Abstract
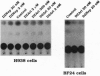
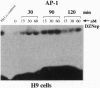
3-Deazaadenosine (DZA), 3-deaza-(+/-)-aristeromycin (DZAri), and 3-deazaneplanocin A (DZNep) are powerful modulators of cellular processes. When tested against H9 cells infected acutely with two different strains of human immunodeficiency virus 1 (HIV-1) and in the chronically infected monocytoid cell lines U1 and THP-1, the 3-deazanucleosides caused a marked reduction in p24 antigen production. Similar reductions in p24 antigen were seen in phytohemagglutinin-stimulated peripheral blood mononuclear cells infected with clinical HIV-1 isolates. Strikingly, in comparing the therapeutic indices between the paired pre- and post-3'-azido-3'-deoxythymidine (AZT) treatment HIV-1 isolates, DZNep and neplanocin A showed an increase of 3- to 18-fold in their potency against AZT-resistant HIV-1 isolates. In H9 cells treated with DZNep and DZAri, the formation of triphosphate nucleotides of DZNep and DZAri was observed. The mode of action of DZNep and DZAri appears complex, at least in part, at the level of infectivity as shown by decreases in syncytia formation in HIV-1-infected H9 cells and at the level of transcription as both drugs inhibited the expression of basal or tat-induced HIV-1 long terminal repeat chloramphenicol acetyltransferase activity in stably transfected cell lines. Since DZNep induced in H9 cells a rapid expression of nuclear binding factors that recognize the AP-1 transcription site, the anti-HIV-1 activity of the DZA analogs could partly be the induction of critical factors in the host cells. Thus, the 3-deazanucleoside drugs belong to an unusual class of anti-HIV-1 drugs, which may have therapeutic potential, in particular against AZT-resistant strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarbakke J., Miura G. A., Prytz P. S., Bessesen A., Slørdal L., Gordon R. K., Chiang P. K. Induction of HL-60 cell differentiation by 3-deaza-(+/-)-aristeromycin, an inhibitor of S-adenosylhomocysteine hydrolase. Cancer Res. 1986 Nov;46(11):5469–5472. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Carotti D., Cantoni G. L. Effects of the S-adenosylhomocysteine hydrolase inhibitors 3-deazaadenosine and 3-deazaaristeromycin on RNA methylation and synthesis. Eur J Biochem. 1986 Oct 15;160(2):245–251. doi: 10.1111/j.1432-1033.1986.tb09963.x. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Brown N. R., Chiang P. K., Cantoni G. L. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology. 1978 Sep;89(2):494–505. doi: 10.1016/0042-6822(78)90191-5. [DOI] [PubMed] [Google Scholar]
- Bennett L. L., Jr, Brockman R. W., Allan P. W., Rose L. M., Shaddix S. C. Alterations in nucleotide pools induced by 3-deazaadenosine and related compounds. Role of adenylate deaminase. Biochem Pharmacol. 1988 Apr 1;37(7):1233–1244. doi: 10.1016/0006-2952(88)90776-9. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Burbelo P. D., Brugh S. A., Gordon R. K., Fukuda K., Yamada Y. Activation of collagen IV gene expression in F9 teratocarcinoma cells by 3-deazaadenosine analogs. Indirect inhibitors of methylation. J Biol Chem. 1992 Mar 5;267(7):4988–4991. [PubMed] [Google Scholar]
- Chiang P. K. Conversion of 3T3-L1 fibroblasts to fat cells by an inhibitor of methylation: effect of 3-deazaadenosine. Science. 1981 Mar 13;211(4487):1164–1166. doi: 10.1126/science.7466386. [DOI] [PubMed] [Google Scholar]
- Flexner C. W., Hildreth J. E., Kuncl R. W., Drachman D. B. 3-Deaza-adenosine and inhibition of HIV. Lancet. 1992 Feb 15;339(8790):438–438. doi: 10.1016/0140-6736(92)90133-n. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Schnittman S., Orenstein J., Poli G., Fauci A. S. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988 Feb 15;140(4):1117–1122. [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Glazer R. I., Hartman K. D., Knode M. C., Richard M. M., Chiang P. K., Tseng C. K., Marquez V. E. 3-Deazaneplanocin: a new and potent inhibitor of S-adenosylhomocysteine hydrolase and its effects on human promyelocytic leukemia cell line HL-60. Biochem Biophys Res Commun. 1986 Mar 13;135(2):688–694. doi: 10.1016/0006-291x(86)90048-3. [DOI] [PubMed] [Google Scholar]
- Japour A. J., Mayers D. L., Johnson V. A., Kuritzkes D. R., Beckett L. A., Arduino J. M., Lane J., Black R. J., Reichelderfer P. S., D'Aquila R. T. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. The RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Chemother. 1993 May;37(5):1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. A., Merrill D. P., Chou T. C., Hirsch M. S. Human immunodeficiency virus type 1 (HIV-1) inhibitory interactions between protease inhibitor Ro 31-8959 and zidovudine, 2',3'-dideoxycytidine, or recombinant interferon-alpha A against zidovudine-sensitive or -resistant HIV-1 in vitro. J Infect Dis. 1992 Nov;166(5):1143–1146. doi: 10.1093/infdis/166.5.1143. [DOI] [PubMed] [Google Scholar]
