Abstract
Ectromelia virus (ECTV), a natural mouse pathogen and an orthopoxvirus, has been used to investigate the correlation between polarized type 1 or type 2 immune responses and resistance to disease in poxvirus infections by using well defined resistant and susceptible mouse strains. Our data show that distinct differences exist in the cytokine profiles expressed in resistant and susceptible mice infected with ECTV. Resistant C57BL/6 mice generate a type 1 cytokine response [IFN-γ, IL-2, and tumor necrosis factor (TNF)], within the first few days of infection, which is associated with strong cytotoxic T lymphocyte response (CTL) and recovery from ECTV infection. Susceptible strains of mice (BALB/c and A/J) on the other hand generate a type 2 cytokine response (IL-4 but little or no IFN-γ and IL-2), which is associated with a weak or an absent CTL response, resulting in uncontrolled virus replication and death. Although deletion of IL-4 function alone did not change the outcome of infection in susceptible mice, the loss of IFN-γ function in resistant mice abrogated natural killer (NK) cell and CTL effector functions resulting in fulminant disease and 100% mortality. Therefore, a clear link exists between the early production of specific type 1 cytokines, in particular, IFN-γ, the nature of the cellular immune response, and disease outcome in this virus model. This finding in the mousepox model raises the possibility that inappropriate cytokine responses may result in increased susceptibility to smallpox in humans.
Variola virus (VARV), a member of the poxvirus family, is the causative agent of smallpox and one of the most virulent human pathogens known. Although smallpox was eradicated more than two decades ago, it continues to pose a threat as a potential agent of bioterrorism (1). The reason for the high mortality rates associated with smallpox, or what constitutes an effective immune response to VARV infection in humans, is not well understood. Indeed, much of our understanding of the pathogenesis of smallpox in humans comes from studies in animal models of poxvirus infections. ECTV, an orthopoxvirus closely related to VARV, is a natural mouse pathogen that has coevolved with its host and causes a generalized infection termed mousepox (2). The mousepox model has been used extensively to study pathogenesis of generalized viral infections, genetic resistance to disease, and viral immunology (2-6).
Cytokines play an important role in orchestrating the responses essential for control and clearance of microbial pathogens. Indeed, the production of cytokines, in particular, IFN-α, IFN-γ, IL-2, and IL-12 p40, early in viral infections, has been associated with the induction of strong cellular immune responses (7) critical for virus clearance and recovery. These cytokines are referred to as type 1 cytokines [profile associated with the type 1 T helper cells (Th1)]. Type 2 cytokines (profile associated with Th2 cells), such as IL-4, IL-5, IL-10, and IL-13, on the other hand, are associated with humoral immunity and promote optimal antibody (Ab) responses (7, 8).
The relevance of polarized type 1 or type 2 responses in terms of disease outcome (resistance or susceptibility) has been extensively studied in models of parasitic but not viral diseases (9). Infection of mice with the protozoan parasite Leishmania major is one of the best characterized models in which uncontrolled parasitism is associated with a polarized Th2 response in the susceptible strains and restricted parasitism is associated with a polarized Th1 response in resistant strains (10). Indeed, the importance of Th1 response in the genetic resistance to mycobacterial infections, first demonstrated in mouse models, has been verified in human studies on individuals with mutations in genes encoding proteins of the Th1 cytokine cascade (11).
Because ECTV is a natural mouse pathogen, the host cytokine response to infection can be studied in a model where virus and host have coevolved. Inbred strains of mice have been classified as resistant or susceptible to infection (6, 12). A/J, BALB/c and DBA/2 strains exhibit 100% mortality to mousepox and are classified as susceptible, whereas C57BL/6 and certain 129 strains have very low mortality and limited pathology and are classified as resistant (refs. 6 and 12; G. K., unpublished work). In susceptible strains, extensive necrosis of major organs, in particular, liver and spleen, is due to massive virus replication that results in death. Resistance to mousepox depends on a number of immune parameters, and at least four genetic loci are involved (12-14). Therefore, the host genetic background and the type of immune response it mounts against the virus are critical to the outcome of an infection.
Recovery of resistant mice from a primary ECTV infection depends on the combined actions of mononuclear phagocytes, interferons, and effector functions of natural killer (NK) cells, CD8+ cytotoxic T lymphocyte (CTL), and Ab (5, 15-20). In addition, we have previously suggested a role for the involvement of type 1 cytokines (21). In this study we have undertaken a detailed analysis of whether resistance or susceptibility to ECTV is related to the production of particular cytokines (type 1 or type 2) in response to virus infection. We have also compared the kinetics of critical immune parameters between resistant and susceptible mice to dissect the influence of cytokines on the type of immune response generated and disease outcome.
Here we show that resistant mice generate a type 1 cytokine response, which is associated with strong cell-mediated immunity and recovery from ECTV infection. In contrast, susceptible strains of mice generate a type 2 cytokine response, which is associated with a weak and delayed or an absent cell-mediated immune response that results in fulminant disease and death.
Materials and Methods
Mice. Female, specific-pathogen-free mice were used at 6-10 weeks of age. A/J, BALB/cJ, and C57BL/6J mice were obtained from National Cancer Institute-Frederick Animal Production Program (Frederick, MD) and Animal Services Division, John Curtin School of Medical Research, Canberra, Australia. The IFN-γ gene knockout mice (22) on a C57BL/6 background, B6.129S7-Ifngtm1Ts, IL-4 gene knockout mice (23), on a BALB/c background, BALB/c-Il4tm2Nnt, and all other strains were bred under specific-pathogen-free conditions at John Curtin School of Medical Research. Experiments were performed according to institutional guidelines for animal care and use.
