Abstract
The reaction of 5-(1-adamantyl)-4-ethyl or allyl-1,2,4-triazoline-3-thione with formaldehyde solution and various 1-substituted piperazines yielded the corresponding N-Mannich bases. The newly synthesized N-Mannich bases were tested for in vitro inhibitory activities against a panel of Gram-positive and Gram-negative bacteria and the yeast-like pathogenic fungus Candida albicans. Six compounds showed potent antibacterial activity against one or more of the tested microorganisms, while two compounds exhibited moderate activity against the tested Gram-positive bacteria. None of the newly synthesized compounds were proved to possess marked activity against Candida albicans. The oral hypoglycemic activity of six compounds was determined in streptozotocin (STZ)-induced diabetic rats. Four compounds produced significant strong dose-dependent reduction of serum glucose levels, compared to gliclazide at 10 mg/kg dose level (potency ratio > 75%).
Keywords: adamantane derivatives; 1,2,4-triazoles; N-Mannich bases; antimicrobial activity; hypoglycemic activity
1. Introduction
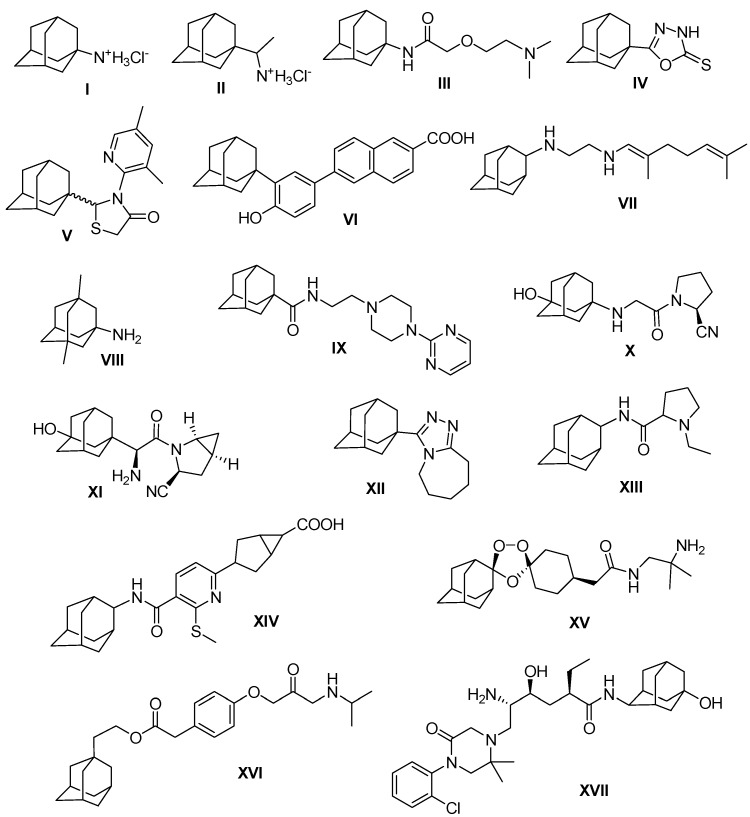
In almost all cases, an adamantyl-bearing compound will be more lipophilic than the des-adamantyl analogue. Beyond increasing partition coefficient, the adamantyl group positively modulates the therapeutic index of many experimental compounds through a variety of mechanisms [1,2]. Several adamantane derivatives have long been known for their diverse biological activities (Figure 1).
Figure 1.
Biologically-active adamantane derivatives.
Amantadine (I) was discovered in 1960 as potent antiviral drug for the treatment of influenza A infection [3,4,5] and as an antiparkinsonian drug [6]. Further studies based on amantadine resulted in the discovery of more potent antiviral drugs such as rimantadine (II) [7], and tromantadine (III) [8]. Several adamantane derivatives were also shown to possess marked inhibitory activity against human immunodeficiency viruses (HIV) [9,10,11,12]. The adamantyl-1,3,4-oxadiazoline-2-thione (IV) [11], and the adamantyl-thiazolidin-4-one (V) [12] displayed potent anti-HIV activity. The synthetic retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid CD437 (VI) was discovered as a potent inducer of apoptosis in human head and neck squamous cell carcinoma [13]. Several adamantane derivatives were recognized as potent bactericidal and fungicidal agents [14,15,16,17,18,19,20,21,22,23]. SQ109 (VII) was approved for use against drug-susceptible and drug-resistant tuberculosis strains [23]. SQ109 also showed excellent inhibitory activity against helicobacter pylori related duodenal ulcers and carcinomas, and Candida glabrata [24]. Memantine (VIII), the dimethyl analogue of amantadine, is used as effective therapy for treatment of moderate to severe Alzheimer’s disease [25]. Adatanserin (IX) is a mixed 5-HTA receptor agonist and also has neuroprotective effects against ischemia-induced glutamatergic excitotoxicity [26]. Vildagliptin (X) [27] and Saxagliptin (XI) [28] are members of a new class of oral hypoglycemic agents known as dipeptidyl peptidase IV (DPP-IV) inhibitors, which were approved for the treatment of type 2 diabetes [29]. Adamantane derivatives constitutes the major class of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitor, which are considered important therapy for controlling non-insulin-dependent diabetes, hyperglycemia, obesity, insulin resistance, hyperlipidemia, hypertension and other symptoms associated with excessive body cortisol [30,31]. MK-544 (XII) [32], PF-877423 (XIII) [33], AZD6925 (XIV) [34], are recently developed drug candidates for the treatment of non-insulin-dependent diabetes and obesity. Moreover, anti-inflammatory activity was reported in several adamantane-containing molecules [19,20,21,22,35,36,37,38]. Arterolane (XV) [39], adaprolol (XVI) [40] and DS-8108b (XVII) [41] are recently developed adamantane-containing drugs for the treatment of malaria, glaucoma and hypertension, respectively.
In continuation of our interest in the chemical and pharmacological properties of adamantane derivatives [11,18,19,20,21,22,35], we report herein the synthesis of new series of 5-(1-adamantyl)-4-substituted-1,2,4-triazole N-Mannich bases, and their in vitro antimicrobial and in vivo hypoglycemic activities.
