Abstract
Extracellular acidification occurs under physiologic and pathologic conditions, such as exercise, ischemia, and inflammation. It has been shown that acidosis has various adverse effects on bone. In recent years there has been increasing evidence which indicates that ovarian cancer G protein-coupled receptor 1 (OGR1) is a pH-sensing receptor and mediates a variety of extracellular acidification-induced actions on bone cells and other cell types. Recent studies have shown that OGR1 is involved in the regulation of osteoclast differentiation, survival, and function, as well as osteoblast differentiation and bone formation. Moreover, OGR1 also regulates acid-induced apoptosis of endplate chondrocytes in intervertebral discs. These observations demonstrate the importance of OGR1 in skeletal development and metabolism. Here, we provide an overview of OGR1 regulation ofosteoclasts, osteoblasts, and chondrocytes, and the molecular actions of OGR1 induced by extracellular acidification in the maintenance of bone health.
Keywords: extracellular acidification, OGR1, osteoclasts, osteoblasts, endplate chondrocytes
1. Introduction
Bone and cartilage are the two primary components that form the skeleton in vertebrates [1]. These two tissues consist of three specific cell types scattered within the extracellular matrix (ECM): Bone-forming osteoblasts, bone-resorbing osteoclasts in bone, and cartilage-forming chondrocytes in cartilage [2]. The macroscopic and microscopic structural changes in bone are influenced by physiologic and pathologic conditions, such as mechanical stress, hypoxia, and acidosis [2,3,4,5]. Because of fractures, hypoxia, inflammation, and tumors, the bone microenvironment has long been known to be acidic [6,7]. Acidic pH can also result from hormonal, growth factor, or cytokine stimulation of bone cell metabolism [8]. It has been shown that parathyroid hormone and insulin-like growth factor-1 (IGF-1) lead to a rapid acid efflux from osteoblasts [9]. The cartilage exists in an extracellular environment where the pH of the interstitial fluid is much more acidic than most other tissues. The avascular nature of cartilage causes hypoxia within the ECM which may lead to acidosis in the cartilage microenvironment [10].
Although the sensing mechanism of extracellular acid remains largely unknown, great breakthroughs have also been made toward understanding the cellular sensory mechanisms by which cells detect changes in the extracellular pH in such a sensitive manner. It has been shown that transient receptor potential V1 (TRPV1) is a calcium-permeable channel which is modulated or activated by extracellular protons [11]. Another family of molecular acid sensors is the acid-sensing ion channels (ASICs), which encode at least six different ASIC subunits, including ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 [12]. Proton-sensing G protein-coupled receptors (GPCRs) are emerging as a new class of acid sensors on a wide range of cell types that transduce signals through heterotrimeric G proteins [13]. The family of GPCRs is involved in cancer cell proliferation, apoptosis, metastasis, angiogenesis, osteoclast differentiation and survival, dendritic cell (DC) activities, alteration of DC functions, and insulin secretion; some of these GPCRs have turned out to be sensors for extracellular acidosis [14]. The transcripts of proton-sensing GPCRs, particularly ovarian cancer G protein-coupled receptor 1 (OGR1), are widely distributed and expressed on bone cells that are involved in the regulation of osteoclast differentiation, survival, and function, osteoblast differentiation and bone formation, as well as apoptosis of endplate chondrocytes in intervertebral discs [13,15,16]. This review will summarize our current knowledge regarding OGR1 in bone, and will highlight recent advances in bone metabolism. It has been showed that in bone cells metabolic acidosis increased [Ca2+]i from intracellular stores through activation of OGR1.