Cell Lines. BS-C-1 (ATCC CCL-26), P815 (H-2d; ATCC TIB-64), MC57G (H-2b; ATCC CRL-2295), and L929 (H-2k; ATCC CCL-1) were maintained in Eagle's MEM (BioWhittaker) supplemented with 2 mM L-glutamine, antibiotics, and 10% FCS.
Virus Infection and Determination of Virus Titer. Virulent ECTV Moscow strain, designated Mos-3-P2 (ATCC VR 1374), was propagated in BS-C-1 cells as described (24, 25). Mice were inoculated with 1 × 103 plaque-forming units (PFUs) of virus in the right and left hind footpads under anesthesia. At various times after infection (p.i.), organs were removed aseptically and processed for determination of virus titer as described (24, 25).
Cytotoxicity Assays and Identification of Effector Populations. Anti-viral NK cell and CTL responses were measured by using pooled popliteal lymph node (PLN) cells from four to six mice or splenocytes from individual animals. Standard 51Cr-release assays were performed as described (26, 27). To detect MHC class I-restricted killing, ECTV-infected and uninfected targets were used. In some experiments CD8+ or CD4+ T cells were depleted from the effector cell populations by using rabbit complement (Cedarlane Laboratories) plus anti-CD8 (clone 3.155) or anti-CD4 (clone RL172), respectively (16).
ELISA for Detection of ECTV-Specific Ab. ECTV-specific IgM and IgG levels in sera were determined by ELISA. U-bottom 96-well Immulon 2 ELISA plates (Dynatech) were coated with purified ECTV (25). Sera from uninfected and infected mice were assayed at dilutions from 1:50 to 1:1,000, and ECTV-specific Ab was detected by using horseradish peroxidase-conjugated goat anti-mouse IgM or IgG (Southern Biotechnology Associates) and color developed with TMB One-Step substrate (Dako Cytomation). Based on this, a serum dilution of 1:200 was selected for determination of relative Ab levels through the course of infection.
Immunohistochemistry and Quantitation of Cytokine-Producing Cells.Spleen and individual PLNs, embedded in small slices of liver tissue, were snap-frozen and stored at -80°C until sectioned as described (28, 29). Sections (8 μM) of organs of all groups within an experiment, for each time point, were placed on the same glass slide. These sections were fixed in acetone containing hydrogen peroxide and then incubated, at 4°C, with optimally diluted primary Ab conjugated to alkaline phosphatase or horseradish peroxidase as described (28, 29). Anti-IFN-γ (clone DB-1 from P. van der Meide, U-CyTech Biosciences, Utrecht, The Netherlands), anti-IL-2 (clone S4B6), anti-IL-4 (clone 11B11), and control rat mAb 2A4 and 1G11 (from A. Zantema, Sylvius Laboratory, Leiden, The Netherlands) were used. Polyclonal rabbit anti-mouse TNF Ab (from P. van der Meide) followed by a secondary anti-Rabbit IgG-horseradish peroxidase (Dako A/S Glostrup, Denmark) was also used. Alkaline phosphatase and horseradish peroxidase activity was visualized as described (28, 29). Sections were counterstained with hematoxylin and mounted in glycerin gelatin.
Cytokine-producing cells were quantitated, and image analysis was performed as described (28). In brief, images of organ sections were acquired with an HTHMX-1 camera (High Technology Holland, Eindhoven, The Netherlands) and image analysis was done by using MCID software (Imaging Research, St. Catherine's, ON, Canada). Stained cells were counted and expressed as the number of cytokine-producing cell per mm2 spleen or whole PLN section (28, 29). Three spleen and three PLN sections from each strain per time point, plus uninfected controls, were analyzed.
ELISA for the Detection of IFN-γ IFN-γ produced by cultured splenocytes from infected mice was determined by ELISA. Splenocytes (4 × 105) were incubated with uninfected or ECTV-infected cells of the appropriate MHC haplotype in 96-well U-bottom plates for 6 h at 37°C in a humidified CO2 incubator at effector to target ratios of 20:1. ELISAs were performed on supernatants by using R4-6A2 (ATCC HB-170) as a capture Ab, and polyclonal rabbit anti-mouse IFN-γ Ab, made in our laboratory, as the detection Ab. Recombinant murine IFN-γ (PeproTech, Rocky Hill, NJ) served as the standard and the sensitivity of detection was 3 units/ml.
In Vivo Neutralization of Cytokines. Neutralization of IFN-γ was achieved by injecting 1 mg of anti-IFN-γ mAb (clone R4-6A2) i.p. on days 0, 2, and 4 p.i (16). Similar treatment regimes were used in experiments where IL-2 was neutralized by using anti-IL-2 (clone S4B6), TNF neutralized by using anti-TNF (clone MP6-XT22), and IL-4 neutralized by using anti-IL-4 (clone 11B11). The neutralizing activities of each mAb was confirmed as described elsewhere for IL-2 neutralization (30).
Results
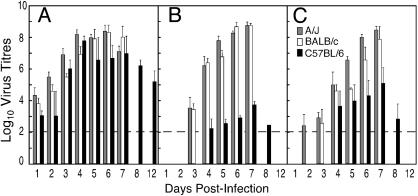
Kinetics of Virus Replication in Organs of Resistant and Susceptible Mice. C57BL/6, A/J, and BALB/c mice were infected with ECTV and on days indicated, groups of four mice from each strain were killed and organs removed for determination of virus titer (Fig. 1). Virus was detectable in PLN of all three strains of mice as early as day 1 p.i., with lowest titers in C57BL/6 PLN (Fig. 1A). Titers increased exponentially in all three strains and plateaued by day 4. Virus infectivity was still high up to the time of death of A/J and BALB/c mice, but in C57BL/6 mice titers declined after day 7. ECTV was eventually cleared from PLN of C57BL/6 mice between days 16 and 18 p.i., coincident with virus clearance from the footpad tissue (data not shown).