2. Results and Discussion
2.1. Chemistry
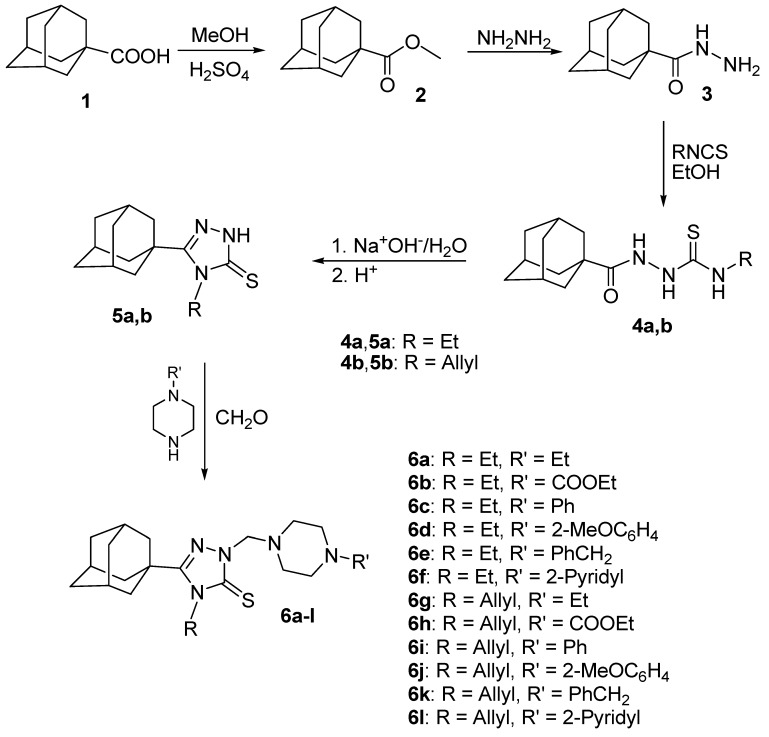
Adamantane-1-carbohydrazide 3, required as starting material, was obtained via esterification of adamantane-1-carboxylic acid 1 with methanol to get the methyl ester 2, which was subsequently heated with hydrazine to afford the target carbohydrazide 3 [35]. The reaction of compound 3 with ethyl or allyl isothiocyanate yielded the intermediate 1-(1-adamantylcarbonyl)-4-substituted thiosemicarbazides 4a and 4b, which were cyclized to the corresponding 5-(1-adamantyl)-4-substituted-1,2,4-triazoline-3-thiones 5a [35] and 5b [19] via heating in 10% aqueous sodium hydroxide. Compounds 5a,b were reacted with the corresponding 1-substituted piperazine and formaldehyde solution in ethanol to yield the corresponding N-Mannich bases 6a–l in good to moderate yields. The reaction was carried out by heating the reactants in ethanol for 15 min to enhance the solubility of compounds 5a,b (Scheme 1, Table 1). The structures of compounds 6a–l were confirmed by elemental analyses, in addition to the 1H NMR, 13C NMR, and electrospray ionization (ESI-MS) or electron impact (EI-MS) mass spectral data which were in full agreement with their structures, and the X-ray spectra of compounds 6c [42], 6d [43] and 6e [44].
Scheme 1.
Synthesis the target N-Mannich bases 6a–l.
Table 1.
Crystallization solvents (Cryst. Solv.), melting points (m.p.), yield percentages, molecular formulae and molecular weights (Mol. Wt.) of compounds 6a–l.
| Comp. No. | R | R' | Cryst. Solv. | m.p. (°C) | Yield (%) | Molecular Formula (Mol. Wt.) |
|---|---|---|---|---|---|---|
| 6a | C2H5 | C2H5 | EtOH/H2O | 158–160 | 77 | C21H35N5S (389.6) |
| 6b | C2H5 | COOC2H5 | EtOH/H2O | 153–155 | 72 | C22H35N5O2S (433.61) |
| 6c | C2H5 | C6H5 | EtOH | 139–141 | 88 | C25H35N5S (437.64) |
| 6d | C2H5 | 2-CH3OC6H4 | EtOH | 204–206 | 92 | C26H37N5OS (467.67) |
| 6e | C2H5 | C6H5CH2 | EtOH | 168–170 | 81 | C26H37N5S (451.67) |
| 6f | C2H5 | 2-Pyridyl | EtOH/H2O | 144–146 | 92 | C24H34N6S (438.63) |
| 6g | CH2=CHCH2 | C2H5 | EtOH/H2O | 109–111 | 66 | C22H35N5S (401.61) |
| 6h | CH2=CHCH2 | COOC2H5 | EtOH/H2O | 114–116 | 60 | C23H35N5O2S (445.62) |
| 6i | CH2=CHCH2 | C6H5 | EtOH | 161–163 | 69 | C26H35N5S (449.65) |
| 6j | CH2=CHCH2 | 2-CH3OC6H4 | EtOH | 208–210 | 74 | C27H37N5OS (479.68) |
| 6k | CH2=CHCH2 | C6H5CH2 | EtOH/H2O | 109–111 | 59 | C27H37N5S (463.68) |
| 6l | CH2=CHCH2 | 2-Pyridyl | EtOH/H2O | 133–135 | 66 | C25H34N6S (450.64) |
2.2. In Vitro Antimicrobial Activity
The newly synthesized compounds 6a–l were tested for their in vitro growth inhibitory activity against the standard strains of the Institute of Fermentation of Osaka (IFO) namely; Staphylococcus aureus IFO 3060, Bacillus subtilis IFO 3007, Micrococcus luteus IFO 3232 (Gram-positive bacteria), Escherichia coli IFO 3301, Pseudomonas aeuroginosa IFO 3448 (Gram-negative bacteria), and the yeast-like pathogenic fungus Candida albicans IFO 0583. The primary screening was carried out using the agar disc-diffusion method using Müller-Hinton agar medium [45]. The results of the preliminary antimicrobial testing of compounds 6a–l (200 μg/disc), the antibacterial antibiotics Ampicillin trihydrate, Gentamicin (100 μg/disc) and the antifungal drug Clotrimazole (100 μg/disc) and the calculated log p values (Clog p) of the tested compounds (calculated using the CS ChemOffice Ultra version 8.0, CambridgeSoft, Cambridge, MA, USA) are shown in Table 2.
Table 2.
Antimicrobial activity of compounds 6a–l (200 μg/8 mm disc), the broad spectrum antibacterial drugs Gentamicin (100 μg/8 mm disc), Ampicillin (100 μg/8 mm disc) and the antifungal drug Clotrimazole (100 μg/8 mm disc) against Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS), Micrococcus luteus IFO 3232 (ML), Escherichia coli IFO 3301 (EC), Pseudomonas aeuroginosa IFO 3448 (PA), and Candida albicans IFO 0583 (CA).
| Comp. No. | Clog p | Diameter of Growth Inhibition Zone (mm) a | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | ||
| 6a | 3.96 | – | – | – | – | – | – |
| 6b | 5.51 | – | – | – | – | – | – |
| 6c | 5.67 | – | – | – | – | – | – |
| 6d | 5.69 | 15 | 17 | 11 | – | – | – |
| 6e | 6.59 | 21 (8) b | 20 (4) b | 17 | 14 | – | – |
| 6f | 4.72 | 15 | 18 (8) b | 11 | – | – | – |
| 6g | 4.21 | 11 | 13 | – | – | – | – |
| 6h | 5.76 | – | 12 | – | – | – | – |
| 6i | 5.92 | 14 | 16 | 12 | – | – | – |
| 6j | 5.82 | 19 (8) b | 18 (16) b | 18 (16) b | – | – | – |
| 6k | 6.84 | 28 (0.5) b | 30 (0.5) b | 19 (8) b | 18 (16) b | 12 | – |
| 6l | 4.97 | 17 | 19 (8) b | 10 | – | – | – |
| Gentamicin | 26 (2) b | 25 (2) b | 18 (2) b | 20 (0.5) b | 19 (1) b | NT | |
| Ampicillin | 23 (2) b | 21 (0.5) b | 19 (2) b | 17 (2) b | 16 (2) b | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 (2) b | |
a (–): Inactive (inhibition zone < 10 mm); NT: Not tested. b The figures shown in parentheses represent the minimal inhibitory concentration (MIC) values (μg/mL).
The results revealed that the tested compounds showed varying degrees of inhibition against the tested microorganisms. Strong antibacterial activity was displayed by the compounds 6d, 6e, 6f, 6j, 6k and 6l which produced growth inhibition zones ≥18 mm against one or more of the tested microorganisms. Meanwhile, the compounds 6c and 6i showed moderate activity (growth inhibition zones 14–17 mm), the compounds 6g and 6h produced marginal activity (growth inhibition zones 10–13 mm) and compounds 6a and 6b were practically inactive (growth inhibition zones < 10 mm) against the tested microorganisms.