2. Proton-Sensing GPCRs
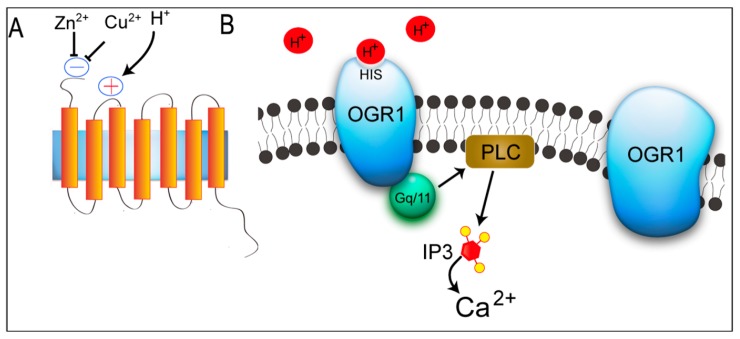
There are four members in the GPCR family (OGR1, G protein-coupled receptor 4 (GPR4), T cell death-associated gene 8 (TDAG8), and G2 accumulation protein (G2A)), which have previously been identified as receptors for lysolipids (sphingosylphosphorylcholine (SPC), lysophosphatidylcholine (LPC), and psychosine (galactosylsphingosine)) [17,18,19,20]. Recent studies, however, have shown that these GPCRs also sense extracellular protons through histidine residues of receptors and are coupled to G-proteins to stimulate intracellular signaling pathways. Ludwig et al. [17] first reported that OGR1 and GPR4 are proton-sensing receptors and coupled to Gq/11 and Gs proteins by regulating activation of the phospholipase C (PLC)/Ca2+ and adenylyl cyclase/Cyclic Adenosine monophosphate (cAMP) signaling pathways, respectively. OGR1 is inactive at pH 7.8, but fully activates inositol phosphate (IP) formation at pH 6.8. Moreover, Ludwig et al. [17] showed that GPR4 senses extracellular protons, but GPR4 activates the Gs-adenylyl cyclase-cAMP signaling pathway; however, they were not able to find any effect of SPC and LPC, which were previously reported to activate OGR1 and GPR4. In 2004, Murakami et al. [21] reported that G2A functions as a proton-sensing GPCR, like OGR1 and GPR4, by regulating multiple classes of G-proteins, including G13 and Gi/Go in signaling. Wang et al. [22] reported that TDAG8 senses extracellular protons and have recently been identified as proton-sensing or extracellular pH-responsive GPCRs, leading to activation of the cAMP signaling pathway. Among these receptors, OGR1is widely distributed and expressed on bone cells that take part in bone metabolism. OGR1, also named as GPR68 (G protein-coupled receptor 68), is a 365 amino acid multi-pass membrane protein that is expressed in testis, spleen, bone, lung, brain and placenta [17,23]. OGR1 selectively binds both protons and bioactive lipids and acts through Gi and Gq proteins-mediated processes [24,25]. Under the acidic conditions, pH-sensing activity of OGR1depends on several His residues that reside in the extracellular domains of this seven-pass transmembrane protein, resulting in the activation of intracellular signaling pathways (Figure 1).
Figure 1.
Activation of ovarian cancer G protein-coupled receptor 1 (OGR1) by extracellular acidification. (A) Proton has been suggested as an agonists of OGR1. Cu2+ and Zn2+ inhibit pH-dependent OGR1 activation; and (B) His residues that have been reported to be involved in proton-sensing process are bolded and underlined in OGR1. The interaction between protons and His residues of OGR1 leads to the activation of Gq/11/PLC/Ca2+ pathway. PLC: phospholipase C; Gq/11: Gq/11 protein; IP3: inositol 1,4,5-trisphosphate.
3. OGR1 and Bone
3.1. Causes of Acidosis
Tissue acidosis can result from a number of systemic and local causes. Systemic acidosis, which is caused by pathologic conditions, such as renal and respiratory disease, anemias, and diabetes, leads to abnormal cell function throughout the body [26]. Systemic acidosis can also be caused by high levels of protein intake (or acid feeding), aging, or the menopause [27]. Localized extracellular acidosis occurs due to ischemia and hypoxia caused by diabetes, wounds, inflammation, infection, and tumors [6]. Extracellular acidosis can also result from hormonal, growth factor, or cytokine stimulation of cell metabolism [8]. The deleterious effect of acidosis on the bone has long been known. In particular, proton-sensing GPCRs, such as OGR1, respond to low pH and play a critical role in the regulation of osteoclast function, osteoblast differentiation, and apoptosis of endplate chondrocytes in intervertebral discs [8,13,16,27,28].