Fig. 1.
ECTV titers in resistant and susceptible mice. Groups of C57BL/6, BALB/c, and A/J mice infected with ECTV were killed on the days indicated. Virus titers in PLN (A), spleen (B), and liver (C) of individual animals were determined. BALB/c and A/J mice did not survive beyond day 7. Virus titers are expressed as mean log10 PFU per g of tissue ± SEM from five mice per group. The limit of detection is 2 log10 PFU (indicated by the dotted line).
In contrast to PLN, only low levels of virus infectivity were observed in spleens of C57BL/6 mice, whereas virus was detected in the spleens of A/J and BALB/c mice as early as day 3 p.i. and titers continued to increase in these strains to >8 log10 PFU just before death (Fig. 1B). Similarly, virus titers in livers of A/J and BALB/c were detectable early in infection and continued to rise till the time of death (Fig. 1C). In resistant C57BL/6 mice, virus was not detected in the liver until later (day 4 p.i.), and despite reaching about 5 log10 PFU at day 7, virus titers declined rapidly thereafter. Kinetics of NK Cell Response. To measure NK cell lytic activity, PLN cells were pooled from groups of five to six mice and used as effectors against 51Cr-labeled YAC-1 target cells. It was necessary to pool the nodes to obtain sufficient numbers of effector cells to measure lytic activity ex vivo. Over the course of infection, no significant NK cell-mediated cytotoxicity was detected in PLN from any of the three strains (data not shown).
Splenocytes from infected animals, on the other hand, showed significant levels of NK cell-mediated cytotoxicity (Fig. 2A). Splenocytes from uninfected A/J and C57BL/6 mice exhibited low levels of cytolytic activity on YAC-1 targets that increased after infection and peaked at day 5; however, this increase was most marked in C57BL/6 mice (an increase of 15-fold). In both strains of susceptible mice, NK activity rapidly declined just before death, whereas the decline was more gradual in C57BL/6 mice.
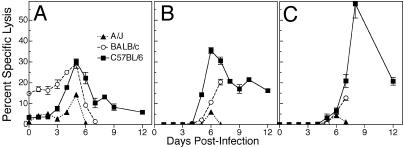
Fig. 2.
NK cell and ECTV-specific CTL activity. Groups of five C57BL/6, BALB/c, and A/J mice were infected with ECTV. Splenic NK cell activity (A) was measured by using 51Cr-labeled YAC-1 targets. Data shown are mean ± SEM of lysis values from five individual mice for each group at effector-to-target-cell ratio of 20:1. Anti-ECTV-specific CTL responses in the PLN (B) and spleen (C) were measured by using 51Cr release assay. Data shown are mean ± SEM of lysis values (lysis of virus-infected syngeneic targets minus lysis of uninfected targets) of pooled PLN from five mice (B) and from five individual spleens (C) at an effector-to-target-cell ratio of 20:1. The results are representative of two separate experiments.
Kinetics of Antiviral CTL response. Antiviral CTL activity was detected ex vivo as early as 5 days p.i. in PLN cells from C57BL/6mice (Fig. 2B). CTL activity peaked at day 6 and declined thereafter, although cytotoxicity was detected even on day 12. In contrast, CTL activity in PLN cells from BALB/c and A/J mice was delayed and significantly lower than the C57BL/6 group.
CTL activity was detected later in the spleen than in PLN in all three strains. In C57BL/6 mice, splenic CTL activity increased rapidly after 6 days, peaked at day 8, and persisted to day 12 p.i. In sharp contrast, CTL activity of splenocytes from BALB/c mice increased only marginally beyond day 6, and A/J mice failed to mount a response. In C57BL/6 mice, the peak in splenic CTL activity (Fig. 2C) coincided with a rapid decline in PLN, splenic and liver virus titers (Fig. 1).
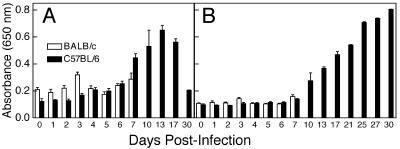
Kinetics of Antiviral Ab Response. Because both susceptible strains showed similar anti-ECTV responses, all subsequent experiments were carried out by using BALB/c mice as the representative susceptible strain. Levels of ECTV-specific IgM (IgM) and IgG were measured. IgM is generally produced early in infection and is independent of T cell help, whereas IgG is produced later and depends on T cell helper function. Groups of five C57BL/6 and BALB/c mice were bled at times indicated and ECTV-specific IgM and IgG levels quantitated in serum samples (Fig. 3). No significant increases in ECTV-specific IgM levels were detected in the first 6 days p.i. in either strain (Fig. 3A). In C57BL/6 mice, these began to rise by day 7 p.i. and peaked at day 13 p.i. BALB/c mice died by day 8 p.i. without mounting a detectable IgM response.
Fig. 3.
ECTV-specific Ab response. Sera were collected from groups of five mice at days indicated p.i. and levels of ECTV-specific IgM (A) and IgG (B) determined for individual animals by ELISA at a serum dilution of 1:200. Data presented are expressed as mean ± SD of one of three separate animal experiments.
Serum levels of anti-ECTV IgG remained unchanged at background levels in both C57BL/6 and BALB/c mice until day 7 p.i. (Fig. 3B). Although the BALB/c group did not survive past this time point, C57BL/6 animals mounted a good anti-ECTV IgG response after day 7 p.i. that continued to rise until day 30 p.i.