The Gram-positive bacteria Bacillus subtilis and Staphylococcus aureus and to a lesser extent Micrococcus luteus are considered the most sensitive among the tested microorganisms. The activity against the tested Gram-negative bacteria was generally lower than that against the Gram-positive bacteria; compound 6d and 6k were strongly active against Escherichia coli and weakly active against Pseudomonas aeuroginosa. The newly synthesized compounds 6a–l lacked inhibitory activity against Candida albicans.
It was observed that the antibacterial activity of the newly synthesized N-Mannich bases 6a–l was mainly dependent on the nature of 4-piperazine substituents regardless the nature of the 1,2,4-triazole substituent at position 4 whether an ethyl or allyl. The presence of 4-aryl, benzyl or 2-pyridyl at the piperazine N-4 was optimistic for antibacterial activity, whereas, the aliphatic substituents (C2H5, COOC2H5) deteriorated the antibacterial activity. The lipophilicity of the compounds 6a–l had limited effect on the antibacterial activity. The minimal inhibitory concentrations (MIC) [46] for the most active compounds 6d, 6e, 6f, 6j, 6k and 6l, which are shown in Table 2, were in accordance with the results obtained in the primary antimicrobial screening.
2.3. In Vivo Hypoglycemic Activity
The oral hypoglycemic activity of compounds 6a, 6c, 6f, 6g, 6j and 6l was determined in streptozotocin (STZ)-induced diabetic rats. The compounds were tested at 10 and 20 mg/kg dose levels. The diabetogenic effect of STZ is the direct result of irreversible damage to the pancreatic beta cells, resulting in degranulation and loss of insulin secretion [47,48].
The results of oral hypoglycemic activity of compounds 6a, 6c, 6f, 6g, 6j and 6l (10 and 20 mg/kg) and the potent hypglycemic drug gliclazide in STZ-induced diabetic rats (10 mg/kg) are listed in Table 3. The highest activity was shown by compounds 6a, 6f, 6g and 6l, which produced significant strong dose-dependent reduction of serum glucose levels in STZ-induced diabetic rats, compared to gliclazide at 10 mg/kg dose level (potency ratio > 75%). Compound 6j displayed good hypoglycemic activity at 20 mg/kg dose level and a weak activity at 10 mg/kg dose level. Compound 6c produced medium activity at 10 mg/kg dose level (potency ratio 40.66%), without significant increase at 20 mg/kg dose level. The structure-hypoglycemic activity relationship of the tested adamantyl 1,2,4-triazoles N-Mannich bases revealed that the hypoglycemic activity is mainly dependent on the nature of the piperazine N-4 substituents. The piperazine N-4 ethyl and 2-pyridyl substituents were optimistic (compounds 6a, 6f, 6g and 6l), while the aromatic substituents (phenyl and 2-methoxyphenyl) were less active at 10 mg/kg dose level and retained the good activity at 20 mg/kg doses (compounds 6c and 6j). In addition, the 4-triazole substituents (ethyl and allyl) showed no significant influence on the hypoglycemic activity.
Table 3.
Oral hypoglycemic activity of compounds 6a, 6c, 6f, 6g, 6j and 6l (10 and 20 mg/kg) and gliclazide (10 mg/kg) in streptozotocin (STZ)-induced diabetic rats.
| Treatment | Results | ||
|---|---|---|---|
| C0 (mg/dL) a | C24 (mg/dL) a | % Glucose Reduction b | |
| Group 1 c | 302.6 ± 11.64 | 287.2 ± 16.85 | 5.09% |
| Group 2 d | 295.4 ± 17.52 | 183.0 ± 13.38 * | 38.05% |
| 6a (10 mg/kg) | 289.0 ± 18.05 | 204.4 ± 7.37 * | 29.27% (76.93%) |
| 6a (20 mg/kg) | 290.4 ± 10.60 | 188.0 ± 8.6 * | 35.26% (46.34%) |
| 6c (10 mg/kg) | 285.8 ± 14.31 | 241.6 ± 19.2 | 15.47% (40.66%) |
| 6c (20 mg/kg) | 297.6 ± 18.57 | 245.4 ± 11.54 | 17.54% (23.05%) |
| 6f (10 mg/kg) | 289.0 ± 14.35 | 197.8 ± 6.13 * | 31.56% (82.94%) |
| 6f (20 mg/kg) | 292.6 ± 10.33 | 189.0 ± 7.42 * | 35.41% (46.53%) |
| 6g (10 mg/kg) | 291.0 ± 5.58 | 190.6 ± 9.05 * | 34.50% (90.67%) |
| 6g (20 mg/kg) | 283.4 ± 10.45 | 184.0 ± 15.28 * | 35.07% (46.09%) |
| 6j (10 mg/kg) | 278.4 ± 15.07 | 228.4 ± 11.41 | 17.96% (47.20%) |
| 6j (20 mg/kg) | 297.4 ± 17.94 | 203.4 ± 15.28 * | 31.61% (41.54%) |
| 6l (10 mg/kg) | 290.4 ± 18.37 | 199.0 ± 18.75 * | 31.47% (82.71%) |
| 6l (20 mg/kg) | 295.4 ± 12.58 | 200.0 ± 18.78 * | 32.30% (42.45%) |
a Results are expressed as mean ± SEM. (n = 5); b The figures shown in parentheses are the relative potency compared with glicalzide; c Treated with a single oral dose of 0.5% (w/v) aqueous CMC solution (5 mL/kg); d Treated with 10 mg/kg gliclazide in 0.5% (w/v) aqueous CMC; and * Significant difference at p < 0.01 compared with the corresponding control.
2.4. Oral Acute Toxicity Testing
The method of Litchfield and Wilcoxon was adopted for measuring the acute oral toxicity of compounds 6a, 6f, 6g and 6l which possessed the highest hypoglycemic activity [49]. The acute toxicity results of compounds 6a, 6f, 6g and 6l in normal albino mice are listed in Table 4. The oral LD50 of gliclazide was reported to be >3000 mg/kg in mice [50]. Although the oral acute toxicity of the tested adamantyl 1,2,4-triazole derivatives is higher than that of gliclazide, the compounds induce their hypoglycemic activity at safe doses.
Table 4.
Oral acute toxicity (mg/kg) of compounds 6a, 6f, 6g and 6l in normal albino mice.
| Comp. No. | LD50 * | LD50 (95% Confidence Limit) |
|---|---|---|
| 6a | 892 ± 38.70 | 892 (844–1089) |
| 6f | 787 ± 41.05 | 787 (721–923) |
| 6g | 833 ± 23.80 | 833 (765–891) |
| 6l | 741 ± 22.50 | 741 (624–806) |
* Results are expressed as mean ± SEM. (n = 6).
3. Experimental Section
3.1. General
Melting points (°C) were measured in open glass capillaries using a Branstead 9100 Electrothermal melting point apparatus (Thermo Fisher Scientific, Waltham, MA, USA) and are uncorrected. NMR spectra were obtained on a Bruker AC 500 Ultra Shield NMR spectrometer (Bruker, Fällanden, Switzerland) operating at 500.13 MHz for 1H and 125.76 MHz for 13C, the chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane (TMS) as internal standard; Coupling constants (J) are expressed in Hz. Electrospray ionization mass spectra (ESI-MS) were recorded on an Agilent 6410 Triple Quad tandem mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) at 4.0 and 3.5 kV for positive and negative ions, respectively. High resolution mass spectra (HR-MS) were recorded on JEOL JMS-700 (JEOL, Tokyo, Japan) using Electron Impact (EI) ionization mode by keeping ionization energy at 70 eV. Elemental analyses (C, H, N and S) were in agreement with the proposed structures within ±0.4% of the theoretical values. Monitoring the reactions and checking the purity of the final products were carried out by thin layer chromatography (TLC) using silica gel precoated aluminum sheets (60 F254, Merck, Darmstadt, Germany) and visualization with ultraviolet light (UV) at 365 and 254 nm. The bacterial strains and Candida albicans fungus were obtained from the Institute of Fermentation of Osaka (IFO), Osaka, Japan. The reference drugs Ampicillin trihydrate (CAS 7177-48-2), Gentamicin sulfate (CAS 1405-41-0), Clotrimazole (CAS 23593-75-1) and Gliclazide (CAS 21187-98-4) were purchased from Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany. The Sprague-Dawley rats and the normal albino mice were purchased from a local animal house (Abu-Rawash, Giza, Egypt). The animal experiments for the determination of the hypoglycemic activity and acute toxicity were performed according to the Ethics Committee of the National Research Centre and in accordance with the recommendations for the proper care and use of laboratory animals “Canadian Council on Animal Care Guidelines, 1984”.