3.2. OGR1 and Osteoclasts
In 2005, Komarova et al. [29] first showed that OGR1 is expressed in osteoclast-like cells differentiated in vitro from RAW 264.7 cells induced by receptor activator of NF-κB ligand (RANKL). In addition, RANKL increases the levels of expression of OGR1 mRNA in RAW 264.7 pre-osteoclast-like cells. Pereverzev et al. [28] also showed that reduction of extracellular pH in osteoclasts resulted in nuclear translocation of NFATc1, a downstream mediator of RANKL differentiation effects, although no specific physiologic role for OGR1 in that process was demonstrated. Localization of OGR1 in the plasma membrane area suggests that OGR1 may act as a functional receptor on these cells. Consistent with the differences in transcript levels, the immunofluorescence staining of OGR1 in differentiated osteoclast-like cells is more intense than undifferentiated RAW 264.7 cells. Yang et al. [16] reported that OGR1 is also significantly up-regulated in tibias and femurs after 2 days of colony stimulating factor-1 (CSF-1) injections based on the ability of CSF-1 to restore osteoclast populations in the CSF-1-null toothless (csf1tl/csf1tl) osteoporotic rat. The expression of OGR1 mRNA and protein was also observed by microarray, real-time RT-PCR, and immunoblotting when mouse bone marrow mononuclear cells (BMMs) were treated with RANKL to induce osteoclast differentiation. Specific inhibition of OGR1 by anti-OGR1 antibody and OGR1-specific RNA interference (RNAi) suppressed RANKL-induced differentiation of both BMMs and RAW 264.7 cells in vitro. It has been proposed that OGR1 is expressed during osteoclastogenesis in vivo and in vitro and is crucial for osteoclast differentiation; however, the molecular mechanism of OGR1 in regulating osteoclast differentiation and function remains unclear.
Pereverzev et al. [28] recently detected extracellular acidification enhances osteoclast survival. This study directly supports the potential activation of OGR1 in mediating osteoclast survival during extracellular acidification. Ca2+ signaling in osteoclasts is crucial for cellular functions, including motility, differentiation, and bone-resorbing activity [16,28,30]. Recent findings suggest that OGR1 is essential for the extracellular acidification-induced increase in [Ca2+]i levels in osteoclasts [28]. More interestingly, OGR1-mediated calcium signaling occurs in osteoclasts during extracellular acidosis, which contributes to acidosis-induced osteoclast survival. OGR1 activation in osteoclast enhances survival by inducing the activation of protein kinase C (PKC) that may affect the phosphorylation status of pro- or anti-apoptotic proteins, or stimulate extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling, which is critical for osteoclast survival [28]. Similar observations were reported in a more recent study in OGR1 deficient mice which were generated by homologous recombination [31]. OGR1 deficiency led to a decrease in osteoclast numbers, suggesting that OGR1 may play an important role in osteoclastogenesis. A pH-dependent survival effect of osteoclasts was also detected. However, overall abnormality in the bones of OGR1 deficient mice was not observed. It is possible that the defect in osteoclast numbers and/or their response to pH changes will affect some biological functions under certain pathological conditions.
3.3. OGR1 and Osteoblasts
Ludwig et al. [17] reported that the expression of OGR1 protein is detected in active osteoblasts, lining cells on the bone surface, and matrix-embedded osteocytes by immunohistochemistry. Moreover, Tomura et al. [32] reported OGR1 is predominantly expressed in human osteoblastic cells (NHOst). Several groups have investigated how acidosis works via OGR1 in osteoblasts [15,17,32,33]. Acidosis activates OGR1 to elevate [Ca2+]i levels via Gq stimulation, inducing cyclooxygenase 2 (COX-2) mRNA and protein expression in human osteoblastic cells. This leads to the production of prostaglandin E2 (PGE2), which is reported to activate osteoblasts to RANKL expression, a key cytokine involved in osteoclast differentiation [32]. Moreover, knocking down OGR1 with siRNA inhibits acidosis-induced COX-2 expression in a human osteoblastic cell line [32]. Tomura et al. [32] used YM-254890, a Gq antagonist that specifically inhibits Gq activation, and PLC inhibitors that significantly inhibit acid-induced COX-2 expression and subsequent PGE2 production, suggesting that the OGR1/Gq/11/PLC pathway is involved in COX-2 expression and PGE2 production in osteoblasts. This cascade from OGR1 to COX-2 and RANKL in osteoblasts might be an event in the induction process by acidic circumstances.