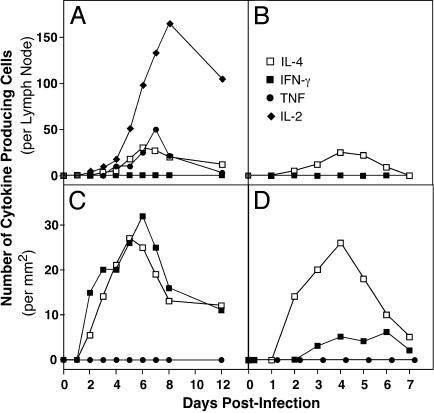
Kinetics of the Cytokine Response. We examined the production of IL-2, IL-4, IFN-γ, and TNF, in response to infection to determine whether there were any differences in cytokine profiles between the strains of mice. By using immunohistochemistry to detect IL-2-, IL-4-, IFN-γ-, and TNF-producing cells (Fig. 6, which is published as supporting information on the PNAS web site) the numbers of cytokine-producing cells were quantified (Fig. 4). Intriguingly, the panel of cytokines expressed in spleens was different from that of the PLN in both C57BL/6 and BALB/c mice (Fig. 4).
Fig. 4.
Kinetics of cytokine production in resistant and susceptible mice. C57BL/6(A and C) and BALB/c(B and D) mice were inoculated with ECTV, and groups of three mice were killed on the days indicated. PLN (A and B) and spleen (C and D) from each mouse were sectioned, stained for cytokine-producing cells, and positive cells were counted. Data presented are expressed as means of three mice from each group.
In PLN of C57BL/6 mice, IL-2-, IL-4-, and TNF-producing cells were detectable by days 3 and 4 p.i (Fig. 4A). The number of IL-2-producing cells increased rapidly, peaked on day 8, and remained high until day 12. The number of cells producing IL-4 or TNF was much lower and peaked on days 6 and 7. No IFN-γ-producing cells were detected in the PLN of C57BL/6 mice throughout the infection. In the spleens of C57BL/6 mice, IFN-γ- and IL-4-producing cells were detectable 2 days p.i. and numbers peaked between 5 and 7 days and declined by day 12 (Fig. 4C). However, no IL-2- or TNF-producing cells were detected in this organ. No cytokine-producing cells were detected in uninfected mice (day 0).
In comparison, in BALB/c mice, no IL-2- or TNF-producing cells were detected in either organ throughout the course of infection (Fig. 4 B and D). BALB/c spleens showed similar numbers and kinetics of IL-4-producing cells to C57BL/6 spleens. However, the numbers of IFN-γ-producing cells were lower and their appearance delayed compared with the C57BL/6 group. A/J mice showed a similar cytokine response to BALB/c mice except that no IFN-γ-producing cells were detected through the course of infection, and a small number of TNF-producing cells were found at days 5 and 6 (data not shown).
In summary, the difference in the cytokine response to infection in resistant and susceptible mice was striking. C57BL/6 mice produced IL-2, IL-4, TNF, and IFN-γ, whereas BALB/c and A/J mice made IL-4 but little or no IFN-γ, IL-2, and TNF.
Identity of T Cell Subsets Producing IFN-γ To identify the subset of T cells producing IFN-γ in spleens of infected mice, splenocytes were depleted of either CD4 or CD8 T cells by using Ab and complement. Splenocytes, thus fractionated, were stimulated with virus-infected syngeneic cells and IFN-γ production measured by ELISA. Splenocytes cultured with uninfected targets were used as controls. Table 1 shows the levels of IFN-γ produced by splenocytes depleted of CD4 cells and the total number of IFN-γ-producing cells in the spleen. As expected, the levels of IFN-γ in supernatants and the numbers of IFN-γ-producing cells in situ were undetectable or very low in BALB/c mice. In contrast, CD4 cell-depleted splenocytes from infected C57BL/6 mice produced high levels of IFN-γ but not until day 6 p.i. This was contemporaneous to the appearance of CTL cytolytic activity. Data from parallel experiments where total splenocytes or splenocytes depleted of CD4, CD8, or both T cell subsets were used, indicated that 80-85% of the total splenic IFN-γ was produced by CD8 T cells (data not shown). Of note, in situ IFN-γ-producing cells could be detected as early as day 2 p.i. in infected C57BL/6 spleens (Fig. 4).
Table 1. Kinetics of CTL activity and IFN-γ production by CD8 splenocytes from ECTV-infected mice.
| C57BL/6
|
BALB/c
|
|||||
|---|---|---|---|---|---|---|
| Days p.i. | Cytolysis* | IFN-γ production† | IFN-γ-producing cells,‡n | Cytolysis | IFN-γ production | IFN-γ-producing cells, n |
| 1 | 0 | <3.0 | 0 | 0 | <3.0 | 0 |
| 2 | 0 | <3.0 | 15 ± 2 | 0 | <3.0 | 0 |
| 3 | 0 | <3.0 | 20 ± 2 | 0 | <3.0 | 3 ± 1 |
| 4 | 0 | <3.0 | 20 ± 2 | 0 | <3.0 | 5 ± 1 |
| 5 | 2.1 ± 0.2 | <3.0 | 22 ± 3 | 0 | <3.0 | 4 ± 1 |
| 6 | 7.2 ± 1.1 | 22.7 ± 2.4 | 32 ± 2 | 5.8 ± 0.8 | <3.0 | 6 ± 2 |
| 7 | 20.9 ± 1.3 | 35.9 ± 2.8 | 25 ± 3 | 13.1 ± 1.9 | <3.0 | 2 ± 1 |
| 8 | 58.0 ± 3.8 | 45.4 ± 5.7 | 16 ± 2 | ND | ND | ND |
| 12 | 24.1 ± 2.3 | 25.1 ± 3.3 | 11 ± 2 | ND | ND | ND |
ND, not done.