3.2. General Procedure for the Preparation of 5-(1-Adamantyl)-4-ethyl or allyl-2-(4-substituted piperazine-1-ylmethyl)-1,2,4-triazoline-3-thiones 6a–l
A mixture of compound 5a or 5b (2.0 mmol), the appropriate N-substituted piperazine (2.0 mmol) and 37% formaldehyde solution (0.5 mL), in ethanol (8 mL), was heated under reflux for 15 min when a clear solution was obtained. Stirring was continued for 12 h at room temperature and the mixture was allowed to stand overnight. Cold water (5 mL) was added and the reaction mixture was stirred for 20 min. The precipitated crude products were filtered, washed with water, dried, and crystallized from ethanol or aqueous ethanol.
6a: 1H NMR (CDCl3): δ 1.29–1.36 (m, 6H, CH2CH3), 1.69–1.72 (m, 6H, Adamantane-H), 1.98 (s, 6H, Adamantane-H), 2.01 (s, 3H, Adamantane-H), 2.60–2.94 (m, 8H, Piperazine-H), 4.14–4.21 (m, 4H, CH2CH3), 5.05 (s, 2H, CH2). 13C NMR: δ 13.75, 16.77 (CH2CH3), 27.97, 35.27, 36.30, 39.82 (Adamantane-C), 41.07, 41.63 (CH2CH3), 48.87, 52.26 (Piperazine-C), 68.34 (CH2), 157.97 (Triazole C-5), 168.73 (C=S). ESI-MS, m/z: 390.3 (M + H)+. EI-HRMS, m/z (Rel. Int.): 391.9701 (15.35), 390.9654 (72.55), 389.9709 (100), 263.1793 (6.89), 233.9379 (16.86), 229.9876 (49.0), 221.9822 (30.64), 220.9799 (16.40), 219.9868 (71.11), 208.0032 (22.56), 205.9923 (66.25), 165.9672 (13.29), 155.9966 (21.69), 135.9874 (20.40), 127.0312 (59.65), 125.0127 (19.34), 112.9976 (12.81), 83.9997 (71.21), 76.9676 (26.56).
6b: 1H NMR (CDCl3): δ 1.17 (t, 3H, CH2CH3, J = 7.0 Hz), 1.33 (t, 3H, CH2CH3, J = 7.0 Hz), 1.69–1.76 (m, 6H, Adamantane-H), 1.98 (s, 6H, Adamantane-H), 2.06 (s, 3H, Adamantane-H), 2.73 (br. s, 4H, Piperazine-H), 3.45 (br. s, 4H, Piperazine-H), 4.02 (q, 2H, CH2CH3, J = 7.0 Hz), 4.17 (q, 2H, CH2CH3, J = 7.0 Hz), 5.07 (s, 2H, CH2). 13C NMR: δ 12.73 (CH2CH3), 13.62 (CH2CH3), 26.92, 34.28, 35.26, 38.82 (Adamantane-C), 40.43 (CH2CH3), 44.93, 49.25 (Piperazine-C), 60.40 (CH2CH3), 71.50 (CH2), 154.38 (C=O), 155.38 (Triazole C-5), 166.33 (C=S). ESI-MS, m/z: 434.3 (M + H)+. EI-HRMS, m/z (Rel. Int.): 433.9816 (12.48), 432.9806 (41.65), 388.7850 (9.89), 276.0345 (7.42), 263.9471 (33.73), 262.9412 (71.36), 189.0572 (22.73), 172.0117 (92.34), 171.0052 (100), 169.9955 (51.80), 135.0313 (47.86), 127.0339 (65.01), 96.9849 (27.69), 83.9934 (10.11), 70.0012 (20.73).
6c: 1H NMR (CDCl3): δ 1.13 (t, 3H, CH2CH3, J = 7.0 Hz), 1.67-1.73 (m, 6H, Adamantane-H), 1.96 (s, 6H, Adamantane-H), 2.03 (s, 3H, Adamantane-H), 2.88 (s, 4H, Piperazine-H), 3.09 (s, 4H, Piperazine-H), 4.17 (q, 2H, CH2CH3, J = 7.0 Hz), 5.08 (s, 2H, CH2), 6.46–6.83 (m, 3H, Ar-H), 7.15–7.17 (m, 2H, Ar-H). 13C NMR: δ 13.81 (CH2CH3), 27.95, 35.24, 36.31, 39.90 (Adamantane-C), 43.43 (CH2CH3), 49.40, 50.37 (Piperazine-C), 68.80 (CH2), 116.32, 119.99, 129.12, 151.27 (Ar-C), 156.10 (Triazole C-5), 168.75 (C=S). ESI-MS, m/z: 438.3 (M + H)+.
6d: 1H NMR (CDCl3): δ 1.32 (t, 3H, CH2CH3, J = 7.0 Hz), 1.71–1.76 (m, 6H, Adamantane-H), 1.98–2.12 (m, 9H, Adamantane-H), 3.08 (s, 8H, Piperazine-H), 3.81 (s, 3H, OCH3), 4.15 (q, 2H, CH2CH3, J = 7.0 Hz), 5.15 (s, 2H, CH2), 6.79–7.01 (m, 4H, Ar-H). 13C NMR: δ 13.76 (CH2CH3), 27.92, 35.32, 36.48, 39.83 (Adamantane-C), 43.83 (CH2CH3), 47.40, 50.18 (Piperazine-C), 55.48 (OCH3), 72.58 (CH2), 111.43, 118.38, 121.12, 123.55, 152.13, 152.26 (Ar-C), 156.57 (Triazole C-5), 167.34 (C=S). ESI-MS, m/z: 468.4 (M + H)+. EI-HRMS, m/z (Rel. Int.): 469.0323 (4.53), 468.0119 (11.48), 263.7015 (23.50), 262.6953 (10.46), 261.6985 (11.70), 205.9870 (70.30), 203.9838 (45.34), 202.9813 (34.01), 190.0338 (54.50), 162.0190 (39.20), 136.0422 (12.45), 135.0388 (82.48), 133.9844 (49.53), 119.9954 (30.67), 107.0433 (6.26), 90.9758 (15.70), 78.9755 (22.69), 76.9620 (19.90), 70.0146 (100), 64.9748 (9.53), 55.9825 (14.75).
