accessorial molecules of the target tumor cells are present at all and are playing a role in such CTL-mediated killing. This observation may result from the fact that a local high concentration of coated MHC-peptide complexes displaying one particular T cell epitope (peptide) is formed on the targeted cells, which greatly exceeds the natural density of such complexes displayed on the surface of cells. Further evidence of this possibility is that MHC tetramers can induce T cell activation by themselves (33); including our recent observation that CTL activation by MHC tetramers without accessorial molecules can be demonstrated at the single-cell level (34). Another possible mode of action of our approach is the induction of Fas/Fas ligand-mediated apoptosis.
In conclusion, the data presented herein clearly demonstrate the usefulness of this approach to recruit active CTLs for tumor cell killing through cancer-specific Ab-guided targeting of scMHC-peptide complexes. This approach may be regarded as a link between antitumor Abs and cell-mediated immunotherapy. These results pave the way for the clinical development of this immunotherapeutic approach based on naturally occurring cellular immune responses that are redirected against the tumor cells.
Acknowledgments
We thank Dr. Ira Pastan (National Cancer Institute, National Institutes of Health, Bethesda) for providing scFv plasmid constructs and Drs. Steven Rosenberg and Mark Dudley (Surgery Branch, National Cancer Institute, National Institutes of Health) for providing gp100 CTL clones. This work was supported by the magneton project administered by the chief scientist office of the Israel Ministry of Trade and Industry.
Abbreviations: i.t., intratumorally; EBV, Epstein-Barr virus; CTL, cytotoxic T lymphocyte; sc, single-chain.
References
- 1.McLaughlin, P., Grillo-Lopez, A. J., Link, B. K., Levy, R., Czuczman, M. S., Williams, M. E., Heyman, M. R., Bence-Bruckler, I., White, C. A., Cabanillas, F., et al. (1998) J. Clin. Oncol. 16, 2825-2833. [DOI] [PubMed] [Google Scholar]
- 2.Cobleigh, M. A., Vogel, C. L., Tripathy, D., Robert, N. J., Scholl, S., Fehrenbacher, L., Wolter, J. M., Paton, V., Shak, S., Lieberman, G. & Slamon, D. J. (1999) J. Clin. Oncol. 17, 2639-2648. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara, N. (2002) Semin. Oncol. 29, 10-14. [DOI] [PubMed] [Google Scholar]
- 4.Pastan, I. (1997) Biochim. Biophys. Acta 1333, C1-C6. [DOI] [PubMed] [Google Scholar]
- 5.Lode, H. N. & Reisfeld, R. A. (2000) Immunol. Res. 21, 279-288. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg, S. A. (2001) Nature 411, 380-384. [DOI] [PubMed] [Google Scholar]
- 7.Offringa, R., van der Burg, S. H., Ossendorp, F., Toes, R. E. & Melief, C. J. (2000) Curr. Opin. Immunol. 12, 576-582. [DOI] [PubMed] [Google Scholar]
- 8.Renkvist, N., Castelli, C., Robbins, P. F. & Parmiani, G. (2001) Cancer Immunol. Immunother. 50, 3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg, S. A., Yang, J. C., Schwartzentruber, D. J., Hwu, P., Marincola, F. M., Topalian, S. L., Restifo, N. P., Dudley, M. E., Schwarz, S. L., Spiess, P. J., et al. (1998) Nat. Med. 4, 321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, P. P., Yee, C., Savage, P. A., Fong, L., Brockstedt, D., Weber, J. S., Johnson, D., Swetter, S., Thompson, J., Greenberg, P. D., et al. (1999) Nat. Med. 5, 677-685. [DOI] [PubMed] [Google Scholar]
- 11.Seliger, B., Maeurer, M. J. & Ferrone, S. (2000) Immunol. Today 21, 455-464. [DOI] [PubMed] [Google Scholar]