Frick et al. [15] previously reported that acidosis also leads to an increase in net Ca2+ efflux from bone. Recent studies have demonstrated that the OGR1 antagonist, Cu2+, significantly decreases acid-induced bone net Ca2+ efflux, a marker of bone resorption, in cultured neonatal mouse calvariae [33]. To further support OGR1 as a prime candidate as an osteoblastic H+ sensor, Frick et al. [33] perfused Chinese hamster ovary (CHO) cells transfected with mouse OGR1 cDNA. Eventually, a rapid increase in the intracellular calcium ([Ca2+]i) levels were also detected in OGR1-transfected CHO cells in response to an acidic medium, which acts as a second messenger to mediate the effects of acidosis on osteoblasts and results in increased osteoclastic bone resorption by inducing increased COX-2 and RANKL expression.
3.4. OGR1 and Chondrocytes
In 2003, Ludwig et al. [17] first reported that the expression of OGR1 is also specifically expressed in chondrocytes of hypertrophic cartilage. Experiments in our laboratory using rat lumbar endplate chondrocytes have been shown that high levels of OGR1 mRNA and low levels of G2A and TDAG8 mRNA in rat endplate chondrocytes were detected by RT-PCR analysis [13]. Interesting results were noted when cultures of rat lumbar endplate chondrocytes were exposed to acidosis; the mRNA levels of OGR1 increased in response to acidosis, whereas the mRNA levels of the other receptors were unchanged. Our data suggest that OGR1 is responsive to pH in endplate chondrocytes [13].
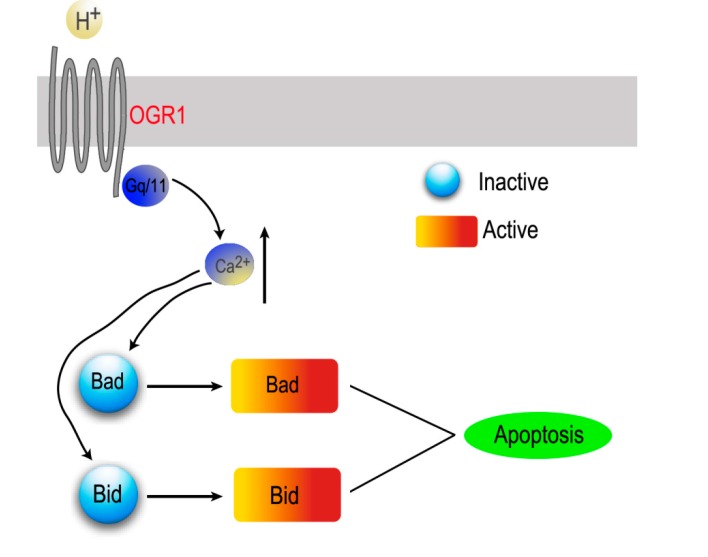
Additionally, we demonstrated that OGR1 is involved in apoptosis of endplate chondrocytes induced by extracellular acid in the rat intervertebral disc. Acid-induced [Ca2+]i increase via OGR1 is responsible for endplate chondrocytes apoptosis. A mechanism for OGR1 in endplate chondrocytes in an acidic environment has been proposed wherein cell apoptosis is related to OGR1-mediated apoptosis via down-regulation of calcium-activated signaling pathways, such as Bid and Bad, and inhibition of caspase-3 and poly(ADP-ribose) polymerase (PARP) activity (Figure 2).
Figure 2.
Schematic diagram of the potential mechanism of proton-sensing receptor OGR1 involved in acid-induced apoptosis of endplate chondrocytes.
4. Conclusions
In the present review, we have discussed extracellular acidification regulating a wide range of cellular functions and their mechanisms, especially focusing on proton-sensing OGR1 in bone cells. Increasing evidence supports a central role for the OGR1 in bone biology and disease; however, there were several limitations to the study that will require further exploration. The role of OGR1 in bone biology and disease should be confirmed in several animal models. It will also be important to determine whether or not there are any developmental changes during bone disease in OGR1-deficient mice. Interestingly, by observing the development of knockout (KO) mice, Li et al.. [31] has elucidated essential roles for OGR1 in regulating osteoclastogenesis. TDAG8 gene mutation in ovariectomized miceresulted in an increase in osteoclastic activity, suggesting an inhibitory role of TDAG8 in osteoclastic bone resorption in osteoporosis [34]. Further investigation into the regulatory mechanisms of OGR1 is necessary for developing effective therapeutic strategies for the treatment of bone diseases.