6e: 1H NMR (CDCl3): δ 1.13 (t, 3H, CH2CH3, J = 6.5 Hz), 1.68–1.73 (m, 6H, Adamantane-H), 1.98–2.10 (m, 9H, Adamantane-H), 2.72–2.76 (m, 4H, Piperazine-H), 3.24–3.26 (m, 4H, Piperazine-H), 3.72 (s, 2H, Benzylic-CH2), 4.15 (q, 2H, CH2CH3, J = 6.5 Hz), 5.02 (s, 2H, CH2), 7.20–7.54 (m, 5H, Ar-H). 13C NMR: δ 12.74 (CH2CH3), 26.90, 34.36, 35.26, 38.82 (Adamantane-C), 40.62 (CH2CH3), 46.01, 50.37 (Piperazine-C), 61.59 (Benzylic-CH2), 66.66 (CH2), 127.40, 128.31, 129.19, 130.57 (Ar-C), 155.55 (Triazole C-5), 167.73 (C=S). ESI-MS, m/z: 452.3 (M + H)+. EI-HRMS, m/z (Rel. Int.): 451.6757 (2.35), 163.8833 (8.26), 262.8768 (27.15), 219.9799 (3.46), 205.9263 (4.21), 190.0603 (35.75), 189.0528 (100), 188.0480 (29.73), 146.0319 (7.87), 135.0343 (17.63), 131.9975 (4.73), 96.9961 (8.44), 91.9824 (8.09), 90.9752 (74.21), 76.9642 (4.83), 64.9756 (7.23).
6f: 1H NMR (CDCl3): δ 1.41 (t, 3H, CH2CH3, J = 7.0 Hz), 1.77–1.83 (m, 6H, Adamantane-H), 2.06 (s, 6H, Adamantane-H), 2.12 (s, 3H, Adamantane-H), 2.92 (t, 4H, Piperazine-H, J = 5.0 Hz), 3.57 (br. s, 4H, Piperazine-H), 4.25 (q, 2H, CH2CH3, J = 7.0 Hz), 5.18 (s, 2H, CH2), 6.62–6.66 (m, 2H, Pyridine-H), 7.48–7.50 (m, 1H, Pyridine-H), 8.18 (d, 1H, Pyridine-H, J = 3.5 Hz). 13C NMR: δ 13.79 (CH2CH3), 27.94, 36.29, 37.09, 39.29 (Adamantane-C), 41.58 (CH2CH3), 45.35, 50.24 (Piperazine-C), 68.92 (CH2), 107.35, 113.25, 137.83, 148.03, 156.11 (Pyridine-C), 158.99 (Triazole C-5), 167.73 (C=S). ESI-MS, m/z: 439.3 (M + H)+. EI-HRMS, m/z (Rel. Int.): 439.8562 (3.34), 438.8099 (10.97), 263.9348 (9.29), 262.9257 (33.99), 229.9813 (5.54), 177.0416 (47.75), 176.0352 (100), 175.02686 (32.67), 147.0295 (37.10), 135.0451 (12.27), 133.0068 (12.67), 121.0062 (48.64), 107.0549 (14.98), 95.0212 (13.15), 78.9646 (9.30), 77.9577 (14.59).
6g: 1H NMR (CDCl3): δ 1.11 (t, 3H, CH2CH3, J = 7.2 Hz), 1.77–1.79 (m, 6H, Adamantane-H), 2.01–2.10 (m, 9H, Adamantane-H), 2.51 (br. s, 8H, Piperazine-H), 2.92 (q, 2H, CH2CH3, J = 7.2 Hz), 4.88–4.93 (m, 2H, –CH2–CH=), 5.01–5.02 (m, 1H, CH2=CH), 5.14–5.20 (m, 3H, CH2=CH & CH2), 5.90–5.92 (m, 1H, CH2=CH). 13C NMR: δ 13.55 (CH2CH3), 28.05, 35.87, 36.37, 40.0 (Adamantane-C), 49.35 (–CH2–CH=), 49.85, 51.65 (Piperazine-C), 71.75 (CH2), 116.95 (CH2=CH), 132.65 (CH2=CH), 155.85 (Triazole C-5), 168.25 (C=S). ESI-MS, m/z: 402.4 (M + H)+.
6h: 1H NMR (CDCl3): δ 1.15 (t, 3H, CH2CH3, J = 7.0 Hz), 1.67–1.74 (m, 6H, Adamantane-H), 1.96 (s, 6H, Adamantane-H), 2.03 (s, 3H, Adamantane-H), 2.67–2.69 (m, 4H, Piperazine-H), 3.39–3.41 (m, 4H, Piperazine-H), 4.02 (q, 2H, CH2CH3, J = 7.0 Hz), 4.83–4.84 (m, 2H, –CH2–CH=), 4.87–4.90 (m, 1H, CH2=CH), 5.06 (s, 2H, CH2), 5.20–5.22 (m, 1H, CH2=CH), 5.80–5.86 (m, 1H, CH2=CH) 13C NMR: δ 14.62 (CH2CH3), 27.90, 35.34, 36.26, 39.91 (Adamantane-C), 43.63, 50.27 (Piperazine-C), 47.63 (–CH2–CH=), 61.53 (CH2CH3), 69.23 (CH2), 117.56 (CH2=CH), 131.62 (CH2=CH), 155.50 (C=O), 156.56 (Triazole C-5), 169.48 (C=S). ESI-MS, m/z: 446.3 (M + H)+.
6i: 1H NMR (CDCl3): δ 1.66–1.74 (m, 6H, Adamantane-H), 1.96–2.09 (m, 9H, Adamantane-H), 3.20 (br. s, 4H, Piperazine-H), 3.49 (br. s, 4H, Piperazine-H), 4.81–4.85 (m, 2H, –CH2–CH=), 4.95–5.01 (m, 1H, CH2=CH), 5.22–5.26 (m, 3H, CH2=CH & CH2), 5.80–5.83 (m, 1H, CH2=CH), 6.90–6.98 (m, 2H, Ar-H), 7.23–7.38 (m, 3H, Ar-H). 13C NMR: δ 27.89, 35.48, 36.26, 39.87 (Adamantane-C), 43.30, 48.07 (Piperazine-C), 48.52 (–CH2–CH=), 72.73 (CH2), 117.47 (CH2=CH), 129.46 (CH2=CH), 115.42, 118.12, 139.46, 149.06 (Ar-C), 156.92 (Triazole C-5), 168.05 (C=S). ESI-MS, m/z: 450.3 (M + H)+.
6j: 1H NMR (CDCl3): δ 1.69–1.74 (m, 6H, Adamantane-H), 1.96–1.98 (m, 6H, Adamantane-H), 2.04 (s, 3H, Adamantane-H), 2.90–3.20 (m, 8H, Piperazine-H), 3.81 (s, 3H, OCH3), 4.79–4.85 (m, 2H, –CH2–CH=), 4.95–5.01 (m, 1H, CH2=CH), 5.17 (s, 2H, CH2), 5.22–5.24 (m, 1H, CH2=CH), 5.80–5.87 (m, 1H, CH2=CH), 6.79–6.99 (m, 4H, Ar-H). 13C NMR: δ 27.94, 35.48, 36.30, 39.86 (Adamantane-C), 43.80, 50.61 (Piperazine-C), 47.56 (–CH2–CH=), 55.48 (OCH3), 72.72 (CH2), 118.12 (CH2=CH), 131.05 (CH2=CH), 110.90, 111.43, 118.98, 121.13, 152.15, 152.26 (Ar-C), 156.91 (Triazole C-5), 168.06 (C=S). ESI-MS, m/z: 480.4 (M + H)+. EI-HRMS, m/z (Rel. Int.): 479.6629 (1.54), 275.6026 (1.07), 240.3820 (2.66), 235.9632 (26.93), 206.0158 (7.68), 203.9816 (6.69), 189.9879 (6.09), 162.0124 (100), 171.0076 (17.38), 160.0030 (8.43), 149.0092 (2.29), 135.0312 (4.97), 133.9789 (3.35), 119.9954 (2.95), 69.9917 (10.89).