CTL responses in the spleen. Data shown are mean ± SEM of percent of specific lysis of syngeneic targets from five mice per group at effector-to-target-cell ratio of 20:1.
IFN-γ production by splenocytes depleted of CD4 cells. Data shown are mean ± SEM units/ml from triplicate cultures for each group of five mice.
Number of IFN-γ-producing cells per mm2 in the spleens of infected mice as determined by immunohistochemistry (Fig. 4).
IFN-γ Production and Resistance to Lethal Infection. The data above suggest that resistance to ECTV infection is associated with early induction of type 1 cytokines like IL-2, IFN-γ, and TNF. In addition, a strong CTL response has been shown to be associated with virus clearance in C57BL/6 mice (5, 18). Conversely, the susceptible mouse strains have weak or absent type 1 cytokine responses and mount a suboptimal CTL response. We therefore tested the effect of blocking IL-2, TNF, or IFN-γ function on the ability of infected C57BL/6 mice to mount an effective CTL response. Neutralizing mAbs against murine IL-2, TNF, or IFN-γ were used to block cytokine function in vivo. Inhibition of IL-2 or TNF did not significantly affect the generation of NK cell and CTL responses (Fig. 5), virus clearance or resistance to ECTV infection (data not shown).
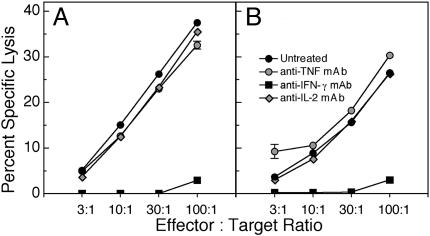
Fig. 5.
Effect of cytokine neutralization on NK cell and ECTV-specific CTL responses. Groups of three C57BL/6 mice were infected with ECTV and untreated or treated with neutralizing mAbs against murine IL-2, TNF, or IFN-γ. Mice were killed at day 6 p.i., and NK cell activity (A) and anti-ECTV-specific CTL responses (B) were measured as for Fig. 2. The results are expressed as mean ± SEM (these fall within the symbols for most data points) of one of two separate experiments.
In contrast, treatment of C57BL/6 mice with mAb to IFN-γ transformed an inapparent infection to fulminant mousepox with 100% mortality and a mean time to death of 7 days, which mimicked the response in susceptible mice (Table 2). Similar results were obtained when IFN-γ gene knockout mice were infected with ECTV. The blockade or absence of IFN-γ in C57BL/6 mice clearly resulted in exacerbated infection; virus titers in all organs examined were similar to those in the susceptible mice (Table 2), which made little or no IFN-γ (Fig. 4). Indeed, the blockade of IFN-γ in infected C57BL/6 mice almost completely abolished the NK cell and CTL responses (Fig. 5). These results clearly indicate the link between IFN-γ production, activation of NK and antiviral CTL responses, and resistance to lethal infection.
Table 2. Enhanced viral load in the absence of IFN-γ.
| Virus titer in organs of mice 7 days p.i. (log10)
|
|||||
|---|---|---|---|---|---|
| Organ | C57BL/6 | C57BL/6 + anti-IFN-γ | B6.IFN-γ−/− | A/J | BALB/c |
| Spleen | 3.7 ± 0.2 | 8.3 ± 0.2 | 8.5 ± 0.2 | 8.9 ± 0.5 | 8.5 ± 0.5 |
| Liver | 5.1 ± 0.3 | 8.2 ± 0.4 | 8.8 ± 0.9 | 8.5 ± 0.5 | 8.3 ± 0.6 |
| Lung | <2.0 | 7.0 ± 0.6 | 7.1 ± 0.4 | 6.9 ± 0.2 | 6.6 ± 0.2 |
| Ovaries | <2.0 | 5.4 ± 0.6 | 5.7 ± 0.4 | 5.1 ± 0.2 | 5.0 ± 0.2 |
| Thymus | <2.0 | 7.0 ± 0.6 | 7.5 ± 0.4 | 7.9 ± 0.9 | 7.3 ± 0.4 |
| Mortality, % (MTD) | 0 | 100 (7 days) | 100 (7 days) | 100 (7 days) | 100 (8 days) |
Data are means ± SEM. MTD, mean time to death.
Depletion of IL-4 Does Not Reverse the Susceptibility to ECTV. Although the production of the type 1 cytokines was minimal or absent in susceptible mouse strains compared with the resistant strain, production of IL-4 was comparable. Because IL-4 is known to down-regulate production of the type 1 cytokines, we tested whether inhibition of IL-4 in susceptible mice could reverse the deficiency in type 1 cytokine production and whether this would result in a concomitant increase in the magnitude of the CTL response.
Groups of BALB/c mice treated with anti-IL-4 or left untreated were infected with ECTV. The treatment had no effect on the susceptibility (100% mortality in both groups) or the mean time to death (7.6 ± 0.5 days for treated and 7.8 ± 0.4 days for untreated group). Not surprisingly then, the inhibition of IL-4 neither reduced virus replication in spleen and liver nor affected CTL response measured at day 7 p.i (data not shown). Similar data were obtained in experiments by using IL-4 gene knockout mice on a BALB/c background. Hence, the inhibition of the type 2 cytokine IL-4 alone did not reverse susceptibility to mousepox.