Acknowledgments
This work was supported by the Shanghai International Science and Technology Partnership Program (11540702700), Natural Science Foundation of China (31170925; 30970718; 81270011), and “Technology Innovation Action Plan” Key Project of Shanghai Science and Technology Commission (12411951300; 12JC1402600).
Author Contributions
Feng-Lai Yuan conceived of the idea and wrote the manuscript; Ming-Dong Zhao, Li-Bo Jiang, Hui-Ren Wang and Lu Cao involved in discussion and helped manuscript preparation; and Xiao-Gang Zhou, Xi-Lei Li and Jian Dong critically reviewed the manuscript. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sgariglia F., Candela M.E., Huegel J., Jacenko O., Koyama E., Yamaguchi Y., Pacifici M. Enomoto-Iwamoto, M. Epiphyseal abnormalities, trabecular bone loss and articular chondrocyte hypertrophy develop in the long bones of postnatal Ext1-deficient mice. Bone. 2013;57:220–231. doi: 10.1016/j.bone.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega N., Behonick D., Stickens D., Werb Z. How proteases regulate bone morphogenesis. Ann. N. Y. Acad. Sci. 2003;995:109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 3.Li X., Wu F.R., Xu R.S., Hu W., Jiang D.L., Ji C., Chen F.H., Yuan F.L. Acid-sensing ion channel 1α-mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opin. Ther. Targets. 2014;18:1–14. doi: 10.1517/14728222.2014.859248. [DOI] [PubMed] [Google Scholar]
- 4.Saito T., Kawaguchi H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthr. Cartil. 2010;18:1552–1556. doi: 10.1016/j.joca.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Su Y.S., Ren D., Wang P.C. Comparison of biomechanical properties of single- and two-segment fusion for Denis type B spinal fractures. Orthop. Surg. 2013;5:266–273. doi: 10.1111/os.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato K., Morita I. Promotion of osteoclast differentiation and activation in spite of impeded osteoblast-lineage differentiation under acidosis: Effects of acidosis on bone metabolism. Biosci. Trends. 2013;7:33–41. [PubMed] [Google Scholar]
- 7.Spector J.A., Mehrara B.J., Greenwald J.A., Saadeh P.B., Steinbrech D.S., Bouletreau P.J., Smith L.P., Longaker M.T. Osteoblast expression of vascular endothelial growth factor is modulated by the extracellular microenvironment. Am. J. Physiol. Cell Physiol. 2001;280:C72–C80. doi: 10.1152/ajpcell.2001.280.1.C72. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi S., Hirukawa K., Togari A. Acidosis inhibits mineralization in human osteoblasts. Calcif. Tissue Int. 2013;93:233–240. doi: 10.1007/s00223-013-9746-2. [DOI] [PubMed] [Google Scholar]
- 9.Santhanagopal A., Dixon S.J. Insulin-like growth factor I rapidly enhances acid efflux from osteoblastic cells. Am. J. Physiol. 1999;277:E423–E432. doi: 10.1152/ajpendo.1999.277.3.E423. [DOI] [PubMed] [Google Scholar]
- 10.Felka T., Schafer R., Schewe B., Benz K., Aicher W.K. Hypoxia reduces the inhibitory effect of IL-1β on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthr. Cartil. 2009;17:1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Solinski H.J., Zierler S., Gudermann T., Breit A. Human sensory neuron-specific Mas-related G protein-coupled receptors-X1 sensitize and directly activate transient receptor potential cation channel V1 via distinct signaling pathways. J. Biol. Chem. 2012;287:40956–40971. doi: 10.1074/jbc.M112.408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Xu R.S., Jiang D.L., He X.L., Jin C., Lu W.G., Su Q., Yuan F.L. Acid-sensing ion channel 1α is involved in acid-induced osteoclastogenesis by regulating activation of the transcription factor NFATc1. FEBS Lett. 2013;587:3236–3242. doi: 10.1016/j.febslet.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Yuan F.L., Wang H.R., Zhao M.D., Yuan W., Cao L., Duan P.G., Jiang Y.Q., Li X.L., Dong J. Ovarian cancer G protein-coupled receptor 1 is involved in acid-induced apoptosis of endplate chondrocytes in intervertebral discs. J. Bone Miner. Res. 2014;29:67–77. doi: 10.1002/jbmr.2030. [DOI] [PubMed] [Google Scholar]