6k: 1H NMR (CDCl3): δ 1.65–1.73 (m, 6H, Adamantane-H), 1.94 (s, 6H, Adamantane-H), 2.02 (s, 3H, Adamantane-H), 2.42 (br. s, 4H, Piperazine-H), 2.78 (br. s, 4H, Piperazine-H), 3.45 (s, 2H, Benzylic CH2), 4.82 (s, 2H, –CH2–CH=), 4.91–4.94 (m, 1H, CH2=CH), 5.19–5.21 (m, 1H, CH2=CH), 5.05 (s, 2H, CH2), 5.80–5.83 (m, 1H, CH2=CH), 7.18–7.23 (m, 5H, Ar-H). 13C NMR: δ 27.92, 33.29, 36.30, 39.90 (Adamantane-C), 48.22, 52.99 (Piperazine-C), 50.20 (–CH2–CH=), 58.31 (Benzylic CH2), 69.12 (CH2), 117.71 (CH2=CH), 127.22, 128.25, 129.43, 137.52 (Ar-C), 131.46 (CH2=CH), 156.36 (Triazole C-5), 169.42 (C=S). ESI-MS, m/z: 464.4 (M + H)+.
6l: 1H NMR (CDCl3): δ 1.65–1.73 (m, 6H, Adamantane-H), 1.93 (s, 6H, Adamantane-H), 2.02 (s, 3H, Adamantane-H), 2.84–2.86 (m, 4H, Piperazine-H), 3.46–3.48 (m, 4H, Piperazine-H), 4.83–4.84 (m, 2H, –CH2–CH=), 4.85–4.89 (m, 1H, CH2=CH), 5.14 (s, 2H, CH2), 5.18–5.20 (m, 1H, CH2=CH), 5.81–5.83 (m, 1H, CH2=CH), 6.53–6.67 (m, 2H, Pyridine-H), 7.38–7.42 (m, 1H, Pyridine-H), 8.10 (d, 1H, Pyridine-H, J = 2.5 Hz). 13C NMR: δ 27.91, 35.32, 36.28, 39.93 (Adamantane-C), 45.33, 50.26 (Piperazine-C), 48.16 (–CH2–CH=), 69.16 (CH2), 117.54 (CH2=CH), 131.41 (CH2=CH), 107.26, 113.32, 137.68, 147.66, 159.29 (Pyridine-C), 156.48 (Triazole C-5), 169.48 (C=S). ESI-MS, m/z: 451.4 (M + H)+. EI-HRMS, m/z (Rel. Int.): 451.8970 (5.24), 450.9609 (15.48), 277.9037 (4.19), 276.9116 (15.45), 275.9136 (52.33), 260.3137 (35.78), 241.9785 (7.15), 177.0405 (67.28), 176.0315 (100), 175.0285 (39.89, 147.0125 (59.59), 144.9953 (7.64), 135.0403 (25.52), 133.0030 (19.26), 121.0062 (71.11), 112.9499 (11.31), 107.0064 (18.36), 94.9993 (13.64), 81.9705 (14.46), 78.9489 (15.17), 77.9388 (25.15), 66.9732 (5.36), 64.9541 (2.41).
3.3. Determination of the in Vitro Antimicrobial Activity (Agar Disc-Diffusion Method)
Sterile filter paper discs (8 mm diameter) were moistened with the compound solution in dimethylsulphoxide of specific concentration (200 μg/disc), the antibacterial antibiotics Gentamicin and Ampicillin trihydrate (100 μg/disc) and the antifungal drug Clotrimazole (100 μg/disc) were carefully placed on the agar culture plates that had been previously inoculated separately with the microorganisms. The plates were incubated at 37 °C, and the diameter of the growth inhibition zones were measured after 24 h in the case of bacteria and 48 h in the case of Candida albicans.
3.4. Determination of the Minimal Inhibitory Concentration (MIC)
Compounds 6d, 6e, 6f, 6j, 6k and 6l Gentamicin and Ampicillin trihydrate were dissolved in dimethylsulphoxide at a concentration of 128 μg/mL. The twofold dilutions of the solution were prepared (128, 64, 32, …, 0.5 μg/mL). The microorganism suspensions at 106 CFU/mL (colony forming unit/mL) concentrations were inoculated to the corresponding wells. The plates were incubated at 36 °C for 24 h. The MIC values were determined as the lowest concentration that completely inhibited visible growth of the microorganism as detected by unaided eye.
3.5. Determination of the in Vivo Hypoglycemic Activity
Animals: Locally bred male Sprague-Dawley rats (250 ± 30 g body weight) were obtained from Abu Rawash, Giza, Egypt. The rats were housed in wire-bottomed cages at 22 ± 2 °C. A standard pellet diet and tap water were supplied ad libitium. The animals were acclimatized to these conditions for 15 days before the experiment.
Induction of experimental diabetes: Rats were fasted for 16 h before the induction of diabetes with STZ (Sigma Chemical Co., St Louis, MO, USA). The animals were injected intraperitoneally with 0.22–0.25 mL of a freshly prepared solution STZ (60 mg/mL in 0.01 M citrate buffer, pH 4.5) at a final dose of 60 mg/kg body weight. Only rats with serum glucose levels greater than 250 mg/dL were used in experiments.
Design of the experiment: Uniform suspensions of the compounds 6a, 6c, 6f, 6g, 6j and 6l and the oral hypoglycemic drug gliclazide (positive control) in 0.5% (w/v) aqueous carboxymethyl cellulose (CMC) solution were prepared at specific concentrations of 10 mg/mL in case of the test compounds and gliclazide. 48 h post STZ injection, the hypoglycemic activity of the compounds 6a, 6c, 6f, 6g, 6j and 6l was assessed, the diabetic rats were fasted for 16 h and divided into 14 groups each of 5 animals (n = 5) and the serum glucose level was determined for each group and considered as initial fasting serum glucose (C0). Group 1, which served as the negative diabetic control group, received only a single oral dose of 0.5% (w/v) aqueous CMC solution (5 mL/kg). Groups 2 was treated with 10 mg/kg gliclazide in 0.5% (w/v) aqueous CMC (positive control). Groups 3–14 were treated with either a single oral dose of the 10 or 20 mg/kg of the test compounds. All treatments were administered by oral gavage. 24 h after treatment, the blood samples were collected and the serum glucose level (C24) was determined for each group.
Determination of serum glucose: Blood samples from the tail vein were collected, allowed to clot, and centrifuged at 2000 rpm for 10 min. The serum was separated and used on the same day for the measurement of serum glucose levels using a commercial glucose oxidase (GO) assay kit (Sigma-Aldrich). Blood glucose levels were expressed in mg/dL as mean ± standard error of mean (SEM). The data were statistically analyzed using ANOVA with Tukey’s multiple comparison test. The values of p < 0.01 were considered as significant. The percentage of serum glucose reduction for each group was calculated in relation to the initial serum glucose level as follows:
| % Serum glucose reduction = [(C0−C24/C0)] × 100 |
where C0 is the mean initial fasting serum glucose level, C24 is the mean serum glucose level 24 h after treatment.
3.6. Determination of the Oral Acute Toxicity of Compounds 6a, 6f, 6g and 6l
Freshly prepared suspensions of compounds 6a, 6f, 6g and 6l in concentrations of 1%, 3%, 4%, 6%, 8% and 12% in 0.5% aqueous carboxymethyl cellulose solution were prepared. Each compound was given to six groups each of six normal albino mice of both sexes by oral intubation in doses of 250, 500, 750, 1000, 1250 and 1500 mg/kg. The percentage mortality was recorded 24 h after compound administration and the oral lethal dose LD50 was calculated.