Discussion
The mousepox model is arguably one of the best available animal models to study pathogenesis of and the immune response to poxvirus infections. Like VARV, which causes smallpox in humans, ECTV encodes various immune-modifying genes, has coevolved with its natural host, and is highly virulent, causing disease with high mortality rates. Indeed, although mortality rates associated with smallpox were reported to be as high as 30%, a significant subset of the infected population recovered (31). The basis of this susceptibility and resistance, and the immune parameters associated with recovery from VARV infection in humans, has not been elucidated. To gain a better understanding of the pathogenetic basis of resistance to poxvirus infections, we have used the mousepox model, where both resistant and susceptible strains are well defined. Resistant (C57BL/6) mice are able to mount a protective immune response to ECTV, and this is reflected in significantly lower virus titers early in infection (Fig. 1) and complete recovery, compared with the susceptible (A/J and BALB/c) mice that have high virus titer and show 100% mortality. Using these strains of mice, we have shown here that the in situ type 1 or type 2 cytokine response (Fig. 4 and Table 2) is clearly associated with resistance or susceptibility to mousepox, respectively.
Cytokines are known to be crucial in the initiation and regulation of an immune response. The production of specific cytokines early in infection not only activates the innate immune response but also directs the development of antigen-specific immunity against the infectious agent. We therefore investigated the cytokine profiles in both resistant and susceptible strains of mice after infection with ECTV. We enumerated cytokine-producing cells through immunohistochemical analyses, as this approach is a definitive means of establishing the presence or absence of specific cytokines in vivo. Cells enumerated in this way would include antigen-specific cells and bystander cells. Our data show that there are distinct differences in the cytokine profiles of resistant and susceptible mice infected with ECTV and that these are clearly associated with the differences in the NK and CTL responses. The resistant C57BL/6 mice expressed IL-2, IL-4, IFN-γ, and TNF protein within the first few days of infection, whereas the susceptible strains (BALB/c and A/J) expressed IL-4, very little IFN-γ but no IL-2 or TNF (Fig. 4).
Intriguingly, the profile of cytokine expression in resistant mice was organ-specific (Fig. 4 A and C). Most strikingly, IL-2 and TNF were expressed in draining PLN but not in the spleen, whereas IFN-γ was expressed in spleen but not in PLN. The compartmentalization of cytokine production may be a reflection of the requirement for distinct cytokines in the priming site and the effector sites. Antigen-specific cells are primed in the PLN where they proliferate and migrate to effector sites such as the spleen, liver, and various other organs. In ECTV-infected mice, PLN become reactive early with evidence of cellular proliferation (16), consistent with a requirement for IL-2. On the other hand, IFN-γ is a critical effector molecule, in the absence of which virus clearance is severely impaired in all major organs except in the PLN (Table 2 and ref. 16).
Although clear differences in cytokine expression in spleen and PLN were observed at the protein level (Fig. 4 and Table 1), the expression of mRNA for IL-2, IL-4, IFN-γ, and TNF in BALB/c mice reached levels almost comparable with C57BL/6 mice, albeit with a delay of 1-2 days (G.K. and R.M.L.B., unpublished observations). Therefore, despite the expression of mRNA for these cytokines in both strains of mice, specific cytokine protein expression was selective, indicating clearly the existence of posttranscriptional regulation of cytokine expression in vivo, a phenomenon that is often overlooked. Moreover, we have also found that PLN from infected C57BL/6 mice that express IFN-γ mRNA, but no IFN-γ-producing cells in vivo, can be restimulated with antigen in short-term 6-h cultures in vitro to express IFN-γ protein (G.K. and R.M.L.B., unpublished observations). These data highlight the importance of the immunohistochemical analyses approach taken here to determine the profile of cytokine expression as an accurate reflection of the situation in situ.
To investigate whether the types of cytokines produced influence the nature of the immune response and disease outcome, we measured critical immune parameters in both resistant and susceptible strains of mice. In C57BL/6 mice, the NK cell response after infection is substantially increased above the background levels in uninfected mice and activity is sustained compared with the susceptible strains (Fig. 2A). The importance of the NK response to protective immunity has been highlighted in genetic studies that have mapped at least one resistance locus (Rmp1) in C57BL/6 mice to the NK cell gene complex (12, 32). Depletion of NK cells in C57BL/6 increases their susceptibility to infection with ECTV (ref. 17 and G.K. and R.M.L.B., unpublished data).
We have also previously shown that CD8+ CTL responses are critical in the recovery of mice from ECTV infection (5). Indeed, high levels of CTL activity can be measured ex vivo from draining PLN and spleens of C57BL/6 mice, compared with the susceptible strains of animals (Fig. 2 B and C). Furthermore, the cytolytic activity of virus-specific CTL was demonstrable at least 1-2 days earlier in the PLN and spleens of the former, compared with the latter. In contrast to the distinct differences in NK and CTL responses, there were no significant differences, between the resistant and susceptible strains, in the ECTV-specific IgM or IgG responses during the first 6 days of infection (Fig. 3). This suggested that Ab had little role to play in the early stages of infection. However, C57BL/6 mice mount substantial IgM and IgG Ab responses after day 7 p.i. and preliminary evidence indicates that this response is important in the later stages of infection for complete recovery (G.K., V.P., and G.C., unpublished work).
IFN-γ-producing cells were detected as early as day 2 p.i. in situ (Fig. 4) in the spleen, but the ex vivo IFN-γ production by splenocytes, after antigenic stimulation, was only evident by day 6 p.i., and this correlated with detectable CTL activity (Table 1). The IFN-γ-producing cells detected at early time points are likely to comprise NK, NKT, and γδ-TCR T cells, whereas the major cellular source of this cytokine from day 6 p.i. is CD8 cells. The peak CTL activity and peak IFN-γ production by MHC class I-restricted CD8+ T cells coincided on day 8 p.i. (Table 2), and this preceded rapid virus clearance in the spleen and liver (Fig. 1).