- Lacey S. F., Reardon J. E., Furfine E. S., Kunkel T. A., Bebenek K., Eckert K. A., Kemp S. D., Larder B. A. Biochemical studies on the reverse transcriptase and RNase H activities from human immunodeficiency virus strains resistant to 3'-azido-3'-deoxythymidine. J Biol Chem. 1992 Aug 5;267(22):15789–15794. [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Li B. Q., Fu T., Yan Y. D., Baylor N. W., Ruscetti F. W., Kung H. F. Inhibition of HIV infection by baicalin--a flavonoid compound purified from Chinese herbal medicine. Cell Mol Biol Res. 1993;39(2):119–124. [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wolfe M. S., Borchardt R. T. Rational approaches to the design of antiviral agents based on S-adenosyl-L-homocysteine hydrolase as a molecular target. Antiviral Res. 1992 Sep;19(3):247–265. doi: 10.1016/0166-3542(92)90083-h. [DOI] [PubMed] [Google Scholar]
- Medzihradsky J. L., Zimmerman T. P., Wolberg G., Elion G. B. Immunosuppressive effects of the S-adenosylhomocysteine hydrolase inhibitor, 3-deazaadenosine. J Immunopharmacol. 1982;4(1-2):29–41. doi: 10.3109/08923978209031073. [DOI] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikovits J. A., Raziuddin, Gonda M., Ruta M., Lohrey N. C., Kung H. F., Ruscetti F. W. Negative regulation of human immune deficiency virus replication in monocytes. Distinctions between restricted and latent expression in THP-1 cells. J Exp Med. 1990 May 1;171(5):1705–1720. doi: 10.1084/jem.171.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Yarchoan R., Broder S. Molecular targets for AIDS therapy. Science. 1990 Sep 28;249(4976):1533–1544. doi: 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- Montgomery J. A., Clayton S. J., Thomas H. J., Shannon W. M., Arnett G., Bodner A. J., Kion I. K., Cantoni G. L., Chiang P. K. Carbocyclic analogue of 3-deazaadenosine: a novel antiviral agent using S-adenosylhomocysteine hydrolase as a pharmacological target. J Med Chem. 1982 Jun;25(6):626–629. doi: 10.1021/jm00348a004. [DOI] [PubMed] [Google Scholar]
- Oxenrider K. A., Bu G., Sitz T. O. Adenosine analogs inhibit the guanine-7-methylation of mRNA cap structures. FEBS Lett. 1993 Feb 1;316(3):273–277. doi: 10.1016/0014-5793(93)81307-l. [DOI] [PubMed] [Google Scholar]
- Prus K. L., Wolberg G., Keller P. M., Fyfe J. A., Stopford C. R., Zimmerman T. P. 3-Deazaadenosine 5'-triphosphate: a novel metabolite of 3-deazaadenosine in mouse leukocytes. Biochem Pharmacol. 1989 Feb 1;38(3):509–517. doi: 10.1016/0006-2952(89)90392-4. [DOI] [PubMed] [Google Scholar]
- Raziuddin, Mikovits J. A., Calvert I., Ghosh S., Kung H. F., Ruscetti F. W. Negative regulation of human immunodeficiency virus type 1 expression in monocytes: role of the 65-kDa plus 50-kDa NF-kappa B dimer. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9426–9430. doi: 10.1073/pnas.88.21.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Bomford R., Gao X. M., Rhodes J. 3-Deazaadenosine--an inhibitor of interleukin 1 production by human peripheral blood monocytes. Int J Immunopharmacol. 1990;12(1):89–97. doi: 10.1016/0192-0561(90)90071-t. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Fenyö E. M., Pavlakis G. N. Rapidly and slowly replicating human immunodeficiency virus type 1 isolates can be distinguished according to target-cell tropism in T-cell and monocyte cell lines. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7200–7203. doi: 10.1073/pnas.86.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Johnson J. A., Turner R. A. Biochemical perturbations of BW 91Y (3-deazaadenosine) on human neutrophil chemotactic potential and lipid metabolism. Int J Tissue React. 1991;13(1):1–18. [PubMed] [Google Scholar]
- Tseng C. K., Marquez V. E., Fuller R. W., Goldstein B. M., Haines D. R., McPherson H., Parsons J. L., Shannon W. M., Arnett G., Hollingshead M. Synthesis of 3-deazaneplanocin A, a powerful inhibitor of S-adenosylhomocysteine hydrolase with potent and selective in vitro and in vivo antiviral activities. J Med Chem. 1989 Jul;32(7):1442–1446. doi: 10.1021/jm00127a007. [DOI] [PubMed] [Google Scholar]
- Wiesmann W. P., Johnson J. P., Miura G. A., Chaing P. K. Aldosterone-stimulated transmethylations are linked to sodium transport. Am J Physiol. 1985 Jan;248(1 Pt 2):F43–F47. doi: 10.1152/ajprenal.1985.248.1.F43. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]