4. Conclusions
In this study, new N-Mannich bases of 5-(1-adamantyl)-4-substituted-1,2,4-triazoline-3-thiones 6a–l were synthesized and their in vitro antimicrobial activity was determined. Compounds 6d, 6e, 6f, 6j, 6k and 6l displayed potent antibacterial activity. In addition, the in vivo oral hypoglycemic activity of compounds 6a, 6c, 6f, 6g, 6j and 6l was determined in streptozotocin (STZ)-induced diabetic rats. Compounds 6a, 6f, 6g and 6l produced marked hypoglycemic activity compared gliclazide. Although, the active compounds are considered to be good candidates as newer antibacterial and hypoglycemic agents, further studies including the exploration of the mechanism of their biological activity are being undertaken.
Acknowledgments
The authors would like to extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this study through the research group project No. RGP-274.
Author Contributions
Ebtehal S. Al-Abdullah and Ali A. El-Emam designed the study; Hanaa M. Al-Tuwaijri, Mogedda E. Haiba, Ebtehal S. Al-Abdullah and Ali A. El-Emam synthesized the intermediate and target compounds and performed the interpretation of spectral data; Hanan M. Hassan conducted the hypoglycemic activity testing; Elsayed E. Habib performed the antimicrobial testing. All the authors participated in the preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lamoureux G., Artavia G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010;17:2967–2978. doi: 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Obando D., Liao V., Lifa T., Codd R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011;46:1949–1963. doi: 10.1016/j.ejmech.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 3.Davies W.L., Grunnert R.R., Haff R.F., McGahen J.W., Neumeyer E.M., Paulshock M., Watts J.C., Wood T.R., Hermann E.C., Hoffmann C.E. Antiviral activity of 1-adamantamine (amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 4.Wendel H.A., Snyder M.T., Pell S. Trial of amantadine in epidemic influenza. Clin. Pharmacol. Ther. 1966;7:38–43. doi: 10.1002/cpt19667138. [DOI] [PubMed] [Google Scholar]
- 5.Vernier V.G., Harmon J.B., Stump J.M., Lynes T.E., Marvel J.P., Smith D.H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol. Appl. Pharmacol. 1969;15:642–665. doi: 10.1016/0041-008x(69)90066-0. [DOI] [PubMed] [Google Scholar]
- 6.Schwab R.S., England A.C., Jr., Poskanzer D.C., Young R.R. Amantadine in the treatment of Parkinson’s disease. J. Am. Med. Assoc. 1969;208:1168–1170. [PubMed] [Google Scholar]
- 7.Hayden F.G., Gwaltney J.M., Jr., van de Castle R.L., Adams K.F., Giordani B. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrob. Agents Chemother. 1981;19:226–233. doi: 10.1128/aac.19.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherm A., Peteri D. Antiviral 1-(aminoethoxyacetylamino)adamantanes. Chem. Abstr. 1971;74:99516. [Google Scholar]
- 9.Van Derpoorten K., Balzarini J., de Clercq E., Poupaert J.H. Anti-HIV activity of N-1-adamantyl-4-aminophthalimide. Biomed. Pharmacother. 1997;51:464–468. doi: 10.1016/s0753-3322(97)82327-x. [DOI] [PubMed] [Google Scholar]
- 10.Burstein M.E., Serbin A.V., Khakhulina T.V., Alymova I.V., Stotskaya L.L., Bogdan O.P., Manukchina E.E., Jdanov V.V., Sharova N.K. Inhibition of HIV-1 replication by newly developed adamantane-containing polyanionic agents. Antivir. Res. 1999;41:135–144. doi: 10.1016/s0166-3542(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 11.El-Emam A.A., Al-Deeb O.A., Al-Omar M.A., Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Balzarini J., Orzeszko B., Mauri J.K., Orzeszko A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007;42:993–1003. doi: 10.1016/j.ejmech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Sun S.Y., Yue P., Chen X., Hong W.K., Lotan R. The synthetic retinoid CD437 selectively induces apoptosis in human lung cancer cells while sparing normal human lung epithelial cells. Cancer Res. 2002;62:2430–2436. [PubMed] [Google Scholar]
- 14.Wang J.J., Wang S.S., Lee C.F., Chung M.A., Chern Y.T. In vitro antitumor and antimicrobial activity of N-substituents of maliemide by adamantane and diamantane. Chemotherapy. 1997;43:182–189. doi: 10.1159/000239557. [DOI] [PubMed] [Google Scholar]
- 15.Antoniadou-Vyza E., Tsitsa P., Hytiroglou E., Tsantili-Kakoulidou A. New adamantan-2-ol and adamantan-1-methanol derivatives as potent antibacterials, synthesis, antibacterial activity and lipophilicity studies. Eur. J. Med. Chem. 1996;31:105–110. [Google Scholar]
- 16.Omar K., Geronikaki A., Zoumpoulakis P., Camoutsis C., Soković M., Ćirić A., Glamoćlija J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010;18:426–432. doi: 10.1016/j.bmc.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Scherman M.S., North E.J., Jones V., Hess T.N., Grzegorzewicz A.E., Kasagami T., Kim I.H., Merzlikin O., Lenaerts A.J., Lee R.E., et al. Screening a library of 1600 adamantyl ureas for anti-Mycobacterium tuberculosis activity in vitro and for better physical chemical properties for bioavailability. Bioorg. Med. Chem. 2012;20:3255–3262. doi: 10.1016/j.bmc.2012.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Emam A.A., Al-Tamimi A.-M.S., Al-Omar M.A., Al-Rashood K.A., Habib E.E. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 2013;68:96–102. doi: 10.1016/j.ejmech.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Al-Deeb O.A., Al-Omar M.A., El-Brollosy N.R., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]acetic acids, 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]propionic acids and related derivatives. Arzneim.-Forsch. 2006;56:40–47. doi: 10.1055/s-0031-1296699. [DOI] [PubMed] [Google Scholar]
- 20.Kadi A.A., El-Brollosy N.R., Al-Deeb O.A., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007;42:235–242. doi: 10.1016/j.ejmech.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Kadi A.A., Al-Abdullah E.S., Shehata I.A., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010;45:5006–5011. doi: 10.1016/j.ejmech.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Al-Abdullah E.S., Asiri H.H., Lahsasni S., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Drug Des. Dev. Ther. 2014;8:505–518. doi: 10.2147/DDDT.S62465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia L., Tomaszewski J.E., Hanrahan C., Coward L., Noker P., Gorman G., Nikonenko B., Protopopova M. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 2005;144:80–87. doi: 10.1038/sj.bjp.0705984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Protopopova M., Hanrahan C., Nikonenko B., Samala R., Chen P., Gearhart J., Einck L., Nacy C.A. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005;56:968–974. doi: 10.1093/jac/dki319. [DOI] [PubMed] [Google Scholar]