- 12.Dudley, M. E., Wunderlich, J. R., Robbins, P. F., Yang, J. C., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., Sherry, R., Restifo, N. P., Hubicki, A. M., et al. (2002) Science 298, 850-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley, M. E., Wunderlich, J. R., Shelton, T. E., Even, J. & Rosenberg, S. A. (2003) J. Immunother. 26, 332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkberg, G., Cohen, C. J., Segal, D., Kirkin, A. F. & Reiter, Y. (2000) Eur. J. Immunol. 30, 3522-3532. [DOI] [PubMed] [Google Scholar]
- 15.Denkberg, G., Cohen, C. J. & Reiter, Y. (2001) J. Immunol. 167, 270-276. [DOI] [PubMed] [Google Scholar]
- 16.Parkhurst, M. R., Salgaller, M. L., Southwood, S., Robbins, P. F., Sette, A., Rosenberg, S. A. & Kawakami, Y. (2001) J. Immunol. 157, 2539-2548. [PubMed] [Google Scholar]
- 17.Lechner, F., Cuero, A. L., Kantzanou, M. & Klenerman, P. (2001) Rev. Med. Virol. 11, 11-22. [DOI] [PubMed] [Google Scholar]
- 18.Uchiyama, T., Broder, S. & Waldmann, T. A. (1981) J. Immunol. 126, 1393-1397. [PubMed] [Google Scholar]
- 19.Chowdhury, P. S., Viner, J. L., Beers, R. & Pastan, I. (1998) Proc. Natl. Acad. Sci. USA 95, 669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tussey, L., Speller, S., Gallimore, A. & Vessey, R. (2000) Eur. J. Immunol. 30, 1823-1829. [DOI] [PubMed] [Google Scholar]
- 21.Porgador, A., Yewdell, J. W., Deng, Y., Bennink, J. R. & Germain, R. N. (1997) Immunity 6, 715-726. [DOI] [PubMed] [Google Scholar]
- 22.Christinck, E. R., Luscher, M. A., Barber, B. H. & Williams, D. B. (1991) Nature 352, 67-70. [DOI] [PubMed] [Google Scholar]
- 23.Withoff, S., Helfrich, W., de Leij, L. F. & Molema, G. (2001) Curr. Opin. Mol. Ther. 3, 53-62. [PubMed] [Google Scholar]
- 24.Renner, C., Jung, W., Sahin, U., Denfeld, R., Pohl, C., Trumper, L., Hartmann, F., Diehl, V., van Lier, R. & Pfreundschuh, M. (1994) Science 264, 833-835. [DOI] [PubMed] [Google Scholar]
- 25.Jain, R. K. (1999) Annu. Rev. Biomed. Eng. 1, 241-263. [DOI] [PubMed] [Google Scholar]
- 26.Ogg, G. S., Dunbar, P. R., Cerundolo, V., McMichael, A. J., Lemoine, N. R. & Savage, P. (2000) Br. J. Cancer 82, 1058-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert, B., Guillaume, P., Luescher, I., Romero, P. & Mach, J. P. (2000) Eur. J. Immunol. 30, 3165-3170. [DOI] [PubMed] [Google Scholar]
- 28.Robert, B., Guillaume, P., Luescher, I., Doucey, M. A., Cerottini, J. C., Romero, P. & Mach, J. P. (2001) Cancer Immun. 1, 2-9. [PubMed] [Google Scholar]
- 29.Savage, P., Cowburn, P., Clayton, A., Man, S., Lawson, T., Ogg, G., Lemoine, N., McMichael, A. & Epenetos, A. (2002) Int. J. Cancer 98, 561-566. [DOI] [PubMed] [Google Scholar]
- 30.Lanzavecchia, A., Lezzi, G. & Viola, A. (1999) Cell 96, 1-4. [DOI] [PubMed] [Google Scholar]
- 31.Bromley, S. K., Burack, W. R., Johnson, K. G., Somersalo, K., Sims, T. N., Sumen, C., Davis, M. M., Shaw, A. S., Allen, P. M. & Dustin, M. L. (2001) Annu. Rev. Immunol. 19, 375-396. [DOI] [PubMed] [Google Scholar]
- 32.Donda, A., Cesson, V., Mach, J. P., Corradin, G., Primus, F. J. & Robert, B. (2003) Cancer Immun. 3, 11-28. [PubMed] [Google Scholar]
- 33.Wang, B., R. Maile, R., Greenwood, E. J., Collins & Frelinger, J. A. (2000) J. Immunol. 164, 1216-1222. [DOI] [PubMed] [Google Scholar]
- 34.Cohen, C. J., Denkberg, G., Schiffenbauer, Y. S., Segal, D., Trubniykov, E., Berke, G. & Reiter, Y. (2003) J. Immunol. Methods 277, 39-52. [DOI] [PubMed] [Google Scholar]