To establish a causal link between the production of type 1 or type 2 cytokines and the resistance or susceptibility to ECTV infection, we tested the effect of blocking IL-2, TNF, or IFN-γ function in resistant mice and, conversely, of blocking IL-4 function in susceptible mice. Our data indicate that neutralizing IL-2 or TNF function individually did not significantly affect virus replication, susceptibility to ECTV, or the generation of antiviral CTL or NK cell responses in C57BL/6 mice (Fig. 5). However, the blockade of IFN-γ abolished the NK and CTL responses in C57BL/6 mice, indicating an important role for this cytokine in the activation/generation of NK cell and CD8 T cell cytolytic effector mechanisms.
IL-4 is known to down-regulate the production of type 1 cytokines. Indeed, infection of C57BL/6 mice with recombinant ECTV encoding IL-4 was shown to enhance virus replication and transformed an inapparent infection to full-blown mousepox (33). This report is consistent with our findings, which has established that the susceptible phenotype of BALB/c and A/J mice is associated with a type 2 cytokine response, with little or no IFN-γ. It was therefore of interest to test whether the absence of IL-4 in susceptible mice could alter the outcome of infection and result in the generation of optimal immune response and recovery from infection. However, our data clearly indicate that both IL-4 gene knockout mice on a BALB/c background and BALB/c mice treated with anti-IL-4 in vivo were similar to BALB/c mice in their response to ECTV infection. The absence of IL-4 did not overcome the susceptible phenotype. Because genetic resistance/susceptibility to mousepox is multigeneic, it is not surprising that inhibition of a single cytokine was insufficient to reverse susceptibility. Indeed, even the inactivation of signal transducer and activator of transcription 6 (STAT6), which is essential for IL-4 signaling and the subsequent development of the type 2 responses, was not sufficient for BALB/c mice to overcome infection with ECTV (34).
In recent years, the identification of a growing number of immune-modulating genes encoded by poxviruses has shed light on the importance of the host immune molecules that are targeted by these genes (35, 36). Not surprisingly, host IFN-γ is strategically targeted at various levels. To attenuate the effects of IFN-γ, ECTV, and other poxviruses like VARV encode a homolog of the ligand-binding α-chain of the host IFN-γ-receptor (IFN-γ-R) that can act as competitive antagonist (37). However, the precise function of the viral (v) IFN-γ-R homolog has not been fully elucidated and at least three lines of evidence support the notion that in ECTV the function of this homolog is not simply to block systemic IFN-γ in the host. First, inactivation of vIFN-γ-R homolog has no effect on virus virulence in A/J, CF1, and ICR mice (R.M.L.B., unpublished work). Second, administration of exogenous IFN-γ, at the site of infection (footpads), has no effect on viral replication locally but results in reduced virus titers in other organs (G.K., unpublished data). Third, administration of anti-IFN-γ mAb, at the site of infection, also has no effect on viral replication locally but results in increased virus titers in other organs (16). These results are consistent with an important role for IFN-γ in the host response and suggest that the vIFN-γ-R homolog may be more important locally for dampening the host response at the primary site of replication rather than for neutralizing systemic IFN-γ. Inactivation of the vIFN-γ-R homolog (B8R) in vaccinia virus resulted in an attenuated phenotype in one study (38) but was found to have no effect on virus virulence in another (39), further illustrating the complex role of the homolog. Because ECTV is a natural mouse pathogen and the vIFN-γ-R homolog it encodes specifically binds to mouse IFN-γ, this is an ideal model to clarify the complex role of this protein in virus-host interactions.
The elucidation of the mechanisms involved in the immune-modulating pathways used by poxviruses, in particular, those relating to host type 1 cytokine production, both at the transcriptional and translational level, will assist in a better understanding of the host response that results in immunity and recovery from a poxvirus infection. Indeed, such information will be necessary to determine whether the high mortality rates seen with smallpox in some human populations are related to host genes that result in a defective production of, or response to, Th1 cytokines, in particular, IFN-γ. More importantly, the identification of this pathway should assist in the development of new targets for preventative or therapeutic intervention.
Acknowledgments
We thank Marjan van Meurs for performing the immunohistochemical staining of organ sections and C. Parish and G. Ada for critically reading the manuscript. This work was supported by grants from the Howard Hughes Medical Institute (to G.K.), the National Health and Medical Research Council (Australia) (to G.K. and G.C.), and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (to R.M.L.B. and G.K.).
Abbreviations: ECTV, ectromelia virus; CTL, cytotoxic T lymphocyte; NK, natural killer; VARV, variola virus; PFU, plaque-forming unit; p.i., after infection; PLN, popliteal lymph node; Th1, type 1 T helper cells.