- 25.Bormann J. Memantine is a potent blocker of N-methyl-d-aspartate (NMDA) receptor channels. Eur. J. Pharmacol. 1989;166:591–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Gharbia M.A., Childers W.E., Fletcher H., McGaughey G., Patel U., Webb M.B., Yardley J., Andree T., Boast C., Kucharik R.J., et al. Synthesis and SAR of adatanserin: Novel adamantly aryl- and heteroarylpiperazines with dual serotonin 5-HT1A and 5-HT2 activity as potential anxiolytic and antidepressant agents. J. Med. Chem. 1999;42:5077–5094. doi: 10.1021/jm9806704. [DOI] [PubMed] [Google Scholar]
- 27.Villhauer E.B., Brinkman J.A., Naderi G.B., Burkey B.F., Dunning B.E., Prasad K., Mangold B.L., Russell M.E., Hughes T.E. 1-(3-Hydroxy-1-adamantyl)aminoacetyl-2-cyano-(S)-pyrrolidine: A potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 2003;46:2774–2789. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- 28.Augeri D.J., Robl J.A., Betebenner D.A., Magnin D.R., Khanna A., Robertson J.G., Wang A., Simpkins L.M., Taunk P., Huang Q., et al. Discovery and preclinical profile of saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005;48:5025–5037. doi: 10.1021/jm050261p. [DOI] [PubMed] [Google Scholar]
- 29.Ahrén B., Landin-Olsson M., Jansson P.A., Svensson M., Holmes D., Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:2078–2084. doi: 10.1210/jc.2003-031907. [DOI] [PubMed] [Google Scholar]
- 30.Joharapurkar A., Dhanesha N., Shah G., Kharul R., Jain M. 11β-Hydroxysteroid dehydrogenase type 1: Potential therapeutic target for metabolic syndrome. Pharmacol. Rep. 2012;64:1055–1065. doi: 10.1016/s1734-1140(12)70903-9. [DOI] [PubMed] [Google Scholar]
- 31.Anagnostis P., Katsiki N., Adamidou F., Athyros V.G., Karagiann A., Kita M., Mikhailidis D.P. 11β-Hydroxysteroid dehydrogenase type 1 inhibitors: Novel agents for the treatment of metabolic syndrome and obesity-related disorders. Metabolism. 2013;62:21–33. doi: 10.1016/j.metabol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Olson S., Aster S.D., Brown K., Carbin L., Graham D.W., Hermanowski-Vosatka A., LeGrand C.B., Mundt S.S., Robbins M.A., Schaeffer J.M., et al. Adamantyl triazoles as selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1. Bioorg. Med. Chem. Lett. 2005;15:4359–4362. doi: 10.1016/j.bmcl.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H., Hoffman J., Le P., Nair S.K., Cripps S., Matthews J., Smith C., Yang M., Kupchinsky S., Dress K., et al. The development and SAR of pyrrolidine carboxamide 11β-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2010;20:2897–2902. doi: 10.1016/j.bmcl.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Cott J.S., Choormun J. 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD-1) inhibitors in developmen. In: Jones R.N., editor. New Therapeutic Strategies for Type-2 Diabetes, Small Molecule Approaches. Royal Society Chemistry; Cambridge, UK: 2012. pp. 109–133. [Google Scholar]
- 35.El-Emam A.A., Ibrahim T.M. Synthesis, anti-inflammatory and analgesic activity of certain 3-(1-adamantyl)-4-substituted-5-mercapto-1,2,4-triazole derivatives. Arzneim.-Forsch. 1991;41:1260–1264. [PubMed] [Google Scholar]
- 36.Kouatly O., Geronikaki A., Kamoutsis C., Hadjipavlou-Litina D., Eleftheriou P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009;44:1198–1204. doi: 10.1016/j.ejmech.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 37.Baxter A., Bent J., Bowers K., Braddock M., Brough S., Fagura M., Lawson M., McInally T., Mortimore M., Robertson M., et al. Hit-to-lead studies: The discovery of potent adamantane amide P2X7 receptor antagonists. Bioorg. Med. Chem. Lett. 2003;13:4047–4050. doi: 10.1016/j.bmcl.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 38.Lee W.G., Lee S.D., Cho J.H., Jung Y., Kim J.H., Hien T.T., Kang K.W., Ko H., Kim Y.C. Structure-activity relationships and optimization of 3,5-dichloropyridine derivatives as novel P2X7 receptor antagonists. J. Med. Chem. 2012;55:3687–3698. doi: 10.1021/jm2012326. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y., Wittlin S., Sriraghavan K., Chollet J., Charman S.A., Charman W.N., Scheurer C., Urwyler H., Tomas J.S., Snyder C., et al. The structure-activity relationship of the antimalarial ozonide arterolane (OZ277) J. Med. Chem. 2010;53:481–491. doi: 10.1021/jm901473s. [DOI] [PubMed] [Google Scholar]
- 40.Boder N., Elkoussi A., Zuobi K., Kovacs P. Synthesis and pharmacological activity of adaprolol enantiomers: A new soft drug for treating glaucoma. J. Ocul. Pharmacol. Ther. 1996;12:115–122. doi: 10.1089/jop.1996.12.115. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y., Fujimoto T., Ogawa Y., Namiki H., Suzuki S., Asano M., Sugita C., Mochizuki A., Miyazaki S., Tamaki K., et al. Lead optimization of 5-amino-6-(2,2-dimethyl-5-oxo-4-phenylpiperazin-1-yl)-4-hydroxyhexanamides to reduce a cardiac safety issue: Discovery of DS-8108b, an orally active renin inhibitor. Bioorg. Med. Chem. 2013;21:3175–3196. doi: 10.1016/j.bmc.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 42.El-Emam A.A., Al-Abdullah E.S., Al-Tuwaijri H.M., Said-Abdelbaky M., García-Granda S. 3-(Adamantan-1-yl)-4-ethyl-1-[(4-phenylpiperazin-1-yl)methyl]-1H-1,2,4-triazole-5(4H)-thione. Acta Cryst. 2012;E68:o2380–o2381. doi: 10.1107/S160053681202990X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Emam A.A., Al-Tuwaijri H.M., Al-Abdullah E.S., Chidan Kumar C.S., Fun H.K. 3-(Adamantan-1-yl)-4-ethyl-1-{[(4-(2-methoxyphenylpiperazin-1-yl)]methyl}-1H-1,2,4-triazole-5(4H)-thione. Acta Cryst. 2014;E70:o25–o26. doi: 10.1107/S1600536813032789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Abdullah E.S., Al-Tuwaijri H.M., El-Emam A.A., Chidan Kumar C.S., Fun H.K. 3-(Adamantan-1-yl)-1-[(4-benzylpiperazin-1-yl)]methyl]-4-ethyl-1H-1,2,4-triazole-5(4H)-thione. Acta Cryst. 2013;E69:o1813–o1814. doi: 10.1107/S1600536813031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods G.L., Washington J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In: Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H., editors. Manual of Clinical Microbiology. American Society of Microbiology; Washington, DC, USA: 1995. [Google Scholar]
- 46.Approved Standard Document M-7A. NCCLS; Villanova, PA, USA: 1985. National Committee for Clinical Laboratory Standards. [Google Scholar]
- 47.Rossini A.A., Like A.A., Chick W.L., Appel M.C., Cahill G.F. Studies of streptozotocin-induced insulitis and diabetes. Proc. Nat. Accad. Sci. USA. 1977;74:2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olajide O.A., Awe S.O., Makinde J.M., Morebise O. Evaluation of the antidiabetic property of Morinda lucida leaves in streptozotocin diabetes rats. J. Pharm. Pharmacol. 1999;51:1321–1324. doi: 10.1211/0022357991776903. [DOI] [PubMed] [Google Scholar]
- 49.Litchfield J.T., Wilcoxon T. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99–104. [PubMed] [Google Scholar]
- 50.Serradas P., Bailbe D., Portha B. Long-term gIiclazide treatment improves the in vitro glucose-induced insulin release in rats with type 2 (non-insulin-dependent) diabetes induced by neonatal streptozotocin. Diabetologia. 1989;55:55–57. doi: 10.1007/BF00285331. [DOI] [PubMed] [Google Scholar]