- 14.Damaghi M., Wojtkowiak J.W., Gillies R.J. pH sensing and regulation in cancer. Front. Physiol. 2013;4:370. doi: 10.3389/fphys.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frick K.K., Krieger N.S., Nehrke K., Bushinsky D.A. Metabolic acidosis increases intracellular calcium in bone cells through activation of the proton receptor OGR1. J. Bone Miner. Res. 2009;24:305–313. doi: 10.1359/jbmr.081015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M., Mailhot G., Birnbaum M.J., MacKay C.A., Mason-Savas A., Odgren P.R. Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J. Biol. Chem. 2006;281:23598–23605. doi: 10.1074/jbc.M602191200. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig M.G., Vanek M., Guerini D., Gasser J.A., Jones C.E., Junker U., Hofstetter H., Wolf R.M., Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 18.Meyer zu Heringdorf D., Jakobs K.H. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Radu C.G., Nijagal A., McLaughlin J., Wang L., Witte O.N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA. 2005;102:1632–1637. doi: 10.1073/pnas.0409415102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Justus C.R., Dong L., Yang L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013;4:354. doi: 10.3389/fphys.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami N., Yokomizo T., Okuno T., Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- 22.Wang J.Q., Kon J., Mogi C., Tobo M., Damirin A., Sato K., Komachi M., Malchinkhuu E., Murata N., Kimura T., et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004;279:45626–45633. doi: 10.1074/jbc.M406966200. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Casey G. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics. 1996;35:397–402. doi: 10.1006/geno.1996.0377. [DOI] [PubMed] [Google Scholar]
- 24.Okajima F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal. 2013;25:2263–2271. doi: 10.1016/j.cellsig.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Im D.S. Two ligands for a GPCR, proton vs. lysolipid. Acta Pharmacol. Sin. 2005;26:1435–1441. doi: 10.1111/j.1745-7254.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 26.Arnett T.R. Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 2010;503:103–109. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Arnett T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 2003;62:511–520. doi: 10.1079/pns2003268. [DOI] [PubMed] [Google Scholar]
- 28.Pereverzev A., Komarova S.V., Korcok J., Armstrong S., Tremblay G.B., Dixon S.J., Sims S.M. Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone. 2008;42:150–161. doi: 10.1016/j.bone.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Komarova S.V., Pereverzev A., Shum J.W., Sims S.M., Dixon S.J. Convergent signaling by acidosis and receptor activator of NF-κB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc. Natl. Acad. Sci. USA. 2005;102:2643–2648. doi: 10.1073/pnas.0406874102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwai K., Koike M., Ohshima S., Miyatake K., Uchiyama Y., Saeki Y., Ishii M. RGS18 acts as a negative regulator of osteoclastogenesis by modulating the acid-sensing OGR1/NFAT signaling pathway. J. Bone Miner. Res. 2007;22:1612–1620. doi: 10.1359/jbmr.070612. [DOI] [PubMed] [Google Scholar]
- 31.Li H., Wang D., Singh L.S., Berk M., Tan H., Zhao Z., Steinmetz R., Kirmani K., Wei G., Xu Y. Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One. 2009;4:e5705. doi: 10.1371/journal.pone.0005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomura H., Wang J.Q., Liu J.P., Komachi M., Damirin A., Mogi C., Tobo M., Nochi H., Tamoto K., Im D.S., et al. Cyclooxygenase-2 expression and prostaglandin E2 production in response to acidic pH through OGR1 in a human osteoblastic cell line. J. Bone Miner. Res. 2008;23:1129–1139. doi: 10.1359/jbmr.080236. [DOI] [PubMed] [Google Scholar]
- 33.Frick K.K., Bushinsky D.A. Effect of metabolic and respiratory acidosis on intracellular calcium in osteoblasts. Am. J. Physiol. Renal Physiol. 2010;299:F418–F425. doi: 10.1152/ajprenal.00136.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hikiji H., Endo D., Horie K., Harayama T., Akahoshi N., Igarashi H., Kihara Y., Yanagida K., Takeda J., Koji T., et al. TDAG8 activation inhibits osteoclastic bone resorption. FASEB J. 2014;28:871–879. doi: 10.1096/fj.13-233106. [DOI] [PubMed] [Google Scholar]