References
- 1.Henderson, D. A. (1999) Science 283, 1279-1282. [DOI] [PubMed] [Google Scholar]
- 2.Fenner, F. (1949) J. Immunol. 63, 341-373. [PubMed] [Google Scholar]
- 3.Blanden, R. V., Doherty, P. C., Dunlop, M. B., Gardner, I. D., Zinkernagel, R. M. & David, C. S. (1975) Nature 254, 269-270. [DOI] [PubMed] [Google Scholar]
- 4.Buller, R. M. & Palumbo, G. J. (1991) Microbiol. Rev. 55, 80-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karupiah, G., Buller, R. M., Van Rooijen, N., Duarte, C. J. & Chen, J. (1996) J. Virol. 70, 8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schell, K. (1960) Aust. J. Exp. Biol. Med. Sci. 38, 289-299. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann, T. R. & Sad, S. (1996) Immunol. Today 17, 138-146. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann, T. R. & Coffman, R. L. (1989) Annu. Rev. Immunol. 7, 145-173. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic, D., Sher, A. & Yap, G. (2001) Curr. Opin. Immunol. 13, 403-409. [DOI] [PubMed] [Google Scholar]
- 10.Sacks, D. & Noben-Trauth, N. (2002) Nat. Rev. Immunol. 2, 845-858. [DOI] [PubMed] [Google Scholar]
- 11.Ottenhoff, T. H., Verreck, F. A., Lichtenauer-Kaligis, E. G., Hoeve, M. A., Sanal, O. & van Dissel, J. T. (2002) Nat. Genet. 32, 97-105. [DOI] [PubMed] [Google Scholar]
- 12.Wallace, G. D., Buller, R. M. & Morse, H. C., III. (1985) J. Virol. 55, 890-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownstein, D., Bhatt, P. N. & Jacoby, R. O. (1989) Arch. Virol. 107, 35-41. [DOI] [PubMed] [Google Scholar]
- 14.Brownstein, D. G., Bhatt, P. N., Gras, L. & Jacoby, R. O. (1991) J. Virol. 65, 1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanden, R. V. (1971) J. Exp. Med. 133, 1074-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karupiah, G., Fredrickson, T., Holmes, K., Khairallah, L. & Buller, R. (1993) J. Virol. 67, 4214-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby, R. O., Bhatt, P. N. & Brownstein, D. G. (1989) Arch. Virol. 108, 49-58. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill, H. C. & Brenan, M. (1987) J. Gen. Virol. 68, 2669-2673. [DOI] [PubMed] [Google Scholar]
- 19.Ramshaw, I. A., Ramsay, A. J., Karupiah, G., Rolph, M. S., Mahalingam, S. & Ruby, J. C. (1997) Immunol. Rev. 159, 119-135. [DOI] [PubMed] [Google Scholar]
- 20.Mullbacher, A., Hla, R. T., Museteanu, C. & Simon, M. M. (1999) J. Virol. 73, 1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karupiah, G. (1998) Vet. Immunol. Immunopathol. 63, 105-109. [DOI] [PubMed] [Google Scholar]
- 22.Dalton, D. K., Pitts-Meek, S., Keshav, S., Figari, I. S., Bradley, A. & Stewart, T. A. (1993) Science 259, 1739-1742. [DOI] [PubMed] [Google Scholar]
- 23.Noben-Trauth, N., Kohler, G., Burki, K. & Ledermann, B. (1996) Transgenic Res. 5, 487-491. [DOI] [PubMed] [Google Scholar]
- 24.Chen, W., Drillien, R., Spehner, D. & Buller, R. M. (1992) Virology 187, 433-442. [DOI] [PubMed] [Google Scholar]
- 25.Scalzo, A. A., Farrell, H. E. & Karupiah, G. (2000) Methods Mol. Biol. 121, 163-177. [DOI] [PubMed] [Google Scholar]
- 26.Karupiah, G., Coupar, B. E., Andrew, M. E., Boyle, D. B., Phillips, S. M., Mullbacher, A., Blanden, R. V. & Ramshaw, I. A. (1990) J. Immunol. 144, 290-298. [PubMed] [Google Scholar]
- 27.Karupiah, G., Woodhams, C. E., Blanden, R. V. & Ramshaw, I. A. (1991) J. Immunol. 147, 4327-4332. [PubMed] [Google Scholar]
- 28.van den Eertwegh, A. J., Fasbender, M. J., Schellekens, M. M., van Oudenaren, A., Boersma, W. J. & Claassen, E. (1991) J. Immunol. 147, 439-446. [PubMed] [Google Scholar]
- 29.van den Eertwegh, A. J., Noelle, R. J., Roy, M., Shepherd, D. M., Aruffo, A., Ledbetter, J. A., Boersma, W. J. & Claassen, E. (1993) J. Exp. Med. 178, 1555-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karupiah, G., Blanden, R. V. & Ramshaw, I. A. (1990) J. Exp. Med. 172, 1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenner, F., Henderson, D. A., Arita, I., Jesek, Z. & Ladnyi, I. D. (1988) Smallpox and Its Eradication (WHO Press, Geneva).
- 32.Delano, M. L. & Brownstein, D. G. (1995) J. Virol. 69, 5875-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson, R. J., Ramsay, A. J., Christensen, C. D., Beaton, S., Hall, D. F. & Ramshaw, I. A. (2001) J. Virol. 75, 1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahalingam, S., Karupiah, G., Takeda, K., Akira, S., Matthaei, K. I. & Foster, P. S. (2001) Proc. Natl. Acad. Sci. USA 98, 6812-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alcami, A. (2003) Nat. Rev. Immunol. 3, 36-50. [DOI] [PubMed] [Google Scholar]
- 36.Seet, B. T., Johnston, J. B., Brunetti, C. R., Barrett, J. W., Everett, H., Cameron, C., Sypula, J., Nazarian, S. H., Lucas, A. & McFadden, G. (2003) Annu. Rev. Immunol. 21, 377-423. [DOI] [PubMed] [Google Scholar]
- 37.Mossman, K., Upton, C., Buller, R. M. & McFadden, G. (1995) Virology 208, 762-769. [DOI] [PubMed] [Google Scholar]
- 38.Verardi, P. H., Jones, L. A., Aziz, F. H., Ahmad, S. & Yilma, T. D. (2001) J. Virol. 75, 11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symons, J. A., Tscharke, D. C., Price, N. & Smith, G. L. (2002) J. Gen. Virol. 83, 1953-1964. [DOI] [PubMed] [Google Scholar]